Abstract
The hallmark of idiopathic pulmonary fibrosis (IPF) is the myofibroblast, the cellular origin of which in the lung is unknown. We hypothesized that alveolar epithelial cells (AECs) may serve as a source of myofibroblasts through epithelial-mesenchymal transition (EMT). Effects of chronic exposure to transforming growth factor (TGF)-β1 on the phenotype of isolated rat AECs in primary culture and a rat type II cell line (RLE-6TN) were evaluated. Additionally, tissue samples from patients with IPF were evaluated for cells co-expressing epithelial (thyroid transcription factor (TTF)-1 and pro-surfactant protein-B (pro-SP-B), and mesenchymal (α-smooth muscle actin (α-SMA)) markers. RLE-6TN cells exposed to TGF-β1 for 6 days demonstrated increased expression of mesenchymal cell markers and a fibroblast-like morphology, an effect augmented by tumor necrosis factor-α (TNF-α). Exposure of rat AECs to TGF-β1 (100 pmol/L) resulted in increased expression of α-SMA, type I collagen, vimentin, and desmin, with concurrent transition to a fibroblast-like morphology and decreased expression of TTF-1, aquaporin-5 (AQP5), zonula occludens-1 (ZO-1), and cytokeratins. Cells co-expressing epithelial markers and α-SMA were abundant in lung tissue from IPF patients. These results suggest that AECs undergo EMT when chronically exposed to TGF-β1, raising the possibility that epithelial cells may serve as a novel source of myofibroblasts in IPF.
Idiopathic pulmonary fibrosis (IPF) is a progressive disease of unknown etiology that continues to be associated with considerable morbidity and mortality.1 Histopathologically, IPF typically shows a pattern of usual interstitial pneumonia, the cardinal features of which are patchy established fibrosis with variable numbers of fibroblastic foci, interspersed with areas of normal or nearly normal lung. IPF is also characterized by a number of minor features, one of which is alveolar epithelial type II (AT2) cell hyperplasia.2 Although initial studies on fibrogenesis focused on the role of inflammation in inciting fibroblast activation and fibrosis, the current paradigm suggests a central role of the epithelium in disease pathogenesis and progression.3,4 Many IPF patients show little evidence of ongoing inflammation, and treatment focused on inhibition of inflammation (eg, steroids and/or immunosuppressive drugs) has had little impact on the disease.1 It has therefore been proposed that IPF is instead the result of epithelial injury and subsequent dysregulated repair, with epithelial cell activation and damage resulting in cytokine release, excess extracellular matrix deposition, and abnormal mesenchymal cell activation and proliferation.3,4
Transforming growth factor-β1 (TGF-β1) is critical to the progression of fibrosis in fibroproliferative acute respiratory distress syndrome (ARDS) and IPF. Knockout mice lacking the integrin αvβ6 are unable to locally activate latent TGF-β1 and are completely protected from pulmonary fibrosis induced by bleomycin.5 Lung fibrosis due to bleomycin was blocked in mice lacking the TGF-β1-dependent Smad3 signaling pathway.6 Transient overexpression of TGF-β1 in rat lung using adenovirus resulted in prolonged and severe pulmonary fibrosis.7 In addition, alveolar macrophages and epithelial cells from patients with IPF and fibroproliferative ARDS express TGF-β1, and it has been found in significant concentrations in bronchoalveolar lavage fluid and serum of patients with these diseases.8–10 TGF-β1 can also cause dramatic changes in morphology and phenotype in many cell types.11
Myofibroblasts are prominent components of fibrosis in many tissues, including the lung.12,13 Myofibroblasts have long been assumed to arise through transdifferentiation of resident pulmonary fibroblasts or migration of blood-borne fibroblasts to areas of fibrosis or injury with subsequent transdifferentiation.14 The possibility that alveolar epithelial cells (AECs) undergo transition toward a myofibroblast phenotype as a result of TGF-β1-induced transdifferentiation has not previously been evaluated.
To investigate this possibility, an AT2 cell line, RLE-6TN, and isolated alveolar epithelial type II (AT2) cells were treated with TGF-β1 for up to 12 days and sub-sequently analyzed for transition toward a myofibro-blast-like phenotype, as determined by morphological changes and expression of mesenchymal markers. Reciprocal loss of epithelial characteristics was evaluated. Combined treatment with tumor necrosis factor-α (TNF-α) was also investigated, because this cytokine potentiates or initiates fibrosis in a number of tissues, including lung.15 Tissue samples from patients with IPF were analyzed for cellular co-expression of epithelial and mesenchymal markers. We demonstrate for the first time using an AT2 cell line, primary AECs, and in vivo specimens that AECs undergo epithelial-mesenchymal transition (EMT), suggesting the possibility that AECs serve as a source of myofibroblasts in IPF.
Materials and Methods
Maintenance and Culture of RLE-6TN Cells
RLE-6TN cells constitute a cell line derived from rat AT2 cells that was obtained from American Type Culture Collection (Manassas, VA). Cells were grown on chamber slides (Falcon; Becton Dickson, Franklin Lakes, NJ) in Dulbecco’s Modified Eagle’s medium, nutrient mixture F-12 Ham supplemented with 10% fetal bovine serum, 40 mmol/L HEPES, 100 U/ml penicillin G, and 100 μg/ml streptomycin. Cells were cultured from the time of plating in medium alone, medium supplemented with either 100 pmol/L (2.5 ng/ml) TGF-β1 or 1 ng/ml recombinant rat TNF-α (R&D Systems, Minneapolis, MN), or medium supplemented with TGF-β1 and TNF-α. The dosage of TGF-β1 was chosen on the basis of previous studies and known physiological levels.16 Cultures were maintained in a humidified 5% CO2 incubator at 37°C, and media were changed every 2 to 3 days.
Cell Isolation and Preparation of AEC Monolayers
AT2 cells were isolated from adult male Sprague-Dawley rats (∼125 to 150 g) by disaggregation with elastase (2.0 to 2.5 U/ml; Worthington Biochemical, Freehold, NJ), followed by differential adherence on IgG-coated bacteriological plates.17 All animals were treated in accordance with the guidelines of and with the approval of the University of Southern California Institutional Animal Care and Use Committee. Freshly isolated AT2 cells were plated in a minimal defined serum-free medium (MDSF)17 on 1.1-cm2 tissue culture-treated polycarbonate (Nucleopore) filter cups (Transwell; Corning Costar, Cambridge, MA). For the first 24 to 48 hours of culture, media were supplemented with 100 μg/ml cis-OH-proline (Sigma, St. Louis, MO) to selectively eliminate fibroblasts from cultures.18 Media were then removed, and cells were subsequently maintained for up to 12 additional days (for a total of 14 days) in MDSF or in MDSF supplemented with 100 pmol/L (2.5 ng/ml) recombinant human TGF-β1 (R&D Systems) ± 1 ng/ml TNF-α in apical and basolateral compartments. Equivalent amounts of vehicle for each supplement were added to unsupplemented cultures. The maximum volume of vehicle, TGF-β1, TNF-α, or cis-OH-proline added to media was 1 μl/ml. Cultures were maintained in a humidified 5% CO2 incubator at 37°C. Media were changed every 2 to 3 days. In one cell preparation, to further exclude the possibility of fibroblast contamination, AT2 cells were further purified by incubation with an antibody (Ab) to lamellar membranes, p180 (Covance, Princeton, NJ), followed by incubation with a secondary Ab conjugated to magnetic beads to yield AT2 cells of 97% purity.
Antibodies
The following Abs were used in immunofluorescence, immunohistochemistry, and Western analyses: mesenchymal cell markers included mouse monoclonal anti-α-smooth muscle actin (Sigma), anti-vimentin (Research Diagnostics, Flanders, NJ), anti-desmin (Becton Dickson Biosciences, San Diego, CA), and rabbit polyclonal anti-collagen I (which recognizes two bands near 115 kd) (Research Diagnostics) Abs; epithelial cell markers included rabbit polyclonal anti-ZO-1 (Zymed Laboratories, South San Francisco, CA) and anti-aquaporin-5 (AQP5) (Chemicon, Temecula, CA), mouse monoclonal anti-pan-cytokeratin (which recognizes cytokeratins 4 to 6, 8, 10, 13, and 18) (Sigma), anti-thyroid transcription factor-1 (TTF-1) (Novocastra Laboratories, Newcastle, UK), and anti-pro-surfactant protein B (pro-SP-B) (Neomarkers, Fremont, CA). Controls included rabbit or mouse IgG at equivalent concentrations.
Western Analyses
AEC monolayers were lysed in 2% SDS sample buffer at 37°C for 15 minutes. Equivalent amounts of protein (5–10 μg) were extracted from control and treated cultures, resolved by SDS-PAGE under reducing conditions using the buffer system of Laemmli,19 and electrophoretically blotted onto Immobilon-P nylon membranes (Millipore) using procedures modified from Towbin et al.20 Eukaryotic initiation factor (eIF) was used as a loading control for all blots, and densitometric analyses were adjusted to account for relative differences in eIF intensity. Protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA) with bovine serum albumin (BSA) as standard. Membranes were blocked overnight at 4°C with 5% nonfat dry milk in Tris-buffered saline (TBS) with 0.1% Tween at pH 7.5. They were then incubated for 2 hours at room temperature (RT) with primary Ab. Finally, blots were incubated with horseradish peroxidase (HRP)-linked anti-mouse or anti-rabbit IgG conjugates for 1 hour at RT. Ab complexes were visualized by enhanced chemiluminescence (Amer-sham, Arlington Heights, IL). Blots were scanned using a Powerlook III color scanner (Umax, Fremont, CA) and Photoperfect 4.4 software (Binuscan, Monte Carlo, Monaco) or directly exposed using a photoimager (Alpha Innotech, San Leandro, CA). Images were analyzed using Photoshop 5.5 (Adobe Systems, San Jose, CA) and Un-Scan-It Gel 4.3 (Silk Scientific Corporation, Orem, UT).
Immunofluorescence
RLE-6TN cells and AEC monolayers maintained in media ± cytokines as detailed above were harvested on days 6 and 14, respectively. Filters or slides were rinsed once with ice-cold phosphate-buffered saline and fixed with 2% paraformaldehyde for 10 minutes at RT, followed by 0.2% Triton X-100 for 10 minutes at RT. After rinsing in phosphate-buffered saline and blocking with 5% BSA at RT for 1 hour, monolayers were incubated with the appropriate primary Ab overnight at 4°C. After extensive washing and blocking with 5% BSA at RT for 30 minutes, filters or slides were incubated with appropriate secondary Abs conjugated to fluorescein isothiocyanate (FITC), rinsed again, and postfixed in 3.7% formalin. They were then treated with Vectashield antifade mounting medium with propidium iodide to stain nuclei (Vector Laboratories, Burlingame, CA) and viewed with an Olympus BX60 microscope equipped with epifluorescence op-tics (Olympus, Melville, NY). Images were captured with a cooled charge-coupled device camera (Magnafire; Olympus) with a barrier filter equipped for simultaneous detection of FITC and propidium iodide. Captured images were imported into Adobe Photoshop (Adobe Systems, San Jose, CA) as TIFF files.
Double-Label Immunofluorescence Time Course in AEC Monolayers
To track changes in cell phenotype over time, AEC monolayers maintained in media ± cytokines as described above were harvested for double-label immunofluorescence on days 6, 8, and 10 in culture. Filters were rinsed and fixed as described above and then blocked using CAS block (Zymed) for 30 minutes at RT. They were then incubated with the first primary Ab (monoclonal anti-α-SMA) overnight at 4°C. After extensive rinsing, monolayers were incubated with biotinylated anti-mouse IgG diluted in CAS blocking solution for 40 minutes at RT. After rinsing again, they were incubated with cell sorter grade fluorescein avidin D conjugate (Vector) for 5 minutes at RT. Antigen retrieval was then performed by heating the filters in an antigen-unmasking solution (Vector), cooling slowly, and rinsing. Monolayers were repermeabilized by incubating in 0.2% Triton X for 10 minutes. Blocking of residual avidin-biotin was performed using an avidin-biotin blocking kit (Vector), followed by a protein block using CAS blocking solution. The second primary Ab (monoclonal anti-TTF-1) was then added, and monolayers were incubated for 2 hours at RT. After extensive rinsing, the monolayers were incubated with biotinylated anti-mouse IgG for 40 minutes at RT. This was followed by incubation at RT for 10 minutes with Cy3-Strepavidin conjugate (Jackson Immunoresearch, West Grove, PA). Filters were then rinsed, mounted on slides using Vectashield mounting medium (without propidium iodide), and coverslipped. Viewing and image capture were performed as described above.
Immunohistochemistry
Paraffin-embedded lung tissue samples from human subjects who fulfilled current clinical criteria for IPF were obtained for immunohistochemical cellular colocalization of epithelial and mesenchymal markers. All patients showed a histological pattern of usual interstitial pneumonia. Samples were fixed and inflated with formalin via injection into the parenchyma. Approval for analysis of archived tissue samples was obtained from the University of Southern California and Royal Brompton Hospital Institutional Review Boards. Tissue was deparaffinized and rehydrated using sequential xylene and ethanol rinses, rinsed with TBS, blocked with CAS block solution (Zymed), and incubated overnight with primary Ab. After washing again in TBS, slides were incubated in 3% H2O2 in TBS for 10 minutes and reacted with biotinylated anti-mouse, rat-adsorbed IgG (Vector) in CAS blocking solution at RT for 40 minutes. After rinsing with TBS, slides were incubated with avidin biotin complex (ABC)-HRP solution (Vectastain HRP Elite ABC, Vector) for 40 minutes at RT. Slides were rinsed and then reacted with 3,3′-diaminobenzidine in H2O2. Color development was stopped with deionized water after 2 to 5 minutes. Slides were then washed and placed in antigen retrieval solution (Vector), heated, and allowed to cool. Slides were then blocked using an avidin-biotin blocking kit (Vector) and CAS solution. Tissue was reacted with the second primary Ab overnight at 4°C. Slides were then washed with TBS with 0.1% Tween and incubated with biotinylated anti-rabbit Ab (Vector) in CAS blocking solution at RT for 40 minutes. After rinsing, slides were incubated in ABC-alkaline phosphatase solution (Vectastain AP Elite ABC, Vector) for 40 minutes at RT. Slides were rinsed and reacted with Fast Red, and the reaction was stopped with deionized water on color development. Tissue was then covered with Crystal Mount and coverslipped. Slides were viewed using Normarsky differential interference contrast microscopy with an Olympus BX60 microscope, and images were captured with a cooled charge-coupled device camera (Magnafire; Olympus). Captured images were imported into Adobe Photoshop (Adobe Systems) as TIFF files. For quantification, 20 high-power fields were examined for each tissue slide for each patient (n = 3). The total number of cells positive for epithelial markers (either pro-SPB or TTF-1) was counted, as well as the number of cells in each field positive for α-SMA and an epithelial marker. The percentage of cells positive for both markers was then expressed as a percentage of the total number of epithelial cells.
Three-Dimensional Deconvolution Microscopy
Using a Zeiss Axiovert 100M microscope equipped with a computer-controlled z-focus drive and a ×100 oil objective with a numerical aperture of 1.4, serial optical sections were acquired at 0.1-μm intervals over a total 3-μm thickness of single cells from five separate fields from each patient (n = 3). Resultant image stacks were deconvolved using an inverse filter method suitable for the analysis of transmitted light brightfield images21 (AutoDeblur 3D 9.3; AutoQuant, Watervliet, NY). Processing was performed with the following settings: modality, transmitted light brightfield; lens NA, 1.4; refractive index, 1.515 (oil); image spacing, 0.067 μm/pixel; low noise level; phase content, expected.
Statistical Analyses
Results of densitometric analyses are expressed as means ± SEM. Significance (P < 0.01) of differences between relative densities was determined by unpaired Student’s t-test.
Results
Effects of TGF-β1 on Cell Morphology and Expression of α-SMA in RLE-6TN Cells
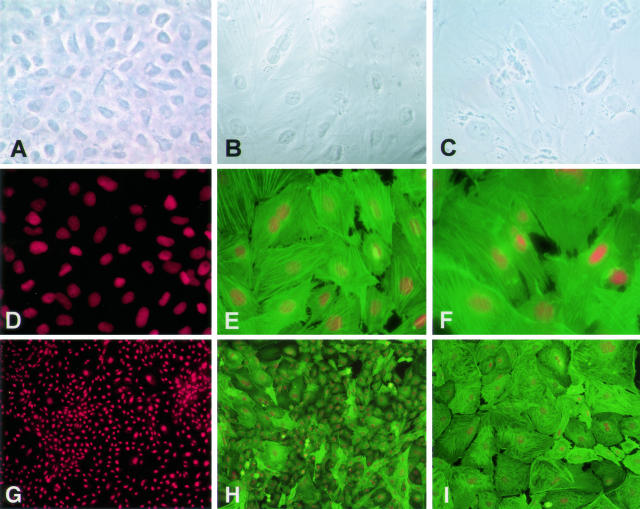
Using indirect immunofluorescence and phase imaging, changes in cell morphology and expression of α-SMA were seen after treatment of RLE-6TN cells with TGF-β1 and TGF-β1 + TNF-α (Figure 1). RLE-6TN cells grown for 6 days in growth media ± TNF-α only had scant α-SMA expression and exhibited an epithelioid, cobblestone appearance (Figure 1, A, D, and G). Cells grown in the presence of TGF-β1 were mixed in phenotype, with large numbers of α-SMA-positive cells with a fibroblast-like morphology, but also some α-SMA-negative, epithelial-like cells (Figure 1, B, E, and H). In the presence of TGF-β1 + TNF-α, these effects were much more marked than those in the presence of TGF-β1 alone. Cells exhibited a homogeneous fibroblast-like morphology, and all expressed high levels of fibril-associated α-SMA (Figure 1, C, F, and I).
Figure 1.
Effects of TGF-β1 ± TNF-α on cell morphology and expression of α-SMA in RLE-6TN cells. Immunoreactivity for α-SMA (green) was assessed by immunofluorescence on day 6, and accompanying phase images of cell morphology were obtained. Expression of α-SMA and assumption of a fibroblast-like morphology was induced in monolayers exposed to TGF-β1 and was seen in 100% of cells in cultures treated with TGF-β1 and TNF-α. A: Phase image of monolayer in media only. B: Phase image of monolayer in media + TGF-β1. C: Phase image of monolayer in media + TGF-β1 + TNF-α. D and G: Monolayer in media only reacted with anti-α-SMA mAb. E and H: Monolayer in media + TGF-β1 reacted with anti-α-SMA mAb. F and I: Monolayer in media + TGF-β1 + TNF-α reacted with anti-α-SMA mAb. Red staining represents propidium iodide-stained nuclei. Photographs are representative of >12 cultures from more than three separate experiments. Original magnification, ×400 for A through F; ×100 for G through I.
Effects of TGF-β1 on Expression of Mesenchymal Cell Markers in Primary AEC Monolayers
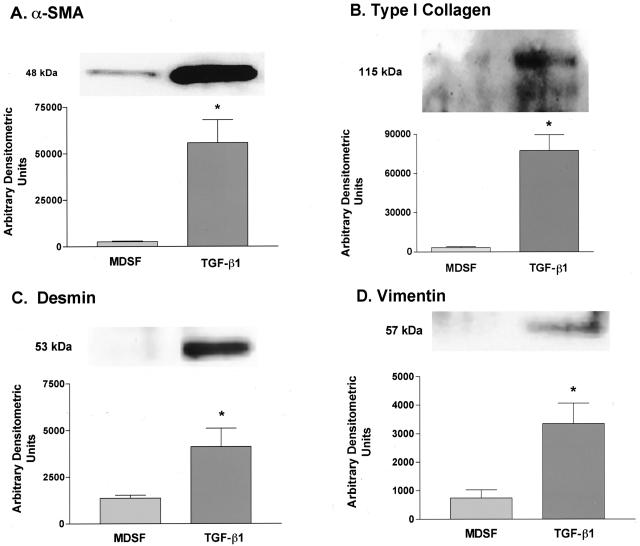
Protein was harvested from adherent AECs cultivated in MDSF ± TGF-β1 and was analyzed by Western blotting. As shown in Figure 2, expression of four mesenchymal cell-specific markers increased significantly with chronic (12-day) exposure to TGF-β1. α-SMA was not expressed by freshly isolated AT2 cells (data not shown) and was increased 21-fold compared with that of untreated cultures on day 14 (Figure 2A). Low-level expression of α-SMA was noted in untreated cells on day 14. Type I collagen (Figure 2B), desmin (Figure 2C), and vimentin (Figure 2D) expression was also increased relative to untreated cells (by 27-fold, threefold, and 4.5-fold, respectively) on day 14.
Figure 2.
Effects of TGF-β1 on expression of mesenchymal cell markers in AECs. Representative Western blots (n = 4 for α-SMA; n = 3 for type I collagen, vimentin, and desmin) and densitometric analyses demonstrate increases in expression of α-SMA, type I collagen, vimentin, and desmin on day 14 in AECs treated with TGF-β1 for 12 days. eIF was used as a loading control for all blots, and densitometric analyses were adjusted to account for relative differences in eIF intensity (data not shown). A: α-SMA expression increased 21-fold over untreated cultures on day 14 in treated AECs. B: Type I collagen expression increased 27-fold over untreated cultures on day 14 in treated AECs. C: Desmin expression increased threefold over untreated cultures on day 14 in treated AECs. D: Vimentin expression increased 4.5-fold over untreated cultures on day 14 in treated AECs. *Significantly different from MDSF.
Effects of TGF-β1 on Expression of Epithelial Markers in AEC Monolayers
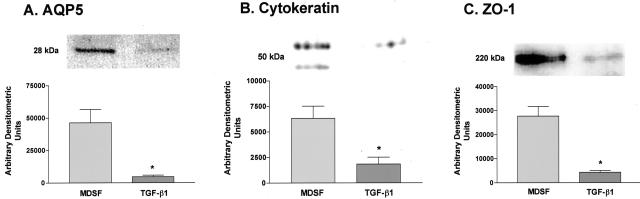
Expression of AQP5, a type I (AT1) cell-specific marker expressed at high levels in AECs in serum-free culture after 4 to 6 days (AT1-like cells), was reduced to about 10% of that in untreated cultures after TGF-β1 treatment (Figure 3A). Expression of two other epithelial cell markers, cytokeratin (Figure 3B) and ZO-1 (Figure 3C), was also markedly reduced on day 14 (to 29 and 6% of untreated cultures, respectively).
Figure 3.
Effects of TGF-β1 on AEC expression of epithelial cell markers. Representative Western blots (n = 4 for AQP5, n = 3 for pancytokeratin and ZO-1) and densitometric analyses demonstrate decreases in expression of AQP5, pancytokeratin, and ZO-1 on day 14 in AECs treated with TGF-β1 for 12 days. eIF was used as a loading control for all blots, and densitometric analyses were adjusted to account for relative differences in eIF intensity (data not shown). Expression of AQP5 (A), cytokeratins (B), and ZO-1 (C) decreased to about 10, 29, and 6% of untreated levels, respectively. *Significantly different from MDSF.
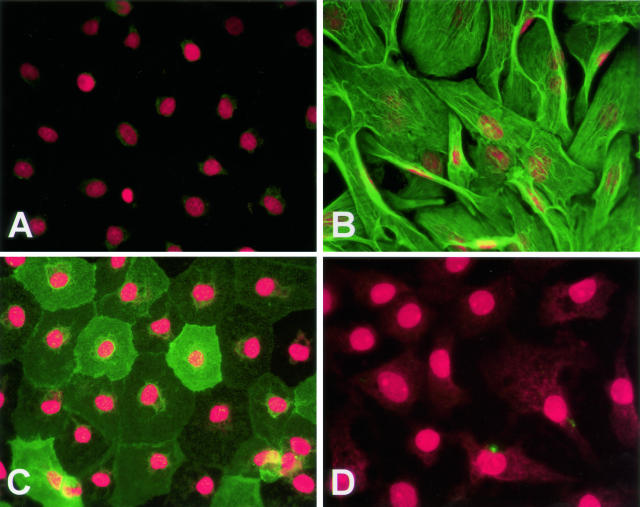
Effects of TGF-β1 on AEC Morphology and Cellular Expression of α-SMA and AQP5
Indirect immunofluorescence was performed to compare cellular morphology and to localize α-SMA and AQP5 on day 14 in culture. AECs cultured in MDSF showed no immunoreactivity for α-SMA and expressed high levels of AQP5, while retaining an epithelial phenotype (Figure 4, A and C). AECs exposed to TGF-β1 for 12 days assumed a fibroblast-like morphology with intense staining for α-SMA and lost any detectable expression of AQP5 (Figure 4, B and D). α-SMA staining occurred in association with intracellular fibrils. In the presence of TGF-β1 + TNF-α, these effects were more marked than those in the presence of TGF-β1 alone (data not shown).
Figure 4.
Effects of TGF-β1 on AEC morphology and associated expression of α-SMA and AQP5. Immunoreactivity for α-SMA (green, A and B) and AQP5 (green, C and D) was assessed by immunofluorescence on day 14. Expression of α-SMA was undetectable in untreated monolayers but was strongly expressed in a fibril-associated pattern in TGF-β1-treated cultures. AQP5 was highly expressed in untreated cultures but was undetectable in monolayers exposed to TGF-β1. A: Monolayer in MDSF reacted with anti-α-SMA mAb. B: Monolayer in MDSF + TGF-β1 reacted with anti-α-SMA mAb. C: Monolayer in MDSF reacted with anti-AQP5 polyclonal Ab. D: Monolayer in MDSF + TGF-β1 reacted with anti-AQP5 polyclonal Ab. Control slides using mIgG revealed no significant staining (not shown). Red staining represents propidium iodide-stained nuclei. Photographs are representative of >12 monolayers from more than five separate experiments. Original magnification, ×400.
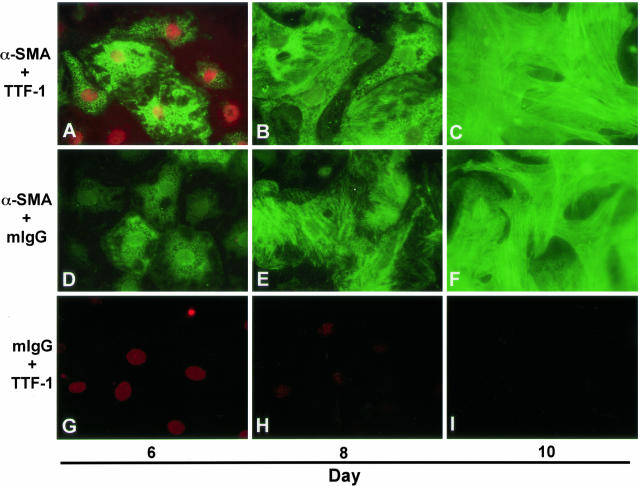
Colocalization of TTF-1 and α-SMA in Primary AEC Monolayers over Time
To assure that AECs and not contaminating mesenchymal cells are the primary contributors to the myofibroblast-like cells seen after 12 days of culture, AEC monolayers exposed to TGF-β1 (2.5 ng/ml) and TNF-α (1 ng/ml) were harvested on days 6, 8, and 10 of culture for double immunofluorescence labeling with TTF-1 and α-SMA. Expression of TTF-1, an epithelial-specific transcription factor, was robust and localized to the nucleus on day 6, but faded by day 10 (Figure 5, A to C and G to I). α-SMA was co-expressed in the cytoplasm of cells expressing TTF-1 on day 6 and grew in intensity over 4 days, forming intracellular fibrils concomitant with a transition in cell morphology to a fibroblast-like phenotype (Figure 5, A to C and D to F).
Figure 5.
Colocalization of α-SMA and TTF-1 in primary AECs during epithelial-mesenchymal transition. Immunoreactivity for α-SMA (green) and TTF-1 (red) was assessed on days 6, 8, and 10 of primary culture of AECs in MDSF + TGF-β1 + TNF-α. On day 6, individual AECs were identified that co-express nuclear TTF-1 and cytoplasmic α-SMA. Expression of α-SMA increased gradually over time in culture (A to C, D to F) and paralleled a concomitant decrease in expression of TTF-1 (A to C, G to I) along with a transition from an epithelial to a fibroblast-like morphology. Photographs are representative of >12 monolayers from more than three separate experiments. Original magnification, ×600.
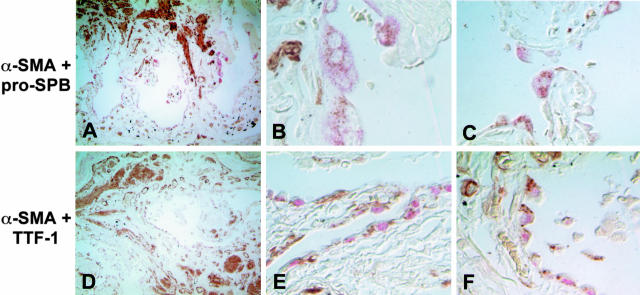
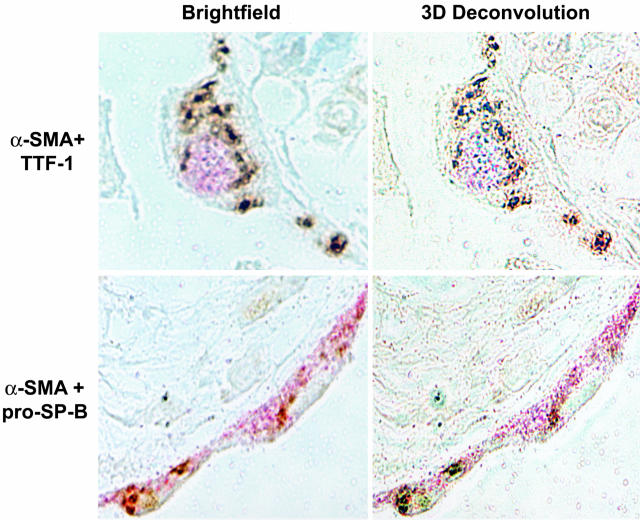
Co-Expression of AEC and Mesenchymal Cell Markers in Vivo
Paraffin-embedded, archived lung tissue samples from three patients with advanced IPF were examined. Double-label immunohistochemical staining for α-SMA and the AT2 cell markers TTF-1 and pro-SP-B was performed. Low-power images revealed severe destruction of lung architecture and extensive fibrosis (Figure 6, A and D). High-power images revealed abundant epithelial cells co-expressing α-SMA and either pro-SP-B (Figure 6, B and C) or TTF-1 (Figure 6, E and F) lining cystic air spaces. To definitively colocalize epithelial and mesenchymal markers within individual cells, series of images were taken of single cells at sequential 0.1-μm z axis depths. The resultant image stacks were analyzed using a three-dimensional deconvolution algorithm.21 At the numerical aperture of the lens used to acquire the data, the depth of focus of each section is on the order of 0.4 μm, which is much less than the diameter of a single cell. As shown in representative images in Figure 7, pro-SP-B and TTF-1 colocalized with α-SMA to single cells in these optical sections. The prevalence of colocalization within biopsy samples was also examined. Twenty high-power fields were examined from each available slide from all three patients. α-SMA colocalized with pro-SPB in 83 ± 8%, and with TTF-1 in 87 ± 9%, of epithelial cells. Tissue from all patients examined exhibited similar findings. Control slides using mouse IgG in place of either or both primary Abs showed no colocalization of staining, whereas staining of normal lung tissue revealed no cells co-expressing both markers (data not shown).
Figure 6.
Co-expression of myofibroblast and AEC markers in AECs of lung tissue from humans with IPF. Low-power images (original magnification, ×100) reveal severe destruction of normal lung architecture due to fibrosis (A and D). Pink color represents Fast Red staining of SP-B (A to C) or TTF-1 (D to F). Brown color represents diaminobenzidine staining of α-SMA. High-power images (original magnification, ×400; two representative fields from within the low-power field shown) reveal abundant epithelial cells adjacent to fibroblastic foci co-expressing SP-B (pink, cytoplasmic) and α-SMA (brown, cytoplasmic) (B and C) or TTF-1 (pink, nuclear) and α-SMA (brown, cytoplasmic) (E and F). Colocalization of both markers was seen in all three patients examined.
Figure 7.
Colocalization of myofibroblast and AEC markers in AECs of lung tissue from humans with IPF using three-dimensional deconvolution microscopy. High-power images (original magnification, ×100) of single epithelial cells were taken at sequential 0.1-μm z axis depths >3 μm. Resultant image stacks were analyzed using a three-dimensional deconvolution algorithm. Pro-SPB and TTF-1 (pink) colocalized with α-SMA (brown) to the same optical section in all cells analyzed.
Discussion
Exposure of rat AECs in primary culture to TGF-β1 resulted in transition from an epithelial to a myofibroblast-like phenotype. Expression of mesenchymal markers (α-SMA, type I collagen, desmin, and vimentin) was significantly increased in treated monolayers, with concomitant reduction in expression of epithelial markers (cytokeratins, ZO-1, and AQP5) and acquisition of a fibroblast-like appearance. Importantly, TTF-1, a lung epithelial-specific marker, was colocalized to cells expressing α-SMA on day 6, establishing the epithelial origin of these myofibroblast-like cells. Similar up-regulation of α-SMA and acquisition of a fibroblast-like morphology was also seen in RLE-6TN cells. This phenotypic transition was markedly augmented by the addition of TNF-α to the growth media. Importantly, examination of human lung tissue from IPF patients revealed the presence of transitional cells lining cystic airspaces that co-expressed alveolar epithelial and mesenchymal markers. These data support our in vitro findings and suggest that epithelial-mesenchymal phenotypic transition may be occurring in the pulmonary fibrosis of usual interstitial pneumonia in vivo. Transition of AECs to a myofibroblast-like phenotype in the presence of TGF-β1, together with identification of cells that co-express markers of epithelial and mesenchymal phenotypes in vitro and in human lung, strongly suggest a novel paradigm in which epithelial cells may serve as a source of myofibroblasts in pulmonary fibrosis, possibly in response to epithelial cell injury.
Several lines of evidence implicate TGF-β1 as a “master switch” of tissue repair, with its disordered or increased expression leading to tissue fibrosis in many organs, including lung.12,22 Progression of fibrosis is associated with activation of type 2 cytokines, including interleukin (IL)-4, IL-5, IL-10, IL-13, and monocyte chemoattractant protein 1, resulting in up-regulation of TGF-β1 and promotion of fibroblast proliferation, collagen gene expression, and collagen synthesis.23,24 In contrast, the type 1 response, including the cytokines interferon-γ, IL-2, IL-12, IL-18, and TNF-β, serves to suppress the fibrogenic response and inhibit the release of type 2 cytokines.23 In this regard, interferon-γ directly inhibits TGF-β1 production and limits fibrosis,25 and it has been used with limited success in the treatment of fibrotic lung disease.26,27 With regard to specific effects on lung epithelial cells, TGF-β1 has been noted to down-regulate surfactant protein and RNA production and to increase extracellular matrix component production in cultured AECs.28–30 These observations suggest that progressive pulmonary fibrosis is a disorder of dysregulated cytokine production and cellular differentiation, rather than one of ongoing inflammation, and they support a role for TGF-β1 in disease pathogenesis.
The importance of TNF-α in lung injury31,32 and its potential role in EMT led us to evaluate its ability to potentiate the effects of TGF-β1. TNF-α levels are elevated in serum and bronchoalveolar lavage fluid samples from patients with pulmonary fibrosis,16 and TNF-α appears to play a role in the pathophysiology of ARDS.33 Overexpression of TNF-α in murine and rat lung results in severe inflammation and fibrogenesis.15,34 Furthermore, TNF-α accelerated TGF-β1-induced EMT in human colonic organoids, and, in conjunction with TGF-β1, stimulated transdifferentiation of hepatic fat-storing cells into myofibroblasts in rat.35,36 In the current study, in AECs and RLE-6TN cells, induction of EMT was augmented by the presence of TGF-β1 and TNF-α. It is likely that other cytokines may play a role in potentiating or inhibiting EMT in AECs, and complete elucidation of the various effects of inflammatory and other cytokine cascades on AECs requires further study.
Several potential cellular sources of myofibroblasts in IPF have been suggested, including resident fibro-blasts, circulating fibroblasts, and circulating progenitor cells.1,14 Although the morphology of epithelial cells in samples of lung tissue from patients with IPF is highly abnormal, with significant alterations in morphology and phenotypic marker expression,4,37 the possibility that AECs may participate in the development of pulmonary fibrosis through transdifferentiation into fibroblasts and/or myofibroblasts has only recently received consideration.3,4,38 Identification of myofibroblasts relies on morphological and ultrastructural analysis, together with expression of panels of characteristic immunocytochemical and biochemical markers. They are large and spindle or stellate shaped, and they can exhibit long cytoplasmic extensions, produce collagen, and contain α-SMA-positive stress fibers running parallel to the long axis of the cell.13 Among available phenotypic markers, α-SMA remains the most reliable marker of myofibroblastic cells,12 and the relative expression of various intermediate filaments (including vimentin, desmin, and smooth muscle myosin heavy chains) have been used to further characterize myofibroblast subphenotypes. The cells we identified after treatment of highly purified, fibroblast-depleted primary AECs with TGF-β1 and TNF-α were extremely large and spindle shaped; contained α-SMA-positive fibers running along the long axis of the cell; expressed α-SMA, type I collagen, vimentin, and desmin in high quantities; and lacked markers of differentiated alveolar epithelial cells (cytokeratins, ZO-1, and AQP5), suggesting transition to a myofibroblast-like phenotype. Furthermore, expression of type I collagen by treated cells suggests that the cells acquired functional characteristics of fibroblasts. Treatment of the cell line RLE-6TN produced cells with similar characteristics. Consistent with these results, recent preliminary data suggested that another lung cell line, L2, could assume a fibroblast-like morphology after exposure to TGF-β1.38
Results of the current study in which we demonstrate transdifferentiation of AECs in vitro and the presence of transitional cells co-expressing AT2 cell markers and α-SMA in biopsy samples of patients with IPF suggest that AECs may serve as a source of myofibroblasts in IPF. Fibroblasts have been shown to differentiate from epithelial cells in fibrotic processes in other tissues,35,39–43 and studies of renal fibrosis suggest that more than one-third of the fibroblasts at sites of injury arise from epithelial cells.44 Our data are highly suggestive that AECs undergo EMT, especially in the setting of chronic lung injury.
Although the possibility exists that colocalization of epithelial markers and α-SMA protein in single cells in patients with IPF (Figures 6 and 7) could be accounted for by endocytosis of debris and apoptotic cells by hyperplastic epithelial cells,45–47 this seems an unlikely explanation for our findings. First, the morphology and cellular localization of α-SMA expression within the epithelium of patients with IPF (Figure 7) is remarkably similar to that seen during EMT in vitro at early time points (Figure 5). Second, because alveolar epithelial uptake of protein from the airspace, while present after injury and in healthy lungs, is a relatively minor component of total alveolar protein clearance,48,49 it is unlikely that nearly 90% of visualized epithelium would contain generous quantities of α-SMA if its origins were solely from endocytotic uptake.
The demonstration of EMT in vitro and in vivo serves to increase the focus on the epithelium as a central factor in the epithelial cell/fibroblast paradigm of IPF. Currently, myofibroblasts that constitute fibroblastic foci have been considered to be central to the pathogenesis of disease. Our observations suggest that they constitute markers of sites of epithelial cell injury and that EMT and other epithelial cell responses to such injury may be the true key to understanding why IPF is often such a relentlessly progressive disease. In this context, the association of increased numbers of fibroblastic foci and a worse prognosis may be due to acceleration in the degree of epithelial cell injury at the time of biopsy.
Several measures were used to exclude the possibility that contamination of primary cultures by fibroblasts contributed to the observed increases in expression of mesenchymal markers and EMT. First, AEC preparations were of the highest possible purity, with one preparation using positive magnetic selection with an AT2 cell-specific Ab that yielded a purity of 97%. In addition, primary cells were grown for 2 days in serum-free medium before treatment with TGF-β1, conditions which are not supportive of fibroblast growth. Monolayers at this stage exhibited 100% epithelial cell morphology, with no detectable expression of mesenchymal markers (data not shown). Growth media were also supplemented for the first 2 days with cis-OH-proline, which eliminates fibroblasts in mixed-cell populations.18 In addition, TGF-β1 has repeatedly been shown to inhibit, not promote, lung fibroblast proliferation at concentrations greater than 2 ng/ml.50–52 Consistent with this, control experiments using primary rat lung fibroblasts (Cell Applications, San Diego, CA) and a rat lung fibroblast cell line (RFL-6; American Type Culture Collection, Manassas, VA) showed that, in culture under the conditions described above, fibroblasts were eliminated within 24 hours (data not shown). Furthermore, our results in primary AECs are supported by our demonstration of similar phenotypic transition in RLE-6TN cells, an epithelial cell line in which there is unlikely to be any fibroblast contamination. Finally, co-expression in the same cell of α-SMA and the epithelial-specific transcription factor TTF-1, during the gradual transition of AECs to a mesenchymal phenotype over time, provides unequivocal evidence of the presence of EMT in AECs.
The mechanism of EMT in AECs remains to be elucidated. TGF-β1 signals through a heteromeric complex of serine/threonine kinase receptors, which in turn phosphorylate ligand-specific Smad proteins.53 Various secondary kinases, including the mitogen-activated protein kinase (MAPK), Rho kinase, Rac1, integrin-linked kinase, and Jun N-terminal kinase can promote Smad-mediated signaling and transcriptional activation. In addition, TGF-β1 can signal through Smad-independent pathways, possibly involving p38 MAPK.54 Rho-dependent mechanisms are critical to EMT in NMuMG and LLC-PK1 cells.53,55 EMT in renal interstitial fibrogenesis is me-diated by integrin-linked kinase.56 Transdifferentiated MDCK cells undergo restoration of epithelial phenotype with inhibition of the MAPK/ERK kinase 1-ERK2 signaling module.57 Bone morphogenetic protein-7 reversed TGF-β1-induced EMT in renal tubular epithelial cells.58 Genetic control of EMT in many tissues is modulated through the Wnt/β-catenin pathway,59 whereas the snail family of transcription factors induce epithelial-mesenchymal transitions through repression of E-cadherin.60 A recent study showed that decreases in expression of Nkx2.1 (TTF-1) correlated with dedifferentiation in lung carcinomas.61 Further study will be required to define the mechanisms and potential for reversibility of EMT in AECs.
In summary, we demonstrate that TGF-β1 ± TNF-α induces an EMT to a myofibroblast-like phenotype in AECs. This process occurred in an alveolar epithelial cell line, RLE-6TN, and primary AECs. Importantly, we now demonstrate the presence of pulmonary cells co-expressing AEC markers and α-SMA in regions of hyperplastic epithelium in human lungs with IPF, suggesting that these cells may be undergoing EMT thereby contributing to fibroblast and myofibroblast accumulation in this disease. These findings are supported by prior reports that AECs are critical to the process of induction of fibrosis,3,4,38 and support the notion that pulmonary fibrosis may be intimately related to an initial injury to the epithelial cell, rather than secondary to inflammation. The potential for reversal or inhibition of this process as a therapeutic strategy in pulmonary fibrosis is enticing, and elucidation of the mechanisms involved is needed to develop specific therapies.
Acknowledgments
We thank Zerlinde Balverde and Juan Ramon Alvarez for expert technical assistance.
Footnotes
Address reprint requests to Zea Borok, M.D., Division of Pulmonary and Critical Care Medicine, University of Southern California, IRD 620, 2020 Zonal Avenue, Los Angeles, CA 90033. E-mail: zborok@usc.edu.
Supported by National Heart, Lung, and Blood Institute grants HL-38578, HL-38621, HL-62569, HL-64365, and HL-72231 and by the Hastings Foundation.
E.D.C. is Hastings Professor and Kenneth T. Norris Jr. Chair of Medicine.
References
- Crystal RG, Bitterman PB, Mossman B, Schwarz MI, Sheppard D, Almasy L, Chapman HA, Friedman SL, King TE, Jr, Leinwand LA, Liotta L, Martin GR, Schwartz DM, Schultz GS, Wagner CR, Musson RA. Future research directions in idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2002;166:236–246. doi: 10.1164/rccm.2201069. [DOI] [PubMed] [Google Scholar]
- Travis WD, King TE, Jr, Bateman ED, Lynch DA. American Thoracic Society/European Respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Resp Crit Care Med. 2002;265:277–304. doi: 10.1164/ajrccm.165.2.ats01. [DOI] [PubMed] [Google Scholar]
- Selman M, Pardo A. The epithelial/fibroblastic pathway in the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:S93–S96. [PubMed] [Google Scholar]
- Chilosi M, Poletti V, Zamo A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C. Aberrant Wnt/β-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162:1495–1502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittet JF, Griffiths MJD, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LAS, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-β is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–1544. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-β-mediated pulmonary fibrosis. J Immunol. 2004;173:2099–2108. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-β1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YD, Hua J, Mui A, O‘Connor R, Grotendorst G, Khalil N. Release of biologically active TGF-β1 by alveolar epithelial cells results in pulmonary fibrosis. Am J Physiol. 2003;285:L527–L539. doi: 10.1152/ajplung.00298.2002. [DOI] [PubMed] [Google Scholar]
- Bergeron A, Soler P, Kambouchner M, Loiseau P, Milleron B, Valeyre D, Hance A, Tazi A. Cytokine profiles in idiopathic pulmonary fibrosis suggest an important role for TGF-beta and IL-10. Eur Respir J. 2003;22:69–76. doi: 10.1183/09031936.03.00014703. [DOI] [PubMed] [Google Scholar]
- Dhainaut J-F, Charpentier J, Chiche J-D. Transforming growth factor-β: a mediator of cell regulation in acute respiratory distress syndrome. Crit Care Med. 2003;31:S258–S264. doi: 10.1097/01.CCM.0000057901.92381.75. [DOI] [PubMed] [Google Scholar]
- Boyer B, Valles AM, Edme N. Induction and regulation of epithelial-mesenchymal transitions. Biochem Pharmacol. 2000;60:1091–1099. doi: 10.1016/s0006-2952(00)00427-5. [DOI] [PubMed] [Google Scholar]
- Serini G, Gabbiani G. Mechanisms of myofibroblast activity and phenotypic modulation. Exp Cell Res. 1999;250:273–283. doi: 10.1006/excr.1999.4543. [DOI] [PubMed] [Google Scholar]
- Schurch W, Seemayer TA, Gabbiani G. The myofibroblast: a quarter century after its discovery. Am J Surg Pathol. 1998;22:141–147. doi: 10.1097/00000478-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sime PJ, Marr RA, Gauldie D, Xing Z, Hewlett BR, Graham FL, Gauldie J. Transfer of tumor necrosis factor-α to rat lung induces severe pulmonary inflammation and patchy interstitial fibrogenesis with induction of transforming growth factor-beta1 and myofibroblasts. Am J Pathol. 1998;153:825–832. doi: 10.1016/s0002-9440(10)65624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Phan S. Cytokines and pulmonary fibrosis. Biol Signals. 1996;5:232–239. doi: 10.1159/000109195. [DOI] [PubMed] [Google Scholar]
- Borok Z, Danto S, Zabski S, Crandall ED. Defined medium for primary culture de novo of rat alveolar epithelial cells. In Vitro Cell Dev Biol Anim. 1994;30A:99–104. doi: 10.1007/BF02631400. [DOI] [PubMed] [Google Scholar]
- Kao WW-Y, Prockop DJ. Proline analogue removes fibroblasts from cultured mixed cell populations. Nature. 1977;266:63–64. doi: 10.1038/266063a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA. 1979;76:43–50. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes TJ, O’Connor NJ. Blind deconvolution of 3D transmitted light brightfield micrographs. J Microsc. 2000;200:114–127. doi: 10.1046/j.1365-2818.2000.00751.x. [DOI] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJD, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin αvβ6 binds and activates latent TGF-β1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Sime PJ, O‘Reilly KMA. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin Immunol. 2001;99:308–319. doi: 10.1006/clim.2001.5008. [DOI] [PubMed] [Google Scholar]
- Pardo A, Selman M. Idiopathic pulmonary fibrosis: new insights in its pathogenesis. Int J Biochem Cell Biol. 2002;34:1534–1538. doi: 10.1016/s1357-2725(02)00091-2. [DOI] [PubMed] [Google Scholar]
- Jaffe HA, Gao Z, Mori Y, Li L, Varga J. Selective inhibition of collagen gene expression in fibroblasts by an interferon-gamma transgene. Exp Lung Res. 1999;25:199–215. doi: 10.1080/019021499270268. [DOI] [PubMed] [Google Scholar]
- Ziesche R, Hofbauer E, Wittman K, Petkov V, Block LH. A preliminary study of long-term treatment with interferon-gamma-1b and low-dose prednisolone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 1999;341:1264–1269. doi: 10.1056/NEJM199910213411703. [DOI] [PubMed] [Google Scholar]
- Raghu G, Brown KK, Bradford WZ, Starko K, Noble PW, Schwartz DM, King TE., Jr A placebo-controlled trial of interferon gamma-1b in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2004;350:125–133. doi: 10.1056/NEJMoa030511. [DOI] [PubMed] [Google Scholar]
- Maniscalco WM, Campbell MH. Transforming growth factor-β induces a chondroitin sulfate/dermatan sulfate proteoglycan in alveolar type II cells. Am J Physiol. 1994;266:L672–L680. doi: 10.1152/ajplung.1994.266.6.L672. [DOI] [PubMed] [Google Scholar]
- Maniscalco WM, Sinkin RA, Watkins RH, Campbell MH. Transforming growth factor-β1 modulates type II cell fibronectin and surfactant protein C expression. Am J Physiol. 1994;267:L569–L577. doi: 10.1152/ajplung.1994.267.5.L569. [DOI] [PubMed] [Google Scholar]
- Li C, Zhu N-LZ, Tan RC, Ballard PL, Derynck R, Minoo P. Transforming growth factor-β inhibits pulmonary surfactant protein B gene transcription through SMAD3 interactions with NHX2.1 and HNF-3 transcription factors. J Biol Chem. 2002;277:38399–38408. doi: 10.1074/jbc.M203188200. [DOI] [PubMed] [Google Scholar]
- Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–156. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- Kelly M, Kolb M, Bonniaud P, Gauldie J. Re-evaluation of fibrogenic cytokines in lung fibrosis. Curr Pharm Des. 2003;9:39–49. doi: 10.2174/1381612033392341. [DOI] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Araki Y, Vesin C, Garcia I, Kapanci Y, Whitsett J, Piguet P, Vassalli P. Expression of a tumor necrosis factor-α transgene in murine lung causes lymphocytic and fibrosing alveolitis: a mouse model of progressive pulmonary fibrosis. J Clin Invest. 1995;96:250–259. doi: 10.1172/JCI118029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachem MG, Sell KM, Melchior R, Kropf J, Eller T, Gressner AM. Tumor necrosis factor alpha and transforming growth factor beta 1 stimulate fibronectin synthesis and the transdifferentiation of fat-storing cells in the rat liver into myofibroblasts. Virchows Arch. 1993;63:123–130. doi: 10.1007/BF02899251. [DOI] [PubMed] [Google Scholar]
- Bates RC, Mercurio AM. Tumor necrosis factor-α stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Mol Biol Cell. 2003;14:1790–1800. doi: 10.1091/mbc.E02-09-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyonaga K, Miyajima M, Suga M, Saita N, Ando M. Alterations in cytokeratin expression by the alveolar lining epithelial cells in lung tissues from patients with idiopathic pulmonary fibrosis. J Pathol. 1997;182:217–224. doi: 10.1002/(SICI)1096-9896(199706)182:2<217::AID-PATH833>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Xu YD, Hua J, O’Connor R, Khalil N. Chronic release of active TGF-β1 by the alveolar epithelial cell (AEC) line, L-2 results in connective tissue (CT) synthesis and conversion of L-2 cells to a fibroblast-like phenotype. Am J Respir Crit Care Med. 2003;67:A572. [Google Scholar]
- Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol. 1994;127:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J-M, Ng Y-Y, Hill PA, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY. Transforming growth factor-β regulates tubular epithelial-myofibroblast transdifferentiation in vitro. Kidney Int. 1999;56:1455–1467. doi: 10.1046/j.1523-1755.1999.00656.x. [DOI] [PubMed] [Google Scholar]
- Fujigaki Y, Sun DF, Fujimoto T, Suzuki T, Goto T, Yonemura K, Morioka T, Yaoita E, Hishida A. Mechanisms and kinetics of Bowman‘s epithelial-myofibroblast transdifferentiation in the formation of glomerular crescents. Nephron. 2002;92:203–212. doi: 10.1159/000064469. [DOI] [PubMed] [Google Scholar]
- Grande M, Franzen A, Karlsson J-O, Ericson LE, Heldin N-E, Nilsson M. Transforming growth factor-β and epidermal growth factor synergistically stimulate epithelial to mesenchymal transition (EMT) through a MEK-dependent mechanism in primary cultured pig thyrocytes. J Cell Sci. 2002;115:4227–4236. doi: 10.1242/jcs.00091. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KJ, Fandy TE, Lee VH, Ann DK, Borok Z, Crandall ED. Net absorption of IgG via FcRn-mediated transcytosis across rat alveolar epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol. 2004;287:L616–L622. doi: 10.1152/ajplung.00121.2004. [DOI] [PubMed] [Google Scholar]
- Widera A, Kim KJ, Crandall ED, Shen WC. Transcytosis of GCSF-transferrin across rat alveolar epithelial cell monolayers. Pharm Res. 2003;20:1231–1238. doi: 10.1023/a:1025005232421. [DOI] [PubMed] [Google Scholar]
- Sexton DW, Blaylock MG, Walsh GM. Human alveolar epithelial cells engulf apoptotic eosinophils by means of integrin- and phosphatidylserine receptor-dependent mechanisms: a process upregulated by dexamethasone. J Allergy Clin Immunol. 2001;108:962–969. doi: 10.1067/mai.2001.119414. [DOI] [PubMed] [Google Scholar]
- Hastings RH, Folkesson HG, Petersen V, Ciriales R, Matthay MA. Cellular uptake of albumin from lungs of anesthetized rabbits. Am J Physiol. 1995;269:L453–L462. doi: 10.1152/ajplung.1995.269.4.L453. [DOI] [PubMed] [Google Scholar]
- Hastings RH, Wright JR, Albertine KH, Ciriales R, Matthay MA. Effect of endocytosis inhibitors on alveolar clearance of albumin, immunoglobulin G, and SP-A in rabbits. Am J Physiol. 1994;266:L544–L552. doi: 10.1152/ajplung.1994.266.5.L544. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Young S. Requirement of transforming growth factor-beta (TGF-beta) type II receptor for TGF-beta-induced proliferation and growth inhibition. J Biol Chem. 1996;271:2369–2372. doi: 10.1074/jbc.271.5.2369. [DOI] [PubMed] [Google Scholar]
- Banner J, Badgett A, Lindroos P, Osornio-Vargas A. Transforming growth factor beta 1 downregulates the platelet-derived growth factor alpha-receptor subtype on human lung fibroblasts in vitro. Am J Respir Cell Mol Biol. 1995;13:496–505. doi: 10.1165/ajrcmb.13.4.7546780. [DOI] [PubMed] [Google Scholar]
- Fine A, Goldstein R. The effect of transforming growth factor-beta on cell proliferation and collagen formation by lung fibroblasts. J Biol Chem. 1987;262:3897–3902. [PubMed] [Google Scholar]
- Bhowmick NA, Ghiassi M, Bakin A, Aakre M, Lundquist CA, Engel ME, Arteaga CL, Moses HL. Transforming growth factor-β1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12:27–36. doi: 10.1091/mbc.12.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Hebert MC, Zhang YE. TGF-β receptor-activated p38 MAP kinase mediates Smad-independent TGF-β responses. EMBO J. 2002;21:3749–3759. doi: 10.1093/emboj/cdf366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masszi A, Di Ciano C, Sirokmany G, Arthur WT, Rotstein OD, Wang J, McCulloch CAG, Rosivall L, Mucsi I, Kapus A. Central role for Rho in TGF-β1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am J Physiol. 2003;284:F911–F924. doi: 10.1152/ajprenal.00183.2002. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang J, Dai C, Wu C, Liu Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112:503–516. doi: 10.1172/JCI17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramek H, Feifel E, Marschitz I, Golochtchapova N, Gstraunthaler G, Montesano R. Loss of active MEK1-ERK1/2 restores epithelial phenotype and morphogenesis in transdifferentiated MDCK cells. Am J Physiol. 2003;285:C652–C661. doi: 10.1152/ajpcell.00463.2002. [DOI] [PubMed] [Google Scholar]
- Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. BMP-7 counteracts TGF-β1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- Kim K, Lu Z, Hay ED. Direct evidence for a role of β-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int. 2002;26:463–476. doi: 10.1006/cbir.2002.0901. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno M, Rodrigo I, Locascio A, Blanco M, del Barr M, Portillo F, Nieto M. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Kang Y, Hebron H, Ozbun L, Mariano J, Minoo P, Jakowlew SB. Nkx2.1 transcription factor in lung cells and a transforming growth factor-β1 heterozygous mouse model of lung carcinogenesis. Mol Carcinog. 2004;40:212–231. doi: 10.1002/mc.20034. [DOI] [PubMed] [Google Scholar]