Abstract
We reported previously that radiocontrast medium induces caspase-dependent apoptosis and that cAMP analogs inhibit cell injury in cultured renal tubular cells. In the present study, cellular me-chanisms underlying the protective effects of cAMP were determined. Ioversol, a radiocontrast medium, caused cell injury accompanied by decreases in Bcl-2, increases in Bax, and caspase activation in LLC-PK1 cells. Both cell injury and cellular events induced by ioversol were inhibited by dibutyryl cAMP and the prostacyclin analog beraprost. Dibutyryl cAMP increased phosphorylation of Akt and CREB, both of which were reversed by H89, wortmannin and the Akt inhibitor SH-6. The protective effect of dibutyryl cAMP was also reversed by these kinase inhibitors. In dominant-negative CREB-transfected cells, dibutyryl cAMP no longer prevented cell injury or inhibited changes in mRNA expression of Bcl-2 and Bax. In mice with unilateral renal occlusion, ioversol increased urinary ex-cretion of N-acetyl-β-d-glucosaminidase with con-comitant decreases in Bcl-2 mRNA, increases in Bax mRNA, activation of caspase-3, and induction of apoptosis in tubular and interstitial cells. Beraprost completely reversed these in vivo effects of ioversol. These findings suggest that elevation of endogenous cAMP effectively prevents radiocontrast nephropathy through activation of A kinase/PI 3-kinase/Akt followed by CREB phosphorylation and enhanced expression of Bcl-2.
Radiocontrast nephropathy is a major complication after radiographical examination with iodinated contrast materials. Although little is known about cellular mechanisms underlying contrast nephropathy, direct toxic action on renal tubular cells1–4 and/or decrease in renal blood flow5,6 are considered to be implicated in the pathogenesis of radiocontrast nephropathy. We have recently shown that a variety of radiocontrast media reduce cell viability in a porcine renal tubular cell line LLC-PK1 cells.7 The cell injury is accompanied by the nuclear fragmentation, increase in the number of cells stained with annexin V, a protein showing high affinity for phosphatidyl serine, and activation of caspases, thereby suggesting that the cell injury is associated with apoptosis. Moreover, ioversol reduces the expression for Bcl-2 mRNA and increased that for Bax mRNA. These intracellular events and apoptosis induced by ioversol are reversed by a non-hydrolysable cAMP analog dibutyryl cAMP (DBcAMP)7 or enhancement of endogenous cAMP synthesis with beraprost,8 a stable prostacyclin analog. We also found that the protective effect of DBcAMP is dependent on the activity of A kinase, phosphatidyl inositol 3 (PI 3)-kinase and Akt. However, it is uncertain how PI 3-kinase/Akt pathway regulates ioversol-induced renal tubular cell apoptosis.
Cyclic AMP response element binding protein (CREB) is one of target proteins that are phosphorylated by A kinase9 and is known as a regulator of diverse stimulus-dependent transcriptional events involving cell survival.10,11 Phosphorylation of CREB at Ser133 binds to the CRE site located on the promoter region of bcl-2 gene and up-regulates Bcl-2 expression.12–14 To determine the role of CREB in cAMP-mediated protection against renal tubular cell injury induced by ioversol, we investigated the effect of DBcAMP on ioversol-induced changes in mRNA expression for Bcl-2 and Bax, and apoptosis in LLC-PK1 cells expressed with dominant negative form of CREB.
Subsequently, we investigated the in vivo effect of beraprost on renal injury and changes in the expression for Bcl-2 and Bax induced by the intravenous injection of ioversol in mice with unilateral renal occlusion.
Materials and Methods
Materials
The following chemicals and drugs were obtained from commercial sources: ioversol (Optiray 350, 350 mg iodine/ml), a non-ionic iodinated radiocontrast medium (Tyco Health care Japan Co., Ltd., Tokyo, Japan), d-2,3-dideoxy-myoinositol 1-[(R)-2-methoxy-3-(octadecyloxy)propyl hydrogen phosphate] (SH-6) and fluorescence-labeled caspase substrates such as Ac-DEVD-7-amino-4-methylcoumarin (AMC) for caspase-3 and Ac-LEHD-AMC for caspase-9 (Alexis Biochemicals, San Diego, CA), wortmannin, forskolin, and caspase inhibitors, including zDEVD-fmk (a caspase-3 specific inhibitor) and zLEHD-fmk (a caspase-9 specific inhibitor) (Calbiochem, San Diego, CA), dibutyryl cAMP (DBcAMP) (Sigma, St. Louis, MO), N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89) (Seikagaku Co., Ltd., Tokyo, Japan). Beraprost sodium was kindly donated from Toray Industries, Inc. (Tokyo, Japan).
Cell Culture
A porcine proximal renal tubular cell line LLC-PK1 cells (American Type Culture Collection, Rockville, MD) were grown in 75 cm2 flask (Corning Inc., Corning, NY) and maintained in Medium 199 (MP Biomedicals, Inc., Irvine, CA) supplemented with 10% fetal bovine serum (FBS; JRH Bioscience, Inc., Lenexa, KS), 60 μg/ml penicillin (Sigma) in an atmosphere of 5% CO2 in air at 37°C. Then, the cells were seeded on 24-well plastic plates (Falcon, Becton Dickinson Co., Ltd., Franklin Lakes, NJ) at the density of 1.0 × 104 cells/cm2 and cultured at 37°C for 24 hours.
Cell Viability
Cells were transiently (30 minutes) exposed to ioversol, then washed twice with phosphate-buffered saline (PBS) and incubated in serum-free culture medium for further 24 hours at 37°C in 5% CO2−95% air. DBcAMP (0.1–1 mmol/L), forskolin (10 μmol/L) or beraprost (1 μmol/L) was added 30 minutes before treatment with the contrast medium and included throughout the experiment. Cell viability was assessed by the mitochondrial activity to reduce 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium (WST-8) to formazan.15 Briefly, the ioversol-treated cells were incubated at 37°C for 1 hour in 210 μl serum-free medium containing 10 μl assay solution (Cell Counting Kit-8, Dojindo Laboratory, Kumamoto, Japan), and aliquots of the incubation medium were transferred to 96-well microplate (Corning Inc.), then absorbance was measured at 450 nm with the reference wavelength of 620 nm using a microplate reader (Immuno Mini NJ-2300, Inter Medical, Osaka, Japan).
Terminal Deoxynucleotidyl Transferase-Mediated dUTP Nick End-Labeling (TUNEL) Stain
Apoptosis was assessed by TUNEL stain, according to the method of Oberhaus.16 Briefly, ioversol-treated cells were washed with PBS and fixed for 30 minutes at a room temperature with 4% (w/v) paraformaldehyde in PBS. Cells were permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate solution. TUNEL stain was carried out using a commercial assay kit (Cell Death Detection kit, Roche Applied Science, Tokyo, Japan), according to the manufacturer’s instructions. The stained cells were visualized with a fluorescence microscope (BX51, Olympus) and a cooled charge coupled device (CCD) camera (DP70, Olympus).
Assay for Caspase Activity
The activities of caspase-9 and caspase-3 were determined fluorometrically by the degradation of the peptide substrates specific for caspase-9 (LEHD-AMC) and caspase-3 (DEVD-AMC), as described previously,7 using the caspase activity assay kit (BioVision, Inc., Mountain View, CA). Briefly, cells were centrifuged at 150 × g for 10 minutes, and the resultant pellets were suspended in 1 ml lysis buffer (BioVision, Inc.) and subjected to caspase activity assay. In a set of experiments where caspase-3 activity was measured in vivo, kidneys were removed 24 hours after ioversol injection and the medulla was dissected. Tissues were homogenized with 1 ml lysis buffer and subjected to the assay for caspase-3. The reaction was started by incubating enzyme extracts with each caspase substrate for 10 minutes in the absence or presence of 10 μmol/L caspase inhibitors, including zLEHD-fmk for caspase-9 and zDEVD-fmk for caspase-3. After centrifugation at 10,000 × g for 10 minutes, the concentration of 7-amino-4-methylcoumarin (AMC) liberated into the supernatant was determined at an excitation wavelength of 380 nm and an emission wavelength of 460 nm using a fluorescence microplate reader (MTP-800AFC, Corona Electric Co., Ltd., Ibaragi, Japan). The protein concentration was measured using bovine serum albumin as the standard, according to the method of Bradford.17 The caspase activity was expressed as nmol of AMC produced per mg protein.
Immunofluorescent Detection for Phosphorylated Akt and Phosphorylated cAMP Responsive Element Binding Protein (CREB)
The immunofluorescent stains for phosphorylated Akt (pAkt) and phosphorylated CREB (pCREB) were carried out, according to the method of Gupta et al18 and Inglefield et al,19 respectively. Briefly, cells were cultured on 8-chamber plastic slides (IWAKI/Asahi Techno Glass Co., Ltd., Chiba, Japan) at the density of 2 × 104 cells/cm2 and incubated for 24 hours. Cells were treated with 0.3 mmol/L DBcAMP for 10 minutes for pAkt assay or 20 minutes for pCREB analysis in the absence or presence of 10 μmol/L H89, 10 nmol/L wortmannin, 1 μmol/L SH-6, and 100 mg iodine/ml ioversol. The chamber slides were rinsed with ice-cold PBS and fixed with 10% (w/v) ice-cold trichloroacetic acid for 30 minutes, at −20°C. The specific rat antibody raised against porcine pCREB (Ser133) (Affinity Bioreagents. Inc., Golden, CO) or rabbit antibody raised against porcine pAkt (Ser473) (Cell Signaling Technology, Inc., Beverly, MA) was diluted (1:50) with phosphate-buffered saline containing 5% (w/v) nonfat dried milk and 0.1% Triton X-100. Cells were incubated with diluted antibody solution overnight in a humidified chamber at 4°C. After washing with PBS, chamber slides were incubated at a room temperature for 2 hours with fluorescent isothiocyanate (FITC)-labeled goat anti-rabbit IgG or anti-rat IgG (1:50 dilution in phosphate-buffered saline) (The Jackson Laboratory, Bar Harbor, ME). Cells were mounted and immunofluorescence was detected by a fluorescence microscope (BX51, Olympus) and a CCD camera (DP70, Olympus).
Transient Expression of Dominant-Negative CREB in LLC-PK1 Cells
The pCG expression vector containing ProCREB, a dominant-negative form of CREB, was constructed by oligonucleotide-directed mutagenesis as described previously.20,21 ProCREB was obtained by substituting Arg by Pro on position 301. LLC-PK1 cells were grown to 70 to 80% confluency in 24-well plates before transfection and 1 μg of plasmid (pCG-ProCREB) were transfected into the cells using SuperFect reagent (Qiagen, Tokyo) according to the manufacturer’s instructions. pCG-UT (empty vector) alone was used as a transfection control (mock). After 48 hours post-transfection, the cells were subject to the experiments. In a parallel experiment, the pEGFP-C1 plasmid (Clontech, Palo Alto, CA) was also transfected using the same method to determine the transfection efficiency. Expression of green fluorescent protein from the pEGFP-C1 plasmid is driven by the CMV promoter. GFP expressing cells were observed under fluorescence microscopy. Transfection efficiency was calculated using the following formula: transfection efficiency (%) = (GFP expressing cells per field)/(total cells per field) ×100. In each experiment, transfection efficiency was 70–80% (data not shown).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
mRNA was isolated from LLC-PK1 cells or murine kidneys using a Quick preparation micro mRNA purification kit (Amersham Biosciences UK Limited, Buckinghamshire, UK). The mRNAs for Bcl-2 and Bax were determined, as described previously.7 Briefly, cells or renal tissues were homogenized with 0.6 ml extraction buffer, and mixed with 0.8 ml elution buffer. After centrifugation at 10,000 × g for 1 minute, the supernatant was applied to the oligo(dT)-cellulose, and washed with a high-salt buffer, followed by a low-salt buffer. The mRNA was eluted with the elution buffer, and quantified from an absorbance at 260 nm. The mRNA solution was diluted with 20 μl RNase-free water, and cDNA was synthesized by using a first-strand cDNA synthesis kit (Amersham Biosciences). A 25-μl aliquot of samples containing cDNA (corresponding to 50 ng of mRNA), 10 pmol/μl of each oligonucleotide primer, 2.5 mmol/L of dNTP and 5 units/μl of Taq polymerase (Nippon Gene, Co., Ltd., Tokyo) was subjected to RT-PCR. Amplification of cDNA obtained from LLC-PK1 cells was carried out for 30 cycles (denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds, and elongation at 72°C for 30 seconds), followed by an additional polymerization at 72°C for 5 minutes, using a Program Temp Control System PC-707 (Aster Co., Ltd., Fukuoka, Japan). The oligonucleotide primers for porcine bcl-2 and bax were designed based on the sequences described previously.22,23 The sequences of PCR primers were as follows: 5′-AGCGTCAACGGGAGATGTC-3′ (sense) and 5′-GTGATGCAAGCTCCCACCAG-3′ (antisense) for bcl-2, and 5′-CAGCTCTGAGCAGATCATGAAGACA-3′ (sense) and 5′-GCCCATCTTCTTCCAGATGGTGAGC-3′ (antisense) for bax. The primers were purchased from Sigma Genosys Japan Co., Ltd. (Sapporo, Japan). Amplification of cDNA obtained from murine renal tissues was carried out for 30 cycles (denaturation at 94°C for 45 seconds, annealing at 53°C for 45 seconds, and elongation at 72°C for 90 seconds), followed by an additional polymerization at 72°C for 7 minutes, using Apoptosis PCR bax/bcl-2 Multiplex Primer Sets (Sigma), according to the manufacture’s instruction. The PCR products were subjected to electrophoresis on 2% agarose gel, and the DNA was visualized by staining with ethidium bromide under ultraviolet irradiation. The intensities of the PCR products were semiquantified densitometrically using the Alpha Imager 2200 (Alpha Innotech Corporation, San Leandro, CA). The mRNA for β-actin or GAPDH was used as the standard.
Measurement of Bcl-2 and Bax Proteins
The protein contents of Bcl-2 and Bax were determined by enzyme immunoassay. Briefly, cells were seeded in 25 cm2 flask, and incubated for 48 hours, then exposed to 100 mg iodine/ml ioversol for 30 minutes in the absence or presence of DBcAMP and specific protein kinase inhibitors, followed by further incubation for 12 hours. After the cells were collected and washed twice with PBS, Bcl-2 and Bax contents were determined using the respective ELISA kit (R&D systems Inc., Minneapolis, MN), according to the manufacturer’s instructions, and the absorbance at O.D. of 450 nm wavelength was measured using a microplate reader (Inter Medical).
Radiocontrast Medium-Induced Nephropathy in Mice
Male ddY mice weighing 30 to 35 g (Kyudo Co., Saga, Japan) were housed in a room maintained on a 12-hour light/dark schedule (lights on at 8:00 a.m.) at a temperature of 23 ± 2°C, and allowed free access to food and water. All in vivo experimental procedures were approved by the Institutional Committee for the Care and Use of Laboratory Animals at Kyushu University Hospital. Mice were anesthetized with 50 mg/kg pentobarbital sodium and subjected to unilateral ligation of the left anterior renal pedicle, including renal artery, renal vein and ureter. The ligated kidney was remarkably shrunken. Seven days after surgical operation, mice were injected i.v. with 4 g iodine/kg ioversol via the tail vein. Beraprost was injected (0.1−0.3 mg/kg, i.p.) 5 minutes before ioversol injection. At 24 hours after ioversol injection, urine samples were collected from bladder under deep ether anesthesia. Serum creatinine and blood urea nitrogen concentrations were determined by Jaffe’s method and the diacetylmonoxime method, respectively, using the respective assay kits (Wako Pure Chemical, Osaka). The activity of N-acetyl-β-d-glucosaminidase (NAG) in urine was determined by the enzymatic degradation of the substrate sodium cresol sulfonephthaleinyl N-acetyl-β-d-glucosaminide using a commercial assay kit (Shionogi Pharmaceutical, Osaka, Japan). NAG activity was expressed as units per g urinary creatinine.
Histochemical Examination of Renal Tissues
The right kidney was dissected and fixed in 20% formalin, then dehydrated in graded concentrations of ethyl alcohol, and embedded in paraffin. The kidney block was cut into 2-μm sections and subjected to periodic acid-Schiff (PAS) stain and TUNEL stain.
Statistical Analysis
Data are expressed as the mean ± SEM and statistically analyzed by one-way analysis of variance followed by Dunnett’s test for multiple comparisons or by Student’s t-test for comparison between two groups, or by the Kruskal-Wallis test combined with a Steel-type multiple comparison tests for non-parametric analysis. Statistical significance was defined as P < 0.05.
Results
Protection by cAMP against Ioversol-Induced Loss of Cell Viability, Apoptosis and Caspase Activation via a Kinase/PI 3-Kinase/Akt Pathway in LLC-PK1 Cells
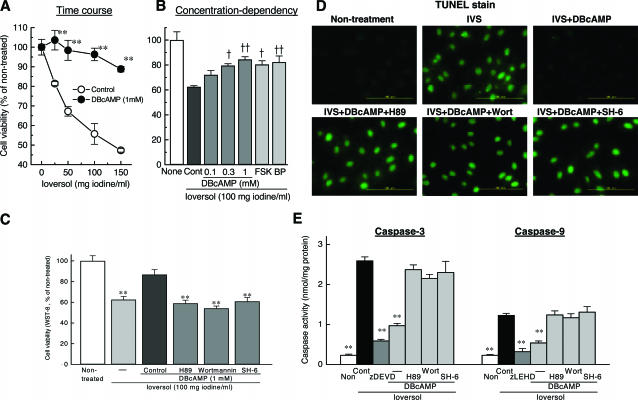
Exposure of LLC-PK1 cells to ioversol for 30 minutes caused a concentration-dependent decrease in cell viability determined at 24 hours by WST-8 assay (Figure 1A). DBcAMP shifted the concentration-response curve for ioversol to the right. Stimulation of cAMP production with forskolin or beraprost, a prostacyclin analog that stimulates adenylate cyclase activity,24 also suppressed ioversol-induced loss of cell viability (Figure 1B). Ioversol markedly enhanced the activities of caspase-9 and caspase-3 (Figure 1E), and caused cell apoptosis as assessed by TUNEL stain (Figure 1D) Moreover, the inhibitory effects of DBcAMP against ioversol-induced loss of cell viability (Figure 1C), apoptosis (Figure 1D), and caspase activation (Figure 1E) were all reversed almost completely by H89, an A kinase inhibitor,25 wortmannin, a PI 3-kinase inhibitor,26 and SH-6, an Akt inhibitor.27
Figure 1.
Effects of DBcAMP, forskolin and a prostacyclin analog beraprost against loss of cell viability induced by ioversol in LLC-PK1 cells (A, B), and involvement of A kinase/PI 3-kinase/Akt pathway in the inhibitory effects of DBcAMP on the decrease in cell viability (C), on the increase in the number of apoptotic cells assessed by TUNEL stain (D), and on the activation of caspase 9 and caspase 3 (E) induced by ioversol in LLC-PK1 cells. A: DBcAMP (1 mmol/L) shifted the concentration-response curves for ioversol in reducing cell viability to the right. **P < 0.01 vs. respective control (Student’s t-test). B: Ioversol-induced loss of cell viability was prevented not only by DBcAMP (0.1–1 mmol/L) but also by forskolin (FSK: 10 μmol/L) and beraprost (BP: 1 μmol/L). †P < 0.05, ††P < 0.01 vs. control (Dunnett’s test). C: The inhibition by DBcAMP (1 mmol/L) of ioversol-induced loss of cell viability was reversed by an A kinase inhibitor H89 (10 μmol/L), PI 3-kinase inhibitor wortmannin (10 nmol/L), and Akt inhibitor SH-6 (1 μmol/L). **P < 0.01 vs. control (Dunnett’s test). D: DBcAMP inhibited ioversol-induced apoptosis via A kinase/PI 3-kinase/Akt-dependent mechanisms. E: Ioversol-induced activations of caspase-9 and caspase-3 were prevented by DBcAMP in the manner dependent on A kinase/PI 3-kinase/Akt signals. **P < 0.01 vs. control (Dunnett’s test).
Phosphorylation of Akt and CREBt by DBcAMP
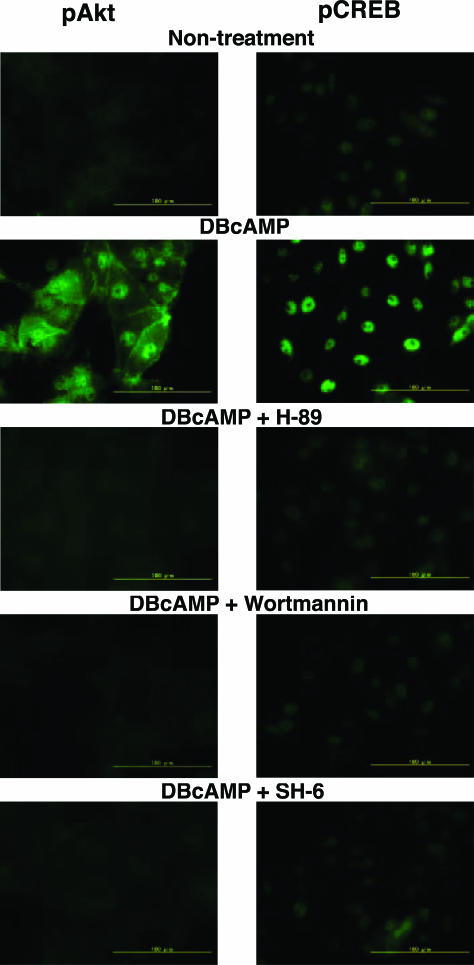
As shown in Figure 2, DBcAMP (0.3 mmol/L) markedly increased the number of cells stained with antiserum against pCREB, and the action of DBcAMP was completely blocked by H89.
Figure 2.
Effect of DBcAMP on the phosphorylation of Akt and CREB in LLC-PK1 cells. DBcAMP (0.3 mmol/L) increased the number of cells stained with anti-pAkt (Ser473) and anti-pCREB (Ser133) antisera. DBcAMP-induced phosphorylations of Akt and CREB were reversed by H89 (10 μmol/L), wortmannin (10 nmol/L), and SH-6 (1 μmol/L).
It has been demonstrated that CREB is phosphorylated not only by A kinase but also by other kinases such as PI 3-kinase/PDK1/Akt.28,29 To determine the role of PI 3-kinase/Akt, the effects of wortmannin and SH-6 on in DBcAMP-mediated CREB phosphorylation were examined. The DBcAMP-induced increase in pCREB-like immunoreactivity was almost completely reversed by 10 nmol/L wortmannin and 1 μmol/L SH-6, thereby indicating that DBcAMP phosphorylates CREB through acti-vation of A kinase/PI 3-kinase/Akt pathway. Indeed, DBcAMP increased the number of cells stained with anti-pAkt antibody, which was completely reversed by wortmannin and SH-6. The DBcAMP-induced increase in pAkt-like immunoreactivity was also completely blocked by H89.
Requirement of CREB Phosphorylation in Protective Effect of DBcAMP
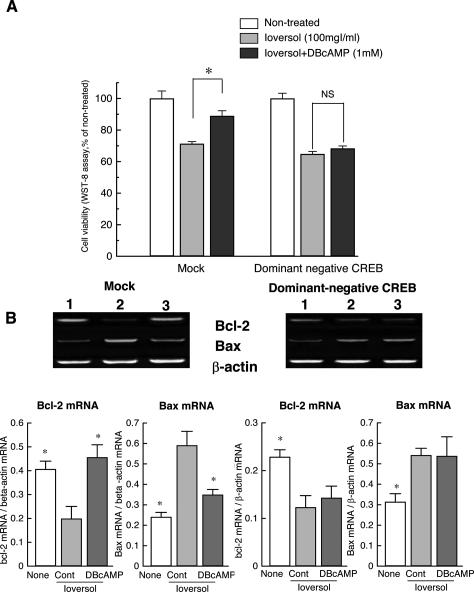
To determine whether phosphorylation of CREB is re-quisite for the protective effect DBcAMP, the effect of DBcAMP was tested in dominant negative form of CREB (ProCREB)-transfected cells. As shown in Figure 3A, the protective effect of DBcAMP, which was observed in mock-transfected LLC-PK1 cells, was no longer observed in dominant negative CREB-transfected cells.
Figure 3.
Lack of effects of DBcAMP on ioversol-induced loss of cell viability (A) and changes in mRNA expression for Bcl-2 and Bax (B) in dominant negative CREB (ProCREB)-transfected LLC-PK1 cells. A: DBcAMP prevented ioversol-induced cell injury in mock cells but not in cells expressed with a dominant negative form of CREB. B: DBcAMP reversed the decrease in Bcl-2 mRNA and increase in Bax mRNA in mock cells but not in ProCREB-transfected cells. *P < 0.05 vs. control (Steel’s test).
We previously reported that ioversol decreases mRNA expression for Bcl-2 and increases that for Bax, which are reversed by DBcAMP.7 The protein contents of Bcl-2 and Bax were also similarly changed after treatment with ioversol in the absence or presence of DBcAMP: Bcl-2 contents (pg/mg protein) were significantly reduced by ioversol from 462.7 ± 36.2 (mean ± SEM, N = 4) in non-treated cells to 232.3 ± 33.8 (N = 4), which was significantly inhibited by 1 mmol/L DBcAMP in a manner dependent on A kinase (411.7 ± 45.3, N = 4, for ioversol+DBcAMP-treated group; 299.4 ± 26.7, N = 4, for ioversol+DBcAMP+ H89-treated group. The Bax contents (pg/mg protein) were significantly elevated by ioversol from 65.3 ± 36.9 (N = 4) in non-treated cells to 834.6 ± 102.2 (N = 4), which was also significantly reversed by DBcAMP via A kinase-dependent mechanism (154.0 ± 54.8, N = 4, for ioversol+DBcAMP-treated group; 526.8 ± 36.2, N = 4, for ioversol+DBcAMP+H89-treated group). Since the changes in mRNA expression for Bcl-2 and Bax were consistent with changes in the contents of these Bcl-2 family proteins, we determined only mRNA expressions for Bcl-2 and Bax in the subsequent study.
In control cells, DBcAMP inhibited the increase in Bcl-2 mRNA and decrease in Bax mRNA induced by ioversol (Figure 3B), whereas, DBcAMP did no longer reverse ioversol-induced changes in mRNA expressions for Bcl-2 and Bax in dominant negative CREB-transfected cells (Figure 3B).
Protective Effect of Beraprost in the in Vivo Model of Radiocontrast Nephropathy in Mice
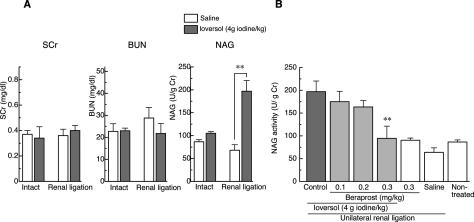
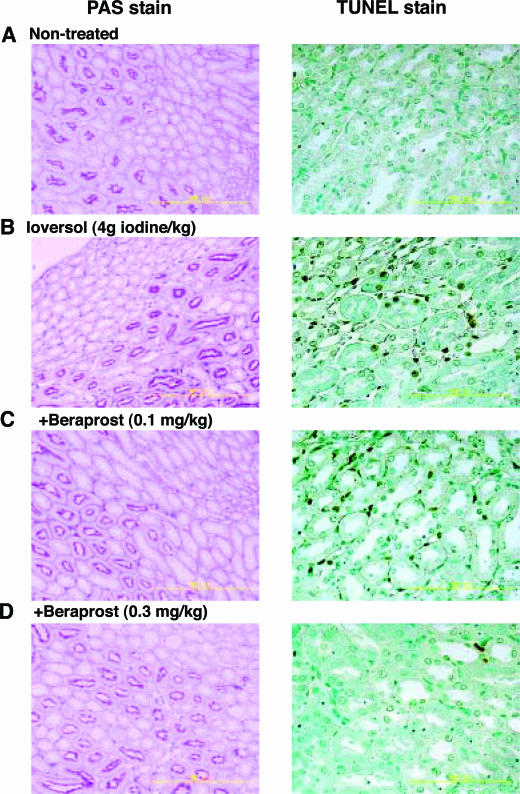
To test the effectiveness of cAMP in the in vivo model of radiocontrast nephropathy, the effect of beraprost on renal injury induced by a systemic injection of ioversol was investigated. In mice with unilateral renal occlusion, intravenous injection of ioversol (4 g iodine/kg) caused a marked increase in the activity of urinary NAG, a specific marker of renal tubular cell injury, at 24 hours after injection (Figure 4B), although the level returned to the baseline at 48 hours after injection. By contrast, ioversol caused no significant effect on either serum level of creatinine or blood urea nitrogen in mice with or without unilateral renal ligation (Figure 4A). Histological observations revealed that a number of TUNEL-positive cells appeared in the outer medulla at 24 hours after injection (Figure 5), indicating that the ioversol-induced kidney injury is associated at least in part with apoptosis. A single administration of beraprost (0.1−0.3 mg/kg, i.p.) attenuated the ioversol-induced elevation of urinary NAG in a dose-dependent manner (Figure 4). The number of TUNEL-positive cells were also reduced by beraprost treatment (Figure 5).
Figure 4.
Changes in the concentrations of serum creatinine (SCr), blood urea nitrogen (BUN) and urinary NAG activity after intravenous injection of ioversol (A) and the effect of beraprost on ioversol-induced elevation of urinary NAG activity (B) in mice. A: Ioversol (4 g iodine/kg, i.v.) increased urinary excretion of NAG without affecting the levels of SCr or BUN in mice with unilateral renal ligation. **P < 0.01 vs. saline (Student’s t-test). B: Beraprost (0.1–0.3 mg/kg, i.p.) reversed the ioversol-induced increase in urinary NAG in a dose-dependent manner. **P < 0.01 vs. control (Dunnett’s test).
Figure 5.
Effect of beraprost on ioversol-induced morphological changes in the kidney of mice with unilateral renal ligation. Beraprost protected renal tissues against ioversol-induced tubular and interstitial cell injury as assessed by PAS stain and TUNEL stain.
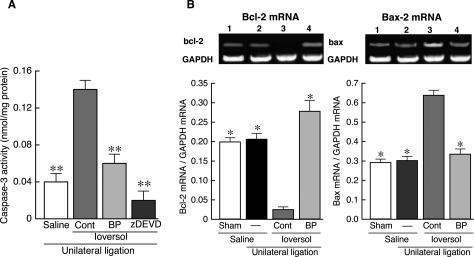
On the other hand, caspase-3 activity in renal medulla was remarkably elevated by ioversol injection (Figure 6A). This activation of caspase-3 was completely abolished by zDEVD, thereby confirming that the response is specific for caspase-3. Interestingly, prior treatment with beraprost (0.3 mg/kg) reversed almost completely the ioversol-induced caspase-3 activation. In addition, the mRNA expression for Bcl-2 dramatically reduced, while that for Bax increased, in renal medulla of mice treated with ioversol (Figure 6B). It was notable that beraprost at 0.3 mg/kg completely reversed the changes in mRNA expression for Bcl-2 and Bax caused by ioversol.
Figure 6.
Effect of beraprost on activation of caspase-3 (A) and changes in mRNA expressions for Bcl-2 and Bax (B) induced by ioversol in renal medulla of mice with unilateral renal ligation. A: Ioversol (4 g iodine/kg, i.v.) significantly increased caspase-3 activity, which was markedly reversed by beraprost (BP: 0.3 mg/kg, i.p.). **P < 0.01 vs. control (Dunnett’s test). B: Ioversol dramatically reduced Bcl-2 mRNA expression, while enhancing Bax mRNA expression, in renal medulla. BP (0.3 mg/kg) completely blocked ioversol-induced changes in mRNA expressions for Bcl-2 and Bax. *P < 0.05 vs. control (Steel’s test).
Discussion
In our previous report,7 a variety of ionic and non-ionic radiocontrast media causes the loss of cell viability, nuclear fragmentation and extrusion of phosphatidyl serine to the outer membrane of LLC-PK1 cells as assessed by annexin V stain, suggesting that radiocontrast medium-induced cell injury is associated with apoptosis. We also found the protective effects of DBcAMP and beraprost against ioversol-induced apoptosis in LLC-PK1 cells.7,8 Consistent with our previous findings, in the present study, ioversol reduced cell viability and induced apoptosis in a manner dependent on the activities of caspase-9 and caspase-3. Moreover, ioversol markedly reduced the mRNA and protein content of Bcl-2 and enhanced those for Bax. Therefore, the activation of caspase-9 followed by caspase-3 induced by ioversol may be due to the reduction in Bcl-2, since the activity of caspase-9 is reported to be regulated by bcl-2 family including Bcl-2 and Bax.30–32 DBcAMP attenuated the ioversol-induced cell injury and shifted the concentration response curve for ioversol-induced loss of cell viability to the right. It was notable that the protective effect of DBcAMP against nephrotoxic action of ioversol was reversed by H89, wortmannin and SH-6. SH-6 is a phosphatidyl-inositol analog that inhibits specifically the Akt activation without affecting other kinases such as 3-phosphoinositide-dependent protein kinase-1 (PDK-1) and MAP kinase.27,33 Therefore, it is suggested that A kinase/PI 3-kinase/Akt pathway is involved in the protective action of DBcAMP. Indeed, DBcAMP increased the phosphorylation of Akt, as assessed by immunofluorescent stain with anti-pAkt antibody. Moreover, DBcAMP-increased Akt phosphorylation was abolished by H89 and wortmannin, suggesting that the Akt phosphorylation is mediated A kinase and PI 3-kinase. It has been demonstrated that cAMP stimulates the activity of PI 3-kinase/Akt in rat hepatocytes.34 Therefore, DBcAMP may cause A kinase-dependent activation of PI 3-kinase, leading to the phosphorylation of Akt.
In the present study, ioversol-induced activations of caspase-9 and caspase-3 were markedly reduced by DBcAMP. In addition, the inhibitory effect of DBcAMP on caspase activation was almost completely reversed by H89, wortmannin and SH-6, thereby suggesting the involvement of A kinase/PI 3-kinase/Akt signals in DBcAMP-induced inhibition of caspase activation. Interestingly, our present data are generally consistent with the data reported by Webster et al34 who reported that cAMP inhibits bile acid-induced apoptosis of rat hepatocyte by activating PI 3-kinase/Akt and subsequent inhibition of caspases 9 and 3.
The activation of caspase-9 is known to be initiated by the release of mitochondrial cytochrome c, which binds to the adapter molecule apoptotic protease activating factor-1 (Apaf-1) and activates pro-caspase-9.35 Moreover, cytochrome c release is regulated by Bax and Bcl-2 on the outer membrane of mitochondria.30–32 In the present study, DBcAMP prevented both the reduction in Bcl-2 mRNA and its protein and the increase in Bax mRNA and its protein induced by ioversol. More interestingly, the inhibitory effects of DBcAMP on ioversol-induced changes in Bcl-2 and Bax proteins were dependent on A kinase/PI 3-kinase/Akt activity. Therefore, the inhibitory effect of DBcAMP on ioversol-induced activation of caspases may be due to the increase in Bcl-2 and decrease in Bax.
It has been demonstrated that phosphorylation of CREB at Ser133 binds to CRE site located on the promoter region of bcl-2 gene and up-regulates Bcl-2 expression.12,36,37 Moreover, Pugazhenthi et al28 have shown that the activation of PI 3-kinase/Akt increases the expression for Bcl-2 by enhancing CREB activity in PC12 cells. Consistent with their findings, in the present study, DBcAMP-induced increase in pCREB immunoreactivity was completely blocked by H89, wortmannin and SH-6, suggesting that A kinase-dependent phosphorylation of CREB is meditated almost exclusively by PI 3-kinase/Akt pathway.
However, it is still uncertain whether phosphorylation of CREB is essential to the protective action of DBcAMP against ioversol-induced apoptotic injury in LLC-PK1 cells. To clarify the role of CREB, we investigated the effect of DBcAMP on ioversol-induced injury in LLC-PK1 cells transfected with a dominant negative form of CREB (Pro CREB). Pro CREB has an Arg-to-Pro amino acid substitution at position 301 in the DNA binding domain and cannot bind to DNA.20 Interestingly, DBcAMP was no longer effective in preventing ioversol-induced cell injury in Pro CREB-transfected cells. Moreover, DBcAMP had no influence on the reduction of Bcl-2 mRNA expression or enhancement of Bax mRNA expression induced by ioversol in these cells. Therefore, it is assumed that phosphorylation of CREB is required for DBcAMP to fulfill its protective action against ioversol-induced apoptosis in LLC-PK1 cells. Taken together, our present findings suggest that DBcAMP stimulates PI 3-kinase/Akt signals via A kinase, and phosphorylates CREB, which, in turn, facilitates Bcl-2 expression and reduces Bax expression, leading to the inhibition of caspase activation and cell protection.
Subsequently, we investigated the effect of beraprost in the in vivo model of radiocontrast nephropathy. It is generally considered that radiocontrast medium causes no marked renal dysfunction, when injected alone in normal animals, however, a slight but significant increase in serum creatinine level is observed when the contrast medium is injected in combination with a nitric oxide synthesis inhibitor NG-nitro-l-arginine and indomethacin.38,39 On the other hand, a more marked increase in urinary NAG activity, a marker of lysosomal activity in renal tubular cells, is observed after treatment with contrast medium in renal artery-clamped rats40 or unilateral nephrectomized rats.41 It has also been reported that a significant elevation of urinary NAG is observed in rats after injection with radiocontrast medium in combination with gentamicin42,43 or in rabbits.44 In humans, urinary NAG excretion is shown to be a more sensitive marker of renal dysfunction than serum creatinine after administration of radiocontrast medium.45 Consistent with these data, in the present study, urinary NAG activity was significantly elevated at 24 hours after injection of 4 g iodine/kg ioversol in mice with unilateral renal occlusion, although serum creatinine or blood urea nitrogen was not significantly changed by ioversol. Moreover, it was noteworthy that a number of TUNEL-positive cells were observed in tubular and interstitial cells of outer medulla after ioversol injection. It was also notable that mRNA expression for Bcl-2 was dramatically reduced, while that for Bax was enhanced, in the outer medulla of the kidney in ioversol-treated mice. These findings, taken together, suggest that renal tubular/interstitial injury induced by systemic injection of ioversol is associated with apoptosis. A single injection of beraprost abolished ioversol-induced enhancement of urinary NAG activity and apoptosis. Moreover, the decrease in mRNA expression for Bcl-2 and increase in that for Bax were reversed completely by beraprost.
In conclusion, we demonstrated here for the first time that phosphorylation of CREB through activation of A kinase/PI 3-kinase/Akt pathway is important to prevent radiocontrast medium-induced apoptosis in renal cells. Moreover, beraprost was highly effective in attenuating the contrast medium-induced renal injury. Beraprost is a stable and orally active prostacyclin analog clinically used for the treatment of Buerger’s disease, arteriosclerosis obliterans and primary pulmonary hypertension. In this respect, this drug may be potentially useful for the prophylaxis of radiocontrast nephropathy.
Footnotes
Address reprint requests to Takahisa Yano, Ph.D., Department of Pharmacy, Kyushu University Hospital, 3–1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan. E-mail: tyano@pharm.med.kyushu-u.ac.jp.
References
- Andersen KJ, Christensen EI, Vik H. Effects of iodinated x-ray contrast media on renal epithelial cells in culture. Invest Radiol. 1994;29:955–962. doi: 10.1097/00004424-199411000-00002. [DOI] [PubMed] [Google Scholar]
- Hardiek K, Katholi RE, Ramkumar V, Deitrick C. Proximal tubule cell response to radiographic contrast media. Am J Physiol Renal Physiol. 2001;280:F61–F70. doi: 10.1152/ajprenal.2001.280.1.F61. [DOI] [PubMed] [Google Scholar]
- Hizoh I, Haller C. Radiocontrast-induced renal tubular cell apoptosis: hypertonic versus oxidative stress. Invest Radiol. 2002;37:428–434. doi: 10.1097/00004424-200208000-00003. [DOI] [PubMed] [Google Scholar]
- Zager RA, Johnson AC, Hanson SY. Radiographic contrast media-induced tubular injury: evaluation of oxidant stress and plasma membrane integrity. Kidney Int. 2003;64:128–139. doi: 10.1046/j.1523-1755.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- Liss P, Nygren A, Hansell P. Hypoperfusion in the renal outer medulla after injection of contrast media in rats. Acta Radiol. 1999;40:521–527. doi: 10.3109/02841859909175578. [DOI] [PubMed] [Google Scholar]
- Lancelot E, Idee JM, Couturier V, Vazin V, Corot C. Influence of the viscosity of iodixanol on medullary and cortical blood flow in the rat kidney: a potential cause of nephrotoxicity. J Appl Toxicol. 1999;19:341–346. doi: 10.1002/(sici)1099-1263(199909/10)19:5<341::aid-jat584>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Yano T, Itoh Y, Sendo T, Kubota T, Oishi R. Cyclic AMP reverses radiocontrast media-induced apoptosis in LLC-PK1 cells by activating A kinase/PI3 kinase. Kidney Int. 2003;64:2052–2063. doi: 10.1046/j.1523-1755.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- Yano T, Itoh Y, Kubota T, Sendo T, Oishi R. A prostacyclin analog beraprost sodium attenuates radiocontrast media-induced LLC-PK1 cells injury. Kidney Int. 2004;65:1654–1663. doi: 10.1111/j.1523-1755.2004.00575.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez GA, Yamamoto KK, Fischer WH, Karr D, Menzel P, Biggs W, 3rd, Vale WW, Montminy MR. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989;337:749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Bonni A, Brunet A, West AE, Datta SR, Takasu MA, Greenberg ME. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science. 1999;286:1358–1362. doi: 10.1126/science.286.5443.1358. [DOI] [PubMed] [Google Scholar]
- Chen W, Yu YL, Lee SF, Chiang YJ, Chao JR, Huang JH, Chiong JH, Huang CJ, Lai MZ, Yang-Yen HF, Yen JJ. CREB is one component of the binding complex of the Ces-2/E2A-HLF binding element and is an integral part of the interleukin-3 survival signal. Mol Cell Biol. 2001;21:4636–4646. doi: 10.1128/MCB.21.14.4636-4646.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson BE, Mochon E, Boxer LM. Induction of Bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol Cell Biol. 1996;16:5546–5556. doi: 10.1128/mcb.16.10.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science. 1999;286:2358–2361. doi: 10.1126/science.286.5448.2358. [DOI] [PubMed] [Google Scholar]
- Rehfuss RP, Walton KM, Loriaux MM, Goodman RH. The cAMP-regulated enhancer-binding protein ATF-1 activates transcription in response to cAMP-dependent protein kinase A. J Biol Chem. 1991;266:18431–18434. [PubMed] [Google Scholar]
- Isobe I, Michikawa M, Yanagisawa K. Enhancement of MTT, a tetrazolium salt, exocytosis by amyloid beta-protein and chloroquine in cultured rat astrocytes. Neurosci Lett. 1999;266:129–132. doi: 10.1016/s0304-3940(99)00282-7. [DOI] [PubMed] [Google Scholar]
- Oberhaus SM. TUNEL and immunofluorescence double-labeling assay for apoptotic cells with specific antigen(s). Methods Mol Biol. 2003;218:85–21896. doi: 10.1385/1-59259-356-9:85. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Gupta AK, McKenna WG, Weber CN, Feldman MD, Goldsmith JD, Mick R, Machtay M, Rosenthal DI, Bakanauskas VJ, Cerniglia GJ, Bernhard EJ, Weber RS, Muschel RJ. Local recurrence in head and neck cancer: relationship to radiation resistance and signal transduction. Clin Cancer Res. 2002;8:885–889. [PubMed] [Google Scholar]
- Inglefield JR, Mundy WR, Meacham CA, Shafer TJ. Identification of calcium-dependent and -independent signaling pathways involved in polychlorinated biphenyl-induced cyclic AMP-responsive element-binding protein phosphorylation in developing cortical neurons. Neuroscience. 2002;115:559–573. doi: 10.1016/s0306-4522(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Belmonte N, Phillips BW, Massiera F, Villageois P, Wdziekonski B, Saint-Marc P, Nichols J, Aubert J, Saeki K, Yuo A, Narumiya S, Ailhaud G, Dani C. Activation of extracellular signal-regulated kinases and CREB/ATF-1 mediate the expression of CCAAT/enhancer binding proteins beta and -delta in preadipocytes. Mol Endocrinol. 2001;15:2037–2049. doi: 10.1210/mend.15.11.0721. [DOI] [PubMed] [Google Scholar]
- Saeki K, Yuo A, Takaku F. Cell-cycle-regulated phosphorylation of cAMP response element-binding protein: identification of novel phosphorylation sites. Biochem J. 1999;338 (Pt 1):49–54. [PMC free article] [PubMed] [Google Scholar]
- Rey C, Mauduit C, Naureils O, Benahmed M, Louisot P, Gasnier F. Up-regulation of mitochondrial peripheral benzodiazepine receptor expression by tumor necrosis factor alpha in testicular leydig cells: possible involvement in cell survival. Biochem Pharmacol. 2000;60:1639–1646. doi: 10.1016/s0006-2952(00)00500-1. [DOI] [PubMed] [Google Scholar]
- Kim MS, Lee DY, Wang T, Schroeder JJ. Fumonisin B (1) induces apoptosis in LLC-PK(1) renal epithelial cells via a sphinganine- and calmodulin-dependent pathway. Toxicol Appl Pharmacol. 2001;176:118–126. doi: 10.1006/taap.2001.9188. [DOI] [PubMed] [Google Scholar]
- Nishio S, Matsuura H, Kanai N, Fukatsu Y, Hirano T, Nishikawa N, Kameoka K, Umetsu T. The in vitro and ex vivo antiplatelet effect of TRK-100, a stable prostacyclin analog, in several species. Jpn J Pharmacol. 1988;47:1–10. doi: 10.1254/jjp.47.1. [DOI] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J. 1993;296(Pt 2):297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuillet EJ, Mahadevan D, Vankayalapati H, Berggren M, Williams R, Coon A, Kozikowski AP, Powis G. Specific inhibition of the Akt1 pleckstrin homology domain by D-3-deoxy-phosphatidyl-myo-inositol analogues. Mol Cancer Ther. 2003;2:389–399. [PubMed] [Google Scholar]
- Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- Kroemer G. The proto-oncogene Bcl-2 and its role in regulating apoptosis. Nat Med. 1997;3:614–620. doi: 10.1038/nm0697-614. [DOI] [PubMed] [Google Scholar]
- Xiang J, Chao DT, Korsmeyer SJ. BAX-induced cell death may not require interleukin 1b-converting enzyme-like proteases. Proc Natl Acad Sci USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozikowski AP, Sun H, Brognard J, Dennis PA. Novel PI analogues selectively block activation of the pro-survival serine/threonine kinase, Akt. J Am Chem Soc. 2003;125:1144–1145. doi: 10.1021/ja0285159. [DOI] [PubMed] [Google Scholar]
- Webster CR, Srinivasulu U, Ananthanarayanan M, Suchy FJ, Anwer MS. Protein kinase B/Akt mediates cAMP- and cell swelling-stimulated Na+/taurocholate cotransport and Ntcp translocation. J Biol Chem. 2002;277:28578–28583. doi: 10.1074/jbc.M201937200. [DOI] [PubMed] [Google Scholar]
- Robertson JD, Orrenius S. Role of mitochondria in toxic cell death. Toxicology. 2002;181–182:491–496. doi: 10.1016/s0300-483x(02)00464-x. [DOI] [PubMed] [Google Scholar]
- Ji L, Mochon E, Arcinas M, Boxer LM. CREB proteins function as positive regulators of the translocated bcl-2 allele in t(14;18) lymphomas. J Biol Chem. 1996;271:22687–22691. doi: 10.1074/jbc.271.37.22687. [DOI] [PubMed] [Google Scholar]
- Freeland K, Boxer LM, Latchman DS. The cyclic AMP response element in the Bcl-2 promoter confers inducibility by hypoxia in neuronal cells. Brain Res Mol Brain Res. 2001;92:98–106. doi: 10.1016/s0169-328x(01)00158-9. [DOI] [PubMed] [Google Scholar]
- Touati C, Idee JM, Deray G, Santus R, Balut C, Beaufils H, Jouanneau C, Bourbouze R, Doucet D, Bonnemain B. Modulation of the renal effects of contrast media by endothelium-derived nitric oxide in the rat. Invest Radiol. 1993;28:814–820. [PubMed] [Google Scholar]
- Wang YX, Jia YF, Chen KM, Morcos SK. Radiographic contrast media induced nephropathy: experimental observations and the protective effect of calcium channel blockers. Br J Radiol. 2001;74:1103–1108. doi: 10.1259/bjr.74.888.741103. [DOI] [PubMed] [Google Scholar]
- Deray G, Dubois M, Martinez F, Baumelou B, Beaufils H, Bourbouze R, Baumelou A, Jacobs C. Renal effects of radiocontrast agents in rats: a new model of acute renal failure. Am J Nephrol. 1990;10:507–513. doi: 10.1159/000168177. [DOI] [PubMed] [Google Scholar]
- Idee JM, Santus R, Beaufils H, Balut C, Huntsman AM, Bourbouze R, Koeltz B, Jouanneau C, Bonnemain B. Comparative effects of low- and high-osmolar contrast media on the renal function during early degenerative gentamicin-induced nephropathy in rats. Am J Nephrol. 1995;15:66–74. doi: 10.1159/000168803. [DOI] [PubMed] [Google Scholar]
- Hofmeister R, Bhargava AS, Gunzel P. The use of urinary N-acetyl-beta-D-glucosaminidase (NAG) for the detection of contrast-media-induced “osmotic nephrosis” in rats. Toxicol Lett. 1990;50:9–15. doi: 10.1016/0378-4274(90)90247-j. [DOI] [PubMed] [Google Scholar]
- Thomsen HS, Dorph S, Larsen S, Horn T, Hemmingsen L, Skaarup P, Golman K, Svendsen O. Urine profiles and kidney histologic findings after intravenous injection of mannitol and iohexol in the degeneration phase of gentamicin nephropathy in rats. Invest Radiol. 1993;28:133–141. doi: 10.1097/00004424-199302000-00010. [DOI] [PubMed] [Google Scholar]
- Leander P, Allard M, Caille JM, Golman K. Early effect of gadopentetate and iodinated contrast media on rabbit kidneys. Invest Radiol. 1992;27:922–926. doi: 10.1097/00004424-199211000-00009. [DOI] [PubMed] [Google Scholar]
- Erley CM, Duda SH, Rehfuss D, Scholtes B, Bock J, Muller C, Osswald H, Risler T. Prevention of radiocontrast-media-induced nephropathy in patients with pre-existing renal insufficiency by hydration in combination with the adenosine antagonist theophylline. Nephrol Dial Transplant. 1999;14:1146–1149. doi: 10.1093/ndt/14.5.1146. [DOI] [PubMed] [Google Scholar]