Abstract
We evaluated the therapeutic efficacy of topical administration of SN50, an inhibitor of nuclear factor-κB, in a corneal alkali burn model in mice. An alkali burn was produced with 1 N NaOH in the cornea of C57BL/6 mice under general anesthesia. SN50 (10 μg/μl) or vehicle was topically administered daily for up to 12 days. The eyes were processed for histological or immunohistochemical examination after bromodeoxyuridine labeling or for semiquantification of cytokine mRNA. Topical SN50 suppressed nuclear factor-κB activation in local cells and reduced the incidence of epithelial defects/ulceration in healing corneas. Myofibroblast generation, macrophage invasion, activity of matrix metalloproteinases, basement membrane destruction, and expression of cytokines were all decreased in treated corneas compared with controls. To elucidate the role of tumor necrosis factor (TNF)-α in epithelial cell proliferation, we performed organ culture of mouse eyes with TNF-α, SN50, or an inhibitor of c-Jun N-terminal kinase (JNK) and examined cell proliferation in healing corneal epithelium in TNF-α−/− mice treated with SN50. An acceleration of epithelial cell proliferation by SN50 treatment was found to depend on TNF-α/JNK signaling. In conclusion, topical application of SN50 is effective in treating corneal alkali burns in mice.
The cornea is a highly organized avascular transparent tissue located in the anterior part of the eye, which must remain transparent to refract light properly. An alkali burn in this tissue may cause a permanent, severe, visual impairment. The postinjury sequence includes acute inflammation and degradation of the matrix of the epithelial basement membrane (BM) and stroma.1–3 These events result in a persistent epithelial defect and subsequent stromal ulceration, and then, even in resurfaced cases, opacification of the stroma and conjunctivalization of the corneal surface occur that impair patients’ vision in the later healing phase.4 Aggressive treatment with anti-inflammatory drugs and surgery in severe cases still may not restore vision in these cases.4–6
Inflammation plays an important role in tissue destruction and remodeling in injured tissue including an alkali-burned cornea.7 Although invasion of monocytes/macrophages is critical in wound healing, excessive infiltration of monocytes/macrophages into the corneal stroma is considered to be unfavorable because they secrete matrix metalloproteinases (MMPs) and other proteins undesirable for tissue healing.7,8 A number of cytokines and growth factors that are up-regulated in corneal cells further contribute to tissue inflammation. A majority of inflammatory cytokines use the nuclear factor (NF)-κB pathway for signaling on ligand binding to cell surface receptors.9,10 We therefore hypothesized that blocking the NF-κB pathway might be beneficial in treating corneal alkali burns as has been shown in other inflammatory diseases.11–17 In the present study a corneal alkali burn model generated by topical alkali administration to the mouse eye was used to evaluate the therapeutic potential of topical administration of SN50,18–21 an inhibitor of NF-κB. Stromal healing was evaluated histologically, as well as by the immunohistochemical detection of expression of α-smooth muscle actin (α-SMA), a hallmark of myofibroblast generation,22–25 immunolocalization of BM components, and evaluation of monocyte/macrophage invasion by detection of F4/80-labeled cells. Expression of soluble factors involved in corneal healing was evaluated by using immunohistochemistry and real-time reverse transcription-polymerase chain reaction (real-time RT-PCR), whereas activity of MMPs was evaluated by using in situ zymography. Epithelial cell proliferation was examined by bromodeoxyuridine (BrdU) labeling. Finally, the role of tumor necrosis factor (TNF)-α in modulation of cell proliferation in regenerated epithelium by the NF-κB inhibitor was examined in TNF-α-null mice26 as well as in organ-culture of a mouse eye with an epithelial defect.
Materials and Methods
Experimental Protocol
All experimental procedures were approved by the DNA Recombination Experiment Committee and Animal Care and Use Committee of Wakayama Medical University, Wakayama, Japan, and conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
A round piece of filter paper, 1.5 mm in diameter, containing 2 μl of 1 N NaOH was applied to the right eye to produce a corneal burn in 4-week-old male C57BL/6 mice under general anesthesia by intraperitoneal pentobarbital. A peptide inhibitor of NF-κB, SN50 (AAVALLPAVLLALLAP-VQRKRQKLMP, catalog no. 25-007; Upstate Biotechnology, Lake Placid, NY)18–21 was dissolved in 0.5% hyaluronan (10 μg/μl) and applied topically (3 μl) to the burned eye daily until the mice were sacrificed 2 hours after injection with BrdU (120 μg/g body weight)27 at days 3, 7, and 12 after wounding. Control animals were treated with the vehicle of 0.5% hyaluronan. Our preliminary experiment showed no difference in the healing process between alkali-burned mouse corneas treated with the control peptide provided by Upstate Biotechnology (catalog no. 25-003) and those with vehicle alone (data not shown). The numbers of eyes (animals) used for preparation of paraffin sections were 8 and 10 at days 3, 15, and 16 and at days 7, 9, 10, and 12 in the control group and treated group, respectively. Eyes from three animals in each of the control or SN50-treatment group were processed for OCT embedding, cryosectioning, and in situ zymography at day 7. Moreover, four specimens of both control and treated groups at day 7 and four uninjured corneas from two mice were processed for real-time RT-PCR for semiquantification of mRNA expression of cytokines and related molecules.
Western Blotting to Examine Signal Transduction Status
Corneas treated with SN50 or vehicle for 7 or 12 days were excised and homogenized in tissue lysis buffer (CelLytic MT; Sigma, St. Louis, MO) containing a proteinase inhibitor (Complete protease inhibitor cocktail tablet; Rosch, Mannheim, Germany) using an ultrasound homogenizer. Four corneas were used for each experimental condition and four uninjured corneas were also included. The samples were centrifuged and mixed with 3× sample buffer. The protein was processed for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and fractionated proteins were transferred to polyvinylidene difluoride membrane for incubation with antibodies against total RelA [1:1000 in phosphate-buffered saline (PBS); Santa Cruz Biotechnology, Santa Cruz, CA], phospho-RelA (1:1000 in PBS; Cell Signaling, Beverly, MA), c-Jun N-terminal kinase (JNK) (Santa Cruz), phospho-JNK (1:1000 in PBS, Cell Signaling), and actin (1:1000 in PBS, Santa Cruz). Detection of phospho-RelA or phospho-JNK was performed on the polyvinylidene difluoride membrane first stained for total RelA to total JNK, respectively, after stripping the antibodies. Immunoreactive bands were visualized on Lumino Analyzer LAS1000 (Fuji Film, Tokyo, Japan) using enhanced chemiluminescence Western blotting detection reagents (Amersham, Buckinghamshire, UK).
Immunohistochemistry
Deparaffinized sections (5 μm) were processed for hematoxylin and eosin (H&E) staining and indirect immunohistochemistry. The following primary antibodies were diluted in PBS; mouse monoclonal anti-phosphorylated p65/RelA of NF-κB (1:50, Cell Signaling), mouse monoclonal anti-phospho-JNK antibody (1:50, Cell Signaling), rat monoclonal anti-laminin antibody (1:25, Sigma), goat polyclonal anti-monocyte/macrophage-chemotactic protein-1 (MCP-1) antibody (1:100, Santa Cruz), goat polyclonal anti-MMP-9 antibody (1:100, Santa Cruz), rabbit polyclonal anti-tissue inhibitor of metalloproteinases-1 (TIMP-1) antibody (1:100, Santa Cruz), goat polyclonal anti-type IV collagen antibody (1:100; Southern Biotechnology, Birmingham AL), rabbit polyclonal anti-laminin antibody (1:25, Sigma), and mouse monoclonal anti-cyclin D1 antibody (1:100, Cell Signaling). For immunostaining for type IV collagen, deparaffinized sections were treated with 1% trypsin before application of primary antibody as previously reported.27 Immunohistochemistry for transforming growth factor (TGF)-β1, β2, and β3 was performed as previously reported.28,29 To evaluate cell proliferation in healing epithelium, the specimens were immunostained with anti-BrdU antibody (1:11 in PBS; Roche Diagnostics, Mannheim, Germany) as previously reported27 and the number of labeled cells in the healing epithelia in the affected cornea was determined. Four specimens were used to obtain the numbers in each group at all intervals and data were analyzed by using the unpaired t-test. The presence of myofibroblasts was examined by using mouse monoclonal anti-α-SMA antibody (1:100; Neomarker, Fremont, CA) and the number of labeled cells in the central cornea (200 μm length) was determined in five corneas in each condition and data were analyzed using the unpaired t-test. The presence of monocytes/macrophages was examined by using rat monoclonal F4/80 anti-macrophage antigen antibody (clone A3-1, 1:400 dilution in PBS; BMA Biomedicals, Augst, Switzerland). The number of labeled cells in the central cornea (200 μm length) was determined in four corneas in each condition and data were analyzed by using the unpaired t-test. Negative controls were performed by omitting each primary antibody.
In Situ Zymography
MMP activity in tissue was evaluated by using an in situ zymography kit (Wako Shin-yaku, Osaka, Japan) according to the manufacturer’s protocol. Unfixed cryosections of day 7 specimens were set on a thin film coated with gelatin and incubated for 24 hours at 37°C in a humidified chamber. Then the sections with the gelatin film were dried and stained with Biebrich Scarlet. To confirm that the digestion of gelatin resulted from MMP activity, the in situ zymography was also done using the gelatin film containing an MMP inhibitor, 1,10-phenanthrolone.
Real-Time RT-PCR
Total RNA was extracted and expression of mRNAs of tgfb1, mcp-1, mmp-9, and tissue inhibitor of metalloproteinases-1 (timp-1) was evaluated by real-time RT-PCR. Total RNA from corneal tissue excised from a burned eye or an uninjured mouse eye was extracted using ISO GENE (Nippon Gene, Tokyo, Japan) according to the manufacturer’s protocol and processed for semiquantitative real-time RT-PCR. Real-time RT-PCR using the TaqMan one-step RT-PCR master mix reagents kit and the Applied Biosystems Prism 7700 (PE Applied Biosystems, Foster City, CA) were used. Primers and oligonucleotide probes used are listed in Table 1 and were designed according to the cDNA sequences in the GenBank database, using the Primers Express software (PE Applied Biosystems, Foster City, CA). The conditions for RT-PCR were as follows: 2 minutes at 50°C (stage 1, reverse transcription), 10 minutes at 95°C (stage 2, reverse transcription inactivation and AmpliTaq Gold activation), and then 40 cycles of amplification for 15 seconds at 95°C and 1 minute at 60°C (stage 3, polymerase chain reaction).
Table 1.
Primers and Oligonucleotide Probes
| Transcript | Sequence |
|---|---|
| mTGFb1 | F: 5′-gca aca tgt gga act cta cca gaa-3′ |
| R: 5′-gac gtc aaa aga cag cca ctc-3′ | |
| P: 5′-acc ttg gta acc ggc tgc tga ccc-3′ | |
| mMCP1 | F: 5′-tgg ctc agc cag atg cag t-3′ |
| R: 5′-cca gcc tac tca ttg gga tca-3′ | |
| P: 5′-ccc cac tca cct gct gct act cat tca-3′ | |
| mMMP9 | F: 5′-aga cca agg gta cag cct gtt c-3′ |
| R: 5′-ggc acg ctg gaa tga tct aag-3′ | |
| P: 5′-cgc acg agt tcg gcc atg cac-3′ | |
| mTIMP-1 | F: 5′-gcc tac acc cca gtc atg ga-3′ |
| R: 5′-ggc ccg tga tga gaa act ctt-3′ | |
| P: 5′-tgg ata tgc cca caa gtc cca gaa cc-3′ |
F, forward primer; R, reverse primer; P, probe.
In Situ Nick-End Labeling of DNA Strand Breaks
To detect programmed cell death in corneal stroma, the terminal dUTP nick-end labeling (TUNEL) method was performed as previously reported.27 In brief, deparaffinized sections were digested with 2 μg/ml of proteinase K (Sigma-Aldrich, St. Louis, MO) for 5 minutes at room temperature. After washes in PBS and distilled water, tissue sections were incubated with 1× TdT (terminal deoxynucleotide transferase) buffer for 2 minutes (Life Technologies, Inc., Gaithersburg, MD). Sections were then treated with 1× TdT buffer containing TdT and biotinylated-dUTP (Boehringer Mannheim, Mannheim, Germany) for 45 minutes at 37°C. After a PBS wash, the sections were allowed to react with streptavidin-peroxidase (1:200 in PBS; DAKO, Carpinteria, CA) for 30 minutes at 37°C. After another wash in PBS, the peroxidase reaction was performed with 3,3′-diaminobenzidine. After counterstaining with methyl green, sections were embedded in balsam and observed by light microscopy.
Effect of Topical SN50 in a TNF-α-Null Mouse
Corneas in the right eyes of 4-week-old TNF-α-null mice on a C57BL/6 background (n = 8)27 received an alkali burn as described above. Four animals were treated with SN50 daily and four with control hyaluronan solution as described above for 7 days at which time the animals were killed 2 hours after an intraperitoneal injection with BrdU. The eyes were processed for immunohistochemistry for BrdU.
Organ Culture of Mouse Eyes with an Epithelial Defect
Organ culture of mouse eyes with a central round epithelial defect (1.5 mm in diameter) was conducted as previously reported.30 In brief, after debridement of the central corneal epithelium the eyeball was enucleated and placed in a well of a 24-well culture plate. Medium was supplemented with either mouse recombinant TNF-α (10 ng/ml; R&D Systems, Minneapolis, MN), SN50 (100 μg/ml), or a peptide JNK inhibitor (10 μmol/L, catalog no., 420116; Calbiochem, San Diego, CA). After 10 hours of incubation the specimens were labeled with BrdU for 2 hours and then fixed in 4% paraformaldehyde. Paraffin sections were immunostained for BrdU as previously reported. Four specimens were prepared for each culture condition and epithelial cells with BrdU-labeled nuclei were counted and statistically analyzed by using the unpaired t-test.
Results
Macrosopic Observation of the Affected Cornea
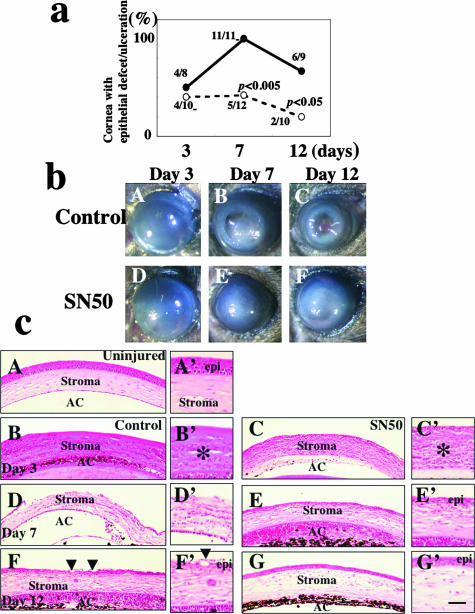
Healing of the corneal surface was evaluated by observing the presence or absence of an epithelial defect/ulceration, and the degree of corneal stromal opacification and the incidences of these findings are summarized in Figure 1a. At days 7 or 12, 100% or 67% of corneas receiving the vehicle showed remaining epithelial defects or stromal ulceration, whereas 42% or 20% of corneas had such disorders in the SN50 inhibitor-treated group, respectively (significant by χ2 test; P < 0.005 or P < 0.05 at days 7 or 12, respectively). Figure 1b shows the appearance of healing corneas in the control or SN50-treated groups. Stromal opacification was observed in corneas of each group even at day 12, although it seemed less in the SN50-treated group as compared with the control group. These results indicate that topical SN50 administration is effective in treating corneal alkali burns, and thus, further analyses were performed to examine the mechanism of its efficacy.
Figure 1.
Healing of an alkali-burned mouse cornea treated with topical SN50. a: Percentage of corneas with epithelial defect (including ulceration) at each healing interval. The incidence of epithelial defect/ulceration is significantly higher in the control group than in the treated group at days 7 and 12 as judged by the χ2 test. b: Macroscopic observation shows similar initial resurfacing in both control (A) and SN50-treated groups (D) at day 3 after alkali burning. Recurrence of the epithelial defect with stromal opacification is observed more frequently in the control group at days 7 (B) and 12 (C) as compared with SN50-treated group (E, F). c: Histology of burned corneas stained with H&E. A: An uninjured cornea. Stratified epithelium and stroma are seen. There is no histological difference between central corneas in the control (B) and SN50-treated group (C) at day 3. The epithelium shows a large defect and many inflammatory cells are observed. At day 7 the burned cornea in the control (D) shows more stromal inflammation, and a large epithelial defect as compared with the SN50-treated corneas that has been resurfaced with a thin epithelium (E). At day 12 the control cornea still shows marked inflammation and hypercellularity in the stroma (F), whereas the treated cornea exhibits a well-regenerated epithelium with a less stromal inflammation (G). Regenerated epithelium in control exhibits conjunctiva-like appearance with goblet cells (arrowheads). A’ to G’ are high-magnification pictures of the central area of the healing corneas in A to G, respectively. Scale bar; 100 μm (A–G), 25 μm (A’–G’).
H&E Histology
H&E-stained sections (Figure 1c) show infiltration of mononuclear cells and morphologically polymorphonuclear cells in the corneal stroma and anterior chamber with an epithelial defect in the central cornea of both control and SN50-treated groups at day 3 (Figure 1c; B, B’, C, C’). At day 7 no corneas were resurfaced in the control group (Figure 1c, D and D’), whereas topical SN50 administration accelerated epithelial regeneration (Figure 1c, E and E’). Inflammation by mononuclear cells in the stroma also seemed less in treated corneas as compared with control corneas. At day 12 the cornea shown in Figure 1c, F and F’, was resurfaced in association with abundant stromal cell repopulation and remaining inflammation, whereas a SN50-treated cornea was well healed with markedly less inflammation in the stroma (Figure 1c, G and G’). Stromal neovascularization was not prominent in the central burned area of specimens of each group throughout the healing interval.
NF-κB Signaling Status in Burned Corneas
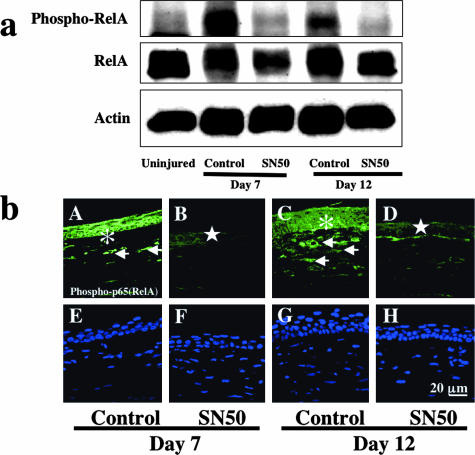
To examine if the SN50 inhibitor suppresses signaling through NF-κB, we performed Western blotting and immunohistochemistry with an antibody against the phosphorylated p65 subunit of NF-κB and total RelA. Western blotting detected bands representing total RelA at 65 kd and phospho-RelA at 85 kd in the control specimen that were decreased with SN50 treatment at both days 7 and 12 (Figure 2a) indicating suppression of NF-κB signaling by SN50.
Figure 2.
Detection of the p65 (RelA) subunit of NF-κB and phosphorylated RelA in burned corneas. a: Western blot for phospho-RelA, RelA, and actin. Western blotting shows up-regulation of phosphorylation of RelA at days 7 and 12 in control specimens with vehicle administration as compared with an uninjured control. This increased phosphorylation is suppressed in SN50-treated specimens at both time points. b: Immunohistochemical detection of phospho-RelA. In a control cornea phospho-p65 is detected in the healing epithelia (asterisks) and stromal cells (arrows) at days 7 (A) and 12 (C), whereas it is very faintly observed in the healing central epithelium (stars) in corneas treated with topical SN50 (B, D). E to H represent nuclear localization by DAPI staining of A to D, respectively. Scale bar, 20 μm.
To further characterize the suppression of RelA/NF-κB signaling in tissue, we performed immunohistochemistry. Epithelium and keratocytes in an uninjured cornea exhibited almost no immunoreactivity for phospho-p65 (data not shown). The results showed that topical administration of SN50 effectively blocked phosphorylation and nuclear translocation of the p65 subunit of NF-κB in both healing epithelial (Figure 2b, asterisks and stars) and stromal cells (Figure 2b, arrows). No specific staining was detected in the negative control (data not shown).
JNK Signaling Status in Burned Corneas
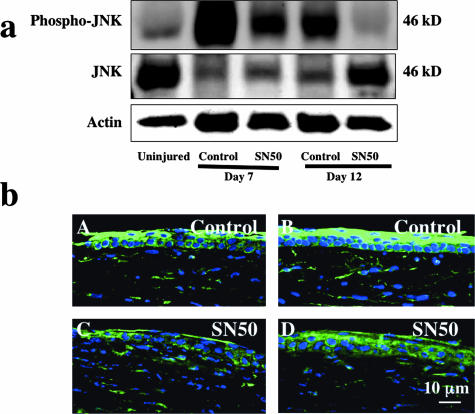
To examine if administration of the SN50 NF-κB inhibitor affects the signaling through JNK, we performed Western blotting and immunohistochemistry with an antibody against the phosphorylated JNK and total JNK. Western blotting detected phospho-JNK at 46 kd in healing burned corneas in control group at days 7 and 12 as compared with that in an uninjured control (Figure 3a). Administration of SN50 suppressed phospho-JNK at both days 7 and 12. Additionally, the level of total JNK was decreased in corneas involved in active wound healing at days 7 and 12 and was restored in a SN50-treated cornea at day 12.
Figure 3.
Detection of c-Jun-N-terminal kinase (JNK) and phosphorylated JNK in burned corneas. a: Western blot for phospho-JNK, JNK, and actin. Western blotting shows up-regulation of phosphorylation of JNK at day 7 in control specimens with vehicle administration as compared with an uninjured control. This increased phosphorylation is suppressed in SN50-treated specimens at both time points. b: Immunohistochemical detection of phospho-JNK. The healing corneal epithelium at the migrating edge (A, C) and also peripheral epithelium (B, D), as well as stromal cells, are markedly labeled with anti-phospho-JNK antibody in nontreated (A, B) corneas while immunoreactivity of phospho-JNK is weaker in the SN50-treated burned cornea (C, D). Scale bar, 10 μm.
To further characterize the effect of SN50 inhibitor on JNK signaling in tissue, we performed immunohistochemistry. Epithelium and keratocytes in an uninjured cornea exhibited almost no immunoreactivity for phospho-JNK (data not shown). Immunoreactivity of phospho-JNK was weaker in epithelium and stromal cells in the SN50-treated burned cornea (Figure 3b, C and D) as compared with nontreated cornea (Figure 3b, A and B). No specific staining was detected in the negative control (data not shown).
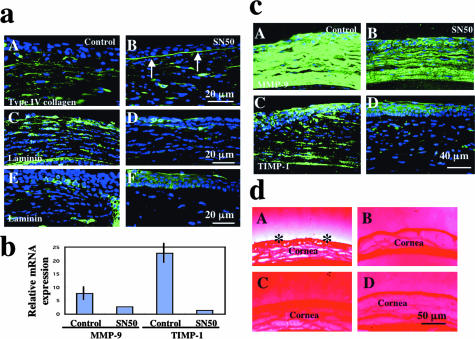
Expression of BM Components, MMP-9 and TIMP-1, and Endogenous MMP Activity Detected by in Situ Zymography
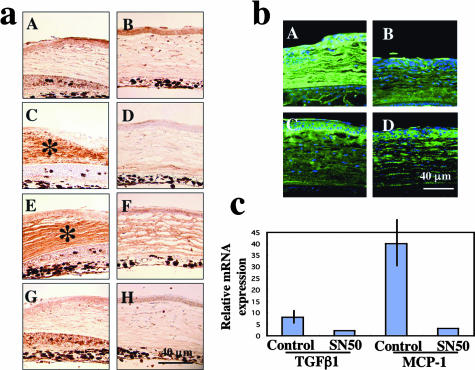
Corneal ulceration is initiated by the destruction of the epithelial BM in an alkali-burned cornea.1–3 It is known that MMPs secreted by activated corneal cells (epithelial cells and keratocytes) and macrophages degrade epithelial BM even though alkali exposure itself does not break it.1–3 We examined the integrity of the epithelial BM by immunohistochemical detection of collagen IV and laminin. Type IV collagen was readily observed in normal epithelial BM. Collagen IV was detected in stroma, but not beneath the regenerated epithelium in control corneas (Figure 4a, A), whereas epithelial BM type IV collagen was maintained in the inhibitor group (Figure 4a, B). At day 12 normal epithelial BM was restored in the inhibitor group, but BM components were strongly expressed in stromal cells with a thick deposition in the subepithelial area of the stroma (not shown). Laminin was detected strongly in stromal cells in control corneas at day 7 (Figure 4a, C) and faintly at day 12 (Figure 4a, E). It was expressed weakly in epithelial BM and stroma at day 7 (Figure 4a, D), but had disappeared by day 12 in the SN50 group (Figure 4a, F).
Figure 4.
Degradation of epithelial BM and expression of matrix-degrading enzymes in burned corneas. a: Expression pattern of BM components. Immunofluorescent detection of type IV collagen and MMP-9 in burned corneas at day 7. The BM zone does not exhibit a linear immunoreactivity for type IV collagen in association with its deposition by stromal keratocytes in a control cornea (A), whereas BM type IV collagen is preserved in a SN50-treated cornea (B, arrows). Laminin was detected strongly in stromal cells in control corneas at day 7 (C) and faintly at day 12 (E). It was seen in epithelial BM at day 7 (D) and never observed in the stroma at day 12 in the SN50 group (F). b: Real-time RT-PCR shows decreased expression of mRNAs of mmp-9 and timp-1 in SN50-treated corneas at day 7. Mean ± bar (SD). c: Expression pattern of matrix-degrading enzymes. MMP-9 expression in healing epithelium and keratocytes is more marked in control cornea (A) as compared with topical SN50 administration (B) at day 7. Although immunoreactivity for TIMP-1 was observed in both healing epithelium and stromal cells in control (C) and SN50 (D) groups at day 12, that in stromal cells was more marked in the control cornea. d: In situ zymography detects MMP activity in the cornea in control group (A, asterisks), but not in the SN50-treated cornea (B) at day 7. C and D: The MMP inhibitor abolishes the degradation of gelatin, indicating that the degradation of gelatin shown in A depends on endogenous MMPs. Scale bars: 20 μm (a); 40 μm (c); 50 μm (d).
Real-time PCR showed up-regulation of mRNA expression of mmp-9 and timp-1 in a burned cornea and suppression of this up-regulation by administration of SN50 (Figure 4b). Similarly, immunoreactivity for MMP-9 was more marked in control corneas (Figure 4c, A) as compared with SN50-treated corneas (Figure 4c, B) at days 7 and 12 (not shown). Although immunoreactivity for TIMP-1 was observed in both healing epithelium and stromal cells in control (Figure 4c, C) and SN50 (Figure 4c, D) groups, that in stromal cells was more marked in the control cornea. In immunostaining no specific staining was detected in the negative control (data not shown).
In situ zymography was performed to examine if SN50 treatment suppresses MMP activity in healing corneas at day 7. A marked MMP activity was detected in the cornea in the control group (Figure 4d, A; asterisks), but not in the SN50-treated cornea (Figure 4d, B). MMP activity was detected in the central cornea during the healing period. The activity was abolished by adding an MMP inhibitor to the assay system, indicating that the gelatinolytic activity is specific for MMP and not other proteases (Figure 4d, C and D).
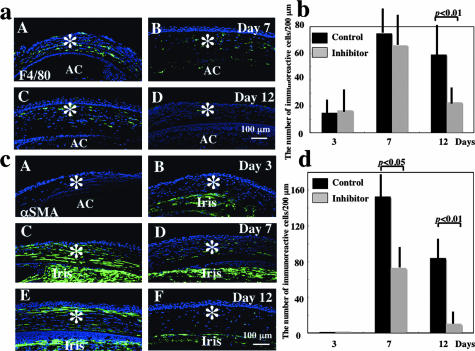
Monocyte/Macrophage Invasion into the Affected Stroma
Invasion of monocytes/macrophages plays an important role in tissue healing in various organs including an alkali-burned cornea.31 We therefore examined the presence and distribution of monocytes/macrophages by using immunohistochemistry with F4/80 antibody in the healing stroma in each group. The results showed that the stroma contained F4/80-labeled cells and there was no obvious difference of the presence of such cells in the stroma at day 3 (data not shown) and day 7 [Figure 5, a (A and B) and b] between control (A) and SN50-treatment (B) groups. However, at day 12, the number of F4/80-labeled monocytes/macrophages was less in NF-κB inhibitor-treated corneas (Figure 5a, D) as compared with control corneas [Figure 5, a (C) and b] (P < 0.01 by unpaired t-test). No specific staining was detected in the negative control (data not shown).
Figure 5.
Immunohistochemical analysis of stromal healing in burned corneas. a: Presence and distribution of F4/80-positive monocytes/macrophages in the burned corneal stroma. Labeled cells are distributed throughout the stroma in corneas in both control and SN50-treated groups at days 7 (A, B), whereas there are significantly fewer cells in the treated group (D) as compared with the control group (C) at day 12. b: Bar chart of the number of monocytes/macrophages in the central stroma determined as described in Materials and Methods. The number of monocytes/macrophages in the stroma of control corneas was similar at days 3 and 7, whereas it was much less in SN50-treated corneas than in control corneas at day 12. Mean ± bar (SD). c: Expression pattern of α-SMA in stromal cells during healing. At day 3 no α-SMA-positive cells are observed in the burned stroma of both groups of corneas (A, B), whereas its expression is more marked in the stroma of a control cornea (C) as compared with that of a treated cornea (D) at day 7. Iris stromal cells are also labeled with anti-α-SMA antibody (Iris). At day 12 α-SMA-expressing cells were still detected in the stroma, especially in the anterior stroma beneath the regenerated epithelium in the control group (E), whereas few cells were labeled in SN50 treatment group (F). d: Bar chart of the number of α-SMA-positive stromal cells (myofibroblasts) in the central stroma determined as described in Materials and Methods. The number of α-SMA-positive stromal cells in control corneas was similar at day 3, whereas it was much less in SN50-treated corneas than in control corneas at days 7 and 12. Mean ± bar (SD). Asterisks, corneal stroma; AC, anterior chamber. Scale bars, 100 μm (b, c).
Myofibroblast Generation in the Affected Stroma
It is well established that during corneal wound healing keratocytes differentiate to myofibroblasts expressing α-SMA, which is one of the hallmarks of scar tissue formation in corneal stromal scarring. We therefore examined the expression pattern of α-SMA in affected corneas. At day 3 no α-SMA-positive cells were detected in the affected stroma in both groups (Figure 5c, A and B). At day 7, many α-SMA-positive cells were observed in corneas of the control group [Figure 5, c (C) and d], whereas only a few stromal cells were labeled for α-SMA [Figure 5, c (D) and d] in the SN50-treatment group. At day 12 α-SMA-expressing cells were still detected in the stroma, especially in the anterior stroma beneath the regenerated epithelium in control eyes [Figure 5, c (E) and d], whereas few cells were labeled in the SN50 treatment group [Figure 5, c (F) and d]. No specific staining was detected in the negative control (data not shown).
Expression of TGF-β and MCP-1
Because TGF-β is a chemoattractant for monocytes/macrophages in healing tissue, we hypothesized that reduced invasion of monocytes/macrophages might result from decreased expression of TGF-β, leading to further suppression of keratocyte activation. Thus, immunohistochemistry and real-time RT-PCR were performed to examine tissue TGF-β levels. Immunohistochemistry detected a similar expression pattern of intracellular TGF-β1 in control (Figure 6a, A) and treated corneas (Figure 6a, B), whereas accumulation of extracellular secreted TGF-β1 [Figure 6a, C (asterisk) and D] and TGF-β2 [Figure 6a, E (asterisk) and F] in burned stroma was more marked in control corneas (Figure 6a, C and E) than in SN50-treated corneas (Figure 6a, D and F). There was no obvious difference of expression of TGF-β3 in healing epithelia and stroma between control (Figure 6a, G) and treated (Figure 6a, H) groups. No specific staining was detected in the negative control (data not shown).
Figure 6.
Expression of cytokines and growth factors in burned corneas. a: Immunohistochemical detection of TGF-βs. Immunohistochemistry detected similar expression of intracellular TGF-β1 in control (A) and SN50-treated (B) corneas, whereas accumulation of extracellular secreted TGF-β1 (C, D) and TGF-β2 (E, F) in burned stroma was more marked in a control cornea (C and E, asterisks) than in a SN50-treated cornea (D, F). There was no obvious difference of expression pattern of TGF-β3 in healing epithelia and stroma between control (G) and treated (H) groups. b: Expression pattern of monocyte/macrophage-chemoattractant protein-1 (MCP-1) in affected corneas at days 7 (A, B) and 12 (C, D). MCP-1 expression is more marked in control corneas (A, C) as compared with SN50-treated corneas (B, D). Mean ± bar (SD). c: Real-time RT-PCR confirmed the suppression of expression of mRNA of tgfb1 and mcp-1. Scale bars, 40 μm (a, b).
Real-Time RT-PCR Confirmed the Suppression of Expression of mRNA of TGF-β1 in Burned Corneas by Topical SN50 (Figure 6c)
Increased TGF-β levels in tissue induce chemoattraction of monocytes/macrophages either directly or indirectly via induction of MCP-1. MCP-1 is a chemokine that attracts macrophages into tissues31 and its expression depends on various signaling pathways, ie, signals via NF-κB and/or TGF-β.32–34 Thus, we hypothesized that both the presence of the SN50 NF-κB inhibitor, as well as suppression of TGF-β expression might result in reduction of MCP-1 expression. Immunohistochemical results showed that MCP-1 expression was decreased in healing epithelium, stromal keratocytes, and stromal matrix in the SN50-treated group (Figure 6b, B and D) compared to the control group (Figure 6b, A and C). No specific staining was detected in the negative control (data not shown). Real-time RT-PCR also showed a marked suppression of expression of MCP-1 mRNA in burned corneas by topical SN50 (Figure 6c).
Cell Proliferation and Cell Death
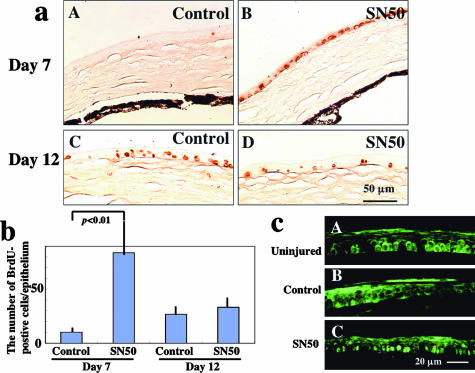
Cell proliferation and cell death were examined by using BrdU labeling and TUNEL staining, respectively, because NF-κB is reportedly involved in such cellular events.35 The number of BrdU-positive cells in healing epithelia was markedly higher in the SN50-treated group (Figure 7a, B), as compared with control corneas (Figure 7a, A) at day 7 (Figure 7b, P < 0.01 by unpaired t-test). At day 12 there was no significant difference in epithelial cell proliferation between groups [Figure 7, a (C and D) and b]. Cyclin D1 expression was low in the cytoplasm and nuclei of uninjured epithelium (Figure 7c, A) and healing epithelium in the control group at days 7 (data not shown) and 12 (Figure 7c, B), but was obviously higher in nuclei of the majority of cells in the healing epithelium in the SN50-group at days 7 (data not shown) and 12 (Figure 7c, C). TUNEL staining detected apoptotic cells in the superficial layer of the regenerated epithelia at days 7 and 12 and there was no difference in the number of positive cells between groups (data not shown).
Figure 7.
Proliferation of healing epithelia of burned corneas as detected by BrdU incorporation. a: Immunohistochemical detection of BrdU-labeled epithelial cells at days 7 (A, B) and 12 (C, D) in control (A, C) and SN50-treated (B, D) corneas. Peripheral healing epithelia contain more BrdU-positive cells in a SN50-treated cornea (B) as compared with a control cornea (A) at day 7. At day 12 the central corneas in both groups have a similar incidence of BrdU-labeled epithelial cells. b: Bar chart of BrdU-positive cells/epithelium in each experimental condition. Mean ± bar (SD). c: Immunofluorescent detection of cyclin D1 in an uninjured (A) or healing (B, C) epithelia at day 12 after alkali burn. Cyclin D1 is observed in the cytoplasm and occasionally detected in nuclei of an uninjured epithelium (A) and healing epithelium in control group at day 12 (B), whereas it is detected in the nuclei of the majority of cells in the healing epithelium in SN50-group at day 12 (C). Scale bars: 50 μm (a); 20 μm (c).
Acceleration of Cell Proliferation in Regenerated Epithelium by SN50 Treatment Is Dependent on TNF-α/JNK Signal
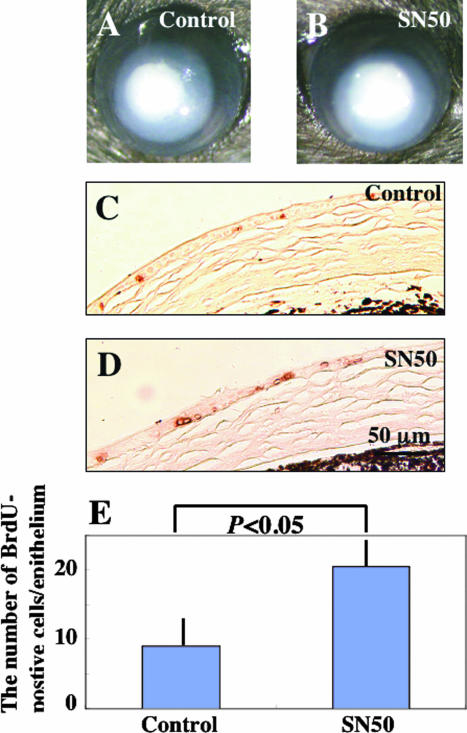
Examination of epithelial cell proliferation in TNF-α-null mice and an organ culture experiment were conducted to elucidate the role of TNF-α-derived signals in acceleration of epithelial cell proliferation by topical SN50 in postalkali burn healing corneal epithelium. There was no significant difference in epithelial defect closure between TNF-α-null corneas treated with SN50 and with vehicle (Figure 8, A and B), although BrdU incorporation in healing epithelium was significantly higher in SN50-treated corneas than in vehicle-treated corneas (Figure 8; C to E). We then compared the level of induction of epithelial cell proliferation by topical SN50 in C57BL/6 wild-type mice (Figure 7b) and in TNF-α-null mice (Figure 8E) and observed that SN50-induced epithelial cell proliferation at day 7 was more marked in wild-type mice than in TNF-α-null mice (P < 0.01 by unpaired t-test in Figure 7b versus Figure 8E). This suggested that TNF-α-derived NF-κB signaling partially suppresses cell proliferation directly or interferes with cellular proliferation-promoting signals in healing corneal epithelium. Moreover, the absence of TNF-α itself did not seem to have a significant effect on epithelial cell proliferation, further suggesting that signals inducing cell proliferation targeted by TNF-α/NF-κB might be also derived from TNF-α.
Figure 8.
Treatment of an alkali burn in the central cornea of TNF-α-null mice with topical SN50 for 7 days. There was no difference in epithelial healing and stromal opacification in a healing, burned, TNF-α-null cornea treated with vehicle (A) or SN50 (B). BrdU immunohistochemistry showed an increase of BrdU-incorporated epithelial cells in a SN50-treated cornea (D) as compared with a vehicle-treated cornea (C), both in the absence of endogenous TNF-α. E: Bar chart of the number of BrdU-labeled cells in regenerated epithelium. Scale bar, 50 μm.
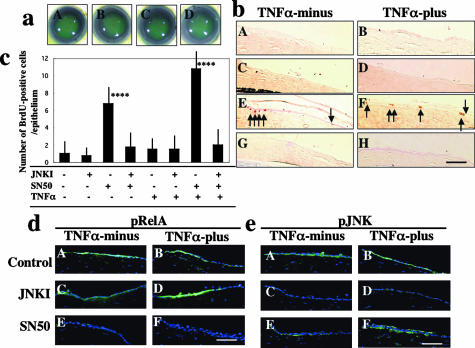
Organ culture experiments were performed to further elucidate the roles of signals derived from TNF-α, ie, NF-κB and JNK. Closure of the epithelial defect was similar among each group (Figure 9a). Adding exogenous JNK inhibitor did not affect epithelial cell proliferation in the healing corneal epithelium, whereas adding SN50 accelerated BrdU incorporation in the regenerated epithelium (Figure 9, b and c). However, SN50-induced cell proliferation acceleration was abolished by the co-presence of the JNK inhibitor (Figure 9, b and c). SN50-induction of epithelial cell proliferation was more marked in the presence of 10 ng/ml of exogenous TNF-α. The effectiveness of inhibition of signals of NF-κB and JNK by specific inhibitors was confirmed by immunohistochemistry using antibodies against phosphorylated forms of the molecules (Figure 9, d and e). No specific staining was detected in the negative control (data not shown). These results indicate that TNF-α/NF-κB signaling blocks the TNF-α/JNK pathway, which then accelerates cell proliferation.
Figure 9.
Organ culture of mouse eyes with a corneal epithelial defect for 12 hours in the presence of TNF-α, SN50 NF-κB inhibitor, or an inhibitor of c-Jun N-terminal kinase (JNKI). a: Fluorescein staining of the remaining epithelial defect at 12 hours in the presence of 10 ng/ml of TNF-α. There is no difference in the size of the remaining defect in cultures treated without inhibitors (A), 10 μmol/L of JNKI (B), 100 μg/ml of SN50 (C), or with both inhibitors (D). Similar results were obtained in a series of culture without exogenous TNF-α (not shown). b: Immunohistochemical detection of BrdU-incorporating cells in the regenerated epithelium. Adding exogenous TNF-α or JNKI does not induce cell proliferation in healing epithelia, whereas adding SN50 induces proliferation in the presence or absence of exogenous TNF-α. Acceleration of epithelial cell proliferation by SN50 is abolished by co-presence of JNKI. A, C, E, and G: Culture without exogenous TNF-α; B, D, F, and H: culture with 10 ng/ml of TNF-α; A and B: control cultures; C and D: with JNKI; E and F: with SN50; G and H: with both JNKI and SN50. c: Bar chart of the number of BrdU-labeled cells in a whole corneal epithelium in each culture condition. ****, P < 0.005 by Student’s unpaired t-test as compared with control. d: Expression of phosphorylated p65 subunit of NF-κB (RelA) in epithelium in each culture condition. Phospho-RelA is readily detected in epithelium in control or JNKI-treated cultures both in the presence or absence of exogenous TNF-α. SN50 suppresses expression of phospho-RelA both in the presence or absence of exogenous TNF-α. A, C, and E: Culture without exogenous TNF-α; B, D, and F: culture with 10 ng/ml of TNF-α; A and B: control cultures; C and D: with JNKI; E and F: with SN50. e: Expression of phosphorylated JNK in epithelium in each culture condition. Phospho-JNK is readily detected in epithelium in control or SN50 culture both in the presence or absence of exogenous TNF-α. Adding JNKI suppresses expression of phospho-JNK both in the presence or absence of exogenous TNF-α. A, C, and E: Culture without exogenous TNF-α; B, D, and F: culture with 10 ng/ml of TNF-α; A and B: control cultures; C and D: with JNKI; E and F: with SN50. Scale bars, 10 μm.
Discussion
In the present study we show that topical administration of SN50, a cell-permeable peptide inhibitor of NF-κB, prevents tissue destruction (epithelial defects/ulceration) in a mouse cornea after a central alkali burn. Histological findings show less inflammation as evaluated by monocyte/macrophage invasion and less scarring as judged by the absence of α-SMA-expressing cells in SN50-treated corneas as compared with control burned corneas at the final time point of day 12 after injury. Although it is not completely understood whether the inhibitory action of SN50 is specific to NF-κB signaling or results from suppression of other signaling cascades,36 our results demonstrated that topical administration of this peptide is beneficial in treating a corneal alkali burn in mice. We have recently determined that transient adenoviral Smad7 gene transfer to an alkali-burned mouse cornea prevents tissue destruction and restores a transparent cornea.37 We showed that exogenous Smad7 blocks not only Smad signaling, but also nuclear translocation of the phosphorylated p65/RelA subunit of NF-κB (S. Saika et al, manuscript in submission). The present study evaluates the contribution of blocking NF-κB signaling to the efficacy of Smad7 gene transfer, as well as establishes a new treatment strategy. Suppression of activation of p65RelA by topical SN50 administration, shown both by immunohistochemistry and Western blotting of tissue extracts, was considered to be due to both the direct effect of SN50 on NF-κB signaling as well as to reduced expression of cytokines concomitant with a reduction of inflammation. Although inhibitory effects of SN50 on signals other than the NF-κB cascade are reported to be very low,18–21 we observed a similar suppression of in vivo JNK signaling by SN50 as we did for in vivo NF-κB signaling, suggesting the acceleration of healing might result in a reduction of cytokines involved in activation of JNK signaling.
Based on our results the mechanism of action of the SN50 NF-κB inhibitor may include its suppression of postburn up-regulation of growth factors, cytokines, chemokines, and metalloproteinases after alkali exposure. Our study showed a suppression of expressions of MMP-9, TGF-βs, and MCP-1 by using immunohistochemistry and real-time RT-PCR in corneas treated with SN50. MMP-9 expression is critical to induce BM degradation and ulceration and expression of many MMP family members, including MMP-9, is modulated by NF-κB signal.38–43 Thus, there is a possibility that expression of other MMPs might also be down-regulated by topical SN50, although we did not examine expression of all members of the MMP family. In situ zymography showed an increased activity of MMP in the control cornea at day 7 that was suppressed with topical SN50. Therefore, an increase in TIMP-1, possibly expressed in response to up-regulation of MMPs, was not enough to counteract the MMP activity because control corneas exhibited a degraded epithelial BM. MCP-1 is a chemokine that is a potent chemoattractant for monocytes/macrophages.31 Macrophages that express various cytokines, ie, TGF-βs, and are involved in tissue damage after alkali exposure of the cornea. The decreased levels of TGF-βs and MCP-1 seen in SN50-treated eyes may contribute to the decreased invasion of monocytes/macrophages in treated alkali-burned stroma, which also results in the down-regulation of cytokine expression, including TGF-β autoinduction, in tissue. It is also possible that inhibition of NF-κB signaling might accelerate apoptosis of macrophages in tissue because this signal is required to maintain mitochondrial homeostasis in macrophages.44,45
We also showed suppression of myofibroblast generation in burned corneal stroma treated with SN50, although it is not clear if expression of α-SMA is directly modulated by signaling through NF-κB. On the other hand, regulation of fibroblast-myofibroblast conversion, a hallmark of wound healing of connective tissues such as dermis or corneal stroma,22–25 by TGF-β/Smad signaling is well-established. Thus, decreased TGF-β levels in treated tissue is a plausible explanation for the suppression of keratocyte activation to myofibroblasts. Degradation of BM might also result in the activation of underlying keratocytes and lead to generation of myofibroblasts.
Finally, our study showed that the SN50 NF-κB inhibitor stimulated cell proliferation in the healing corneal epithelium, a process that is modulated by a complex autocrine and paracrine interaction of cytokines. Nevertheless, it is known that NF-κB signaling can modulate cell proliferation and cell death.36,46–48 One NF-κB-related signaling mechanism believed to modulate cell proliferation is regulation (either stimulation or suppression) of activation of cyclin D1.49–52 Furthermore, overexpression of dominant-negative NF-κB or gene knockout of Ik-kinase is reported to result in embryonic epidermal hyperproliferation.53–55 Recently it was reported that TNF-α-derived NF-κB signaling counteracts the JNK’s effects on acceleration of epithelial cell proliferation.56,57 Because the corneal epithelium is of a lineage similar to the epidermal keratinocyte, a similar mechanism involving TNF-α/NF-κB regulation of cell-cycle machinery might be involved, although, dissimilar to epithelial cell proliferation in a developing embryo, cell proliferation in the healing corneal epithelium is considered to be modulated by many signaling cascades other than JNK. In the present study we compared the level of induction of epithelial cell proliferation by topical SN50 in C57BL/6 wild-type mice with that in TNF-α-null mice on a C57BL/6 background. Although SN50 induced epithelial cell proliferation in a healing alkali-burned cornea in TNF-α-null mice, this induction was much less in TNF-α-null mice as compared with in wild-type mice. This suggests that epithelial cell proliferation is partially counteracted by TNF-α-derived NF-κB signaling, as well as by other cell proliferation-suppressing cytokines, ie, TGF-β. To further examine the roles of signals derived from TNF-α, ie, NF-κB and JNK, we used an organ-cultured mouse eye that is free from inflammatory cells from the systemic circulation. As expected, the absence of either TNF-α or JNK signaling abolished the cell proliferation-promoting effect of SN50 on healing corneal epithelium. These results indicate that TNF-α/NF-κB signaling blocks the TNF-α/JNK pathway which then accelerates cell proliferation. In vivo induction of cell proliferation by SN50 in TNF-α-null mice appears to depend on reduced invasion of inflammatory cells from the systemic circulation and subsequent reduction of proliferation-inhibitory cytokines by topical SN50 administration. In vivo, the corneal epithelial defect is first covered with a thin regenerated migrating epithelium that lacks proliferative potential and then once the defect has been resurfaced, the epithelium begins to proliferate to re-establish stratification.31 We have shown that a significant acceleration of cell proliferation in migrating corneal epithelium by exogenous Sonic hedgehog was not sufficient to promote closure of an epithelial defect in organ-cultures of mouse corneas.58 Cell proliferation acceleration by exogenous Sonic hedgehog was much more marked than that observed by adding exogenous TNF-α and SN50 consistent with our observation that SN50-induced cell proliferation did not promote closure of an epithelial defect in an organ-cultured cornea. Nevertheless, it may not be excluded that the mechanisms of accelerated healing in vivo in the presence of the SN50 NF-κB inhibitor might include direct induction of JNK-driven epithelial cell proliferation and its suppression of up-regulation of TGF-βs that often inhibit cell proliferation. Blocking NF-κB could also potentially affect apoptotic signaling by affecting the c-Jun N-terminal kinase cascade,46–48 but the present study detected no alteration of cell death in healing epithelium.
The role of NF-κB signaling in wound healing appears to be tissue-specific. For example, blocking this signal suppresses tissue damage in inflammatory disorders in joints and the skeletal system,11–17 whereas it retards local healing in an inflammatory bowel disease model.59,60 Further work is needed to study the effects of blocking NF-κB signaling in the repair of the conjunctiva because the present alkali burn model is limited to the cornea with the conjunctiva remaining intact.
Corticosteroid and nonsteroidal anti-inflammatory drugs administered to reduce corneal inflammation possess activity to block NF-κB signaling. However these drugs are not selective for this pathway and have many side effects. To target a specific signaling pathway involved in tissue inflammation may decrease undesirable effects, keeping a potent anti-inflammatory action in a burned cornea. Further study is needed to establish the clinical utility of blocking NF-κB signaling in the treatment of corneal alkali burn.
Footnotes
Address reprint requests to Shizuya Saika, M.D., Ph.D., Department of Ophthalmology, Wakayama Medical University, 811-1 Kimiidera, Wakayama, 641-0012, Japan. E-mail: shizuya@wakayama-med.ac.jp.
Supported by the Ministry of Education, Science, Sports, and Culture of Japan (to S.S.); Uehara Memorial Foundation (to S.S.); Wakayama Medical University (research grant on priority areas to S.S., Y.M., and A.O.); National Institutes of Health (grant EY 13755); Research to Prevent Blindness; and the Ohio Lions Eye Research Foundation (to W.W.-Y.K.).
References
- Ishizaki M, Zhu G, Haseba T, Shafer SS, Kao WW-Y. Expression of collagen I, smooth muscle α-actin, and vimentin during the healing of alkali-burned and lacerated corneas. Invest Ophthalmol Vis Sci. 1993;34:3320–3328. [PubMed] [Google Scholar]
- Saika S, Kobata S, Hashizume N, Okada Y, Yamanaka O. Epithelial basement membrane in alkali-burned corneas in rats. Immunohistochemical study. Cornea. 1993;12:383–390. doi: 10.1097/00003226-199309000-00003. [DOI] [PubMed] [Google Scholar]
- Saika S, Uenoyama K, Hiroi K, Tanioka H, Takase K, Hikita M. Ascorbic acid phosphate ester and wound healing in rabbit corneal alkali burns: epithelial basement membrane and stroma. Graefes Arch Clin Exp Ophthalmol. 1993;231:221–227. doi: 10.1007/BF00918845. [DOI] [PubMed] [Google Scholar]
- Brodovsky SC, McCarty CA, Snibson G, Loughnan M, Sullivan L, Daniell M, Taylor HR. Management of alkali burns: an 11-year retrospective review. Ophthalmology. 2000;107:1829–1835. doi: 10.1016/s0161-6420(00)00289-x. [DOI] [PubMed] [Google Scholar]
- Sridhar MS, Bansal AK, Sangwan VS, Rao GN. Amniotic membrane transplantation in acute chemical and thermal injury. Am J Ophthalmol. 2000;130:134–137. doi: 10.1016/s0002-9394(00)00500-6. [DOI] [PubMed] [Google Scholar]
- Meller D, Pires RT, Mack RJ, Figueiredo F, Heiligenhaus A, Park WC, Prabhasawat P, John T, McLeod SD, Steuhl KP, Tseng SC. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107:980–989. doi: 10.1016/s0161-6420(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Pfister RR, Haddox JL, Sommers CI. Injection of chemoattractants into normal cornea: a model of inflammation after alkali injury. Invest Ophthalmol Vis Sci. 1998;39:1744–1750. [PubMed] [Google Scholar]
- Sotozono C, He J, Tei M, Honma Y, Kinoshita S. Effect of metalloproteinase inhibitor on corneal cytokine expression after alkali injury. Invest Ophthalmol Vis Sci. 1999;40:2430–2434. [PubMed] [Google Scholar]
- Schmidt C, Peng B, Li Z, Sclabas GM, Fujioka S, Niu J, Schmidt-Supprian M, Evans DB, Abbruzzese JL, Chiao PJ. Mechanisms of proinflammatory cytokine-induced biphasic NF-κB activation. Mol Cell. 2003;12:1287–1300. doi: 10.1016/s1097-2765(03)00390-3. [DOI] [PubMed] [Google Scholar]
- Chen LF, Greene WC. Shaping the nuclear action of NF-κB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- Seetharaman R, Mora AL, Nabozny G, Boothby M, Chen J. Essential role of T cell NF-κB activation in collagen-induced arthritis. J Immunol. 1999;163:1577–1583. [PubMed] [Google Scholar]
- Baldwin AS., Jr Series introduction: the transcription factor NF-κB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP, Gerlag DM, Aupperle KR, van de Geest DA, Overbeek M, Bennett BL, Boyle DL, Manning AM, Firestein GS. Inhibitor of nuclear factor κB kinase β is a key regulator of synovial inflammation. Arthritis Rheum. 2001;44:1897–1907. doi: 10.1002/1529-0131(200108)44:8<1897::AID-ART328>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. Role of the NF-κB pathway in the pathogenesis of human disease states. Curr Mol Med. 2001;1:287–296. doi: 10.2174/1566524013363816. [DOI] [PubMed] [Google Scholar]
- Jimi E, Aoki K, Saito H, D’Acquisto F, May MJ, Nakamura I, Sudo T, Kojima T, Okamoto F, Fukushima H, Okabe K, Ohya K, Ghosh S. Selective inhibition of NF-κB blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10:617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-κB by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- Tergerson TR, Colosia AD, Donahue JP, Lin Y-Z, Hawiger JH. Regulation of NF-kB, AP-1, NFAT, and STAT1 nuclear import in T lymphocytes by noninvasive delivery of peptide carrying the nuclear localization sequence of NF-kB p50. J Immunol. 1998;161:6084–6092. [PubMed] [Google Scholar]
- Kolenko V, Bloom T, Rayman P, Bukowski R, His E, Finke J. Inhibition of NF-kB activity in human T lymphocytes induces caspase-dependent apoptosis without detectable activation of caspase-1 and -3. J Immunol. 1999;163:590–598. [PubMed] [Google Scholar]
- Kelly A, Vereker E, Nolan Y, Brady M, Barry C, Loscher CE, Mills KH, Lynch MA. Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1β on long term potentiation in rat dentate gyrus. J Biol Chem. 2003;278:19453–19462. doi: 10.1074/jbc.M301938200. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Jester JV, Huang J, Petroll WM, Cavanagh HD. TGFβ induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFβ, PDGF and integrin signaling. Exp Eye Res. 2002;75:645–657. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Darby IA, Gabbiani G. Normal and pathologic soft tissue remodeling: role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab Invest. 2003;83:1689–707. doi: 10.1097/01.lab.0000101911.53973.90. [DOI] [PubMed] [Google Scholar]
- Garrett Q, Khaw PT, Blalock TD, Schultz GS, Grotendorst GR, Daniels JT. Involvement of CTGF in TGF-β1-stimulation of myofibroblast differentiation and collagen matrix contraction in the presence of mechanical stress. Invest Ophthalmol Vis Sci. 2004;45:1109–1116. doi: 10.1167/iovs.03-0660. [DOI] [PubMed] [Google Scholar]
- Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNFα-deficient mice: a critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397–1411. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Saika S, Liu CY, Azhar M, Sanford LP, Doetschman T, Gendron RL, Kao CW, Kao WW. TGFβ2 in corneal morphogenesis during mouse embryonic development. Dev Biol. 2001;240:419–432. doi: 10.1006/dbio.2001.0480. [DOI] [PubMed] [Google Scholar]
- Flanders KC, Thompson NL, Cissel DS, Van Obberghen-Schilling E, Baker CC, Kass ME, Ellingsworth LR, Roberts AB, Sporn MB. Transforming growth factor-β1: histochemical localization with antibodies to different epitopes. J Cell Biol. 1989;108:653–660. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson NL, Flanders KC, Smith JM, Ellingsworth LR, Roberts AB, Sporn MB. Expression of transforming growth factor-β1 in specific cells and tissues of adult and neonatal mice. J Cell Biol. 1989;108:661–669. doi: 10.1083/jcb.108.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Okada Y, Miyamoto T, Yamanaka O, Ohnishi Y, Ooshima A, Liu CY, Weng D, Kao WW. Role of p38 MAP kinase in regulation of cell migration and proliferation in healing corneal epithelium. Invest Ophthalmol Vis Sci. 2004;45:100–109. doi: 10.1167/iovs.03-0700. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Yoshida A, Matsui H, Takada Y, Ishibashi T. Involvement of macrophage chemotactic protein-1 and interleukin-1β during inflammatory but not basic fibroblast growth factor-dependent neovascularization in the mouse cornea. Lab Invest. 2003;83:927–938. doi: 10.1097/01.lab.0000075642.11787.83. [DOI] [PubMed] [Google Scholar]
- Ueda A, Ishigatsubo Y, Okubo T, Yoshimura T. Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-κB sites and NF-κB/Rel subunit specificity. J Biol Chem. 1997;272:31092–31099. doi: 10.1074/jbc.272.49.31092. [DOI] [PubMed] [Google Scholar]
- Denk A, Goebeler M, Schmid S, Berberich I, Ritz O, Lindemann D, Ludwig S, Wirth T. Activation of NF-κB via the IκB kinase complex is both essential and sufficient for proinflammatory gene expression in primary endothelial cells. J Biol Chem. 2001;276:28451–28458. doi: 10.1074/jbc.M102698200. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M, Denholm EM, Phan SH. Costimulation of fibroblast collagen and transforming growth factor β1 gene expression by monocyte chemoattractant protein-1 via specific receptors. J Biol Chem. 1996;271:17779–17784. doi: 10.1074/jbc.271.30.17779. [DOI] [PubMed] [Google Scholar]
- Chen F, Castranova V, Shi X. New insights into the role of nuclear factor-κB in cell growth regulation. Am J Pathol. 2001;159:387–397. doi: 10.1016/s0002-9440(10)61708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby M. Specificity of SN50 for NF-κB? Nat Immunol. 2001;2:471–472. doi: 10.1038/88652. [DOI] [PubMed] [Google Scholar]
- Saika S, Ikeda K, Yamanaka O, Miyamoto T, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Nakajima Y, Kao WW-Y, Flanders KC, Roberts AB. Expression of Smad7 in mouse eyes accelerates healing of corneal tissue following exposure to alkali. Am J Pathol. 2005;166:1405–1418. doi: 10.1016/S0002-9440(10)62358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-kappa B. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- Bond M, Baker AH, Newby AC. Nuclear factor κB activity is essential for matrix metalloproteinase-1 and -3 upregulation in rabbit dermal fibroblasts. Biochem Biophys Res Commun. 1999;264:561–567. doi: 10.1006/bbrc.1999.1551. [DOI] [PubMed] [Google Scholar]
- Han YP, Tuan TL, Wu H, Hughes M, Garner WL. TNF-α stimulates activation of pro-MMP2 in human skin through NF-κB mediated induction of MT1-MMP. J Cell Sci. 2001;114:131–139. doi: 10.1242/jcs.114.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YP, Tuan TL, Hughes M, Wu H, Garner WL. Transforming growth factor-β- and tumor necrosis factor-α-mediated induction and proteolytic activation of MMP 9 in human skin. J Biol Chem. 2001;276:22341–22350. doi: 10.1074/jbc.M010839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Aggarwal BB. Betulinic acid suppresses carcinogen-induced NF-κB activation through inhibition of I κB alpha kinase and p65 phosphorylation: abrogation of cyclooxygenase-2 and matrix metalloprotease-9. J Immunol. 2003;171:3278–3286. doi: 10.4049/jimmunol.171.6.3278. [DOI] [PubMed] [Google Scholar]
- Takahra T, Smart DE, Oakley F, Mann DA. Induction of myofibroblast MMP-9 transcription in three-dimensional collagen I gel cultures: regulation by NF-κB, AP-1 and Sp1. Int J Biochem Cell Biol. 2004;36:353–363. doi: 10.1016/s1357-2725(03)00260-7. [DOI] [PubMed] [Google Scholar]
- Pagliari LJ, Perlman H, Liu H, Pope RM. Macrophages require constitutive NF-κB activation to maintain A1 expression and mitochondrial homeostasis. Mol Cell Biol. 2000;20:8855–8865. doi: 10.1128/mcb.20.23.8855-8865.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Ma Y, Pagliari LJ, Perlman H, Yu C, Lin A, Pope RM. TNF-alpha-induced apoptosis of macrophages following inhibition of NF-κB: a central role for disruption of mitochondria. J Immunol. 2004;172:1907–1915. doi: 10.4049/jimmunol.172.3.1907. [DOI] [PubMed] [Google Scholar]
- Chen F, Lu Y, Zhang Z, Vallyathan V, Ding M, Castranova V, Shi X. Opposite effect of NF-κB and c-Jun N-terminal kinase on p53-independent GADD45 induction by arsenite. J Biol Chem. 2001;276:11414–11419. doi: 10.1074/jbc.M011682200. [DOI] [PubMed] [Google Scholar]
- Liu H, Lo CR, Czaja MJ. NF-kappaB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology. 2002;35:772–778. doi: 10.1053/jhep.2002.32534. [DOI] [PubMed] [Google Scholar]
- Tang F, Tang G, Xiang J, Dai Q, Rosner MR, Lin A. The absence of NF-κB-mediated inhibition of c-Jun N-terminal kinase activation contributes to tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol. 2002;22:8571–8579. doi: 10.1128/MCB.22.24.8571-8579.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce D, Bouzahzah B, Fu M, Albanese C, D’Amico M, Steer J, Klein JU, Lee RJ, Segall JE, Westwick JK, Der CJ, Pestell RG. Integration of Rac-dependent regulation of cyclin D1 transcription through a nuclear factor-κB-dependent pathway. J Biol Chem. 1999;274:25245–25249. doi: 10.1074/jbc.274.36.25245. [DOI] [PubMed] [Google Scholar]
- Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-κB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690–2698. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-κB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz CS, Lin Q, Deng H, Khavari PA. Alterations in NF-κB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-κB. Proc Natl Acad Sci USA. 1998;95:2307–2312. doi: 10.1073/pnas.95.5.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Limb and skin abnormalities in mice lacking IKKα. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKalpha subunit of IkappaB kinase. Science. 1999;84:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Green CL, Tao S, Khavari PA. NF-κB RelA opposes epidermal proliferation driven by TNFR1 and JNK. Genes Dev. 2004;18:17–22. doi: 10.1101/gad.1160904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugasyan R, Voss A, Varigos G, Thomas T, Grumont RJ, Kaur P, Grigoriadis G, Gerondakis S. The transcription factors c-rel and RelA control epidermal development and homeostasis in embryonic and adult skin via distinct mechanisms. Mol Cell Biol. 2004;24:5733–5745. doi: 10.1128/MCB.24.13.5733-5745.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Muragaki Y, Okada Y, Miyamoto T, Ohnishi Y, Ooshima A, Kao WW-Y. Sonic hedgehog expression and role in healing corneal epithelium. Invest Ophthalmol Vis Sci. 2004;45:2577–2585. doi: 10.1167/iovs.04-0001. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Fujita T, Yamamoto A. Role of nuclear factor-κB in gastric ulcer healing in rats. Am J Physiol. 2001;280:G1296–G1304. doi: 10.1152/ajpgi.2001.280.6.G1296. [DOI] [PubMed] [Google Scholar]
- Chen LW, Egan L, Li ZW, Greten FR, Kagnoff MF, Karin M. The two faces of IKK and NF-κB inhibition: prevention of systemic inflammation but increased local injury following intestinal ischemia-reperfusion. Nat Med. 2003;9:575–581. doi: 10.1038/nm849. [DOI] [PubMed] [Google Scholar]