Abstract
Damage to the cornea from chemical burns is a serious clinical problem that often leads to permanent visual impairment. Because transforming growth factor (TGF)-β has been implicated in the response to corneal injury, we evaluated the effects of altered TGF-β signaling in a corneal alkali burn model using mice treated topically with an adenovirus (Ad) expressing inhibitory Smad7 and mice with a targeted deletion of the TGF-β/activin signaling mediator Smad3. Expression of exogenous Smad7 in burned corneal tissue resulted in reduced activation of Smad signaling and nuclear factor-κB signaling via RelA/p65. Resurfacing of the burned cornea by conjunctival epithelium and its differentiation to cornea-like epithelium were both accelerated in Smad7-Ad-treated corneas with suppressed stromal ulceration, opacification, and neovascularization 20 days after injury. Introduction of the Smad7 gene suppressed invasion of monocytes/macrophages and expression of monocyte/macrophage chemotactic protein-1, TGF-β1, TGF-β2, vascular endothelial growth factor, matrix metalloproteinase-9, and tissue inhibitors of metalloproteinase-2 and abolished the generation of myofibroblasts. Although acceleration of healing of the burned cornea was also observed in mice lacking Smad3, the effects on epithelial and stromal healing were less pronounced than those in corneas treated with Smad7. Together these data suggest that overexpression of Smad7 may have effects beyond those of simply blocking Smad3/TGF-β signaling and may represent an effective new strategy for treatment of ocular burns.
The cornea is a highly organized avascular transparent tissue located in the anterior part of the eye. It must remain transparent to refract light properly. Ocular trauma in the form of an alkali burn to the cornea is a serious clinical problem and may cause severe and permanent visual impairment.1 Activation of corneal cells, ie, keratocytes and epithelial cells, and influx of inflammatory cells such as monocytes/macrophages, are both involved in the pathogenesis of injury after alkali tissue damage in the cornea and can lead to persistent epithelial defects.2,3 Moreover, breakdown of the basement membrane by matrix metalloproteinases (MMPs, gelatinases) secreted by these cells contributes to the pathogenic ulceration and perforation of the stroma.4–9 Conjunctivalization of the corneal surface on the loss of limbal stem cells together with opacification and neovascularization of the corneal stroma all impair the patients’ vision in the later healing phases.10,11 Surgical transplantation of autografts or allografts of limbal epithelium containing corneal epithelial stem cells is used in some cases.12,13 Although such allografting can be effective in cases with alkali injury to both eyes, this therapy requires long-term immunosuppression by drugs, potentially increasing risks of infection and producing other unwanted side effects. Moreover, even this treatment is not effective in severe cases, leading to loss of vision, and emphasizing the need for development of new, more effective, treatment strategies.
A number of growth factors and cytokines are believed to be involved in the tissue destruction and late scarring that occur in the cornea after alkali burn. One prime candidate is transforming growth factor (TGF)-β, which has been shown not only to promote migration of corneal epithelial cells and keratocytes, but also to be chemotactic to monocytes/macrophages and to induce transdifferentiation of keratocytes to myofibroblasts.14–17 Overexpression of TGF-β in the burned cornea exacerbates damage of the injured tissue, in part by its ability to induce expression of other cytokines, such as vascular endothelial growth factor (VEGF) and monocyte/macrophage chemotactic protein-1 (MCP-1),18,19 which are believed to be involved in local neovascularization and inflammation, respectively.20–27
TGF-β activates multiple signaling cascades including those involving the mitogen-activated protein kinase (MAPK), c-Jun-N-terminal kinase, p38MAPK, and Smads.28–31 Of these, the Smad2/3/4 pathway is uniquely specific for the TGF-β/activin signaling.28–31 In this pathway, receptor-activated Smad proteins, Smad2 or Smad3, are phosphorylated directly by the TGF-β type I receptor kinase (TβRI), partner with the common mediator, Smad4, and translocate to the nucleus where they play a prominent role in activation of TGF-β/Smad-dependent gene targets. Smad3 signaling has been shown to be critical in healing of cutaneous wounds and in injury-induced fibrosis in many tissues, such as skin, kidney, and the lens and retina of the eye.17,32–40 Smad7 is an inhibitory Smad, inducible not only by TGF-β but also by inflammatory cytokines.28–31 It both blocks Smads2/3 signaling41,42 and has other putative Smad-independent effects.43 Transient introduction of the Smad7 gene by using an adenovirus vector has been reported to be effective in treating tissue inflammatory/fibrotic disorders in lung, kidney, liver, and lens of the eye.44–47 Crosstalk between the Smad and nuclear factor (NF)-κB signal transduction pathways has also been reported.48–51 In particular, Smad7 has been shown to exert an inhibitory effect on NF-κB signaling independent of its action on Smad signaling,52 and might contribute to modulation of the inflammatory reaction triggered by various cytokines. Based on this, we hypothesized that overexpression of Smad7 might simultaneously modulate TGF-β and NF-κB signaling pathways and prevent the tissue destruction resulting from alkali injury to the cornea.
To test this hypothesis, we used a mouse model of total ocular surface burn in which the eye is exposed to alkali. Here we show for the first time the therapeutic efficacy of Smad7 adenoviral-mediated cDNA transfer to the alkali-burned mouse cornea. Evaluation of epithelial healing, stromal repair, influx of inflammatory cells, and patterns of cytokine expression all suggest that gene transfer of Smad7 improves the healing of the injured tissue, and reduces scarring and neovascularization. Combined in vivo and in vitro data demonstrate effects of Smad7 overexpression on both Smad2/3 and NF-κB signaling. The better outcome of eyes treated with Smad7 compared to eyes in Smad3-null mice after alkali injury to the cornea suggests that Smad7 likely affects multiple signaling pathways in addition to those mediated by Smad3.
Materials and Methods
All experimental procedures were approved by the DNA Recombination Experiment Committee and Animal Care and the Use Committee of Wakayama Medical University, Wakayama, Japan, and conducted in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Adenovirus Vector Construction and Virus Purification
We used the adenovirus Cre/LoxP-regulated expression vector set (no. 6151; Takara, Tokyo, Japan) to make recombinant adenovirus expressing Smad7 as previously reported.44 Each adenoviral vector was used at the concentration of 1.0 × 107 PFU/μl.
Alkali Burn in C57BL/6 Mice and Treatment with Smad7 Gene Transfer for Histological Evaluation
Three μl of 1 N sodium hydroxide solution was applied to the right eye of adult C57BL/6 mice (n = 87) to produce an ocular surface alkali burn under both general and topical anesthesia.49 A mixture of recombinant adenoviruses carrying CAG (cytomegalovirus enhancer, chicken β-actin promoter plus a part of 3′ untranslated region of rabbit β-globin) promoter-driven Cre (Cre-Ad) and mouse Smad7 cDNA (Smad7-Ad) was administered at 2 and 24 hours and days 5, 10, and 15 after an alkali exposure (Smad7-Ad group). Cre recombinase expressed via the CAG promoter deletes the stuffer PolyA through the Cre/LoxP system. Preliminary experiments showed that there was no obvious difference in the histology or in healing at the macroscopic level in an alkali-burned mouse eye with (Cre-Ad group) or without application of adenovirus carrying Cre (no vector group). Thus corneas of the Cre-Ad group were used as controls. After the evaluation of the corneal surface with fluorescein staining of the injured epithelium, the eye globe was enucleated 2 hours after labeling with bromodeoxyuridine (BrdU)53 and processed for histological examination in either paraffin or cryosections at days 3, 5, 10, and 20. Fluorescein staining of the cornea allows surface defects to be visualized as green. The numbers of eyes for paraffin sections were 4 and 4 (day 3), 6 and 6 (day 5), 14 and 17 (day 10), or 8 and 8 (day 20) in the Cre-Ad and Smad7-Ad groups, respectively. The numbers of eyes for cryosection were two and two (day 3), three and two (day 5), four and three (day 10), or two and two (day 20) in Cre-Ad group and Smad7-Ad group, respectively. The efficiency of gene transfer was evaluated by co-infection of Cre-Ad and GFP (green fluorescein protein) under control of the Cre/LoxP system. Expression of GFP was evaluated in unfixed cryosections using fluorescent microscopy.
Alkali Burn in Smad3-Knockout Mice
To compare the effects of Smad7 gene transfer and loss of Smad3 on the healing process after alkali burn in a cornea, a similar alkali burn was administered to corneas of Smad3 knockout mice (a mixture of three strains; C57BL/6/NIH Swiss/SV129). Twenty-four eyes total of 12 Smad3+/+ mice and 12 Smad3−/− mice were examined at days 5, 10, or 20 after alkali burn. The enucleated eyes were processed for paraffin sections as described above. For assay of expression of mRNAs of cytokines, real-time reverse transcriptase (RT)-polymerase chain reaction (PCR) was performed as described above. Alkali-burned corneas of Smad3+/+ and Smad3−/− mice were obtained at days 10 and 20 after burn (n = 4 at each time point) and processed for tRNA extraction and real-time RT-PCR as described below. Four uninjured corneas of two Smad3+/+ mice and six of three Smad3−/− mice were included to obtain the basal expression level of each cytokine.
Immunohistochemistry
Deparaffinized sections (5 μm thick) or cryosections (7 μm thick) fixed in cold acetone for 5 minutes were processed for indirect immunohistochemistry. The following antibodies were used; rabbit polyclonal anti-Smad3 antibody [(1:100 dilution in phosphate-buffered saline (PBS); Zymed, South San Francisco, CA)], goat polyclonal anti-Smad7 antibody (1:300 in PBS; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit polyclonal anti-phosphorylated p65/RelA of NF-κB (1:50 dilution in PBS; Cell Signaling, Beverly, MA), rabbit polyclonal anti-keratin 12 antibody (1 μg/ml in PBS), mouse monoclonal anti-α-SMA antibody (1:100 dilution in PBS; Neomarker, Fremont, CA), rat monoclonal anti-laminin antibody (1:25 dilution in PBS; Sigma, St. Louis, MO), goat polyclonal anti-MCP-1 (1:100 dilution in PBS, Santa Cruz), goat polyclonal anti-connective tissue growth factor (CTGF) antibody (1:100 dilution in PBS, Santa Cruz), goat polyclonal anti-VEGF (1:100 dilution in PBS, Santa Cruz), goat polyclonal anti-type IV collagen antibody (1:100 in PBS; Southern Biotechnology, Birmingham AL), and rat monoclonal anti-CD31 (PECAM) antibody (1:100 in PBS, Santa Cruz). The rat monoclonal F4/80 anti-macrophage antigen antibody (clone A3-1, 1:400 dilution in PBS; BMA Biomedicals, Augst, Switzerland) was used to detect monocytes/macrophages. The number of labeled cells in the central cornea (200 μm in length) was determined in four corneas in each treatment group. Fluorescein isothiocyanate-conjugated specific secondary antibodies were used for detection of the primary antibody and 4,6-diamidino-2-phenylindole (DAPI) was used for nuclear counterstaining. Cell proliferation in the healing epithelium was determined by immunostaining with an anti-BrdU antibody (1:11 in PBS; Roche Diagnostics, Mannheim, Germany) and diaminobenzidine reaction with hematoxylin counterstaining as previously reported53 followed by counting the number of labeled cells in healing epithelia in the affected cornea. Specimens were treated with 2 N HCl for 60 minutes at 37°C before antibody application. Immunohistochemistry for TGF-βs was performed as previously reported.54,55
Expression of mRNAs of TGF-β1, TGF-β2, MCP-1, VEGF, MMP-2, MMP-9, Smad7, and Tissue Inhibitors of Metalloproteinases (TIMP)-1 and -2 in Burned Corneas by Using Real-Time RT-PCR
For extraction of RNA, alkali-burned corneas from seven mice were obtained from each treatment group [C57BL/6 mice (n = 28), Smad3+/+ mice (n = 8), or Smad3−/− mice (n = 8)] at days 10 or 20 and stored at −80°C. Untreated corneas of three C57BL/6 mice, two Smad3+/+ mice, and six Smad3−/− mice were also included to get the baseline mRNA expression. Total RNA from corneal tissue excised from a burned eye was extracted using ISO GENE (Nippon Gene, Tokyo, Japan) according to the manufacturer’s protocol and processed for semiquantitative real-time RT-PCR for mRNAs of mouse TGF-b1, TGF-b2, mcp-1, vegf, Smad7, mmp-9, and timp-2. For the real-time RT-PCR, the TaqMan one-step RT-PCR master mix reagents kit and the Applied Biosystems Prism 7700 (PE Applied Biosystems, Foster City, CA) were used as previously described. Primers and oligonucleotide probes were designed according to the cDNA sequences in the GenBank database using the Primers Express software (PE Applied Biosystems) and are listed in Table 1. RT-PCR conditions were as follows: 2 minutes at 50°C (stage 1, reverse transcription), 10 minutes at 95°C (stage 2, reverse transcription inactivation and AmpliTaq Gold activation), and then 40 cycles of amplification for 15 seconds at 95°C and 1 minute at 60°C (stage 3, PCR).
Table 1.
Primers and Olignucleotide Probes
| Transcript | Sequence |
|---|---|
| mTGF-β1 | F: 5′-gca aca tgt gga act cta cca gaa-3′ |
| R: 5′-gac gtc aaa aga cag cca ctc-3′ | |
| P: 5′-acc ttg gta acc ggc tgc tga ccc-3′ | |
| mTGF-β2 | F: 5′-acc ttt ttg ctc ctg cat ctg-3′ |
| R: 5′-gtc gag ggt gct gca ggt a-3′ | |
| P: 5′-tcc cgg tgg cgc tca gtc tgt-3′ | |
| mMCP1 | F: 5′-tgg ctc agc cag atg cag t-3′ |
| R: 5′-cca gcc tac tca ttg gga tca-3′ | |
| P: 5′-ccc cac tca cct gct gct act cat tca-3′ | |
| MVEGF | F: 5′-agc gga gaa agc att tgt ttg-3′ |
| R: 5′-caa cgc gag tct gtg ttt ttg-3′ | |
| P: 5′-cca aga tcc gca gac gtg taa atg ttc c-3′ | |
| mMMP9 | F: 5′-aga cca agg gta cag cct gtt c-3′ |
| R: 5′-ggc acg ctg gaa tga tct aag-3′ | |
| P: 5′-cgc acg agt tcg gcc atg cac-3′ | |
| mTIMP-2 | F: 5′-gtc cca tga tcc ctt gct aca-3′ |
| R: 5′-tgc cca ttg atg ctc ttc tct-3′ | |
| P: 5′-ctc ccc gga tga gtg cct ctg ga-3′ | |
| mSmad7 | F: 5′-gac tcc agg acg ctg ttg gt-3′ |
| R: 5′-cca tgg ttg ctg cat gaa ct-3′ | |
| P: 5′-agt gtt ccc tgg ttt ctc cat caa ggc t-3′ |
F, Forward primer; R, reverse primer; P, probe.
Effect of Exogenous Smad7 on NF-κB Signaling in Cultured Cells
Simian virus (SV)-40 large T-antigen-immortalized human corneal epithelial cells (Araki-Sasaki cells)56 provided by the Riken Cell Bank (Tokyo, Japan) were used to examine the effect of overexpression of exogenous Smad7 on NF-κB signaling. The cells (2.0 × 105/six-well culture plate or 2.0 × 104/16-well chamber slides; Nalge Nunc Int., Naperville, IL) were cultured in Dulbecco’s modified Eagle’s medium/F-12 mixture medium supplemented with 10% fetal bovine serum, 5 μg/ml cholera toxin, 5 mg/ml insulin, 10 ng/ml human epidermal growth factor, and antibiotics for 24 hours until confluent. Cells were then incubated for 2 hours in a serum-free medium containing Cre-Ad or both Cre-Ad and Smad7-Ad at a concentration of 4 × 103 PFU/ml, and then incubated for another 48 hours in Dulbecco’s modified Eagle’s medium/F-12 medium supplemented with 10% fetal bovine serum, 5 μg/ml cholera toxin, and antibiotics. Cells were then exposed to 10 ng/ml of recombinant human tumor necrosis factor (TNF)-α (R&D Systems, Minneapolis, MN) for 30 minutes, 1 hour, and 2 hours and were processed for immunocytochemistry and Western blotting.
For Western blotting, cells in six-well plates were homogenized in a lysis buffer (100 ml of CelLytic-M mammalian cell lysis/extraction reagent; Sigma) supplemented with a cocktail of proteinase inhibitors (Complete protease inhibitor cocktail tablet; Rosch, Mannheim, Germany). The cell lysate was centrifuged, mixed with 3× sample buffer, run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membrane, and Western blotted for Smad7 (Santa Cruz), total RelA (Santa Cruz), phospho-RelA (Cell Signaling), and β-actin (Santa Cruz). Phospho-RelA was determined on polyvinylidene difluoride membranes first stained for total RelA and stripped. To determine nuclear proteins, cells were fractionated into cytoplasm and nuclei by using ProteoExtract subcellular proteome extraction kit (Calbiochem, Darmstadt, Germany) according to the manufacturer’s protocol and processed as above. Immunoreactive bands were visualized on Lumino analyzer LAS1000 (Fujifilm, Tokyo, Japan), using enhanced chemiluminescence Western blotting detection reagents (Amersham Bioscience, Piscataway, NJ). For immunocytochemistry, cells in Nunc 16-well chamber slides were immunostained with antibody against Smad7, total RelA, or phospho-RelA and detected as above.
Results
Efficiency of Adenoviral Gene Transfer into a Burned Mouse Cornea
The efficiency of gene transfer was evaluated by co-infection of adenoviruses carrying Cre under control of the CAG promoter and GFP under control of the Cre/LoxP system. Our preliminary experiments in uninjured mouse corneas or corneas with a central epithelial 2-mm debridement showed that although adenovirus infected conjunctival epithelium efficiently, it infected corneal epithelium only weakly, and did not infect keratocytes in agreement with previously published studies.57 In contrast, in the total ocular surface alkali burn model used in the present study, we administered the GFP-expressing adenovirus at 2 and 24 hours and day 5 and the cryosections of day 5 or day 10 specimens were observed under fluorescence microscope without fixation. GFP expression was easily detected in conjunctival epithelium, subconjunctival fibroblasts, keratocytes, and healing epithelium at day 5 and very weakly in epithelium at day 10 (data not shown).
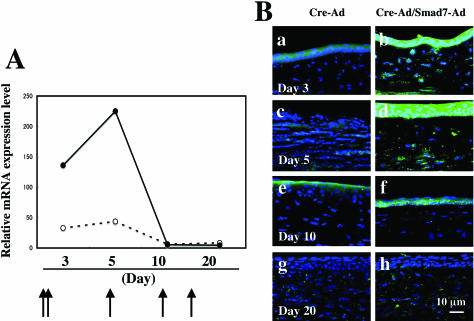
A mixture of Cre-Ad and a recombinant adenovirus carrying mouse Smad7 cDNA under control of the Cre/LoxP system was administered at 2 and 24 hours, and days 5, 10, and 15 after an alkali exposure (hereafter referred to as Smad7-Ad). Cre recombinase expressed via the CAG promoter deletes the stuffer PolyA through the Cre/LoxP system, leading to expression of Smad7. Real-time RT-PCR showed up-regulation of Smad7 mRNA at days 3 and 5 in both Cre-Ad and Smad7-Ad groups (Figure 1a), with expression levels being much higher in Smad7-Ad group as compared with Cre-Ad group at these time points. In both groups Smad7 mRNA expression rapidly declined at day 10 (Figure 1A), suggesting that administration of Smad7-Ad to the healing cornea at days 10 and 15 had no effect, consistent with the nearly complete extent of healing at this time point (Figure 2A). Immunohistochemical analysis showed that almost no Smad7 was detected in uninjured corneal epithelium and keratocytes (or corneal fibroblasts) (data not shown). At days 3 to 5 a faint immunoreactivity for endogenous Smad7 was detected in healing epithelium or keratocytes in corneas of the Cre-Ad control group (Figure 1B, a and c). On the other hand, marked expression of exogenous Smad7 was seen in healing epithelium on the burned cornea and keratocytes in the Smad7-Ad group (Figure 1B, b and d). At day 10, weak Smad7 staining was still detected in the regenerated epithelium in the Smad7-Ad-treated cornea (Figure 1B, f), but it was still more intense than that in a Cre-Ad group cornea (Figure 1B, e). Smad7 was barely detectable at day 20 in both groups (Figure 1B, g and h), consistent with the RT-PCR results.
Figure 1.
Detection of adenovirally introduced Smad7 in the healing mouse cornea after alkali burn. A: Real-time RT-PCR showed a marked up-regulation of Smad7 mRNA at days 3 and 5 after injury in Smad7-Ad groups, compared to the Cre-Ad group. In both groups levels of Smad7 mRNA were strongly reduced at day 10. Corneas were treated topically with Smad7-Ad at 2 and 24 hours, and again at days 5, 10, and 15 as indicated. Data represent a typical result in one experiment of four done at each time point. Arrows indicate the points of viral vector administration. B: Immunohistochemical analysis showed that a faint immunoreactivity for endogenous Smad7 was detected in healing epithelium or keratocytes in corneas of the Cre-Ad control group at days 3 to 5 (a, c). On the other hand, marked expression of exogenous Smad7 was seen in healing epithelium and keratocytes in the Smad7-Ad group (b, d). At day 10, weak Smad7 staining was still detected in the regenerated epithelium in the Smad7-Ad-treated cornea (f), but it was more intense than that in a Cre-Ad group cornea (e). It was then barely detectable at day 20 in both groups (g, h), Nuclei are counterstained with DAPI. Scale bar, 10 μm.
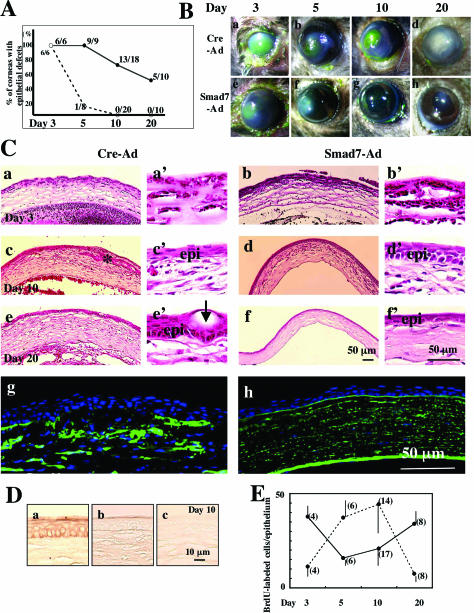
Figure 2.
Healing of an alkali-burned mouse cornea treated with either Smad7-Ad or Cre-Ad. A: Scoring of the percent incidence of corneas of C57BL/6 mice with an epithelial defect at each time point in Cre-Ad (solid line) or Smad7-Ad-treated (dotted line) groups. At day 10 all of the corneas in the Smad7-Ad group were re-epithelialized, whereas at day 20, 50% of the corneas in the Cre-Ad group still showed defects. B: Macroscopic observation of the corneal defects as visualized by staining with fluorescein to allow visualization of cornea defects in green shows the extent of the ocular surface burn in the Cre-Ad- (a) and Smad7-Ad-treated (e) groups at day 3. In the Cre-Ad group, epithelial defects with stromal opacification are observed at days 5 (b) and 10 (c), with re-epithelialization and dense stromal opacification at day 20 (d). Defects seen in corneas treated with Smad7-Ad were typically smaller at day 5 (f) and the cornea is almost transparent at days 10 (g) and 20 (h). C: Histology of burned corneas stained with H&E. No histological differences were seen in the central corneas of the Cre-Ad group (a, a’) and Smad7-Ad group (b, b’) at day 3. Bleeding and inflammation in the anterior chamber are more marked in the Cre-Ad group as compared with the Smad7-Ad group. Higher magnification pictures show the majority of cells in the burned stroma to be inflammatory cells (a’, b’). At day 10 Cre-Ad-treated corneas often show epithelial in-growth into the stroma (asterisk, c, c’), whereas Smad7-Ad-treated corneas exhibit a regenerated epithelium with nearly normal stratification (epi) (d, d’). At day 20 corneas in the Cre-Ad group still show a significant hypercellularity (presumed to be both inflammatory cells and corneal stromal cells), conjunctivalization of resurfaced epithelium and thicker stroma (e, e’), whereas corneas in the Smad7-Ad group exhibit a well-regenerated epithelium with a less cellularity in the stroma (f, f’). Higher magnification pictures show the presence of many goblet cells (arrows) in conjunctival epithelium resurfacing a Cre-Ad-treated cornea (e’), whereas the epithelium in a Smad7-Ad-treated cornea exhibits a cornea-like morphology (f’). Immunofluorescence staining for type IV collagen shows a disrupted basement membrane and ectopic type IV collagen deposition in the stroma in a Cre-Ad-treated cornea at day 20 (g), whereas the basement membrane remains intact in a Smad7-Ad-treated cornea (h). epi, healing epithelium. Nuclei are counterstained with DAPI in g and h. D: Whereas the uninjured mouse cornea stains positively for keratin 12 (a), regenerated epithelium in burned corneas is conjunctiva-derived and keratin 12-negative in both a Cre-Ad cornea (b) and a Smad7-Ad-treated cornea (c) at day 10 after injury. E: Cell proliferation in either Cre-Ad-treated (dotted line) or Smad7-Ad-treated (solid line) corneas at days 3 to 20 after injury as detected by BrdU incorporation. Bar, SD of the number (in parentheses) of corneas examined. Scale bars: 50 μm (C); 10 μm (D).
Alkali-Burned Corneas Treated with Adenoviral Gene Transfer of Smad7 Show Improved Healing
Because preliminary experiments showed no obvious differences in either the histology or the rate of healing of alkali-burned mouse corneas treated with either Cre-Ad alone or no vector, we used corneas of the Cre-Ad group as controls. Green fluorescein staining was used to evaluate healing of the corneal surface as quantitated by the presence or absence of an epithelial defect/ulceration (Figure 2A), and the degree of macroscopic corneal stromal opacification (Figure 2B). At day 10 after injury, 13 of 18 corneas of the Cre-Ad group still showed an epithelial defect or stromal ulceration, whereas 0 of 20 corneas treated with Smad7-Ad showed any defects. At this same time point, corneas in the Cre-Ad group that remained free of epithelial defects (5 of 18) exhibited dense stromal opacification, compared to minimal stromal opacification in all of the corneas of Smad7 group (Figure 2B, c versus g). At day 20 after injury, 50% of the corneas in the Cre-Ad group still exhibited epithelial defects/ulceration and the remaining corneas showed dense stromal opacification (Figure 2B, d). In contrast, all corneas in the Smad7-Ad group exhibited only minor opacification without epithelial defects (Figure 2B, h).
Hematoxylin and eosin (H&E) staining (Figure 2C) showed that although burned corneas of both Cre-Ad and Smad7-Ad groups showed epithelial defects and stromal inflammation at day 3 (Figure 2C; a, a’, b, b’), at day 10, corneas in the Cre-Ad group were characterized by a less organized epithelium with invasion into the underlying stroma (Figure 2C; c, c’, d, d’). The absence of staining for keratin 12, which is expressed in corneal, but not conjunctival epithelium (see uninjured cornea in Figure 2D, a), showed that the regenerated epithelium in both Cre-Ad- (Figure 2D, b) and Smad7-Ad-treated groups (Figure 2D, c) was of conjunctival origin, regardless of the more organized appearance in the Smad7-Ad-treated eyes. At day 20, corneas in the Cre-Ad group were, on average, thicker than corneas in the Smad7-Ad group, which had remodeled to a nearly normal thickness (Figure 2C; e, e’, f, f’). Importantly, the conjunctival epithelium resurfacing the corneas in the Smad7-Ad group transdifferentiated into cornea-like epithelium, while that resurfacing the healing corneas in the Cre-Ad group showed conjunctivalization (Figure 2C, e’ versus f’), as indicated by the presence of many mucin-secreting goblet cells (arrows).
Development of corneal ulceration after alkali burn is characterized by degradation of the epithelial basement membrane by MMPs expressed by healing epithelial cells and keratocytes as well as by inflammatory cells. Immunostaining of healing corneas in the Cre-Ad control group for type IV collagen, showed disruption of the basement membrane and loss of epithelial integrity beginning at day 5 (data not shown) and continuing through day 20 (Figure 2C, g). In contrast, corneas in the Smad7-Ad group showed a linear pattern of subepithelial immunoreactivity indicating an intact basement membrane (Figure 2C, h).
Smad7 Expression Alters the Proliferative Profile of the Healing Epithelium
Proliferation and migration of regenerated epithelium are critical to corneal healing after alkali injury, and both are known to be responsive to TGF-β/Smad signaling. Such epithelial cell proliferation is considered to be modulated in a complex way by both autocrine and paracrine signaling. Quantitation of BrdU staining showed that treatment with Smad7-Ad (solid line, Figure 2E) profoundly alters the time course of proliferation of conjunctival epithelium, enhancing proliferation at early times after injury but suppressing it from days 5 to 10 after injury, compared to Cre-Ad-treated eyes (dotted line, Figure 2E). At the latest time point, day 20 after injury, epithelial proliferation in the Smad7-Ad group was again enhanced compared to controls (Figure 2E).
Smad Signaling in the Injured Cornea Is Inhibited by Exogenous Smad7
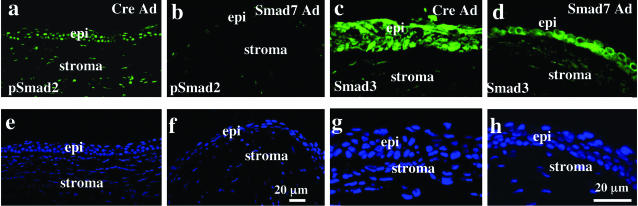
To investigate whether treatment with Smad7-Ad blocks Smad signaling in alkali-burned corneas, specimens were immunostained with antibodies against phosphorylated Smad2, as an indicator of activation of Smad signaling. At day 5 after injury, when expression of exogenous Smad7 was high, phospho-Smad2 was detected in the nuclei of healing epithelium and keratocytes in the Cre-Ad-treated group, but to a greatly reduced extent in eyes treated with Smad7-Ad (Figure 3, a versus b). Similarly, at day 5 after burn, Smad3 was detected in the cytoplasm and nuclei of many epithelial cells in Cre-Ad group cornea, whereas it mainly localized to the cytoplasm of epithelial cells in Smad7-Ad group (Figure 3, c versus d).
Figure 3.
Smad signaling status in alkali-burned corneas treated with Cre-Ad or Smad7-Ad at day 5. At day 5 after injury, staining for nuclear phospho-Smad2 is detected in the healing epithelium and stromal cells in the peripheral cornea treated with Cre-Ad (a), whereas it is not seen in the healing epithelium and stromal cells of a Smad7-Ad-treated cornea (b). Smad3 is detected in the cytoplasm and nuclei of the healing epithelium and faintly in stromal cells in a cornea treated with Cre-Ad (c), whereas Smad3 is located mainly in the cytoplasm of healing epithelium and stromal cells in the Smad7-Ad group (d). e to h: The nuclei of a to d as visualized by DAPI staining. epi, epithelium. Scale bars, 20 μm.
Effect of Loss of Smad3 on Corneal Healing
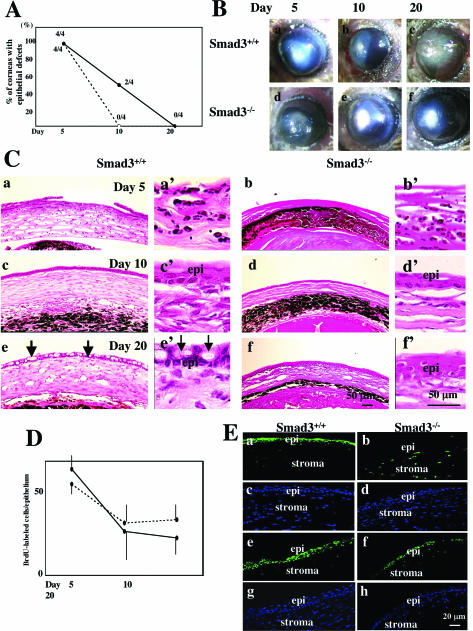
Because Smad2/3 signaling is known to be inhibited by Smad741,42 and because we have previously shown enhanced healing of an incisional skin wound in mice in which Smad3 has been deleted,17 we evaluated healing of corneas of Smad3-knockout mice after alkali burn. Throughout the time period of observation, the degree of tissue damage and inflammation from the alkali injury was less severe in the mixed C57BL/6/NIH Swiss/SV129 genetic background of the Smad3+/+ mice, compared to that of the C57BL/6 mice used for the adenoviral study, with or without viral vector application. Although corneas from Smad3−/− mice showed a lesser incidence of epithelial defects and stromal thickening at day 10 than Smad3+/+ controls (Figure 4A), corneas of both Smad3+/+ and Smad3−/− were free from ulceration [Figure 4, A and B (b, e)]. However, at day 20, corneas from both Smad3+/+ and Smad3−/− mice remained opaque (Figure 4B, c and f), in contrast to those C57BL/6 mice treated with Smad7-Ad, which were almost transparent at this time point (Figure 2B, h).
Figure 4.
Healing of alkali-burned corneas of Smad3-knockout mice. A: The percent incidence of corneas with an epithelial defect at each time point in Smad3+/+ (n = 4, solid line) and Smad3−/− (n = 4, dotted line) mice. Epithelial resurfacing is accelerated in Smad3−/− mice. B: Macroscopic observation shows total ocular surface burn in both Smad3+/+ (a) and Smad3−/− (d) mice at day 5. At day 10, an epithelial defect with stromal opacification is observed in a Smad3+/+ cornea (b), whereas the burned cornea has been resurfaced in Smad3−/− (e) mice. Although all of the corneas in both Smad3+/+ (c) and Smad3−/− (f) mice have been resurfaced by day 20, stromal opacification is more marked in Smad3+/+ mice. C: Histology of burned corneas of Smad3-knockout mice stained with H&E. An epithelial defect is observed in both Smad3+/+ (a, a’) and Smad3−/− (b, b’) mice at day 5. The burned stroma is thinner in a Smad3−/− mouse (b, b’) as compared with that in a Smad3+/+ mouse (a, a’). Higher magnification pictures (a’, b’) suggest that the majority of cells in the burned stroma are inflammatory cells at day 5. At day 10 the burned corneas in both Smad3+/+ and Smad3−/− mice have been resurfaced (c, c’, d, d’) and stroma is again thinner in a Smad3−/− mouse (c versus d). At day 20, the corneal surface is covered with conjunctival epithelium with abundant goblet cells in a Smad3+/+ mouse (e and e’, arrows), whereas the epithelium in Smad3−/− mice is cornea-like (f, f’). The thickened stroma of a Smad3+/+ mouse compared to that of a Smad3−/− mouse persists at this time point. D: Cell proliferation in healing corneas as detected by BrdU incorporation. There is no difference of the number of BrdU-labeled epithelial cells between Smad3+/+ mice (solid line) and Smad3−/− mice (dotted line) at each healing interval. Bar, SD. E: Immunofluorescent staining for phospho-Smad2 (a, b, e, f) in the central (a to d) or peripheral (e to h) corneas of Smad3+/+ (a, c, e, g) and Smad3−/− (b, d, f, h) mice at day 10 after alkali burn. Nuclear staining for phospho-Smad2 is more marked in the healing corneal epithelium (epi) of Smad3+/+ mice than in Smad3−/− mice. c, d, g, and h: DAPI nuclear staining of the area seen in a, b, e, and f, respectively. Scale bars: 20 μm (E); 50 μm (C).
Examination of the histology of healing corneas of Smad3−/− mice confirmed accelerated healing compared to eyes of Smad3+/+ littermates (Figure 4C). Stromal edema and infiltration of inflammatory cells were more prominent in Smad3+/+ mice (Figure 4C; a, a’, c, c’, e, e’) than in Smad3−/− littermates (Figure 4C; b, b’, d, d’, f, f’) at each time point examined. Even though these mice showed less severe injury than the C57BL/6 mice, at day 20, healed corneas in Smad3−/− mice still exhibited a thickened edematous, less-organized, stroma (Figure 4C, f), as compared with burned corneas of C57BL/6 mice treated with Smad7-Ad (Figure 2C, f). As in both the Smad7-Ad and Cre-Ad-treated groups of C57BL/6 mice, the resurfacing epithelium of Smad3-knockout mice was negative for keratin 12 regardless of the Smad3 genotype (data not shown). Also consistent with the Smad7-Ad treated corneas, regenerated epithelia in Smad3+/+ mice exhibited a conjunctival phenotype as evidenced by the presence of many goblet cells (arrows), whereas that in Smad3−/− mice lacked goblet cells at day 20 (Figure 4C, e and f). In contrast to the effects seen with Smad7-Ad treatment of C57BL/6 mice, epithelial cell proliferation as evaluated by BrdU incorporation was not affected by the Smad3 genotype (Figure 4D). Assessment of the effects of loss of Smad3 on Smad2 signaling in the healing cornea showed markedly less staining for phospho-Smad2 in both the central (Figure 4E, a and b) and peripheral cornea of Smad3−/− mice compared to Smad3+/+ littermates day10 after injury (Figure 4E, e and f).
Formation of Myofibroblasts Is Associated with the Poorer Healing in Cre-Ad and Smad3+/+ Corneas
One of the hallmarks of scar tissue formation and opacification of corneal stroma is the well-established transdifferentiation of keratocytes into α-SMA-positive myofibroblasts.58–61 Examination of the expression pattern of α-SMA in injured corneas showed that, at day 10 after injury, many stromal cells in injured corneas of Cre-Ad-treated mice and Smad3+/+ mice expressed α-SMA (Figure 5A, b and h, respectively), but that this was substantially reduced in Smad7-Ad-treated and Smad3−/− mice (Figure 5A, e and k, respectively). Although these differences persisted at day 20 after wounding (Figure 5A; c, f, i, and l), it is important to note that α-SMA staining was still detectable (arrows in Figure 5A, l) in corneas of Smad3−/− mice at this time point, whereas it was below the level of detection in corneas treated with Smad7-Ad (Figure 5A, f), consistent with the better outcome in that group.
Figure 5.
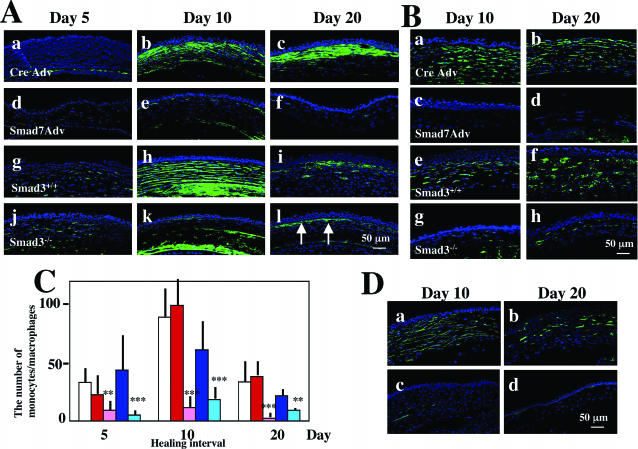
Analysis of stromal healing in burned corneas. A: The expression pattern of α-smooth muscle actin (α-SMA) as detected by immunofluorescence in stromal cells at days 5 (a, d, g, j), 10 (b, e, h, k), and 20 (c, f, i, l) after alkali burn of either Cre-Ad (a to c) or Smad7-Ad-treated (d to f) C57BL/6 mice. The same analysis was done in Smad3+/+ (g to i) and Smad3−/− (j to l) mice. At day 5 no α-SMA-positive cells are observed in the burned stroma of corneas treated with either Cre-Ad (a) or Smad7-Ad (d). In burned corneas treated with Cre-Ad, stromal cells expressing α-SMA are observed at days 10 (b) and 20 (c), whereas such cells are seen only occasionally at day 10 (e) and are absent at day 20 (f) in corneas treated with Smad7-Ad. In Smad3+/+ mice (g to i) and Smad3−/− mice (j to l) stromal cells expressing α-SMA are observed throughout the healing interval up to day 20 with a peak at day 10 (h, k). However, α-SMA expression is more marked in Smad3+/+ mice (h, i) as compared with that in Smad3−/− mice (k, l) at both days 10 (h, k) and 20 (i, l). B: The presence and distribution of F4/80-positive monocytes/macrophages as detected by immunofluorescence in representative areas of the burned corneal stroma at days 10 (a, c, e, g) and 20 (b, d, f, h) in either Cre-Ad (a, b) or Smad7-Ad-treated (c, d) C57BL/6 mice or in Smad3+/+ (e, f) and Smad3−/− mice (g, h). Labeled cells (monocytes/macrophages) are distributed throughout the stroma in corneas treated with Cre-Ad (a, b), whereas a few, if any, labeled cells are seen in the stroma of corneas treated with Smad7-Ad (c, d) at days 10 (a, c) and 20 (b, d). Similarly, the burned stromas of Smad3+/+ mice show many monocytes/macrophages at days 10 (e) and 20 (f), whereas many fewer cells are detected in the Smad3−/− mice (g, h). C: Bar graph of the number of monocytes/macrophages in the central stroma at days 5 to 20 after injury determined as described in Materials and Methods: C57BL/6 mice untreated (white bar), treated with Cre-Ad (red bar), or treated with Smad7-Ad (pink bar); Smad3+/+ mice (dark blue bar), Smad3−/− mice (light blue bar). The number of monocytes/macrophages in the stroma of corneas treated with Smad7-Ad is significantly reduced compared to that of corneas treated with Cre-ad at all time points examined. Whereas levels of monocytes/macrophages in Smad3+/+ mice is less than in C57BL/6 mice at days 10 and 20 (dark blue bar), loss of Smad3 in the mice of this background further reduces the number of such cells at all time points (light blue bar). ** or *** indicate P < 0.05 or P < 0.01, respectively, for comparisons of Smad7-Ad against Cre-Ad and of Smad3−/− against Smad3+/+ data. D: Stromal neovascularization after alkali burn as detected by immunofluorescent staining using an antibody against PECAM (CD31). At each time point, stromal neovascularization was suppressed by application of Smad7-Ad vector (c, d) as compared with Cre-Ad (a, b). Nuclei are counterstained with DAPI in A, B, and D. Scale bars, 50 μm.
Smad7-Ad-Ttreated and Smad3−/− Corneas Exhibit Less Damage to the Stromal Compartment
Because invasion of monocytes/macrophages plays an important role in tissue damage after injury, including alkali burning in the cornea, we used the F4/80 antibody to quantify the number of monocytes/macrophages in the central stroma of alkali-injured corneas (Figure 5, B and C). At all time points examined there was a substantial reduction in the number of F4/80-positive cells in corneas of Smad7-Ad-treated and Smad3−/− mice [Figure 5, B (c, d and g, h) and C] compared to their respective Cre-Ad and Smad3+/+ controls [Figure 5, B (a, b and e, f)and C ]. As expected, there was no significant difference in the number of monocytes/macrophages in untreated burned corneas compared to the Cre-Ad-treated group (Figure 5C, white versus red bars).
Neovascularization of the corneal stroma likely contributes to stromal opacification and is associated with inflammation. Examination of stromal neovascularization using an antibody to CD31 (PECAM) (Figure 5D) showed marked staining in Cre-Ad-treated corneas at all time points examined (Figure 5D, a and b) which was substantially reduced in eyes treated with Smad7-Ad (Figure 5D, c and d). Similar differences were observed in Smad3+/+ as compared to Smad3−/− eyes (not shown).
Smad7-Ad-Treated and Smad3−/− Corneas Show Reduced Expression of Growth Factors and Metalloproteases after Alkali Injury Compared to Their Controls
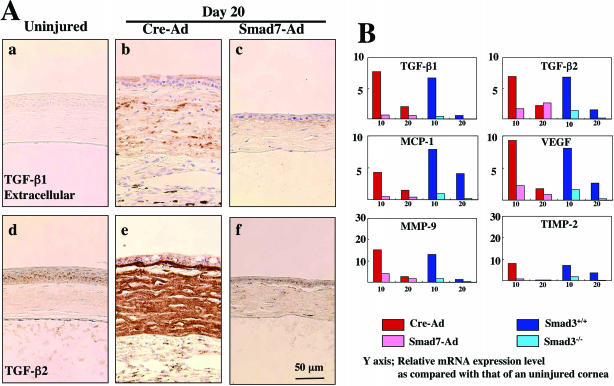
Because various cytokines and matrix-degrading enzymes induced after injury are known to be regulated, in part, through the TGF-β/Smad signaling pathway, we examined the effects of Smad7-Ad treatment and loss of Smad3 on expression of such target genes implicated in the tissue destruction in alkali-burned corneas. It is known that Smad3 signaling contributes to autoinduction of TGF-β1 in many injured tissues, and that this results in elevated levels of peptide that typically persist long after the initial injury. Immunohistochemical staining showed that TGF-β1 and TGF-β2 were both expressed in the epithelium of the uninjured cornea (Figure 6A). Although the level of expression of intracellular TGF-β1 in epithelium did not change substantially after injury (not shown) levels of extracellular TGF-β1 were significantly elevated at day 10 (not shown) and persisted at day 20 after injury in Cre-Ad-treated eyes (Figure 6A, b). A similar pattern was observed for TGF-β2, with stromal expression of TGF-β2 being particularly intense at day 20 (Figure 6A, e). This up-regulation of TGF-β1 and TGF-β2 was suppressed by Smad7-Ad treatment (Figure 6A, c and f). The expression patterns of both TGF-β1 and TGF-β2 in Smad3−/− mice compared to Smad3+/+mice were similar to that seen in corneas treated by Smad7-Ad compared to Cre-Ad (data not shown).
Figure 6.
Expression of cytokines and growth factors in burned corneas. A: Immunohistochemical detection of extracellular TGF-β1 (a) and TGF-β2 (b) in representative uninjured corneas and in burned corneas treated with either Cre-Ad (b, e) or Smad7-Ad (c, f) at day 20 after injury. Accumulation of extracellular TGF-β1 and of TGF-β2 is increased in the burned stroma of the Cre-Ad group (b, e) as compared with that in Smad7-Ad group (c, f) at day 20. Nuclei are counterstained with hematoxylin. B: Quantitation of mRNAs of TGF-β1, TGF-β2, MCP-1, MMP-9, and TIMP-2 by real-time RT-PCR at days 10 and 20 after injury in corneas of C57BL/6 mice treated with either Cre-Ad (red) or Smad7-Ad (pink) or in corneas of Smad3+/+ (blue) or Smad3−/− mice (light blue). Real-time RT-PCR shows a marked up-regulation mRNAs of TGF-β1, TGF-β2, MCP-1, MMP-9, and TIMP-2 in burned corneas of Cre-Ad-treated C57BL/6 mice and in those of Smad3+/+ mice as compared to levels in the uninjured cornea, and this up-regulation is dramatically suppressed by Smad7 gene transfer or in corneas of mice lacking Smad3 at days 10 and 20. Data represent a typical result in one experiment of three to four done at each time point. Scale bar, 50 μm.
MCP-1, VEGF, and CTGF were all detected immunohistochemically in epithelium and stromal cells in healing postalkali-burned corneas in the Cre-Ad group, and expression of these cytokines was markedly reduced by Smad7-Ad treatment (data not shown). Real-time RT-PCR also showed a marked up-regulation mRNAs of TGF-β1, TGF-β2, mcp-1, mmp-9, and timp-2 in burned corneas of Cre-Ad-treated C57BL/6 mice and those of Smad3+/+ mice, compared to their respective controls at days 10 and 20 (Figure 7B).
Figure 7.
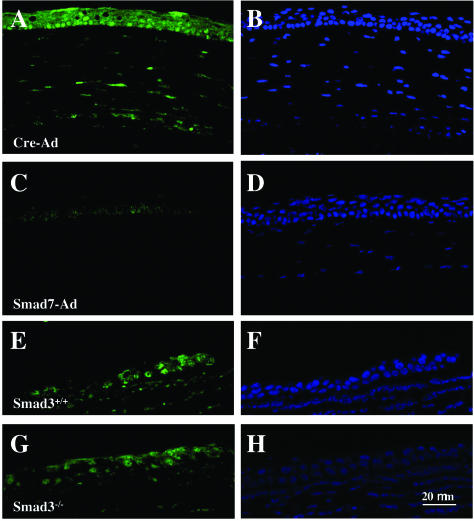
Effect of Smad7 expression on signal transduction through the NF-κB pathway. In a representative cornea treated with Cre-Ad, phospho-p65/RelA is detected in the nuclei of healing peripheral epithelium and stromal cells (A), whereas it can be seen only faintly in the healing central epithelium in a cornea treated with Smad7-Ad (C). In contrast, no difference is seen in peripheral corneas of Smad3+/+ compared to Smad3−/− mice. B, D, F, and H represent DAPI nuclear staining corresponding to A, C, E, and G, respectively. Nuclei are counterstained with DAPI. Scale bar, 20 μm.
Reduction in NF-κB Signaling Distinguishes Smad7-Ad-Treated Corneas from Corneas of Smad3−/− Mice after Alkali Injury
NF-κB mediates signaling from many inflammatory cytokines. It was recently reported that Smad7 inhibits NF-κB signaling independently of its effects on Smad2/3 phosphorylation.52 Indeed, evaluation of the status of NF-κB signaling in burned corneas showed that the phospho-p65/RelA subunit of NF-κB was detected in epithelial cells and stromal cells of Cre-Ad-treated corneas (Figure 7A), but not in corneas of the Smad7-Ad group (Figure 7C). Importantly, epithelial staining for phospho-p65/RelA was not reduced in Smad3−/− corneas as compared to Smad3+/+ corneas, suggesting that Smad3 is not involved in the regulation of NF-κB signaling in this model (Figure 7, E and G).
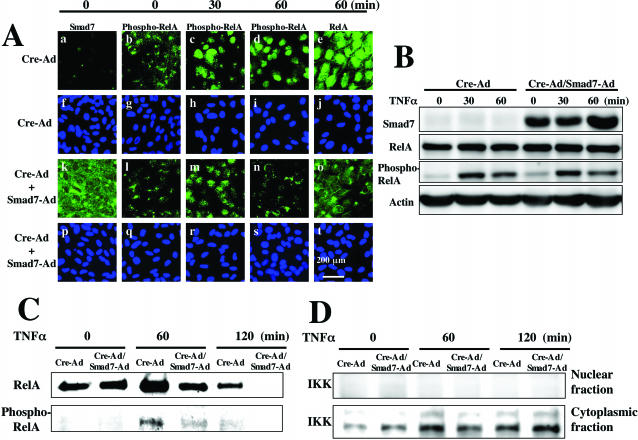
To further confirm the suppressive effect of ectopic Smad7 on NF-κB signaling, we performed immunocytochemistry and Western blotting using an SV40-immortalized human corneal epithelial cell line (Araki-Sasaki’s cell) treated with either Cre-Ad or Smad7-Ad. Immunostaining showed that exogenous Smad7 did not affect immunolocalization of RelA and phospho-RelA at 30 minutes after TNF-α addition (Figure 8A, c and m) despite strongly increased immunoreactivity for Smad7 protein (Figure 8A, a compared to k), but suppressed nuclear translocation of phospho-RelA and expression RelA at 60 minutes (Figure 8A; d, n, and e, o). In contrast, Western blotting of the total cell lysate showed that overexpression of exogenous Smad7 did not affect the levels of RelA and phospho-RelA up to 60 minutes (Figure 8B). Because phospho-RelA is translocated into the nucleus, we then analyzed the effect of Smad7 overexpression on its nuclear translocation. Western blotting of the nuclear fraction for RelA and phospho-RelA (Figure 8C) showed that nuclei of cells treated with Smad7-Ad contained substantially less RelA and phospho-RelA than those of cells treated with Cre-Ad at both 60 and 120 minutes after addition of TNF-α. The purity of the nuclear fractions were confirmed by the absence of staining for the cytoplasmic protein I κ kinase α (Figure 8D). These results indicate that ectopic Smad7 does not alter phosphorylation of NF-κB/RelA in these cells, but attenuates nuclear localization of both RelA and phospho-RelA.
Figure 8.
Suppression of NF-κB signaling by Smad7 overexpression in SV40-immortalized corneal epithelial cells. A: Immunocytochemistry confirms the high level of Smad7 expression in cells treated with Smad7-Ad (k). There is no obvious difference in expression of phospho-RelA between Cre-Ad and Smad7-Ad-treated cultures up to 30 minutes after TNF-α addition (b versus l, c versus m). At 60 minutes of exposure to TNF-α, staining for phospho-RelA (d versus n) and total RelA (e versus o) in the nuclei is reduced in the cells treated with Smad7-Ad as compared with Cre-Ad. f to j and p to t: Nuclear localization as stained with DAPI corresponding to a to e and k to o. Nuclei are counterstained with DAPI. B: Western blotting of total cell lysates shows that overexpression of exogenous Smad7 did not affect the level of RelA and phospho-RelA up to 60 minutes after TNF-α addition. C: Western blotting of nuclear fractions shows that total RelA, but not phospho-RelA can be detected in untreated Cre-Ad and Smad7-Ad cultures (time 0), At 60 and 120 minutes after TNF-α addition, Smad7-Ad suppresses TNF-α-induced up-regulation of nuclear translocation of total RelA and phospho-RelA. D: Immunodetection of I κ kinase (IKK) in the nuclear fractions used in C and the corresponding cytoplasmic fractions derived from each cell culture. IKK is detected in the cytoplasmic fraction, but not in nuclear fraction, indicating the fraction is not contaminated. Scale bar, 200 μm.
Discussion
In the present study we show that adenoviral gene transfer of Smad7 prevents tissue destruction in a mouse cornea burned with a topical application of 1 N NaOH. Corneas of the control Cre-Ad group exhibited ulceration, scarring, and neovascularization, as well as conjunctivalization of the resurfaced epithelium at the final time point of day 20. In contrast, burned corneas treated with Smad7-Ad were resurfaced with a cornea-like epithelium and healed with no, or minimal, scarring as evidenced by restored transparency at day 20. Expression of exogenous Smad7 at early times after injury (up to day 5) was sufficient to improve healing as expression fell to control levels at later times, despite repeated infection at days 10 and 20. Our immunohistochemical data suggest that the mechanisms whereby overexpression of Smad7 improves healing of alkali-burned corneas involve blocking not only of Smad2/3 signaling, but possibly also of signaling leading to activation and nuclear translocation of NF-κB, a dimeric transcription factor activated most commonly by proinflammatory cytokines including TNF-α and interleukin-1.62–65 At a cellular level, Smad7-Ad likely prevents corneal ulceration and stromal opacification directly by modulating Smad and/or NF-κB signaling in conjunctival epithelial cells, keratocytes/myofibroblasts and indirectly by reducing their expression of growth factors and cytokines/chemokines that both feedback on these two target pathways and contribute in other ways to the outcome.
Effect of Smad7 on Stromal Wound Healing in the Injured Cornea
Transient conversion of keratocytes to myofibroblasts, as characterized by expression of α-SMA,58–61 is one of the most important elements of corneal stromal wound healing after exposure to alkali and is associated with up-regulation of matrix components involved in stromal scarring. Although in vitro studies have shown that expression of α-SMA in dermal fibroblasts is regulated by cytokines and extracellular matrix, ie, fibronectin ED-A54, TGF-β/Smad2 signaling is essential for it.66–69 In the present study, treatment with Smad7-Ad completely abolished expression of α-SMA in keratocytes in burned stroma, whereas it was only partially reduced in corneas lacking Smad3. Although the data suggest that blocking of both Smad2 and Smad3 signaling may be necessary to completely inhibit expression of α-SMA, the reduction of expression of TGF-β1 and of matrix proteins that modulate α-SMA expression in Smad3−/− mice likely contributes indirectly to its down-regulation. These mechanisms are distinct from those implicated in myofibroblast differentiation after injury to the kidney, lens, or retina, where myofibroblasts are generated by epithelial-mesenchymal transition (EMT) of renal tubular epithelial cells, lens epithelial cells, or retinal pigment epithelial cells, respectively.36–38,44 In those cases, an unidentified early step in the differentiation is completely blocked in the absence of Smad3.
Stromal scarring and opacification are believed to be dependent, in part, on up-regulation of TGF-β1, causing stromal cells to express extracellular matrix components qualitatively and quantitatively different from those found in uninjured stroma. Up-regulation of TGF-β1 in the burned cornea was completely blocked by loss of Smad3 or by treatment of injured eyes with Smad7-Ad, consistent with its dependence on Smad3. Regulation of MMP/TIMP levels, important in degradation and remodeling of stromal extracellular matrix, has been implicated in modulation of stromal opacification.6–9 Expression of mmp-9 and timp-2 were both suppressed in corneas of Smad7-Ad-treated or Smad3−/− mice, compared to their respective controls, suggesting an altered pattern of remodeling (turnover) of matrix macromolecules in corneas in which Smad3 signaling is blocked.
Neovascularization of corneal stroma also potentially contributes to opacification. Suppression of the up-regulation of VEGF expression by treatment of alkali-burned corneas with Smad7-Ad correlates with reduced stromal neovascularization. Moreover, in corneas treated with Smad7-Ad, reduced levels of VEGF, as well as TGF-β and MCP-1, each of which is capable of recruiting monocytes/macrophages to sites of injury likely contribute to the reduced recruitment and invasion of inflammatory cells, which themselves are capable of VEGF production. Our unpublished data showing suppression of neovascularization after alkali burn by topical administration of an adenoviral vector expressing the soluble VEGF receptor (S. Saika, K. Ikeda, O. Yamanaka, unpublished data) further support the possible role of the reduction of VEGF level in suppression of stromal neovascularization.
Effect of Interference with Smad Signaling on Epithelial Wound Healing in an Alkali Burned Cornea
In the burn model we have described here, defects in the corneal epithelium resulting from alkali injury were replaced by conjunctival epithelium lacking keratin 12, similar to that seen in the clinical setting.1–3 Importantly, histological analysis showed a transdifferentiation to a cornea-like epithelium in resurfaced corneas of the Smad7-Ad group and Smad3−/− mice, while the resurfaced epithelium in the Cre-Ad group and in Smad3+/+ mice retained its conjunctival nature, as evidenced by the presence of goblet cells (conjunctivalization). The similar effect of overexpression of Smad7 and loss of Smad3 on the epithelial phenotype, suggest that this particular effect of Smad7 is likely dependent on its ability to block the Smad3 pathway. This difference in the phenotype of the conjunctival epithelium may also contribute to the lack of effectiveness of repeated Smad7-Ad applications at days 10 and 15, because conjunctival epithelium is more susceptible to adenoviral infection than corneal epithelium.56 It was recently reported that differentiation of conjunctival epithelium resurfacing the injured corneal surface depends on the level of VEGF in the tissue.22 The suppressed levels of VEGF shown here in alkali-injured corneas treated with Smad7-Ad or in Smad3−/− burned corneas, together with our demonstration that adenoviral gene transfer of soluble VEGF receptor suppressed conjunctivalization of the healing epithelium in the same type of mouse corneal alkali burn (S. Saika, K. Ikeda, O. Yamanaka, unpublished data) suggest that a reduction of VEGF might contribute to the cornea-like differentiation of resurfacing conjunctival epithelium as well as to reduced neovascularization of the injured stroma.
Expression of Smad7 Blocks Not Only Smad2/3 Signaling but Also Signaling via NF-κB
Direct comparisons between the adenoviral treated C57BL6 mice and the Smad3 mice are complicated both by effects of the mixed background of the latter and by the different approaches involving transient expression of ectopic genes versus genetic loss. Nonetheless, the strikingly similar effects of overexpression of Smad7 and of loss of Smad3 on epithelial phenotype, inflammation, neovascularization, and expression of various cytokine mediators of the response to injury clearly implicate the blocking of Smad3 in the mechanism of Smad7 action and suggest that deleterious effects of Smad signaling are most prominent in the early stages of the response to injury. Yet, other effects such as suppression of α-SMA expression and effects on proliferation of the corneal epithelium clearly distinguish the mechanisms of action of these two signaling molecules. In this regard, we have demonstrated here for the first time that ectopic expression of Smad7 not only blocks Smad signaling, but also signaling through NF-κB in vivo, suggesting that blocking of both of these pathways contributes to accelerated corneal wound healing with reduced scarring/opacification. In vitro studies using an immortalized human corneal epithelial cell line confirmed that nuclear translocation of both RelA and phospho-RelA were reduced in cells treated with Smad7-Ad, similar to results previously reported in cultured renal podocytes.70 Interestingly, this effect seems to be cell type-specific because no effect of Smad7 overexpression was seen on nuclear phospho-RelA in the human HaCat keratinocytes cell line (L. Lyakh, personal communication). Our finding of reduced NF-κB activation in corneas treated with Smad7-Ad likely contributes both to the repressed inflammation in response to alkali injury and to the suppressed expression of MMPs in cells,71,72 and amplifies the effects of Smad7 on interference with Smad2/3 signaling in the burned cornea. Again, while avoiding a direct comparison, the reduced effect on healing of loss of Smad3 compared to Smad7-Ad-treated corneas is consistent with the lack of effect of loss of Smad3 on NF-κB signaling and with our data on the effectiveness of topical administration of a cell permeable NF-κB inhibitor ligand (Upstate Biotechnology, Lake Placid, NY) on the healing of a mouse corneal alkali burn.73 Whether the reduced phosphorylation of the p65/RelA subunit of NF-κB in corneas treated with Smad7-Ad results from direct effects of Smad7 on the pathway or indirectly from modulated expression of cytokines capable of activating the NF-κB pathway, cannot be determined in this model. Regardless, our data lead us to suggest that development of Smad7 delivery vectors may open new opportunities for therapeutic intervention in treatment of chemically burned corneas.
Footnotes
Address reprint requests to Shizuya Saika, M.D., Ph.D., Department of Ophthalmology, Wakayama Medical University, 811-1 Kimiidera, Wakayama, 641-0012, Japan. E-mail: shizuya@wakayama-med.ac.jp.
Supported by the Ministry of Education, Science, Sports, and Culture of Japan [grants C15591871 and C16590150 (to K.I.)]; the Uehara Memorial Foundation (to S.S.); the Wakayama Medical University (research grant on priority areas to S.S., Y.M., and A.O); the National Institutes of Health (grant EY 13755); Research to Prevent Blindness; and the Ohio Lions Eye Research Foundation (to W.W.-Y.K.).
References
- Brodovsky SC, McCarty CA, Snibson G, Loughnan M, Sullivan L, Daniell M, Taylor HR. Management of alkali burns: an 11-year retrospective review. Ophthalmology. 2000;107:1829–1835. doi: 10.1016/s0161-6420(00)00289-x. [DOI] [PubMed] [Google Scholar]
- Saika S, Kobata S, Hashizume N, Okada Y, Yamanaka O. Epithelial basement membrane in alkali-burned corneas in rats. Immunohistochemical study. Cornea. 1993;12:383–390. doi: 10.1097/00003226-199309000-00003. [DOI] [PubMed] [Google Scholar]
- Ishizaki M, Zhu G, Haseba T, Shafer SS, Kao WW-Y. Expression of collagen I, smooth muscle α-actin, and vimentin during the healing of alkali-burned and lacerated corneas. Invest Ophthalmol Vis Sci. 1993;34:3320–3328. [PubMed] [Google Scholar]
- Ravanti L, Kahari VM. Matrix metalloproteinases in wound repair. Int J Mol Med. 2000;6:391–407. [PubMed] [Google Scholar]
- Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- Ye HQ, Azar DT. Expression of gelatinases A and B, and TIMPs 1 and 2 during corneal wound healing. Invest Ophthalmol Vis Sci. 1998;39:913–921. [PubMed] [Google Scholar]
- Kato T, Kure T, Chang JH, Gabison EE, Itoh T, Itohara S, Azar DT. Diminished corneal angiogenesis in gelatinase A-deficient mice. FEBS Lett. 2001;508:187–190. doi: 10.1016/s0014-5793(01)02897-6. [DOI] [PubMed] [Google Scholar]
- Sivak JM, Fini ME. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog Retin Eye Res. 2002;21:1–14. doi: 10.1016/s1350-9462(01)00015-5. [DOI] [PubMed] [Google Scholar]
- Daniels JT, Geerling G, Alexander RA, Murphy G, Khaw PT, Saarialho-Kere U. Temporal and spatial expression of matrix metalloproteinases during wound healing of human corneal tissue. Exp Eye Res. 2003;77:653–664. doi: 10.1016/j.exer.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Kao WW, Liu CY, Converse RL, Shiraishi A, Kao CW, Ishizaki M, Doetschman T, Duffy J. Keratin 12-deficient mice have fragile corneal epithelia. Invest Ophthalmol Vis Sci. 1996;37:2572–2584. [PubMed] [Google Scholar]
- Moyer PD, Kaufman AH, Zhang Z, Kao CW, Spaulding AG, Kao WW. Conjunctival epithelial cells can resurface denuded cornea, but do not transdifferentiate to express cornea-specific keratin 12 following removal of limbal epithelium in mouse. Differentiation. 1996;60:31–38. doi: 10.1046/j.1432-0436.1996.6010031.x. [DOI] [PubMed] [Google Scholar]
- Sridhar MS, Bansal AK, Sangwan VS, Rao GN. Amniotic membrane transplantation in acute chemical and thermal injury. Am J Ophthalmol. 2000;130:134–137. doi: 10.1016/s0002-9394(00)00500-6. [DOI] [PubMed] [Google Scholar]
- Meller D, Pires RT, Mack RJ, Figueiredo F, Heiligenhaus A, Park WC, Prabhasawat P, John T, McLeod SD, Steuhl KP, Tseng SC. Amniotic membrane transplantation for acute chemical or thermal burns. Ophthalmology. 2000;107:980–989. doi: 10.1016/s0161-6420(00)00024-5. [DOI] [PubMed] [Google Scholar]
- Saika S, Okada Y, Miyamoto T, Yamanaka O, Ohnishi Y, Ooshima A, Liu CY, Weng D, Kao WW. Role of p38 MAP kinase in regulation of cell migration and proliferation in healing corneal epithelium. Invest Ophthalmol Vis Sci. 2004;45:100–109. doi: 10.1167/iovs.03-0700. [DOI] [PubMed] [Google Scholar]
- Andresen JL, Ehlers N. Chemotaxis of human keratocytes is increased by platelet-derived growth factor-BB, epidermal growth factor, transforming growth factor-alpha, acidic fibroblast growth factor, insulin-like growth factor-I, and transforming growth factor-beta. Curr Eye Res. 1998;17:79–87. doi: 10.1076/ceyr.17.1.79.5261. [DOI] [PubMed] [Google Scholar]
- You L, Kruse FE. Differential effect of activin A and BMP-7 on myofibroblast differentiation and the role of the Smad signaling pathway. Invest Ophthalmol Vis Sci. 2002;43:72–81. [PubMed] [Google Scholar]
- Ashcroft GS, Yang X, Glick AB, Weinstein M, Letterio JL, Mizel DE, Anzano M, Greenwell-Wild T, Wahl SM, Deng C, Roberts AB. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. doi: 10.1038/12971. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Li JH, Garcia G, Mu W, Piek E, Bottinger EP, Chen Y, Zhu HJ, Kang DH, Schreiner GF, Lan HY, Johnson RJ. TGF-β induces proangiogenic and antiangiogenic factors via parallel but distinct Smad pathways. Kidney Int. 2004;66:605–613. doi: 10.1111/j.1523-1755.2004.00780.x. [DOI] [PubMed] [Google Scholar]
- Abraham S, Sawaya BE, Safak M, Batuman O, Khalili K, Amini S. Regulation of MCP-1 gene transcription by Smads and HIV-1 Tat in human glial cells. Virology. 2003;309:196–202. doi: 10.1016/s0042-6822(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Edelman JL, Castro MR, Wen Y. Correlation of VEGF expression by leukocytes with the growth and regression of blood vessels in the rat cornea. Invest Ophthalmol Vis Sci. 1999;40:1112–1123. [PubMed] [Google Scholar]
- Lai CM, Spilsbury K, Brankov M, Zaknich T, Rakoczy PE. Inhibition of corneal neovascularization by recombinant adenovirus mediated antisense VEGF RNA. Exp Eye Res. 2002;75:625–634. doi: 10.1006/exer.2002.2075. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Poulaki V, Mitsiades N, Stechschulte SU, Kirchhof B, Dartt DA, Fong GH, Rudge J, Wiegand SJ, Yancopoulos GD, Adamis AP. VEGF-dependent conjunctivalization of the corneal surface. Invest Ophthalmol Vis Sci. 2003;44:117–123. doi: 10.1167/iovs.01-1277. [DOI] [PubMed] [Google Scholar]
- Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996;87:3336–3343. [PubMed] [Google Scholar]
- Yang ZF, Poon RT, Luo Y, Cheung CK, Ho DW, Lo CM, Fan ST. Up-regulation of vascular endothelial growth factor (VEGF) in small-for-size liver grafts enhances macrophage activities through VEGF receptor 2-dependent pathway. J Immunol. 2004;173:2507–2515. doi: 10.4049/jimmunol.173.4.2507. [DOI] [PubMed] [Google Scholar]
- Tesch GH, Schwarting A, Kinoshita K, Lan HY, Rollins BJ, Kelley VR. Monocyte chemoattractant protein-1 promotes macrophage-mediated tubular injury, but not glomerular injury, in nephrotoxic serum nephritis. J Clin Invest. 1999;103:73–80. doi: 10.1172/JCI4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, Kovacs EJ, DiPietro LA. Wound healing in MIP-1alpha(−/−) and MCP-1(−/−) mice. Am J Pathol. 2001;159:457–463. doi: 10.1016/s0002-9440(10)61717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister RR, Haddox JL, Sommers CI. Injection of chemoattractants into normal cornea: a model of inflammation after alkali injury. Invest Ophthalmol Vis Sci. 1998;39:1744–1750. [PubMed] [Google Scholar]
- Massague J, Wotton D. Transcriptional control by the TGF-β/Smad signaling system. EMBO J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustakas A, Pardali K, Gaal A, Heldin CH. Mechanisms of TGF-β signaling in regulation of cell growth and differentiation. Immunol Lett. 2002;82:85–91. doi: 10.1016/s0165-2478(02)00023-8. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Goumans MJ, Itoh F, Itoh S. Regulation of cell proliferation by Smad proteins. J Cell Physiol. 2002;191:1–16. doi: 10.1002/jcp.10066. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Roberts AB, Piek E, Bottinger EP, Ashcroft G, Mitchell JB, Flanders KC. Is Smad3 a major player in signal transduction pathways leading to fibrogenesis? Chest. 2001;120(Suppl 1):43S–47S. doi: 10.1378/chest.120.1_suppl.s43-a. [DOI] [PubMed] [Google Scholar]
- Schnabl B, Kweon YO, Frederick JP, Wang XF, Rippe RA, Brenner DA. The role of Smad3 in mediating mouse hepatic stellate cell activation. Hepatology. 2001;3:89–100. doi: 10.1053/jhep.2001.25349. [DOI] [PubMed] [Google Scholar]
- Flanders KC, Sullivan CD, Fujii M, Sowers A, Anzano MA, Arabshahi A, Major C, Deng C, Russo A, Mitchell JB, Roberts AB. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol. 2002;160:1057–1068. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-β1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders KC, Major CD, Arabshahi A, Aburime EE, Okada MH, Fujii M, Blalock TD, Schultz GS, Sowers A, Anzano MA, Mitchell JB, Russo A, Roberts AB. Interference with transforming growth factor-β/ Smad3 signaling results in accelerated healing of wounds in previously irradiated skin. Am J Pathol. 2003;163:2247–2257. doi: 10.1016/s0002-9440(10)63582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Kono-Saika S, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Flanders KC, Yoo J, Anzano M, Liu CY, Kao WW, Roberts AB. Smad3 signaling is required for epithelial-mesenchymal transition of lens epithelium after injury. Am J Pathol. 2004;164:651–663. doi: 10.1016/S0002-9440(10)63153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders KC. Smad3 as a mediator of the fibrotic response. Int J Exp Pathol. 2004;85:47–64. doi: 10.1111/j.0959-9673.2004.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol. 2004;173:2099–2108. doi: 10.4049/jimmunol.173.3.2099. [DOI] [PubMed] [Google Scholar]
- Saika S, Kono-Saika S, Tanaka T, Yamanaka O, Ohnishi Y, Sato M, Muragaki Y, Ooshima A, Yoo J, Flanders KC, Roberts AB. Smad3 is required for dedifferentiation of retinal pigment epithelium following retinal detachment in mice. Lab Invest. 2004;84:1245–1258. doi: 10.1038/labinvest.3700156. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA, Jr, Wrana JL, Falb D. The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P. Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- Pulaski L, Landstrom M, Heldin CH, Souchelnytskyi S. Phosphorylation of Smad7 at Ser-249 does not interfere with its inhibitory role in transforming growth factor-beta-dependent signaling but affects Smad7-dependent transcriptional activation. J Biol Chem. 2001;276:14344–14349. doi: 10.1074/jbc.M011019200. [DOI] [PubMed] [Google Scholar]
- Nakao A, Fujii M, Matsumura R, Kumano K, Saito Y, Miyazono K, Iwamoto I. Transient gene transfer and expression of Smad7 prevents bleomycin-induced lung fibrosis in mice. J Clin Invest. 1999;104:5–11. doi: 10.1172/JCI6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan HY, Mu W, Tomita N, Huang XR, Li JH, Zhu HJ, Morishita R, Johnson RJ. Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J Am Soc Nephrol. 2003;14:1535–1548. doi: 10.1097/01.asn.0000067632.04658.b8. [DOI] [PubMed] [Google Scholar]
- Dooley S, Hamzavi J, Breitkopf K, Wiercinska E, Said HM, Lorenzen J, Ten Dijke P, Gressner AM. Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology. 2003;125:178–191. doi: 10.1016/s0016-5085(03)00666-8. [DOI] [PubMed] [Google Scholar]
- Saika S, Ikeda K, Yamanaka O, Sato M, Muragaki Y, Ohnishi Y, Ooshima A, Nakajima Y, Namikawa K, Kiyama H, Flanders KC, Roberts AB. Transient adenoviral gene transfer of Smad7 prevents injury-induced epithelial-mesenchymal transition of lens epithelium in mice. Lab Invest. 2004;84:1259–1270. doi: 10.1038/labinvest.3700151. [DOI] [PubMed] [Google Scholar]
- Bitzer M, von Gersdorff G, Liang D, Dominguez-Rosales A, Beg AA, Rojkind M, Bottinger EP. A mechanism of suppression of TGF-β/SMAD signaling by NF-κB/RelA. Genes Dev. 2000;14:187–197. [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rovira T, Chalaux E, Rosa JL, Bartrons R, Ventura F. Interaction and functional cooperation of NF-kappa B with Smads. Transcriptional regulation of the junB promoter. J Biol Chem. 2000;275:28937–28946. doi: 10.1074/jbc.M909923199. [DOI] [PubMed] [Google Scholar]
- Monteleone G, Mann J, Monteleone I, Vavassori P, Bremner R, Fantini M, Del Vecchio Blanco G, Tersigni R, Alessandroni L, Mann D, Pallone F, MacDonald TT. A failure of transforming growth factor-beta1 negative regulation maintains sustained NF-κB activation in gut inflammation. J Biol Chem. 2004;279:3925–3932. doi: 10.1074/jbc.M303654200. [DOI] [PubMed] [Google Scholar]
- Lallemand F, Mazars A, Prunier C, Bertrand F, Kornprost M, Gallea S, Roman-Roman S, Cherqui G, Atfi A. Smad7 inhibits the survival nuclear factor kappaB and potentiates apoptosis in epithelial cells. Oncogene. 2001;20:879–884. doi: 10.1038/sj.onc.1204167. [DOI] [PubMed] [Google Scholar]
- Saika S, Shiraishi A, Liu CY, Funderburgh JL, Kao CW, Converse RL, Kao WW. Role of lumican in the corneal epithelium during wound healing. J Biol Chem. 2000;275:2607–2612. doi: 10.1074/jbc.275.4.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saika S, Saika S, Liu CY, Azhar M, Sanford LP, Doetschman T, Gendron RL, Kao CW, Kao WW. TGFβ2 in corneal morphogenesis during mouse embryonic development. Dev Biol. 2001;240:419–432. doi: 10.1006/dbio.2001.0480. [DOI] [PubMed] [Google Scholar]
- Flanders KC, Thompson NL, Cissel DS, Van Obberghen-Schilling E, Baker CC, Kass ME, Ellingsworth LR, Roberts AB, Sporn MB. Transforming growth factor-β1: histochemical localization with antibodies to different epitopes. J Cell Biol. 1989;108:653–660. doi: 10.1083/jcb.108.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanders KC, Ludecke G, Engels S, Cissel DS, Roberts AB, Kondaiah P, Lafyatis R, Sporn MB, Unsicker K. Localization and actions of transforming growth factor-βs in the embryonic nervous system. Development. 1991;113:183–191. doi: 10.1242/dev.113.1.183. [DOI] [PubMed] [Google Scholar]
- Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–621. [PubMed] [Google Scholar]
- Tsubota K, Inoue H, Ando K, Ono M, Yoshino K, Saito I. Adenovirus-mediated gene transfer to the ocular surface epithelium. Exp Eye Res. 1998;67:531–538. doi: 10.1006/exer.1998.0557. [DOI] [PubMed] [Google Scholar]
- Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142:873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Jester JV, Huang J, Petroll WM, Cavanagh HD. TGFβ induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFβ, PDGF and integrin signaling. Exp Eye Res. 2002;75:645–657. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Darby IA, Gabbiani G. Normal and pathologic soft tissue remodeling: role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab Invest. 2003;83:1689–1707. doi: 10.1097/01.lab.0000101911.53973.90. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr Series introduction: the transcription factor NF-κB and human disease. J Clin Invest. 2001;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada T, Yoshimura A. Regulation of cytokine signaling and inflammation. Cytokine Growth Factor Rev. 2002;13:413–421. doi: 10.1016/s1359-6101(02)00026-6. [DOI] [PubMed] [Google Scholar]
- Piek E, Ju WJ, Heyer J, Escalante-Alcalde D, Stewart CL, Weinstein M, Deng C, Kucherlapati R, Bottinger EP, Roberts AB. Functional characterization of transforming growth factor β signaling in Smad2- and Smad3-deficient fibroblasts. J Biol Chem. 2001;276:19945–19953. doi: 10.1074/jbc.M102382200. [DOI] [PubMed] [Google Scholar]
- Itoh S, Thorikay M, Kowanetz M, Moustakas A, Itoh F, Heldin CH, ten Dijke P. Elucidation of Smad requirement in transforming growth factor-β type I receptor-induced responses. J Biol Chem. 2003;278:3751–3761. doi: 10.1074/jbc.M208258200. [DOI] [PubMed] [Google Scholar]
- Yang YC, Piek E, Zavadil J, Liang D, Xie D, Heyer J, Pavlidis P, Kucherlapati R, Roberts AB, Bottinger EP. Hierarchical model of gene regulation by transforming growth factor β. Proc Natl Acad Sci USA. 2003;100:10269–10274. doi: 10.1073/pnas.1834070100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RA, Tian YC, Steadman R, Phillips AO. TGF-β1-mediated fibroblast-myofibroblast terminal differentiation—the role of Smad proteins. Exp Cell Res. 2003;282:90–100. doi: 10.1016/s0014-4827(02)00015-0. [DOI] [PubMed] [Google Scholar]
- Schiffer M, Bitzer M, Roberts ISD, Kopp JB, ten Dijke P, Mundel P, Böttinger EP. Apoptosis in podocytes induced by TGF-β and Smad7. J Clin Invest. 2001;108:807–816. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond M, Fabunmi RP, Baker AH, Newby AC. Synergistic upregulation of metalloproteinase-9 by growth factors and inflammatory cytokines: an absolute requirement for transcription factor NF-κB. FEBS Lett. 1998;435:29–34. doi: 10.1016/s0014-5793(98)01034-5. [DOI] [PubMed] [Google Scholar]
- Bond M, Baker AH, Newby AC. Nuclear factor κB activity is essential for matrix metalloproteinase-1 and -3 upregulation in rabbit dermal fibroblasts. Biochem Biophys Res Commun. 1999;264:561–567. doi: 10.1006/bbrc.1999.1551. [DOI] [PubMed] [Google Scholar]
- Saika S, Miyamoto T, Yamanaka O, Kato T, Ohnishi Y, Flanders KC, Ikeda K, Nakajima Y, Kao WW-Y, Sdato M, Muragaki Y, Ooshima A. Therapeutic effect of topical administration of SN50, an inhibitor of NF-κB, in treatment of corneal alkali burns in mice. Am J Pathol. 2005;166:1393–1403. doi: 10.1016/s0002-9440(10)62357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]