Abstract
Microglia accumulation at the site of amyloid plaques is a strong indication that microglia play a major role in Alzheimer’s disease pathogenesis. However, how microglia affect amyloid-β peptide (Aβ) deposition remains poorly understood. To address this question, we developed a novel bigenic mouse that overexpresses both amyloid precursor protein (APP) and monocyte chemotactic protein-1 (MCP-1; CCL2 in systematic nomenclature). CCL2 expression, driven by the glial fibrillary acidic protein promoter, induced mononuclear phagocyte (MP; monocyte-derived macrophage and microglial) accumulation in the brain. When APP/CCL2 transgenic mice were compared to APP mice, a fivefold increase in Aβ deposition was present despite increased MP accumulation around hippocampal and cortical amyloid plaques. Levels of full-length APP, its C-terminal fragment, and Aβ-degrading enzymes (insulin-degrading enzyme and neprilysin) in APP/CCL2 and APP mice were indistinguishable. Sodium dodecyl sulfate-insoluble Aβ (an indicator of fibrillar Aβ) was increased in APP/CCL2 mice at 5 months of age. Apolipoprotein E, which enhances Aβ deposition, was also increased (2.2-fold) in aged APP/CCL2 as compared to APP mice. We propose that although CCL2 stimulates MP accumulation, it increases Aβ deposition by reducing Aβ clearance through increased apolipoprotein E expression. Understanding the mechanisms underlying these events could be used to modulate microglial function in Alzheimer’s disease and positively affect disease outcomes.
Accumulating evidence supports a prominent role for brain inflammation in the pathogenesis of Alzheimer’s disease (AD). That nonsteroidal anti-inflammatory drugs (NSAIDs) can positively affect the onset and progression of AD supports this notion.1 Indeed, NSAIDs suppress glial activation and regulate amyloid precursor protein (APP) processing.2–5 However, a recent AD cooperative study showed no clinical improvement in AD symptoms with either a cyclooxygenase-2 inhibitor (rofecoxib) or a nonselective NSAID (naproxen) when the drugs were administered for 1 year.6 Disease outcomes after longer NSAID treatment regimens await further study. The different reported clinical outcomes may result from differences in how the drugs affect microglial inflammation. Microglia play critical roles in inciting inflammation while serving to clear damaged brain tissue. Nonetheless, their precise role in disease pathogenesis remains uncertain.7–9 In addition, microglia accumulate at the site of senile plaques in AD brains, are activated by APP processing products, such as secreted APP and amyloid-β peptide (Aβ), and induce neurotoxicity.10,11 They also affect Aβ deposition and neurodegenerative processes including synaptic and neuronal cell loss. Elucidation of microglial function and its effect on cognitive impairment in AD is critical for understanding the mechanisms underlying disease pathogenesis.
Tg2576 transgenic APP mice mimic many aspects of human disease including microglial-induced brain inflammation. Importantly, APP transgenic mice replicate many aspects of AD neuropathology including (Aβ plaque formation, dystrophic neurites, astrogliosis, and microglial activation) along with deficits in learning and memory.12,13 Tg2576 mouse lines have also been instrumental in clarifying the importance of microglial cells in AD. Tg2576 mice deficient for CD40 ligand, a signaling molecule participating in T-cell-microglial immune responses, show a marked reduction in Aβ deposition, microglial reactions, astrogliosis, and APP β-processing.14 However, APP mice deficient in complement component C1q show reduced microgliosis without significant changes in amyloid deposition.15 Furthermore, APP mice overexpressing C3 inhibitor, soluble complement receptor-related protein y, show reduced microglial activities and enhanced Aβ deposition.16
CCL2 is a member of the β chemokine subfamily and a signaling ligand for the seven-transmembrane spanning G-protein-coupled receptor, CCR2.17 Activated astrocytes and mononuclear phagocytes (MP; perivascular and parenchymal macrophages and microglia) express CCL2 in the brain.18,19 CCL2 is present in senile plaques, reactive microglia,20,21 and microvessels22 in AD brains. Because astrocytes are the main source of CCL2,23 a transgenic mouse (JE-95) expressing the murine CCL2 under the control of the human glial fibrillar acidic protein (GFAP) promoter was generated. The constructed animals overexpress CCL2 in an astroglial activation-dependent manner.24 JE-95 mice develop a pertussis toxin-induced reversible encephalopathy dependent on CCL2 overexpression.24 With chronic overexpression of CCL2, aged JE-95 mice develop delayed spontaneous neurodegeneration dependent on MCP-1 (CCL2) overexpression (DESMO), characterized by motor impairment and a reduced life span.25 The pathological features of DESMO include modest perivascular cell infiltrates and blood-brain barrier compromise, with widespread microglia activation shown by ionized calcium-binding adaptor molecule 1 (IBA-1) immunohistochemistry. In the central nervous system of DESMO mice there was no demyelination, and a reduction in the numbers of neurons, axons, and synapses. The great majority of MP cells in the central nervous system of aged JE-95 mice were CD45dim microglia. Despite their activated morphology, these microglia did not express the surface markers of antigen-presenting cells, and were grossly deficient in acquiring amoeboid morphology in cortical slice preparations. We demonstrate that APP/CCL2 mice show increased numbers of MP and diffuse Aβ plaque deposition in both the hippocampus and the cortex. Biochemical studies demonstrate that sodium dodecyl sulfate (SDS)-insoluble Aβ accumulates in the hippocampus by 5 months of age. In contrast, Aβ production, APP processing, and Aβ-degrading enzyme levels remain unchanged. These data support the idea that enhanced Aβ deposition is due to reduced Aβ clearance. To investigate how brain MP may affect amyloid deposition during AD, APP/CCL2 bigenic mice were generated and analyzed.
Materials and Methods
APP/CCL2 Bigenic Mice
Tg2576 mice expressing the Swedish mutation of human APP695 were obtained from Drs. G. Carlson and K. Ashe through Mayo Medical Venture.12 Male Tg2576 mice were crossed with CCL2 (JE-95 strain) females generating APP/CCL2 mice and wild-type (WT) littermates, and then backcrossed at least four times to 129/Sve mice to develop a 129/Sve strain. Animals used for this study were WT (APP and CCL2 transgene-negative, 14 months; three males and three females), CCL2 (APP transgene-negative and CCL2 transgene-positive, 14 months; three males and three females), APP (APP transgene-positive and CCL2 transgene-negative, 5 months, two males and one female; 14 months, three males and three females), and APP/CCL2 (APP and CCL2 transgene-positive, 5 months, two males and one female; 14 months, three males and three females) mice. The APP, APP/CCL2, WT, and CCL2 mice are littermates.
Genotyping Protocol
DNA samples were prepared from cut tail tips (<1.0 cm) of individual pups, and genomic DNA was extracted using the Easy DNA kit (Invitrogen, Carlsbad, CA). Polymerase chain reaction (PCR) was performed as described previously to identify APP transgene-positive mice.26 The human GFAP promoter murine CCL2 transgene was confirmed by PCR of genomic DNA with primers JE-5 (5′-TTC CTG GGC ACA GGC TGA ATA GAG) and JE-3 (5′-ATT GAG CAG GGG GCT TGC ATT G), which amplify a 150-bp DNA fragment from the transgene, but not the endogenous murine CCL2 gene.
Protein Extraction and Enzyme-Linked Immunosorbent Assay (ELISA)
For immunoblotting and CCL2 ELISA, brain tissues were homogenized in solubilization buffer [50 mmol/L Tris-Cl, pH 7.5, 100 mmol/L NaCl, 2 mmol/L ethylenediamine tetraacetic acid-Na, 1% Triton X-100, and protease inhibitor cocktails (Roche Applied Science, Indianapolis, IN)] and centrifuged at 100,000 × g for 1 hour at 4°C. The protein concentration of the supernatant was quantified by BCA assay kit (Pierce, Rockford, IL) and subjected to immunoblotting and CCL2 ELISA (mouse JE/CCL2; R&D Systems, Minneapolis, MN).27 For total Aβ ELISA, brain tissues were homogenized in 5 mol/L guanidine isothiocyanate to prepare a protein extract28 and subjected to Aβ40 and Aβ42 ELISA (Biosource, Camarillo, CA). For SDS-insoluble Aβ ELISA, brain tissue was homogenized in 2% SDS-containing solubilization buffer, followed by centrifugation at 100,000 × g for 1 hour. The supernatant fraction was used to measure SDS-soluble Aβ. The pellet was resuspended in 5 mol/L guanidine isothiocyanate and used for SDS-insoluble Aβ measurements.28
Quantitative Real-Time Reverse Transcriptase (RT)-PCR for CCL2 mRNA Levels
Total RNA was isolated from brain tissues using TRIzol (Invitrogen), and subjected to real-time RT-PCR tests using TaqMan predeveloped murine CCL2 gene expression assay reagents and the ABI Prism 7000 sequence detection system (both from Applied Biosystems, Foster City, CA) and the mRNA levels were quantified as previously described.29
Immunohistochemistry
Animals were euthanized with isoflurane and perfused transcardially with 25 ml of normal (0.9%) saline as described.2 The brains were rapidly removed and bisected sagitally. The left hemispheres were immersed in freshly depolymerized 4% paraformaldehyde for 48 hours and the frontal cortex were dissected and frozen. The left hemispheres were cryoprotected by successive 24-hour immersions in 10%, 20%, and 30% sucrose in Sorenson’s phosphate buffer immediately before sectioning. Fixed, cryoprotected brains were frozen and sectioned in the horizontal plane at 10 μm using a Cryostat (Leica, Bannockburn, IL), with sections collected serially. The right hemispheres were embedded in paraffin block and sectioned as well. Immunohistochemistry was performed using specific antibodies to identify cellular and molecular markers for Aβ, GFAP, CD11b/Mac-1, CD11c, IBA-1,30 MHC class II (MHC-II), CD45, phosphotyrosine, and synaptophysin antibodies as listed in Table 1, which also includes the antibodies, specificities, dilutions, and references. Brain sections stained for microglial markers were additionally stained with thioflavin-S (TS; Sigma, St. Louis, MO) to localize deposits of amyloid in a β-sheet configuration. Two regions were examined quantitatively using a stereological system: the hippocampus and the cortex. Alternatively, paraffin-embedded brains were sectioned at 5 μm for Aβ, TS, or GFAP staining. Systematic uniform random sets of sections with 300-μm spacing were used for staining. All immunohistochemistry was visualized using avidin-biotin-horseradish peroxidase with 3,3′-diaminobenzidine for color development (Vector Laboratories, Burlingame, CA). Envision Plus (DAKO, Carpinteria, CA) was used instead of avidin-biotin-horseradish peroxidase for Aβ staining.
Table 1.
Summary of Antibodies Used for Immunohistochemistry and Immunoblotting
| Antibody | Epitope protein/amino acids | Type | Dilution | Company | Reference |
|---|---|---|---|---|---|
| Aβ | Aβ1-30 | P | 1:200 | Zymed | 52 |
| β-Actin | Slightly modified synthetic β-cytoplasmic actin N-terminal peptide | M | 1:100,000 | Sigma | 56 |
| ApoE | Purified mouse apoE from pooled mouse plasma high- and very low-density lipoproteins | P | 1:1000 | Biodesign | 55 |
| APP C-terminal | C-terminal 20 amino acids residue (751 to 770) of human APP | P | Calbiochem | 57 | |
| CD10 | Prokaryotic recombinant fusion protein corresponding to the external domain of the CD10 glycoprotein | M | 1:100 | Novocastra | 58 |
| CD11b/Mac-1 | T cell-enriched splenocytes from B10 mice | R | 1:50 | Serotec | 15 |
| CD11c | Purified from mouse ascites fluid | H | 1:100 | eBioscience | 59 |
| CD45 | Purified B cells from mouse lymph nodes | R | 1:500 | Serotec | 41 |
| 6E10 | Aβ3-8 | M | 1:500 | Signet | 56 |
| GFAP | GFAP isolated from cow spinal cord | P | 1:1000 | DAKO | 53 |
| IBA-1 | C-terminal portion of IBA-1 | P | 1:500 | * | 54 |
| IDE | Recombinant GST-fusion protein containing amino acids 97 to 273 of IDE | P | 1:1000 | Oncogene | 60 |
| MHC-II (I-A/I-E) | Activated C57BL/6 mouse spleen cells | M | 1:100 | BD PharMingen | 15 |
| PY-20 | phosphotyrosine-protein conjugate | M | 1:400 | Sigma | 56 |
| Synaptophysin | synaptosome preparation from rat retina | M | 1:1000 | Sigma | 57 |
Antibody from Dr. S. Kohsaka.
M, mouse monoclonal antibody; P, rabbit polyclonal antibody; R, rat monoclonal antibody; H, hamster polyclonal antibody.
Image Analysis
Images were captured with a digital camera (DVC-1310C; DVC Company) attached to an Eclipse TE-300 Nikon microscope using C-View v1.2 software (DVC).31 Ten to twenty images, covering the entire cortical and hippocampal areas, were taken per each 5- or 10-μm section (10 sections per brain, 300-μm spacing) at ×200 magnification. Because the measured outcome of total volume occupied by TS, Aβ, GFAP, Mac-1, and IBA-1 immunohistochemical reaction were readily discernable by visual inspection. These were quantified by image software (NIH Image 1.62). The number of Aβ plaques and immunopositive cells (GFAP, Mac-1, and IBA-1 staining) were counted manually. The intensity of synaptophysin was determined by semiquantification of digitally captured image by Image-Pro Plus 4.0 (Media Cybernetics, Inc., Silver Spring, MD). Data are reported as a percentage of the total stained area divided by the total cortical or hippocampal area, or the total number of immunostained cells divided by the total cortical or hippocampal area for each animal. Immunohistochemical tests were analyzed by M.Y. and M.H. in a blind manner.
Immunoblots
Brain lysates (30 μg) were precleared with 10 μl of protein G-Sepharose FF (Amersham Pharmacia, Arlington Heights, IL) to remove endogenous mouse IgG and subjected to standard immunoblotting using 4 to 15% Precast gel SDS-polyacrylamide gel electrophoresis (Bio-Rad, Richmond, CA).11 Proteins were transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA), and subjected to immunoblotting using primary antibodies (listed in Table 1). Alkaline phosphatase-conjugated secondary antibodies were used against mouse and rabbit IgG (1:2000 dilution; Vector Laboratories, Burlingame, CA), and developed using NBT/BCIP solution (Roche Applied Science).11 The images were digitally captured by a computer scanner (Canon), and the band intensities of apoE, APP, APP C-terminal fragments (CTF), β-actin, GFAP, IBA-1, insulin-degrading enzyme (IDE), and neprilysin were measured by NIH Image 1.62 software program. Data were presented as a ratio of target band intensity/β-actin band intensity.
Tissue Culture and Recombinant Adenovirus Infection
PC12 cells (1 × 105 cells/well in poly-d-lysine-coated 24-well plates) were differentiated by nerve growth factor (100 ng/ml, R&D Systems) for 5 days, and infected with recombinant adenovirus expressing Swedish APP mutation (multiplicity of infection = 10) as described previously.11
Statistics
All data were normally distributed. In case of multiple mean comparisons, data were analyzed by analysis of variances, followed by Newman-Keuls multiple comparison test using statistics software (Prism 3.0 and 4.0; Graphpad Software, Inc., San Diego, CA). In case of single mean comparison, data were analyzed by Student’s t-test. A P value of <0.05 was regarded as significant.
Results
CCL2 Enhances Diffuse Amyloid Deposition
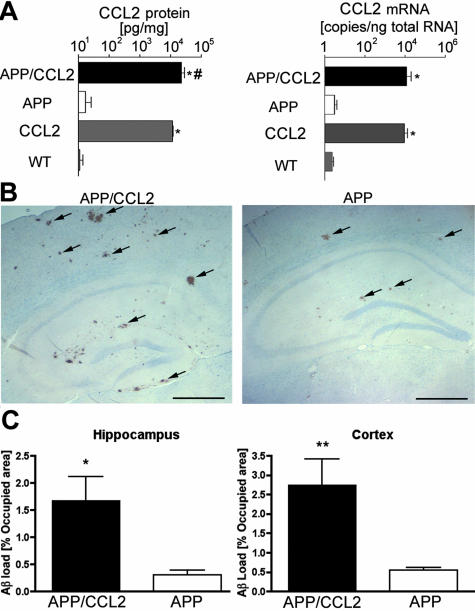
Neuropathological changes and clearance of Aβ in the APP/CCL2 bigenic mice were followed throughout time and compared to APP and CCL2 transgenics and control littermates (WT). A 1000-fold increase in levels of CCL2 protein was observed in the frontal cortex of APP/CCL2 and CCL2 mice (34.6 and 18.1 ng/mg, respectively) compared to APP and WT mice (34.0 and 21.6 pg/mg, respectively; Figure 1A, left). Murine CCL2 mRNA levels, analyzed by quantitative real-time RT-PCR, were significantly increased in APP/CCL2 and CCL2 mice (10,127 and 9048 copies/ng total RNA, respectively) compared to APP and WT mice (3.7 and 2.3 copies/ng total RNA, respectively; Figure 1A, right). APP/CCL2 mice showed significantly more Aβ deposition than did their APP littermates as determined by Aβ immunostaining of both hippocampal and cortical diffuse plaques at 14 months of age (Figure 1B). Quantitative immunohistochemical assays showed a 5.6- and a 5.0-fold increase in Aβ deposition in the hippocampus and cortex, respectively, of APP/CCL2 compared to APP mice (Figure 1C). Measures of total Aβ40 and Aβ42 by ELISA confirmed these results (Table 2). In both SDS-soluble and -insoluble fractions, Aβ40 and Aβ42 were significantly increased in APP/CCL2 mice as compared to their APP littermates, although the Aβ42/40 ratio remained unchanged. There was no difference in the number of compact plaques stained by TS between APP and APP/CCL2 animals (Figure 2; A to C). This suggested that the Aβ deposition of only diffuse plaques was increased in APP/CCL2 mice. In addition, TS-positive plaques at 10 and 12 months of age were detected in both APP and APP/CCL2 mice (data not shown), suggesting that the onset of amyloid plaque formation remained unchanged by CCL2 transgene expression.
Figure 1.
CCL2 levels and Aβ deposition in APP/CCL2 mice. A: Frontal cortex of APP/CCL2, APP, CCL2, and WT mice at 14 months of age (n = 6) were dissected and subjected for protein extraction in solubilization buffer or total RNA preparation. Murine CCL2 protein and mRNA levels were determined using ELISA and real-time RT-PCR as described in the Material and Methods, respectively. *, P < 0.0001 between APP/CCL2 versus APP or CCL2 versus WT; #, P < 0.05 between APP/CCL2 versus CCL2. B: Ten slides of 5-μm-thick brain sections of APP or APP/CCL2 mice were immunostained with anti-Aβ antibody, developed with DAB, and counterstained with hematoxylin. The arrows indicate immunopositive Aβ deposits. C: Average percent area occupied by Aβ deposits in the hippocampus (left) and the cortex (right) as quantified by image analyses of immunostained slides (n = 6). * and **, P < 0.05 and P < 0.01 versus APP mice as determined by Student’s t-test, respectively. Original magnifications, ×40. Scale bar, 250 μm.
Table 2.
Aβ Level in APP and APP/CCL2 Transgenic Mouse Brain
| Genotype | Age (months) | n | Aβ | 2% SDS Aβ ng/mg protein* | 5 mol/L guanidine Aβ ng/mg protein† | Total Aβ ng/mg protein‡ | Total Aβ40 + Αβ42 ng/mg protein3 |
|---|---|---|---|---|---|---|---|
| APP | 14 | 6 | Aβ40 | 38.3 ± 8.4 | 130.7 ± 17.0 | 54.1 ± 8.6 | 61.0 ± 9.0 |
| Aβ42 | 7.1 ± 2.2 | 8.1 ± 0.8 | 6.9 ± 0.6 | ||||
| Aβ42/Aβ40 | 21.2 ± 3.7 | 6.8 ± 1.2 | 14.0 ± 2.0 | ||||
| APP/CCL2 | 14 | 6 | Aβ40 | 70.5 ± 13.1§ | 298.5 ± 54.6§ | 102.5 ± 15.4§ | 117.3.±17.1§ |
| Aβ42 | 13.7 ± 2.1¶ | 22.6 ± 2.8|| | 14.8 ± 2.1¶ | ||||
| Aβ42/Aβ40 | 18.7 ± 1.7 | 8.3 ± 1.0 | 14.5 ± 1.3 |
Brain cerebrum has been homogenized in 2% SDS buffer and ultracentrifuged as described in the Material and Methods. SDS-soluble Aβ40 and Aβ42 levels in the supernatant fraction were presented as 2% SDS values. The SDS-insoluble fraction was solubilized in 5 mol/L guanidine buffer and its Aβ40 and Aβ42 values were presented as 5 mol/L guanidine values.
Total protein in SDS soluble fraction.
Total protein in SDS insoluble fraction.
Total protein in SDS soluble and insoluble fraction.
§, ¶, and || in APP/CCL2 group denotes P < 0.05, 0.01, and 0.001 versus corresponding Aβ value of APP group as determined by Student’s t-test, respectively.
Figure 2.
TS staining of APP/CCL2 mouse brains. A: Mice at 14 months of age were tested for compact amyloid plaques by TS staining. Images show 10-μm-thick coronal sections of the hippocampal region. B: Quantitative analysis of the number of TS-positive plaques in hippocampus and cortex. C: Quantitative analysis of the area occupied by TS-positive plaques in hippocampus and cortex. N.S. in B and C denotes no statistical significance as determined by Student’s t-test. Original magnifications, ×40.
CCL2 Enhances Mononuclear Phagocyte Accumulation in Brain
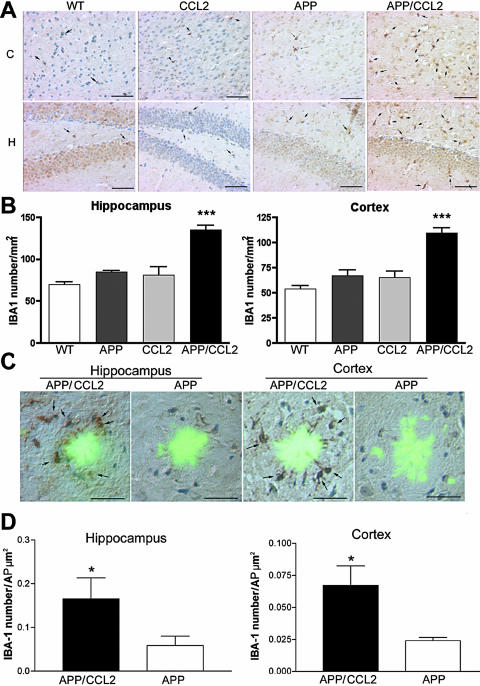
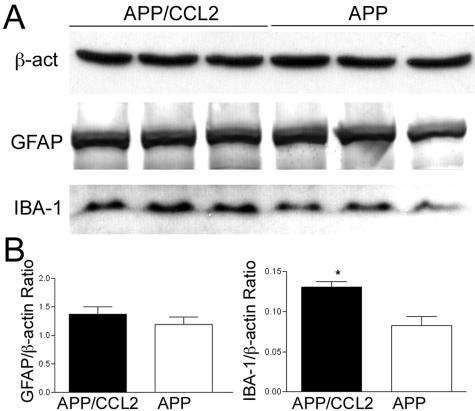
Increased Aβ deposition correlated with increased microglial numbers in both the cortex and the hippocampus of APP/CCL2 mice when assessed by IBA-1 immunostaining (Figure 3, A and B). In APP/CCL2 bigenic mice, MP accumulated at the TS-positive compact plaques in both the hippocampus and the cortex to a significantly greater extent than what was observed in APP mice (Figure 3, C and D). Similar results were obtained for microglia by Mac-1 staining (data not shown). These data demonstrated that not just the total number (Figure 3B) but also the plaque-associated microglia (Figure 3D) were increased in APP/CCL2 mice as compared to APP mice in both the hippocampus and the cortex. Such microglia showed a nonactivated phenotype as determined by diminished CD45, MHC class II, CD11c, or phosphotyrosine immunostaining (data not shown). The intensity of astrogliosis, as determined by quantitative immunohistochemistry and immunoblotting of GFAP, was similar in APP and APP/CCL2 mice (Figure 4, A and B). GFAP immunoblotting showed increased astrogliosis in APP and APP/CCL2 mice when compared to WT and CCL2 mice (Figure 4C). No differences in synaptic density were seen in APP/CCL2 and APP mice by synaptophysin immunostaining (Figure 5). Such findings showed that MP numbers, rather than the level of astrogliosis, were enhanced by CCL2 and associated with Aβ deposition.
Figure 3.
Microglial accumulation in APP/CCL2 mice. A: Ten-μm-thick slices of cortical (C) and hippocampal (H) regions of mice at 14 months of age (n = 6) were immunostained with anti-IBA-1 antibody and counterstained with hematoxylin. Arrows indicate IBA-1-positive cells in the brain regions. B: Quantification of IBA-1-positive cells in brain regions. *, P < 0.05 versus other groups as determined by analysis of variance and Newman-Keuls post hoc. C: TS-positive amyloid plaques (AP) were surrounded by IBA-1-positive microglial cells in both the hippocampal and cortical regions of APP/CCL2 or APP mice. D: Quantification of IBA-1-positive cells per μm2 AP. *, P < 0.05 versus APP mice (n = 6) as determined by Student’s t-test. Original magnifications: ×200 (A); ×400 (C). Scale bars, 25 μm.
Figure 4.
Astrogliosis in APP/CCL2 mouse brains. A: APP and APP/CCL2 mice at 14 months of age (n = 3) were tested for astrogliosis by anti-GFAP staining. Images show 5-μm-thick coronal sections of the hippocampal region. B: Quantitative analysis of the number of GFAP-positive astrocytes in the hippocampus and the cortex of APP/CCL2 and APP mice. No statistical significance was observed. C: Immunoblotting analysis of GFAP at 14 months of age (n = 3 per group). *, Statistical differences (P < 0.05) between WT versus APP and CCL2 versus APP/CCL2 as determined by analysis of variance and Newman-Keuls post hoc. Original magnifications, ×40.
Figure 5.
Synaptic density in APP/CCL2 mouse brains. A: Mice at 14 months of age were tested for synaptic density by anti-synaptophysin staining. Images show 5-μm-thick coronal section of hippocampal region. B: Quantitative analysis of the density of synaptophysin staining in the hippocampus and the cortex. No statistical significance was observed among the group in either brain subregion. Original magnifications, ×40.
CCL2 Does Not Alter APP Processing but Enhances Aβ Aggregation
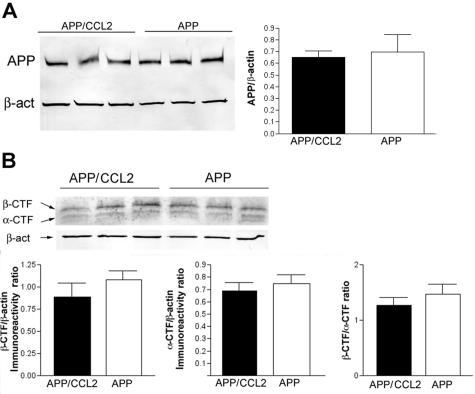
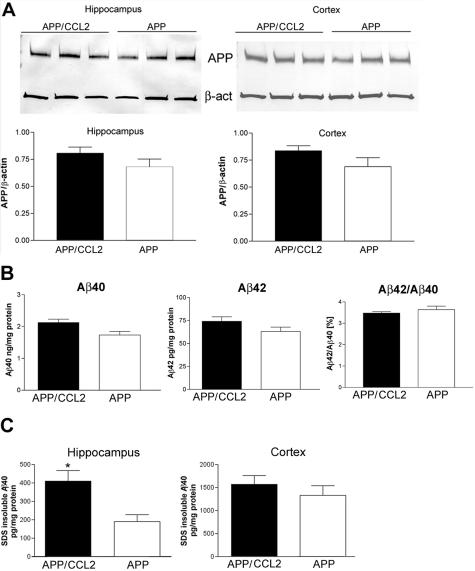
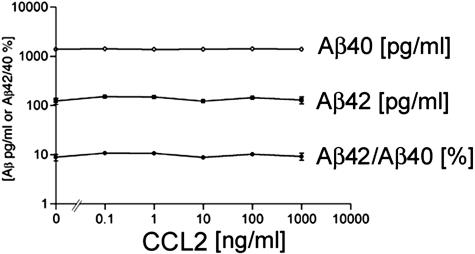
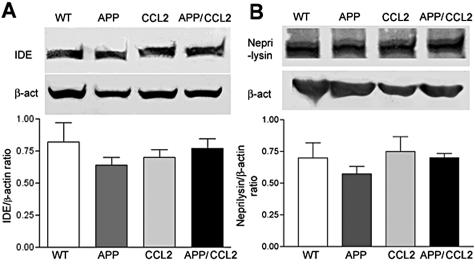
Whether Aβ deposition occurs is determined by the balance between three processes: Aβ production, aggregation, and clearance. Although Aβ production was up-regulated in APP/CCL2 mice at 14 months of age, no significant differences were found in APP expression between APP and APP/CCL2 mice in the cortex (Figure 6A). APP C-terminal fragments are processing products of APP at the α-site (α-CTF) or β-site (β-CTF, Figure 6B). Neither α/β-processing nor the ratio of α/β processing was altered in APP/CCL2 as compared to APP mice. The SDS-insoluble fraction of Aβ40 was increased in the hippocampus of APP/CCL2 mice as compared to APP mice at 5 months of age (Figure 7C). Total amounts of Aβ40, Aβ42, or the Aβ42/40 ratio were unchanged in brains at 5 months of age (Figure 7; A to C). Although there is no difference in GFAP expression, there is a significant increase in IBA-1 expression in APP/CCL2 mice at 5 months of age (Figure 8, A and B), suggesting increased MP accumulation in brain tissues. CCL2 had no direct effect on APP processing when PC12 cells expressing the Swedish APP mutant were treated with increasing doses of CCL2 (Figure 9). Taken together, these data suggest that increased Aβ deposition in APP/CCL2 mice was not secondary to enhanced APP or Aβ production. Thus, we next investigated whether Aβ clearance was affected in the APP/CCL2 mice. In these experiments the Aβ degradation enzymes, IDE and neprilysin,32,33 were examined. The levels for each enzyme in the frontal cortex of APP/CCL2, APP, CCL2, and WT mice were not significantly different (Figure 10, A and B). Because this reflects the extracellular levels of Aβ-degrading enzyme, the data suggested that Aβ degradation was not affected by CCL2 expression in the various regions of the brain. Finally, we examined the expression of apolipoprotein E (apoE), which has been shown to be important in Aβ clearance. The apoE4 allele has been genetically linked to the incidence of sporadic and late-onset AD.34,35 Although there was no significant difference in apoE levels between APP/CCL2 and APP mice at 5 months of age, there was a 2.2-fold increase of apoE in APP/CCL2 mice as compared to APP mice at 14 months of age (Figure 11, A and B). Because endogenous murine apoE has been shown to induce Aβ deposition in vivo,36 and all apoE isoforms can propagate Aβ aggregation in vitro,37 this suggests that the accumulation of Aβ in APP/CCL2 mice may be linked to increased apoE levels. Because apoE is mainly secreted from microglia instead of astrocytes in vitro,38 the increased apoE level is likely because of the enhanced accumulation of microglia seen in the APP/CCL2 mouse brain. These data suggest that chronic up-regulation of CCL2 enhances MP accumulation and amyloid deposition due to the reduced amyloid clearance. This reduced clearance may be because of increased Aβ aggregation caused by increased apoE expression in the mouse brain.
Figure 6.
APP expression and processing. A: Protein extracts from the cortex and the hippocampus of APP/CCL2 and APP mice (n = 3) were subjected to immunoblotting for APP and β-actin using anti-Aβ (6E10) and anti-β-actin monoclonal antibodies. The APP-immunoreactive band intensity was normalized by β-actin band intensity. No statistical significance was observed. B: APP α- and β-CTF protein levels were quantified by immunoblotting using anti-APP 751-770 antibody and normalized by β-actin protein levels in cortex (n = 3). No statistical significance was observed.
Figure 7.
APP and Aβ in young mouse brains. A and B: APP, total Aβ40, Aβ42, and Aβ42/Aβ40 ratio in cortex quantified by specific Aβ ELISA at 5 months of age (n = 3 per group). No statistical significance was observed. C: SDS-insoluble Aβ40 ELISA in hippocampus and cortex of APP/CCL2 and APP mice at 5 months of age (n = 3). *P < 0.05 versus APP mice as determined by Student’s t-test.
Figure 8.
GFAP and IBA-1 expression in young mouse brains. A: Protein extracts (30 μg/lane) from the frontal cortex of APP/CCL2 and APP mice at 5 months of age (n = 3 per group) were subjected to immunoblotting for β-actin, GFAP, and IBA-1. B: Quantification of immunoreactive bands in A. *, P < 0.05 versus APP as determined by Student’s t-test.
Figure 9.
Effect of CCL2 on APP processing in vitro. Nerve growth factor-treated differentiated PC12 cells (1 × 105 cells/well in poly-d-lysine-coated 24-well plate) were infected with recombinant adenovirus expressing Swedish FAD APP695 mutant11 and subsequently stimulated with increasing doses of recombinant murine CCL2 for 24 hours. Media was collected and both Aβ40 and Aβ42 levels were determined by specific Aβ ELISA. Data were presented as pg/ml, ng/ml, or percent ratio of Aβ42/Aβ40.
Figure 10.
Aβ-degrading enzymes in brains. A and B: Protein extracts (30 μg/lane) from the frontal cortex of four mouse groups at 14 months of age (n = 3) were subjected to immunoblotting using anti-IDE (A), anti-neprilysin (B), and anti-β-actin monoclonal antibodies (top). The band intensity of IDE and neprilysin was normalized by the β-actin signal and the average intensity ratios were presented (bottom). No statistical significance was observed.
Figure 11.
ApoE levels in brains of young and aged animals. A: Protein extracts (30 μg/lane) from the frontal cortex of APP and APP/CCL2 mice at 5 and 14 months of age (n = 3 per group per age) were subjected to immunoblotting using anti-apoE (Biodesign) and anti-β-actin monoclonal antibodies. B: The band intensity of apoE was normalized by the β-actin signal and the average intensity ratios were presented (filled bar, APP/CCL2 group; open bar, APP group). *, P < 0.05 versus APP at 14 months of age as determined by Student’s t-test.
Discussion
These data show a significant role for CCL2 in the development of the AD-like pathology seen in APP mouse brain tissue. This role for CCL2 is supported by a number of experimental observations. First, CCL2 enhances MP accumulation, although astrogliosis remains unchanged. Second, MP accumulation is accompanied by enhanced Aβ deposition. Third, the differences in Aβ deposition were due to an increase in diffuse plaques in APP/CCL2 mice because the numbers of TS-positive compact plaques were similar between APP/CCL2 and APP mice at 14 months of age. This indicated that microglial accumulation enhances diffuse but not compact plaque formation. Such observations were noted in both the hippocampus and the cortex. Fourth, CCL2 overexpression significantly enhanced the formation of SDS-insoluble Aβ in the hippocampus at 5 months of age although its amount is slightly but not significantly increased in the cortex. This suggested that Aβ deposition in the hippocampus precedes its deposition in the cortex, probably due to the perforant pathway-mediated hippocampal amyloid deposition mechanism.39 Both diffuse and compact plaque formation starts as early as 10 months of age (data not shown), although the number of TS-positive compact plaques was similar to age-matched APP mice at 14 months of age. Thus, CCL2 overexpression enhanced SDS-insoluble Aβ formation but did not accelerate the onset of Aβ deposition in the APP mouse brain. Because there is no difference in APP expression, α/β-processing, or Aβ-degrading enzyme levels between APP and APP/CCL2 groups, it is likely that the enhanced Aβ deposition was due to reduced Aβ clearance possibly caused by deficits in phagocytosis or in the vascular endothelial export from central nervous system.
How MP accumulation leads to reduce Aβ clearance is unknown. It is known that suppression of inflammatory reaction by NSAIDs reduces the Aβ load in APP mouse brain.2,3,40,41 NSAIDs have also been shown to directly lower Aβ42 when administered at high concentrations.4,42 On the other hand, lipopolysaccharide stimulation activates microglia and enhances Aβ clearance in APP/PS-1 mouse brain,43 whereas nonactivated microglia (by dexamethasone) are inefficient in clearing Aβ plaques even after passive immunization with Aβ antibody.44,45 The accumulated MP around Aβ plaques in APP/CCL2 are incompletely activated despite their morphology because they are negative for microglial activation markers, such as MHC class II, phosphotyrosine; further, these cells were deficient for acquiring amoeboid morphology in a cortical slice preparation assay (data not shown). Thus, it is likely that the nonactivated status of MP contributes to the reduced clearance seen in the APP/CCL2 mouse brain. Our data suggest that the effect of NSAIDs on APP mice may also be due to suppression of CCL2 and/or MP accumulation. Another APP bigenic mouse model that overexpresses transforming growth factor-β1 (TGF-β1) showed enhanced microglial Aβ clearance and reduced deposition in the brain parenchyma.46 The major difference between the APP/CCL2 and the APP/TGF-β1 mouse model is the lower level of microglial activation seen in APP/CCL2 mice, as compared to the high level of microglial phagocytosis seen in APP/TGF-β1 mice.46 These studies suggest that the microglia associated with plaques require specific stimulating factors, such as antibodies or TGF-β1, to initiate Aβ phagocytosis. Without this response, microglia are inefficient in the clearance of Aβ. There are several studies in the literature that support this idea. It is known that degradation of ingested Aβ by primary cultured, unstimulated, microglia in vitro is slow as compared to acetylated low-density lipoprotein.47 There was little evidence of internalized Aβ found in the microglia of AD brains.48 A similar observation is seen in Tg2576, where activated microglia may not be engaged in Aβ removal or plaque degradation, but may be enhancing plaque formation.49 Three-dimensional reconstitution of an ultrastructural study showed that microglia do not engulf Aβ inclusions in APP23 mice.50 Thus, microglial amyloid plaque phagocytosis is not evident in AD or APP mouse brains without administration of amyloid immune therapy. Another possible explanation for this finding is that accumulated microglia increase Aβ deposition to reduce its toxicity. It is known that soluble oligomer Aβ is detrimental to neurophysiological function, and that aggregation of the soluble oligomer Aβ as condensed Aβ deposits might be beneficial to the brain. To address this issue, we are examining the effect of CCL2 expression on APP mice by behavioral and electrophysiological analyses.
Accumulating evidence suggests that endogenous apoE in mouse brain enhances Aβ deposition in APP animal models.36,37 Although many studies concluded that astrocytes are the main apoE-producing cell in brain,51 recent reports suggest that microglial cells are also a significant source of apoE.38,52 ApoE secretion does not require microglial activation, because lipopolysaccharide stimulation rather reduces apoE secretion in vitro.38 Thus, although microglia could contribute to the increase in apoE in the double-transgenic mice, astrocytes are an abundant source of apoE in the central nervous system and they might also contribute.53,54 The more than twofold increase of apoE levels seen in APP/CCL2 mice is significant, because APP/apoE−/+ mice showed more than an 80% reduction in Aβ deposition as compared to APP/apoE+/+ mice.36 ApoE has also been shown to enhance Aβ aggregation in vitro.55 These data indicate that microglia may enhance Aβ aggregation through apoE secretion. Thus, the accumulated microglia around TS+ plaques may be involved in the acceleration of diffuse plaque enlargement in situ. It would be worthwhile to examine apoE levels in the other APP transgenic animal models to see if alterations in Aβ deposition correlate with changes in apoE levels in the brain.
In summary, we have demonstrated that APP/CCL2 bigenic mice show enhanced Aβ plaque deposition, MP accumulation, and increased apoE levels. APP/CCL2 bigenic mice also show SDS-insoluble Aβ accumulation and increased IBA-1 levels at a younger age. Our data suggests that MP plays a critical role not only in the regulation of Aβ clearance but as a central player in a novel mechanism for AD progression, in which chronic accumulation of MP leads to the acceleration of amyloid deposition because of reduced phagocytic function and increased apoE levels. Anti-chemokine interventions that reduce MP accumulation may prove useful as a way to prevent or treat this aspect of AD.
Acknowledgments
We thank Drs. G. Carlson and K. Ashe for providing Tg2576 mice, Dr. S. Barger for critical reading of the manuscript, Dr. S. Kohsaka for IBA-1 antibody, Drs. L. Poluektova and Y. Persidsky for assistance in immunohistochemistry, and Dr. X. Luo and G. Weber for the transgenic mouse colony.
Footnotes
Address reprint requests to Tsuneya Ikezu, M.D., Ph.D., Center for Neurovirology and Neurodegenerative Disorders, 985880 Nebraska Medical Center, University of Nebraska Medical Center, Omaha, NE 68198-5880. E-mail: tikezu@unmc.edu.
Supported by the Vada Oldfield Alzheimer Research Foundation (to T.I.); the University of Nebraska Medical Center (departmental start-up grant to T.I., and the David T. Purtilo Chair in Pathology and Microbiology to H.E.G.); the Nancy Davis MS Center Without Walls (to R.M.R.); and the National Institutes of Health (grants P01 NS043985 to H.E.G. and T.I., 2 R37 NS36126 to H.E.G., and RO1 NS32151 to R.M.R.).
References
- McGeer P, Schulzer M, McGeer E. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- Jantzen PT, Connor KE, DiCarlo G, Wenk GL, Wallace JL, Rojiani AM, Coppola D, Morgan D, Gordon MN. Microglial activation and beta-amyloid deposit reduction caused by a nitric oxide-releasing nonsteroidal anti-inflammatory drug in amyloid precursor protein plus presenilin-1 transgenic mice. J Neurosci. 2002;22:2246–2254. doi: 10.1523/JNEUROSCI.22-06-02246.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GP, Yang F, Chu T, Chen P, Beech W, Teter B, Tran T, Ubeda O, Ashe KH, Frautschy SA, Cole GM. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J Neurosci. 2000;20:5709–5714. doi: 10.1523/JNEUROSCI.20-15-05709.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Su Y, Li B, Liu F, Ryder JW, Wu X, Gonzalez-DeWhitt PA, Gelfanova V, Hale JE, May PC, Paul SM, Ni B. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science. 2003;302:1215–1217. doi: 10.1126/science.1090154. [DOI] [PubMed] [Google Scholar]
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA. 2003;289:2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- Cotman C, Su J. Mechanisms of neuronal death in Alzheimer’s disease. Brain Pathol. 1996;6:493–506. doi: 10.1111/j.1750-3639.1996.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Griffin W, Sheng J, Roberts G, Mrak R. Interleukin-1 expression in different plaque types in Alzheimer’s diseases: significance in plaque evolution. J Neuropathol Exp Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Sheng JG, Griffin WST. Glial cytokines in Alzheimer’s disease: review and pathogenic implications. Hum Pathol. 1995;26:816–823. doi: 10.1016/0046-8177(95)90001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger S, Harmon A. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;388:878–881. doi: 10.1038/42257. [DOI] [PubMed] [Google Scholar]
- Ikezu T, Luo X, Weber GA, Zhao J, McCabe L, Buescher JL, Ghorpade A, Zheng J, Xiong H. Amyloid precursor protein-processing products affect mononuclear phagocyte activation: pathways for sAPP- and Abeta-mediated neurotoxicity. J Neurochem. 2003;85:925–934. doi: 10.1046/j.1471-4159.2003.01739.x. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Yang F, Irrizarry M, Hyman B, Saido TC, Hsaio K, Cole GM. Microglial response to amyloid plaques in APPsw transgenic mice. Am J Pathol. 1998;152:307–317. [PMC free article] [PubMed] [Google Scholar]
- Tan J, Town T, Crawford F, Mori T, DelleDonne A, Crescentini R, Obregon D, Flavell RA, Mullan MJ. Role of CD40 ligand in amyloidosis in transgenic Alzheimer’s mice. Nat Neurosci. 2002;5:1288–1293. doi: 10.1038/nn968. [DOI] [PubMed] [Google Scholar]
- Fonseca MI, Zhou J, Botto M, Tenner AJ. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer’s disease. J Neurosci. 2004;24:6457–6465. doi: 10.1523/JNEUROSCI.0901-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Yan F, Lin AH, Lambris JD, Alexander JJ, Quigg RJ, Masliah E. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer’s mice. Proc Natl Acad Sci USA. 2002;99:10837–10842. doi: 10.1073/pnas.162350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci USA. 1994;91:2752–2756. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo CF, Yoshimura T, Gelman M, Mallat M. Production of monocyte chemotactic protein-1 by rat brain macrophages. Eur J Neurosci. 1996;8:1725–1734. doi: 10.1111/j.1460-9568.1996.tb01316.x. [DOI] [PubMed] [Google Scholar]
- Glabinski AR, Balasingam V, Tani M, Kunkel SL, Strieter RM, Yong VW, Ransohoff RM. Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J Immunol. 1996;156:4363–4368. [PubMed] [Google Scholar]
- Ishizuka K, Kimura T, Igata-yi R, Katsuragi S, Takamatsu J, Miyakawa T. Identification of monocyte chemoattractant protein-1 in senile plaques and reactive microglia of Alzheimer’s disease. Psychiatry Clin Neurosci. 1997;51:135–138. doi: 10.1111/j.1440-1819.1997.tb02375.x. [DOI] [PubMed] [Google Scholar]
- Xia MQ, Hyman BT. Chemokines/chemokine receptors in the central nervous system and Alzheimer’s disease. J Neurovirol. 1999;5:32–41. doi: 10.3109/13550289909029743. [DOI] [PubMed] [Google Scholar]
- Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol Aging. 2001;22:837–842. doi: 10.1016/s0197-4580(01)00276-7. [DOI] [PubMed] [Google Scholar]
- Ransohoff RM, Hamilton TA, Tani M, Stoler MH, Shick HE, Major JA, Estes ML, Thomas DM, Tuohy VK. Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J. 1993;7:592–600. doi: 10.1096/fasebj.7.6.8472896. [DOI] [PubMed] [Google Scholar]
- Huang D, Tani M, Wang J, Han Y, He TT, Weaver J, Charo IF, Tuohy VK, Rollins BJ, Ransohoff RM. Pertussis toxin-induced reversible encephalopathy dependent on monocyte chemoattractant protein-1 overexpression in mice. J Neurosci. 2002;22:10633–10642. doi: 10.1523/JNEUROSCI.22-24-10633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DR, Wujek JR, Kidd G, He TT, Cardona A, Sasse ME, Stein EJ, Kish J, Tani M, Charo IF, Proudfoot AE, Rollins BJ, Handel T, Ransohoff RM: Chronic expression of monocyte chemoattractant protein-1 in the central nervous system causes delayed encephalopathy and impaired microglial function in mice. FASEB J (in press) [DOI] [PubMed] [Google Scholar]
- Hsiao KK, Borchelt DR, Olson K, Johannsdottir R, Kitt C, Yunis W, Xu S, Eckman C, Younkin S, Price D, Iadecola C, Brent Clark H, Carlson G. Age-related CNS disorder and early death in transgenic FVB/N mice overexpressing Alzheimer amyloid precursor proteins. Neuron. 1995;15:1203–1218. doi: 10.1016/0896-6273(95)90107-8. [DOI] [PubMed] [Google Scholar]
- Karpus WJ, Lukacs NW, McRae BL, Strieter RM, Kunkel SL, Miller SD. An important role for the chemokine macrophage inflammatory protein-1 alpha in the pathogenesis of the T cell-mediated autoimmune disease, experimental autoimmune encephalomyelitis. J Immunol. 1995;155:5003–5010. [PubMed] [Google Scholar]
- Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Khan K, Gordon M, Tan H, Games D, Lieberburg I, Schenk D, Seubert P, McConlogue L. Amyloid precursor protein processing and A beta42 deposition in a transgenic mouse model of Alzheimer disease. Proc Natl Acad Sci USA. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson KA, Limoges J, Pohlman GD, Poluektova LY, Langford D, Masliah E, Ikezu T, Gendelman HE. OTK18 expression in brain mononuclear phagocytes parallels the severity of HIV-1 encephalitis. J Neuroimmunol. 2004;150:186–198. doi: 10.1016/j.jneuroim.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996;224:855–862. doi: 10.1006/bbrc.1996.1112. [DOI] [PubMed] [Google Scholar]
- Luo X, Weber GA, Zheng J, Gendelman HE, Ikezu T. C1q-calreticulin induced oxidative neurotoxicity: relevance for the neuropathogenesis of Alzheimer’s disease. J Neuroimmunol. 2003;135:62–71. doi: 10.1016/s0165-5728(02)00444-7. [DOI] [PubMed] [Google Scholar]
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Morishima M, Lee HJ, Hama E, Sekine-Aizawa Y, Saido TC. Identification of the major Abeta1–42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi H, Sugihara S, Ogawa A, Oshima N, Ihara Y. Alzheimer beta amyloid deposition enhanced by apoE epsilon4 gene precedes neurofibrillary pathology in the frontal association cortex of nondemented senior subjects. J Neuropathol Exp Neurol. 2001;60:731–739. doi: 10.1093/jnen/60.7.731. [DOI] [PubMed] [Google Scholar]
- Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, Ghetti B, Paul SM. Apolipoprotein E is essential for amyloid deposition in the APP(V717F) transgenic mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 1999;96:15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski M, Pankiewicz J, Scholtzova H, Ripellino JA, Li Y, Schmidt SD, Mathews PM, Fryer JD, Holtzman DM, Sigurdsson EM, Wisniewski T. A synthetic peptide blocking the apolipoprotein E/beta-amyloid binding mitigates beta-amyloid toxicity and fibril formation in vitro and reduces beta-amyloid plaques in transgenic mice. Am J Pathol. 2004;165:937–948. doi: 10.1016/s0002-9440(10)63355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saura J, Petegnief V, Wu X, Liang Y, Paul SM. Microglial apolipoprotein E and astroglial apolipoprotein J expression in vitro: opposite effects of lipopolysaccharide. J Neurochem. 2003;85:1455–1467. doi: 10.1046/j.1471-4159.2003.01788.x. [DOI] [PubMed] [Google Scholar]
- Lazarov O, Lee M, Peterson DA, Sisodia SS. Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J Neurosci. 2002;22:9785–9793. doi: 10.1523/JNEUROSCI.22-22-09785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Kajdasz ST, Christie RH, Carter C, Games D, Seubert P, Schenk D, Hyman BT. Imaging of amyloid-beta deposits in brains of living mice permits direct observation of clearance of plaques with immunotherapy. Nat Med. 2001;7:369–372. doi: 10.1038/85525. [DOI] [PubMed] [Google Scholar]
- Yan Q, Zhang J, Liu H, Babu-Khan S, Vassar R, Biere AL, Citron M, Landreth G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J Neurosci. 2003;23:7504–7509. doi: 10.1523/JNEUROSCI.23-20-07504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen JL, Sagi SA, Smith TE, Weggen S, Das P, McLendon DC, Ozols VV, Jessing KW, Zavitz KH, Koo EH, Golde TE. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J Clin Invest. 2003;112:440–449. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herber DL, Roth LM, Wilson D, Wilson N, Mason JE, Morgan D, Gordon MN. Time-dependent reduction in Abeta levels after intracranial LPS administration in APP transgenic mice. Exp Neurol. 2004;190:245–253. doi: 10.1016/j.expneurol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, Munireddy SK, Rosenthal A, Ugen KE, Gordon MN, Morgan D. Microglial activation facilitates Abeta plaque removal following intracranial anti-Abeta antibody administration. Neurobiol Dis. 2004;15:11–20. doi: 10.1016/j.nbd.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, DiCarlo G, Henderson D, Jackson J, Clarke K, Ugen KE, Gordon MN, Morgan D. Intracranially administered anti-Abeta antibodies reduce beta-amyloid deposition by mechanisms both independent of and associated with microglial activation. J Neurosci. 2003;23:3745–3751. doi: 10.1523/JNEUROSCI.23-09-03745.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Lin C, Yan F, Yu GQ, Rohde M, McConlogue L, Masliah E, Mucke L. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7:612–618. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- Paresce DM, Chung H, Maxfield FR. Slow degradation of aggregates of the Alzheimer’s disease amyloid beta-protein by microglial cells. J Biol Chem. 1997;272:29390–29397. doi: 10.1074/jbc.272.46.29390. [DOI] [PubMed] [Google Scholar]
- Ard MD, Cole GM, Wei J, Mehrle AP, Fratkin JD. Scavenging of Alzheimer’s amyloid beta-protein by microglia in culture. J Neurosci Res. 1996;43:190–202. doi: 10.1002/(SICI)1097-4547(19960115)43:2<190::AID-JNR7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Wegiel J, Wang KC, Imaki H, Rubenstein R, Wronska A, Osuchowski M, Lipinski WJ, Walker LC, LeVine H. The role of microglial cells and astrocytes in fibrillar plaque evolution in transgenic APP(SW) mice. Neurobiol Aging. 2001;22:49–61. doi: 10.1016/s0197-4580(00)00181-0. [DOI] [PubMed] [Google Scholar]
- Stalder M, Deller T, Staufenbiel M, Jucker M. 3D-Reconstruction of microglia and amyloid in APP23 transgenic mice: no evidence of intracellular amyloid. Neurobiol Aging. 2001;22:427–434. doi: 10.1016/s0197-4580(01)00209-3. [DOI] [PubMed] [Google Scholar]
- Danik M, Chabot JG, Michel D, Quirion R, editors. Austin: RG Landes Company; Clusterin and Apolipoprotein E Gene Expression in the Adult Brain. 1999:pp 17–34. [Google Scholar]
- Mori K, Yokoyama A, Yang L, Maeda N, Mitsuda N, Tanaka J. L-serine-mediated release of apolipoprotein E and lipids from microglial cells. Exp Neurol. 2004;185:220–231. doi: 10.1016/j.expneurol.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987;917:148–161. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- LaDu MJ, Gilligan SM, Lukens JR, Cabana VG, Reardon CA, Van Eldik LJ, Holtzman DM. Nascent astrocyte particles differ from lipoproteins in CSF. J Neurochem. 1998;70:2070–2081. doi: 10.1046/j.1471-4159.1998.70052070.x. [DOI] [PubMed] [Google Scholar]
- Golabek AA, Soto C, Vogel T, Wisniewski T. The interaction between apolipoprotein E and Alzheimer’s amyloid beta-peptide is dependent on beta-peptide conformation. J Biol Chem. 1996;271:10602–10606. doi: 10.1074/jbc.271.18.10602. [DOI] [PubMed] [Google Scholar]
- Christensen MA, Zhou W, Qing H, Lehman A, Philipsen S, Song W. Transcriptional regulation of BACE1, the beta-amyloid precursor protein beta-secretase, by Sp1. Mol Cell Biol. 2004;24:865–874. doi: 10.1128/MCB.24.2.865-874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP, Uljon SN, Fraser PE, Fauq A, Lookingbill HA, Findlay KA, Smith TE, Lewis PA, McLendon DC, Wang R, Golde TE. Presenilin 1 regulates pharmacologically distinct gamma-secretase activities. Implications for the role of presenilin in gamma-secretase cleavage. J Biol Chem. 2000;275:26277–26284. doi: 10.1074/jbc.M002812200. [DOI] [PubMed] [Google Scholar]
- Wang DS, Iwata N, Hama E, Saido TC, Dickson DW. Oxidized neprilysin in aging and Alzheimer’s disease brains. Biochem Biophys Res Commun. 2003;310:236–241. doi: 10.1016/j.bbrc.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Fischer HG, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166:2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- Morelli L, Llovera R, Gonzalez SA, Affranchino JL, Prelli F, Frangione B, Ghiso J, Castano EM. Differential degradation of amyloid beta genetic variants associated with hereditary dementia or stroke by insulin-degrading enzyme. J Biol Chem. 2003;278:23221–23226. doi: 10.1074/jbc.M300276200. [DOI] [PubMed] [Google Scholar]