Abstract
Mechanisms that control the proliferation capability of the initiated cells during hepatocarcinogenesis are still largely unclear. We investigated the role of a pro-death Bcl-2 family protein, Bid, in liver tumor development using a neonatal diethylnitrosamine model. Diethylnitrosamine was administrated to 15-day-old wild-type and bid-null mice. The development of microfoci at the early stage and of gross tumors at the later stage was compared between the two groups of mice. Both microfoci and gross tumor development were significantly retarded in the bid-null mice, despite reduced cell death as measured by TUNEL staining. Further studies indicated that there were significantly less proliferating cells in diethylnitrosamine-treated bid-null livers. The regulation of cell proliferation by Bid was confirmed in two other systems not involving carcinogenesis. Hepatocyte proliferation following partial hepatectomy and T lymphocyte proliferation following anti-CD3 stimulation were both retarded in bid-null mice. Thus, these studies revealed a previously undisclosed function of Bid in regulating cell proliferation, which can be important to tumor development. Furthermore, the role of Bid in promoting hepatocarcinogenesis is in contrast to its reported role in suppressing myeloid leukemia and thus suggests an organ- and/or etiology-specific role of the Bcl-2 family proteins in regulating oncogenesis.
Hepatocellular carcinoma is a worldwide health concern with diverse etiology. Viral hepatitis, alcohol liver disease, and carcinogen exposures are the major causes.1 Rodents have been traditionally used to study carcinogen-induced liver cancers and to screen potential carcinogens. The mouse models have become increasingly important with the availability of various transgenic/knockout mice, which offer a definite way to dissect genetic pathways in the carcinogenesis. Diethylnitrosamine (DEN) is one of the most extensively studied carcinogens for the liver.2,3 It is a complete genotoxic carcinogen, and it can induce liver tumors at a proper dosage without any promoting agents, particularly in neonatal mice, whose proliferating hepatocytes are particularly susceptible to it.3 The molecular mechanism of how DEN induces liver cancer is not completely understood. It is likely that the mutations accumulated in the genome eventually lead to oncogenic transformation and tumor development. Histologically, numerous microfoci appear in the first several months after a single DEN injection at the neonatal stage, followed by the development of adenomas and carcinomas by 8 to 12 months.2
Genetic elements that control and regulate DEN-induced carcinogenesis are still in large part unknown, although it seems that many different genes can affect the process. Mice overexpressing c-myc or TGF-α in the liver developed spontaneous hepatocellular carcinoma and were also more susceptible to DEN-induced hepatocarcinogenesis, although their actual contributions under non-overexpression conditions are less clear.4 On the other hand, deletion of cyclin G15 or c-Jun6 did not seem to increase spontaneous liver cancer, but did reduce DEN-induced liver carcinogenesis, indicating their importance in the natural history of the DEN-induced tumor development.5,6 Not surprisingly, these genes have been shown to regulate cellular proliferation and/or cell death, the two critical events controlling cellular homeostasis. Similar events may also determine the different susceptibility to DEN carcinogenesis among different strains of mice and between neonatal and adult mice.7,8
The Bcl-2 family of proteins are key molecules regulating apoptosis during development and homeostasis maintenance.9 Family members share significant sequence homology in one to four functional domains (BH1, 2, 3, and 4 domains), which are the structural basis for their functions as well as for the interactions between the members. This large family of proteins can be divided into the anti-apoptosis group (including Bcl-2, Bcl-xL etc) and the pro-apoptosis group. The latter can be further divided into the multidomain subfamily (including Bax), which has BH1–3 domains, and the BH3-only subfamily (including Bid, Bad, Bim etc). Overexpression of Bcl-2 in mouse livers suppressed DEN-induced liver carcinogenesis,10 and TGF-α,11 c-myc12 or SV40-T-Ag12 overexpression-induced tumor development, in contrast to its tumor-promoting role in other systems.13 It was speculated that Bcl-2 might play an antiproliferation role in regulating liver tumor development.10,14 However, whether Bcl-2 normally participates in tumorigenesis in the liver is not known, since it is not expressed in the normal mouse liver.14
Bid is a BH3-only pro-death Bcl-2 family molecule.15 It is normally expressed in mouse livers and is critical to liver injury and hepatocyte apoptosis under a number of pathophysiological conditions.15–18 Deletion of Bid can cause chronic myelomonocytic leukemia in aged mice,19 suggesting that it can regulate homeostasis. However, it was not known whether Bid might be also involved in tumor development in the liver and how Bid might regulate oncogenesis. We therefore examined the role of Bid in DEN-induced hepatic carcinogenesis using bid-deficient mice. Surprisingly, we found that DEN-induced liver tumor development was significantly impeded in the bid-deficient mice. Further studies revealed a novel function of Bid in promoting cell proliferation, which may be a more dominant activity than its pro-death activity in determining hepatocarcinogenesis.
Materials and Methods
Mice and the Hepatic Carcinogenesis Model
All mice were maintained in a specific pathogen-free environment according to NIH and University of Pittsburgh guidelines. bid-deficient mice16 have been backcrossed to C57BL/6 for 12 generations. Wild-type mice were littermates in C57BL/6 background. The neonatal DEN model of hepatocarcinogenesis was established as previously described.2 Briefly, male mice 15 days old were given intraperitoneally a single injection of 15 mg/kg DEN. They were then analyzed at designated time points.
Histology and Morphometric Analysis
Mouse livers were dissected and examined for gross changes by the naked eye. Observable tumors were recorded and the diameters were measured. Liver tissues were fixed in 10% neutral buffered formalin and paraffin embedded. Sections were cut at 5 μm in thickness and stained with hematoxylin and eosin (H-E). Images were taken with a digital camera (SPOT, Diagnostic Instruments, Sterling Heights, MI) under a light microscope (Nikon Eclipse TE200, Melville, CA). Tumorigenic foci were counted from at least four sections from all five lobes and their sizes were determined using the morphometric tools of the SPOT software.
For cell proliferation analysis, mice were given intraperitoneally 30 mg/kg BrdU 2 hours before sacrifice. Livers were formalin-fixed, paraffin-embedded, and sectioned. The sections were deparaffinized, rehydrated, and boiled in a microwave oven for 10 minutes in 0.01 mol/L sodium citrate buffer (pH 6.0). The endogenous peroxidase activity was inactivated by the treatment with 0.73% H2O2 in methanol for 30 minutes. After sequential rinses in tap water and in Tris-buffered saline (TBS, pH 7.6), the sections were treated with 0.1% pronase E in TBS for 15 minutes. A primary mouse antibody against BrdU (Dako Cytomation, Carpinteria, CA) was applied to the sections for 1 hour at room temperature, followed by wash and the application of a biotinylated rabbit-anti-mouse antibody (Dako Cytomation) for 30 minutes. After the incubation with the StreptABComplex/HRP (Dako Cytomation), a substrate mixture containing 0.06% DAB and 0.03% H2O2 in TBS was applied. The sections was counterstained with hematoxylin, dehydrated, and mounted.
For cell death analysis, we used the method of terminal deoxynucleotidyl transferase mediated dUTP-biotin nick end labeling (TUNEL) with a commercial kit (ApopTag kit, Chemicon International, Temecula, CA) according to the manufacturer’s instructions. BrdU or TUNEL positive cells were quantified digitally from at least 5 sections including all five lobes at high power (×200), which covered at least 2000 hepatocytes. The results were expressed as the number of positive cells per high power field (HPF).
Partial Hepatectomy
This was performed as previously described20 by removing two-thirds of the liver, including the left lateral, the right and left median lobes of the liver. Mice were approximately 2 to 3 months old, and the procedure was conducted in the morning between 9 and 12 a.m. Mice were then sacrificed at designated time point and BrdU (30 mg/kg) was given 2 hours before sacrifice. Liver sections, BrdU staining and analysis were conducted as described above, except that the number of BrdU positive cells was expressed as the percentage of total cells counted.
Analysis of T Cell Proliferation
This was conducted as previously described.21 Mouse splenic T cells were enriched using nylon-wool columns in the flow-through portion. Flow cytometry analysis indicated that more than 90% of isolated cells were CD3 positive. T cells (3–5 × 105/well) were then cultured in 24-well plates pre-coated with the anti-CD3 antibody (clone 2C11, BD PharMingen, San Diego, CA; 500 ng/well, overnight at 4°C). Cells were harvested at designated time after anti-CD3 stimulation and incubated with the Krishan’s reagent (0.1 mg/ml propidium iodide, 0.02 mg/ml RNase A, 0.3% NP-40, 0.1% sodium citrate) for 30 minutes followed by flow cytometry analysis.
Western Blot Analysis
Liver lysates were prepared using RIPA buffer (150 mmol/L NaCl, 50 mmol/L Tris-HCl, pH 7.4, 1 mmol/L EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) with standard protease inhibitors (1 μg/ml aprotinin, 1 μg/ml pepstatin A and 1 μg/ml leupeptin and 20 mg/ml PMSF). About 50 μg of protein were separated on a 12% SDS-PAGE, transferred to PVDF membranes, and analyzed with an anti-cyclin E (Upstate, Charlottesville, VA), anti-cyclin D1 (BD PharMingen) or anti-p27Kip1 (Santa Cruz Biotechnology, Santa Cruz, CA) antibody.
Results
Development of DEN-Induced Microfoci and Large Tumor Lesions Were Impeded in bid-Deficient Livers
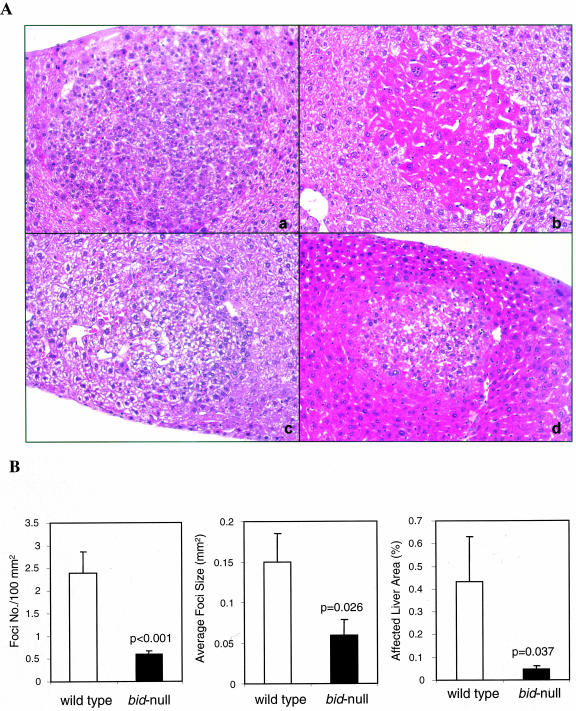
We used a commonly used neonatal DEN-induced hepatocarcinogenesis model,2 in which a single dose of 15 mg/kg DEN was intraperitoneally injected into 15-day-old wild-type and bid −/− mice. The mice were sacrificed 4, 8, or 10–12 months later. DEN-induced microfoci could be readily observed under light microscope in wild-type liver by 4 months (Figure 1A). The most common foci were basophilic cell type, followed by clear cell type and the mixed type containing both types of cells, as previously described.22 Eosinophilic cell type was rare, but could be observed (Figure 1A). These foci were glucose-6-phosphatase (G-6-P) negative (data not shown), consistent with previous report.22,23 Both basophilic and clear cell type foci were observed in bid-deficient livers and were not apparently different from those in the wild-type mice. However, there were significant differences in the number of foci and the size of foci between the two types of mice (Figure 1B). Intriguingly, there were much fewer foci found in the bid −/− livers and the foci were also much smaller. Thus the percentage of areas affected by the lesions was much smaller in the bid-deficient livers (Figure 1B). These data indicate that in the bid-deficient livers the microfoci development was impeded.
Figure 1.
Impeded development of microfoci in DEN-treated bid-deficient livers. A: Typical morphology of microfoci detected in the wild-type (a-c) and bid-deficient (d) livers 4 months after DEN treatment (×200). Shown are basophilic cell type (a, d), eosinophilic cell type (b) and clear cell type (c). Intracytoplasmic inclusions could also be observed in some of the foci (a, c, d). Note the size difference in the foci between wild-type (a-c) and bid-deficient livers (d). B: Analysis of the foci number and size in wild-type (open bar) and bid-deficient (closed bar) livers treated with DEN for 4 months. Foci in bid-deficient livers are significantly lower in number and smaller in size. Data are from five liver sections of each of the 10 wild-type mice and 13 bid-deficient mice and shown as mean ± SE The P values are based on Student’s t-test.
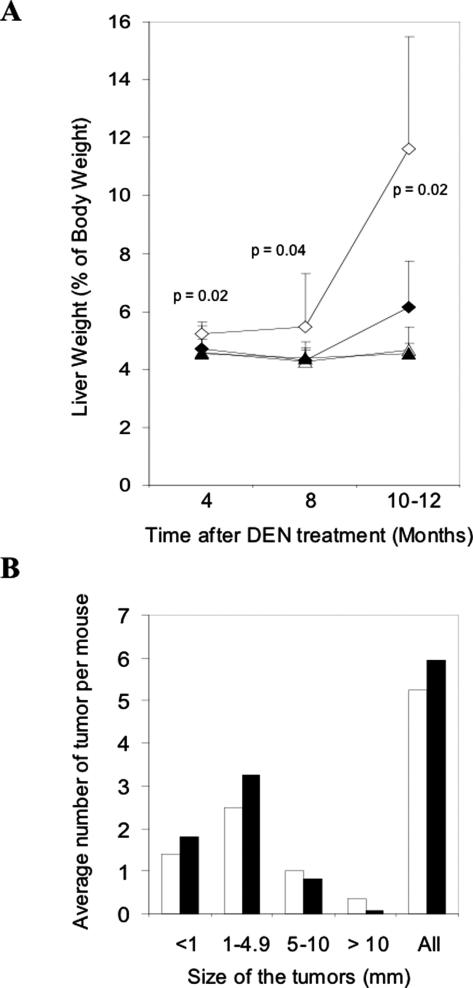
Like the microfoci development, the gross tumor development in the wild-type mice seemed to be also more advanced. The relative liver weight versus body weight in treated wild-type mice was always larger than that of treated bid-deficient mice, which was most prominent by 10 to 12 months post-DEN treatment (Figure 2A), suggesting that the overall tumor burden was larger in wild-type mice. However, a more detailed analysis of the size distribution of gross lesions suggested a more complicated pattern of tumor development in these mice (Figure 2B).
Figure 2.
Reduced gross tumor development in DEN-treated bid-deficient livers. A: Liver weight in percentage of body weight. Livers of male DEN-treated and age-matched control wild-type and bid-deficient mice were weighted at different times after DEN administration. The ratios of the liver to body weight were determined and plotted as the percentage (open diamond, DEN-treated wild-type mice; closed diamond, DEN-treated bid-deficient mice; open triangle, untreated wild-type mice; closed triangle, untreated bid-deficient mice). Data are means ± SD from 4 to 13 mice per group. P values are obtained from Student’s t-test (treated wild-type versus treated bid-null). B: Size distribution of gross lesions. Gross lesions were countered and their sizes were measured in DEN-treated wild-type (open bar) and bid-deficient (closed bar) livers. The lesions are categorized to four different size groups based on the largest diameters and the average number of lesions per mouse in each size group is shown. Data are obtained from 17 to 20 mice of each genotype treated with DEN for 8–12 months.
Grossly observed liver lesions (white spots or bigger lesions) could be detected in 10 of 13 wild-type mice and in 11 of 13 bid-deficient mice by 8 months and in all mice (n = 17–20) by 10 to 12 months after DEN treatment. The number of lesions and the size of the lesion were variable among the mice. However, it seemed that lesions in the bid-deficient livers tended to be smaller and there were more large lesions (>5.0 mm) in the wild-type livers (25.8% vs. 14.7% in bid-deficient livers), although the total number of lesions was not significantly different between the two types of mice (Figure 2B). The size of the tumor was represented by the largest diameter across the observable area.24 For tumors that could be individually measured, the average largest diameter of the tumors was 3.7 ± 3.5 mm (n = 89 from 14 mice) in the wild-type livers and 2.5 ± 2.3 mm (n = 95 from 14 mice) in the bid-null livers, which was statistically significant (P = 0.007 by Student’s t-test). Furthermore, by 10 to 12 months, about 43% wild-type mice had tumors all over the liver, which could not be counted as individual ones, whereas only 25% bid-deficient mice had tumors at this stage. These data were consistent with the microfoci development at the early stage in the two types of mice and suggested that tumor development in the absence of Bid was delayed, rather than accelerated as it might be expected from the loss of the pro-apoptosis function.
All mice developed histological changes by 8 months after treatment, even gross lesions might not be observed. These changes were much more extensive than those in the 4-month livers, which were focal and limited, and were essentially as described previously.22,24,25 Hepatocyte dysplasia was apparent with increased nuclear/cytoplasm ratio, pleomorphism, vacuolization and clear cell change. Hepatocellular carcinomas were present with the typical trabecular structure containing more than three layers of cells. In general, while variations existed, there were no apparent differences in the histological changes between the wild-type and bid-deficient livers (data not shown).
Reduced Proliferation and Cell Death in bid-Deficient Livers during DEN-Induced Carcinogenesis
The impeded development of microfoci and tumor lesions in bid-deficient livers could not be simply explained by the known pro-apoptosis function of Bid. We thus examined cell proliferation in the DEN-treated livers.
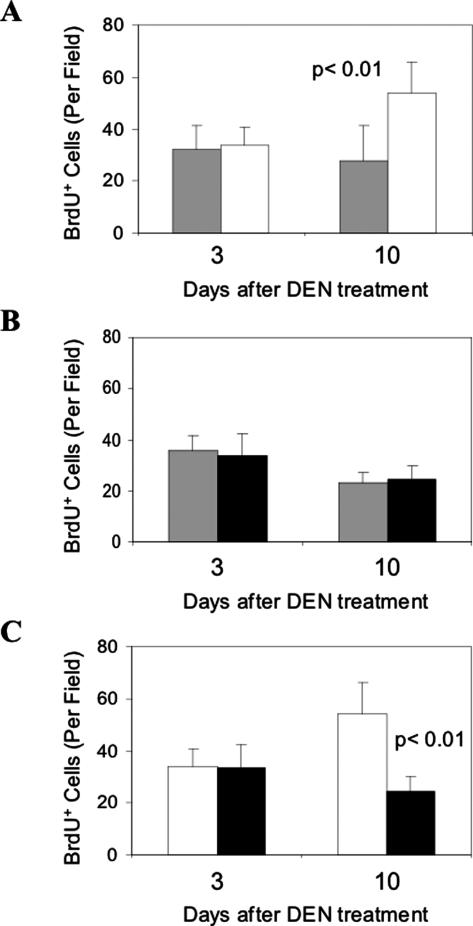
Enhanced hepatocyte proliferation could be observed soon after DEN administration. We examined the BrdU labeling in wild-type and bid-deficient mice at 3 days and 10 days after the DEN administration (Figure 3). Hepatocyte proliferation was evident at this young age, but an apparent increase in proliferation could be observed 10 days after the DEN treatment in wild-type livers. However, such an increase was not observed in bid-deficient livers, suggesting that bid deficiency led to a suppression of such a reaction in hepatocyte proliferation.
Figure 3.
Reduced cell proliferation in bid-deficient livers soon after DEN administration. A: Fifteen-day-old wild-type mice (open bar) were treated with DEN. Three and 10 days later, these mice and their non-treated age-matched littermates (gray bar) were injected with BrdU and sacrificed. BrdU incorporation in the liver was determined by anti-BrdU immunohistochemical staining and BrdU positive cells were counted in high power fields (×200). At least five fields were counted for each of the three mice in each group. B: DEN-treated 15-day-old bid-deficient mice (closed bar) and their non-treated age-matched littermates (gray bar) were analyzed in the same way as the wild-type mice. C: Comparison of BrdU incorporation in wild-type (open bar) and bid-deficient (closed bar) mouse livers at 3 days and 10 days after DEN treatment. Significant increase of BrdU incorporation could be detected in treated wild-type livers by day 10 (A) but not in bid-deficient (B) livers and the difference between the treated wild-type and bid-null mice are significant (C). The P values are based on Student’s t-test.
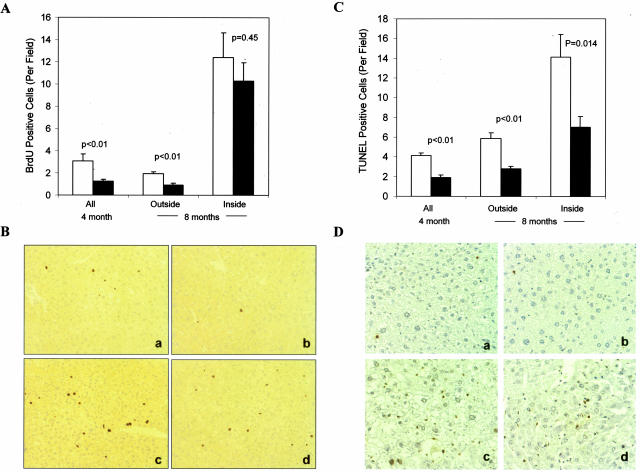
Examination of BrdU incorporation in mice treated with DEN for 4 months indicated that there were significantly fewer proliferating cells in the bid-deficient livers (Figure 4A). By 8 months, larger lesions were developed in both wild-type and bid-deficient livers. BrdU incorporation within the lesions was still lower in the bid-deficient livers but not significantly (Figure 4A). However, BrdU labeling outside the lesions remained to be significantly lower in the bid-deficient liver, which might represent proliferation of those initiated cells that were still at their early phase of expansion (a situation similar to the 4-month livers). These data suggest that there was a proliferation defect in the bid-deficient livers, which was prominently present in the early phase of tumor development, but less so at the advanced stage, when other pro-growth oncogenes might be actively involved.
Figure 4.
Reduced cell proliferation and cell death in long-term DEN-treated bid-deficient livers. A: Wild-type (open bar) and bid-deficient (closed bar) mice treated with DEN for 4 or 8 months were sacrificed following BrdU labeling. BrdU incorporation in the liver was determined by anti-BrdU immunohistochemical staining and BrdU positive cells were counted in high power fields (×200). The BrdU positive cells in 8-month livers were further grouped into those inside and those outside histological lesions. At least five fields were counted for each liver section and 10–13 mice were included in each group. Data are presented as mean ± SE and the P value are based on Student’s t-test. B: Representative figures of BrdU positive cells (brown) outside (a, b) and inside (c, d) of the lesions in wild-type (a, c) and bid-deficient (b, d) livers 8 months after DEN treatment. C: Wild-type (open bar) and bid-deficient (closed bar) mice treated with DEN for 4 or 8 months were sacrificed. Cell death in the liver was determined by TUNEL and TUNEL positive cells were counted in high power fields (×200). The positive cells in 8-month livers were further grouped into those inside and those outside histological lesions. At least five fields were counted for each liver section and 10 to 13 mice were included in each group. Data are presented as means ± SE and the P values are based on Student’s t-test. D: Representative figures of TUNEL positive cells (arrows) outside (a, b) and inside (c, d) of the lesions in wild-type (a, c) and bid-deficient (b, d) livers 8 months after DEN treatment.
As a pro-death molecule, Bid was anticipated to negatively regulate tumor development, particularly at the later stage when apoptosis rate could constitute an important factor determining tumor expansion. We examined the status of cell death in DEN-treated livers by TUNEL staining. Indeed, cell death in bid-deficient livers was significantly lower at both 4 and 8 months after DEN treatment (Figure 4B). This suggests that Bid did participate in the cell death process. The contribution of this Bid function to DEN-induced tumor development might be more significant at the later stage (>8 months), as the difference in cell proliferation between the wild-type and bid-deficient livers became smaller by that time (Figure 4A). However, overall, the pro-proliferation function of Bid seemed to outweigh its pro-death function in determining the expansion of DEN-induced tumors in the mice (Figure 2).
Bid Possesses an Intrinsic Activity in Regulating Cell Cycle Progression
The reduced BrdU labeling in DEN-treated bid-deficient livers might be due to the endogenous difference in the capability of proliferation between wild-type and bid-deficient cells. To examine this hypothesis, we determined the effects of Bid on cell proliferation in conditions other than tumorigenesis.
We first compared the regenerative hepatocyte proliferation induced by two-thirds partial hepatectomy (PH) in wild-type and bid-deficient mice. The physical removal of a large portion of liver should provide the same strength of growth stimulation to the regenerative proliferation of the remaining hepatocytes, thus eliminating the potential impact of Bid on cell death and allowing an independent assessment of its ability to regulate cell proliferation. The mainly synchronous regenerative proliferation would also facilitate the determination of the role of Bid in cell cycle progression.
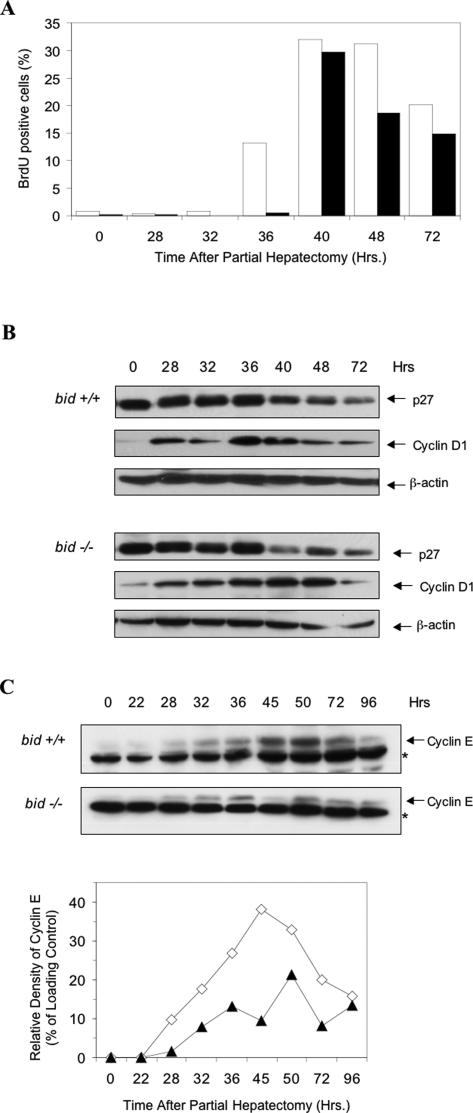
We found that wild-type livers began to show significant DNA synthesis around 36 hours after PH based on BrdU incorporation, suggesting the entry of the quiescent hepatocytes into the S phase (Figure 5A). However bid-deficient hepatocytes did not show obvious DNA synthesis until 40 hours later, which suggested a delay in S phase transition. The percentage of BrdU positive cells remained relatively low in bid-deficient livers after 40 hours, consistent with the notion that fewer bid-deficient cells entered into S phase during this period of time.
Figure 5.
Regulation of regenerative hepatocyte proliferation by Bid. A: Wild-type (open bar) and bid-deficient (closed bar) mice were subjected to two-thirds partial hepatectomy. Mice were then sacrificed at designated time points after 2 hours of BrdU labeling. BrdU positive hepatocytes were counted and expressed as the percentage of total hepatocytes. At least 500 positive cells were counted. B: Liver lysates from mice treated as in A were separated on SDS-PAGE, followed by immunoblot analysis using antibodies against p27Kip1, cyclin D1 or β-actin. C: Liver lysates from mice treated as in A were separated on SDS-PAGE, followed by immunoblot analysis using an anti-cyclin E antibody (upper panel). Asterisks indicate a nonspecific cross-reactive band, which serves as a surrogate loading control. The lower panel shows densitometric analysis of the cyclin E bands and the surrogate loading control bands. Results were expressed as the relative cyclin E density in percentage of that of the loading control. Cyclin E expression was reduced and delayed in the bid-null regenerating livers. All data are representative of three or four experiments performed with three or four mice at each time point.
G1-S phase transition is driven by many factors, particularly by cyclin D/CDK4/6, and by cyclin E/CDK2.26 Previous work on the similar effects of Bcl-2 overexpression on hepatocyte proliferation had found alterations in the kinetics of cyclin E expression, but not in cyclin D expression.14 We therefore examined the kinetics of cyclin D1 and cyclin E expression by immunoblot analysis in wild-type and bid-deficient livers. We found that cyclin D1 expression was increased in both wild-type and bid-deficient livers following PH, but there were no apparent differences in the expression levels (Figure 5B). In contrast, cyclin E expression in the bid-deficient livers was reduced in amount and delayed in both first appearance and peak time (Figure 5C). Cyclin E could be detected in the wild-type regenerating livers at 28 hours after PH and its level peaked around 45 hours. It became observable in the bid-deficient regenerative livers at 32 hours with a peak at 50 hours after PH. These results were consistent with the BrdU labeling analysis (Figure 5A) and suggested that deficits in cyclin E expression contributed to the reduced proliferation in bid-null livers. It did not seem that the delayed expression of cyclin E was due to a higher p27Kip1 expression in the bid-deficient livers, as the expression level of p27Kip1 in both normal livers and regenerating livers were compatible in the two types of mice (Figure 5B). Taken together, these data indicate that Bid does have an endogenous function in regulating cell proliferation.
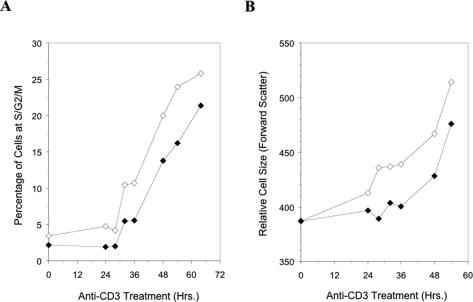
To further evaluate this novel function of Bid, we took a second approach examining the proliferative response of T lymphocytes to mitogenic stimulation. Resting splenic T cells were isolated and stimulated with anti-mouse CD3 antibodies. Flow cytometry analysis indicated that bid-deficient T cells were slower in entering S phase in response to anti-CD3 stimulation and the numbers of proliferating cells were always lower in the bid-deficient population than in the wild-type population (Figure 6A). Cell size changes as cells enter into S phase. Correspondingly, wild-type T cells enlarged more quickly than bid-deficient T cells (Figure 6B).
Figure 6.
Delayed cell cycle progression in stimulated bid-deficient T cells. A: Splenic T cells from wild-type (open diamond) and bid-deficient (closed diamond) were stimulated with anti-CD3 antibodies for different times as indicated and then analyzed by flow cytometry for the DNA profiles. The percentage of S and G2/M T cells were plotted. There were less proliferating bid-deficient T cells at all time points. B: The relative cell size as determined by forward scatter analysis was also smaller for anti-CD3-stimulated bid-deficient cells (closed diamond) than wild-type cells (open diamond). All data are representative of three experiments performed with three or four mice at each time point.
These data clearly indicate that the regulation of cell proliferation by Bid is intrinsically inherited and is global, regardless of cell type (hepatocytes or lymphocytes) and stimulation (DEN, PH or anti-CD3). This function of Bid can affect tumor development as shown in the DEN-induced hepatic carcinogenesis.
Discussion
Hepatic carcinogenesis is a complicated process and despite numerous efforts the early molecular events are still largely unknown. DEN is a commonly used liver carcinogen and causes DNA ethylation, which can thus induce mutations in genes key to oncogenesis. The early step of DEN-induced carcinogenesis would include the expansion of the mutated cells (the initiated cells), which would be critically affected by the proliferation capability of these cells. Susceptibility of mice to DEN-induced liver carcinogenesis varies between neonatal and adult mice and among different strains, which has been attributed to the variations in the proliferation capability of the hepatocytes.7,8 These studies indicate the importance of various genetic factors that contribute to hepatocyte proliferation in liver tumor development. We have now demonstrated that Bid is one such gene based on a loss-of-function mouse model.
Bid is normally expressed in the mouse liver,15,16 but it had not been recognized to be able to function in cell proliferation. In contrast, it has been identified as a pro-apoptosis molecule of the Bcl-2 family, whose functions are better known to regulate apoptosis. Nevertheless, several Bcl-2 family proteins have been recently shown to regulate cell proliferation.9 Early studies on T cell activation found that T cells from bcl-2 transgenic mice proliferated more slowly than the wild-type cells,21,27,28 whereas T cells from bcl-2-deficient mice moved into cell cycle more rapidly than the wild-type cells.21 The anti-proliferation effects of Bcl-2 could be also observed in re-entry of serum-deprived cells into cell cycle in vitro27–29 and in entry of quiescent hepatocytes into cell cycle after partial hepatectomy.14 Conversely, the pro-death molecules, Bad30,31 and Bax32 could promote cell proliferation and antagonize the effects of Bcl-228,29,33 or Bcl-xL.30,34 We have found that the ability of the pro-death molecule Bid to promote proliferation is intrinsic to the cell, regardless of cell type and simulation. We have documented the effect of Bid on hepatocyte proliferation induced by DEN or partial hepatectomy and on T lymphocyte proliferation induced by anti-CD3 antibodies. Thus Bcl-2 family proteins with opposing functions in regulating cell death seem to have also opposing functions in regulating cell proliferation.
How Bcl-2 family proteins regulate cellular proliferation is not as clear as their regulation of apoptosis. However, it seems that at least some of the biochemical activities of the Bcl-2 family proteins may be shared in the regulation of both apoptosis and proliferation, since all important structural features required for apoptosis regulation are also required for proliferation regulation.33,34 Thus it seems which function will be manifested would be dependent on whether the cells are stimulated with a death signal or a proliferation signal. The effect of Bcl-2 family proteins in cell proliferation is most prominent in the entry of quiescent cells14,21,27,28,31,33 (Figure 6) or the re-entry of growth arrested cells into the cycle,27–30,34 although effects on continuously cycling cells were also reported.29 Thus the Bcl-2 family proteins could regulate the transition into S phase. In non-hepatocytes, overexpression of Bcl-2 or Bcl-xL induced a prolonged presence of G0 markers following mitogenic stimulations, such as smaller cell size, lower RNA content, and higher p27Kip1 expression,21,33–36 and a delayed appearance of G1 markers, such as the phosphorylation of Rb27 and CDK2 activation.27,36,37 In hepatocytes, overexpression of Bcl-2 did not affect significantly the kinetics of cyclin D expression, but did delay the expression of cyclins E and A, and CDK2 activity following PH.14 How these events are affected by the Bcl-2 family proteins is not known. However, our analysis of bid-deficient hepatocytes following PH was in agreement with these observations, showing delayed and reduced cyclin E expression but normal cyclin D1 expression in bid-deficient livers. Interestingly, expression of p27Kip1 in both normal livers and regenerating livers showed overall no apparent differences between the two types of mice. Thus future endeavor will be devoted to the understanding of how exactly Bcl-2 family proteins regulate the S phase transition, which may be influenced by specific cell type and the context of growth stimulation.
How do the Bcl-2 family proteins regulate tumorigenesis while possessing two functions that seem to have conflicting effects on tumor development? In the current study, it seems that the effect of Bid on DEN-induced liver carcinogenesis is more correlated with its regulation on cell proliferation. Our data indicated that DEN-induced liver carcinogenesis was delayed and reduced in the absence of Bid, rather than being promoted as it would be expected based on its orthodox pro-death function. The development of both microfoci at the early stage and the grossly observable tumors at the later stage was greatly delayed in bid-deficient mice, suggesting that expansion of the tumorigenic cells was hindered in the absence of Bid. This notion is supported by the finding that bid-deficient livers displayed significantly reduced proliferation capability following DEN treatment.
We noted that the effect of Bid on cell death was still present. The bid-deficient livers still manifested less cell death following DEN treatment, particularly at the later stage (>8 months), when apoptosis could be a more influencing factor for tumor expansion. It is likely that the pro-death function of Bid still plays a role in DEN-induced tumor development, but this effect seems to be out-weighted by the more prominent pro-proliferation function of Bid at the early stage of tumor initiation.
Results from studies using gene overexpression models, while less physiologically relevant, are largely consistent with those based on the bid-deletion model. Overexpression of Bcl-2 in the liver, which does not normally express this molecule,14 did not by itself lead to tumor formation but did delay tumor development induced by DEN,10 or by overexpression of c-myc,12 SV40-T-Ag,12 or TGF-α.11 In these cases, the antiproliferative effects of Bcl-2 were clearly documented and seemed to out-weight its anti-apoptosis function in tumor development.14 However, it was concerned that Bcl-2 was not normally expressed in the liver and therefore the overexpression results might not be relevant to its normal role in regulating tumorigenesis. Our studies with the bid-deletion model now firmly indicate that the function of the Bcl-2 family proteins in cell proliferation regulation does seem to be important to tumorigenesis. Moreover, we did not observe any histological alterations in tumors developed in bid-deficient livers, suggesting that it is likely the progression of tumors rather than the alteration of tumor profile that is affected by the function of Bcl-2 family proteins in DEN-induced liver carcinogenesis.
It seems that the overall negative effect of Bid deficiency (this study) and Bcl-2 overexpression10–12 on tumor development may be liver-specific. In fact, bid deficiency by itself led to an increased incidence of chronic myelomonocytic leukemia in aged mice.19 This is similar to bad deficiency in which aged mice developed more frequently lymphoid tumors either spontaneously or in response to sublethal γ-irradiation.38 In addition, while loss of Bax led to few spontaneous tumors, it enhanced tumor development induced by SV40 T antigen in brain choroid plexus39 and by c-myc in B cells.40 Similarly, loss of Bim did not lead to increased tumor incidence, but could collaborate with c-myc to promote B cell leukemia.41 On the other hand, overexpression of the anti-death members, Bcl-2 or Bcl-xL, clearly promoted tumor development in the lymphoid cells,42–45 in the myeloid cells46 and in the pancreatic β cells.47,48 It seems that in all these settings, the Bcl-2 family proteins affect tumorigenesis mainly via their regulation on apoptosis with the result of overexpression of Bcl-2 being equivalent to the loss of p53.40,41,45,47,48
The effect of Bcl-2 family proteins on mammary tumors seems to be varied with the oncogenic stimulations. Loss of one copy of Bax seemed sufficient to enhance tumor development induced by SV40 T antigen in mammary epithelium.49 Similarly, overexpression of Bcl-2 in combination of c-myc overexpression also promoted mammary tumor development.50 However, when the tumor was induced by the carcinogen, dimethylbenz(a)anthracene, overexpressed Bcl-2 actually led to tumor development at a much slow pace than that in the non-transgenic mice and significantly inhibited cell proliferation.51
What may determine such a tissue or etiology specific effect of the Bcl-2 family proteins on tumorigenesis is not clear. We speculate that the control and regulation of cellular proliferation and apoptosis could be different in a tissue specific way, eg, due to the expression variations of relevant molecules. Thus the relative importance of the two functions of the Bcl-2 family proteins to tumorigenesis would depend on the actual cellular environment and specific tumor induction regime. In the case of liver tumorigenesis, cell proliferation might be more severely affected by the Bcl-2 family proteins than in other cases. Future experiments would be directed to test these hypotheses.
In conclusion, using a loss-of-function genetic model, we demonstrated convincingly for the first time that a pro-death Bcl-2 family protein could have a significant role in the natural history of hepatocellular carcinomas induced by a chemical carcinogen. We have discovered a novel function of Bid in regulating cell proliferation, which seems to be more important than its pro-death function in affecting liver tumor development. In contrast to its tumor-promoting effect in the myeloid cell lineage, loss of Bid in the hepatocytes impeded tumorigenesis. Notably, previous studies with enforced overexpression of Bcl-2 in hepatocytes, although arguably being artificial to some extent, came with largely consistent results10–12,14. Together, these studies suggest that the impact of the Bcl-2 family proteins on oncogenesis would be affected not only by their regulation on cell death, but also by their regulation on cell proliferation. We further suggest that there is a tissue-specific and/or etiology-specific choice for which of the two functions would be more critical in determining the ultimate fate of the tumor development in a particular setting.
Footnotes
Address reprint requests to Xiao-Ming Yin, M.D., Ph.D., Department of Pathology, University of Pittsburgh School of Medicine, Scaife Hall, 7th Floor, Room S739, 3550 Terrace Street, Pittsburgh, PA 15261. E-mail: xmyin@pitt.edu.
Supported in part by the Howard Temin Award (National Institutes of Health grant K01 CA 74885) and National Institutes of Health grant R01 CA 83817 (to X.-M.Y). L.B. and H.-M.N. were recipients of postdoctoral fellowships from the Department of Pathology, University of Pittsburgh School of Medicine.
References
- Bruix J, Boix L, Sala M, Llovet JM. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–219. doi: 10.1016/s1535-6108(04)00058-3. [DOI] [PubMed] [Google Scholar]
- Vesselinovitch SD, Mihailovich N. Kinetics of diethylnitrosamine hepatocarcinogenesis in the infant mouse. Cancer Res. 1983;43:4253–4259. [PubMed] [Google Scholar]
- Vesselinovitch SD, Koka M, Mihailovich N, Rao KV. Carcinogenicity of diethylnitrosamine in newborn, infant, and adult mice. J Cancer Res Clin Oncol. 1984;108:60–65. doi: 10.1007/BF00390974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fausto N. Mouse liver tumorigenesis: models, mechanisms, and relevance to human disease. Semin Liver Dis. 1999;19:243–252. doi: 10.1055/s-2007-1007114. [DOI] [PubMed] [Google Scholar]
- Jensen MR, Factor VM, Fantozzi A, Helin K, Huh CG, Thorgeirsson SS. Reduced hepatic tumor incidence in cyclin G1-deficient mice. Hepatology. 2003;37:862–870. doi: 10.1053/jhep.2003.50137. [DOI] [PubMed] [Google Scholar]
- Eferl R, Ricci R, Kenner L, Zenz R, David JP, Rath M, Wagner EF. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- Diwan BA, Rice JM, Ohshima M, Ward JM. Interstrain differences in susceptibility to liver carcinogenesis initiated by N-nitrosodiethylamine and its promotion by phenobarbital in C57BL/6NCr, C3H/HeNCrMTV- and DBA/2NCr mice. Carcinogenesis. 1986;7:215–220. doi: 10.1093/carcin/7.2.215. [DOI] [PubMed] [Google Scholar]
- Drinkwater NR, Ginsler JJ. Genetic control of hepatocarcinogenesis in C57BL/6J and C3H/HeJ inbred mice. Carcinogenesis. 1986;7:1701–1707. doi: 10.1093/carcin/7.10.1701. [DOI] [PubMed] [Google Scholar]
- Cory S, Huang DC, Adams JM. The Bcl-2 family: roles in cell survival and oncogenesis. Oncogene. 2003;22:8590–8607. doi: 10.1038/sj.onc.1207102. [DOI] [PubMed] [Google Scholar]
- Pierce RH, Vail ME, Ralph L, Campbell JS, Fausto N. Bcl-2 expression inhibits liver carcinogenesis and delays the development of proliferating foci. Am J Pathol. 2002;160:1555–1560. doi: 10.1016/S0002-9440(10)61101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vail ME, Pierce RH, Fausto N. Bcl-2 Delays and Alters Hepatic Carcinogenesis Induced by Transforming Growth Factor a. Cancer Res. 2001;61:594–601. [PubMed] [Google Scholar]
- de La Coste A, Mignon A, Fabre M, Gilbert E, Porteu A, Van Dyke T, Kahn A, Perret C. Paradoxical inhibition of c-myc-induced carcinogenesis by Bcl-2 in transgenic mice. Cancer Res. 1999;59:5017–5022. [PubMed] [Google Scholar]
- Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693s–1700s. [PubMed] [Google Scholar]
- Vail ME, Chaisson ML, Thompson J, Fausto N. Bcl-2 expression delays hepatocyte cell cycle progression during liver regeneration. Oncogene. 2002;21:1548–1555. doi: 10.1038/sj.onc.1205212. [DOI] [PubMed] [Google Scholar]
- Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- Li S, Zhao Y, He X, Kim T-H, Kuharsky DK, Rabinowich H, Chen J, Du C, Yin X-M. Relief of extrinsic pathway inhibition by the Bid-dependent mitochondrial release of Smac in Fas-mediated hepatocyte apoptosis. J Biol Chem. 2002;277:26912–26920. doi: 10.1074/jbc.M200726200. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Ding WX, Qian T, Watkins S, Lemasters JJ, Yin XM. Bid activates multiple mitochondrial apoptotic mechanisms in primary hepatocytes after death receptor engagement. Gastroenterology. 2003;125:854–867. doi: 10.1016/s0016-5085(03)01066-7. [DOI] [PubMed] [Google Scholar]
- Zinkel SS, Ong CC, Ferguson DO, Iwasaki H, Akashi K, Bronson RT, Kutok JL, Alt FW, Korsmeyer SJ. Proapoptotic BID is required for myeloid homeostasis and tumor suppression. Genes Dev. 2003;17:229–239. doi: 10.1101/gad.1045603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins G, Anderson R. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- Linette GP, Li Y, Roth K, Korsmeyer SJ. Cross talk between cell death and cell cycle progression: bCL-2 regulates NFAT-mediated activation. Proc Natl Acad Sci USA. 1996;93:9545–9552. doi: 10.1073/pnas.93.18.9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM. Morphology of potential preneoplastic hepatocyte lesions and liver tumors in mice and a comparison with other species. Washington: Hemisphere Publishing Corp; 1984:pp 1–26. [Google Scholar]
- Moore MR, Drinkwater NR, Miller EC, Miller JA, Pitot HC. Quantitative analysis of the time-dependent development of glucose-6-phosphatase-deficient foci in the livers of mice treated neonatally with diethylnitrosamine. Cancer Res. 1981;41:1585–1593. [PubMed] [Google Scholar]
- Ward JM, Vlahakis G. Evaluation of hepatocellular neoplasms in mice. J Natl Cancer Inst. 1978;61:807–811. [PubMed] [Google Scholar]
- Vesselinovitch SD, Mihailovich N, Rao KV. Morphology and metastatic nature of induced hepatic nodular lesions in C57BL x C3H F1 mice. Cancer Res. 1978;38:2003–2010. [PubMed] [Google Scholar]
- Ekholm SV, Reed SI. Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol. 2000;12:676–684. doi: 10.1016/s0955-0674(00)00151-4. [DOI] [PubMed] [Google Scholar]
- Mazel S, Burtrum D, Petrie H. Regulation of cell division cycle progression by bcl-2 expression: a potential mechanism for inhibition of programmed cell death. J Exp Med. 1996;183:2219–2226. doi: 10.1084/jem.183.5.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly LA, Huang DC, Strasser A. The cell death inhibitor Bcl-2 and its homologues influence control of cell cycle entry. EMBO J. 1996;15:6979–6990. [PMC free article] [PubMed] [Google Scholar]
- Borner C. Diminished cell proliferation associated with the death-protective activity of Bcl-2. J Biol Chem. 1996;271:12695–12698. doi: 10.1074/jbc.271.22.12695. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A, Chiang CW, Yang E. BAD/BCL-[X(L)] heterodimerization leads to bypass of G0/G1 arrest. Oncogene. 2001;20:4507–4518. doi: 10.1038/sj.onc.1204584. [DOI] [PubMed] [Google Scholar]
- Mok CL, Gil GmG, Williams O, Coles M, Taga S, Tolaini M, Norton T, Kioussis D, Brady HJM. Bad can act as a key regulator of T cell apoptosis and T cell development. J Exp Med. 1999;189:575–586. doi: 10.1084/jem.189.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson CM, Johnson GM, Lin Y, Korsmeyer SJ. Bax accelerates tumorigenesis in p53-deficient mice. Cancer Res. 2001;61:659–665. [PubMed] [Google Scholar]
- Cheng N, Janumyan YM, Didion L, Van Hofwegen C, Yang E, Knudson CM. Bcl-2 inhibition of T-cell proliferation is related to prolonged T-cell survival. Oncogene. 2004;23:3770–3780. doi: 10.1038/sj.onc.1207478. [DOI] [PubMed] [Google Scholar]
- Janumyan YM, Sansam CG, Chattopadhyay A, Cheng N, Soucie EL, Penn LZ, Andrews D, Knudson CM, Yang E. Bcl-xL/Bcl-2 coordinately regulates apoptosis, cell cycle arrest and cell cycle entry. EMBO J. 2003;22:5459–5470. doi: 10.1093/emboj/cdg533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vairo G, Soos TJ, Upton TM, Zalvide J, DeCaprio JA, Ewen ME, Koff A, Adams JM. Bcl-2 retards cell cycle entry through p27(Kip1), pRB relative p130, and altered E2F regulation. Mol Cell Biol. 2000;20:4745–4753. doi: 10.1128/mcb.20.13.4745-4753.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greider C, Chattopadhyay A, Parkhurst C, Yang E. BCL-x(L) and BCL2 delay Myc-induced cell cycle entry through elevation of p27 and inhibition of G1 cyclin-dependent kinases. Oncogene. 2002;21:7765–7775. doi: 10.1038/sj.onc.1205928. [DOI] [PubMed] [Google Scholar]
- Gil-Gomez G, Berns A, Brady HJ. A link between cell cycle and cell death: bax and Bcl-2 modulate Cdk2 activation during thymocyte apoptosis. EMBO J. 1998;17:7209–7218. doi: 10.1093/emboj/17.24.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP, Kutok JL, Le Beau MM, Greenberg ME, Korsmeyer SJ. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci USA. 2003;100:9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Knudson CM, Korsmeyer SJ, Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385:637–640. doi: 10.1038/385637a0. [DOI] [PubMed] [Google Scholar]
- Eischen CM, Roussel MF, Korsmeyer SJ, Cleveland JL. Bax loss impairs Myc-induced apoptosis and circumvents the selection of p53 mutations during Myc-mediated lymphomagenesis. Mol Cell Biol. 2001;21:7653–7662. doi: 10.1128/MCB.21.22.7653-7662.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egle A, Harris AW, Bouillet P, Cory S. Bim is a suppressor of Myc-induced mouse B cell leukemia. Proc Natl Acad Sci USA. 2004;101:6164–6169. doi: 10.1073/pnas.0401471101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, Harris AW, Bath ML, Cory S. Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature. 1990;348:331–333. doi: 10.1038/348331a0. [DOI] [PubMed] [Google Scholar]
- McDonnell TJ, Korsmeyer SJ. Progression from lymphoid hyperplasia to high-grade malignant lymphoma in mice transgenic for the t(14; 18). Nature. 1991;349:254–256. doi: 10.1038/349254a0. [DOI] [PubMed] [Google Scholar]
- Linette GP, Hess JL, Sentman CL, Korsmeyer SJ. Peripheral T-cell lymphoma in lckpr-bcl-2 transgenic mice. Blood. 1995;86:1255–1260. [PubMed] [Google Scholar]
- Schmitt CA, Fridman JS, Yang M, Baranov E, Hoffman RM, Lowe SW. Dissecting p53 tumor suppressor functions in vivo. Cancer Cell. 2002;1:289–298. doi: 10.1016/s1535-6108(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Kogan SC, Brown DE, Shultz DB, Truong BT, Lallemand-Breitenbach V, Guillemin MC, Lagasse E, Weissman IL, Bishop JM. BCL-2 cooperates with promyelocytic leukemia retinoic acid receptor alpha chimeric protein (PMLRARalpha) to block neutrophil differentiation and initiate acute leukemia. J Exp Med. 2001;193:531–543. doi: 10.1084/jem.193.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik P, Karrim J, Hanahan D. The rise and fall of apoptosis during multistage tumorigenesis: down-modulation contributes to tumor progression from angiogenic progenitors. Genes Dev. 1996;10:2105–2116. doi: 10.1101/gad.10.17.2105. [DOI] [PubMed] [Google Scholar]
- Pelengaris S, Khan M, Evan GI. Suppression of Myc-induced apoptosis in beta cells exposes multiple oncogenic properties of Myc and triggers carcinogenic progression. Cell. 2002;109:321–334. doi: 10.1016/s0092-8674(02)00738-9. [DOI] [PubMed] [Google Scholar]
- Shibata MA, Liu ML, Knudson MC, Shibata E, Yoshidome K, Bandey T, Korsmeyer SJ, Green JE. Haploid loss of bax leads to accelerated mammary tumor development in C3(1)/SV40-TAg transgenic mice: reduction in protective apoptotic response at the preneoplastic stage. EMBO J. 1999;18:2692–2701. doi: 10.1093/emboj/18.10.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager R, Herzer U, Schenkel J, Weiher H. Overexpression of Bcl-2 inhibits alveolar cell apoptosis during involution and accelerates c-myc-induced tumorigenesis of the mammary gland in transgenic mice. Oncogene. 1997;15:1787–1795. doi: 10.1038/sj.onc.1201353. [DOI] [PubMed] [Google Scholar]
- Murphy KL, Kittrell FS, Gay JP, Jager R, Medina D, Rosen JM. Bcl-2 expression delays mammary tumor development in dimethylbenz(a)anthracene-treated transgenic mice. Oncogene. 1999;18:6597–6604. doi: 10.1038/sj.onc.1203099. [DOI] [PubMed] [Google Scholar]