Abstract
In most cases, small-cell carcinoma of the urinary bladder is admixed with other histological types of bladder carcinoma. To understand the pathogenetic relationship between the two tumor types, we analyzed histologically distinct tumor cell populations from the same patient for loss of heterozygosity (LOH) and X chromosome inactivation (in female patients). We examined five polymorphic microsatellite markers located on chromosome 3p25-26 (D3S3050), chromosome 9p21 (IFNA and D9S171), chromosome 9q32-33 (D9S177), and chromosome 17p13 (TP53) in 20 patients with small-cell carcinoma of the urinary bladder and concurrent urothelial carcinoma. DNA samples were prepared from formalin-fixed, paraffin-embedded tissue sections using laser-assisted microdissection. A nearly identical pattern of allelic loss was observed in the two tumor types in all cases, with an overall frequency of allelic loss of 90% (18 of 20 cases). Three patients showed different allelic loss patterns in the two tumor types at a single locus; however, the LOH patterns at the remaining loci were identical. Similarly, the same pattern of nonrandom X chromosome inactivation was present in both carcinoma components in the four cases analyzed. Concordant genetic alterations and X chromosome inactivation between small-cell carcinoma and coexisting urothelial carcinoma suggest that both tumor components originate from the same cells in the urothelium.
Small-cell carcinoma of the urinary bladder histologically resembles that occurring in the lung and has been reported with an increasing frequency in recent years.1–10 It has been estimated to represent 0.5% of bladder malignancies and develops more frequently in older men, with hematuria as the most common presenting symptom.8 Small-cell carcinoma of the urinary bladder behaves aggressively, often with locally advanced or metastatic disease at the time of presentation.11
Over the years, three principal theories have been proposed to account for the development of small-cell carcinoma in the urinary bladder. The first theory is that small-cell carcinomas originate from multipotential, undifferentiated cells or stem cells in the urothelium.5,8,12,13 The frequent association of this tumor with coexisting urothelial carcinoma supports this theory. The second theory is that these tumors arise from neuroendocrine cells within normal or metaplastic urothelium.14 The third theory is that small-cell carcinomas are derived from an undefined population of submucosal neuroendocrine cells.1 In this study, we investigated the clonal relationships between small-cell carcinoma and coexisting urothelial carcinoma using loss of heterozygosity (LOH) and X chromosome inactivation analysis.
Materials and Methods
Patients
Twenty patients with small-cell carcinoma of the urinary bladder and concurrent urothelial carcinoma were included in our study. Archival materials from the 20 cases were retrieved from the surgical pathology files. Patients ranged in age from 58 to 83 years of age, with a mean age of 69 years. Ten patients were pathological stage pT2; nine were pathological stage pT3; and one was pathological stage pT4. Tumors were diagnosed by light microscopy with each case fulfilling the criteria established for urothelial carcinoma and small-cell carcinoma according to the World Health Organization classification system.15 The 2002 tumor, lymph node, and metastasis (TNM) classification system was used for pathological staging.16 This research was approved by the Indiana University Institutional Review Board
Tissue Samples and Microdissection
Histological sections were prepared from formalin-fixed, paraffin-embedded tissue and were stained with hematoxylin and eosin (H&E) for microscopic evaluation. From these slides, the two different tumors (urothelial carcinoma and small-cell carcinoma) were identified. Laser-assisted microdissection of the two components was performed (Figure 1) on the unstained sections using a PixCell II Laser Capture Microdissection system (Arcturus Engineering, Mountain View, CA), as previously described.17–19 Approximately 400 to 1000 cells of each component were microdissected from the 5-μm histological sections. Normal tissue from each case was microdissected as a control.
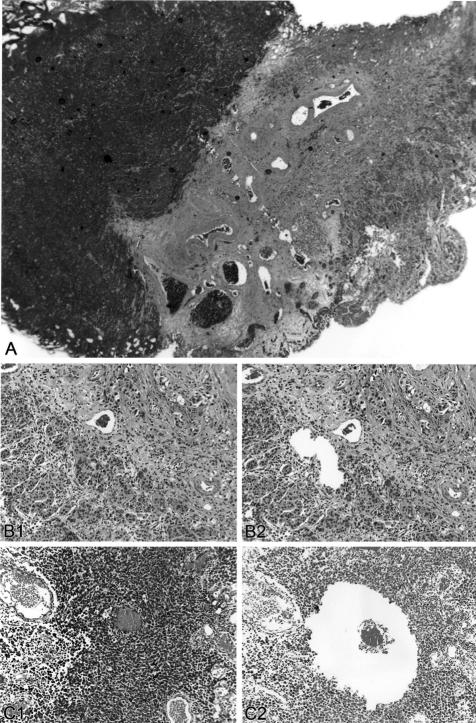
Figure 1.
Laser microdissection of concordant small-cell and urothelial carcinoma of the urinary bladder. A: Low-power view of a tumor with coexsting small-cell carcinoma and urothelial carcinoma components. B: Urothelial carcinoma before microdissection (B1) and after microdissection (B2). C: Urothelial carcinoma before microdissection (C1) and after microdissection (C2).
Detection of LOH
The dissected cells were de-paraffinized with xylene and ethyl alcohol. Polymerase chain reaction (PCR) was used to amplify genomic DNA at various specific loci on chromosome 3p25-26 (D3S3050), chromosome 9p21 (D9S171 and IFNA), chromosome 9q32-33 (D9S177), and chromosome 17p13 (TP53). Previous studies demonstrated frequent allelic instability on these chromosomes in urothelial carcinoma.20–26 D3S3050 is located in the region near the Von-Hippel Lindau tumor suppressor gene locus. IFNA and D9S171 include regions of the putative tumor suppressor gene p16. D9S177 is located within a putative tumor suppressor gene involved in the carcinogenesis of squamous cell carcinomas and urothelial carcinomas. TP53 contains the tumor suppressor p53 locus. Polymerase chain reaction amplification and gel electrophoresis were performed as previously described.17,20,27–33 Polymerase chain reactions for each polymorphic microsatellite marker were repeated at least twice from the same DNA preparations, and the same results were obtained.
Analysis of Allelic Loss Pattern
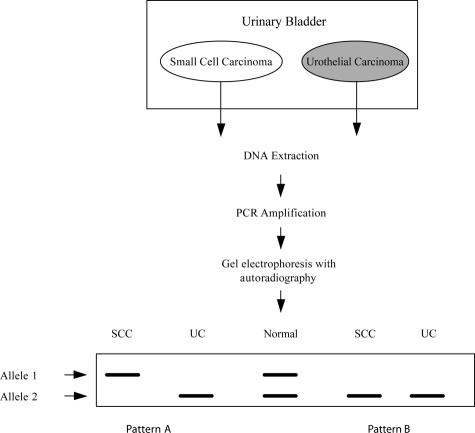
When the genetic material in a patient was found to be homozygous for the polymorphic markers (ie, showing only one allele in the normal control tissue), the case was considered noninformative. Patients with genetic material that was informative (ie, showing two alleles in the normal control tissue) were divided into two categories.34 Their DNA may show no allelic deletions in the tumor, retaining two different alleles of similar intensity on autoradiographs, or show absence of one allele. Allelic loss pattern analysis is illustrated in Figure 2. DNA sampled from separate small-cell and urothelial carcinoma cells demonstrating identical allelic loss patterns is compatible with a common clonal origin, whereas different patterns of allelic deletions are compatible with independent clonal origins of these tumors (Figure 2).17,19,20,25,28,29,32
Figure 2.
Analysis of allelic loss pattern in concordant small-cell and urothelial carcinoma of bladder. Cells from the distinctly separate small-cell and urothelial components were obtained by tissue microdissection, DNA was prepared, and the polymerase chain reaction was used to amplify DNA. DNA sampled from separate small cell and urothelial cells demonstrating identical allelic loss pattern (pattern B) is compatible with a common clonal origin, whereas different patterns of allelic deletions (pattern A) are compatible with independent clonal origins of these two components.
X Chromosome Inactivation Analysis
X chromosome inactivation analysis was performed on the urothelial and small-cell carcinomas from four female patients, as previously described.25,30–32 DNA samples were prepared from the two tumor types from the same patient. The microdissected tissue was placed in 15 μl of buffer (ie, 10 mmol/L Tris, 1 mmol/L ethylenediamine tetraacetic acid, 1% Tween 20, and 0.2 mg/ml proteinase K, pH 8.3) and incubated overnight at 37°C for DNA extraction. Eight-microliter aliquots of the DNA extract were digested overnight with 1 U of HhaI restriction endonuclease (New England Biolabs, Inc., Beverly, MA) in a total volume of 10 μl. Control reactions for each sample were incubated in the digestion buffer without HhaI endonuclease. Primers used in this reaction were: AR-sense, 5′-TCC AGA ATC TGT TCC AGA GCG TGC-3′; and AR-antisense, 5′-GCT GTG AAG GTT GCT GTT CCT CAT-3′. Three microliters of digested or nondigested DNA was amplified in a 25-μl polymerase chain reaction volume containing 0.1 μl of α-32P-labeled deoxyadenosine triphosphate (3000 Ci/mmol), 4 μmol/L AR-sense primer, 4 μmol/L AR-antisense primer, 4% dimethyl sulfoxide, 2.5 mmol/L MgCl2, 300 μmol/L deoxycytidine triphosphate, 300 μmol/L deoxythymidine triphosphate, 300 μmol/L deoxyguanosine triphosphate, 300 μmol/L deoxyadenosine triphosphate, and 0.13 U of AmpliTaq Gold DNA polymerase (Perkins-Elmer Cetus, Foster City, CA). PCR amplification was performed with an initial denaturation step of 95°C for 8 minutes followed by 32 cycles as follows: 95°C for 40 seconds, 63°C for 40 seconds, 72°C for 60 seconds, and the final extension step at 72°C for 10 minutes. The PCR products were then diluted with 4 μl of loading buffer containing 95% deionized formamide, 20 mmol/L ethylenediamine tetraacetic acid, 0.05% bromophenol, and 0.05% xylene cyanole FF (Sigma Chemical Co., St. Louis, MO). The samples were heated to 95°C for 5 minutes and then placed on ice. Three microliters of this mixture was loaded onto 6.5% polyacrylamide denaturing gels without formamide. Electrophoresis was performed at 1600V for 4 to 7 hours, followed by autoradiography with Kodak X-OMAT AR film (Eastman Kodak Company, Rochester, NY) for 8 to 16 hours.
Analysis of X Chromosome Inactivation
The cases were considered informative if the control sample displayed two alleles after PCR amplification without HhaI digestion. Nonrandom inactivation of the X chromosomes was defined as a complete or nearly complete absence of one or the other allele after HhaI digestion, indicating predominance of one androgen receptor allele.25,30–32
The clonality of the samples was evaluated on the basis of a polymorphism of the X-linked human androgen receptor gene (HUMARA) locus.25,30–32 The technique is dependent on digestion of DNA with the methylation-sensitive restriction enzyme HhaI, polymerase chain reaction (PCR) amplification of the HUMARA locus, and detection of methylation of this locus. With this method, only the methylated HUMARA allele is selectively amplified by PCR. The random inactive status of X chromosomes is established in all female somatic cells early in embryogenesis.32 Normal female tissues should be a cellular mosaic, with an equal distribution of cells containing maternal or paternal-derived inactivated X chromosomes. Tumors arising from a single transformed progenitor cell should contain the same inactive X chromosome in each tumor cell. Different patterns of X chromosome inactivation are consistent with independent origin of the cells.
Immunohistochemistry
Neuroendocrine marker immunostainings were performed on formalin-fixed, paraffin-embedded sections using the avidin-biotin complex technique, as previously described.35–37 Primary rabbit polyclonal antibodies were used for evaluation of chromogranin A expression (DAKO, Carpinteria, CA; prediluted antibody).
Results
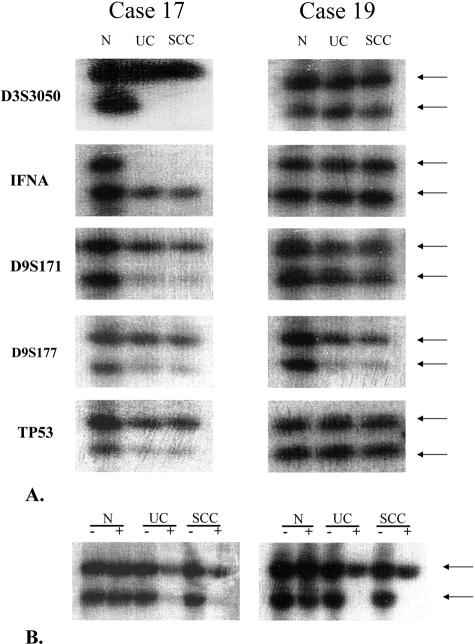
Eighteen of 20 patients (90%) with small-cell and urothelial carcinoma showed allelic loss in both components (Table 1). All 18 of these cases showed loss of heterozygosity (LOH) in at least 1 locus in each tumor type. The number of specific loci lost ranged from one to five in both tumor types. Figure 3 shows representative LOH results (cases 17 and 19). Two patients did not show allelic loss at any of the five loci examined in either tumor type.
Table 1.
Comparison of Allelic Loss in Concurrent Urothelial Carcinoma and Small-Cell Carcinoma of the Urinary Bladder
| Case no. | Microsatellite markers
|
X-Chromosome inactivation
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D3S3050
|
IFNA
|
D9S171
|
D9S177
|
TP53
|
HUMARA
|
||||||||
| UC | SCC | UC | SCC | UC | SCC | UC | SCC | UC | SCC | UC | SCC | ||
| 1 | v | v | u | u | v | v | v | v | t | t | |||
| 2 | v | v | v | v | v | v | v | v | t | t | |||
| 3 | v | v | u | u | t | t | t | t | t | t | |||
| 4 | t | t | t | t | v | v | v | v | v | v | |||
| 5 | t | t | v | v | v | v | v | v | u | u | |||
| 6 | NI | NI | NI | NI | t | t | v | v | v | v | |||
| 7 | v | v | u | u | v | v | NI | NI | t | t | |||
| 8 | t | t | v | v | v | v | v | v | v | v | |||
| 9 | v | v | u | u | v | t | v | v | NI | NI | |||
| 10 | v | v | v | v | v | v | u | u | v | v | |||
| 11 | t | t | v | v | v | v | v | v | NI | NI | |||
| 12 | v | v | t | t | u | u | t | t | NI | NI | |||
| 13 | v | v | v | v | t | t | v | v | v | v | |||
| 14 | t | v | v | v | v | v | t | t | v | v | |||
| 15 | t | t | t | t | t | t | t | t | t | t | |||
| 16 | v | v | v | v | v | v | v | v | v | v | |||
| 17 | t | t | u | u | t | t | t | t | t | t | t | t | |
| 18 | t | t | t | t | v | v | t | t | v | t | t | t | |
| 19 | v | v | v | v | v | v | t | t | v | v | t | t | |
| 20 | v | v | v | v | v | v | v | v | v | v | t | t | |
U, urothelial carcinoma component; S, small-cell carcinoma component; t, loss of lower allele; u, loss of upper allele; v, both alleles present; NI, noninformative; HUMARA, human androgen receptor gene.
Figure 3.
Representative results of loss of heterozygosity (LOH) (A) and X chromosome inactivation analysis (B) (cases 17 and 19). DNA was prepared from normal tissue (N), small-cell carcinoma (SCC), and urothelial carcinoma (UC) of the combined tumor; amplified by polymerase chain reaction using polymorphic markers D3S3050, IFNA, D9S171, D9S177, and TP53; and separated by gel electrophoresis. Arrows: allelic bands. −: without HhaI digestion; +: with HhaI digestion.
Nearly identical patterns of allelic loss were present in the small-cell and the urothelial carcinoma components in all of the tumors (Table 1). The frequency of allelic loss in the urothelial carcinoma components of informative cases was 47% (9 of 19) with D3S3050, 47% (9 of 19) with IFNA, 30% (6 of 20) with D9S171, 42% (8 of 19) with D9S177, and 41% (7 of 17) with TP53. The frequency of allelic loss in the small-cell carcinoma components of informative cases was 42% (8 of 19) with D3S3050, 47% (9 of 19) with IFNA, 35% (7 of 20) with D9S171, 42% (8 of 19) with D9S177, and 47% (8 of 17) with TP53. Three patients showed different allelic loss patterns in the two tumor types at a single locus. However, in all three of these patients, the LOH patterns at the other loci were identical. Of these three patients, one showed LOH at the D9S171 locus in the small-cell carcinoma but not in the urothelial carcinoma; one showed allelic loss at the D3S3050 locus in the urothelial carcinoma but not in the small-cell carcinoma; and one showed LOH at the TP53 locus in only the small-cell carcinoma. These were the only discrepancies from an otherwise identical LOH pattern between the tumor types in all of the patients examined. Identical LOH patterns in both tumor types were seen at the IFNA and D9S177 loci.
X chromosome inactivation analysis was performed on the two tumor types in four female patients (Table 1; Figure 3). The same pattern of nonrandom X chromosome inactivation in the urothelial carcinoma and the small-cell carcinoma tumors was seen in the four cases analyzed, providing further evidence of common origin.
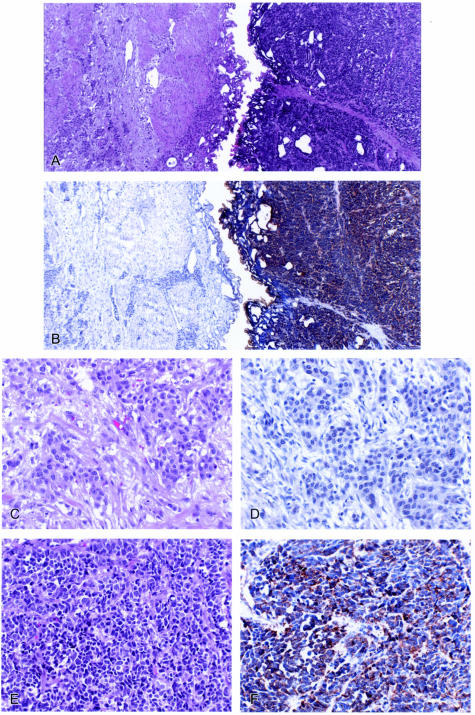
Immunostaining for markers of neuroendocrine differentiation was performed in eight cases, and all showed positive staining in the small-cell carcinoma component. The coexisting urothelial carcinoma components were negative (Figure 4).
Figure 4.
Neuroendocrine marker (chromogranin A) staining in small-cell carcinoma with coexisting urothelial carcinoma. A: Left, urothelial carcinoma; right, small-cell carcinoma (H&E staining, original magnification, ×40); B: chromogranin A staining (original magnification, ×40); C: urothelial carcinoma (H&E staining, original magnification, ×200); D: chromogranin A was negative in urothelial carcinoma component (original magnification, ×200); E: small-cell carcinoma (H&E staining, original magnification, ×200); F: small-cell carcinoma component showed positive cytoplasmic staining for chromogranin A (original magnification, ×200).
Discussion
Small-cell carcinoma of the urinary bladder often coexists with urothelial carcinoma in the same patient.11 Detailed characterization and comparison of genetic alterations in biologically and morphologically distinct tumor cell populations may provide information about the clonal evolution of concurrent small-cell and urothelial carcinoma of the urinary bladder. Loss of heterozygosity (LOH) has been found at various chromosomal loci in many types of human cancers,38 including urothelial carcinomas.20–26 The chromosomal regions in which LOH has been detected are thought to contain specific genes that when disrupted lead to either neoplastic transformation or progression. In this study, we analyzed the pattern of allelic loss in coexisting small-cell and urothelial carcinomas in 20 patients using five polymorphic microsatellite markers: DS3050, IFNA, D9S171, D9S177, and TP53. We also assessed the clonality of these tumor types using X chromosome inactivation analysis in four women.
We found a nearly identical pattern of allelic loss in the small-cell carcinoma and the concurrent urothelial carcinoma in all informative cases. In addition, the X chromosome analysis data are consistent with a common origin for these two tumor types. These findings do not support the hypothesis of a nonurothelial endocrine cell as the precursor for small-cell carcinoma of the bladder. Rather, they suggest that the small-cell component and the urothelial component share the same cell of origin, that of a multipotential, undifferentiated cell or stem cell present in the urothelium. Three patients demonstrated LOH at a single locus in one of the tumor types that was not present in the other coexisting tumor type; however, in each of these three cases, identical allelic loss patterns were present at the other loci, and in one of these cases, X chromosome inactivation analysis was performed and demonstrated an identical pattern of nonrandom X chromosome inactivation in each of the two tumor types. Thus, despite these minor differences in LOH pattern, the data overwhelmingly support a common clonal origin for urothelial and small-cell carcinomas of the urinary bladder. These three differences are most likely attributable to clonal divergence during tumor progression.
The identification of components of different biological aggressiveness within a single neoplasm is a common finding in pathology. These variable components are thought to result from tumor cell dedifferentiation or transformation, with the subsequent evolution of different subpopulations of tumor cells, a concept that is exemplified by the co-existence of small cell and urothelial carcinoma of the bladder. Our study provided evidence of biphenotypic differentiation in the tumorigenesis of small-cell carcinoma of the urinary bladder. We found that neuroendocrine biomarkers were positive in the small-cell carcinoma component but negative in the non-small-cell (urothelial) carcinoma component in the same patient in all cases studied (Figure 4). Thus, the tumor likely undergoes biphenotypic differentiation after carcinogenesis is initiated, a hypothesis that is further supported by our X chromosome inactivation data. The same pattern of X chromosome inactivation (between small-cell carcinoma and coexisting urothelial carcinoma components) was seen in all four women, suggesting a common clonality. The stage of carcinogenesis in which the pathways of differentiation diverge remains to be resolved.
The existence of pure carcinoids of bladder39 suggests that there are neuroendocrine cells of the bladder that can become neoplastic. Oesterling et al1 speculated that neuroendocrine stem (Kultschitzky type) cells with neurosecretory granules may exist within the urothelium that could give rise to neuroendocrine tumors, such as small-cell carcinoma. They also suggested that small-cell carcinoma of the bladder may derive from a poorly defined submucosal cell of neural crest origin, the same cells from which pheochromocytomas/paragangliomas40 and neurofibromas41 arise in the urinary bladder. Cramer et al14 suggested that small-cell carcinomas in the bladder arise from a cell that is present in the urothelium as a result of metaplasia. They cited the frequent findings of glandular metaplasia as well as adenocarcinoma and squamous cell carcinoma of the urinary bladder as evidence for their hypothesis. A large fraction of patients with small-cell carcinoma of the urinary bladder have concomitant foci of urothelial carcinoma.2,6,11 Our data did not support the notion that the small-cell tumor cells develop from neuroendocrine cells within normal or metaplastic urothelium or from an undefined population of submucosal neuroendocrine cells. However, the possibility remains that the small-cell carcinoma is a result of progression of urothelial carcinoma by an unknown mechanism of carcinogenesis. The current chemotherapeutic management of these tumors is in part predicated on the concept that these tumors should respond as if they are derived from neuroendocrine cells. Our findings raise doubts about this premise. Nonetheless, caution is warranted in interpreting our data. Further delineation of tumorigenesis of these cases would involve not only molecular studies of the malignant neuroendocrine cell population, but also functional studies involving induction of carcinogenesis in non-neoplastic neuroendocrine cells and investigation of the molecular changes that occur in these cells during carcinogenesis.
In summary, concordant genetic alterations between small-cell carcinoma and coexisting urothelial carcinoma as well as X chromosome inactivation analysis data suggest that urothelial and small-cell carcinoma components originate from the same cells in the urothelium.
Footnotes
Address reprint requests to Liang Cheng, M.D., Department of Pathology and Laboratory Medicine, Indiana University Medical Center, University Hospital 3465, 550 North University Boulevard, Indianapolis, IN 46202. E-mail: lcheng@iupui.edu.
References
- Oesterling JE, Brendler CB, Burgers JK, Marshall FF, Epstein JI. Advanced small cell carcinoma of the bladder: successful treatment with combined radical cystoprostatectomy and adjuvant methotrexate, vinblastine, doxorubicin, and cisplatin chemotherapy. Cancer. 1990;65:1928–1936. doi: 10.1002/1097-0142(19900501)65:9<1928::aid-cncr2820650910>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Partanen S, Asikainen U. Oat cell carcinoma of the urinary bladder with ectopic adrenocorticotropic hormone production. Hum Pathol. 1985;16:313–315. doi: 10.1016/s0046-8177(85)80020-4. [DOI] [PubMed] [Google Scholar]
- Blomjous CE, Vos W, Schipper NW, De Voogt HJ, Baak JP, Meijer CJ. Morphometric and flow cytometric analysis of small cell undifferentiated carcinoma of the bladder. J Clin Pathol. 1989;42:1032–1039. doi: 10.1136/jcp.42.10.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes CV, Soneru I. Small cell carcinoma of the urinary bladder with hypercalcemia. Cancer. 1985;56:2530–2533. doi: 10.1002/1097-0142(19851115)56:10<2530::aid-cncr2820561035>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Podesta AH, True LD. Small cell carcinoma of the bladder: report of five cases with immunohistochemistry and review of the literature with evaluation of prognosis according to stage. Cancer. 1989;64:710–714. doi: 10.1002/1097-0142(19890801)64:3<710::aid-cncr2820640324>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Mills SE, Wolfe JT, III, Weiss MA, Swanson PE, Wick MR, Fowler JE, Jr, Young RH. Small cell undifferentiated carcinoma of the urinary bladder: a light-microscopic, immunocytochemical, and ultrastructural study of 12 cases. Am J Surg Pathol. 1987;11:606–617. doi: 10.1097/00000478-198708000-00004. [DOI] [PubMed] [Google Scholar]
- Ordonez NG, Khorsand J, Ayala AG, Sneige N. Oat cell carcinoma of the urinary tract: an immunohistochemical and electron microscopic study. Cancer. 1986;58:2519–2530. doi: 10.1002/1097-0142(19861201)58:11<2519::aid-cncr2820581127>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Blomjous CE, Vos W, De Voogt HJ, Van der Valk P, Meijer CJ. Small cell carcinoma of the urinary bladder: a clinicopathologic, morphometric, immunohistochemical, and ultrastructural study of 18 cases. Cancer. 1989;64:1347–1357. doi: 10.1002/1097-0142(19890915)64:6<1347::aid-cncr2820640629>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Cheng C, Nicholson A, Lowe DG, Kirby RS. Oat cell carcinoma of urinary bladder. Urology. 1992;39:504–507. doi: 10.1016/0090-4295(92)90002-e. [DOI] [PubMed] [Google Scholar]
- Grignon DJ, Ro JY, Ayala AG, Shum DT, Ordonez NG, Logothetis CJ, Johnson DE, Mackay B. Small cell carcinoma of the urinary bladder: a clinicopathologic analysis of 22 cases. Cancer. 1992;69:527–536. doi: 10.1002/1097-0142(19920115)69:2<527::aid-cncr2820690241>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Cheng L, Pan C, Yang XJ, Lopez-Beltran A, MacLennan GT, Lin H, Kuzel TM, Papavero V, Tretiakova M, Nigro K, Koch MO, Eble JN. Small cell carcinoma of the urinary bladder: a clinicopathologic analysis of 64 patients. Cancer. 2004;101:957–962. doi: 10.1002/cncr.20456. [DOI] [PubMed] [Google Scholar]
- Davis BH, Ludwig ME, Cole SR, Pastuszak WT. Small cell neuroendocrine carcinoma of the urinary bladder: report of three cases with ultrastructural analysis. Ultrastruct Pathol. 1983;4:197–204. doi: 10.3109/01913128309140790. [DOI] [PubMed] [Google Scholar]
- Kim CK, Lin JI, Tseng CH. Small cell carcinoma of urinary bladder: ultrastructural study. Urology. 1984;24:384–386. doi: 10.1016/0090-4295(84)90220-6. [DOI] [PubMed] [Google Scholar]
- Cramer SF, Aikawa M, Cebelin M. Neurosecretory granules in small cell invasive carcinoma of the urinary bladder. Cancer. 1981;47:724–730. doi: 10.1002/1097-0142(19810215)47:4<724::aid-cncr2820470417>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Eble JN, Sauter G, Epstein JI, Sesterhenn IAE. Lyon: IARC Press,; World Health Organization Classification of TumoursPathology and Genetics of Tumours of the Urinary System and Male Genital Organs. 2004 [Google Scholar]
- Greene FL, Page DL, Flemming ID, Fritz AG, Balch CM, Haller DG, Morrow M. New York: Springer-Verlag,; American Joint Committee on Cancer Staging Manual. 2002:pp 337–346. [Google Scholar]
- Brandli DW, Ulbright TM, Foster RS, Cummings OW, Zhang S, Sweeney CJ, Eble JN, Cheng L. Stroma adjacent to metastatic mature teratoma after chemotherapy for testicular germ cell tumors is derived from the same progenitor cells as the teratoma. Cancer Res. 2003;63:6063–6068. [PubMed] [Google Scholar]
- Cheng L, MacLennan GT, Zhang S, Wang M, Pan CX, Koch MO. Laser capture microdissection analysis reveals frequent allelic losses in papillary urothelial neoplasm of low malignant potential of the urinary bladder. Cancer. 2004;101:183–188. doi: 10.1002/cncr.20343. [DOI] [PubMed] [Google Scholar]
- Kernek KM, Ulbright TM, Zhang S, Billings SD, Cummings OW, Henley JD, Michael H, Brunelli M, Martignoni G, Eble JN, Cheng L. Identical allelic loss in mature teratoma and different histologic components of malignant mixed germ cell tumors of the testis. Am J Pathol. 2003;163:2477–2484. doi: 10.1016/S0002-9440(10)63602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Bostwick DG, Li G, Zhang S, Vortmeyer AO, Zhuang Z. Conserved genetic findings in metastatic bladder cancer: a possible utility of allelic loss of chromosomes 9p21 and 17p13 in diagnosis. Arch Pathol Lab Med. 2001;125:1197–1199. doi: 10.5858/2001-125-1197-CGFIMB. [DOI] [PubMed] [Google Scholar]
- Orlow I, Lacombe L, Hannon GJ, Serrano M, Pellicer I, Dalbagni G, Reuter VE, Zhang ZF, Beach D, Cordon-Cardo C. Deletion of the p16 and p15 genes in human bladder tumors. J Natl Cancer Inst. 1995;87:1524–1529. doi: 10.1093/jnci/87.20.1524. [DOI] [PubMed] [Google Scholar]
- Williamson MP, Elder PA, Shaw ME, Devlin J, Knowles MA. p16 (CDKN2) is a major deletion target at 9p21 in bladder cancer. Hum Mol Genet. 1995;4:1569–1577. doi: 10.1093/hmg/4.9.1569. [DOI] [PubMed] [Google Scholar]
- Sidransky D, Von Eschenbach A, Tsai YC, Jones P, Summerhayes I, Marshall F, Paul M, Green P, Hamilton SR, Frost P, Vogelstein B. Identification of p53 gene mutations in bladder cancers and urine samples. Science. 1991;252:706–709. doi: 10.1126/science.2024123. [DOI] [PubMed] [Google Scholar]
- Cairns P, Tokino K, Eby Y, Sidransky D. Homozygous deletions of 9p21 in primary human bladder tumors detected by comparative multiplex polymerase chain reaction. Cancer Res. 1994;54:1422–1424. [PubMed] [Google Scholar]
- Paterson RF, Ulbright TM, MacLennan GT, Zhang S, Pan CX, Sweeney CJ, Moore CR, Foster RS, Koch MO, Eble JN, Cheng L. Molecular genetic alterations in the laser-capture-microdissected stroma adjacent to bladder carcinoma. Cancer. 2003;98:1830–1836. doi: 10.1002/cncr.11747. [DOI] [PubMed] [Google Scholar]
- Miyao N, Tsai YC, Lerner SP, Olumi AF, Spruck CHI, Goñzalez-Zulueta M, Nichols PW, Skinner DG, Jones PA. Role of chromosome 9 in human bladder cancer. Cancer Res. 1993;53:4066–4070. [PubMed] [Google Scholar]
- Cheng L, Shan A, Cheville JC, Qian J, Bostwick DG. Atypical adenomatous hyperplasia of the prostate: a premalignant lesion? Cancer Res. 1998;58:389–391. [PubMed] [Google Scholar]
- Cheng L, Song SY, Pretlow TG, Abdul-Karim FW, Kung HJ, Dawson DV, Park WS, Moon YW, Tsai ML, Linehan WM, Emmert-Buck MR, Liotta LA, Zhuang Z. Evidence of independent origin of multiple tumors from patients with prostate cancer. J Natl Cancer Inst. 1998;90:233–237. doi: 10.1093/jnci/90.3.233. [DOI] [PubMed] [Google Scholar]
- Cheng L, Bostwick DG, Li G, Wang Q, Hu N, Vortmeyer AO, Zhuang Z. Allelic imbalance in the clonal evolution of prostate carcinoma. Cancer. 1999;85:2017–2022. doi: 10.1002/(sici)1097-0142(19990501)85:9<2017::aid-cncr20>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Cheng L, Gu J, Eble JN, Bostwick DG, Younger C, MacLennan GT, Abdul-Karim FW, Geary WA, Koch MO, Zhang S, Ulbright TM. Molecular genetic evidence for different clonal origin of components of human renal angiomyolipomas. Am J Surg Pathol. 2001;25:1231–1236. doi: 10.1097/00000478-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Cheng L, Gu J, Ulbright TM, MacLennan GT, Sweeney CJ, Zhang S, Sanchez K, Koch MO, Eble JN. Precise microdissection of human bladder carcinomas reveals divergent tumor subclones in the same tumor. Cancer. 2002;94:104–110. doi: 10.1002/cncr.10151. [DOI] [PubMed] [Google Scholar]
- Gu J, Roth LM, Younger C, Michael H, Abdul-Karim FW, Zhang S, Ulbright TM, Eble JN, Cheng L. Molecular evidence for the independent origin of extra-ovarian papillary serous tumors of low malignant potential. J Natl Cancer Inst. 2001;93:1147–1152. doi: 10.1093/jnci/93.15.1147. [DOI] [PubMed] [Google Scholar]
- McCarthy RP, Zhang S, Bostwick DG, Qian J, Eble JN, Wang M, Lin H, Cheng L. Molecular genetic evidence for different clonal origins of epithelial and stromal components of phyllodes tumor of the prostate. Am J Pathol. 2004;165:1390–1400. doi: 10.1016/S0002-9440(10)63397-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z, Merino MJ, Chuaqui R, Liotta LA, Emmert-Buck MR. Identical allelic loss on chromosome 11q13 in microdissected in situ and invasive human breast cancer. Cancer Res. 1995;55:467–471. [PubMed] [Google Scholar]
- Cheng L, Pan C, Zhang JT, Zhang S, Kinch MS, Li L, Baldridge LA, Wade C, Hu Z, Koch MO, Ulbright TM, Eble JN. Loss of 14-3-3sigma in prostate cancer and its precursors. Clin Cancer Res. 2004;10:3064–3068. doi: 10.1158/1078-0432.ccr-03-0652. [DOI] [PubMed] [Google Scholar]
- Cheng L, Nagabhushan M, Pretlow TP, Amini SB, Pretlow TG. E-cadherin expression in primary and metastatic prostate cancer. Am J Pathol. 1996;148:1375–1380. [PMC free article] [PubMed] [Google Scholar]
- Zeng G, Hu Z, Kinch MS, Pan CX, Flockhart DA, Kao C, Gardner TA, Zhang S, Li L, Baldridge LA, Koch MO, Ulbright TM, Eble JN, Cheng L. High-level expression of EphA2 receptor tyrosine kinase in prostatic intraepithelial neoplasia. Am J Pathol. 2003;163:2271–2276. doi: 10.1016/S0002-9440(10)63584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Cano SJ, Blanes A, Wolfe HJ. PCR techniques for clonality assays. Diagn Mol Pathol. 2001;10:24–33. doi: 10.1097/00019606-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Martignoni G, Eble JN. Carcinoid tumors of the urinary bladder: immunohistochemical study of 2 cases and review of the literature. Arch Pathol Lab Med. 2003;127:e22–e24. doi: 10.5858/2003-127-e22-CTOTU. [DOI] [PubMed] [Google Scholar]
- Cheng L, Leibovich BC, Cheville JC, Ramnani DM, Sebo TJ, Neumann RM, Nascimento AG, Zincke H, Bostwick DG. Paraganglioma of the urinary bladder: can biologic potential be predicted? Cancer. 2000;88:844–852. doi: 10.1002/(sici)1097-0142(20000215)88:4<844::aid-cncr15>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Cheng L, Scheithauer BW, Leibovich BC, Ramnani DM, Cheville JC, Bostwick DG. Neurofibroma of the urinary bladder. Cancer. 1999;86:505–513. [PubMed] [Google Scholar]