Abstract
During the process of malignant transformation, nascent melanoma cells escape keratinocyte control through down-regulation of E-cadherin and instead communicate among themselves and with fibroblasts via N-cadherin-based cell-cell contacts. The zonula occludens (ZO) protein-1 is a membrane-associated component of both the tight and adherens junctions found at sites of cell-cell contact. In most cancers, levels of ZO-1 are typically down-regulated, leading to increased motility. Here we report the novel observation that ZO-1 expression is up-regulated in melanoma cells and is located at adherens junctions between melanoma cells and fibroblasts. Immunofluorescence and co-immunoprecipitation studies showed co-localization of ZO-1 with N-cadherin. Down-regulation of ZO-1 in melanoma cells through RNA interference produced marked changes in cell morphology—leading to a less-dendritic, more rounded phenotype. Consistent with a role in N-cadherin-based adhesion, RNAi-treated melanoma cells were less adherent and invasive when grown in a collagen gel. These data provide the first evidence that increased ZO-1 expression in melanoma contributes to the oncogenic behavior of this tumor and further illustrate that protein products of genes, such as ZO-1, can function in either a pro- or anti-oncogenic manner when expressed in different cellular contexts.
Under normal homeostasis, melanocyte growth is regulated by the surrounding keratinocytes through a variety of paracrine growth factors and cell-cell adhesion molecules.1–4 During oncogenesis, this keratinocyte control is lost after down-regulation of E-cadherin.5 Loss of E-cadherin is typically accompanied by increased N-cadherin expression, the so-called cadherin-switch, that enables melanoma cells to communicate among themselves and with the surrounding dermal fibroblasts.4,6,7 The switching of E-cadherin to N-cadherin most likely provides the early tumor with important motility and survival advantages.6,8
Cadherins are part of larger protein complexes that link cell adhesion to control of cell morphology, motility, and intracellular signaling events.9 Direct roles for cadherin-based adhesion in signaling have been demonstrated by the activation of the small GTPases Rho, Rac, and Cdc4210–12 and PI3-kinase/Akt8 after homotypic cadherin adhesion. Some of the best known binding partners of the cadherins are the catenin family of proteins, which include α-catenin, β-catenin, and plakoglobin.13 The catenins bind to the cytoplasmic tail of the cadherins and link to the actin cytoskeleton.14
Another protein that associates with the cadherin/catenin complex is ZO-1, which is a member of the membrane-associated guanylate kinase homolog family (MAGUKS).15 ZO-1 exists in at least two alternately spliced isotypes, ZO-1α+ and ZO-1α−, which confer differences in junctional dynamics.16–18 Although ZO-1 associates with claudins and occludins, and was first described as a component of epithelial tight junctions,19,20 it has also been identified in nonepithelial cells, such as fibroblasts, where it instead associates with adherens junctions.21–23 In these cadherin-based adherens junctions, ZO-1 functions as a cross-linker between α-catenin and the actin cytoskeleton.21 There is also evidence that ZO-1 is involved in the regulation of cell-cell contacts through direct interaction with the Ras target AF-6.24
The multiple PDZ domains of ZO-1 enables it to form protein-protein complexes with other molecules involved in cell-cell communication, such as the gap junction-forming connexins.25–27 Recent work has shown that ZO-1 can migrate between the cell membrane and the nucleus and may have a direct role in cell signaling. At least part of ZO-1’s putative signaling function is mediated via a SH3 domain-binding serine protein kinase, ZAK,28 and a novel SH3 domain-interacting Y-box transcription factor, ZONAB.29,30 The ZO-1/ZONAB interaction appears to be critical for controlling cell density in MDCK cell epithelial sheets through regulation of CDK4 localization.30
Although the role of ZO-1 in cancer has been little studied, its structural similarity to the Drosophila tumor suppressor gene, Dlg may suggest some anti-cancer role. In agreement with this idea, it has been demonstrated that ZO-1 expression is reduced or lost (along with E-cadherin) in up to 69% of breast carcinomas.31 Here we demonstrate for the first time that expression of ZO-1 is up-regulated in both melanoma cell lines and melanoma samples, where it functionally associates with N-cadherin. RNAi studies reveal that the strength of cell-cell adhesion is reduced when ZO-1 is knocked down. ZO-1 loss is associated with altered cytoskeletal organization, lack of long pseudopodia, and reduced invasion into collagen gels. It therefore seems that after cadherin switching, recruitment of increased levels of ZO-1 to N-cadherin-based junctions may lead to greater invasion.
Materials and Methods
Unless otherwise stated all reagents were purchased from Sigma-Aldrich (St. Louis, MO). Rabbit polyclonal anti-ZO-1, occludin, and claudin-7 antibodies were from Zymed (San Francisco, CA). Mouse anti-N-cadherin antibodies were from either BD Pharmingen (San Diego, CA) or from DAKO Cytomation (clone M3613; Glostrup, Denmark). Mouse anti-E-cadherin, mouse anti-ZO-1, anti-α-catenin, anti-β-catenin, anti-γ-catenin (plakoglobin), and anti-phospho p120catenin monoclonal antibodies were from BD Pharmingen. Anti-GAPDH antibody was from Abcam (Cambridge, UK). Texas Red phalloidin, Alexa 488 anti-rabbit and anti-mouse secondary antibodies, and Alexa 594 anti-mouse and anti-rabbit secondary antibodies were from Molecular Probes (Eugene, OR). Texas Red-conjugated anti-mouse secondary antibody was from Vector Laboratories (Burlingame, CA). Isotype controls were purchased from DAKO Cytomation. Normal human skin was obtained during the routine clinical removal of tumors and epidermal cysts; the samples were localized at least 2 cm from the respective lesions. Their usage was approved by the medical ethics committee of Hamburg (no. 060900). Samples of nevi and melanoma were also obtained from the clinical removal of the tumor. In agreement with the medical ethics committee in Hamburg (no. OB-008/04) the samples were used after diagnostic procedures had been completed. All patients gave their informed consent. In all cases, unless otherwise stated, data shown is representative of at least three independent experiments.
Cell Culture
Human melanoma cells were isolated and cultured as described.32 After establishment of continuous growth, cells were maintained in 2% melanoma media, a 4:1 mixture of MCDB153 and L15, supplemented with 2 mmol/L Ca2+, heat-inactivated fetal bovine serum (2%), and insulin (5 μg/ml) in a 37°C, 5% CO2 atmosphere at constant humidity. Primary human dermal fibroblasts were initiated as explant cultures from trypsin-treated and epidermis-stripped neonatal foreskin and maintained in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum. Human melanocytes were isolated from foreskin and maintained in MCDB153 medium supplemented with 2% fetal bovine serum, endothelin-3, basic fibroblast growth factor, and stem cell factor. Primary human skin keratinocytes were routinely cultured in EpiLIFE media (Cascade Biologicals, Portland, OR), containing growth factors.
RNA Extraction and Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR)
Total RNA was extracted from plates of 80% confluent cells using the RNeasy kit from Qiagen (Stanford, CA). The RNA samples were DNase-treated using a commercial on-column DNase treatment kit (Qiagen). Two μg of total RNA was used to generate cDNA by reverse-transcribing for 1 hour at 43°C using SuperScript II (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. PCR was conducted using 1/20th of the cDNA reaction as template using Taq polymerase (Promega, Madison, WI) consisting of 26 cycles of denaturing at 94°C (30 seconds), annealing at 52°C (30 seconds), and extension at 72°C (30 seconds), and with a final extension at 72°C (5 minutes). The constitutively expressed housekeeping gene, GAPDH, (sense: CCA CCC ATG GCA AAT TCC ATG GCA; anti-sense: TCT AGA CGG CAG GTC AGG TCC ACC) was used as a positive control and with total RNA as a negative control. ZO-1 primers (sense: GCA GCC ACA ACC AAT TCA TAG; anti-sense: GCA GAC GAT GTT CAT AGT TTC G) were designed encompassing the splice variant giving a PCR product size of 529 bp for variant one (α+), 290 bp for variant two (α−). N-cadherin primers (sense: CAT CCC TCC AAT CAA CTT GC; anti-sense: ATG TGC CCT CAA ATG AAA CC) and E-cadherin primers (sense: GCA GGA TTG CAA ATT CCT GCC; and anti-sense: CCT TCA TAG TCA AAC ACG AGC) were used as described above. Ten μL of PCR products were separated on 2.0% agarose gels with 0.1% ethidium bromide.
Confocal/Immunofluorescence Microscopy
Melanoma cells were seeded onto glass coverslips in six-well plates and incubated overnight. Cells were then fixed in 4% formaldehyde solution (Electron Microscopy Systems, Hatfield, PA) and permeabilized with Triton X-100 (0.2% v/v) before being blocked in phosphate-buffered saline (PBS) containing 1% bovine serum albumin. Primary antibody incubations (range, 1:50 to 1:200) were performed at 37°C in a humidified atmosphere for 1 hour. Coverslips were then washed three times in PBS, before being incubated with secondary antibodies for 1 hour under similar conditions to the primary antibody (dilution factor of 1:250). Coverslips were then further washed in PBS and sterile water before being treated with VectorShield anti-fade (Vector Laboratories) and analyzed using either immunofluorescence or confocal microscopy.
Immunofluorescence on Frozen Sections of Human Skin and Malignant Melanoma
Five-μm-thick cryostat sections of frozen tissues were fixed in −20°C acetone for 10 minutes. They were blocked for unspecific binding sites with 2% normal goat serum and 0.1% Triton X-100 in PBS for 15 minutes at room temperature. Primary antibodies were applied for 30 minutes at room temperature (ZO-1, 1:1500 in PBS; N-cadherin, 1:20 in PBS), followed by washing the samples three times for 10 minutes. Afterward secondary antibodies were applied for 30 minutes at room temperature (Alexa 488 F(ab′)2-anti-rabbit, 1:600 in PBS; Alexa 594 (F(ab′)2-anti-mouse, 1:1250), followed by another washing step. For negative controls we used the appropriate matched isotype controls. Finally the slides were washed twice with ddH2O, and coverslips mounted with Fluoromount (Southern Biotechnology Associates, Inc., Birmingham, AL). An Axiophot II microscope (Carl Zeiss, Jena/Oberkochen, Germany), a charge-coupled device camera (Hamamatsu Photonics, Hamamatsu City, Japan), and Openlab 2.0.4 software (Improvision, Coventry, UK) were used to visualize and evaluate the stained sections.
Western Blot Analysis
Cells were solubilized in 200 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer (4% sodium dodecyl sulfate, 60 mmol/L Tris, pH 6.8, 5% glycerol, 0.01% bromophenol blue, and 50 mmol/L mercaptoethanol), and heated to 95°C for 5 minutes. Whole cell protein extracts (20 to 40 μg) were separated on 6 to 15% sodium dodecyl sulfate-polyacrylamide gels. Proteins were electrophoretically transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA) for 1 hour, washed in Tris-buffered saline (TBS; 100 mmol/L Tris-HCl, pH 7.5, and 150 mmol/L NaCl), before being blocked for 1 hour in TBS containing 0.1% Tween-20 and 5% milk (TBST-milk). Primary antibody incubations were performed overnight at 4°C in TBST-milk followed by washing and then a 1-hour incubation with either anti-rabbit or anti-mouse horseradish peroxidase-conjugated secondary antibody diluted in TBST-milk (Amersham Pharmacia, Little Chalfont, UK). Immunocomplexes were visualized using the enhanced chemiluminescence system (Amersham Pharmacia) and detected on photographic film (Kodak, Rochester, NY). After analysis, Western blots were stripped once and reprobed to demonstrate even protein loading.
Immunoprecipitation
For immunoprecipitation, confluent cells were scraped off a confluent 10-cm plate (equivalent to 2 to 3 mg of protein per plate), washed with PBS, and extracted into 1 ml of PBS containing 1% Triton X-100, 1% Nonidet P-40, 1 mmol/L sodium orthovanadate, 1 mmol/L phenylmethyl sulfonyl fluoride, and protease inhibitors (leupeptin, aprotinin, and pepstatin). Immunoprecipitation experiments were performed on 0.5 ml of the protein extract using either anti-ZO-1 antibody or nonimmune mouse IgG at a final concentration of 5 μg per reaction overnight at 4°C with shaking. Protein A Sepharose CL-4B beads (Pharmacia Biotech, Uppsala, Sweden) were added and incubated for another 4 hours. Samples were washed three times with lysis buffer, boiled in Laemmli buffer containing β-mercaptoethanol (10%), and subjected to electrophoresis on an 8% sodium dodecyl sulfate-polyacryl-amide gel. Separated proteins were transferred onto polyvinylidene difluoride membrane and immunoblotted with anti-N-cadherin antibodies and peroxidase-conjugated secondary antibody.
Calcium-Switching Experiments
This assay was performed as described in Li and colleagues6 with modifications. Briefly, cells were cultured overnight in regular 2% melanoma media on glass coverslips. Some of the cells were fixed in 4% paraformaldehyde. Other coverslips were treated with media containing 4 mmol/L EGTA for 15 minutes, before being formaldehyde fixed. The rest of the coverslips were then washed twice in 2% melanoma media to remove the EGTA and then left to re-equilibrate for a further 30 minutes before being fixed. All of the fixed coverslips were then stained with antibodies to both N-cadherin and ZO-1 as described above.
Three-Dimensional Spheroid Growth
Melanoma spheroids were prepared using the liquid overlay method. Briefly, 200 μl of melanoma cells (25,000 cells per ml) were added to a 96-well plate coated in 1.5% agar (Difco, Sparks, MD). Plates were left to incubate for 48 hours, by which time cells had organized into three-dimensional spheroids. Spheroids were implanted into a gel of bovine collagen I containing essential modified Eagle’s medium, l-glutamine, and 2% fetal bovine serum. Normal 2% melanoma media was overlaid on top of the collagen and the spheroids were left to grow for 96 hours. After this time, pictures of the invading spheroids were taken using a Nikon-300 inverted microscope (Nikon, Melville, NY).
Lentiviral Vector Construction
Our RNA interference (RNAi) lentiviral system is a series of four plasmids. The siRNA sequences were selected from the open reading frame region of the ZO-1 cDNA sequence, 100 nucleotides downstream from the start codon. The 959-bp subsequence comprising nucleotides 4448 to 5407 of NM_003257 was chosen based on the susceptibility of this region for hybridization by anti-sense oligonucleotides. Several 19- to 21-bp sequences beginning with AAG, preferably ending with C, and containing greater than 30% GC content from the accessible region were selected as possible target sequences. BLAST searches were performed to ensure low sequence homology to other human genes. The RNA-induced silencing complex preferentially accepts the strand of the siRNA that presents the less stable 5′ ends. Therefore, we introduced a thermodynamically unstable 5′ end wobble to the anti-sense strand by changing the aforementioned end C to a T to increase the incorporation of the anti-sense strand of siRNA to the RNA-induced silencing complex. When possible, an internal C to T wobble was also incorporated into the sense strand of siRNA to further increase the incorporation of the anti-sense strand of siRNA to the RNA-induced silencing complex. Sequences were also generated to include the restriction enzyme overhang (BamH1-XhoI) and a loop region according to the template [5′ template: GAT CCC N19–21(SENSE) TTC AAG AGA N19–21 (ANTISENSE) TTT TTC; 3′ template: TCG AGA AAA A N19–21(SENSE) TCT CTT GAA N19–21 (ANTISENSE) GGG]. The selected sequences were inserted into the sites (BamH1-XhoI) immediately downstream of the H1 promoter in H1UG1 (kindly provided by Xiao-Feng Qin, M.D. Anderson, Houston, TX) to drive the expression of the shRNA. This vector has been designed to contain an enhanced GFP gene, whose expression is independently driven by the UbiC promoter, as a marker. The lentivirus was produced by co-transfection of human embryonic kidney 293T cells with four plasmids, including a packaging defective helper construct (pMDLg/pRRE), a Rev plasmid (RSV-Rev), a plasmid coding for a heterologous (pCMV-VSV-G) envelope protein, and the H1UG-1 vector construct harboring a selected siRNA sequence. Of the three sequences tested, one sequence (5′ GGT GAA ACA CTG TTG AGT CC) decreased protein level greater than 90%. A mutated control RNAi sequence was generated to that which gave >90% knockdown, by mutating the center three nucleotides, to give the sequence (GGT GAA ACC AGG TTG AGT CC). Transduced target cells were assessed by their level of GFP expression and gene knockdown was seen 48 hours after infection.
Results
ZO-1 Is Expressed at the RNA and Protein Levels in Melanoma Cells, Keratinocytes, and Fibroblasts
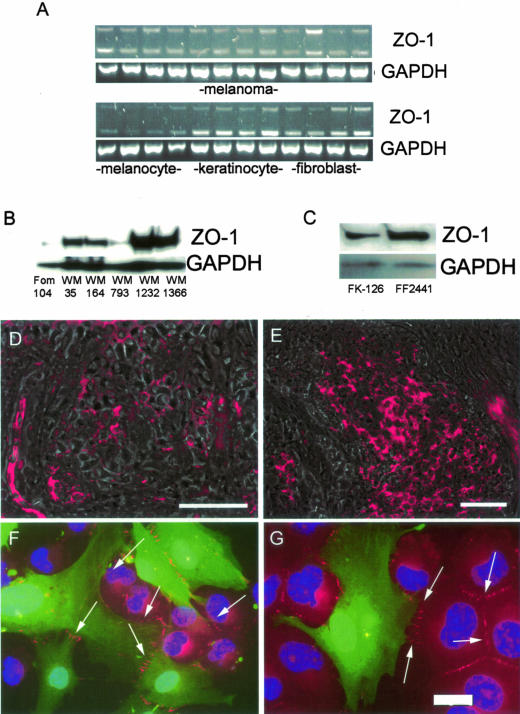
ZO-1 has been identified as a component of both tight and adherens junctions in both epithelial and nonepithelial cells. Little is known about the expression of ZO-1 in melanocytic cells. To determine whether ZO-1 is expressed in human skin cells, RNA was harvested from 35 human melanoma cell lines of various stages, primary human melanocytes, keratinocytes, and dermal fibroblasts. RT-PCR analysis revealed significant ZO-1 RNA levels in 29 of 35 melanoma cell lines, all of the keratinocytes, and all of the dermal fibroblasts (representative data shown in Figure 1A). The top panel of the RT-PCR data in Figure 1A, shows 12 melanoma cell lines: WM35, WM39, WM278, 451Lu, WM9, WM164, WM239B, WM266-2, WM793, WM852, WM902, and WM266-4. There was little ZO-1 RNA in any of the four primary human melanocytes examined (Figure 1A). Most of the melanoma cell lines and the dermal fibroblasts expressed both of the α+ and α− splice variants of ZO-1. In contrast, the keratinocytes appeared to express more of the α− variant of ZO-1. Western blotting experiments revealed increased expression of ZO-1 protein in melanoma cells compared to FOM 104 melanocytes (Figure 1B). ZO-1 expression was found to be up-regulated in all of the melanoma cell lines examined (Figure 1B). Levels of ZO-1 were also high in both primary human keratinocytes and dermal fibroblasts (Figure 1C). The up-regulation of ZO-1 in human melanocytic nevi (n = 4; Figure 1D) and melanoma (n = 12; Figure 1E) was demonstrated by immunostaining frozen sections. In all cases, the lesions were diagnosed by a dermatopathologist before inclusion in this study. One of the nevi was negative, three were weakly positive for ZO-1 (Figure 1D). One of the melanomas was negative, two were weakly positive, and nine were strongly positive for ZO-1 (Figures 1E and 2D). Of the melanomas studied, four were of radial growth phase (RGP), seven were vertical growth phase (VGP), and one was metastatic. There was no clear correlation between level of ZO-1 expression and tumor stage.
Figure 1.
ZO-1 expression in skin cells and melanoma. A: RT-PCR analysis of ZO-1 RNA in melanoma cells, melanocytes, skin keratinocytes, and skin fibroblasts. The upper band shows the α+ splice variant of ZO-1 and the lower band shows the α− splice variant. Equal RNA loading was confirmed by performing RT-PCR to GAPDH. B: Western blot analysis of ZO-1 protein in melanocytes (FOM 104) and melanoma (WM35-WM1366). Even protein loading was confirmed by reprobing the membrane with antibody to GAPDH. C: Western blot showing expression of ZO-1 in keratinocytes (FK126) and fibroblasts (FF2441). D and E: Immunofluorescence microscopy (overlay of epifluorescence and corresponding phase contrast) of ZO-1 in a junctional nevus (D) and a VGP melanoma (E). F and G: Immunofluorescence microscopy of ZO-1 staining (red, indicated with arrows) between melanoma cells (red) and GFP-tagged human dermal fibroblasts (green). Scale bars: 50 μm (D, E); 20 μm (G).
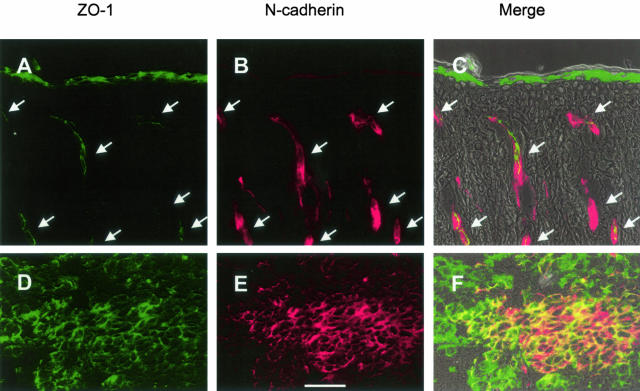
Figure 2.
Expression of ZO-1 in human skin and metastatic melanoma. A–C: Localization of ZO-1 and N-cadherin in human scalp skin. D–F: Localization of ZO-1 and N-cadherin in melanoma metastasis. Immunofluorescence microscopy of ZO-1 is shown in green (A, C, D, F). Expression of N-cadherin is shown in red (B, C, E, F). The corresponding overlay of epifluorescence and phase contrast is shown in C and F. The arrows shown in A to C denote blood vessels. Scale bar, 50 μm.
During the oncogenic process, melanoma cells acquire the ability to communicate with fibroblasts.6 Staining of mixed fibroblast-melanoma cultures showed the recruitment of ZO-1 to adhesions between fibroblast-fibroblast, melanoma-melanoma, and fibroblast-melanoma (Figure 1, F and G). In contrast, melanocytes expressed no ZO-1 at their cell-cell contacts (data not shown). To determine whether ZO-1-based contacts would be formed in co-culture, melanocytes and keratinocytes were grown in mixed culture for 48 hours. In these experiments, it was shown that ZO-1 was only found on contacts between keratinocytes and keratinocytes and not between melanocytes and keratinocytes (data not shown).
There was co-localization between N-cadherin and ZO-1 in the endothelia of blood vessels in human scalp skin (Figure 2; A to C). In agreement with published work, there was also expression of ZO-1 in the stratum granulosum, the transition layer of the epidermis, and, in a heterogeneous and weaker manner, in the upper layers of the stratum spinosum.33,34 ZO-1 was found to be highly expressed in the metastatic melanoma (Figure 2; D to F), and there was strong co-localization between ZO-1 and N-cadherin throughout the tumor.
ZO-1 Associates with N-Cadherin-Based Adherens Junctions in Melanoma
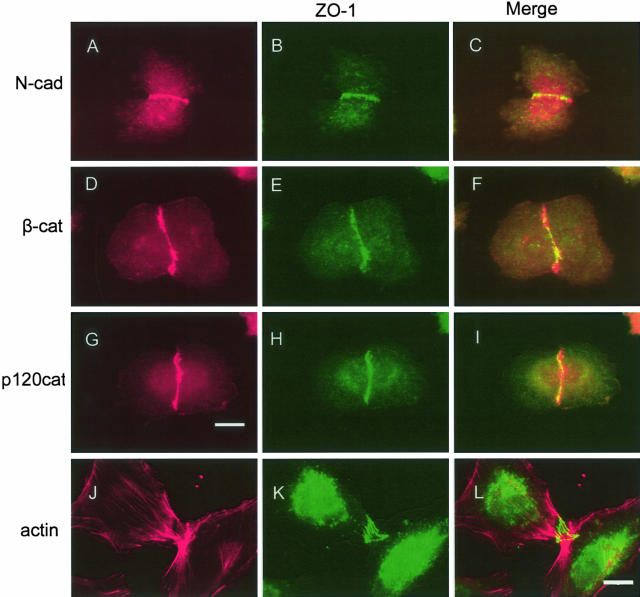
As tumor cells are not thought to form tight junctions, we investigated whether ZO-1 was associated with the adherens junctions. In both the WM35 (RGP) and WM1366 (VGP) melanoma cell lines, ZO-1 was found to co-localize with N-cadherin, β-catenin and phospho-p120 catenin (data shown for WM1366: Figure 3, A to I). There was also evidence that some ZO-1 was also found in the cytoplasm, perinuclear areas and the nucleus, as well as some cytoplasmic localization of N-cadherin. There was no co-localization between ZO-1 and actin stress fibers in either WM1232 or WM1366 melanoma cells (data shown for WM1232: Figure 3, J to L).
Figure 3.
Co-localization of ZO-1 with adherens junction proteins in human melanoma cells. Cells were stained in red with antibodies to N-cadherin (N-Cad; A, C), β-catenin (β-cat; D, F), p120catenin (p120cat; G, I), or actin (J, L). B, C, E, F, H, I, K, L: The same cells were also co-stained in green with antibodies to ZO-1. Scale bars: 20 μm (A–I); 4 μm (J–L).
Western blotting showed that three melanoma cell lines of different stages, WM35 (RGP), WM1366 (VGP) and 1205Lu (metastatic) expressed α-, β-, and p120 catenin (Figure 4). None of the melanoma cell lines tested expressed plakoglobin (γ-catenin). Surprisingly, one of the melanoma cell lines (WM1366) with an epithelial morphology and phenotype, was found to express occludin. Human primary keratinocytes expressed all four of the catenins, occludin and claudin-7. Fibroblasts, like the melanoma, also lacked expression of the tight junction proteins occludin and claudin-7, as well as plakoglobin.
Figure 4.
Expression of adherens and tight-junction proteins in melanoma, keratinocytes, and fibroblasts. Western blot analysis of protein extracts from melanoma cells (WM35, WM1366, and 1205Lu), keratinocytes (FK126), and fibroblasts (FF2441). Blots were probed with antibodies to α-catenin, β-catenin, plakoglobin (γ-catenin), p120catenin, occludin, claudin-7, and GAPDH. Experiments were performed at least three times and the figure shows representative blots. In each case, blots were stripped and reprobed for GAPDH to demonstrate equal protein loading.
N-Cadherin-Based Cell-Cell Adhesions Are Required for ZO-1 Recruitment
To investigate the role of N-cadherin in ZO-1 recruitment to the cell membrane in melanoma cells, calcium switching experiments were performed. Under normal calcium conditions, both N-cadherin and ZO-1 are recruited to cell-cell adhesions (Figure 5; A to C). Switching to a calcium-free media led to disruption of both N-cadherin and ZO-1 and loss of cell-cell contacts (Figure 5; D to F). Restoration of calcium resulted in the renewed association of both N-cadherin and ZO-1 with cell-cell adhesions (Figure 5; G to I). Immunoprecipitation of melanoma cell extracts using a ZO-1-specific antibody, pulled down N-cadherin, demonstrating physical interactions between the two proteins (Figure 5J). Control experiments, using similar concentrations of a matched IgG did not result in pull-down of N-cadherin (Figure 5J).
Figure 5.
Localization of both N-cadherin and ZO-1 to cell-cell adhesions requires the presence of calcium. A and C: WM1366 cells grown in 2% melanoma media were stained in red with antibodies to N-cadherin. B and C: Cells were also co-stained in green with antibodies to ZO-1. After a 15-minute treatment with calcium-free EDTA containing melanoma media both N-cadherin (D, F) and ZO-1 (E, F) disappeared from the points of cell-cell adhesion. After being returned to normal, calcium-containing media for 30 minutes both N-cadherin (G, I) and ZO-1 (H, I) returned to the points of cell-cell adhesion. J: Physical interaction between ZO-1 and N-cadherin, as demonstrated by co-immunoprecipitation. Whole cell melanoma protein extracts were subject to pull down using antibodies to either ZO-1 (ZO-1) or matched IgG (control), subsequent blots were then probed for N-cadherin. Scale bar, 20 μm.
In Melanoma Cells that Express E- and N-Cadherin, ZO-1 Only Associates with N-Cadherin at Cell-Cell Contacts
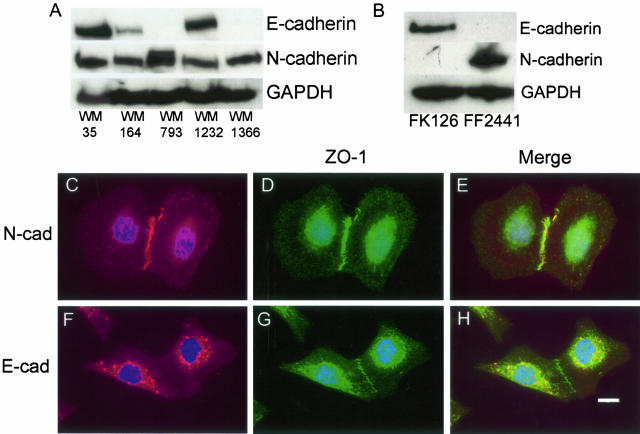
RT-PCR studies on 35 melanoma cell lines, revealed a number that expressed both E- and N-cadherin at the RNA level (data not shown). Most of the lines that expressed E-cadherin at the RNA level were from the RGP and early VGP. From these, a number of melanomas that expressed E-cadherin at the RNA level were tested for E-cadherin protein expression. Three out of the selected five melanoma cell lines, WM35, WM164, and WM1232 were found to express both E- and N-cadherin (Figure 6A). In contrast, it was demonstrated that keratinocytes expressed only E-cadherin and dermal fibroblasts expressed only N-cadherin (Figure 6B). To determine whether the E-cadherin was functionally active in the melanoma cell lines, and whether it associated with ZO-1, co-localization experiments were performed. It was found in the WM35 (Figure 6; C to E) and WM1232 cell lines that only N-cadherin was located at cell-cell adhesions, and that this co-localized with ZO-1. The expressed E-cadherin was primarily restricted to cytoplasmic pools and did not localize with ZO-1 at cell-cell adhesions (Figure 6; F to H). However, there was evidence of co-localization of E-cadherin and ZO-1 in the cytoplasm, although it is not known whether these two proteins interact in a functional manner.
Figure 6.
Expression and co-localization of E- and N-cadherin with ZO-1 in melanoma cell lines. A: Western blots showing the expression of both E- and N-cadherin in selected melanoma cells. B: Western blot showing the expression of E- and N-cadherin in skin keratinocytes (FK126) and dermal fibroblasts (FF2441). Blots are representative of three independent experiments. In each case, blots were stripped and reprobed with anti-GAPDH to confirm equal protein loading. Co-localization of N-cadherin (C, E), with ZO-1 in WM1232 melanoma cells (D, E). Co-localization of E-cadherin (F, H) with ZO-1 in WM1232 melanoma cells (G, H). Scale bar, 20 μm.
Specific RNAi Knockdown Leads to Loss of ZO-1 at the RNA and Protein Level
Endogenous ZO-1 was knocked-down in WM35 melanoma cells using a lentiviral RNAi expression system. Incorporation of the lentivirus into the WM35 cells was confirmed by expression of GFP in-infected cells. Typical infection rates were more than 95%, with uniform levels of GFP detected throughout the cell population. Lentiviruses expressing three RNAi sequences were screened, and one (Z11) was found to give good levels of ZO-1 knockdown (>90%). Control cells were transfected with the empty H1UG1 GFP-viral construct (H1UG1) or a three-point mutation of the Z11 RNAi sequence called N3. Extraction of RNA from H1UG1- and Z11-infected WM35 cells, followed by RT-PCR showed a good suppression of ZO-1 (Figure 7A). Western blotting (Figure 7, B and C) and immunofluorescence (Figure 7; D to F) showed loss of ZO-1 both at the protein level and from the cell-cell junctions and the cytoplasm of Z11-infected cells, but not H1UG1- or N3-treated cells.
Figure 7.
Characterization of a RNAi to knockdown ZO-1. A: RT-PCR showing knockdown of ZO-1 RNA in WM35 melanoma cells treated with lentivirus encoding Z11 clone RNAi, RT-PCRs for GAPDH were included as a control. B: Western blot showing knockdown of ZO-1 protein in Z11-infected WM35 cells, note the lack of knockdown in closely related proteins E- and N-cadherin and GAPDH. C: Reversal of the RNAi effect of the Z11 clone lentivirus through introduction of 3-point mutations into the sequence (N3). D and E: Immunofluorescence microscopic pictures showing expression of ZO-1 at cell-cell adhesions (indicated with arrows) in H1UG1 vector control cells and N3 mutant cells. F: Loss of ZO-1 expression at cell-cell adhesions in cells treated with the lentivirus for Z11 clone RNAi. Scale bar, 20 μm.
Down-Regulation of ZO-1 Using RNAi Leads to a Rounded Morphology
Treatment with the Z11 lentiviral clone led to marked changes in the morphology of the WM35 cells (Figure 8; D to F). The cells were less dendritic, and lacked the actin stress fibers of the H1UG1 (Figure 8; A to C) and N3 (data not shown) control cells. In each case, the level of lentiviral infection was confirmed by the GFP expression (Figure 8, C and F). Confocal microscopy confirmed these morphological changes, and also indicated from the z-series that the RNAi-treated cells were taller than control WM35 cells (average, 15 μm versus 5 μm) (Figure 8; G to J).
Figure 8.
Knockdown of ZO-1, using RNAi, alters the morphology of WM35 cells. Cells infected with lentivirus for the H1UG1 vector control sequence exhibit a normal dendritic morphology when stained for cytoskeletal actin (A) or DIC (B). C: GFP staining demonstrates viral infection. D and E: In contrast to controls, Z11-infected cells are more rounded. F: GFP image demonstrates viral infection. G and I: Confocal microscopic pictures show the spreading, dendritic appearance of H1UG1 vector control cells that is lacking in the Z11 RNAi-infected cells (H, J). Scale bar, 20 μm.
Knockdown of ZO-1, Using RNAi, Does Not Alter Localization of E- or N-Cadherin but Reduces Both Adhesion Strength and Collagen Invasion
Immunostaining of Z11-infected cells for E- and N-cadherin did not reveal any changes in the localization of these proteins relative to the N3 sequence control (Figure 9; A to D). Levels of E- and N-cadherin protein were unaffected by Z11 RNAi treatment as demonstrated by Western blotting (Figure 7B). Previous reports have suggested that ZO-1 loss in breast carcinoma is linked to reduced cell-cell adhesion.31 Cell aggregation assays were performed in which cells were plated onto nonadherent agar-coated plates and left to grow as spheroids for 72 hours (Figure 9, E and F). After this time, the spheroids were subjected to agitation, in the form of gentle pipetting. It was noted that the Z11 spheroids (Figure 9F) were more fragile and less adhesive than the N3 (Figure 9E) and H1UG1 (not shown) controls and broke-up into several pieces.
Figure 9.
Knockdown of ZO-1 in melanoma cells fails to alter localization of E- or N-cadherin but reduces melanoma invasion and strength of cell-cell adhesion. A and B: Immunofluorescence images showing similar localization of E-cadherin in both control H1UG1 (not shown), N3 and Z11lentivirus-infected cells. C and D: The localization of N-cadherin is also similar in H1UG1 (not shown), N3 and Z11 lentivirus-infected WM35 cells (indicated by arrows). E: Gentle agitation of WM35 spheroids has no effect on N3-infected WM35 cells. F: However, Z11-infected WM35 spheroids are more easily disrupted. G: Preformed H1UG1 (not shown) and N3-infected WM35 control spheroids invade into the surrounding collagen after 96 hours. H: In contrast Z11-infected WM35 cell spheroids exhibit much reduced invasion into the collagen matrix. Scale bar, 100 μm.
To investigate whether increased ZO-1 expression was important for invasion we implanted preformed 72-hour-old WM35 spheroids, infected with either the Z11 or N3 lentivirus into a collagen I gel. After 96 hours of growth in collagen there was marked invasion of the N3 (Figure 9G) and H1UG1 (not shown) control spheroids into the collagen gel, whereas there was significantly less invasion of the Z11 RNAi-treated cells (Figure 9H).
Discussion
The involvement of junction proteins in the pathogenesis of malignant melanoma has been little investigated. Here, we report for the first time, that the ZO-1 is up-regulated in melanoma, where it co-localizes to N-cadherin-based junctions. RT-PCR analysis revealed an up-regulation in ZO-1 levels in 83% of the melanoma cell lines examined. ZO-1 exists in two forms, termed α+ and α−, which are the result of alternative RNA splicing.16 The two isoforms are so-called as they differ by an internal 80-amino acid domain α, which is present in the α+ and absent in the α− isoforms.17 The pattern of expression of the two isoforms was heterogeneous across the melanoma lines, with some such as WM3248 expressing more of the α+ isoforms, and others such as WM3438 expressing predominantly the α− isoform. Differential expression analysis has demonstrated that the ZO-1 α− isoform is restricted to specialized junctions in endothelial cells and Sertoli cells, whereas ZO-1 α+ is located in epithelial cells.17 There is some correlation between expression of the two ZO-1 isoforms and junction turnover, with ZO-1 α− being expressed in more dynamic junctions, and the α+ isoform being located at less dynamic junctions.17 In contrast to melanoma cells, melanocytes tested express very little ZO-1 RNA of either isoform. Keratinocytes express higher levels of α− than α+, and dermal fibroblasts have equal RNA levels of both isoforms. The expression of ZO-1 at the protein level was similar to that of RNA, with melanocytes expressing very low levels, and keratinocytes, fibroblasts, and melanoma expressing high levels. These findings are in agreement with a number of previous studies that have demonstrated the expression of ZO-1 in epidermal keratinocytes, where it associates with occludin and claudins.33–35
Immunostaining experiments show that ZO-1 is highly expressed throughout samples of human melanoma (RGP, VGP, and metastatic) as well as nevi. The up-regulation of ZO-1 in nevi suggests that this is an early event in transformation that persists throughout tumor progression. The early appearance of progression markers in nevi is well known. In particular the high incidence of BRAF V600E mutations in benign nevi is thought to contribute to the later oncogenic behavior of VGP and metastatic melanomas.36
ZO-1 is also found in normal human skin where it is expressed primarily in the stratum granulosum, the transitional layer of the epidermis, and weakly in the upper layers of the stratum spinosum as well as the endothelial cells of blood vessels of the dermis. In the stratum granulosum and in the endothelia, the ZO-1 is likely involved in the formation of tight junctions.33–35,37 This is in contrast to malignant melanoma, in which ZO-1 is found to co-localize and interact with N-cadherin and is involved in the formation of adherens junctions.
The increased expression of ZO-1 in melanoma is somewhat surprising because studies have demonstrated that ZO-1 expression is typically lost in gastrointestinal adenocarcinoma,38 breast cancer,31 and colorectal carcinoma.39 In breast carcinoma, loss of ZO-1 is closely paralleled by loss of E-cadherin and may therefore be linked to reduced cell-cell adhesion and tumor progression.31 However, the comparison of breast carcinoma to melanoma can be often misleading, particularly with regard to adhesion proteins. As one example, the adhesion molecule Mel-CAM (CD146, MUC18), is up-regulated in melanoma and is implicated in disease progression, whereas in breast carcinoma this molecule has tumor suppressor properties.40 In melanoma cells, ZO-1 associates with cell-cell contacts between melanoma-melanoma cells, melanoma-fibroblasts, and fibroblasts-fibroblasts. Co-localization experiments revealed that ZO-1 localizes with N-cadherin, β-catenin, and p120catenin, strongly suggesting that ZO-1 is being recruited to N-cadherin-based adherens junctions in these cells. Previous studies have shown the localization of ZO-1 with E- and P-cadherin-based adherens junctions in nonpolarized fibroblasts.41 Western blotting studies demonstrated that the three melanoma cells tested expressed most of the expected adherens junctions proteins, with the exception of plakoglobin (γ-catenin). Plakoglobin loss in cancer, particularly in neuroblastoma, is often associated with an adverse outcome.42 A possible role for plakoglobin loss in the metastatic phenotype is dem-onstrated by studies showing that tumorigenicity is suppressed by transfection of plakoglobin into renal carcinoma cells lacking catenin expression.43
The Src-substrate, p120catenin, is known to play a role in stabilizing cadherins at the cell membrane, through control of their turnover.44–47 p120catenin exists in multiple isoforms, which derive from the same gene through alternative splicing and multiple translational initiation codons. There were significant differences in the expression patterns of the different isoforms of p120catenin between melanoma cells, keratinocytes, and fibroblasts, possibly a reflection of the different strengths of junctions between these cell types. Previous studies have reported that melanocytes and melanoma express the long isoform 1A, whereas keratinocytes express the shorter isoforms of p120, such as 3A.48 In this study we used the antibody to phospho-p120 catenin, which recognizes all four isoforms of p120catenin, when phosphorylated at tyrosine 228 by Src.49
To investigate whether the increased ZO-1 expressed was associated with tight junctions, melanoma cells were probed for the expression of occludin and claudin-7 by Western blotting and immunofluorescence. Claudin-7 was selected because it has been previously identified throughout the human epidermis.50 In addition, our own microarray studies have suggested that this particular claudin was expressed in skin keratinocytes (data not shown). Only one of the three melanoma cell lines tested expressed occludin. None of the melanoma cell lines tested expressed any claudin-7. The expression of occludin in the WM1366 cell line was unexpected, but consistent with the epithelial phenotype and cobblestone morphology of this cell line. To our knowledge this is the first ever report of a tight junction molecule being expressed in human melanoma, and as yet its functional consequences are unknown.
Co-staining of the actin cytoskeleton with ZO-1 did not reveal any co-localization in melanoma cells, and rather the ZO-1 was located toward the ends of the actin stress fibers at the sites of cell-cell contact. However, in tight junction-expressing epithelial cells, ZO-1 is known to simultaneously bind claudin through its first PDZ domain and F-actin through its C-terminal tail.21,50–53 ZO-1 is also able to bind directly to the actin cytoskeleton, and may be a functional linker between the cadherin-based adhesion system and the actin cytoskeleton.21,52 In nonepithelial cells, ZO-1 also associates with actin-binding α-catenin, suggesting that the association of ZO-1 with the actin cytoskeleton can be both indirect and direct.21,54
Calcium-switching experiments demonstrated that both N-cadherin and ZO-1 are recruited to cell-cell adhesions under high calcium conditions. More evidence of a direct interaction between N-cadherin and ZO-1 was demonstrated by co-immunoprecipitation experiments, in which the anti-ZO-1 antibody pulled down N-cadherin from melanoma cell extracts.
Although all melanoma cells, irrespective of their stage, were found to express N-cadherin, some cell lines still expressed E-cadherin. RT-PCR studies demonstrated that a number of melanoma cell lines expressed some E-cadherin at the RNA level. A preselected sample of these melanoma cell lines, were also found to express E-cadherin at the protein level. There was also evidence of cytoplasmic co-localization between E-cadherin and ZO-1. However, it appeared that this E-cadherin was nonfunctional; immunofluorescence studies revealed a cytoplasmic localization of this protein. Indeed, only the N-cadherin was found at the sites of cell-cell contact. Co-culture of E-cadherin-expressing melanoma cells with keratinocytes did not result in the formation of E-cadherin/E-cadherin contacts between keratinocytes and melanoma cells, further suggesting that E-cadherin was functionally inactive in these melanoma cells (data not shown). The finding that many of the melanoma cell lines still express E-cadherin, suggests that there may be multiple mechanisms responsible for its functional loss, and that often E-cadherin may be still expressed, albeit in an inactive state. To date, the best characterized mechanism of E-cadherin loss in melanoma is via the up-regulation of Snail family transcription factors that suppress E-cadherin expression.55,56 Another recently identified mechanism of E-cadherin down-regulation is via Hakai, a Cbl-related E3-ubiquitin ligase.57 Hakai binds to tyrosine-phosphorylated E-cadherin and mediates its internalization and destruction.57 Thus it has been proposed that Hakai disrupts E-cadherin-mediated cell-cell adhesion and may contribute to the metastatic phenotype.57
To properly assess the role of ZO-1 in melanoma behavior a number of lentiviral vectors encoding for a short interfering RNA (siRNA) were constructed. Three interfering RNA sequences were designed and tested for knockdown activity. One of the sequences, clone Z11, gave good knockdown of ZO-1 at both the RNA and protein level in WM35 cells. Knockdown of ZO-1 did not affect the localization of either N-cadherin to the cell-cell contacts, or the cytoplasmic localization of E-cadherin in WM35 cells. This suggests that ZO-1 was recruited to N-cadherin-based cell contacts, and not the other way around. It also demonstrated that up-regulated ZO-1 was not involved in the suppression and aberrant localization of E-cadherin in this cell line. Knockdown of ZO-1 appeared to weaken cell-cell adhesion between melanoma cells. Z11 clone-infected WM35 cells were not as strongly adhesive under anchorage-independent spheroid conditions as N3 clone-infected control cells. This finding is in agreement with studies on MCF-7 breast carcinoma cells, where ZO-1 anti-sense was found to reduce cell-cell adhesion, resulting in the formation of smaller spheroids.58 However, it is worth noting that the role of ZO-1 in MCF-7 cells is different to that of WM35 melanoma cells. In the MCF-7 cell line, increased ZO-1 expression was associated with high E-cadherin expression.58 Indeed, the authors go as far to suggest that ZO-1 expression is anti-metastatic in breast cancer.58
The observation that ZO-1 is up-regulated in a high proportion of melanoma lines, including those that are highly metastatic, suggests that ZO-1 plays a pro-oncogenic role in melanoma cell lines. Studies have shown the interaction of ZO-1 with the actin cytoskeleton,21 in agreement with this melanoma cells expressing the Z11 RNAi clone were found to exhibit a different morphology than control cells. Instead of being flattened and dendritic, the Z11-infected cells were found to be smaller and rounder, and lacked the characteristic actin stress fibers. Organization of both tubulin and vimentin were unaffected (data not shown). Because N-cadherin expression is associated with a more motile and invasive phenotype it is likely that increased ZO-1 expression in melanoma is involved in this process. Indeed, we provide evidence that the interaction of N-cadherin with ZO-1 enhances invasion in melanoma cells. WM35 cells infected with the Z11 clone RNAi lentivirus exhibit markedly less invasion into collagen I gels when compared to control WM35 cells infected with the N3 three-point mutated siRNA lentivirus. It is worth noting that the inhibition of RNAi-treated WM35 cell invasion is not total, and there is still a low level of invasion in the RNAi-treated cells. This possibly suggesting that there may be some functional redundancy between cytoskeletal crosslinking proteins and other mechanisms can compensate for loss of ZO-1 after RNAi treatment. It seems counterintuitive to suggest that reduced adhesion can impair invasion, but this is indeed the case. Tumor invasion requires the carefully orchestrated adhesion of the cell to the surrounding matrix to generate traction, inhibiting this adhesion can therefore block cell motility.
Here we have demonstrated for the first time that, unlike other cancers, ZO-1 is up-regulated in human melanoma. It seems likely that the increased expression of this molecule may contribute toward the functional consequences of E- to N-cadherin switching. It is hoped that a greater understanding of the processes of this cadherin switch will lead to improved therapeutic approaches to this currently deadly disease.
Acknowledgments
We thank James Hayden for microscopy support, Ewa Wladykowski for technical assistance, and Xiao-Feng Qin for the modified H1UG1 vector.
Footnotes
Address reprint requests to Meenhard Herlyn, Wistar Institute, 3601 Spruce St., Philadelphia, PA 19104. E-mail: herlynm@wistar.upenn.edu.
Supported by the National Institutes of Health (grants CA 76674, CA 25874, CA 10815, CA 93372) and the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health.
References
- Tang A, Eller MS, Hara M, Yaar M, Hirohashi S, Gilchrest BA. E-cadherin is the major mediator of human melanocyte adhesion to keratinocytes in vitro. J Cell Sci. 1994;107:983–992. doi: 10.1242/jcs.107.4.983. [DOI] [PubMed] [Google Scholar]
- Hsu MY, Andl T, Li G, Meinkoth JL, Herlyn M. Cadherin repertoire determines partner-specific gap junctional communication during melanoma progression. J Cell Sci. 2000;113:1535–1542. doi: 10.1242/jcs.113.9.1535. [DOI] [PubMed] [Google Scholar]
- Hsu MY, Meier FE, Nesbit M, Hsu JY, Van Belle P, Elder DE, Herlyn M. E-cadherin expression in melanoma cells restores keratinocyte-mediated growth control and down-regulates expression of invasion-related adhesion receptors. Am J Pathol. 2000;156:1515–1525. doi: 10.1016/S0002-9440(10)65023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass NK, Smalley KSM, Herlyn M. The role of altered cell-cell communication in melanoma progression. J Mol Histol. 2004;35:309–318. doi: 10.1023/b:hijo.0000032362.35354.bb. [DOI] [PubMed] [Google Scholar]
- Hirohashi S. Inactivation of the E-cadherin-mediated cell adhesion system in human cancers. Am J Pathol. 1998;153:333–339. doi: 10.1016/S0002-9440(10)65575-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001;61:3819–3825. [PubMed] [Google Scholar]
- Li G, Satayamoorthy K, Meier F, Berking C, Bogenrieder T, Herlyn M. Function and regulation of melanoma-stromal fibroblast interactions: when seed meets soil. Oncogene. 2003;22:3162–3171. doi: 10.1038/sj.onc.1206455. [DOI] [PubMed] [Google Scholar]
- Tran NL, Adams DG, Vaillancourt R, Heimark RL. Signal transduction from N-cadherin increases Bcl-2. Regulation of the phosphatidylinositol 3-kinase/Akt pathway by homophilic adhesion and actin cytoskeletal organization. J Biol Chem. 2002;277:32905–32914. doi: 10.1074/jbc.M200300200. [DOI] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Ann Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- Fukata M, Kuroda S, Nakagawa M, Kawajiri A, Itoh N, Shoji I, Matsuura Y, Yonehara S, Fujisawa H, Kikuchi A, Kaibuchi K. Cdc42 and Rac1 regulate the interaction of IQGAP1 with beta-catenin. J Biol Chem. 1999;274:26044–26050. doi: 10.1074/jbc.274.37.26044. [DOI] [PubMed] [Google Scholar]
- Fukata M, Nakagawa M, Kuroda S, Kaibuchi K. Cell adhesion and Rho small GTPases. J Cell Sci. 1999;112:4491–4500. doi: 10.1242/jcs.112.24.4491. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Fukata M, Nakagawa M. Regulation of cadherin-mediated cell-cell adhesion by the Rho family GTPases. Curr Opin Cell Biol. 1999;11:591–596. doi: 10.1016/s0955-0674(99)00014-9. [DOI] [PubMed] [Google Scholar]
- Connacci-Sorrell M, Zhurinsky J, Ben-Ze’ev A. The cadherin-catenin system in signalling and cancer. J Clin Invest. 2002;109:987–991. doi: 10.1172/JCI15429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Nose A, Hatta K, Kawakami A, Takeichi M. Calcium-dependent cell-cell adhesion molecules (cadherins): subclass specificities and possible involvement of actin bundles. J Cell Biol. 1987;105:2501–2510. doi: 10.1083/jcb.105.6.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol. 2000;11:315–324. doi: 10.1006/scdb.2000.0178. [DOI] [PubMed] [Google Scholar]
- Willott E, Balda MS, Heintzelman M, Jameson B, Anderson JM. Localization and differential expression of two isoforms of the tight junction protein ZO-1. Am J Physiol. 1992;262:C1119–C1124. doi: 10.1152/ajpcell.1992.262.5.C1119. [DOI] [PubMed] [Google Scholar]
- Balda MS, Andersen JM. Two classes of tight junctions are revealed by ZO-1 isoforms. Am J Physiol. 1993;264:C918–C924. doi: 10.1152/ajpcell.1993.264.4.C918. [DOI] [PubMed] [Google Scholar]
- Sheth B, Fesenko I, Collins JE, Moran B, Wild AE, Anderson JM, Fleming TP. Tight junction assembly during mouse blastocyst formation is regulated by late expression of ZO-1 alpha+ isoform. Development. 1997;124:2027–2037. doi: 10.1242/dev.124.10.2027. [DOI] [PubMed] [Google Scholar]
- Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Stevenson BR, Jesaitism LA, Goodenough DA, Mooseker MS. Characterization of ZO-1, a protein component of the tight junction from mouse liver and Madin-Darby canine kidney cells. J Cell Biol. 1998;106:1141–1149. doi: 10.1083/jcb.106.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giepmans BN, Moolenaar WH. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol. 1998;8:931–934. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Tachibana K, Nakanishi H, Yamamoto Y, Irie K, Mandai K, Nagafuchi A, Monden M, Takai Y. Alpha-catenin-independent recruitment of ZO-1 to nectin-based cell-cell adhesion sites through afadin. Mol Biol Cell. 2001;12:1595–1609. doi: 10.1091/mbc.12.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Harada N, Kano K, Taya S, Canaani E, Matsuura Y, Mizoguchi A, Ide C, Kaibuchi K. The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J Cell Biol. 1997;139:785–795. doi: 10.1083/jcb.139.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing JG, Manley-Markowski RN, Koval M, Civitelli R, Steinberg TH. Connexin45 interacts with zonula occludens-1 in osteoblastic cells. Cell Commun Adhes. 2001;8:209–212. doi: 10.3109/15419060109080725. [DOI] [PubMed] [Google Scholar]
- Nielsen PA, Baruch A, Shestopalov VI, Giepmans BN, Dunia I, Benedetti EL, Kumar NM. Lens connexins alpha3Cx46 and alpha8Cx50 interact with zonula occludens protein-1 (ZO-1). Mol Biol Cell. 2003;14:2470–2481. doi: 10.1091/mbc.E02-10-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kausalya PJ, Reichert M, Hunziker W. Connexin45 directly binds to ZO-1 and localizes to the tight junction region in epithelial MDCK cells. FEBS Lett. 2001;505:92–96. doi: 10.1016/s0014-5793(01)02786-7. [DOI] [PubMed] [Google Scholar]
- Balda MS, Anderson JM, Matter K. The SH3 domain of the tight junction protein ZO-1 binds to a serine protein kinase that phosphorylates a region C-terminal to this domain. FEBS Lett. 1996;399:326–332. doi: 10.1016/s0014-5793(96)01352-x. [DOI] [PubMed] [Google Scholar]
- Balda MS, Matter K. The tight junction protein ZO-1 and an interacting transcription factor regulate ErbB-2 expression. EMBO J. 2000;19:2024–2033. doi: 10.1093/emboj/19.9.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003;160:423–432. doi: 10.1083/jcb.200210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover KB, Liao SY, Bryant PJ. Loss of the tight junction MAGUK ZO-1 in breast cancer: relationship to glandular differentiation and loss of heterozygosity. Am J Pathol. 1998;153:1767–1773. doi: 10.1016/S0002-9440(10)65691-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyamoorthy K, DeJesus E, Linnenbach AJ, Kraj B, Kornreich DL, Rendle S, Elder DE, Herlyn M. Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma Res. 1997;7(Suppl 2):S35–S42. [PubMed] [Google Scholar]
- Pummi K, Malminen M, Aho H, Karvonen SL, Peltonen J, Peltonen S. Epidermal tight junctions: ZO-1 and occludin are expressed in mature, developing, and affected skin and in vitro differentiating keratinocytes. J Invest Dermatol. 2001;117:1050–1058. doi: 10.1046/j.0022-202x.2001.01493.x. [DOI] [PubMed] [Google Scholar]
- Brandner JM, Kief S, Grund C, Rendl M, Houdek P, Kuhn C, Tschachler E, Franke WW, Moll I. Organization and formation of the tight junction-system in human epidermis and cultured keratinocytes. Eur J Cell Biol. 2002;81:253–263. doi: 10.1078/0171-9335-00244. [DOI] [PubMed] [Google Scholar]
- Malminen M, Koivukangas V, Peltonen J, Karvonen SL, Oikarinen A, Peltonen S. Immunohistological distribution of the tight junction components ZO-1 and occludin in regenerating human epidermis. Br J Dermatol. 2003;149:255–260. doi: 10.1046/j.1365-2133.2003.05438.x. [DOI] [PubMed] [Google Scholar]
- Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, Robbins CM, Moses TY, Hostetter G, Wagner U, Kakareka J, Salem G, Pohida T, Heenan P, Duray P, Kallioniemi O, Hayward NK, Trent JM, Meltzer PS. High frequency of BRAF mutations in nevi. Nat Genet. 2003;33:19–20. doi: 10.1038/ng1054. [DOI] [PubMed] [Google Scholar]
- Langbein L, Grund C, Kuhn C, Praetzel S, Kartenbeck J, Brandner JM, Moll I, Franke WW. Tight junctions and compositionally related junctional structures in mammalian stratified epithelia and cell cultures derived therefrom. Eur J Cell Biol. 2002;81:419–435. doi: 10.1078/0171-9335-00270. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Shiozaki H, Hirao M, Maeno Y, Doki Y, Inoue M, Monden T, Ando-Akatsuka Y, Furuse M, Tsukita S, Monden M. Expression of occludin, tight-junction-associated protein, in human digestive tract. Am J Pathol. 1997;151:45–54. [PMC free article] [PubMed] [Google Scholar]
- Kaihara T, Kawamata H, Imura J, Fujii S, Kitajima K, Omotehara F, Maeda N, Nakamura T, Fujimori T. Redifferentiation and ZO-1 reexpression in liver-metastasized colorectal cancer: possible association with epidermal growth factor receptor-induced tyrosine phosphorylation of ZO-1. Cancer Sci. 2003;94:166–172. doi: 10.1111/j.1349-7006.2003.tb01414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih IM. The role of CD146 (Mel-CAM) in biology and pathology. J Pathol. 1999;189:4–11. doi: 10.1002/(SICI)1096-9896(199909)189:1<4::AID-PATH332>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Yonemura S, Itoh M, Nagafuchi A, Tsukita S. Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J Cell Sci. 1995;108:127–142. doi: 10.1242/jcs.108.1.127. [DOI] [PubMed] [Google Scholar]
- Amitay R, Nass D, Meitar D, Goldberg I, Davidson B, Trakhtenbrot L, Brok-Simoni F, Ben-Ze‘ev A, Rechavi G, Kaufmann Y. Reduced expression of plakoglobin correlates with adverse outcome in patients with neuroblastoma. Am J Pathol. 2001;159:43–49. doi: 10.1016/S0002-9440(10)61671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simcha I, Geiger B, Yehuda-Levenberg S, Salomon D, Ben-Ze’ev A. Suppression of tumorigenicity by plakoglobin: an augmenting effect of N-cadherin. J Cell Biol. 1996;133:199–209. doi: 10.1083/jcb.133.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AB, Kanner SB, Wang HC, Parsons JT. Stable association of activated pp60src with two tyrosine-phosphorylated cellular proteins. Mol Cell Biol. 1989;9:3951–3958. doi: 10.1128/mcb.9.9.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AB, Herbert L, Cleveland JL, Berg ST, Gaut JR. p120, a novel substrate of protein tyrosine kinase receptors and of p60v-src, is related to cadherin-binding factors β-catenin, plakoglobin and armadillo. Oncogene. 1992;7:2439–2445. [PubMed] [Google Scholar]
- Ireton RC, Davis MA, van Hengel J, Mariner DJ, Barnes K, Thoreson MA, Anastasiadis PZ, Matrisian L, Bundy LM, Sealy L, Gilbert B, van Roy F, Reynolds AB. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002;159:465–476. doi: 10.1083/jcb.200205115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aho S, Levansuo L, Montonen O, Kari C, Rodeck U, Uitto J. Specific sequences in p120ctn determine subcellular distribution of its multiple isoforms involved in cellular adhesion of normal and malignant epithelial cells. J Cell Sci. 2002;115:1391–1402. doi: 10.1242/jcs.115.7.1391. [DOI] [PubMed] [Google Scholar]
- Mariner DJ, Anastasiadis P, Keilhack H, Bohmer FD, Wang J, Reynolds AB. Identification of Src phosphorylation sites in the catenin p120ctn. J Biol Chem. 2001;276:28006–28013. doi: 10.1074/jbc.M102443200. [DOI] [PubMed] [Google Scholar]
- Brandner JM, Proksch E. Epidermal barrier function: role of tight junctions. Elias PM, Feingold KR, editors. New York: Marcel Dekker Inc.,; Skin Barrier. 2004 [Google Scholar]
- Itoh M, Morita K, Tsukita S. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin. J Biol Chem. 1999;274:5981–5986. doi: 10.1074/jbc.274.9.5981. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Ma TY, Anderson JM. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J. 2002;16:1835–1837. doi: 10.1096/fj.02-0121fje. [DOI] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Yonemura S, Kitani-Yasuda T, Tsukita S, Tsukita S. The 220-kD protein colocalizing with cadherins in non-epithelial cells is identical to ZO-1, a tight junction-associated protein in epithelial cells: cDNA cloning and immunoelectron microscopy. J Cell Biol. 1993;121:491–502. doi: 10.1083/jcb.121.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Poser I, Dominguez D, de Herreros AG, Varnai A, Buettner R, Bosserhoff AK. Loss of E-cadherin expression in melanoma cells involves up-regulation of the transcriptional repressor Snail. J Biol Chem. 2001;276:24661–24666. doi: 10.1074/jbc.M011224200. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HE, Behrens J, Sommer T, Birchmeier W. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol. 2002;4:222–231. doi: 10.1038/ncb758. [DOI] [PubMed] [Google Scholar]
- Mauro L, Bartucci M, Morelli C, Ando S, Surmacz E. IGF-I receptor-induced cell-cell adhesion of MCF-7 breast cancer cells requires the expression of junction protein ZO-1. J Biol Chem. 2001;276:39892–39897. doi: 10.1074/jbc.M106673200. [DOI] [PubMed] [Google Scholar]