Abstract
Pneumocystis spp. pneumonia (PCP) in humans and in surrogate animal species typically occurs in the absence of CD4 T cells, as takes place during acquired immune deficiency syndrome. However, patients treated with highly active anti-retroviral therapy sometimes exhibit an exacerbation of diseases such as PCP that coincides with resurgent CD4 T cells, a phenomenon known as immune reconstitution disease. We used an animal model of PCP using the B-cell-deficient μMT mouse together with antibody-mediated depletion of various T-cell subsets to examine the role of CD4 and CD8 T cells in the development of pathology in PCP. Although overt pathology occurs in the presence of CD4 T cells only, CD8 T cells only, or both, pulmonary injury occurs via different paths, depending on the complement of T cells present. Surprisingly, profound damage occurred when only CD4 T cells were present, and this pathology coincided with enhanced recruitment and activation of eosinophils and strong type 2 cytokine polarization in the alveolar environment. In addition, CD8 T cells can act to moderate this CD4 T cell-mediated pathology, possibly by increasing the ratio of putative CD25+ suppressor CD4 T cells to CD25− effector CD4 T cells.
Because the respiratory system is constantly exposed to potentially pathogenic microorganisms, it possesses the capability of mounting vigorous innate and acquired immune responses. However, it is these same vigorous responses that are often the source of pulmonary impairments and damage to the respiratory epithelium during microbial infections.1–3 This type of inflammation-related damage is evident in pneumonia caused by the opportunistic pathogen Pneumocystis, which typically only occurs in individuals with some form of immunosuppression.4 In immunocompetent patients or experimental animals, Pneumocystis infection is easily cleared without significant ensuing pathology. However, in patients with reduced CD4 lymphocyte numbers [such as acquired immune deficiency syndrome (AIDS) patients], and in mice in which CD4 lymphocytes have been experimentally depleted, steady growth of Pneumocystis occurs in the lung despite the accumulation of inflammatory cells, including macrophages, CD8 lymphocytes, and, in later stages, neutrophils.5–7 The considerable pathology that ensues typically coincides with the accumulation of these inflammatory cells; indeed SCID mice that are deficient in lymphocytes demonstrate less respiratory impairment than mice that are only deficient in CD4 cells, even though they have comparable loads of Pneumocystis.8
These observations have led to studies that examined the type(s) of inflammatory cells potentially involved in the host tissue damage seen in Pneumocystis pneumonia (PCP). Although the influx of neutrophils into the alveolar space coincides with the onset of severe pathology in PCP, we have recently presented evidence that this is only correlational and that the neutrophil defense mechanisms may not be directly involved in the development of lung pathology.9 In contrast, there is stronger evidence that the presence of CD8 lymphocytes is required for the full development of respiratory impairment in PCP,10 although the mechanisms by which this damage occurs are not yet clear. It is overly simplistic, however, to think of pulmonary damage in a disease state occurring because of the unmodulated actions of one cell type alone. Not only are many types of inflammatory cell types capable of producing host tissue damage, but there is also abundant evidence of significant interactions between inflammatory cell types that can potentially enhance or depress the potential for inflammatory damage by one the of cells in question.11,12 These types of interactions may be relevant in immune reconstitution disease (IRD), which occurs in some AIDS patients in whom highly active anti-retroviral therapy results in markedly increased levels of CD4 lymphocytes. In some of these individuals, intense inflammatory responses can occur in the lungs (involving CD4 cells and residual Pneumocystis organisms13,14) and requires vigorous therapeutic intervention. This clinical syndrome highlights the potential interaction of multiple inflammatory cell types and mechanisms that may be involved in the pathology of PCP. Furthermore, this scenario also points to the need for a well-characterized animal model of PCP in which the functions of CD4 lymphocytes can be investigated.
To address these questions we examined the onset of Pneumocystis-related pathology in an animal model of PCP using the B-cell-deficient μMT mouse. Because these mice cannot produce functional antibodies, they develop full-blown PCP despite the presence of CD4 lymphocytes.15 By depletion of specific lymphocyte subsets using antibody-mediated depletions, we examined the type and onset of pathology during PCP in μMT mice that were deficient in CD4 lymphocytes, CD8 lymphocytes, or both CD4 and CD8 lymphocytes, as well as mice that were replete in both CD4 and CD8 lymphocytes. We report here that significant inflammatory damage occurs in both CD4-depleted and CD8-depleted μMT mice during Pneumocystis infection, although it may occur via different mechanisms. We also report evidence that there is interaction between CD4 and CD8 cells when both are present during Pneumocystis infection, which alters the subsets of lymphocytes present, the cytokine profile, and the type of pathology present at the inflammatory site.
Materials and Methods
Animals
Breeder mice (μMT; stock number 002249) were obtained from Jackson Laboratories (Bar Harbor, ME) and bred in our facility at Montana State University. Control C57BL/6 mice were obtained from the National Cancer Institute (Fredrick, MD). Mice were kept in ventilated cages, so that incoming air was HEPA filtered, and given autoclaved mouse chow and acidified water.
Depletion of T-Cell Subsets and Infection with Pneumocystis
For depletion of CD4 T lymphocytes, mice were given intraperitoneal injections of 300 μg of the anti-CD4 antibody GK1.5 (American Type Culture Collection, Manassas, VA) twice weekly. Depletion of CD8 T cells was done using twice weekly injection of 300 μg of the anti-CD8 antibody TIB-210 (American Type Culture Collection). Some animals received double depletions with twice weekly injections of 300 μg each of both GK1.5 and TIB-210.
Respiratory Measurements
Before tissue sampling, respiratory rates and tidal volumes were measured on conscious mice using a whole-body plethysmograph (Buxco Electronics, Sharon, CT). Arterial blood gases were determined after warming the mice for 5 minutes at 39°C, carefully nicking the ventral tail artery, and then collecting 150 μl of blood into a heparinized capillary tube. The blood was mixed using a small steel flea and magnet, and the samples were analyzed on a clinical blood gas analyzer (Omni AVL; Roche Diagnostics, Indianapolis, IN) within 1 hour of sampling.
Bronchoalveolar Lavage
Mice were sacrificed by pentobarbital anesthesia (100 μg/g body weight) followed by exsanguination. The trachea of each mouse was nicked with fine scissors, and then a tube was inserted. Five 1-ml aliquots of Hanks’ balanced salt solution with 3 mmol/L ethylenediaminetetraacetic acid were then applied to lavage the alveolar contents.16 Samples from each 5-ml pooled lavage were spun onto a slide using a cytospin centrifuge and then stained with Diff-Quick dye (Dade Behring, Newark, DE). Proportions of each cell type were determined microscopically using a ×100 objective lens. The bronchoalveolar lavage fluid (BALF) supernatant was collected by centrifugation at 200 × g for 10 minutes, and aliquots were used for the following assays. Albumin and lactate dehydrogenase (LDH) concentrations were determined with commercial kits (631-2; Sigma Diagnostics, St. Louis, MO, and CytoTox 96: Promega, Madison, WI). Eosinophil peroxidase (EPO) activity in the BALF was measured using a modification of the method of Duguet and colleagues17; briefly, aliquots of BALF were incubated either with O-phenylenediamine, H2O2, and the specific inhibitor 3-amino-1,2,3-triazole or with O-phenylenediamine and H2O2 alone. After 30 minutes of incubation at room temperature, absorbance at 405 nm was measured. EPO-specific activity was estimated as the absorbance measured in wells without the inhibitor 3-amino-1,2,3-triazole minus the absorbance in wells containing the same sample with 3-amino-1,2,3-triazole.
Flow Cytometry and Cytokine Analysis
Bronchoalveolar lavage (BAL) cells were resuspended in a minimal volume of phosphate-buffered saline with 2% calf serum and an anti-mouse Fc receptor antibody (Trudeau Institute, Saranac Lake, NY) to block nonspecific binding. These cells were then stained with a cocktail of fluorophore-conjugated antibodies against the mouse CD antigens CD4, CD8, CD28, and either CD25 or CD62L (PharMingen, San Diego, CA) and analyzed on a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA). Lymphocytes obtained from spleens of control uninfected mice were used as a basis of comparison. Flow cytometry-based bead ELISA kits (mouse Th1-Th2 and Mouse Inflammation; Becton Dickinson) were used to measure cytokine concentrations in undiluted samples of BALF.
Enumeration of Pneumocystis
After lavage, the trachea was ligated and two-thirds of the lung were removed, placed into 5 ml of sterile Hanks’ balanced salt solution, and homogenized by pushing through a metal mesh screen. An aliquot of this material was diluted 1:20 and applied to a glass slide using a cytospin centrifuge. After drying, the slides were stained for an extended period of time in Diff-Quick dye (40 to 60 minutes). Pneumocystis nuclei (both cysts and trophozoites) were then enumerated in a minimum of five ×60 fields, up to a maximum of 50 (if nuclei were not readily apparent). The average counts were then converted to log Pneumocystis nuclei per lung. With this technique in this laboratory, the limit of detection was (log) 4.43 when 50 fields were counted. Although the previous lavage removes some Pneumocystis organisms from the lung, it is a small fraction of what is present in the lung (<10%), and this was consistent among different groups of mice.
Histology
One-third of the lung (the large left lobe) was fixed for 24 hours in phosphate-buffered formalin, embedded in paraffin, sectioned at 5 μm, and stained with either hematoxylin and eosin (H&E) or Alcian Blue with periodic acid-Schiff using standard histological techniques.
Statistical Analysis
The software program Graph Pad Prism (San Diego, CA) was used for all statistical tests of significance (to P ≤ 0.05). Where greater than two groups were being compared, one-way analysis of variance with Tukey’s post hoc pairwise comparisons were made. When only two groups were compared, we used a two-sided t-test, with Welch’s correction if the groups had unequal variances. In cases in which a deviation from a normal distribution was observed, a nonparametric test (Mann-Whitney test) was also applied. In those cases, we found that both the t-test and Mann-Whitney test indicated the same result (ie, both indicated significance or nonsignificance); however, typically one test gave a more conservative P value (larger, but still <0.05). The P values we report are always the conservative values.
Results
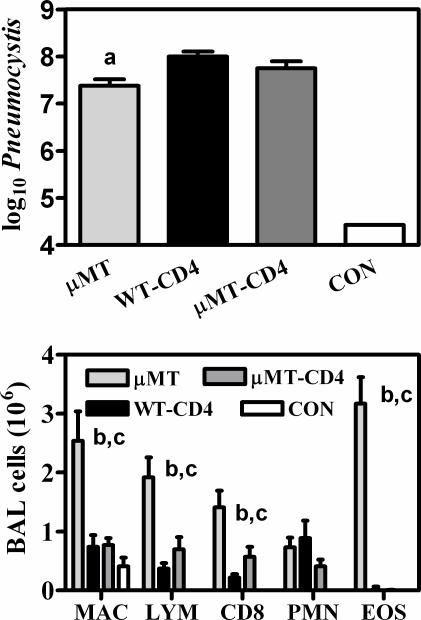
Although the common mouse model of Pneumocystis pneumonia (PCP) is that of wild-type mice depleted of CD4 lymphocytes, it has previously been shown that μMT mice, which are deficient in B-cell and antibody production, also develop serious and progressive infections with Pneumocystis, even with normal numbers of CD4 lymphocytes.15 We first compared these two models and found that at 28 days after inoculation μMT mice exhibited a burden of Pneumocystis that is only slightly lower than that seen in CD4+ depleted wild-type mice infected at the same time (Figure 1). However, there were some subtle differences in the Pneumocystis-related pathology found in these two models. Although there were no statistical differences in the indicators of BAL albumin, BAL LDH, or respiratory rates, arterial blood oxygen was significantly lower in μMT mice (Table 1). μMT mice also recruited considerably more cells into the alveolar compartment than did CD4-depleted wild-type mice. These included lymphocytes (mostly CD8 lymphocytes) and most strikingly, large numbers of eosinophils (Figure 1). The CD4-depleted wild-type mice had fewer accumulated CD8 lymphocytes in their lungs and, as would be expected, had no CD4 lymphocytes and few eosinophils in the BAL during Pneumocystis infection (Figure 1). However, if CD4 lymphocytes were depleted from μMT mice, then the Pneumocystis disease took on characteristics very similar to that of CD4-depleted wild-type mice. This was true with respect to Pneumocystis burden (Figure 1), physiological impairments (Table 1), and cellular influx into the lung (Figure 1).
Figure 1.
Pneumocystis organism burden and inflammatory cell influx in lungs of μMT and wild-type (WT; C57BL/6) mice. Top: Mice were inoculated with 107 Pneumocystis organisms, and 28 days later, lungs were homogenized and aliquots were used for microscopic quantification. Groups with −CD4 in the name were depleted of CD4 T cells before inoculation and during the entire incubation period. CON indicates uninfected WT mice. A log of 4.4 is the limit of detection of this technique. Bottom: Cell counts in BALF from the same mice were multiplied by the percentage of each cell type as determined from differential microscopic examination, with lymphocyte subset percentages determined by flow cytometric evaluation. Cell types shown are alveolar macrophages (MAC), total lymphocytes (LYM), CD8 lymphocytes (CD8), neutrophils (PMN), and eosinophils (EOS). Values are means ±SEM, n = 5; a = significantly lower than WT-CD4 mice; b = significantly higher than WT-CD4 mice; c = significantly higher than μMT-CD4 mice (all at P ≤ 0.05).
Table 1.
Measurements of Pneumocystis-Related Pulmonary Pathology in μMT and Wild-Type Mice with CD4 T-Cell Depletion
| Parameter | μMT | WT-CD4 | μMT-CD4 | Control |
|---|---|---|---|---|
| Respiratory rate (breaths/minute) | 520.0 ± 11.7 (4) | 505.2 ± 14.4 (5) | 441.6 ± 17.9 (5) | 316.1 ± 10.1 (5) |
| PaO2 (mmHg) | 42.33 ± 2.55 (4)* | 63.72 ± 2.16 (5) | 54.78 ± 4.37 (4) | 87.40 ± 0.72 (5) |
| BAL albumin (mg/ml) | 0.098 ± 0.01 (4) | 0.13 ± 0.03 (4) | 0.13 ± 0.04 (5) | 0.01 ± 0.01 (5) |
| BAL LDH (U/ml) | 0.125 ± 0.034 (4) | 0.195 ± 0.062 (4) | 0.100 ± 0.008 (4) | 0.060 ± 0.008 (4) |
μMT and wild-type (WT; C57BL/6) mice were inoculated with 107 Pneumocystis organisms. −CD4 groups were depleted of CD4+ cells with twice weekly injections of GK1.5. Control mice were neither inoculated nor depleted. Twenty-eight days after inoculation, respiratory rates were measured and arterial blood was sampled for measurement of O2 partial pressure (PaO2). Mice were sacrificed and bronchoalveolar lavage (BAL) performed to obtain samples for measurement of albumin and LDH. Values are means ± SEM (n).
Indicates value is significantly different from all other groups in same row (P < 0.05).
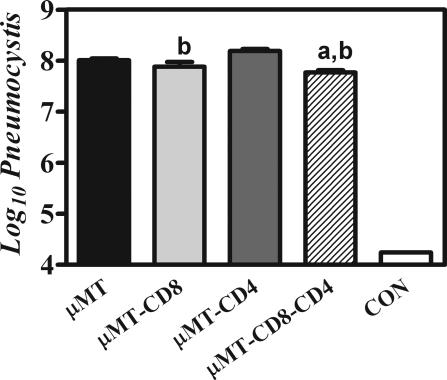
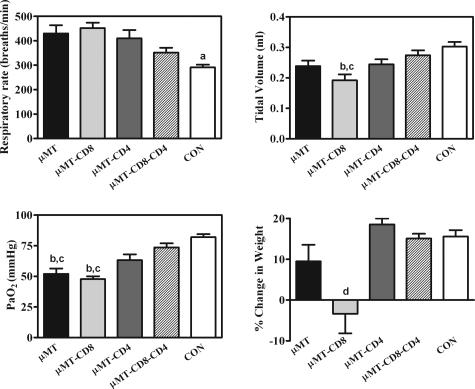
Previous research has implicated CD8 lymphocytes as a cause of host tissue damage in Pneumocystis, and this initial study would seem to agree, as both B-cell-deficient and B- and CD4-cell-deficient mice had extensive host tissue damage. To examine further the role of the CD8 cells, we infected μMT mice that were depleted of CD4 lymphocytes, CD8 lymphocytes, or both CD4 and CD8 lymphocytes as well as undepleted μMT mice with Pneumocystis. We found that there was little significant difference in the burden of Pneumocystis after 30 days of infection among any of the groups of μMT mice; mice in which both CD4 T cells and CD8 T cells were depleted had slightly lower levels of Pneumocystis (Figure 2). However, there were some surprising observations of physiological parameters in Pneumocystis-infected mice within these groups. Although we expected Pneumocystis-infected μMT mice that were CD8 depleted to have reduced host tissue damage, they in fact exhibited physiological indicators of damage (increased respiratory rate, reduced tidal volume, and low PaO2), which were equal to CD4 T-cell-depleted μMT mice, and actually showed the greatest decrease in body mass throughout the course of the infection (Figure 3). Conversely, when both CD4 and CD8 lymphocytes were depleted in Pneumocystis-infected μMT mice, there was very little physiological impairment (despite a high Pneumocystis burden); PaO2 and tidal volumes were not statistically different from that of uninfected control mice, although respiratory rates were marginally higher than those of control mice (Figure 3).
Figure 2.
Pneumocystis organism burden in lungs of μMT mice with various complements of T cells. Mice were inoculated with 107 Pneumocystis organisms, and 28 days later, lungs were homogenized and aliquots were used for microscopic quantification. Groups with −CD4 in the name were depleted of CD4 T cells before inoculation and during the entire incubation period. Groups with −CD8 in the name were depleted of CD8 cells before inoculation and during the entire incubation period. CON indicates uninfected WT mice. A log of 4.4 is the limit of detection of this technique. Values are the mean Pneumocystis organisms per lung ± SEM, n = 5; a = significantly less than μMT mice; b = significantly less than μMT-CD4 mice (all at P ≤ 0.05).
Figure 3.
Physiological assessments of pulmonary damage in μMT mice with various complements of T cells 28 days after inoculation with Pneumocystis. Experimental groups are as described in Figure 2. Respiratory rate (top left) and tidal volume (top right) were measured using whole body plethysmography and are expressed as breaths per minute and ml per breath, respectively. Partial pressure of oxygen was measured in arterial blood taken from the tail artery of mice, and is expressed in mm of mercury (mmHg; bottom left). Body weight of experimental animals was measured at the time of inoculation and at 28 days, and changes in weight are expressed as a percent change of total body mass (bottom right). Values are means ± SEM, n = 5. a = Control mice respiratory rates are significantly lower that all other groups (P ≤ 0.05); b = significantly different from the same parameter in μMT-CD4-CD8 mice (P ≤ 0.05); c = significantly different from the same parameter in control mice (P ≤ 0.05); d = significantly lower than same parameter in all other groups of mice (P ≤ 0.01).
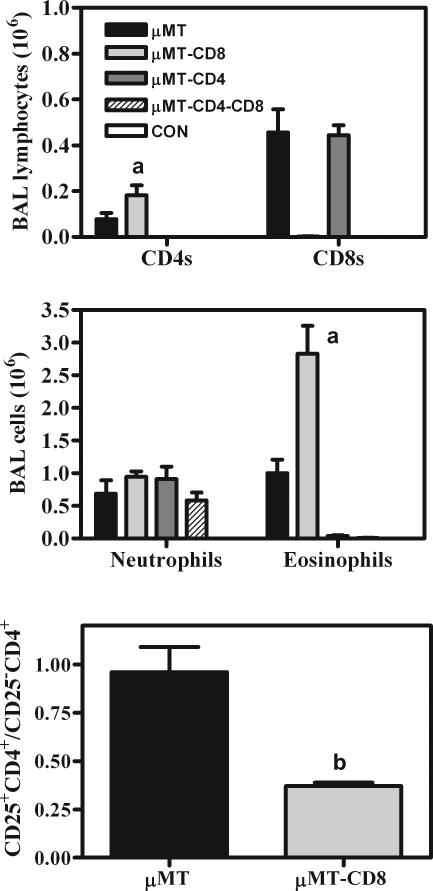
The relative proportions of inflammatory cells in the lung were also very different in the various depletion models, as one would expect. When μMT mice were depleted of CD4 lymphocytes during Pneumocystis infection, they had a total lack of CD4 T cells and eosinophils in the BAL (Figure 4). Conversely, when μMT mice were depleted of CD8 lymphocytes, there was a tendency for higher levels of both CD4 lymphocytes and eosinophils to be present in the BAL, compared to that seen in undepleted μMT, (Figure 4), although the degree of elevation of these cell types was somewhat variable between experimental replications. When both CD4 and CD8 lymphocytes were depleted, there were no eosinophils (and of course no CD4 or CD8 cells), and the numbers of neutrophils (Figure 4) and macrophages (data not shown) were similar to that of undepleted μMT mice. Flow cytometry was also used to probe for the presence of putative regulatory T cells (CD28−CD8+ T cells and CD25+ CD4+ T cells). Although the CD8 T cells exhibited a spectrum of CD28 staining, there were no clearly demarcated populations of CD28-negative and -positive cells, although the CD28 staining for some of these cells was at or below the level of background staining, indicating that CD28−CD8+ T cells were present (data not shown). However, CD25+CD4+ T cells could be clearly demarcated from CD25−CD4+ cells. Although both Pneumocystis-infected μMT mice and Pneumocystis-infected CD8 T-cell-depleted μMT mice had similar numbers of CD25+CD4+ T cells, the mice that were depleted of CD8 T cells had greater numbers of CD25−CD4+ T cells, so that the ratio of CD4 T cells that includes potential regulatory cells (CD25+CD4+) and putative effector cells (CD25−CD4+) was significantly lower in Pneumocystis-infected CD8 T-cell-depleted μMT mice (Figure 4).
Figure 4.
Inflammatory cells in the BALF of μMT with various complements of T cells 28 days after inoculation with Pneumocystis. See Figure 1 legend for methodology. Experimental groups are as described in Figure 2. Top: Cell types shown are CD8 lymphocytes (CD8) and CD4 lymphocytes (CD4). Middle: Cell types shown are neutrophils and eosinophils. Bottom: Ratio of putative CD4 suppressor T cells to CD4 effector T cells (total numbers of CD25+ CD4 lymphocytes divided by total numbers of CD25-CD4 lymphocytes) in the BALF of μMT mice with and without CD8 T cells being present 28 days after inoculation with Pneumocystis. Values are means ± SEM, n = 5 for each group; a = significantly greater than in μMT mice (P ≤ 0.05); b = significantly greater than in μMT mice (P ≤ 0.05).
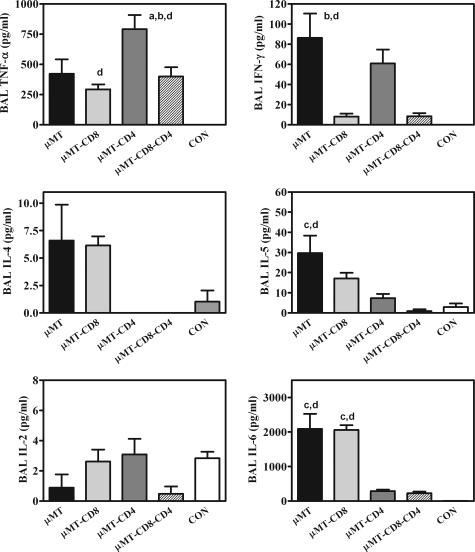
Additionally, the profile of some important inflammatory cytokines in the BAL of Pneumocystis-infected mice was very dissimilar in the different depletion models. In undepleted, Pneumocystis-infected μMT mice, BAL samples contained elevated levels of both type 1 [tumor necrosis factor (TNF)-α and interferon (IFN)-γ] and type 2 [interleukin (IL)-4 and IL-5] cytokines (Figure 5). When the μMT mice were depleted of CD4 lymphocytes, the levels of IL-4, IL-5, and IL-6 in the BALF were reduced, while TNF-α and IFN-γ remained elevated (Figure 5). An opposite change occurs with CD8 depletion: IL-4, IL-5, and IL-6 were elevated, while IFN-γ was decreased (Figure 5). When CD4 and CD8 lymphocytes were depleted, IL-4, IL-5, and IFN-γ were all low, while TNF-α remained at levels similar to undepleted, infected μMT mice (Figure 5).
Figure 5.
Cytokine profiles of BALF in μMT with various complements of T cells 28 days after inoculation with Pneumocystis. Experimental groups are as described in Figure 2. Values are mean cytokine concentrations (in pg per ml of lavage fluid) ± SEM, n = 5. a = Significantly different from levels in μMT mice; b = significantly different from levels in μMT-CD8 mice; c = significantly different from levels in μMT-CD4 mice; d = significantly different from levels in μMT-CD8-CD4 mice (all at P ≤ 0.05).
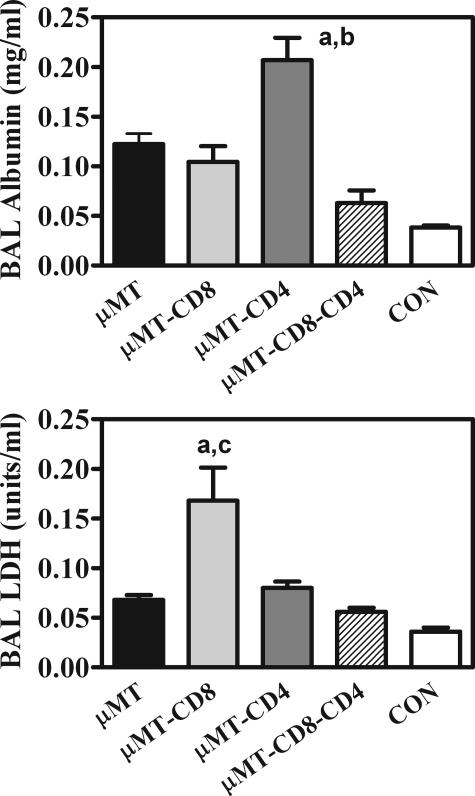
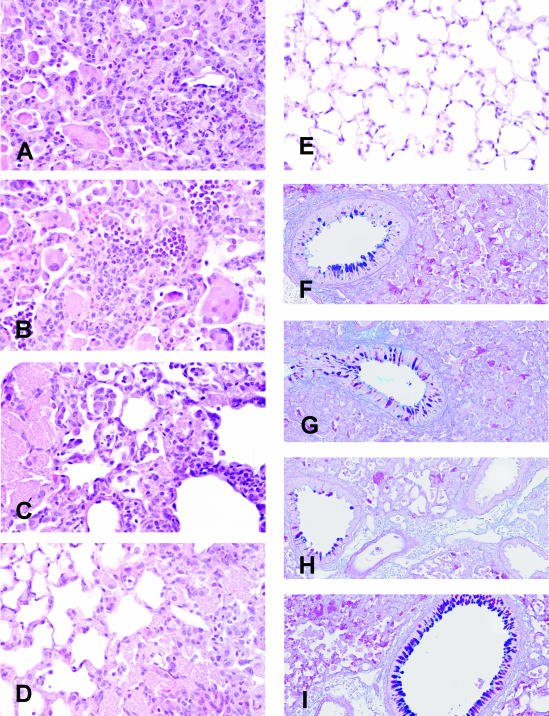
It is apparent that there are different conditions leading up to similar levels of pathology in CD8-depleted versus CD4-depleted μMT mice. Albumin in the BAL, indicative of increased permeability, was moderately elevated in all of the infected groups (Figure 6; although the albumin in μMT mice with CD4 T cells depleted was significantly higher in the experiment shown, this was not always the case). However, LDH in the BAL, indicative of local cell death, was consistently twofold higher in CD8-depleted mice than in μMT and CD4-depleted μMT mice (Figure 6). The different forms of pathology were also evident histologically: multinucleated giant cells were visible in the alveoli of Pneumocystis-infected, undepleted μMT mice and were present in even greater numbers in CD8-depleted μMT mice (Figure 7). However, they were absent in all other groups. It is also noteworthy that, whereas all of the μMT groups had moderate mucous cell hypertrophy in their airways after Pneumocystis infection, μMT mice depleted of both CD8 and CD4 T cells exhibited marked mucous cell hyperplasia in their airways (Figure 7).
Figure 6.
Biochemical indicators of pulmonary pathology in μMT mice with various complements of T cells 28 days after inoculation with Pneumocystis. Experimental design is as described in Figure 2. Albumin (a marker of endothelial and/or epithelial leakage) is expressed as mg per ml of lavage fluid (top). LDH (a marker of local cell death) is expressed as U of dehydrogenase activity per ml of lavage fluid (bottom). Values are means ± SEM, n = 5. a = significantly different from the same indicator in μMT mice; b = significantly different from the same indicator in μMT-CD8 mice; c = significantly different from the same indicator in μMT-CD4 mice (all at P ≤ 0.05).
Figure 7.
Histological assessment of pulmonary pathology in μMT mice with various complements of T cells 28 days after inoculation with Pneumocystis. A–E: H&E-stained sections. F–I: Alcian Blue/PAS-stained sections (to highlight mucus-containing airway cells). Lungs from μMT mice are shown in A and F, B and G are from μMT-CD8 mice, C and H are from μMT-CD4 mice, and D and I are from μMT-CD8-CD4 mice. A section from the lung of an uninfected control mouse is shown for comparison in E. Large pink clumps of Pneumocystis organisms can be seen in A–D; multinucleate giant cells can also be seen in μMT and μMT-CD8 mice (A, B). Airway cells staining positive for mucus can be found in all of the Pneumocystis-infected groups, although they are consistently more numerous in μMT-CD8-CD4 mice. Original magnifications: ×600 (A–E); ×200 (F–I).
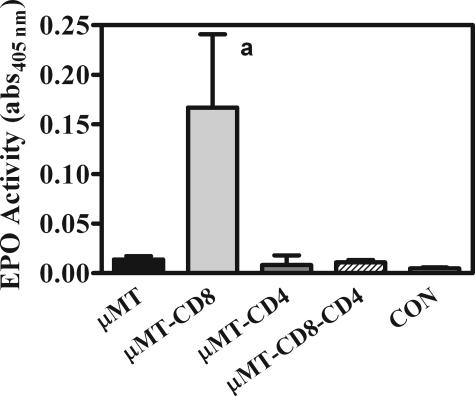
Finally, because of the high numbers of eosinophils in CD8-depleted μMT mice and their potential role as effector cells in host tissue damage, we examined whether these cells had been activated and undergone degranulation. Levels of the enzyme EPO were measured in the BALF of all groups, and the activity was significantly elevated only in CD8-depleted μMT mice (Figure 8).
Figure 8.
Activity of the EPO enzyme in BALF in μMT mice with various complements of T cells 28 days after inoculation with Pneumocystis. Experimental design is as described in Figure 3. Specific EPO activity is expressed as the absorbance at 405 nm of the peroxidase substrate O-phenylenediamine of aliquots of BALF. Values are means ± SEM, n = 5; a = significantly different from all other groups (P ≤ 0.05).
Discussion
A variety of studies in animal models have implicated the host inflammatory response as a major cause of pathology in PCP.4–6 Some of these studies have shown that neutrophils may have a correlative but not a causative relationship to this pathology,9 whereas other studies have pointed to a causative role for CD8 T cells in pulmonary damage.10,18 Because CD4 T cells are deficient in many humans with Pneumocystis and absent in common animal models of PCP, their involvement in Pneumocystis-related pathology has not been addressed. However, the clinical syndrome of IRD, during which AIDS patients being treated with highly active anti-retroviral therapy develop serious pulmonary inflammation (often Pneumocystis related) that coincides with resurgent CD4 lymphocytes, raises some interesting questions. For example, can CD4 T cells, if present at sufficient levels, also be involved in pulmonary damage in PCP? Or, can interactions between CD8 and CD4 lymphocytes occur to potentiate the damaging actions of CD8 cells? To begin to address these questions, we have examined Pneumocystis-related pathology in μMT mice in which the absence of antibody-producing B cells allows for the development of PCP in the presence of various combinations of CD4 and CD8 T cells. Our results show that there can be multiple pathways of host tissue damage, depending on the complement of T cells present, and that interactions between CD4 and CD8 T cells probably occur in these models of PCP, but such interactions are more complex than we initially envisioned.
Our results with μMT mice in which both CD4 and CD8 T cells have been depleted throughout the course of infection with Pneumocystis reaffirms previous observations that lymphocyte-related inflammatory responses cause much of the pulmonary pathology, as evidenced by physiological lung function that is only marginally worse than uninfected animals, despite a high PC burden. This lack of damage occurs even in the presence of moderately elevated TNF-α; this confirms previous observations that the TNF-α receptor is implicated in the development of pathology in PCP, although much of this pathology occurs through T-cell-related pathways.18
That CD8 T cells are essential to host tissue damage in mice in which CD4 T cells have been depleted was also confirmed in this study; Pneumocystis-infected μMT mice that were depleted of CD4 but not CD8 T cells show almost identical pathology as wild-type mice in which CD4 T cells were depleted. It was somewhat of a surprise to find that equivalent pathology (or more severe, if weight loss is taken into account) occurred in μMT mice in which CD8 T cells but not CD4 T cells had been depleted. However, it was also apparent that although the physiological outcomes of the inflammatory damage (increased respiratory rate, reduced tidal volumes, reduced PaO2) were qualitatively the same in μMT mice whether they possessed only CD4 T cells or only CD8 T cells, the pathways to this damage were somewhat different. In μMT mice that possessed only CD8 T cells, there were elevated concentrations of albumin in the BALF, indicative of leakage of the endothelial and/or epithelial barrier of the alveoli. Furthermore, the cytokine analysis of the BALF indicated a type1 cytokine skewed profile, in which TNF-α and IFN-γ were elevated while IL-4 and IL-5 were absent. However, in μMT mice possessing only CD4 T cells, BALF albumin levels were modestly elevated, and BALF LDH was sharply elevated (and this was only observed in this group), indicating local cell death. The cytokine profile of BALF in these mice indicated type 2 cytokine skewing, with elevated IL-4, IL-5, and IL-6.
In the case of μMT mice with only CD4 T cells, there are diverse effector mechanisms that could account for increased local cell death. A skewed Th2 phenotype has been implicated with pathology in allergic disorders11,19 and parasitic infections.20 Eosinophilia is sometimes present in those conditions as well, as it was in CD8 T-cell-depleted μMT mice in this report. Not only did these mice have abundant eosinophils in the alveolar space, there was also greater activation and degranulation of the eosinophils, as indicated by higher levels of EPO in the BALF. Eosinophil granule proteins such as EPO and MBP are known to be cytotoxic in vitro,21 and there is also some evidence for their involvement in pulmonary pathology in vivo (although at least in asthma models, there are clear differences in this respect between mice and humans24,25).17,22,23 The possible effector mechanisms in μMT mice in which only CD8 T cells were present are not as clear. Although perforin/granzyme-mediated cell lysis has been shown to be a cytotoxic mechanism used by some CD8 cells, this pathway is probably not important in PCP26 (N.N. Meissner, manuscript submitted).
In many pulmonary pathologies, including cryptococcal infection27 and respiratory allergies,2,25 eosinophil influx into the alveolar space is CD4 T cell-dependent, and this is obviously the case here, as indicated by the absence of alveolar eosinophils in μMT mice that were CD4 depleted. However, it is apparent from our data that the CD4-dependent recruitment and activation of eosinophils, as well as type 2 cytokine skewing, is moderated during Pneumocystis infection when CD8 T cells are present. This is most evident in the group of μMT mice that have been depleted of CD8 T cells, resulting in a twofold greater recruitment of eosinophils as well as increased eosinophil degranulation. The possibility that CD8 T cells (or more likely, a subpopulation of CD8 T cells) could modulate CD4 T cell functions is well established. Exacerbation of inflammatory damage has been shown to occur with CD8 depletion in murine Mycoplasma pneumonia.28 Th2-associated pathology found in experimental transplantation models is also enhanced when CD8 T cells are depleted.29 Additionally, accumulation of eosinophils in the lung is exacerbated by CD8 depletion in mouse models of RSV infection30 and allergic asthma.31 Observations such as these have lead to a resurgence in the study of putative CD8-positive regulatory or suppressor cells.32–34 A major focus in these studies has been CD28−CD8+ cells, which are believed to induce tolerance in transplantation models via contact-dependent mechanisms involving antigen-presenting cells and subsequently T helper CD4 cells.33,35
Whether or not CD8 suppressor cells are involved in this modulation, it is clear that when CD8 T cells are absent in Pneumocystis-infected μMT mice, a relative deficit of CD25+CD4+ T cells occurs. Extensive research into this subset of T cells in recent years has shown them to have complex suppressive effects in a variety of disease states,36–38 some of which have similar characteristics to Pneumocystis infection. For example, the type 2 cytokine polarization and alveolar influx of eosinophils that is seen in Pneumocystis-infected μMT mice, which possess CD4 T cells, is also seen in mouse models of ovalbumin-induced asthma, and these responses are modulated by CD25+CD4+ T cells.39 And, in a study of PCP in RAG-2−/− mice, transfer of CD25−CD4+ T cells alone resulted in much more severe pathology than when both CD25−CD4+ and CD25+CD4+ T cells are transferred (although eosinophil levels were not reported in that study).40 These observations, together with data we report here, support the hypothesis that the ratio of CD25−CD4+ to CD25+CD4+ T cells is instrumental in the enhanced eosinophil-related pathology seen in Pneumocystis-infected CD8-depleted μMT mice.
If a subset of CD8 T cells is modulating CD4 T-cell effector functions in our model of PCP, either directly or via CD25+CD4+ T cells, the question arises as to the mechanism of interaction of this modulation. In some instances, such as with CD8+CD28− suppressor cells discussed above, it is believed that a physical interaction must occur between the CD8 suppressors and other cells (APCs and CD4 T cells).34,41 However, in other experimental models, it is believed that soluble mediators secreted from a group of CD8 T cells are sufficient to change CD4 T cell function at sites of inflammation. Studies in several disease models have provided evidence that reduction in secretion of IFN-γ (at least in part from CD8 T cells) is associated with CD4-related eosinophilia occurring in the lung.30,31,42,43 In our system, IFN-γ secretion is markedly reduced when CD8 cells are depleted and lung eosinophilia is greatest, so it is feasible that this mechanism of interaction is what is occurring in our experimental model. Studies are currently underway to explore this possibility.
One caveat that must be considered in the interpretation of this study is the absence of B cells in the μMT mice. This immunological defect is of course what makes these mice susceptible to Pneumocystis disease in the presence of CD4 cells because an effective acquired immune response against Pneumocystis cannot be mounted without antibody production. In AIDS, an effective acquired immune response against Pneumocystis is also absent, but this is due to the lack of CD4 cells (as in the conventional mouse model of Pneumocystis disease, in which CD4 cells are depleted). It is well known that, in addition to antibody production, B cells also have other important immunological functions such antigen presentation and modulation of other lymphocyte functions.44–47 However, because several aspects of B-cell function are perturbed in varying degrees in HIV infection,48 the model presented here remains useful in exploring the development of pathology in PCP, even though it is not a complete recreation of what is occurring in HIV-infected humans.
Indeed, although we show here that host pulmonary damage can occur via both CD8-mediated and CD4-mediated pathways during PCP in μMT mice, these models represent, by necessity, extremes as far as relative proportions of CD4 and CD8 T cells present during PCP. In humans with Pneumocystis-related IRD, there is probably a wide range of proportions of CD4 versus CD8 T cells at the sites of inflammation. Clinical reports make clear that IRD is a syndrome involving many pathogen-specific inflammatory responses, as well as other noninfectious processes.13,49 Even in cases in which there is clear evidence of involvement of Pneumocystis, there is a diverse range of findings regarding the types of inflammatory processes occurring in the lungs.14,50–52 The common factor in all these cases is that symptoms coincide with the reoccurrence of CD4 T cells, and that, when examined, CD4 T cells are present in the lung at sites of inflammation. The proportions of inflammatory cells are quite variable in those cases in which this was examined and may indicate a preponderance of lymphocytes or alveolar macrophages; however, in only one case were significant numbers of eosinophils reported in the BALF.14 Because none of these cases reported dramatic reductions of CD8 T cells, they do not approach the extreme case of PCP in the presence of CD4 T cells with absence of CD8 T cells in which we found severe pathology in our μMT mice. Therefore, there is no evidence to suggest that the inflammatory pathology seen in IRD is due exclusively to products from activated eosinophils. What this does suggest is that the presence of CD4 T cells influences pathology in IRD through other mechanisms. One possibility is via antibody-mediated mechanisms, which would not be reflected in our B-cell-deficient mouse model. Another possibility that would be relevant to the μMT model is that the presence of CD4 T cells influences the pathology by promoting a type 2 cytokine polarization. Although this has not been examined in Pneumocystis-related reconstitution disease, there is evidence that this occurs in IRD associated with other pathogens. For example, patients with a history of IRD related to cytomegalovirus exhibit both a predominant Th2 cytokine environment and a delayed recovery of CD8 T-cell numbers.53 Another study looking at patients with IRD related to a variety of pathogens showed that individuals susceptible to IRD had significantly higher levels of IL-6 (which was also consistently high in Pneumocystis-infected μMT mice possessing CD4 T cells).54
In summary, although CD8 T cells are believed to be the primary effector cell in host tissue inflammatory damage in PCP, we have shown that when CD4 T cells are present, but CD8 T cells and antibody production is absent, CD4 T cells will mediate significant host damage, although the path to this damage may be different from when only CD8 T cells are present. This pathology is associated with increased production of type 2 cytokines (IL-4, IL-5, and IL-6). Another likely effector in this CD4 T-cell-dependent response is BAL eosinophils, which are only recruited and activated to the alveolar space in the presence of CD4 T cells. Interestingly, CD8 T cells (or a subset thereof) actually moderate these CD4 T-cell-dependent processes, in part by increasing the ratio of CD25+CD4+ regulatory/suppressor to CD25−CD4+ effector T cells. Therefore, host tissue damage in PCP can occur through several pathways, but there are also naturally occurring mechanisms of modulation of these processes. Elucidating what these mechanisms are may provide potential strategies for ameliorating inflammatory pulmonary damage in PCP and other pulmonary diseases.
Acknowledgments
We thank Gayle Callis, Pete Degel, Ann Harmsen, Soo Han, Melanie Higgins, Quinton King, Tammy Marcottte, Lynelle McNamee, Katie Shampeny, Sarah Sheldon, Mike Tighe, and Jim Wiley for expert assistance.
Footnotes
Address reprint requests to Steve D. Swain, Department of Veterinary Molecular Biology, Montana State University, 960 Technology Blvd., Bozeman, MT 59718. E-mail: uvsss@montana.edu.
Supported by the National Institutes of Health (grants RO1HL55002, PO1HL71659, and COBRE 5P20RR020185).
References
- Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990;141:471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- Lee NA, Gelfand EW, Lee JJ. Pulmonary T cells and eosinophils: coconspirators or independent triggers of allergic respiratory pathology? J Allergy Clin Immunol. 2001;107:945–957. doi: 10.1067/mai.2001.116002. [DOI] [PubMed] [Google Scholar]
- Delclaux C, Azoulay E. Inflammatory response to infectious pulmonary injury. Eur Respir J Suppl. 2003;42:10s–14s. doi: 10.1183/09031936.03.00420203. [DOI] [PubMed] [Google Scholar]
- Hahn PY, Limper AH. The role of inflammation in respiratory impairment during Pneumocystis carinii pneumonia. Semin Respir Infect. 2003;18:40–47. doi: 10.1053/srin.2003.50004. [DOI] [PubMed] [Google Scholar]
- Su TH, Martin WJ., II Pathogenesis and host response in Pneumocystis carinii pneumonia. Annu Rev Med. 1994;45:261–272. doi: 10.1146/annurev.med.45.1.261. [DOI] [PubMed] [Google Scholar]
- Walzer PD. Immunological features of Pneumocystis carinii infection in humans. Clin Diagn Lab Immunol. 1999;6:149–155. doi: 10.1128/cdli.6.2.149-155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen AG, Stankiewicz M. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med. 1990;172:937–945. doi: 10.1084/jem.172.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Mills JW, Harmsen AG. Development and resolution of Pneumocystis carinii pneumonia in severe combined immunodeficient mice: a morphological study of host inflammatory responses. Int J Exp Pathol. 1992;73:709–720. [PMC free article] [PubMed] [Google Scholar]
- Swain SD, Wright TW, Degel PM, Gigliotti F, Harmsen AG. Neither neutrophils nor reactive oxygen species contribute to tissue damage during Pneumocystis pneumonia in mice. Infect Immun. 2004;72:5722–5732. doi: 10.1128/IAI.72.10.5722-5732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright TW, Gigliotti F, Finkelstein JN, McBride JT, An CL, Harmsen AG. Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia. J Clin Invest. 1999;104:1307–1317. doi: 10.1172/JCI6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- Dayer JM. How T-lymphocytes are activated and become activators by cell-cell interaction. Eur Respir J Suppl. 2003;44:10s–15s. doi: 10.1183/09031936.03.00000403b. [DOI] [PubMed] [Google Scholar]
- Shelburne SA, III, Hamill RJ. The immune reconstitution inflammatory syndrome. AIDS Rev. 2003;5:67–79. [PubMed] [Google Scholar]
- Wislez M, Bergot E, Antoine M, Parrot A, Carette MF, Mayaud C, Cadranel J. Acute respiratory failure following HAART introduction in patients treated for Pneumocystis carinii pneumonia. Am J Respir Crit Care Med. 2001;164:847–851. doi: 10.1164/ajrccm.164.5.2007034. [DOI] [PubMed] [Google Scholar]
- Marcotte H, Levesque D, Delanay K, Bourgeault A, de la Durantaye R, Brochu S, Lavoie MC. Pneumocystis carinii infection in transgenic B cell-deficient mice. J Infect Dis. 1996;173:1034–1037. doi: 10.1093/infdis/173.4.1034. [DOI] [PubMed] [Google Scholar]
- Harmsen AG. Role of alveolar macrophages in lipopolysaccharide-induced neutrophil accumulation. Infect Immun. 1988;56:1858–1863. doi: 10.1128/iai.56.8.1858-1863.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguet A, Iijima H, Eum SY, Hamid Q, Eidelman DH. Eosinophil peroxidase mediates protein nitration in allergic airway inflammation in mice. Am J Respir Crit Care Med. 2001;164:1119–1126. doi: 10.1164/ajrccm.164.7.2010085. [DOI] [PubMed] [Google Scholar]
- Wright TW, Pryhuber GS, Chess PR, Wang Z, Notter RH, Gigliotti F. TNF receptor signaling contributes to chemokine secretion, inflammation, and respiratory deficits during Pneumocystis pneumonia. J Immunol. 2004;172:2511–2521. doi: 10.4049/jimmunol.172.4.2511. [DOI] [PubMed] [Google Scholar]
- Romagnani P, Annunziato F, Piccinni MP, Maggi E, Romagnani S. Th1/Th2 cells, their associated molecules and role in pathophysiology. Eur Cytokine Netw. 2000;11:510–511. [PubMed] [Google Scholar]
- Jarnicki AG, Fallon PG. T helper type-2 cytokine responses: potential therapeutic targets. Curr Opin Pharmacol. 2003;3:449–455. doi: 10.1016/s1471-4892(03)00077-8. [DOI] [PubMed] [Google Scholar]
- Kroegel C, Warner JA, Virchow JC, Jr, Matthys H. Pulmonary immune cells in health and disease: the eosinophil leucocyte. Eur Respir J. 1994;7:743–760. doi: 10.1183/09031936.94.07040743. [DOI] [PubMed] [Google Scholar]
- Walsh GM. Eosinophil granule proteins and their role in disease. Curr Opin Hematol. 2001;8:28–33. doi: 10.1097/00062752-200101000-00006. [DOI] [PubMed] [Google Scholar]
- Shinkai K, Mohrs M, Locksley RM. Helper T cells regulate type-2 innate immunity in vivo. Nature. 2002;420:825–829. doi: 10.1038/nature01202. [DOI] [PubMed] [Google Scholar]
- Malm-Erjefalt M, Persson CG, Erjefalt JS. Degranulation status of airway tissue eosinophils in mouse models of allergic airway inflammation. Am J Respir Cell Mol Biol. 2001;24:352–359. doi: 10.1165/ajrcmb.24.3.4357. [DOI] [PubMed] [Google Scholar]
- Gonzalo JA, Lloyd CM, Kremer L, Finger E, Martinez AC, Siegelman MH, Cybulsky M, Gutierrez-Ramos JC. Eosinophil recruitment to the lung in a murine model of allergic inflammation. The role of T cells, chemokines, and adhesion receptors. J Clin Invest. 1996;98:2332–2345. doi: 10.1172/JCI119045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AN, Mohammed AZ, Rice WR, Fiedeldey DT, Liebermann JS, Whitsett JA, Braciale TJ, Enelow RI. Perforin-independent CD8(+) T-cell-mediated cytotoxicity of alveolar epithelial cells is preferentially mediated by tumor necrosis factor-alpha: relative insensitivity to Fas ligand. Am J Respir Cell Mol Biol. 1999;20:849–858. doi: 10.1165/ajrcmb.20.5.3585. [DOI] [PubMed] [Google Scholar]
- Huffnagle GB, Lipscomb MF, Lovchik JA, Hoag KA, Street NE. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol. 1994;55:35–42. doi: 10.1002/jlb.55.1.35. [DOI] [PubMed] [Google Scholar]
- Jones HP, Tabor L, Sun X, Woolard MD, Simecka JW. Depletion of CD8+ T cells exacerbates CD4+ Th cell-associated inflammatory lesions during murine mycoplasma respiratory disease. J Immunol. 2002;168:3493–3501. doi: 10.4049/jimmunol.168.7.3493. [DOI] [PubMed] [Google Scholar]
- Adams B, Nagy N, Paulart F, Vanderhaeghen ML, Goldman M, Flamand V. CD8+ T lymphocytes regulating Th2 pathology escape neonatal tolerization. J Immunol. 2003;171:5071–5076. doi: 10.4049/jimmunol.171.10.5071. [DOI] [PubMed] [Google Scholar]
- Hussell T, Baldwin CJ, O’Garra A, Openshaw PJ. CD8+ T cells control Th2-driven pathology during pulmonary respiratory syncytial virus infection. Eur J Immunol. 1997;27:3341–3349. doi: 10.1002/eji.1830271233. [DOI] [PubMed] [Google Scholar]
- Allakhverdi Z, Lamkhioued B, Olivenstein R, Hamid Q, Renzi PM. CD8 depletion-induced late airway response is characterized by eosinophilia, increased eotaxin, and decreased IFN-gamma expression in rats. Am J Respir Crit Care Med. 2000;162:1123–1131. doi: 10.1164/ajrccm.162.3.9910001. [DOI] [PubMed] [Google Scholar]
- Zimring JC, Kapp JA. Identification and characterization of CD8+ suppressor T cells. Immunol Res. 2004;29:303–312. doi: 10.1385/IR:29:1-3:303. [DOI] [PubMed] [Google Scholar]
- Cortesini R, LeMaoult J, Ciubotariu R, Cortesini NS. CD8+CD28− T suppressor cells and the induction of antigen-specific, antigen-presenting cell-mediated suppression of Th reactivity. Immunol Rev. 2001;182:201–206. doi: 10.1034/j.1600-065x.2001.1820116.x. [DOI] [PubMed] [Google Scholar]
- Chess L, Jiang H. Resurrecting CD8+ suppressor T cells. Nat Immunol. 2004;5:469–471. doi: 10.1038/ni0504-469. [DOI] [PubMed] [Google Scholar]
- Molteni M, Rossetti C, Scrofani S, Bonara P, Scorza R, Kohn LD. Regulatory CD8+ T cells control thyrotropin receptor-specific CD4+ clones in healthy subjects. Cancer Detect Prev. 2003;27:167–174. doi: 10.1016/s0361-090x(03)00023-0. [DOI] [PubMed] [Google Scholar]
- Martin B, Banz A, Bienvenu B, Cordier C, Dautigny N, Becourt C, Lucas B. Suppression of CD4+ T lymphocyte effector functions by CD4+CD25+ cells in vivo. J Immunol. 2004;172:3391–3398. doi: 10.4049/jimmunol.172.6.3391. [DOI] [PubMed] [Google Scholar]
- Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10:801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- Jaffar Z, Sivakuru T, Roberts K. CD4+CD25+ T cells regulate airway eosinophilic inflammation by modulating the Th2 cell phenotype. J Immunol. 2004;172:3842–3849. doi: 10.4049/jimmunol.172.6.3842. [DOI] [PubMed] [Google Scholar]
- Hori S, Carvalho TL, Demengeot J. CD25+CD4+ regulatory T cells suppress CD4+ T cell-mediated pulmonary hyperinflammation driven by Pneumocystis carinii in immunodeficient mice. Eur J Immunol. 2002;32:1282–1291. doi: 10.1002/1521-4141(200205)32:5<1282::AID-IMMU1282>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Filaci G, Suciu-Foca N. CD8+ T suppressor cells are back to the game: are they players in autoimmunity? Autoimmun Rev. 2002;1:279–283. doi: 10.1016/s1568-9972(02)00065-4. [DOI] [PubMed] [Google Scholar]
- Humphreys IR, Edwards L, Walzl G, Rae AJ, Dougan G, Hill S, Hussell T. OX40 ligation on activated T cells enhances the control of Cryptococcus neoformans and reduces pulmonary eosinophilia. J Immunol. 2003;170:6125–6132. doi: 10.4049/jimmunol.170.12.6125. [DOI] [PubMed] [Google Scholar]
- Iwamoto I, Nakajima H, Endo H, Yoshida S. Interferon gamma regulates antigen-induced eosinophil recruitment into the mouse airways by inhibiting the infiltration of CD4+ T cells. J Exp Med. 1993;177:573–576. doi: 10.1084/jem.177.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble A, Zhao ZS, Cantor H. Suppression of immune responses by CD8 cells. II. Qa-1 on activated B cells stimulates CD8 cell suppression of T helper 2 responses. J Immunol. 1998;160:566–571. [PubMed] [Google Scholar]
- Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- Crow MK. Costimulatory molecules and T-cell-B-cell interactions. Rheum Dis Clin North Am. 2004;30:175–191, vii–viii. doi: 10.1016/S0889-857X(03)00111-X. [DOI] [PubMed] [Google Scholar]
- Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr Dir Autoimmun. 2005;8:25–54. doi: 10.1159/000082086. [DOI] [PubMed] [Google Scholar]
- De Milito A. B lymphocyte dysfunctions in HIV infection. Curr HIV Res. 2004;2:11–21. doi: 10.2174/1570162043485068. [DOI] [PubMed] [Google Scholar]
- Stoll M, Schmidt RE. Adverse events of desirable gain in immunocompetence: the immune restoration inflammatory syndromes. Autoimmun Rev. 2004;3:243–249. doi: 10.1016/j.autrev.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Koval CE, Gigliotti F, Nevins D, Demeter LM. Immune reconstitution syndrome after successful treatment of Pneumocystis carinii pneumonia in a man with human immunodeficiency virus type 1 infection. Clin Infect Dis. 2002;35:491–493. doi: 10.1086/341974. [DOI] [PubMed] [Google Scholar]
- Barry SM, Lipman MC, Deery AR, Johnson MA, Janossy G. Immune reconstitution pneumonitis following Pneumocystis carinii pneumonia in HIV-infected subjects. HIV Med. 2002;3:207–211. doi: 10.1046/j.1468-1293.2002.00115.x. [DOI] [PubMed] [Google Scholar]
- Chen F, Sethi G, Goldin R, Wright AR, Lacey CJ. Concurrent granulomatous Pneumocystis carinii and Mycobacterium xenopi pneumonia: an unusual manifestation of HIV immune reconstitution disease. Thorax. 2004;59:997–999. doi: 10.1136/thx.2003.012567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SF, Price P, Tay-Kearney ML, French MA. Cytomegalovirus (CMV) retinitis immune restoration disease occurs during highly active antiretroviral therapy-induced restoration of CMV-specific immune responses within a predominant Th2 cytokine environment. J Infect Dis. 2002;185:1813–1817. doi: 10.1086/340636. [DOI] [PubMed] [Google Scholar]
- Stone SF, Price P, Keane NM, Murray RJ, French MA. Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV Med. 2002;3:21–27. doi: 10.1046/j.1464-2662.2001.00096.x. [DOI] [PubMed] [Google Scholar]