Abstract
The transforming growth factor-β superfamily member bone morphogenetic protein-2 (BMP-2) is up-regulated in atherosclerotic arteries; however, its effects on the endothelium are not well characterized. Using microdissected coronary arterial endothelial cells (CAECs) and cultured primary CAECs, we demonstrated endothelial mRNA expression of BMP-2 and BMP-4. The proinflammatory cytokine tumor necrosis factor-α and H2O2 significantly increased endothelial expression of BMP-2 but not BMP-4. In organ culture, BMP-2 substantially decreased relaxation of rat carotid arteries to acetylcholine and increased production of reactive oxygen species, events inhibited by pharmacologically blocking protein kinase C (PKC) or NAD(P)H oxidase. BMP-2 activated nuclear factor-κΒ in CAECs, and BMP-2 and BMP-4 substantially increased adhesion of monocytic THP-1 cells, which was reduced by pharmacologically inhibiting p42/44 MAP kinase pathway (also by siRNA down-regulating ERK-1/2) or PKC. Incubation of rat carotid arteries with BMP-2 ex vivo also increased adhesion of mononuclear cells to the endothelium, requiring p42/44 MAP kinase and PKC. Western blotting showed that in CAECs and carotid arteries BMP-2 elicited phosphorylation of p42/44 MAP kinase, which was reduced by blocking MAP kinase kinase and PKC. Collectively, expression of BMP-2 is regulated by proinflammatory stimuli, and increased levels of BMP-2 induce endothelial dysfunction, oxidative stress, and endothelial activation. Thus, the proinflammatory effects of BMP-2 may play a role in vascular pathophysiology.
The cytokine bone morphogenetic protein-2 (BMP-2), a transforming growth factor superfamily member, was originally detected in cartilage and bone1; however, recent studies demonstrated that vascular endothelial and smooth muscle cells are also a significant source of BMPs.2–7 Genetic analysis of patients with primary pulmonary hypertension indicated that a vascular BMP-2/BMP receptor system plays an important role in vascular physiology.8,9 BMP-2 is known to regulate a host of cell-ular functions,2,4,5,10 including cardiovascular development,10 neovascularization in bone7 and tumors,11 and smooth muscle cell chemotaxis.2 Endothelium-derived BMP-2 is osteoinductive5,7 and hypotheses have been put forward that BMPs may contribute to vascular calcification.3,5,12 Despite evidence for the physiological/pathophysiological importance of BMP-2 the regulation of BMP-2 expression and the effects exerted by BMP-2 on endothelial function and phenotype have yet to be clearly elucidated.
Previously, we have demonstrated that in coronary arteries in hyperhomocysteinemia vascular inflammation and up-regulation of tumor necrosis factor (TNF)-α is associated with an increased vascular BMP-2 expression.13 Importantly, recent studies confirmed a striking up-regulation of BMPs in atherosclerotic lesions.2–4 BMP-4 (which is related to BMP-2 by its amino acid sequence but is transcribed from an entirely different gene)4,6 was shown to exert proinflammatory effects by enhancing monocyte adhesion to the endothelium. On the basis of the aforementioned findings we hypothesized that expression of BMP-2 in endothelial cells is regulated by inflammatory stimuli14 and/or that BMP-2 itself exerts proinflammatory, proatherogenic effects.
Materials and Methods
Animals
Male Wistar rats (n = 30; Taconic Biotechnology, Germantown, NY) were sacrificed by injection of sodium pentobarbital (50 mg/kg i.p.), and the carotid artery was isolated using microsurgery instruments. In some experiments the aorta, the septal coronary artery, femoral and brachial arteries, and the middle cerebral artery were also harvested.
Studies on Endothelial Cell Cultures
Primary rat coronary arterial endothelial cells (CAECs; Celprogen, San Pedro, CA), rat aortic vascular smooth muscle cells (VSMCs; Cell Applications Inc., San Diego, CA), and SV-40-immortalized rat aortic smooth muscle cells (SV40-SMC, no. CRL-2018; American Type Culture Collection, Manassas, VA) were maintained in culture as described.15 After passage 4 cells were treated with recombinant TNF-α (1 or 10 ng/ml for 24 hours)15 or H2O2 (100 μmol/L for 24 hours).
Microdissection of Arterial Endothelium
Microdissection was performed using the PALM microlaser technology (PALM GmbH, Bernried, Germany) on frozen sections (10 μm thick, stored at −80°C) of coronary vessels stained by hematoxylin as described.16 For RNA analyses, the endothelial and the smooth muscle layers of multiple vessels were selectively dissected and catapulted into 20 μl of catapulting buffer and stored at −80°C. RNA was extracted using the RNAqueous-Micro kit (Ambion, Austin, TX) as described.16 In separate experiments, endothelial and smooth muscle cells from TNF-α-treated (10 ng/ml for 24 hours) untreated control aortae were microdissected.
Quantitative Real-Time Polymerase Chain Reaction (PCR)
Total RNA from the arteries was isolated with Mini RNA isolation kit (Zymo Research, Orange, CA) and was reverse-transcribed using Superscript II RT (Invitrogen, Carlsbad, CA) as described previously.16,17 Real-time reverse transcriptase (RT)-PCR was used to analyze mRNA expression using the Stratagene MX3000, as reported.13,15–17 Samples were run in triplicates. Efficiency of the PCR reaction was determined using dilution series of a standard vascular sample. Quantification was performed using the ΔΔCt method. The housekeeping gene β-actin or GAPDH was used for internal normalization. Oligonucleotides used for real-time QRT-PCR are listed in Table 1. Fidelity of the PCR reaction was determined by melting temperature analysis and visualization of product on a 2% agarose gel.
Table 1.
Oligonucleotides for Real-Time RT-PCR
| mRNA targets | Sense | Anti-sense |
|---|---|---|
| Rat BMP-2 | 5′-TCAAGCCAAACACAAACAG-3′ | 5′-CGCTAAGCTCAGTGGG-3′ |
| Rat BMP-4 | 5′-GAATAAGAACTGCCGTCG-3′ | 5′-CCTTGTCGTACTCGTCC-3′ |
| Rat β-actin | 5′-GAAGTGTGACGTTGACAT-3′ | 5′-ACATCTGCTGGAAGGTG-3′ |
Immunolabeling
Rat hearts were embedded in OCT medium and 8-μm sections were cut using a cryostat. Dual immunofluorescent labeling was performed using a primary antibody against BMP-2 (R&D Systems, Minneapolis, MN) and α-smooth muscle actin as described.17
Western Blotting
Western blotting was performed as described,16–18 using a primary antibody that recognizes both the more abundant glycosylated (18 kd) and the nonglycosylated (13 to 15 kd) forms of BMP-2 (R&D Systems). Anti-β-actin (Novus Biologicals, Littletown, CO) was used for normalization purposes.
Vessel Culture and Functional Studies
Isolated carotid arteries were maintained in vessel culture under sterile conditions in F12 medium (Life Technologies, Inc., Grand Island, NY) containing antibiotics (100 UI/L penicillin, 100 mg/L streptomycin) and supplemented with 5% fetal calf serum (Gibco/Invitrogen), as previously described.13,15,19,20 Arteries were treated with BMP-2 (1 to 100 ng/ml) for 24 hours. In some experiments vessels were treated with 10 ng/ml of BMP-2 in the presence of a neutralizing antibody (mAb 3552, 20 μg/ml; R&D Systems).
Endothelial function was assessed as previously described.21 In brief, cultured arteries were cut into ring segments 2 mm in length and mounted on 40-μm stainless steel wires in the myograph chambers (Danish Myo Technology A/S, Inc., Atlanta, GA) containing Krebs buffer solution (118 mmol/L NaCl, 4.7 mmol/L KCl, 1.5 mmol/L CaCl2, 25 mmol/L NaHCO3, 1.1 mmol/L MgSO4, 1.2 mmol/L KH2PO4, and 5.6 mmol/L glucose, at 37°C, gassed with 95% air and 5% CO2) for measurement of isometric tension. After an equilibration period of 1 hour during which an optimal passive tension of 0.5 g was applied to the rings (as determined from the vascular length-tension relationship), the vessels were contracted by KCl (30 mmol/L) and relaxations to acetylcholine (from 10−9 to 10−4 mol/L) and the NO donor S-nitrosopenicillamine (from 10−9 to 3 × 10−5 mol/L) were obtained.
Measurement of Reactive Oxygen Species
Production of O2.− in segments of the same carotid arteries that were used for functional studies was assessed using the dihydroethidine staining method, as described.13,16,19,20 In addition, O2.− generation was measured by the lucigenin (10 μmol/L) chemiluminescence method, as described.13,16,19,20
In CAECs reactive oxygen species (ROS) production was measured using the methods of Werner,22 after the following treatments: BMP-2 (100 ng/ml), BMP-2 plus diphenyleneiodonium [DPI, 10−5 mol/L, an inhibitor of flavoprotein-containing oxidases, including NAD(P)H oxidases] or BMP-2 plus apocynin (3 × 10−4 mol/L, which inhibits NAD(P)H oxidases19,20) or BMP-2 plus chelerythrine (an inhibitor of total protein kinase C activity; 10 μmol/L). Cells were incubated with an assay mix consisting of homovanillic acid (100 μmol/L), horseradish peroxidase (5 U/ml), and superoxide dismutase (SOD; 200 U/ml, to convert O2.− to H2O2) in HEPES-buffered salt solution (pH 7.5) at 37°C for 1 hour. The reaction was stopped with 80 μl of glycine solution (0.1 mol/L, pH 10, 0°C). H2O2-induced fluorescent product was assessed using a fluorimeter (excitation, 321 nm; emission, 421 nm), and the background-corrected fluorescent signal was normalized to the cell count. Calibration curve was constructed using 0.01 to 100 μmol/L H2O2 standards in assay mix (1 hour at 37°C) with or without catalase (200 U/ml).
Transient Transfection and Luciferase Assays
Effect of BMP-2 and TNF-α on nuclear factor (NF)-κΒ activity in CAECs and VSMCs was tested by a reporter gene assay. We used a NF-κΒ reporter comprised of a NF-κB response element upstream of firefly luciferase (NF-κΒ-Luc; Stratagene, La Jolla, CA) and a Renilla luciferase plasmid under the control of the CMV promoter (as an internal control). All transfections were performed with Novafector (Venn Nova LLC, Pompano Beach, FL) following the manufacturer’s protocols. Firefly and Renilla luciferase activities were assessed after 42 hours using the Dual Luciferase Reporter Assay kit (Promega, Madison, WI) and a luminometer. Pyrrolidine dithiocarbamate (10−5 mol/L), an inhibitor of NF-κB activation, was used as control.
Monocyte Adhesion Assays: Inhibition of MAP Kinase Pathways by Pharmacological and Molecular Methods
We measured adhesion of fluorescently labeled human monocytic (THP-1) cells to confluent monolayers of HCAECs using a microplate-based assay. In brief, HCAECs were grown to confluence in 96-well plates and were treated with BMP-2 or BMP-4 (0.1 to 30 ng/ml; incubation time, 2 hours at 37°C) in the absence or presence (30 minutes of preincubation) of the protein kinase C (PKC) inhibitors chelerythrine (10 μmol/L) and staurosporine (10−6 mol/L), or PD 98059 (10 μmol/L) and U0126 [10 μmol/L; two structurally unrelated inhibitors of p42/44 (ERK1/2) MAP kinase activation by inhibition of its phosphorylation by MAP kinase kinase] or SB 203580 (5 μmol/L, a pyridinyl imidazole inhibitor of the p38 MAP kinase)23 or DPI (10−5 mol/L) or apocynin (3 × 10−4 mol/L) or the free radical scavengers SOD plus catalase (200 U/ml). TNF-α was used as positive control. THP-1 cells were labeled with the fluorescent dye BCECF (5 μmol/L final concentration; Molecular Probes, Eugene, OR; in serum-free RPMI medium for 45 minutes at 37°C). Then the cells were washed twice with prewarmed (37°C) RPMI. PMA (phorbol myristate acetate, 10−6 mol/L)-pretreated fluorescently labeled THP-1 cells (5 × 105/well) were added the microplate wells containing confluent HCAECs (medium removed; incubation time of 45 minutes at 37°C). Nonadherent THP-1 cells were removed by careful washing (three times with prewarmed RPMI). Then, 200 μl of phosphate-buffered saline (PBS) was added to each well, and fluorescence was measured using an Flx-800 (Bio-Tek Instruments) fluorescent plate reader (excitation, 485 nm; emission, 528 nm). Controls included measurement of total fluorescence of labeled cells before adhesion, controls for measuring autofluorescence of unlabeled cells, and measurement of monocyte adhesion to endothelial cell-free microplate wells.
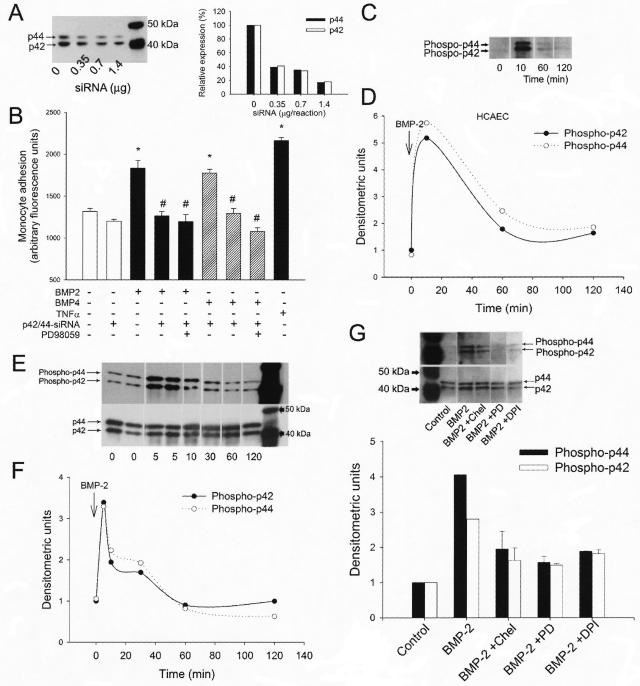
In separate experiments down-regulation of the p42 and p44 MAP kinases in HCAECs was achieved by RNA interference using the proprietary p42 and p44 siRNA sequences (Cell Signaling, Beverly, MA) and the Amaxa Nucleofector technology (Amaxa, Gaithersburg, MD), as we have previously reported.15,18 Cell density at transfection was 30%. Specific gene silencing was verified with Western blotting as described.15 Monocyte adhesion assay was performed using HCAECs transfected with anti-p42 and anti-p44 siRNA on day 2 after the transfection, when gene silencing was optimal.
In other experiments, monocyte-enriched peripheral blood mononuclear cells were isolated from rats, and BCECF-labeled mononuclear cell binding to the endothelium of carotid arteries was determined according to the methods of Sorescu and colleagues.4 Some vessel segments were pretreated with chelerythrine, PD 98059, and/or SB 203580. After treatment with BMP-2 (10 to 100 ng/ml for 2 hours) or TNF-α (10 ng/ml for 2 hours) vessels were cut open (en face) and incubated with PMA (10−6 mol/L)-pretreated BCECF-loaded monocytes. After a 1-hour incubation at 37°C under, unbound monocytes were washed out. Bound monocytes were quantified by counting the cells under a fluorescent microscope.
Detection of BMP-2-Induced p42/44 MAPK Phosphorylation
Phosphorylation of p42/44 MAP kinase was detected by Western blotting as described.23 In brief, HCAECs were treated with BMP (10 ng/ml) for 0 to 120 minutes. The cells were rinsed with PBS and then hot lyses buffer was added (10 mmol/L Tris, pH 7.4, 1 mmol/L sodium orthovanadate, 1% sodium dodecyl sulfate). The lysate was microwaved for 5 seconds. In separate experiments HCAECs pretreated with PD 98059, DPI, and chelerythrine were compared.
In other experiments rat carotid arterial segments were treated with BMP (10 ng/ml) for 0 to 120 minutes and then snap-frozen in liquid nitrogen. Protein samples were prepared, as previously described.23 Na3VO4 (1 mmol/L) and phosphatase inhibitor cocktail I and II (10 μl; Sigma Chemical Co., St. Louis, MO) were added to the homogenization buffer to inhibit phosphatases. Equal amounts of protein (50 μg) were electrophoresed on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gel, transferred to a PVDH membrane with a semidry blotting system, and labeled with phosphorylation-specific primary antibody for p42/44 MAP kinase (Cell Signaling) in 1:1000 dilution for 24 hours at 4°C. The membranes were washed with PBS and incubated for 1 hour with sheep anti-rabbit IgG horseradish peroxidase or donkey anti-mouse IgG horseradish peroxidase (Amersham, Arlington Heights, IL) at the final titer of 1:4000. The membranes were developed with ECL-Plus (Amersham).
Data Analysis
Data were normalized to the respective control mean values. Data are expressed as mean ± SEM. Statistical analyses of data were performed by Student’s t-test or by two-way analysis of variance followed by the Tukey post hoc test, as appropriate. P < 0.05 was considered statistically significant.
Results
Expression of BMP2/4 in Endothelial and Smooth Muscle Cells
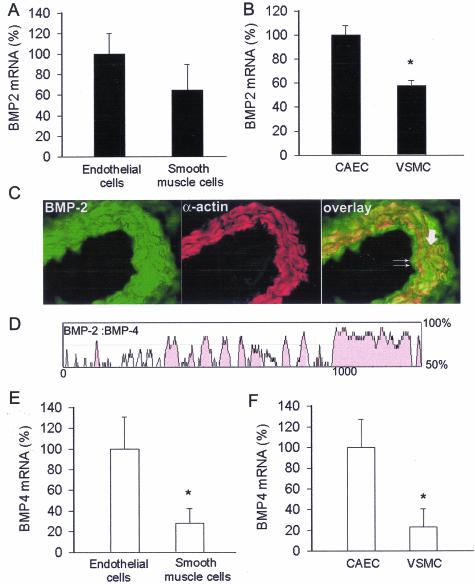
Both endothelial and smooth muscle cells express BMP-2 mRNA. Expression of BMP-2 mRNA was more pronounced in cultured CAECs than in VSMCs. Although BMP-2 mRNA expression tended to be greater in microdissected endothelial cells, the difference did not reach statistical significance because of the greater variance in the samples (Figure 1, A and B). Comparison of BMP-2 mRNA levels in aorta, coronary, cerebral, femoral, and brachial arteries suggest that BMP-2 is uniformly expressed in the systemic circulation (Table 2). Similar results were obtained in mouse aorta, carotid, mesentery, femoral, and renal arteries as well (not shown). Immunolabeling (Figure 1C) and Western blotting (Figure 2) confirmed that BMP-2 protein is present both in endothelial and smooth muscle cells. Full-length BMP-2 is a 396-amino acid glycosylated polypeptide that has a 19-amino acid signal sequence, a 263-amino acid proregion, and a 114-amino acid mature segment. BMP-4 is a related cytokine consisting of a 408-amino acid prepropeptide with a 19-amino acid signal sequence, a 273-amino acid proregion, and a 116-amino acid mature segment. We used rVISTA (http://www-gsd.lbl.gov/vista), a tool for comparative sequence analysis,24 to show that the mRNA sequences encoding the mature forms of the rat BMP-2 and BMP-4 exhibit a high degree of homology (Figure 1D). BMP-4 mRNA expression was more pronounced both in microdissected CAECs and cultured endothelial cells than in smooth muscle cells (Figure 1, E and F).
Figure 1.
A and B: Expression of BMP-2 in endothelial and smooth muscle cells microdissected from coronary arteries (A) and in cultured primary rat CAECs and VSMCs (B). Analysis of mRNA expression was performed by real-time QRT-PCR. GAPDH was used for normalization. Data are mean ± SEM (n = 3 to 5 for each group). *P < 0.05. C: Fluorescent photomicrographs showing that immunolabeling for BMP-2 (green) is present in endothelial cells (double arrows) and medial smooth muscle cells (bold arrow, smooth muscle cells show red immunofluorescent labeling for α-smooth muscle actin). D: VISTA plot showing the percentage of conservation between the mRNAs transcribed from the rat BMP-2 and BMP-4 genes. E and F: Expression of BMP-4 in endothelial and smooth muscle cells microdissected from coronary arteries (E) and in cultured CAECs and VSMCs (F). Data are mean ± SEM (n = 3 to 5 for each group). *P < 0.05.
Table 2.
BMP-2 mRNA Expression in Different Vascular Beds
| BMP-2 mRNA (%) | |
|---|---|
| Aorta | 100 ± 36 |
| Coronary artery | 109 ± 20 |
| Middle cerebral artery | 79 ± 40 |
| Femoral artery | 127 ± 39 |
| Brachial artery | 148 ± 36 |
Expression of BMP-2 mRNA in different vascular beds in male Wistar rats. β-Actin mRNA was used for normalization purposes. Data are mean ± SD (n = 4 in each group). n.d.: not determined. Values are normalized to the mean of BMP:β-actin ratios in the aorta.
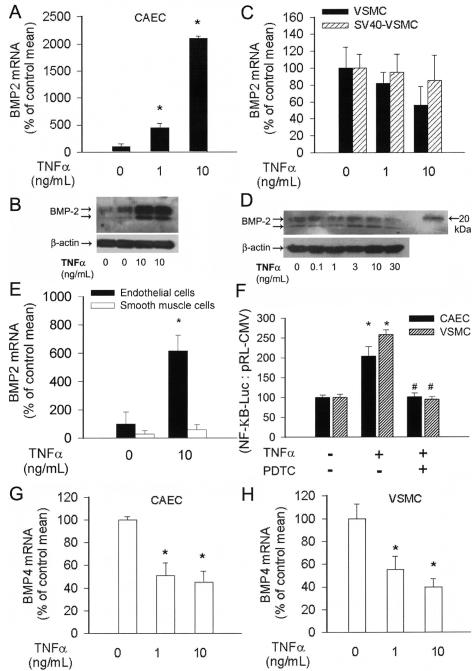
Figure 2.
Effect of TNF-α (1 or 10 ng/ml, for 24 hours) on the expression of BMP-2 mRNA (A, C: QRT-PCR) and protein (B, D: Western blotting) in cultured primary rat CAECs and VSMCs. Data are mean ± SEM (n = 4 to 8 for each group). *P < 0.05 versus untreated control. E: Expression of BMP-2 mRNA in endothelial and smooth muscle cells microdissected from control and TNF-α-treated (10 ng/ml, for 24 hours) cultured rat aortas. Data are mean ± SEM. *P < 0.05 versus untreated control. F: Reporter gene assay showing the effects of TNF-α (10 ng/ml) on NF-κΒ reporter activity in CAECs and VSMCs. Cells were transiently co-transfected with NF-κΒ-driven firefly luciferase and CMV-driven Renilla luciferase constructs followed by TNF-α stimulation. Cells were then lysed and subjected to luciferase activity assay. After normalization relative luciferase activity was obtained from seven independent transfections. In control experiments PDTC was used to inhibit NF-κB activity. Data are mean ± SEM. *P < 0.05 versus control. G: Effect of TNF-α (1 or 10 ng/ml, for 24 hours) on the expression of BMP-4 mRNA (QRT-PCR) in CAECs and VSMCs (H). Data are mean ± SEM. *P < 0.05 versus untreated control.
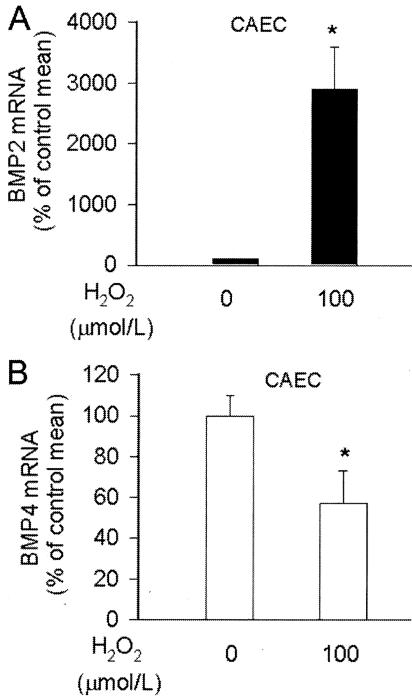
To gain some insight in the regulation of vascular BMP expression, we investigated the effects of known proinflammatory stimuli in vitro in cultured endothelial and smooth muscle cells. In CAECs TNF-α elicited substantial increases in both the mRNA and protein expression of BMP-2, extending our recent findings18 (Figure 2, A and B). Unexpectedly, although TNF-α elicited similar NF-κΒ activation in CAECs and VSMCs (Figure 2F), it did not increase BMP-2 mRNA and protein expression in VSMCs (Figure 2, C and D). This effect was not cell line-dependent because identical findings were obtained in SV-40-immortalized aortic smooth muscle cells (Figure 2C) and in microdissected smooth muscle cells from TNF-α-treated rat aortas (Figure 2E). In contrast to BMP-2, expression of BMP-4 mRNA significantly decreased in TNF-α-treated CAECs and VSMCs (Figure 2, G and H). Inhibition of NF-κB activation with Pyrrolydine dithiocarbamate did not affect TNF-α-induced down-regulation of BMP-4 in CAECs (not shown). H2O2 also elicited substantial up-regulation of BMP-2, but not BMP-4, in CAECs (Figure 3, A and B), which is consistent with the view that both H2O2 and TNF-α regulate BMP-2 expression by activating a common signaling pathway.18
Figure 3.
Effect of H2O2 on BMP-2 (A) and BMP-4 (B) mRNA expression in CAECs (QRT-PCR). Data are mean ± SEM (n = 5 for each group). *P < 0.05 versus untreated control.
We used rVISTA to search for evolutionarily conserved regions of the human and rat BMP-2 and BMP-4 promoters. This analysis revealed that despite the significant homology between the coding sequences for BMP-2 and BMP-4, the 5′ flanking regulatory regions of the BMP-2 and BMP-4 genes are markedly different. Importantly, the highly conserved NF-κΒ-binding site that is present in the BMP-2 promoter region18,25–27 is absent in the 5′ flanking region of the BMP-4 gene, which may explain the opposite regulation of the two genes by TNF-α and H2O2 in endothelial cells.
Effect of BMP-2 on Endothelial Vasodilator Function and ROS Production
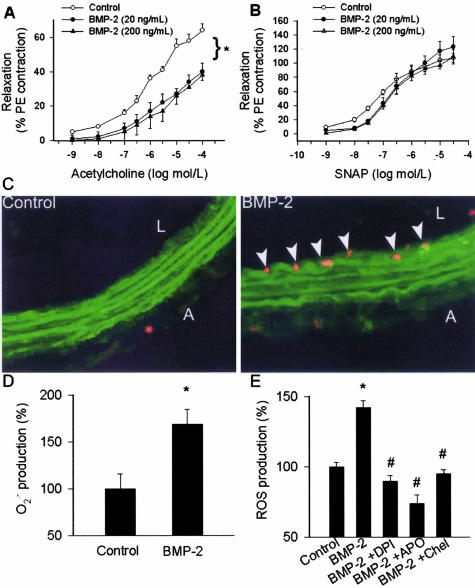
BMP-2 treatment (in all concentrations tested) resulted in a significant impairment of acetylcholine-induced relaxation of cultured rat carotid arteries (Figure 4A). In contrast, BMP-2 elicited only minor alterations of vascular relaxations to the NO donor S-nitrosopenicillamine (Figure 4B). In BMP-2-treated vessels there was an intensive ethidium bromide staining, localized to the endothelial cells (Figure 4C), and a significantly increased lucigenin chemiluminescent signal (Figure 4D), indicating that BMP-2 promotes endothelial O2.− generation. In CAECs BMP-2 also elicited substantial increases in ROS generation that were inhibited by apocynin, DPI, and chelerythrine (Figure 4E). Acetylcholine-induced relaxations (10−5 mol/L; control, 55 ± 4%; BMP-2, 27 ± 3%; BMP-2 + neutralizing antibody, 48 ± 8%) and endothelial ethidium bromide staining (fluorescence units; control, 0.6 ± 0.2; BMP-2, 13.5 ± 1.5; BMP-2 + neutralizing antibody, 1.2 ± 0.5) did not differ between untreated vessels and vessels co-incubated with BMP-2 and a BMP neutralizing antibody, confirming that the observed effects were specific to BMP-2 activity.
Figure 4.
Relaxations to acetylcholine (A) and the NO donor S-nitrosopenicillamine (B) in ring preparations of rat carotid arteries maintained in vessel culture (for 24 hours) in the absence and presence of recombinant BMP-2 (20 or 200 ng/ml). Data are mean ± SEM (n = 4 to 6 for each group). *P < 0.05. C: Compared to the untreated controls (left), BMP-2 elicited significant increases in endothelial O2.− production, as indicated by the intensive red fluorescent staining of the endothelial nuclei by ethidium bromide (right). Green autofluorescence is shown for orientation purposes (L, lumen; A, adventitia). D: Demonstration of increased O2.− production in BMP-2-treated cultured carotid arteries using lucigenin chemiluminescence (mean ± SEM, n = 5 for each group; *P < 0.05 versus untreated control). E: BMP-2 induced generation of reactive oxygen species in CAECs (assessed by a homovanillic acid/horseradish peroxidase method in the presence of SOD, to convert O2.− to H2O2) in the absence and presence of the NAD(P)H oxidase inhibitor DPI and apocynin (APO) or the PKC inhibitor chelerythrine. Results are normalized to the control mean values (n = 5 for each group; *P < 0.05 versus untreated control, #P < 0.05 versus BMP-2 treatment, data are mean ± SEM).
Demonstration of BMP-2-Induced Activation of NF-κΒ in Endothelial Cells
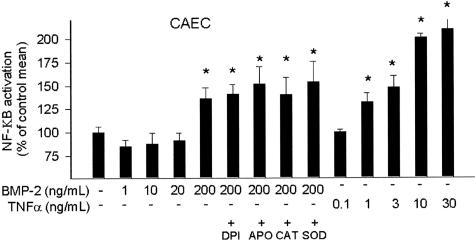
We demonstrated that BMP-2 at a concentration of 200 ng/ml (but not at 20 ng/ml) significantly enhanced the transcriptional activity of NF-κΒ in CAECs (as indicated by an increase in the luciferase activity; Figure 5). Interestingly, BMP-2-induced NF-κΒ activity could not be inhibited by catalase, SOD, DPI, or apocynin (Figure 5), suggesting that NAD(P)H oxidase-derived H2O2 production may not play a key role in BMP-2-induced NF-κΒ activation in CAECs.
Figure 5.
Reporter gene assay showing the effects of NAD(P)H oxidase inhibitors (3 × 10−4 mol/L APO, 10−5 DPI), SOD (200 U/ml), or catalase (200 U/ml) on BMP-2-induced NF-κΒ reporter activity in CAECs. Concentration-dependent TNF-α-induced NF-κΒ reporter activity is shown for comparison. Endothelial cells were transiently co-transfected with NF-κΒ-driven firefly luciferase and CMV-driven Renilla luciferase constructs followed by BMP-2 or TNF-α stimulation. Cells were then lysed and subjected to luciferase activity assay. After normalization relative luciferase activity was obtained from four to seven independent transfections. Data are mean ± SEM. *P < 0.05 versus control.
Demonstration of BMP-2/4-Induced Monocyte Adhesion: Role of ROS, PKC, and MAP Kinases
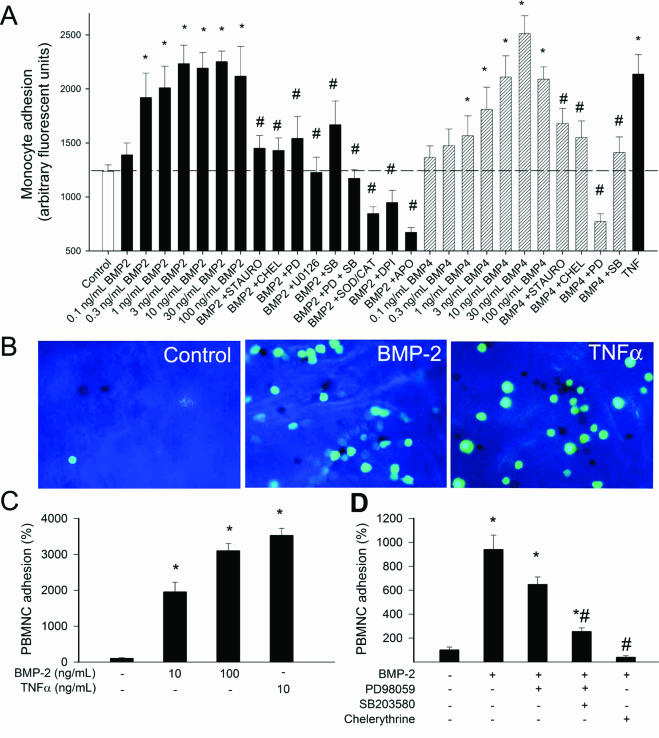
BMP-2 significantly increased monocyte adherence to cultured HCAECs in a concentration-dependent manner (Figure 6A). BMP-4 also increased monocyte adherence to HCAECs in a similar concentration range. Pretreatment of the vessels with pharmacological inhibitors of PKC, p38, and p42/44 MAP kinases, and NAD(P)H oxidase significantly reduced or prevented BMP-2- and BMP-4-induced monocyte adhesion (Figure 6A). BMP-2 significantly increased monocyte adherence to the endothelium of intact aortae (Figure 6, B and C). Pretreatment of the vessels with PD 98059, SB203580, and chelerythrine significantly decreased BMP-2-induced monocyte adhesion (Figure 6D). We also found that pretreatment of HCAECs with anti-p42/44 MAP kinase siRNAs prevented BMP-2-induced increases in monocyte adhesiveness (Figure 7B). Western blotting was used to show the efficiency of the siRNA treatment (Figure 7A).
Figure 6.
A: Results of monocyte adhesion assay (see Materials and Methods). Treatment of primary human CAECs with increasing concentrations of BMP-2 and BMP-4 (2 hours) significantly increased the adhesion of fluorescently labeled PMA-stimulated monocytes. TNF-α (10 ng/ml) was used as positive control. The effects of 10 ng/ml BMP-2 and BMP-4 were also assessed after pretreatment with inhibitors on p42/44 MAP kinase (10 μmol/L PD 98059, 10 μmol/L U0126), p38 MAP kinase [10 μmol/L SB203580, PKC (10 μmol/L chelerythrine (CHEL), 10 μmol/L staurosporine (STAURO)], NAD(P)H oxidase (10 μmol/L DPI, 3 × 10−4 mol/L APO), and the free radical scavenger SOD plus catalase (200 U/ml). Data are mean ± SEM (n = 8 for each group). *P < 0.05 versus untreated control. #P < 0.05 versus BMP treatment. B: Representative fluorescent images showing the effect of BMP-2 and TNF-α treatments (2 hours) on the adhesion of activated monocytes (green fluorescence) to the endothelium of rat aortic segments (en face preparation). C: Summary bar graphs are shown (data are normalized to control mean values). D: Effects of PD 98059, SB203580, and chelerythrine on BMP-2 induced monocyte adhesion to the aortic endothelium. Data are mean ± SEM. *P < 0.05 versus control, #P < 0.05 versus BMP-2 treatment. Original magnifications, ×40.
Figure 7.
A: Representative Western blot (left) and densitometric data (right) showing the effect of increasing concentrations of anti-p42/p44 siRNAs on the expression of p42/44 MAP kinase in primary human coronary arterial endothelial cells (HCAECs). B: Effect of pretreatment with anti-p42/p44 siRNAs on BMP-2- and BMP-4- (10 ng/ml, for 2 hours) induced adhesion of fluorescently labeled PMA-stimulated monocytes to HCAECs. PD98059 (30 minutes, 10 μmol/L) was used to pharmacologically inhibit MAP kinase activity. TNF-α (10 ng/ml) was used as positive control. Data are mean ± SEM. *P < 0.05 versus control, #P < 0.05 versus BMP-2/4 treatment. C–F: Representative Western blots (C, E) and densitometric data (D, F) showing the time course of p42/44 MAP kinase phosphorylation in BMP-2 (10 ng/ml)-treated HCAECs (C, D) and rat carotid arterial segments (E, F). G: Representative Western blot (top) and densitometric data (bottom) showing BMP-2 (10 ng/ml, 10 minutes)-induced phosphorylation of p42/44 MAP kinase in HCAECs pretreated with DPI, chelerythrine, and PD98059.
Demonstration of BMP-Induced MAP Kinase Activation in Endothelial Cells
In carotid arterial segments BMP-2 elicited rapid increases in phosphorylation of p42/44 MAP kinase (Figure 7, E and F). Similar time course of p42/44 MAP kinase phosphorylation was observed in BMP-2-treated HCAECs (Figure 7, C and D), suggesting that BMP-2 activates MAP kinase pathways in the endothelium, which promotes monocyte adhesiveness (Figures 6A and 7A). BMP-2-induced phosphorylation of p42/44 MAP kinase was decreased in HCAECs pretreated with DPI and chelerythrine (Figure 7G).
Discussion
There are three important findings in this study. First, we have shown that vascular endothelial and smooth muscle cells, both in vivo and in culture, express BMP-2 mRNA and protein (Figure 1). Interestingly, endothelial cells tend to express higher levels of BMP-2 as well as BMP-4 (which exhibit a high degree of similarity with BMP-2) than smooth muscle cells (Figure 1). Our data suggest that in endothelial cells expression of BMP-2 is regulated by proinflammatory stimuli, such as TNF-α and H2O2 (Figures 2A and 3A). These findings are in line with recent results showing that NF-κΒ signaling plays a central role in regulation of BMP-2 expression.18 Importantly, endothelial NF-κΒ activation28 and enhanced co-expression of BMP-2 and TNF-α13; have been recently demonstrated in hyperhomocysteinemia, a pathophysiological condition that promotes vascular inflammation and atherosclerosis.
Interestingly, we also found that in smooth muscle cells TNF-α did not increase BMP-2 expression (Figure 2, C–E). Because TNF-α elicited comparable NF-κB activation in endothelial and smooth muscle cells (Figure 2F), we speculate that differences in factors that lie downstream from NF-κB (eg, differential expression of cell-specific co-activators) are responsible for this phenomenon. It is of note that vascular endothelial growth factor was also recently shown to up-regulate BMP-2 in cultured human dermal microvascular endothelial cells but not in lymphatic endothelial cells.29 Although this finding raised the possibility that regulation of BMP-2 expression may also differ between endothelial cells from different organs, we found that basal BMP-2 expression was comparable in vessels from various systemic vascular beds (Table 2). Also, we have found that TNF-α elicited similar BMP-2 induction in human umbilical vein endothelial cells and CAECs.18
Although BMP-2 and BMP-4 exhibit a high degree of sequence similarity and likely act on the same receptor(s), the biological role of the two cytokines may be different. Importantly, our data show that proinflammatory stimuli have an opposite effect on endothelial expression of BMP-4 and BMP-2 (Figures 2G and 3B). Accordingly, the promoter regions of the human and mouse BMP-2 and BMP-4 genes contain markedly different regulatory elements.26 Alignment of the DNA sequences of the 5′ flanking regions of the human, mouse, and/or rat genes revealed that a highly conserved NF-κΒ binding site is present in the BMP-2 promoter region,18,25–27 but it is missing from the BMP-4 promoter, which provides an explanation for the different regulation of the two related cytokines by TNF-α and H2O2.
The second significant finding in this study was that BMP-2 elicited endothelial dysfunction (Figure 4A) and substantial NAD(P)H oxidase-derived ROS production in the endothelial cells (Figure 4, C and D). The finding that chelerythrine inhibited BMP-2-induced ROS generation suggests that it is mediated by PKC activation (Figure 4E). Indeed, our previous studies showed that endothelial NAD(P)H oxidase activity is regulated by PKC in a calcium-dependent manner.19,20 Further, there is evidence for the association of the BMP receptor complex with PKC.30 BMP-4 was also reported to induce oxidative stress in human umbilical vein endothelial cells6; however, further studies are needed to establish the role of PKC in this process. One of the transcriptional mechanisms that mediate proinflammatory phenotypic changes in blood vessels is an activation of NF-κΒ. NF-κΒ may be activated by both redox-dependent and -independent mechanisms.6 We found that BMP-2, especially at higher concentrations, elicits NF-κΒ activation in endothelial cells (Figure 5). Yet, because NF-κΒ activation was unaffected by inhibition of NAD(P)H oxidase or scavenging of ROS, we propose that NAD(P)H oxidase-derived ROS are unlikely to mediate BMP-2-induced NF-κΒ activation in endothelial cells (Figure 5). It is also yet to be determined which cellular programs are induced by BMP-2 via NF-κΒ.
The third interesting finding was that BMP-2 plays an important role in endothelial activation. We have demonstrated that the monocyte adhesiveness both to cultured endothelial cells and vascular endothelial cells in situ was substantially increased by BMP-2 as well as BMP-4 (Figure 6, A–C). Because monocyte adhesion was substantially reduced by pharmacological and molecular inhibition of p42/44 and p38 MAP kinases (Figure 6, A and D, and Figure 7A) and abolished by inhibition of PKC in endothelial cells both in culture and in intact vessels (Figure 6, A and D), we propose that in endothelial cells BMP-2/4 activates a PKC- and MAP kinase-dependent pathway,11,31,32 which results in endothelial activation. This view is supported by previous findings demonstrating that the BMP receptor complex is associated with components of PKC and MAP kinase pathways, which can be activated on BMP-2 binding to its receptor.11,30–35 Because inhibition of PKC decreased BMP-2-induced NAD(P)H oxidase-derived ROS generation (Figure 4E) and inhibition of NAD(P)H oxidase prevented BMP-2 induced endothelial activation (Figure 6A), we posit that NAD(P)H oxidase activation represents a link between PKC and downstream signaling mechanisms, including activation of MAP kinase(s). Indeed, we have evidence that BMP-2 induces p42/44 MAP kinase activation both in cultured endothelial cells (Figure 7, C and D) and intact vessels (Figure 7, E and F), and this effect can be reduced by inhibition of PKC and the NAD(P)H oxidase (Figure 7G).
In conclusion, it seems that in endothelial cell inflammatory stimuli (TNF-α, H2O2) regulate BMP-2 expression. BMP-2 itself can elicit oxidative stress in endothelial cells and promote vasodilator dysfunction. In addition, BMP-2 may induce NF-κΒ and promote proatherogenic endothelial activation via PKC/MAP kinase-dependent pathway(s).4,6 Thus, we propose that BMP-2 acts as a proinflammatory cytokine in the systemic circulation. More information on the phenotypic consequences of BMP-2 induction should improve our understanding of pathological vascular remodeling and development of early atherosclerosis.
Footnotes
Address reprint requests to Zoltan Ungvari, M.D., Ph.D., Department of Physiology, New York Medical College, Valhalla, New York 10595. E-mail: zoltan_ungvari@nymc.edu.
Supported by the American Heart Association (grants 0430108N and 0435140N), the American Health Assistance Foundation (grant H2004-024), the American Federation for Aging Research, Philip Morris USA, and the National Institutes of Health (PO-1-HL-43023).
References
- Nomura S, Takano-Yamamoto T. Molecular events caused by mechanical stress in bone. Matrix Biol. 2000;19:91–96. doi: 10.1016/s0945-053x(00)00050-0. [DOI] [PubMed] [Google Scholar]
- Willette RN, Gu JL, Lysko PG, Anderson KM, Minehart H, Yue T. BMP-2 gene expression and effects on human vascular smooth muscle cells. J Vasc Res. 1999;36:120–125. doi: 10.1159/000025634. [DOI] [PubMed] [Google Scholar]
- Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, Tordoir JH, Spronk HM, Vermeer C, Daemen MJ. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, Boyd N, Boo YC, Vega JD, Taylor WR, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem. 2003;278:31128–31135. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- Shin V, Zebboudj AF, Bostrom K. Endothelial cells modulate osteogenesis in calcifying vascular cells. J Vasc Res. 2004;41:193–201. doi: 10.1159/000077394. [DOI] [PubMed] [Google Scholar]
- Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a Nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- Kaigler D, Krebsbach PH, West ER, Horger K, Huang YC, Mooney DJ. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. FASEB J. 2005;19:665–667. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- De Caestecker M, Meyrick B. Bone morphogenetic proteins, genetics and the pathophysiology of primary pulmonary hypertension. Respir Res. 2001;2:193–197. doi: 10.1186/rr57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M. The mouse through the looking glass: a new door into the pathophysiology of pulmonary hypertension. Circ Res. 2004;94:1001–1004. doi: 10.1161/01.RES.0000128079.89263.68. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- Langenfeld EM, Langenfeld J. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res. 2004;2:141–149. [PubMed] [Google Scholar]
- Griethe W, Schmitt R, Jurgensen JS, Bachmann S, Eckardt KU, Schindler R. Bone morphogenic protein-4 expression in vascular lesions of calciphylaxis. J Nephrol. 2003;16:728–732. [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, Koller A. Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2003;23:418–424. doi: 10.1161/01.ATV.0000061735.85377.40. [DOI] [PubMed] [Google Scholar]
- Fukui N, Zhu Y, Maloney WJ, Clohisy J, Sandell LJ. Stimulation of BMP-2 expression by pro-inflammatory cytokines IL-1 and TNF-alpha in normal and osteoarthritic chondrocytes. J Bone Joint Surg Am. 2003;85-A(Suppl 3):59–66. doi: 10.2106/00004623-200300003-00011. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Proinflammatory phenotype of coronary arteries promotes endothelial apoptosis in aging. Physiol Genom. 2004;17:21–30. doi: 10.1152/physiolgenomics.00136.2003. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Edwards JG, Kaminski PM, Wolin MS, Koller A, Kaley G. Aging-induced phenotypic changes and oxidative stress impair coronary arteriolar function. Circ Res. 2002;90:1159–1166. doi: 10.1161/01.res.0000020401.61826.ea. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Ungvari Z, Koller A, Edwards JG, Kaley G. Aging-induced proinflammatory shift in cytokine expression profile in rat coronary arteries. FASEB J. 2003;17:1183–1185. doi: 10.1096/fj.02-1049fje. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Smith K, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of Bone Morphogenetic Protein-2 Expression in Endothelial Cells: Role of Nuclear Factor-κB Activation by Tumor Necrosis Factor-α, H2O2, and High Intravascular Pressure. Circulation. 2005;111:2364–2372. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Chronic high pressure-induced arterial oxidative stress: involvement of protein kinase C-dependent NAD(P)H oxidase and local renin-angiotensin system. Am J Pathol. 2004;165:219–226. doi: 10.1016/S0002-9440(10)63290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation. 2003;108:1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- Gupte SA, Arshad M, Viola S, Kaminski PM, Ungvari Z, Rabbani G, Koller A, Wolin MS. Pentose phosphate pathway coordinates multiple redox-controlled relaxing mechanisms in bovine coronary arteries. Am J Physiol. 2003;285:H2316–H2326. doi: 10.1152/ajpheart.00229.2003. [DOI] [PubMed] [Google Scholar]
- Werner E. Determination of cellular H2O2 production. Sci STKE. 2003;168:PL3. doi: 10.1126/stke.2003.168.pl3. [DOI] [PubMed] [Google Scholar]
- Massett MP, Ungvari Z, Csiszar A, Kaley G, Koller A. Different roles of PKC and MAP kinases in arteriolar constrictions to pressure and agonists. Am J Physiol. 2002;283:H2282–H2287. doi: 10.1152/ajpheart.00544.2002. [DOI] [PubMed] [Google Scholar]
- Frazer KA, Pachter L, Poliakov A, Rubin EM, Dubchak I. VISTA: computational tools for comparative genomics. Nucleic Acids Res. 2004;32:W273–W279. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T. Cloning and functional characterization of the 5′-flanking region of the human bone morphogenetic protein-2 gene. Biochem J. 1999;338:433–440. [PMC free article] [PubMed] [Google Scholar]
- Helvering LM, Sharp RL, Ou X, Geiser AG. Regulation of the promoters for the human bone morphogenetic protein 2 and 4 genes. Gene. 2000;256:123–138. doi: 10.1016/s0378-1119(00)00364-4. [DOI] [PubMed] [Google Scholar]
- Feng JQ, Xing L, Zhang JH, Zhao M, Horn D, Chan J, Boyce BF, Harris SE, Mundy GR, Chen D. NF-kappaB specifically activates BMP-2 gene expression in growth plate chondrocytes in vivo and in a chondrocyte cell line in vitro. J Biol Chem. 2003;278:29130–29135. doi: 10.1074/jbc.M212296200. [DOI] [PubMed] [Google Scholar]
- Au-Yeung KK, Woo CW, Sung FL, Yip JC, Siow YL, Karmin O. Hyperhomocysteinemia activates nuclear factor-kappaB in endothelial cells via oxidative stress. Circ Res. 2004;94:28–36. doi: 10.1161/01.RES.0000108264.67601.2C. [DOI] [PubMed] [Google Scholar]
- Veikkola T, Lohela M, Ikenberg K, Makinen T, Korff T, Saaristo A, Petrova T, Jeltsch M, Augustin HG, Alitalo K. Intrinsic versus microenvironmental regulation of lymphatic endothelial cell phenotype and function. FASEB J. 2003;17:2006–2013. doi: 10.1096/fj.03-0179com. [DOI] [PubMed] [Google Scholar]
- Hassel S, Eichner A, Yakymovych M, Hellman U, Knaus P, Souchelnytskyi S. Proteins associated with type II bone morphogenetic protein receptor (BMPR-II) and identified by two-dimensional gel electrophoresis and mass spectrometry. Proteomics. 2004;4:1346–1358. doi: 10.1002/pmic.200300770. [DOI] [PubMed] [Google Scholar]
- Hassel S, Schmitt S, Hartung A, Roth M, Nohe A, Petersen N, Ehrlich M, Henis YI, Sebald W, Knaus P. Initiation of Smad-dependent and Smad-independent signaling via distinct BMP-receptor complexes. J Bone Joint Surg Am. 2003;85-A(Suppl 3):44–51. doi: 10.2106/00004623-200300003-00009. [DOI] [PubMed] [Google Scholar]
- Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem. 2002;277:5330–5338. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- Lemonnier J, Ghayor C, Guicheux J, Caverzasio J. Protein kinase C-independent activation of protein kinase D is involved in BMP-2-induced activation of stress mitogen-activated protein kinases JNK and p38 and osteoblastic cell differentiation. J Biol Chem. 2004;279:259–264. doi: 10.1074/jbc.M308665200. [DOI] [PubMed] [Google Scholar]
- Hay E, Lemonnier J, Fromigue O, Marie PJ. Bone morphogenetic protein-2 promotes osteoblast apoptosis through a Smad-independent, protein kinase C-dependent signaling pathway. J Biol Chem. 2001;276:29028–29036. doi: 10.1074/jbc.M011265200. [DOI] [PubMed] [Google Scholar]
- Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J. Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res. 2003;18:2060–2068. doi: 10.1359/jbmr.2003.18.11.2060. [DOI] [PubMed] [Google Scholar]