Abstract
Smooth muscle cell (SMC) migration from the tunica media to the intima, a key event in neointimal formation, requires proteolytic degradation of elastin-rich extracellular matrix barriers. Although cathepsin S (Cat S) is overexpressed in atherosclerotic and neointimal lesions, its exact role in SMC behavior remains primarily unresolved. We examined the involvement of Cat S on SMC migration through an extracellular matrix barrier and its localization in SMCs. A selective Cat S inhibitor and the endogenous inhibitor cystatin C significantly attenuated SMC invasion across elastin gel. Western blotting and cell surface biotinylation analysis demonstrated localization of the 28-kd active form of Cat S on the SMC surface, consistent with its role in the proteolysis of subcellular matrices. Treatment with interferon-γ or interleukin-β1 significantly augmented the ability of SMC membranes to degrade elastin along with a significant increase in the level of active Cat S compared with controls. Immunofluorescence and confocal microscopy showed a punctuated pattern of Cat S clusters at the periphery of SMCs; further studies demonstrated partial co-localization of Cat S and integrin ανβ3 at the cell surfaces. These findings demonstrate that active Cat S co-localizes with integrin ανβ3 as a receptor on the SMC surface, playing an important role in the invasive behavior of SMCs.
Under normal conditions, vascular smooth muscle cells (SMCs) in the tunica media of blood vessels are quiescent and are embedded in a network of elastin-rich extracellular matrix (ECM) that acts as a barrier to SMC migration and proliferation.1,2 Early in the formation of the thickened intima, as in atherosclerotic and neointimal lesions, SMCs that migrate from the tunica media into the developing intima must penetrate the internal elastic lamina. Destruction of the aortic media and supporting lamina through degradation of elastin is also an important mechanism in the formation and expansion of aortic aneurysms.3 SMCs in the arterial wall are believed to be involved in this vascular remodeling through the production of various proteases. Degradation of the elastin component is believed to be the result of a proteolytic cascade that involves the cooperation of serine proteases (SPs),4 matrix metalloproteinases (MMPs),5 and cysteine proteases (CPs).6,7 Of the various proteases present in vascular diseases, the members of the MMP family and SPs, mostly plasminogen and its activators, have received much attention, and substantial evidence supports the involvement of these proteases in the process of vascular remodeling.8,9
Recent studies have shown that cathepsin (Cat) S and Cat K are overexpressed in SMCs of atherosclerotic and neointimal lesions in humans and animals.6,10,11 It has also been reported that Cat S has potent elastolytic activity as well as collagenolytic activity, and Cat S-deficiency markedly reduced content of intimal SMCs and fragmentation of elastic lamina in aortas of atherosclerotic lesions.6,7,10–12 These observations may indicate that the interaction of Cat S released from SMCs with ECM proteins is involved in SMC migration or vascular remodeling, a process that likely occurs in the development of atherosclerotic and intimal lesions. However, it remains unclear whether cathepsins released from SMCs really contribute to the SMC migration through ECM proteins. Generally, during the process of cell migration through ECM proteins, the proteolysis is counterproductive for the cell migration. Therefore, these enzymes are usually localized to receptors, adhesion sites, or invasive protrusions of cells where ECM degradation takes place. This localization concentrates their activity in close proximity to their substrates. By concentrating proteolytic events at or near the cell surface, these processes can be effective even in the presence of high concentrations of inhibitors.13,14 At present it remains unknown how SMC-derived Cat S interacts with ECM components during SMC migration through ECM. In addition, it remains unresolved whether cathepsins are associated with the SMC surface close to the substrates or whether they are localized to the specific receptors.
In the present study, we examined whether cathepsins derived from SMCs are involved in the SMC invasion through collagen and elastin substrates. In addition, we further analyzed the intracellular distribution of cathepsins in cultured SMCs and tried to identify their localization on the SMC surface as well as their molecular functions.
Materials and Methods
Inhibitors and Antibodies
Morpholinurea-leucine-homophenylalanine-vinylsulfone-phenyl (LHVS) was purchased from Arris Pharmaceutical Inc. Trans-epoxysuccinyl-l-leucylamido-(4-guanidino) butane (E64) was purchased from Molecular Probes Inc., Eugene, OR. Peptide hydroxamic acid (GM6001) and Alexa Fluor goat anti-mouse IgG (H+L) were purchased from Calbiochem, La Jolla, CA. Phenylmethyl sulfonyl fluoride (PMSF) and monoclonal anti-Cat L (mAb Cat L) were purchased from Sigma-Aldrich, St. Louis, MO. Recombinant human cystatin C (Cyst C) was purchased from Biotrend Chemikalien GmbH (Köln, Sweden). Mouse monoclonal anti-ανβ3 (mAb LM609) was purchased from Chemicon International Inc., Temecula, CA Antibodies to human Cat S and Cat K were raised from rabbits as previously described (for Western blot, immunofluorescence, immunoprecipitation).6 Rabbit polyclonal anti-Cat B (pAb Cat B) was purchased from Upstate Biotechnology, Lake Placid, NY. Goat polyclonal anti-Cat D (pAb Cat D) and anti-integrin β5 (pAb β5) were purchased from Santa Cruz Biotechnology, Santa Cruz, CA. Anti-goat and anti-rabbit IgG (H+L)-FITC were purchased from Medical & Biological Laboratories Co., Ltd. (Nagoya, Japan). Mouse monoclonal anti-integrin αν (mAb αν), β1 (mAb β1), and β3 (mAb β1) were purchased from BD Biosciences Pharmingen.
Cell Culture
Human aortic SMCs (HSMCs, BioWhittaker, Walkersville, MD) were subcultured at passages 4 to 6 for the following experiments in SmBM supplemented with SmGM-2 Single Quots (both from Cambrex BioScience, Walkersville, MD). Both rat and bovine SMCs (RSMCs and BSMCs) were obtained from the media of rat and calf aortas by the tissue explant method and were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum.15
Migration and Invasion Assay
SMC migration and invasion were assessed with a modified Boyden chamber and platelet-derived growth factor-BB (Peprotech Ltd., London, UK) as a chemoattractant (10 ng/ml).8 For the migration assay, the membrane was coated with 100 μg/ml of type I collagen (BD Biosciences) or Etna-Elastin (Elastin Products Co., Inc.); for the invasion assay, the membrane was coated with 20 μl of type I collagen (1 mg/ml) or Etna-Elastin (15 mg/ml) solution. HSMC migration (6 hours) and invasion assays (12 hours) were performed in the presence or absence of a selective Cat S inhibitor (LHVS, 5 nmol/L), a broader-spectrum CP inhibitor (E64, 20 μmol/L), an endogenous CP inhibitor (Cyst C, 1 μg/ml), an MMP inhibitor (GM6001, 10 μmol/L), and an SP inhibitor (PMSF, 2 mmol/L) for 6 hours (12 hours for PMSF). Migrated and invaded cells were counted in eight randomly chosen fields of duplicate chambers (n = 10) at ×200 magnification for each sample.
Cell Adhesion Assay
The 96-well plates were coated with type I collagen, denatured type I collagen (each 5 μg/50 μl in 0.02 N acetic acid per well), vitronectin [1 μg/50 μl phosphate-buffered saline (PBS) per well] or Etna-Elastin (1 μg/50 μl Dulbecco’s modified Eagle’s medium per well), and nonspecific binding was blocked with 10% bovine serum albumin (BSA). HSMC adhesion was performed in the presence or absence of several protease inhibitors as described above. Procedural details are described elsewhere.16 To examine the effect of the soluble human recombinant active form of Cat S (R&D Systems, Inc., Minneapolis, MN) on the attachment of HSMCs to matrix proteins, HSMCs were pretreated in the presence or absence of the active form of Cat S (0 to 5 μg/ml) for 30 minutes at 37°C and then seeded onto matrix protein-coated wells, followed by adhesion assay as described above.
Isolation of Plasma Membrane
After being cultured in the serum-free condition (24 hours, 90% confluent), cells were solubilized in 25 mmol/L Tris-HCl (pH 7.4) containing 8.5% sucrose, 50 mmol/L NaCl, and 1 mmol/L PMSF (lysis buffer) and sonicated. After removal of the insolubilized material by centrifugation at 500 × g for 5 minutes, the supernatant was then centrifuged at 100,000 × g for 2 hours at 4°C. The supernatants were concentrated with YM-10 Centricon filters (Millipore Corp., Bedford, MA) as the cytosol fractions for the following Western blot analysis and elastase assay. The pellets was resuspended in lysis buffer without sucrose and separated further on a discontinuous sucrose gradient (20, 30, 50, and 60% sucrose in water) centrifuged at 100,000 × g for 2 hours. The plasma membrane-enriched fraction, appearing as a visible band at the 30/50% sucrose interface, was collected as previously described,17 pelleted at 100,000 × g for 2 hours, and then suspended at a concentration of 4 to 6 mg/ml protein for Western blotting analysis and elastase assay.
Biotinylation of Cell Surface Protein and Avidin-Agarose Extraction
SMCs (2 × 107 cells) cultured in serum-free media for 24 hours were washed with ice-cold PBS twice and incubated with 0.5 mg/ml EZ Link Sulfo-NHS-LC-Biotin (Pierce, Rockford, IL) at 4°C for 30 minutes. After biotinylation of the cell surface, the cells were washed extensively using quenching buffer (20 mmol/L Tris-HCl and 120 mmol/L NaCl, pH 7.4) to remove excess biotin, sonicated, and centrifuged at 500 × g for 5 minutes. After the addition of avidin-agarose beads to the supernatant (Pierce) and incubation overnight at 4°C, the proteins that bound to the avidin-agarose beads were washed with RIPA buffer (Upstate Biotechnology) six times and were solubilized in the sample buffer for Western blotting analysis.
Immunoprecipitation
Immunoprecipitation was performed as previously described.18 In brief, after rinsing with PBS, SMCs were solubilized at 107 cells/ml in lysis buffer [40 mmol/L Tris-HCl, pH 7.4, 10 mmol/L NaCl, 0.5 (v/v) Triton X-100, 1 mmol/L PMSF, 2 mmol/L ethylenediaminetetraacetic acid] and sonicated on ice. Insolubilized material was removed by centrifugation as described above. After preclearing with protein A-Sepharose or protein G-Sepharose (both from Sigma-Aldrich), the resulting samples (250 μl) were incubated with 3 μg of mAb LM609, mAb β1, mAb β3, pAb β5, or pAb Cat S. The immune complexes were collected on protein G-Sepharose (for ανβ3, αν, β1, and β3) or protein A-Sepharose (for β5 and Cat S) during 2 hours of incubation at room temperature. After being washed for six times, the beads were treated with reducing sample buffer and boiled. Eluted proteins were analyzed by Western blot.
Western Blot Analysis
The procedures for preparing SMC plasma membrane fractions and cytosol fractions were identical to those described above. The conditioned media from serum-free cultured SMCs (24 hours) were collected and concentrated with YM-10 Centricon filters. Equal amounts of protein from each sample were separated on 6% (for integrins αν and β3) or 15% (for Cat S, K, B, L, and D) sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto polyvinylidene difluoride membrane as previously described.10,19 Immunoblotting used antibodies specific to integrins (αν and β3) and cathepsins (Cat S, K, B, L, D). We also examined the Cat S and Cat K content of fractions of the plasma membrane, cytosol, and media of HSMCs stimulated with and without interferon-γ (IFN-γ, 400 U/ml) or interleukin-1β (IL-1β, 10 ng/ml; Genzyme Corp., Cambridge, MA), respectively.
Immunocytofluorescence
For single-staining, after the attachment of SMCs (cells density at 2 × 104/ml) to coverslips precoated with type I collagen, SMCs were fixed for 10 minutes with 8% paraformaldehyde in PBS, washed, and permeabilized for 6 minutes with 0.5% Triton X-100. The cells were subsequently treated as follows. After washing with PBS-glycerol and blocking with 3% BSA/PBS, they were incubated with primary antibodies (1:100, Cat S, K, B, L, and D) for 40 minutes at room temperature, washed with 0.1% BSA/PBS, incubated with fluorescein isothiocyanate (FITC) green-conjugated (1:400, for Cat S, K, D, and B) or Alexa Fluor red-conjugated (1:200, for Cat L and integrin ανβ3) secondary antibodies for 40 minutes at room temperature, and washed with PBS. We also performed single immunostaining for SMCs stimulated with rat, bovine, and human recombinant IFN-γ (400 U/ml, R&D Systems, Inc.; Serotec Ltd., Oxford, UK) for 24 hours. For double staining, the cells were treated with a mixture of two primary antibodies (Cat S and integrin ανβ3) and stained as described above. For negative controls, rabbit and goat IgG were used as first antibodies. Coverslips were treated with Prolong mounting medium (Molecular Probes) and visualized by confocal microscopy.
Cat S Binding Assay
Cat S binding assays were performed as described,19 with modifications. Briefly, wells of 96-well plates were precoated with 75 μl of human recombinant integrin ανβ3 (Chemicon International, Inc.) and BSA at 2 μg/ml in binding buffer (20 mmol/L Tris-HCl, 150 mmol/L NaCl, and 1 mmol/L CaCl2, pH 7.4) overnight at 4°C. After being washed three times with PBS, the plates were blocked with 10% BSA in PBS for 1 hour and then washed with PBS. A total of 100 μl of the human recombinant active form of Cat S (4 μg/ml) in 1% BSA/PBS was added to the precoated wells. After overnight incubation at 4°C, the wells were washed three times with PBS. Bound material was solubilized with sodium dodecyl sulfate reducing sample buffer (25 μl/well). The contents of three wells were combined and analyzed by Western blotting using Cat S antibody.
Elastase and Collagenase Assay
Protease assays were was performed as described,15,20 with minor modification. The procedures of preparing the lysates, plasma membranes, cytosols, and conditioned media were identical to those described above. Total protein (100 μg) from each sample was incubated with 1 mg/ml of Elastin-Congo Red (24 hours, for elastase assay) or 500 μg/ml of FITC-labeled type I collagen (6 hours, collagenase assay). Reactions were performed in the absence or presence of LHVS (5 nmol/L), E64 (20 μmol/L), Cyst C (1 μg/ml), and GM6001 (10 μmol/L). Procedural details are described elsewhere.6 We also prepared the plasma membrane from HSMCs cultured for 24 hours in the presence or absence of 400 U/ml of IFN-γ and 10 ng/ml of IL-1β to examine their effect on elastolytic activity in the presence or absence of 5 nmol/L of LHVS.
Quantitative Real-Time Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR)
After being cultured in serum-free Dulbecco’s modified Eagle’s medium, the HSMCs were stimulated with or without 400 U/ml of IFN-γ and 10 ng/ml of IL-1β for 24 hours. After extraction of total RNA, 20 ng of cellular RNA was analyzed for the Cat S mRNA by measuring the specific fluorescence signal from the TaqMan probe during quantitative real-time RT-PCR as previously described.6 The primer and probe used here included forward primer (5′-AGGGAACTCATCAAAGACATCACTT-3′), reverse primer (5′-GGGAATGCACTCATACGATCTG-3′), and probe (5′-TCACTGGTCATGTCTCCCAGGT-GGTTC-3′). Each mRNA quantity was normalized in regard to its respective GAPDH mRNA quantity.
Statistical Analysis
Values were expressed as means ± SE. Statistical analysis was performed by one-way analysis of variance followed by Scheffé’s multiple comparison. A value of P < 0.05 was considered statistically significant.
Results
Cat S Involved in SMC Invasion, Not Adhesion and Migration
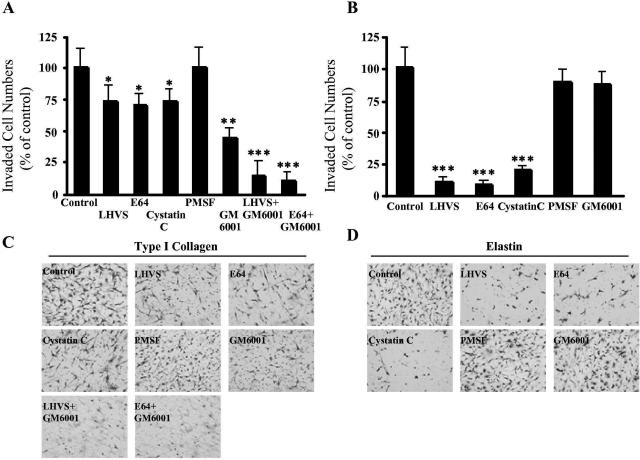
To examine the function of Cat S in SMC invasion across the reconstituted ECM barrier, an SMC invasion assay was performed in the presence or absence of the selective Cat S inhibitor LHVS.10 As shown in Figure 1A, 5 nmol/L of LHVS significantly inhibited platelet-derived growth factor-BB-directed HSMC invasion through type I collagen gel (27.9%). Similar to the LHVS, a synthetic inhibitor for a broader spectrum of CPs, 20 μmol/L of E64 showed a comparable level of inhibition of HSMC invasion (29.5%, Figure 1A). Endogenous inhibitor Cyst C inhibits a spectrum of CPs but has the highest affinity for Cat S.21 In this in vitro assay, Cyst C at a physiological concentration of 1 μg/ml significantly inhibited HSMC invasion (26.5%, Figure 1A). In contrast, 5 nmol/L of LHVS, 20 μmol/L of E64, and 1 μg/ml of Cyst C almost completely inhibited HSMC invasion through elastin gel by 91.5%, 92.1%, and 84.6%, respectively (Figure 1B). The MMP inhibitor GM6001 (10 μmol/L) significantly blocked HSMC invasion through type I collagen gel (56.5%, Figure 1A) much more strongly than LHVS and E64 did, although GM6001 had no effect on HSMC invasion through elastin gel (Figure 1B). In addition, we observed that either LHVS (5 nmol/L) or E64 (20 μmol/L) combined with GM6001 (10 μmol/L) significantly increased the inhibition of HSMC invasion through type I collagen gel compared to that of the inhibitory effect by LHVS or E64 alone (83.9% and 87.5% inhibition, respectively) (Figure 1A). There was no effect on the HSMC invasion through either type I collagen gel or elastin gel in the presence of the SP inhibitor PMSF (2 mmol/L) (Figure 1, A and B). Compared with control, the addition of LHVS (5 nmol/L), E64 (20 μmol/L), or Cyst C (1 μg/ml) demonstrated no significant differences in HSMC adhesion onto the type I collagen, denatured type I collagen, fibronectin, elastin, or vitronectin (data not shown). In addition, we also observed that these CP inhibitors had no effect on HSMC migration across type I collagen elastin thin-coated filters (data not shown).
Figure 1.
Effects of protease inhibitors on HSMC invasion through collagen gel (A and C) or elastin gel (B and D). SMC invasions were conducted in the presence or absence of a selective Cat S inhibitor (LHVS, 5 nmol/L), a broad-spectrum CP inhibitor (E64, 20 μmol/L), an endogenous CP inhibitor (Cyst C, 1 μg/ml), a MMP inhibitor (GM6001, 10 μmol/L), and an SP inhibitor (PMSF, 2 mmol/L). The effects of GM6001 in combination with LHVS or E64 were also examined for HSMC invasion through collagen gel. Data represent quantitative analysis of six independent experiments. The values are means ± SE and are expressed as a percentage of control values. Representative photos are shown at bottom. *P < 0.05, **P < 0.01, ***P < 0.0001 versus control. Original magnifications, ×200.
Cell Surface Distribution of Cat S in SMCs
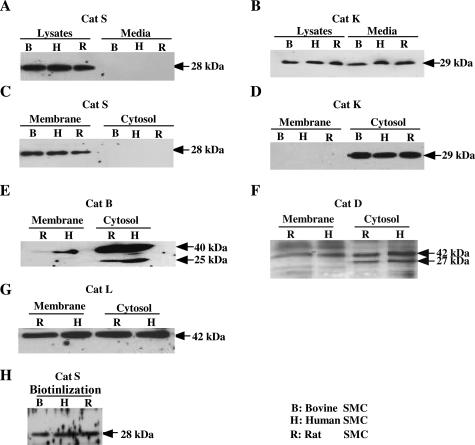
We examined the distribution of Cat S and K in SMCs derived from various species (HSMCs, RSMCs, and BSMCs). As shown in Figure 2, A and C, the 28-kd active form of Cat S10 was detected in plasma membrane fractions as well as whole lysates from SMCs but not in the cell-cultured media or cytosol fractions. In contrast, the 29-kd active form of Cat K10 was detected in the whole cell lysates, cultured media, and cytosol fractions but not in the plasma membrane fractions (Figure 2, B and D). Western blot analysis of the plasma membrane and cytosol fractions from HSMCs and RSMCs for Cat B, L, and D showed that their latent forms (40 kd for Cat B, 42 kd for Cat L, and 42 kd for Cat D) were detected both in the plasma membrane and cytosol fractions, and the 25-kd active forms of Cat B and 27-kd active forms of Cat D were detected only in their cytosol fractions (Figure 2, E–G). To test the hypothesis that Cat S is localized at the cell surface, SMCs were subjected to surface biotinylation in conjunction with Western blotting using the pAb to Cat S. As shown in Figure 2H, the active form of Cat S was precipitated with avidin-agarose after the cell surface was labeled using biotin. The latent forms of Cat B and Cat L were also precipitated with avidin-agarose after the cell surface was labeled using biotin (data not shown).
Figure 2.
Distribution of Cats in intracellular and extracellular SMCs. Western blot analysis of protein extracts (lysates, membranes, cytosols, and conditioned media) prepared by ultracentrifugation (A–G) from cultured SMCs. Equal amounts of protein for each sample was loaded and immunoblotted using the pAbs to Cat S, K, B, and D and the mAb to Cat L. H: The surface deposition of Cat S was analyzed for SMCs by performing surface biotinylation in conjunction with Western blot analysis using antibody to Cat S.
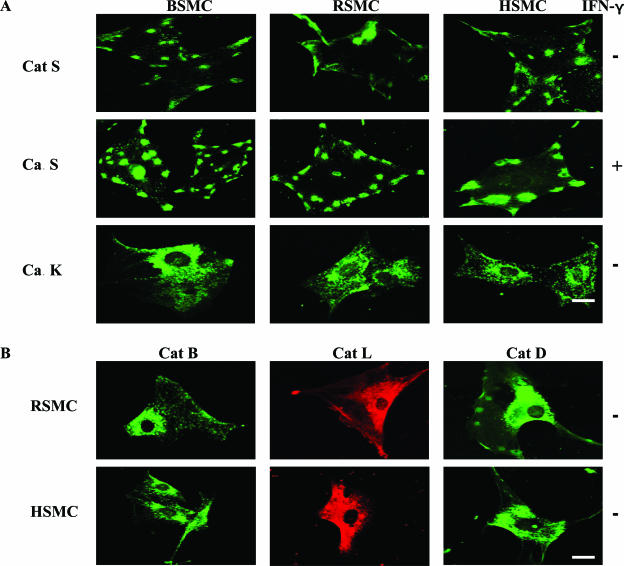
We next used immunofluorescence staining and confocal microscopy for the visualization of Cat S localization on SMCs. As shown in Figure 3A, a punctuated pattern representing clusters of Cat S was observed on the surface of HSMCs, BSMCs, and RSMCs (Figure 3A, top). Usually three to five patches were observed on each cell, although their number varied from 0 to 7 in unstimulated SMCs. In contrast, Cat K staining showed a completely different pattern in HSMCs, BSMCs, and RSMCs with positive spots uniformly distributed along the cell body (Figure 3A, bottom). As shown in Figure 3B, Cat B, L, and D staining was concentrated in the perinuclear region in HSMCs and RSMCs. The staining was specific because no signal was detected using secondary antibody alone or the corresponding nonimmune antibody (data not shown).
Figure 3.
Distribution of Cats in SMC intracellular region visualized by immunofluorescence. After being attached to coverslips precoated with type I collagen, cells were cultured in the presence or absence of IFN-γ for 24 hours, fixed, and then stained as described in the Materials and Methods section. Single immunostaining was performed with the pAbs (FITC, green) to Cat S (A: top, no treatment; middle, treated with IFN-γ), Cat K (A, bottom), Cat B (B, left), Cat D (B, right), or the mAb to Cat L (Alexa, red; B, middle). Scale bars, 10 μm.
Elastolytic and Collagenolytic Activities of Cat S on SMC Plasma Membrane
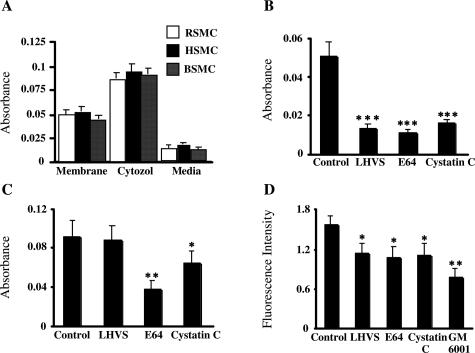
As shown in Figure 4A, the conditioned media from SMCs cultured in serum-free medium for 24 hours exhibited little or no elastolytic activity, whereas the SMC plasma membranes as well as cytosol fractions showed potential elastolytic activity (Figure 4A). Furthermore, LHVS, E64, or Cyst C significantly reduced the degradation of elastin by incubation with membrane fractions from HSMCs by 74%, 78.6%, or 69%, respectively (Figure 4B). The degradation of elastin by the plasma membrane was slightly reduced by GM6001 (data not shown). In addition, we observed that LHVS had no effect on the elastolytic activity of the cytosol fraction, although E64 and Cyst C significantly attenuated its activity (Figure 4C). We also observed that LHVS as well as E64 and Cyst C significantly reduced the degradation of type I collagen induced by the membrane fractions from HSMCs by 28%, 31.8%, and 29.3%, respectively, although they did so to a lesser extent than they inhibited the elastin degradation (Figure 4D). GM6001 significantly inhibited the collagenolytic activity of the HSMC plasma membrane (57.9%, Figure 4D).
Figure 4.
Protease activities of SMCs and cultured media. Protein (100 μg) from each sample was incubated with 1 mg/ml of elastin-Congo Red (24 hours) or 500 μg/ml of FITC-labeled type I collagen (6 hours) for the elastase assay (A–C) and the collagenase assay (D), respectively. A: The fractions (membranes, cytosols, and conditioned media) prepared from RSMCs, BSMCs, and HSMCs were analyzed for elastin degradation. Elastolytic activities of both the membrane (B) and cytosol (C) fractions and the collagenolytic activity of the membrane fraction (D) from HSMCs were analyzed in the presence or absence of LHVS (5 nmol/L), E64 (20 μmol/L), Cyst C (1 μg/ml), and GM6001 (10 μmol/L). The relative amounts of elastin and collagen degradation are represented by the absorbance and fluorescence intensity determined in the experiments. Data are derived from quantitative analysis of five independent experiments. *P < 0.05, **P < 0.01, ***P < 0.0001 versus control.
Effects of Proinflammatory Cytokines on Expression of Cat S mRNA and Protein and Elastolytic Activity of SMC Plasma Membrane
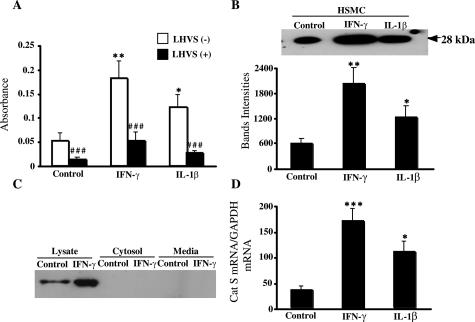
As shown in Figure 5A, the plasma membrane fractions obtained from HSMCs stimulated with IFN-γ (400 U/ml) or IL-1β (10 ng/ml) significantly augmented the degradation of elastin (3.6-fold and 2.4-fold, respectively) compared with an unstimulated control. Interestingly, the increased elastolytic activity stimulated by IFN-γ or IL-1β was significantly reduced by treatment with LHVS (70% or 78%, respectively; Figure 5A). To further determine whether the response of SMCs to proinflammatory cytokines was representative of increasing the active form of Cat S on the cell surface, the content of Cat S in the plasma membrane fractions was determined after treatment of HSMCs with IFN-γ or IL-1β. As shown in Figure 5B, the treatment with IFN-γ (400 U/ml) or IL-1β (10 ng/ml) for 24 hours significantly increased the level of the active form of Cat S protein by 3.4- or 2.1-fold, respectively, compared with the control. Furthermore, it was reconfirmed by Western blot of the lysate that much greater levels of the active form of Cat S were detected in the lysates (Figure 5C). However, we did not detect the active form of Cat S in the fractions of cytosol and media (Figure 5C). It is consistent with the data from immunofluorescence staining and confocal microscopy analysis that IFN-γ increased the numbers of patches representing clusters of Cat S between 10 to 20 on HSMC surface compared with unstimulated control cells (Figure 3A, middle). The data from real-time RT-PCR demonstrated that the Cat S mRNA was expressed in the unstimulated HSMCs, and both IFN-γ (400 U/ml) and IL-1β (10 ng/ml) significantly increased its expression (5.0-fold and 3.4-fold, respectively; Figure 5D).
Figure 5.
Regulation of Cat S mRNA and protein and elastase activity of the cell membrane fractions. Cell membranes prepared from HSMCs stimulated with or without IL-1β and IFN-γ were incubated with 1 mg/ml of elastin-Congo Red for 24 hours to determine the elastolytic activity in the presence or absence of LHVS (A) or analyzed by Western blot using antibody to Cat S (B). The data in B are combined with data on Cat S protein level. The fractions of cytosol and media from HSMCs stimulated with or without IFN-γ were analyzed for Cat S levels by Western blot (C). The expression of Cat S mRNA was detected by quantitative real-time RT-PCR in HSMCs cultured in presence or absence of IL-1β and IFN-γ. Data are derived from quantitative analysis of four independent experiments. *P < 0.05 and **P < 0.01 versus control; ###P < 0.0001 versus LHVS (−).
Co-Localization of Active Form of Cat S with Integrins
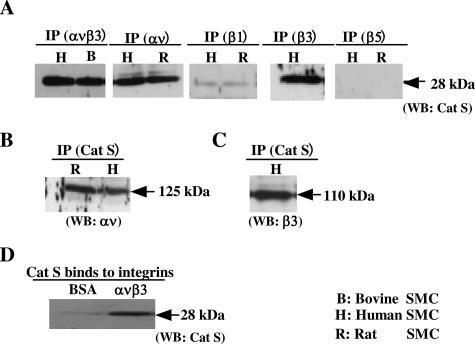
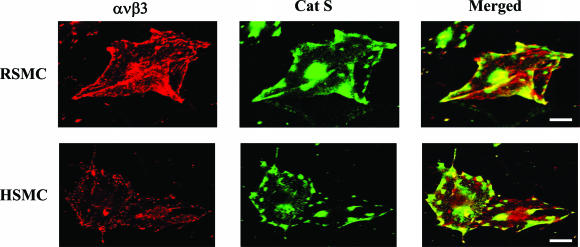
Previous studies had demonstrated that integrins ανβ3 and β1 on the SMC surface not only promote cell migration but also serve as receptors for proteases such as MMP-2, facilitating their expression in a functionally active form.19,22,23 These findings prompted us to hypothesize that the localization of Cat S on the surface of the SMCs may be mediated by integrins. To assess this idea, we first analyzed cell lysates prepared from HSMCs and RSMCs by sequential immunoprecipitation with antibodies against integrins such as ανβ3, β1, β3, or β5, followed by immunoblotting with antibody to Cat S. As shown in Figure 6A, a 28-kd immunoreactive protein from the immunoprecipitation using the antibodies to ανβ3, αν, and β3 was detected on the Western blot, corresponding to the predicted size of the active form of Cat S. The results from immunoprecipitation using antibody to Cat S was analyzed by Western blot using antibodies to αν and β3. As shown in Figure 6, B and C, αν and β3 integrins were precipitated with Cat S antibody. To strengthen this finding, double-label immunofluorescence staining using confocal microscopy was performed in RSMCs and HSMCs. As shown in Figure 7, we observed that the greater part of the clustered ανβ3 integrin was co-localized with Cat S on the cell surface. The data from Cat S binding assay showed that the active form of Cat S was capable of binding to the immobilized integrin ανβ3 (Figure 6D). We next assessed whether the active form of Cat S that binds to the integrins could affect SMC adhesion on ECM. The pretreatment of SMCs with an exogenous active form of Cat S at concentrations from 1 to 5 μg/ml had no effect on SMC adhesion to the type I collagen-, denatured-type I collagen-, elastin-, and vitronectin-coated plates compared with untreated controls (data not shown).
Figure 6.
Co-localization of Cat S with integrin ανβ3 on SMC surface. A: The co-localization of the active form of Cat S and ανβ3 integrin was analyzed by immunoprecipitation (IP, mAb LM609, mAb αν, mAb β1, mAb β3, and pAb β5) in conjunction with Western blot analysis using antibody to Cat S. B and C: After immunoprecipitation with antibodies to Cat S, Western blot was performed using antibodies to αν and β3. D: Soluble active form of Cat S binding to integrins. After 96-well plates were precoated with 75 μl of soluble integrin ανβ3 and BSA at 2 μg/ml and blocked with 10% BSA in PBS, a total of 100 μl of the active form of Cat S (4 μg/ml) was added to the wells. After overnight incubation the wells were washed and solubilized with sodium dodecyl sulfate reducing sample buffer. The contents of three wells were combined and analyzed by Western blotting using Cat S antibody.
Figure 7.
Cat S and ανβ3 integrin are partially co-localized on the RSMCs (top) and HSMCs (bottom). Double immunolocalization was performed with the pAb to Cat S (FITC, green) and the mAb to integrin ανβ3 (Alexa, red). Scale bars, 10 μm.
Discussion
The degradation of ECM in vivo is confined to the immediate pericellular environment and is achieved by proteolytic enzymes localized at discrete areas of cell-matrix contact. These enzymes include the MMPs19,22 and the plasminogen/plasmin system.24 In this study, we demonstrated for the first time that an active form of Cat S, a CP with potent elastolytic activity, was localized on the SMC plasma membrane surface and contributed to SMC elastolytic activity as well as SMC invasive behavior through ECM.
It has been demonstrated by immunostaining observations that cells migrating into and accumulating within developing intimal plaques in humans synthesize the potent elastase Cat S.10 Recent studies have shown that Cat S is overexpressed in atherosclerotic and neointimal lesions in human and animal models, and Cat S deficiency prevents the degradation of elastic lamina and type I collagen in aortal atherosclerotic lesion of LDL receptor-deficient mice.6,10,25 These findings suggest that Cat S may participate in ECM degradation and SMC migration during the development of intimal plaque or hyperplasia. To establish the role of the proteases in the SMC movement into the subendothelial space in the vascular wall, in vitro models of chemoattractant-directed SMC migration across ECM-coated porous filters, which function as ECM barriers, in the presence or absence of the associated inhibitors have frequently been used.15,16 In the present study we demonstrated that a selective Cat S inhibitor, LHVS,10 as well as a synthetic broad spectrum CP inhibitor, E64, and a natural inhibitor, Cyst C,21 blocked SMC invasion across type I collagen gel, although with a lesser inhibitory effect than that of an MMP inhibitor, GM6001. Interestingly, LHVS almost completely inhibited SMC invasion across an elastin barrier, which was comparable to the action of E64 and Cyst C. In contrast to the Cat S inhibitor, a SP inhibitor, PMSF, had no effect on SMC invasion across type I collagen gel or elastin gel. These data suggest that among SMC-derived CP, Cat S in particular may play an important role in SMC migration from the media into the subendothelial space by facilitating the proteolytic degradation of the elastin-rich matrix. Additionally, the combination of GM6001 with LHVS or E64 has been demonstrated to inhibit the invasion of SMCs through a collagen lattice barrier more effectively than each inhibitor alone inhibits such invasions. This result suggested that not only MMPs but also Cat S are involved in SMC migration through type I collagen and that the combination of MMP and CP inhibitor, particularly the Cat S inhibitor, may provide a more effective and useful method of experimental and clinical therapy of restenosis after angioplasty. As in this study, the inhibition of Cat S had no effect on SMC adhesion to ECM or on SMC migration through an ECM thin-coated membrane. This result suggests that Cat S likely has a role in mediating the proteolytic process during SMC invasion through elastin or collagen matrix but does not have a role in mediating the migration process itself.
Evidence from several studies indicates that cells can concentrate proteases such as pro-MMP-219,22 and urokinase-type plasminogen activator24 at specific sites on their surface, allowing for the degradation of ECM components. Here, we demonstrated for the first time, based on the observations of Western blotting analysis of subcellular fractions, that the active form of Cat S exists on the SMC plasma membrane but not in the cytosol. In contrast, the active forms of Cat K, B, L, and D were not detected on the SMC plasma membranes. Immunofluorescence staining with cathepsin-specific antibodies revealed punctate labeling of Cat S on the periphery of the SMCs, in contrast with Cat K in which positive spots were uniformly distributed along the cell body. Cat B, L, and D staining was concentrated in the intracellular region, particularly the perinuclear region in SMCs. These results suggest that Cat B, D, K, and L have different spatial regulation and molecular functions during SMC adhesion, migration, and invasion.
Previous immunostaining data of Sukhova and colleagues on Cat S10 suggest that Cat S has a lysosomal distribution. However, they did not show any direct evidence to illustrate intracellular localization of Cat S. In addition, the results of Western blotting analysis by Sukhova and colleagues10 reported the secretion of Cat S by SMCs from the saphenous vein into the media and the presence of precursor of Cat S in the cell lysate (applied 0.3 mg protein). In this study, we have reported that the active form of Cat S was detected in the membrane fractions of aortic SMCs (human, rat, and bovine) but not in the media (applied 0.06 mg protein). The data from Watari’s group26 had demonstrated that the active form of Cat S (28 kd) was detected in cell lysates from control and tumor necrosis factor-α-treated cells, whereas pro-Cat S (∼37 kd) protein was detected only in conditioned medium from cultures treated with tumor necrosis factor-α. It has also been reported that both pro- and active form of Cat S were secreted into the culture medium after 5 days in culture.27 Therefore the different results between Sukhova and colleagues10 previous and our present studies regarding the distribution of Cat S seem to be attributable to the different applied amounts of total protein for Western blotting assay. It is also possible that the different results might be contributable to the different source of SMCs (saphenous vein or aorta).
The findings of the biotinylation studies on the cell membrane support and extend the existence of the active form of Cat S outside the surface of the SMCs. Interestingly, we observed that the SMC membrane fractions showed potential elastolytic and collagenolytic activities, and their abilities were significantly reduced by LHVS as well as E64. Additionally, we also demonstrated that there was no effect of LHVS on the elastolytic activity of the cytosol fraction from SMCs. These results, together with the immunofluorescence staining of SMCs, suggested that Cat S is a major contributor to the elastolytic activity of SMC membrane but not to that of the cytosol. In addition, these findings indicate that the active form of Cat S on the SMC surface, where the cell makes contact with the ECM, facilitates ECM degradation and SMC migration.
Several lines of investigation demonstrated that among CPs, Cat S expression and activity were up-regulated by inflammatory cytokines, such as IFN-γ, in mice peritoneal macrophage, and human cervical SMCs.26–28 The present study shows that IL-1β and IFN-γ up-regulated vascular SMC expression of the active form of Cat S protein and accumulation on the cell surface and enhanced its elastolytic activity. This result suggests that overexpression and accumulation of Cat S stimulated with the inflammatory cytokine on SMC plasma membrane may promote the tunica medial SMC degradation of ECM and migration through the elastic lamina to the intimal place during the inflammatory arterial diseases.
Earlier results from our laboratory and from others demonstrated that the ανβ3 integrin that was expressed on the surface as a receptor for several proteases was involved in SMC adhesion and migration.16,19,22 In this study, we clearly illustrated the distinctive cell surface localization of Cat S at the periphery of the cell, often at cell protrusions. Apparently, the clustering of Cat S and ανβ3 integrin, demonstrated in this study by confocal microscopy and co-precipitation, may represent a means to most efficiently and specifically decorate discrete regions of SMCs with the mature Cat S enzyme in its proteolytically active form. However, it should be noted that Cat S and ανβ3 integrin are partially co-localized and clustered at SMC surfaces because double-staining and confocal microscopy confirmed that there was no full overlap of the red and green colors in each cell. In fact, we observed that immunoprecipitation of SMC lysates with monoclonal antibody to β1 integrin precipitated the small amount of the active form of Cat S, suggesting that among the integrins, ανβ3 integrin is the most efficient but not unique in its ability to associate with the active form of Cat S. To our surprise, we failed to observe any effect of the pretreatment of HSMCs with the active form of Cat S on SMC adhesion to vitronectin or denatured-type I collagen on which SMC attaches through ανβ3 integrin.16 These findings, in combination with an earlier report that Cat S lacks an arginine-glycine-aspartic acid (RDG) sequence29 commonly found in ligands for ανβ3 integrin, indicates that the ability of Cat S to associate with ανβ3 integrin appears to be distinct from other ανβ3 integrin-directed ligands. Although it is not clear how ανβ3 integrin interacts with the domain of Cat S, throughout the past few years, various other unrelated non-RGD ligands of ανβ3 integrin have also been recognized, including CD31/PECAM-130 and MMP-2.19,22 The co-localization of Cat S and ανβ3 integrin on SMCs is consistent with the recent evidence that proteases and their receptors may be functionally associated with integrin. Indirectly supporting this concept is the present observation from the Cat S binding assay that the soluble human recombinant active form of Cat S is capable of binding to immobilized receptor of integrin ανβ3. However, no biochemical evidence exists to suggest that these receptors are structurally linked on the cell surface.
In summary, our data show that Cat S and its proposed receptor integrin ανβ3 are both targeted to the same membrane microdomains in vascular SMCs. This finding provides an elegant mechanism to restrict the proteolysis of the ECM to a limited microenvironment of the cell. This study also raises numerous topics for further investigation, such as the secretion and maturation of cathepsins, and underscores the need to consider Cat S as a molecular therapeutic target in vascular diseases such as atherosclerosis and restenosis after angioplasty.
Footnotes
Address reprint requests to Masafumi Kuzuya, M.D., Ph.D., Department of Geriatrics, Nagoya University Graduate School of Medicine, 65 Tsurumai-cho, Showa-ku, Nagoya 466-8550, Japan. E-mail: kuzuya@med.nagoya-u.ac.jp.
Supported in part by the Ministry of Education, Science, and Culture, Japan (Scientific Research Fund grant no. 13671182).
References
- Brenner CA, Adler RR, Rappolee DA, Pedersen RA, Werb Z. Genes for extracellular-matrix-degrading metalloproteinases and their inhibitor, TIMP, are expressed during early mammalian development. Genes Dev. 1989;3:848–859. doi: 10.1101/gad.3.6.848. [DOI] [PubMed] [Google Scholar]
- Pauly RR, Passaniti A, Bilato C, Monticone R, Cheng L, Papadopoulos N, Gluzband YA, Smith L, Weinstein C, Lakatta EG, Crow MT. Migration of cultured vascular smooth muscle cells through a basement membrane barrier requires type IV collagenase activity and is inhibited by cellular differentiation. Circ Res. 1994;75:41–54. doi: 10.1161/01.res.75.1.41. [DOI] [PubMed] [Google Scholar]
- Lawrence DM, Singh RS, Franklin DP, Carey DJ, Elmore JR. Rapamycin suppresses experimental aortic aneurysm growth. J Vasc Surg. 2004;40:334–338. doi: 10.1016/j.jvs.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Mignatti P, Robbins E, Rifkin DB. Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell. 1986;47:487–498. doi: 10.1016/0092-8674(86)90613-6. [DOI] [PubMed] [Google Scholar]
- Russell RE, Thorley A, Culpitt SV, Dodd S, Donnelly LE, Demattos C, Fitzgerald M, Barnes PJ. Alveolar macrophage-mediated elastolysis: roles of matrix metalloproteinases, cysteine, and serine proteases. Am J Physiol. 2002;283:L867–L873. doi: 10.1152/ajplung.00020.2002. [DOI] [PubMed] [Google Scholar]
- Cheng XW, Kuzuya M, Sasaki T, Arakawa K, Kanda S, Sumi D, Koike T, Maeda K, Tamaya-Mori N, Shi GP, Saito N, Iguchi A. Increased expression of elastolytic cysteine proteases, cathepsins S and K, in the neointima of balloon-injured rat carotid arteries. Am J Pathol. 2004;164:243–251. doi: 10.1016/S0002-9440(10)63114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi GP, Sukhova GK, Kuzuya M, Ye Q, Du J, Zhang Y, Pan JH, Lu ML, Cheng XW, Iguchi A, Perrey S, Lee AM, Chapman HA, Libby P. Deficiency of the cysteine protease cathepsin S impairs microvessel growth. Circ Res. 2003;92:493–500. doi: 10.1161/01.RES.0000060485.20318.96. [DOI] [PubMed] [Google Scholar]
- Kuzuya M, Kanda S, Sasaki T, Tamaya-Mori N, Cheng XW, Itoh T, Itohara S, Iguchi A. Deficiency of gelatinase a suppresses smooth muscle cell invasion and development of experimental intimal hyperplasia. Circulation. 2003;108:1375–1381. doi: 10.1161/01.CIR.0000086463.15540.3C. [DOI] [PubMed] [Google Scholar]
- Falkenberg M, Tom C, DeYoung MB, Wen S, Linnemann R, Dichek DA. Increased expression of urokinase during atherosclerotic lesion development causes arterial constriction and lumen loss, and accelerates lesion growth. Proc Natl Acad Sci USA. 2002;99:10665–10670. doi: 10.1073/pnas.162236599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576–583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhova GK, Zhang Y, Pan JH, Wada Y, Yamamoto T, Naito M, Kodama T, Tsimikas S, Witztum JL, Lu ML, Sakara Y, Chin MT, Libby P, Shi GP. Deficiency of cathepsin S reduces atherosclerosis in LDL receptor-deficient mice. J Clin Invest. 2003;111:897–906. doi: 10.1172/JCI14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yasuda Y, Li W, Bogyo M, Katz N, Gordon RE, Fields GB, Bromme D. Regulation of collagenase activities of human cathepsins by glycosaminoglycans. J Biol Chem. 2004;279:5470–5479. doi: 10.1074/jbc.M310349200. [DOI] [PubMed] [Google Scholar]
- Chapman HA, Jr, Stone OL, Vavrin Z. Degradation of fibrin and elastin by intact human alveolar macrophages in vitro. Characterization of a plasminogen activator and its role in matrix degradation. J Clin Invest. 1984;73:806–815. doi: 10.1172/JCI111275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee C, Shiu RP. Degradation of endothelial basement membrane by human breast cancer cell lines. Cancer Res. 1986;46:1835–1839. [PubMed] [Google Scholar]
- Cheng XW, Kuzuya M, Sasaki T, Kanda S, Tamaya-Mori N, Koike T, Maeda K, Nishitani E, Iguchi A. Green tea catechins inhibit neointimal hyperplasia in a rat carotid arterial injury model by TIMP-2 overexpression. Cardiovasc Res. 2004;62:594–602. doi: 10.1016/j.cardiores.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Kanda S, Kuzuya M, Ramos MA, Koike T, Yoshino K, Ikeda S, Iguchi A. Matrix metalloproteinase and alphavbeta3 integrin-dependent vascular smooth muscle cell invasion through a type I collagen lattice. Arterioscler Thromb Vasc Biol. 2000;20:998–1005. doi: 10.1161/01.atv.20.4.998. [DOI] [PubMed] [Google Scholar]
- Strongin AY, Marmer BL, Grant GA, Goldberg GI. Plasma membrane-dependent activation of the 72-kDa type IV collagenase is prevented by complex formation with TIMP-2. J Biol Chem. 1993;268:14033–14039. [PubMed] [Google Scholar]
- Puyraimond A, Fridman R, Lemesle M, Arbeille B, Menashi S. MMP-2 colocalizes with caveolae on the surface of endothelial cells. Exp Cell Res. 2001;262:28–36. doi: 10.1006/excr.2000.5069. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Li Z, Greenbaum D, Bogyo M, Weber E, Bromme D. Cathepsin V: a novel and potent elastolytic activity expressed in activated macrophages. J Biol Chem. 2004;279:36761–36770. doi: 10.1074/jbc.M403986200. [DOI] [PubMed] [Google Scholar]
- Hall A, Ekiel I, Mason RW, Kasprzykowski F, Grubb A, Abrahamson M. Structural basis for different inhibitory specificities of human cystatins C and D. Biochemistry. 1998;37:4071–4079. doi: 10.1021/bi971197j. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Ratnikov B, Monosov E, Postnova TI, DiScipio R, Smith JW, Strongin AY. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263:209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- Ellerbroek SM, Fishman DA, Kearns AS, Bafetti LM, Stack MS. Ovarian carcinoma regulation of matrix metalloproteinase-2 and membrane type 1 matrix metalloproteinase through beta1 integrin. Cancer Res. 1999;59:1635–1641. [PubMed] [Google Scholar]
- Testa JE, Quigley JP. The role of urokinase-type plasminogen activator in aggressive tumor cell behavior. Cancer Metastasis Rev. 1990;9:353–367. doi: 10.1007/BF00049524. [DOI] [PubMed] [Google Scholar]
- Jormsjö S, Wuttge DM, Sirsjo A, Whatling C, Hamsten A, Stemme S, Eriksson P. Differential expression of cysteine and aspartic proteases during progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2002;161:939–945. doi: 10.1016/S0002-9440(10)64254-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watari M, Watari H, DiSanto ME, Chacko S, Shi GP, Strauss JF., III Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. Am J Pathol. 1999;154:1755–1762. doi: 10.1016/S0002-9440(10)65431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punturieri A, Filippov S, Allen E, Caras I, Murray R, Reddy V, Weiss SJ. Regulation of elastinolytic cysteine proteinase activity in normal and cathepsin K-deficient human macrophages. J Exp Med. 2000;192:789–799. doi: 10.1084/jem.192.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers C, Honey K, Fink S, Forbush K, Rudensky A. Differential regulation of cathepsin S and cathepsin L in interferon gamma-treated macrophages. J Exp Med. 2003;197:169–179. doi: 10.1084/jem.20020978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi GP, Webb AC, Foster KE, Knoll JH, Lemere CA, Munger JS, Chapman HA. Human cathepsin S: chromosomal localization, gene structure, and tissue distribution. J Biol Chem. 1994;269:11530–11536. [PubMed] [Google Scholar]
- Piali L, Hammel P, Uherek C, Bachmann F, Gisler RH, Dunon D, Imhof BA. CD31/PECAM-1 is a ligand for alpha v beta 3 integrin involved in adhesion of leukocytes to endothelium. J Cell Biol. 1995;130:451–460. doi: 10.1083/jcb.130.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]