Abstract
Chronic cholestatic liver diseases are characterized by impaired balance between proliferation and death of cholangiocytes, as well as vanishing of bile ducts and liver failure. Ursodeoxycholic acid (UDCA) is a bile acid widely used for the therapy of cholangiopathies. However, little is known of the cytoprotective effects of UDCA on cholangiocytes. Therefore, UDCA and its taurine conjugate tauroursodeoxycholic acid (TUDCA) were administered in vivo to rats simultaneously subjected to bile duct ligation and vagotomy, a model that induces cholestasis and loss of bile ducts by apoptosis of cholangiocytes. Because these two bile acids act through Ca2+ signaling, animals were also treated with BAPTA/AM (an intracellular Ca2+ chelator) or Gö6976 (a Ca2+-dependent protein kinase C-α inhibitor). The administration of UDCA or TUDCA prevented the induction of apoptosis and the loss of proliferative and functional responses observed in the bile duct ligation-vagotomized rats. These effects were neutralized by the simultaneous administration of BAPTA/AM or Gö6976. UDCA and TUDCA enhanced intracellular Ca2+ and IP3 levels, together with increased phosphorylation of protein kinase C-α. Parallel changes were observed regarding the activation of the MAPK and PI3K pathways, changes that were abolished by addition of BAPTA/AM or Gö6976. These studies provide information that may improve the response of cholangiopathies to medical therapy.
Cholangiocytes are the epithelial cells that line the intrahepatic biliary tree.1 Cholangiocytes are the target of chronic cholestatic liver diseases, also termed cholangiopathies, a group of disorders responsible for more than 20% of liver transplants among adults and 50% among pediatric patients in the United States.2 Whatever the primary cause may be, cholangiopathies are almost invariably characterized by an abnormal balance between cholangiocyte proliferation and death, thus leading to chronic cholestasis, ductopenia, and liver failure.1,3
The autonomic nervous system plays a crucial role in hepatic regeneration and metabolic regulation of liver cells.4,5 After liver transplantation, nerve fibers are resected. Despite the fact that an increasing body of knowledge exists on the impact of transplantation on liver allograft, the functional consequences of the loss of autonomic innervation have been neglected.4–6 Little is known of the importance of liver innervation on the final outcome of transplantation, but it has been shown that adjuvant treatment with the bile acid ursodeoxycholic acid (UDCA) reduces allograft rejection.7
Cholangiocytes are normally mitotically dormant8; however, their proliferation can be triggered in several experimental models, the most validated of which is the bile duct ligated (BDL) rat model.9,10 The growth of the biliary tree after extrahepatic biliary obstruction by BDL is associated with a marked enhancement of cholangiocyte functional activity, eg, secretin-induced choleresis and intracellular cAMP synthesis.11–13 Cholinergic innervation of the liver is required for these proliferative and functional responses. We have demonstrated that severing the vagus nerve, which does not affect cholangiocyte growth or survival in the normal rat, abolishes the growth of the biliary tree after BDL.13–15 In addition, vagotomy in BDL rats triggers cholangiocyte apoptosis and abolishes their functional activity. Together, the loss of proliferation and enhanced apoptosis lead to a significant reduction in the number of bile ducts.13,15
Bile acids play a major role in the regulation of cholangiocyte biology. Bile acids enter cholangiocytes by a specific transporter localized at the apical pole of the cell, thus altering cholangiocyte functional activity, proliferation, and apoptosis.15–18 In particular, we have shown that cholinergic nerves and bile acids cooperatively modulate cholangiocyte survival. In fact, taurocholic acid feeding protects from the above-described loss of bile ducts induced by vagotomy.15 UDCA is a hydrophilic bile acid that is widely used for the treatment of various chronic cholestatic diseases.19 Despite several mechanisms that have been postulated,19 the cytoprotective effects of UDCA on cholangiocytes remains poorly understood. We have previously shown that feeding of UDCA or its taurine conjugate tauroursodeoxycholic acid (TUDCA) inhibits the proliferative response induced by BDL in rats.20 Furthermore, TUDCA reduces the growth of cholangiocellular neoplastic cell lines.21 Such effects are mostly determined by the UDCA- and TUDCA-induced activation of Ca2+-dependent protein kinase C-α (PKC-α).20,21 However, the effects of UDCA on cholangiocyte survival in the course of cholestasis, associated with the loss of bile ducts, is unknown.
To address this, we posed the following questions. 1) Do UDCA and TUDCA protect the biliary tree from damage induced by vagotomy in BDL rats? 2) Are the effects of UDCA and TUDCA associated with changes in Ca2+ signaling? 3) Does in vivo blockage of Ca2+ signaling neutralize the protective effects of UDCA and TUDCA on the vagotomy-induced damage of the biliary tree in the BDL rat? Finally, because the cytoprotective effect of UDCA and TUDCA on hepatocytes is mediated by the mitogen-activated protein kinase (MAPK) and by phosphatidyl-inositol-3-kinase (PI3K),22,23 we asked the following additional question: 4) Do the UDCA/TUDCA-induced changes in Ca2+ signaling subsequently modify the MAPK and PI3K pathways?
Materials and Methods
Materials
Reagents were purchased from Sigma Chemical Co. (St. Louis, MO), unless differently indicated. Rat chow, containing either 1% UDCA (275 μmol/day), 1% TUDCA (275 μmol/day), or control diet (AIN-76), was prepared by Dyets Inc., Bethlehem, PA. Control chow (AIN 76) has the same composition of rat chow containing UDCA or TUDCA but does not contain bile acids. A radioimmunoassay kit for the determination of intracellular cAMP levels was purchased from Amersham (Arlington Heights, IL). The d-myo-inositol 1,4,5, trisphosphate (IP3) [3H] kit, for the determination of intracellular IP3 levels, was purchased from Amersham. Antibodies for immunohistochemistry or immunoblotting were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA), unless otherwise reported. BAPTA/AM and Gö6976 were purchased from Calbiochem (La Jolla, CA).
Animal Models
Male Fischer 344 rats (150 to 175 g), purchased from Charles River (Wilmington, MA), were used in the study. Animals were kept in a temperature-controlled environment (20 to 22°C) with a 12-hour light-dark cycle with free access to drinking water and standard rat chow. As shown in Table 1, the study was mostly performed in eight experimental groups (groups 2 to 9) composed of 10 rats each. On day 0 (D0) for each group, six animals were subjected to BDL and four to BDI (bile duct incannulation).10,15,20 The six BDL rats were used either to obtain liver sections (n = 3) or for cholangiocyte purification (n = 3).15,20 The four BDI animals were used for bile collection.10,15,20 To induce loss of bile ducts, animals of groups 3 to 9 (Table 1) were subjected to vagotomy.13,15,24 This additional surgical procedure was performed on D0, immediately after BDL or BDI.
Table 1.
Animal Models
| Group | Surgery* | No. of animals | Treatment† |
|---|---|---|---|
| 1-NR | None | 6 | Control diet + control injections |
| None | 4 | ||
| 2-BDL | BDL | 6 | Control diet + control injections |
| BDI | 4 | ||
| 3-BDL + vagotomy | BDL + vagotomy | 6 | Control diet + control injections |
| BDI + vagotomy | 4 | ||
| 4-BDL + vagotomy + UDCA | BDL + vagotomy | 6 | UDCA feeding + control injections |
| BDI + vagotomy | 4 | ||
| 5-BDL + vagotomy + UDCA + BAPTA/AM | BDL + vagotomy | 6 | UDCA feeding + BAPTA/AM injections |
| BDI + vagotomy | 4 | ||
| 6-BDL + vagotomy + UDCA + Gö6976 | BDL + vagotomy | 6 | UDCA feeding + Gö6976 injections |
| BDI + vagotomy | 4 | ||
| 7-BDL + vagotomy + TUDCA | BDL + vagotomy | 6 | TUDCA feeding + control injections |
| BDI + vagotomy | 4 | ||
| 8-BDL + vagotomy + TUDCA + BAPTA/AM | BDL + vagotomy | 6 | TUDCA feeding + BAPTA/AM injections |
| BDI + vagotomy | 4 | ||
| 9-BDL + vagotomy + TUDCA + Gö6976 | BDL + vagotomy | 6 | TUDCA feeding + Gö6976 injections |
| BDI + vagotomy | 4 |
All the surgical procedures were performed on day 0 (D0); BDL was performed to obtain liver sections and to isolate cholangiocytes; BDI for bile collection.
All the treatments were started immediately after surgery and lasted one week.
Groups 2 to 3 (Table 1) received a control diet. In contrast, to study whether UDCA or TUDCA protects bile ducts from the damage observed in the BDL+ vagotomy model, groups 4 to 6 and 7 to 9 (Table 1) were fed a diet enriched with 1% UDCA or 1% TUDCA, respectively.20 The bile acid feeding started on D0, immediately after surgery, and lasted 1 week. Groups 2 to 4 and 7 (Table 1) received control injections. To verify whether the effects of UDCA and TUDCA were mediated by the Ca2+-dependent PKC signaling, groups 5 and 8 (Table 1) were also treated with daily intraperitoneal injections of BAPTA/AM (an intracellular Ca2+ chelator, 6 mg/kg body weight/day),25 whereas groups 6 and 9 were treated with daily intraperitoneal injections of Gö6976 (a Ca2+-dependent PKC-α inhibitor, 2.35 mg/kg body weight, by a 0.05 mmol/L stock solution in 0.1% dimethyl sulfoxide).26 Treatments with injections started on D0, immediately after surgery, and lasted 1 week. As baseline control, we used 10 normal rats (NR, group 1) that did not undergo any surgery or treatment for 1 week (Table 1). Four of these animals were subjected to BDI on day 7 (D7), immediately before starting bile collection.
BDL was performed as previously described.27 Briefly, under pentobarbital anesthesia (50 mg/kg, i.p.), the common bile duct was exposed through a paramedian incision, ligated twice (silk suture, 0.5 cm) and finally severed between the ligations. BDI was performed as previously described by us.10 Briefly, under pentobarbital anesthesia (50 mg/kg, i.p.), the common bile duct was cannulated through a small abdominal incision. After free flow of bile was established, the bile duct cannula (4 to 5 cm long) was sealed at its open end with a flame and secured to the abdominal wall with several ligatures. The abdomen was then sutured, and the animal allowed to recover and kept in a standard rat cage. Total vagotomy was performed as described previously.15,17,24 Briefly, a midline incision was made just anterior to the nasopharyngeal opening and anterior to the thoracic outlet, and the right carotid artery was located and isolated. The vagus nerve was then blunt dissected and ligated. The incision was closed with 3-O Vicryl suture (Ethicon, Somerville, NJ) in an interrupted vertical mattress pattern. As internal controls, BDL rats were subjected to vagotomy, fed a control diet, and treated daily with BAPTA/AM or Gö6976, diluted in 0.1% dimethyl sulfoxide, as described above. We have previously shown that chronic intraperitoneal injections of dimethyl sulfoxide does not alter cholangiocyte apoptosis, proliferation, and secretion of BDL rats.15,17 In a previous study we have widely characterized the control groups of rats subjected to BDL and fed either a UDCA- or TUDCA-enriched diet.20 Therefore, we did not use these groups of animals in the study. The animals were fasted overnight before each experiment.17 Before each procedure, animals were anesthetized with sodium pentobarbital (50 mg/kg i.p.). Study protocols were performed in compliance with institutional guidelines.
Purification of Cholangiocytes
Pure cholangiocytes were obtained from the selected group of animals using a monoclonal antibody that selectively binds to an unidentified antigen expressed by intrahepatic rat cholangiocytes,17,28 as previously described.10,17,18,28 Purity of cholangiocytes was evaluated by histochemistry for γ-GT,29 a specific marker for cholangiocytes.10 Cell viability was determined by trypan blue exclusion and was found to be greater than 97%.
Evaluation of Cholangiocyte Apoptosis
Cholangiocyte apoptosis was evaluated by 1) quantitative terminal dUTP nick-end labeling (TUNEL) analysis15 in liver sections, 2) caspase 3 activity assay15 and 3) immunoblots for cleaved pro-caspase 3 expression30 in purified cholangiocytes. TUNEL analysis was performed using a commercially available kit (Wako Chemicals, Tokyo, Japan), as previously described.15 After counterstaining with hematoxylin solution, liver sections (three for each group of treatment) were examined by light microscopy with a BX-40 microscope (Olympus, Tokyo, Japan) equipped with a camera. Approximately 100 cells per slide were counted in a coded manner in 10 nonoverlapping fields.
Caspase 3 activity was measured in pure cholangiocytes purified from the selected group of animals by an enzymatic kit (Medical & Biological Laboratories Co., Ltd., Nagoya, Japan), as previously described by us,15 according to the instructions of the vendor. The assay is based on the photometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the specific substrate (DEVD-pNA). The pNA light emission was quantified using a microtiter plate reader at 406 nm. The expression of cleaved pro-caspase 3 was assayed by immunoblot using as primary antibody the rabbit anti-caspase 3 (Oncogene Research Products, Boston, MA), as previously described.30
Evaluation of Cholangiocyte Proliferation
Cholangiocyte proliferation was studied both by quantitative immunohistochemistry for cytokeratin-19 (CK-19, a specific marker of cholangiocytes)31 or proliferating cell nuclear antigen (PCNA)31 in liver sections and by immunoblots for PCNA protein expression in pure cholangiocyte lysates15 from the selected groups of animals. After staining with specific antibodies for either PCNA or CK-19, sections were counterstained with hematoxylin and examined with a microscope (BX 40, Olympus).31 Data were expressed as number of PCNA- or CK-19-positive cholangiocytes per 100 cholangiocytes counted in seven different fields. DNA replication was evaluated by measurement of PCNA protein expression by immunoblot of pure cholangiocyte lysates, as previously described.15
Evaluation of Cholangiocyte Functional Activity
Cholangiocyte functional activity was studied measuring the basal and secretin-stimulated bile flow and bicarbonate secretion in BDI rats and intracellular cAMP synthesis in pure cholangiocytes from the selected group of animals. After anesthesia, animals were surgically prepared for bile collection as previously described by us.20 After steady spontaneous bile flow was reached (60 to 70 minutes from the infusion of Krebs-Ringer-Henseleit), animals were infused for 30 minutes with secretin (100 nmol/L) followed by a final infusion of Krebs-Ringer-Henseleit for 30 minutes. Bile was collected every 10 minutes, put in preweighed tubes, and immediately stored at −70°C. Biliary bicarbonate concentration (measured as total CO2) was determined by an ABL 520 blood gas system (Radiometer Medical A/S, Copenhagen, Denmark).
Intracellular cAMP levels were measured in pure cholangiocytes isolated from the selected groups of animals, after a 1-hour incubation at 37°C to regenerate membrane proteins damaged by proteolytic enzymes during the purification.8,11,32 Cells (1 × 105) were incubated at room temperature11,17 with 0.2% bovine serum albumin (basal) or 100 nmol/L secretin with 0.2% bovine serum albumin. Intracellular cAMP levels were determined by commercially available radioimmunoassay kits (Amersham Corp.), according to the instructions of the vendor.
Measurement of Intracellular Ca2+ Levels
Calcium fluorescence measurements in cholangiocytes isolated from the selected group of animals were performed using fluo-3 AM (Molecular Probes, Eugene, OR) and a Fluoroskan Ascent FL (Thermolabsystems, Helsinki, Finland) microplate reader equipped with three injectors, as previously described by us.33 Briefly, cholangiocytes were loaded for 1 hour at room temperature with 5 μmol/L of fluo-3 AM in Tyrode’s salt solution with 0.1% Pluronic F-127 (Molecular Probes). After two washes, cells were subsequently resuspended in Tyrode’s salt solution and incubated for an additional 30 minutes at room temperature. The loaded cells were then pelleted and resuspended at 4 × 104 cells per 100 μl of Tyrode’s salt solution and added to the well of a 96-well black microplate. The baseline fluorescence was measured three times at 20-second intervals at the wavelength of 538 nm. The excitation wavelength was 485 nm. Intracellular calcium [Ca2+]i concentration was calculated as a mean of the three baseline measurements with a minimum of six independent samples for each treatment group evaluated as follows: [Ca2+]i = Kd(F − Fmin)/(Fmax − F). Fmax refers to fluorescence intensity measured after permeabilization of the cells with 1% Nonidet P-40. Then, 0.1 mol/L EGTA was added to chelate Ca2+, and minimum fluorescence intensity (Fmin) was obtained. The kd of fluo-3 AM was 390 nm.33,34
Measurement of the Inositol-3-Phosphate (IP3) Intracellular Levels
After purification, cholangiocyte (5 × 106) intracellular IP3 levels were measured by the IP3 [3H] assay system according to the instructions supplied by the vendor (Amersham Corp.).33,35
Evaluation of PKC-α, ERK1/2, and AKT Phosphorylation
PKC-α phosphorylation was assayed by immunoblot using the rabbit anti-phospho-PKC-α or the mouse anti-PKC-α. ERK1/2 phosphorylation was estimated by immunoblot using as primary antibodies the mouse anti-phospho-ERK1/2 and either the goat anti-ERK1 or the goat anti-ERK2. The phosphorylation of AKT, an immediate downstream of PI3K,15 was studied using the rabbit anti-phospho-AKT (Ser 473) and goat anti-AKT antibodies.
Immunoblotting
Pure cholangiocytes were resuspended in lysis buffer (20 mmol/L Tris-HCl, pH 7.4, 150 mmol/L NaCl, 5 mmol/L ethylenediaminetetraacetic acid, 1% Nonidet P-40, 1 mmol/L aprotinin, 1 mmol/L phenylmethyl sulfonyl fluoride, and 1 mmol/L leupeptin) and sonicated three times (10-second bursts). Proteins (50 μg/lane) were resolved by sodium dodecyl sulfate-7.5% polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. After blocking, the membrane was incubated overnight at 4°C with primary antibody followed by incubation with the corresponding secondary antibody. The comparability of the protein used was determined by immunoblot for β-actin; the β-actin antibody was purchased from Sigma Chemical Co. Proteins were visualized using chemiluminescence (ECL Plus kit, Amersham). The intensity of the bands was determined by scanning video densitometry using the ChemiImager 4000 low-light imaging system (Alpha Innotech, San Leandro, CA).
Statistical Analysis
All data are expressed as mean ± SEM. The differences between groups were analyzed by Student’s t-test when two groups were analyzed or by analysis of variance if more than two groups were analyzed. P < 0.05 was accepted as significant.
Results
UDCA and TUDCA Feeding Prevents Vagotomy-Induced Reduction of Proliferation and Increased Apoptosis in Cholangiocytes from BDL Rats
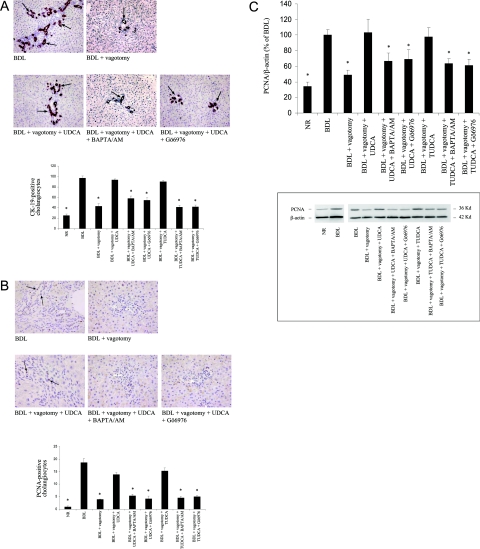
In BDL rats, vagotomy induces apoptosis of cholangiocytes. In fact, similar to what we have previously shown11,15 there was a marked increase in the number of TUNEL-positive cholangiocytes in liver sections, in the caspase 3 activity, and in the expression of cleaved pro-caspase 3 in purified cholangiocytes (Figure 1, A–C, respectively). Simultaneous feeding with either UDCA or TUDCA prevented the vagotomy-induced increase in cholangiocyte apoptosis (Figure 1, A–C). No significant changes in cholangiocyte apoptosis between normal rats (NR) and BDL rats were observed. As expected, BDL induced a marked increase in cholangiocyte proliferation when compared to NR (Figure 2, A–C). Moreover, immunohistochemical studies showed that vagotomy, in BDL rats, reduced the number of CK-19- and PCNA-positive cholangiocytes (Figure 2, A and B, respectively). Diminished expression of PCNA protein expression in isolated cholangiocytes was observed by immunoblotting (Figure 2C), demonstrating a significant reduction of cholangiocyte proliferation. UDCA and TUDCA counteracted the effect of vagotomy, demonstrated by the return of the number of CK-19- and PCNA-positive cells and PCNA protein expression to levels similar to BDL rat cholangiocytes (Figure 2, A–C). UDCA and TUDCA exerted a protective effect on cholangiocyte functional activity. Bile acid feeding restored the secretin response of bile flow (Table 2), bicarbonate secretion (Table 2), and intracellular cAMP synthesis (Table 2), responses that are lost after vagotomy (Table 2).13,15
Figure 1.
Evaluation of cholangiocyte apoptosis. A: Quantitative TUNEL analysis in liver sections obtained from rats of the different experimental groups. Top: Representative images of the histochemical reaction. Positive cholangiocytes are indicated by arrows. Bottom: The quantitative analysis (n = 3). B: Caspase 3 activity in cholangiocytes freshly isolated from the different experimental groups (n = 3). C: Cleaved pro-caspase 3 expression in cholangiocytes freshly isolated from rats of the different experimental groups. Top: Quantitative data of the changes in cleaved pro-caspase 3 protein expression (to compare NR cholangiocyte values, obtained in different experimental sets, data are expressed as percentage of BDL value, n = 3). Bottom: Representative images of the immunoblots for pro-caspase 3 and β-actin. Data are mean ± SEM of three experiments. *P < 0.05 versus NR BDL, BDL + vagotomy + UDCA, and BDL + vagotomy + TUDCA.
Figure 2.
Evaluation of cholangiocyte proliferation. A: Quantitative CK-19 immunohistochemistry in liver sections obtained from rats of the different experimental groups. Top: Representative images of the immunohistochemical reaction. Positive bile ducts are denoted by arrows. Bottom: The quantitative analysis (n = 3). B: Quantitative PCNA immunohistochemistry in liver sections from rats of the different experimental groups. The top part displays representative images of the immunohistochemical reaction; positive cholangiocytes are denoted by arrows (n = 3). C: PCNA protein expression, measured by immunoblot, in cholangiocytes freshly isolated from rats of the different experimental groups. Top: Quantitative data of the changes in PCNA protein expression (to compare NR cholangiocyte values, obtained in different experimental sets, data are expressed as percentage of BDL value, n = 3). Bottom: Representative images of the immunoblots PCNA and β-actin. Data are mean ± SEM of three experiments. *P < 0.05 versus BDL, BDL + vagotomy + UDCA, and BDL + vagotomy + TUDCA. Original magnifications: ×250 (A); ×1000 (B).
Table 2.
Evaluation of Cholangiocyte Functional Activity
| Bile Flow (μl/min/kg body weight)
|
Bicarbonate secretion (μEq/min/kg body weight)
|
cAMP levels (fmol/100,000 cells)
|
||||
|---|---|---|---|---|---|---|
| Basal | Secretin | Basal | Secretin | Basal | Secretin | |
| NR | 72.8 (± 4.4) | 73.5 (± 5.3) | 1.9 (± 0.1) | 1.9 (± 0.2) | 14.2 (± 1.6) | 39.3* (± 3.4) |
| BDL | 94.1 (± 6.7) | 126.9* (± 9.3) | 3.3 (± 0.6) | 6.4* (± 0.8) | 39.0 (± 6.8) | 74.5* (± 6.7) |
| BDL + vagotomy | 90.1 (± 3.8) | 93.4 (± 8.9) | 3.3 (± 0.2) | 3.5 (± 0.1) | 39.4 (± 9.4) | 40.8 (± 4.5) |
| BDL + vagotomy + UDCA | 95.7 (± 3.7) | 128.1* (± 3.4) | 3.4 (± 0.1) | 5.8* (± 0.2) | 45.7 (± 3.5) | 67.3* (± 4.7) |
| BDL + vagotomy + UDCA + BAPTA/AM | 103.7 (± 9.1) | 114.3 (± 14.4) | 3.7 (± 0.3) | 3.9 (± 0.4) | 29.1 (± 5.4) | 32.8 (± 4.1) |
| BDL + vagotomy + UDCA + Gö6976 | 105.1 (± 10.4) | 105.9 (± 2.5) | 3.8 (± 0.3) | 3.5 (± 0.2) | 25.1 (± 2.1) | 30.7 (± 6.9) |
| BDL + vagotomy + TUDCA | 112.9 (± 14.0) | 155.1* (± 8.6) | 3.9 (± 0.4) | 6.0* (± 0.5) | 30.4 (± 2.9) | 51.7* (± 3.7) |
| BDL + vagotomy + TUDCA + BAPTA/AM | 99.7 (± 12.3) | 105.7 (± 15.7) | 4.1 (± 0.2) | 3.8 (± 0.3) | 27.0 (± 1.5) | 27.1 (± 1.2) |
| BDL + vagotomy + TUDCA + Gö6976 | 115.5 (± 15.8) | 120.3 (± 14.6) | 4.4 (± 0.4) | 4.6 (± 0.4) | 29.5 (± 2.9) | 27.9 (± 5.7) |
Cholangiocyte functional activity was measured as response to secretin of the bile flow and bicarbonate secretion in vivo and the in vitro response to secretin of the intracellular cAMP synthesis in cholangiocytes isolated from animals of the different experimental groups. The protective effects of UDCA and TUDCA on cholangiocyte functional activity, that is lost when the BDL rat is subjected to vagotomy, is dependent on the Ca2+-PKC-α signaling, since they were no longer evident in animals injected with either BAPTA/AM or Gö6976. Data are mean ± SEM of six experiments.
P < 0.05 versus the corresponding basal value.
The Protective Effects of UDCA and TUDCA on Vagotomy-Induced Damage of the Biliary Tree Are Associated with the Restoration of IP3-Ca2+-PKC-α Signaling
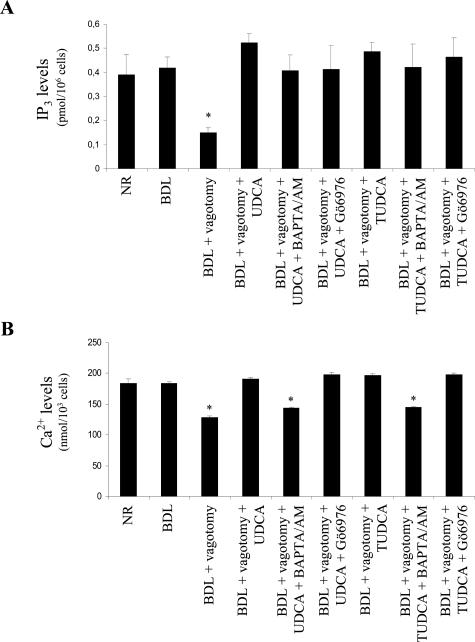
In BDL cholangiocytes, IP3 and Ca2+ levels and PKC-α phosphorylation were not significantly different from those in NR cholangiocytes (Figures 3 and 4). Compared to BDL, intracellular IP3 levels in cholangiocytes isolated from BDL-vagotomized animals were markedly reduced, which was prevented by UDCA and TUDCA feeding (Figure 3A). In a similar manner, vagotomy induced a decrease in Ca2+ levels in pure cholangiocytes (Figure 3B). This effect was reversed by UDCA and TUDCA feeding (Figure 3B). UDCA and TUDCA also prevented the decreased phosphorylation of PKC-α (Figure 4). In contrast, no changes in the expression of the constitutive PKC-α were observed.
Figure 3.
Evaluation of Ca2+ and IP3 levels. A: Measurement of intracellular IP3 levels in cholangiocytes freshly isolated from rats of the different experimental groups, using the IP3 [3H] assay (n = 6). Data are mean ± SEM of six experiments. *P < 0.05 versus the other groups. B: Assay of the intracellular Ca2+ levels in cholangiocytes freshly isolated from rats of the different experimental groups measured using fluo-3 AM and a Fluoroskan Ascent FL microplate reader (n = 6). Data are mean ± SEM of six experiments. *P < 0.05 versus NR, BDL, BDL + vagotomy + UDCA ± Gö6976, and BDL + vagotomy + TUDCA Gö6976.
Figure 4.
Evaluation of PKC-α phosphorylation. PKC-α phosphorylation was studied by immunoblot of whole cell lysates of cholangiocytes freshly isolated from rats of the different experimental groups. Top: Quantitative data of the changes in PKC-α phosphorylation (to compare NR cholangiocyte values, obtained in different experimental sets, data are expressed as percentage of BDL value, n = 3). Bottom: Representative images of the immunoblot for phosphorylated and constitutive PKC-α and β-actin. Data are mean ± SEM of three experiments. *P < 0.04 versus BDL, BDL + vagotomy + UDCA, and BDL + vagotomy + TUDCA.
Blockage of the Ca2+-PKC Signaling Neutralizes the Cytoprotective Effects of UDCA and TUDCA on the Vagotomy-Induced Damage of the Biliary Tree in the BDL Rat
The anti-apoptotic effect of UDCA and TUDCA was no longer evident when the Ca2+-PKC-α signal was blocked. The number of TUNEL-positive cholangiocytes (Figure 1A), the level of caspase 3 activity (Figure 1B), and the expression of cleaved pro-caspase 3 (Figure 1C) were all significantly increased when UDCA and TUDCA feeding were associated with the simultaneous administration of BAPTA/AM or Gö6976, thus returning to levels not statistically different from those of the BDL vagotomized animals. Simultaneous treatment with BAPTA/AM or Gö6976 blocked the restoration of cholangiocyte proliferation induced by UDCA and TUDCA in BDL-vagotomized rats. Indeed, immunohistochemistry showed that the number of CK-19- and PCNA-positive cells was markedly diminished (Figure 2, A and B) after treatment with BAPTA/AM or Gö6976 compared to UDCA or TUDCA feeding. A similar trend was observed in PCNA protein expression measured in pure cholangiocytes by immunoblot (Figure 2C). Finally, administration of either BAPTA/AM or Gö6976 neutralized the protective effect of UDCA and TUDCA on the response to secretin in bile flow, bicarbonate secretion, and intracellular cAMP synthesis (Table 2).
Administration of BAPTA/AM or Gö6976 Abolishes the UDCA- and TUDCA- Induced Increases in Ca2+ Levels or Ca2+-Dependent PKC-α Phosphorylation but Not IP3 Levels
The simultaneous administration of BAPTA/AM or Gö6976 did not modify the increase of IP3 levels induced by either UDCA or TUDCA feeding (Figure 3A). BAPTA/AM, but not Gö6976, blocked the enhancement of intracellular Ca2+ levels observed after UDCA or TUDCA feeding (Figure 3B). However, both BAPTA/AM and Gö6976 administration blocked the stimulatory effect of UDCA and TUDCA feeding on PKC-α phosphorylation (Figure 4).
UDCA and TUDCA Prevent the Vagotomy-Induced ERK1/2 and AKT Dephosphorylation in a Ca2+-PKC-Dependent Manner
As expected, both ERK1/2 and AKT phosphorylation were strongly increased in BDL cholangiocytes compared to NR cholangiocytes (Figure 5). Vagotomy stimulated a marked decrease in the ERK1/2 phosphorylation (Figure 5A). This effect was neutralized by the simultaneous administration of either BAPTA/AM or Gö6976. As shown in Figure 5B, a similar trend in the different experimental conditions was found for AKT phosphorylation. There was no change in the expression of total ERK1/2 or AKT in any of the experimental conditions observed.
Figure 5.
Evaluation of ERK1/2 and AKT phosphorylation. A: Measurement of ERK1/2 phosphorylation by immunoblot of whole cell lysates of cholangiocytes freshly isolated from rats of the different experimental groups. Top: Quantitative data of the changes in ERK1/2 phosphorylation (to compare NR cholangiocyte values, obtained in different experimental sets, data are expressed as percentage of BDL value, n = 3). Bottom: Representative images of the immunoblots for phosphorylated and constitutive ERK1/2 and β-actin. Data are mean ± SEM of three experiments. #P < 0.05 versus pERK1 of BDL, BDL + vagotomy + UDCA, and BDL + vagotomy + TUDCA; §P < 0.05 versus pERK2 of BDL, BDL + vagotomy + UDCA, and BDL + vagotomy + TUDCA. B: Measurement of AKT phosphorylation by immunoblot of whole cell lysates of cholangiocytes freshly isolated from the different experimental groups. Top: Quantitative data of the changes in AKT phosphorylation (to compare NR cholangiocyte values, obtained in different experimental sets, data are expressed as percentage of BDL value, n = 3). Bottom: Representative images of the immunoblot for phosphorylated and constitutive AKT and β-actin. Data are mean ± SEM of three experiments. *P < 0.05 versus pAKT of BDL, BDL + vagotomy + UDCA, and BDL + vagotomy + TUDCA.
Discussion
The results of this study demonstrate that 1) UDCA and TUDCA prevent the loss of bile ducts induced by vagotomy in the BDL rat; 2) the cytoprotective effects of UDCA and TUDCA are associated with enhancement of the Ca2+ signaling, the blockage of which 3) neutralizes the protective effect of such bile acids on the vanishing of bile ducts induced by vagotomy in the BDL rat, 4) UDCA and TUDCA feeding results in the activation of the MAPK and PI3K pathways, and 5) blockage of Ca2+ signaling inhibits the activation of such survival pathways.
Cholangiopathies are a wide array of disorders that recognize a large number of different etiopathogenic factors.1,3,36,37 On the other hand, they are known to share similar features, such as the abnormal balance between proliferation and death of cholangiocytes.1,3,36 In particular, vanishing of bile ducts seems to result from enhanced apoptosis and impaired proliferative capacity of the biliary epithelium.1,3,36 However, what drives this balance in cholangiocytes is still primarily unknown. Limited knowledge and poor understanding of how to manipulate cholangiocyte survival pathways in vivo results in a lack of effective pharmacological therapies for cholangiopathies.1–3,19 UDCA is the only drug commonly used in the medical therapy of cholangiopathies with a certain degree of success.19 Therefore, the principal aim of our study was to verify whether UDCA and its taurine conjugate TUDCA have cytoprotective effects in cholangiocytes in an in vivo model of marked loss of bile ducts.
In previous studies we have shown that the interruption of cholinergic innervation impairs cholangiocyte proliferation and functions in response to BDL and also triggers apoptosis.13,15 The BDL + vagotomy rat model, thus, reproduces a condition of cholestasis, cholangiocyte apoptosis, and loss of proliferative capacity and functional activity.13,15 Even though this model does not recreate all of the features of cholangiopathies, eg, inflammation and immune-mediated damage,1,3,36,37 it certainly represents an interesting tool to study the factors that govern the balance between cholangiocyte death and proliferation in the course of chronic cholestasis. Therefore, we evaluated the changes in cholangiocyte apoptosis, proliferation, and functional activity in rats simultaneously subjected to BDL and vagotomy and subsequently fed for 1 week with UDCA or TUDCA. The administration of these bile acids prevented the induction of apoptosis by vagotomy (Figure 1). In addition, UDCA and TUDCA feeding also restored cholangiocyte proliferation (Figure 2) and maintained a proper functional activity of the biliary epithelium (Table 2), which was lost after vagotomy.13,15 These data demonstrate that UDCA and TUDCA prevent the loss of cholangiocytes and maintain their functional activity in a condition characterized by marked loss of ductal mass. After vagotomy, there are no changes in the biliary bile acid composition or in the inflammation within the liver.13 Such observations support the concept that the protective effects of UDCA and TUDCA should be ascribed to their direct action on cholangiocytes.
UDCA and TUDCA effects in liver cells (cholangiocytes included)13,20,21 are mediated by the Ca2+-dependent PKC19,38–40; in addition, recent evidence shows that the loss of Ca2+ signaling is a unifying feature of the last stages of cholangiopathies.41 Such a feature is reproduced by the BDL + vagotomy model, which is featured by the loss of Ca2+-agonistic M3 acetylcholine receptors in cholangiocytes after the interruption of cholinergic innervation.13,14 In the current study, we observed that vagotomy blunted the intracellular IP3 and Ca2+ levels and Ca2+-dependent PKC-α phosphorylation, events that were prevented by UDCA and TUDCA feeding. To provide a mechanistic significance to our findings, we then determined whether in vivo administration of BAPTA/AM (an intracellular Ca2+-chelator) or Gö6976 (a Ca2+-dependent PKC inhibitor) neutralizes the protective effects of UDCA and TUDCA. The use of such inhibitors has been successfully used to block Ca2+ signaling in vivo.25,26 Simultaneous treatments with either BAPTA/AM or Gö6976 determined a complete reversal of the protective effects of UDCA and TUDCA against the loss of bile ducts and their functional activity. Despite the fact that bile acids can affect cell biology by many intracellular pathways,17,42–44 these data strongly confirm what has been suggested by previous studies in biliary cells20,21: Ca2+ signaling is necessary for the properties of these bile acids on cholangiocytes. In Figure 3, it is also shown that the administration of BAPTA/AM did not block the enhancement of IP3 levels induced by bile acids; similarly, Gö6976 did not alter IP3 and Ca2+ levels. If these results were expected, given the fact that IP3 is upstream of Ca2+ and Ca2+ of PKC,45 they are highly suggestive of a direct effect of BAPTA/AM and Gö6976 on cholangiocytes, rather than a generalized, nonspecific effect.
Last we aimed to clarify how UDCA/TUDCA-activated Ca2+ signaling affects intracellular events responsible for survival, growth, and functional activity of cholangiocytes. We found that UDCA and TUDCA feeding prevented the inhibitory effect of vagotomy on the phosphorylation of ERK1/2 and AKT, indices of the activation state of the MAPK and PI3K pathways, respectively, known to be major determinants of cell growth and survival.15,46 In contrast, such effects were blunted by the blockage of the Ca2+ signaling. Similarly, BAPTA/AM and Gö6976 neutralized the restoration of the secretin response of intracellular synthesis of cAMP, a key molecule in the regulation of cholangiocyte functional activity.12 Together, these data indicate that chronic administration of UDCA and TUDCA prevents the reduction of bile ducts by sustaining survival pathways via the stimulation of Ca2+ signaling. This, together with the Ca2+-dependent enhancement of adenylyl cyclase activity, allows such bile acids also to maintain cholangiocyte choleresis.
These data provide several answers on the significance of UDCA and its derivate TUDCA for the pathophysiology of chronic cholestasis. Apoptosis is the major form of cholangiocyte death observed in the most common of cholangiopathies such as primary biliary cirrhosis.19 Anti-apoptotic properties of UDCA have been described in hepatocytes both in vivo and in vitro.23,47–50 However, the current study provides evidence of a direct in vivo effect of UDCA in preventing cholangiocyte apoptosis. In addition, UDCA and TUDCA reduce the bile duct loss also sustaining cholangiocyte growth. In a previous investigation,20 we found that when UDCA or TUDCA are fed to BDL rats, an opposite effect occurs, cholangiocyte proliferation and functional activity was reduced rather than enhanced. Such a major difference might be ascribed to a diverse status of the biliary tree in the two experimental models. In the BDL rat, cholangiocytes undergo an intense proliferative reaction, whereas in the model used here there is a marked loss of bile ducts coupled with apoptotic death of the biliary epithelium, not present in the former. It can be postulated that, in the course of liver injury, UDCA and its conjugate TUDCA exert protective effects on the biliary tree that are heterogeneous and mostly depend on the features of the damage itself.
This study demonstrates that such effects of UDCA and TUDCA in cholangiocytes are determined by the activation of the Ca2+-dependent PKC-α signaling, confirmed by the fact that the simultaneous administration of either BAPTA/AM or Gö6976 neutralizes the effects of the studied bile acids. In particular, we found that the UDCA/TUDCA stimulation of Ca2+ signaling activates the MAPK and PI3K cascades, in agreement with what has been recently described in hepatocytes.23,24 These investigations proposed a direct activation of MAPK and PI3K signaling by bile acids. In contrast, in our study we demonstrate that in cholangiocytes the effects of UDCA and TUDCA on such pathways are neutralized by the simultaneous administration of BAPTA/AM and Gö6976, thus suggesting that the modulation of MAPK and PI3K is mostly dependent on the Ca2+ signaling. Furthermore, the fact that the simultaneous administration of BAPTA/AM and Gö6976 abolished all of the protective effects of the tested bile acids represents the first mechanistic demonstration of the relationship between UDCA and TUDCA and Ca2+ signaling in cholangiocytes in vivo. This observation is significant, because it warns the researcher and the clinician to consider the possible negative effects of the use of drugs that affect such a signal when UDCA-based treatment for chronic cholestasis has been started.
The actual role of the PKC isoforms in the pathogenesis of cholestasis has been recently questioned. A recent study reported that Ca2+-dependent isoforms induce cholestasis, retrieving canalicular bile acid transporters in cultured hepatocytes.51 Apparently this does not happen in cholangiocytes, where the bile acid-induced activation of Ca2+-dependent PKC (Figure 4) is associated with potent enhancement of cholangiocyte secretory activity (Table 2). Similar observations, however, have been previously demonstrated both in hepatocytes38–40 and in cholangiocytes themselves,21 thus supporting the concept that Ca2+ signaling plays a fundamental role in mediating the procholeretic properties of UDCA and TUDCA.
In summary, we found that administration of UDCA or TUDCA to rats with chronic cholestasis and loss of bile ducts protects from apoptosis induction, enhances growth and maintains functional activity of the biliary epithelium. Such effects are attributable to the activation of Ca2+ signaling and the consequent stimulation of the MAPK and PI3K pathways. These findings provide direct evidence of the cytoprotective effects of such bile acids on cholangiocytes and a mechanistic description of the intracellular events by which this happens. In particular, the current data demonstrate the efficacy of the bile acid-based prevention of the loss of bile ducts can be significantly diminished by the simultaneous treatment with drugs that interfere with Ca2+ signaling. Such information may represent an important tool to design future strategies to improve the response of cholangiopathies to medical therapy.
Footnotes
Address reprint requests to Shannon Glaser, M.S., Dept. of Medicine, Division of R&E, Scott and White Memorial Hospital and The Texas A&M University System Health Science Center College of Medicine, MRB, 702 South West H.K. Dodgen Loop, Temple, Texas 76504. E-mail: sglaser@neo.tamu.edu.
Supported by the Scott and White Hospital (grants to S.G., H.F., and G.A.), the Veterans Administration (research scholar award and a merit award to G.A.), the National Institutes of Health (grants DK58411 and DK062975 to G.A.), the Health and Labour Sciences (research grants from the Ministry of Health, Labour, and Welfare of Japan to Y.U.), the Research on Measures for Intractable Diseases (to Y.U.), Japan Society for the Promotion of Science (grant-in-aid for scientific research C, 16590573, to Y.U.), and by the Università Politecnica delle Marche, Ancona, Italy (grant MIUR 2003060137_004 to the Department of Gastroenterology).
References
- Alpini G, McGill JM, LaRusso NF. The pathobiology of biliary epithelia. Hepatology. 2002;35:1256–1268. doi: 10.1053/jhep.2002.33541. [DOI] [PubMed] [Google Scholar]
- 2001 Annual Report of the US Organ Procurement and Transplantation Network and the Scientific Registry for Transplant Recipients: Transplant Data 1991–2000. Rockville, Department of Health and Human Services, Health Resources and Services Administration, Office of Special Programs, Division of Transplantation, 2001 [Google Scholar]
- Desmet VJ. Vanishing bile duct disorders. Prog Liver Dis. 1992;10:89–121. [PubMed] [Google Scholar]
- Bioulac-Sage P, Lafon ME, Saric J, Balabaud C. Nerves and perisinusoidal cells in human liver. J Hepatol. 1990;10:105–112. doi: 10.1016/0168-8278(90)90080-b. [DOI] [PubMed] [Google Scholar]
- Kato H, Shimazu T. Effect of autonomic denervation on DNA synthesis during liver regeneration after partial hepatectomy. Eur J Biochem. 1983;134:473–478. doi: 10.1111/j.1432-1033.1983.tb07591.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto I, Takahashi T, Kakita A, Hayashi I, Majima M, Yamashina S. Experimental study on hepatic reinnervation after orthotopic liver transplantation in rats. J Hepatol. 2002;37:814–823. doi: 10.1016/s0168-8278(02)00283-0. [DOI] [PubMed] [Google Scholar]
- Friman S, Persson H, Schersten T, Svanvik J, Karlberg I. Adjuvant treatment with ursodeoxycholic acid reduces acute rejection after liver transplantation. Transpl Int. 1992;5(Suppl 1):S187–S189. doi: 10.1007/978-3-642-77423-2_58. [DOI] [PubMed] [Google Scholar]
- LeSage G, Glaser S, Gubba S, Robertson WE, Phinizy JL, Lasater J, Rodgers R, Alpini G. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal bile secretion. Gastroenterology. 1996;111:1633–1644. doi: 10.1016/s0016-5085(96)70027-6. [DOI] [PubMed] [Google Scholar]
- Alpini G, Glaser SS, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, LaRusso NF. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol. 1998;274:G767–G775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- Alpini G, Lenzi R, Sarkozi L, Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser SS, Rodgers RE, Phinizy JL, Robertson WE, Lasater J, Caligiuri A, Tretjak Z, LeSage GD, Alpini G. Gastrin inhibits secretin-induced ductal secretion by interaction with specific receptors on rat cholangiocytes. Am J Physiol. 1997;273:G1061–G1070. doi: 10.1152/ajpgi.1997.273.5.G1061. [DOI] [PubMed] [Google Scholar]
- Kanno N, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol. 2001;281:G612–G625. doi: 10.1152/ajpgi.2001.281.3.G612. [DOI] [PubMed] [Google Scholar]
- LeSage G, Alvaro D, Benedetti A, Glaser S, Marucci L, Eisel W, Caligiuri A, Baiocchi L, Rodgers R, Phinizy JL, Francis H, Alpini G. Cholinergic system modulates growth, apoptosis and secretion of cholangiocytes from bile duct ligated rats. Gastroenterology. 1999;117:191–199. doi: 10.1016/s0016-5085(99)70567-6. [DOI] [PubMed] [Google Scholar]
- Alvaro D, Alpini G, Jezequel AM, Bassotti C, Francia C, Fraioli F, Romeo R, Marucci L, Le Sage G, Glaser SS, Benedetti A. Role and mechanisms of action of acetylcholine in the regulation of rat cholangiocyte secretory functions. J Clin Invest. 1997;100:1349–1362. doi: 10.1172/JCI119655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzioni M, LeSage G, Glaser S, Patel T, Marienfeld C, Ueno Y, Francis H, Alvaro D, Phinizy JL, Tadlock L, Benedetti A, Marucci L, Baiocchi L, Alpini G. Taurocholate prevents the loss of intrahepatic bile ducts due to vagotomy in bile duct ligated rats. Am J Physiol. 2003;284:G837–G852. doi: 10.1152/ajpgi.00398.2002. [DOI] [PubMed] [Google Scholar]
- Alpini G, Glaser S, Robertson W, Phinizy JL, Rodgers RE, Caligiuri A, LeSage G. Bile acids stimulate proliferative and secretory events in large but not small cholangiocytes. Am J Physiol. 1997;273:G518–G529. doi: 10.1152/ajpgi.1997.273.2.G518. [DOI] [PubMed] [Google Scholar]
- Alpini G, Glaser SS, Ueno Y, Rodgers R, Phinizy JL, Francis H, Baiocchi L, Holcomb LA, Caligiuri A, LeSage GD. Bile acid feeding induces cholangiocyte proliferation and secretion: evidence for bile acid-regulated ductal secretion. Gastroenterology. 1999;116:179–186. doi: 10.1016/s0016-5085(99)70242-8. [DOI] [PubMed] [Google Scholar]
- Alpini G, Ueno Y, Glaser SS, Marzioni M, Phinizy JL, Francis H, LeSage G. Bile acid feeding increased proliferative activity and apical bile acid transporter expression in both small and large rat cholangiocytes. Hepatology. 2001;34:868–886. doi: 10.1053/jhep.2001.28884. [DOI] [PubMed] [Google Scholar]
- Paumgartner G, Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525–531. doi: 10.1053/jhep.2002.36088. [DOI] [PubMed] [Google Scholar]
- Alpini G, Baiocchi L, Glaser S, Ueno Y, Marzioni M, Francis H, Phinizy JL, Angelico M, LeSage G. Ursodeoxycholate and tauroursodeoxycholate inhibit cholangiocyte growth and secretion of BDL rats through activation of PKC alpha. Hepatology. 2002;35:1041–1052. doi: 10.1053/jhep.2002.32712. [DOI] [PubMed] [Google Scholar]
- Alpini G, Kanno N, Phinizy JL, Glaser S, Francis H, Taffetani S, LeSage G. Tauroursodeoxycholate inhibits human cholangiocarcinoma growth via Ca2+-, PKC-, and MAPK-dependent pathways. Am J Physiol. 2004;286:G973–G982. doi: 10.1152/ajpgi.00270.2003. [DOI] [PubMed] [Google Scholar]
- Qiao L, Yacoub A, Studer E, Gupta S, Pei XY, Grant S, Hylemon PB, Dent P. Inhibition of the MAPK and PI3K pathways enhances UDCA-induced apoptosis in primary rodent hepatocytes. Hepatology. 2002;35:779–789. doi: 10.1053/jhep.2002.32533. [DOI] [PubMed] [Google Scholar]
- Schoemaker MH, Conde de la Rosa L, Buist-Homan M, Vrenken TE, Havinga R, Poelstra K, Haisma HJ, Jansen PL, Moshage H. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology. 2004;39:1563–1573. doi: 10.1002/hep.20246. [DOI] [PubMed] [Google Scholar]
- Powley TL, Prechtl JC, Fox EA, Berthoud HR. Anatomical considerations for surgery of the rat abdominal vagus: distribution, paraganglia and regeneration. J Auton Nerv Syst. 1983;9:79–97. doi: 10.1016/0165-1838(83)90133-9. [DOI] [PubMed] [Google Scholar]
- Jang YJ, Ryu HJ, Choi YO, Kim C, Leem CH, Park CS. Improvement of insulin sensitivity by chelation of intracellular Ca(2+) in high-fat-fed rats. Metabolism. 2002;51:912–918. doi: 10.1053/meta.2002.33351. [DOI] [PubMed] [Google Scholar]
- Biswas DK, Dai SC, Cruz A, Weiser B, Graner E, Pardee AB. The nuclear factor kappa B (NF-kappa B): a potential therapeutic target for estrogen receptor negative breast cancers. Proc Natl Acad Sci USA. 2001;98:10386–10391. doi: 10.1073/pnas.151257998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner JW, Carruthers JS, Kalifat SR. The ductular cell reaction of rat liver in extrahepatic cholestasis. I. Proliferated biliary epithelial cells. Exp Mol Pathol. 1962;1:162–185. doi: 10.1016/0014-4800(62)90019-9. [DOI] [PubMed] [Google Scholar]
- Ishii M, Vroman B, LaRusso NF. Isolation and morphological characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989;97:1236–1247. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- Rutenburg AM, Kim H, Fishbein JW, Hanker JS, Wasserkrug HL, Seligman AM. Histochemical and ultrastructural demonstration of g-glutamyl transpeptidase activity. J Histochem Cytochem. 1969;17:517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- Alpini G, Ueno Y, Tadlock L, Glaser SS, LeSage G, Francis H, Taffetani S, Marzioni M, Alvaro D, Patel T. Increased susceptibility of cholangiocytes to tumor necrosis factor-alpha cytotoxicity after bile duct ligation. Am J Physiol. 2003;285:C183–C194. doi: 10.1152/ajpcell.00497.2002. [DOI] [PubMed] [Google Scholar]
- LeSage G, Glaser S, Ueno Y, Alvaro D, Baiocchi L, Kanno N, Phinizy JL, Francis H, Alpini G. Regression of cholangiocyte proliferation after cessation of ANIT feeding is associated with increased apoptosis. Am J Physiol. 2001;281:G182–G190. doi: 10.1152/ajpgi.2001.281.1.G182. [DOI] [PubMed] [Google Scholar]
- Kato A, Gores GJ, LaRusso NF. Secretin stimulates exocytosis in isolated bile duct epithelial cells by a cyclic AMP-mediated mechanism. J Biol Chem. 1992;267:15523–15529. [PubMed] [Google Scholar]
- Glaser S, Alvaro D, Roskams T, Phinizy JL, Stoica G, Francis H, Ueno Y, Barbaro B, Marzioni M, Mauldin J, Rashid S, Mancino MG, LeSage G, Alpini G. Dopaminergic inhibition of secretin-stimulated choleresis by increased PKC-gamma expression and decrease of PKA activity. Am J Physiol. 2003;284:G683–G694. doi: 10.1152/ajpgi.00302.2002. [DOI] [PubMed] [Google Scholar]
- Kao JP. Practical aspects of measuring [Ca2+] with fluorescent indicators. Methods Cell Biol. 1994;40:155–181. doi: 10.1016/s0091-679x(08)61114-0. [DOI] [PubMed] [Google Scholar]
- LeSage GD, Marucci L, Alvaro D, Glaser SS, Benedetti A, Marzioni M, Patel T, Francis H, Phinizy JL, Alpini G. Insulin inhibits secretin-induced ductal secretion by activation of PKC alpha and inhibition of PKA activity. Hepatology. 2002;36:641–651. doi: 10.1053/jhep.2002.35537. [DOI] [PubMed] [Google Scholar]
- Lazaridis KN, Strazzabosco M, LaRusso NF. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Sherlock S. Overview of chronic cholestatic conditions in adults: terminology and definitions. Clin Liver Dis. 1998;2:217–233. doi: 10.1016/s1089-3261(05)70004-0. [DOI] [PubMed] [Google Scholar]
- Beuers U, Bilzer M, Chittattu A, Kullak-Ublick GA, Keppler D, Paumgartner G, Dombrowski F. Tauroursodeoxycholic acid inserts the apical conjugate export pump, Mrp2, into canalicular membranes and stimulates organic anion secretion by protein kinase C-dependent mechanisms in cholestatic rat liver. Hepatology. 2001;33:1206–1216. doi: 10.1053/jhep.2001.24034. [DOI] [PubMed] [Google Scholar]
- Beuers U, Nathanson MH, Isales CM, Boyer JL. Tauroursodeoxycholic acid stimulates hepatocellular exocytosis and mobilizes extracellular Ca2+ mechanisms defective in cholestasis. J Clin Invest. 1993;92:2984–2993. doi: 10.1172/JCI116921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuers U, Throckmorton DC, Anderson MS, Isales CM, Thasler W, Kullak-Ublick GA, Sauter G, Koebe HG, Paumgartner G, Boyer JL. Tauroursodeoxycholic acid activates protein kinase C in isolated rat hepatocytes. Gastroenterology. 1996;110:1553–1563. doi: 10.1053/gast.1996.v110.pm8613063. [DOI] [PubMed] [Google Scholar]
- Shibao K, Hirata K, Robert ME, Nathanson MH. Loss of inositol 1,4,5-trisphosphate receptors from bile duct epithelia is a common event in cholestasis. Gastroenterology. 2003;125:1175–1187. doi: 10.1016/s0016-5085(03)01201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellentani S. Immunomodulating and anti-apoptotic action of ursodeoxycholic acid: where are we and where should we go? Eur J Gastroenterol Hepatol. 2005;17:137–140. doi: 10.1097/00042737-200502000-00001. [DOI] [PubMed] [Google Scholar]
- Miura T, Ouchida R, Yoshikawa N, Okamoto K, Makino Y, Nakamura T, Morimoto C, Makino I, Tanaka H. Functional modulation of the glucocorticoid receptor and suppression of NF-kappaB-dependent transcription by ursodeoxycholic acid. J Biol Chem. 2001;276:47371–47378. doi: 10.1074/jbc.M107098200. [DOI] [PubMed] [Google Scholar]
- Nathanson MH, Burgstahler AD, Masyuk A, LaRusso NF. Stimulation of ATP secretion in the liver by therapeutic bile acids. Biochem J. 2001;358:1–5. doi: 10.1042/0264-6021:3580001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Latif AA. Calcium-mobilizing receptors, polyphosphoinositides, generation of second messengers and contraction in the mammalian iris smooth muscle: historical perspectives and current status. Life Sci. 1989;45:757–786. doi: 10.1016/0024-3205(89)90170-7. [DOI] [PubMed] [Google Scholar]
- Alvaro D, Onori P, Metalli VD, Svegliati-Baroni G, Folli F, Franchitto A, Alpini G, Mancino MG, Attili AF, Gaudio E. Intracellular pathways mediating estrogen-induced cholangiocyte proliferation in the rat. Hepatology. 2002;36:297–304. doi: 10.1053/jhep.2002.34741. [DOI] [PubMed] [Google Scholar]
- Botla R, Spivey JR, Aguilar H, Bronk SF, Gores GJ. Ursodeoxycholate (UDCA) inhibits the mitochondrial membrane permeability transition induced by glycochenodeoxycholate: a mechanism of UDCA cytoprotection. J Pharmacol Exp Ther. 1995;272:930–938. [PubMed] [Google Scholar]
- Rodrigues CM, Fan G, Ma X, Kren BT, Steer CJ. A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest. 1998;101:2790–2799. doi: 10.1172/JCI1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues CM, Ma X, Linehan-Stieers C, Fan G, Kren BT, Steer CJ. Ursodeoxycholic acid prevents cytochrome c release in apoptosis by inhibiting mitochondrial membrane depolarization and channel formation. Cell Death Differ. 1999;6:842–854. doi: 10.1038/sj.cdd.4400560. [DOI] [PubMed] [Google Scholar]
- Rodrigues CM, Sola S, Sharpe JC, Moura JJ, Steer CJ. Tauroursodeoxycholic acid prevents Bax-induced membrane perturbation and cytochrome c release in isolated mitochondria. Biochemistry. 2003;42:3070–3080. doi: 10.1021/bi026979d. [DOI] [PubMed] [Google Scholar]
- Kubitz R, Saha N, Kuhlkamp T, Dutta S, vom Dahl S, Wettstein M, Haussinger D. Ca2+-dependent protein kinase C isoforms induce cholestasis in rat liver. J Biol Chem. 2004;279:10323–10330. doi: 10.1074/jbc.M306242200. [DOI] [PubMed] [Google Scholar]