Abstract
Vascular remodeling in chronic hypoxic pulmonary hypertension includes marked fibroproliferative changes in the pulmonary artery (PA) adventitia. Although resident PA fibroblasts have long been considered the primary contributors to these processes, we tested the hypothesis that hypoxia-induced pulmonary vascular remodeling requires recruitment of circulating mesenchymal precursors of a monocyte/macrophage lineage, termed fibrocytes. Using two neonatal animal models (rats and calves) of chronic hypoxic pulmonary hypertension, we demonstrated a dramatic perivascular accumulation of mononuclear cells of a monocyte/macrophage lineage (expressing CD45, CD11b, CD14, CD68, ED1, ED2). Many of these cells produced type I collagen, expressed α-smooth muscle actin, and proliferated, thus exhibiting mesenchymal cell characteristics attributed to fibrocytes. The blood-borne origin of these cells was confirmed in experiments wherein circulating monocytes/macrophages of chronically hypoxic rats were in vivo-labeled with DiI fluorochrome via liposome delivery and subsequently identified in the remodeled pulmonary, but not systemic, arterial adventitia. The DiI-labeled cells that appeared in the vessel wall expressed monocyte/macrophage markers and procollagen. Selective depletion of this monocytic cell population, using either clodronate-liposomes or gadolinium chloride, prevented pulmonary adventitial remodeling (ie, production of collagen, fibronectin, and tenascin-C and accumulation of myofibroblasts). We conclude that circulating mesenchymal precursors of a monocyte/macrophage lineage, including fibrocytes, are essential contributors to hypoxia-induced pulmonary vascular remodeling.
Significant fibroproliferative changes in the pulmonary artery (PA) adventitia are prominent characteristics of chronic pulmonary hypertension of many causes, including hypoxia. These processes occur in both large and small PAs as a result of adventitial cell proliferation, production/deposition of extracellular matrix proteins, especially collagens, and accumulation of myofibroblasts [α-smooth muscle actin (α-SMA)-expressing fibroblasts].1–5 These fibroproliferative changes have long been assumed to be due to activation, proliferation, and differentiation of resident adventitial fibroblasts. However, the possibility that nonresident cells may contribute directly to the hypoxia-induced pulmonary remodeling process emerges from recent reports in various disease models (including those of the lung) that demonstrate recruitment of circulating, bone marrow-derived cells capable of assuming a mesenchymal/fibroblast phenotype at the site of tissue injury.6–9 Among different types of circulating mesenchymal progenitors, mononuclear cells (MNCs) of a monocyte/macrophage lineage, and specifically a subpopulation of MNCs termed “fibrocytes,” have recently received significant attention with regard to their potential role in various fibroproliferative diseases.10–13 Fibrocytes comprise a subpopulation of circulating MNCs of a monocyte/macrophage lineage (CD11b+, CD13+, CD14+) that can exhibit fibroblast properties at the site of tissue injury (collagen production, differentiation into myofibroblasts).10–15 Fibrocytes have been shown to be an important source of tissue fibroblasts in asthma and lung fibrosis.12,13,16 However, to our knowledge, there are no studies demonstrating the contribution of circulating fibrocytes or other types of mesenchymal precursors of a mononuclear origin to pulmonary vascular remodeling in the setting of hypoxic pulmonary hypertension. We thus tested the hypothesis that chronic hypoxic exposure induces recruitment of circulating fibrocytes to the pulmonary circulation, and that these cells contribute directly and substantially to pulmonary vascular remodeling.
Two neonatal animal models (rat and calf) of chronic hypoxia-induced pulmonary hypertension were used to test this hypothesis in an effort to determine how generalized and consistent the response was across species. In these models we sought to 1) identify the contribution of MNCs and fibrocytes to PA adventitial thickening using immunofluorescence analysis with a panel of cell type-specific antibodies; 2) delineate the direct contribution of these cells to fibroproliferative processes (collagen production, proliferation, accumulation of myofibroblasts) in hypoxic pulmonary vascular remodeling using double-label immunofluorescent staining for leukocyte antigens, markers of fibrosis (procollagen), cell replication [5-bromo-2′-deoxyuridine (BrdU)], and myofibroblasts (α-SMA); 3) assess the blood-borne origin of these cells in the remodeled pulmonary adventitia of chronically hypoxic animals by in vivo labeling of these cells in the circulation and subsequent determination of their localization in the lung and systemic vasculature; and 4) evaluate the role of these cells in hypoxic pulmonary vascular remodeling process by selectively depleting them and subsequently assessing the impact on pulmonary adventitial fibrosis and myofibroblast accumulation.
Materials and Methods
Animal Models
Two animal species were used as models of hypoxia-induced neonatal pulmonary hypertension: weanling rats and, to confirm the findings in a large mammalian species, calves. The advantages of using the hypoxic calf model have been described before1 and include remarkable thickening of PA adventitia, which resembles the pathological picture in human neonatal pulmonary hypertension.2–4
Rats were used in this study as an animal model of hypoxic pulmonary hypertension because these rodents, in contrast to mice, develop marked PA adventitial thickening17 and because certain experimental manipulations can be performed in rats but are not economically feasible in calves. The Wistar-Kyoto rat strain was chosen because it develops more severe hypoxic pulmonary hypertension than the Sprague-Dawley strain.18
Weanling (4 weeks old) male Wistar-Kyoto rats were purchased from Charles River Laboratories (Wilmington, MA). Experimental hypoxic groups of rats (n = 4 to 6, each time point) were exposed to hypobaric hypoxia (PB = 380 mmHg) for 24 hours to 4 weeks. Age-matched controls were kept at ambient altitude. One-day-old male Holstein calves were purchased from Laluna dairy farm (Fort Collins, CO). The experimental hypoxic group (n = 7) was exposed for 2 weeks to hypobaric hypoxia (PB = 445 mmHg), while age-matched controls (n = 6) were kept at ambient altitude (Denver, CO; PB = 640 mmHg).1
Animals of both species were euthanized by overdose of sodium pentobarbital (160 mg/kg body weight). Standard veterinary care was used following institutional guidelines: for rats, at the University of Colorado Health Sciences Center for Laboratory Animal Care (Denver, CO) in compliance with Institutional Animal Care and Use Committee-approved protocols; for calves, at the Department of Physiology, School of Veterinary Medicine, Colorado State University (Fort Collins, CO).
Tissue Samples and Antibodies
Freshly obtained tissue samples were embedded in O.C.T. (Sakura Finetek, Torrance, CA) and frozen at −70°C until use. The following antibodies against leukocyte antigens were used in the study: bovine-specific monoclonal antibodies (mAbs) against CD45, CD11b, CD14 (15 μg/ml; VMRD Inc., Pullman, WA), CD68 (EBM11, 1:100; DakoCytomation Corp., Carpinteria, CA); rat-specific mAbs against CD45, CD11b (OX-1, OX-42, 1:100; Chemicon Int., Temecula, CA), mAbs against ED1, ED2 (1:10, 1:50; BD Pharmingen, San Diego, CA), and anti-granulocyte mAbs (RK-4, 1:50; Cedarline Laboratories, Ontario, Canada). The following antibodies against markers of fibrosis were used: mAbs against type I procollagen (SP1.D8, 1:10; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), mAbs against collagen-prolyl-4-hydroxylase-α (6–6H2, 1:50; Medicorp, Montreal, Canada), mAbs against ED-A-FN (ist-9, 1:10; Oxford Biotechnology, Oxfordshire, UK), and rabbit polyclonal anti-tenascin-C Abs (1:100, Chemicon Int.). To define the expression of a smooth muscle marker α-SMA, mAb 1A4 was used (1:100; Sigma Chemical Co., St. Louis, MO). Proliferating cells were identified using fluorescein isothiocyanate-conjugated anti-BrdU mAbs (1:3, BD Pharmingen).
Immunofluorescent Labeling
Tissue cryosections (5 μm) were fixed in methanol:acetone (1:1). Nonspecific binding was blocked with fetal bovine serum:phosphate-buffered saline (PBS) (1:1), and sections were incubated overnight with primary Abs at 4°C. Secondary biotinylated Abs (Vector Laboratories, Burlingame, CA), streptavidin-Alexa-594 (red) and streptavidin-Alexa-488 (green) (Molecular Probes, Eugene, OR) were used at dilutions recommended by the manufacturers. Immunolabeled sections were mounted in VectaShield/DAPI (Vector Laboratories) and examined under a Zeiss fluorescent microscope with an AxioVision digital imaging system (Carl Zeiss MicroImaging, Inc., Thornwood, NY). For double-label confocal microscopy, labeling with antibodies against CD45, CD11b, CD14, CD68, and ED1 was accomplished first. Next, anti-procollagen or α-SMA mAbs, directly labeled with Zenon-Alexa-fluor kit (Molecular Probes), were applied for 1 to 2 hours. Images were analyzed on DeltaVision digital deconvolution confocal microscope (Olympus America Inc., Melville, NY). Photographs were taken with a Photometrics Quantix cooled digital charge-coupled device camera (Olympus). Quantitative analysis of the percentage of monocytic cells co-expressing procollagen, BrdU, and α-SMA was performed on 12 to 18 stained lung sections that were obtained from four to six animals (rats and calves, respectively).
Time Course Analysis
Rats were exposed to hypoxia for 24 hours, 48 hours, 72 hours, 96 hours, and 1, 2, 3, and 4 weeks. Lung cryosections were immunolabeled for a monocyte/macrophage marker CD11b, and the perimeter of the free PA wall (contiguous to alveoli) was examined within the radial distance of ≥150 μm outward from the external elastic lamina. CD11b+ cell number was assessed per unit volume (10-μm radial distance by 500-μm perimeter length).
BrdU Injections
Rats (72-hour hypoxic or normoxic) were injected with BrdU (100 μg/kg body weight) at 24, 18, and 2 hours before euthanization.
DiI-Liposome, Clodronate-Liposome, and Gadolinium Chloride Injections
Liposomes were prepared as previously described.19 For liposome-mediated in vivo DiI-labeling of circulating monocytes/macrophages, liposomes containing red fluorochrome, DiI, were introduced into the circulation via a series of intravenous injections (see below). These liposomes are readily taken up via phagocytosis by circulating phagocytic MNCs of a monocyte/macrophage lineage (but not by other subsets of MNCs), and DiI-fluorochrome is incorporated into the cell membrane, resulting in selective labeling of circulating monocytes/macrophages.19,20 DiI liposomes were intravenously injected (0.1 ml/10 g body weight) into anesthetized rats (n = 5) every third day during the 3-week hypoxic (or normoxic) exposure, as well as on 3 consecutive days before the end of the experiment before euthanasia. Tissues were frozen in O.C.T., and unfixed cryosections were examined for the red DiI fluorescence. After capturing the image demonstrating DiI fluorescence, the cryosection was fixed in acetone:methanol, which completely bleached DiI fluorescence, and then labeled with antibodies against CD11b, and/or ED1, and/or ED2.
To deplete circulating MNCs of a monocyte/macrophage lineage, we used two separate approaches: serial intravenous injections of liposomes containing clodronate (Cl2MBP; gift of Roche Diagnostics GmbH, Mannheim, Germany) or intravenous injections of gadolinium chloride (GdCl3, Sigma Chemical Co.). These methods are commonly used to target and deplete phagocytic cells. Clodronate has an extremely short half-life in the circulation and therefore is not toxic.19 However, when delivered into phagocytic cells using liposomes as vehicles, clodronate accumulates in the cell and induces apoptosis after exceeding a threshold concentration.19 Importantly, Cl2MBP-liposomes do not cross vascular endothelial barriers and thus would not be taken up by resident tissue phagocytic cells. The selective action of GdCl3 on monocytes/macrophages is based on the fact that this compound is readily dissolved in normal saline; however, when injected into the bloodstream, it rapidly aggregates into relatively large colloidal particles at neutral pH. Similar to liposomes, the particles of GdCl3 are taken up exclusively by circulating phagocytic MNCs but not by other cells. Once inside the phagocytic cell and after exceeding the threshold concentration, GdCl3 causes cell apoptosis.21
Experimental hypoxic and normoxic rats (n = 8, each group) were injected intravenously with Cl2MBP-liposomes bi-weekly for 4 weeks (first injection was at 0.1 ml/10 g body weight, subsequent injections were at 0.05 ml/10 g body weight). Control hypoxic and normoxic rats were intravenously injected with PBS liposomes (n = 6) on the same schedule. GdCl3 (2 mg/ml, 10 mg/kg body weight) was administered as intravenous injections bi-weekly for 4 weeks of hypoxic or normoxic exposure (n = 6, each), and control rats were injected with PBS (n = 8).
Data Analysis
Data are presented as mean ± SEM. Statistical analysis used Student’s t-test and one-way analysis of variance; significance accepted at P < 0.05.
Results
Hypoxia Induces Robust Accumulation of MNCs of a Monocyte/Macrophage Lineage in the PA Adventitia
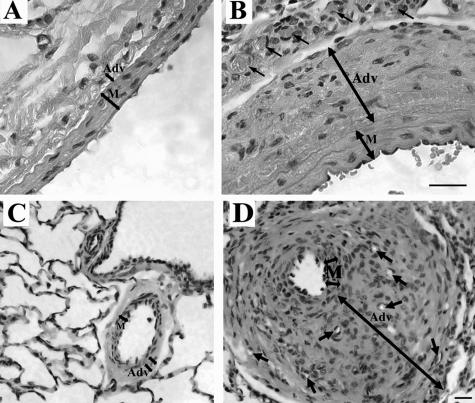
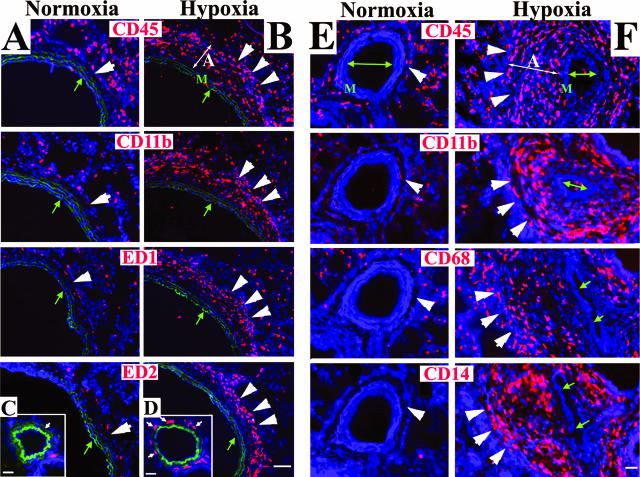
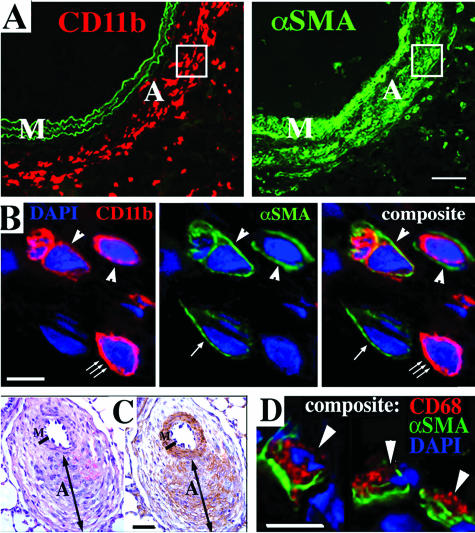
In normoxic animals of both species (rats and calves), the PA adventitia was thin [Figure 1, A (rat) and C (calf)], whereas in animals exposed to chronic hypobaric hypoxia (rats for 4 weeks and calves for 2 weeks), the PA adventitia was remarkably thickened [Figure 1, B (rat) and D (calf)]. We first examined whether MNCs of a monocyte/macrophage lineage were recruited to the PA in response to chronic hypoxic exposure using a panel of antibodies to monocyte/macrophage antigens (in rats, CD45, CD11b, ED1, ED2; in calves, CD45, CD11b, CD14, CD68). In normoxic animals (both rats and calves), the adventitia contained very few cells expressing these antigens [Figure 2, normoxia columns, A and C (rat) and E (calf)]. However, in chronically hypoxic animals of both species, numerous cells expressing monocyte/macrophage antigens were observed in the thickened adventitia of both large and small PAs [Figure 2, hypoxia columns, B and D (rat) and F (calf)]. Accumulation of these cells was specific to the lung vasculature, because the aorta, carotid, and femoral arteries of hypoxic animals were devoid of such cells (data not shown). Moreover, neutrophils were not identified in the PA adventitia of either hypoxic calves (at 2 weeks of hypoxic exposure) or hypoxic rats (starting from 24 hours and up to 4 weeks of hypoxic exposure) (data not shown).
Figure 1.
Chronic hypoxia induces PA adventitial thickening. H&E staining of pulmonary arteries from rats (A, B) and calves (C, D). The adventitial layer (Adv) is very thin in normoxic rats (A) and calves (C). In chronically hypoxic animals of both species, the PA adventitia is remarkably thickened (B, rats; D, calves). Small arrows point to newly formed capillaries of the vasa vasorum. M, tunica media. Scale bars, 20 μm.
Figure 2.
Hypoxia induces a robust appearance of MNCs in the PA adventitia. Cryosections of PAs from rats (A–D) and calves (E, F) were labeled with antibodies against MNC/macrophage antigens (red) and cell nuclei (DAPI, blue). Few MNCs are present in the adventitia of normoxic rats and calves (single large arrowheads in the normoxia columns). In the adventitia of chronically hypoxic rats and calves, abundant MNCs of a monocyte/macrophage phenotype are present (triple large arrowheads in the hypoxia columns). For rats, micrographs A and B show main PAs, whereas C and D show distal PAs (80 to 100 μm in diameter). Green autofluorescence of the elastic lamellae in A--D defines the boundaries of the PA tunica media. The PA lumens are marked with small green arrows. M, media; A, adventitia. Scale bars: 100 μm (A, B); 20 μm (C–F).
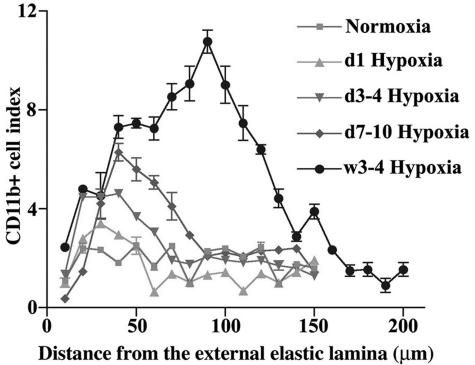
A kinetic analysis demonstrated a progressive accumulation of CD11b+ monocytes/macrophages in the pulmonary perivascular space of hypoxic (24 hours to 4 weeks) rats with a peak accumulation index of 11 at 4 weeks of hypoxic exposure compared to 2.5 in normoxic rats (Figure 3). Furthermore, a progressive increase in the distance from the external elastic lamellae (x axis) of the CD11b+ cell peak was observed, which correlated with the progressive increase in the thickening of PA adventitia in response to chronic exposure to hypoxia.
Figure 3.
Chronic hypoxia causes a progressive accumulation of MNCs in the PA adventitia. Time course analysis of accumulation of MNCs expressing CD11b antigen (CD11b+ cell index) in the PA adventitia of chronically hypoxic (1 day to 4 weeks) rats demonstrates a significant and progressive increase in the CD11b+ cell index throughout time, compared to normoxic controls. Furthermore, a progressive increase in the distance from the external elastic lamellae (x axis) of the CD11b+ cell peak was observed that correlated with the progressive increase in the thickening of PA adventitia in response to chronic exposure to hypoxia.
MNCs, Accumulating in the Remodeled PA Adventitia, Exhibit a Fibrocyte Phenotype
Extensive collagen deposition, accumulation of myofibroblasts, and cell proliferation in the PA adventitia are prominent features of hypoxia-induced pulmonary vascular remodeling.1–5,22 We thus evaluated whether any of the MNCs of a monocyte/macrophage lineage accumulating in the pulmonary perivascular space in response to chronic hypoxia exhibited a phenotype characteristic of a fibrocyte (ie, produced collagen, expressed α-SMA, proliferated).10,11
Collagen Production
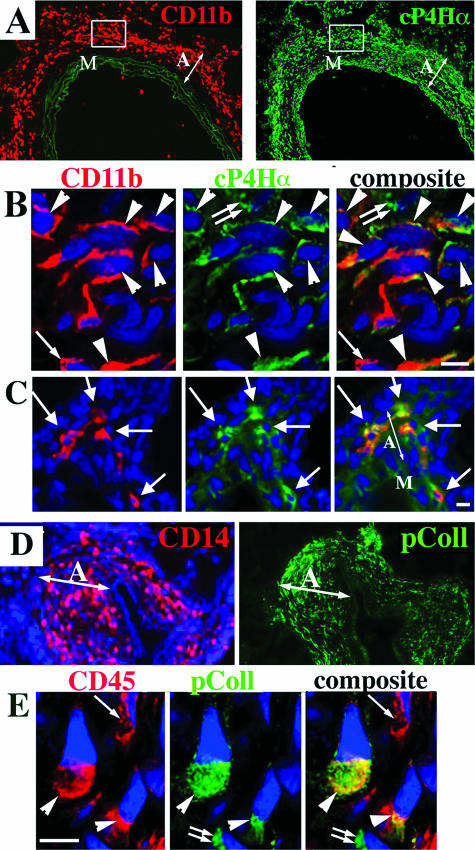
To identify fibrocytes in the PA adventitia, double-label immunostaining was performed for MNC-macrophage antigens (in rat, CD45, CD11b, ED1, ED2; in calf, CD45, CD11b, CD14, CD68) and intracellular collagen precursor molecules [in rat, collagen-prolyl-4-hydroxylase-α (cP4Hα); in calf, α-I procollagen (pColl)]. Routine and confocal fluorescence microscopy demonstrated that a substantial proportion of MNCs, accumulating in the remodeled PA adventitia of hypoxic animals, was comprised of collagen-expressing fibrocytes (64.3 + 4.3% in rats, and 38.1 + 3.6% in calves) [Figure 4, A–C (rat) and D–E (calf)]. No fibrocytes were identified in the PA adventitia of normoxic calves and/or rats (not shown)
Figure 4.
Monocytic cells in the remodeled PA adventitia express procollagen. A: Serial tissue sections of the rat PA labeled with antibodies against a monocytic cell marker CD11b (red, left) and collagen precursor molecule, collagen-prolyl-hydroxylase-α (cP4Hα) (green, right). Elastic lamellae of the tunica media (left) exhibit green autofluorescence, which is shown to better define the tunica media boundaries. B: Micrograph of the adventitial area shown within the frame in A. Double-label immunofluorescent staining followed by deconvolution confocal microscopy demonstrates that the remodeled PA adventitia of chronically hypoxic rats contains numerous cells that co-express (arrowheads) CD11b (red fluorescence) and cP4Hα (green fluorescence). There is a cell (small single arrow) that expresses a monocytic antigen CD11b (red) but not cP4Hα, and another cell (small double arrow) expresses cP4Hα but not CD11b. DAPI (blue) labels all cell nuclei. C: Routine immunofluorescence microscopy of the small distal PA of chronically hypoxic rat shows adventitial cells that also co-express CD11b (red) and cP4Hα (green) (arrows). D: Serial sections of the distal PA of chronically (2 week) hypoxic calf was labeled with CD14 Abs (red) and type I procollagen (pColl) Abs (green). E: Double-label immunofluorescent staining followed by deconvolution confocal microscopy demonstrates adventitial cells from the 2-week hypoxic calf that co-express (arrowheads) CD45 (red) and pColl (green). There is also a cell that expresses CD45 but not pColl (small single arrow), and a cell that expresses pColl but not CD45 (small double arrows). M, media; A, adventitia. Scale bars, 5 μm.
α-SMA Expression
We determined if any MNCs co-expressed α-SMA and thus contributed directly to the accumulation of myofibroblasts (α-SMA-expressing fibroblasts) in the PA adventitia of chronically hypoxic animals. Analysis of double-label immunostaining demonstrated that, in hypoxic animals of both animal species, numerous adventitial cells co-expressed macrophage antigens (CD11b in rats and CD68 in calves) and α-SMA [Figure 5, A and B (rat) and C and D (calf)]. Deconvolution confocal microscopy showed that, among all MNCs within the remodeled adventitia, 19.2 ± 4.3% MNCs (in rats) and 7.8 ± 3.2% MNCs (in calves) co-expressed α-SMA. No α-SMA-expressing MNCs were identified in the PA adventitia of normoxic calves and rats (not shown).
Figure 5.
Monocytic cells in the remodeled PA adventitia express α-SMA. A: Serial tissue sections of the rat PA labeled with antibodies against a monocytic cell marker CD11b (red, left) and α-SMA (αSMA, green, right). Elastic lamellae of the tunica media (left) exhibit green autofluorescence, which is shown to better define the tunica media boundaries. B: Micrograph of the adventitial area shown within the frame in A. Double-label immunofluorescent staining and deconvolution confocal microscopy demonstrate adventitial cells that co-express (arrowheads) CD11b (red) and α-SMA (αSMA, green). There is a cell (small triple arrow) that expresses a monocytic antigen CD11b (red) but not α-SMA, and another cell (small single arrow) expresses α-SMA but not CD11b. DAPI (blue) labels cell nuclei. C: Distal PA of the chronically (2 week) hypoxic calf was labeled with H&E (left) or with peroxidase-conjugated antibodies against α-SMA (brown, right). PA adventitia is remarkably thickened (similar to image in Figure 1D), and α-SMA immunoreactivity (brown) is observed not only in the tunica media but also in some areas of the adventitia. D: Double-label immunofluorescent staining and deconvolution confocal microscopy demonstrate cells from the adventitia of a hypoxic calf that co-express (arrowheads) a macrophage marker CD68 (red) and α-SMA (green). DAPI (blue) labels cell nuclei. M, media; A, adventitia. Scale bars: 50 μm (A, C); 5 μm (B, D).
Proliferation
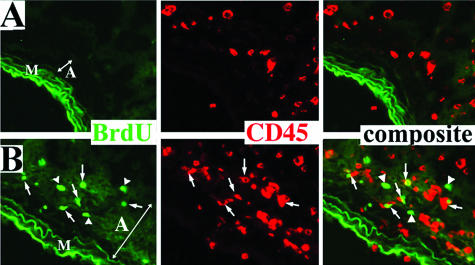
The PA adventitia of hypoxic rats demonstrated marked increases in nuclear BrdU incorporation (Figure 6B), whereas no proliferating adventitial cells were observed in normoxic rats (Figure 6A). Analysis of double-label immunofluorescent staining of lung tissues from chronically hypoxic rats demonstrated that a significant number (15.4 ± 2.3%) of MNCs expressing CD45 and/or ED2 had incorporated BrdU (Figure 6B, arrows; CD45 is shown). Cells other than MNCs, potentially resident fibroblasts, were also observed to have incorporated BrdU (Figure 6B, arrowheads). The relative contribution of cells expressing MNC markers to the overall BrdU labeling index in the PA adventitia at 72 hours of hypoxic exposure was 28.6 ± 4.1%. Thus, the MNCs, accumulating within the remodeled PA adventitia, contributed significantly to the overall increases in cell proliferation observed in the PA adventitia of hypoxic animals.
Figure 6.
Monocytic cells actively replicate in the remodeled PA adventitia. Double-label immunofluorescent staining of the remodeled PA from the chronically (72 hours) hypoxic rats (B) demonstrates numerous adventitial cells that co-express (arrows) MNC antigens (CD45, red) and proliferation-associated marker, BrdU (green). Several cells that have also incorporated BrdU, but do not express MNC antigen, CD45, are marked by arrowheads. Adventitial cells in the PA of age-matched normoxic rats (A) do not demonstrate BrdU incorporation. Elastic lamellae of the PA media exhibit green autofluorescence and are shown to define the medial-adventitial border. M, media; A, adventitia.
Fibrocytes, Accumulating in the PA Adventitia, Originate from the Circulation
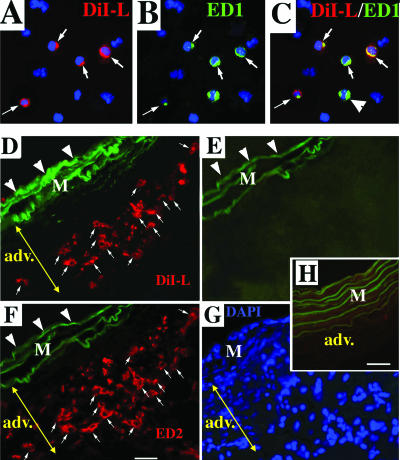
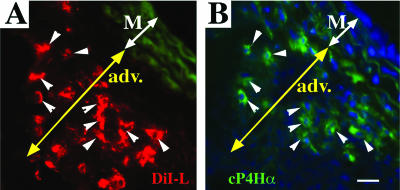
Based on expression of hematopoietic markers (including CD45), we hypothesized that fibrocytes, accumulating in the remodeled PA adventitia, were recruited from the circulation. We sought to determine the blood-borne origin of fibrocytes using a liposome-mediated in vivo labeling approach, wherein phagocytic MNCs could be selectively labeled via liposome delivery of DiI fluorochrome directly in the circulation of live rats (Materials and Methods).19,20 Figure 7, A–C, demonstrates that peripheral blood MNCs, exclusively of a monocytic origin (ED1+), take up liposomes loaded with red DiI fluorochrome. The DiI-labeled circulating cells were subsequently identified in the PA adventitia of chronically hypoxic (Figure 7, D–G) but not normoxic (Figure 7H) rats as follows. Lung and systemic vascular tissues from hypoxic and normoxic rats, which were intravenously injected with DiI liposomes, were examined for the presence of DiI-labeled cells within the vessel wall. In hypoxic animals, numerous DiI-labeled cells were identified in the PA adventitia (Figure 7D). No DiI-labeled cells were observed around aortas or carotid arteries of hypoxic rats (Figure 7H, aorta is shown) or around PA of normoxic rats (not shown). To determine the phenotype of DiI-labeled cells, immunofluorescent labeling of monocyte/macrophage antigens (CD11b, ED1, ED2) was performed on the same tissue section that was first bleached of fluorescence (Figure 7E) and then labeled with anti-MNC antibodies (Figure 7F, also see Materials and Methods). Most DiI-labeled cells expressed MNC antigens (Figure 7F, ED2 immunoreactivity is shown). Importantly, many of the DiI-labeled MNCs in the adventitia also expressed type I procollagen (Figure 8), a phenotype consistent with that of a fibrocyte.
Figure 7.
Fibrocytes, which have accumulated in the remodeled PA adventitia, originate from the circulation. A–C: Cytospins of MNCs isolated from peripheral blood of rats that were exposed to hypoxia for 96 hours and were intravenously injected with DiI liposomes 2 hours before drawing blood. A: MNCs that phagocytized DiI liposomes (DiI-L) exhibit red fluorescence. B: MNCs labeled with antibodies against a monocytic marker ED1 exhibit green fluorescence. C: Composite image demonstrates that DiI liposomes are taken up in the circulation by MNCs of exclusively monocytic lineage (ED1+) (small arrows). An arrowhead points to one of the ED1+ MNCs that was not DiI-labeled in the circulation. D: Monocytic cells labeled in the circulation with DiI (red fluorochrome) via liposome-mediated delivery (see Materials and Methods and A–C above) are subsequently observed in the remodeled PA adventitia of chronically (4 week) hypoxic rats (small arrows). E and F: The DiI+ cells (D) express monocyte/macrophage phenotype. E and F: After the images of DiI+ cells (seen in D) were acquired, DiI fluorescence was completely bleached by fixing this tissue cryosection in acetone/methanol (E), and next immunolabeling for a macrophage marker ED2 (red) was performed on the same cryosection (F). Small arrows in D and F point to numerous cells that show both DiI-labeling (D) and ED2 expression (F). Large arrowheads in D–F point to elastic lamellae of the tunica media (M), which exhibit green autofluorescence (shown to define the boundaries of the medial layer). Yellow double-headed arrows mark the PA adventitia (adv). G: Cell nuclei on the same tissue section are shown by DAPI immunoreactivity (blue). H: Aorta from the chronically hypoxic rat (same one as in D–G) that was intravenously injected with DiI liposomes, demonstrates the absence of DiI+ cells. Scale bars, 10 μm.
Figure 8.
Circulating MNCs that have accumulated in the remodeled PA adventitia express a fibrocyte phenotype. A and B: MNCs that were labeled with DiI (red fluorescence) in the circulation (see Materials and Methods and Figure 7, A–C), are subsequently found in the remodeled PA adventitia of chronically (4 week) hypoxic rat (A), and express cP4Hα (procollagen) (green fluorescence) (B). Arrowheads point to fibrocytes that demonstrate both DiI-labeling and expression of cP4Hα. M, media; A, adventitia. Scale bars, 20 μm.
Selective Depletion of Circulating Fibrocytes Attenuates Hypoxia-Induced PA Adventitial Remodeling
Having established that circulating fibrocytes appear in the PA adventitia of chronically hypoxic animals, we sought to evaluate their overall contribution to the remodeling process by selectively depleting these cells in circulation. Because we showed that a selective ED1+ subpopulation of circulating MNCs (monocytes) takes up DiI liposomes and is subsequently found within the hypoxic PA, we specifically targeted these cells for depletion using serial intravenous injections of liposomes containing clodronate (instead of DiI), a technique successfully used by us (NVR) and others.19,20,23–26
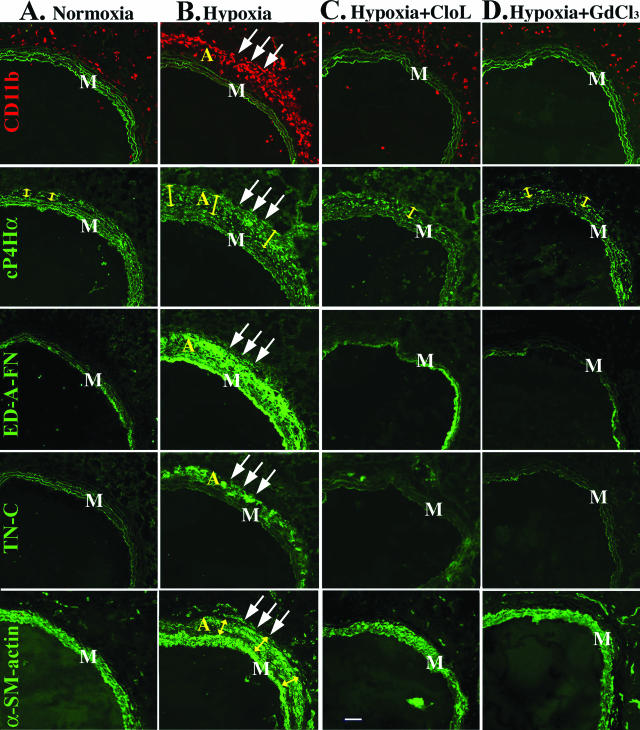
Chronically hypoxic rats injected with PBS-containing liposomes (control hypoxic group) showed abundant perivascular accumulation of MNCs, markedly thickened PA adventitia, and a high degree of adventitial fibrosis (as assessed by expression of procollagen, fibronectin, tenascin-C) and myofibroblast (α-SMA-positive fibroblast) accumulation, similar to untreated hypoxic animals (Figure 9B, hypoxia column). In chronically hypoxic rats intravenously injected with clodronate-liposomes (Cl2MBP-liposomes), influx and accumulation of MNCs in the PA adventitia was markedly diminished, as were adventitial thickening, development of fibrosis (expression of procollagen, ED-A-fibronectin, tenascin-C), and accumulation of myofibroblasts (Figure 9C, hypoxia+CloL column).
Figure 9.
Depletion of fibrocytes in the circulation of chronically hypoxic rats attenuates PA adventitial remodeling. A: Normoxia column: row CD11b: the PA adventitia of normoxic rats demonstrates few CD11b+ MNCs (red). Elastic lamellae of the tunica media exhibit green autofluorescence and are shown to define the boundaries of the tunica media. Row cP4Hα: The PA adventitia of normoxic rats is comprised of resident adventitial fibroblasts expressing cP4Hα (green fluorescence within the adventitia). Adventitia in normoxic rats is thin (yellow arrows). Rows ED-A-FN and TN-C: Resident fibroblasts in the PA adventitia of normoxic rats do not express cellular ED-A-fibronectin (ED-A-FN) or tenascin-C (TN-C). Row α-SM-actin: Resident fibroblasts in the PA adventitia of normoxic rats do not express α-SMA [SMC of the tunica media (M) express α-SMA]. B: Hypoxia column: rows CD11b and cP4Hα: the remodeled PA adventitia (triple arrows) of chronically (4 week) hypoxic rats demonstrates numerous CD11b+ cells (row CD11b, red), and markedly thickened adventitia (marked by yellow arrows in row cP4Hα) with numerous cP4Hα-expressing cells (green fluorescence within the adventitia). Rows ED-A-FN and TN-C: The remodeled PA adventitia of chronically hypoxic rats demonstrates deposition of ED-A-fibronectin and tenascin-C (rows ED-A-FN and TN-C, green fluorescence). Row α-SM-actin: Numerous α-SMA-expressing myofibroblasts are observed in the PA adventitia of chronically hypoxic rats (triple arrows). C and D: Hyp+Cl2MBP-L and Hyp+GdCl3 columns: selective depletion (see Materials and Methods and Results sections) of monocytic cells in the circulation of chronically hypoxic rats by either clodronate-liposomes (C, Hypoxia+Cl2MBP-L) or gadolinium chloride (D, Hypoxia+GdCl3) results in diminished accumulation of CD11+ cells in the PA adventitia (row CD11b, red), reduced adventitial thickness (row cP4Hα, yellow arrows), prevention of adventitial fibrosis [absence of fibronectin (row ED-A-FN) and tenascin-C (row TN-C) deposition], as well as prevention of α-SMA expression by adventitial fibroblasts (row α-SMA). M, tunica media. Scale bar, 100 μm.
To confirm this result we used a second approach to deplete circulating monocytic cells, intravenous injections of gadolinium chloride (GdCl3), a method previously used successfully in other animal models involving monocyte/macrophage accumulation.21,27 In GdCl3-treated rats, the accumulation of fibrocytes in the PA adventitia was markedly reduced, as were adventitial thickening, fibrosis (expression of procollagen, ED-A-fibronectin, tenascin-C), and myofibroblast accumulation (Figure 9D, hypoxia+GdCl3 column).
Discussion
In the present study, we tested the hypothesis that a circulating pool of mesenchymal precursors termed “fibrocytes” plays an essential role as precursors of mesenchymal cells in hypoxia-induced pulmonary vascular remodeling. Using two neonatal animal models (rat and bovine) of chronic hypoxia-induced pulmonary hypertension, we found that pulmonary adventitial remodeling, at least in the neonatal period, is due to the robust recruitment of nonresident, blood-borne MNCs of a monocyte/macrophage lineage that can produce collagen and express α-SMA, thus representing a subpopulation termed “fibrocytes.” The critical role of these cells in the remodeling process, as compared to that of resident adventitial fibroblasts, was demonstrated by experiments in which in vivo depletion of these cells in circulation of chronically hypoxic rats led to markedly attenuated adventitial thickening and substantial diminution of fibrosis and myofibroblast accumulation.
Most studies of fibrotic diseases have focused on the mechanisms and pathways through which resident fibroblasts are stimulated to undergo a phenotypic switch into a fibrotic phenotype. However the possibility that nonlocal cells, including circulating fibrocytes, give rise to tissue fibroblasts has recently been suggested in wound healing, asthma, and bleomycin-induced lung injury.10–12,16 Yet, there is a paucity of data regarding the contribution of circulating fibrocytes to the pulmonary perivascular fibrosis observed in various forms of pulmonary hypertension. The present study demonstrates that a large proportion of cells contributing to PA adventitial thickening and fibrosis is comprised of fibrocytes.
The appearance of myofibroblasts (α-SMA-expressing fibroblasts) is a characteristic feature of various fibroproliferative diseases including hypoxic pulmonary hypertension and has long been considered to be due to differentiation of resident fibroblasts.1–5,17,22 The present study suggests MNCs of a monocyte/macrophage lineage as yet another source of adventitial myofibroblasts in hypoxic pulmonary hypertension. Whether these myofibroblasts originated from fibrocytes or from a distinct subpopulation of MNCs requires further determination. Several studies in the setting of wound healing and asthma suggest that fibrocytes can differentiate into myofibroblasts.10,11,15,16 These studies base their conclusions on the demonstration of the co-expression of α-SMA and a certain MNC antigen; however, this experimental approach cannot establish whether the cells expressing MNC antigens are actually fibrocytes. Macrophages have been proposed as precursors of myofibroblasts in models of granulation tissue formation and neointimal formation after coronary arterial injury.28–30 At the same time, fibrocytes are known to express most of the monocyte/macrophage antigens, and thus, can be potential candidates for the cellular source of myofibroblasts in these injury models as well. Importantly, fibrocytes and other MNCs of a monocytic lineage have not been described to continue their differentiation into smooth muscle cells (SMCs). Therefore, they seem to comprise a distinct population of circulating mesenchymal progenitors that differs from the circulating SMC precursors described by Simper and colleagues.31
It should be noted that there were many MNCs in the PA perivascular areas of chronically hypoxic animals that lacked expression of the fibrocyte marker, type 1 collagen, and the myofibroblast marker α-SMA. It was not entirely surprising that hypoxia was capable of causing leukocytic invasion into the lung. Increased numbers of macrophages and neutrophils have been described in murine and rat lungs in response to both acute and chronic hypoxia.32–34 Interestingly, in our model of chronic hypoxia, at least beginning at 24 hours after hypoxic exposure, no influx of neutrophils into the perivascular areas was observed. Increased numbers of macrophages have also been documented under various pathological conditions associated with hypoxia, such as in ischemic areas of dermal wounds, in atherosclerotic plaques, malignant tumors, and in eyes with proliferative retinopathy.35–37 MNC/macrophage influx and accumulation has also been reported in primary pulmonary hypertension as well as in secondary forms of pulmonary hypertension, including high-flow congenital heart disease, scleroderma, and pulmonary hypertension-associated acute respiratory distress syndrome.38,39 However, in none of the aforementioned studies was the potential for the MNCs to give rise to mesenchymal-like cells described. The accumulation of monocytes, macrophages, and fibrocytes could have significant indirect effects on the structure and function of lung blood vessels. Activated fibrocytes act as inflammatory cells (macrophages) and release a variety of proinflammatory cytokines capable of causing vasoconstriction and increasing vascular permeability.32,40 In addition, both macrophages and fibrocytes are potent producers of promitogenic and proangiogenic factors.32,33,40,41 Thus they may play critical roles not only in inducing proliferation and phenotypic modulation of resident SMCs and fibroblasts but also in promoting neovascularization of the arterial vasa vasorum,37 a finding consistent with our previously published observation of a marked PA adventitial neovascularization in chronically hypoxic calves (Figure 1, small arrows pointing to new vasa vasorum capillaries).7 The expanded network of PA vasa vasorum may act as a conduit for continuous delivery of circulating cells to the PA adventitia, creating a feed-forward loop of pathological remodeling.
Because the vascular adventitia itself has recently been suggested as a source of mesenchymal progenitor cells,42 we sought to confirm a circulating, nonresident origin of fibrocytes accumulating in the remodeled PA adventitia by using a liposome-mediated in vivo DiI-labeling strategy. This approach is based on the numerous previous studies demonstrating that cells of a monocyte/macrophage lineage could be selectively labeled with red DiI-fluorochrome encapsulated into liposomes, which are taken up exclusively by phagocytic MNCs.19,20 Using this strategy we demonstrated that the DiI-labeled MNCs accumulating in the PA adventitia of chronically hypoxic rats originated from circulation (Figure 7, A–D, F), and comprised a subpopulation of fibrocytes (co-expressed MNC antigens and procollagen) (Figure 8). Interestingly, expression of CD45 (a common leukocyte antigen) by fibrocytes within the remodeled PA adventitia (Figure 2) not only points to the hematopoietic lineage of these cells but also to their potential origin from the bone marrow. Several reports demonstrate a bone marrow origin of circulating cells in injury-induced remodeling in various tissues.43–46 Data from the Hashimoto’s group8 demonstrate that a large proportion of interstitial lung fibroblasts in a bleomycin-induced mouse model of pulmonary fibrosis originates from the bone marrow. Most recently Hayashida and colleagues9 reported the bone marrow origin of adventitial myofibroblasts in a mouse model of hypoxic pulmonary hypertension.
The critical role of circulating fibrocytes in the pulmonary vascular remodeling process, as compared to that of resident adventitial fibroblasts, was demonstrated in the present study in experiments using a liposome-mediated in vivo depletion approach, ie, selectively targeting and depleting circulating phagocytic MNCs using Cl2MBP-liposomes. This depletion approach has been commonly used by other investigators in models of various diseases and experimental settings of vascular injury.19,20,24–27 In the present study marked attenuation of vascular remodeling was achieved with this liposome-mediated depletion strategy. In the absence of circulating MNCs and fibrocytes, yet with an intact resident adventitial fibroblast population, the development of pulmonary vascular remodeling (adventitial thickening, fibrosis, accumulation of myofibroblasts) was prevented. These findings were confirmed using a separate and distinct strategy, ie, intravenous injections of gadolinium chloride (GdCl3), the strategy that has been commonly used by other investigators to deplete circulating monocytes and resident macrophages in various organs, including the lung.21,28,48 Combined, the results of Cl2MBP-liposome and GdCl3 treatment provide experimental support for the novel concept that recruitment of circulating fibrocytes is critical to the hypoxia-induced vascular remodeling process.
In conclusion, our findings provide experimental evidence for an essential pathophysiological role of circulating fibrocytes in the development of hypoxia-induced pulmonary vascular remodeling. These observations may have important implications for understanding the pathogenesis of hypoxic forms of pulmonary hypertension in the developing lung circulation and for the future development of therapeutic approaches aimed at ameliorating vascular remodeling.
Acknowledgments
We thank Kenneth G. Morris for measuring PA pressure in rats; Drs. Russ Anthony and Jan Rhodes for their invaluable assistance in the neonatal bovine model system; Drs. Peter L. Jones and William E. Huffer for valuable discussion; and the Developmental Studies Hybridoma Bank for providing antibodies against type I procollagen (clone SP1.D8) generated by Dr. Heinz Furthmayr, developed under the auspices of the National Institute of Child Health and Human Development, maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA.
Footnotes
Address reprint requests to Maria G. Frid, Ph.D., Developmental Lung Biology Research Laboratory, Department of Pediatrics, University of Colorado Health Sciences Center, 4200 E. 9th Ave., Denver, CO 80262. E-mail: maria.frid@uchsc.edu.
Supported by the National Institutes of Health (grants SCOR HL-57144-8 and PPG HL-14985-32).
This study is dedicated to the memory of John T. Reeves, who passed away.
References
- Stenmark KR, Mecham R. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol. 1997;59:89–144. doi: 10.1146/annurev.physiol.59.1.89. [DOI] [PubMed] [Google Scholar]
- Jeffery T, Morrell NW. Molecular and cellular basis of pulmonary vascular remodeling in pulmonary hypertension. Prog Cardiovasc Dis. 2002;45:173–202. doi: 10.1053/pcad.2002.130041. [DOI] [PubMed] [Google Scholar]
- Rabinovitch M. Pulmonary vascular remodeling in hypoxic pulmonary hypertension. Yuan JX-J, editor. Dordrecht: Kluwer Academic Publishers,; Hypoxic Pulmonary VasoconstrictionCellular and Molecular Mechanisms. 2004:pp 403–418. [Google Scholar]
- Meyrick B. The pathology of pulmonary artery hypertension. Clin Chest Med. 2001;22:393–404. doi: 10.1016/s0272-5231(05)70279-3. [DOI] [PubMed] [Google Scholar]
- Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43(Suppl S):13S–24S. doi: 10.1016/j.jacc.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Pereira R, Halford K, O’Hara M, Leeper D, Sokolov B, Pollard M, Bagasra O, Prockop D. Cultured adherent cells from marrow can serve as long-lasting precursor cells for bone, cartilage, and lung in irradiated mice. Proc Natl Acad Sci USA. 1995;92:4857–4861. doi: 10.1073/pnas.92.11.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie NJ, Crossno JT, Frid MG, Hofmeister SE, Reeves JT, Hyde DM, Carpenter TC, Brunetti JA, McNiece IK, Stenmark KR. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: contribution of progenitor cells. Am J Physiol. 2004;286:L668–L678. doi: 10.1152/ajplung.00108.2003. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Jin H, Liu T, Chensue S, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Fujita J, Miyake Y, Kawada H, Ando K, Ogava S, Fukuda K. Bone marrow-derived cells contribute to pulmonary vascular remodeling in hypoxia-induced pulmonary hypertension. Chest. 2005;127:1793–1798. doi: 10.1378/chest.127.5.1793. [DOI] [PubMed] [Google Scholar]
- Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Metz CN. Fibrocytes: a unique cell population implicated in wound healing. Cell Mol Life Sci. 2003;60:1342–1350. doi: 10.1007/s00018-003-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B, Kolodsick J, Thannickal V, Cooke K, Moore T, Hogaboam C, Wilke C, Toews G. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling D, Buckley CD, Salmon M, Gomer RH. Inhibition of fibrocyte differentiation by serum amyloid P. J Immunol. 2003;171:5537–5546. doi: 10.4049/jimmunol.171.10.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Scott PG, Giuffre J, Shankowsky HA, Ghahary A, Tredget EE. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82:1183–1192. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;170:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- Sobin SS, Chen PC. Ultrastructural changes in the pulmonary arterioles in acute hypoxic pulmonary hypertension in the rat. High Alt Med Biol. 2000;1:311–322. doi: 10.1089/15270290050502381. [DOI] [PubMed] [Google Scholar]
- Bochnowicz S, Osborn RR, Luttmann MA, Louden C, Hart T, Hay DW, Underwood DC. Differences in time-related cardiopulmonary responses to hypoxia in three rat strains. Clin Exp Hypertens. 2000;22:471–492. doi: 10.1081/ceh-100100085. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanisms of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Danenberg HD, Fishbein I, Gao J, Mönkkönen J, Reich R, Gati I, Moerman E, Golomb G. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106:599–605. doi: 10.1161/01.cir.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- Singh B, Pearce JW, Gamage LN, Janardhan K, Caldwell S. Depletion of pulmonary intravascular macrophages inhibits acute lung inflammation. Am J Physiol. 2004;286:L363–L372. doi: 10.1152/ajplung.00003.2003. [DOI] [PubMed] [Google Scholar]
- Short M, Nemenoff RA, Zawada WM, Stenmark KR, Das M. Hypoxia induces differentiation of pulmonary artery adventitial fibroblasts into myofibroblasts. Am J Physiol. 2004;286:C416–C425. doi: 10.1152/ajpcell.00169.2003. [DOI] [PubMed] [Google Scholar]
- Hoch JR, Stark VK, van Rooijen N, Kim JL, Nutt MP, Warner TF. Macrophage depletion alters vein graft intimal hyperplasia. Surgery. 1999;126:428–437. [PubMed] [Google Scholar]
- Westerhuis R, Straaten S, van Dixhoorn M, van Rooijen N, Verhagen N, Dijkstra C, de Heer E, Daha M. Distinctive roles of neutrophils and monocytes in anti-Thy-1 nephritis. Am J Pathol. 2000;156:303–310. doi: 10.1016/S0002-9440(10)64731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderkotter C, Nikolic T, Dillon M, van Rooijen N, Stehling M, Drevets D, Leenen P. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- Galeazzi F, Haapala EM, Van Rooijen N, Vallance BA, Collins SM. Inflammation-induced impairment of enteric nerve function in nematode-infected mice is macrophage dependent. Am J Physiol. 2000;278:G259–G265. doi: 10.1152/ajpgi.2000.278.2.G259. [DOI] [PubMed] [Google Scholar]
- Jankov RP, Luo X, Belcastro R, Copland I, Frndova H, Lye SJ, Hoidal JR, Post M, Transwell AK. Gadolinium chloride inhibits pulmonary macrophage influx and prevents O(2)-induced pulmonary hypertension in the neonatal rat. Pediatr Res. 2001;50:172–183. doi: 10.1203/00006450-200108000-00003. [DOI] [PubMed] [Google Scholar]
- Bayes-Genis A, Campbell J, Carlson P, Holmes D, Schwartz R. Macrophages, myofibroblasts and neointimal hyperplasia after coronary artery injury and repair. Atherosclerosis. 2002;163:89–98. doi: 10.1016/s0021-9150(01)00771-7. [DOI] [PubMed] [Google Scholar]
- Jabs A, Moncada G, Nichols C, Waller E, Wilcox J. Peripheral blood mononuclear cells acquire myofibroblast characteristics in granulation tissue. J Vasc Res. 2005;42:174–180. doi: 10.1159/000084406. [DOI] [PubMed] [Google Scholar]
- Campbell JH, Efendy JL, Han CL, Campbell GR. Blood vessels from bone marrow. Ann NY Acad Sci. 2000;902:224–229. doi: 10.1111/j.1749-6632.2000.tb06317.x. [DOI] [PubMed] [Google Scholar]
- Simper D, Stalboerger P, Panetta C, Wang S, Caplice N. Smooth muscle progenitor cells in human blood. Circulation. 2002;106:1199–1204. doi: 10.1161/01.cir.0000031525.61826.a8. [DOI] [PubMed] [Google Scholar]
- Murdoch C, Giannoudis A, Lewis CE. Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood. 2004;104:2224–2234. doi: 10.1182/blood-2004-03-1109. [DOI] [PubMed] [Google Scholar]
- Madjdpour C, Jewell UR, Kneller S, Ziegler U, Schwendener R, Booy C, Klausli L, Pasch T, Schimmer RC, Beck-Schimmer B. Decreased alveolar oxygen induces lung inflammation. Am J Physiol. 2003;284:L360–L367. doi: 10.1152/ajplung.00158.2002. [DOI] [PubMed] [Google Scholar]
- Minamino T, Christou H, Hsieh C-M, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA. 2001;98:8798–8803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther M, Brown NJ, Bishop ET, Lewis CE. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol. 2002;70:478–490. [PubMed] [Google Scholar]
- Esser P, Heimann K, Wiedemann P. Macrophages in proliferative vitreoretinopathy and proliferative diabetic retinopathy: differentiation of subpopulations. Br J Ophthalmol. 1993;77:731–733. doi: 10.1136/bjo.77.11.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo K-M, Gillies S, Javaherian K, Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci USA. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto R, de Lourdes Higuchi M, Aiello V. Decreased numbers of T-lymphocytes and predominance of recently recruited macrophages in the walls of peripheral pulmonary arteries from 26 patients with pulmonary hypertension secondary to congenital cardiac shunts. Cardiovasc Pathol. 2004;13:268–275. doi: 10.1016/j.carpath.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Dorfmuller P, Perros F, Balabanian K, Humbert M. Inflammation in pulmonary arterial hypertension. Eur Respir J. 2003;22:358–363. doi: 10.1183/09031936.03.00038903. [DOI] [PubMed] [Google Scholar]
- Hartlapp I, Abe R, Saeed RW, Peng T, Voelter W, Bucala R, Metz CN. Fibrocytes induce angiogenic phenotype in cultured endothelial cells and promote angiogenesis in vivo. FASEB J. 2001;15:2215–2224. doi: 10.1096/fj.01-0049com. [DOI] [PubMed] [Google Scholar]
- Ishida S, Usui T, Yamashiro K, Kaji Y, Amano S, Ogura Y, Hida T, Oguchi Y, Ambati J, Miller JW, Gragoudas ES, Ng Y-S, D’Amore PA, Shima DT, Adamis AP. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med. 2003;198:483–489. doi: 10.1084/jem.20022027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med. 2003;9:702–712. doi: 10.1038/nm0603-702. [DOI] [PubMed] [Google Scholar]
- Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summe RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development. 2001;128:5181–5188. doi: 10.1242/dev.128.24.5181. [DOI] [PubMed] [Google Scholar]
- Wang G, Bunnell B, Painter R, Quiniones B, Tom S, Lanson N, Spees J, Bertucci D, Peister A, Weiss D, Valentine V, Prockop D, Kolls J. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci USA. 2005;102:186–191. doi: 10.1073/pnas.0406266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyaizu T, Okada Y, Shoji W, Matsumura Y, Shimada K, Sado T, Sato M, Kondo T. Reduction of recipient macrophages by gadolinium chloride prevents development of obliterative airway disease in a rat model of heterotopic tracheal transplantation. Transplantation. 2003;76:1214–1220. doi: 10.1097/01.TP.0000088672.48259.F1. [DOI] [PubMed] [Google Scholar]