Abstract
Activated B cells function in antibody production and antigen presentation, but whether they perform any pathophysiological functions at sites of inflammation is not fully understood. Here, we report that intravenous injection of an agonistic anti-CD40 monoclonal antibody (αCD40) causes a biphasic inflammatory liver disease in inbred mice. The late phase of disease was suppressed in B-cell-deficient mice and by the depletion of macrophages, but not T cells or natural killer cells. We also report that SCID mice were not susceptible to αCD40-induced liver disease unless they were reconstituted with normal B cells and that B cells as well as macrophages played key roles in αCD40-induced late phase of liver inflammation. Finally, liver disease and the recruitment of inflammatory cells into the liver were mediated by interferon-γ and tumor necrosis factor-α, but not by Fas. In conclusion, these results indicate that CD40 ligation can trigger a B-cell-mediated inflammatory response that can have pathogenic consequences for the liver.
B cells produce antibodies that prevent the spread of infections and perform additional effector functions including opsonization, and complement fixation.1 B cells also function as antigen-presenting cells and can secrete inflammatory cytokines and chemokines under various conditions.2 Recently, B-cell subsets (termed B effector 1 and 2 cells) that possess distinct cytokine production profiles have been identified.3 These activities suggest that B cells may be able to regulate the inflammatory response and attract other inflammatory cells to sites of infection. Indeed, it has been demonstrated that B cells participate in the induction of rheumatoid arthritis4,5 and contribute to experimental autoimmune encephalomyelitis.6 Moreover, centrilobular and portal B-cell infiltrates occur both in autoimmune hepatitis and chronic viral hepatitis C,7,8 implying that B cells may also contribute to the pathogenesis of liver injury in those diseases. The latter hypothesis has not been tested, however, because a suitable animal model has not been available.
CD40 is a 50-kd glycoprotein that is present on the surface of B cells, follicular dendritic cells, monocytes, and some endothelial, epithelial, and cancer cells.9–11 CD40 plays a crucial role in B-cell proliferation; immunoglobulin secretion and differentiation;11 T-cell activation;10 and monocyte, macrophage, and dendritic cell functions, including their survival and ability to secrete several inflammatory cytokines.9,12 Recent studies have shown that anti-CD40 (αCD40) therapy may have a place in the treatment of infectious diseases and cancer. For example, αCD40 induced marked isotype switching and protective antibody responses to a polysaccharide antigen,13 and indirectly activated natural killer (NK) cells resulting in significant anti-tumor and anti-metastatic effects.14 Furthermore, CD40 ligation has been shown to trigger an inflammatory response in the lungs secondary to activation of bone marrow-derived CD40-positive cells.15,16 Finally, CD40 ligation has been shown to induce the secretion of antiviral cytokines that inhibit hepatitis B virus replication in the liver of HBV transgenic mice.17
We recently showed that CD40 ligation induces a biphasic inflammatory disease in the mouse liver that peaks on day 1 and again on day 5,17 and that nuclear factor (NF)-κB signaling controls this liver inflammation.18 In the former study, we focused on the mechanisms responsible for the early (day 1) phase of the disease, and demonstrated that it was mainly triggered by activated APCs and mediated by interferon (IFN)-γ and tumor necrosis factor (TNF)-α.17 In the current study, we focused on the mechanisms responsible for the late (day 5) phase of the disease, and discovered a hitherto unexpected role for activated B cells in the pathogenesis of liver injury, although once again the disease is mediated by IFN-γ and the cells it recruits. Our results indicate that the late phase of inflammatory liver disease induced by CD40 ligation is a macrophage- and B-cell-dependent process, and provide new insight into the pathogenic roles of B cells as effectors of the immune response.
Materials and Methods
Mice
CB6F1/J and C57BL/6 mice were purchased from Japan SLC (Shizuoka, Japan). SCID and C.B.17 mice were obtained from Japan Clea (Shizuoka, Japan). μMT mice were obtained from Jackson Laboratory (Bar Harbor, ME). Fas KO mice19 were generously provided by Dr. S. Nagata (Osaka University, Osaka, Japan). All animals were housed in pathogen-free rooms under strict barrier conditions, and received humane care according to the guidelines of the Animal Care Committee of Gifu University School of Medicine.
Anti-CD40 and Anti-Cytokine Antibodies
The FGK45 hybridoma producing a rat IgG2a monoclonal antibody (mAb) against mouse CD40 (αCD40) was kindly provided by Dr. A. Rolink (Basel Institute for Immunology, Basel, Switzerland). Mice were injected intravenously with either 100 μg of αCD40 or 100 μg of purified rat IgG2a (BD Pharmingen, San Diego, CA) as a control. To neutralize IFN-γ and TNF-α, mice were injected intraperitoneally (250 μg/mouse) on days 0 and +2 with 1) hamster mAb H22 specific for murine IFN-γ20; 2) hamster mAb TN3 19.12 specific for murine TNF-α21 (both generously provided by Dr. R. Schreiber, Washington University, St. Louis, MO); or 3) control hamster IgG (Jackson ImmunoResearch, West Grove, PA).
Enzyme-Linked Immunosorbent Assay
Serum IFN-γ and TNF-α concentrations were assayed using enzyme-linked immunosorbent assay kits (Genzyme Techne Co., Minneapolis, MN) according to the manufacturer’s protocols.
In Vivo Depletion of CD4+ and CD8+ T Cells, NK Cells, and Macrophages
To deplete CD4+ and CD8+ T cells, mice were injected intravenously with rat anti-mouse CD4 (YTS191.1) and rat anti-mouse CD8 (YTS169.4) mAb,22 both kindly provided by Dr. R. Zinkernagel (University of Zurich, Zurich, Switzerland). To deplete NK cells in the liver, mice were injected intravenously with rabbit anti-mouse asialo-GM1 antibody (50 μg/mouse) (Wako Pure Chemical, Osaka, Japan) on days −1 and +2 relative to the αCD40 injection. Purified rabbit IgG (Sigma-Aldrich, St. Louis, MO) was used as a negative control. To deplete macrophages in the liver, mice were injected intravenously (100 μl/mouse) with liposome-encapsulated dichloromethylene diphosphonate (L-MDP)23 on days −1 and +2 relative to the αCD40 injection, kindly provided by Dr. M. Naito (Niigata University School of Medicine, Niigata, Japan).
Tissue DNA and RNA Analyses
Frozen liver was mechanically pulverized under liquid nitrogen and total RNAs were isolated for RNase protection assays (RPA) as previously described.17 All reagents for RPA were purchased from BD Pharmingen. Specific signals were detected using a BAS-2500 imaging analyzer (Fuji Film, Nakanuma, Japan) and a FLA-3000 phosphoimager (Fuji Film). The mRNA expression levels were calculated as relative percentage values of the L32 housekeeping gene expression by using Image J soft ware.
Biochemical and Histological Analyses
The extent of hepatocellular injury was monitored biochemically by measuring the serum alanine aminotransferase (sALT) activity at multiple time points using a standard clinical automatic analyzer. For histological analysis, liver tissue was fixed in 10% zinc-buffered formalin, embedded in paraffin, sectioned (3 μm), and stained with hematoxylin and eosin (H&E).
Immunohistochemistry
For immunofluorescent microscopic analyses, liver sections were fixed with acetone at 4°C for 10 minutes, and preincubated with 10 μg/ml of rat anti-CD16/32 antibody (clone 2.4G2, BD Pharmingen) for 30 minutes. The sections were then incubated with rat anti-mouse B220-PE (clone RA3-6B2, BD Pharmingen) followed by rat anti-mouse IgM-FITC (clone R6-60.2, BD Pharmingen) for 1 hour each at room temperature. After each step of the staining, the sections were washed three times with phosphate-buffered saline (PBS) for 10 minutes each. Finally, the sections were observed using a DMRA fluorescence microscope and the QFISH software (Leica Microsystems Imaging Solutions, Cambridge, UK).
Liver tissues were treated with biotin conjugated with rat anti-mouse B220 mAb (BD Pharmingen) followed by streptavidin biotin-horseradish peroxidase complex (DAKO, Glostrup, Denmark). The reaction was visualized with 0.035% H2O2 and 0.03% 3,3′-diaminobenzidine (WAKO, Tokyo, Japan) in 50 mmol/L Tris-HCl (pH 7.6) for 2 to 3 minutes. After 4% formaldehyde fixation, specimens were counterstained with hematoxylin and observed.
IHLs
To isolate intrahepatic leukocytes (IHLs), single-cell suspensions were prepared from liver perfused with PBS via the inferior vena cava and were digested in 10 ml of RPMI 1640 (Life Technologies, Gaithersburg, MD) containing 0.02% (w/v) collagenase IV (Sigma-Aldrich) and 0.002% (w/v) DNase I (Sigma-Aldrich) for 40 minutes at 37°C. Cells were overlaid on 24% (w/v) metrizamide (Sigma-Aldrich) in PBS. After centrifugation for 20 minutes at 1500 × g, IHLs were isolated at the interface.
Fluorescence-Activated Cell Sorting (FACS) Analysis
To examine cytokine production in IHLs, isolated IHLs were cultured ex vivo for 4 hours in Brefeldin A (BD Pharmingen) and then stained with anti-CD3, anti-NK1.1, anti-CD11b, anti-CD11c, or anti-B220 (all from BD Pharmingen). After fixation, the cells were permeabilized for 30 minutes in 25 μl of PBS plus 0.5% saponin. Anti-mouse IFN-γ, TNF-α-PE, or allophycocyanin (APC) or isotype control mAb were added at a final dilution of 1/100, and the cells were incubated for 30 minutes at room temperature. The cells were washed and re-suspended in 1 ml of FACS buffer for analysis using a FACScalibur flow cytometer (BD Immunocytometry Systems, San Jose, CA).
Isolation of B Cells
B cells were purified by negative selection using a B-cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. The purified cells were routinely >95% B220+.
Data Analysis
All values in the figures and text are expressed as the mean ± SD. The significance of differences among mean values was evaluated according to the Mann-Whitney U-test.
Results
A Single Injection of αCD40 Causes a Biphasic Inflammatory Liver Disease
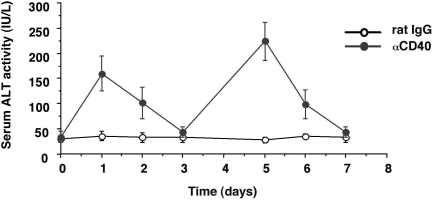
To determine whether αCD40 causes liver injury in inbred mice, four groups of age- and sex-matched CB6F1 mice (three mice/group) received an intravenous injection of αCD40 (100 μg/mouse) or rat IgG2a (rat IgG). As shown in Figure 1, sALT activity was mildly elevated (160 ± 32 U/L) at day 1, returned to normal (44 ± 12 U/L) at day 3, and then rebounded (242 ± 36 U/L) at day 5 after the injection.
Figure 1.
Serum ALT activity. CB6F1 mice were injected intravenously with 100 μg of αCD40 or 100 μg of rat IgG as a control, and sacrificed at the indicated time points. The mean sALT activity measured at the time of autopsy is indicated for each group and expressed in IU/L.
CD40 Ligation Induces Inflammatory Liver Disease
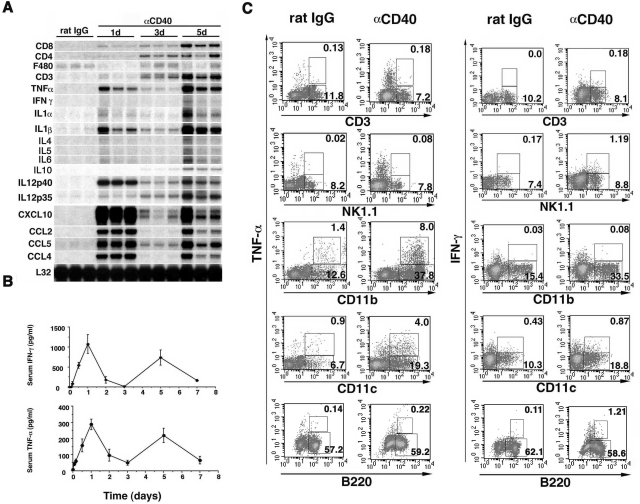
To evaluate inflammatory cytokine and chemokine expressions in the liver after CD40 ligation, RPA analysis of total liver RNA revealed that αCD40 injection induced CD3, CD4, and CD8 mRNA expression in the liver on day 1, and progressively increased on days 3 and 5 (Figure 2A). These changes were accompanied by clear biphasic increases in IFN-γ and TNF-α levels in the serum (Figure 2B) and hepatic interleukin (IL)-1, IL-12, CXCL10, CCL2, CCL4, and CCL5 expression (Figure 2A) on days 1 and 5, reflecting the biphasic increase in sALT activity described above (Figure 1), although the hepatic IFN-γ mRNA levels were too low to have confidence in their kinetics. Further, we found that other inflammatory cytokines, Th2 type cytokines, IL-4, IL-5, and IL-6 mRNA expressions were increased faintly on day 5 although IL-10 mRNA expression was gradually elevated until day 5. We also found that the expression of F4/80 mRNA (a macrophage marker24 that is down-regulated on activation24–26) was reduced on days 1 and 5 compared with the rat IgG control mice, suggesting that intrahepatic macrophages were also activated by αCD40 at these time points.25,26 To determine which cell population produced IFN-γ and TNF-α on day 5 after the αCD40 injection, we stained intrahepatic macrophages (CD11b+), dendritic cells (CD11c+), NK cells (NK1.1+), T cells (CD3+), and B cells (B220+) with antibodies against IFN-γ and TNF-α. As shown in Figure 2C, macrophages and dendritic cells were found to produce TNF-α, in contrast, IFN-γ was produced by NK cells and at lower levels by T and B cells. We also found that intrahepatic inflammatory cells, including macrophages, dendritic cells, and B cells were detected in the liver, indicating that CD40 ligation induced migration and proliferation of these cells (data not shown).
Figure 2.
A: Injection of αCD40 causes liver injury. CB6F1 mice were injected intravenously with 100 μg of αCD40 and sacrificed at the indicated time points. Total hepatic RNA was analyzed for various cytokines and chemokines by RPA, as indicated. B: Serum IFN-γ and TNF-α concentrations. The serum IFN-γ and TNF-α concentrations were analyzed at the indicated time points after αCD40 injection in CB6F1 mice. The data are expressed as the mean ± SD. C: Intracellular cytokine expressions of IHLs. IHLs were stained with anti-mouse CD3, NK1.1, CD11b, CD11c, and B220-FITC, or anti-mouse IFN-γ and TNF-α-PE or APC, and analyzed using a FACScalibur system.
Depletion Experiments
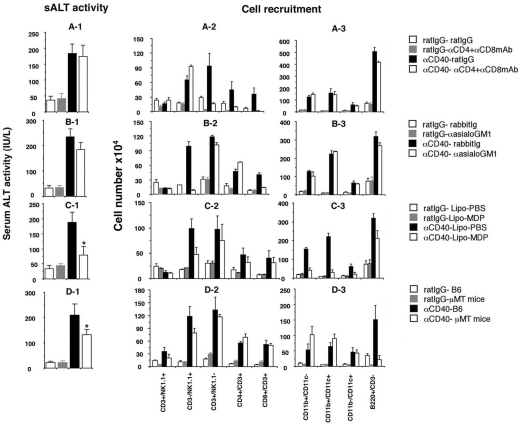
To determine the role of T cells in the late (day 5) phase of αCD40-induced liver injury, mice were treated with αCD4 and αCD8 mAb or control rat IgG before the αCD40 injection. As shown in Figure 3A-1, αCD40 caused liver injury irrespective of the presence or absence of T cells, suggesting that intrahepatic CD4+ and CD8+ T cells did not contribute to the late-phase liver injury or affect the recruitment of NK cells, macrophages, dendritic cells, and B cells to the liver by αCD40 (Figure 3, A-2 and A-3). Consistent with the result of liver injury we found that depletion of T cells did not affect the cytokine and chemokine expression in the liver after injection (Figure 4A).
Figure 3.
Depletion experiment. CB6F1 mice were injected with anti-mouse CD4 mAb (αCD4), anti-mouse CD8 mAb (αCD8), or control (rat IgG) antibodies at days −1 and +2 relative to the αCD40 injection and then sacrificed at day 5. The mean sALT activity expressed in IU/L is indicated for each group (A-1). IHLs from these animals were isolated and the absolute cell numbers were calculated (A-2, A-3). CB6F1 mice were injected with rabbit anti-mouse asialo-GM1 or control (rabbit Ig) antibodies at days −1 and +2 relative to the αCD40 injection and then sacrificed at day 5. sALT activity (B-1) and FACS (B-2, B-3) analyses were performed as described above. CB6F1 mice were injected with L-MDP or L-PBS at day −1 relative to the αCD40 injection and then sacrificed at day 5. sALT activity (C-1) and FACS (C-2, C-3) analyses were performed. μMT mice and C57BL/6 mice were injected with αCD40 or rat IgG and then sacrificed at day 5. sALT activity (D-1) and FACS (D-2, D-3) analyses were performed. All data are expressed as the mean ± SD for three mice. *P < 0.05.
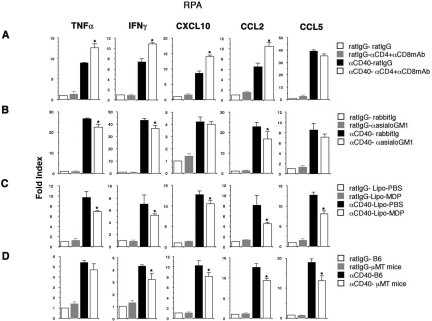
Figure 4.
Cytokine and chemokine expression. Total hepatic RNA (20 μg) was isolated from the livers at various time points and analyzed for cytokine and chemokine expressions by RPA. To quantify the differences in the mRNA expressions at various groups, the mRNA expression levels were calculated as the relative percentage values of the L32 housekeeping gene expression. All data are expressed as the mean ± SD for three mice. *P < 0.05.
Next, to determine whether αCD40-induced late-phase liver injury requires NK cells, mice were treated with αasialo-GM1 antibody at days −1 and +2 relative to the αCD40 injection. We found that NK cell depletion also had little or no effect on total IHL recruitment (Figure 3, B-2 and B-3) or the severity of the liver disease (Figure 3B-1). Further, as shown in Figure 4B, we found that NK cell depletion significantly reduced IFN-γ, TNF-α, and CCL2 mRNA expression in the liver though CXCL10 and CCL5 mRNA expression revealed the similar levels. As we showed NK cells produced IFN-γ at this time point (Figure 2C), this result is consistent with the finding that NK cells can produce IFN-γ.
To evaluate the role of macrophages in this process, mice were treated with L-MDP, which induces apoptosis of macrophages in the liver in vivo.23 One day later, the mice were injected intravenously with αCD40 or rat IgG. As shown in Figure 3C-1, elevation of sALT activity was significantly reduced in the L-MDP-treated mice, indicating that macrophages are necessary for liver injury on day 5 after αCD40 injection (*P < 0.05). However, note that the sALT elevation was not completely blocked despite near total macrophage depletion (Figure 3C-1), suggesting that inflammatory cells other than macrophages may be involved in liver inflammation. Also note that a B-cell infiltrate was observed in the liver despite macrophage depletion, demonstrating that hepatic B-cell recruitment is primarily macrophage-independent.
Because the intrahepatic B-cell population increased massively after αCD40 injection, we investigated the role of B cells in the pathogenesis of the liver disease. μMT mice, which lack mature B cells because of a germline deletion of the membrane exon of the immunoglobulin μ heavy chain,27 and C57BL/6 mice were treated with αCD40 or rat IgG, and sALT activity and IHL infiltration were monitored 5 days later. As shown in Figure 3D-1, μMT mice displayed less liver disease (sALT activity: 138 ± 18 U/L) than normal control mice (sALT activity: 212 ± 23 U/L) after αCD40 injection, and this difference was statistically significant (*P < 0.05), suggesting that B cells contribute to the pathogenesis of αCD40-induced late-phase liver disease. Interestingly, macrophage and dendritic cell recruitment were increased in μMT mice (Figure 3, D-2 and D-3), perhaps accounting for the relatively modest reduction in the severity of liver disease in the B-cell knockout animals. In addition, importantly we found that IFN-γ, CXCL10, CCL2, and CCL5 mRNA expression reduced in the liver with μMT mice although TNF-α expression revealed the same level irrespective of B cell absence or presence (Figure 4D). These results suggested that B cells contribute to induction of IFN-γ and chemokines at late phase after αCD40 injection.
Histological Analysis
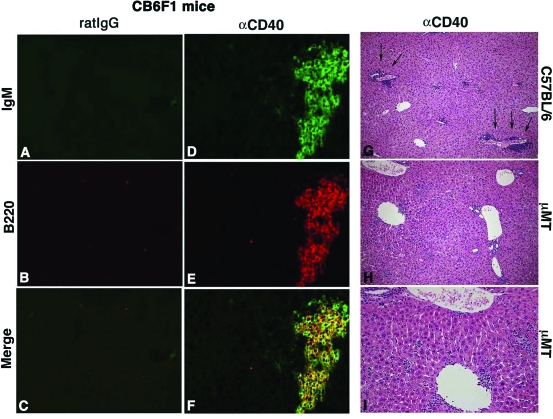
To determine the distributions of B cells in the liver after αCD40 injection, we performed H&E staining and immunohistological staining. Histological analysis revealed that lymphocytes had infiltrated around the portal tract (Figure 5, arrow) and liver parenchyma with wild-type mice. In contrast, αCD40-treated μMT mice did not show infiltration of these cells around the portal tract (Figure 5, right). Immunohistochemical analysis revealed that huge numbers of B220+/IgM+ B cells had infiltrated into the parenchyma and around the portal tract and central vein on day 5 after the injection in wild-type mice (Figure 5, left).
Figure 5.
Histological analysis. Liver sections obtained from C57BL/6 mice sacrificed at day 5 after injection of αCD40 or rat IgG were stained with anti-mouse B220-PE and anti-mouse IgM-FITC antibodies (A–F). Liver sections were obtained from C57BL/6 or μMT mice sacrificed at day 5 after αCD40 injection and stained with H&E (H–I).
Transfer of B Cells into SCID Mice
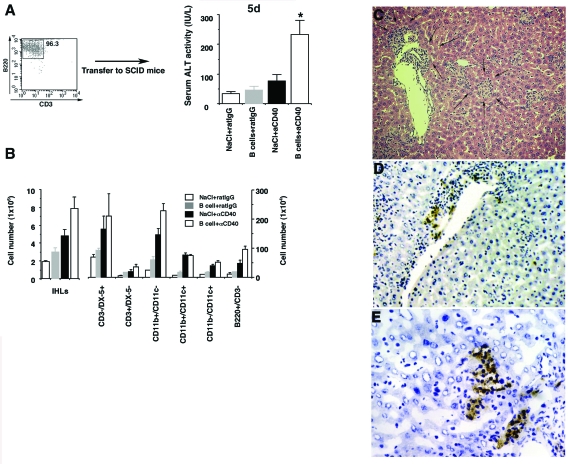
To confirm the involvement of B cells in this model, we adoptively transferred 1 × 107 purified B cells isolated from the spleens of C.B.17 mice (Figure 6A) into SCID mice, and then injected αCD40 or rat IgG 1 day later. As shown in Figure 6A, sALT activity was significantly elevated in B-cell-reconstituted mice that received αCD40 compared with the NaCl-treated and rat IgG control groups (*P < 0.05). Further, we also showed that total number of IHLs increased in B-cell-reconstituted mice as compared with NaCl-treated mice after αCD40 injection (Figure 6B). To evaluate which cell population is affected by adoptively transferred B cells, we analyzed the phenotype of IHLs by FACS. As shown in Figure 6B, we used anti-mouse CD49b/Pan-NK cells mAb (clone DX-5) instead of anti-mouse NK1.1 mAb (clone PK136) as a marker of NK cells in this experiment because the PK136 antibody does not stain NK cells in C.B.17 and SCID mice (data not shown).28 Interestingly, the number of CD3−/DX-5+ cells (NK cells) and macrophages increased in B-cell-reconstituted mice, demonstrating that αCD40 activated B cells can induce the proliferation and recruitment of these inflammatory cells (Figure 6B). Consistent with the result of sALT activity, histological analysis revealed the presence of a necroinflammatory liver disease (Figure 6C, arrows) consisting of B cells that infiltrated the liver parenchyma (Figure 6, D and E). These results strongly support the hypothesis that B cells play a key role in αCD40-induced liver injury.
Figure 6.
Adoptive transfer of B cells into SCID mice. SCID mice received isolated B cells (1 × 107) from the spleens of C.B.17 mice. A: One day later, αCD40 or rat IgG was injected into the recipient mice and the sALT activity was measured at day 5. Data are expressed as the mean ± SD for three mice. *P < 0.05. B: The numbers in each cell subset in the liver were calculated by multiplying the total number of IHLs by the frequency of each subset in the IHL population by FACS analysis. Data are expressed as the mean ± SD for three mice. Liver sections obtained from mice sacrificed at day 5 after injection of αCD40 with B cells were stained with H&E (C) or anti-mouse B220 antibody (D, E).
Determination of What Molecules Mediate αCD40-Induced Liver Injury
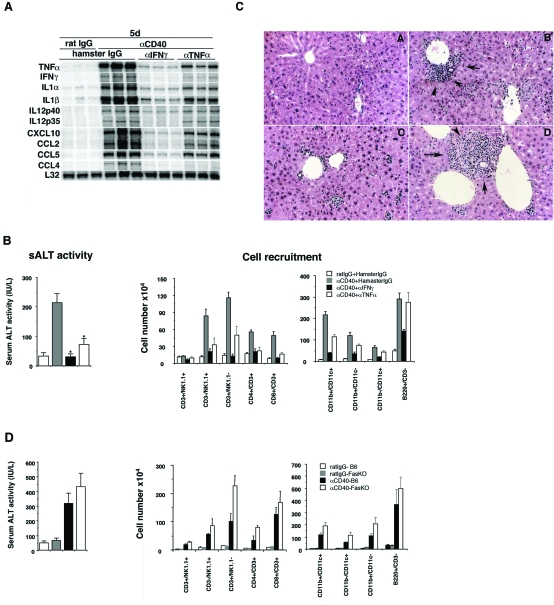
First, to determine whether IFN-γ or TNF-α contribute to αCD40-induced late-phase liver injury, we injected CB6F1 mice with αIFN-γ or αTNF-α mAb, or an irrelevant hamster IgG before αCD40 administration. The αIFN-γ mAb completely blocked the induction of liver injury (*P < 0.05) and greatly attenuated the intrahepatic inflammatory cell infiltrate (Figure 7, B and C) and cytokine/chemokine expression (Figure 7A and Supplemental Figure S1; for supplemental figure, see http://ajp.amjpathol.org) in the same animals, indicating that IFN-γ is primarily responsible for all these events.
Figure 7.
Liver injury induced by αCD40 is mediated by IFN-γ and TNF-α, but not by Fas. A: CB6F1 mice were injected with αIFN-γ or αTNF-α mAb, or control hamster IgG, before the injection of αCD40 or rat IgG. Total hepatic RNA was analyzed as described in the legend for Figure 2. The mean sALT activity expressed in IU/L is indicated for each group. B: IHLs from these animals were isolated and the effects of IFN-γ and TNF-α mAb, or a control antibody, on mice treated with αCD40 or rat IgG were analyzed. The numbers in each cell subset in the liver were calculated by multiplying the total number of IHLs by the frequency of each subset in the IHL population by FACS analysis. All data are expressed as the mean ± SD for three mice. *P < 0.05. Histology. Liver sections obtained from mice sacrificed at day 5 after injection of rat IgG (C-A) or αCD40 with hamster IgG (C-B), αIFN-γ (C-C), or αTNF-α (C-D) were stained with H&E. D: The role of Fas. Fas KO and C57BL/6 mice were injected with αCD40 and then sacrificed at day 5. The mean sALT activity expressed in IU/L is indicated for each group. IHLs from these animals were isolated and the absolute cell numbers were calculated.
In contrast, αTNF-α mAb treatment provided substantial but partial protection against late-phase liver injury (Figure 7B, left), which corresponded with its ability to only partially block the recruitment of NK and T cells into the liver (Figure 7B) and the induction of inflammatory cytokines and chemokines (Figure 7A). These results suggest that TNF-α also contributes to the pathogenetic activity of B cells and macrophages in the late phase of liver injury induced by αCD40.
Furthermore, to determine whether the Fas-Fas ligand pathway plays an important role in the liver injury, we injected αCD40 into Fas KO and C57BL/6 mice and then sacrificed them on day 5 for analysis. As shown in Figure 7D, we found that Fas KO mice displayed wild-type levels of serum ALT activity and inflammatory cell recruitment, indicating that the αCD40-induced liver disease is independent of the Fas pathway.
Discussion
The results presented here demonstrate that CD40-activated B cells and macrophages can cause a necroinflammatory response in the liver by inducing inflammatory cytokines (especially IFN-γ and TNF-α) and chemokines that recruit additional inflammatory cells that damage the liver. We have previously shown that αCD40 causes a biphasic, IFN-γ-, and TNF-α-dependent liver disease,17 the first wave of which requires intrahepatic macrophages and NK cells. In the current study, we examined the pathophysiology of the second wave of the liver injury induced by CD40 ligation. Having observed that the liver injury occurred in the absence of macrophages, T cells, and NK cells after CD40 ligation while a large number of B cells infiltrated the portal and central veins in the liver during the delayed phase of the liver disease, we hypothesized that B cells might contribute to the pathogenesis of this disease.
On testing this hypothesis, we found that the late phase of αCD40-induced liver injury was suppressed in μMT mice and normal mice treated with L-MDP, but that depletion of T or NK cells had no effect. We confirmed the involvement of B cells in this model by showing that αCD40 treatment could cause liver injury after adoptive transfer of B cells into SCID mice (Figure 6). Based on these results, we conclude that αCD40-activated B cells and macrophages mediate the second wave of liver injury in this model. Although the potential of B cells to produce a variety of cytokines and chemokines is well demonstrated in vitro,3,29 the ability of B cells to perform effector functions at inflammatory sites in vivo is not well defined. In the current article, we demonstrate that activated B cells can contribute to liver inflammation. Nonetheless, several questions remain to be elucidated.
First, how do αCD40-activated B cells cause liver injury? One possible clue is our observation that αCD40 treatment causes liver injury and cell recruitment in Fas knockout mice as well as in wild-type mice (Figure 7). This result demonstrates that the liver disease is not Fas-Fas ligand-dependent, although CD40 ligation increases Fas expression in B cells.11 Furthermore, we showed that the liver disease is mediated by inflammatory cytokines, especially IFN-γ and TNF-α. However, several points remain obscure. First, NK cell depletion by αasialo-GM1 treatment did not mitigate the liver disease or inflammatory cell recruitment, yet NK cells are the main producers of IFN-γ (Figure 2C). This suggests that cells other than NK cells, possibly B, T, and dendritic cells, produce the pathogenetic IFN-γ. Actually, we detected IFN-γ mRNA in the liver after αCD40 injection in NK cell-depleted mice (Figure 4) and also showed IFN-γ mRNA expression was suppressed in the liver with μMT mice (Figure 4). In support of this hypothesis, it has been reported that αCD40-activated B cells could produce IFN-γ and induce IFN-γ production on CD4 T cells.3,30 Because IFN-γ is well known as a potent activator of macrophages,31,32 and TNF-α (produced by macrophages) is also known to cause liver injury,33 we hypothesize that αCD40 induced B cell and secondary activated other inflammatory cell-derived IFN-γ may be the inducer in the cascade leading to liver inflammation, and that IFN-γ activates macrophages and other inflammatory cells that serve as effectors in this disease. This scenario is supported by the findings that IFN-γ blockade completely protects against αCD40-induced liver injury and cell recruitment, whereas TNF-α blockade confers only partial protection. Importantly, as shown in Figure 3, we found that αCD40 treatment induced the migration of macrophages, dendritic cells, and NK cells in μMT mice, indicating that these cells can be recruited into the liver without B cell help, and suggesting that they also contribute to the residual late phase in the B-cell knockout animals.
Furthermore, we found that liver injury was observed in μMT mice and reduced in L-MDP-treated mice, indicating that macrophages also contribute to liver inflammation in this model. Notably, we confirmed that αCD40-induced liver injury was completely prevented by depletion of both B cells and macrophages in the liver (Supplemental Figure S2 at http://ajp.amjpathol.org). It is well known that macrophages can induce liver injury through cytokine production.34–36 Accordingly, the interaction of macrophages and B cells in this liver injury model is interesting. We found that co-culture of B cells and macrophages under αCD40 treatment increased IFN-γ or TNF-α production, respectively, as compared with single cells (data not shown). These findings suggested that B cells and macrophages could stimulate each other by cell-to-cell or cytokine under some conditions. However, we have shown here that B cells infiltrate the liver in macrophage-depleted animals after CD40 ligation. In contrast, macrophages were also recruited to the liver in μMT mice after stimulation (Figure 3). Consistent with the fact that CD40 ligation induces macrophage and B cell activation and proliferation independently11,37,38 we consider that these cells work to establish liver inflammation independently in this system.
Interestingly, despite showing that B cells play an important role in the late-phase liver injury, we demonstrated that B cells do not contribute to the early phase liver disease (Supplemental Figure S3 at http://ajp.amjpathol.org). Furthermore, we found that the increase of intrahepatic B cells was small on day 1 after αCD40 injection, indicating that recruitment and proliferation in B cells was not enough to function as effector cells at this time. With regard to effector functions of B cells, we have to exclude the possibility that CD40 ligation induced antibody-dependent effector functions because CD40 ligation can induce autoreactive antibody.12,39,40 To evaluate this hypothesis, we examined the number of antibody-producing cells, plasma cell populations in IHLs and spleen after αCD40 injection. As shown in Supplemental Figure S4 at http://ajp.amjpathol.org, we found antibody-secreting cells (B220+/CD138+) in IHLs and spleen peaked at day 3 after injection despite liver injury were not observed at this time, suggesting that antibody-dependent mechanisms are not responsible for liver injury in this model.
Recently antibody-independent contributions of B cells have been demonstrated in murine models of autoimmune disease. Chan and colleagues41 have shown that MLR/lpr mice that are deficient in their ability to secrete Ig develop interstitial nephritis, vasculitis, and mortality when compared with secretary sufficient MLR/lpr. Based on this precedent, we suggest that CD40-activated B cells behave as effector cells in an antibody-independent manner in the current model.
Finally, we have also an interest for the role of NKT cells in this liver inflammation because NKT cells are abundant in the liver and recovered to base line at the late phase after αCD40 injection (data not shown). A current report shows NKT cells recognize an endogenous ligand presented by CD1d on B cells and regulate B-cell proliferation and effector functions.42 This result suggests that NKT cells also might activate B cells and accelerate liver injury in this model.
In summary, we have shown here that αCD40-activated B cells and macrophages produce inflammatory cytokines and contribute to the pathogenesis of a necroinflammatory liver disease. The present data illustrate that activated B cells can both cause and amplify tissue injury, and provide new evidence that B cells can function in a pathogenic manner at sites of tissue inflammation.
Supplementary Material
Acknowledgments
We thank Dr. Antonius Rolink (Basel Institute for Immunology) for providing the anti-mouse agonistic CD40 monoclonal antibody, Dr. Rolf Zinkernagel (University of Zurich) for providing the anti-mouse CD4 and CD8 antibodies, Dr. Robert Schreiber (Washington University) for providing the anti-mouse IFN-γ and TNF-α antibodies, Dr. Shigekazu Nagata (Osaka University) for providing the Fas KO mice, and Dr. Kazuhiro Kakimi (Tokyo Medical University School of Medicine) for critical advice.
Footnotes
Address reprint requests to Kiminori Kimura, M.D., Ph.D., Center for Emerging Infectious Diseases, Gifu University, Gifu-shi, Gifu 501-1194, Japan. E-mail: kkimura@cc.gifu-u.ac.jp.
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- Schaniel C, Pardali E, Sallusto F, Speletas M, Ruedl C, Shimizu T, Seidl T, Andersson J, Melchers F, Rolink AG, Sideras P. Activated murine B lymphocytes and dendritic cells produce a novel CC chemokine which acts selectively on activated T cells. J Exp Med. 1998;188:451–463. doi: 10.1084/jem.188.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- Maccioni M, Zeder-Lutz G, Huang H, Ebel C, Gerber P, Hergueux J, Marchal P, Duchatelle V, Degott C, van Regenmortel M, Benoist C, Mathis D. Arthritogenic monoclonal antibodies from K/BxN mice. J Exp Med. 2002;195:1071–1077. doi: 10.1084/jem.20011941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, Takahashi K, Holers VM, Walport M, Gerard C, Ezekowitz A, Carroll MC, Brenner M, Weissleder R, Verbeek JS, Duchatelle V, Degott C, Benoist C, Mathis D. Arthritis critically dependent on innate immune system players. Immunity. 2002;16:157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- Donaldson P, Doherty D, Underhill J, Williams R. The molecular genetics of autoimmune liver disease. Hepatology. 1994;20:225–239. doi: 10.1016/0270-9139(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Peters M, Vierling J, Gershwin ME, Milich D, Chisari FV, Hoofnagle JH. Immunology and the liver. Hepatology. 1991;13:977–994. [PubMed] [Google Scholar]
- Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- Grewal IS, Borrow P, Pamer EG, Oldstone MB, Flavell RA. The CD40-CD154 system in anti-infective host defense. Curr Opin Immunol. 1997;9:491–497. doi: 10.1016/s0952-7915(97)80100-8. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C, Liu YJ, Rousset F, Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Grewal IS, Flavell RA. A central role of CD40 ligand in the regulation of CD4+ T-cell responses. Immunol Today. 1996;17:410–414. doi: 10.1016/0167-5699(96)10030-x. [DOI] [PubMed] [Google Scholar]
- Dullforce P, Sutton DC, Heath AW. Enhancement of T cell-independent immune responses in vivo by CD40 antibodies. Nat Med. 1998;4:88–91. doi: 10.1038/nm0198-088. [DOI] [PubMed] [Google Scholar]
- Turner JG, Rakhmilevich AL, Burdelya L, Neal Z, Imboden M, Sondel PM, Yu H. Anti-CD40 antibody induces antitumor and antimetastatic effects: the role of NK cells. J Immunol. 2001;166:89–94. doi: 10.4049/jimmunol.166.1.89. [DOI] [PubMed] [Google Scholar]
- Wiley JA, Geha R, Harmsen AG. Exogenous CD40 ligand induces a pulmonary inflammation response. J Immunol. 1997;158:2932–2938. [PubMed] [Google Scholar]
- Wiley JA, Harmsen AG. Bone marrow-derived cells are required for the induction of a pulmonary inflammatory response mediated by CD40 ligation. Am J Pathol. 1999;154:919–926. doi: 10.1016/S0002-9440(10)65339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Kakimi K, Wieland S, Guidotti LG, Chisari FV. Activated intrahepatic antigen-presenting cells inhibit hepatitis B virus replication in the liver of transgenic mice. J Immunol. 2002;169:5188–5195. doi: 10.4049/jimmunol.169.9.5188. [DOI] [PubMed] [Google Scholar]
- Kimura K, Nagaki M, Takai S, Satake S, Moriwaki H. Pivotal role of nuclear factor κB signaling in anti-CD40-induced liver injury in mice. Hepatology. 2004;40:1180–1189. doi: 10.1002/hep.20432. [DOI] [PubMed] [Google Scholar]
- Adachi M, Suematsu S, Kondo T, Ogasawara J, Tanaka T, Yoshida N, Nagata S. Targeted mutation in the Fas gene causes hyperplasia in peripheral lymphoid organs and liver. Nat Genet. 1995;11:294–300. doi: 10.1038/ng1195-294. [DOI] [PubMed] [Google Scholar]
- Schreiber RD, Hicks LJ, Celada A, Buchmeier NA, Gray PW. Monoclonal antibodies to murine gamma-interferon which differentially modulate macrophage activation and antiviral activity. J Immunol. 1985;134:1609–1618. [PubMed] [Google Scholar]
- Sheehan KC, Ruddle NH, Schreiber RD. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989;142:3884–3893. [PubMed] [Google Scholar]
- Cobbold SP, Martin G, Qin S, Waldmann H. Monoclonal antibodies to promote marrow engraftment and tissue graft tolerance. Nature. 1986;323:164–166. doi: 10.1038/323164a0. [DOI] [PubMed] [Google Scholar]
- Naito M, Nagai H, Kawano S, Umezu H, Zhu H, Moriyama H, Yamamoto T, Takatsuka H, Takei Y. Liposome-encapsulated dichloromethylene diphosphonate induces macrophage apoptosis in vivo and in vitro. J Leukoc Biol. 1996;60:337–344. doi: 10.1002/jlb.60.3.337. [DOI] [PubMed] [Google Scholar]
- Leenen PJ, de Bruijn MF, Voerman JS, Campbell PA, van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Ezekowitz RA, Gordon S. Down-regulation of mannosyl receptor-mediated endocytosis and antigen F4/80 in bacillus Calmette-Guerin-activated mouse macrophages. Role of T lymphocytes and lymphokines. J Exp Med. 1982;155:1623–1637. doi: 10.1084/jem.155.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austyn JM, Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981;11:805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Kitamura D, Roes J, Kuhn R, Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991;350:423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Yokoyama WM, Seaman WE. The Ly-49 and NKR-P1 gene families encoding lectin-like receptors on natural killer cells: the NK gene complex. Annu Rev Immunol. 1993;11:613–635. doi: 10.1146/annurev.iy.11.040193.003145. [DOI] [PubMed] [Google Scholar]
- Pistoia V. Production of cytokines by human B cells in health and disease. Immunol Today. 1997;18:343–350. doi: 10.1016/s0167-5699(97)01080-3. [DOI] [PubMed] [Google Scholar]
- Duddy ME, Alter A, Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol. 2004;172:3422–3427. doi: 10.4049/jimmunol.172.6.3422. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- Bradham CA, Plumpe J, Manns MP, Brenner DA, Trautwein C. Mechanisms of hepatic toxicity. I. TNF-induced liver injury. Am J Physiol. 1998;275:G387–G392. doi: 10.1152/ajpgi.1998.275.3.G387. [DOI] [PubMed] [Google Scholar]
- Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- Dambach DM, Watson LM, Gray KR, Durham SK, Laskin DL. Role of CCR2 in macrophage migration into the liver during acetamino-phen-induced hepatotoxicity in the mouse. Hepatology. 2002;35:1093–1103. doi: 10.1053/jhep.2002.33162. [DOI] [PubMed] [Google Scholar]
- Wolf D, Schumann J, Koerber K, Kiemer AK, Vollmar AM, Sass G, Papadopoulos T, Bang R, Klein SD, Brune B, Tiegs G. Low-molecular-weight hyaluronic acid induces nuclear factor-κB-dependent resistance against tumor necrosis factor α-mediated liver injury in mice. Hepatology. 2001;34:535–547. doi: 10.1053/jhep.2001.27218. [DOI] [PubMed] [Google Scholar]
- van Kooten C, Banchereau J. CD40-CD40 ligand. J Leukoc Biol. 2000;67:2–17. doi: 10.1002/jlb.67.1.2. [DOI] [PubMed] [Google Scholar]
- Calderhead DM, Kosaka Y, Manning EM, Noelle RJ. CD40-CD154 interactions in B-cell signaling. Curr Top Microbiol Immunol. 2000;245:73–99. doi: 10.1007/978-3-642-59641-4_4. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Hirose S, Abe M, Yagita H, Okumura K, Shirai T. CD40-mediated stimulation of B1 and B2 cells: implication in autoantibody production in murine lupus. Eur J Immunol. 1996;26:3061–3065. doi: 10.1002/eji.1830261236. [DOI] [PubMed] [Google Scholar]
- Hirose S, Yan K, Abe M, Jiang Y, Hamano Y, Tsurui H, Shirai T. Precursor B cells for autoantibody production in genomically Fas-intact autoimmune disease are not subject to Fas-mediated immune elimination. Proc Natl Acad Sci USA. 1997;94:9291–9295. doi: 10.1073/pnas.94.17.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–1648. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli G, Nuti S, Tavarini S, Galli-Stampino L, De Lalla C, Casorati G, Dellabona P, Abrignani S. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J Exp Med. 2003;197:1051–1057. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.