Abstract
Connective tissue growth factor (CTGF), a downstream mediator of transforming growth factor-β1, mediates mesangial cell/fibroblast proliferation and extracellular matrix production by renal cells. Here, we show that renal tubular epithelial cells from patients with minimal change nephritic syndrome produced CTGF after glucocorticoid treatment. In addition, the glucocorticoid dexamethasone (DEX) increased CTGF mRNA levels in the kidneys of C57B6 but not SJL mice and produced intermediate CTGF mRNA levels in the kidneys of F1 (C57B6 × SJL) mice, midway between the levels found for parental strains. DEX also increased CTGF mRNA levels in cultured tubular epithelial cells derived from C57B6 (mProx24) but not SJL (MCT) mice via transcriptional up-regulation of CTGF mRNA. Transient transfection experiments using luciferase reporter constructs bearing CTGF promoter fragments revealed that the −897- to −628-bp fragment contained DEX-responsive positive regulatory elements, which were active in mProx24 but not MCT cells. Long-term DEX treatment resulted in fibronectin deposition in the kidneys of C57B6 but not SJL mice, and this effect was inhibited by co-administration of CTGF anti-sense oligodeoxynucleotides. Thus, glucocorticoid-induced renal fibrogenesis seems to be influenced by genetic background, with the critical DEX-responsive elements in the −897- to −628-bp region of the CTGF promoter.
Connective tissue growth factor (CTGF) is a member of the CTGF, cyr 61/cef 10, nov (CCN) protein family, which participates in a wide variety of biological processes such as embryonic development and tissue repair.1,2 CTGF itself is a potent and ubiquitously expressed growth factor for fibroblasts, chondrocytes and vascular endothelial cells.2 CTGF is also a downstream mediator of transforming growth factor (TGF)-β1, a key cytokine involved in renal fibrogenesis, ie, glomerulosclerosis and interstitial fibrosis. As such CTGF plays a unique role in mediating mesangial cell/fibroblast proliferation and extracellular matrix production by renal cells.3,4 Glomerular visceral/parietal epithelial cells, mesangial cells, fibroblasts, and endothelial cells have been shown to produce CTGF in fibrosing kidneys.4–6 We previously showed that tubular epithelial cells are potent producers of CTGF and that CTGF itself probably plays a role in renal fibrogenesis, as determined using the remnant kidney model.7,8
Minimal change nephrotic syndrome is a benign disease that rarely leads to renal failure.9 Although its frequency remains to be clarified, however, minimal change nephrotic syndrome follows a steroid-dependent course, and relapsing patients sometimes develop renal functional impairment as a result of glomerulosclerosis and/or interstitial fibrosis that is thought to be attributable to long-term protein overload.10 As an alternative hypothesis, we proposed that these changes might be attributable to long-term exposure to glucocorticoids, which have been reported to be potent inducers of CTGF in the kidney.11 In this study, we immunohistochemically analyzed human renal biopsy material and mouse kidneys to determine which kidney cells expressed CTGF in response to glucocorticoid treatment. Our results showed that glucocorticoid induced CTGF expression in tubular epithelial cells. We then examined the molecular mechanisms responsible for this effect using cultured mouse tubular epithelial cells. Finally, we determined whether glucocorticoid treatment itself induced renal fibrogenesis.
Materials and Methods
Cell Culture
Cultured mouse proximal tubular epithelial cell lines, mProx2412 and MCT,13 derived from C57B6 and SJL mice, respectively, were maintained in Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin. For experimental purposes, the cells were seeded in six-well plates (1 × 105 cells/well) and incubated overnight in growth medium, after which the medium was replaced with modified K-1 medium (50:50 Ham’s F-12/Dulbecco’s modified Eagle’s medium with 5 μg/ml of transferrin and 5 μg/ml of insulin). After 48 hours, various doses of dexamethasone (DEX; Sigma, St. Louis, MO) or rhTGF-β1 (R&D Systems, Minneapolis, MN) were added to the wells, and the cells were harvested for mRNA extraction as described below after various incubation times. To examine the mechanism of DEX-induced CTGF expression in these cells, neutralizing anti-TGF-β (Genzyme Corp., Cambridge, MA) and anti-CTGF7 antibodies, as well as rmTNF-α (10 ng/ml; R&D Systems), the AP-1 inhibitor curcumin (4.0 μmol/L; Sigma), the PKC inhibitor H7 (1.0 μg/ml; Sigma), the MEK inhibitor U0126 (10 μmol/L; Sigma), or the tyrosine kinase inhibitor genistein (10 μmol/L; Sigma) were added to the cultures 30 minutes before DEX (1000 nmol/L). After an incubation of 3 hours, the cells were harvested. To determine the CTGF mRNA stability of these DEX-treated cultured cells, those that had been incubated in the presence or absence of DEX (1000 nmol/L) for 3 hours were treated with the transcriptional inhibitor actinomycin-D (5 μg/ml; Sigma) for varying periods of time, after which they were harvested for RNA extraction.
In a separate experiment, cells were harvested from collagenase-digested fragments of proximal tubules obtained from the cortices of both C57B6 and SJL mice using a modification of a previously described procedure.14 Briefly, cortices were minced and incubated with 0.5 mg/ml of collagenase (type IV-S; Sigma) and 0.5 mg/ml of soybean trypsin inhibitor (Sigma) in Hanks’ salt solution for 30 minutes at 37°C. After the large undigested fragments were removed by gravitation, the suspension was mixed with an equal volume of Hanks’ solution containing 10% horse serum and centrifuged at 500 rpm for 7 minutes at room temperature. The pellets were washed once with Dulbecco’s modified Eagle’s medium by centrifugation, after which they were resuspended in the modified K-1 medium. Cells isolated using this technique were previously shown to be predominantly proximal tubular cells.14 The response of these cells to DEX and rhTGF-β1 was determined as described above for the mProx24 and MCT cell lines.
Treatment of Mice with DEX
Animal care and treatment were provided in conformity with institutional guidelines. Male C57B6, SJL, F1(C57B6xSJL), Balb/C, and 129 mice (5 to 7 weeks of age) were injected intraperitoneally with 1 mg of DEX in phosphate-buffered saline (PBS) per kg body weight; control mice were injected with PBS alone. These animals were then serially sacrificed after 1, 3, 12, 24, or 48 hours, and their kidneys, liver, and lungs were harvested and processed for RNA isolation and immunohistochemistry.
Separate cohorts of C57B6 and SJL mice were injected intraperitoneally daily for 2 weeks with 1 mg/kg of DEX. We previously reported that when a given anti-sense oligodeoxynucleotide (ODN) was injected intravenously into rodents, it was absorbed into the proximal tubular epithelium where it was retained for nearly 48 hours and during which time it could block transcription of a target gene.7,15 Thus, these mice were similarly injected intravenously with the CTGF anti-sense (5′-TGCGACGGAGGCGAGCAT-3′)7 or mutated anti-sense ODNs (5′-TGCAGTGGAAATGAGTGC-3′) at a concentration of 1.0 mg/kg every 2 days from day 0 to day 14. The experimental groups consisted of C57B6 mice that were treated with DEX and anti-sense ODN (C57-AS, n = 8), C57B6 mice treated with DEX and mutated anti-sense ODN (C57-mAS, n = 8), SJL mice treated with DEX and anti-sense ODN (SJL-AS, n = 8), SJL mice treated with DEX and mutated anti-sense ODN (SJL-mAS, n = 8), and negative control mice of both strains (C57-NC, n = 8; SJL-NC, n = 8). After 12 hours or 2 weeks of treatment, the renal tissues from these animals were harvested for RNA extraction and immunohistochemistry.
Immunohistochemistry
Twelve renal biopsy specimens from patients with minimal change nephrotic syndrome, six of whom had just received steroid pulse therapy (500 mg/24 hours of methyl-prednisolone for 3 days) and six who did not, were examined for the presence of CTGF. The specimens, all of which were confirmed cases of minimal change nephrotic syndrome, came from patients who underwent renal biopsy at our hospital between 1996 and 2002 for the evaluation of nephrotic range proteinuria. The protocol for the use of human materials was approved by the ethics committee of the institution. The specimens from these patients, as well as renal samples from the mice described above, were fixed in 4% paraformaldehyde overnight and processed for paraffin embedding. Sections (4 μm) were cut from these blocks, after which they were deparaffinized, rehydrated, and treated with proteinase K. The sections were then boiled in citrate buffer in a microwave to unmask antigenic sites. Endogenous biotin was blocked by using a biotin blocking system (X0590; DAKO Corp., Carpinteria, CA). The sections were then immersed in 3% H2O2 in methanol to inhibit endogenous peroxidase and incubated with 1% nonfat milk in PBS to block nonspecific binding. Rabbit polyclonal anti-CTGF (1:200) or anti-fibronectin (anti-FN; Life Technologies, Inc., Grand Island, NY) (1:400) primary antibodies were then applied to the sections, after which the sections were washed and then incubated with a biotin-conjugated anti-rabbit IgG secondary antibody. A catalyzed signal amplification system peroxidase (K1500; DAKO) was used to visualize the antibody reactions.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) and Real-Time RT-PCR
Total RNA was extracted from the homogenates of kidney tissues and cultured cells using TRIzol (Life Technologies, Inc.) according to the manufacturer’s instructions. All RNA samples were pretreated with RNase-free DNase I (Qiagen, Basel, Switzerland). The RT-PCR assay was performed to detect the expression of the glucocorticoid receptor gene using glucocorticoid receptor primers, forward 5′-CAAAGCCGTTTCACTGTCC-3′ and reverse 5′-ACAATTTCACACTGCCACC-3′.16 The PCR protocol was as follows: 94°C for 2 minutes, followed by 30 cycles at 94°C for 1 minute, 56°C for 1 minute, and 72°C for 1 minute, and ending with 72°C for 7 minutes. Real-time quantitative one-step RT-PCR assay was performed to quantify CTGF, TGF-β1, fibronectin EIIIA isoform (FN-EIIIA), α1 chain of type I procollagen (α1COLI), angiotensinogen, and GAPDH mRNAs using QuantiTect SYBR Green RT-PCR (Qiagen) and an ABI Prism 7700 sequence detection system (Applied Biosystems, Tokyo, Japan). The primers used for real-time RT-PCR were as follows: CTGF primers, forward 5′-GTGGAATATTGCCGGTGCA-3′, reverse 5′-CCATTGAAGCATCTTGGTTCG-3′; TGF-β1 primers, forward 5′-TCGTGGAACTGCCCTACCAG-3′, reverse 5′-ATGTTGGTGAGGGCGGAGAG-3′; FN-EIIIA primers, forward 5′-ATCCGGGAGCTTTTCCCTG-3′, reverse 5′-TGCAAGGCAACCACACTGAC-3′; α1COLI primers, forward 5′-TGTAAACTCCCTCCACCCCA-3′, reverse 5′-TCGTCTGTTTCCAGGGTTGG-3′; angiotensinogen primers, forward 5′-GAGGCAAATCTGAGCAACATTG-3′, reverse 5′-GAGTTCGAGGAGGATGCTATTGA-3′; and GAPDH primers, forward 5′-TGCAGTGGCAAAGTGGAGATT-3′, reverse 5′-TTGAATTTGCCGTGAGTGGA-3′. All of these oligonucleotides were designed by using Primer Express software (Perkin Elmer, Foster City, CA). Preliminary RT-PCR experiments in which these primer sets were used yielded appropriately sized, single products.
Luciferase Reporter Constructs and Transient Transfection Assay
A series of firefly luciferase reporter minigenes were constructed bearing various 5′ fragments of the CTGF gene. The plasmids, pCT-897.L, pCT-628.L, pCT-483.L, and pCT-202.L contained genomic DNA −897, −628, −483, and −202 bp upstream of the transcription start site, respectively. In these plasmids, genomic fragments were placed 5′ of the firefly luciferase cDNA in pGL3b (Promega, Madison, WI). The accuracy of all constructed plasmids was verified by sequencing.
Transient transfections were performed using TransFast (Promega) according to the manufacturer’s instructions. The Dual Luciferase system (Promega) was used for the sequential measurement of firefly and Renilla luciferase activity using the specific substrates of beetle luciferin and coelenterazine, respectively. One μg of pCT-897.L or isomolar amounts of other firefly luciferase constructs were co-transfected with 0.5 μg of Renilla luciferase control plasmid (pRL-TK; Promega) into 1.0 × 105 cells plated in each well of a six-well plate. The medium was changed 12 hours later, and the cells were incubated with or without 1000 nmol/L DEX in K-1 medium for another 36 hours. Quantification of luciferase activity and the calculation of relative ratios were performed manually using a luminometer (TD-20/20; Turner Designs, Sunnyvale, CA). The genomic sequences from −897 to −628 bp upstream of the transcription start site of the CTGF gene in C57B6 and SJL mice were determined using capillary sequence methods.
Statistical Analysis
Values are presented as the means ± SE. Statistical differences between groups were evaluated using a Bonferroni/Dunnett’s test; P values that were ≤0.05 were considered to be statistically significant.
Results
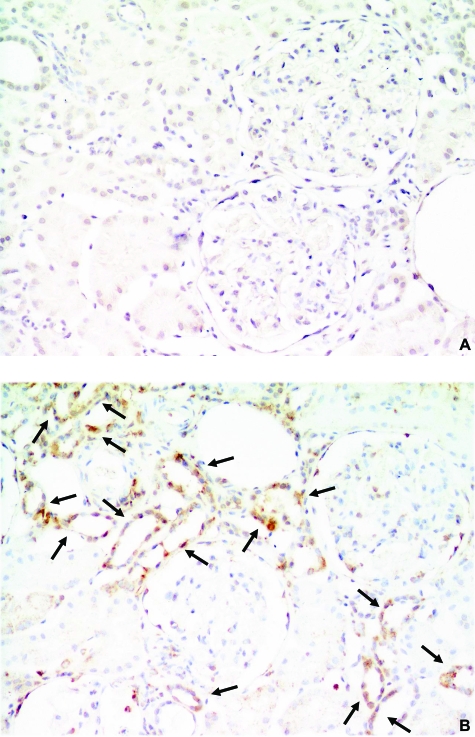
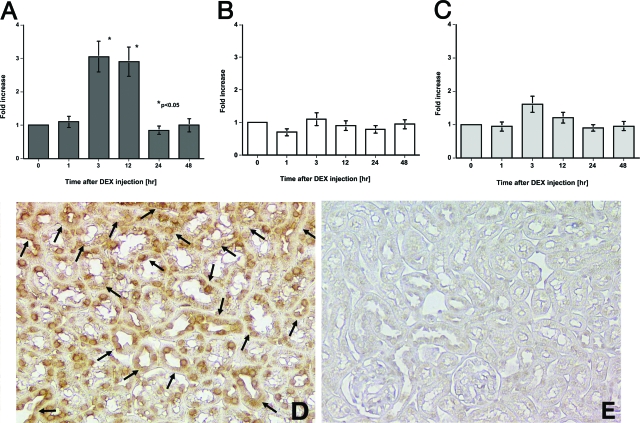
Demographic data for the 12 patients whose biopsies were examined in this study are shown in Table 1. Although no CTGF protein expression was detected in the kidneys from the six untreated minimal change nephrotic syndrome patients (Figure 1A), CTGF protein localized in the tubular epithelial cells in four of the six kidney tissues from minimal change nephrotic syndrome patients who had been treated or who had just finished their treatment with steroid pulse therapy (Figure 1B). There were no differences in the demographic data between the CTGF-positive and -negative minimal change nephrotic syndrome patients (data not shown). In light of these findings, we next examined the effects of DEX on the expression of CTGF mRNA in the kidneys of different mouse strains. DEX treatment significantly increased CTGF mRNA levels in the kidneys of C57B6 mice (Figure 2A), had no effect in the kidneys of SJL mice (Figure 2B), and slightly increased CTGF mRNA levels in the kidneys of Balb/C and 129 mice (data not shown). Interestingly, DEX significantly increased CTGF mRNA levels in the lungs of C57B6 (4.3 ± 0.5-fold, P < 0.05) but not SJL mice after 3 hours. Regardless of DEX treatment, however, CTGF mRNA levels in the liver were significantly lower than those in the kidneys and lungs in both strains of mice. DEX slightly, but not significantly, increased CTGF mRNA levels in the kidneys of F1 (C57B6 × SJL) mice to levels that were approximately midway between the levels found in C57B6 and SJL mice (Figure 2C). Immunohistochemistry using an anti-CTGF antibody localized CTGF protein exclusively in the renal tubular epithelial cells of DEX-treated, but not untreated, C57B6 mice (Figure 2, D and E).
Table 1.
Demographic Data of Patients with Minimal Change Nephrotic Syndrome at Biopsy
| Case | Gender (M/F) | Age | Pulse therapy (−/+) | Oral PSL (mg/day) | Proteinuria (mg/day) | CTGF in TEC (−/+) |
|---|---|---|---|---|---|---|
| 1 | M | 23 | − | 0 | 3500 | − |
| 2 | M | 36 | − | 0 | 6200 | − |
| 3 | M | 28 | − | 0 | 4800 | − |
| 4 | M | 33 | − | 0 | 6800 | − |
| 5 | M | 40 | − | 0 | 9000 | − |
| 6 | F | 22 | − | 0 | 5500 | − |
| 7 | M | 17 | + | 50 | 8100 | − |
| 8 | F | 22 | + | 40 | 7900 | − |
| 9 | M | 23 | + | 60 | 9500 | + |
| 10 | M | 27 | + | 60 | 6800 | + |
| 11 | M | 27 | + | 60 | 5600 | + |
| 12 | F | 19 | + | 40 | 4500 | + |
Pulse therapy; methyl prednisolone 500 mg/day i.v. for 3 days, PSL, prednisolone; TEC, tubular epithelial cells.
Figure 1.
Localization of CTGF protein in the kidneys of patients with minimal change nephrotic syndrome. A: An untreated patient. No CTGF expression was detected. B: A patient who just completed steroid pulse therapy. CTGF protein was found exclusively in tubular epithelial cells (arrows). Figures are representative sections from six samples each. DAB stain. Original magnifications, ×200.
Figure 2.
DEX induction of CTGF expression in the mouse kidney. A: CTGF mRNA levels in DEX-treated C57B6 mice. B: CTGF mRNA levels in DEX-treated SJL mice. C: CTGF mRNA levels in DEX-treated F1 (C57B6 × SJL) mice. CTGF protein localization in the kidneys of DEX-treated C57B6 mice 24 hours after treatment (arrows) (D) and in the kidneys of untreated C57B6 mice (E). Each of the data in A, B, and C were obtained from four mice and data in D and E are representative of the sections from four mice in each case. DAB stain. Original magnifications, ×100.
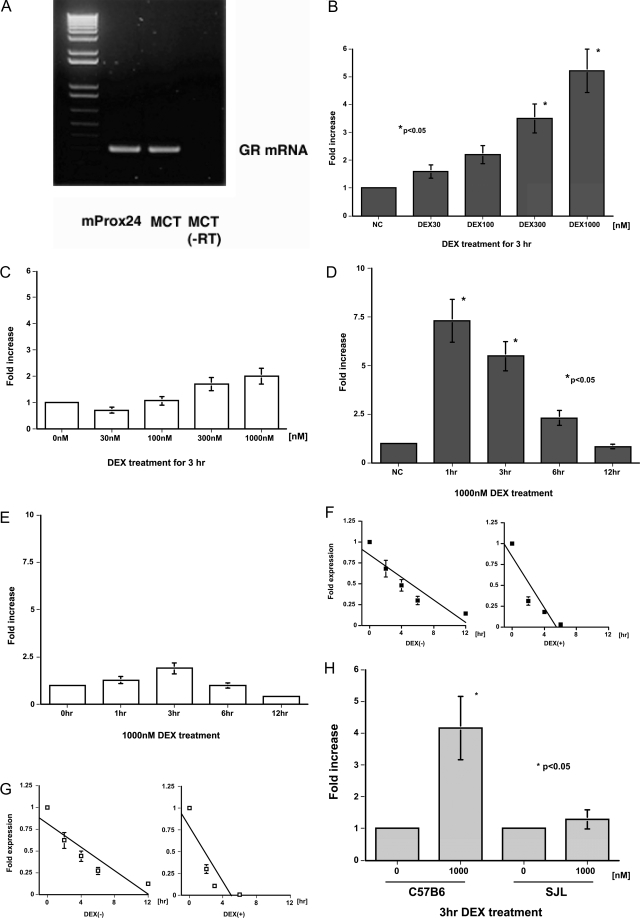
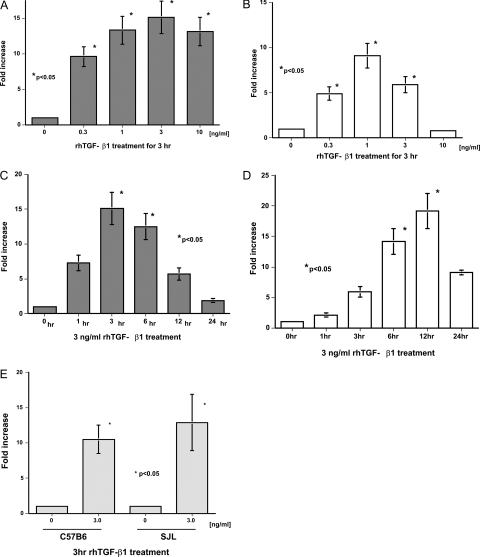
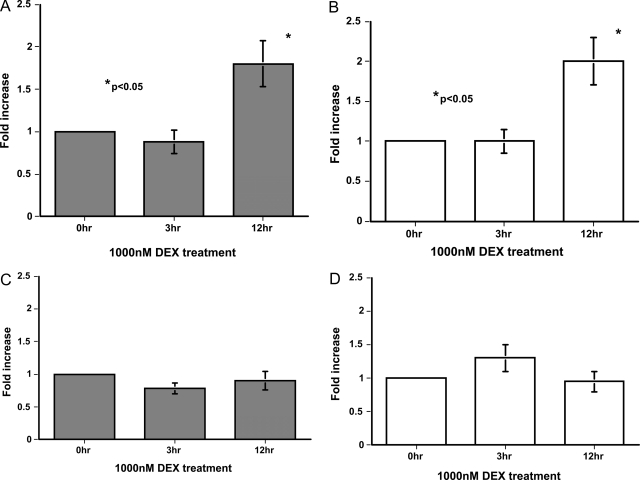
The mProx24 and MCT tubular epithelial cell lines derived from C57B6 and SJL mice, respectively, were used to examine the effects of DEX on the induction of CTGF. RT-PCR revealed that both cell lines were positive for glucocorticoid receptor mRNA (Figure 3A). As expected from our in vivo findings, DEX increased CTGF mRNA levels in mProx24 cells in a dose-dependent manner (Figure 3B), with levels at 1 and 3 hours after DEX treatment being significantly greater than controls (Figure 3D). Because DEX facilitated CTGF mRNA degradation in mProx24 cells (Figure 3F), it was likely that this increase was attributable to the up-regulation of CTGF gene transcription by DEX. In contrast, DEX did not increase CTGF mRNA levels in MCT cells (Figure 3, C and E). Because DEX facilitated CTGF mRNA degradation in MCT cells to the same degree as in mProx24 cells (Figure 3G), it seems reasonable that DEX does not up-regulate CTGF gene transcription in MCT cells. These findings were unlikely to have been attributable to the spontaneous transformation of both cell lines in culture because DEX also significantly increased CTGF mRNA levels in primary cultured cells derived from C57B6, but not SJL, mice (Figure 3H). CTGF mRNA levels increased in both cell lines as well as both primary cultured cells in response to rhTGF-β1 (Figure 4), suggesting that they possessed the capacity to synthesize CTGF. DEX was reported to induce the expression of angiotensinogen and FN mRNA in certain cultured tubular epithelial cells.17,18 We also found that DEX significantly increased angiotensinogen but not FN-EIIIA mRNA levels in both mProx24 and MCT cells (Figure 5, A–D), indicating that the glucocorticoid receptor binds to DEX and transduces signals into the nucleus to promote target transcription in both cell lines.
Figure 3.
DEX induction of CTGF mRNA expression in C57B6-derived mProx24 and SJL-derived MCT renal tubular epithelial cells. A: Glucocorticoid receptor mRNA expression in mProx24 and MCT cells. Dose dependency of DEX induction of CTGF mRNA expression at 3 hours in mProx24 (B) and MCT cells (C). Time course of DEX (1000 nmol/L) induction of CTGF mRNA expression in mProx24 (D) and MCT cells (E). Effect of DEX treatment (1000 nmol/L, 3 hours) on CTGF mRNA stability in mProx24 (F) and MCT cells (G). CTGF mRNA levels were normalized against GAPDH levels and expressed relative to the initial values. In both the mProx24 (F) and MCT cells (G), the rate of degradation of CTGF mRNA was significantly and similarly enhanced by DEX treatment. H: Effect of DEX treatment (1000 nmol/L, 3 hours) on CTGF mRNA levels in the primary cultured cells derived from C57B6 and SJL mice. The data in B to H were each obtained from four independent experiments.
Figure 4.
TGF-β1 induction of CTGF mRNA expression in mProx24 and MCT cells. Dose dependency of TGF-β1 induction of CTGF mRNA expression at 3 hours in mProx24 (A) and MCT cells (B). Time course of TGF-β1 (3 ng/ml) induction of CTGF mRNA expression in mProx24 (C) and MCT cells (D). E: Effect of TGF-β1 (3 ng/ml, 3 hours) on CTGF mRNA levels in the primary cultured cells derived from C57B6 and SLJ mice. The data in A to E were each obtained from four independent experiments.
Figure 5.
DEX induction of mRNA expression of angiotensinogen and FN-EIIIA in mProx24 and MCT cells. DEX (1000 nmol/L) induction of angiotensinogen mRNA in mProx24 (A) and MCT cells (B). DEX (1000 nmol/L) induction of FN-EIIIA mRNA in mProx24 (C) and MCT cells (D). The data in A to D were each obtained from four independent experiments.
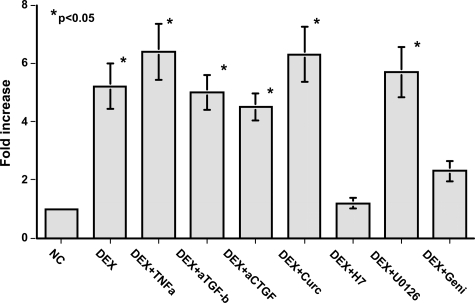
The induction of CTGF mRNA expression by TGF-β1 was attenuated by tumor necrosis factor (TNF)-α in fibroblasts, an effect that was blocked by the PKC inhibitor Go6983, the MEK inhibitor U0126, and the tyrosine kinase inhibitor genistein in mesangial cells. DEX induction of CTGF mRNA expression was not affected by TNF-α or U0126, or by neutralizing anti-TGF-β or anti-CTGF antibodies (Figure 6). However, the PKC inhibitor H7 or genistein significantly attenuated DEX induction of CTGF mRNA expression in mProx24 cells (Figure 6), suggesting that PKC and tyrosine kinase pathways played a role in this process.
Figure 6.
Effects of various substances on DEX induction of CTGF mRNA expression in mProx24 cells. Both the PKC inhibitor H7 and the tyrosine kinase inhibitor genistein significantly inhibited DEX induction of CTGF mRNA expression in mProx24 cells, suggesting the involvement of PKC and tyrosine kinase. However, treatment with TNF-α, neutralizing anti-TGF-β, and anti-CTGF antibodies, curcumin and U-0126, was without effects. The data were obtained from four independent experiments in each case.
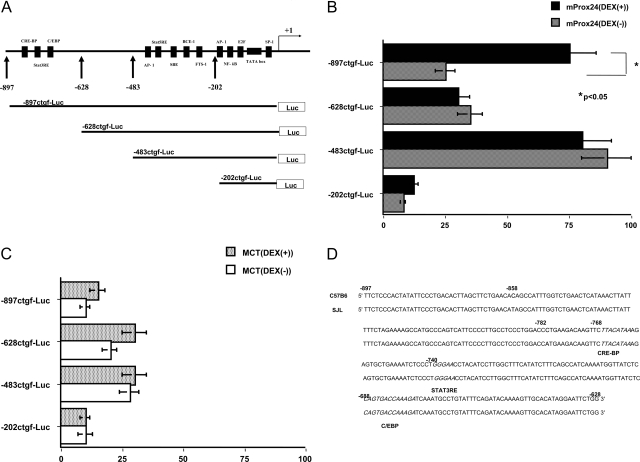
GenBank data analysis revealed a number of consensus motifs in the promoter sequence of the mouse CTGF gene (representatives are shown in Figure 7A), although it was found to lack consensus glucocorticoid responsive cis-elements (GRE). Transient transfection experiments with firefly luciferase reporter constructs bearing CTGF promoter fragments revealed that there were universal positive and negative regulatory elements in the −483- to −202-bp and −628- to −483-bp fragments, respectively, elements that were not reactive to DEX (Figure 7, B and C). In contrast, the −897- to −628-bp fragment was found to contain positive regulatory elements that were responsive to DEX but were active only in mProx24, but not MCT, cells (Figure 7, B and C). We also identified two polymorphisms in the genomic DNA sequence of the −897- to −628-bp fragment between C57B6 and SJL mice (at −858 and −782 bp; Figure 7D) that were outside of the consensus motifs (ie, STAT3RE at −740 to −736 bp, and C/EBP at −688 to −676 bp).
Figure 7.
DEX-responsive elements in the CTGF promoter. A: Consensus motifs in the mouse CTGF promoter and firefly luciferase reporter constructs. Promoter activity of CTGF promoter fragments with or without DEX treatment in mProx24 (B) and MCT cells (C). D: The genomic DNA sequence at the −897 to −628 bp of CTGF promoter. Polymorphisms between C57B6 and SJL mice are shown in bold (at −858 and −782 bp), and consensus motifs (STAT3RE and C/EBP) are shown in italic. The data in B and C were each obtained from four independent experiments.
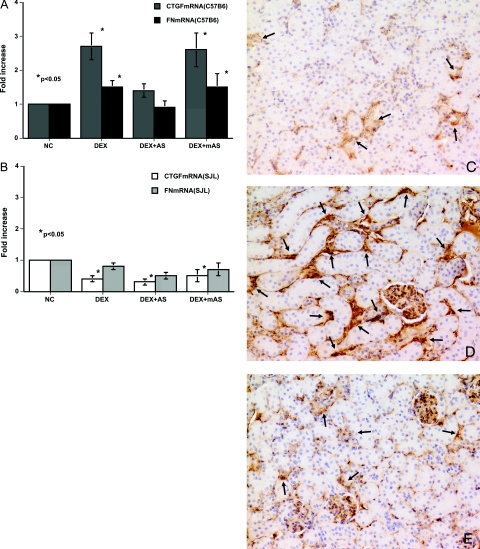
To determine the pathophysiological role of glucocorticoid in CTGF-mediated renal fibrogenesis, we treated glucocorticoid-susceptible (C57B6) and nonsusceptible (SJL) mice with DEX. Twelve hours after DEX treatment, both FN-EIIIA and CTGF mRNA levels were elevated in the kidneys of C57B6 mice (Figure 8B). Treatment of C57B6 mice with CTGF anti-sense ODN at the same time that they received DEX suppressed these elevations in both CTGF and FN-EIIIA mRNA levels (Figure 8A), supporting the notion that DEX-induced increases in CTGF mRNA levels indirectly resulted in elevations in FN-EIIIA mRNA levels. On the other hand, DEX treatment reduced CTGF and FN-EIIIA mRNA levels in SJL mice (Figure 8B). DEX treatment resulted in a significant induction in FN protein deposition in the kidneys of C57B6, but not SJL, mice 2 weeks after treatment (Figure 8D).
Figure 8.
Fibronectin deposition in the kidneys of DEX-treated animals is mediated by CTGF. DEX increased the mRNA expression of CTGF and FN-EIIIA in the kidneys of C57B6 (A) and SJL mice (B) after 12 hours. A: Co-treatment with DEX and CTGF-AS significantly inhibited DEX-induced CTGF and FN-EIIIA mRNA expression in the kidneys of C57B6 mice, but there were no significant effects by co-treatment with CTGF-mAS. B: DEX decreased CTGF and FN-EIIIA mRNA levels in the kidneys of SJL mice regardless of co-treatments. C: Minimal deposition of fibronectin protein (arrows) was seen in control C57B6 mouse kidneys (C57-NC). D: C57B6 mice that were treated for 2 weeks with DEX and CTGF-mAS (C57-mAS) displayed fibronectin protein deposition in their kidneys (arrows). E: In contrast, co-treatment of C57B6 mice with CTGF-AS (C57-AS) suppressed DEX-induced fibronectin protein deposition (arrows). Each of the data in A and B was obtained from four mice, and data in C to E are representative of the sections obtained from four mice in each case. DAB stain. Original magnifications, ×100.
Discussion
DEX induction of CTGF expression in the mouse kidney was previously reported by Dammeier.11 In our study, we found that tubular epithelial cells produced CTGF in response to DEX in vivo and in vitro. We also found the DEX-induced CTGF mRNA expression in the kidney is mouse strain-specific, with C57B6 mice responding to treatment in this way whereas SJL mice did not. A biopsy sample from a kidney of a minimal change nephrotic syndrome patient who had been treated with high doses of glucocorticoids revealed the presence of CTGF in tubular epithelial cells. This finding was not disease-specific because similar staining was demonstrated in the kidneys of glucocorticoid-treated lupus nephritis patients (data not shown). TGF-β1 is a strong inducer of CTGF in tubular epithelial cells8,19 as well as in a variety of cells such as fibroblasts, mesangial cells, endothelial cells, and chondrocytes3; TGF-β1/Smad-responsive regulatory elements were found in the CTGF promoter regions of some of these cells.20–24 In contrast, although CTGF induction by glucocorticoid has similarly been detected in fibroblasts, osteoblasts, and chondrocytes,11,25,26 the molecular mechanisms that control this process have not as yet been elucidated.
We were surprised to discover that the mouse strain-specific responsiveness to DEX that we uncovered was seemingly attributable to relatively simple genetics because F1 (C57B6 × SJL) mice showed responsiveness that was midway between the levels seen in responsive C57B6 mice and unresponsive SJL mice; DEX responsiveness was observed not only in renal cells but also in lung cells of C57B6 mice. It has been reported that glucocorticoid facilitates mRNA degradation and down-regulates the expression of some genes via the 3′-UTR.27 Similarly, DEX was shown to inhibit TNF-α gene expression via adenosine/uridine-rich elements in its 3′-UTR.28 Interestingly, the CTGF gene bears adenosine/uridine-rich elements in its 3′-UTR, and DEX shortened the half-life of CTGF mRNA to the same degree in both cell lines, suggesting that the observed differences in DEX-induced CTGF expression were regulated at the transcriptional level. Our luciferase reporter assay results suggested the presence of strain-specific regulatory elements at position −897 to −628 bp in the mouse CTGF gene promoter that were active in mProx24 (C57B6-derived) but not in MCT (SJL-derived) cells. Because both mProx24 and MCT cell lines had glucocorticoid receptors and increased their angiotensinogen and CTGF mRNA levels in response to DEX and rhTGF-β1, respectively, the presence of these regulatory elements likely accounted for the strain-specific CTGF gene responsiveness to DEX.
Regulatory elements that were constitutively active and responsive to TGF-β1 were found at −483 to +1 bp in the CTGF gene promoter,20–24 which was recognized as the prototypical CTGF promoter. The prototypical promoter in NIH3T3 fibroblasts reportedly responded to DEX, with the relevant DEX-responsive elements likely being present at −292 to +22 bp in the human CTGF gene promoter.26 The activity found at −897 to −628 bp of the CTGF gene promoter in response to DEX in mProx24 cells in our study has not been previously reported but is consistent with data collected in human chondrocytes, which demonstrated DEX-responsive elements at −802 to −292 bp in their CTGF gene promoter.26 The glucocorticoid receptor generally has three functional domains, ie, a DNA-binding domain, a transcriptional activity domain, and a ligand (glucocorticoid)-binding domain. The C-terminal ligand-binding domain undergoes a conformational change after its binding to glucocorticoid in the cytoplasm, enabling the glucocorticoid-glucocorticoid receptor complex to bind to a series of co-activator proteins, after which the glucocorticoid-glucocorticoid receptor complex enters the nucleus and binds to GRE to regulate target genes.27 In our study, DEX increased angiotensinogen mRNA expression in both cell lines, possibly via the GRE in its 5′ promoter sequence.18 However, 5′ and 3′ sequences of the mouse CTGF gene do not contain consensus GRE (half-) site sequences (GGTACAnnnTGTTCT).27 Instead, a CCAAT/enhancing binding protein site (C/EBP site; −688 to −676 bp) found in the −897- to −628-bp fragment is thought to tether GRE, to which the glucocorticoid-glucocorticoid receptor complex indirectly binds other transcriptional factors in a larger regulatory complex.27 Transcriptional cooperation with the glucocorticoid-glucocorticoid receptor complex was demonstrated to be specific for C/EBPα for CYP3A1,29 C/EBPβ for phosphoenolpyruvate carboxykinase,30 and β-casein.31 Induction of the transcription of these genes by glucocorticoid does not require binding of the glucocorticoid-glucocorticoid receptor complex to the consensus GRE. In addition, a STAT3-responsive element (−740 to −736 bp) exists in the −897 to −628 bp fragment of the mouse CTGF promoter, which may also be involved in the tethering of GRE because phosphorylated STAT3 associates with the glucocorticoid-glucocorticoid receptor complex to form a transactivating/signaling complex. This complex was reported to increase the transcription of α2-macroglobulin and fibrinogen genes via STAT3-responsive elements in their 5′ promoter sequences.32,33 PKC inhibitors and tyrosine kinase inhibitors universally inhibited the activity of the prototypical CTGF promoter in fibroblasts and mesangial cells20,21 as well as DEX induction of CTGF in the tubular epithelial cells in this study. If phosphorylated STAT3 is truly involved in this signaling process, then a tyrosine kinase most likely plays a direct role because it phosphorylates STAT3.34
The observation has often been made that sclerotic and fibrotic reactions occur at the same time that inflammatory and immune reactions subside in the diseased kidney after glucocorticoid treatment35; our above findings may provide a possible explanation for this. Such processes are likely mediated by high concentrations of glucocorticoids because patients with sustained exposure to low levels of glucocorticoids, such as those with Cushing’s syndrome and asthmatics treated with glucocorticoids, have not been reported to develop renal fibrosis. It is well established that organ fibrogenesis is significantly affected by genetic background, and the C57B6 mouse is recognized as a fibrosis-prone strain that is sensitive not only to DEX-induced renal fibrosis but also to TGF-β1-induced lung fibrosis.36 The fact that four of our six patients with minimal change nephrotic syndrome expressed CTGF in their kidneys after steroid pulse therapy suggests that, at the very least, Japanese individuals may be high responders to glucocorticoids. We are thus conducting experiments to find some differences in the postreceptive pathway after DEX binding between C57B6 and SJL mice and to locate C57B6 strain-specific DEX-responsive elements at −897 to −628 bp in the mouse CTGF promoter. Such information should contribute to our understanding of the genetics of fibrosis susceptibility.
Acknowledgments
We thank M. Otobe for her technical assistance.
Footnotes
Address reprint requests to Hiromichi Suzuki, M.D., Ph.D., Department of Nephrology, Saitama Medical School, 38 Morohongo, Moroyamamachi, Irumagun, Saitama 350-0495, Japan. E-mail: iromichi@saitamamed.ac.jp.
A portion of this work was presented at the 36th Annual Meeting of the American Society of Nephrology in St. Louis, MO, in 2004.
References
- Moussad EE, Brigstock DR. Connective tissue growth factor: what’s in a name? Mol Genet Metab. 2000;71:276–292. doi: 10.1006/mgme.2000.3059. [DOI] [PubMed] [Google Scholar]
- Takigawa M. CTGF/Hcs24 as a multifunctional growth factor for fibroblasts, chondrocytes and vascular endothelial cells. Drug News Perspect. 2003;16:11–21. doi: 10.1358/dnp.2003.16.1.829302. [DOI] [PubMed] [Google Scholar]
- Blom IE, Goldschmeding R, Leask A. Gene regulation of connective growth factor: new targets for antifibrotic therapy? Matrix Biol. 2002;21:473–482. doi: 10.1016/s0945-053x(02)00055-0. [DOI] [PubMed] [Google Scholar]
- Gupta S, Clarkson MR, Duggan J, Brady HR. Connective tissue growth factor: potential role in glomerulosclerosis and tubulointerstitial fibrosis. Kidney Int. 2000;58:1389–1399. doi: 10.1046/j.1523-1755.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- Riser BL, Denichilo M, Cortes P, Baker C, Grondini JM, Yee J, Narins RG. Regulation of connective tissue growth factor activity in cultured rat mesangial cells and its expression in experimental diabetic glomerulosclerosis. J Am Soc Nephrol. 2000;11:25–38. doi: 10.1681/ASN.V11125. [DOI] [PubMed] [Google Scholar]
- Yokoi H, Mukoyama M, Nagae T, Mori K, Suganami T, Sawai K, Yoshioka T, Koshikawa M, Nishida T, Takigawa M, Sugawara A, Nakao K. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J Am Soc Nephrol. 2004;15:1430–1440. doi: 10.1097/01.asn.0000130565.69170.85. [DOI] [PubMed] [Google Scholar]
- Okada H, Kikuta T, Kobayashi T, Inoue T, Kanno Y, Takigawa M, Sugaya T, Kopp JB, Suzuki H. Connective tissue growth factor expressed in tubular epithelium plays a pivotal role in renal fibrogenesis. J Am Soc Nephrol. 2005;16:133–143. doi: 10.1681/ASN.2004040339. [DOI] [PubMed] [Google Scholar]
- Inoue T, Okada H, Kobayashi T, Watanabe Y, Kanno Y, Kopp JB, Nishida T, Takigawa M, Ueno M, Nakamura T, Suzuki H. Hepatocyte growth factor counteracts transforming growth factor-β1, through attenuation of connective tissue growth factor induction, and prevents renal fibrogenesis in 5/6 nephrectomized mice. FASEB J. 2003;17:268–270. doi: 10.1096/fj.02-0442fje. [DOI] [PubMed] [Google Scholar]
- Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003;362:629–639. doi: 10.1016/S0140-6736(03)14184-0. [DOI] [PubMed] [Google Scholar]
- Remuzzi G, Ruggenenti P, Perico N. Chronic renal diseases: renoprotective benefits of renin-angiotensin system inhibition. Ann Intern Med. 2002;136:604–615. doi: 10.7326/0003-4819-136-8-200204160-00010. [DOI] [PubMed] [Google Scholar]
- Dammeier J, Beer HD, Brauchle M, Werner S. Dexamethasone is a novel potent inducer of connective tissue growth factor expression. J Biol Chem. 1998;273:18185–18190. doi: 10.1074/jbc.273.29.18185. [DOI] [PubMed] [Google Scholar]
- Takaya K, Koya D, Isono M, Sugimoto T, Sugaya T, Kashiwagi A, Haneda M. Involvement of ERK pathway in albumin-induced MCP-1 expression in mouse proximal tubular cells. Am J Physiol. 2003;284:F1037–F1045. doi: 10.1152/ajprenal.00230.2002. [DOI] [PubMed] [Google Scholar]
- Okada H, Danoff TM, Kalluri R, Neilson EG. Early role of Fsp1 in epithelial-mesenchymal transformation. Am J Physiol. 1997;273:F563–F574. doi: 10.1152/ajprenal.1997.273.4.F563. [DOI] [PubMed] [Google Scholar]
- Sheridan A, Schwartz J, Kroshian V, Tercyak A, Laraia J, Masino S, Lieberthal W. Renal mouse proximal tubular cells are more susceptible than MDCK cells to chemical anoxia. Am J Physiol. 1993;265:F323–F350. doi: 10.1152/ajprenal.1993.265.3.F342. [DOI] [PubMed] [Google Scholar]
- Okada H, Moriwaki K, Kalluri R, Imai H, Ban S, Takahama M, Suzuki H. Inhibition of monocyte chemoattractant protein-1 expression in tubular epithelium attenuates tubulointerstitial alteration in rat Goodpasture syndrome. Kidney Int. 2000;57:927–936. doi: 10.1046/j.1523-1755.2000.00909.x. [DOI] [PubMed] [Google Scholar]
- Gupta V, Wagner BJ. Expression of the functional glucocorticoid receptor in mouse and human lens epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:2041–2046. doi: 10.1167/iovs.02-1091. [DOI] [PubMed] [Google Scholar]
- Viedt C, Bruger A, Hansch GM. Fibronectin synthesis in tubular epithelial cells: up-regulation of the EDA splice variant by transforming growth factor β. Kidney Int. 1995;48:1810–1817. doi: 10.1038/ki.1995.479. [DOI] [PubMed] [Google Scholar]
- De Haij S, Adcock IM, Bakker AC, Gobin SJ, Daha MR, van Kooten C. Steroid responsiveness of renal epithelial cells. Dissociation of trans-repression and transactivation. J Biol Chem. 2003;278:5091–5098. doi: 10.1074/jbc.M209836200. [DOI] [PubMed] [Google Scholar]
- Inoue T, Okada H, Kobayashi T, Watanabe Y, Kikuta T, Kanno Y, Takigawa M, Suzuki H. TGF-β1 and HGF coordinately facilitate collagen turnover in subepithelial mesenchyme. Biochem Biophys Res Commun. 2002;297:255–260. doi: 10.1016/s0006-291x(02)02192-7. [DOI] [PubMed] [Google Scholar]
- Leask A, Holmes A, Black CM, Abraham DJ. Connective tissue growth factor gene regulation. J Biol Chem. 2003;278:13008–13015. doi: 10.1074/jbc.M210366200. [DOI] [PubMed] [Google Scholar]
- Chen Y, Blom IE, Sa S, Goldschmeding R, Abraham DJ, Leask A. CTGF expression in mesangial cells: involvement of SMADs, MAP kinase, and PKC. Kidney Int. 2002;62:1149–1159. doi: 10.1111/j.1523-1755.2002.kid567.x. [DOI] [PubMed] [Google Scholar]
- Grotendorst GR, Okochi H, Hayashi N. A novel transforming growth factor beta response element controls the expression of the connective tissue growth factor gene. Cell Growth Differ. 1996;7:469–480. [PubMed] [Google Scholar]
- Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, Leask A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem. 2001;276:10594–10601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Kimura Y, Tamura T, Ichikawa H, Yamaai Y, Sugimoto T, Takigawa M. Cloning of a mRNA preferentially expressed in chondrocytes by differential display-PCR from a human chondrocytic cell line that is identical with connective tissue growth factor mRNA. Biochem Biophys Res Commun. 1997;234:206–210. doi: 10.1006/bbrc.1997.6528. [DOI] [PubMed] [Google Scholar]
- Pereira RC, Durant D, Canalis E. Transcriptional regulation of connective tissue growth factor by cortisol in osteoblasts. Am J Physiol. 2000;279:E570–E576. doi: 10.1152/ajpendo.2000.279.3.E570. [DOI] [PubMed] [Google Scholar]
- Kubota S, Moritani NH, Kawaki H, Mimura H, Minato M, Takigawa M. Transcriptional induction of connective tissue growth factor/hypertrophic chondrocyte-specific 24 gene by dexamethasone in human chondrocytic cells. Bone. 2003;33:694–702. doi: 10.1016/s8756-3282(03)00227-8. [DOI] [PubMed] [Google Scholar]
- Schoneveld OJLM, Gaemers IC, Lamers WH. Mechanisms of glucocorticoid signalling. Biochim Biophys Acta. 2004;1680:114–128. doi: 10.1016/j.bbaexp.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis D, Pasparakis M, Pizarro TT, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- Rodrigues E, Vilarem MJ, Ribeiro V, Maurel P, Lechner MC. Two CCAAT/enhancer binding protein sites in the cytochrome P4503A1 locus. Eur J Biochem. 2003;270:556–564. doi: 10.1046/j.1432-1033.2003.03413.x. [DOI] [PubMed] [Google Scholar]
- Yamada K, Duong DT, Scott DK, Wang JC, Granner DK. CCAAT/enhancer-binding protein b is an accessory factor for the glucocorticoid response from the cAMP response element in the rat phosphoenolpyruvate carboxykinase gene promoter. J Biol Chem. 1999;274:5880–5887. doi: 10.1074/jbc.274.9.5880. [DOI] [PubMed] [Google Scholar]
- Wyszomierski SL, Rosen JM. Cooperative effects of STAT5 and C/EBPβ on β-casein gene transcription are mediated by the glucocorticoid receptor. Mol Endocrinol. 2005;15:228–240. doi: 10.1210/mend.15.2.0597. [DOI] [PubMed] [Google Scholar]
- Lerner L, Henriksen MA, Zhang X, Darnell JE., Jr STAT3-dependent enhancesome assembly and disassembly: synergy with GR for full transcriptional increase of the α2-macroglobulin gene. Gene Dev. 2003;17:2564–2577. doi: 10.1101/gad.1135003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller GM, Zhang Z. Transcriptional control mechanism of fibrinogen gene expression. Ann NY Acad Sci. 2001;936:469–479. doi: 10.1111/j.1749-6632.2001.tb03534.x. [DOI] [PubMed] [Google Scholar]
- Takeda T, Kurachi H, Yamamoto T, Nishio Y, Nakatsuji Y, Morishige K, Miyake A, Murata Y. Crosstalk between the interleukin-6 (IL-6)-JAK-STAT and the glucocorticoid-nuclear receptor pathway: synergistic activation of IL-6 response element by IL-6 and glucocorticoid. J Endocrinol. 1998;159:323–330. doi: 10.1677/joe.0.1590323. [DOI] [PubMed] [Google Scholar]
- Balow JE, Austin HAI, Muenz LR, Joyce KM, Antonovych TT, Klippel JH, Steinberg AD, Plotz PH, Decker JL. Effect of treatment on the evolution of renal abnormalities in lupus nephritis. N Engl J Med. 1984;311:491–495. doi: 10.1056/NEJM198408233110802. [DOI] [PubMed] [Google Scholar]
- Kolb M, Bonniaud P, Galt T, Sime PJ, Kelly MM, Margetts PJ, Gauldie J. Differences in the fibrogenic response after transfer of active transforming growth factor-β1 gene to lungs of “fibrosis-prone” and “fibrosis-resistant” mouse strains. Am J Respir Cell Mol Biol. 2002;27:141–150. doi: 10.1165/ajrcmb.27.2.4674. [DOI] [PubMed] [Google Scholar]