Abstract
Recent studies demonstrate roles for activation of caspases and cleavage of cellular proteins within neurons of the Alzheimer’s disease (AD) brain. To determine whether a similar role for caspases also occurs within glial cells in AD, we designed a site-directed caspase-cleavage antibody specific to glial fibrillary acidic protein (GFAP), a cytoskeleton protein specifically expressed in astrocytes. In vitro characterization of this antibody using both a cell-free system and a cell model system of apoptosis demonstrated that the antibody (termed GFAP caspase-cleavage product antibody or GFAP-CCP Ab) immunolabeled the predicted caspase-cleavage fragment, but not full-length GFAP, by Western blot analysis. To determine whether caspases cleave GFAP in vivo, tissue sections from control and AD brains were examined by immunohistochemistry using the GFAP-CCP Ab. Two prominent features of staining were evident: immunolabeling of degenerating astrocytes in proximity to blood vessels and staining within plaque-rich regions of the AD brain. Furthermore, co-localization of the GFAP-CCP Ab and an antibody specific to active caspase-3 was demonstrated within damaged astrocytes of the AD brain. These data suggest that the activation of caspases and cleavage of cellular proteins such as GFAP may contribute to astrocyte injury and damage in the AD brain.
Alzheimer’s disease (AD) is characterized by memory loss as well as difficulties with language, visuospatial abilities, and other cognitive domains.1,2 The diagnosis of AD is based on the extent of senile plaque and neurofibrillary tangle accumulation.3 Senile plaques contain the β-amyloid peptide (Aβ), and evidence suggests that Aβ deposition precedes neurofibrillary tangle formation and may be the earliest event that triggers subsequent downstream molecular events leading to neuronal death and synaptic loss.4 Aβ is known to be toxic to neurons, and one putative mechanism is through the activation of apoptosis.5,6 Apoptosis is characterized by plasma membrane blebbing, nuclear fragmentation, and cell shrinkage7 and is initiated by the proteolytic enzymes, caspases.8 Numerous studies have documented the activation of caspases in the AD brain as well as the cleavage of critical cellular proteins.9–13 In addition, recent studies have implicated the activation of caspases and cleavage of tau as early events in the disease process that may link Aβ and neurofibrillary tangle in AD.14–18 These studies suggest that it is the caspase-mediated cleavage of important cellular proteins, per se, and not the full execution of apoptosis that may be important for driving the pathology associated with AD.
Although a role for caspase activation within neurons of the AD brain has been established, whether a similar role can be attributed to caspases in astrocytes has not been explored. Astrocytes outnumber neurons in the brain and play many roles essential for normal brain function including ion buffering, glutamate uptake, and participation in the formation of the blood-brain barrier.19 During the progression of AD, astrocytes undergo both morphological and functional changes, giving rise to the term “reactive gliosis.” Reactive gliosis is characterized by the hypertrophy of astrocytes as well as by proliferation and up-regulation of the intermediate filament protein glial fibrillary acidic protein (GFAP).19 In AD, reactive astrocytes accumulate in the vicinity of senile plaques and may contribute to maturation from a diffuse to a cored plaque by releasing inflammatory mediators.20
Because previous studies have demonstrated a role for caspases in neurodegeneration and possible neurofibrillary tangle formation in AD, we hypothesized that a similar role for caspases may occur within astrocytes and contribute to astrocytic degeneration. The present study hypothesized that caspase-mediated cleavage of GFAP occurs selectively within reactive astrocytes of the AD brain. A site-directed antibody specific to a putative caspase-cleavage consensus site within GFAP was synthesized and tested for its utility in several in vitro model systems of apoptosis. Application of this antibody to AD brain sections revealed immunolabeling within damaged astrocytes in plaque-rich regions and along blood vessels of the AD brain that co-localized with active caspase-3. These findings not only underscore the involvement of caspases in astrocytic injury but may also provide a possible mechanism for why the blood-brain barrier is compromised in AD.21,22
Methods and Methods
Materials
Purified GFAP, recombinant human caspase-3 and -7, and staurosporine (SST) were purchased from Calbiochem (La Jolla, CA). The sulfolink coupling kit used to affinity purify antibodies was purchased from Pierce (Rockford, IL). β-Amyloid (1-42) (Aβ) peptide was from Biosource International Inc. (Camarillo, CA). Concanavalin type VI (Con A) was from Sigma (St. Louis, MO). The monoclonal anti-active caspase-3 antibody was from BD Pharmingen (La Jolla, CA). The mouse anti-GFAP antibody (mAb 3402) was purchased from Chemicon International (Temecula, CA). The mouse anti-6E10 (anti-Aβ) antibody was from Senetek PLC (Maryland Heights, MS). Z-Val-Ala-Ala-Asp (OMe)-FMK (Z-VAD) was from Enzyme Systems Products (Livermore, CA).
Generation of Polyclonal Antibodies
Two sets of polyclonal antibodies were synthesized based on a putative caspase cleavage consensus site (DLTD266) within GFAP: one to the amino-terminal upstream fragment and the other to the downstream carboxy-terminal cleavage fragment that would be generated after cleavage by caspases. For the amino-terminal site, a 16-mer peptide (CGGGGGGRSKFADLTD) corresponding to the upstream neoepitope fragment that would be generated after cleavage was synthesized, coupled to keyhole limpet hemocyanin and injected into rabbits. Resulting sera were used to affinity purify antibodies using a sulfolink column coupled with the peptide CRSKFADLTD. Peptide synthesis and generation of polyclonal antibodies was contracted out to Chemicon International. This antibody is referred to as amino terminal GFAP caspase-cleavage product (nGFAP-CCP) in the current study. For the carboxy-terminal site, an 8-mer peptide (AAARNAEC) was synthesized and injected into rabbits after conjugation to keyhole limpet hemocyanin. This antibody, termed cGFAP-CCP Ab, was purified using a sulfolink column coupled with the peptide AAARNAEC. For this antibody, synthesis of peptides, injections of immunogens, and collection of antisera were contracted out to Bethyl Laboratories (Montgomery, TX). Unless noted otherwise, the cGFAP-CCP Ab was used in all experiments.
Cell Culture
Human U-87 MG glioblastoma-astrocytoma cells (American Type Culture Collection, Manassas, VA) were grown in Eagle’s minimal essential medium (no. 30-2003, American Type Culture Collection) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin.
Preparation of Human Brain Lysates
Case demographics are presented in Table 2. Frozen human temporal cortex tissues from control or AD cases were homogenized in a Tris extraction buffer consisting of 1% sodium dodecyl sulfate and a protease inhibitor cocktail (catalog no. 158837; Biomedicals Inc.). After homogenization, samples were centrifuged for 1 hour at 4°C at 130,000 × g, and the resulting supernatant was collected (representing the soluble fractions). Protein content was measured using the BCA method (Pierce Biotechnology Inc.), and equal protein amounts were then analyzed by Western blot.
Table 2-6787.
Case Demographics for Western Blot Analysis
| Case | Group | Age (years) | Sex | PMI (hours) | Braak and Braak stage | MMSE |
|---|---|---|---|---|---|---|
| 1 | Control | 83 | M | 2.3 | II | N/A |
| 2 | Control | 88 | F | 5 | II | 29 |
| 3 | Control | 85 | F | 4.3 | III | 29 |
| 4 | Early AD | 81 | F | 7 | V | 23 |
| 5 | AD | 79 | M | 4.5 | V | Too impaired |
| 6 | AD | 96 | F | 3.3 | V | Too impaired |
| 7 | AD | 77 | M | 5 | VI | Too impaired |
| 8 | AD | 76 | F | 4.5 | VI | Too impaired |
PMI, post mortem interval; MMSE, Mini Mental State Examination.
Western Blot Analysis
Purified human GFAP, U-87 cell extracts, or human brain lysates were processed for Western blot analysis. Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose. Membranes were incubated in either cGFAP-CCP (1:500) or anti-GFAP (1:500), and primary antibody was visualized using either goat anti-rabbit or anti-mouse horseradish peroxidase-linked secondary antibody (1:5000; Jackson Laboratory, West Grove, PA), followed by enhanced chemiluminescence detection.
Cell-Free Digestion of GFAP
To examine whether executioner caspases can cleave GFAP, 15 μg of purified human GFAP was incubated with active human recombinant caspase-3 or -7 (at equivalent specific activities) in 2× reaction buffer containing 10 mmol/L dithiothreitol overnight at 37°C. Reactions were terminated by the addition of 5× sample buffer and analyzed by Western blot.
Treatment Protocols for U-87 Cell Experiments
Con A was made as a 25 μmol/L stock in serum-free medium and filter sterilized before use. SST was made as a 5 mmol/L stock in sterile dimethyl sulfoxide and diluted 1:100 in bovine serum albumin/phosphate-buffered saline before addition to cell cultures. Z-VAD was prepared as a 50 mmol/L stock in sterile dimethyl sulfoxide. To permit adequate cellular loading, Z-VAD was added 1 hour before insult. Fibrillar Aβ was prepared by freeze-thawing (three times) followed by incubation at room temperature overnight. After various treatments, U-87 cell extracts were prepared by adding ice-cold lysis buffer (50 mmol/L Tris-HCl, 150 mmol/L NaCl, 1% Nonidet P-40, 0.25% deoxycholate, 1 mmol/L EGTA, pH 7.4, and protease inhibitor cocktail), followed by centrifugation and addition of 5× sample buffer. For immunocytochemistry, cells were plated on poly-d-lysine/laminin-coated chamber slides (BD Biosciences), treated with various insults (SST, Con A, or Aβ), and then fixed in ice-cold methanol for 2 minutes. Fluorescence immunocytochemistry was as previously described23 using the cGFAP-CCP Ab (1:500). Bound primary antibody was detected using a biotinylated anti-rabbit ABC peroxidase kit (Vector Laboratories, Burlingame, CA) followed by visualization using a tyramide signal amplification kit (Molecular Probes, Eugene, OR) consisting of Alexa Fluor 488-labeled tyramide (excitation/emission = 495/519). To visualize apoptotic cells, the DNA intercalator propidium iodide was used, resulting in red fluorescence at 488 nm. For microscopic observation and photomicrography of fluorescently labeled cells, an Olympus BX60 fluorescence microscope equipped with a PM-10AD system for photomicrography was used.
Human Patients
Autopsy brain tissues from the hippocampus and entorhinal cortex of eight neuropathologically confirmed AD cases and six nondemented cases diagnosed as normal were studied. Five of six of the normal cases were identified as having diffuse senile plaques and/or mild Braak/Braak changes within the hippocampus and entorhinal cortex as a final neuropathological diagnosis. Case demographics are presented in Table 1. Age at death was not significantly different between AD (mean, 79.9 ± 5.74 years) and controls (mean, 81.3 ± 10.2 years). Human brain tissues used in this study were provided by the Institute for Brain Aging and Dementia Tissue Repositories at the University of California, Irvine.
Table 1.
Case Demographics for Immunohistochemical Analysis
| Case | Group | Age (years) | Sex | PMI | MMSE | Clinical diagnosis | Neuropathological diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | AD | 75 | M | 4.5 | 21 | Probable AD | AD |
| 2 | AD | 80 | F | 4 | 9 | Probable AD | AD |
| 3 | AD | 73 | M | 4 | 14 | Probable AD | AD |
| 4 | AD | 87 | F | 2.67 | 18 | Probable AD | AD |
| 5 | AD | 77 | M | 3.58 | 22 | Probable AD | AD |
| 6 | AD | 85 | M | 5.25 | 4 | Probable AD | AD |
| 7 | AD | 87 | F | 5 | 9 | Probable AD | AD |
| 8 | AD/PD | 75 | F | 2.5 | N/A | Probable AD/PD | AD/PD |
| 9 | Ctl | 84 | F | 7.1 | N/A | N/A | Normal (MPC) |
| 10 | Ctl | 74 | F | 2.75 | N/A | Normal | Normal (MPC) |
| 11 | Ctl | 68 | F | 6 | N/A | N/A | Normal (MPC) |
| 12 | Ctl | 77 | M | 6.5 | N/A | Normal | Normal (MPC) |
| 13 | Ctl | 90 | F | 6.5 | N/A | Normal | Normal (MPC) |
| 14 | Ctl | 95 | M | 3.67 | N/A | Normal | Normal |
PMI, postmortem interval; MMSE, Mini Mental State Examination; MPC, mild pathological changes consistent with Braak and Braak stage I/II; N/A, not available.
Immunohistochemistry, Immunofluorescence, and Confocal Microscopy
Free-floating 40-μm-thick serial sections were used for immunohistochemical and immunofluorescence studies as previously described.14 Antibody dilutions were the following: cGFAP-CCP or nGFAP-CCP (1:100), anti-GFAP (1:500), mAb 6E10 (anti-Aβ, 1:10,000). Antigen visualization was determined using ABC complex (ABC Elite immunoperoxidase kit, Vector Laboratories), followed by diaminobenzidine or Blue SG substrate (Vector Laboratories). For immunofluorescence studies, antigen visualization was accomplished using an Alexa Fluor 488-labeled tyramide (green, excitation/emission = 495/519) or streptavidin Cy-3 (red, 1:200). Confocal images were collected on an Olympus IX70 inverted microscope using both a ×20 and ×40 objective for image analysis and barrier filters at 510 and 605 nm.
Results
Characterization of the cGFAP-CCP Ab by Western Blot Analysis
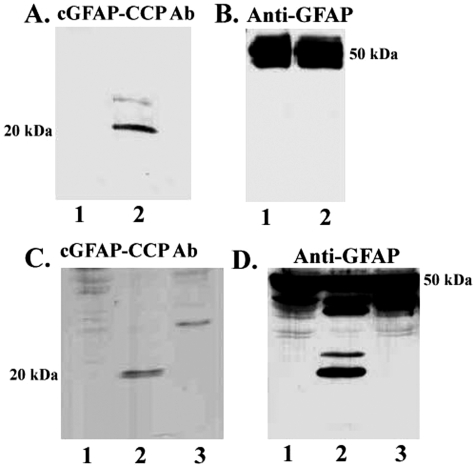
The goal of the present study was to determine whether GFAP, a 50-kd protein expressed specifically in astrocytes, is cleaved by caspases in the AD brain. Because caspases are specific in that they cleave only after aspartic residues,24 cleavage will reveal new, antigenically distinct sites. Examination of the GFAP protein sequence indicated a single putative caspase-cleavage consensus site, DLTD266, that would produce two major predicted fragments on cleavage: an amino-terminal fragment of ∼30 kd and a carboxy-terminal fragment of ∼20 kd. As an initial approach, we synthesized antibodies to the carboxy-terminal (C-terminal) caspase-cleavage site within GFAP and tested its validity as a specific probe for GFAP CCPs using a cell-free system. Purified human GFAP was incubated with or without caspase-3, and samples were immunoblotted with the cGFAP-CCP Ab. Although no immunoreactivity to the antibody was evident in nondigested samples (Figure 1A, lane 1), a prominent band at 20 kd was evident after digestion of GFAP with caspase-3 (Figure 1A, lane 2). To verify that full-length GFAP was indeed present, the same blots were stripped and reprobed with an anti-GFAP antibody that recognizes full-length GFAP. As shown in Figure 1B, strong immunolabeling was evident using this antibody indicating the presence of full-length GFAP in these samples. At longer exposures, we were able to faintly detect the 20-kd fragment of cleaved GFAP using this antibody (data not shown). These initial results suggest that the cGFAP-CCP Ab recognizes the C-terminal fragment of GFAP but does not immunoreact with full-length GFAP.
Figure 1-6787.
Characterization of cGFAP-CCP antibody by Western blot analysis. A and B: Cell-free system using purified human GFAP incubated without (lanes 1) or with caspase-3 (lanes 2). Samples were probed with cGFAP-CCP Ab (A) or a commercially available antibody to GFAP, which recognizes full-length GFAP (B). C and D: An in vitro model system consisting of U-87 glial cells treated with the apoptotic insult, SST (lanes 2), or SST and the caspase inhibitor, Z-VAD (lanes 3). Samples were probed as described for A and B.
To further characterize the cGFAP-CCP Ab, experiments were performed using human U-87 astrocytoma cells. U-87 cells were incubated overnight in the absence or presence of the classical apoptotic insult SST, and cell extracts were analyzed by Western blot using cGFAP-CCP Ab. A single band corresponding to ∼20 kd was evident after treatment of U-87 cells with SST (Figure 1C, lane 2). The appearance of this SST-induced cleavage fragment was prevented after pretreatment of cells with the caspase inhibitor Z-VAD (Figure 1C, lane 3). As in the cell-free system, the cGFAP-CCP Ab did not appear to strongly label full-length GFAP in U-87 cells even though there were ample levels of full-length GFAP present in these samples (Figure 1D). In addition, the anti-GFAP antibody strongly labeled several caspase cleavage fragments of GFAP, including one at 20 kd (Figure 1D, lane 2), that were completely prevented after preincubation of cells with the caspase inhibitor Z-VAD (Figure 1D, lane 3).
In Situ Detection of GFAP CCPs in a Model System of Apoptosis
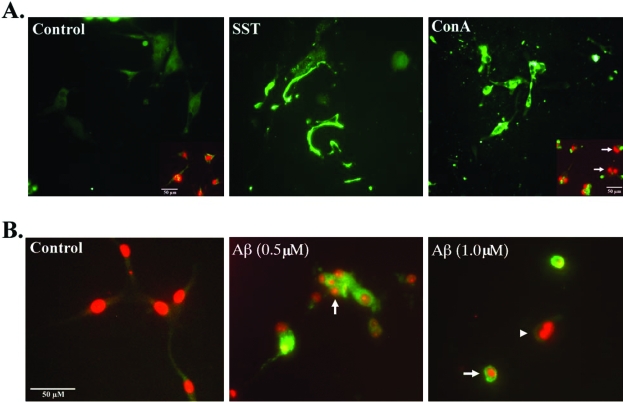
Experiments were performed to characterize the cGFAP-CCP Ab by immunocytochemistry. U-87 cells were treated with SST (500 nmol/L) or Con A (1 μmol/L), which have previously been demonstrated to be effective apoptotic stimuli.25,26 Treatment of U-87 cells with either SST or Con A resulted in the telltale morphological signs of apoptosis including cell shrinkage and nuclear condensation and fragmentation (Figure 2). Application of the cGFAP-CCP Ab resulted in little staining in nontreated cells (Figure 2A). In contrast, strong labeling of fragmented processes and cell bodies was apparent in SST- or Con A-treated cells (Figure 2A). Staining with the DNA intercalator propidium iodide indicated that labeled cells had condensed, fragmented nuclei in contrast to untreated cells (Figure 2A, inset). In a similar set of experiments, in situ detection of the cGFAP-CCP was evident after treatment of U-87 cells with Aβ (Figure 2B). U-87 cells treated with fibrillar Aβ exhibited features of apoptosis including cell shrinkage and nuclear condensation, actions of Aβ that have been previously reported in neuronal cells.27,28 The immunoreactivity distribution between SST-treated cells and that of Aβ-treated was different. Whereas cGFAP-CCP staining was more confined to the cell membrane in SST-treated cells, it appeared more cytoplasmic in Aβ-treated cells (Figure 2B). We are unsure of the reason for this difference, but it is possible that the resultant cell shrinkage after treatment of U-87 cells with Aβ may have contributed to the more limited distribution of cGFAP-CCP immunoreactivity.
Figure 2-6787.
Caspase-mediated cleavage of GFAP after treatment of U-87 glial cells with various apoptotic insults. A: U-87 glial cells were treated with SST (500 nmol/L) or Con A (1 μmol/L) for 24 hours, fixed in methanol, and immunostained with the cGFAP-CCP Ab (1:500, green). Insets represent double-immunofluorescence images with cGFAP-CCP Ab (green) and the nuclear stain propidium iodide (red). B: U-87 glial cells treated with fibrillar Aβ (1-42); at the indicated concentrations for 24 hours and subsequently immunostained with cGFAP-CCP (1:500, green) and propidium iodide (red). U-87 cells treated with Aβ exhibited condensed, fragmented nuclei (arrows) characteristic of apoptosis. Arrowhead indicates an apoptotic cell that did not label with the cGFAP Ab. Scale bars 50 μm.
Detection of GFAP CCPs in a Transgenic Mouse Model of AD
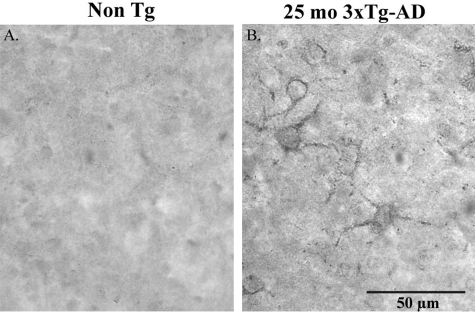
To further verify that cGFAP-CCP Ab can detect CCPs of GFAP, immunohistochemical analysis was performed using tissue sections from triple-transgenic mice (3xTg-AD). The 3xTg-AD mice develop Aβ and tau pathology that closely follows the pathological development of AD in human brain.29,30 In addition, by 18 months of age, 3xTg-AD mice begin to show signs of reactive gliosis in plaque-rich regions.29 No immunoreactivity to the cGFAP-CCP Ab was seen in non-TG control mice (Figure 3A). In contrast, staining of a subset of astrocytes with fragmented processes was observed in the cortex of 25-month-old 3xTg-AD mice after application of the cGFAP-CCP Ab (Figure 3B). Taken together, the results presented in Figures 1 to 3 support the conclusion that the cGFAP-CCP Ab is an effective marker for the detection of caspase-cleaved GFAP and therefore, may serve as a useful tool to examine caspase-mediated cleavage of GFAP in the human AD brain.
Figure 3-6787.
Evidence for caspase-mediated cleavage of GFAP within astrocytes in a triple-transgenic model of AD. Immunohistochemical analysis using the cGFAP-CCP Ab (1:100) revealed the presence of GFAP-CCP-positive astrocytes with fragmented processes in the entorhinal cortex of 3xTg-AD mice at 25 months of age (B), whereas no labeling was evident in non-Tg control mice (A). Scale bar, 50 μm.
The cGFAP-CCP Ab as a Marker for Caspase-Mediated Cleavage of GFAP in the AD Brain
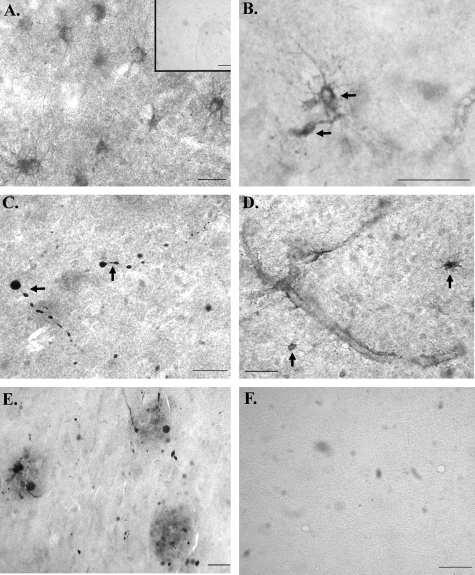
After confirmation of the cGFAP-CCP Ab as a specific probe for the C-terminal caspase-cleavage fragment of GFAP, immunohistochemical experiments were performed using hippocampal sections from AD or age-matched control brains. In control cases examined, we observed staining of astrocytes after application of the cGFAP-CCP Ab (Figure 4A). However, when staining was observed in a normal control case (defined as nondemented at the time of death) the neuropathological diagnosis was inevitably described as having senile degenerative changes with the presence of scattered diffuse senile plaques. In those control patients in which there was a total absence of AD pathology, there was a corresponding lack of immunoreactivity to the cGFAP-CCP Ab (Figure 4A, inset). In contrast, widespread labeling of degenerating astrocytes was observed in all AD brain sections examined after application of the cGFAP-CCP Ab (Figure 4, B–E). In general, staining with the cGFAP-CCP Ab was very heterogeneous in AD cases, for example occurring within beaded astrocytic processes, in cell bodies only, or within small fragmented processes. Evidence for cGFAP-CCP-positive astrocytes was found both in white and gray matter as well as in the hippocampus proper and entorhinal cortex. In AD cases, GFAP-CCP-positive astrocytes displayed many hallmark features of apoptosis including cell shrinkage, swollen varicosities, and fragmented processes (Figure 4, B and C). In addition, in AD sections there were two common features associated with cGFAP-CCP Ab immunoreactivity: staining along blood vessels (Figure 4D) and within plaques (Figure 4E). Figure 4F illustrates that staining with the cGFAP-CCP Ab was completely prevented after preabsorption with free peptide, illustrating the specificity of the cGFAP-CCP Ab.
Figure 4-6787.
cGFAP-CCP immunoreactivity in AD. A: Representative cGFAP-CCP staining in a normal case with a final neuropathological diagnosis of senile degenerative changes within hippocampus and amygdala. Inset of A is a representative staining from a control case characterized as having no histological changes as a final neuropathological diagnosis. B–E: Representative staining after application of the cGFAP-CCP Ab (1:100) in four different AD cases. Staining was evident within damaged astrocytes exhibiting beaded processes (B, C), along blood vessels (D), and within plaque-rich regions (E) of the AD brain. F: An AD case showing an absence of labeling after application with preadsorbed cGFAP-CCP antibody with immunizing peptide. Scale bars, 50 μm.
The presence of cGFAP-CCP Ab staining in association with senile plaques supports the hypothesis that extracellular Aβ serves as a stimulus to activate apoptotic pathways in astrocytes as they are recruited to plaque-rich areas. These results demonstrate that GFAP is cleaved by caspases in the AD brain and is confined to those astrocytes that appear damaged.
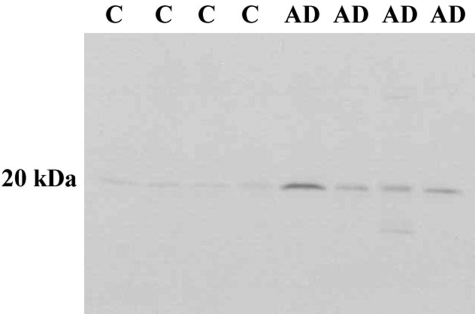
To confirm the immunohistochemical results, Western blot experiments were performed using temporal cortex brain lysates from control or AD brains (see Table 2 for case demographics). Cases were carefully selected based on MMSE (Mini Mental State Examination) scores to clearly define controls from ADs. As depicted in Figure 5, we were able to detect the C-terminal caspase-cleaved fragment of GFAP after application of the cGFAP-CCP Ab in four of four AD cases with only faint labeling of the same band in control cases. It is noteworthy the high degree of specificity exhibited by the cGFAP-CCP Ab by Western blot analysis, further supporting our immunohistochemical findings and suggesting that the C-terminal caspase-cleaved fragment of GFAP is present in the AD brain.
Figure 5-6787.
Detection of the C-terminal caspase-cleaved fragment of GFAP in the AD brain by Western blot analysis. Human brain lysates from control or AD cases were separated on 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, transferred to nitrocellulose, and then probed with the cGFAP-CCP Ab (1:500). A prominent band at ∼20 kd was detected in all four AD cases whereas only faint labeling was evident in control cases.
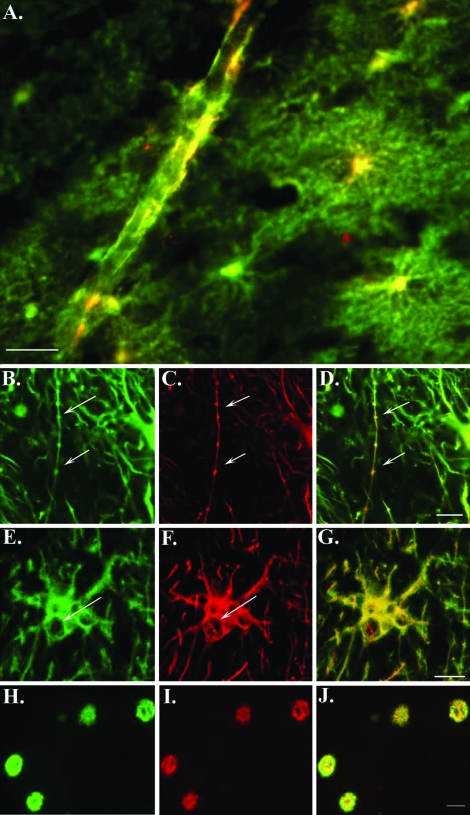
Double-label immunofluorescence experiments were also performed to verify that the labeling with the cGFAP-CCP Ab was indeed specific for astrocytes. AD sections were double-labeled with the cGFAP-CCP Ab and the anti-GFAP antibody, which labels full-length GFAP. Co-localization of both antibodies was evident along blood vessels (Figure 6A), within beaded processes (Figure 6, B–D; arrows) and in astrocytes containing what appeared to be autophagic cytoplasmic vacuoles (Figure 6, E–G; arrows). Although co-localization was evident, there was a clear spatial resolution between the two antibodies in labeled structures, indicating recognition of distinct epitopes within GFAP. Additionally, we were able to determine the co-localization of caspase-cleaved GFAP within Aβ plaques after double-label immunofluorescence with the cGFAP-CCP Ab and the monoclonal antibody 6E10, which detects Aβ (Figure 6, H–J). These data provide further support for the caspase-mediated cleavage of GFAP within damaged astrocytes in the AD brain.
Figure 6-6787.
Caspase-cleaved GFAP is found predominantly within damaged astrocytes and plaque-rich regions of the AD brain. A: Overlap immunofluorescence image using a monoclonal antibody to full-length GFAP (1:500, green) and cGFAP-CCP (1:100, red). Yellow/orange coloring indicates areas where markers are overlapping. Labeling is evident along a blood vessel and within several degenerating astrocytes in the hippocampal region of the AD brain. B–D: Double-label confocal immunofluorescence images with full-length GFAP antibody (1:500, green), cGFAP-CCP (1:100, red), and the two images overlapped in D. Arrows denote labeling within a beaded astrocytic process. E–G: Full-length GFAP antibody (1:500, green), cGFAP-CCP (1:100, red), and the two images overlapped in G. Arrows denote vacuoles within a GFAP-CCP-positive astrocyte. H–J: Double-label immunofluorescence images for 6E10 (anti-Aβ, 1:10,000, green) and GFAP-CCP (1:100, red) illustrating co-localization of GFAP caspase cleavage products together with Aβ in plaque-rich regions of the entorhinal cortex in AD (overlap, J). Scale bars, 50 μm (A, H–J); 10 μm (B–G).
Caspase-Cleaved GFAP Co-Localizes with Active Caspase-3 in the AD Brain
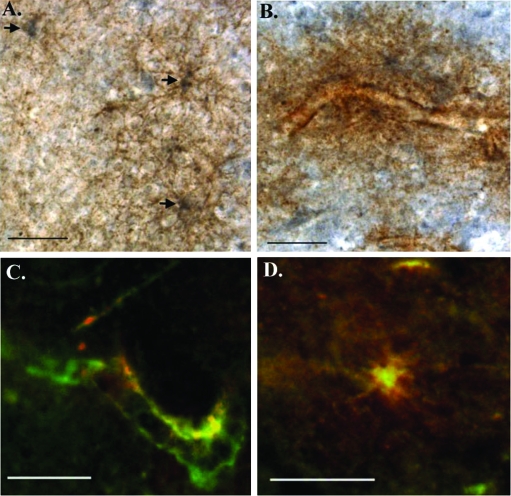
The in vitro experiments performed in Figures 1 and 2 suggest that caspase-3 may cleave GFAP, generating a 20-kd fragment that is detected by the cGFAP-CCP Ab. To examine a possible relationship between cleaved GFAP and caspase-3 activation in the AD brain, double-labeling experiments were performed using an anti-active caspase-3 antibody and cGFAP-CCP Ab. As shown in Figure 7, co-localization of these two markers was evident within damaged astrocytes along blood vessels. In general, the staining pattern revealed by these experiments consisted of labeling of the cGFAP-CCP Ab within cell bodies whereas that of active caspase-3 was more homogeneous being found both in the cell body and in astrocytic processes (Figure 7, A and D). In addition, many of the astrocytes appeared to have damaged processes that were fragmented or disintegrating (Figure 7, A, B, and D). These data showing a relationship of cGFAP-CCP immunoreactivity with a marker for apoptosis support the hypothesis that GFAP is indeed cleaved by caspases in the AD brain and this cleavage event occurs within damaged astrocytes.
Figure 7-6787.
Co-localization of active caspase-3 with caspase-cleaved GFAP in damaged astrocytes of the AD brain. A and B: Double-labeling immunohistochemical analysis demonstrating co-localization of active caspase-3 (brown, diaminobenzidine) and cGFAP CCP (blue, SG) within astrocytes of the AD brain. Arrows in A denote the labeling of three astrocytes showing labeling of cGFAP CCP mainly within the cell body and active caspase-3 in damaged processes. C and D: Overlap immunofluorescence images using a monoclonal antibody to active caspase-3 (1:500, green) and cGFAP-CCP (1:100, red). Yellow/orange coloring indicates areas where markers are overlapping. Co-localization of the two antibodies is evident along a blood vessel (C) and within a damaged astrocyte (D) of the hippocampus. Scale bars, 50 μm.
The Amino-Terminal Fragment of Caspase-Cleaved GFAP Is Present in the AD Brain
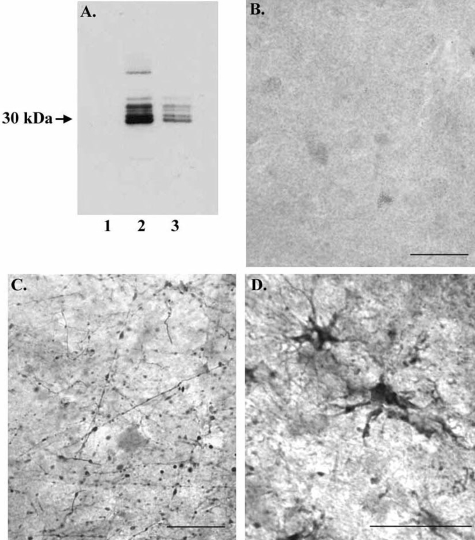
In a final set of experiments, we determined whether the upstream, amino-terminal (N-terminal) fragment of GFAP, nGFAP-CCP, could be detected in the AD brain. To accomplish this, an antibody was designed to the upstream neoepitope region that would be revealed after cleavage of GFAP at D266 (see Materials and Methods). To confirm that this antibody, termed nGFAP-CCP Ab, preferentially recognizes the N-terminal fragment, Western blot analysis was performed after digestion of full-length GFAP with either caspase-3 or -7. In this case, the nGFAP-CCP Ab labeled the predicted 30-kd fragment after GFAP digestion (Figure 8A, lanes 2 and 3) but did not immunoreact with full-length GFAP (Figure 8A, lane 1). The nGFAP-CCP Ab labeled several bands ∼30 kd, most likely representing incomplete cleavage fragments of GFAP. Application of the nGFAP-CCP Ab to control or AD brain sections gave similar results to those of the cGFAP-CCP Ab: a relative paucity of staining in control cases with a total absence of any AD pathology (Figure 8B) and prominent labeling of damaged astrocytes exhibiting beaded processes in AD cases (Figure 8, C and D).
Figure 8-6787.
Evidence for the amino-terminal caspase-cleaved GFAP fragment in AD. A: Characterization of the nGFAP-CCP Ab by Western blot analysis. Purified human GFAP (15 μg) was incubated in the absence (lane 1) or presence of either caspase-3 (lane 2) or caspase-7 (lane 3) for 24 hours, separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels, transferred to nitrocellulose, and then probed with an antibody directed toward the amino-terminal caspase-cleavage site DLTD266 of GFAP. The antibody did not react with full-length GFAP (lane 1) but did immunolabel the predicted 30-kd fragment after cleavage by executioner caspases (lanes 2 and 3). B–D: Application of the nGFAP-CCP Ab (1:100) to a control case (A) or two different AD cases (C, D) revealed staining of damaged astrocytes with beaded processes. Scale bars, 50 μm.
Discussion
In the present study, we demonstrate that executioner caspases can cleave GFAP in vitro and that the resulting N- and C-terminal fragments could be detected using two new site-directed caspase-cleavage antibodies. In addition, identification of the C-terminal GFAP CCP was found in astrocytes in culture after incubation of U-87 cells with apoptotic stimuli and Aβ. Lastly, using these GFAP-CCP antibodies, we observed caspase-cleavage fragments of GFAP in damaged astrocytes that co-localized with active caspase-3 in the AD brain.
The few available studies that have examined a role for apoptosis in astrocytes in AD have been contradictory and, for the most part, inconclusive. Thus, although some studies have supported a role for apoptosis occurring in astrocytes in the AD brain,31,32 others have not.33,34 Evidence for apoptotic cell death in all of these studies consisted of in situ detection of fragmented DNA by terminal dUTP nick-end labeling techniques. Interpretation of terminal dUTP nick-end labeling-positive labeling is a challenge and has come under criticism as to whether or not it is a specific marker for apoptosis,15,35 possibly contributing to the contradictory conclusions regarding whether apoptosis occurs in astrocytes of the AD brain.
An alternative approach for detecting apoptosis is to detect the activation of caspases or their target protein fragments after cleavage. Because caspases are specific in that they cleave only after aspartic residues, cleavage reveals new antigentically distinct sites. These consensus caspase-cleavage sites, therefore, represent desirable targets for cleavage site-directed antibodies. Using such antibodies, numerous studies have demonstrated the activation of caspases10,14,36,37 and the caspase-mediated cleavage of important neuronal proteins in the AD brain, including actin,12 fodrin,11 the amyloid precursor protein,9 and tau.14,16 In the present study, we designed a caspase-cleavage site-directed antibody to GFAP, a protein specifically expressed in astrocytes. Examination of the GFAP sequence revealed a single caspase consensus sequence, DLTD266, based on the general tetrapeptide motif DXXD recognized by caspases.8 Based on this site, we synthesized an antibody to the downstream predicted fragment that would be generated after cleavage of full-length GFAP by caspases at this site. After characterization of this antibody, application to AD brain sections resulted in the labeling of astrocytes that were damaged and displayed features characteristic of apoptosis.
Staining with the cGFAP-CCP Ab occurred within cell bodies, in beaded processes and/or in fragmented processes and co-localized with active caspase-3. It is important to note that the demonstration of caspase-mediated cleavage of GFAP and the activation of caspase-3 do not imply that astrocytes are dying through apoptosis. Indeed, an alternative explanation is that the cleavage of GFAP by caspases reflects the turnover of GFAP in reactive astrocytes, rather than overt apoptosis. This alternative should be considered based on the fact that apoptotic bodies are not observed frequently in sections of the AD brain, and considerable controversy exists on whether cells are in fact dying by this pathway.15 In support of this is the fact that many processes labeled by the GFAP-CCP Abs were not fragmented but were intact and not degenerated. However, the morphological appearance of GFAP-CCP-labeled astrocytes suggested that these cells were severely damaged and exhibited features characteristic of apoptosis. Thus, many of the cGFAP-CCP-positive astrocytes had a punctate appearance with the processes reduced to clusters or lines of dots radiating outward from the cell body. Moreover, many of the labeled astrocytes contained what appeared to be autophagic cytoplasmic vacuoles, another feature indicative of apoptosis. Therefore, taken together, our results suggest that caspase-mediated cleavage of GFAP, activation of apoptotic pathways and degeneration of astrocytes may be linked. This hypothesis is consistent with a previous report that demonstrated a role for astrocytic degeneration, activation of caspase-3, and DNA fragmentation in frontotemporal dementia.38
Evidence for caspase-mediated cleavage of GFAP was also found in control cases, although labeled astrocytes were comparatively less damaged and occurred in those cases that had a final neuropathological diagnosis of senile degenerative changes with mild Braak/Braak changes. The appearance of GFAP-CCP-positive astrocytes in control cases should not be surprising considering that age-related glial changes, which likely represent the normal wear and tear of the aging process, occur in normal elderly individuals.39
An additional finding of the present study was the labeling of GFAP-CCP-positive astrocytes along blood vessels in the AD brain. Many of these labeled astrocytes exhibited processes that were damaged in appearance. One role for astrocytes is in forming the blood-brain barrier. Astrocytes confer a protective role on the blood-brain barrier against hypoxia and aglycemia by extending numerous, long cytoplasmic processes that terminate in end feet and encapsulate brain capillaries.40 Therefore, astrocytes in this role allow the blood-brain barrier to act as a physical and metabolic barrier between the central nervous system and the systemic circulation, regulating and protecting the microenvironment of the brain. Damage of astrocytic processes after activation of apoptotic pathways and cleavage of cytoskeletal proteins, such as GFAP as demonstrated in the present study, may be one of many factors that contribute to the compromised blood-brain barrier observed in AD.21,41
In summary, caspase-mediated cleavage of GFAP, activation of caspase-3, and damage to astrocytes were common features found together in the AD brain. The link between reactive gliosis, caspase activation, and cytoskeletal protein cleavage may arise from extracellular Aβ acting to stimulate the activation of apoptotic pathways within astrocytes. The consequences of caspase activation and cleavage of cytoskeletal proteins in astrocytes may lead to the degeneration of astrocytes and loss of blood-brain barrier integrity. Astrocyte degeneration may not only be a common feature found in certain neurodegenerative diseases, including AD and frontotemporal dementia, but may also represent normal age-related processes, although to a lesser degree.
Footnotes
Address reprint requests to Troy T. Rohn, Department of Biology, Science/Nursing Building, Room 228, Boise State University, Boise, ID 83725. E-mail: trohn@boisestate.edu.
Supported by the National Institutes of Health (National Center for Research Resources grant P20RR016454 to T.T.R.) and the Alzheimer’s Disease Research Center, University of California at Irvine (grant P50 AG16573).
References
- Morris JC, Mohs RC, Rogers H, Fillenbaum G, Heyman A. Consortium to establish a registry for Alzheimer’s disease (CERAD) clinical and neuropsychological assessment of Alzheimer’s disease. Psychopharmacol Bull. 1988;24:641–644. [PubMed] [Google Scholar]
- Carter J, Lippa CF. Beta-amyloid, neuronal death and Alzheimer’s disease. Curr Mol Med. 2001;1:733–737. doi: 10.2174/1566524013363177. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brounlee LM, Vogel FS, Hughes JP, VanBelle G, Berg L. The consortium to establish a registry for Alzheimer’s disease (CERAD) part II. Standardization of the neuropathological assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Cummings BJ, Cotman CW. Beta-amyloid induces neuritic dystrophy in vitro: similarities with Alzheimer pathology. Neuroreport. 1992;3:769–772. doi: 10.1097/00001756-199209000-00012. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Anderson AJ. A potential role for apoptosis in neurodegeneration and Alzheimer’s disease. Mol Neurobiol. 1995;10:19–45. doi: 10.1007/BF02740836. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson DW. Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 1999;6:1028–1042. doi: 10.1038/sj.cdd.4400598. [DOI] [PubMed] [Google Scholar]
- Gervais FG, Xu D, Robertson GS, Vaillancourt JP, Zhu Y, Huang J, LeBlanc A, Smith D, Rigby M, Shearman MS, Clarke EE, Zheng H, Van Der Ploeg LH, Ruffolo SC, Thornberry NA, Xanthoudakis S, Zamboni RJ, Roy S, Nicholson DW. Involvement of caspases in proteolytic cleavage of Alzheimer’s amyloid-beta precursor protein and amyloidogenic A beta peptide formation. Cell. 1999;97:395–406. doi: 10.1016/s0092-8674(00)80748-5. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Head E, Nesse WH, Cotman CW, Cribbs DH. Activation of caspase-8 in the Alzheimer’s disease brain. Neurobiol Dis. 2001;8:1006–1016. doi: 10.1006/nbdi.2001.0449. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Head E, Su JH, Anderson AJ, Bahr BA, Cotman CW, Cribbs DH. Correlation between caspase activation and neurofibrillary tangle formation in Alzheimer’s disease. Am J Pathol. 2001;158:189–198. doi: 10.1016/S0002-9440(10)63957-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Sun X, Beech W, Teter B, Wu S, Sigel J, Vinters HV, Frautschy SA, Cole GM. Antibody to caspase-cleaved actin detects apoptosis in differentiated neuroblastoma and plaque-associated neurons and microglia in Alzheimer’s disease. Am J Pathol. 1998;152:379–389. [PMC free article] [PubMed] [Google Scholar]
- Su JH, Kesslak JP, Head E, Cotman CW. Caspase-cleaved amyloid precursor protein and activated caspase-3 are co-localized in the granules of granulovacuolar degeneration in Alzheimer’s disease and Down’s syndrome brain. Acta Neuropathol (Berl) 2002;104:1–6. doi: 10.1007/s00401-002-0548-2. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Rissman RA, Davis MC, Kim Y-E, Cotman C, Head E. Caspase-9 activation and caspase cleavage of tau in the Alzheimer’s disease brain. Neurobiol Dis. 2002;11:341–354. doi: 10.1006/nbdi.2002.0549. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Rissman RA, Head E, Cotman CW. Caspase activation in the Alzheimer’s disease brain: tortuous and torturous. Drug News Perspect. 2002;15:549–557. doi: 10.1358/dnp.2002.15.9.740233. [DOI] [PubMed] [Google Scholar]
- Rissman RA, Poon WW, Blurton-Jones M, Oddo S, Reidun T, Vitek MP, LaFerla FM, Rohn TT, Cotman CW. Caspase-cleavage of tau is an early event in Alzheimer’s disease pathology. J Clin Invest. 2004;114:121–130. doi: 10.1172/JCI20640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Albrecht S, Bourdeau M, Petzke T, Bergeron C, LeBlanc AC. Active caspase-6 and caspase-6-cleaved tau in neuropil threads, neuritic plaques, and neurofibrillary tangles of Alzheimer’s disease. Am J Pathol. 2004;165:523–531. doi: 10.1016/S0002-9440(10)63317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamblin TC, Chen F, Zambrano A, Abraha A, Lagalwar S, Guillozet AL, Lu M, Fu Y, Garcia-Sierra F, LaPointe N, Miller R, Berry RW, Binder LI, Cryns VL. Caspase cleavage of tau: linking amyloid and neurofibrillary tangles in Alzheimer’s disease. Proc Natl Acad Sci USA. 2003;100:10032–10037. doi: 10.1073/pnas.1630428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Unger JW. Glial reaction in aging and Alzheimer’s disease. Microsc Res Tech. 1998;43:24–28. doi: 10.1002/(SICI)1097-0029(19981001)43:1<24::AID-JEMT4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- Farkas E, Luiten PG. Cerebral microvascular pathology in aging and Alzheimer’s disease. Prog Neurobiol. 2001;64:575–611. doi: 10.1016/s0301-0082(00)00068-x. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Cusack SM, Kessinger SR, Oxford JT. Caspase activation independent of cell death is required for proper cell dispersal and correct morphology in PC12 cells. Exp Cell Res. 2004;295:215–225. doi: 10.1016/j.yexcr.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Nicholson DW, Thornberry NA. Caspases: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- Cribbs DH, Kreng VM, Anderson AJ, Cotman CW. Crosslinking of membrane glycoproteins by concanavalin A induces apoptosis in cortical neurons. Neuroscience. 1996;75:173–185. doi: 10.1016/0306-4522(96)80001-p. [DOI] [PubMed] [Google Scholar]
- Rohn TT, Ivins KJ, Bahr BA, Cotman CW, Cribbs DH. A Monoclonal antibody to amyloid precursor protein induces neuronal apoptosis. J Neurochem. 2000;74:2331–2342. doi: 10.1046/j.1471-4159.2000.0742331.x. [DOI] [PubMed] [Google Scholar]
- Ivins KJ, Thornton PL, Rohn TT, Cotman CW. Neuronal apoptosis induced by beta-amyloid is mediated by caspase-8. Neurobiol Dis. 1999;6:440–449. doi: 10.1006/nbdi.1999.0268. [DOI] [PubMed] [Google Scholar]
- Loo DT, Copani A, Pike CJ, Whittemore ER, Walencewicz AJ, Cotman CW. Apoptosis is induced by beta-amyloid in cultured central nervous system neurons. Proc Natl Acad Sci USA. 1993;90:7951–7955. doi: 10.1073/pnas.90.17.7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- LaFerla FM, Oddo S. Alzheimer’s disease: abeta, tau and synaptic dysfunction. Trends Mol Med. 2005;11:170–176. doi: 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Hayashi M, Nakano H, Fukutani Y, Sasaki K, Shimazaki M, Koshino Y. Apoptosis of astrocytes with enhanced lysosomal activity and oligodendrocytes in white matter lesions in Alzheimer’s disease. Neuropathol Appl Neurobiol. 2002;28:238–251. doi: 10.1046/j.1365-2990.2002.00390.x. [DOI] [PubMed] [Google Scholar]
- Li WP, Chan WY, Lai HW, Yew DT. Terminal dUTP nick end labeling (TUNEL) positive cells in the different regions of the brain in normal aging and Alzheimer patients. J Mol Neurosci. 1997;8:75–82. doi: 10.1007/BF02736774. [DOI] [PubMed] [Google Scholar]
- Brown WR, Moody DM, Thore CR, Challa VR. Cerebrovascular pathology in Alzheimer’s disease and leukoaraiosis. Ann NY Acad Sci. 2000;903:39–45. doi: 10.1111/j.1749-6632.2000.tb06348.x. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Reeves M, McKinney M. Topographic associations between DNA fragmentation and Alzheimer’s disease neuropathology in the hippocampus. Neurochem Int. 1997;31:275–281. doi: 10.1016/s0197-0186(96)00158-1. [DOI] [PubMed] [Google Scholar]
- Charriaut-Marlangue C, Ben-Ari Y. A cautionary note on the use of the TUNEL stain to determine apoptosis. Neuroreport. 1995;7:61–64. [PubMed] [Google Scholar]
- Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Bruck W, Jellinger K, Lassmann H. Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer’s disease. Evidence for apoptotic cell death. Am J Pathol. 1999;155:1459–1466. doi: 10.1016/S0002-9440(10)65460-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JH, Zhao M, Anderson AJ, Srinivasan A, Cotman CW. Activated caspase-3 expression in Alzheimer’s and aged control brain: correlation with Alzheimer pathology. Brain Res. 2001;898:350–357. doi: 10.1016/s0006-8993(01)02018-2. [DOI] [PubMed] [Google Scholar]
- Martin JA, Craft DK, Su JH, Kim RC, Cotman CW. Astrocytes degenerate in frontotemporal dementia: possible relation to hypoperfusion. Neurobiol Aging. 2001;22:195–207. doi: 10.1016/s0197-4580(00)00231-1. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26:349–354. doi: 10.1016/j.neurobiolaging.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC, Wegiel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging. 2004;25:663–674. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Berzin TM, Zipser BD, Rafii MS, Kuo-Leblanc V, Yancopoulos GD, Glass DJ, Fallon JR, Stopa EG. Agrin and microvascular damage in Alzheimer’s disease. Neurobiol Aging. 2000;21:349–355. doi: 10.1016/s0197-4580(00)00121-4. [DOI] [PubMed] [Google Scholar]