Abstract
Capillaries expressing the laminin α2 chain in basement membranes may be considered early developing vessels in normal and neoplastic human tissues. Therefore, we investigated whether up-regulation of this extracellular matrix protein favors transendothelial migration of neoplastic cells and then metastasis. In lung small and large cell neuroendocrine carcinomas, which exhibit a stronger metastatic tendency among carcinomas, laminin α2 chain-positive vessels were more numerous than in carcinoid tumors and supraglottis, breast, and lung non-small cell carcinomas, suggesting a direct relationship between these vessels and metastasis. In vitro studies showed that epidermal growth factor (EGF) induced a more efficient migration of the AE-2 lung neuroendocrine carcinoma cell line through the purified laminin α2 chain rather than through the laminin β1 chain and fibronectin. AE-2 cells constitutively expressed all EGF receptors and the α6β1 integrin, which is one of the laminin α2 chain receptors. EGF up-regulated α6β1 expression in several tumors. In this regard, we show that EGF increased the chemo-kinetic migration of AE-2 cells through EAHY endothelial monolayers, which was inhibited by the anti-α6 integrin chain monoclonal antibody. These data indicate that laminin α2 chain and α6β1 may be mutually involved in EGF-dependent migration of AE-2 cells and that laminin α2 chain-positive vessels may favor metastasis of EGF-dependent tumors.
Metastasis is the leading cause of death in cancer patients and involves a complex multistep process including detachment of tumor cells from the primary cancer, invasion of surrounding tissue, entry into the circulatory system, reinvasion, and proliferation at a distant secondary site. A wide variety of factors contributing to the spread of tumor cells includes cytokines, hormones, growth factors, cell adhesion molecules, and extracellular matrix proteins (ECMPs) such as laminins. Laminins are a family of α-β-γ heterotrimeric ECMPs, commonly present in basement membranes of the epithelium and endothelium. These molecules promote a number of functions in normal and neoplastic tissues including cell adhesion and migration via integrins, cell proliferation, differentiation, and cell shape.1 More than 12 isoforms are presently known and can be distinguished by their arrangements of α, β, and γ subunits, physical properties, and tissue and cell distribution; they are differentially recognized by several integrins.1–6 Some epithelial laminin isoforms provide specific contributions to promote local tumor invasion, as reported for laminin-10 in lung carcinomas,3 laminin γ2 chain in esophageal carcinomas,5 and laminin-5 in other human solid tumors.6 Laminin α2 chain represents the α2 chain of laminin-21; in normal human tissues the distribution of the laminin α2 chain is restricted to the sarcolemma, nerve sheaths, placenta, and basement membranes of small vessels of the central nervous system. Moreover, in reactive nonneoplastic conditions, a proportion of capillaries of granulation tissue consists of endothelial cells and basement membranes positive for the laminin α2 chain.1 In neoplastic conditions this ECMP is expressed in hemangiomas. α1β1, α2β1, α3β1, α6β1, α6β4, and α7β1 represent the integrin receptors for this ECMP.4,7–12 Furthermore, we have previously demonstrated in glioblastoma multiforme that solid glomeruloid endothelial cell proliferations consist of endothelial cells and basement membranes that, respectively, produce and contain the laminin α2 chain.13 The restriction of the laminin α2 chain expression to endothelial sprout-like structures, such as solid endothelial cell proliferations, and to early developing capillary-like structures, such as those of granulation tissue,1,14 suggested that the up-regulation of this ECMP might be related to early phases of development of new vessels and, therefore, it might be considered as a marker of early angiogenesis.13,14 We have also supported this hypothesis by providing evidence that, on early endothelial single cell cultures, gene and protein expression of the laminin α2 chain was stronger and present in a greater number of cells than for the laminin β1 chain.14 Furthermore, we have reported that in supraglottis carcinomas, laminin α2 chain-positive vessels were distributed either in the stroma or in neoplastic parenchyma, in close contact with neoplastic cells producing vascular endothelial growth factor (VEGF); moreover, in breast and non-small cell lung carcinomas these vessels were predominantly distributed in the stroma where mononuclear cells produce VEGF, fibroblast growth factor-2 (FGF2), and transforming growth factor-β1. These data suggested again that laminin α2 chain-positive vessels may represent early developing vascular structures in human solid tumors.14 It has been reported that angiopoietin 2 and VEGF increase permeability of the endothelium of previously existing and newly formed vessels during angiogenesis15; moreover, remodeling of ECMPs in basement membranes of vessels is observed during angiogenesis, and penetration of newly-formed vessels occurs during tumor invasion and metastasis. In this regard, the metastatic tendency of several human solid tumors has been directly related to the number of cells producing VEGF rather than to the number of vessels present in the neoplastic tissue.16,17 Therefore, we investigated whether and how laminin α2 chain expression during angiogenesis may favor transendothelial migration of neoplastic cells and, possibly, metastasis. To address in vitro studies on the prognostic significance of laminin α2 chain-positive vessels we have evaluated ex vivo, at tissue level, the presence of these vessels in lung small cell carcinomas (SCCs) and lung large cell neuroendocrine carcinomas (LCNCs), which represent a human solid tumor with a rate of metastasis that is higher than other carcinomas.18–20 Moreover, we have performed test migration assays on a lung neuroendocrine carcinoma cell line, named AE-2, and on a breast carcinoma cell line from the American Type Culture Collection, Rockville, MD, named MDA-MB231, through a purified laminin α2 chain and other ECMPs present in vascular basement membranes to establish their role in transendothelial migration of neoplastic cells and, therefore, in metastasis.
Materials and Methods
Tissues and Procedures
Tissues
Four atypical carcinoid tumors (Table 1), five SCCs, and four LCNCs (Table 2) were collected at surgery during frozen section procedures. Small samples from these tumors were immediately embedded in OCT compound, snap-frozen in liquid nitrogen to avoid RNA degradation, and stored at −80°C. Tumors were classified and graded according to the 1999 World Health Organization nomenclature.21
Table 1.
Laminin α2 Chain-Positive Vessels in Atypical Carcinoid of the Lung
| Case | VD | FVIIIRA | α2 | α5 | α6 | β1 | β4 | Fibronectin | Tenascin | Laminin β1 chain | Laminin α2 chain | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SV | 15 | 100 | 0 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 23 |
| PV | 85 | 70 | 0 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 50 | |
| Neoplastic cells | 0 | 5 | 0 | 2 | 100 | 0 | 0 | 0 | 0 | 0 | ||
| 2 | SV | 10 | 100 | 0 | 100 | 100 | 0 | 70 | 100 | 100 | 100 | 10 |
| PV | 90 | 80 | 0 | 100 | 30 | 0 | 100 | 100 | 100 | 100 | 30 | |
| Neoplastic cells | 0 | 2 | 0 | 5 | 100 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | SV | 10 | 100 | 0 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 10 |
| PV | 90 | 70 | 0 | 100 | 100 | 0 | 90 | 100 | 100 | 100 | 100 | |
| Neoplastic cells | 0 | 4 | 0 | 4 | 100 | 0 | 0 | 0 | 0 | |||
| 4 | SV | 100 | 0 | 100 | 100 | 100 | 0 | 100 | 100 | 100 | 25 | |
| PV | 100 | 80 | 0 | 100 | 85 | 0 | 85 | 100 | 100 | 100 | 25 | |
| Neoplastic cells | 0 | 5 | 0 | 6 | 100 | 0 | 0 | 0 | 0 | 0 | ||
The results are expressed as percentage of positive vessels of 200 vessels counted.
VD, vascular distribution; SV, stromal vessels; PV, parenchymal vessels.
Table 2.
Laminin α2 Chain-Positive Vessels in Large Cell Neuroendocrine Carcinomas (LCNCs) and in Small Cell Carcinomas (SCCs) of the Lung
| Case | VD | FVIIIRA | α2 | α5 | α6 | β1 | β4 | Fibronectin | Tenascin | Laminin β1 chain | Laminin α2 chain | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 LCNC | SV | 20 | 100 | 0 | 20 | 100 | 0 | 100 | 100 | 100 | 100 | 25 |
| PV | 80 | 85 | 0 | 10 | 100 | 0 | 100 | 100 | 100 | 100 | 40 | |
| Neoplastic cells | 0 | 5 | 0 | 15 | 100 | 0 | 0 | 0 | 0 | 0 | ||
| 2 LCNC | SV | 20 | 100 | 0 | 20 | 100 | 0 | 100 | 100 | 100 | 100 | 20 |
| PV | 80 | 85 | 0 | 10 | 100 | 0 | 100 | 100 | 100 | 100 | 35 | |
| Neoplastic cells | 0 | 5 | 0 | 15 | 100 | 0 | 0 | 0 | 0 | 0 | ||
| 3 LCNC | SV | 20 | 100 | 0 | 20 | 100 | 0 | 100 | 100 | 100 | 100 | 100 |
| PV | 80 | 85 | 0 | 10 | 100 | 0 | 100 | 100 | 100 | 100 | 100 | |
| Neoplastic cells | 0 | 5 | 0 | 25 | 100 | 0 | 0 | 0 | 0 | 0 | ||
| 4 LCNC | SV | 25 | 100 | 0 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 10 |
| PV | 75 | 75 | 0 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 30 | |
| Neoplastic cells | 0 | 7 | 0 | 20 | 100 | 0 | 0 | 0 | 0 | 0 | ||
| 5 SCC | SV | 10 | 100 | 0 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 30 |
| PV | 90 | 75 | 0 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 80 | |
| Neoplastic cells | 0 | 6 | 0 | 15 | 100 | 0 | 0 | 0 | 0 | 0 | ||
| 6 SSC | SV | 10 | 100 | 0 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 10 |
| PV | 90 | 100 | 0 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 50 | |
| Neoplastic cells | 0 | 4 | 0 | 25 | 100 | 0 | 0 | 0 | 0 | 0 | ||
| 7 SSC | SV | 35 | 100 | 0 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 0 |
| PV | 65 | 30 | 0 | 30 | 100 | 0 | 100 | 100 | 100 | 100 | 30 | |
| Neoplastic cells | 0 | 5 | 0 | 60 | 100 | 0 | 0 | 0 | 0 | 0 | ||
| 8 SSC | SV | 20 | 100 | 0 | 100 | 100 | 0 | 25 | 100 | 100 | 100 | 60 |
| PV | 80 | 80 | 0 | 70 | 100 | 0 | 0 | 100 | 100 | 100 | 100 | |
| Neoplastic cells | 0 | 6 | 0 | 25 | 100 | 0 | 0 | 0 | 0 | 0 | ||
| 9 SSC | SV | 30 | 100 | 0 | 100 | 100 | 0 | 100 | 100 | 100 | 100 | 70 |
| PV | 70 | 20 | 0 | 30 | 100 | 0 | 20 | 100 | 100 | 100 | 100 | |
| Neoplastic cells | 0 | 5 | 0 | 20 | 100 | 0 | 0 | 0 | 0 | 0 | ||
The results are expressed as percentage of positive vessels of 200 vessels counted.
VD, vascular distribution; SV, stromal vessels; PV, parenchymal vessels.
Immunohistochemistry
Five-μm-thick cryostat sections were cut from neoplastic tissue. The sections were fixed in acetone, preincubated with normal serum to prevent nonspecific binding, and incubated with optimal dilutions of the following monoclonal antibodies specific for FVIIIRA, CD31 (Dako A/S, Glostrup, Denmark), fibronectin (clone120.5, IgG1mouse), tenascin (cloneT2, IgG1mouse), α2 (CD49b), α5 (CD49e), α6 (CD49f) (from Immunotech, Marseilles, France), laminin α2 chain (merosin M chain) (clone5H2, IgG1 mouse), and laminin clone 4E10 IgG1 κ mouse (MAB1921), which detects a conformational epitope of B1 heterodimer (β1 chain) (Chemicon International Inc., Temecula, CA). The immunoreaction products were developed using the avidin-biotin-peroxidase complex method. Negative control sections were obtained after incubation with nonimmune isotype Ig of the same class of each antibody and by omission of the primary antibody. Slides were counterstained with hematoxylin and mounted for microscopic examination. The expression of the antigens was evaluated independently by two investigators.
Evaluation of Vascularity and ECMP Expression
The number and distribution of tumor vessels were evaluated on contiguous sections and immunostained using immunohistochemistry with monoclonal antibodies specific for endothelial markers such as von Willebrand factor, FVIIIRA, CD31, and endothelial integrin chains such as α4 (CD49d), α5 (CD49e), α6 (CD49f), and β4 (from Immunotech). Moreover, distribution of tumor vessels was cross-checked using monoclonal antibodies specific for fibronectin (clone120.5, IgG1mouse), tenascin (cloneT2, IgG1mouse) (from Immunotech), laminin α2 chain (merosin M chain, clone5H2, IgG1 mouse), and laminin (β1chain, clone 4E10 IgG1 κ mouse) MAB1921 (from Chemicon International Inc.), which are known to be ECMPs present in endothelial basement membranes. Therefore, vascularity in neoplastic tissues was expressed as the relative number of vessels distributed in the stroma and parenchyma of a total of 200 vessels counted in randomly chosen fields at ×400 magnification.
Cell Lines and Procedures
EAHY Endothelial Cell Line
EAHY endothelial cells22 (1 × 106/ml) were maintained in 250-ml plastic tissue culture flasks in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS), sodium bicarbonate solution, l-glutamine, HAT, and penicillin-streptomycin and were incubated in 5% CO2 and 95% air at 37°C.
AE-2 Cell Line
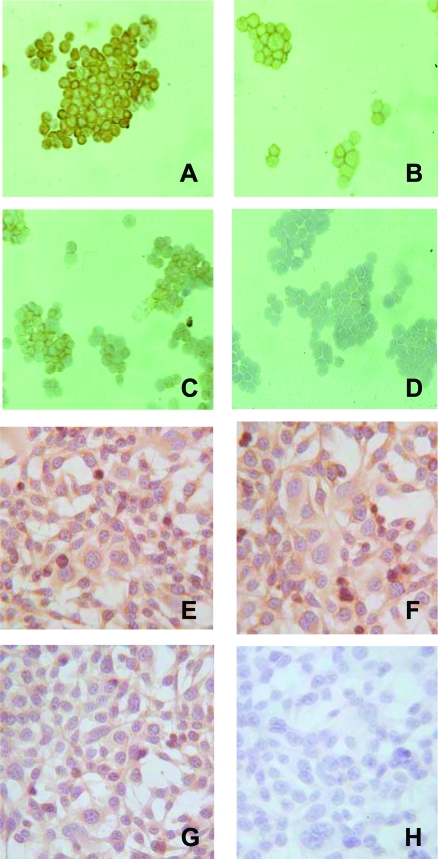
AE-2 is the name given to a neuroendocrine lung carcinoma cell line that was kindly provided by Dr. Pier Giorgio Natali (Istituto Regina Elena, Rome, Italy). This cell line consists of epithelial cells measuring 20 to 35 μm in size at their greatest dimension and present a high nucleus:cytoplasm ratio. The AE-2 cell line was maintained in 250-ml plastic tissue culture flasks in RPMI supplemented with 10% FCS, and incubated in 5% CO2 and 95% air at 37°C. The AE-2 cells are characterized by a weak adhesion to plastic; therefore they float in the medium either as single cells or as clusters resembling rosette-like structures (Figure 3). The cells may be suspended in the medium using a light trypsin treatment.
Figure 3.
Cytosmears of AE-2 cells immunostained with mAbs specific for chromogranin A (A), NCAM (B), α6 integrin chain (C), and laminin α2 chain (D), using the ABC method, and counterstained with hematoxylin. AE-2 cells display a granular, cytoplasmic staining for chromogranin A (A); moreover, they display cell membrane immunoreactivity for NCAM (B) and α6 integrin chain (C). AE-2 cells are laminin α2 chain-negative (D). Adherent MDA-MB231 cells immunostained with mAbs specific for α2 integrin chain (E), α5 integrin chain (F), α6 integrin chain (G), and laminin α2 chain (H), using the ABC method, and counterstained with hematoxylin. MDA-MB231 cells display cell membrane immunoreactivity for all these integrins and are laminin α2 chain-negative. Original magnifications, ×250.
MDA-MB231 and MDA-MB435
MDA-MB231 and MDA-MB435 cell lines were obtained from American Type Culture Collection, Rockville, MD. Cells were maintained in DMEM containing 10% FCS. Cells were lysed using RIPA buffer (1% Triton X-100, 0.5% deoxycholic acid, 0.05% sodium dodecyl sulfate, 200 mmol/L NaCl, 25 mmol/L Tris, pH 7.4, 1 mmol/L phenylmethyl sulfonyl fluoride, 1 mmol/L NaOV4).
Antigen Expression on AE-2 and MDA-MB231 Cell Lines
AE-2 and MDA-MB231 cell lines were maintained in RPMI supplemented with 10% FCS, washed twice in phosphate-buffered saline (PBS), and then suspended at 1 × 106 cells/ml in RPMI medium alone. AE-2 and MDA-MB231 cell suspensions were immediately used to prepare cytosmears using a Shandon Cytospin 3. The cytosmears were dried overnight at room temperature and then fixed in acetone, preincubated with normal serum to prevent nonspecific binding, and incubated with optimal dilutions of monoclonal antibodies specific for cytokeratin (clone MNF116), NCAM (neuronal cell adhesion molecule), chromogranin A, CD31 (from Dako A/S), fibro-nectin (clone120.5, IgG1 mouse), tenascin (cloneT2, IgG1mouse), α2 (CD49b), α3 (CD49c), α4 (CD49d), α5 (CD49e), α6 (CD49f) (from Immunotech), and laminin α2 chain (merosin M chain, clone 5H2, IgG1), laminin (β1chain, clone 4E10, IgG1 κ mouse, catalogue number MAB1921; Chemicon International Inc.). The immunoreaction products were developed using the avidin-biotin-peroxidase complex method. Negative controls were obtained after incubation with nonimmune Ig of the same isotype class of each specific antibody and by omission of the primary antibody. The slides were counterstained with hematoxylin and mounted for microscopic examination. The expression of antigens was evaluated independently by two investigators (Figure 3).
Western Blotting Analysis of Epidermal Growth Factor (EGF) Receptors on AE-2 and MDA-MB231 Cell Lines
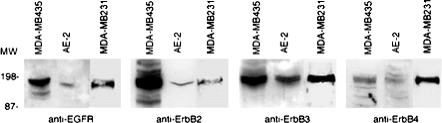
For Western blotting analysis, 100 μg of protein lysate derived from MDA-MB435 (control), AE-2, and MDA-MB231 cell lines were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as previously described.23 After electrophoresis, the proteins were transferred to a nitrocellulose membrane at 40 V for 1 hour. Membranes were treated with specific polyclonal antibodies (1:100) specific for EGFR, ErbB2, ErbB3, and ErbB4, purchased from Santa Cruz Biotechnology, Santa Cruz, CA. After washing, membranes were incubated with horseradish peroxidase conjugated with goat anti-rabbit serum (Santa Cruz Biotechnology). Bound antibody was visualized by a Supersignal West Pico chemiluminescence kit (Pierce, Rockford, IL) (Figure 4).
Figure 4.
Expression of ErbB receptors in the AE-2 cell line. Western blotting analysis demonstrates low levels of expression of EGFR, ErbB2, and ErbB4 and a discrete amount of ErbB3. MDA-MB231 cells display a good expression of EGFR, Erb-B3, and Erb-B4, and only a detectable expression of Erb-B2. MDA-MB435 cells were used as a positive control.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis of Gene Expression Specific for EGF Receptors on AE-2 Cell Line
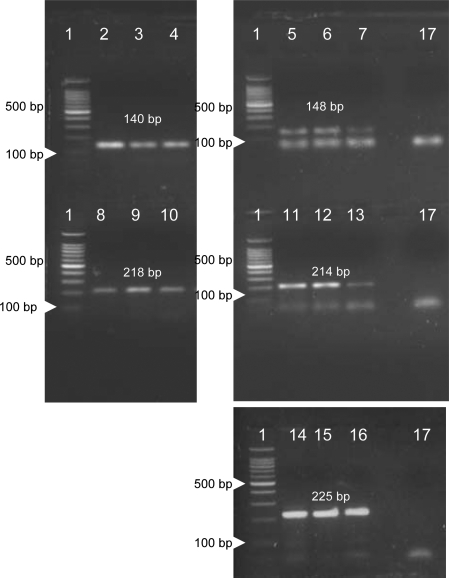
AE-2 cell suspensions (1 × 106/ml) were maintained in 250-ml plastic tissue culture flasks in RPMI supplemented with 50 ng/ml EGF or with 10% FCS (control), and incubated in 5% CO2 and 95% air at 37°C. Ten-ml samples of EGF-treated and of non-EGF-treated AE-2 cells were harvested after 30 and 90 minutes in culture, were centrifuged and the AE-2 cell pellets were homogenized by using 1 ml of the RNA Fast RNA isolation system (Molecular System Co., San Diego, CA). The amount of the RNA yield was measured by optical density reading, and only RNA samples showing an A260/A280 ratio from 1.8 to 2.0 were used to obtain cDNA. cDNA samples were generated by reverse transcription of 10 μg of total RNA in a solution containing 10 mmol/L Tris-HCl (pH 8.3 at room temperature), 1.5 mmol/L MgCe2, 100 μg/ml of bovine serum albumin, fraction V, a mixture of four dNTPs, at a concentration of 2.5 mmol/L each, oligo (dT) primers (5 μg/ml), 20 U of placental RNase inhibitor, 100 U of Moloney murine leukemia virus reverse transcriptase and H2O up to a 20-μl final total volume. The number of 40 cycles of PCR was assessed to synthesize the optimal yield product using 3 μl of the cDNA solutions, obtained from the RT products, and optimal concentration of forward and reverse oligonucleotide primers as reported by NCBI on-line service primer information: RH12420-EGFR (140 bp): 5′: TCG,GTG,TAA,ACG,TTG,CAA,AA and 3′:GAC,CAC,GGA,GGA,TAG,TAT,GAG,C; GDB:181407-Erb-B2 (148 bp): 5′: TCC,GTT,TCC,TGC,AGC, AGT,CTC,CGC,A and 3′: AGA,GAG,CCA,GCC,CTC,TGA,CGT,CCA,T; STS-M34309-Erb-B3 (218 bp): 5′: AAT,TCT,TAT,GGT,ATG,TAG,CCA,GC and 3′:TTG,ACA,GTC,TGA,TGG,GAA,AC; RH68995-Erb-B4 (214 bp): 5′: ACC,TGG,CAG,ATA,CTC,AGA,AAT,G and 3′: CAT,AGT,CCC,TGG,ATA,CCG,TTG; M10277-human β-actin (225 bp): 5′: AGC,ACA,GAG,CCT,CGC,CTT,TG and 3′: CGC,CCA,CAT,AGG,AAT,CCT,TC.
PCR assays for β-actin were performed as a control to assess the cDNA yield obtained from each RNA sample enrolled in the present study. The 50-μl PCR solution contained optimal MgCl2 (from 1 to 2.5 mmol/L) and dNTP concentrations, previously tested dilutions of both up- and downstream oligonucleotide primers and 0.3 U of TaqDNA polymerase (no. 801.0046; Perkin-Elmer-Cetus). The size of the PCR products was evaluated by electrophoresis on a 2.5% agarose gel (Figure 5).
Figure 5.
RT-PCR analysis of the AE-2 cell line cultured in non-EGF conditioned medium (lanes 2, 5, 8, 11, and 14) and in EGF-conditioned medium for 30 minutes (lanes 3, 6, 9, 12, and 15) and 90 minutes (lanes 4, 7, 10, 13, and 16). Lane 1: Marker, bp 100. Gene expression for β-actin was detected in AE-2 cells in all conditions under investigation (lanes 14–16). Lane 17: Negative controls. Both EGF-untreated and EGF-treated AE-2 cells show gene expression for Erb-B1 (lanes 2–4), Erb-B2 (lanes 5–7), Erb-B3 (lanes 8–10), and Erb-B4 (lanes 11–13).
Kinetics of Laminin α2 Chain, Laminin β1 Chain, and FVIIIRA Expression in the Adherent EAHY Endothelial Cell Line
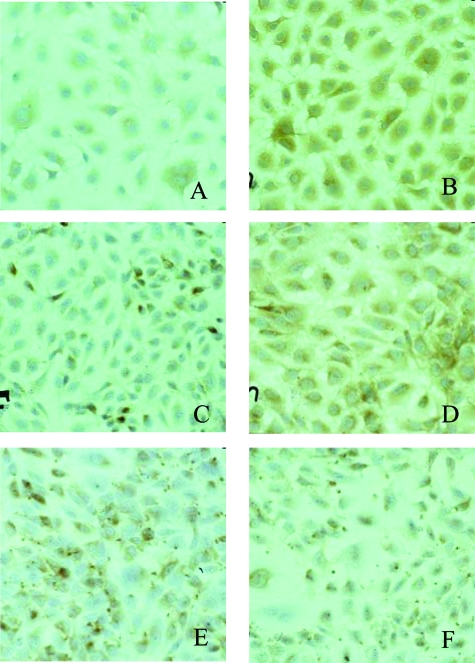
As previously reported,14 aliquots of cells (1 × 106/ml) were harvested in three different assays and then transferred onto tissue chamber slides with nonsupplemented DMEM, supplemented DMEM, supplemented DMEM and 10 ng/ml of VEGF, supplemented DMEM and 10 ng/ml of FGF2, supplemented DMEM and 10 ng/ml of VEGF and FGF2. Tissue chamber slides were harvested after 1, 3, 18, 24, 48, and 96 hours in culture for immunocytochemical analysis. The proportion of adherent cells immunostained for the laminin α2 chain, laminin β1 chain, and FVIIIRA was determined by counting 200 cells at ×400 magnification in randomly chosen fields. According to the results of this kinetic study, after 18 hours in culture adhering EAHY cells were at the same time confluent, laminin α2 chain-positive and laminin β1 chain-negative. Therefore, this time of culture was chosen to obtain EAHY-coated filters in Transwell chambers to perform test migration assays on the AE-2 line (Figure 6).
Figure 6.
Time course of positive immunostaining for laminin β1 chain (A, C, E) and laminin α2 chain (B, D, F) in EAHY single cell cultures after 5 (A, B), 24 (C, D), and 48 (E, F) hours. At the indicated time periods living cultures were fixed, immunostained, counterstained with hematoxylin, and photographed. Staining for laminin α2 chain was cytoplasmic, diffuse, and stronger than that for laminin β1 chain at the earliest time intervals, acquiring a dot-spot appearance after 48 hours in culture.
Immunofluorescence and Fluorescence-Activated Cell Sorting (FACS) Analysis of Laminin α2 Chain Expression in the Nonadherent EAHY Endothelial Cell Line
Confluent EAHY cells were trypsinized, washed, suspended with supplemented DMEM with 10 ng/ml of VEGF at the concentration of 1.5 × 106 cells per ml and transferred into polycarbonate tubes. Therefore, these endothelial cells were treated for different time periods (3, 12, 18, and 24 hours) with 10 ng/ml of VEGF, harvested, and then aliquots were incubated with an appropriate dilution of anti-laminin α2 chain monoclonal antibody (merosin M chain), clone 5H2-IgG1, laminin (β1chain; clone 4E10 IgG1 κ mouse, catalogue number MAB1921; Chemicon International Inc.). After washing, cells were stained with fluorescein isothiocyanate-conjugated goat anti-mouse IgG and fluorescence was analyzed by a FACScalibur cytofluorimeter (BD Biosciences, San Jose, CA) following the observation of 10,000 events using CellQuest software.
Migration Test Assays of AE-2 and MDA-MB231 Cell Lines through Purified ECMPs
Four independent assays were performed as described.24 In brief, blind well chemotaxis chambers (Boyden chamber) with 13-mm diameter polyvinylpyrrolidone-free polycarbonate filters, 8-μm pore size, were used. The filters were coated with human laminin-purified protein (catalog no. AG56P, Chemicon International Inc.), which consists of a mixture of β1 chains from human laminins, principally laminin-10. This preparation is immunologically and biologically identical to intact human laminins. Moreover, these polyvinylpyrrolidone-free filters were coated with purified laminin α2 chain (catalogue no. CC085, Chemicon International Inc.), and purified human fibronectin (catalogue no. F1904, Chemicon International Inc.). AE-2 and MDA-MB231 cell line cells were lightly trypsinized, washed, resuspended in serum-free DMEM at the proper concentration of 0.5 × 106/ml and used as follows: 200-μl volumes of each cell suspension, untreated and pretreated with 50 ng/ml EGF from Sigma-Aldrich Co. (St. Louis, MO), and were loaded in the upper compartment of the Boyden chambers. In addition, 50 ng/ml of EGF were used as a chemoattractant in those assays in which EGF pretreated AE-2 and MDA-MB231 were used, and placed in the lower compartment of the Boyden chambers. After 3 hours of incubation at 37°C in 5% CO2, the cells were removed from the upper side of the filters; the filters were then fixed and stained with Diff-Quick. The results of four independent experiments are reported as the mean ± SD of the numbers of migrated AE-2 and MDA-MB231 cells counted in 10 high-power fields (×400) on the lower side of the polycarbonate filters (Figure 7).
Figure 7.
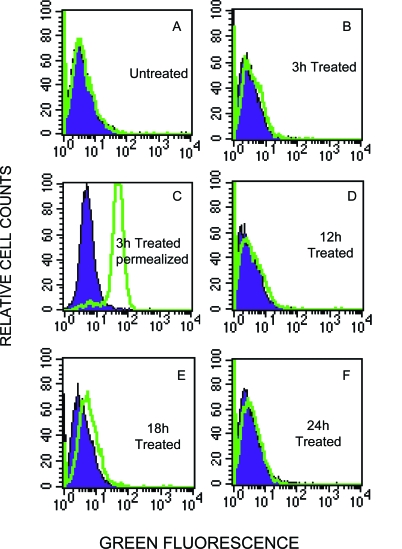
Expression of laminin α2 chain by flow cytometry in EAHY endothelial cells. Cells stained with anti-laminin α2 chain mAb followed by fluorescein isothiocyanate-conjugated goat anti-mouse IgG were analyzed on 10,000 events acquired. Each histogram represents the overlay between positive expression (green histograms) of laminin α2 chain and negative control (blue histograms). No detectable level of laminin α2 chain was observed on the surface of endothelial cells after 3 hours of stimulation (B), which is however synthesized by the cells, but still inside, as demonstrated by permeabilized cells (C). E: Expression of laminin α2 chain on the surface of EAHY cells after a VEGF treatment of 18 hours; hence, this laminin isoform is still present but with a tendency to decline after 24 hours of treatment.
Transwell Test Migration Assay of AE-2 and MDA-MB231 Cell Lines through EAHY-Coated Filters
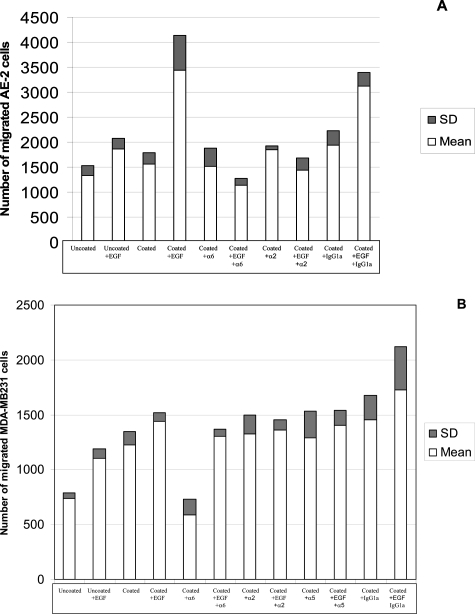
Four independent experiments were performed as already described on AE-2 and MDA-MB231 cell lines.24 Seventy percent confluent EAHY cells were trypsinized, washed, resuspended with supplemented DMEM at the concentration of 1.5 × 106 cells per ml, transferred onto the upper side of polyvinylpyrrolidone-free polycarbonate filters (8-μm pore size), of 14 wells of Transwell chambers (Costar catalogue no. 3403; Corning Inc., Corning, NY) and finally incubated in 5% CO2 and 95% air at 37°C. Two sets of polycarbonate membranes were, respectively, prepared for the AE-2 and MDA-MB231 cell lines. Polycarbonate membranes of four Transwell chambers remained uncoated and loaded with DMEM medium alone. The EAHY endothelial cells loaded on polycarbonate filters were checked after 18 hours of incubation using an inverted microscope and their laminin α2 chain and laminin β1 chain expression was assessed on two polycarbonate filters of two Transwell chambers using specific monoclonal antibodies with the avidin-biotin-peroxidase complex method (ABC). Transwell test migration assays were performed only when these cells reached more than 90% of confluence and were at the same time laminin α2 chain-positive and laminin β1 chain-negative after 18 hours in culture (Figure 6). The DMEM medium was removed and upper and lower chambers of the Trans-wells were washed twice with PBS. Each experiment of test migration assays was performed by evaluating each of the following experimental conditions in duplicate sets: 1) the lower chambers of two Transwells with uncoated filters were filled with 2 ml of RPMI supplemented with 10% FCS; 2) the lower chambers of two Transwells with uncoated filters were filled with 2 ml of RPMI supplemented with 10% FCS and 50 ng/ml of EGF; 3) the lower chambers of two Transwells with EAHY-coated filters were filled with 2 ml of RPMI supplemented with 10% FCS; 4) the lower chambers of two Transwells with EAHY-coated filters were filled with 2 ml of RPMI supplemented with 10% FCS and 50 ng/ml of EGF; 5) the lower chambers of two Transwells with EAHY-coated filters were filled with 2 ml of RPMI supplemented with 10% FCS and 5 μg of anti-α6 integrin/CD49f clone BQ16 isotype IgG1 mouse (Dako); 6) the lower chambers of two Transwells with EAHY-coated filters were filled with 2 ml of RPMI supplemented with 10% FCS and 50 ng/ml EGF and 5 μg of anti-α6 integrin/CD49f clone BQ16 isotype IgG1 mouse (Dako); 7) the lower chambers of two Transwells with EAHY-coated filters were filled with 2 ml of RPMI supplemented with 10% FCS and 5 μg of anti-α2 integrin/CD49b clone BQ16 isotype IgG1 mouse (Dako); 8) the lower chambers of two Transwells with EAHY-coated filters were filled with 2 ml of RPMI supplemented with 10% FCS and 50 ng/ml EGF and 5 μg of anti-α2 integrin/CD49b clone BQ16 isotype IgG1 mouse (Dako); 9) the lower chambers of two Transwells with EAHY-coated filters were filled with 2 ml of RPMI supplemented with 10% FCS and 5 μg of anti-α5 integrin/CD49e clone BQ16 isotype IgG1 mouse (Dako); and 10) the lower chambers of two Transwells with EAHY-coated filters were filled with 2 ml of RPMI supplemented with 10% FCS and 50 ng/ml EGF and 5 μg of anti-α5 integrin/CD49e clone BQ16 isotype IgG1 mouse (Dako). Finally, the upper chambers of two sets of Transwells were loaded with 200 μl of 0.5 × 106/ml of AE-2 and MDA-MB231 suspensions (Figure 8). Controls consisted of consecutive tests performed as previously described in points 5, 6, 7, 8, 9, and 10, omitting the anti-α6, anti-α2, and anti-α5 integrin mAbs that were replaced with nonimmune isotype IgG1 mouse (Dako). The test migration assays were harvested after 3 hours of incubation at 37°C in 5% CO2 and 95% air. Each experimental condition was scored as the total amount of cells migrated in two Transwell chambers. The results are reported as the mean ± SD of the numbers of migrated AE-2 and MDA-MB231 cells on the lower chamber of Transwells in four independent experiments; each experiment includes duplicate sets (Figure 8).
Figure 8.
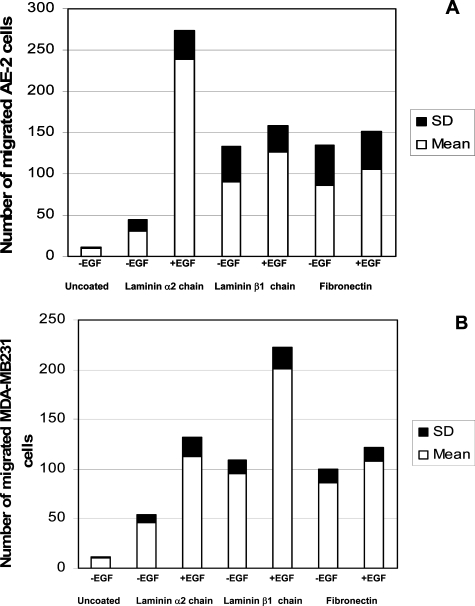
Three-hour migration test assay of AE-2 neuroendocrine carcinoma cell line (A) and MDA-MB231 cell line (B) through nucleopore membranes coated with purified laminin α2 chain, laminin β1 chain, and fibronectin. Results are expressed as mean ± SD of four independent experiments evaluating each of the following experimental conditions: uncoated: migration tests were performed on unstimulated cell lines through uncoated polycarbonate filters (control); −EGF/laminin α2 chain: migration tests were performed on unstimulated cell lines through polycarbonate filters coated with laminin α2 chain; +EGF/laminin α2 chain: migration tests were performed on EGF-stimulated cell lines through polycarbonate filters coated with laminin α2 chain; −EGF/laminin β1 chain: migration tests were performed on unstimulated cell lines through polycarbonate filters coated with laminin β1 chain; +EGF/laminin β1 chain: migration tests were performed on EGF-stimulated cell lines through polycarbonate filters coated with laminin β1 chain; −EGF/fibronectin: migration tests were performed on unstimulated cell lines through polycarbonate filters coated with fibronectin; +EGF/fibronectin: migration tests were performed on EGF-stimulated cell lines through polycarbonate filters coated with fibronectin.
Statistical Analysis
The results are expressed as the mean ± SD. The statistical evaluation of the data were performed using the Student’s test for paired samples, with values of P < 0.05 representing the minimum level of statistical significance.
Results
Atypical Carcinoids
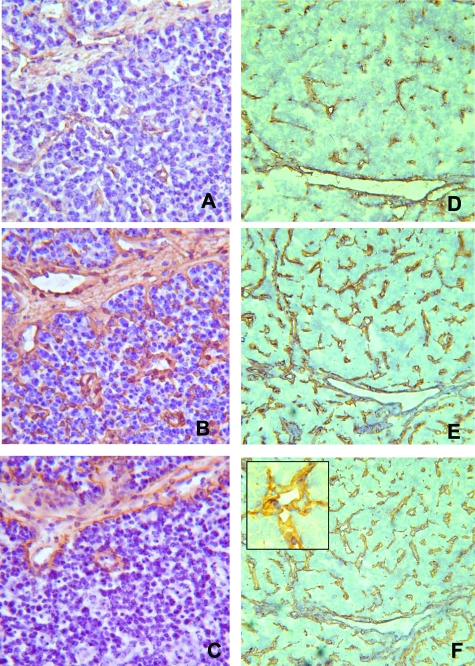
The four cases of lung atypical carcinoid tumor under investigation consisted of peripheral tumors with rather well-defined desmoplastic borders pushing the surrounding tissue. The majority of the vessels (85%) was distributed within the parenchyma being represented by capillary-like structures in close contact with neoplastic cells, whereas only a few vessels (15%) were present in the peritumoral stroma. Immunohistochemistry demonstrated that all of the stromal vessels were positive for FVIIIRA; on the contrary, only 70 to 80% of parenchymal vessels were immunoreactive for this antigen. Crosschecking on contiguous sections demonstrated that all of the stromal and parenchymal vessels had endothelial cells immunostained by α5, α6, and β4 integrins; furthermore, their basement membranes were always immunoreactive for fibronectin, tenascin, and laminin β1 chain. As far as laminin α2 chain was concerned, 23%, 10%, 10%, and 25% of stromal vessels were positive for this ECMP; moreover, 50%, 30%, 100%, and 25% of parenchymal vessels were positive for this ECMP (Figure 1, A–C). It is worth noting that in these tumors laminin α2 chain-positive vessels expressed this ECMP in endothelial cells and in their adjacent basement membranes. Furthermore, in all cases, all of the neoplastic cells were immunoreactive for the β1 chain of integrins, whereas only a small proportion of neoplastic cells was immunoreactive for the α2 and α6 chains of integrins. Finally, neoplastic cells were negative for the α5 chain of integrins, endothelial markers, and all ECMPs including the laminin α2 chain (Table 1).
Figure 1.
Cryostat serial sections of an atypical carcinoid and of a small cell lung carcinoma immunostained with anti-FVIIIRA (A and D), anti-laminin β1 chain (B and E), and anti-laminin α2 chain (C and F) mAbs, using the ABC method, and counterstained with hematoxylin. In the atypical carcinoid, only a proportion of vessels positive for FVIIIRA and laminin β1 chain also express the laminin α2 chain, whereas in the small cell lung carcinoma, all FVIIIRA and laminin β1 chain-positive vessels are positive for the laminin α2 chain. This ECMP was expressed both in endothelial cells and basement membranes. Original magnifications: ×250 (A–C); ×100 (D–F); ×400 (F, inset).
Neuroendocrine Carcinomas
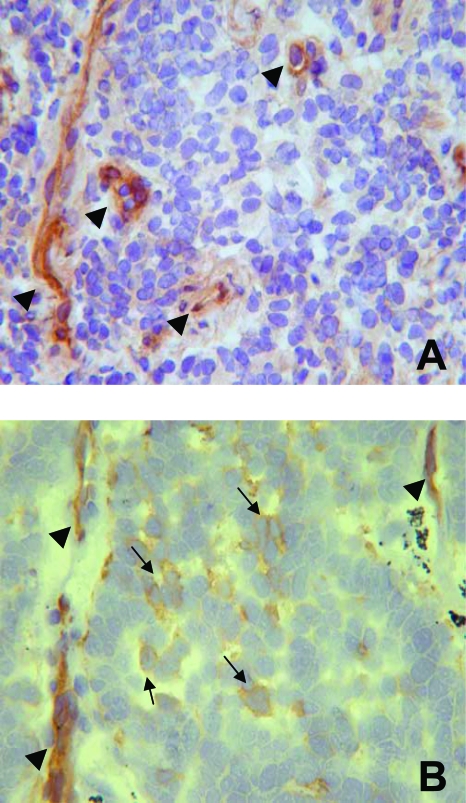
The five cases of SCCs consisted of tumors with poorly defined borders, infiltrating mediastinal lymph nodes and soft tissues, whereas, the four cases of LCNCs consisted of parenchymal tumors with rather well-defined borders and foci of desmoplastic reaction. In all these neuroendocrine carcinomas a large proportion of vessels ranging from 75 to 90% was distributed within the neoplastic parenchyma and was represented by capillary-like structures in close contact with neoplastic cells. In these tumors, all stromal vessels were positive for FVIIIRA, whereas only a proportion of parenchymal vessels, ranging from 20 to 85%, was immunoreactive for this antigen. Crosschecking on contiguous sections demonstrated that all stromal and parenchymal vessels had endothelial cells positive for α5, α6, and β4 chains of integrins, as well as basement membranes immunoreactive for fibronectin, tenascin, and laminin β1 chain. Moreover, as far as the laminin α2 chain was concerned, in eight of nine cases 25%, 20%, 10%, 30% 10%, 60%, 70%, and 100% of stromal vessels were positive for this laminin isoform, whereas in the remaining case stromal vessels were negative for the laminin α2 chain. In three of nine cases of neuroendocrine carcinomas, all of the parenchymal vessels were laminin α2 chain-positive; in the remaining six cases this laminin isoform was expressed in 40%, 35%, 30%, 80%, 50%, and 30% of the vessels (Figure 1, D–F). It is worth noting that in all these carcinomas laminin α2 chain-positive vessels expressed this ECMP in endothelial cells and in their adjacent basement membranes (Figure 1F, inset). Moreover, all of the neoplastic cells expressed the β1 chain of integrins; only a proportion of them, ranging from 15 to 60%, and predominantly distributed around capillary-like structures, was immunoreactive for the α6 chain of integrins (Figure 2). Few scattered neoplastic cells immunoreactive for the α2 chain of integrins were observed. Finally, in all tumors neoplastic cells were negative for the α5 chain of integrins, endothelial markers, and all ECMPs including the laminin α2 chain (Table 2).
Figure 2.
Cryostat sections of an atypical carcinoid (A) and of a small cell lung carcinoma (B) immunostained with anti-α6 integrin chain mAb, using the ABC method, and counterstained with hematoxylin. In the atypical carcinoid only capillary-like structures (arrowheads) are immunostained, whereas in small cell lung carcinoma numerous neoplastic cells are positive (arrows) and are distributed around the α6 chain-positive capillary-like structures (arrowheads). Original magnifications, ×400.
Cytology, Antigen, and Gene Expression of AE-2 and MDA-MB231 Cell Lines
The AE-2 cell line consists of epithelial cells 20 μm to 35 μm in size at their greatest dimension, showing a high nucleus:cytoplasm ratio. All these cells were immunoreactive for cytokeratin, clone MNF116, NCAM, and α2 and β1 chains of integrins. Moreover, 65% and 85% of these cells were positive for chromogranin A and the α6 chain of integrins. NCAM and chromogranin A expression is consistent with the neuroendocrine differentiation of this lung tumor-derived cell line (Figure 3). Western blot analysis demonstrated that AE-2 cells express Erb-B2 and Erb-B3 and decreased levels of EGFR and Erb-B4 (Figure 4). Western blot analysis was further supported by gene expression for all EGFRs (Figure 5). The MDA-MB231 cell line from American Type Culture Collection consists of epithelial cells 30 μm to 55 μm in size at their greatest dimension, showing a nucleus:cytoplasm ratio lower than that of AE-2 cells. All these cells were immunoreactive for cytokeratin, clone MNF116, α2, α5, α6 (Figure 3), and β1 chains of integrins. Western blot analysis demonstrated that MDA-MB231 cells express EGFR, Erb-B3, and Erb-B4 and decreased levels of Erb-B2 (Figures 4 and 5).
Kinetics of the Laminin α2 Chain Expression in EAHY Cell Cultures
Adherent EAHY Cells
In cultures incubated with DMEM alone, adherent EAHY cells were observed only after 24 hours, whereas in those incubated with DMEM and 10% FCS, and 10% FCS supplemented with VEGF, FGF2, and VEGF + FGF2, adherent cells were observed as early as after 1 hour. The proportion of adherent cells immunostained was determined by counting 200 cells at ×400 in randomly chosen fields and was independent of the medium used. Positive immunostaining for FVIIIRA and the laminin α2 chain was demonstrated in adherent EAHY cells as early as after 1 hour in culture. FVIIIRA displayed a finely granular, paranuclear immunostaining pattern whereas the staining for the laminin α2 chain was cytoplasmic, diffuse, and coarsely granular until 48 hours in culture. Furthermore, at the earliest intervals, the cytoplasmic immunoreactivity for the laminin α2 chain (Figure 6B) was stronger than that for the laminin β1 chain (Figure 6A), and after 48 and 96 hours in culture (Figure 6, C and D) displayed a cell membrane dot-spot pattern. According to the results of this kinetic study adhering EAHY cells were at the same time confluent, laminin α2 chain-positive, and laminin β1 chain-negative after 18 hours in culture; therefore, this time of culture was chosen to obtain EAHY-coated filters in Transwell chambers to perform test migration assays on the AE-2 line (Figure 6).
Nonadherent EAHY Cells
To further assess the synthesis of the laminin α2 chain in the EAHY endothelial cell line within 24 hours in culture, the expression of this laminin isoform was evaluated in nonadherent EAHY cells either untreated or treated with 10 ng/ml of VEGF at different time intervals. Using immunofluorescence and FACS analysis, we were able to detect the laminin α2 chain expression level on the surface of VEGF-treated EAHY cells after 18 hours in culture (Figure 7E). Moreover, this laminin isoform was still present, but with a tendency to decline after 24 hours in culture (Figure 7F). No detectable level of laminin α2 chain expression was observed on the surface of endothelial cells after 3 hours of stimulation (Figure 7C); however, this isoform was synthesized inside the cells, as demonstrated by permeated cells. Therefore, despite the nonadherent condition of culture, these observations indicate that EAHY endothelial cells are able to synthesize and express the laminin α2 chain on their cell surface; this property can be directly involved in the process of chemokinetic migration of AE-2 and MDA-MB231 cells through EAHY-coated filters.
Test Migration Assays of AE-2 and MDA-MB231 Cell Lines on Purified ECMPs
As shown in Figure 8A, AE-2 cells displayed a low migration activity through uncoated membranes. The unstimulated AE-2 cell line migrated more efficiently through the laminin β1 chain and fibronectin than through the laminin α2 chain. Nevertheless, EGF-treated AE-2 cells displayed a more efficient migration through the laminin α2 chain-coated membranes than through the laminin β1 chain- and fibronectin-coated ones (P < 0.05). As shown in Figure 8B, EGF-untreated MDA-MB231 cells displayed a low migration activity through control uncoated membranes. This breast carcinoma cell line migrated more efficiently through the laminin β1 chain- and fibronectin-coated membranes than through the laminin α2 chain-coated ones. However, EGF treatment increased the rate of MDA-MB231 cell migration through all these purified ECMPs (P < 0.05), although the chemokinetic improvement through the laminin β1 chain was higher than that through the laminin α2 chain and fibronectin (P < 0.05).
Transwell Test Migration Assay of AE-2 and MDA-MB231 Cell Lines on EAHY-Coated Filters
As shown in Figure 9A, unstimulated AE-2 cells displayed a basal migration activity through EAHY-uncoated membranes that was slightly increased by EGF, but similar to that observed through EAHY-coated membranes in the absence of EGF stimulation. On the contrary, EGF stimulation induced an evident increase of AE-2 cell migration through EAHY-coated membranes, which was significantly higher than that induced through uncoated membranes (P < 0.05). This indicates that EAHY endothelial cells played a crucial role in the EGF-mediated migration of AE-2 cells. It is worth noting that the EGF-dependent migration of AE-2 cells through EAHY-coated membranes was significantly inhibited (P < 0.05) by anti-α6 integrin mAb, although the anti-α2 integrin mAb also induced a less efficient inhibition. Nevertheless, neither the anti-α6 integrin nor the anti-α2 integrin mAb significantly modified AE-2 cell migration through EAHY-coated membranes in the absence of EGF stimulation (P > 0.05). Altogether these observations indicate a pivotal role of α6β1 and α2β1 in the cooperative action of endothelial cells in AE-2 cell migration induced by EGF. In this regard, the replacement of anti-α6 and α2β1 integrin mAbs with a nonspecific IgG1a isotype mAb did not significantly inhibit the migration either of unstimulated or EGF-stimulated AE-2 cells through EAHY-coated membranes (P > 0.05).
Figure 9.
Three-hour migration test assays of AE-2 neuroendocrine cell carcinoma line (A) and MDA-M B231 cell line (B) through confluent laminin α2 chain-positive EAHY endothelial cells. Results are expressed as mean ± SD of four independent experiments and each experiment includes duplicate sets. The tests were performed evaluating each of the following experimental conditions: uncoated: Transwells with EAHY-uncoated filters and filled with the cell suspension maintained in RPMI supplemented with 10% FCS alone; uncoated + EGF: Transwells with EAHY-uncoated filters and filled with the cell suspension maintained in RPMI supplemented with 10% FCS and 50 ng/ml of EGF; coated: Transwells with EAHY-coated filters and filled with the cell suspension maintained in RPMI supplemented with 10% FCS alone; coated + EGF: Transwells with EAHY-coated filters and filled with the cell suspension maintained in RPMI supplemented with 10% FCS and 50 ng/ml of EGF; coated + anti-α6 integrin: Transwells with EAHY-coated filters and filled with the cell suspension maintained in RPMI supplemented with 10% FCS and 5 μg of the mAb specific for the α6 chain of integrins; coated + EGF + anti-α6 integrin: Transwells with EAHY-coated filters and filled with the cell suspension maintained in RPMI supplemented with 10% FCS, 50 ng/ml EGF, and 5 μg of the mAb specific for the α6 chain of integrins; coated + anti-α2 integrin: Transwells with EAHY-coated filters and filled with the cell suspension maintained in RPMI supplemented with 10% FCS and 5 μg of the mAb specific for the α2 chain of integrins; coated + EGF + anti-α2 integrin: Transwells with EAHY-coated filters and filled with the cell suspension maintained in RPMI supplemented with 10% FCS, 50 ng/ml EGF, and 5 μg of the mAb specific for the α2 chain of integrins; *coated + anti-α5 integrin: Transwells with EAHY-coated filters and filled with the cell suspension maintained in RPMI supplemented with 10% FCS and 5 μg of the mAb specific for the α5 chain of integrins; *coated + EGF + anti-α5 integrin: Transwells with EAHY-coated filters and filled with the cell suspension maintained in RPMI supplemented with 10% FCS, 50 ng/ml EGF, and 5 μg of the mAb specific for the α5 chain of integrins; coated + anti-IgG1: Transwells with EAHY-coated filters and filled with the cell suspension maintained in RPMI supplemented with 10% FCS and 5 μg of nonimmune isotype IgG1 mouse; coated + EGF + anti-IgG1: Transwells with EAHY-coated filters and filled with the cell suspension maintained in RPMI supplemented with 10% FCS, 50 ng/ml of EGF, and 5 μg of nonimmune isotype IgG1 mouse. *Not determined in AE-2 cells because they do not express the α5 chain of integrins.
As shown in Figure 9B, unstimulated MDA-MB231 cells displayed a basal migration activity through EAHY-uncoated membranes that was significantly increased either by EGF stimulation or by EAHY coating (P < 0.05). Moreover, the migration of cells through the EAHY-coated membrane was further increased by EGF stimulation (P < 0.05) suggesting a cooperative role between EAHY endothelial cells and EGF in this process. It is worth noting that the anti-α6 integrin mAb significantly inhibited the migration of EGF-untreated MDA-MB231 cells through EAHY-coated membranes (P < 0.05); on the contrary, in presence of EGF stimulation, this antibody produced a lower inhibitory effect on MDA-MB231 cell migration through EAHY. Altogether these observations indicate that EGF and the α6 chain of integrins are able to improve the migration of MDA-MB231 cells according to independent mechanisms that may sum up their positive effects. This is demonstrated by the positive effect of EGF alone on cell migration through uncoated membranes, and by the evidence that the inhibitory effect of the anti-α6 chain mAb on the migration of MDA-MB231 cells through coated membranes, was primarily reversed by EGF stimulation. Finally, the replacement of the anti-α6 chain mAb either with anti-α2 chain or anti-α5 chain mAbs or with the nonspecific IgG1a isotype mAb, did not significantly inhibit the migration either of untreated or EGF-treated MDA-MB231 cells through EAHY-coated membranes (P > 0.05).
Discussion
It is well known that up-regulation of different epithelial laminin isoforms provides specific contributions to tumor growth and progression.3–6 In this regard, it has been previously reported that in tumors, as well as in normal tissues, basement membranes of blood vessels express α4, α5, β1, and γ1 chains indicating the presence of laminin-8 and laminin-10, which are known to be endothelial-specific laminin isoforms.1 Accordingly, in the present study we have shown that in all SCCs and LCNCs under investigation all stromal and parenchymal vessels were immunoreactive for the laminin β1 chain that is a subunit shared by laminin-1, laminin-8, and laminin-10. Moreover, despite the relatively low number of SCCs and LCNCs under investigation due to diagnosis usually made on small bronchial biopsies frequently not suitable for further frozen sampling, in all of the neuroendocrine carcinomas we have demonstrated the presence of a high percentage of laminin α2 chain-positive vessels. It is worth noting that the number of laminin α2 chain-positive vessels present in SCCs and LCNCs was significantly higher than the number previously reported in stroma and parenchyma of supraglottis (P < 0.01), breast (P < 0.05), and non-small cell lung carcinomas (P < 0.05),14 all displaying a lower rate of metastasis than lung neuroendocrine carcinomas. Altogether, these ex vivo observations suggest a possible direct relationship between laminin α2 chain-positive vessels and the metastatic tendency of these human solid tumors. This is further supported by the evidence that atypical carcinoid tumors, despite their numerous vessels predominantly distributed in close contact with neoplastic cells, are characterized by a rate of metastasis and a percentage of laminin α2 chain-positive vessels that are lower than those of SCCs and LCNCs, but higher than those of supraglottis, breast, and non-small cell lung carcinomas.14 Therefore, these ex vivo observations prompted us to perform in vitro migration test assays to assess whether the expression of the laminin α2 chain may favor neoplastic vessel invasion more efficiently than other laminin isoforms present in endothelial basement membranes, such as laminin-1, laminin-8, and laminin-10, sharing laminin β1 chain, but different α chains such as α1, α4, and α5 chains.1,17,25 In four consecutive experiments we observed that EGF increased chemokinetic migration of the AE-2 cell line more efficiently through laminin α2 chain than through laminin β1 chain and fibronectin (P < 0.05). Moreover, EGF significantly increased also chemokinetic migration of the MDA-MB231 control cell line through laminin α2 chain and laminin β1 chain (P < 0.05). These ECMPs represent the ligands of α1β1, α2β1, α3β1, α6β1, and α7β1, which are up-regulated or constitutively expressed in several human solid tumors. Therefore, these observations suggest that during angiogenesis in human solid tumors the expression of the laminin α2 chain may increase the availability of the specific ligands for integrins in basement membranes of newly formed vessels. In turn, laminin α2 chain-positive vessels may favor the adhesion of neoplastic cells and their trans-endothelial migration more efficiently than resting vessels, expressing only other laminin isoforms and ECMPs constitutively present in their basement membranes. EGF is produced by neoplastic cells, acting as an autocrine stimulus, and by stromal and endothelial cells as well, acting as a paracrine stimulus.25–27 EGF plays a crucial role in tumor growth and progression28–36 and up-regulates the expression of α6β1 and α2β1 integrins in neoplastic cells of several human carcinomas.37,38 AE-2 and MDA-MB231 cell lines represent in vitro models of lung neuroendocrine and breast carcinomas. These cell lines are able to express all four EGF receptors and integrins such as α6β1 and α2β1 that are receptors for the laminin 2 isoform.1 As previously described in this laboratory in supraglottis, breast, and non-small cell lung carcinomas,14 we have hereby reported that in lung neuroendocrine carcinomas, laminin α2 chain-positive vessels express this laminin subunit in both basement membranes and adjacent endothelial cells. Therefore, we performed migration tests on AE-2 and MDA-MB231 cell lines through monolayers of laminin α2 chain-positive EAHY endothelial cells, to simulate laminin α2 chain-positive vessels in vivo. These experiments have shown that EGF-treated AE-2 cells migrated significantly better through EAHY-coated membranes than through uncoated ones (P < 0.05), indicating a pivotal role of these endothelial cells in the EGF-dependent migration of AE-2 cells. Moreover, EGF-dependent migration of AE-2 cells through EAHY-coated membranes was significantly inhibited by anti-α6 (P < 0.05) and, at least in part, by anti-α2 integrin chain mAbs. These observations indicate a positive role of α6β1 and α2β1 integrins in the cooperative action of EGF and endothelial cell monolayers favoring migration of AE-2 cells; this may be related to the ability of these integrins to bind their ligand laminin α2 chain expressed on endothelial cell monolayers. Altogether, our in vitro observations strongly suggest a further cooperative role of EGF with the laminin α2 chain on the chemokinetic migration of the AE-2 neuroendocrine carcinoma cell line, and a critical role of α6β1 and α2β1 integrins in this process as well. In all SCCs and LCNCs under investigation a significant proportion of neoplastic cells was α6β1-positive and frequently distributed around vessels; on the contrary, in atypical carcinoid tumors, characterized by a lower metastatic tendency, only few neoplastic cells were positive for this integrin. It has been previously described in vitro that laminin-10 enhances basal and EGF-stimulated motility of colon carcinoma cells via α3β1 and α6β4 integrins.24,38 Likewise, our in vitro model of lung neuroendocrine carcinomas suggests that in some EGF-dependent human solid tumors,28–32 the concomitant up-regulation of α6β1 and α2β1 integrins and the expression of the laminin α2 chain in newly formed vessels, may improve the adhesion of neoplastic cells to vessels, therefore contributing to those mechanisms promoting neoplastic vessel invasion and metastasis. The migration of the MDA-MB231 cell line through uncoated membranes was increased either by EGF or by the EAHY endothelial coating. Moreover, the anti-α6 integrin chain did significantly inhibit the migration of the MDA-MB231 cell line through endothelial-coated membranes only in absence of EGF stimuli. These observations indicate that EGF and laminin α2 chain-positive endothelial cells may independently promote the chemokinetic activity of this cell line. This hypothesis is supported by the evidence that the anti-α6 integrin chain mAb did not significantly inhibit the migration of EGF-treated MDA-MB231 cells through endothelial-coated membranes. However, these observations also suggest that, independently of an EGF-dependent up-regulation of α6β1, EGF stimulation and laminin α2 chain-positive vessels may sum up their effects, thus increasing the metastatic potential of breast carcinomas. This phenomenon could be related to the described pathogenic role of HER-2/NEU-EGF receptor up-regulation and to the constitutive high expression of α6β1 in these tumors. We therefore propose that, according to the neoplastic model hereby discussed, the cooperative role of EGF and laminin α2 chain-positive vessels may account for the metastatic tendency of human solid tumors, either dependently or independently of integrin up-regulation. Further studies at tissue level and on other neoplastic models must be performed to establish to what extent laminin α2 chain-positive vessels may be considered as a new prognostic indicator. EGF induces a VEGF release from neoplastic cells; therefore, both factors mutually support tumor angiogenesis, growth, and progression.39,40 Accordingly, new therapeutic approaches are on trial to assess the therapeutic effects of recombinant mAbs specific for EGFRs,41–45 VEGFRs,46–48 and VEGF49–51 in the treatment of human solid tumors such as lung, ovarian, renal, and colorectal cancer. The possible cooperative role of EGF and laminin α2 chain in the metastatic process may contribute to the rationale of new therapies of the metastatic disease in carcinomas.
Acknowledgments
We thank Dr. A. Santoni for supplying the EAHY cells and Mrs. M.G. Saladino and Dr. Francesca Cancellario d’Alena for their help in manuscript preparation.
Footnotes
Address reprint requests to Domenico Vitolo, II Cattedra di Anatomia ed Istologia Patologica, Dipartimento di Medicina Sperimentale e Patologia, Universita’ degli Studi di Roma “La Sapienza,” Viale Regina Elena, 324, 00161, Rome, Italy. E-mail: domenico.vitolo@uniroma1.it.
Supported by grants from the Italian Ministry of University and Scientific and Technological Research (MURST 2002 to D.V.), Associazione Italiana per la Ricerca sul Cancro (to C.D.B.), COFIN 2004 granted to D.V. and L.M., and Istituto Pasteur-Fondazione Cenci-Bolognetti (to C.D.B.).
D.V. is Second Chair of Pathological Anatomy of the Department of Experimental Medicine and Pathology.
References
- Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin Cancer Biol. 2002;12:197–207. doi: 10.1016/S1044-579X(02)00023-8. [DOI] [PubMed] [Google Scholar]
- Sorokin LM, Paush F, Frieser M, Kroger S, Ohage E, Deutzmann R. Developmental regulation of the laminin α5 chain suggests a role in epithelial and endothelial cell maturation. Dev Biol. 1997;189:285–300. doi: 10.1006/dbio.1997.8668. [DOI] [PubMed] [Google Scholar]
- Kikkawa Y, Sanzen N, Sekiguchi K. Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells—laminin-10/11 mediates cell adhesion through integrin α3β1. J Biol Chem. 1998;25:15854–15859. doi: 10.1074/jbc.273.25.15854. [DOI] [PubMed] [Google Scholar]
- Tani T, Lehto VP, Virtanen I. Expression of laminins 1 and 10 in carcinoma cells and comparison of their roles in cell adhesion. Exp Cell Res. 1999;248:115–121. doi: 10.1006/excr.1999.4399. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Itoh F, Iku S, Hosokawa M, Imai K. Expression of the gamma(2) chain of laminin-5 at the invasive front is associated with recurrence and poor prognosis in human esophageal squamous cell carcinoma. Clin Cancer Res. 2001;7:896–900. [PubMed] [Google Scholar]
- Lohi J. Laminin-5 in the progression of carcinomas. Int J Cancer. 2001;94:763–767. doi: 10.1002/ijc.1539. [DOI] [PubMed] [Google Scholar]
- Leivo I, Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striate muscle and trophoblast, is expressed late in nerve and muscle development. Proc Natl Acad Sci USA. 1988;85:1544–1561. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivo I, Engvall E, Laurila P, Miettinen M. Distribution of merosin, a laminin-related tissue-specific basement membrane protein, in human Schwann cell neoplasms. Lab Invest. 1989;61:426–432. [PubMed] [Google Scholar]
- Ehrig K, Leivo I, Agraves WS, Ruoslahti E, Engvall E. Merosin, a tissue specific basement membrane protein, is a laminin-like protein. Proc Natl Acad Sci USA. 1990;87:3264–3268. doi: 10.1073/pnas.87.9.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky CH, Fitzgeraald ML, Fischer SJ. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway in vivo. J Clin Invest. 1992;89:210–222. doi: 10.1172/JCI115565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachon PH, Loechel F, Xu H, Wewer UM, Engvall E. Merosin and laminin in myogenesis; specific requirement for merosin in myotube stability and survival. J Cell Biol. 1996;134:1483–1497. doi: 10.1083/jcb.134.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagoe-Suzuki Y, Nakagawa M, Takeda S. Merosin and congenital muscular dystrophy. Microsc Res Tech. 2000;48:181–191. doi: 10.1002/(SICI)1097-0029(20000201/15)48:3/4<181::AID-JEMT6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Vitolo D, Paradiso P, Uccini S, Ruco LP, Baroni CD. Expression of adhesion molecules and extracellular matrix proteins in glioblastomas: relation to angiogenesis and spread. Histopathology. 1996;28:521–528. doi: 10.1046/j.1365-2559.1996.d01-471.x. [DOI] [PubMed] [Google Scholar]
- Vitolo D, Ciocci L, Cicerone E, Rossi C, Tiboni F, Ferrauti P, Gallo A, Baroni CD. Laminin α2 chain (merosin M chain) distribution and VEGF, FGF2 and TGFβ1 gene expression in angiogenesis of supraglottis, lung and breast carcinomas. J Pathol. 2001;195:197–208. doi: 10.1002/path.938. [DOI] [PubMed] [Google Scholar]
- Gupta MK, Qin RY. Mechanism and its regulation of tumor-induced angiogenesis. World J Gastroenterol. 2003;9:1144–1155. doi: 10.3748/wjg.v9.i6.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaussin M, Sartelet H, Robert C, Moro D, Clara ZC, Brambilla C, Brambilla E. Expression of vascular endothelial growth factor (VEGF) and its two receptors (VEGF-R1-FLT1 and VEGF-R2-KDR) in non-small cell lung carcinomas: correlation with angiogenesis and survival. J Pathol. 1999;188:369–377. doi: 10.1002/(SICI)1096-9896(199908)188:4<369::AID-PATH381>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Vesweber D. Molecular mechanisms that control endothelial cell contacts. J Pathol. 2000;190:281–291. doi: 10.1002/(SICI)1096-9896(200002)190:3<281::AID-PATH527>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Colby TV, Koss MN, Travis WD. Tumors of the lower respiratory tract. Armed Forces Institute of Pathology: Washington DC,; Atlas of Tumor Pathology, third series, fascicle 13. 1995:235–315. [Google Scholar]
- Mills SE, Gaffey M, Frierson HF. Tumors of the upper aerodigestive tract and ear. Armed Forces Institute of Pathology: Washington DC,; Atlas of Tumor Pathology, third series, fascicle 26. 2000:45–117. [Google Scholar]
- Roset PP, Oberman HA. Tumors of the mammary gland. Armed Forces Institute of Pathology: Washington DC,; Atlas of Tumor Pathology, third series, fascicle 7. 1993:157–244. [Google Scholar]
- Travis TW, Colby V, Corrin B, Shimosato Y, Brambilla E. In Collaboration with Sobin LH and Pathologists in 14 Countries. Germany, Berlin: Springer-Verlag,; Histological Typing of Lung and Pleural Tumors. (3rd ed.) 1999 [Google Scholar]
- Meri S, Mattila P, Renkonen R. Regulation of CD59 expression on the human endothelial cell line EA.hy 926. Eur J Immunol. 1993;23:2511–2516. doi: 10.1002/eji.1830231020. [DOI] [PubMed] [Google Scholar]
- Bei R, Masuelli L, Moriconi E, Visco V, Moretti A, Kraus MH, Muraro R. Immune responses to all ErbB family receptors detectable in serum of cancer patients. Oncogene. 1999;18:1267–1275. doi: 10.1038/sj.onc.1202442. [DOI] [PubMed] [Google Scholar]
- Pouliot N, Nice EC, Burgess AW. Laminin-10 mediate basal and EGF-stimulate motility of human colon carcinoma cells via alpha (3) beta (1) and alpha (6) beta (4) integrins. Exp Cell Res. 2001;266:1–10. doi: 10.1006/excr.2001.5197. [DOI] [PubMed] [Google Scholar]
- Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- Alroy I, Yarden Y. The ErbB signaling network in embryogenesis and oncogenesis: signal diversification through combinatorial ligand-receptor interactions. FEBS Lett. 1997;410:83–86. doi: 10.1016/s0014-5793(97)00412-2. [DOI] [PubMed] [Google Scholar]
- Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerizaton in development and cancer. EMBO J. 2000;19:3159–3167. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damstrup L, Rude Voldborg B, Spang-Thomsen M, Brunner N, Skovgaard Poulsen H. In vitro invasion of small-cell lung cancer cell lines correlates with expression of epidermal growth factor receptor. Br J Cancer. 1998;78:631–640. doi: 10.1038/bjc.1998.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandis JR, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, Drenning SD, Tweardy DJ. Levels of TGF-α and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90:824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor α and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck. Cancer Res. 1993;53:3579–3584. [PubMed] [Google Scholar]
- Deugnier MA, Faraldo MM, Janji B, Rousselle P, Thiery JP, Glukhova MA. EGF controls the in vivo developmental potential of a mammary epithelial cell line possessing progenitor properties. J Cell Biol. 2002;159:453–463. doi: 10.1083/jcb.200207138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch V, Baselga J, Cordon-Cardo C, Orazem J, Zaman M, Hoda S, McIntosh J, Kurie J, Dmitrovsky E. Differential expression of the epidermal growth factor receptor and its ligands in primary non-small cell lung cancers and adjacent benign lung. Cancer Res. 1993;53:2379–2385. [PubMed] [Google Scholar]
- Wells A, Kassis J, Solava J, Turner T, Lauffenburger DA. Growth factor-induced cell motility in tumor invasion. Acta Oncol. 2002;41:124–130. doi: 10.1080/028418602753669481. [DOI] [PubMed] [Google Scholar]
- Damstrup L, Pedersen Wandahl M, Bastholm L, Elling F, Poulsen Skovgaard H. Epidermal growth factor receptor mutation type III transfected into a small cell lung cancer cell line is predominantly localized at the cell surface and enhances the malignant phenotype. Int J Cancer. 2002;97:7–14. doi: 10.1002/ijc.1572. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Tanaka S, Haruma K, Kitadai Y, Yoshihara M, Sumii K, Kajiyama G, Shimamoto F. Growth characteristics of rectal carcinoid tumors. Oncology. 2000;59:229–237. doi: 10.1159/000012166. [DOI] [PubMed] [Google Scholar]
- Franklin WA, Veve R, Hirsh FR, Helfrich BA, Bunn PA., Jr Epidermal growth factor receptor family in lung cancer and premalignancy. Semin Oncol. 2002;29:3–14. doi: 10.1053/sonc.2002.31520. [DOI] [PubMed] [Google Scholar]
- Bello-DeOcampo D, Kleinmann HK, Webber MM. The role of alpha 6 beta 1 integrin and EGF in normal and malignant acinar morpho-genesis of human prostatic epithelium. Mutat Res. 2001;480–481:209–217. doi: 10.1016/s0027-5107(01)00201-9. [DOI] [PubMed] [Google Scholar]
- Poullot N, Connoly LM, Moritz RC, Simpson RJ, Burgess WA. Colon cancer cells adhesion and spreading on autocrine laminin-10 is mediated by multiple integrin receptors and modulated by EGF receptor stimulation. J Pathol. 1999;188:361–368. doi: 10.1006/excr.2000.5065. [DOI] [PubMed] [Google Scholar]
- Goldman CK, Kim J, Wong WL, King V, Brock T, Gillespie GY. Epidermal growth factor stimulates vascular endothelial growth factor production by human malignant glioma cells: a model of glioblastoma multiforme pathophysiology. Mol Biol Cell. 1993;4:121–133. doi: 10.1091/mbc.4.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y, Nakamura S, Nasu K, Narahara H, Miyakawa I. Effect of epidermal growth factor on vascular endothelial growth factor secretion by endometrial stromal cells. Clin Exp Med. 2002;2:69–75. doi: 10.1007/s102380200009. [DOI] [PubMed] [Google Scholar]
- Baselga J. Why the epidermal growth factor receptor? The rationale for cancer therapy. Oncologist. 2002;7:2–8. doi: 10.1634/theoncologist.7-suppl_4-2. [DOI] [PubMed] [Google Scholar]
- Bookman MA, Darcy KM, Pearson-Clarke D, Boothby RA, Horowitz IR. Evaluation on monoclonal humanized anti-HER 2 antibody, Trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol. 2003;21:283–290. doi: 10.1200/JCO.2003.10.104. [DOI] [PubMed] [Google Scholar]
- Fujiuchi S, Ohsaky, Kikuchi K. Suramin inhibits the growth of non-small-cell cancer cells that express the epidermal growth factor receptor. Oncology. 1997;54:134–140. doi: 10.1159/000227677. [DOI] [PubMed] [Google Scholar]
- Bunn PA, Jr, Franklin W. Epidermal growth factor receptor expression, signal pathway inhibitors in non-small cell lung cancer. Semin Oncol. 2002;29:38–44. doi: 10.1053/sonc.2002.35646. [DOI] [PubMed] [Google Scholar]
- Gainet M, Guardiola E, Dufresne A, Pivot X. Epidermal growth factor receptors (EGFR): a new target for anticancer therapy. Cancer Radiother. 2003;7:195–199. doi: 10.1016/s1278-3218(03)00019-2. [DOI] [PubMed] [Google Scholar]
- Presta LG, Chen H, O’Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- Prewett M, Huber J, Li Y, Santiago A, O’Connor W, King K, Overholser J, Hooper A, Pytowski B, Witte L, Bohlen P, Hicklin DJ. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- Klement G, Baruchel S, Rak J, Man S, Clark K, Hicklin DJ, Bohlen P, Kerbel RS. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity. J Clin Invest. 2000;105:15–24. doi: 10.1172/JCI8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD, Rudge JS. VEGF-trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil(FU)/leucovorin(LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]