Abstract
The nonreceptor protein tyrosine kinase Src is overexpressed in 70% of pancreatic adenocarcinomas. Here, we describe the effect of molecular and pharmacological down-regulation of Src on incidence, growth, and metastasis of pancreatic tumor cells in an orthotopic model. Src expression in L3.6pl human pancreatic tumor cells was reduced by stable expression of a plasmid encoding small interfering RNA (siRNA) to c-src. In stable siRNA clones, Src expression was reduced >80%, with no change in expression of the related kinases c-Yes and c-Lyn, and proliferation rates were similar in all clones. Phosphorylation of Akt and p44/42 Erk mitogen-activated protein kinase and production of VEGF and IL-8 in culture supernatants were also reduced (P < 0.005). On orthotopic implantation of varying cell numbers into nude mice, tumor incidence was unchanged; however, in the siRNA clones, large tumors failed to develop, and incidence of metastasis was significantly reduced, suggesting that c-Src activity is critical to tumor progression. To examine this possibility further, animals bearing established wild-type tumors were treated with the Src/Abl-selective inhibitor BMS-354825 (dasatinib). Tumor size was decreased, and incidence of metastases was significantly reduced in treated mice compared with controls. These results demonstrate that Src activation contributes to pancreatic tumor progression in this model, offering Src as a candidate for targeted therapy.
Adenocarcinoma of the exocrine pancreas is the fourth most common cause of cancer death in developed countries with more than 30,000 estimated deaths in 2004 in the United States alone.1 Of the 5% of patients who present with resectable disease, only 12% survive 1 year after diagnosis and less than 5% survive 5 years.2–4 Metastasis to the lymphatics, liver, and vessel walls leads to widespread disease, resulting in a severe wasting condition that accounts for approximately 80% of deaths in advanced pancreatic cancer.5 Even when potentially curative surgery is performed, approximately 80 to 90% of patients develop disease recurrence with standard chemotherapeutic agents having marginal effect on patient survival. Because of the high mortality associated with pancreatic adenocarcinoma and early systemic disease, it is essential that therapeutic regimens be developed to inhibit tumor progression and metastasis.
The progression of pancreatic adenocarcinoma has been associated with deregulation of several signaling molecules.6 One of the potential therapeutic targets receiving considerable recent attention is activation of c-Src, a nonreceptor protein tyrosine kinase. c-Src is a 60-kd prototype of a nine-member family of structurally related Src family kinases (SFKs). In normal cells, SFKs regulate diverse biological processes by associating with multiple signaling and structural molecules. Overexpression of SFKs occurs in many solid tumors, often at later stages of disease,7 and can be predictive of poor prognosis.8 In addition, Src activation can be associated with chemoresistance. Thus, Gleevec-resistant chronic myeloid leukemia (CML)9 and taxol-resistant ovarian cancer cells10 are frequently associated with increased expression of SFKs.
In pancreatic adenocarcinomas, Src is activated in more than 70% of primary tumors.11 Several recent reports have implicated this activity as important to properties of tumor progression. Ito et al12 demonstrated that inhibition of Src resulted in a 90% decrease in in vitro pancreatic cancer cell invasiveness by inhibiting Src-dependent matrix metalloproteinases MMP 2 and MMP 9. We have recently demonstrated that Src is a critical regulator of pro-angiogenic molecules.13–15 Duxbury et al16 have provided evidence that gemcitabine resistance correlates with increased Src activity, and Src inhibition overcomes this resistance. Recently, Src inhibition with a novel Src family kinase inhibitor has demonstrated significant antitumor and antimetastatic activity in a pancreatic cancer orthotopic nude mouse model.17 These data support a potential role for Src inhibitors in the treatment of pancreatic cancer.
However, signal transduction inhibitors affect multiple targets, and “off-target” inhibition can be responsible for antitumor effects. Additionally, SFKs have overlapping functions in multiple signaling pathways. Therefore, we first used molecular strategies to examine the specific role of c-Src in pancreatic tumor growth in vitro and in vivo. We then determined whether dasatinib, a dual Src/Abl inhibitor,18 would give results similar to those of the molecular approach. The data in this study strongly support a role for activation of c-Src, as opposed to other SFK members, in pancreatic tumor progression in a relevant mouse model and suggest that Src-selective inhibitors could have efficacy in preventing or delaying pancreatic tumor metastasis.
Materials and Methods
Cell Lines
The L3.6pl pancreatic cancer cell line was obtained from Dr. Lee Ellis (University of Texas-MD Anderson Cancer Center). The L3.6pl cell line was derived from a repeated cycle of injecting COLO-357 cells into the pancreas of nude mice, selecting for liver metastases, and re-injecting into the pancreas.19 The cells were plated on 10-cm tissue culture dishes, grown as monolayer cultures, and maintained in culture in minimal essential media supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT), 2 mmol/L L-glutamine, and 0.6% penicillin/streptomycin and 5% CO2/95% air at 37°C.
Cell Lysis and Protein Extraction
Cells (1 × 106) were plated in 10-cm dishes and maintained in minimal essential media with 10% FBS. At 70 to 80% confluence, the cells were washed with Dulbecco’s phosphate buffered saline (D-PBS) at 37°C and maintained in serum-free media (10 ml) for 24 hours. The cells and supernatants were harvested at 24 hours. The cells were washed with ice-cold 1× D-PBS, scraped from the plates, lysed, and harvested on ice in radio-immune precipitation assay (RIPA-B) buffer (20 mmol/L sodium phosphate buffer, 150 mmol/L NaCl, 5 mmol/L EDTA, 1% Triton X-100, and 0.5% sodium deoxycholate) supplemented with one tablet complete mini-EDTA protease inhibitor cocktail (Roche Diagnostic, Mannheim, Germany) and sodium orthovanadate (1 mmol/L, pH 7.4). Harvested orthotopic pancreatic tumors were homogenized in RIPA-B buffer using a tissue homogenizer. The homogenates were clarified by centrifugation at 15,000 × g for 15 minutes at 4°C and prepared for Western analysis and immunoprecipitation. Metastases were isolated from normal liver, frozen in liquid nitrogen, and lysed in RIPA-B via mortar and pestle.
Creation of Small Interfering RNA (siRNA) Expression Plasmids for Silencing Src Gene Expression
siRNA expression plasmids were created as described elsewhere,13 using the Ambion pSilencer 1.0-U6 (Austin, TX) according to manufacturer’s directions. Briefly, c-Src-specific target sequences were designed using the Ambion siRNA Web design tool. The two target sequences used were (52 to 71 bp) 5′-AACAAGAG CAAGCCCAAGGAT-3′ and (226 to 244 bp) 5′-AAGCTGTTCGGAGGCTTCAAC-3′. Oligonucleotides corresponding to these sequences with flanking ApaI (5′) and EcoR1 (3′) ends were purchased from Invitrogen/Life Technologies (Carlsbad, CA) and ligated into the expression plasmid at compatible sites. Constructs were confirmed by DNA sequencing. L3.6pl cells were then transfected with 0.5 ng of each siRNA plasmid and 10 ng of pcDNA G418 resistance promoterless plasmid for selection of transfectants. Cells were then grown in selective media containing G418 as previously described.20 Negative controls were transfected with empty vector target sequences and pcDNA plasmids at identical concentrations. Total c-Src expression levels in siRNA clones were determined by Western blot analysis.
Cell Proliferation Assay
Cell proliferation was quantified by 3-(4,5-dimethyl-2-thiazol-2-yl) 2,5-diphenyltetrazolium bromide assay (Trivegen, Gaithersburg, MD). Cells were seeded into 96-well plates at 1 × 103 cells per well and allowed to adhere overnight in medium containing 10% FBS. The cells were maintained in standard culture conditions, and cellular proliferation and viability were assayed at different time points. Plates were read using spectrophotometric analysis at a wavelength of 570 nm using the TECAN Genios plate reader (TECAN US, Durham, NC) and Magellan version 4.0 software. Twelve samples were used for each cell clone, and the experiments were performed in triplicate.
Immunoblot Analysis
Total protein concentrations were determined via the Bio-Rad Dc protein assay protocol (Bio-Rad, Hercules, CA) followed by spectrophotometric analysis using the TECAN Genios plate reader and Magellan version 4.0 software. Equal amounts of protein (50 μg) were loaded in each well, separated via 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and electroblotted onto Immobilon-P membranes (Millipore, Billerica, MA). The membranes were blocked with Tris-buffered saline/Tween (0.1%) + 5% dried milk for 30 minutes and probed with desired primary antibody diluted 1:1000 in blocking buffer overnight at 4°C. Membranes were probed with polyclonal antibodies to phospho-AktS473 (Cell Signaling Technology, Beverly, MA), phospho-p44/42 ErkT202/Y204 (Cell Signaling Technology), and total p44/42 Erk mitogen-activated protein kinase (MAPK) (Ab-2; Calbiochem, San Diego, CA) and monoclonal antibodies to total Src (Calbiochem), c-Yes (Wako Chemicals, Richmond, VA), Lyn (Santa Cruz Biotechnology, Santa Cruz, CA), Akt (5G3; Cell Signaling Technology), and vinculin (Sigma-Aldrich, St. Louis, MO). Primary antibody incubation was followed by incubation with a horseradish peroxidase-conjugated secondary antibody (Bio-Rad goat anti-mouse and sheep anti-rabbit) diluted 1:2000 in blocking buffer for 1 hour at room temperature with gentle rocking. Western blot analyses of actin and vinculin expression were performed as a loading control using anti-actin and anti-vinculin monoclonal antibodies (Sigma-Aldrich). Proteins were visualized by incubation with ECL detection reagents (Perkin-Elmer, Boston, MA) and exposed to film (Kodak Biomax MR, Rochester, NY). Membranes were stripped and reprobed.
Immunoprecipitation
For detection of c-Yes expression in tumor samples, 500 μg of the samples in 650 μl of RIPA buffer was incubated by rotation with 6 μl of antibody to total c-Yes overnight at 4°C. Fifty μL of a 1:1 slurry of protein G agarose in RIPA-B buffer was added and incubated with rotation for 1 additional hour at 4°C. Bound proteins were pelleted by centrifugation, washed three times with RIPA-B buffer, and eluted by boiling in 1× Laemmli’s sample buffer with subsequent immunoblotting with antibodies against c-Yes (1:1000).
Determination of Interleukin 8 (IL-8) and Vascular Endothelial Growth Factor (VEGF) Levels
Culture supernatants were centrifuged for 1 minute at 15,000 rpm to pellet debris and transferred to microcentrifuge tubes. Supernatants not assayed immediately were frozen at −80°C. Quantitative measurements of IL-8 and VEGF in the cell supernatants were determined using enzyme-linked immunosorbent assay (ELISA) kits (Quantikine Human IL-8 Immunoassay; R&D Systems, Minneapolis, MN; and Human VEGF Immunoassay kit; Biosource International, Inc., Camarillo, CA) following the manufacturers’ instructions. The detection limits of the IL-8 and VEGF ELISAs were 37 and 23.4 pg/ml, respectively. IL-8 and VEGF concentrations (picograms/milliliter) were determined spectrophotometrically at 450 nm using a TECAN Genios plate reader and Magellan version 4.0 software and normalized against total protein levels in the corresponding cell lysate. The results are presented as the means of triplicate determinations (±SD).
Animals
Specific, pathogen-free male nude mice (strain NCR-NU) were purchased from the Animal Production Area of the National Cancer Institute-Frederick Cancer Research and Development Center (Frederick, MD). The mice were housed and maintained in specific, pathogen-free conditions. The facilities have been approved by the American Association for Accreditation of Laboratory Animal Care and meet all current regulations and standards of the U.S. Department of Agriculture, the U.S. Department of Health and Human Services, and the National Institutes of Health. The mice were used between the ages of 8 and 12 weeks, in accordance with institutional guidelines.
Orthotopic Pancreatic Injections
For in vivo injection, cells were harvested from 10-cm tissue culture dish by a 2- to 3-minute treatment with 1× trypsin followed by suspension in a D-PBS solution. Only single-cell suspensions of greater than 90% viability, as determined by trypan blue exclusion, were used for injection. Male nude mice were anesthetized with methoxyflurane. A small left abdominal flank incision was made, and the spleen and pancreas were exteriorized. Tumor cells (5 × 105), including siRNA clones, vector, and wild-type parental controls, in D-PBS were injected subcapsularly into a region of the pancreas just beneath the spleen with a 27-gauge needle and 1-ml disposable syringe. To prevent intraperitoneal leakage, a cotton swab was held for 1 minute over the site of injection. Both layers of the abdominal wound were closed with wound clips (Autoclip; Clay Adams, Parsippany, NJ). A successful subcapsular intrapancreatic injection of tumor cells was identified by the appearance of a fluid bleb without intraperitoneal leakage. Mice were sacrificed via cervical dislocation 6 weeks after orthotopic injections.
Treatment of Established Human Pancreatic Tumors with Dasatinib
For these studies, we used dasatinib, a dual Src/Abl inhibitor currently in clinical trials for CML. Fourteen days after orthotopic injection of wild-type L3.6pl pancreatic tumor cells, the mice were randomized into two groups: treatment and control (n = 8). The treatment group received 15 mg · kg−1 · day−1 dasatinib, solubilized in a sodium citrate/citric acid buffer diluent, by oral gavage. The control group received citrate buffer diluent alone. All mice were sacrificed by cervical dislocation on day 42. Tumor volume (measured by caliper), weight, and incidence of regional (celiac or para-aortal) lymph node and liver metastases were recorded. Tissue not homogenized immediately for Western blot analysis was snap-frozen in liquid nitrogen and immediately frozen at −80°C. For immunohistochemical staining, a part of the tumor was embedded in OCT compound (Sakura Fineter, Torrance, CA), snap-frozen in liquid nitrogen, and stored at −80°C.
Immunohistochemical Determination of CD31/PECAM-1, Src, Phospho-Akt, and Phospho-Erk 44/42
Frozen tissues used for identification of CD31/PECAM-1 and Src (green fluorescence) were sectioned (8 to 10 μm), mounted on positively charged Plus slides (Fisher Scientific, Loughborough, UK), and air-dried for 30 minutes. The sections were fixed in cold acetone for 5 minutes, followed by 1:1 acetone:chloroform (v/v) for 5 minutes, and then acetone for 5 minutes. The sections were washed with PBS, and immunohistochemical staining for CD31 was performed as previously described.21 A positive reaction was visualized by incubating the slides in stable 3,3′-diaminobenzidine (DAB) for 10 to 20 minutes. The sections were rinsed with distilled water, counterstained with Gill’s hematoxylin for 1 minute, and mounted with Universal Mount (Research Genetics, Huntsville, AL). Control samples were exposed to secondary antibody alone and demonstrated no specific staining. Sections analyzed for Src were pretreated with goat anti-mouse IgG F(Ab)2 fragment (1:10 dilution in PBS) for 4 to 6 hours before incubation with the primary antibody. The samples were then incubated at 4°C for 18 hours with a 1:200 dilution of monoclonal mouse anti-human antibody for Src (Calbiochem). The samples were then rinsed three times for 3 minutes each with PBS and incubated at room temperature for 1 hour with a 1:200 dilution of secondary Alexa Fluor 488-conjugated anti-mouse antibody, avoiding exposure to light. All samples were washed twice with PBS containing 0.1% Brij and washed with PBS for 5 minutes, and nuclear staining was performed by incubating the samples with 300 mg/ml Hoechst dye diluted in PBS for 2 minutes. The nuclei were identified by blue staining, and Src was identified by green fluorescence. Control samples were exposed to secondary antibody alone and demonstrated no specific staining.
Paraffin-embedded tissues were used for identification of Src (DAB), phospho-Akt, and phospho-Erk 44/42. Sections (4 to 6 μm thick) were mounted on positively charged Superfrost slides (Fischer Scientific, Co., Houston, TX) and dried overnight. Sections were deparaffinized in xylene, then treated with a graded series of alcohol [100, 95, and 80% ethanol (v/v) in double distilled H2O], and rehydrated in PBS (pH 7.5). Sections were treated with 10 mmol/L citrate buffer, pH 6.0, and microwaved 10 minutes for antigen retrieval. Sections were blocked with 3% H2O2 in PBS for 12 minutes and washed with PBS (3 × 5 minutes). The sections were blocked with 4% fish gel for 20 minutes and then incubated with the appropriate primary antibody, anti-Src (Cell Signaling Technology), anti-phospho-Akt (Cell Signaling Technology), or anti-phospho-Erk 44/42 (Cell Signaling Technology) overnight at 4°C. Immunohistochemistry for Src was performed using Avidin-Biotin blocking (Biocare Medical, Concord, MA), followed by goat anti-rabbit biotinylation (Biocare Medical) and streptavadin-horseradish peroxidase incubation for 30 minutes each at room temperature. Immunohistochemistry for phospho-Akt and phospho-Erk 44/42 was performed using goat anti-rabbit biotinylation and streptavadin-horseradish peroxidase (DAKO, Glostrup, Denmark) incubation for 30 minutes each at room temperature. A positive reaction was visualized by incubating the slides in stable DAB for 10 to 20 minutes. The sections were rinsed with distilled water, counterstained with Gill’s hematoxylin for 1 minute, and mounted with Universal Mount (Research Genetics, Huntsville, AL). Control samples were exposed to secondary antibody alone and demonstrated no specific staining.
Immunofluorescence Microscopy
Immunofluorescence microscopy was performed using an epifluorescence microscope equipped with narrow band pass excitation filters mounted in a filter wheel (Ludl Electronic Products, Hawthorne, NY) to select for green fluorescence. Images were captured using a 3CCD camera (Photometrics, Tuscon, AZ) mounted on a Zeiss universal microscope (Carl Zeiss, Thornwood, NY) and Optimas Image Analysis software (Bioscan, Edmond, WA) installed on a Compaq computer with Pentium chip, frame grabber, an optical disk storage system, and a Mavigraph UP-D7000 digital color printer (Sony, Tokyo, Japan). Images were additionally processed using Adobe Photoshop software (Adobe Systems, Mountain View, CA). For the quantification of CD31 staining, 10 random 0.159-mm2 fields at ×100 magnification were captured for each tumor, and microvessels were quantified according to the method described previously.22,23
Statistical Analyses
The significance of differences in IL-8 and VEGF cytokine expression between cells was determined using a Student’s t-test (two tailed). The significance of differences in primary tumor growth and metastases was determined using Student’s t-test (two tailed), and vessel density in siRNA tumor samples versus control samples was determined using a Mann-Whitney U-test. A value of P < 0.05 was deemed significant.
Results
Expression of siRNA Specifically Reduces Src Levels
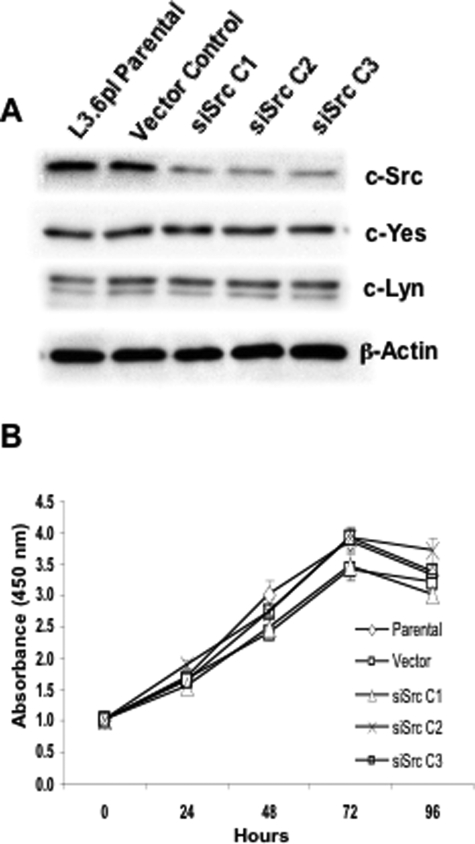
To directly determine the role of Src in regulating pancreatic tumor growth and metastasis, we used L3.6pl pancreatic cancer cells, a variant of COLO-357 selected for its high metastatic potential in orthotopic nude mouse models. We also examined subclones of these cells that expressed reduced c-Src levels due to stable transfection of a pSILENCER vector expressing a Src siRNA, as described in Materials and Methods. Several colonies from single cells were expanded, and SFK expression was determined. As shown in Figure 1A, Src levels were significantly reduced (4- to 5-fold) in each siRNA clone (siSrc C1, siSrc C2, and siSrc C3) when compared with parental and vector control cells. In contrast, there was no change in expression of the most related SFK member c-Yes or of c-Lyn. To examine whether cell proliferation in vitro was affected by decreased c-Src expression, proliferation rates were determined as described in Materials and Methods. As shown in Figure 1B, no significant differences were observed in proliferation rates of cells in log phase growth. Therefore, other SFKs likely play redundant roles to Src in regulating in vitro cell proliferation.
Figure 1.
c-Src siRNA causes a specific reduction in c-Src expression without affecting in vitro growth rates. A: Stable G418-resistant L3.6pl clones expressing either c-src-targeted siRNA (siSrc C1, C2, and C3) or vector controls were generated from parental L3.6pl cells as described in Materials and Methods. Fifty micrograms of RIPA cell lysates were resolved by 8% SDS-PAGE and Western blot analysis performed using anti-Src monoclonal antibody 327, anti-Yes, or anti-Lyn antibodies. Membranes were stripped and re-probed for β-actin as a loading control. Blots are representative of three experiments. B: siSrc C1, C2, and C3; parental L3.6pl cells; or cells expressing empty vector were plated on day 0 as described in Materials and Methods. 3-(4,5-Dimethyl-2-thiazol-2-yl) 2,5-diphenyltetrazolium bromide assays were performed 1, 24, 48, 72, and 96 hours after plating. Values represent the mean of three experiments.
Effects of Src siRNA on Signaling Intermediates
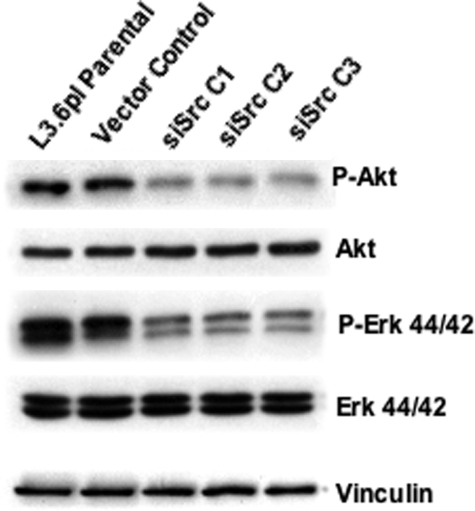
Several previous studies have demonstrated that Src activity can directly or indirectly regulate members of the MAPK family as well as phosphatidylinositol 3-kinase (PI3 kinase) activity. Therefore, the phosphorylation of p44/42 Erk and Akt (as a phosphatidylinositol 3-kinase substrate) was determined from cell lines expressing c-src-targeted siRNA. As seen in Figure 2, decreases in constitutive Akt and p44/42 Erk phosphorylation were observed in the Src siRNA clones relative to parental L3.6pl cells and vector controls, with no change in expression of these signaling enzymes.
Figure 2.
Reduced c-Src expression results in decreased activation of Akt and Erk 44/42. Parental L3.6pl cells or clonal variants expressing empty vector or c-src-targeted siRNA (siSrc C1 and C2) were plated and maintained as described in Figure 1. Twenty-four hours after plating, cells were serum-starved for 24 hours, and cell lysates were harvested as described in Materials and Methods. Expression of phosphorylated Akt and Erk 44/42 was determined by Western blot analysis. Blots were stripped and re-probed for total Akt and Erk 44/42, respectively. Anti-vinculin Western blot analysis was performed as a loading control. Blots are representative of three independent experiments.
c-Src Regulates IL-8 and VEGF Expression in L3.6pl Cells
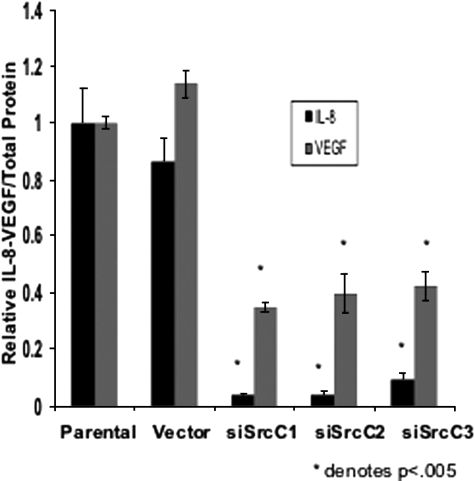
Recent evidence has suggested that c-Src may contribute to tumor growth/progression through regulation of pro-angiogenic molecules.24 To examine the specific role of c-Src in regulating IL-8 and VEGF expression (the most abundant pro-angiogenic molecules secreted by pancreatic tumor cells), expression of these cytokines was compared in siRNA clones and parental cells. IL-8 and VEGF levels were significantly reduced by approximately 14- and 3-fold, respectively, in comparison with parental cells and cells expressing vector alone (P < 0.005; Figure 3), corresponding with reduced Src expression. These results are consistent with those obtained by pharmacological inhibition of SFKs (data not shown), indicating that c-Src is required for maximal constitutive production of IL-8 and VEGF in L3.6pl pancreatic cancer cells.
Figure 3.
siRNA-mediated c-Src knockdown results in decreased expression of IL-8 and VEGF. siSrc C1, C2, and C3; parental L3.6pl cells; and vector controls were plated and maintained as described in Materials and Methods. Twenty-four hours after plating, the cell culture medium was replaced with serum-free medium and allowed to incubate for an additional 24 hours, at which point cell lysates and culture supernatants were harvested. IL-8 and VEGF levels were measured by ELISA as described in Materials and Methods and expressed relative to total cellular protein (picograms/milligram). Expression levels of both IL-8 (black bars) and VEGF (gray bars) were reduced significantly (P < 0.005, n = 3) relative to controls.
Effects of Decreased c-Src Expression on Tumor Incidence, Growth, and Metastatic Potential
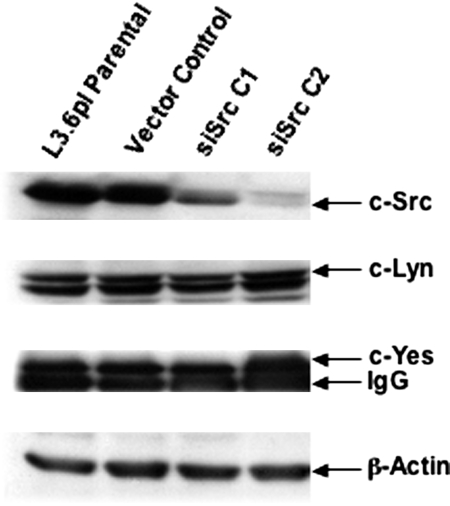
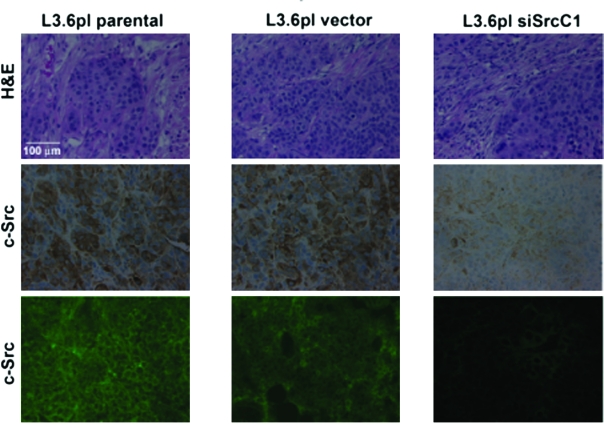
To examine effects on primary tumor incidence and tumor growth between parental, vector, and siRNA clones, serial dilutions of 1.25, 2.5, and 5.0 × 105 cells were injected into the pancreas as described in Materials and Methods. After 42 days, mice were sacrificed, and tumor incidence and size were determined. Tumors were removed and processed for Western blotting, immunofluorescence, and immunohistochemistry as described in Materials and Methods. To determine whether the tumors induced by siRNA clones maintained reduced Src expression, we performed immunoblotting on lysates from primary tumors (Figure 4) and immunofluorescence and immunohistochemistry for total Src expression (Figure 5). As observed by Western blotting, Src expression remained low in tumors, whereas protein levels of fellow Src family kinases Lyn and c-Yes were unchanged. These results demonstrate that expression of siRNA in primary tumor cells was stable and c-Src expression was specifically reduced over the period analyzed. Immunofluorescence and immunohistochemical staining of tumor samples indicated that the reduced levels of c-Src expression occurred specifically in tumor cells (Figure 5).
Figure 4.
siRNA-mediated reduction in c-Src expression is maintained in tumor cells grown in vivo. siSrc clones, parental L3.6pl cells, and vector controls were injected into the pancreases of nude mice (5 × 105 cells/mouse) on day 0. After 6 weeks, tumors were harvested and frozen in liquid nitrogen. Tumors were homogenized in RIPA, tumor cell lysates were quantitated, and 50 μg of total protein was resolved by 8% SDS-PAGE. Blots were probed for total c-Src and Lyn as described in Materials and Methods. Because of low expression of c-Yes, 500 μg of tumor lysates was immunoprecipitated with the anti-Yes antibody, followed by anti-Yes Western blot analysis. Anti-β-actin Western blot analysis was performed as a total protein loading control. Results are representative of at least three independent Western blots. A specific reduction in total c-Src levels was observed in the siRNA clones after 6 weeks growth in vivo in the absence of G418.
Figure 5.
Src expression is decreased in tumors produced from siRNA-expressing clones. L3.6pl parental, vector, and siSrc tumor samples were harvested and prepared for staining as described in Materials and Methods. Presence of tumors was confirmed by hematoxylin and eosin staining (top panels); Src expression was examined by immunohistochemistry (middle panels) and immunofluorescence (bottom panels) as described in Materials and Methods. The results displayed in the figure are representative of sections of all tumors obtained from L3.6pl parental (left panels), vector-transfected (middle panels), and siSrc (right panels; siSrcC1 pictured) tumors, in which Src was reduced by expression of an siRNA as described in Materials and Methods.
As shown in Table 1, at every cell number used as inoculum, no significant differences were observed in tumor incidence. These results suggest that reduction of Src expression was insufficient to inhibit tumor formation of L3.6pl cells. At lower inocula (1.25 × 105 cells), tumor sizes of parental and siRNA clones were relatively similar. However, whereas tumor size in parental cells increased proportionally to the increased number of cells implanted, this was not observed in tumors from the siRNA clones. Rather, the siRNA clones achieved a maximum tumor size at 2.5 × 105 cells injected, with an increased number of cells injected having no further effect on tumor size.
Table 1.
Effects of Cell-Dependent c-Src-Targeted siRNA on in Vivo Growth and Progression of Pancreatic Adenocarcinoma Cells
| Primary pancreatic tumors
|
Metastases
|
|||||
|---|---|---|---|---|---|---|
| Incidence | Mass (mg)
|
Lymph node | Liver | |||
| Mean | Median | Range | ||||
| 1.25 × 105 | ||||||
| Parental | 6/6 | 1250 | 1050 | 400 to 2700 | 6/6 | 1/6 |
| Vector | 7/7 | 2114 | 1900 | 800 to 4200 | 5/7 | 1/7 |
| siSrc | 7/7 | 1271 | 1100 | 700 to 2100 | 1/7* | 0/7 |
| 2.5 × 105 | ||||||
| Parental | 8/8 | 2075 | 1850 | 900 to 4000 | 7/8 | 4/8 |
| Vector | 5/5 | 3120 | 2900 | 1500 to 7500 | 5/5 | 3/5 |
| siSrc | 9/9 | 1700 | 1900 | 400 to 2600 | 2/9* | 0/9* |
| 5.0 × 105 | ||||||
| Parental | 7/7 | 2786 | 2800 | 1600 to 5300 | 6/7 | 3/7 |
| Vector | 6/6 | 1260 | 1600 | 500 to 1700 | 4/6 | 3/6 |
| siSrc | 10/10 | 1660 | 1450 | 400 to 2600 | 2/10* | 1/10* |
L3.6pl control cells or clonal variants stably expressing c-Src-targeted siRNA (1.25, 2.5, and 5.0 × 105 cells/mouse) were injected into the pancreases of nude mice on day 0. After 6 weeks, all mice were sacrificed and evaluated for primary tumors and liver and lymph node metastases.
P < 0.05, relative to controls.
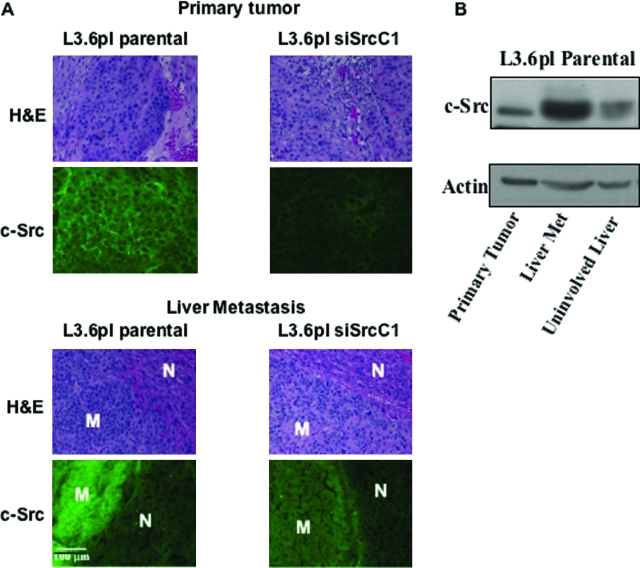
In mice injected with parental cells, 90% developed lymph node metastases, and 40% developed liver metastases (Table 1). Similar results were observed in vector controls (80% lymph node metastases; 40% liver metastases) (Table 1). In contrast, only 19% of mice injected with siRNA Src clones developed lymph node metastases (P < 0.05), and only 3% developed liver metastases (P < 0.05) (Table 1). The decreased incidence of metastasis was not due to tumor size, because the siRNA Src clones were still significantly reduced in incidence of metastasis at inocula of 1.25 × 105, where primary tumor sizes were similar between siRNA clones and control (Table 1). These results demonstrate that Src expression and/or activity regulate the ability of L3.6pl cells to metastasize. Immunofluorescence staining for Src expression in primary tumors and metastases is presented in Figure 6A. In liver metastases arising from parental cells, Src expression was substantially increased relative to that observed in primary tumors, consistent with changes in Src expression and activity observed in human colon tumors.25 This result was corroborated by anti-Src Western blot analysis of primary tumor samples, liver metastases, and uninvolved liver, demonstrating that total c-Src expression in L3.6pl liver metastases was substantially higher than in primary tumor or the surrounding uninvolved liver (Figure 6B). There was insufficient tissue from siSrc liver metastases to perform Western blot analysis. However, when metastases from siSrc clones were examined for Src expression via immunofluorescence, an increase was observed relative to that of primary tumors, although the expression was not as high as observed in metastases from parental cells (Figure 6A). These results suggest that some of the metastatic potential of the siSrc C1 clone may be due to escape of Src down-regulation by the siRNA expression vector.
Figure 6.
Comparison of c-Src expression in primary tumors and liver metastases. L3.6pl parental and siSrc primary tumor and liver samples (with and without metastases) were harvested and prepared for staining or Western blot analysis as described in Materials and Methods. A: Hematoxylin and eosin staining was performed to verify the presence of tumors and metastases, compared with uninvolved tissue. Src expression was examined by immunofluorescence. All staining was performed as described in Materials and Methods. For liver metastases, a comparison of Src staining in metastasis (M) is shown relative to uninvolved liver (N). The images shown are representative of sections obtained from at least five tumor samples. Results are shown for siSrc C1. B: Anti-Src Western blot analysis was performed on lysates of L3.6pl parental primary tumors, liver metastases, and normal liver as described in Materials and Methods.
c-Src siRNA Decreases Tumor Vascularity and Inhibits Activation of Signaling Intermediates Regulating Expression of Angiogenic Proteins
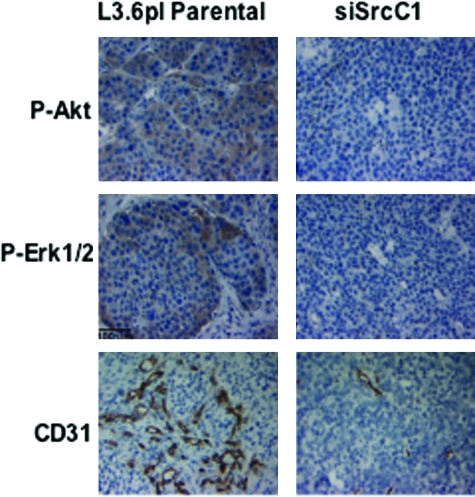
Vessel density in tumors induced by L3.6pl parental cells, vector-transfected cells, and stably transfected cells (siSrc C1) were also examined, as described in Materials and Methods. Consistent with the in vitro results demonstrating reduction of expression of pro-angiogenic molecules in vitro, vessels in tumors from siSrc clones, as determined by CD31/PECAM-1 staining, were significantly reduced (Figure 7). Parental L3.6pl tumors produced a mean vessel count of 14 ± 6 vessels/field compared with 16 ± 4 vessels/field for L3.6pl vector tumors and 5 ± 3 vessels/field for L3.6pl siSrc C1 tumors (n = 10; P < 0.0001). Immunofluorescence and immunohistochemistry were also performed for phospho-Akt and phospho-Erk 44/42 MAPK (Figure 7). Again, consistent with the in vitro results, phospho-Erk 44/42 and phospho-Akt levels were reduced in tumors produced from siSrc clones. Immunohistochemical staining verified that levels of phospho-Erk 44/42 and phospho-Akt were reduced specifically in siRNA-expressing tumor cells.
Figure 7.
Phospho-Akt, phospho-Erk 44/42, and CD31+ vessel infiltration are reduced in tumors from siRNA-expressing clones. L3.6pl parental and siSrc tumor samples were harvested and prepared for staining as described in Materials and Methods. Tumor samples were stained with phosho-Akt (top panels), phospho-p44/42 Erk (middle panels), or CD31 (bottom panels) antibodies to reveal activation of Akt, p44/42 Erk, and CD31+ vessel infiltration, respectively. Immunohistochemical staining was performed as described in Materials and Methods. The images shown are representative of sections obtained from at least five tumor samples. Results are shown for siSrc C1.
Treatment of Tumor-Bearing Mice with Dasatinib Inhibits Tumor Growth and Metastasis
Recently, the dual Src/Abl inhibitor dasatinib has been demonstrated to show efficacy against CML cells in vitro and in vivo.18 We therefore sought to determine whether this clinically relevant inhibitor would have efficacy in pancreatic tumor cells, and if so, whether it would have similar effects on development of metastases as observed in siRNA clones. In contrast to the siRNA clones, dasatinib inhibited activity of all Src family members in vitro (data not shown), consistent with previous findings on this pharmacological agent.26 To examine effects in vivo, 14 days after inoculating mice with 1 × 106 L3.6pl cells, mice were treated daily by oral gavage with dasatinib. As shown in Table 2, all mice developed primary tumors, but tumor size in the pancreas was significantly reduced (P < 0.05) as was incidence of metastasis, with only 14% of treated mice developing metastases compared with 60% of vehicle control mice. These results correspond with those obtained with the siRNA Src clones, suggesting that c-Src and/or Src family kinases are the likely therapeutic targets for dasatinib in this model.
Table 1.
Effects of c-Src-Targeted siRNA or the Src Family Kinase Selective Inhibitor Dasatinib on in Vivo Growth and Progression of Pancreatic Adenocarcinoma Cells
| Primary pancreatic tumors
|
Metastases
|
|||||
|---|---|---|---|---|---|---|
| Incidence | Mass (mg)
|
Lymph node | Liver | |||
| Mean | Median | Range | ||||
| Vehicle | 9/9 | 1486 | 1634 | (570 to 2600) | 5/9 | 3/9 |
| Dasatinib | 7/7 | 754* | 606 | (370 to 1900) | 1/7* | 0/7* |
L3.6pl cells were injected into the pancreas of nude mice (5 × 105 cells/mouse) on day 0. On day 14, 200 μl of dasatinib (15 mg/kg) or an equal volume of citrate buffer vehicle was administered by oral gavage. Treatments continued daily for 28 days. Mice were sacrificed on day 42 and evaluated for primary pancreatic tumors and liver and lymph node metastases.
P < 0.05, relative to controls.
Discussion
Despite aggressive treatment for pancreatic adenocarcinoma, the prognosis remains poor. The high mortality is in part due to micrometastatic disease that is not detected at the time of surgery. Thus, therapeutic strategies that control the spread of pancreatic carcinomas are especially critical to potential control of this disease. In this study, we have shown that Src activation affects pancreatic tumor progression through activation of several signaling molecules that are known to contribute to tumor cell survival and increased metastatic potential. To examine the specific role of Src in pancreatic tumor growth and progression, we first used an siRNA approach whereby Src was specifically and stably reduced in the highly metastatic L3.6pl cells. Whereas tumors develop in siRNA clones, even in equivalent sized tumors, the incidence of metastasis was much higher in wild-type and vector controls than in siRNA clones or in mice treated with dasatinib. These results suggest that expression and/or activation of Src contributes directly to metastatic potential.
Although it is likely that multiple pathways regulated by Src contribute to its role in invasion and metastasis, we have focused on the effect of Src on pro-angiogenic molecules. Recently, we have demonstrated that Src regulates expression of IL-8 and VEGF,13,14 both of which contribute to angiogenesis and tumor progression through paracrine effects on endothelial cells. Consistent with these results, Bruns et al27 demonstrated decreased growth and metastasis of L3.6pl cells in an orthotopic model by the EGF-R inhibitor PKI166, correlating with decreased IL-8 and VEGF expression.
Recently, Weis et al28 demonstrated another potential role for Src in regulation of angiogenesis critical to metastasis. Their results suggest that Src facilitates extravasation of tumor cells from its environment through disruption of the endothelial cell barrier function that potentiates tumor cell metastasis. In src-null mice, a significant reduction in VEGF-induced vascular permeability led to significant decreases in metastases in experimental and spontaneous lung tumor metastasis models.29 Thus, Src affects several properties consistent with the phenotype observed in this study, ie, development of small tumors impaired in growth and metastasis.
Other Src functions are also associated with development of metastasis. Src is a critical regulator of migration, and Src−/− cells are deficient in this process.29 Ito et al12 demonstrated that Src family kinases regulate expression of matrix metalloproteinases in pancreatic cancer cell lines and that decreasing SFK decreases invasiveness of these cells in vitro. Src activity also correlates with the loss of epithelial differentiation and cell adhesion system leading to increased metastatic potential of tumor cells.30,31 All of these properties are more consistent with Src regulating tumor progression rather than tumor development and are consistent with our results in the pancreatic cancer model used in this study.
In contrast, pharmacological inhibitors against Src family kinases have shown a combined effect on primary tumor growth as well as metastasis.17 Whether these are due to the pharmacological inhibition of other Src family members, because SFK function is required for proliferation, or reflect impairment of tumors to grow beyond a given size remains to be determined. Our results with dasatinib show that it acts very similarly to siRNA clones in which Src alone is reduced with respect to inhibition of metastases. It should be noted, however, that treatment with dasatinib resulted in a significant decrease in primary tumor size relative to controls, whereas siRNA clones were not significantly smaller than controls. This result is likely due to inhibition of all SFKs expressed in the tumor cells by dasatinib, although “off-target” inhibition that affects proliferation cannot be excluded. Nevertheless, the data demonstrate that Src-selective inhibitors may show efficacy in inhibiting tumor progression.
In summary, the data presented in this study suggest that Src plays an important role in pancreatic tumor metastases. Recently, Src has emerged as an attractive candidate molecule for targeted therapies, with development of several small molecule inhibitors of Src family kinases18,32 that may be of use in targeting pancreatic tumor growth and metastases, with an emphasis on combination therapies with standard chemotherapeutic agents.16,17 As shown by Duxbury et al,16 c-Src inhibition may serve the dual function of increasing the sensitivity of pancreatic tumors to established chemotherapeutic agents and inhibiting the ability of these tumors to metastasize. Together with the results presented here, these data suggest the possibility that c-Src represents an important candidate for targeted therapy in pancreatic cancer.
Footnotes
Address reprint requests to Gary E. Gallick, Ph.D., Department of Cancer Biology-179, the University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd., Houston, TX 77030. E-mail: ggallick@mdanderson.org.
Supported in part by grants from the National Institutes of Health (2RO1 CA65527, U54 CA 090810, and P20-CA10193 to G.E.G.; and T32 CA 09599 to J.G.T.), The Lockton Fund, and the Eleanor B. Pillsbury Fellowship-University of Illinois Hospital (to J.G.T.).
J.G.T. and J.M.S. contributed equally to this work.
References
- Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- Ward S, Morris E, Bansback N, Calvert N, Crellin A, Forman D, Larvin M, Radstone D. A rapid and systematic review of the clinical effectiveness and cost-effectiveness of gemcitabine for the treatment of pancreatic cancer. Health Technol Assess. 2001;5:1–70. doi: 10.3310/hta5240. [DOI] [PubMed] [Google Scholar]
- Maisey N, Chau I, Cunningham D, Norman A, Seymour M, Hickish T, Iveson T, O’Brien M, Tebbutt N, Harrington A, Hill M. Multicenter randomized phase III trial comparing protracted venous infusion (PVI) fluorouracil (5-FU) with PVI 5-FU plus mitomycin in inoperable pancreatic cancer. J Clin Oncol. 2002;20:3130–3136. doi: 10.1200/JCO.2002.09.029. [DOI] [PubMed] [Google Scholar]
- Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161–167. doi: 10.1038/sj.bjc.6600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palesty JA, Dudrick SJ. What we have learned about cachexia in gastrointestinal cancer. Dig Dis. 2003;21:198–213. doi: 10.1159/000073337. [DOI] [PubMed] [Google Scholar]
- Windham TC, Parikh NU, Siwak DR, Summy JM, McConkey DJ, Kraker AJ, Gallick GE. Src activation regulates anoikis in human colon tumor cell lines. Oncogene. 2002;21:7797–7807. doi: 10.1038/sj.onc.1205989. [DOI] [PubMed] [Google Scholar]
- Summy JM, Gallick GE. Src family kinases in tumor progression and metastasis. Cancer Metastasis Rev. 2003;22:337–358. doi: 10.1023/a:1023772912750. [DOI] [PubMed] [Google Scholar]
- Aligayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE. Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer. 2002;94:344–351. doi: 10.1002/cncr.10221. [DOI] [PubMed] [Google Scholar]
- Donato NJ, Wu JY, Stapley J, Gallick G, Lin H, Arlinghaus R, Talpaz M. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood. 2003;101:690–698. doi: 10.1182/blood.V101.2.690. [DOI] [PubMed] [Google Scholar]
- Chen T, Pengetnze Y, Taylor CC. Src inhibition enhances paclitaxel cytotoxicity in ovarian cancer cells by caspase-9-independent activation of caspase-3. Mol Cancer Ther. 2005;4:217–224. [PubMed] [Google Scholar]
- Coppola D. Molecular prognostic markers in pancreatic cancer. Cancer Control. 2000;7:421–427. doi: 10.1177/107327480000700504. [DOI] [PubMed] [Google Scholar]
- Ito H, Gardner-Thorpe J, Zinner MJ, Ashley SW, Whang EE. Inhibition of tyrosine kinase Src suppresses pancreatic cancer invasiveness. Surgery. 2003;134:221–226. doi: 10.1067/msy.2003.224. [DOI] [PubMed] [Google Scholar]
- Trevino JG, Summy JM, Gray MJ, Nilsson MB, Lesslie DP, Baker CH, Gallick GE. Expression and activity of Src regulate interleukin-8 expression in pancreatic adenocarcinoma cells: implications for angiogenesis. Cancer Res. 2005;65:7214–7222. doi: 10.1158/0008-5472.CAN-04-3858. [DOI] [PubMed] [Google Scholar]
- Summy JM, Trevino JG, Baker CH, Gallick GE. c-Src regulates constitutive and EGF-mediated VEGF expression in pancreatic adenocarcinoma cells through activation of phosphatidyl inositol-3 kinase and p38 MAPK. Pancreas. 2005;31:263–274. doi: 10.1097/01.mpa.0000178280.50534.0c. [DOI] [PubMed] [Google Scholar]
- Summy JM, Trevino JG, Lesslie DP, Baker CH, Shakespeare WC, Wang Y, Sundaramoorthi R, Metcalf CA, III, Keats JA, Sawyer TK, Gallick GE. AP23846, a novel and highly potent Src family kinase inhibitor, reduces vascular endothelial growth factor and interleukin-8 expression in human solid tumor cell lines and abrogates downstream angiogenic processes. Mol Cancer Ther. 2005;4:1900–1911. doi: 10.1158/1535-7163.MCT-05-0171. [DOI] [PubMed] [Google Scholar]
- Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. Inhibition of SRC tyrosine kinase impairs inherent and acquired gemcitabine resistance in human pancreatic adenocarcinoma cells. Clin Cancer Res. 2004;10:2307–2318. doi: 10.1158/1078-0432.ccr-1183-3. [DOI] [PubMed] [Google Scholar]
- Yezhelyev MV, Koehl G, Guba M, Brabletz T, Jauch KW, Ryan A, Barge A, Green T, Fennell M, Bruns CJ. Inhibition of SRC tyrosine kinase as treatment for human pancreatic cancer growing orthotopically in nude mice. Clin Cancer Res. 2004;10:8028–8036. doi: 10.1158/1078-0432.CCR-04-0621. [DOI] [PubMed] [Google Scholar]
- Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- Bruns CJ, Harbison MT, Kuniyasu H, Eue I, Fidler IJ. In vivo selection and characterization of metastatic variants from human pancreatic adenocarcinoma by using orthotopic implantation in nude mice. Neoplasia. 1999;1:50–62. doi: 10.1038/sj.neo.7900005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad SA, Liu W, Jung YD, Fan F, Wilson M, Reinmuth N, Shaheen RM, Bucana CD, Ellis LM. The effects of angiopoietin-1 and -2 on tumor growth and angiogenesis in human colon cancer. Cancer Res. 2001;61:1255–1259. [PubMed] [Google Scholar]
- Rak J, Filmus J, Kerbel RS. Reciprocal paracrine interactions between tumour cells and endothelial cells: the ‘angiogenesis progression’ hypothesis. Eur J Cancer. 1996;32A:2438–2450. doi: 10.1016/s0959-8049(96)00396-6. [DOI] [PubMed] [Google Scholar]
- Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90:447–454. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- Radinsky R, Risin S, Fan D, Dong Z, Bielenberg D, Bucana CD, Fidler IJ. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin Cancer Res. 1995;1:19–31. [PubMed] [Google Scholar]
- Lesslie DP, Gallick GE. Src family kinases as regulators of angiogenesis: therapeutic implications. Curr Cancer Therapy Rev. 2005;1:45–50. [Google Scholar]
- Talamonti MS, Roh MS, Curley SA, Gallick GE. Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer. J Clin Invest. 1993;91:53–60. doi: 10.1172/JCI116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo LJ, Lee FY, Chen P, Norris D, Barrish JC, Behnia K, Castaneda S, Cornelius LA, Das J, Doweyko AM, Fairchild C, Hunt JT, Inigo I, Johnston K, Kamath A, Kan D, Klei H, Marathe P, Pang S, Peterson R, Pitt S, Schieven GL, Schmidt RJ, Tokarski J, Wen ML, Wityak J, Borzilleri RM. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)-thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- Bruns CJ, Solorzano CC, Harbison MT, Ozawa S, Tsan R, Fan D, Abbruzzese J, Traxler P, Buchdunger E, Radinsky R, Fidler IJ. Blockade of the epidermal growth factor receptor signaling by a novel tyrosine kinase inhibitor leads to apoptosis of endothelial cells and therapy of human pancreatic carcinoma. Cancer Res. 2000;60:2926–2935. [PubMed] [Google Scholar]
- Weis S, Cui J, Barnes L, Cheresh D. Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol. 2004;167:223–229. doi: 10.1083/jcb.200408130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscuoli ML, Nguyen M, Eliceiri BP. Tumor metastasis but not tumor growth is dependent on Src-mediated vascular permeability. Blood. 2005;105:1508–1514. doi: 10.1182/blood-2004-06-2246. [DOI] [PubMed] [Google Scholar]
- Nam JS, Ino Y, Sakamoto M, Hirohashi S. Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin Cancer Res. 2002;8:2430–2436. [PubMed] [Google Scholar]
- Boyer B, Bourgeois Y, Poupon MF. Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene. 2002;21:2347–2356. doi: 10.1038/sj.onc.1205298. [DOI] [PubMed] [Google Scholar]
- Tsygankov AY, Shore SK. Src: regulation, role in human carcinogenesis and pharmacological inhibitors. Curr Pharm Des. 2004;10:1745–1756. doi: 10.2174/1381612043384457. [DOI] [PubMed] [Google Scholar]