Abstract
Pre-existing amyloid fibrils can induce further polymerization of endogenous precursor proteins in vivo. Thus, transmission of amyloid fibrils (AApoAII) may induce a conformational change in endogenous apolipoprotein A-II and accelerate amyloid deposition in mouse senile amyloidosis. To characterize transmissibility, we examined amyloidosis in the offspring of AApoAII-injected mother mice that possessed the amyloidogenic Apoa2c allele of the apolipoprotein A-II gene. At 4 months of age, amyloid deposits were detected in the intestines of offspring born from and nursed by amyloid fibril-injected mothers, with intensity of deposition increasing thereafter. No amyloid deposits were detected in the offspring of noninjected control mothers. Accelerated amyloidosis was also observed in offspring born from mothers without injection but nursed by amyloid fibril-injected mothers. However, this was not observed in offspring born from amyloid fibril-injected mothers but nursed by control mothers. This fostering excluded vertical transmission through the placenta, suggesting the presence of factors that accelerate amyloidosis during the nursing period. In addition, milk obtained from amyloid fibril-injected mothers induced AApoAII amyloidosis in young mice, and transmission electron microscopy detected noodle-like amyloid fibrils in milk of amyloid fibril-injected mothers. These results provide important insight into the etiology and pathogenesis of amyloid diseases.
Amyloidoses are a group of diseases caused by the structural disorder of proteins in which normally soluble proteins are deposited in tissues as highly ordered, insoluble amyloid fibrils made up of β-pleated sheets.1 Several serious human diseases are associated with amyloid fibril deposition, such as Alzheimer’s disease, type II diabetes, prion diseases, and familial amyloid polyneuropathy (FAP).2,3 Many factors, such as aging, primary sequences and mutations of amyloid proteins, and the genetic background of patients, and epigenetic factors, including the composition of food and conditions of rearing, may influence fibril formation and deposition in tissues. Nucleation-dependent polymerization or seeding is postulated as a model of fibril formation in several kinds of amyloidoses, including prion diseases.4,5 Prions, abnormal form of the host cellular prion protein (PrPC), are responsible for transmissible spongiform encephalopathies. These include scrapie in sheep, bovine spongiform encephalopathy, and human Creutzfeldt-Jakob disease.6,7 The now widely accepted prion protein hypothesis suggests that the amyloidogenic and protease-resistant form (PrPsc) of the protein propagates itself by inducing a conformational change in the normal, protease-sensitive PrPC.8,9 Thus, transmission of amyloid fibrils may influence fibril formation as an important epigenetic factor.10
In mice, spontaneous senile amyloidosis has been found in many strains.11 We isolated a unique amyloid fibril protein from the liver of senescence-accelerated mouse prone-1 (SAMP1) mice. In this strain, apolipoprotein A-II (apoA-II), the second most abundant apoprotein of serum high density lipoprotein, is deposited systemically in the form of amyloid fibrils (AApoAII), although not in the brain.12,13 Three major alleles (Apoa2a, Apoa2b, and Apoa2c) of the apoA-II gene encode three variants of the apoA-II protein.14,15 Mice such as SAMP1, A/J, SJL/J, SM/J, and the congenic R1.P1-Apoa2c strain,16 which have an Apoa2c encoding C-type apoA-II (APOAIIC, Gln5, and Ala38), show a high incidence of severe senile amyloidosis.11,17 Previously, we described the prion-like transmission of AApoAII amyloidosis in which intravenous and peripheral injection of AApoAII fibrils markedly accelerated amyloid deposition in young R1.P1-Apoa2c mice.18 We also reported a study in which young R1.P1-Apoa2c mice fed with AApoAII amyloid fibrils or those reared in the same cage as older R1.P1-Apoa2c mice with severe amyloid deposits proceeded to develop amyloid deposits.19 Injection of AApoAII fibrils also induced amyloidosis in less amyloidogenic mouse strains with Apoa2a or Apoa2b alleles.20,21 Prion-like transmission was also reported in mouse inflammation-associated amyloid A (AA) amyloidosis.22,23
Natural scrapie in sheep has been shown to transmit laterally to goats when they are closely confined for long time periods with a succession of natural sheep scrapie cases.24 The likelihood of maternal transmission of natural sheep scrapie from an infected ewe to her lamb has also been shown.25,26 It is also believed that scrapie is an endemic disease of goats that appears to propagate at least partly through maternal transmission.27 However, a recent report showed that experimentally induced bovine spongiform encephalopathy did not transmit via goat embryos,28 indicating the complex nature of this issue. On the other hand, genetic anticipation was observed in Met30-transthyretin-related type I FAP from Portugal, Sweden, and Japan.29–31 A tendency toward an earlier age of onset and increased severity of clinical symptoms among younger generations has been recognized in families. Notably, descendants of affected mothers appear to be more prone to anticipation than descendants of affected fathers. The molecular basis of anticipation in FAP has not yet been elucidated.
The purpose of the study presented here was to examine whether AApoAII amyloid deposition could be accelerated in offspring born and nursed from amyloidosis-laden R1.P1-Apoa2c mice. We also examined the amyloidosis-inducing effects of milk obtained from mothers with amyloidosis. These phenomena provide new insight into the process of transmission of amyloidosis.
Materials and Methods
Animals
R1.P1-Apoa2c mice, a congenic strain of mice with the amyloidogenic Apoa2c allele of the SAMP1 strain on the genetic background of the SAMR1 strain,16 were maintained by brother-sister mating in the Division of Laboratory Animal Research, Research Center for Human and Environmental Science, Shinshu University. Mice were raised under specific pathogen-free conditions at 24 ± 2°C with a light-controlled regimen (12-hour light/dark cycle). A commercial diet (MF; Oriental Yeast, Tokyo, Japan) and tap water were provided ad libitum. All experiments were performed with the consent of the Animal Care and Use Committee of Shinshu University School of Medicine and conducted in accordance with the guidelines for the use of laboratory animals at Shinshu University.
Isolation of AApoAII Amyloid Fibrils from Mouse Tissues
The AApoAII amyloid fibril fraction was isolated as a water suspension from the livers of 20-month-old R1.P1-Apoa2c mice as described previously.18 Isolated amyloid fibril fractions were further purified by ultracentrifugation.20 Purified amyloid fibrils were resuspended at a concentration of 1.0 mg/ml in distilled water (DW). One milliliter of this solution was put into an Eppendorf tube and sonicated on ice for 30 seconds with an ultrasonic homogenizer VP-5S (Tietech Co., Ltd., Tokyo, Japan) at power 4. This procedure was repeated five times at 30-second intervals. Sonicated amyloid samples (100 μg) were then immediately injected into the tail vein of mice to induce AApoAII amyloidosis.
Detection of Amyloid Deposition
Deposition of amyloid fibrils was identified by the appearance of green birefringence in Congo Red-stained sections under polarizing microscopy. Amyloid fibril proteins, AApoAII and AA proteins, were identified immunohistochemically using the avidin-biotinylated horseradish peroxidase complex method with specific antiserum against mouse apoA-II or AA.13 The intensity of AApoAII amyloid deposition was determined semiquantitatively using the amyloid index (AI). The AI was determined by taking the mean value of the scores of amyloid deposition (graded 0 to 4) in the seven major tissues (liver, spleen, tongue, heart, intestine, stomach, and skin) stained with Congo Red as described previously.19
Evaluation of Maternal and Paternal Transmission
In the first experiment, 2-month-old female R1.P1-Apoa2c mice were amyloidosis-induced by intravenous injection with AApoAII amyloid fibrils. Control mice were injected with DW. Three months after injection, amyloid fibril-injected and control mice were mated with normal male mice until pregnancy was confirmed. Offspring born from amyloid fibril-injected or control mother mice were nursed for 21 days and then weaned. Offspring were sacrificed at 4, 6, and 8 months of age by cardiac puncture under diethyl ether anesthesia, and the amount of amyloid deposition was determined. Part of the seven major tissues were fixed in 10% neutral buffered formalin, embedded in paraffin, and cut into 4-μm sections for Congo Red staining and immunohistochemstry (Figure 1A). In the second experiment, offspring born from amyloid fibril-injected and control mothers were exchanged just after their birth. Thus, offspring of amyloid fibril-injected mothers were nursed by control mothers, and offspring of control mothers were nursed by amyloid fibril-injected mothers. Weanlings were separated from their foster mothers after 21 days of lactation and sacrificed at 4, 6, and 8 months of age, and their tissues were fixed in 10% neutral buffered formalin.
Figure 1.
A schematic of events leading from the induction of amyloidosis in parental mice until the determination of amyloid deposition in offspring. Solid and dotted lines represent parents with and without induction of amyloidosis, respectively. Offspring (thinner solid lines) were sacrificed at the ages shown by arrowheads. A: Analysis of maternal transmission. 1, female 2-month-old R1.P1-Apoa2c mice were amyloidosis induced by intravenous injection with AApoAII amyloid fibrils. Control mice were injected with DW. Three months after injection, mice were mated with normal male mice. Offspring were nursed for 21 days, weaned, and sacrificed at 4, 6, 8, and 12 months of age. 2, offspring born from amyloid fibril-injected and control mothers were exchanged just after birth. B: Paternal transmission. Male 2-month-old R1.P1-Apoa2c mice were induced for amyloidosis. Three months after induction, the male mice were mated with normal female mice. The offspring were separated from their parents at the age of 21 days. C: Analysis of induction by milk. Milk of amyloid fibril-injected mother was injected into 1-month-old R1.P1-Apoa2c mice intraperitoneally.
Male 2-month-old R1.P1-Apoa2c mice were induced for amyloidosis by intravenous injection with AApoAII amyloid fibrils. Three months after injection, the male amyloid fibril-injected mice were mated with normal female mice. Male mice were kept with female mice throughout the entire period of pregnancy, parturition, and nursing. The offspring were separated from their parents at the age of 21 days. Offspring were sacrificed at 4, 6, and 8 months of age, and amyloid deposition was determined histochemically (Figure 1B).
Induction of Amyloidosis by Milk
Female 2-month-old R1.P1-Apoa2c mice were induced for amyloidosis by intravenous injection with amyloid fibrils. Three months after injection, amyloid fibril-injected female mice mated with normal male mice, delivered litters, and nursed them. During the lactation period (7 to 10 days after delivery), mother mice were anesthetized by intraperitoneal injection of 1.0 mg of pentobarbital sodium salt (Nakarai Tesque, Kyoto, Japan) after an 8-hour separation from their pups. Milk was collected by suction with a vacuum pump (KNF Neuberger, Freiburg, Germany) after subcutaneous injection of 0.18 U of oxytocin (Wako Chemical, Tokyo, Japan). Ten microliters of milk from amyloid fibril-injected or control mothers was injected intraperitoneally into 1-month-old R1.P1-Apoa2c mice. Three months after injection, mice were sacri-ficed, and tissues were treated as described above (Figure 1C).
Examination of Induction of Amyloidosis by Denatured Milk
Milk from amyloid fibril-injected mothers was denatured in a solution of 6 mol/L guanidine hydrochloride, 0.1 mol/L Tris-HCl (pH 10.0), and 50 mmol/L dithiothreitol (1.0 mg/ml) for 24 hours with gentle stirring at room temperature. Denatured milk was dialyzed quickly against 10 mmol/L NH4HCO3 solution. The solution was used immediately for injection. Three months after injection, mice were sacrificed, and tissues were treated as described above.
Isolation of AApoAII Amyolid Fibrils from Milk
AApoAII (C) amyloid fibril fractions were isolated from the milk of amyloid fibril-injected mothers using the method of Pras.32 Aliquots of 50 μl of milk were diluted with 450 μl of 0.15 mol/L NaCl solution. After centrifugation at 40,000 × g at 4°C for 20 minutes, the pellet was obtained. The pellet was rinsed with distilled water, and centrifugation was repeated at 40,000 × g at 4°C for 20 minutes. The amyloid fraction in the supernatant was obtained and used for Western blotting and transmission electron microscopy analysis.
Analysis of Milk and Urine Proteins
Milk was obtained from amyloid fibril-injected, lactating R1.P1-Apoa2c mice 4 months after injection and stored frozen at −70°C. Four months after injection of AApoAII amyloid fibrils, R1.P1-Apoa2c mice were housed overnight in a metabolic cage (Natsume, Tokyo, Japan) for urine collection from 8:00 p.m. to 9:00 a.m. of the following day. Food and water were supplied ad libitum. Urine was collected in the morning and centrifuged for 5 minutes at 3000 rpm to discard any food or cellular debris. The urine samples were then frozen at −80°C. Samples were centrifuged at 100,000 × g for 1 hour at 4°C after dialysis against phosphate-buffered saline. Pellets were then resuspended in sterile water. Tris-Tricine/sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed on 16.5% acrylamide gels.32 The duplicated part of the gel was electrophoretically transferred to a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Hercules, CA). Protein bands reacting with anti-apoA-II33 antibody were visualized using biotinylated pig anti-rabbit immunoglobulin, avidin combined with horseradish peroxidase, and 3,3-diaminobenzidine.21
Induction of Amyloidosis by Urine
Samples of 10 μl of urine from amyloid fibril-injected mothers were injected intraperitoneally into 1-month-old R1.P1-Apoa2c mice. Three months after injection, mice were sacrificed, and tissues were treated as described above.
Transmission Electron Microscopy
Aliquots of 5 μl of amyloid fibril fraction isolated from milk were diluted with 45 μl of distilled water, and 20-μl aliquots of the diluted fraction were applied to 400-mesh collodion-coated copper grids (Nissin EM Co., Ltd., Tokyo, Japan) for 1 minute and subjected to negative staining with 20 μl of 1% phosphotungstic acid (pH 7.0) for 1 minute. The negatively stained samples were observed with an electron microscope (1200 EX; JEOL, Tokyo, Japan) operated at 80 kV.
Statistical Analysis
We used the StatView software package (Abacus Concepts, Berkley, CA). Significant differences in the value of amyloid indices among the various groups of mice were examined using the Mann-Whitney U-test.
Results
Maternal Transmission of AApoAII Amyloidosis
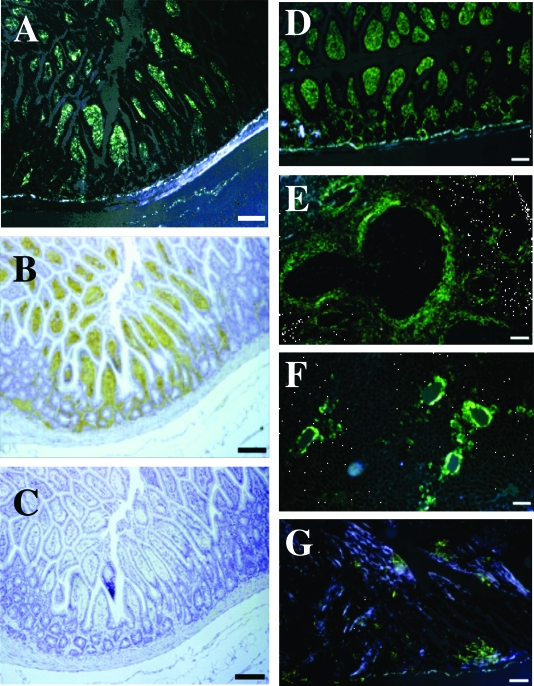
To determine whether amyloidosis in mothers predisposes offspring to development of amyloidosis and to study the factors that may play a role in this possible linkage, we compared the progress of amyloidosis in various offspring. At 4 months of age, mild amyloid deposition was detected by the presence of green birefringence in Congo Red-stained tissue from the intestines of several mice (7 of 10) born from amyloid fibril-injected mothers (Figure 2A). Amyloid fibrils were observed in the intestinal villi, which stained positively with anti-apoA-II antiserum and negatively with anti-AA antiserum (Figure 2, B and C). Amyloid deposits were observed at 6 and 8 months of age in all offspring from amyloid fibril-injected mothers. Amyloid deposits were first observed in the intestine and then were seen to extend to the tongue, liver, spleen, heart, and stomach (Figure 2, D–G). The intensity of amyloid deposition in these mice increased with age (Figure 3A). By contrast, no deposition was observed in offspring born from control mothers at 4 or 6 months of age. Up until 8 months of age, the degree of amyloidosis in offspring of amyloid fibril-injected mothers was significantly higher than that of offspring from control mothers. The degree of amyloidosis in offspring nursed by amyloid fibril-injected or control foster mothers was compared (Figure 3B). Amyloid fibril deposition was detected in the intestine of offspring from control mothers fostered to amyloid fibril-injected mothers at 4 months of age in 5 of 10 animals, and the degree of deposition became more severe with increasing age. On the other hand, in offspring from amyloid fibril-injected mothers fostered to control mothers, none had any amyloid deposits at 4 months of age and only one of nine had amyloid deposits at 6 months of age. Thus, significant acceleration of amyloidosis was observed at 4, 6, and 8 months of age (P < 0.05) in offspring of control mothers nursed by amyloid fibril-injected foster mothers. However, no significant difference could be found between the degree of amyloidosis in mice born from or nursed by amyloid fibril-injected mothers and those born from normal mothers and nursed by amyloid fibril-injected mothers.
Figure 2.
Amyloid deposition in mice born from amyloid fibril-injected mothers. Amyloid deposition was histologically detected by green birefringence in Congo Red-stained sections (A, D, E, F, and G), and amyloid proteins were immunohistochemically identified with anti-apoA-II (B) and anti-AA antiserum (C). A: Amyloid deposit (grade 2) in the intestine of a 4-month-old offspring. B: AApoAII deposits identified in the intestine by staining with anti-apoA-II antiserum. C: No AA was detected in the intestine by staining with anti-AA antiserum. Amyloid deposition in the small intestine (D) (grade 4), spleen (E) (grade 3), liver (F) (grade 3), and heart (G) (grade 2) of 8-month-old offspring. Scale bars = 100 μm.
Figure 3.
. Intensity of amyloid deposition in mice born from amyloid fibril-injected mothers. A: Intensity of amyloid deposition was determined in offspring mice of amyloid fibril-injected (•) or control mothers (▵). Litters of 10, 7, and 11 offspring from amyloid fibril-injected mothers and 7, 3, and 9 offspring from control mothers were sacrificed at the ages of 4, 6, and 8 months, respectively. AI was determined from Congo Red-stained sections from seven tissues, plotted versus age. *P < 0.05; **P < 0.01. B: Intensity of amyloid deposition in R1.P1-Apoa2c mice born from control mothers and nursed by amyloid fibril-injected foster mothers (•) and mice born from amyloid fibril-injected mothers and nursed by control foster mothers (○). Ten, 7, and 8 offspring nursed by amyloid fibril-injected mothers and 5, 9, and 5 offspring nursed by control mothers were sacrificed at the age of 4, 6, and 8 months, respectively. AI determined from Congo Red-stained sections from seven tissues was plotted versus age. *P < 0.05; **P < 0.01.
Amyloidosis in Offspring of Amyloid Fibril-Injected Male Mice
At 4 months of age, 20% of offspring (2 of 10) born from amyloid fibril-injected fathers and nursed by normal mothers in the same cage with the father mice had slight amyloid deposits in the intestine. However, no significant difference was observed in the degree of amyloidosis compared with control mice born from parents without amyloidosis (Table 1). The intensity of amyloidosis in offspring from amyloid fibril-injected fathers was significantly lower than that of offspring from amyloid fibril-injected mothers (P = 0.023).
Table 1.
AApoAII Amyloid Deposits in Offspring of Amyloid Fibril-Injected Parent Mice
| Amyloid fibril-injected parents | Case/at risk | AI | P value (AI versus mother) |
|---|---|---|---|
| Mother | 7/10 | 0.22 | |
| Male | 2/10 | 0.04 | P < 0.05 |
| Control* | 0/10 | 0 | P< 0.01 |
Amyloid deposits in the offspring at 4 months of age. Offspring were born from mother or father mice injected with amyloid fibrils. They were nursed for 21 days then weaned.
Ten control mice were born from uninjected normal parental mice.
Induction of Amyloidosis by Milk of Amyloid Fibril-Injected Mothers
Milk was collected from amyloid fibril-injected mothers and injected into 1-month-old R1.P1-Apoa2c mice. At 3 months after injection, amyloid deposition was observed in the tongue, lungs, and intestine (AI = 0.27) in 9 of 11 mice (Table 2). In contrast, three mice injected with denatured milk from amyloid fibril-injected mothers and five control mice injected with milk from mothers without amyloidosis showed no amyloid deposition. The degree of amyloid deposition in mice injected with amyloid fibril-injected mothers’ milk was significantly higher than that in mice injected with normal milk (P = 0.011).
Table 2.
AApoAII Amyloid Deposits in Mice Injected with Milk or Urine of Amyloid Fibril-Injected Mother Mice
| Milk* | Case/at risk | AI | P value (AI versus control) |
|---|---|---|---|
| Amyloidosis | 9/11 | 0.27 | P< 0.01 |
| Control† | 0/5 | 0 | |
| Denatured‡ | 0/3 | 0 | |
| Urine | 0/3 | 0 |
Milk was obtained from amyloid fibril-injected mother mice or normal control mice. Urine was obtained from amyloid fibril-injected mothers. R1.P1-Apoa2cmice at 1 month of age were injected with milk or urine intraperitoneally. Mice were killed at the age of 4 months, and amyloid deposits were determined.
Five control mice were injected with milk of normal mother mice.
Three mice were injected with denatured milk of amyloid fibril-injected mothers.
Induction of Amyloidosis by Urine of Amyloid Fibril-Injected Mothers
To investigate the effects of urine on amyloid induction, we injected the urine of amyloid fibril-injected mothers. No amyloid deposition was observed at 4 months of age after injection (Table 2).
AApoAII Fibrils in the Milk and Urine
The milk and isolated amyloid fraction were subjected to 16.5% Tris-Tricine/SDS-PAGE. Several bands were detected in the Coomassie Brilliant Blue R-250-stained gels. Subsequent Western blotting analysis showed major immunoreactive bands with molecular weights of approximately 8 and 16 kd, corresponding to apoA-II monomer and dimer, respectively, in milk from amyloid fibril-injected mothers. In contrast, no bands were detected in milk from control mothers (Figure 4A). ApoA-II monomers and dimers were concentrated in the amyloid fibril fraction from amyloid fibril-injected mothers. Urine was collected from amyloid fibril-injected and control mice, dialyzed against phosphate-buffered saline, and centrifuged at 100,000 × g for 1 hour. No obvious bands were found to react specifically with anti-apoA-II antisera in whole urine samples or pellets from urine samples of control or amyloid fibril-injected mice (Figure 4B).
Figure 4.
Detection of apoA-II protein in milk and urine. Western blotting analysis of milk and urine from amyloid fibril-injected R1.P1-Apoa2c mice, detected with anti-apoA-II antiserum. A: Lane 1, kaleidoscope prestained standard markers. Lanes 2 and 3, 1 μl of Coomassie Brilliant Blue R 250-stained milk from an amyloid fibril-injected mother (6 months old) and control mother (6 months old), respectively. Lanes 4, 5, 6, 7, 8, 9, and 10, Western blotting analysis of milk reacted with anti-apoA-II antiserum. Lane 4, 1 μl of milk from an amyloid fibril-injected mother; Lane 5, 1 μl of milk from a control mother. Lane 6, 50 μg of proteins from 0.15 mol/L NaCl supernatant centrifuged at 40,000 × g from the milk from an amyloid fibril-injected mother. Lane 7, 20 μg of proteins from the water suspension (amyloid fibril) fraction of milk from an amyloid fibril-injected mother. Lane 8, 50 μg of proteins from the 0.15 mol/L NaCl supernatant of milk from a control mother. Lane 9, 20 μg of proteins from the water suspension fraction of milk from a control mother. Lane 10, AApoAII amyloid fibril. B: Lane 1, 100 μg of proteins from lyophilized whole urine of amyloid fibril-injected mice; lane 2, 100 μg of proteins from lyophilized whole urine of control mice; lane 3, pellets centrifuged at 100,000 × g from 4.0 ml of urine from amyloid fibril-injected mice; lane 4, pellets from urine of control mice; and lane 5, AApoAII amyloid fibril.
Transmission Electron Microscopy of Amyloid Fibrils in Milk
To confirm the existence of amyloid fibrils in milk, we observed negatively stained milk from amyloid fibril-injected mothers by transmission electron microscopy. The noodle-like amyloid fibrils appeared as ∼10 nm in width and ∼135 nm in length (Figure 5).
Figure 5.
Transmission electron microscopy images of amyloid fibrils extracted from milk of an amyloid fibril-injected mother. Scale bar = 100 nm.
Discussion
In this study, we induced amyloidosis in female mice by injection of AApoAII amyloid fibrils to evaluate the possibility of the vertical transmission of amyloidosis. Amyloid deposition was significantly accelerated in offspring from amyloid fibril-injected mothers. Initial amyloid deposition was found in the intestines of these offspring. We previously observed initial amyloid deposition in the intestines after oral injection of amyloid fibrils and in the tongue and lungs after intravenous injection.19,35 These results suggested the possibility of oral transmission of AApoAII fibrils to offspring. Thus, we synchronized the pregnancy of amyloid fibril-injected and control female mice and exchanged their pups immediately after delivery. Acceleration of amyloidosis was seen only in the offspring that were nursed by amyloid fibril-injected mice. These findings confirmed lateral postnatal events rather than in utero transmission. Relatively large amounts of amyloid deposits were observed mainly around vessels in the uterus and ovaries, but no amyloid deposits were observed in the placenta or fetus (data not shown).
Before this study, maternal or lateral transmission of nonprion amyloidosis had not been observed. However, tendencies for an earlier age of onset and increased severity of clinical symptoms among younger rather than older generations have been recognized in type I FAP. Simple intake of amyloid fibril transthyretin may not explain the differences in the mean age at onset or the progression of amyloidosis in FAP.36,37 Offspring of mothers with AA amyloidosis do not show a predisposition to developing AA amyloidosis.38 Transmission from mother to child has not been observed in human Creutzfeldt-Jakob disease. However, offspring of scrapie-affected ewes show a higher risk of developing scrapie than those from asymptomatic ewes.27 Recent studies have contradicted the theory that transmission occurs via embryos29 and suggested an early lateral postnatal event as the pathway of scrapie transmission from ewes to lambs.39 Our observation that the degree of amyloid deposits was significantly greater in offspring from amyloid fibril-injected mothers compared with male mice encouraged us to evaluate the amyloidogenicity of milk from amyloid fibril-injected mothers. In SDS-PAGE and Western blotting analyses, we only detected apoA-II protein in amyloid fibril fraction isolated from milk of amyloid fibril-injected mice. Injection of milk collected from amyloid fibril-injected mothers into young R1.P1-Apoa2c mice induced amyloidosis, whereas denatured milk did not. In addition, the noodle-like amyloid fibrils in milk from amyloid fibril-injected mothers were detected by transmission electron microscopy. These results indicated that the amyloid fibrils in milk served as a seed for the acceleration of amyloidosis. The AApoAII amyloid deposition found in the intralobular tissue in the mammary glands of R1.P1-Apoa2c mice suggests a direct origin from the mammary glands (data not shown).
We revealed the lateral transmission of AApoAII amyloidosis from amyloidosis-laden aged mice to young R1.P1-Apoa2c mice and proposed a theory of oral-fecal transmission of amyloid fibrils.19 In this study, pups were nursed by amyloid fibril-injected mothers for 21 days and weaned. The possibility that pups ate or licked feces containing amyloid fibrils from the mothers should be considered. When pups of normal mothers were nursed for 21 days and then nursed for 5 more days by amyloid fibril-injected mothers, there was no observable acceleration in amyloid deposition (data not shown). Thus, the contribution of oral-fecal transmission of amyloidosis observed here might be minimal.
A protease-resistant prion protein isoform was reported in the urine from scrapie-infected hamsters, but this isoform did not cause clinical signs of prion disease after inoculation.40 Because amyloid deposits were found in the glomeruli, tubules and papillae of the kidneys from R1.P1-Apoa2c mice, we collected urine of amyloid fibril-injected mice and used Western blotting analysis to detect apoA-II. We did not detect any protein bands corresponding to apoA-II protein in whole urine or centrifuged pellets of dialyzed urine samples in which amyloid fibrils should be concentrated. In the present study, no amyloid deposition was observed in mice after injection of urine. The results confirmed that no amyloid fibrils exist in the urine of amyloid fibril-injected mice.
It is possible that inflammation induced by injection of amyloid fibrils may accelerate amyloidosis. However, we confirmed previously that injection of several proteins, such as albumin, transthyretin, and nonamyloid apoA-II, did not induce AApoAII amyloidosis.18,35 In the present study, we did not observe AA amyloid deposition, and no obvious inflammation was detected in mothers or offspring mice. In addition, injection of milk from control mothers or milk denatured with guanidine hydrochloride did not accelerate amyloidosis.
Amyloidosis, including prion diseases, results from a conformational change in a protein. It is this conversion that makes the disease transmissible. Recently, this protein-only transmission hypothesis was demonstrated in yeast and human prion strains.41–43 However, transmission of prion or amyloid agents between individuals under natural conditions is still poorly understood. To date, more than 25 amyloid fibrils associated with amyloidosis have been identified in humans and animals. Among them, mouse AApoAII amyloidosis has the following characteristics: 1) precursor apoA-II protein constitutively circulates in the blood and forms amyloid fibrils without degradation or modification; 2) amyloid fibrils deposit throughout the whole body; and 3) the addition of pre-existing amyloid fibrils into a solution of amyloid protein monomer accelerates fibril formation under neutral pH.44 Thus, mouse AApoAII amyloidosis is one of the interesting and suitable models for amyloidosis transmission.
In summary, we first demonstrated the transmission of amyloid fibrils from mother to offspring using mouse AApoAII amyloidosis. This transmission occurs postnatally rather than by in utero innovation of amyloid fibrils. The further characterization of mechanisms of vertical acceleration in AApoAII amyloidosis has proven helpful for identification of the epigenetics of amyloidosis, yielding results that are applicable to other protein-folding diseases as well.
Acknowledgments
We thank Kiyoshi Matsumoto and Hiroshi Tomozawa for taking care of mice and Kiyokazu Kametani for assistance with the histological studies.
Footnotes
Address reprint requests to Xiaoying Fu, Department of Aging Biology, Institute on Aging and Adaptation, Shinshu University Graduate School of Medicine, Asahi 3-1-1, Matsumoto 390-8621, Japan. E-mail: fuxy@sch.md.shinshu-u.ac.jp.
Supported in part by grants-in-aid for priority areas (17028018), scientific research (B) (17390111), and a Japan Society for the Promotion of Science fellowship (17–05268) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and by a grant from the Ministry of Health, Labor and Welfare of Japan.
References
- Selkoe DJ. Folding proteins in fatal ways. Nature. 2003;426:900–904. doi: 10.1038/nature02264. [DOI] [PubMed] [Google Scholar]
- Glenner GG. Amyloid deposits and amyloidosis: the beta-fibrilloses. N Engl J Med. 1980;302:1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]
- Jarrett JT, Lansbury PT., Jr Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- Rochet JC, Lansbury PT., Jr Amyloid fibrillogenesis: themes and variations. Curr Opin Struct Biol. 2000;10:60–68. doi: 10.1016/s0959-440x(99)00049-4. [DOI] [PubMed] [Google Scholar]
- Weissmann C. Molecular genetics of transmissible spongiform encephalopathies. J Biol Chem. 1999;274:3–6. doi: 10.1074/jbc.274.1.3. [DOI] [PubMed] [Google Scholar]
- Paushkin SV, Kushnirov VV, Smirnov VN, Ter-Avanesyan MD. In vitro propagation of the prion-like state of yeast Sup 35 protein. Science. 1997;277:381–383. doi: 10.1126/science.277.5324.381. [DOI] [PubMed] [Google Scholar]
- Prusiner SB. Prion diseases and the BSE crisis. Science. 1991;252:1515–1522. doi: 10.1126/science.278.5336.245. [DOI] [PubMed] [Google Scholar]
- Pan KM, Baldwin M, Nguyen J, Gasset M, Serban A, Groth D, Mehlhorn I, Huang Z, Fletterick RJ, Cohen FE, Prusiner SB. Conversion of alpha-helices into β-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci USA. 1993;90:10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Higuchi K. Amyloid fibril proteins. Mech Ageing Dev. 2002;123:1625–1636. doi: 10.1016/s0047-6374(02)00098-2. [DOI] [PubMed] [Google Scholar]
- Higuchi K, Hosokawa M, Takeda T. Senescence-accelerated mouse. Methods Enzymol. 1999;309:674–686. doi: 10.1016/s0076-6879(99)09044-8. [DOI] [PubMed] [Google Scholar]
- Higuchi K, Yonezu T, Kogishi K, Matsumura A, Takeshita S, Kohono A, Matsushita T, Hosokawa M, Takeda T. Purification and char-acterization of a senile amyloid-related antigenic substance (apoSASSAM) from mouse serum. apoSASSAM is an apoA-II apo-lipoprotein of mouse high density lipoproteins. J Biol Chem. 1986;261:12834–12840. [PubMed] [Google Scholar]
- Higuchi K, Matsumura A, Honma A, Takeshita S, Hashimoto K, Hosokawa M, Yasuhira K, Takeda T. Systemic senile amyloid in senescence-accelerated mice: a unique fibril protein demonstrated in tissues from various organs by the unlabeled immunoperoxidase method. Lab Invest. 1983;48:231–240. [PubMed] [Google Scholar]
- Higuchi K, Kitagawa K, Naiki H, Hanada K, Hosokawa M, Takeda T. Polymorphism of apolipoprotein A-II (apoA-II) among inbred strains of mice: relationship between the molecular type of apoA-II and mouse senile amyloidosis. Biochem J. 1991;279:427–433. doi: 10.1042/bj2790427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Wang J, Mastushita T, Kogishi K, Hosokawa M, Fu X, Guo Z, Mori M, Higuchi K. Polymorphisms of mouse apolipoprotein A-II: seven alleles found among 41 inbred strains of mice. Amyloid. 2003;10:207–214. doi: 10.3109/13506120309041737. [DOI] [PubMed] [Google Scholar]
- Higuchi K, Naiki H, Kitagawa K, Kitado H, Kogishi K, Matsusita T, Takeda T. Apolipoprotein A-II gene and development of amyloidosis and senescence in a congenic strain of mice carrying amyloidogenic ApoA-II. Lab Invest. 1995;72:75–82. [PubMed] [Google Scholar]
- Naiki H, Higuchi K, Shimada A, Takeda T, Nakakuki K. Genetic analysis of murine senile amyloidosis. Lab Invest. 1993;68:332–337. [PubMed] [Google Scholar]
- Higuchi K, Kogishi K, Wang J, Chen X, Chiba T, Matsushita T, Hoshii Y, Kawano H, Ishihara T, Yokota T, Hosokawa M. Fibrilization in mouse senile amyloidosis is fibril conformation-dependent. Lab Invest. 1998;78:1535–1542. [PubMed] [Google Scholar]
- Xing Y, Nakamura A, Chiba T, Kogishi K, Matsushita T, Fu L, Guo Z, Hosokawa M, Mori M, Higuchi K. Transmission of mouse senile amyloidosis. Lab Invest. 2001;81:493–499. doi: 10.1038/labinvest.3780257. [DOI] [PubMed] [Google Scholar]
- Xing Y, Nakamura A, Korenaga T, Guo Z, Yao J, Fu X, Matsushita T, Kogishi K, Hosokawa M, Kametani F, Mori M, Higuchi K. Induction of protein conformational change in mouse senile amyloidosis. J Biol Chem. 2002;277:33164–33169. doi: 10.1074/jbc.M111570200. [DOI] [PubMed] [Google Scholar]
- Korenaga T, Fu X, Xing Y, Matsusita T, Kuramoto K, Syumiya S, Hasegawa Z, Naiki H, Ueno M, Ishihara T, Hosokawa M, Mori M, Higuchi K. Tissue distribution, biochemical properties and transmission of mouse type A AApoAII amyloid fibrils. Am J Pathol. 2004;164:1597–1606. doi: 10.1016/S0002-9440(10)63718-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundmark K, Westermark GT, Nystrom S, Murphy CL, Solomon A, Westermark P. Transmissibility of systemic amyloidosis by a prion-like mechanism. Proc Natl Acad Sci USA. 2002;99:6979–6984. doi: 10.1073/pnas.092205999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D, Kawano H, Takahashi M, Hoshii Y, Setoguchi M, Gondo T, Ishihara T. Acceleration of murine AA amyloidosis by oral administration of amyloid fibrils extracted form different species. Pathol Int. 2002;52:40–45. doi: 10.1046/j.1440-1827.2002.01309.x. [DOI] [PubMed] [Google Scholar]
- Brotherston JG, Renwick CC, Stamp JT, Zlotnik I, Pattison IH. Spread of scrapie by contact to goats and sheep. J Comp Pathol. 1968;78:9–17. doi: 10.1016/0021-9975(68)90107-2. [DOI] [PubMed] [Google Scholar]
- Dickinson AG, Stamp JT, Renwick CC. Maternal and lateral transmission of scrapie in sheep. J Comp Pathol. 1974;84:19–25. doi: 10.1016/0021-9975(74)90023-1. [DOI] [PubMed] [Google Scholar]
- Elsen JM, Amigues Y, Schelcher F, Ducrocq V, Andreoletti O, Eychenne F, Khang JV, Poivey JP, Lantier F, Laplanche JL. Genetic susceptibility and transmission factors in scrapie: detailed analysis of an epidemic in a closed flock of Romanov. Arch Virol. 1999;144:431–445. doi: 10.1007/s007050050516. [DOI] [PubMed] [Google Scholar]
- Hourrigan JL, Klingsporn AL, Clark WW, De Camp M. Epidemiology of scrapie in the United States. Prusiner SB, Hadlow WJ, editors. New York: Academic Press,; Slow Transmissible Disease of the Nervous System. 1979:pp 331–356. [Google Scholar]
- Foster J, McKelvey W, Fraser H, Chong A, Ross A, Parnham D, Goldmann W, Hunter N. Experimentally induced bovine spongiform encephalopathy did not transmit via goat embryos. J Gen Virol. 1999;80:517–524. doi: 10.1099/0022-1317-80-2-517. [DOI] [PubMed] [Google Scholar]
- Soares M, Buxbaum J, Sirugo G, Coelho T, Sousa A, Kastner D, Saraiva M. Genetic anticipation in Portuguese kindreds with familial amyloidotic polyneuropathy is unlikely to be caused by triplet repeat expansions. J Hum Genet. 1999;104:480–485. doi: 10.1007/s004390050991. [DOI] [PubMed] [Google Scholar]
- Drugge U, Andersson R, Chizari F, Danielsson M, Holmgren G, Sandgren O, Sousa A. Familial amyloidotic polyneuropathy in Sweden: a pedigree analysis. J Med Genet. 1993;30:388–392. doi: 10.1136/jmg.30.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Ikeda S, Hanyu N, Takeda S, Yanagisawa N. A pedigree analysis with minimised ascertainment bias shows anticipation in Met30-transthyretin related familial amyloid polyneuropathy. J Med Genet. 1998;35:23–30. doi: 10.1136/jmg.35.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pras M, Schubert M, Zucker-Franklin D, Rimon A, Franklin EC. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968;47:924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagger H, Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Higuchi K, Matsumura A, Honma A, Toda K, Takeshita S, Matsushita M, Yonezu T, Hosokawa M, Takeda T. Age-related changes of serum apoprotein SASSAM, apoprotein A-I and low-density lipoprotein levels in senescence accelerated mouse (SAM). Mech Ageing Dev. 1984;26:311–326. doi: 10.1016/0047-6374(84)90103-9. [DOI] [PubMed] [Google Scholar]
- Fu X, Korenaga T, Fu L, Xing Y, Guo Z, Matsushita T, Hosokawa M, Naiki H, Baba S, Kawata Y, Ikeda S, Ishihara T, Mori M, Higuchi K. Induction of AApoAII amyloidosis by various heterogeneous amyloid fibrils. FEBS Lett. 2004;563:179–184. doi: 10.1016/S0014-5793(04)00295-9. [DOI] [PubMed] [Google Scholar]
- Wei L, Kawano H, Fu X, Cui D, Ito S, Yamamura K, Ishihara T, Tokuda T, Higuchi K, Maeda S. Deposition of transthyretin amyloid is not accelerated by the same amyloid in vivo. Amyloid. 2004;11:113–120. doi: 10.1080/13506120410001726344. [DOI] [PubMed] [Google Scholar]
- Tagoe CE, French D, Gallo G, Buxbaum JN. Amyloidogenesis is neither accelerated nor enhanced by injections of preformed fibrils in mice transgenic for wild-type human transthyretin: the question of infectivity. Amyloid. 2004;11:21–26. doi: 10.1080/13506120410001674982. [DOI] [PubMed] [Google Scholar]
- Shtrasburg S, Pras M, Brezniak N, Dolitzki M, Livneh A. Offspring of amyloidotic mice are not predisposed to develop amyloidosis. J Lab Clin Med. 1999;134:168–172. doi: 10.1016/s0022-2143(99)90122-1. [DOI] [PubMed] [Google Scholar]
- Andreoletti O, Lacroux C, Chabert A, Monnereau L, Tabouret G, Lantier F, Berthon P, Eychenne F, Lafond-Benestad S, Elsen JM, Schelcher F. PrPSc accumulation in placentas of ewes exposed to natural scrapie: influence of foetal PrP genotype and effect on ewe-to-lamb transmission. J Gen Virol. 2002;83:2607–2616. doi: 10.1099/0022-1317-83-10-2607. [DOI] [PubMed] [Google Scholar]
- Shaked GM, Shaked Y, Kariv-Inbal Z, Halimi M, Avraham I, Gabizon RA. A protease-resistant prion protein isoform is present in urine of animals and humans affected with prion diseases. J Biol Chem. 2001;276:31479–31482. doi: 10.1074/jbc.C100278200. [DOI] [PubMed] [Google Scholar]
- King CY, Diaz-Avalos R. Protein-only transmission of three yeast prion strains. Nature. 2004;428:319–323. doi: 10.1038/nature02391. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Chien P, Naber N, Cooke R, Weissman JS. Conformational variations in an infectious protein determine prion strain differences. Nature. 2004;428:323–328. doi: 10.1038/nature02392. [DOI] [PubMed] [Google Scholar]
- Legname G, Baskakov IV, Nguyen HO, Riesner D, Cohen FE, DeArmond SJ, Prusiner SB. Synthetic mammalian prions. Science. 2004;305:673–676. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- Naiki H, Higuchi K, Nakakuki K, Takeda T. Kinetic analysis of amyloid fibril polymerization in vitro. Lab Invest. 1991;65:104–110. [PubMed] [Google Scholar]