Abstract
Perivascular macrophages are uniquely situated at the intersection between the nervous and immune systems. Although combined myeloid marker detection differentiates perivascular from resident brain macrophages (parenchymal microglia), no single marker distinguishes perivascular macrophages in humans and mice. Here, we present the macrophage scavenger receptor CD163 as a marker for perivascular macrophages in humans, monkeys, and mice. CD163 was primarily confined to perivascular macrophages and populations of meningeal and choroid plexus macrophages in normal brains and in brains of humans and monkeys with human immunodeficiency virus or simian immunodeficiency virus (SIV) encephalitis. Scattered microglia in SIV encephalitis lesions and multinucleated giant cells were also CD163 positive. Consistent with prior findings that perivascular macrophages are primary targets of human immunodeficiency virus and SIV, all SIV-infected cells in the brain were CD163 positive. Using fluorescent dyes that definitively and selectively label perivascular macrophages in vivo, we confirmed that dye-labeled simian perivascular macrophages were CD163 positive and able to repopulate the central nervous system within 24 hours. Flow cytometric studies demonstrated a subset of monocytes (CD163+CD14+CD16+) that were immunophenotypically similar to brain perivascular macrophages. These findings recognize CD163+ blood monocytes/macrophages as a source of brain perivascular macrophages and underscore the utility of this molecule in studying the biology of perivascular macrophages and their precursors in humans, monkeys, and mice.
Brain macrophages are normal constituents of the central nervous system (CNS) and central players in many CNS pathologies.1,2 They are heterogeneous in their location, turnover, and function, although they are all of common developmental origin. Perivascular macrophages, located in the perivascular (Virchow-Robin) space of cerebral microvessels, are continuously repopulated by blood-derived monocytes/macrophages, unlike the resident brain macrophages, microglia.3–5 Perivascular macrophages play distinct roles, as opposed to parenchymal microglia, in normal CNS functions and in CNS inflammatory diseases such as bacterial meningitis, experimental allergic encephalomyelitis (EAE), and simian immunodeficiency virus (SIV) and human immunodeficiency virus (HIV) encephalitis (SIVE and HIVE).6–13 Because the relative contribution of perivascular macrophages and parenchymal microglia differs in normal CNS physiology and disease progression, phenotypic and functional differentiation between these two brain macrophages becomes important. The rat macrophage-associated antigen ED2 selectively identifies perivascular macrophages, but no such marker exists in humans.14–17 In addition, a mannose receptor specific for perivascular macrophages in mouse brain was recently reported,18 but a human equivalent has yet to be identified. At present, no single phenotypic marker, equivalent or comparable with ED2, that solely identifies perivascular macrophages is available in humans. In humans and monkeys, perivascular macrophages can be distinguished from parenchymal microglia based on levels of CD14 and CD45.8,10,19–21 Although such methods of distinction are powerful, results can vary between laboratories depending on the sensitivity of immunohistochemical detection methods and the antigen retrieval techniques used.22,23
CD163 (also known as M130) is a newly identified member of the scavenger receptor cysteine-rich superfamily group B.24–29 This protein (primarily molecular weight 130 kd) is expressed exclusively on all circulating monocytes and on subpopulations of tissue macrophages.30,31 CD163 mediates removal of hemoglobin-heptaglobin complexes by monocytes/macrophages, and its soluble form regulates inflammation.32 Unlike other monocyte-associated markers such as CD14, CD16, and CD64 that label other leukocytes, CD163 is reportedly expressed specifically on human monocytes in which expression is highest on a CD14+CD16+ subset.33 Recently, Dijkstra and colleagues34 suggested that ED2 may be a rat homolog of human CD163; however, definitive data for this has yet to be published. Although it has been demonstrated that CD163 identifies perivascular macrophages in human CNS tissues,35,36 whether CD163 is a marker of perivascular macrophages remains open.
In the present study, we examined CD163 expression on perivascular macrophages in normal human, monkey, and mouse brains and in human and monkey brains infected with HIVE and SIVE. We report that CD163 is a marker for perivascular macrophages in humans, monkeys, and mice in the normal CNS and that CD163+ perivascular macrophages are a prime target of SIV infection in monkeys. Using dextran amine dyes injected into the cerebrospinal fluid (CSF) of live monkeys, we show that perivascular macrophages selectively in-corporate dye and are all CD163 positive. Correspondingly, we observed a subset of blood monocytes (CD163+CD14+CD16+) that are immunophenotypically similar to the HIV- or SIV-infected perivascular macrophages found in encephalitic brains. This subset may represent precursors to specific macrophage lineage subpopulations in the CNS that are CD163 positive.
Materials and Methods
Tissue Samples
Human Brain Tissue
Formalin-fixed, paraffin-embedded sections of frontal white and gray matter and pons were obtained from the Manhattan HIV Brain Bank.8 A total of four HIVE cases, two HIV-1-positive cases without encephalitis, and two seronegative controls were analyzed. CNS pathology was diagnosed by neuropathological examination initially performed by S. Morgello.
Rhesus Monkey Tissue
Necropsy specimens of brain and lymph node from 24 adult rhesus macaques (Macaca mulatta) were used in the present study. Twenty-one of these animals were infected with SIVmac251 (20 ng of SIV p27) by intravenous injection and killed at peak viremia (n = 5) or when they developed AIDS (n = 16). Nine of the monkeys with AIDS had evidence of SIVE, defined by the presence of SIV in the brain and the accumulation of macrophages and multinucleated giant cells (MNGCs).37,38 Three normal, age-matched, uninfected animals were used as controls. All animals were anesthetized with ketamine-HCl, euthanized by intravenous pentobarbital overdose, and exsanguinated. Tissues were either collected in 10% neutral buffered formalin and embedded in paraffin or blocked in optimum cutting temperature compound (Miles Scientific, Elkhart, IN) and snap-frozen. Formalin-fixed, paraffin-embedded tissues were cut into 6-μm-thick sections, and frozen tissues were sectioned at 10 μm.
Mouse Brain Tissue
Brain and spleen were obtained from two 13-week-old C3H mice. Whole-brain tissue was frozen in optimum cutting temperature compound (Miles Scientific) and stored at −80°C until use. Sagittal sections of 10-μm thickness were cut, dried, and acetone fixed for 10 minutes on ice.
Injection of Dextran Amines
Hydrophilic fluorescent dyes injected into the lateral cerebral ventricle selectively label perivascular macrophages.39 We used the same approach to rhesus monkeys to visualize perivascular macrophages. A total of four normal uninfected rhesus macaques were used: three animals received either fluorescein-conjugated dextran (Fluoro-Emerald; Molecular Probes, Eugene, OR), rhodamine-conjugated dextran (Fluoro-Ruby; Molecular Probes), or biotinylated dextran amine (BDA-10,000; Molecular Probes); one received Fluoro-Emerald and BDA-10,000 sequentially. Before injections, animals were initially tranquilized with ketamine or telazol and anesthetized with sodium pentobarbital. One milliliter of 5% dextran amines dissolved in 0.9% NaCl saline was injected into the cerebellomedullary cistern. Injections were performed over a 5-minute period using a stereotaxic apparatus. These animals were killed 1 day after Fluoro-Emerald, Fluoro-Ruby, or BDA-10,000 injection. To follow the sequential traffic of new macrophages into perivascular spaces, one animal was injected with a second dye BDA-10,000 7 days after the first Fluoro-Emerald injection and killed 24 hours later. Excess fluorescent dye that was not taken up by perivascular macrophages was completely cleared from the CSF within 24 hours. Because of this, newly immigrated perivascular macrophages can be identified by the second dye label.
Antibodies
Primary antibodies used are listed in Table 1. To examine CD163 expression in paraffin-embedded human brain tissue, several monoclonal antibodies (mAbs) directed against human CD163 antigen were initially tested with or without antigen retrieval pretreatments (microwave or heat treatment in 0.01 mol/L sodium citrate buffer, pH 6.0). Two clones, EDHu-1 (Serotec, Oxford, UK) and 10D6 (Novocastra, Newcastle on Tyne, UK), were selected as most effective in formalin-fixed, paraffin-embedded human tissues.29,40 Clone 10D6 was effective on paraffin-embedded tissues only after pretreatment; however, clone EDHu-1 was effective on paraffin-embedded tissues without pretreatment and also functioned on frozen tissues. Because of this, EDHu-1 was the primary antibody used for immunohistochemical and fluorescence studies. The cross-species reactivity of these anti-human CD163 mAbs with rhesus macaque tissue was evaluated by immunohistochemistry and immunoblot. To verify results obtained with paraffin-embedded tissues after antigen retrieval, which can result in the generation of neo-antigens, we used EDHu-1 and additional anti-human CD163 clones GHI/61 (PharMingen, San Diego, CA) and 5C6-FAT (BMA Biomedicals, Augst, Switzerland), which function on frozen tissues.28 Two polyclonal antibodies, one against the N-terminal region of mouse CD163 (G-17; Santa Cruz Biotechnology; Santa Cruz, CA) and another against its internal region (K-18; Santa Cruz Biotechnology), which had not been previously assessed by immunohistochemistry, were also tested on mouse CNS tissues. Clones MAC 2-158 (Trillium Diagnostics, Scarborough, ME), EDHu-1, GHI/61, and 5C6-FAT were used for flow cytometry studies of CD163 expression on monocytes and monocyte-derived macrophages as described below.
Table 1.
Antibodies Used in the Present Study
| Antigen | Clone | Isotype | Reactivity | Manufacturer | Application |
|---|---|---|---|---|---|
| CD14 | AML-2-23 | Mouse IgG2b | Hu, Mk | Medarex | IHC(F), IF(F) |
| CD14 | 7 | Mouse IgG2a | Hu | Novocastra | IHC(P), IF(P) |
| CD14 | RMO52 | Mouse IgG2a | Hu, Mk | Immunotech | FC |
| CD16 | 2H7 | Mouse IgG2a | Hu, Mk | Novocastra | IHC(P), IF(P) |
| CD16 | 3G8 | Mouse IgG1 | Hu, Mk | PharMingen | FC |
| CD163 | EDHu-1 | Mouse IgG1 | Hu, Mk | Serotec | IHC(F,P), IF(F,P), FC |
| CD163 | 10D6 | Mouse IgG1 | Hu, Mk | Novocastra | IHC(P), IF(P) |
| CD163 | GHI/61 | Mouse IgG1 | Hu, Mk | PharMingen | IHC(F), FC |
| CD163 | 5C6-FAT | Mouse IgG1 | Hu, Mk | BMA | IHC(F), FC |
| CD163 | MAC 2-158 | Mouse IgG1 | Hu, Mk | Trillium | FC |
| CD163 | Polyclonal G-17 | Goat IgG | Ms | Santa Cruz | IHC(F), IF(F) |
| CD163 | Polyclonal K-18 | Goat IgG | Ms | Santa Cruz | IHC(F), IF(F) |
| Glut-1 | Polyclonal | Rabbit IgG | Hu, Mk, Rt | Chemicon | IHC(F,P), IF(F,P) |
| HLA-DR | CD3/43 | Mouse IgG1 | Hu, Mk | DAKO | IHC(F,P) |
| HLA-DR | L243 | Mouse IgG2a | Hu, Mk | Becton Dickinson | FC |
Hu, human; Mk, monkey; Ms, mouse; Rt, rat; IHC, immunohistochemistry; IF, immunofluorescence; FC, flow cytometry; F, frozen; P, paraffin.
Immunohistochemistry
Deparaffinized sections and acetone-fixed frozen sections were assessed by immunohistochemistry for CD163. Immunohistochemistry was performed using avidin-biotin peroxidase complex (Vectastain kit; Vector Laboratories, Burlingame, CA) or peroxidase-conjugated polymer reagent (SuperPicTure kit; Zymed, San Francisco, CA) according to the manufacturers’ instructions. Before primary antibody incubation, serum or nonserum protein block was applied for 2 hours at room temperature or overnight at 4°C. The color reaction product was developed using 3,3′-diaminobenzidine tetrahydrochloride (DAB; DAKO) as the chromogenic substrate for horseradish peroxidase. The sections were counterstained with hematoxylin and then dehydrated and mounted. Controls consisted of omission of the primary antibody or addition of isotype-matched immunoglobulin. To detect CD163+ cells and Glut-1+ endothelial cells in human and monkey brains, double-label immunohistochemistry was performed using the DAKO Double Stain System, according to the manufacturer’s instructions. The color reaction product was developed using DAB and Fast Red (DAKO) for CD163 and Glut-1 staining, respectively. The sections were mounted using Faramout aqueous mounting medium (DAKO).
In Situ Hybridization for SIV RNA
In situ hybridization for SIV RNA was performed using digoxigenin-labeled antisense riboprobes obtained from Lofstrand Labs (Gaithersburg, MD; with permission from Dr. V. Hirsch and C. Brown [National Institute of Health, Rockville, MD]). In situ hybridization was performed with a modification of previously published procedures.41
For simultaneous detection of viral nucleic acid and CD163 in the same tissue section, in situ hybridization for SIV RNA was followed by immunohistochemistry or immunofluorescence for CD163, using previously described methods10 and as described below.
Confocal Microscopy
Immunofluorescence confocal microscopy was used to identify CD163+ cells in brain, to examine whether CD163+ cells were SIV RNA positive, and to determine whether dextran dye-labeled perivascular macrophages were CD163 positive. Deparaffinized sections or acetone-fixed frozen sections were washed with phosphate-buffered saline containing 0.2% fish skin gelatin (PBS/FSG) at room temperature and blocked with 10% normal goat serum diluted in PBS/FSG (2% normal rabbit serum was used for goat polyclonal anti-mouse CD163 antibody). Primary antibodies were diluted in blocking solution and incubated for 1 hour at room temperature or overnight at 4°C. CD163 immunofluorescence was revealed using goat anti-mouse or rabbit anti-goat secondary antibodies labeled with Alexa Fluor 488 or 568 (Molecular Probes). Glut-1 was followed by goat anti-rabbit Alexa Fluor 633 (Molecular Probes). Sections were washed with PBS/FSG before the addition of the next primary or secondary antibody. Secondary antibodies were also diluted in blocking solution and incubated for 30 minutes. Triple-label immunofluorescence was performed to colocalize CD163 with CD14 or CD16 on perivascular macrophages with Glut-1+ CNS microvessels. After incubation with primary mAbs to CD14 or CD16 and biotinylated isotype-specific secondary antibodies (PharMingen), sections were visualized with streptavidin-conjugated Alexa Fluor 568 (Molecular Probes).
Confocal microscopy was performed using a Leica TCS SP laser scanning microscope equipped with three lasers. Individual optical slices represent 0.2 μm, and 32 to 62 optical slices were collected at 512 × 512 pixel resolution. The fluorescence of individual fluorochromes was captured separately in sequential mode after optimization to reduce bleed-through between channels (photomultiplier tubes) using Leica software. NIH Image version 1.62 and Adobe Photoshop version 7 software were used to assign correct colors of up to four channels collected, including the fluorochromes Alexa Fluor 488 (green), Alexa Fluor 568 (red), and Alexa Fluor 633 (blue) and the differential interference contrast image (gray scale). Some images were rendered with Volocity 3.6 Software (Improvision, Lexington, MA). Colocalization of antigens is indicated by the addition of colors as indicated in the figure legends.
Flow Cytometry
To examine the expression of CD163 on circulating monocytes, EDTA-treated fresh whole venous blood from normal uninfected humans and monkeys was tested by four-color flow cytometry. Prior studies using anti-CD163 mAbs GHI/61, EDHu-1, and MAC 2-158 reported extreme inconsistencies in the level of expression per cell or percentage of circulating monocytes expressing CD163 (reported percentage of positive cells ranged from 0 to 90% depending on the clones and methods).28,29,42 Initially, we also found great variability among blood samples and mAbs when using heparinized blood. However, using EDTA as a coagulant, we found that more than 90% of peripheral blood monocytes expressed CD163 regardless of the clone used. For multiparameter flow cytometry staining, 100-μL aliquots of whole blood were mixed with fluorescein isothiocyanate-conjugated anti-CD163 (MAC 2-158 or 5C6-FAT) or phycoerythrin-conjugated anti-CD163 (GHI/61) in combination with fluorescein isothiocyanate- or phycoerythrin-conjugated anti-CD16 (3G8; PharMingen), energy-coupled dye-PE-Texas Red-conjugated CD14 (RMO52; Beckman Coulter, Miami, FL), and Allophycocyanin-conjugated anti-HLA-DR (L243; Becton Dickinson, San Jose, CA). Samples were incubated for 30 minutes at room temperature. For CD163 detection using unconjugated EDHu-1, a secondary antibody conjugated with Alexa Fluor 488 was used. After staining, erythrocytes were lysed using ImmunoPrep Reagent System. The samples were then washed with PBS, resuspended in 2% formaldehyde in PBS, and analyzed on a FACSCalibur flow cytometer (Becton Dickinson).
In addition, the regulation of CD163 expression was assessed in vitro using monocyte-derived macrophages. Peripheral blood mononuclear cells were isolated from heparinized whole blood using Ficoll-Hypaque. Peripheral blood mononuclear cells were washed twice with Dulbecco’s PBS, resuspended in RPMI with 2% human AB serum, and incubated for 2 hours at 37°C and 5% CO2. Nonadherent cells were removed by gentle washing with RPMI, and the remaining adherent monocytes were cultured in RPMI with 2% human AB serum in the presence of granulocyte-macrophage colony-stimulating factor (500 U/ml; PharMingen) for 5 days. Flow cytometry was performed as described above with 1 × 106 granulocyte-macrophage colony-stimulating factor-derived cultured macrophages.
Results
CD163 Selectively Identifies Perivascular Macrophages in Normal Brains of Monkeys, Humans, and Mice
Following the suggestion that CD163 is equivalent to the perivascular macrophage marker ED2 in rodents,34 we sought to determine whether CD163 detects perivascular macrophages in normal human and monkey brains. Initially, mAbs to human CD163 were evaluated with formalin-fixed, paraffin-embedded tissue sections from normal rhesus macaques (Table 2). Two clones (EDHu-1 and 10D6) were selected because they functioned well in paraffin-embedded human tissues and, importantly, cross-react to rhesus monkey. These clones functioned and yielded similar patterns of reactivity on lymph nodes, specifically staining macrophages (data not shown). In the normal human CNS, CD163-labeled perivascular macrophages represented flattened and elongated cells and were found exclusively around small- to medium-sized blood vessels (Figures 1A and 2A). In the normal monkey brain, these antibodies stained perivascular macrophages and populations of macrophages in the meninges and choroid plexus (Figure 2A; data not shown). To confirm the CD163 specificity found in paraffin sections, we also used clones EDHu-1, GHI/61, and 5C6-FAT, which were effective on frozen tissues and reacted with the same populations (data not shown). In addition to these antibodies functioning in human and nonhuman primate CNS tissues, polyclonal anti-mouse CD163 antibodies (G-17 and K-18) specifically labeled perivascular macrophages in mouse brain, similar to the mAbs in humans and monkeys (data not shown). Selective expression of CD163 on perivascular macrophages and meningeal and choroid plexus macrophages, but not microglia, clearly demonstrates the existence of distinct subpopulations of macrophages in the CNS.
Table 2.
Summary of CD163 Immunohistochemistry on HIVE and SIVE Brains
| Clone | Tissue | Dilution | Pretreatment* | ABC or Polymer† | Staining |
|---|---|---|---|---|---|
| EDHu-1 | Frozen SIVE | 1:500 | None | ABC or polymer | Perivascular |
| Paraffin SIVE | 1:250 | None | ABC | Perivascular | |
| Paraffin SIVE | 1:250 | Microwave | ABC | Perivascular; scattered parenchymal | |
| Paraffin SIVE | 1:250 | None | Polymer | Perivascular; scattered parenchymal | |
| Paraffin HIVE | 1:250 | None | ABC | Perivascular; scattered parenchymal | |
| Paraffin HIVE | 1:250 | None | Polymer | Perivascular, scattered parenchymal | |
| 10D6 | Paraffin SIVE | 1:100 | None | ABC | Perivascular (very weak) |
| Paraffin SIVE | 1:100 | Boiling | ABC | Perivascular | |
| Paraffin SIVE | 1:100 | Microwave | ABC | Perivascular; scattered parenchymal | |
| Paraffin SIVE | 1:100 | Boiling | Polymer | Perivascular; scattered parenchymal | |
| Paraffin HIVE | 1:100 | Boiling | ABC | Perivascular; scattered parenchymal | |
| Paraffin HIVE | 1:100 | Boiling | Polymer | Perivascular; scattered parenchymal | |
| GHI/61 | Frozen SIVE | 1:500 | None | ABC | Perivascular |
| 5C6-FAT | Frozen SIVE | 1:1000 | None | ABC or polymer | Perivascular |
Pretreatment was performed either by microwave antigen retrieval in 0.01 mol/L sodium citrate buffer, pH 6.0, for 20 minutes or by heating in 0.01 mol/L sodium citrate buffer, pH 6.0, for 30 minutes at 95°C.
ABC, avidin/biotin complex; polymer, peroxidase-labeled polymer conjugated with secondary antibodies.
Figure 1.
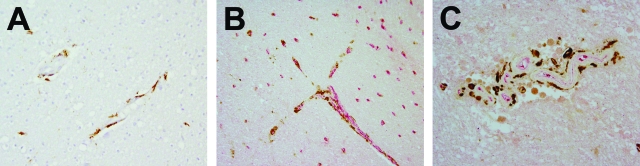
CD163 expression in HIV encephalitis brains. A: Perivascular macrophages in brains of control uninfected humans selectively labeled with anti-human CD163 antibody. B: Association of CD163+ cells (DAB; brown) with Glut-1+ CNS vessels (Fast Red; red) in HIVE brains. C: Accumulation of CD163+ cells in HIVE lesions. CD163 immunoreactivity (DAB; brown) was found on CNS perivascular macrophages and intimately associated with Glut-1+ CNS vessels (Fast Red; red). MNGCs and populations of macrophages in the meninges and choroid plexus were also stained with anti-CD163 antibody (data not shown). Original magnifications, ×400 (A and C) and ×200 (B).
Figure 2.
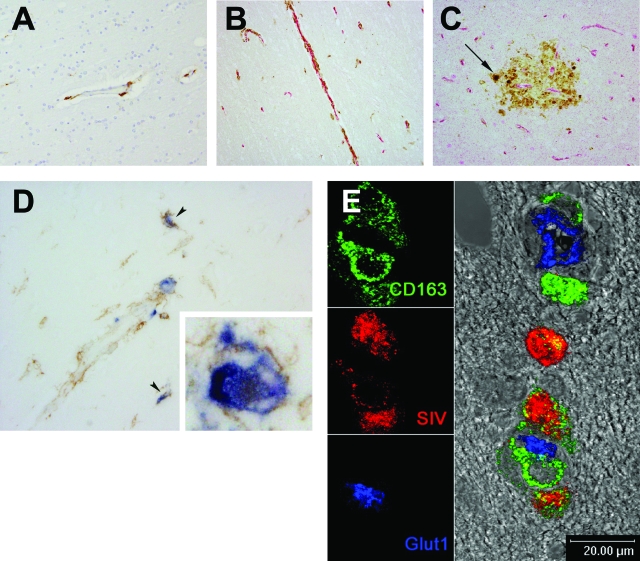
SIV-infected cells in the CNS are CD163 positive. A: Brains of control uninfected macaques stained with anti-human CD163 antibody. B: Association of CD163+ cells with CNS vessels in SIVE brains. C: Accumulation of CD163+ cells in SIVE lesions. Virtually all CD163+ cells, although morphologically diverse, were found intimately associated with Glut-1+ vessels (Fast Red; red). Note the presence of CD163+ MNGCs in the SIVE lesions in C (arrow). CD163 expression levels and the number of CD163+ cells in SIVE brains were higher than those found in the CNS of noninfected controls. D: In situ hybridization for SIV RNA combined with immunohistochemistry for CD163. CD163+ (brown) and SIV RNA+ (blue) perivascular macrophages (arrowheads) and MNGCs (inset). Virtually all SIV RNA+ productively infected cells detected were CD163 positive. E: In situ hybridization for SIV RNA combined with immunofluorescence for CD163. A representative confocal image of SIV RNA+ (red), CD163+ (green) perivascular macrophages near a CNS vessel (Glut-1; blue).
CD163+ Cells Are Associated with Blood Vessels in Encephalitic Brains
Because HIVE and SIVE are characterized by perivascular accumulation of macrophages and MNGCs, we investigated whether CD163 also labels macrophages in the perivascular cuffs of the CNS of HIV-infected humans or SIV-infected monkeys. Our findings of CD163-positive cells in HIVE and SIVE brains are summarized in Table 2. We found CD163 reactivity by perivascular macrophages with elongated morphology in HIV-infected patients without HIVE, in SIV-infected animals sacrificed at peak viremia (14 days after infection) and in animals that died of AIDS and without SIVE (data not shown). No difference in levels of CD163 expression or number of CD163-reactive cells was found among patients or animals that were noninfected or infected with HIV or SIV without encephalitis (data not shown). In CNS tissues from patients or animals with AIDS and encephalitis, we observed a substantial increase in the number of CD163-reactive cells as well as the level of CD163 expression (Figures 1 and 2). Cells expressing CD163 were found almost exclusively in the perivascular cuffs and were present in HIVE and SIVE lesions (Figures 1 and 2). In addition to the elongated perivascular macrophages found in normal brains, CD163 labeled large, round macrophages in the perivascular cuffs often with a short process. MNGCs were also labeled, and CD163 immunoreactivity was detected mainly within the cytoplasm (Figure 2, C and D). In some cases, CD163 immunoreactivity was detected on scattered cells in the parenchyma that were morphologically consistent with ramified microglia. When found in the parenchyma, CD163 staining in our experience was mainly intracytoplasmic rather than membranous. To address the association of these CD163+ perivascular macrophages with CNS vessels, we performed double-label immunohistochemistry with another brain endothelial marker, Glut-1.43 Although morphologically diverse, CD163+ cells were mainly found in association with Glut-1+ CNS vessels (Figures 1 and 2).
SIV-Infected Cells in the Brain Are CD163-Positive Perivascular Macrophages
Using either CD14 or CD45 with Glut-1 to immunophenotypically define the perivascular macrophage population, we have previously shown that perivascular macrophages are a major cell target of productive infection in the CNS of SIV-infected macaques.10 Because we found that CD163 is preferentially expressed on perivascular macrophages in the CNS, we sought to determine whether CD163 functions as a marker of SIV-infected cells in the CNS. Combined in situ hybridization and immunohistochemistry or immunofluorescence for SIV RNA, CD163, and Glut-1 showed that virtually all SIV RNA-positive, productively infected cells, including perivascular macrophages and MNGCs, were CD163 positive (Figure 2, D and E). However, not all CD163-positive macrophages were positive for SIV RNA. These observations are similar to previous studies using CD14, CD45, and PCNA as markers of infected perivascular macrophages.10,41 In these studies, a majority of SIV-infected cells were positive for CD14, CD45, and PCNA, but many noninfected CD14+CD45+ macrophages were in the lesions.
Fluorescent Dye-Labeled Cells in the Monkey Brain Express CD163 and Turnover
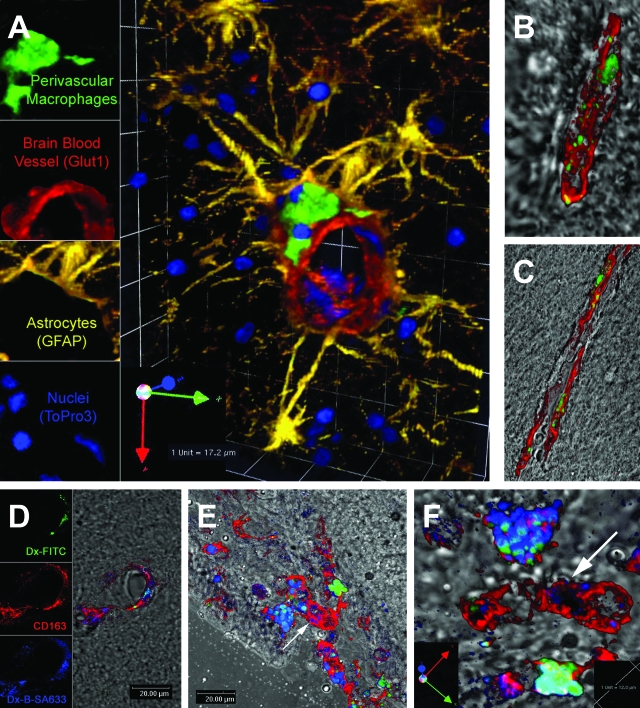
Intracerebroventricular injection of fluorescent dextran amines in the rat labels perivascular macrophages but not parenchymal microglia.39 We have used the ability of perivascular macrophages to phagocytose dextran amines as a definitive marker for perivascular macrophages. Using confocal microscopy, we sought to examine whether the dextran dye-labeled perivascular macrophages are CD163-positive (Figure 3). Fluorescein-conjugated dextran amine (Fluoro-Emerald) was injected into the CSF of normal uninfected monkeys, which were sacrificed 1 day later. In multiple tissue sections examined, perivascular macrophages were consistently dye-labeled (Figure 3). Next, double-label confocal microscopy was performed to confirm further that the dye-labeled cells were CD163+ and CD14+ perivascular macrophages. All perivascular macrophages were dye-labeled, and were all CD163 (Figure 3B) and CD14 positive (Figure 3C). In addition, CD163+ meningeal macrophages were also labeled with the dextran amine dye (Figure 3, E and F). To examine the turnover of these cells, we injected biotinylated dextran amine (BDA-10,000) (Figure 3, D–F) 7 days after the Fluoro-Emerald injection and sacrificed this animal 24 hours later. Because the first dye was completely cleared from the CSF within 24 hours because of rapid turnover of total CSF (about 5 hours for humans), the second injected dye labeled cells that were in the perivascular space at the time of the first injection and new cells that had trafficked to this space within the last 24 hours. Sequential labeling identified two populations: perivascular and meningeal macrophages that had taken up both dyes (green and blue) and newly arrived macrophages that were labeled only with the second dye (blue). Although most perivascular macrophages were double positive (green and blue), approximately 1 of 500 dye-labeled cells was single-positive for the second dye injected (blue). This turnover rate is strikingly consistent with reports of perivascular macrophage turnover in rodents in similar dye experiments.39 All of the dye-labeled cells, whether single or double labeled, were CD163 positive (Figure 3), indicating that CD163 is a definitive marker of perivascular macrophages in uninfected CNS and that turnover of such CD163+ dye-labeled cells can be detected in noninflammatory conditions within as little as 24 hours.
Figure 3.
Localization and turnover of CD163+ perivascular and meningeal macrophages labeled by dextran amine dye injected into the CSF of live monkeys. Confocal microscopy studies of brain sections from normal noninfected rhesus macaques injected intracisternally with fluorescent dextran amine dye (Fluoro-Emerald; green), showing localization of dye-labeled CD14+CD163+ perivascular macrophages in the perivascular space (A, B, and C). A: Dextran amine dye specifically labels CNS perivascular macrophages in normal noninfected rhesus macaques. Perivascular macrophages (green) adjacent to a CNS microvessel (Glut-1; red). Astrocytes (GFAP; yellow) and cell nuclei (ToPro3; blue). B: Dye-labeled perivascular macrophages (green) are CD163 positive (red) in CNS of a normal noninfected rhesus macaque. C: Dye-labeled perivascular macrophages (green) are CD14 positive (red). Confocal images from a noninfected animal subjected to consecutive injections of Fluoro-Emerald (green) followed by biotinylated dextran amine (BDA-10,000; visualized with streptavidin-Alexa Fluor 633; blue) 7 days later (D, E, and F). The animal was sacrificed at 24 hours after BDA-10,000 injection. D: Sequential labeling of perivascular macrophages by consecutive injections of Fluoro-Emerald (green; top leftpanel) followed 7 days later by biotinylated dextran (blue; bottom leftpanel) detects double-positive (green and blue) and single-positive (blue) perivascular macrophages, both of which are CD163 positive (red). The sequential labeling identifies two populations of CD163+ perivascular macrophages in perivascular spaces: perivascular macrophages that do not turnover in a 7-day period (green-blue) and perivascular macrophages that have recently arrived in the CNS (blue only). E: Sequential labeling by consecutive injections of Fluoro-Emerald (green) followed 7 days later by biotinylated dextran (blue) demonstrates double-positive (green-blue) and single-positive (blue) meningeal macrophages, all of which express CD163. The majority of cells are double positive, with few single-positive cells that have recently arrived. F: Magnification of E. High-power magnification view demonstrates double-positive (green-blue) and single-positive (blue) meningeal macrophages in the same field of meninges (horizontally flipped rear view image of E). Arrows point to the identical CD163+ meningeal macrophage that does not contain Fluoro-Emerald but BDA-10,000 (blue only).
A subset of blood monocytes has been hypothesized to be potential precursors of perivascular macrophages.44,45 To identify a subset of monocytes that may represent such precursors, we have characterized phenotypes of blood monocytes by flow cytometry using anti-CD163, anti-CD14, anti-CD16, and anti-HLA-DR antibodies. Although greater than 80% of CD163+ monocytes co-expressed CD14 and HLA-DR, only ∼10% of CD163+ cells were CD16+ (data not shown). Monkey monocyte expression of CD163 with CD14 or CD16 increased after differentiation of monocytes to macrophages in culture and after acute infection with SIV (data not shown). These findings are consistent with previous studies in humans and suggest that mature and/or activated CD163+CD14+CD16+ cells in blood may represent precursors to CNS perivascular macrophages. It is possible that a population of these cells may traffic to the CNS to replace perivascular macrophages.
CD163+ Perivascular Macrophages Co-Express CD14 and CD16
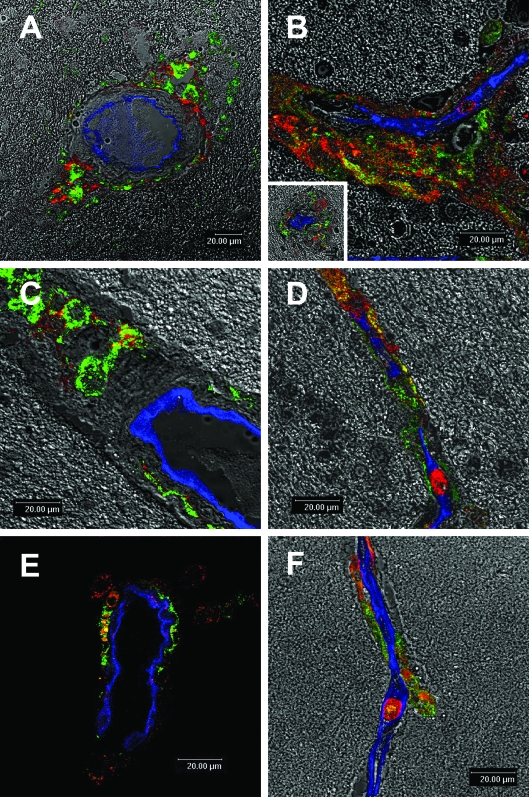
Although perivascular macrophages and parenchymal microglia are derived from bone marrow, perivascular macrophages are considered to be of more recent monocytic origin than their parenchymal counterparts. We have previously shown that perivascular macrophages exhibit an immune phenotype that is similar to a subset of monocytes (CD14+CD16+) that are expanded in blood of SIV-infected monkeys.45 Such cells may traffic to the CNS and become perivascular macrophages. Because CD163 is expressed on perivascular brain macrophages that are CD14 and CD16 positive in the human, we examined CD14, CD16, and HLA-DR expression in the monkey brain by single-label immunohistochemistry (Table 3). Although low CD16 and HLA-DR expression was largely restricted to perivascular macrophages in normal brains, this was up-regulated on perivascular macrophages and was induced de novo on parenchymal microglia in encephalitic brains. CD14, CD16, and HLA-DR all were easily detected on MNGCs. The distribution of CD14+ cells resembled that of CD163+ cells: all were closely associated with vessels in normal and encephalitic brain. Triple-label confocal microscopy studies confirmed CD163 as a marker for perivascular macrophage and demonstrated that CD14 or CD16 was co-expressed on CD163+ perivascular macrophages located adjacent to Glut-1+ CNS vessels (Figure 4). Because activation antigens including CD16 and HLA-DR are inducible on CD163−-activated microglia and are constitutively expressed on CD163+ perivascular macrophages in normal and encephalitic brain, it is likely that, similar to CD14, CD163 is a lineage-specific marker expressed by subpopulations of brain macrophages and an activation marker on these cells.
Table 3.
Immune Phenotype of CD163+ Perivascular Macrophages
| Perivascular macrophages
|
Parenchymal microglia
|
|||
|---|---|---|---|---|
| Control brain | Encephalitis lesion | Control brain | Encephalitis lesion | |
| CD14 | +++* | +++ | − | − |
| CD16 | +++ | +++ | ± | ++ |
| HLA-DR | +++ | +++ | ± | + |
| CD163 | +++ | +++ | − | ± |
, −, no staining present; ±, (<5 to 10%); +, (30 to 60%); ++, (60 to 90%); +++, (>90%).
Figure 4.
Co-expression of CD14 and CD16 on CD163+ perivascular macrophages in human and monkey brains. Triple-label confocal microscopic studies of HIVE brains (A, B, C, and D) and SIVE brains (E and F) demonstrate perivascular macrophages (CD163, green) that are CD14 positive (red; A, C, and E) and CD16 positive (red; B, D, and F) next to a CNS vessel (Glut-1, blue). A large number of CD163+ perivascular macrophages were positive for CD14 and CD16. These CD163+CD14+CD16+ perivascular macrophages accumulated in the perivascular space, which was markedly enlarged (A, B, and C). B, inset: A typical HIVE lesion with a collapsed vessel at its center. CD163+CD14+CD16+ cells were often found within the lumen of a vessel (D and F). It is possible that such peripheral blood monocytes/macrophages were in the process of trafficking to the perivascular space.
Discussion
Located in the perivascular (Virchow-Robin) space of cerebral microvessels, perivascular macrophages are situated at the interface between the nervous and immune systems and likely connect the two.46 Although several studies of human CNS disease demonstrate the importance of these cells, studies of the biology of perivascular macrophages in the human CNS has been hampered by the lack of a selective marker. The biology of these cells in vivo has been studied in the rat using the ED2 antigen, which selectively identifies perivascular macrophages, but not microglia, in normal and diseased brains.47,48 Recently, Dijkstra and colleagues34 suggested that human CD163 is homologous or even identical to rat ED2 based on peptide sequence analysis. We originally aimed to investigate whether CD163 is a specific marker for human and macaque perivascular macrophages in normal and HIV- and SIV-infected brains. Our results show that CD163 selectively identifies perivascular macrophages in normal human, monkey, and mouse brains. In our experience, CD163 was mainly restricted to perivascular macrophages in brains of HIVE and SIVE. However, scattered cells with a microglial morphology were found to be CD163 positive, consistent with previous reports.40,49 These findings suggest that CD163 may be a lineage-specific marker, distinguishing perivascular macrophages and subpopulations of macrophages in the meninges and choroid plexus from brain parenchymal microglia. ED2 expression by the same macrophage populations in the CNS has been reported.48,50 In some ways, our observations suggest that populations of CD163 monocytes, all of which are positive in blood, replace some but not all macrophage populations in the CNS. These findings also suggest that monocytes/macrophages in the meninges and choroid plexus are heterogeneous with respect to CD163 expression and that some of these cells are similar to perivascular macrophages.51 Bone marrow chimera studies have shown that the biology and kinetics of perivascular macrophage turnover are similar to some macrophages in the meninges and choroid plexus.4,5
CD163 is a recently designated CD molecule that is a member of the scavenger receptor cysteine-rich superfamily. CD163 expression is restricted to cells of the monocyte/macrophage lineage. It functions as an endocytotic receptor for hemoglobin-heptaglobin complexes, and its soluble form regulates inflammation.32 Ex vivo analysis of monocytes and enzyme-linked immunosorbent assay for soluble CD163 showed that cell surface expression and shedding of CD163 are increased in inflammatory disorders including Gaucher disease and reactive hemophagocytic syndrome.52,53 A similar shedding of soluble CD14 by monocytes, which might play an immune regulatory role, has been demonstrated.54–56
Although several mAbs that recognize the CD163 molecule have become available, they were tested mostly in non-CNS tissues, and therefore little is known about CD163 expression in the CNS. A previous study of phagocytic macrophages in the CNS of multiple sclerosis patients showed that perivascular macrophages, but not microglia, in normal white matter and in active lesions were stained with Ber-MAC3 antibody.57 This antibody was later found to recognize CD163.58 Recently, two other studies have shown that perivascular macrophages express CD163 in the normal human CNS, whereas human parenchymal microglia do not.29,35 Rezaie and Male35 stated in their brief report that this marker is largely absent on parenchymal microglia in neurodegenerative disorders including Alzheimer’s disease, diffuse Lewy body disease, and multiple sclerosis. Our results are in agreement with these studies and further substantiate the use of CD163 as a marker for perivascular macrophages in both normal and diseased brains, in multiple species. Our data extend these observations to SIV-infected perivascular macrophages.
Very recently, Fox and colleagues40,49 showed that CD163 labels not only perivascular macrophages but also activated microglia and a small subset of ramified microglia in gray matter in SIVE and HIVE brains, whereas only perivascular macrophages were stained in normal human and monkey brains and brains of variant Creutzfeldt-Jacob disease and Alzheimer’s disease. In some cases, we also found intracellular CD163 immunoreactivity in ramified microglia cells. Such expression may be interpreted in two ways, either 1) de novo expression on activated microglia within SIV and HIV lesions or 2) the recent migration of CD163-positive cells from blood into the brain where such cells take up residence and differentiate into cells with a ramified morphology. Interestingly, in our experience, parenchymal microglial cells mainly expressed CD163 intracellularly. One reason might be that these cells reflect a transitional state from monocytes/macrophages to ramified microglia. It has been shown that a small population of parenchymal microglia is repopulated in the brains of adult rodents and that these cells are replaced by bone marrow-derived cells during CNS inflammation.59 Because we and others have shown that the majority of blood monocytes are CD163 positive, it is possible that CD163+ cells reported by Fox and colleagues40,49 are recent CNS immigrants. Rappaport and colleagues8,60 have suggested that HIV-infected CD16+ microglia in the CNS of patients with HIVE might be derived from monocytes that have recently trafficked to the CNS and lost CD14 expression. Our data partly underscore this possibility. It should be noted that there are reports of scattered ED2 immunoreactivity on populations of parenchymal microglia.61,62 Lastly, a population of juxtavascular microglia have been described that are morphologically similar to parenchymal microglia.62 Because these cells are situated adjacent to CNS vessels and can quickly migrate from vessels to sites of injury, they might be a source of the CD163-positive, dye-labeled cells we identified with parenchymal microglial morphology.
Phenotypic heterogeneity between parenchymal microglia and perivascular macrophages suggests that they may represent two separate lineages of macrophage populations in the CNS.10,21,63–65 Parenchymal microglia are derived from fetal precursors early in development and originate in the yolk sac, although it is not clear whether bone marrow elements or circulating monocytes are differentiated into parenchymal microglia during development.66–69 In contrast, perivascular macrophages appear later when the vasculature develops.67,70 Previous studies in adult rodents have shown that macrophages in the perivascular, meningeal, and choroid plexus turnover regularly, whereas the repopulation of microglia by bone marrow precursors is limited, at least under normal conditions.3,71–73 Therefore, perivascular macrophages in adult and developing CNS are undoubtedly of monocytic origin. Two distinct populations of brain macrophages revealed by CD163 expression support the existence of two separate lineages of cells (CD163+ and CD163−). Using fluorochrome-conjugated dextrans that label only perivascular macrophage, we have shown for the first time in primates that CD163+ perivascular macrophages turnover on a time scale of a day, consistent with studies in rodents.39 Because a large majority of circulating monocytes are CD163 and CD14 positive, this supports the notion that perivascular macrophages are replaced by blood monocytes from the circulation. In this study, we have also shown that the perivascular macrophages co-expressing CD16 resemble a subset of blood monocytes that are positive for CD14, CD16, CD163, and SIV RNA and DNA and may have a higher potential for trafficking to the CNS to become perivascular macrophages.45
Increasing evidence points to the importance of the subset of monocytes/macrophages expressing CD14 and CD16 as mediators of HIV-related CNS disease. It has been shown that this subset expands in response to HIV infection and that its increased percentages correlate with HIV-associated dementia.74–77 In this study, we found that perivascular CD163 expression is upregulated and the number of CD163+ cells, including MNGCs, increases in HIVE and SIVE brains. CD163 is not a “classical” activation marker per se, because peripheral blood monocytes and most tissues macrophages of normal uninfected controls all express it and because in vitro proinflammatory stimuli largely down-regulate its expression. Monocyte-derived macrophages in culture show increased expression of CD163. It has been shown in vivo that CD163 expression on peripheral macrophages can be differentially regulated.25,78,79 These findings suggest that CD163 expression is regulated in association with a certain stage of differentiation. Although regulation of CD163 expression on a subset of monocytes expressing CD14 and CD16 at different phases of HIV infection is not known, its expression is highest in the CD14+CD16+ subset.33 It is likely that during HIV neuropathogenesis, a subset of monocytes increasingly infiltrate the CNS to become perivascular macrophages. Similarly, it has been shown that ED2 expression is up-regulated in EAE, facial nerve axotomy, and spinal cord injury.11,80 Interestingly, early up-regulation of ED2 expression on perivascular macrophages precedes the onset of EAE,11 suggesting that perivascular macrophages sense activating stimuli before certain CNS inflammation and/or that activated perivascular macrophages are the first infiltrating cells in the CNS with inflammation.
In this study, we have shown that perivascular macrophages express CD163 and are immunophenotypically distinct from parenchymal microglia, which are largely CD163 negative. We have also shown that CD163-positive perivascular macrophages are the primary cell type infected with SIV in encephalitic brains. This marker will be of great use to address the trafficking and differentiation of blood monocytes in the CNS as well as the respective contribution of perivascular macrophages to the development of CNS disease.
Acknowledgments
Human brain tissues were kindly provided by Susan Morgello from the Manhattan HIV Brain Bank (1R24MH59724).
Footnotes
Address reprint requests to Dr. Kenneth C. Williams, Division of Viral Pathogenesis, Beth Israel Deaconess Medical Center, 330 Brookline Avenue, RE-113, Boston, MA 02215. E-mail: Kenneth_Williams@jhms.harvard.edu.
Supported in part by Public Health Service grants NS37654 and NS40237 (to K.W.), RR00164 (to X.A.), CIHR37857 (to J.M.), and RR00150 (to S.W.).
References
- Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu Rev Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–247. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Vass K, Lassmann H. Bone marrow-derived elements in the central nervous system: an immunohistochemical and ultrastructural survey of rat chimeras. J Neuropathol Exp Neurol. 1992;5:246–256. doi: 10.1097/00005072-199205000-00002. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Schmied M, Vass K, Hickey WF. Bone marrow derived elements and resident microglia in brain inflammation. Glia. 1993;7:19–24. doi: 10.1002/glia.440070106. [DOI] [PubMed] [Google Scholar]
- Bauer J, Huitinga I, Zhao W, Lassmann H, Hickey WF, Dijkstra CD. The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia. 1995;15:437–446. doi: 10.1002/glia.440150407. [DOI] [PubMed] [Google Scholar]
- Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult rat microglia separated from other CNS macrophages by flow cytomteric sorting: phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- Polfliet MM, Zwijnenburg PJ, van Furth AM, van der Poll T, Dopp EA, Renardel de Lavalette C, van Kesteren-Hendrikx EM, van Rooijen N, Dijkstra CD, van den Berg TK. Meningeal and perivascular macrophages of the central nervous system play a protective role during bacterial meningitis. J Immunol. 2001;167:4644–4650. doi: 10.4049/jimmunol.167.8.4644. [DOI] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, deBakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J Exp Med. 2001;193:905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polfliet MM, van de Veerdonk F, Dopp EA, van Kesteren-Hendrikx EM, van Rooijen N, Dijkstra CD, van den Berg TK. The role of perivascular and meningeal macrophages in experimental allergic encephalomyelitis. J Neuroimmunol. 2002;122:1–8. doi: 10.1016/s0165-5728(01)00445-3. [DOI] [PubMed] [Google Scholar]
- Schiltz JC, Sawchenko PE. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J Neurosci. 2002;22:5606–5618. doi: 10.1523/JNEUROSCI.22-13-05606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G, Banati R. Brain microglia and blood-derived macrophages: molecular profiles and functional roles in multiple sclerosis and animal models of autoimmune demyelinating disease. Brain Res Brain Res Rev. 2004;46:261–281. doi: 10.1016/j.brainresrev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Dijkstra CD, Dopp EA, Joling P, Kraal G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in rat recognized by monoclonal antibodies ED1, ED2 and ED3. Adv Exp Med Biol. 1985;186:409–419. doi: 10.1007/978-1-4613-2463-8_50. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ, Kreutzberg GW. Identity of ED2-positive perivascular cells in rat brain. J Neurosci Res. 1989;22:103–106. doi: 10.1002/jnr.490220114. [DOI] [PubMed] [Google Scholar]
- Honda H, Kimura H, Silvers WK, Rostami A. Perivascular location and phenotypic heterogeneity of microglial cells in the rat brain. J Neuroimmunol. 1990;29:183–191. doi: 10.1016/0165-5728(90)90161-f. [DOI] [PubMed] [Google Scholar]
- Kida S, Steart PV, Zhang ET, Weller RO. Perivascular cells act as scavengers in the cerebral perivascular spaces and remain distinct from pericytes, microglia and macrophages. Acta Neuropathol. 1993;85:646–652. doi: 10.1007/BF00334675. [DOI] [PubMed] [Google Scholar]
- Galea I, Palin K, Newman TA, Van Rooijen N, Perry VH, Boche D. Mannose receptor expression specifically reveals perivascular macrophages in normal, injured, and diseased mouse brain. Glia. 2005;49:375–384. doi: 10.1002/glia.20124. [DOI] [PubMed] [Google Scholar]
- Becher B, Antel J. Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia. 1996;18:1–10. doi: 10.1002/(SICI)1098-1136(199609)18:1<1::AID-GLIA1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Dick AD, Pell M, Brew BJ, Foulcher E, Sedgwick JD. Direct ex vivo flow cytometric analysis of human microglial cell CD4 expression: examination of central nervous system biopsy specimens from HIV-seropositive patients and patients with other neurological disease. AIDS. 1997;11:1699–1708. doi: 10.1097/00002030-199714000-00006. [DOI] [PubMed] [Google Scholar]
- Walker WS. Separate precursor cells for macrophages and microglia in mouse brain: immunophenotypic and immunoregulatory properties of the progeny. J Neuroimmunol. 1999;94:127–133. doi: 10.1016/s0165-5728(98)00237-9. [DOI] [PubMed] [Google Scholar]
- Mattiace LA, Davies P, Dickson DW. Detection of HLA-DR on microglia in the human brain is a function of both clinical and technical factors. Am J Pathol. 1990;136:1101–1114. [PMC free article] [PubMed] [Google Scholar]
- Cosenza MA, Zhao ML, Si Q, Lee SC. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 2002;12:442–455. doi: 10.1111/j.1750-3639.2002.tb00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzun HJ, Kreipe H, Bodewadt S, Hansmann ML, Barth J, Parwaresch MR. Ki-M8 monoclonal antibody reactive with an intracytoplasmic antigen of monocyte/macrophage lineage. Blood. 1987;69:1320–1327. [PubMed] [Google Scholar]
- Zwadlo G, Voegeli R, Osthoff KS, Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Exp Cell Biol. 1987;55:295–304. doi: 10.1159/000163432. [DOI] [PubMed] [Google Scholar]
- Morganelli PM, Guyre PM. IFN-gamma plus glucocorticoids stimulate the expression of a newly identified human mononuclear phagocyte-specific antigen. J Immunol. 1988;140:2296–2304. [PubMed] [Google Scholar]
- Backe E, Schwarting R, Gerdes J, Ernst M, Stein H. Ber-MAC3: new monoclonal antibody that defines human monocyte/macrophage differentiation antigen. J Clin Pathol. 1991;44:936–945. doi: 10.1136/jcp.44.11.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulford K, Micklem K, McCarthy S, Cordell J, Jones M, Mason DY. A monocyte/macrophage antigen recognized by the four antibodies GHI/61, Ber-MAC3, Ki-M8 and SM4. Immunology. 1992;75:588–595. [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel MM, Tensen CP, van As JH, Van den Berg TK, Fluitsma DM, Dijkstra CD, Dopp EA, Droste A, Van Gaalen FA, Sorg C, Hogger P, Beelen RH. Regulation of CD 163 on human macrophages: cross-linking of CD163 induces signaling and activation. J Leukoc Biol. 1999;66:858–866. doi: 10.1002/jlb.66.5.858. [DOI] [PubMed] [Google Scholar]
- Lau SK, Chu PG, Weiss LM. CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol. 2004;122:794–801. doi: 10.1309/QHD6-YFN8-1KQX-UUH6. [DOI] [PubMed] [Google Scholar]
- Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163. Immunobiology. 2005;210:153–160. doi: 10.1016/j.imbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- Buechler C, Ritter M, Orso E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- van den Berg TK, Puklavec MJ, Barclay AN, Dijkstra CD. Monoclonal antibodies against rat leukocyte surface antigens. Immunol Rev. 2001;184:109–116. doi: 10.1034/j.1600-065x.2001.1840110.x. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Male D. Microglia in fetal and adult human brain can be distinguished from other mononuclear phagocytes through their lack of CD163 expression. Neuroembryology. 2003;2:130–133. [Google Scholar]
- Fabriek BO, Van Haastert ES, Galea I, Polfliet MM, Dopp ED, Van Den Heuvel MM, Van Den Berg TK, De Groot CJ, Van Der Valk P, Dijkstra CD. CD163-positive perivascular macrophages in the human CNS express molecules for antigen recognition and presentation. Glia. 2005;51:297–305. doi: 10.1002/glia.20208. [DOI] [PubMed] [Google Scholar]
- Budka H. Multinucleated giant cells in brain: a hallmark of the acquired immune deficiency syndrome (AIDS). Acta Neuropathol. 1986;69:253–258. doi: 10.1007/BF00688301. [DOI] [PubMed] [Google Scholar]
- Lackner AA, Smith MO, Munn RJ, Martfeld DJ, Gardner MB, Marx PA, Dandekar S. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol. 1991;139:609–621. [PMC free article] [PubMed] [Google Scholar]
- Bechmann I, Kwidzinski E, Kovac AD, Simburger E, Horvath T, Gimsa U, Dirnagl U, Priller J, Nitsch R. Turnover of rat brain perivascular cells. Exp Neurol. 2001;168:242–249. doi: 10.1006/exnr.2000.7618. [DOI] [PubMed] [Google Scholar]
- Roberts ES, Zandonatti MA, Watry DD, Madden LJ, Henriksen SJ, Taffe MA, Fox HS. Induction of pathogenic sets of genes in macrophages and neurons in NeuroAIDS. Am J Pathol. 2003;162:2041–2057. doi: 10.1016/S0002-9440(10)64336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Schwartz A, Corey S, Orandle M, Kennedy W, Thompson B, Alvarez X, Brown C, Gartner S, Lackner AA. Proliferating cellular nuclear antigen expression as a marker of perivascular macrophages in simian immunodeficiency virus encephalitis. Am J Pathol. 2002;161:575–585. doi: 10.1016/S0002-9440(10)64213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulahian TH, Hogger P, Wahner AE, Wardwell K, Goulding NJ, Sorg C, Droste A, Stehling M, Wallace PK, Morganelli PM, Guyre PM. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000;12:1312–1321. doi: 10.1006/cyto.2000.0720. [DOI] [PubMed] [Google Scholar]
- Pardridge WM, Boado RJ, Farrell CR. Brain-type glucose transporter (GLUT-1) is selectively localized to the blood-brain barrier: studies with quantitative Western blotting and in situ hybridization. J Biol Chem. 1990;265:18035–18040. [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Kim WK, Corey S, Alvarez X, Williams K. Monocyte/macrophage traffic in HIV and SIV encephalitis. J Leukoc Biol. 2003;74:650–656. doi: 10.1189/jlb.0503207. [DOI] [PubMed] [Google Scholar]
- Williams K, Alvarez X, Lackner AA. Central nervous system perivascular cells are immunoregulatory cells that connect the CNS with the peripheral immune system. Glia. 2001;36:156–164. doi: 10.1002/glia.1105. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ, Buringer D. Identity of ED2-positive perivascular cells in rat brain. J Neurosci Res. 1989;28:236–243. doi: 10.1002/jnr.490220114. [DOI] [PubMed] [Google Scholar]
- Bauer J, Ruuls SR, Huitinga I, Dijkstra CD. The role of macrophage subpopulations in autoimmune disease of the central nervous system. Histochem J. 1996;28:83–97. doi: 10.1007/BF02331413. [DOI] [PubMed] [Google Scholar]
- Roberts ES, Masliah E, Fox HS. CD163 identifies a unique population of ramified microglia in HIV encephalitis (HIVE). J Neuropathol Exp Neurol. 2004;63:1255–1264. doi: 10.1093/jnen/63.12.1255. [DOI] [PubMed] [Google Scholar]
- Perry VH, Lawson LJ, Reid DM. Biology of the mononuclear phagocyte system of the central nervous system and HIV infection. J Leukoc Biol. 1994;56:399–406. doi: 10.1002/jlb.56.3.399. [DOI] [PubMed] [Google Scholar]
- Hickey WF, Williams KC. Mononuclear phagocyte heterogeneity and the blood-brain barrier: a model for HIV-1 neuropathogenesis. Gendelman HE, Lipton SA, L Epstein, Swindells S, editors. New York: Chapman and Hall; The Neurology of AIDS. 1998:pp 61–72. [Google Scholar]
- Moller HJ, Aerts H, Gronbaek H, Peterslund NA, Hyltoft Petersen P, Hornung N, Rejnmark L, Jabbarpour E, Moestrup SK. Soluble CD163: a marker molecule for monocyte/macrophage activity in disease. Scand J Clin Lab Invest Suppl. 2002;237:29–33. doi: 10.1080/003655102762377466. [DOI] [PubMed] [Google Scholar]
- Schaer DJ, Schleiffenbaum B, Kurrer M, Imhof A, Bachli E, Fehr J, Moller HJ, Moestrup SK, Schaffner A. Soluble hemoglobin-haptoglobin scavenger receptor CD163 as a lineage-specific marker in the reactive hemophagocytic syndrome. Eur J Haematol. 2005;74:6–10. doi: 10.1111/j.1600-0609.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- Bazil V, Strominger JL. Shedding as a mechanism of down-modulation of CD14 on stimulated human monocytes. J Immunol. 1991;147:1567–1574. [PubMed] [Google Scholar]
- Nockher WA, Bergmann L, Scherberich JE. Increased soluble CD14 serum levels and altered CD14 expression of peripheral blood monocytes in HIV-infected patients. Clin Exp Immunol. 1994;98:369–374. doi: 10.1111/j.1365-2249.1994.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien E, Aukrust P, Sundan A, Muller F, Froland SS, Espevik T. Elevated levels of serum-soluble CD14 in human immunodeficiency virus type 1 (HIV-1) infection: correlation to disease progression and clinical events. Blood. 1998;92:2084–2092. [PubMed] [Google Scholar]
- Li H, Newcombe J, Groome NP, Cuzner ML. Characterization and distribution of phagocytic macrophages in multiple sclerosis plaques. Neuropathol Appl Neurobiol. 1993;19:214–223. doi: 10.1111/j.1365-2990.1993.tb00431.x. [DOI] [PubMed] [Google Scholar]
- IUIS/WHO Subcommittee on CD Nomenclature CD antigens 1996: updated nomenclature for clusters of differentiation on human cells. Bull World Health Organ. 1997;75:385–387. [PMC free article] [PubMed] [Google Scholar]
- Flugel A, Bradl M, Kreutzberg GW, Graeber MB. Transformation of donor-derived bone marrow precursors into host microglia during autoimmune CNS inflammation and during the retrograde response to axotomy. J Neurosci Res. 2001;66:74–82. doi: 10.1002/jnr.1198. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Adeniyi A, Rybicka K, Morgello S, Khalili K, Rappaport J. Macrophage/microglial accumulation and proliferating cell nuclear antigen expression in the central nervous system in human immunodeficiency virus encephalopathy. Am J Pathol. 2004;164:2089–2099. doi: 10.1016/S0002-9440(10)63767-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka T, Baba T, Black KL, Streit WJ. Immunophenotypic analysis of infiltrating leukocytes and microglia in an experimental rat glioma. Acta Neuropathol. 1992;83:590–597. doi: 10.1007/BF00299407. [DOI] [PubMed] [Google Scholar]
- Grossmann R, Stence N, Carr J, Fuller L, Waite M, Dailey ME. Juxtavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia. 2002;37:229–240. [PubMed] [Google Scholar]
- de Groot CJ, Huppes W, Sminia T, Kraal G, Dijkstra CD. Determination of the origin and nature of brain macrophages and microglial cells in mouse central nervous system, using non-radioactive in situ hybridization and immunoperoxidase techniques. Glia. 1992;6:301–309. doi: 10.1002/glia.440060408. [DOI] [PubMed] [Google Scholar]
- Mato M, Ookawara S, Sakamoto A, Aikawa E, Ogawa T, Mitsuhashi U, Masuzawa T, Suzuki H, Honda M, Yazaki Y, Watanabe E, Luoma J, Yla-Herttuala S, Fraser I, Gordon S, Kodama T. Involvement of specific macrophage-lineage cells surrounding arterioles in barrier and scavenger function in brain cortex. Proc Natl Acad Sci USA. 1996;93:3269–3274. doi: 10.1073/pnas.93.8.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Nakazato Y, Ogawa A, Sugihara S. The immunophenotype of perivascular cells in the human brain. Pathol Int. 1996;46:15–23. doi: 10.1111/j.1440-1827.1996.tb03528.x. [DOI] [PubMed] [Google Scholar]
- Cuadros MA, Rodriguez-Ruiz J, Calvente R, Almendros A, Marin-Teva JL, Navascues J. Microglia development in the quail cerebellum. J Comp Neurol. 1997;389:390–401. [PubMed] [Google Scholar]
- Andjelkovic AV, Nikolic B, Pachter JS, Zecevic N. Macrophages/microglial cells in human central nervous system during develop-ment: an immunohistochemical study. Brain Res. 1998;814:13–25. doi: 10.1016/s0006-8993(98)00830-0. [DOI] [PubMed] [Google Scholar]
- Alliot F, Godin I, Pessac B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res Dev Brain Res. 1999;117:145–152. doi: 10.1016/s0165-3806(99)00113-3. [DOI] [PubMed] [Google Scholar]
- Kaur C, Hao AJ, Wu CH, Ling EA. Origin of microglia. Microsc Res Tech. 2001;54:2–9. doi: 10.1002/jemt.1114. [DOI] [PubMed] [Google Scholar]
- Rezaie P, Dean A, Male D, Ulfig N. Microglia in the cerebral wall of the human telencephalon at second trimester. Cereb Cortex. 2004;15:938–949. doi: 10.1093/cercor/bhh194. [DOI] [PubMed] [Google Scholar]
- Kennedy DW, Abkowitz JL. Kinetics of central nervous system microglial and macrophage engraftment: analysis using a transgenic bone marrow transplantation model. Blood. 1997;90:986–993. [PubMed] [Google Scholar]
- Vallieres L, Sawchenko PE. Bone marrow-derived cells that populate the adult mouse brain preserve their hematopoietic identity. J Neurosci. 2003;23:5197–5207. doi: 10.1523/JNEUROSCI.23-12-05197.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DC, Abe T, Hill WD, Studdard AM, Carothers J, Masuya M, Fleming PA, Drake CJ, Ogawa M. Hematopoietic origin of microglial and perivascular cells in brain. Exp Neurol. 2004;186:134–144. doi: 10.1016/j.expneurol.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Allen JB, Wong HL, Guyre PM, Simon GL, Wahl SM. Association of circulating receptor Fc gamma RIII-positive monocytes in AIDS patients with elevated levels of transforming growth factor-beta. J Clin Invest. 1991;87:1773–1779. doi: 10.1172/JCI115196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher C, Vanham G, Kestens L, Kruger M, Ceuppens JL, Vingerhoets J, Gigase P. Expression patterns of Fc gamma receptors, HLA-DR and selected adhesion molecules on monocytes from normal and HIV-infected individuals. Clin Exp Immunol. 1994;98:115–122. doi: 10.1111/j.1365-2249.1994.tb06616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–3424. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- Zwadlo-Klarwasser G, Neubert R, Stahlmann R, Schmutzler W. Influence of dexamethasone on the RM 3/1-positive macrophages in the peripheral blood and tissues of a New World monkey (the marmoset Callithrix jacchus). Int Arch Allergy Immunol. 1992;97:178–180. doi: 10.1159/000236115. [DOI] [PubMed] [Google Scholar]
- Boven LA, van Meurs M, Boot RG, Mehta A, Boon L, Aerts JM, Laman JD. Gaucher cells demonstrate a distinct macrophage phenotype and resemble alternatively activated macrophages. Am J Clin Pathol. 2004;122:359–369. doi: 10.1309/BG5V-A8JR-DQH1-M7HN. [DOI] [PubMed] [Google Scholar]
- Satake K, Matsuyama Y, Kamiya M, Kawakami H, Iwata H, Adachi K, Kiuchi K. Nitric oxide via macrophage iNOS induces apoptosis following traumatic spinal cord injury. Brain Res Mol Brain Res. 2000;85:114–122. doi: 10.1016/s0169-328x(00)00253-9. [DOI] [PubMed] [Google Scholar]