Abstract
The prototypic pituitary hormone prolactin (PRL) exerts a wide variety of bioregulatory effects in mammals and is also found in extrapituitary sites, including murine skin. Here, we show by reverse transcriptase-polymerase chain reaction and immunohistology that, contrary to a previous report, human skin and normal human scalp hair follicles (HFs), in particular, express both PRL and PRL receptors (PRL-R) at the mRNA and protein level. PRL and PRL-R immunoreactivity can be detected in the epithelium of human anagen VI HFs, while the HF mesenchyme is negative. During the HF transformation from growth (anagen) to apoptosis-driven regression (catagen), PRL and PRL-R immunoreactivity appear up-regulated. Treatment of organ-cultured human scalp HFs with high-dose PRL (400 ng/ml) results in a significant inhibition of hair shaft elongation and premature catagen development, along with reduced proliferation and increased apoptosis of hair bulb keratinocytes (Ki-67/terminal dUTP nick-end labeling immunohistomorphometry). This shows that PRL receptors, expressed in HFs, are functional and that human skin and human scalp HFs are both direct targets and sources of PRL. Our data suggest that PRL acts as an autocrine hair growth modulator with catagen-promoting functions and that the hair growth-inhibitory effects of PRL demonstrated here may underlie the as yet ill-understood hair loss in patients with hyperprolactinemia.
The polypeptide hormone prolactin (PRL) belongs to the PRL/growth hormone/placental lactogen gene family. The PRL gene is 10 kb in size, and transcription of the PRL gene is regulated by two different promoter regions. The proximal 5000-bp region directs pituitary-specific expression, whereas the more upstream promoter region is responsible for extrapituitary expression.1 PRL has been shown to exert an exceptionally wide variety of bioactivities. Beyond lactation and reproduction, PRL is now recognized to modulate immune responses, osmoregulation, angiogenesis via induction of vascular endothelial growth factor, development, and hair growth.2–10 It has been speculated that the mammotropic actions of PRL in humans may have evolved from more generalized actions of this versatile biomediator on integumental structures in other vertebrates, such as the epidermis of amphibians, the feathers of birds, or hair and sebaceous glands in mammals.11 Released most prominently by the pituitary gland and binding to specific receptors in the skin, PRL has been hypothesized to act as a neuroendocrine modulator of epithelial proliferation and of the skin immune system.9,12
The role of PRL in hair growth regulation has been intensely studied in mammals with seasonally dependent cycles of pelage replacements. PRL has been shown to stimulate hair growth, moulting, and shedding in sheep and mink, and contradictory data report of induction of both anagen (hair growth) and catagen (HF regression) in seasonal dependent HFs by PRL.13–18 Although PRL and melatonin stimulate hair shaft elongation in culture in cashmere goats,16 increased levels of PRL after experimentally increased photoperiods have been shown to decrease hair growth in vitro.7,19 Increasing PRL levels in spring was even shown to reactivate telogen HFs and induce anagen in cashmere goats.20
PRL likely also plays a role in seasonally independent hair cycles, as they are characteristic for mice and man.21 We have recently demonstrated that PRL and its receptor are expressed in a hair cycle-dependent manner in HF keratinocytes in mice in vivo. Treatment of murine anagen HFs with PRL leads to HF regression (catagen) accompanied by decreased proliferation in murine skin organ culture.22 Also, disruption of the PRL receptor (PRL-R) gene in mice results in hair cycle perturbations: PRL-R knockout mice show premature fur molting and premature entry of their HFs into the next hair cycle.23
The role of PRL in human hair growth control is still unclear. Hyperprolactinemia is accompanied by an androgenetic alopecia-like hair loss pattern, amenorrhea, infertility, acne vulgaris, and hirsutism.24–26 This may be related to the fact that PRL can increase adrenal androgen production, although it can attenuate 5-α-reductase activity both in vivo and in vitro.27 However, in men presenting premature balding before the age of 30, a recent study has reported subnormal PRL serum levels.28 In women, hair loss (telogen effluvium) may also be seen as a side-effect of treatment with bromocriptine, a dopaminergic inhibitor of pituitary PRL secretion.29–31
A particularly intriguing issue is from where the ligands arise that stimulate cutaneous PRL-Rs. It is now recognized that, besides the pituitary gland, a number of extrapituitary tissues (such as placenta, uterus, mammary gland, brain, and lymphocytes) can synthesize PRL.1,32 The PRL receptor (PRL-R) is a single-pass membrane-bound protein that belongs to the cytokine receptor family and transduces its signal by binding Janus kinases (JAKs) and by activating signal transducers and activators of transcription (Stat) proteins. Crosslinking of PRL-Rs by PRL brings the JAK2 that is bound to the cytoplasmic tail of each PRL-R together, leading to PRL-R phosphorylation. Subsequently, this activation of the PRL-R by JAK2 turns the receptor into a receptor tyrosine kinase, which phosphorylates inactive STATs and causes them to dimerize and translocate as activated transcription factors into the nucleus, where they bind to specific DNA regions and stimulate transcription of PRL target genes.32–34 In addition, the biological activity of PRL is triggered via a hormone-induced receptor homodimerization process that is regulated by tertiary features of the hormone. This feature plays an important role in the regulation of these systems by producing binding surfaces with dramatically different binding affinities to the receptor.35 Such PRL-R are expressed by human epidermal keratinocytes in vitro36; in the dermal papilla, matrix, outer root sheath, lower regions of the inner root sheath, and connective tissue sheath in wool follicles of sheep37,38; and in the outer root sheath of anagen HFs of mice.22,23 This expression pattern suggests that PRL can directly alter skin and HF functions by targeting cognate, locally expressed receptors. In human skin, PRL mRNA has been reported to be expressed in and released by dermal fibroblasts and sweat glands in vitro.39 Another group found PRL-R to be expressed in differentiated human keratinocytes in vitro.36 However, it has been recently reported that PRL RNA cannot be detected in truncal skin by reverse transcriptase (RT)-polymerase chain reaction (PCR).40
In this study we, therefore, wished to clarify further the role of PRL in human skin, with emphasis on its role in human hair growth. We investigated by immunohistology where exactly PRL and its receptor are expressed in HFs and by RT-PCR whether PRL is even synthesized in HFs. In addition, we wanted to know whether PRL is able to modulate human hair growth in vitro and whether it shows any influence on follicular apoptosis and proliferation. We show for the first time that PRL mRNA and protein are expressed in human skin and isolated organ-cultured HFs. In addition, we show that PRL induces premature catagen in isolated anagen scalp HFs. These data support the hypothesis that PRL is locally produced in the skin and acts directly as a hormonal regulator of HF regression in human anagen scalp HFs, possibly as a cutaneous response to stress or as a part in the pathogenesis of androgenetic alopecia in females.
Materials and Methods
Materials
Williams E medium (Life Technologies, Inc., Rockville, MD) was supplemented with l-glutamine, penicillin, and streptomycin. Human recombinant PRL was purchased from R&D Systems (Minneapolis, MN). Goat anti-human PRL antibody was obtained from Santa Cruz (Santa Cruz Biotechnology, Santa Cruz, CA) and sheep anti-human PRL-R from DFC, Biermann GmbH (Bad Nauheim, Germany).
PRL and PRL-R Immunohistochemistry
Cryosections from isolated human HFs were fixed in acetone, washed in Tris-buffered saline, and incubated with 3% H2O2, followed by avidin and biotin application. Additionally, cryosections from full-thickness human scalp skin were treated the same way to look for PRL protein expression in the skin and without the wounding trauma of microdissected HFs. The samples were blocked with 10% donkey serum and 3% bovine serum albumin for 20 minutes and incubated with goat anti-human PRL antibody (1:100, Santa Cruz Biotechnology) overnight at 4°C (polyclonal goat antibody raised against a peptide mapping near the carboxy terminus of PRL of human origin). After further washing biotin-marked donkey anti-goat secondary antibody (1:200: Jackson ImmunoResearch, Hamburg, Germany) was applied for 45 minutes. Washes and incubation with ABC-Kit (Vector Laboratories, Burlingame, CA) for 30 minutes followed. AEC+ was used as substrate (DAKO, Hamburg, Germany), and sections were counterstained with hematoxylin and mounted using Kaiser’s glycerol gelatin. Human pituitary gland sections were used as positive controls. Sections without primary antibody served as negative controls.
For detecting PRL-R, cryosections were treated the same way as for the anti-PRL staining. Blocking solution of 10% rabbit serum and 3% bovine serum albumin were applied for 20 minutes, followed by incubation of the primary antibody sheep anti-human PRL-R (1:100; DFC, Biermann) overnight at 4°C. Biotin-marked rabbit anti-sheep IgG (1:200, Jackson ImmunoResearch) was used as secondary antibody and incubated for 45 minutes at room temperature. AEC+ (DAKO) was used as substrate. Human mammary gland sections served as positive control. Sections omitting the primary antibody served as negative controls.
Isolation and Culture of Human Hair Follicles
Excess anagen HFs from occipital human scalp skin, obtained with informed consent during routine hair transplant or face-lift surgery, were isolated and cultured within 24 hours after surgery as previously described by Philpott and colleagues.41 The total number of organ-cultured HFs in anagen VI stage was 180, derived from 12 different individuals 25 to 55 years of age. After separation of epidermis and dermis from subcutaneous fat under a binocular dissecting microscope, anagen HFs were isolated from the subcutis by using watchmaker’s forceps.
HFs were then cultured under serum-free conditions in a 24-well plate containing 500 μl of Williams E medium supplemented with insulin, l-glutamine, hydrocortisone, streptomycin, and penicillin. Three follicles per well were incubated for 8 days at 5% CO2 with addition of 400 ng/ml of human recombinant PRL (R&D Systems). Medium was changed every second day. Cultured HFs without PRL served as vehicle controls. After 4 days in culture, human HFs were washed in phosphate-buffered saline and embedded in OCT for cryosectioning. Normal PRL levels in humans vary between nonpregnant females (30 to 80 ng/ml), pregnant females (150 to 600 ng/ml), and males (5 to 20 ng/ml).5,8,42
Statistical Analysis and Quantitative Histomorphometry
Hair shaft length was measured every second day using a binocular dissecting microscope and hair-cycle stages were assessed according to defined morphological criteria and photodocumented by light microscopy. Longitudinally cut HFs (n = 60/group) were counted, and the hair-cycle stage of each HF was assessed according to defined morphological criteria, classified by morphological criteria, and assigned to their respective hair-cycle stages, following quantitative hair-cycle histomorphometry techniques described for murine catagen development.43,44 The hair-cycle score (HCS) was assessed and calculated as described.45,46 The data of all experiments were pooled and statistical analysis was calculated by Mann-Whitney U-test for unpaired samples.
Ki-67/Terminal dUTP Nick-End Labeling (TUNEL) Immunohistomorphometry
To demonstrate proliferating and apoptotic cells at the same time, we combined the established protocols for Ki-67 (Dianova, Hamburg, Germany) and TUNEL (Apop-tag; Oncor Appligene, Heidelberg, Germany) immunohistochemistry.46–48 Briefly, 5-μm cryosections of human HFs were air-dried, fixed in 1% paraformaldehyde, and postfixed in an ethanol:acetic acid mixture (2:1) at −20°C. After incubation with TdT enzyme for 1 hour at 37°C, TUNEL-positive cells were visualized by an anti-digoxigenin fluorescein antibody. Subsequently, tissue sections were preincubated with 10% goat serum, followed by an application of mouse anti-human Ki-67 antiserum (1:20, Dianova). To detect Ki-67 immunoreactivity, rhodamine-conjugated goat anti-mouse secondary antibody (1:200, Jackson ImmunoResearch) was used. Sections were then counterstained with 4,6-diamidino-2-phenylindole (DAPI) (1:5000, Hoechst 33342).
Negative controls for the TUNEL staining were made by omitting TdT enzyme, and murine spleen sections served as positive control. For Ki67, positive controls were run by comparison with tissue sections from the back skin of mice in anagen VI stage of the depilation-induced hair cycle. Sections were examined under a Zeiss Axioscope microscope, using the appropriate excitation-emission filter systems for studying the fluorescence induced by DAPI, fluorescein, and rhodamine. The number of cells positive for Ki-67 and TUNEL immunoreactivity was counted per hair bulb and statistical significance was calculated by Mann-Whitney U-test for unpaired samples.
RT-PCR
Total RNA was isolated from ∼1 g of each frozen scalp skin by grinding to powder under liquid nitrogen in a freezer mill (SPEX 7700; Glen Creston Ltd., Middlesex, UK) and extraction with TRIzol reagent (Life Technologies, Inc.) according to the manufacturer’s instructions. RNA concentration was measured by spectrophotometry at 260 nm, and RNA integrity was verified by Northern blotting. A surgically obtained full-thickness human scalp skin, 30 freshly isolated human HFs and human pituitary gland (positive control) were snap-frozen in liquid nitrogen and homogenized using an electronic homogenizer. Total RNA was isolated with an RNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. cDNA was synthesized by reverse transcription of 1 μg of total RNA using First Strand cDNA synthesis kit for RT-PCR (AMV) (Roche, Mannheim, Germany). The following sets of oligonucleotide primers were used: hPRL forward, 5′-CCC TTG CCC ATC TGT CCC GGC G-3′; hPRL reverse, 5′-ATC GCA ATA TGC TGA CTA TCA G-3′; 5-GAPDH, 5′-TGGGTGTGAACCATGAGAAG-3′; 3-GAPDH: 5′-GCTAAGCAGTTGGTGGTGC-3′.
Primers for PRL are located in different exons according to the reported sequences in GenBank (accession number, NM000948). Amplification was performed using PCR core kit (Qiagen) for more than 40 cycles using an automated thermal cycler (Biometra, Göttingen, Germany). Each cycle consisted of denaturing at 94°C (30 seconds), annealing at 63°C (1 minute), and extension at 72°C (1 minute). PCR conditions for GAPDH (GenBank accession number, AY340484.1), which gave a 168-bp PCR product, involved 94°C (5 minutes); 28 cycles of 94°C (1minute), 58°C (1 minute), and 72°C (1 minute); and 72°C (10 minutes). PCR products were analyzed by agarose gel electrophoresis. PCR fragment identity was verified with digestion with restriction enzyme EcoRI and NcoI (Roche), which gave expected sized digested fragments through gel electrophoresis (data not shown).
Results
Human Scalp Hair Follicles Express PRL and PRL-R-Like Immunoreactivity in Vivo and in Organ Culture
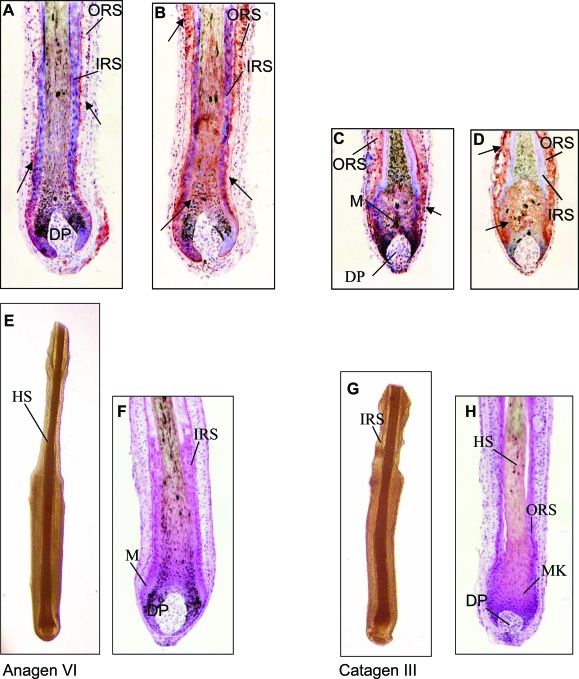
In isolated human anagen VI HFs, PRL protein was expressed in a thin layer of keratinocytes between inner and outer root sheath. Matrix keratinocytes and dermal papilla were negative (Figure 1A). Prolactin receptor (PRL-R)-like immunoreactivity was detected in the outer root sheath and additionally in the proximal inner root sheath and matrix keratinocytes. No immunoreactivity could be observed in the dermal papilla (Figure 1B). The positive and negative controls confirmed the sensitivity and specificity of these immunostaining results. Sections from full-thickness human scalp skin showed a similar expression pattern for PRL and PRL-R as in microdissected, organ-cultured human HFs. Besides the HF immunoreactivity, PRL and PRL-R were seen in the epidermis and arrector pili muscle. The capsule of the sebaceous gland stained positive for PRL-R, while the immunoreactivity of the receptor could be detected in the whole sebaceous gland (data not shown).
Figure 1.
A–D: PRL-like immunoreactivity (A, C) and PRL-R-like immunoreactivity (B, D) in isolated human anagen (A, B) and catagen (C, D) HFs were stained using the ABC method with AEC+ as substrate and hematoxylin (blue) for counterstaining. IRS, inner root sheath; ORS, outer root sheath; M, melanocytes; DP, dermal papilla; HS, hair shaft; MK, matrix keratinocytes. Arrows designate PRL and PRL-R staining. E–H: Macroscopic and histological demonstration of human HFs after 8 days in culture. E and G: Light microscopy; E and F: vehicle control; F and H: H&E staining (anagen VI); G and H: 400 ng/ml PRL (catagen III). PRL induced cessation of pigmentation, the shape of the dermal papilla changed into a more condensed shape, the volume of the hair matrix diminishes, and the pigmented lower end of the hair shaft moved upward in isolated HFs. All are characteristic events in catagen development.
Interestingly, in catagen III HFs, the immunoreactivity for PRL and its receptor was more intense than in anagen VI HFs. PRL and PRL-R-like protein could be detected in keratinocytes of the outer root sheath and matrix, while the inner root sheath and dermal papilla were negative (Figure 1, C and D) in contrast to anagen VI HFs, where the proximal inner root sheath was positive (Figure 1, A and B). Although this could not be further quantitated, this suggests a slight up-regulation of intrafollicular PRL/PRL-R signaling during the anagen-catagen transformation of human scalp HFs.
PRL Inhibits Hair Shaft Elongation in Human Organ-Cultured Hair Follicles
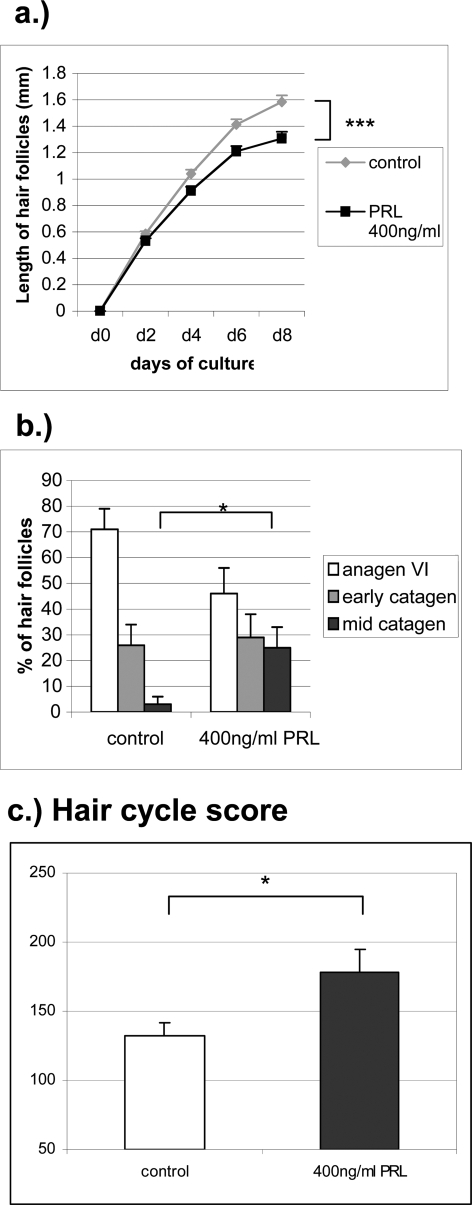
In addition, we investigated whether PRL directly exerts growth-modulating effects on human HFs. PRL was added to the microdissected hair bulbs of organ-cultured human anagen VI HFs. In the current study, we cultured human anagen VI HFs from male occipital scalp skin up to 6 days and observed a hair shaft elongation of ∼0.3 mm per day during this time (Figures 1E and 2a). Human HFs treated with 400 ng/ml of PRL every other day showed a significantly reduced hair shaft elongation rate compared to vehicle controls (P < 0001). We could demonstrate an inhibition of hair shaft elongation due to PRL treatment (Figure 1G) compared to control HFs after 8 days in culture (Figure 1E). In the control group, there was an increase in hair shaft length of ∼1.6 mm after 8 days in contrast to only 1.3 mm in the PRL-treated group (Figure 2a). In our experiments, concentrations of 200 ng and 400 ng of PRL were used, reasoning that physiological PRL levels in human males are unlikely to be associated with experimentally discernable hair growth abnormalities. Normal PRL levels in humans vary between nonpregnant females (30 to 80 ng/ml), pregnant females (150 to 600 ng/ml), and males (5 to 20 ng/ml).5,8,42 These supraphysiological doses of PRL were purposely chosen to imitate the increased PRL levels (200 to 400 ng/ml) that are seen in patients with telogen effluvium and/or hirsutism associated with hyperprolactemia, as it occurs with prolactinoma.1
Figure 2.
a: Hair shaft elongation of PRL-treated HFs compared to vehicle control (n = 63 HFs per group) after 0, 2, 4, 6, and 8 days in culture. ***P < 0001. b: Percentage of HFs at defined hair cycle stages after 8 days in culture were assessed and statistical significance was calculated using Mann-Whitney U-test. *P < 0.05. c: Calculation of the HCS. All HFs of each group were staged and each stage of the hair cycle has been scored as follows: anagen VI = 100, early catagen = 200, mid catagen = 300. The HCS indicates the mean of the stages of all HFs per group. *P < 0.05.
PRL Prematurely Induces a Catagen-Like Stage in Organ-Cultured Human Hair Follicles
Quantitative histomorphometry of hematoxylin and eosin-stained HF sections revealed that PRL was able to accelerate spontaneous catagen development in human anagen follicles in vitro. PRL induced cessation of pigmentation, the shape of the dermal papilla changed into a more condensed shape, the volume of the hair matrix diminished, and the pigmented lower end of the hair shaft moved upward in isolated HFs. Although more than 70% of control follicles were still in anagen VI and only 3% had entered mid-catagen stage (Figure 1F), only 45% of PRL-treated HFs remained in anagen VI, and 25% had already approached mid-catagen after 8 days of organ culture under serum-free conditions (Figures 1H and 2b). This was confirmed by calculation of the HCS, which allows one to summarily assess and compare the full range of catagen stages between experimental groups.45,46 The HCS showed a high value for the group treated with 400 ng of PRL (Figure 2c) and a lower value for control HFs, indicating that catagen development in PRL-treated HFs was significantly advanced compared to vehicle-treated HFs. Interestingly, immunohistology of the potent catagen inductor transforming growth factor (TGF)-β2 in the HF after PRL treatment did not show any changes in location or intensity (data not shown).
PRL Decreases Proliferation and Up-Regulates Apoptosis of Keratinocytes in Human Hair Follicles
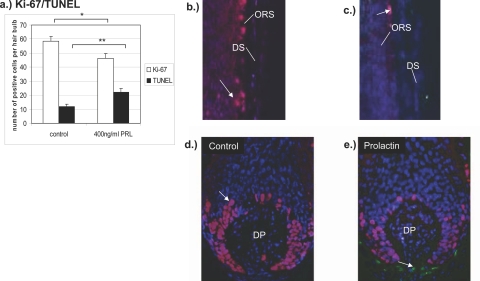
To investigate the influence of PRL on proliferation and apoptosis of follicular cells quantitative immunohistomorphometry of Ki-67+ or TUNEL+ keratinocytes in the hair bulb was performed. Evaluating the number of proliferating and apoptotic cells per hair bulb a significant down-regulation of Ki-67-positive cells (P < 0.05) and a significant increase of TUNEL-positive cells (P < 0.01) were found in the PRL-treated group (400 ng/ml) (Figure 3a). Control follicles displayed a high amount of proliferating matrix cells around the dermal papilla and only very few apoptotic cells (Figure 3, b and d; left). In contrast, there were fewer Ki-67+ matrix keratinocytes and more TUNEL+ cells in the outer root sheath and dermal papilla of PRL treated HFs (Figure 3, c and e; right). The calculation of Ki-67-positive cells per hair bulb showed a significant decrease of 47% proliferating cells in the PRL-treated HFs compared to 59% proliferating cells in the control group per hair bulb. The number of apoptotic cells in the PRL group was significantly higher than in the control group (Figure 3a).
Figure 3.
a: Total mean number of Ki-67+ and TUNEL+ cells after 8 days in culture. PRL-treated HFs show a down-regulation of proliferating cells and an increase of apoptotic cells. *P < 0.05, **P < 0.01. b: Outer root sheath of a control HF. c: Outer root sheath of a HF of the PRL-treated group. d: Hair bulb vehicle control. e: Hair bulb PRL-treated group. Ki-67/TUNEL double staining of anagen VI follicles after 8 days in culture. Red fluorescence, Ki-67+ cells; green fluorescence, TUNEL+ cells; blue fluorescence, DAPI counterstaining.
The PRL Gene Is Transcribed in Human Skin and Hair Follicles
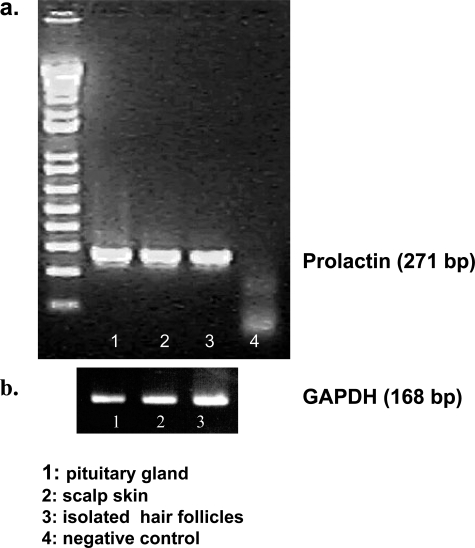
To investigate whether the PRL-like immunoreactivity in human HFs corresponds to actual PRL gene transcription in human skin, we checked for the presence of PRL mRNA by RT-PCR. Human PRL transcripts were indeed found both in human scalp skin and in microdissected isolated human HFs in the expected size (271 bp), as well as in the pituitary gland (positive controls) (Figure 4). Our two primers are located in different exons, and this 271-bp PCR fragment cannot be amplified from contaminating genomic DNA because genomic DNA contains a long intron between the two primers, this 271-bp PCR fragment can only be amplified from the cDNA, not from the genomic DNA).
Figure 4.
RT-PCR of PRL (a) and GAPDH (b). Total RNA was extracted from a pituitary gland, 30 freshly isolated human HFs, and full-thickness scalp skin. Lane 1, specific PCR signal using pituitary gland cDNA; lane 2, specific PCR signal with scalp skin cDNA; lane 3, isolated human HFs; lane 4, negative control.
Discussion
Here, we provide the first evidence that human scalp HFs not only express functional PRL-R but also serve as an important extrapituitary site of PRL expression on the gene and protein level (Figure 1, A–D, and Figure 4). Given that human skin has been calculated to display ∼5 million HFs,49 this calls attention to a very substantial, newly identified source of potential PRL synthesis in humans. This deserves further scrutiny and characterization, eg, in healthy versus inflamed human skin, and definition of the quantity of HF-derived PRL that is actually secreted systemically and thus exerts genuine endocrine, rather than autocrine or paracrine activities.
Although our finding of intracutaneous transcription of the PRL gene in human skin in situ is well in line with the previous finding of PRL transcription in murine skin in vivo22,23 and in human cultured dermal fibroblasts, keratinocytes, and sweat glands in vitro,36,39 it conflicts with the report of Slominski and colleagues40 who could not detect PRL mRNA in human skin by RT-PCR. In our experiments, we detected PRL transcripts of the expected length both in human full-thickness skin and in isolated human HFs, using pituitary gland as positive control, and confirmed our data by sequencing the PRL RT-PCR product. The negative PRL expression data of Slominski and colleagues40 may be related to the fact that these investigators studied sun-exposed truncal skin (containing primarily vellus HFs, approximately half of which are in the telogen stage of the hair cycle), whereas we analyzed scalp skin, which is unusually rich in very large terminal HFs, 80 to 90% of which are in anagen VI HF.49 In addition, we used different primer sequences and PCR conditions than these investigators, who may well have identified an alternatively spliced PRL mRNA variant that could not be detected because of the exonal location of their primers. The sense primer used by Slominski and colleagues40 was located in exon 3, and the anti-sense primer contained both the end of exon 4 sequences and the initial part of exon 5 sequences. In contrast, our sense primer is in exon 2 and anti-sense primer is in exon 4.
PRL mRNA as well as PRL and PRL-R immunoreactivity can be detected within the same epithelial human HF compartments (Figure 1, A–D, and Figure 4), and culture of microdissected, denervated, and avascular HFs in the presence of exogenous PRL exerts significant growth-modulatory effects (Figure 1, E–H, and Figure 2, a and b). This supports the hypothesis that PRL acts in an autocrine and/or paracrine manner on locally expressed high-affinity receptors and functions as a catagen-promoting signal in human HFs just as it does in mouse HFs.22,23 The strictly epithelial immunoreactivity pattern of PRL and PRL-R identified here for human scalp HFs corresponds well to the one previously described in mice.22,23 However, in ovine HFs, PRL-R expression has also been detected in the dermal papilla.7,37,50,51 Thus, expression of PRL-R seems to be differentially regulated in seasonally dependent HFs (ovine) compared to seasonally independent HFs (mouse, human).
Steroid hormones stimulate cognate receptors in the HF epithelium and mesenchyme and change the secretion of potent hair growth modulators such as TGF-β,46,52 which then act back on the epithelium. In contrast, the polypeptide hormone PRL seems capable of signaling more directly within the HF epithelium as an autocrine and/or paracrine promoter of apoptosis-driven HF regression. However, our currently available data do not allow us to exclude that the observed HF effects of PRL were mediated at least in part also indirectly. This could happen via the recognized effects of PRL on peripheral androgen27 and estrogen metabolism,53,54 and/or via induction of changes in the intrafollicular expression of PRL-sensitive growth factors, cytokines, and enzymes with recognized hair growth-modulatory functions,21 such as TGF-β1,55 vascular endothelial growth factor,2 IGF-2,56 interferon-γ,57 and ornithine decarboxylase.58
Treatment of isolated human HFs in culture with PRL results in apoptosis-driven HF regression (catagen), decreased proliferation, and increased apoptosis of follicular keratinocytes (Figure 3). These data correspond well to the rapid, premature induction of apoptosis-driven catagen development in murine anagen skin organ culture22 and to the reported catagen induction by PRL in sheep in vivo.59 However, PRL has also been shown to exert anti-apoptotic functions, eg, in cultured human breast cancer cell lines in vitro,60 to act as a larval growth hormone and to be required for limb regeneration in amphibians.61 Therefore, the anti-proliferative and proapoptotic properties of PRL in HF epithelium may not extend to all epithelial-mesenchymal interaction systems and may be developmentally controlled.
Although it remains to be clarified how PRL exerts its activities on human HFs, we show that PRL is a potent catagen-promoter of human HFs in vitro, with efficacy comparable to that of TGF-β2,62 yet is lower than that of interferon-γ.63 We also show that the catagen-promoting activity of PRL is independent of the hypothalamus-pituitary-adrenal axis and systemic hormone levels. It applies to HFs of a mammalian species with mosaic and seasonally independent HF cycling (=human scalp HF).49,64 PRL has long been recognized to play a role in hair growth control in seasonally dependent coat changes, because both rising and falling daily plasma PRL levels can induce moulting.13,19,59 The current human data fit well with the previous reports that PRL induces premature catagen in the, also seasonally independent, murine hair cycle22 and that murine PRL-R-null mutants show longer and coarser hair as well as hair cycle perturbations.23 The present data, therefore, underscore the importance of PRL as a hair growth modulator for both seasonally dependent and independent HF cycling across different mammalian species.
PRL has also been implicated in the pathogenesis of androgenetic alopecia25 by modulation of androgens, and hyperprolactemia is associated with an androgenetic alopecia-type hair loss pattern, along with hirsutism (in females).25,26 Usually, occipital scalp HFs are insensitive to hormones such as androgens. In our experiments we used mostly occipital scalp HFs and additionally frontal HFs. It is therefore particularly interesting that PRL was able to induce catagen in these hormone-insensitive HFs. It is important to mention that PRL may have distinct functions on distinct areas of scalp and body HFs and that this will be an interesting issue to investigate in the future. Recently, it has been shown that neuroendocrine factors mediate stress-induced acne. HFs and the sebaceous glands express functional receptors for stress-related hormones, which are able to modulate androgen metabolism in the sebaceous gland. These up-regulated androgens in the sebaceous gland could also be involved in stress-induced hair loss. Therefore, it will be interesting to investigate whether PRL is able to modulate androgen receptor expression and/or androgen metabolism in the human pilosebaceous unit.65,66
In summary, our study shows that human anagen scalp HFs are very sensitive for inhibitory PRL-R-mediated signals. This is clinically relevant, because it provides a reasonable mechanism to explain the, as yet ill-understood, telogen effluvium associated with hyperprolactinemia.25 It also points to novel therapeutic strategies for the management of stress-related and hormonal hair loss in men and women,60 eg, by use of recently developed PRL-R antagonists.67–70
Acknowledgments
We thank Gundula Pilnitz-Stolze and Silvia Wegerich for their excellent technical support and Bernhard Gerstmayer for his support and helpful discussion of the results.
Footnotes
Address reprint requests to Kerstin Foitzik, M.D., Dept. of Dermatology, University Hospital Hamburg-Eppendorf, University of Hamburg, Martinistr.52, D-20246 Hamburg, Germany. E-mail: kfoitzik@yahoo.com.
Supported in part by grants from the German Research Foundation (Pa 345/11-2) and the Federal Ministry of Education and Research (to R.P.).
References
- Freeman M, Kanycisca B, Lerant A, Nagy G. Prolactin: structure, function and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Goldhar AS, Vonderhaar BK, Trott JF, Hovey RC. Prolactin-induced expression of vascular endothelial growth factor via Egr-1. Mol Cell Endocrinol. 2005;232:9–19. doi: 10.1016/j.mce.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Gootwine E. Placental hormones and fetal-placental development. Anim Reprod Sci. 2004;82–83:551–566. doi: 10.1016/j.anireprosci.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Soares MJ. The prolactin and growth hormone families: pregnancy-specific hormones/cytokines at the maternal-fetal interface. Reprod Biol Endocrinol. 2004;2:51. doi: 10.1186/1477-7827-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Philadelphia: Saunders; Williams Textbook of Endocrinology. (ed 10.) 2003 [Google Scholar]
- Goffin V, Binart N, Touraine P, Kelly PA. Prolactin: the new biology of an old hormone. Annu Rev Physiol. 2002;64:47–67. doi: 10.1146/annurev.physiol.64.081501.131049. [DOI] [PubMed] [Google Scholar]
- Nixon AJ, Ford CA, Wildermoth JE, Craven AJ, Pearson AJ. Regulation of prolactin receptor expression in ovine skin in relation to circulating prolactin and wool follicle growth status. J Endocrinol. 2002;172:605–614. doi: 10.1677/joe.0.1720605. [DOI] [PubMed] [Google Scholar]
- Felig P, Frohmann L. Columbus: McGraw-Hill; Endocrinology and Metabolism. (ed 4) 2001 [Google Scholar]
- Malley BWO. London: Academic Press; 1998 [Google Scholar]
- Paus R. Does prolactin play a role in skin biology and pathology? Med Hypoth. 1991;36:33–42. doi: 10.1016/0306-9877(91)90161-q. [DOI] [PubMed] [Google Scholar]
- Hadley MC. Englewood Cliffs: Prentice Hall; Endocrinology. (ed 2) 1988 [Google Scholar]
- Slominski A, Wortsman J. Neuroendocrinology of the skin. Endocr Rev. 2000;5:457–487. doi: 10.1210/edrv.21.5.0410. [DOI] [PubMed] [Google Scholar]
- Martinet L, Allain D, Weiner C. Role of prolactin in the photoperiodic control of moulting in the mink (Mustela vison). J Endocrinol. 1984;103:9–15. doi: 10.1677/joe.0.1030009. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Goldman BD. Hormonal regulation of the annual pelage colour cycle in the Djungarian hamster, Phodopus sungorus. II. Role of prolactin. J Exp Zool. 1984;203:97–103. doi: 10.1002/jez.1402300113. [DOI] [PubMed] [Google Scholar]
- Rougeot J, Allain D, Martinet L. Photoperiodic and hormonal control of seasonal coat changes in mammals with special reference to sheep and mink. Acta Zool Fennica. 1984;171:13–18. [Google Scholar]
- Ibraheem M, Galbraith H, Scaife J, Ewen S. Growth of secondary hair follicles of the cashmere goat in vitro and their response to prolactin and melatonin. J Anat. 1994;185:135–142. [PMC free article] [PubMed] [Google Scholar]
- Alonso LC, Rosenfield RL. Molecular genetic and endocrine mechanisms of hair growth. Horm Res. 2003;60:1–13. doi: 10.1159/000070821. [DOI] [PubMed] [Google Scholar]
- Puchala R, Pierzynowski SG, Wuliji T, Goetsch AL, Soto-Navarro SA, Sahlu T. Effects of prolactin administered to a perfused area of the skin of Angora goats. J Anim Sci. 2003;81:279–284. doi: 10.2527/2003.811279x. [DOI] [PubMed] [Google Scholar]
- Pearson AJ, Parry AL, Ashby MG, Choy VJ, Wildermoth JE, Craven AJ. Inhibitory effect of increased photoperiod on wool follicle growth. J Endocrinol. 1996;148:157–166. doi: 10.1677/joe.0.1480157. [DOI] [PubMed] [Google Scholar]
- Dicks P. The role of prolactin and melatonin in regulating the timing of the spring moult in the cashmere goat. Eur Fine Fibre Netw Occasional Publ. 1994;2:109–125. [Google Scholar]
- Stenn K, Paus R. Controls of hair follicle cycling. Physiol Rev. 2001;81:449–494. doi: 10.1152/physrev.2001.81.1.449. [DOI] [PubMed] [Google Scholar]
- Foitzik K, Krause K, Nixon AJ, Ford CA, Ohnemus U, Pearson AJ, Paus R. Prolactin and its receptor are expressed in murine hair follicle epithelium, show hair cycle-dependent expression, and induce catagen. Am J Pathol. 2003;162:1611–1621. doi: 10.1016/S0002-9440(10)64295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven A, Ormandy C, Robertson F, Kelly P, Nixon A, Pearson A. Prolactin signalling influences the timing mechanism of the hair follicle: analysis of hair growth cycles in prolactin receptor knockout mice. Endocrinology. 2001;142:2533–2539. doi: 10.1210/endo.142.6.8179. [DOI] [PubMed] [Google Scholar]
- Moltz L. Hormonal diagnosis in so-called androgenetic alopecia in the female. Geburtshilfe Frauenheilkd. 1988;48:203–214. doi: 10.1055/s-2008-1026489. [DOI] [PubMed] [Google Scholar]
- Schmidt JB. Hormonal basis of male and female androgenic alopecia: clinical relevance. Skin Pharmacol. 1994;7:61–66. doi: 10.1159/000211275. [DOI] [PubMed] [Google Scholar]
- Schmidt JB, Lindmaier A, Trenz A, Schurz B, Spona J. Hormone studies in females with androgenic hair loss. Gynecol Obstet Invest. 1991;31:235–239. doi: 10.1159/000293166. [DOI] [PubMed] [Google Scholar]
- Serafini P, Lobo RA. Prolactin modulates peripheral androgen metabolism. Fertil Steril. 1986;45:41–46. [PubMed] [Google Scholar]
- Starka L, Cermakova I, Duskova M, Hill M, Dolezal M, Polacek V. Hormonal profile of men with premature balding. Exp Clin Endocrinol Diabetes. 2004;112:24–28. doi: 10.1055/s-2004-815723. [DOI] [PubMed] [Google Scholar]
- Fabre N, Montastruc JL, Rascol O. Alopecia: an adverse effect of bromocriptine. Clin Neuropharmacol. 1993;16:266–268. [PubMed] [Google Scholar]
- Blum I, Leiba S. Increased hair loss as the side-effect of bromocriptine treatment. N Engl J Med. 1980;303:1418. doi: 10.1056/nejm198012113032416. [DOI] [PubMed] [Google Scholar]
- Sinclair RD, Banfield CC, Dawber RPR. Oxford: Blackwell Science; Handbook of Diseases of the Hair and Scalp. 1999 [Google Scholar]
- Harris J, Stanford PM, Oakes SR, Ormandy CJ. Prolactin and the prolactin receptor: new targets of an old hormone. Ann Med. 2004;36:414–425. doi: 10.1080/07853890410033892. [DOI] [PubMed] [Google Scholar]
- Kelly PA, Binart N, Freemark M, Lucas B, Goffin V, Bouchard B. Prolactin receptor signal transduction pathways and actions determined in prolactin receptor knock-out mice. Biochem Soc Trans. 2001;29:48–52. doi: 10.1042/0300-5127:0290048. [DOI] [PubMed] [Google Scholar]
- Ormandy CJ, Binart N, Helloco C, Kelly PA. Mouse prolactin receptor gene: genomic organization reveals alternative promoter usage and generation of isoforms via alternative 3′-exon splicing. DNA Cell Biol. 1998;17:761–770. doi: 10.1089/dna.1998.17.761. [DOI] [PubMed] [Google Scholar]
- Kossiakoff AA. The structural basis for biological signaling, regulation, and specificity in the growth hormone-prolactin system of hormones and receptors. Adv Protein Chem. 2004;68:147–169. doi: 10.1016/S0065-3233(04)68005-3. [DOI] [PubMed] [Google Scholar]
- Poumay Y, Jolivet G, Pittelkow MR, Herphelin F, De Potter IY, Mitev V, Houdebine LM. Human epidermal keratinocytes upregulate expression of the prolactin receptor after the onset of terminal differentiation, but do not respond to prolactin. Arch Biochem Biophys. 1999;364:247–253. doi: 10.1006/abbi.1999.1132. [DOI] [PubMed] [Google Scholar]
- Choy VJ, Nixon J, Pearson AJ. Distribution of PRL receptor immunoreactivity in ovine skin and changes during the wool follicle growth cycle. J Endocrinol. 1997;155:265–275. doi: 10.1677/joe.0.1550265. [DOI] [PubMed] [Google Scholar]
- Choy VJ, Wildermoth JE, Nixon AJ, Pearson AJ: Localisation of target tissues in ovine skin. Proc Endocr Soc Aust 1994, pp NZ12 [Google Scholar]
- Richards RG, Hartman SM. Human dermal fibroblast cells express prolactin. J Invest Dermatol. 1996;106:1250–1255. doi: 10.1111/1523-1747.ep12348944. [DOI] [PubMed] [Google Scholar]
- Slominski A, Malarkey WB, Wortsman J, Asa SL, Carlson A. Human skin expresses growth hormone but not the prolactin gene. J Lab Clin Med. 2000;6:476–481. doi: 10.1067/mlc.2000.110605. [DOI] [PubMed] [Google Scholar]
- Philpott MP, Sanders D, Westgate GE, Kealey T. Human hair growth in vitro: a model for the study of hair follicle biology. J Dermatol Sci. 1994;7:S55–S72. doi: 10.1016/0923-1811(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Hadley ME. Upper Saddle River: Simon and Schuster; Endocrinology. (ed 4) 1996 [Google Scholar]
- Muller-Rover S, Handjiski B, van-der-Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Kligman AM. The human hair cycle. J Invest Dermatol. 1959;5:307–316. doi: 10.1038/jid.1959.156. [DOI] [PubMed] [Google Scholar]
- Maurer M, Handjiski B, Paus R. Hair growth modulation by topical immunophilin ligands: induction of anagen, inhibition of massive catagen development, and relative protection from chemotherapy-induced alopecia. Am J Pathol. 1997;150:1433–1441. [PMC free article] [PubMed] [Google Scholar]
- Foitzik K, Spexard T, Nakamura M, Halsner U, Paus R. Towards dissecting the pathogenesis of retinoid-induced hair loss: all-trans retinoic acid induces premature hair follicle regression (catagen) by upregulation of TGF-β2 in the dermal papilla. J Invest Dermatol. 2005;124:1119–1126. doi: 10.1111/j.0022-202X.2005.23686.x. [DOI] [PubMed] [Google Scholar]
- Lindner G, Botchkarev VA, Botchkareva NV, Ling G, van der Veen C, Paus R. Analysis of apoptosis during hair follicle regression. Am J Pathol. 1997;1:1601–1617. [PMC free article] [PubMed] [Google Scholar]
- Foitzik K, Lindner G, Müller-Röver S, Maurer M, Botchkareva N, Botchkarev V, Handjiski B, Metz M, Hibino T, Soma T, Dotto G, Paus R. Control of murine hair follicle regression (catagen) by TGF-beta1 in vivo. FASEB J. 2000;14:752–760. doi: 10.1096/fasebj.14.5.752. [DOI] [PubMed] [Google Scholar]
- Paus R, Peker S. Biology of hair and nail. Bologna JL, Jorizzo JL, Rapini RP, editors. London: Mosby; Dermatology. 2003:pp 1007–1032. [Google Scholar]
- Ouhtit A, Morel G, Kelly P. Visualization of gene expression of short and long forms of prolactin receptor in the rat. Endocrinology. 1993;133:135–144. doi: 10.1210/endo.133.1.8319561. [DOI] [PubMed] [Google Scholar]
- Rose J, Oldfield J, Stormshak F. Apparent role of melatonin and prolactin in initiating winter fur growth in mink. Gen Comp Endocrinol. 1987;65:212–215. doi: 10.1016/0016-6480(87)90168-7. [DOI] [PubMed] [Google Scholar]
- Inui S, Fukuzato Y, Nakajima T, Yoshikawa K, Itami S. Androgen-inducible TGF-beta1 from balding dermal papilla cells inhibits epithelial cell growth: a clue to understand paradoxical effects of androgen on human hair growth. FASEB J. 2002;16:1967–1969. doi: 10.1096/fj.02-0043fje. [DOI] [PubMed] [Google Scholar]
- Gutzman JH, Miller KK, Schuler LA. Endogenous human prolactin and not exogenous human prolactin induces estrogen receptor alpha and prolactin receptor expression and increases estrogen responsiveness in breast cancer cells. J Steroid Biochem Mol Biol. 2004;88:69–77. doi: 10.1016/j.jsbmb.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Frasor J, Gibori G. Prolactin regulation of estrogen receptor expression. Trends Endocrinol Metab. 2003;14:118–123. doi: 10.1016/s1043-2760(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Philips N, McFadden K. Inhibition of transforming growth factor-beta and matrix metalloproteinases by estrogen and prolactin in breast cancer cells. Cancer Lett. 2004;206:63–68. doi: 10.1016/j.canlet.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Brisken C, Ayyannan A, Nguyen C, Heineman A, Reinhardt F, Tan J, Dey SK, Dotto GP, Weinberg RA. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev Cell. 2002;3:877–887. doi: 10.1016/s1534-5807(02)00365-9. [DOI] [PubMed] [Google Scholar]
- Breidthardt T, Frohn C, Luhm J, Kirchner H, Brand JM. Prolactin induces enhanced interferon gamma release in peripheral whole blood after stimulation with either PHA or LPS. Immunobiology. 2002;206:424–431. doi: 10.1078/0171-2985-00191. [DOI] [PubMed] [Google Scholar]
- Gonzalez SI, Suescun MO, Rulli SB, Estivariz F, Calandra RS. Modulation of ornithine decarboxylase activity by prolactin in seminal vesicles of the rat. Int J Androl. 1994;17:143–148. doi: 10.1111/j.1365-2605.1994.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Pearson AJ, Ashby MG, Wildermoth JE, Craven AJ, Nixon AJ. Effect of exogenous prolactin on the hair growth cycle. Exp Dermatol. 1999;8:358–360. [PubMed] [Google Scholar]
- Perks CM, Keith AJ, Goodhew KL, Savage PB, Winters ZE, Holly JM. Prolactin acts as a potent survival factor for human breast cancer cell lines. Br J Cancer. 2004;91:305–311. doi: 10.1038/sj.bjc.6601947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert SF. Sunderland: Sinauer; Developmental Biology. (ed 7) 2003 [Google Scholar]
- Soma T, Tsuji Y, Hibino T. Involvement of transforming growth factor-beta2 in catagen induction during the human hair cycle. J Invest Dermatol. 2002;118:993–997. doi: 10.1046/j.1523-1747.2002.01746.x. [DOI] [PubMed] [Google Scholar]
- Ito T, Ito N, Saathoff M, Bettermann A, Takigawa M, Paus R. Interferon-gamma is a potent inducer of catagen-like changes in cultured human anagen hair follicles. Br J Dermatol. 2005;152:623–631. doi: 10.1111/j.1365-2133.2005.06453.x. [DOI] [PubMed] [Google Scholar]
- Paus R, Foitzik K. In search of the hair cycle clock: a guided tour. Differentiation. 2004;72:489–511. doi: 10.1111/j.1432-0436.2004.07209004.x. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol. 2004;22:360–366. doi: 10.1016/j.clindermatol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Bohm M. Neuroendocrine regulation of sebocytes—a pathogenetic link between stress and acne. Exp Dermatol. 2004;13(Suppl 4):S31–S35. doi: 10.1111/j.1600-0625.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- Bernichtein S, Kayser C, Dillner K, Moulin S, Kopchick JJ, Martial JA, Norstedt G, Isaksson O, Kelly PA, Goffin V. Development of pure prolactin receptor antagonists. J Biol Chem. 2003;208:11–21. doi: 10.1074/jbc.M305687200. [DOI] [PubMed] [Google Scholar]
- Kuo CB, Coss D, Walker AM. Prolactin receptor antagonists. Endocrine. 1998;9:121–131. doi: 10.1385/endo:9:2:121. [DOI] [PubMed] [Google Scholar]
- Peirce SK, Chen WY. Human prolactin and its antagonist, hPRL-G129R, regulate bax and bcl-2 gene expression in human breast cancer cells and transgenic mice. Oncogene. 2004;2312:1248–1255. doi: 10.1038/sj.onc.1207245. [DOI] [PubMed] [Google Scholar]
- Cataldo L, Chen NY, Yuan Q, Li W, Ramamoorthy P, Wagner TE, Sticca RP, Chen WY. Inhibition of oncogene STAT3 phosphorylation by a prolactin antagonist, hPRL-G129R, in T-47D human breast cancer cells. Int J Oncol. 2000;17:1179–1185. doi: 10.3892/ijo.17.6.1179. [DOI] [PubMed] [Google Scholar]