Abstract
The aquaporins represent a family of transmembrane water channel proteins that play a major role in trans-cellular and transepithelial water movement. Most tumors have been shown to exhibit high vascular permeability and interstitial fluid pressure, but the transport pathways for water within tumors remain unknown. Here, we tested 10 non-small cell lung cancer cell lines of various origins by reverse transcriptase-polymerase chain reaction and Western blot analysis and identified clear expression of aquaporin 1 (AQP1) in seven cell lines. We next examined the distribution of the AQP1 protein in several types of primary lung tumors (16 squamous cell carcinomas, 21 adenocarcinomas, and 7 bronchoalveolar carcinomas) by immunohistochemical staining. AQP1 was overexpressed in 62% (13 of 21) and 75% (6 of 8) of adenocarcinoma and bronchoalveolar carcinoma, respectively, whereas all cases of squamous cell carcinoma and normal lung tissue were negative. Forced expression of full-length AQP1 cDNA in NIH-3T3 cells induced many phenotypic changes characteristic of transformation, including cell proliferation-enhancing activity by the MTT assay and anchorage-independent growth in soft agar. Although further details on the molecular function of AQP1 related to tumorigenesis remain to be elucidated, our results suggest a potential role of AQP1 as a novel therapeutic target for the management of lung cancer.
Until recently, water transport across cell membranes was thought to occur by simple diffusion. However, the rate of water transport across erythrocyte and kidney tubule cell membranes is higher than would be expected by simple diffusion alone.1 The reason for this discrepancy was disclosed by the discovery of a family of water channel proteins, the aquaporins (AQPs) that facilitate the passage of water across cell membranes.1,2 To date, 10 distinct AQPs have been characterized in human, and more than 30 other family members have been described in amphibians, insects, plants, and bacteria (PubMed database, http://www.pubmed.com).
Human AQP1 (hAQP1) belongs to the major intrinsic protein family and consists of a functional monomer with six transmembrane helices.3–5 hAQP1 is naturally expressed in erythrocytes and in many epithelial and endothelial tissues including kidney, choroids plexus, bile duct, gall bladder, eye lens, brain, and placenta.3–5 Evidence of human diseases resulting from alterations in AQP gene expression or regulation is limited. AQP1 expression is preferentially associated with microvessels of multiple myeloma, and its overexpression parallels angiogenesis in multiple myeloma progression.6 Overexpression of AQP1 has been identified in brain tumors.7 In addition, reports have shown that AQP1 is involved in cell-cycle control,8,9 suggesting that the AQP1 gene could play a part in uncontrolled cell replication.
Most recently, we reported that the expression of AQP1 and AQP5 is induced in the early stages of colorectal carcinogenesis.10 Other reports have alluded to the role of other AQPs in the development of human cancer. For example, expression of AQP3 was increased in renal cell cancer, as was AQP5 in pancreatic cancer.11,12 As a first step in examining the role of AQPs in human lung carcinogenesis, we studied AQP1 expression during the development of non-small cell lung cancer (NSCLC). We initially used reverse transcriptase-polymerase chain reaction (RT-PCR) to screen the expression profiles of human AQP1 in 10 NSCLC cell lines, and confirmed our results by Western blot analysis. Based on this information, we further studied the expression of AQP1 in primary tumor by performing immunohistochemical staining on multiple tissue sections from 44 resected tumor samples and 8 normal lung tissues. Forced expression of hAQP1 in NIH-3T3 cell lines produced a neoplastic phenotype characterized by anchorage-independent growth and increased cell proliferation, which is partially because of their resistance to apoptosis. Our results present a clear and novel example of AQP1 expression in NSCLC and provide functional evidence demonstrating novel oncogenic properties of AQP1.
Materials and Methods
Cell Lines
Ten NSCLC cell lines (H1299, H1437, H1563, H1650, H1703, H1944, H1975, H2030, H23, and H838) were maintained in RPMI 1640 medium with 10% fetal bovine serum.
RT-PCR Analysis of hAQPs Expression in Human Tumor Cell Lines
Total RNA extracted using an RNeasy total RNA kit (Qiagen, Valencia, CA) from cell lines was reverse-transcribed by random primer and Superscript II reverse transcriptase (Life Technologies, Inc., Grand Island, NY). The resulting cDNA was subjected to PCR with the following primer sequences AQP1 forward, 5′-CGCAGAGTGTGGGCCACATCA-3′ and AQP1 reverse, 5′-CCCGAGTTCACACCATCAGCC-3′, amplifying a 220-bp product. The PCR reaction was performed for 40 cycles using the following conditions: 10 seconds at 94°C, 50 seconds at 63°C, and 50 seconds at 72°C. GAPDH mRNA amplification was performed on the cDNA as a positive control for reaction efficiency and was done for 25 cycles. The PCR product was introduced into the pCRII-TOPO plasmid vector (Invitrogen, Carlsbad, CA) using the TOPO TA cloning system, and the AQP1-specific sequence was confirmed.
Western Blot Analysis
Cells were lysed with ice-cold RIPA buffer (50 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate) containing 1 mmol/L phenylmethyl sulfonyl fluoride. The lysate was incubated on ice for 20 minutes, followed by sonication for 5 seconds, and further incubated for another 20 minutes on ice. The lysate was then centrifuged at 10,000 rpm in a microcentrifuge tube for 10 minutes and the supernatant was saved at −80°C. Protein concentration was determined by the Bio-Rad DC protein assay kit (Bio-Rad, Hercules, CA). Equal amounts of protein (50 μg) were separated on 14% sodium dodecyl sulfate-polyacrylamide gels. The protein was transferred onto nitrocellulose membrane and incubated in blocking solution [5% nonfat dry milk in 1× phosphate-buffered saline (PBS)] for 1 hour at room temperature. Then, the filters were incubated for 1 hour at room temperature in 1× PBS containing the primary antibody. After being washed twice in 5-minute intervals in 1× PBS, the filters were incubated for 1 hour at room temperature in secondary antibody. After again washing twice at 5-minute intervals with 1× PBS, immunoreactivity was visualized using the ECL system (Amersham, Arlington Heights, IL). The primary antibody was rabbit anti-AQP1 polyclonal antibody (Chemicon International, Temecula, CA) at a 1:100 dilution. The secondary antibody was horseradish peroxidase-conjugated donkey anti-rabbit antibody (Amersham) at a 1:3000 dilution. The membranes were then washed to remove attached antibody and reacted with antibody against β-actin (1:5000) as an internal control.
Tissues
A total of 44 archival paraffin-embedded specimens from lung cancer patients (15 squamous cell carcinoma, 21 adenocarcinoma, and 8 bronchoalveolar carcinoma) and 8 nonneoplastic lung tissue samples from the different patients were obtained from the Department of Pathology, Asan Medical Center, University of Ulsan, College of Medicine, Seoul, South Korea. This study was granted an exemption from the Asan Medical Center institutional review board because samples were evaluated without any identifiers. The tumors were staged according to the tumor-node-metastasis classification, and histologically classified according to the World Health Organization guidelines.
Tissue Microarray Construction, AQP1 Immunohistochemistry, and Fluorescence in Situ Hybridization (FISH)
Formalin-fixed, paraffin-embedded tumor samples of representative tumor regions were used for preparation of tissue microarray blocks. Briefly, blocks were selected after the sections were stained with hematoxylin and eosin and were evaluated for tumor viability. The tissue microarrays were assembled using Beecher tissue-arraying device. Two parallel tissue microarray blocks were made by using a 2.0-mm-diameter core biopsy needle. Eight other control specimens were included in each of the tissue array blocks. The histological diagnoses were reviewed by a professional pathologist (S.J.J.). Both tissue microarray blocks were screened for AQP1 expression. Subsequently, 5-μm sections were cut from the array blocks, transferred to adhesive-coated slides using the adhesive-coated tape sectioning system, and exposed to ultraviolet light for 60 seconds to seal the sections to the slides.
Microarrayed sections were deparaffinized. Antigen retrieval was performed using heat-induced epitope retrieval with 10 mmol/L citrate buffer. Tissue sections were incubated with a previously characterized affinity-purified rabbit antibody raised against the 19-amino acid sequence (amino acids 251 to 269) of the COOH-terminus of human AQP1 (Alpha Diagnostic, San Antonio, TX) at a 1:50 dilution. The AQP1 antibody was visualized using the avidin-biotin-peroxidase technique (DAKO LSAB kit; DAKO Cytomation, Carpinteria, CA). The samples were graded either as positive (in which 100% of tumor cell expressed AQP1), focal positive (in which 5 to 30% expressed AQP1), or negative (<5% which was confined only in vascular endothelium or no staining). Omitting the primary or secondary antibody abolished staining.
An additional 60 NSCLCs were reassembled in two tissue microarrays for FISH analysis. Sections (5 μm thick) of the tissue microarray slides were deparaffinized and pretreated for FISH. The probe for AQP1 detection was derived from Homo sapiens PAC clone RP5–877J2 from 7p14-p15 containing the whole AQP1 gene (GenBank accession no. AC005155), and labeled with rhodamine (Macrogen, Seoul, Korea). α-satellite DNA probe for human chromosome 7 was labeled with FITC was used as control probe (Macrogen). FISH was performed using Vysis reagents according to the manufacturer’s protocols (Vysis, Downers Grove, IL). Slides were counterstained with 4,6-diamidino 2-phenylindole (DAPI) for microscopy. The signal enumeration was performed under ×1000 magnification.
Vector Construction, Transfection, and Isolation of AQP1-Expressing Clones
For the transfection of NIH-3T3 cells, we used the pcDNA3 mammalian expression vector (Invitrogen). This vector contains a cytomegalovirus promoter allowing efficient transcription of the recombinant AQP1-cDNA, and the neomycin resistance gene for easy selection of the transfected cells. The full-length sequence of hAQP1 cDNA was isolated from the recombinant TA plasmid using the following primers: 5′-CAAGGTACCCGCCACCATGGCCAGCGAGTT-3′ and 5′-GTGTTCTAGAGGCTGGTTTAGTGGTAACCAGAGGG-3′ (including KpnI and XbaI site). The amplified hAQP1 full-length cDNA was subsequently subcloned into a eukaryotic expression vector, pcDNA3 (Invitrogen). Transfection of hAQP1-cDNA and empty vector was performed by Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Selection was performed via the addition of 1000 μg/ml G418 (Life Technologies, Inc.) at final concentration. To detect the expression of hAQP1, stable transfectants were lysed in the sample buffer, and equal amounts of protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subjected to Western blotting using rabbit anti-AQP1 polyclonal antibody (Chemicon International) at a 1:100 dilution.10 Five positive clones and three control clones were picked after 17 days selection. Among these, three positive clones and one control clone were selected for further functional experiments.
NIH-3T3 Transformation Assays
Anchorage-Independent Proliferation
To determine the ability of AQP1 to confer anchorage-independent growth, colony formation in soft agar was assessed as described.13 Cells (5 × 103) from each stable clone, mock clone, and parental NIH-3T3 were seeded in 1 ml of 0.3% low-melting agarose over a 0.6% agar bottom layer in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum and 1000 μg/ml G418. The medium was changed every 3 days and the clones were allowed to grow for 17 days. Each assay was performed in triplicate on two independent occasions. Colony formation (2 to 3 mm) was counted for each cell clone.
Focus Formation Assay
pcDNA3-AQP1, pcDNA3 (vector alone), or H-Ras were transfected into NIH-3T3 cells. Three hundred ng, 600 ng, and 900 ng of pcDNA3-AQP1 expression vectors and empty vector were used separately. Fifty ng of H-ras expression vector was used as a positive control. The transfected cells were plated in a 100-mm culture dish in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum. The medium was changed and the cells were ringed with medium 24 hours after transfection, and then maintained in Dulbecco’s modified Eagle’s medium plus 5% fetal bovine serum. The medium was changed every 3 days thereafter. Photographs were taken at day 21, and no foci were seen in mock-transfected NIH-3T3.
Measurement of in Vitro Growth Rate
hAQP1-cDNA and blank vectors transfectants were plated in triplicate in six-well plates (3 × 103 cells per well). Cells were counted every other day throughout a 14-day period by means of the MTT assay. The MTT assay is an index of cell viability and cell growth, which is based on the ability of viable cells to reduce MTT from a yellow water-soluble dye to a purple-insoluble formazan product. After the appropriate time of incubation, culture media was replaced with fresh media. One hundred μl of MTT solution was added to each well and incubated at 37°C and 5% CO2. Four hours after incubation, 1 ml of solubilization buffer was added and the mixture was incubated for 3 hours at 37°C to allow complete solubilization. Two hundred μl of the mixture was aliquoted onto a 96-well plate. Spectrophotometric readings (A550 nm to A650 nm) were obtained on a Spectra Max 250 96-well plate reader (Molecular Devices, Sunnyvale, CA). The ATCC-MTT cell proliferation assay kit was used for all experiments.
Terminal dUTP Nick-End Labeling (TUNEL) Assay
For each stable cell line, 2 × 103 cells per well were plated on Lab-Tek II chamber slide (Nunc, Naperville, IL). The medium was removed and replaced with fresh culture medium supplemented with 10% fetal bovine serum every 3 days for 15 days. Apoptosis was analyzed at the same time following the manufacturer’s instruction (The DeadEnd fluorometric TUNEL system; Promega, Madison, WI). For the starvation experiment, cells were plated and incubated overnight. The medium then was removed and replaced with fresh culture medium without serum and apoptosis was analyzed at 24 hours and 48 hours after serum withdrawal. Total cell numbers were counted with 4,6-diamidino-2-phenylindole (DAPI)-stained cells and the fluorescein-12-dUTP-labeled DNA indicating apoptotic cells were visualized directly by fluorescence microscopy. The entire area of each well was observed for DAPI and apoptotic cells in low-power field.
Measurement of ERK Activity
hAQP1-cDNA and mock transfectants were plated on six-well culture plates (1 × 105 cells per well) and incubated overnight. The medium was replaced with fresh culture medium without serum and incubated for 24 hours. Starved cells were stimulated with serum and lysed in Nonidet P-40 lysis buffer (10 mmol/L Tris-Cl, pH 7.4, 137 mmol/L NaCl, 10% glycerol, and 1% Nonidet P-40) in an inhibitor cocktail containing 10 mmol/L β-glycerol phosphate, 1 mmol/L phenylmethyl sulfonyl fluoride, 10 mmol/L NaF, 10 mmol/L Na orthovanadate, 4.5 U/ml aprotinin (Sigma, St. Louis, MO), and 1 μg/ml leupeptin (Sigma) at the indicated time point. Equal amounts of proteins were visualized by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with anti-phospho-ERK (Santa Cruz Biotechnologies, Santa Cruz, CA). After stripping the blot, the same membrane was blotted with anti-ERK antibody (Santa Cruz).
Results
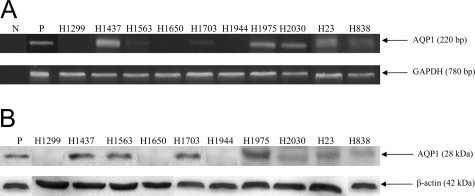
Ten NSCLC cell lines of various origins were screened with RT-PCR. Seven of the ten cell lines displayed expression of AQP1 (Figure 1A). Western blot analysis demonstrated that AQP1 was expressed concurrently in all of the tested cancer cell lines (Figure 1B), confirming the RT-PCR results. Moreover, when the NSCLC cell line H23 was treated with siRNA designed against AQP1, preliminary data after 5 days of treatment demonstrated a statistically significant decrease in the cell proliferation rate of the treated cells (data not shown). These results led us to investigate further the role of AQP1 in NSCLC.
Figure 1.
Expression of AQP1 in NSCLC cell lines. A: RT-PCR was performed using sense and anti-sense primers for AQP1 for 10 NSCLC cancer cell lines (H1299, H1437, H1563, H1650, H1703, H1944, H1975, H2030, H23, and H838). The name of each cell line is marked on the top of each lane, and the size of PCR product is marked with an arrow. NIH-3T3 cells transfected with AQP1 expression constructs were used as positive controls (P) and PCR mixtures without templates were used as negative controls (N). GAPDH was the loading control. Each PCR product was cloned and its sequence confirmed. Seven of the ten cell lines demonstrated expression of AQP1. B: Western blot analysis was performed using antibodies for AQP1 for the same 10 NSCLC cell lines. The name of each cell line is marked on the top of each lane, and the size of the protein is marked with an arrow. NIH-3T3 cells transfected with AQP1 expression constructs were used as positive controls for Western blots (P). β-Actin was the loading control.
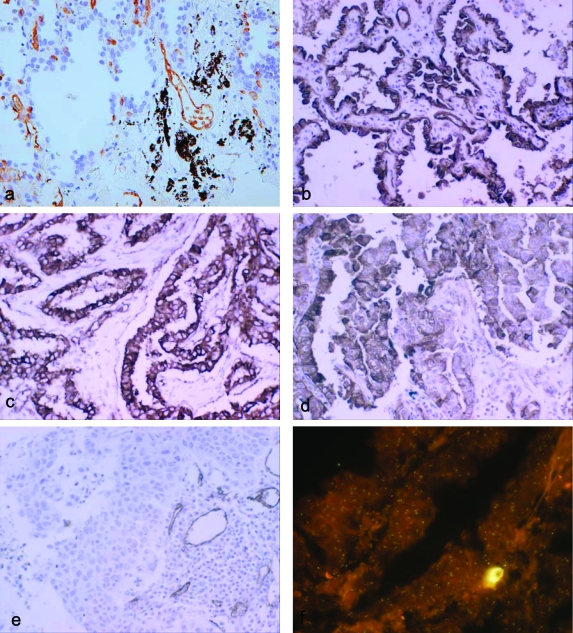
We then tested the expression of AQP1 by immunohistochemical staining on tumor tissue sections from 44 different patients (15 squamous cell carcinomas, 21 adenocarcinomas, and 8 bronchoalveolar carcinomas). Demographic and clinical information of these patients is summarized in Table 1. Eighteen of forty-four (41%) of the examined samples showed strong (100%) or focal (5 to 30%) expression of AQP1. Both membranous and cytoplasmic staining was observed in tumor cells. In most cases, the staining intensity in tumor cells is stronger than in the endothelia of tumor vasculature that consistently expresses AQP1 and was, therefore, used as an internal positive control. A summary of the immunohistochemical results is shown in Table 1. When we stratified the tissue samples by histological subtype, 13 of 21 (62%) of adenocarcinomas and 6 of 8 (75%) of bronchoalveolar carcinomas showed immunoreactivity, whereas none of squamous cell carcinomas showed any expression (Table 2). By the Fisher exact test, it was determined that the increased expression of AQP1 in adenocarcinoma and bronchoalveolar carcinoma compared to squamous cell carcinoma is statistically significant (P = 0.0003 and P = 0.0002, respectively). To evaluate the AQP1 expression in the normal lung, we examined eight age-matched nonneoplastic lung samples. Consistent with previous reports, immunoreactivity was found only in vascular endothelial cells.14,15 Examples of AQP1 immunoreactivity of each of the cancer types and normal lung tissues examined are shown in Figure 2. In normal lung tissue, alveolar lining epithelial cells are negative for AQP1 (Figure 2a) and strong immunostaining for AQP1 in bronchioloalveolar carcinoma (Figure 2b), well-differentiated adenocarcinoma (Figure 2c), and poorly differentiated adenocarcinoma (Figure 2d). No expression of AQP1 in lung squamous cell carcinoma was observed in contrast to the clear expression of AQP1 in vascular endothelium (Figure 2e). In addition to immunohistochemical analysis, we had examined the mRNA expression of AQP1 at least in some cases of NSCLC tissues as a part of our previous work.16 In both adenocarcinoma and bronchioloalveolar carcinoma (BAC), in situ hybridization clearly showed AQP1 mRNA expression consistent with immunohistochemical finding (data not shown).
Table 1.
Clinicopathological Characteristics of Cases and Controls and Summary of Immunohistochemistry
| Histology | Age (years)/sex | Stage | Lymph node status | AQP1 immunoreactivity | |
|---|---|---|---|---|---|
| 1 | Adeno | 76/M | 1B | N0 | Positive |
| 2 | Adeno | 57/M | 1B | N0 | Negative |
| 3 | Adeno | 74/M | IA | N0 | Negative |
| 4 | Adeno | 48/M | 1B | N0 | Focal positive |
| 5 | Adeno | 70/F | IA | N0 | Positive |
| 6 | Adeno | 71/F | 1B | N0 | Negative |
| 7 | Adeno | 65/M | 1B | N0 | Focal positive |
| 8 | Adeno | 65/F | IA | N0 | Positive |
| 9 | Adeno | 62/M | IA | N0 | Focal positive |
| 10 | Adeno | 54/M | lllA | N2 | Negative |
| 11 | Adeno | 70/M | llA | N1 | Positive |
| 12 | Adeno | 61/M | llB | N1 | Positive |
| 13 | Adeno | 58/M | IA | N0 | Focal positive |
| 14 | Adeno | 53/M | IA | N0 | Focal positive |
| 15 | Adeno | 45/F | llA | N1 | Negative |
| 16 | Adeno | 40/F | IA | N0 | Positive |
| 17 | Adeno | 64/F | 1B | N0 | Negative |
| 18 | Adeno | 79/M | 1B | N0 | Positive |
| 19 | Adeno | 62/M | IA | N0 | Positive |
| 20 | Adeno | 68/M | IA | N0 | Negative |
| 21 | Adeno | 67/M | 1B | N0 | Negative |
| 22 | SCC | 76/M | 1B | N0 | Negative |
| 23 | SCC | 47/M | 1B | N0 | Negative |
| 24 | SCC | 68/M | llB | N0 | Negative |
| 25 | SCC | 68/M | llB | N0 | Negative |
| 26 | SCC | 65/M | IA | N0 | Negative |
| 27 | SCC | 65/M | IA | N0 | Negative |
| 28 | SCC | 65/M | IA | N0 | Negative |
| 29 | SCC | 62/M | llA | N0 | Negative |
| 30 | SCC | 62/M | IA | N0 | Negative |
| 31 | SCC | 66/M | llB | N1 | Negative |
| 32 | SCC | 66/M | IA | N0 | Negative |
| 33 | SCC | 65/M | 1B | N0 | Negative |
| 34 | SCC | 72/M | 1B | N0 | Negative |
| 35 | SCC | 62/M | IA | N0 | Negative |
| 36 | SCC | 62/M | llA | N1 | Negative |
| 37 | BAC | 56/M | IA | N0 | Positive |
| 38 | BAC | 72/M | IA | N0 | Positive |
| 39 | BAC | 52/M | 1B | N0 | Positive |
| 40 | BAC | 61/M | IA | N0 | Positive |
| 41 | BAC | 69/M | IA | N0 | Positive |
| 42 | BAC | 43/F | IA | N0 | Negative |
| 43 | BAC | 58/F | 1B | N0 | Negative |
| 44 | BAC | 78/M | IA | N0 | Positive |
SCC, Squamous cell carcinoma; BAC, bronchoalveolar carcinoma; Adeno, adenocarcinoma.
Table 2.
Association of AQP1 Staining with Histology for the 44 Primary Lung Cancers
| Number of positive or negative cases/cases examined (%)
|
||
|---|---|---|
| Positive | Negative | |
| Total | 18/44 (41%) | 26/44 (59%) |
| Histology | ||
| Adenocarcinoma | 12/21 (57%) | 9/21 (43%) |
| Squamous cell carcinoma | 0/15 (0%) | 15/15 (100%) |
| Bronchoalveolar carcinoma | 6/8 (75%) | 2/8 (25%) |
Figure 2.
Immunohistochemical staining and FISH for AQP1. a: In normal lung tissue, alveolar lining epithelial cells are negative for AQP1 in contrast to endothelia of blood vessels and red blood cells. Strong immunostaining for AQP1 in bronchioloalveolar carcinoma (b), well-differentiated adenocarcinoma (c), and poorly differentiated adenocarcinoma (d). No expression of AQP1 in lung squamous cell carcinoma in contrast to the clear expression of AQP1 in vascular endothelium (e). f: In the FISH analysis, no genomic amplification is identified in any of 60 NSCLCs tested, and one representative example is shown (green color, FITC-labeled control probe; red color, rhodamine-labeled AQP1 probe). Original magnifications: ×200 (a–e); ×1000 (f).
To investigate whether the AQP1 gene is amplified or not in the lung cancers with AQP1 immunoreactivity, we performed FISH analysis using two tissue microarrays, which included 12 bronchioloalveolar carcinomas, 30 adenocarcinomas, and 18 squamous cell carcinomas. We did not find evidence of genomic amplifications in this analysis (Figure 2f).
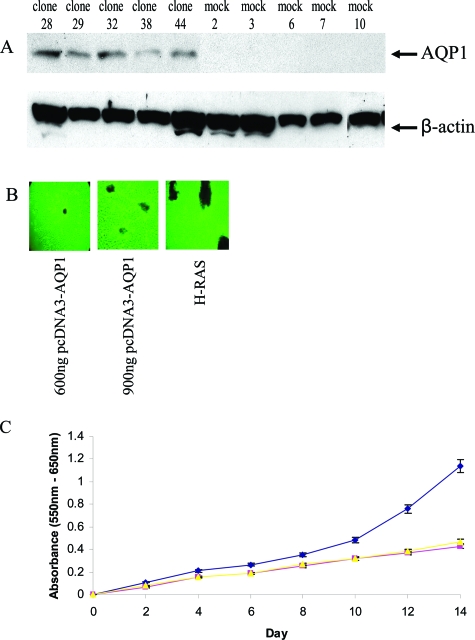
To investigate the effects of forced expression of AQP1, we constructed an AQP1 expression vector, in which wild-type AQP1 was cloned into the pcDNA3 vector under the CMV promoter and transfected into NIH-3T3 cells. Stable transfectants were selected and Figure 3A shows the results of Western blot analysis of the parental NIH-3T3, empty vector, and AQP1-transfected clones. The expression of endogenous AQP1 was undetectable in NIH-3T3 cell lines and the expression level varied among the transfected clones (Figure 3A). The phase morphology of clones overexpressing AQP1 was not appreciably changed (data not shown). From these transfected clones and related controls, we made the following observations: first, AQP1 overexpression markedly promoted colony formation in soft agar relative to NIH-3T3 cells transformed with empty vector or in parental cells. In fact, 18 days after seeding on soft agar, colonies were present only in clones with AQP1 overexpression, supporting the notion that AQP1 can facilitate anchorage-independent growth (Table 3). Second, to further assess whether AQP1 has oncogenic potential, the loss of contact inhibition was monitored by using a standard transformation assay in NIH-3T3 cells. We transfected NIH-3T3 cells with an increasing amount of pcDNA3 empty vector and AQP1-expressing plasmid. Three weeks after transfection, there were no foci in the control plates whereas numerous foci were apparent in the AQP1-transfected plates (Figure 3B). When transfected with pcDNA3-AQP1, NIH-3T3 cells began to form foci by day 14. There was no focus formation in nontransformed NIH-3T3 or pcDNA3-transfected NIH-3T3 cells, even beyond 4 weeks after transfection (data not shown). Finally, to evaluate the in vitro growth rate of AQP1-transfected cells, we performed the MTT assay. This assay is based on the ability of viable cells to have sufficient mitochondrial dehydrogenase to reduce MTT, and MTT absorbance correlates with the number of viable cells. The MTT assay of NIH-3T3 cells transfected with AQP1 in the recombinant pcDNA3 construct, driven by the CMV promoter, showed significant enhanced cellular survival after 14 days (Figure 3C). Thus, NIH-3T3 cells overexpressing AQP1 demonstrate many properties consistent with a transformed phenotype.
Figure 3.
Oncogenic properties of AQP1. A: Western blot identification of NIH-3T3 cells stably transfected with the mammalian expression vector pcDNA3 with full-length AQP1 cDNA and empty pcDNA3vector (mock). Clones 28, 29, 32, 38, and 44 expressed AQP1 at various levels. There is no detectable AQP1 in mock-transfected clones and parental NIH-3T3 cells. AQP1-expressing clones 28, 29, and 32, mock 3, and parental NIH-3T3 cells were used for further studies. B: Focus formation assay. NIH-3T3 cells (2 × 105) were transfected with pcDNA3-AQP1, only pcDNA3 (600 ng and 900 ng), or H-Ras (50 ng). Twenty-four hours after transfection, cells were split 1:5 in a 100-mm culture dish in Dulbecco’s modified Eagle’s medium supplemented with 5% fetal bovine serum. The medium was changed every 3 days thereafter, and the formation of foci was inspected visually. Foci started to appear in pcDNA3-AQP1-transfected cells after 8 days. The photographs shown were taken at day 21. H-Ras was used as positive control. There were no foci in pcDNA3, only transfected NIH-3T3 cells (data not shown). C: Cell growth rate is expressed as absorbance at 550 to 650 nm. Clone 44 (♦), mock 3 (▴), and parental (▪) NIH-3T3. Data are representative of two independent experiments (n = 6 for each experiment).
Table 3.
Cloning Efficiency of AQP1-Transfected Cells in the Soft-Agar Assay
| AQP1 no. 28 | AQP1 no. 29 | AQP1 no. 32 | Mock 3 | Parental NIH3T3 | |
|---|---|---|---|---|---|
| Number of clone per 5000 cells | 81.33 ± 10.59 | 66.66 ± 10.96 | 82.33 ± 17.89 | 0 | 0 |
Results are the mean of two independent experiments done in triplicate and represent the number of clones after 18 days.
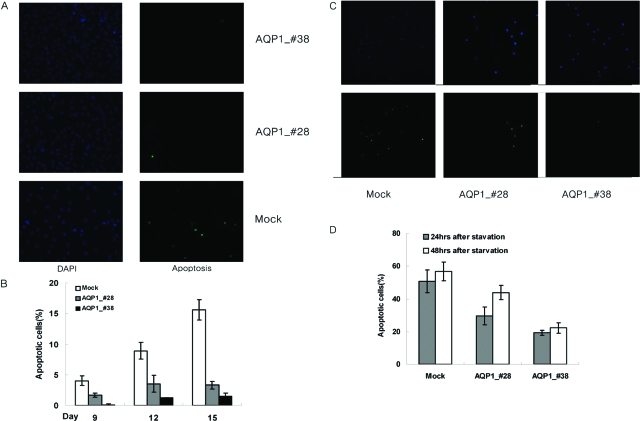
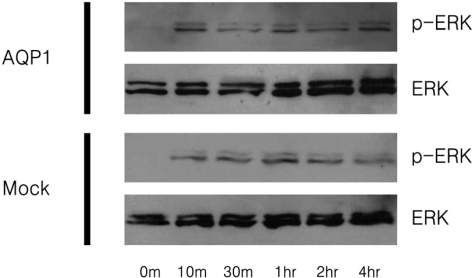
To investigate the mechanisms of transformation by AQP1, apoptotic activity and ERK activity were examined in the NIH-3T3 cells stably expressing AQP1. Using the TUNEL assay, the apoptotic activity of AQP1 stable cell lines was examined. Interestingly, by day 15, it is clear that there is decreased apoptosis among cells stably expressing AQP1 compared to the mock transfectants (Figure 4). In addition, this resistance to apoptosis was found to be proportional to AQP1 expression. These results seem to indicate that the enhanced cell proliferation observed in AQP1 stable cell lines is partially because of their resistance to apoptosis. It has also been reported that the anchorage-independent growth and cell proliferation has been associated with the continuous activation of the ERK pathway. Using Western blot analysis, the activation state of ERK/MAP kinase was assayed in AQP1 stable cell lines 24 hours after serum starvation. The intensity of activation was not significantly different between AQP1 stable cell lines and the mock transfectants (Figure 5). Thus, the anchorage-independent and cell proliferative properties of the AQP1 stable cell lines are not because of the activation of the ERK signal cascade, but are properties of their resistance to apoptosis.
Figure 4.
Apoptosis assay for cells stably expressing AQP1. A: Immunofluorescent terminal dUTP nick-end labeling assay of NIH-3T3 cells carrying AQP1 and mock. Left: DAPI staining for total number of cells. Right: Apoptotic cells at 15th day after seeding under normal culture condition. B: The ratio of apoptotic versus total number of cells was evaluated by TUNEL at the 3rd, 6th, 9th, 12th, and 15th day after seeding. No significant differences were observed between cells carrying AQP1 and mock at 3rd and 6th days (data not shown), and clear differences were observed at 9th, 12th, and 15th day after seeding. C: Immunofluorescent TUNEL assay of each stable cell at 48 hours after serum starvation. Top: DAPI staining for total number of cells. Bottom: Apoptotic cells. D: The ratio of apoptotic versus total number of cells was evaluated at 24 and 48 hours after serum starvation. Cells expressing AQP1 exhibit a significant decrease in the percentage of apoptosis at each time point and the resistance to apoptosis is proportional to AQP1 expression.
Figure 5.
Analysis of ERK activation in serum-stimulated cells expressing AQP1 and mock after 24 hours of starvation. The activation state of ERK/MAP kinase from cell lysates in various time points was assessed by Western blot. Top: Phospho-ERK and total ERK from cells expressing AQP1 (marked as AQP1). Bottom: Phosphor-ERK and total ERK from cells carrying mock (marked as mock). The intensity of activation is not significantly different, although minimal activation of phosphor-ERK at 0 minutes was observed in cells expressing AQP1, not in cells carrying the empty vector.
Discussion
This report provides a novel example of the involvement of water channel proteins in human lung cancer. Thus far, AQP4 is the major AQP known to be expressed in the basal layer of bronchial epithelium9,17 whereas a consistent expression of AQP1 in the human bronchial epithelium has not been reported. Western blot analysis showed that AQP1 was expressed concurrently in all of the tested cancer cell lines. Interestingly enough, the rabbit anti-AQP1 polyclonal antibody used for Western blot (Chemicon International) did not give a satisfactory immunohistochemical staining. Therefore, we used affinity-purified rabbit antibody raised against the 19-amino acid sequence (amino acids 251 to 269) of the COOH-terminus of human AQP1 (Alpha Diagnostic) for immunohistochemistry. This particular 19-amino acid sequence was found unique to AQP1 without significant homology to any other known eukaryotic protein and therefore, we believe that the finding of our immunohistochemistry study using this particular antibody is fairly specific to AQP1 expression.12,14,15
Overall, we demonstrated that the majority of NSCLC tumors exhibited either strong (32%) or focal level (11%) of AQP1 protein expression showing either a membranous and/or a cytoplasmic labeling, and there was a significant correlation between AQP1 protein expression and histological subtypes, with the highest expression in BAC and no expression in squamous cell carcinoma. Furthermore, the study showed that AQP1 protein expression was more prominent in well-differentiated than in the poorly differentiated histology (Table 1). Overexpression of AQP1 in adenocarcinoma and BAC and the absence of expression in squamous cell carcinoma may suggest some important roles of AQP1 in the genesis of adeno and BAC types of lung cancer. Specifically, acquisition of AQP1 expression plays a certain role in fluid secretion or absorption during the development of adeno and BAC types of NSCLC. Future studies including genetics analysis of AQP1 promoter in various types of NSCLC samples would give some clues to better understand the molecular mechanism responsible for AQP1 expression specific to the adenocarcinoma cell type.18,19
The signals that induce AQP1 expression in the later types of lung cancer is unknown, but might include vascular endothelial growth factor, which is produced by tumor cells and is known to produce vascular permeability. The presence of AQP1 immunoreactivity in both cell membrane and cytoplasm suggests a high turnover of AQP1 protein. However, we were not able to determine whether there is any association of localization of AQP1 and clinical parameters because of the small sample size in each group (data not shown).
We had previously reported that AQP1 is expressed in infiltrating lymphocytes and dendritic cells near lung and other cancers.16 Because we used a tumor tissue microarray for immunohistochemistry only limited cases contained infiltrating lymphocytes and dendritic cells. For identification of dendritic cells, a double-immunostaining technique is needed using a dendritic cell marker and AQP1. Unfortunately, those works were not in the scope of this study and we did not pursue this. Also, as seen in Figure 2, red bloods cells consistently expressed AQP1,1,2 and it is very clear that the tumor cells, which are morphologically distinct from red bloods cells, lymphocytes, and dendritic cells, clearly express AQP1.10
Overexpression of several aquaporins has been reported in different types of human cancer7,10 indicating a possible role for water channels in tumor growth and spread. While this manuscript was in preparation, Saadoun and colleagues20 reported that AQP1 deletion impairs tumor microvessel proliferation, which produces extensive tumor necrosis. Our data are unique in part because they provide the first evidence that high levels of AQP1 are found in the adeno and BAC subtype of lung cancer. Members of the AQP family have been shown to have contrasting effects on cell proliferation and tumorigenic potential in cell lines of different origins. For example, AQP1 has been linked to tumorigenesis in lung cancer. However, AQP3 has no implication in promotion of cell proliferation in lung cancer cell lines (unpublished data).
The genetic amplification of growth-enhancing genes plays a key role in the development of human malignancy. An important task in understanding oncogenesis is the identification of those genes whose copy number and expression increase during tumorigenesis. As seen in one of the examples in Figure 2, data from FISH in 60 NSCLCs did not show evidence of genetic amplification of AQP1. Additionally, the overexpression of AQP1 in NSCLC is not because of a mutation. Sequencing of the entire AQP1 cDNA of all 10 NSCLC cell lines and resected tumor samples did not demonstrate a mutation that led to an amino acid change (data not shown). Based on previous promoter study results, expression of AQP1 in NSCLC cells may be induced by the binding of immediate early response gene products to AQP1’s promoter binding sites, including oncogenes such as Fos/Jun.18,19
During the cell cycle, as cell volume needs to increase rapidly by absorbing water from the immediate external cellular environment using a minimal amount of energy, expression of several types of AQPs in the tumor cells may be advantageous compared to normal cells with limited AQP expression. Also, tumor cells may require several AQPs for high metabolic turnover or tumor-specific metabolic pathways needed for survival, as hinted at previously for human brain tumors.7 Ectopic expression of full-length cDNA of AQP1 induced many phenotypic changes characteristic of transformation including enhanced cell survival and anchorage-independent growth, the hallmarks of cancer.21 Furthermore, stable expression of AQP1 increased the resistance of NIH-3T3 cells to apoptosis. Although the differences in biology between rodent cells and human cells have led some to question the validity of the NIH-3T3 model, the evidence of human cancer cell lines and primary tumor tissue support the hypothesis that AQP1 may play a significant role in human lung cancer development. Future transfection studies using squamous cell carcinoma-derived cells or adenocarcinoma-derived cells might provide a more realistic model with different biological behaviors, which will likely facilitate to understand the role of AQP1 in the development of NSCLC.
In summary, based on tumor-specific overexpression, we propose that increased levels of AQP1 may play a role in tumorigenesis. Furthermore, we provide functional evidence supporting novel oncogenic properties of AQP1. Further studies on the regulation of the AQP1 signaling transduction pathway may provide insights leading to the design of novel therapeutic approaches.
Acknowledgments
We thank Cherry Junn for her editorial advice.
Footnotes
Address reprint requests to Chulso Moon, M.D., Ph.D., The Head and Neck Cancer Research Division, Department of Otolaryngology, The Johns Hopkins School of Medicine, 818 Ross Research Building, 720 Rutland Ave., Baltimore, MD 21205-2196. E-mail: cmoon5@jhmi.edu.
Supported in part by the National Cancer Institute Specialized Programs of Research Excellence (grant P50 CA96784-01 to C.M.), the American Cancer Society (grant RPG-98-054 to L.M.), the Fondation de France, AP-HP (to J-C.S.), the Lilly Fondation (to J-C.S.), Cancer Center (grant P30 CA16620 to M. D. Anderson Cancer Center), the Tobacco Research Fund from the State of Texas (to M. D. Anderson Cancer Center), Cancer research grant from Pyung-Ya Foundation (to C.M.), and a research grant from the Korea Science and Engineering Foundation (to S.J.J.).
References
- Verkman AS, van Hoek AN, Ma T, Frigeri A, Skach WR, Mitra A, Tamarappoo BK, Farinas J. Water transport across mammalian cell membranes. Am J Physiol. 1996;270:C12–C30. doi: 10.1152/ajpcell.1996.270.1.C12. [DOI] [PubMed] [Google Scholar]
- King LS, Agre P. Pathophysiology of the aquaporin water channels. Annu Rev Physiol. 1996;58:619–648. doi: 10.1146/annurev.ph.58.030196.003155. [DOI] [PubMed] [Google Scholar]
- Agre P, Preston GM, Smith BL, Jung JS, Raina S, Moon C, Guggino WB, Nielsen S. Aquaporin CHIP: the archetypal molecular water channel. Am J Physiol. 1993;265:F463–F476. doi: 10.1152/ajprenal.1993.265.4.F463. [DOI] [PubMed] [Google Scholar]
- Cheng A, van Hoek AN, Yeager M, Verkman AS, Mitra AK. Three-dimensional organization of a human water channel. Nature. 1997;387:627–630. doi: 10.1038/42517. [DOI] [PubMed] [Google Scholar]
- Heymann JB, Agre P, Engel A. Progress on the structure and function of aquaporin 1. J Struct Biol. 1998;121:191–206. doi: 10.1006/jsbi.1997.3951. [DOI] [PubMed] [Google Scholar]
- Vacca A, Frigeri A, Ribatti D, Nicchia GP, Nico B, Ria R, Svelto M, Dammacco F. Microvessel overexpression of aquaporin 1 parallels bone marrow angiogenesis in patients with active multiple myeloma. Br J Haematol. 2001;113:415–421. doi: 10.1046/j.1365-2141.2001.02738.x. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Davies DC, Bell BA, Krishna S. Increased aquaporin 1 water channel expression in human brain tumours. Br J Cancer. 2002;87:621–623. doi: 10.1038/sj.bjc.6600512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C, Williams JB, Preston GM, Copeland NG, Gilbert DJ, Nathans D, Jenkins NA, Agre P. The mouse aquaporin-1 gene. Genomics. 1995;30:354–357. doi: 10.1006/geno.1995.0029. [DOI] [PubMed] [Google Scholar]
- Ma T, Verkman AS. Aquaporin water channels in gastrointestinal physiology. J Physiol. 1999;517:317–326. doi: 10.1111/j.1469-7793.1999.0317t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C, Soria JC, Jang SJ, Lee J, Obaidul Hoque M, Sibony M, Trink B, Chang YS, Sidransky D, Mao L. Involvement of aquaporins in colorectal carcinogenesis. Oncogene. 2003;22:6699–6703. doi: 10.1038/sj.onc.1206762. [DOI] [PubMed] [Google Scholar]
- Kageyama Y, Sasaki S, Yamamura Y, Oshima H, Ikawa Y. Water channel protein subtype suggests the origin of renal cell carcinoma. J Urol. 1996;156:291–295. [PubMed] [Google Scholar]
- Burghardt B, Elkaer ML, Kwon TH, Racz GZ, Varga G, Steward MC, Nielsen S. Distribution of aquaporin water channels AQP1 and AQP5 in the ductal system of the human pancreas. Gut. 2003;52:1008–1016. doi: 10.1136/gut.52.7.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark GJ, Cox AD, Graham SM, Der CJ. Biological assays for Ras transformation. Methods Enzymol. 1995;255:395–412. doi: 10.1016/s0076-6879(95)55042-9. [DOI] [PubMed] [Google Scholar]
- Mobasheri A, Marples D. Expression of the AQP-1 water channel in normal human tissues: a semiquantitative study using tissue microarray technology. Am J Physiol. 2004;286:C529–C537. doi: 10.1152/ajpcell.00408.2003. [DOI] [PubMed] [Google Scholar]
- Mobasheri A, Airley R, Hewitt SM, Marples D. Heterogeneous expression of the aquaporin 1 (AQP1) water channel in tumors of the prostate, breast, ovary, colon and lung: a study using high density multiple human tumor tissue microarrays. Int J Oncol. 2005;26:1149–1158. [PubMed] [Google Scholar]
- Moon C, Rousseau R, Soria JC, Hoque MO, Lee J, Jang SJ, Trink B, Sidransky D, Mao L. Aquaporin expression in human lymphocytes and dendritic cells. Am J Hematol. 2004;75:1–6. doi: 10.1002/ajh.10476. [DOI] [PubMed] [Google Scholar]
- Verkman AS. Role of aquaporin water channels in kidney and lung. Am J Med Sci. 1998;316:310–320. doi: 10.1097/00000441-199811000-00004. [DOI] [PubMed] [Google Scholar]
- Moon C, Preston GM, Griffin CA, Jabs EW, Agre P. The human aquaporin-CHIP gene. Structure, organization, and chromosomal localization. J Biol Chem. 1993;268:15772–15778. [PubMed] [Google Scholar]
- Moon C, King LS, Agre P. Aqp1 expression in erythroleukemia cells: genetic regulation of glucocorticoid and chemical induction. Am J Physiol. 1997;273:C1562–C1570. doi: 10.1152/ajpcell.1997.273.5.C1562. [DOI] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Hara-Chikuma M, Verkman AS. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nature. 2005;434:786–792. doi: 10.1038/nature03460. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]