Abstract
Alcohol hepatic toxicity in heavy drinkers is associated with high endotoxin blood levels and increased intestinal permeability. Because endotoxins can cross damaged mucosa, we investigated the mechanisms through which ethanol impairs the colonic epithelium of rats submitted to acute alcohol intake. Colonic permeability to 51Cr-ethylenediamintetraacetic acid was increased 24 hours after 3.0 g/kg ethanol intake (3.2 ± 0.2% versus 2.2 ± 0.2%) and was associated with significant endotoxemia. Antibiotics and doxantrazole (a mast cell membrane stabilizer) significantly inhibited the effect of ethanol. Two hours after intake, plasma concentrations of ethanol were twofold higher in antibiotic-treated rats than in controls (155.8 ± 9.3 mg/dl versus 75.7 ± 7.6 mg/dl, P < 0.001). Lumenal concentrations of acetaldehyde were markedly increased after ethanol intake (132.6 ± 31.6 μmol/L versus 20.8 ± 1.4 μmol/L, P < 0.05) and antibiotics diminished this increase (86.2 ± 10.9 μmol/L). In colonic samples mounted in Ussing chambers, acetaldehyde but not ethanol increased dextran flux across the mucosa by 54%. Doxantrazole inhibited the effect of acetaldehyde. This study demonstrates that an acute and moderate ethanol intake alters the epithelial barrier through ethanol oxidation into acetaldehyde by the colonic microflora and downstream mast cell activation. Such alterations that remain for longer periods could result in excessive endotoxin passage, which could explain the subsequent endotoxemia frequently observed in patients with alcoholic liver disease.
Ethanol exerts several deleterious effects on the liver through its metabolism in situ into acetaldehyde by alcohol dehydrogenase and, particularly under chronic alcoholism, cytochrome P450 2E1 (CYP2E1). Acetaldehyde produced in the liver may interact with proteins to generate adducts that are able to activate immune cells.1 Although the toxicity of hepatic acetaldehyde is clearly established, the presence of endotoxins in the blood appears to be a prerequisite to cirrhosis, which develops in 10 to 20% of chronic alcoholics.2 Indeed, several studies have revealed that endotoxemia was strongly associated with the occurrence and severity of cirrhosis in humans.3,4 Moreover, endotoxins are strong activators of Kupffer cells,5 which are immune cells derived from the macrophage lineage involved in liver inflammation. The intestine, characterized by abundant resident microflora and food bacteria, is the main reservoir of endotoxins. Basically, the intestinal epithelium acts as a continuous barrier to avoid the penetration of lumenal elements, but some endogenous or exogenous factors may alter the barrier function of the gut. This leaky gut, assessed by an increased permeability to several markers and/or a decreased transepithelial resistance (TER), has been observed both in humans and animals after alcohol consumption6,7 and is associated with endotoxemia. Moreover, restoring a normal permeability by dietary components decreases endotoxemia and hepatic injury after ethanol intake in rats.7 The underlying mechanism through which ethanol may be deleterious for the intestinal epithelium remains primarily unknown. On one hand, some studies demonstrated that ethanol addition on intestinal epithelial cell lines induces barrier alteration.8 However, the high concentrations needed (5% ethanol) and the weak observed effect (10% decrease of TER) suggest that a direct effect of ethanol on epithelial cells is not the main mechanism involved in vivo after alcohol consumption. On the other hand, acetaldehyde may be produced in the colon because ethanol diffuses easily from blood to the colonic lumen9 and some bacterial species of the microflora are able to metabolize ethanol.10 Atkinson and Rao11 have shown that concentrations of acetaldehyde that are observed in colonic content after alcohol intake disrupts tight junctions of Caco-2 monolayers through a tyrosine kinase-dependent mechanism, with subsequent alterations of tight junction protein assembly. Finally, several studies, which do not concern alcohol effects, have pointed to a key role for mast cell in the disruption of the epithelial barrier.12,13 Activated mast cells release mediators that can directly alter the epithelial barrier function or activate other immune cells which in turn can release mediators impairing the gut barrier. Moreover, acetaldehyde is known to activate mast cells at concentrations frequently observed after alcohol ingestion.14 Consequently, the aim of the present study was to investigate in rodents whether the increase in intestinal permeability associated with alcohol ingestion depends on the metabolism of ethanol into acetaldehyde by the resident microflora and whether mast cells are involved in this effect.
Materials and Methods
Animals
Sprague-Dawley rats (200 to 250 g) and C3H/He mice were obtained from Janvier (Elevage Janvier, Le Genest St-Isle, France), kept at a constant temperature (23 ± 1°C) in a pathogen-free animal facility, and maintained on a 12-hour light/dark cycle. Food and water were available ad libitum. Germ-free C3H/He mice (25 to 30 g) were bred and maintained in isolators.
Measurement of Intestinal Permeability in Rats
Rats were placed in individual metabolic cages 24 hours before experiments. On the day of the experiment, rats were given ethanol, isocaloric amounts of dextrose by gavage, or water. Thirty minutes later, 0.7 μCi of 51Cr-ethylenediaminetetraacetic acid (EDTA) (Perkin Elmer Life Sciences, Courtaboeuf, France) in 0.5 ml of saline was orally administered. Urine was collected for 24 hours, then radioactivity was measured with a gamma counter. Intestinal permeability was expressed as the percentage of total radioactivity administered.
Measurement of Blood Endotoxins
Rats were anesthetized with pentobarbital 90 minutes after ethanol intake. A laparotomy was performed, and the portal vein was localized and punctured. Blood was centrifuged (10 minutes, 2500 rpm) and serum was recovered. The endotoxin level was measured using a limulus amoebocyte lysate-based kit (Cambrex, Verviers, Belgium). Samples were diluted 1:4 with sterile apyrogen water and heated 10 minutes at 70°C. Standards and samples were incubated 10 minutes at 37°C with limulus amoebocyte lysate and then 6 minutes with colorimetric substrate. The reaction was stopped with 25% acetic acid, and absorbance was read at 405 nm.
Blood Ethanol Assay
Two hours after ethanol administration by gavage, animals were anesthetized with ketamine (100 mg/kg) and acepromazine (0.5 mg/kg). After laparotomy, the abdominal aorta was isolated, and a blood puncture was performed using heparinized tubes. Blood samples were centrifuged at 1200 × g for 10 minutes, plasma was recovered and immediately analyzed. Ethanol was assayed by an enzymatic in vitro test (Roche Diagnostics, Meylan, France).
Colonic Acetaldehyde Determination by GC-MS
After sacrifice, samples of luminal content from the cecum and proximal colon were harvested, and acetaldehyde was assayed as described by Gukovskaya and colleagues.15 Homogenate (300 μl) was mixed with an equal volume of 6 mol/L HCl, containing 500 μmol/L of butyraldehyde as internal standard and 4 mg/ml of 2,4-dinitrophenylhydrazine (DNPH) to form a Schiff base. Samples were then incubated at 40°C for 1 hour, extracted with 3 ml of n-heptane, and vacuum-dried.
The residue was dissolved in 1 ml of n-hexane, and a 20 μl aliquot was diluted in 1 ml of n-hexane. One μl of the resulting solution was injected in the splitless mode into a Finnigan Polaris Q gas chromatograph-ion trap mass spectrometer fitted with an AS2000 autoinjector (Thermo Electron, Les Ulis, France). The chromatographic separation was achieved on a BPX5 capillary column (25 m × 0.22 mm ID, 0.25-μm film thickness) from SGE (Courtaboeuf, France). Helium was used as the carrier gas at a flow rate of 1 ml/minute. The oven temperature was programmed as follows: 100°C for 1 minute, then 100°C to 200°C at 20°C/minute, hold time of 5 minutes at 200°C, 200°C to 220°C at 5°C/minute and 220°C to 280°C at 30°C/minute with a final hold time of 4 minutes at 280°C. The injector and GC-MS interface temperatures were set at 250°C. EI mass spectra were generated at 70 eV at a source temperature of 220°C. Spectra were acquired from m/z 60 to m/z 350. Due to the formation of syn/anti-isomers during derivatization, DNPH-acetaldehyde was eluted in two peaks at 13.6 and 14.2 minutes, whereas DNPH-butyraldehyde gave two peaks at 16.3 and 16.7 minutes. Ion currents at m/z 224 and 252 were extracted for integration of the peaks corresponding to DNPH-acetaldehyde and DNPH-butyraldehyde, respectively. Acetaldehyde determinations were performed by internal standard calibration. The calibration curve was linear from 4 to 200 pg of acetaldehyde, and the limit of detection was estimated at 600 fg of acetaldehyde.
Ussing Chamber Experiments
Immediately after sacrifice, portions of proximal colon (exposed area, 0.5 cm2) were mounted into Ussing chamber (Physiological Instruments, San Diego, CA), each side containing 5 ml of Krebs buffer (NaCl, 118 mmol/L; KCl, 4.7; KH2PO4, 1.2; MgSO4, 1.2; NaHCO3, 25; glucose, 8.3; CaCl2, 2.5), continuously gassed with 95% O2/5% CO2. TER was monitored throughout the experiment to assess the viability of the tissue. After 20 minutes, fluorescein isothiocyanate-labeled 4-kd dextran was added at the mucosal side (2.2 mg/ml as final concentration). At the same time, ethanol (17 mmol/L), acetaldehyde (from 40 to 160 μmol/L), or buffer were added either in the serosal compartment (ethanol) or in the mucosal compartment (acetaldehyde). Fluorescence was measured at the serosal side after 60 minutes. Results were expressed as the flux of dextran crossing the epithelial barrier (nmol/hour/cm2).
Experimental Design
To determine the effects of ethanol on intestinal permeability, three groups of eight rats were given 1.5, 3.0, or 4.5 g/kg ethanol, three other groups received isocaloric amounts of dextrose. The role of the microflora was investigated on two groups of eight rats receiving antibiotics (1.0 g/L ampicillin and 0.5 g/L neomycin in drinking water) for 12 days. Ampicillin and neomycin are two broad-spectrum antibiotics that are not (neomycin) or weakly absorbed and, thus, are able target the commensal microbes. On the 12th day, one group was given 3.0 g/kg ethanol, the other was given dextrose, and the permeability to 51Cr-EDTA was assessed.
The involvement of mast cells was demonstrated on two groups of 16 rats, injected with either a mast cell membrane stabilizer (doxantrazole, 20 mg/kg i.p.) or its vehicle (NaHCO3, 5% w/v) 30 minutes before ethanol intake. In each group, eight animals received oral administration of 3.0 g/kg ethanol, eight other animals received dextrose. Ethanol blood levels were determined in two groups of conventional rats 2 hours after a 3.0 g/kg ethanol intake, one group being pretreated with antibiotics. Similar experiments were conducted in one group of germ-free C3H/He mice and one group of conventional C3H/He receiving 3.0 g/kg ethanol. Endotoxin blood levels were measured in three groups of rats. One group served as control, two groups were submitted to a 3.0 g/kg ethanol intake, one being pretreated with antibiotics. Colonic acetaldehyde content was determined on four groups of 10 rats. Two groups received 3.0 g/kg ethanol with or without pretreatment with antibiotics. Two other groups received dextrose, with or without pretreatment with antibiotics. Ussing chamber experiments were conducted on rats (n = 5 to 8 per group) treated with doxantrazole (20 mg/kg i.p.) or its vehicle (NaHCO3, 5% w/v) 30 minutes before sacrifice.
Chemicals
Chemicals were obtained from Sigma (Saint Quentin Fallavier, France). Doxantrazole [3-(1H-tetrazol-5-yl)-9H-thioxanten-9-one 10,10-dioxide monohydrate] was a gift from the Wellcome Trust (London, UK).
Statistics
Data were analyzed by one-way analysis of variance, followed by posthoc Dunnet’s multiple comparison test or Student’s t-test where appropriate. Differences were considered significant at P < 0.05.
Results
Ethanol Increases Intestinal Permeability in Vivo
Basal intestinal paracellular permeability to 51Cr-EDTA in rats was 2.3 ± 0.3%. Oral administration of dextrose at concentrations that mimic the caloric intake of ethanol (10.5, 21.0, and 31.5 kcal/kg) was devoid of effect (Figure 1). The lowest dose of ethanol (1.5 g/kg) did not induce any significant change in paracellular permeability (2.3 ± 0.1% versus 2.4 ± 0.2% in controls, P > 0.05). Higher ethanol intake at doses of 3.0 and 4.5 g/kg increased intestinal permeability by 45% (3.2 ± 0.2% versus 2.2 ± 0.2%, P < 0.05) and 59% (3.5 ± 0.4% versus 2.2 ± 0.1%, P < 0.05), respectively (Figure 1). Because a significant permeability increase was observed with 3.0 g/kg ethanol, this concentration was used thereafter.
Figure 1.
Intestinal paracellular permeability to 51Cr-EDTA of rats receiving by gavage: ethanol (black bars), isocaloric amounts of dextrose (white bars), or water (gray bar). Data (means ± SEM) are expressed as the percentage of total radioactivity recovered in urine for 24 hours after intake. *P < 0.05.
Role of the Microflora
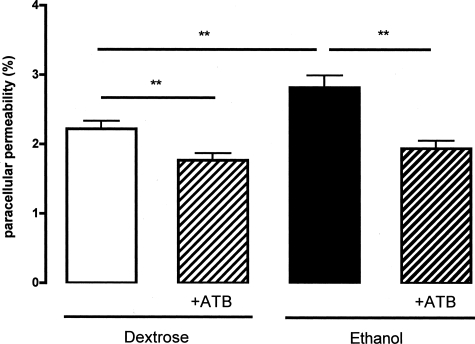
A treatment with antibiotics significantly diminished basal intestinal permeability to 51Cr-EDTA (1.8 ± 0.1% versus 2.2 ± 0.1%, P < 0.01). Similarly, the ethanol-induced increase of intestinal permeability was abolished by antibiotic treatment (1.9 ± 0.1% versus 2.8 ± 0.2%, P < 0.01; Figure 2). Ethanol blood concentration reached 75.7 ± 7.6 mg/dl 2 hours after a 3.0 g/kg oral ethanol intake. With antibiotic treatment, ethanolemia was nearly twofold for the same intake (155.8 ± 9.3 mg/dl, P < 0.001; Figure 3). Similarly, for a 3.0 g/kg ethanol intake, plasma concentrations of ethanol were significantly increased in C3H/He germ-free mice (212.2 ± 17.7 mg/dl), compared with conventional mice (93.4 ± 6.5 mg/dl, P < 0.001; Figure 3).
Figure 2.
Effect of antibiotics (ATB) on intestinal paracellular permeability to 51Cr-EDTA of rats receiving by gavage: 3.0 g/kg ethanol, an isocaloric amount of dextrose. Data (means ± SEM) are expressed as the percentage of total radioactivity recovered in urine for 24 hours after intake. **P < 0.01.
Figure 3.
Blood ethanol concentrations measured 2 hours after a 3.0 g/kg ethanol intake in conventional and germ-free C3H/He mice, and in Sprague-Dawley rats pretreated or not with antibiotics (ATB). Data (means ± SEM) are expressed as mg/dl. ***P < 0.001.
Endotoxin Levels after Ethanol Intake and Antibiotic Treatment
Ethanol 3.0 g/kg induced a significant increase of endotoxin levels in the portal blood (789.3 ± 73.7 UI/L versus 382.2 ± 34.2 UI/L for controls, P < 0.01; Figure 4). A treatment with antibiotics inhibited the effects of ethanol, the endotoxin concentrations returning to basal values (433.8 ± 42.5 UI/L, P < 0.01 versus ethanol; Figure 4).
Figure 4.
Portal blood endotoxin concentration measured 90 minutes after a 3.0 g/kg ethanol intake, pretreated or not with antibiotics. Data (means ± SEM) are expressed in IU/L. **P < 0.01.
Acetaldehyde Production into the Colon
Two hours after oral administration of dextrose (21.0 kcal/kg), the concentration of acetaldehyde in luminal content was 20.8 ± 1.4 μmol/L. Two hours after 3.0 g/kg ethanol intake, colonic concentration of acetaldehyde reached 132.6 ± 31.6 μmol/L (P < 0.05). However, the amount of acetaldehyde produced in situ strongly varied from one animal to another (Figure 5). Antibiotics partially inhibited the production of acetaldehyde (86.2 ± 10.9 μmol/L), and strongly reduced distribution data scattering.
Figure 5.
Lumenal acetaldehyde concentration in colonic contents of rats receiving 3.0 g/kg ethanol or an isocaloric amount of dextrose, and pretreated or not with antibiotics for 12 days. Data are expressed as μmol/L (median ± range). ns, not significant.
Involvement of Mast Cells
The mast cell stabilizer doxantrazole (20 mg/kg i.p.) reduced the effect of a single 3.0 g/kg ethanol intake on intestinal permeability (2.5 ± 0.1% versus 3.0 ± 0.1%, P < 0.05; Figure 6). This inhibition was partial because intestinal permeability after doxantrazole treatment remained higher than in dextrose-receiving animals (2.2 ± 0.1%, P < 0.05). Doxantrazole itself did not affect significantly basal permeability (P = 0.15).
Figure 6.
Effect of the mast cell membrane stabilizer doxantrazole (hatched bars) on intestinal paracellular permeability to 51Cr-EDTA of rats receiving by gavage ethanol (black bars) or isocaloric amounts of dextrose (white bars). *P < 0.05 and ***P < 0.001 versus dextrose-treated group; #P < 0.05 versus ethanol-treated group.
In Vitro Experiments
A concentration of 17 mmol/L ethanol, mimicking the ethanol blood level 2 hours after a single 3.0 g/kg intake, added on the serosal side of rat colonic samples mounted in Ussing chambers had no effect on dextran flux across the tissue (Table 1). Conversely, 133 μmol/L acetaldehyde, a concentration similar to the one observed in the colonic lumen after ethanol ingestion, increased the dextran flux by 54% compared with control tissues (P < 0.05, Table 1). Acetaldehyde also markedly induced a dose-dependent increase of the dextran flux across the epithelium, reaching significance at 120 μmol/L (Figure 7). In samples harvested from rats treated with doxantrazole, basal permeability to dextran was significantly reduced when compared with controls (P < 0.05). Acetaldehyde addition on colonic samples from doxantrazole-treated rats had no effect (Table 1).
Table 1.
Dextran Fluxes Across Segments of Proximal Colon Mounted in Ussing Chamber, Expressed in nmol/hour/cm2 (Means ± SEM)
| Controls | Doxantrazole-treated rats | |
|---|---|---|
| Buffer | 1.52 ± 0.11 | 0.68 ± 0.15* |
| Ethanol, 17 mmol/L | 1.68 ± 0.15 | n.d. |
| Acetaldehyde, 133 μmol/L | 3.25 ± 0.56* | 0.67 ± 0.22† |
Treatments were added in the mucosal compartment of chambers.
P < 0.05 versus control rats treated with buffer.
P < 0.05 versus doxantrazole-treated rats treated with buffer.
n.d., not determined.
Figure 7.
Dose-dependent effect of acetaldehyde on the dextran flux across the epithelium of rat colonic strips mounted in Ussing chambers. Acetaldehyde was added in the mucosal compartment of the chamber. Results (means ± SEM) are expressed as nmol of fluorescein isothiocyanate-dextran crossing 1 cm2 of epithelium in 1 hour (nmol·h−1·cm−2).
Discussion
Our results indicate that impairment of the intestinal barrier function by ethanol is associated with endotoxemia and appears to be dependent of its metabolism into acetaldehyde by the intestinal resident microflora. Moreover, we have shown that a direct effect of ethanol on the epithelium is unlikely at nontoxic concentration. Our data also pointed out that acetaldehyde may trigger an increase in paracellular permeability through the local activation of mast cells.
Alterations of the intestinal barrier in patients suffering alcoholic liver disease (ALD) are clearly established.4 An intact epithelium is necessary to prevent bacteria and endotoxin translocation into the internal milieu. Here, we show that a single alcoholization is sufficient to produce a significant increase in intestinal paracellular permeability to 51Cr-EDTA in rats. Although similar observations have been reported in humans,6 the leaky gut of alcoholics was first considered as a consequence of the liver disease. The hypothesis was that portal hypertension associated with ALD would be responsible for an acute venous congestion, edema, and ischemia, leading to mucosal damage.16 Our results are in agreement with a new concept proposing that mucosal damage is a prerequisite to develop ALD.4
Several underlying mechanisms through which ethanol may impair the mucosal barrier can be hypothesized. First, ethanol could act directly on epithelial cells. In 1982, Levitt and colleagues9 showed that ethanol concentrations after acute ingestion were identical in blood and intestinal lumen. This diffusion allows a direct contact between ethanol and enterocytes. Some studies have focused on this hypothesis using cell culture experiments by measuring TER and permeability to markers of cell monolayers. These studies revealed that ethanol concentrations of 5 to 10% must be reached to decrease TER and to increase permeability.8,17 Such concentrations might be present in the stomach and proximal duodenum, immediately after ethanol consumption, but could not be reached in the colonic lumen from diffusion from blood. In our model of explanted colonic tissue mounted in Ussing chamber, we did not observe any modification of permeability after ethanol addition, at a concentration mimicking the ethanol blood levels 2 hours after intake.
Second, the effect of ethanol could be related to acetaldehyde, which is produced by ethanol oxidation. In the colon, acetaldehyde can be generated either through cytochrome P450 2E1,18 or by indigenous bacterial species that are able to oxidize ethanol through an alcohol dehydrogenase activity.19 Moreover, some antibiotics have been found to decrease significantly the whole body ethanol metabolism.20 Our present data support these observations because a twofold increase of ethanol blood concentration was observed after antibiotic treatment in rats or in germ-free mice, in comparison with conventional animals. These results highlight a significant role for the gut microflora in ethanol metabolism, which may be responsible for half of the whole ethanol metabolic activity. Antibiotic treatment also blocks the effects of ethanol on gut permeability despite high ethanol blood concentration. In addition, the increase of portal blood endotoxins observed 90 minutes after alcohol intake is absent in antibiotic-treated animals. These results reinforce the hypothesis of an indirect effect of ethanol. Moreover, we have shown that significant acetaldehyde concentrations are present in the colonic content after ethanol intake. However, as observed by others,21 there were great interindividual variations in colonic acetaldehyde concentration. Although not significant, antibiotic treatment tends to reduce intracolonic concentration of acetaldehyde, and strongly diminishes the data scattering. Acetaldehyde is known to disrupt epithelial tight junctions of cell monolayers, through a mechanism involving a loss of phosphatase activity and an increased tyrosine kinase activity,11 resulting in an inhibition of the regulation of the phosphorylation-dephosphorylation balance of tight junction proteins. Interestingly, acetaldehyde may act on the epithelial barrier at concentration levels similar to those observed after ethanol intake. Visapaa and colleagues21 observed that colonic acetaldehyde may reach concentrations as high as 3 mmol/L, whereas in vitro studies have shown that 0.1 mmol/L acetaldehyde was able to impair the epithelial barrier.11 Our results show that 120 μmol/L acetaldehyde, ie, slightly lower that the mean luminal concentration after ethanol intake, increased the permeability to dextran of colonic samples mounted in Ussing chambers.
One study indicates that acetaldehyde at low concentration is a potent mast cell degranulator in vitro.14 Mast cells are immunocytes in close relationship with epithelia and receive signals from their host tissue, other immunocytes, and nerves. Their activation lead to degranulation and release of several mediators such as histamine, eicosanoids, cytokines, and chemokines.22 Several studies have shown that mast cells play a key role in the regulation of the epithelial physiology, especially regarding intestinal permeability.12,13,23 This is corroborated by our present observation, showing that in rats treated with the mast cell stabilizer doxantrazole, the in vitro basal permeability to dextran was significantly lower than for control animals. Doxantrazole is able to stabilize the mast cell membrane, thus inhibiting its degranulation, through still poorly understood mechanisms, despite its discovery in 1975.24 One study by Sadeghi-Hashjin and colleagues25 showed in isolated alveolar macrophages that doxantrazole was also a scavenger of free radicals. In our present study, such a mechanism appears unlikely because acetaldehyde has never been found associated with macrophage activation within the gut. Doxantrazole was also found to reduce slightly but not significantly basal permeability to 51Cr-EDTA in vivo. We demonstrated herein that the effects of ethanol intake on the increase of intestinal permeability to 51Cr-EDTA are blocked by a mast cell stabilizer in vivo. In addition, we also found that in doxantrazole-treated rats, acetaldehyde did not increase permeability to dextran in colonic samples mounted in Ussing chambers. It is noteworthy that a direct effect of acetaldehyde on tight junctions may occur independently of mast cells. Atkinson and Rao11 have indeed observed an increased phosphorylation of ZO-1, E-cadherin, and β-catenin after 5 hours of contact between acetaldehyde and epithelial cell line monolayers.
In summary, our data suggest an original mechanism of action for alcohol-induced impairment of the gut mucosal barrier function. The deleterious effects of ethanol require the presence of the resident microflora, which is able to oxidize ethanol into acetaldehyde in situ. Acetaldehyde will thereafter stimulate mast cells, resulting in an alteration of the mucosal barrier function, which in turn may lead to a crossing of bacteria or other lumenal antigens as endotoxins toward the internal milieu.
Footnotes
Address reprint requests to Laurent Ferrier, Ph.D., Unité de Neuro-Gastroentérologie et Nutrition, Institut National de la Recherche Agronomique, 180 Chemin de Tournefeuille, B.P. 3, 31931 Toulouse Cedex 9, France. E-mail: ferrier@toulouse.inra.fr.
Supported by the Institut National de la Recherche Agronomique and the Institut de Recherche Scientifique sur les Boissons.
References
- Willis MS, Klassen LW, Tuma DJ, Sorrell MF, Thiele GM. Adduction of soluble proteins with malondialdehyde-acetaldehyde (MAA) induces antibody production and enhances T-cell proliferation. Alcohol Clin Exp Res. 2002;26:94–106. [PubMed] [Google Scholar]
- Arteel G, Marsano L, Mendez C, Bentley F, McClain CJ. Advances in alcoholic liver disease. Best Pract Res Clin Gastroenterol. 2003;17:625–647. doi: 10.1016/s1521-6918(03)00053-2. [DOI] [PubMed] [Google Scholar]
- Pascual S, Such J, Esteban A, Zapater P, Casellas JA, Aparicio JR, Girona E, Gutierrez A, Carnices F, Palazon JM, Sola-Vera J, Perez-Mateo M. Intestinal permeability is increased in patients with advanced cirrhosis. Hepatogastroenterology. 2003;50:1482–1486. [PubMed] [Google Scholar]
- Rao RK, Seth A, Sheth P. Recent advances in alcoholic liver disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol. 2004;286:G881–G884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- Wheeler MD. Endotoxin and Kupffer cell activation in alcoholic liver disease. Alcohol Res Health. 2003;27:300–306. [PMC free article] [PubMed] [Google Scholar]
- Parlesak A, Schafer C, Schutz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J Hepatol. 2000;32:742–747. doi: 10.1016/s0168-8278(00)80242-1. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, Jakate S, Fields JZ. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther. 2001;299:442–448. [PubMed] [Google Scholar]
- Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol. 1999;276:G965–G974. doi: 10.1152/ajpgi.1999.276.4.G965. [DOI] [PubMed] [Google Scholar]
- Levitt MD, Doizaki W, Levine AS. Hypothesis: metabolic activity of the colonic bacteria influences organ injury from ethanol. Hepatology. 1982;2:598–600. doi: 10.1002/hep.1840020514. [DOI] [PubMed] [Google Scholar]
- Baraona E, Julkunen R, Tannenbaum L, Lieber CS. Role of intestinal bacterial overgrowth in ethanol production and metabolism in rats. Gastroenterology. 1986;90:103–110. doi: 10.1016/0016-5085(86)90081-8. [DOI] [PubMed] [Google Scholar]
- Atkinson KJ, Rao RK. Role of protein tyrosine phosphorylation in acetaldehyde-induced disruption of epithelial tight junctions. Am J Physiol. 2001;280:G1280–G1288. doi: 10.1152/ajpgi.2001.280.6.G1280. [DOI] [PubMed] [Google Scholar]
- Santos J, Yang PC, Soderholm JD, Benjamin M, Perdue MH. Role of mast cells in chronic stress induced colonic epithelial barrier dysfunction in the rat. Gut. 2001;48:630–636. doi: 10.1136/gut.48.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci USA. 2003;100:7761–7766. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto T, Kaihovaara P, Salaspuro M. Acetaldehyde induces histamine release from purified rat peritoneal mast cells. Life Sci. 1999;64:183–190. doi: 10.1016/s0024-3205(98)00550-5. [DOI] [PubMed] [Google Scholar]
- Gukovskaya AS, Mouria M, Gukovsky I, Reyes CN, Kasho VN, Faller LD, Pandol SJ. Ethanol metabolism and transcription factor acti-vation in pancreatic acinar cells in rats. Gastroenterology. 2002;122:106–118. doi: 10.1053/gast.2002.30302. [DOI] [PubMed] [Google Scholar]
- Hashimoto N, Ohyanagi H. Effect of acute portal hypertension on gut mucosa. Hepatogastroenterology. 2002;49:1567–1570. [PubMed] [Google Scholar]
- Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther. 1999;291:1075–1085. [PubMed] [Google Scholar]
- Rosenberg DW, Mankowski DC. Induction of cyp2e-1 protein in mouse colon. Carcinogenesis. 1994;15:73–78. doi: 10.1093/carcin/15.1.73. [DOI] [PubMed] [Google Scholar]
- Jokelainen K, Siitonen A, Jousimies-Somer H, Nosova T, Heine R, Salaspuro M. In vitro alcohol dehydrogenase-mediated acetaldehyde production by aerobic bacteria representing the normal colonic flora in man. Alcohol Clin Exp Res. 1996;20:967–972. doi: 10.1111/j.1530-0277.1996.tb01932.x. [DOI] [PubMed] [Google Scholar]
- Tillonen J, Homann N, Rautio M, Jousimies-Somer H, Salaspuro M. Ciprofloxacin decreases the rate of ethanol elimination in humans. Gut. 1999;44:347–352. doi: 10.1136/gut.44.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visapaa JP, Jokelainen K, Nosova T, Salaspuro M. Inhibition of intracolonic acetaldehyde production and alcoholic fermentation in rats by ciprofloxacin. Alcohol Clin Exp Res. 1998;22:1161–1164. [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Barreau F, Ferrier L, Fioramonti J, Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004;53:501–506. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor JF, Follenfant MJ, Garland LG, Gorvin JH, Green AF, Hodson HF, Hughes DT, Tateson JE. Doxantrazole, an antiallergic agent orally effective in man. Lancet. 1975;1:1169–1170. doi: 10.1016/s0140-6736(75)93141-4. [DOI] [PubMed] [Google Scholar]
- Sadeghi-Hashjin G, Nijkamp FP, Henricks PA, Folkerts G. Sodium cromoglycate and doxantrazole are oxygen radical scavengers. Eur Respir J. 2002;20:867–872. doi: 10.1183/09031936.02.00382002. [DOI] [PubMed] [Google Scholar]