Abstract
Aspiration of gastric acid commonly injures airway epithelium and, if severe, can lead to respiratory failure from acute respiratory distress syndrome. Recently, we identified cyclooxygenase-2 (COX-2)-derived prostaglandin E2 (PGE2) and lipoxin A4 (LXA4) as pivotal mediators in vivo for resolution of acid-initiated acute lung injury. To examine protective mechanisms for these mediators in the airway, we developed an in vitro model of acid injury by transiently exposing well-differentiated normal human bronchial epithelial cells to hydrochloric acid. Transmission electron microscopy revealed selective injury to superficial epithelial cells with disruption of cell attachments and cell shedding. The morphological features of injury were substantially resolved within 6 hours. Acid triggered and early marked increases in COX-2 expression and PGE2 production, and acid-induced PGE2 significantly increased epithelial LXA4 receptor (ALX) expression. LXA4 is generated in vivo during acute lung injury, and we observed that nanomolar quantities increased basal epithelial cell proliferation and potently blocked acid-triggered interleukin-6 release and neutrophil transmigration across well-differentiated normal human bronchial epithelial cells. Expression of recombinant human ALX in A549 airway epithelial cells uncovered ALX-dependent inhibition of cytokine release by LXA4. Together, these findings indicate that injured bronchial epithelial cells up-regulate ALX in a COX-2-dependent manner to promote LXA4-mediated resolution of airway inflammation.
The airway mucosa is lined by a continuous epithelium that forms a vital protective barrier. More than a mechanical interface, epithelial cells also have pivotal regulatory roles in inflammation1 and host defense.2 Aspiration of gastric acid can directly injure the upper respiratory tract epithelium, leading to disruption of the epithelial barrier, cell shedding, and acute inflammation.3 Acid aspiration is a common cause of clinical illness, including acute lung injury and acute respiratory distress syndrome4 —diseases with excess morbidity and mortality and no available treatment. Therefore, identification of bronchial epithelium responses to acid that promote restitution and limit acute inflammation is needed for new therapeutic insights.
Acute inflammation is regulated in part by lipid mediators generated from arachidonic acid (AA).1 Proinflammatory eicosanoids—prostaglandins (PGs) and leukotrienes (LTs)—are generated by cyclooxygenases (COXs) and 5-lipoxygenase (5-LO)5 to participate in the pathophysiology of acid-induced acute lung injury.6 Resolution of inflammation in acute lung injury is characterized by clearance of neutrophils [polymorphonuclear leukocytes (PMNs)] from the lung and restoration of epithelial barrier function.7 Natural resolution of inflammation occurs via the synthesis of braking signals such as lipoxins (LXs) at sites of inflamed tissue.8 LXs are locally produced via cell-cell interactions between leukocytes and resident cells during multicellular host responses to injury, inflammation, and microbial invasion.9 LXs display diverse counterregulatory actions, including inhibition of PMN functional responses, T-cell activation, and cytokine signaling and release, plus stimulation of macrophage clearance of apoptotic PMNs. Together, these properties of LXs serve to promote resolution of acute inflammation.10 LXA4 exerts many of its bioactions through its cognate receptor ALX, which is expressed on leukocytes11 and airway epithelial cells.12
Unlike many other tissues, the lung constitutively expresses COX-2,12 and COX-2-derived mediators promote in vivo resolution in several models of thoracic inflammation, including allergic pleuritis,13 carrageenan-induced pleurisy,14 and acid-triggered acute lung injury.12 COX-2-derived PGE2 triggers eicosanoid class switching in PMN by decreasing 5-LO-catalyzed LT formation and increasing 15-LO expression and LX biosynthesis.15 In a murine model of acid-initiated acute lung injury, levels of PGE2, LXA4, and ALX expression increase to dampen lung inflammation and injury, suggesting direct roles for LX signaling in the resolution of airway epithelial injury. Here, we present evidence for regulation of injured human bronchial epithelial cell function by COX-2-dependent increases in epithelial ALX that limit proinflammatory responses to acid and promote a return to homeostasis.
Materials and Methods
Materials
LXA4 was obtained from Calbiochem (San Diego, CA). PGE2, the COX-2 selective inhibitor NS398 and anti-COX-2 polyclonal antibody were acquired from Cayman Chemical (Ann Arbor, MI), and human recombinant tumor necrosis factor (TNF)-α, rabbit IgG, and fluorescein isothiocyanate-conjugated anti-rabbit IgG antibodies were from BD Pharmingen (San Diego, CA). Anti-ALX (also named FPRL-1) polyclonal antibody was from Origene (Rockville, MD). Horseradish peroxidase-conjugated anti-rabbit IgG was purchased from Amersham Pharmacia Biotech (Piscataway, NJ). The polyclonal antibody against β-actin was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Methods
Airway Epithelial Cell Culture
Primary normal human bronchial epithelial (NHBE) cells (Clonetics-BioWhittaker, San Diego, CA) were cultured in an air/liquid interface system.12 In brief, cells were expanded on tissue culture-treated plastic in bronchial epithelial growth medium (Clonetics-BioWhittaker) supplemented with bovine serum albumin (1.5 μg/ml) and retinoic acid (50 nmol/L) and plated on uncoated nucleopore membranes (24-mm diameter, 0.4-μm pore size, Transwell Clear; Costar, Cambridge, MA) in a 1:1 mixture of bronchial epithelial growth medium and Dulbecco’s modified Eagle’s medium (Invitrogen Corp., Carlsbad, CA) applied at the basal side only to establish an air/liquid interface. Cells were maintained in culture for 21 days to obtain a differentiated cell population with a mucociliary phenotype. In some experiments, NHBE cells were exposed (37°C, 5% CO2) to LXA4 (100 nmol/L, 15 minutes), PGE2 (0.1 or 10 nmol/L, 15 minutes), or NS398 (10 μmol/L, 1 hour) before acid injury. After pH neutralization, fresh medium was added to the bottom chamber.
Human type II alveolar epithelial A549 cells were grown in RPMI 1640 medium (Invitrogen Corp.) with 10% heat-inactivated fetal bovine serum (Sigma Aldrich Corp., St. Louis, MO), penicillin (100 U/ml), and streptomycin (100 μg/ml) (Invitrogen Corp.). Full-length recombinant human ALX (rhALX) cDNA was cloned into HindIII/XbaI sites of pcDNA3 vector. A549 cells (6 × 105 cells in 60-mm dishes) were transfected with 5 mg of either pcDNA3 or pcDNA3-hALX using Superfect transfection reagent according to the manufacturer’s instructions (Qiagen, Chatsworth, CA). Neomycin-resistant clones were selected (750 mg/ml) and expanded. In some experiments transfected A549 cells were exposed (15 minutes, 37°C, 5% CO2) to LXA4 (0.01 to 10 nmol/L) or vehicle before HCl (0.1 N, pH 2.0) or TNF-α (1 ng/ml). After 6 hours, supernatants were removed and assayed for cytokine release.
Experimental Model of Airway Epithelial Acid Injury
Hydrochloric acid (HCl, 0.1 N, pH 1.5, 500 μl/well) was gently applied drop-by-drop onto the apical side of NHBE cell cultures and incubated for 5 minutes at 37°C. Then, acid was removed and pH neutralized with phosphate-buffered saline (PBS) (until pH ≥ 7). Fresh medium was added to the bottom of the wells and NHBE cells were returned to 37°C, 5% CO2. The pH of the acid applied to nondifferentiated epithelial cells (ie, A549 cells) was 2.0 rather than 1.5 because these cells were more sensitive to acid injury than the well-differentiated NHBE cells.
Transmission Electron Microscopy
NHBE cells on inserts were fixed with 2.5% glutaraldehyde (Electron Microscopy Sciences, Fort Washington, PA) in 0.1 mol/L sodium cacodylate buffer for 1 hour at room temperature and kept at 4°C until processing. Cells were then rinsed in cacodylate buffer and postfixed in 1.25% osmium tetroxide (Electron Microscopy Sciences) for 1 hour at room temperature. After rinsing in 15% ethanol, cells were stained in 4% aqueous uranyl acetate (Electron Microscopy Sciences) for 1 hour, dehydrated through graded ethanols, and embedded in Polybed 812 (PolySciences, Inc., Warrington, PA). Tissue was thin sectioned on a Reichert-Jung Ultra Cut (Leica, Inc., Deerfield, IL), poststained in uranyl acetate and lead citrate, viewed on a Zeiss 902 electron microscope (Carl Zeiss SMT, Thornwood, NY), and recorded with Kodak E.M. film (Eastman Kodak Co., Rochester, NY).
Mediator Measurements
Mediators were determined in cell supernatants by sensitive and specific enzyme-linked immunosorbent assays for PGE2 (Cayman Chemical), interleukin (IL)-6, and IL-8 (Diaclone, Stamford, CT).
Determination of Gene Expression
Total RNA was purified from cell lysates (RNeasy; Qiagen, Valencia, CA), and cDNA were synthesized (Ready-to-Go RT-PCR beads; Amersham, Piscataway, NJ). Semiquantitative human ALX gene expression was determined using specific primers for human ALX (sense primer, 5′-TT GCT CTA GTC CTT ACC TTG C-3′, and anti-sense primer, 5′-GC AAG TAC AAA ATC ATT GAC ATC-3′) and β-actin (internal control, sense primer, 5′-GCTGGAGCATGCCCGTATT-3′, and anti-sense primer, 5′-ACC CTG CTG TGC TGA GTGTC-3′). After electrophoresis, densitometry was performed using Scion Image software.
Quantitative polymerase chain reaction reactions were performed using SYBR Green Master Mix (Applied Biosystems, Foster City, CA) in an ABI Prism 5700 (Applied Biosystems). Fold changes were calculated relative to control treatment using the deltadelta Ct method.16 β-Actin expression was used as reference standard. The respective forward and reverse primers for COX-2 were 5′-CCT TGA CCA TGA TGG CCA G-3′ and 5′-TGG AGG GCA GTG CTG TTT G-3′.
For protein determination, whole cells were lysed in 50 mmol/L Tris-HCl (pH 7.4), 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 0.5% Nonidet P-40, 0.25% deoxycholic acid sodium salt, 1 mmol/L phenyl-methyl sulfonyl fluoride, and protease inhibitors (Complete cocktail tablets; Roche Applied Science, Indianapolis, IN). Total protein (50 μg) was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 8% Tris-HCl gels and then transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The membranes were blocked [4% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20 (TBST), 1 hour, room temperature] and probed with rabbit polyclonal anti-human COX-2 antibody (1:1000 dilution) overnight at 4°C. After serial washes with TBST, membranes were incubated with horseradish peroxidase-conjugated donkey anti-rabbit IgG (1:20,000 dilution). The polyclonal antibody against β-actin (1:500 dilution) was used as internal control. Visualization was performed by chemiluminescence (Pierce Biotechnology, Inc., Rockford, IL) followed by autoradiography.
Cell Proliferation
Cell proliferation was determined using reduction of tetrazolium dye (MTT; Chemicon Int., Temecula, CA) to blue formazan to measure living cells. After 24 hours in epidermal growth factor (EGF)-free medium, NHBE cells (∼3 × 103 cells/well) were seeded on 96-well plates and cultured in bronchial epithelial growth medium for 24 hours (37°C, 5% CO2) in the presence of LXA4 (10, 100, and 1000 nmol/L), PGE2 (200 nmol/L), EGF (0.5 ng/ml), or vehicle (medium without EGF). MTT solution (10 μl) was then added to each well and incubated for 4 hours (37°C). Dye was extracted from cells in 100 μl of isopropanol: 1 N HCl (96:4, v:v), and absorbance at 570 nm was determined. A linear relationship was determined for MTT reduction (absorbance at 570 nm) and NHBE cell number (y = 4 × 10−5(x) + 0.0532, r2 = 0.97).
PMN Transmigration Across Well-Differentiated NHBE Cells
NHBE cells were grown on inverted polycarbonate filters with a surface area of 0.33 cm2 (Costar inserts; Costar Corp.) to study basal to apical transmigration.17 The cells were cultured for 14 days at an air/liquid interface. Human PMNs were isolated from healthy patients who denied taking any medications for at least 2 weeks and had given written informed consent to a protocol approved by Brigham and Women’s Hospital’s Human Research Committee. PMNs were isolated from whole blood as previously described15 and resuspended in modified HBSS (lacking Ca2+ and Mg2+) at 25 × 106 cells/ml. PMNs (1 × 106/well) were exposed to LXA4 (0.1, 1, 10, 100, or 1000 nmol/L) or vehicle for 15 minutes at 37°C before addition to the upper chambers. The chemoattractant LTB4 (1 μmol/L in HBSS containing Ca2+ and Mg2+) was added to the lower chambers, and transmigration was allowed to proceed for 60 minutes (37°C, 5%CO2). PMN number was determined by measurement of MPO activity.17
Analysis of ALX Surface Expression by Flow Cytometric Analysis
A549 cells (500,000 cells) were incubated (4°C, 30 minutes) in fluorescence-activated cell sorting buffer (DPBS + 5% fetal calf serum + 0.01% sodium azide) with 1 mg of rabbit anti-human ALX or with class-matched irrelevant rabbit IgG1. Each sample was washed, resuspended in fluorescence-activated cell sorting buffer containing a fluorescein isothiocyanate-conjugated goat anti-rabbit IgG antibody (1:100 final dilution), and incubated for 30 minutes (4°C). After washing, samples were analyzed by cytofluorometer (FACScan; Becton Dickinson, Mountain View, CA) with Cellquest software. Data were collected on 10,000 cells per sample.
Statistical Analysis
Values represent the mean ± SEM. Comparisons among groups were performed by Student’s t-test. For all analyses, findings were considered significant when P < 0.05.
Results
Ultrastructural Changes in Differentiated NHBE Cells after Acid Exposure
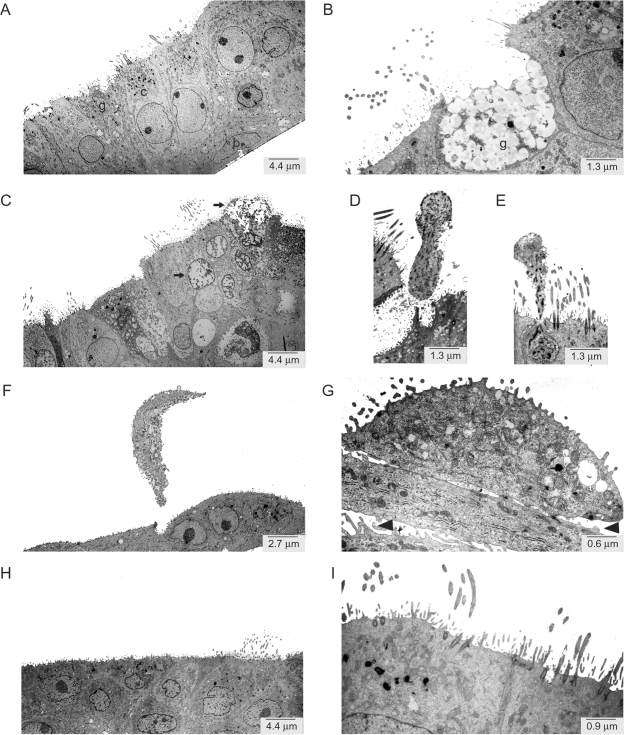
To determine the effect of acid injury on epithelial cells, we examined NHBE cell morphology by transmission electron microscopy. Before acid injury, the NHBE cells displayed typical features of a well-differentiated bronchial epithelium with basal and ciliated cells (Figure 1A) as well as goblet cells that secreted an apical coating of mucus (Figure 1B). Within 5 minutes, acid damaged select superficial cells with nuclear and cytoplasmic changes of necrosis (Figure 1C). Dying cells were released from the apical surface of the epithelium (Figure 1, D and E). Two hours after acid injury, the entire superficial layer of ciliated and goblet cells was apically shed (Figure 1F) with disruption of cellular attachments (Figure 1G). The acid injury did not appear to alter basal cell layer morphology. Within 6 hours the superficial epithelial layer was primarily restored (Figure 1H), albeit goblet cell numbers were still decreased compared to cells cultured in the absence of acid and regeneration of cilia was incomplete (Figure 1I).
Figure 1.
Transmission electron micrographs of well-differentiated NHBE cells exposed to acid. A and B: NHBE cells were differentiated in air-liquid interface culture for 21 days. The epithelium comprised ciliated (c), goblet (g), and basal (b) cells. C, D, and E: NHBE cells were transiently exposed to 0.1 N HCl (pH 1.5, 5 minutes) and incubated in fresh medium at neutral pH for 2 hours (F, G) and 6 hours (H, I). Arrows indicate examples of cell injury (C) and arrowheads indicate loss of cell attachments after acid injury (G). Magnifications are indicated.
Transient Acid Exposure Stimulated COX-2 Expression and Activity
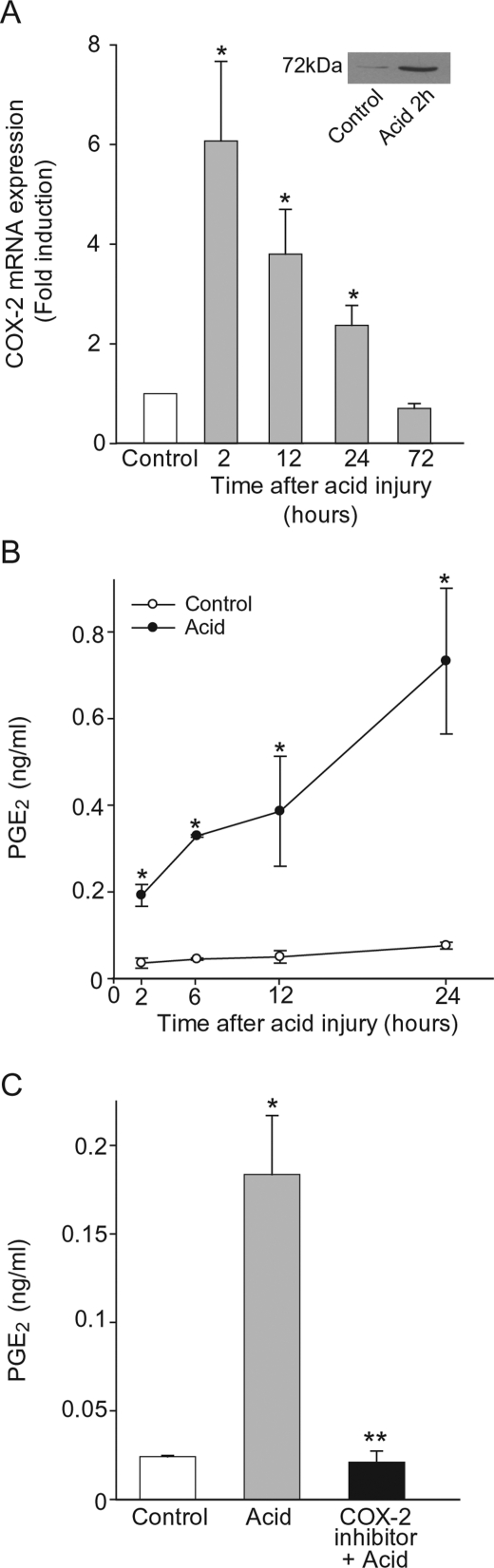
Because COX-2 is pivotal to resolution in vivo of acid-initiated acute lung injury,12 we investigated the time-dependent expression and activity of COX-2 in well-differentiated NHBE cells after in vitro acid injury. COX-2 mRNA and protein were significantly increased, with maximal levels occurring 2 hours after acid injury (6.1 ± 1.6-fold increase) and baseline levels returning by 72 hours. COX-2 protein expression was also increased 2 hours after acid injury (Figure 2A, inset). In addition, PGE2 generation was significantly increased within 2 hours (0.2 ± 0.025 ng/ml) and continued to increase during the first 24 hours (0.73 ± 0.17 ng/ml at 24 hours) (Figure 2B). Epithelial PGE2 was COX-2-derived as a COX-2 selective inhibitor completely blocked acid-induced PGE2 release (Figure 2C).
Figure 2.
Injury increases NHBE cell COX-2 expression and activity. A: Time course for COX-2 expression in well-differentiated NHBE cells after transient acid exposure (see Methods). Results show fold induction of COX-2 mRNA by quantitative polymerase chain reaction compared to cells without acid for each time point. COX-2 protein expression was also analyzed 2 hours after acid injury by immunoblot (inset). B: Time-dependent PGE2 production after acid injury. C: NHBE cells were exposed for 1 hour to a COX-2 selective inhibitor or vehicle, before acid injury (see Methods) and incubated with fresh medium for an additional 2 hours. Results are the mean ± SEM for n = 3, *P < 0.05 (acid compared to control) and **P < 0.05 (COX-2 inhibitor compared to vehicle).
Acid Injury Up-Regulates NHBE Cell ALX Expression
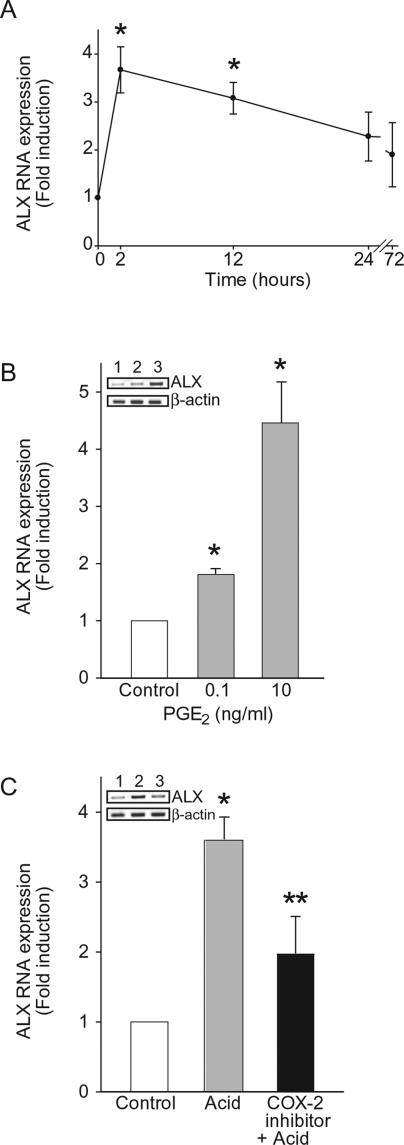
Because a component of the in vivo protective effects of COX-2 during acid-mediated acute lung injury occurred via enhanced LX signaling,12 we next determined NHBE cell ALX expression in vitro after acid injury. ALX mRNA expression significantly increased 2 hours after acid injury (3.7 ± 0.5-fold) and, similar to COX-2, returned to baseline within 72 hours (Figure 3A). To determine whether PGE2 directly modulates ALX expression, NHBE cells were cultured in the presence of PGE2 in amounts similar to those present in NHBE cell supernatants early (within 2 hours) after acid injury (Figure 2B) and those present during in vivo experimental acute lung injury.12 PGE2 significantly increased ALX mRNA 1.8 ± 0.1- and 4.5 ± 0.7-fold with 0.1 ng/ml and 10 ng/ml PGE2, respectively. To determine whether COX-2-derived PGE2 from NHBE cells can directly increase ALX expression, we exposed NHBE cells to acid in the presence of a selective COX-2 inhibitor (Figure 3C). Acid-induced increases in ALX expression were significantly decreased with the COX-2 inhibitor (69.2 ± 13.4% inhibition). Together, these results indicate that acid triggers NHBE cell expression of ALX in part via COX-2-dependent generation of PGE2.
Figure 3.
Effect of acid and PGE2 exposure on ALX expression in NHBEs. A: Time course of ALX mRNA expression in well-differentiated NHBEs after transient exposure to acid. Results show fold induction of ALX mRNA expression compared to cells without acid for each time point. B: NHBEs were incubated with PGE2 or vehicle for 2 hours, and ALX mRNA expression was determined by semiquantitative polymerase chain reaction (see Methods). Inset is a representative gel of three independent experiments; 1, control; 2, PGE2 0.1 ng/ml; 3, PGE2 10 ng/ml. C: NHBEs were incubated for 1 hour with a selective COX-2 inhibitor or vehicle and then transiently exposed to acid and incubated with fresh medium for 2 hours. Inset is a representative gel of three independent experiments; 1, control; 2, acid; 3, COX-2 inhibitor + acid. *P < 0.05 (compared to control) and **P < 0.05 (COX-2 inhibitor compared to vehicle) (n = 3).
LXA4 Promotes Basal NHBE Cell Proliferation and Inhibits Inflammatory Responses
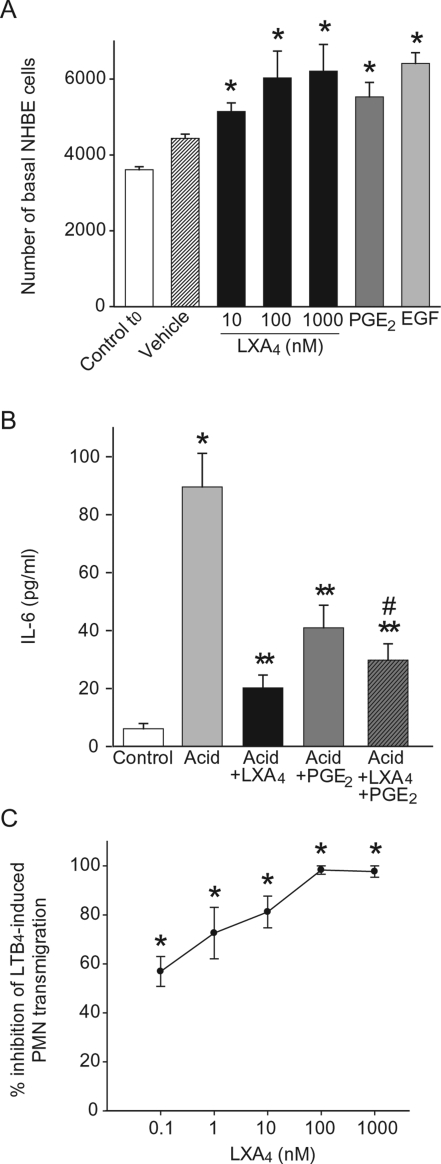
LXA4 is an ALX ligand that is generated in vivo during acute lung injury,12 so we next examined its impact on NHBE cell functional responses to injury. Because cell proliferation is an early event in restoring airway epithelial integrity, we first assessed the impact of LXA4 on basal NHBE cell proliferation. LXA4 induced a concentration-dependent increase in basal cell number (Figure 4A) that was similar in potency to PGE2 (200 nmol/L) and EGF (0.5 ng/ml), known proliferative agonists for bronchial epithelial cells.18 No significant changes in basal NHBE cell shape or size were observed with LXA4. Because IL-6 is a pleiotropic cytokine produced by epithelial cells during acute inflammation, acute lung injury, and acute respiratory distress syndrome,19,20 we next determined the effect of LXA4 on NHBE cell IL-6 release (Figure 4B). Levels of IL-6 were markedly increased in NHBE cell supernatants after acid injury (89.6 ± 11.6 pg/ml compared to untreated cells 6.1 ± 1.9 pg/ml). LXA4 (100 nmol/L) and PGE2 (200 nmol/L) dramatically inhibited IL-6 release from acid-injured NHBE cells (81.5 ± 6.0% inhibition and 59.8 ± 6.1% inhibition, respectively). Incubation of acid-injured NHBE cells with both LXA4 and PGE2 also markedly inhibited IL-6 release (72.3. ± 5.6%), and such inhibition was significantly greater than that seen with PGE2 alone (n ≥ 3, P < 0.05). No significant differences in IL-6 inhibition were observed for the combination of LXA4 and PGE2 compared to LXA4 alone (Figure 4B). LXA4 inhibits PMN transmigration across epithelial monolayers21 and PMN accumulation in acid-injured lung in vivo,12 so we next determined its effect on PMN trans-epithelial migration across well-differentiated NHBE cells. Pathophysiological roles have been assigned to the PMN agonist LTB4 in acute lung injury,6,22 so we used LTB4 as a PMN chemoattractant for transmigration. LXA4 gave potent and concentration-dependent inhibition of PMN transmigration (Figure 4C). At 0.1 nmol/L, LXA4 displayed 56.9 ± 6.1% inhibition, and maximum inhibition (98.3 ± 2.9%) was achieved with 100 nmol/L LXA4. Together, these results indicate that LXA4 mediates counterregulatory actions on human bronchial epithelial cells by promoting basal cell proliferation and inhibiting proinflam-matory events, such as cytokine release and PMN transmigration.
Figure 4.
Impact of LXA4 on epithelial cell proliferation and inflammatory responses. A: LXA4, PGE2 (200 nmol/L), EGF (0.5 ng/ml), or vehicle were added to basal NHBE cells and cell number was determined after 24 hours (see Methods). Values are the mean ± SEM, n ≥ 3, d = 3. B: Cells were exposed (15 minutes) to LXA4 (100 nmol/L), PGE2 (200 nmol/L), or vehicle before acid injury, and then incubated in fresh medium for 6 hours (see Methods). IL-6 levels were measured in cell-free supernatants by enzyme-linked immunosorbent assay. Results are expressed as mean ± SEM (n = 3). C: PMNs were exposed (15 minutes, 37°C) to LXA4, and transmigration toward the chemoattractant LTB4 (1 μmol/L) was determined by myeloperoxidase activity (see Methods). Results are expressed as percent inhibition of LTB4-induced PMN transmigration and represent mean ± SEM for n = 3. *P < 0.05 (compared to control), **P < 0.05 (compared to acid), and #P < 0.05 (compared to acid + PGE2).
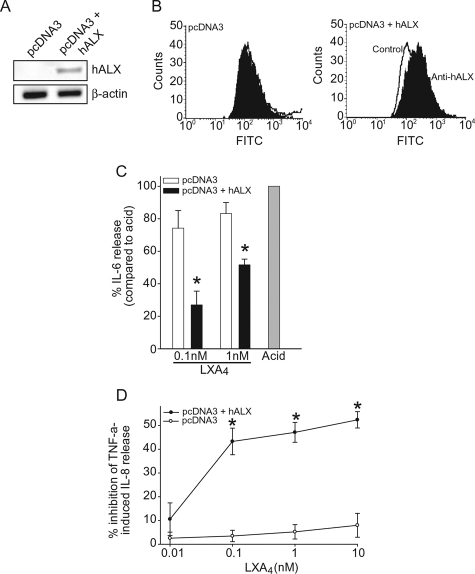
LXA4 Mediates Epithelial Cell Actions via ALX
To investigate whether LXA4’s anti-inflammatory effects on airway epithelial cells are mediated by its cognate G protein-coupled receptor ALX, we used A549 epithelial cells, which do not express ALX,23,24 and generated stable expression of rhALX by transfection (Figure 5). ALX expression was verified in the A549 stable transfectants by reverse transcriptase-polymerase chain reaction (Figure 5A) and flow cytometric analysis, which indicated that rhALX protein was expressed on cell surfaces (Figure 5B). A549 cells with and without ALX expression were exposed to acid in the presence or absence of LXA4. IL-6 release was significantly reduced by LXA4 (0.1 and 1 nmol/L) in cells expressing rhALX, but these concentrations of LXA4 had little effect on acid-injured A549 cells without ALX expression (Figure 5C). In addition to IL-6, TNF-α and IL-8 have also been associated with PMN lung recruitment and activation in acute lung injury,19,20 so we determined the impact of ALX expression on TNF-α-induced IL-8 secretion. LXA4 yielded concentration-dependent inhibition of IL-8 release in TNF-α-activated A549 cells expressing rhALX in sharp contrast to cells not expressing the receptor (Figure 5D).
Figure 5.
ALX signals LXA4-mediated anti-inflammatory actions for airway epithelial cells. A: Expression of ALX mRNA in A549 cells transfected with rhALX cDNA. B: Determination by flow cytometry of rhALX surface expression on A549 cells transfected with pcDNA3 vector alone (left) or vector containing rhALX cDNA (right). hALX on cell surfaces is expressed as a logarithmic increase in mean index of fluorescent intensity when A549 cells were incubated with either anti-hALX (black) or control antibody (white). The x axis represents log increase in fluorescence (fluorescein isothiocyanate), and the y axis represents relative cell number (counts). C: A549 cells transfected with pcDNA3 vector alone or rhALX cDNA were exposed to LXA4 before acid injury and incubated in fresh medium for 6 hours. IL-6 levels in cell-free supernatants were measured by enzyme-linked immunosorbent assay. Results are expressed as percentage of IL-6 release compared to acid injury alone (100%) and represent mean ± SEM for n ≥ 3. *P < 0.05 when compared with A549 cells exposed to acid. D: A549 transfected with pcDNA3 vector alone (○) or with vector containing rhALX cDNA (•) were exposed to LXA4 before TNF-α (1 ng/ml) and incubated for 6 hours. IL-8 release was determined by enzyme-linked immunosorbent assay. Results are expressed as percent inhibition of TNF-α-induced IL-8 release and represent mean ± SEM for n = 3. *P < 0.05 compared to vehicle.
Discussion
LXs are produced locally in the lung to regulate inflammatory cells and promote resolution of acute inflammation. The present results provide the first evidence for potential anti-inflammatory and proresolving roles for LXs on human bronchial epithelial cells. In a new experimental model of acid aspiration injury, well-differentiated NHBE cells engage lipid mediator signaling pathways to regulate cell responses and restore mucosal integrity, most notably by COX-2-dependent PGE2 formation and ALX expression. In nanomolar quantities, LXA4 stimulates basal NHBE cell proliferation and inhibits cytokine release and PMN transmigration via interactions with ALX. Together, these actions would serve to facilitate the resolution of airway injury.
Acid aspiration into the proximal airways leads to necrosis and sloughing of the superficial airway epithelium.3 Within the desquamated and necrotic epithelium, inflammatory cells accumulate and release potentially toxic agents, such as reactive oxygen species and digestive enzymes, into surrounding tissues.3 Driven by both peptide and lipid mediators, this inflammatory response can inadvertently amplify inflammation and lead to tissue injury.25 After acid injury, epithelial cellular debris is cleared via the airways, and basal cells proliferate to regenerate the airway mucosal barrier. Here, transient exposure to acid in vitro initiated epithelial morphological changes similar to in vivo aspiration events3 with necrosis of superficial cells, disruption of cellular attachments, and cell shedding from the apical surface of the culture. Notably, transmission electron microscopy revealed that basal epithelial cells were protected from injury and the epithelium was regenerated within hours. Epithelial cells that did not generate mucus in culture (eg, A549) were more sensitive to acid, suggesting that the mucus coating on apical surfaces of differentiated NHBE cells provided a protective barrier from injury. Thus, our in vitro model for acid injury of bronchial epithelia recapitulated many of the in vivo cellular events ascribed to aspiration of gastric acid.
In response to acid injury, epithelial cells rapidly increased COX-2 expression and PGE2 levels. COX-2-derived mediators display counterregulatory actions in the lung because COX-2−/− mice have increased inflammatory responses.26 PGE2 induces airway epithelial cell wound closure18 and can switch PMN phenotype from proinflammatory effector to generator of anti-inflammatory signals.12,15 During experimental acid-induced acute lung injury, decreasing COX-2 activity by pharmacological inhibition or gene disruption markedly increases PMN infiltration and delays resolution.12 Similarly, gastric epithelial cells display a rapid induction of COX-2 when exposed to acid after aspirin or indomethacin administration,27 and COX-2-derived mediators can protect the stomach from acid injury,28,29 in part via LX generation.30 Here, we have uncovered that COX-2-derived PGE2, in addition to increasing LX biosynthetic circuits,15 also directly induced ALX expression in bronchial epithelial cells. Therefore, in response to acid injury, PGE2 can augment LX signaling by up-regulating both ligand (LXA4) and receptor (ALX).
In addition to their actions on leukocytes, LXs also regulate epithelial cell responses. LXs inhibit chemokine and cytokine secretion from cultured intestinal epithelial cells,31–33 enhance mucosal anti-microbial protection by stimulating bactericidal/permeability-increasing protein (BPI) expression in oral and intestinal epithelial cells,2 and accelerate epithelial wound closure in murine cornea.34 Here, we provide the first evidence for direct anti-inflammatory and proresolving effects of LXA4 on human airway epithelial cells. Similar to its stimulation of re-epithelialization of injured cornea,34 LXA4 increased basal airway epithelial proliferation. These properties for LXA4 on nondifferentiated epithelial cells are distinct from its capacity to block malignant epithelial cell proliferation35 and the ability of renal mesangial cells and fibroblasts to respond to mitogens.36,37 In addition, LXA4 regulated airway epithelial release of IL-6 and IL-8, two potent proinflammatory cytokines that have recently been linked to mortality and prolonged mechanical ventilation in acute lung injury/acute respiratory distress syndrome.20 Similar to its inhibitory properties for PMN transmigration across intestinal epithelial monolayers,21 LXA4 was also a potent inhibitor of basal to apical PMN transmigration across well-differentiated bronchial epithelial cells. Epithelial transmigration for PMN is a multistep process with CD11b/CD18 playing a pivotal role.17 PMN CD11b/CD18 and l-selectin expression is regulated by LXA438 and is likely central to LXA4’s inhibitory actions in our system. Thus, LXA4 directly regulates several functional responses by injured human bronchial epithelial cells, including basal cell proliferation, cytokine release, and PMN transmigration that together promote resolution of injury and acute inflammation.
ALX is LXA4’s cognate receptor that mediates its leukocyte selective actions. LXA4 can also compete with LTD4 for binding to CysLT1 receptors, serving as a CysLT1 receptor level antagonist,39 but this receptor was not present on the differentiated NHBE cells (data not shown). Epithelial ALX was induced by acid injury, providing a mechanism for LXA4’s epithelial cell actions. Unlike NHBE cells, A549 cells do not express ALX, thus providing a useful model system to investigate rhALX-dependent epithelial cell responses. A549 cells expressing rhALX matched LXA4’s protective actions for acid-triggered cytokine release by NHBE cells. Several potential targets have been described for LXA4’s regulation of cytokine production including inhibition of nuclear factor-κB-mediated transcriptional activation of proinflammatory genes32 and increased expression of suppressors of cytokine signaling (SOCS)-1 and -2.40 In addition to its leukocyte selective actions, LXA4 signaling via ALX also displays diverse regulatory properties in the aerodigestive tract epithelium.
In conclusion, our findings have uncovered a role for lipid mediators, such as PGE2 and LXA4, in promoting resolution of acid-initiated human bronchial epithelium injury and inflammatory responses. In particular, LXA4 is a potent anti-inflammatory agonist in airway epithelium via interactions with ALX. Augmenting LX signaling circuits would facilitate restitution of airway epithelial homeostasis in the resolution of acute lung injury/acute respiratory distress syndrome from acid aspiration and represents a potential new therapeutic strategy in these common yet devastating clinical conditions.
Acknowledgments
We thank Dr. Makoto Arita for assistance with ALX cDNA transfection, Dr. Dan Tschumperlin for advice in the culture of differentiated NHBE cells, and the Department of Cell Biology and Physiology at the Washington University School of Medicine for the generous use of their electron microscope.
Footnotes
Address reprint requests to Bruce D. Levy, Pulmonary and Critical Care Medicine, Brigham and Women’s Hospital, 75 Francis St., Boston, MA 02115. E-mail: blevy@partners.org.
Supported in part by the National Institutes of Health (grants HL68669, DE016191, AI0608084), La Fondation de la Recherche Medicale, Pfizer, and Uehara Memorial Research Foundation.
References
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Canny G, Levy O, Furuta GT, Narravula-Alipati S, Sisson RB, Serhan CN, Colgan SP. Lipid mediator-induced expression of bactericidal/ permeability-increasing protein (BPI) in human mucosal epithelia. Proc Natl Acad Sci USA. 2002;99:3902–3907. doi: 10.1073/pnas.052533799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne JW, Ramphal R, Hood CI. Tracheal mucosal damage after aspiration. A scanning electron microscope study. Am Rev Respir Dis. 1981;124:728–732. doi: 10.1164/arrd.1981.124.6.728. [DOI] [PubMed] [Google Scholar]
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- Nagase T, Uozumi N, Ishii S, Kume K, Izumi T, Ouchi Y, Shimizu T. Acute lung injury by sepsis and acid aspiration: a key role for cytosolic phospholipase A2. Nat Immunol. 2000;1:42–46. doi: 10.1038/76897. [DOI] [PubMed] [Google Scholar]
- Matthay MA, Zimmerman GA. Acute lung injury and the acute respiratory distress syndrome: four decades of inquiry into pathogenesis and rational management. Am J Respir Cell Mol Biol. 2005;33:319–327. doi: 10.1165/rcmb.F305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy DW, Lawrence T, Perretti M, Rossi AG. Inflammatory resolution: new opportunities for drug discovery. Nat Rev Drug Discov. 2004;3:401–416. doi: 10.1038/nrd1383. [DOI] [PubMed] [Google Scholar]
- Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005;73:141–162. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- McMahon B, Godson C. Lipoxins: endogenous regulators of inflammation. Am J Physiol. 2004;286:F189–F201. doi: 10.1152/ajprenal.00224.2003. [DOI] [PubMed] [Google Scholar]
- Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol. 2005;174:5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- Bandeira-Melo C, Serra MF, Diaz BL, Cordeiro RS, Silva PM, Lenzi HL, Bakhle YS, Serhan CN, Martins MA. Cyclooxygenase-2-derived prostaglandin E2 and lipoxin A4 accelerate resolution of allergic edema in Angiostrongylus costaricensis-infected rats: relationship with concurrent eosinophilia. J Immunol. 2000;164:1029–1036. doi: 10.4049/jimmunol.164.2.1029. [DOI] [PubMed] [Google Scholar]
- Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nat Med. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Parkos CA, Delp C, Arnaout MA, Madara JL. Neutrophil migration across a cultured intestinal epithelium. Dependence on a CD11b/CD18-mediated event and enhanced efficiency in physiological direction. J Clin Invest. 1991;88:1605–1612. doi: 10.1172/JCI115473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savla U, Appel HJ, Sporn PH, Waters CM. Prostaglandin E(2) regulates wound closure in airway epithelium. Am J Physiol. 2001;280:L421–L431. doi: 10.1152/ajplung.2001.280.3.L421. [DOI] [PubMed] [Google Scholar]
- Cromwell O, Hamid Q, Corrigan CJ, Barkans J, Meng Q, Collins PD, Kay AB. Expression and generation of interleukin-8, IL-6 and granulocyte-macrophage colony-stimulating factor by bronchial epithelial cells and enhancement by IL-1 beta and tumour necrosis factor-alpha. Immunology. 1992;77:330–337. [PMC free article] [PubMed] [Google Scholar]
- Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, Wheeler AP. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005;33:1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230–232. [DOI] [PubMed] [Google Scholar]
- Colgan SP, Serhan CN, Parkos CA, Delp-Archer C, Madara JL. Lipoxin A4 modulates transmigration of human neutrophils across intestinal epithelial monolayers. J Clin Invest. 1993;92:75–82. doi: 10.1172/JCI116601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman G, Welbourn R, Kobzik L, Valeri CR, Shepro D, Hechtman HB. Synergism between leukotriene B4 and thromboxane A2 in mediating acid-aspiration injury. Surgery. 1992;111:55–61. [PubMed] [Google Scholar]
- Gronert K, Gewirtz A, Madara JL, Serhan CN. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)-13 and interferon gamma and inhibits tumor necrosis factor alpha-induced IL-8 release. J Exp Med. 1998;187:1285–1294. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnans C, Mainprice B, Chanez P, Bousquet J, Urbach V. Lipoxin A4 stimulates a cytosolic Ca2+ increase in human bronchial epithelium. J Biol Chem. 2003;278:10879–10884. doi: 10.1074/jbc.M210294200. [DOI] [PubMed] [Google Scholar]
- Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Bonner JC, Rice AB, Ingram JL, Moomaw CR, Nyska A, Bradbury A, Sessoms AR, Chulada PC, Morgan DL, Zeldin DC, Langenbach R. Susceptibility of cyclooxygenase-2-deficient mice to pulmonary fibrogenesis. Am J Pathol. 2002;161:459–470. doi: 10.1016/S0002-9440(10)64202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NM, Sharkey KA, Asfaha S, Macnaughton WK, Wallace JL. Aspirin causes rapid up-regulation of cyclo-oxygenase-2 expression in the stomach of rats. Aliment Pharmacol Ther. 1997;11:1101–1108. doi: 10.1046/j.1365-2036.1997.00247.x. [DOI] [PubMed] [Google Scholar]
- Maricic N, Ehrlich K, Gretzer B, Schuligoi R, Respondek M, Peskar BM. Selective cyclo-oxygenase-2 inhibitors aggravate ischaemia-reperfusion injury in the rat stomach. Br J Pharmacol. 1999;128:1659–1666. doi: 10.1038/sj.bjp.0702966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretzer B, Maricic N, Respondek M, Schuligoi R, Peskar BM. Effects of specific inhibition of cyclo-oxygenase-1 and cyclo-oxygenase-2 in the rat stomach with normal mucosa and after acid challenge. Br J Pharmacol. 2001;132:1565–1573. doi: 10.1038/sj.bjp.0703955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza MH, de Lima OM, Jr, Zamuner SR, Fiorucci S, Wallace JL. Gastritis increases resistance to aspirin-induced mucosal injury via COX-2-mediated lipoxin synthesis. Am J Physiol. 2003;285:G54–G61. doi: 10.1152/ajpgi.00525.2002. [DOI] [PubMed] [Google Scholar]
- Gewirtz AT, McCormick B, Neish AS, Petasis NA, Gronert K, Serhan CN, Madara JL. Pathogen-induced chemokine secretion from model intestinal epithelium is inhibited by lipoxin A4 analogs. J Clin Invest. 1998;101:1860–1869. doi: 10.1172/JCI1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz AT, Collier-Hyams LS, Young AN, Kucharzik T, Guilford WJ, Parkinson JF, Williams IR, Neish AS, Madara JL. Lipoxin A4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. 2002;168:5260–5267. doi: 10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- Kucharzik T, Gewirtz AT, Merlin D, Madara JL, Williams IR. Lateral membrane LXA4 receptors mediate LXA4’s anti-inflammatory actions on intestinal epithelium. Am J Physiol. 2003;284:C888–C896. doi: 10.1152/ajpcell.00507.2001. [DOI] [PubMed] [Google Scholar]
- Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, Laniado Schwartzman M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- Claria J, Lee MH, Serhan CN. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Mol Med. 1996;2:583–596. [PMC free article] [PubMed] [Google Scholar]
- Wu SH, Wu XH, Lu C, Dong L, Chen ZQ. Lipoxin A4 inhibits proliferation of human lung fibroblasts induced by CTGF. Am J Respir Cell Mol Biol. 2006;34:65–72. doi: 10.1165/rcmb.2005-0184OC. [DOI] [PubMed] [Google Scholar]
- Rodgers K, McMahon B, Mitchell D, Sadlier D, Godson C. Lipoxin A4 modifies platelet-derived growth factor-induced profibrotic gene expression in human renal mesangial cells. Am J Pathol. 2005;167:683–694. doi: 10.1016/S0002-9440(10)62043-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore S, Serhan CN. Lipoxin A4 receptor activation is distinct from that of the formyl peptide receptor in myeloid cells: inhibition of CD11/18 expression by lipoxin A4-lipoxin A4 receptor interaction. Biochemistry. 1995;34:16678–16686. doi: 10.1021/bi00051a016. [DOI] [PubMed] [Google Scholar]
- Gronert K, Martinsson-Niskanen T, Ravasi S, Chiang N, Serhan CN. Selectivity of recombinant human leukotriene D(4), leukotriene B(4), and lipoxin A(4) receptors with aspirin-triggered 15-epi-LXA(4) and regulation of vascular and inflammatory responses. Am J Pathol. 2001;158:3–9. doi: 10.1016/S0002-9440(10)63937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieran NE, Doran PP, Connolly SB, Greenan MC, Higgins DF, Leonard M, Godson C, Taylor CT, Henger A, Kretzler M, Burne MJ, Rabb H, Brady HR. Modification of the transcriptomic response to renal ischemia/reperfusion injury by lipoxin analog. Kidney Int. 2003;64:480–492. doi: 10.1046/j.1523-1755.2003.00106.x. [DOI] [PubMed] [Google Scholar]