Abstract
Neuroinflammatory disorders (including human immunodeficiency virus-1 encephalitis, HIVE) are associated with oxidative stress and inflammatory brain injury, and excessive alcohol use can exacerbate tissue damage. Using a murine model of HIVE, we investigated the effects of alcohol abuse on the clearance of virus-infected macrophages and neuroinflammation. Severe combined immunodeficient mice were reconstituted with human lymphocytes, and encephalitis was induced by intracranial injection of HIV-1-infected monocyte-derived macrophages (HIV-1+ MDM). Animals were fed an ethanol-containing diet beginning 2 weeks before lymphocyte engraftment and for the entire duration of the experiment. Lymphocyte engraftment was not altered by ethanol exposure. Alcohol-mediated immunosuppression in ethanol-fed mice was manifested by a significant decrease in CD8+/interferon-γ+ T lymphocytes, a fivefold increase in viremia, and diminished expression of immunoproteasomes in the spleen. Although both groups showed similar amounts of CD8+ T-lymphocyte infiltration in brain areas containing HIV-1+ MDMs, ethanol-fed mice featured double the amounts of HIV-1+ MDMs in the brain compared to controls. Ethanol-exposed mice demonstrated higher microglial reaction and enhanced oxidative stress. Alcohol exposure impaired immune responses (increased viremia, decreased immunoproteasome levels, and prevented efficient elimination of HIV-1+ MDMs) and enhanced neuroinflammation in HIVE mice. Thus, alcohol abuse could be a co-factor in progression of HIV-1 infection of the brain.
The independent effects of alcohol and human immunodeficiency virus (HIV)-1 in the central nervous system (CNS) are multifaceted and complex. Profound neurocognitive deficits are observed in alcoholics and patients with HIV-1-associated dementia.1,2 HIV-1 encephalitis [(HIVE) neuropathological correlate of HIV-1-associated dementia] is characterized by macrophage infiltration, diffuse activation of resident brain macrophages (microglia), productive HIV-1 infection of macrophages, and neuronal damage.3,4 Neuropathological examination of brain tissue from chronic alcoholics suggests that alcohol abuse results in neuronal degeneration, ranging from minor dendritic structural change and synaptic changes to neuronal cell death in the CNS.5 Postmortem examination of brain tissues from individuals with HIVE and alcohol abusers suggests similar neuropathological changes in both conditions.6 It has been suggested that the CNS suffers the additive effects of alcohol abuse and HIV-1 infection7,8 ; however, mechanistic studies assessing combined CNS injury are lacking. Interestingly, microglial activation (an important neuropathological correlate of HIV-1-associated dementia) was noted in animals chronically exposed to alcohol.9 In vitro data and results obtained in animal models suggest that alcohol abuse could increase HIV-1 replication through yet unknown pathways.10–12
Recent studies suggest that peripheral adaptive immune responses, including virus-specific CTL, control HIV-1 replication in the CNS.13,14 It is plausible to suggest that alcohol impairs immune responses, leading to increased viral replication, enhanced neuroinflammation, and neurodegeneration. Combined effects of alcohol abuse and HIV-1 on the CNS are difficult to investigate because of multiple confounding factors in human studies. Therefore, we tested whether alcohol abuse could affect clearance of virus-infected macrophages from the brain in a murine model of HIVE. To address our hypothesis, we used our previously developed mouse model of HIVE in hu-PBL-NOD/SCID HIVE mice. This model recapitulates the cellular immune responses against HIV-1-infected brain macrophages that occur in humans during progressive disease.15 Hu-PBL-NOD/SCID mice with focally induced HIVE and fed an ethanol-containing diet, demonstrated inefficient elimination of HIV-1-infected cells from the brain, augmented viremia, and increased microglial responses, suggesting that alcohol abuse can exacerbate HIV-1 CNS infection.
Materials and Methods
Cell Isolation and Viral Infection
Monocytes and peripheral blood lymphocytes (PBLs) were obtained by countercurrent centrifugal elutriation of leukopheresis packs from HIV-1, HIV-2, and hepatitis B-seronegative donors. Monocytes were cultivated in suspension culture using Teflon flasks in Dulbecco’s modified Eagle’s medium (Sigma, St. Louis, MO) supplemented with 10% heat-inactivated pooled human serum, 1% glutamine, 50 μg/ml gentamicin, 10 μg/ml ciprofloxacin (Sigma), and 1000 U/ml highly purified recombinant human macrophage colony-stimulating factor (a generous gift from Genetics Institute, Cambridge, MA). After 7 days in culture, MDMs were infected with HIV-1ADA at a multiplicity of infection of 0.01.16
Chronic Ethanol Administration in hu-PBL-NOD/SCID Mice with HIVE
Four-week-old male NOD/C.B-17 SCID mice were purchased from Jackson Laboratory (Bar Harbor, ME). Animals were maintained in sterile microisolator cages under pathogen-free conditions in accordance with ethical guidelines for care of laboratory animals at the University of Nebraska Medical Center and National Institutes of Health. Animals were weight-matched and randomly assigned to an alcohol-containing diet or control group. The experimental groups were fed a Lieber-DeCarli liquid diet (Dyets Inc., Bethlehem, PA) containing 4% (v/v) ethanol (providing ∼22% ethanol-derived calories ad libitum, whereas the control groups were fed an isocaloric diet lacking ethanol.17 The animals were monitored twice weekly for their body weight. Ethanol levels were detected in peripheral blood twice a week by assay kit (Diagnostic Chemicals Ltd., Charlottetown, PE, Canada).
Two initial experiments (n = 60, Table 1) were performed to establish the chronic ethanol murine HIVE model (pair-feeding with Lieber-DeCarli diet) and to evaluate levels/intensity of neuroinflammatory responses in animals inoculated stereotactically with HIV-1-infected MDMs without human PBL reconstitution. The animals were fed with ethanol diet 2 weeks before MDM intracerebral inoculation and for the entire duration of the experiment. HIV-1ADA-infected MDMs (3 × 105 cells in 5 μl) were injected intracerebrally.18 These experiments assured the absence of ethanol effects on MDM viability or level of HIV-1 infection in MDMs.
Table 1.
Testing of Ethanol Effects in Mouse Model for HIVE
| Treatment groups | Weeks of sacrifice
|
|
|---|---|---|
| Week 1 (n) | Week 2 (n) | |
| Control | 19* (13) | 26* (17) |
| Ethanol | 19* (13) | 24* (17) |
Total number of mice used in the study. The number of mice in the first two experiments (without PBL reconstitution) is shown in parentheses.
The third experiment (n = 28) was designed to investigate the role of excessive alcohol use in HIV-1 CNS infection, including human lymphocyte reconstitution, immune responses, and neuropathological alterations. All animals were injected intraperitoneally with rat anti-mouse CD122 (0.25 mg/mouse) 3 days before PBL transplantation and twice with rabbit anti-mouse asialo-GM1 antibodies (0.2 mg/mouse) (Wako, Richmond, VA) 1 day before and 3 days after PBL injection (20 × 106cells/mouse) to deplete mouse natural killer cells, and in turn, facilitate the engraftment of human lymphocytes. Animals were fed a Lieber-DeCarli diet 2 weeks before lymphocyte engraftment and for the duration of the experiment. HIV-1ADA-infected MDMs (3 × 105 cells in 5 μl) were injected intracerebrally14 8 days after PBL reconstitution, thus generating hu-PBL-NOD/SCID HIVE mice. Mice were sacrificed at 7 and 14 days after intracerebral injection with human MDMs. PBLs were evaluated by flow cytometry for engraftment, and plasma was assayed for HIV-1 p24 antigen levels by enzyme-linked immunosorbent assay. A total of three independent experiments were performed using three different human leukocyte donors.
Fluorescence-Activated Cell Sorting (FACS) of Mononuclear Cells from Blood and Spleen of hu-PBL-NOD/SCID HIVE Mice
Two-color FACS analysis was performed on blood and splenocytes at weeks 1 and 2 after intracerebral injection of human MDMs. Cells were incubated with fluorochrome-conjugated monoclonal antibodies (mAbs) specific to human CD4, CD8, CD56, CD3, and interferon (IFN)-γ (eBioscience, San Diego, CA) for 30 minutes at 4°C. To evaluate the cellular immune response, IFN-γ intracellular staining was performed in combination with anti-human CD8 and fluorescein isothiocyanate-conjugated anti-mouse CD45 antibodies (Abs) to exclude murine cells. Staining was performed as per the recommendation of the National Institutes of Health/National Institute of Allergy and Infections Disease, National Tetramer Core Facilities (Atlanta, GA). Data were analyzed with a FACSCalibur system using CellQuest software (Becton Dickinson Immunocytometry System, San Jose, CA).
Western Blot Analysis
Western blot assays were performed on whole cell protein extracts of spleen and human brain homogenates (basal ganglia) using primary Abs or α-actin. Briefly, 15 to 20 μg of lysate protein was loaded onto 1.5-mm-thick 4 to 15% gradient polyacrylamide precast gels (Bio-Rad, Hercules, CA) and was electrophoresed. Proteins from the gels were transblotted onto nitrocellulose membranes (0.45-μm pore size) at 60 V for 1 hour at room temperature. The membranes were blocked with superblock (Bio-Rad) containing 2% nonfat dry milk for 40 minutes at room temperature. Blots were incubated for 1 hour at room temperature with respective primary antibody diluted 1/10 in superblock solution in 20 mmol/L phosphate-buffered saline, pH 7.4, containing 0.1% Tween-20 (PBST). Primary antibody-reacted blots were washed in three 5-minute washes of PBST. Immunoreactive bands were detected by luminol detection kit (Pierce, Rockford, IL) after exposure to Kodak X-ray film (Eastman-Kodak, Rochester, NY). The bands were quantified densitometrically on GelExpert as arbitrary volume integration units using the Molecular Dynamics ImageQuant software (Sunnyvale, CA). Results were expressed as ratios of intensities for target proteins to α-actin for normalization. The following Abs were used for Western blotting: monoclonal Abs to LMP2 or LMP7 (Affinity Research Products, Mamhead, UK), CD45 (Santa Cruz Biotechnology, Santa Cruz, CA), and nitrotyrosine (Upstate Cell Signaling Solutions, Lake Placid, NY).
Histopathology and Image Analysis
Histopathology and image analyses were performed as described previously.14 Briefly, brain tissue was collected at days 7 and 14 after intracerebral injection of MDMs, fixed in 4% phosphate-buffered paraformaldehyde, and embedded in paraffin or frozen at −80°C for later use. In brief, coronal brain sections were cut to identify the injection site of HIV-1-infected MDMs. Serial 5-μm-thick sections (n = 30 to 100) covering the entire area of human MDM injection were cut for each mouse, and three to seven matched sections (10 sections apart) were analyzed. Immunohistochemical staining was performed using a basic indirect staining protocol. After deparaffinization, slides were heated for 30 minutes in 0.01mol/L citrate buffer at 95°C for antigen retrieval. Anti-vimentin mAb (1:50, clone 3B4; DAKO, Carpinteria, CA), which identifies all human leukocytes, was used to detect human cells in mouse brains. Human MDMs and CD8+ T lymphocytes were detected with CD68 (1:50 dilution, clone KP 1) and CD8 mAbs (1:50 dilution, clone 144B), respectively. Virus-infected cells were detected with mAb to HIV-1 p24 (1:10, clone Kal-1; DAKO). Reactive murine microglial cells were detected with ionized calcium-binding adapter molecule 1 (Iba1) antibody (1:500, Wako). Reactive astrocytes were labeled with antibodies to glial fibrillary acidic protein (GFAP) (1:1500, DAKO). Primary antibodies were detected with the appropriate biotinylated secondary antibodies and visualized with avidin-biotin complexes (Vectastain Elite ABC kit, Vector Laboratories, Burlingame, CA) or horseradish peroxidase-coupled dextran polymer (EnVision, DAKO). Immunostained sections were counterstained with Mayer’s hematoxylin. Deletion of primary antibody or irrelevant IgG isotype served as controls.
The numbers of CD8+ lymphocytes, CD68+ MDMs, and HIV-1 p24+ MDMs in each section were counted in a blinded manner by three independent observers. Mean number of stained cells per section within the injected hemisphere was calculated for each mouse (three to seven sections/mouse) and a total of 13 to 17 mice per group were examined at weeks 1 and 2 after MDM injection. Light microscopic examination was performed with an Eclipse 800 microscope (Nikon Instruments Inc., Melville, NY) equipped with ×20/0.5 objective lens. Images were acquired with a digital CCD Color View II camera (Soft Imaging Systems, Lakewood, CO). Semiquantitative analysis (percentage of area occupied per section by immunostaining) for Iba1 and GFAP were performed by computer-assisted image analysis (Image-ProPlus; Media Cybernetics, Silver Spring, MD).14
Statistical Analysis
Data were analyzed using Prism (GraphPad) with Student’s t-test for comparisons and analysis of variance. P values <0.05 were considered significant. All results are presented as mean plus or minus (±) SEM.
Results
EtOH-hu-PBL NOD/SCID HIVE Mouse Model
The goal of this study was to investigate whether chronic alcohol abuse is a co-factor for progression of HIVE. To that end, hu-PBL-NOD/SCID HIVE mice were fed either Lieber-DeCarli liquid diet containing 4% ethanol or an isocaloric control diet. The mice fed an ethanol diet maintained a blood alcohol level of 0.13 to 0.22% (data not shown). Mice were engrafted with human PBLs to generate hu-PBL-NOD/SCID animals. Eight days after lymphocyte engraftment, encephalitis was induced by stereotactic injection of syngeneic human HIV-1ADA-infected MDMs (derived from the same donor as PBLs) into the basal ganglia and caudate generating hu-PBL-NOD/SCID HIVE mice. FACS analysis of blood and spleen (weeks 1 to 2) was performed in control and ethanol-fed hu-PBL-NOD/SCID HIVE mice to assess the level of human lymphocyte engraftment. Similar numbers of human CD3+, CD4+, and CD8+ T lymphocytes were found in the blood and spleens of control and ethanol-fed mice, suggesting equal levels of engraftment in both the groups. Table 2 shows results of two-color staining for human CD3+, CD4+, and CD8+ cells in peripheral blood samples of control and ethanol-fed hu-PBL-NOD/SCID HIVE mice. Our results show that ethanol-containing diet did not change the level of lymphocyte engraftment in our model.
Table 2-6819.
Human Lymphocytes and Plasma Levels of HIV-1 p24 in the Peripheral Blood of Ethanol-Fed Versus Pair-Fed Control Mice
| Weeks* | Treatment groups | Lymphocytes population (%) (mean ± SEM)
|
HIV-1 p24 pg/ml (mean ± SEM) | ||
|---|---|---|---|---|---|
| CD3+† | CD4+† | CD8+† | |||
| Week 1 | Control | 44.99 ± 1.06 | 15.32 ± 1.08 | 22.25 ± 0.79 | 2.07 ± 0.59 |
| Ethanol | 44.21 ± 1.13 | 16.27 ± 0.67 | 18.02 ± 0.32 | 5.84 ± 1.08 | |
| Week 2 | Control | 62.86 ± 0.35 | 32.31 ± 0.37 | 26.97 ± 0.25 | 24.6 ± 14.43 |
| Ethanol | 65.01 ± 0.68 | 30.53 ± 0.46 | 31.32 ± 0.26 | 132.13 ± 58.47‡ | |
Days after MDM injection.
No significant difference in percentage of CD3+/CD8+/CD4+ cells between the groups.
P < 0.032, compared with control.
Ethanol Administration Enhanced Viremia and Suppressed Immune Response in hu-PBL-NOD/SCID HIVE Mice
Chronic ethanol abuse in humans leads to a variety of immunomodulatory events that can alter resistance to infectious agents. To study ethanol effect on viremia, HIV-1 p24 blood levels were measured in control and ethanol-fed hu-PBL-NOD/SCID HIVE mice. HIV-1 p24 levels in ethanol-fed mice were fivefold greater compared to control mice (132 ± 58.47 pg/ml in ethanol versus 24.6 ± 14.43 pg/ml in control mice, P < 0.03; Table 2). Immunosuppressive effects of alcohol abuse have been associated with ethanol-induced impairment of cellular responses. To determine whether alcohol exposure affects immune responses in our model, we compared levels of IFN-γ-positive effector CD8+ T lymphocytes in peripheral blood by FACS analysis (Figure 1A). The percentage of IFN-γ-positive cells was significantly lower in ethanol-fed mice (P < 0.05) compared to controls (Figure 1B). These results suggest that chronic ethanol administration diminished induction of anti-viral cellular immune responses.
Figure 1.
Effects of chronic ethanol abuse on the number of IFN-γ-positive human CD8+ cells and IPR expression. A and B: Number of CD8+/IFN-γ-secreting cells in the peripheral blood of control and ethanol (EtOH)-fed hu-PBL-NOD-SCID HIVE mice. Representative histogram of human CD8+/IFN-γ+ cells in control and EtOH-fed mice are shown in A. Percentage of CD8+/IFN-γ-secreting cells (n = 15 for control and n = 13 for EtOH mice) in peripheral blood is shown in B. Levels of LMP2 (C) and LMP7 (D) subunits of IPRs detected by Western blot in spleen homogenates from control and EtOH-fed mice. The expressions of IPR subunits (LMP2 and LMP7) are normalized to that of CD45 levels. Results are expressed as normalized LMP2 or LMP7 level to that of internal standard α-actin. Error bars indicate SEM. *P < 0.05 (EtOH versus control); **P < 0.04 (EtOH versus control).
Proteasomes (PRs) are multicatalytic protease complexes that degrade polyubiquitinated cellular proteins. PR constitutive subunits have chymotrypsin-like (X), trypsin-like (Y), and peptidyl-glutamyl-peptide hydrolase (Z) activities.19 Stimulation of PRs constitutes subunits (X, Y, and Z) by proinflammatory cytokines such as IFN-γ, tumor necrosis factor-α, and interleukin-6 are, respectively, replaced by subunits LMP2, LMP7, and MELC-1 which are now referred as inducible PR subunits.20,21 PRs with inducible PR subunits are known as immunoproteasomes (IPRs), which process antigenic peptides for antigen presentation on major histocompatibility complex (MHC) class I molecules. We demonstrated that both HIV-1 and ethanol diminish PR activity and IPR content in MDMs, and therefore, may contribute to dysfunction in the HIV-infected host.22 To further study the effects of ethanol on IPR content in vivo, we preformed Western blot analysis of IPR subunits, LMP2, and LMP7 on protein extracts derived from spleen. The IPR expression (LMP2 and LMP7) was adjusted to human CD45 (reflecting number of human cells) in the samples and expressed as the ratio of target protein immunoreactivity to that of internal standard α-actin. The levels of LMP2 and LMP7 were significantly lower as compared to mice on control diet (P < 0.04; Figure 1, C and D). These results indicate that chronic ethanol consumption affects IPR protein levels in vivo.
Chronic Ethanol Exposure Did Not Affect MDM Viability or Level of HIV-1 Infection in MDMs
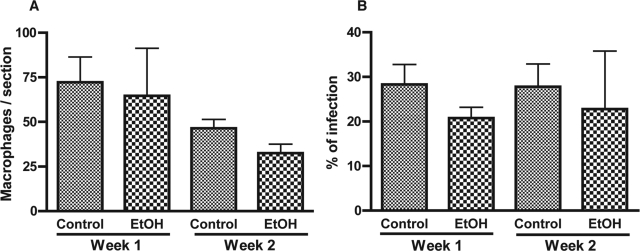
Initial experiments were performed to establish the ethanol model (pair-feeding with Lieber-DeCarli diet) to evaluate the in vivo effects of ethanol on MDM viability and HIV-1 infection in nonreconstituted animals inoculated intracerebrally with HIV-1-infected MDMs (Table 1). Serial brain sections were immunostained for CD68 and HIV-1 p24. Mean numbers of stained cells per section within the injected hemisphere were calculated for each mouse (three to seven sections/mouse) and mean numbers of cells for 13 to 17 mice per each group were evaluated at weeks 1 and 2 after MDM injection. Equal numbers of MDMs were detected in the brain tissue of controls and ethanol-fed mice (72.2 ± 14.2 versus 64.6 ± 26.7 at week 1 and 46 ± 14.9 versus 32.6 ± 5 at week 2; P > 0.05) indicating that ethanol feeding did not significantly affect MDM viability in the brain (Figure 2A). Ethanol exposure did not significantly change the levels of HIV-1 infection in both groups (percentage of HIV-1 p24+ MDMs, 28 ± 4.5% versus 20.8 ± 2.4% at week 1 and 27.8 ± 5.1% versus 22.8 ± 13% at week 2, P > 0.05; Figure 2B). These experiments assured that ethanol feeding had no effect on HIV-1 MDM infection or cell viability in the brain.
Figure 2.
Ethanol exposure did not affect HIV-1 infection and MDM viability in animals without PBL reconstitution. CD68+ MDMs (A) and percentage of CD68+ cells infected (B) were assessed in brain tissue sections of control and ethanol-fed mice at weeks 1 and 2 after intracerebral inoculation of HIV-1-infected MDMs. Value represents mean ± SEM per 5-μm section, six to nine sections were analyzed for each animal.
Chronic Ethanol Exposure Increased Neuroinflammation in hu-PBL-NOD/SCID HIVE Mice
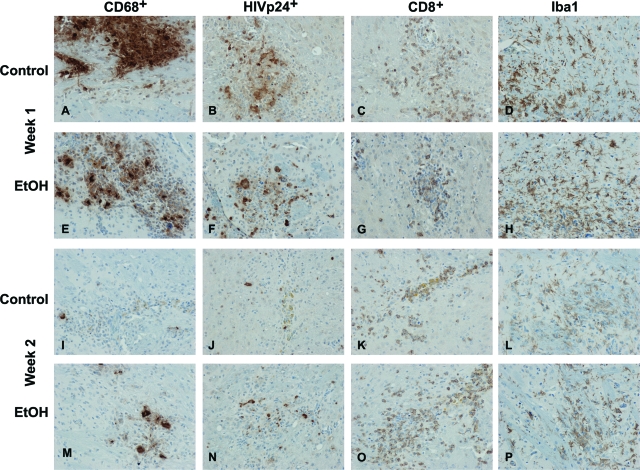
To analyze neuroinflammatory responses in the hu-PBL-NOD/SCID mice brain, serial sections were immunostained for human CD68 (marker for human MDMs), HIV-1 p24 (viral antigen), human CD8 (T cells), and Iba1 (mouse microglial marker). Cells were counted in a blinded manner by three investigators, and the number of positive cells was averaged for the area covering the entire left hemisphere (inoculated with MDMs). At week 1, comparable numbers of CD68+ MDMs (Figure 3, A and E) and CD8+ lymphocytes (Figure 3, C and G) were seen in the brains of control and ethanol mice. Ethanol-fed mice demonstrated more HIV-1 p24+ MDMs (Figure 3, B and F) and greater levels of microglial reaction (number of cells and activated phenotype; Figure 3, D and H) compared with controls.
Figure 3.
Neuroinflammatory responses in control and ethanol-fed hu-PBL-NOD/SCID HIVE mice. Comparable numbers of CD68+ MDMs were found in the brains of control and ethanol-fed (EtOH) mice at week 1 (A, E), but increased numbers of CD68+ MDMs were present in EtOH-fed mice versus controls at week 2 (I, M). More HIV-1 p24+ MDMs were found in EtOH-fed mice at week 1 (F) and week 2 (N) as compared to controls (B, J). Similar levels of CD8+ lymphocytes were detected in the brain tissue in both groups at weeks 1 and 2 (C, G, K, O). Microglial reaction was consistently higher in PBL-reconstituted EtOH mice (H and P) as compared to control mice (Dand L). Primary Abs were detected by Vectastain Elite kit with DAB as a substrate. Original magnifications, ×200.
By week 2, brains of ethanol-fed mice contained more CD68+ MDMs (Figure 3, I and M) and HIV-1 p24+ MDMs (Figure 3, J and N). Enhanced microglial reaction (Figure 3, L and P) were seen in ethanol-fed animals compared with controls. Similar to week 1, numbers of CD8+ lymphocytes detected in brain tissues from both groups were comparable (Figure 3, K and O).
Chronic Ethanol Exposure Abridges Clearance of Autologous Infected MDMs from Brains of hu-PBL-NOD/SCID HIVE Mice
To explore the effects of ethanol on elimination of virus-infected macrophages, CD68+, HIV-1 p24+, and CD8+ lymphocytes were counted in brain tissue in a blinded manner by three independent observers. Mean numbers of stained cells per section within the injection hemisphere were calculated for each mouse (three to seven sections/mouse) and the mean numbers of cells for six to nine mice per each group were evaluated at weeks 1 and 2 after MDM injection. As shown in Figure 4A, the mean numbers of CD68+ MDMs was lower, although not statistically significant from the control group, in the ethanol group (154 ± 34 versus 102.83 ± 20, P > 0.05) at week 1. At week 2 the amount of CD68+ MDMs in ethanol group was significantly higher than in the control mice (19 ± 7 versus 5 ± 3, P < 0.03). Although comparable numbers of CD8+ T cells were observed at weeks 1 and 2 in ethanol and control mice (Figure 4B), more HIV-1 p24+ MDMs were found in the ethanol mice at week 1 (42 ± 8 versus 21 ± 6; P < 0.04) and week 2 (7.57 ± 2.16 versus 3 ± 0.957, P < 0.02) as compared with control mice (Figure 4C). These results suggest inefficient clearance of HIV-1-infected p24+ MDMs (main target for CTL) from the brains of ethanol mice.
Figure 4.
Elimination of autologous HIV-1-infected MDMs from brains of ethanol versus control hu-PBL-NOD/SCID HIVE mice. CD68+ MDMs (A), CD8+ lymphocytes (B), and HIV-1 p24+ MDMs (C) were counted in brain tissue sections of control and ethanol (EtOH)-fed hu-PBL-NOD-SCID HIVE mice at weeks 1 and 2 after intracerebral inoculation of HIV-1-infected MDMs. EtOH-fed mice demonstrated inefficient elimination of MDM/HIV-1 p24+ MDMs (C) compared with pair-fed controls. Value represents mean ± SEM per 5-μm section; six to nine sections were analyzed for each animal. *P < 0.03; **P < 0.04; #P < 0.02 (EtOH versus control).
Chronic Ethanol Exposure Induces Neuroinflammation and Oxidative Stress in Vivo
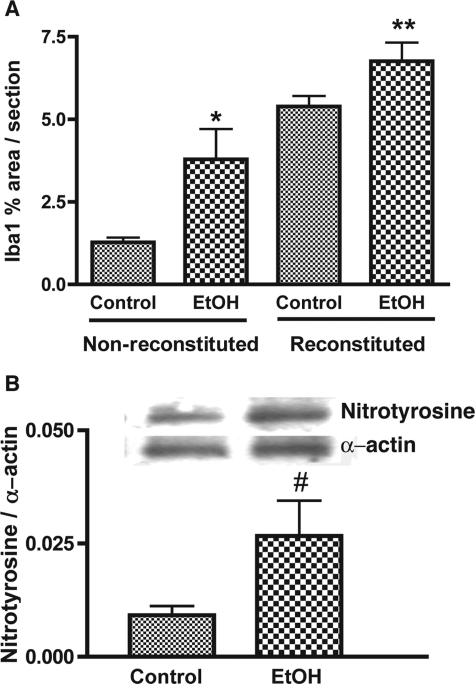
Induction of an inflammatory response in the brain was determined by measurement of microglial reaction in brain tissue (assessed by digital image analysis and expressed as percentage of the area occupied by Iba1 immunostaining). Microglial reaction was threefold higher in nonreconstituted ethanol-fed animals (P < 0.008; Figure 5A) compared to control mice. When microglial reaction was assessed in PBL-reconstituted and uninfected MDM-injected mice, as expected, lymphocyte infiltration significantly enhanced Iba1 reaction (fourfold as compared to nonreconstituted mice). Microglial responses were further amplified by 20% in reconstituted ethanol-fed animals (P < 0.02; Figure 5A) compared to those of reconstituted animals fed an isocaloric diet.
Figure 5.
Chronic ethanol abuse enhanced microglial reaction and caused oxidative stress. A: Microglial reaction (detected by Iba1 staining) was consistently higher in nonreconstituted ethanol (EtOH)-fed mice. PBL reconstitution and CTL brain infiltration further enhanced microglial responses in EtOH-fed mice. Semiquantitative analysis for Iba1 was performed by computer-assisted image analysis (Image-ProPlus; Media Cybernetics, Silver Spring, MD). B: Western blot analysis of nitrotyrosine levels in brain homogenates from control and EtOH-fed mice. Representative immunoblots of nitrotyrosine and internal standard α-actin are shown in blots and the ratios of nitrotyrosine staining to α-actin are shown in the histogram. Error bars represent SEM. *P < 0.008; **P < 0.02; #P < 0.05 (EtOH versus control).
One recognized mechanism of chronic alcohol abuse is enhanced oxidative stress.23 The degree of nitrotyrosine expression provides an index of the production of reactive nitrogen species and potential cell damage throughout a period of time. Nitrotyrosine analysis was performed by Western blot analysis on protein extracts from brain tissue of ethanol and control mice. The level of nitrotyrosine in ethanol-fed mice was significantly (P < 0.05) higher than in control mice (Figure 5B). These results suggest that additive effects of HIV-1-associated neuroinflammation and ethanol exposure could be mediated in part by oxidative stress.
Discussion
Mounting evidence demonstrates the adverse effects of alcohol abuse on the immune system and progression of HIV-1 infection, including development of HIV-1-associated cognitive impairment.24–26 Using an ethanol HIVE mouse model, we demonstrated that chronic alcohol administration enhanced viremia, suppressed adaptive anti-viral immune responses, led to inefficient elimination of virus-infected macrophages from the brain, and increased neuroinflammation. Mice that were not reconstituted with PBLs but injected with HIV-1-infected MDMs clearly demonstrated amplification of HIV-1-induced neuroinflammation by chronic alcohol exposure. Microglial reactions were further augmented in PBL-reconstituted mice because of CD8+ lymphocyte infiltration into the brain, thus, mimicking HIVE in humans.14
Because many neurodegenerative diseases (including HIVE) are associated with oxidative damage and neuronal injury caused by inflammatory responses in the brain,27 we hypothesized that excessive alcohol use could exacerbate the progression of these disorders. Given the multiple confounding factors in the human host, such processes can be dissected only in relevant in vivo models (like hu-PBL-NOD/SCID mouse model) that reproduce the induction of virus-specific cellular immune responses.15 Previous studies using the hu-PBL-NOD/SCID HIVE mouse model proved it to be an excellent tool for quantitative evaluation of immune responses and neuroinflammatory events that occur during ongoing HIV-1 CNS infections.14 In the present study, introduction of ethanol exposure allowed us to demonstrate that alcohol abuse could be an exacerbating factor of HIV-1 CNS infection. To our knowledge, this is the first time the synergistic effects of alcohol-induced immune dysregulation and neuroinflammation in the setting of HIVE in an animal model were studied.
Mice were fed a Lieber-DeCarli liquid diet, which supplied 22% of caloric intake as ethanol, or an isocaloric control diet, which was shown optimal for immunological studies using murine models. Such diets maximize ethanol consumption and maintain animal body weight.28 The blood ethanol levels (0.13 to 0.22%) achieved in HIVE mice were comparable to ones seen in the peripheral blood of moderately to severely intoxicated humans29,30 (consuming ∼7 to 12 drinks per hour) and in mouse models exploring effects of alcohol abuse on immune responses against infectious agents.31 Chronic alcohol administration did not change levels of PBL engraftment in spleen/blood or viability of CD68+/HIV-1 p24+ MDMs in brains of nonreconstituted animals. Thus, chronic ethanol exposure had no effect on the validity of the model.
Conflicting results have been obtained regarding the effects of alcohol on HIV-1 infection, replication, and course of disease.32–36 Our study showed significantly higher levels of HIV-1 p24 in the plasma of hu-PBL-NOD/SCID HIVE mice chronically exposed to ethanol as compared to controls fed an isocaloric diet (Table 2). Although the effects of ethanol on HIV-1 infection still remain inconclusive, our results parallel observations in which chronic alcohol exposure in macaques infected with simian immunodeficiency virus (animal model for HIV-1 infection) resulted in high plasma viral load.11,12 However, the precise mechanisms by which ethanol affects HIV-1 replication remain unresolved.
Alcohol exposure causes immunosuppressive effects in several types of immune cells including T lymphocytes,37–39 neutrophils,40,41 monocytes/macrophages, and dendritic cells.42–44 Acute and chronic alcohol consumption results in marked alteration of host immunity. Ethanol impairs T- and B-lymphocyte function and alters immunoglobulin production and secretion of proinflammatory cytokines such as tumor necrosis factor-α, interleukin-1β, and interleukin-6.45–48 Our findings that chronic alcohol exposure decreased levels of human IFN-γ-producing CD8+ T lymphocytes in blood could be one mechanism for augmented viremia in ethanol-fed HIVE mice. One possible mechanism of inefficient elimination of HIV-1-infected MDMs may be associated with decreased migration of effector CD8+ lymphocytes into the brain.15 Indeed, acute ethanol exposure resulted in diminished leukocyte migration to the sites of inflammatory responses in vivo.49–51 However, this appears not to be the case in the chronically exposed hu-PBL-NOD/SCID mouse model in which equal levels of CD8+ lymphocytes were found in brain areas of HIVE mice fed an ethanol or isocaloric diet.
Chronic alcohol abuse is associated with increased morbidity and mortality because of infections; however, specific effects on the immune system in such a setting are controversial. Studies in murine models showed that splenocytes exposed to ethanol had impaired humoral and cellular immune responses.52 One plausible mechanism of ethanol immunosuppressive effects on the host immune system is impaired antigen presentation by antigen-presenting cells such as macrophages and dendritic cells.53 Proteasomes (PRs) are multicatalytic proteinase complexes degrading intercellular proteins. Immune activation and stimulation with IFN-γ result in up-regulation of activity and expression of inducible PR subunits, known as immunoproteasomes (IPRs).20,21 IPR-cleaved peptides are most suitable for subsequent conjugation with peptides of MHC class I molecules, and they are important to initiation of cellular immune responses by antigen-presenting cells.21,54 The protective role of IPRs through generation of CTL responses was demonstrated in experimental viral and bacterial liver infections.21 Both HIV-1 and ethanol inhibited IPR expression and activity in human MDMs in vitro.22 In vivo studies showed decreased PR activity after administration of high doses of ethanol,55 and a reduced amount of CD8+ T cells and impaired immune responses were seen in LMP2- and LMP7-deficient mice.56,57 Similarly in the present in vivo study, ethanol-fed hu-PBL-NOD/SCID mice with HIVE showed decreased LMP2 and LMP7 levels in the spleen, suggesting IPR dysfunction. Thus, decreased IPR expression may be one reason for ineffective elimination of HIV-1 p24+ MDMs in the brain.
The adverse effects of alcohol abuse are also exerted through generation of reactive oxygen species and enhanced oxidative stress in brain.58,59 IPR activity is susceptible to inhibition by reactive oxygen species.60 Reactive oxygen species generation was linked to ethanol metabolism in neural cells61 and macrophages resulting in IPR dysfunction.22 Indeed, brain tissue of ethanol-fed mice featured higher levels of nitrotyrosine indicating enhanced oxidative damage. Activation of brain macrophages is a process involving environmental cues (like systemic or brain infections) and cell signals from injured neurons or other neural cells.62 Diffuse microglial activation is thought to be an underlying process in HIV-1-mediated neurodegeneration.4 Importantly, chronic ethanol exposure can result in microglial activation and accumulation in animal models.9 HIV-1 proteins and ethanol appear to produce synergistic neurotoxic effects.23,63 Our data indicates that chronic ethanol administration enhances microglial reaction more than three times as compared to one caused by HIV-1-infected MDMs alone. Augmentation of neuroinflammation because of CTL infiltration further amplified microglial reaction suggesting the concerted effects of both factors promote neuronal demise.
Alcohol has been linked to heightened susceptibility to and progression of HIV-1 infection and HIV-1-mediated CNS disease. The current study explores several mechanisms leading to progression of HIV-1 CNS infection including immunosuppressive effects of alcohol abuse and enhanced neuroinflammation. We demonstrated that chronic ethanol exposure in HIVE mice resulted in increased viremia and inefficient elimination of HIV-1-infected macrophages indicating defective immune responses. Ethanol-fed animals featured augmented microglial reaction and evidence of oxidative stress in PBL reconstituted mice, demonstrating that alcohol abuse could be an exacerbating factor in HIV-1 CNS infection. Based on our observations, novel treatment strategies (including anti-inflammatory and anti-oxidative compounds) should be included in the treatment schemes for HIV-1 infection in alcohol abusers in addition to anti-retroviral drugs.
Acknowledgments
We thank Ms. Robin Taylor for excellent editorial support and Dr. Lee Mosley for critical reading of the manuscript.
Footnotes
Address reprint requests to Yuri Persidsky, M.D., Ph.D., Center for Neurovirology and Neurodegenerative Disorders, Departments of Pathology/Microbiology and Pharmacology and Experimental Neuroscience, 985215 Nebraska Medical Center, Omaha, NE 68198-5215. E-mail: ypersids@unmc.edu.
Supported in part by the National Institutes of Health (grants AA013846 and AA15913 to Y.P.).
References
- Rosenbloom MJ, O’Reilly A, Sassoon SA, Sullivan EV, Pfefferbaum A. Persistent cognitive deficits in community-treated alcoholic men and women volunteering for research: limited contribution from psychiatric comorbidity. J Stud Alcohol. 2005;66:254–265. doi: 10.15288/jsa.2005.66.254. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Brew BJ, Nath A. Neurological complications of HIV infection. Lancet Neurol. 2005;4:543–555. doi: 10.1016/S1474-4422(05)70165-4. [DOI] [PubMed] [Google Scholar]
- Adamson P, Etienne S, Couraud P, Calder V, Greenwood J. Lymphocyte migration through brain endothelial cell monolayers involves signaling through endothelial ICAM-1 via a rho-dependent pathway. Immunology. 1999;162:2964–2973. [PubMed] [Google Scholar]
- Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukoc Biol. 2003;74:691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- Harper C. The neuropathology of alcohol-specific brain damage, or does alcohol damage the brain? J Neuropathol Exp Neurol. 1998;57:101–110. doi: 10.1097/00005072-199802000-00001. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Ashida N, Arai H, Yamasaki M, Kita T. Distinct signaling pathways for MCP-1-dependent integrin activation and chemotaxis. J Biol Chem. 2001;276:16555–16560. doi: 10.1074/jbc.M009068200. [DOI] [PubMed] [Google Scholar]
- Meyerhoff D, Bloomer C, Cardenas V, Norman D, Weiner M, Fein G. Elevated subcortical choline metabolites in cognitively and clinically symptomatic HIV+ patients. Neurology. 1999;52:995–1003. doi: 10.1212/wnl.52.5.995. [DOI] [PubMed] [Google Scholar]
- Riikonen J, Jaatinen P, Rintala J, Porsti I, Karjala K, Hervonen A. Intermittent ethanol exposure increases the number of cerebellar microglia. Alcohol Alcohol. 2002;37:421–426. doi: 10.1093/alcalc/37.5.421. [DOI] [PubMed] [Google Scholar]
- Wang X, Douglas SD, Metzger DS, Guo CJ, Li Y, O’Brien CP, Song L, Davis-Vogal A, Ho WZ. Alcohol potentiates HIV-1 infection of human blood mononuclear phagocytes. Alcohol Clin Exp Res. 2002;26:1880–1886. doi: 10.1097/01.ALC.0000042148.50808.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby GJ, Stoltz DA, Zhang P, Kolls JK, Brown J, Bohm RP, Jr, Rockar R, Purcell J, Murphey-Corb M, Nelson S. The effect of chronic binge ethanol consumption on the primary stage of SIV infection in rhesus macaques. Alcohol Clin Exp Res. 2003;27:495–502. doi: 10.1097/01.ALC.0000057947.57330.BE. [DOI] [PubMed] [Google Scholar]
- Kumar R, Perez-Casanova AE, Tirado G, Noel RJ, Torres C, Rodriguez I, Martinez M, Staprans S, Kraiselburd E, Yamamura Y, Higley JD, Kumar A. Increased viral replication in simian immunodeficiency virus/simian-HIV-infected macaques with self-administering model of chronic alcohol consumption. J Acquir Immune Defic Syndr. 2005;39:386–390. doi: 10.1097/01.qai.0000164517.01293.84. [DOI] [PubMed] [Google Scholar]
- Kim WK, Corey S, Chesney G, Knight H, Klumpp S, Wuthrich C, Letvin N, Koralnik I, Lackner A, Veasey R, Williams K. Identification of T lymphocytes in simian immunodeficiency virus encephalitis: distribution of CD8+ T cells in association with central nervous system vessels and virus. J Neurovirol. 2004;10:315–325. doi: 10.1080/13550280490505382. [DOI] [PubMed] [Google Scholar]
- Potula R, Poluektova L, Knipe B, Chrastil J, Heilman D, Dou H, Takikawa O, Munn DH, Gendelman HE, Persidsky Y. Inhibition of indoleamine 2,3-dioxygenase (IDO) enhances elimination of virus-infected macrophages in an animal model of HIV-1 encephalitis. Blood. 2005;106:2382–2390. doi: 10.1182/blood-2005-04-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poluektova LY, Munn DH, Persidsky Y, Gendelman HE. Generation of cytotoxic T cells against virus-infected human brain macrophages in a murine model of HIV-1 encephalitis. J Immunol. 2002;168:3941–3949. doi: 10.4049/jimmunol.168.8.3941. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, Wahl LA, Lane HC, Fauci AS, Burke DS, Meltzer MS. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrells TR, Smith W, Eckardt MJ. Murine model of ethanol-induced immunosuppression. Alcohol Clin Exp Res. 1990;14:546–550. doi: 10.1111/j.1530-0277.1990.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Limoges J, McComb R, Bock P, Baldwin T, Tyor W, Patil A, Nottet HS, Epstein L, Gelbard H, Flanagan E, Reinhard J, Pirruccello SJ, Gendelman HE. Human immunodeficiency virus encephalitis in SCID mice. Am J Pathol. 1996;149:1027–1053. [PMC free article] [PubMed] [Google Scholar]
- Piccinini M, Tazartes O, Mostert M, Musso A, DeMarchi M, Rinaudo MT. Structural and functional characterization of 20S and 26S proteasomes from bovine brain. Brain Res Mol Brain Res. 2000;76:103–114. doi: 10.1016/s0169-328x(99)00337-x. [DOI] [PubMed] [Google Scholar]
- Fruh K, Gruhler A, Krishna RM, Schoenhals GJ. A comparison of viral immune escape strategies targeting the MHC class I assembly pathway. Immunol Rev. 1999;168:157–166. doi: 10.1111/j.1600-065x.1999.tb01290.x. [DOI] [PubMed] [Google Scholar]
- Khan S, van den Broek M, Schwarz K, de Giuli R, Diener PA, Groettrup M. Immunoproteasomes largely replace constitutive proteasomes during an antiviral and antibacterial immune response in the liver. J Immunol. 2001;167:6859–6868. doi: 10.4049/jimmunol.167.12.6859. [DOI] [PubMed] [Google Scholar]
- Haorah J, Heilman D, Diekmann C, Osna N, Donohue TM, Jr, Ghorpade A, Persidsky Y. Alcohol and HIV decrease proteasome and immunoproteasome function in macrophages: implications for impaired immune function during disease. Cell Immunol. 2004;229:139–148. doi: 10.1016/j.cellimm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Flora G, Pu H, Lee YW, Ravikumar R, Nath A, Hennig B, Toborek M. Proinflammatory synergism of ethanol and HIV-1 Tat protein in brain tissue. Exp Neurol. 2005;191:2–12. doi: 10.1016/j.expneurol.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, Pretsell DO, Chiswick A, Egan V, Brettle RP. The Edinburgh cohort of HIV-positive injecting drug users at 10 years after infection: a case-control study of the evolution of dementia. AIDS. 1996;10:431–440. doi: 10.1097/00002030-199604000-00012. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ. Effects of alcohol and HIV infection on the central nervous system. Alcohol Res Health. 2001;25:288–298. [PMC free article] [PubMed] [Google Scholar]
- Rothlind JC, Greenfield TM, Bruce AV, Meyerhoff DJ, Flenniken DL, Lindgren JA, Weiner MW. Heavy alcohol consumption in individuals with HIV infection: effects on neuropsychological performance. J Int Neuropsychol Soc. 2005;11:70–83. doi: 10.1017/S1355617705050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boven LA, Gomes L, Hery C, Gray F, Verhoef J, Portegies P, Tardieu M, Nottet HS. Increased peroxynitrite activity in AIDS dementia complex: implications for the neuropathogenesis of HIV-1 infection. J Immunol. 1999;162:4319–4327. [PubMed] [Google Scholar]
- Monahan CM, Padgett EL, Biber KL, Moscatello KM, Johnston FL, Wolcott RM. Dose response to ethanol-containing liquid diets for use in a murine model for studies of biological effects due to ethanol consumption. Alcohol Clin Exp Res. 1997;21:1092–1099. [PubMed] [Google Scholar]
- Deutch SR, Christian C, Hoyer S, Christensen EF, Dragsholt C, Hansen AC, Kristensen IB, Hougaard K. Drug and alcohol use among patients admitted to a Danish trauma centre: a prospective study from a regional trauma centre in Scandinavia. Eur J Emerg Med. 2004;11:318–322. doi: 10.1097/00063110-200412000-00004. [DOI] [PubMed] [Google Scholar]
- Cherpitel CJ, Ye Y, Bond J. Attributable risk of injury associated with alcohol use: cross-national data from the emergency room collaborative alcohol analysis project. Am J Public Health. 2005;95:266–272. doi: 10.2105/AJPH.2003.031179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason CM, Dobard E, Zhang P, Nelson S. Alcohol exacerbates murine pulmonary tuberculosis. Infect Immun. 2004;72:2556–2563. doi: 10.1128/IAI.72.5.2556-2563.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagasra O, Bachman SE, Jew L, Tawadros R, Cater J, Boden G, Ryan I, Pomerantz RJ. Increased human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells induced by ethanol: potential immunopathogenic mechanisms. J Infect Dis. 1996;173:550–558. doi: 10.1093/infdis/173.3.550. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Kajdacsy-Balla A, Lischner HW, Pomerantz RJ. Alcohol intake increases human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells. J Infect Dis. 1993;167:789–797. doi: 10.1093/infdis/167.4.789. [DOI] [PubMed] [Google Scholar]
- Liu X, Zha J, Nishitani J, Chen H, Zack JA. HIV-1 infection in peripheral blood lymphocytes (PBLs) exposed to alcohol. Virology. 2003;307:37–44. doi: 10.1016/s0042-6822(02)00031-4. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Han YC, Wu WJ. A brief review of immunomodulation caused by acute administration of ethanol: involvement of neuroendocrine pathways. Alcohol Alcohol Suppl. 1994;2:431–437. [PubMed] [Google Scholar]
- Fong IW, Read S, Wainberg MA, Chia WK, Major C. Alcoholism and rapid progression to AIDS after seroconversion. Clin Infect Dis. 1994;19:337–338. doi: 10.1093/clinids/19.2.337. [DOI] [PubMed] [Google Scholar]
- Watson RR, Prabhala RH, Abril E, Smith TL. Changes in lymphocyte subsets and macrophage functions from high, short-term dietary ethanol in C57/BL6 mice. Life Sci. 1988;43:865–870. doi: 10.1016/0024-3205(88)90514-0. [DOI] [PubMed] [Google Scholar]
- Kaplan DR. A novel mechanism of immunosuppression mediated by ethanol. Cell Immunol. 1986;102:1–9. doi: 10.1016/0008-8749(86)90320-5. [DOI] [PubMed] [Google Scholar]
- Chang MP, Norman DC, Makinodan T. Immunotoxicity of alcohol in young and old mice. I. In vitro suppressive effects of ethanol on the activities of T and B immune cells of aging mice. Alcohol Clin Exp Res. 1990;14:210–215. doi: 10.1111/j.1530-0277.1990.tb00474.x. [DOI] [PubMed] [Google Scholar]
- Greenberg SS, Ouyang J, Zhao X, Parrish C, Nelson S, Giles TD. Effects of ethanol on neutrophil recruitment and lung host defense in nitric oxide synthase I and nitric oxide synthase II knockout mice. Alcohol Clin Exp Res. 1999;23:1435–1445. [PubMed] [Google Scholar]
- Tamura DY, Moore EE, Partrick DA, Johnson JL, Offner PJ, Harbeck RJ, Silliman CC. Clinically relevant concentrations of ethanol attenuate primed neutrophil bactericidal activity. J Trauma. 1998;44:320–324. doi: 10.1097/00005373-199802000-00015. [DOI] [PubMed] [Google Scholar]
- Chen GJ, Huang DS, Watzl B, Watson RR. Ethanol modulation of tumor necrosis factor and gamma interferon production by murine splenocytes and macrophages. Life Sci. 1993;52:1319–1326. [PubMed] [Google Scholar]
- Arbabi S, Garcia I, Bauer GJ, Maier RV. Alcohol (ethanol) inhibits IL-8 and TNF: role of the p38 pathway. J Immunol. 1999;162:7441–7445. [PubMed] [Google Scholar]
- Szabo G, Catalano D, White B, Mandrekar P. Acute alcohol consumption inhibits accessory cell function of monocytes and dendritic cells. Alcohol Clin Exp Res. 2004;28:824–828. doi: 10.1097/01.alc.0000127104.80398.9b. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Howeedy A, Dorio R, Kajdacsy-Balla A. Functional analysis of T-cell subsets in chronic experimental alcoholism. Immunology. 1987;61:63–69. [PMC free article] [PubMed] [Google Scholar]
- Mili F, Flanders WD, Boring JR, Annest JL, DeStefano F. The associations of alcohol drinking and drinking cessation to measures of the immune system in middle-aged men. Alcohol Clin Exp Res. 1992;16:688–694. doi: 10.1111/j.1530-0277.1992.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Jerrells TR, Marietta CA, Bone G, Weight FF, Eckardt MJ. Ethanol-associated immunosuppression. Adv Biochem Psychopharmacol. 1988;44:173–185. [PubMed] [Google Scholar]
- Kaelin RM, Semerjian A, Center DM, Bernardo J. Influence of ethanol on human T-lymphocyte migration. J Lab Clin Med. 1984;104:752–760. [PubMed] [Google Scholar]
- Nelson S, Bagby GJ, Bainton BG, Summer WR. The effects of acute and chronic alcoholism on tumor necrosis factor and the inflammatory response. J Infect Dis. 1989;160:422–429. doi: 10.1093/infdis/160.3.422. [DOI] [PubMed] [Google Scholar]
- Kawakami M, Switzer BR, Herzog SR, Meyer AA. Immune suppression after acute ethanol ingestion and thermal injury. J Surg Res. 1991;51:210–215. doi: 10.1016/0022-4804(91)90096-5. [DOI] [PubMed] [Google Scholar]
- Gentilello LM, Cobean RA, Walker AP, Moore EE, Wertz MJ, Dellinger EP. Acute ethanol intoxication increases the risk of infection following penetrating abdominal trauma. J Trauma. 1993;34:665–674. doi: 10.1097/00005373-199305000-00009. [DOI] [PubMed] [Google Scholar]
- Friedman H. Alcohol effects on cytokine responses by immunocytes. Alcohol Clin Exp Res. 1998;22:184S–187S. doi: 10.1111/j.1530-0277.1998.tb03997.x. [DOI] [PubMed] [Google Scholar]
- Szabo G, Verma B, Catalano D. Selective inhibition of antigen-specific T lymphocyte proliferation by acute ethanol exposure: the role of impaired monocyte antigen presentation capacity and mediator production. J Leukoc Biol. 1993;54:534–544. doi: 10.1002/jlb.54.6.534. [DOI] [PubMed] [Google Scholar]
- Groettrup M, Soza A, Kuckelkorn U, Kloetzel PM. Peptide antigen production by the proteasome: complexity provides efficiency. Immunol Today. 1996;17:429–435. doi: 10.1016/0167-5699(96)10051-7. [DOI] [PubMed] [Google Scholar]
- Bardag-Gorce F, Yuan QX, Li J, French BA, Fang C, Ingelman-Sundberg M, French SW. The effect of ethanol-induced cytochrome p4502E1 on the inhibition of proteasome activity by alcohol. Biochem Biophys Res Commun. 2000;279:23–29. doi: 10.1006/bbrc.2000.3889. [DOI] [PubMed] [Google Scholar]
- Fehling HJ, Swat W, Laplace C, Kuhn R, Rajewsky K, Muller U, von Boehmer H. MHC class I expression in mice lacking the proteasome subunit LMP-7. Science. 1994;265:1234–1237. doi: 10.1126/science.8066463. [DOI] [PubMed] [Google Scholar]
- Van Kaer L, Ashton-Rickardt PG, Eichelberger M, Gaczynska M, Nagashima K, Rock KL, Goldberg AL, Doherty PC, Tonegawa S. Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity. 1994;1:533–541. doi: 10.1016/1074-7613(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M, Romero FJ, Garcia-Verdugo JM. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci USA. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun AY, Sun GY. Ethanol and oxidative mechanisms in the brain. J Biomed Sci. 2001;8:37–43. doi: 10.1007/BF02255969. [DOI] [PubMed] [Google Scholar]
- Amici M, Lupidi G, Angeletti M, Fioretti E, Eleuteri AM. Peroxynitrite-induced oxidation and its effects on isolated proteasomal systems. Free Radic Biol Med. 2003;34:987–996. doi: 10.1016/s0891-5849(02)01369-2. [DOI] [PubMed] [Google Scholar]
- Hansson T, Tindberg N, Ingelman-Sundberg M, Kohler C. Regional distribution of ethanol-inducible cytochrome P450 IIE1 in the rat central nervous system. Neuroscience. 1990;34:451–463. doi: 10.1016/0306-4522(90)90154-v. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Thrash JC, Lo D. Analysis of microglial gene expression: identifying targets for CNS neurodegenerative and autoimmune disease. Am J Pharmacogenomics. 2004;4:321–330. doi: 10.2165/00129785-200404050-00005. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Neafsey EJ, Collins MA. Human immunodeficiency virus type 1 gp120 and ethanol coexposure in rat organotypic brain slice cultures: curtailment of gp120-induced neurotoxicity and neurotoxic mediators by moderate but not high ethanol concentrations. J Neurovirol. 2003;9:45–54. doi: 10.1080/13550280390173409. [DOI] [PubMed] [Google Scholar]