Abstract
Lipid rafts/caveolae are membrane platforms for signaling molecules that regulate various cellular functions, including cell survival. To better understand the role of rafts in tumor progression and therapeutics, we investigated the effect of raft disruption on cell viability and compared raft levels in human cancer cell lines versus their normal counterparts. Here, we report that cholesterol depletion using methyl-β cyclodextrin caused anoikis-like apoptosis, which in A431 cells involved decreased raft levels, Bcl-xL down-regulation, caspase-3 activation, and Akt inactivation regardless of epidermal growth factor receptor activation. Cholesterol repletion replenished rafts on the cell surface and restored Akt activation and cell viability. Moreover, the breast cancer and the prostate cancer cell lines contained more lipid rafts and were more sensitive to cholesterol depletion-induced cell death than their normal counterparts. These results indicate that cancer cells contain increased levels of rafts and suggest a potential use of raft-modulating agents as anti-cancer drugs.
Lipid rafts are low-density, detergent-resistant microdomains of plasma membrane that are enriched in cholesterol and glycosphingolipids. Caveolae, a subclass of rafts, are characterized by flask-like invaginations of the plasma membrane that are distinguished from bulk lipid rafts by the presence of caveolin-1. Rafts/caveolae are known to be abundant in various signaling molecules, such as cell surface receptors and intracellular signaling molecules, and thus, these microdomains have been involved in many cellular functions, including the regulation of apoptosis and cell proliferation.1 Growth factor receptors, T-cell receptors, and the tumor necrosis factor receptor superfamily have been shown to interact with rafts/caveolae, and some intracellular signaling molecules are redistributed to rafts/caveolae after the activation of those receptors.2–6 Moreover, this redistribution plays an important role in the regulation of receptor-mediated cellular function, and accordingly, the disruption of rafts/caveolae results in the impairment of signaling events and receptor function. Therefore, it has been proposed that rafts/caveolae serve as molecular platforms that spatially organize appropriate molecules for specific signaling pathways.7
Cholesterol is an abundant component of the plasma membranes of eukaryotic cells and plays an essential role in maintaining membrane integrity and fluidity.8 It is also critical for liquid-ordered raft/caveolae formation by serving as a spacer between the hydrocarbon chains of sphingolipids.9,10 Therefore, it has been speculated that alterations in the cholesterol contents of cells should modify the properties of these domains. In fact, several lines of study have demonstrated that the depletion of cholesterol from the plasma membrane causes disruption of rafts/caveolae and release of raft/caveolae constituents into a non-raft/caveola membrane, which renders them nonfunctional.9,11 These studies indicate that cholesterol is crucial for maintaining intact raft/caveola structure and function.
The cholesterol contents of cell membranes are tightly regulated, and this process involves the uptake of cholesterol-rich low-density lipoprotein both from plasma and from synthetic pathways. Interestingly, cholesterol accumulation has been reported in various solid tumors, including prostate cancer and oral cancer.12,13 In addition, cholesterol metabolism is dysregulated in many malignancies, including myeloid leukemia, lung, and breast cancers.14–17 For example, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase is the rate-limiting enzyme in cholesterol biosynthesis that catalyzes mevalonate formation, and HMG-CoA reductase activity is up-regulated in certain tumors. Moreover, malignant cells have been reported to have elevated levels of mevalonate, a cholesterol precursor, and mevalonate treatment was found to promote tumor growth in vivo and to stimulate the proliferation of breast cancer cells.15
Akt/protein kinase B (PKB) is a serine/threonine kinase that is a critical regulator for cell survival and proliferation, especially in human malignant cancer. Activated Akt phosphorylates pro-apoptotic proteins, thereby inactivating their activities. Akt activation also up-regulates anti-apoptotic genes such as Bcl-xL and FLICE-inhibitory protein (FLIP).18–25 Akt activation involves phosphorylation of Ser473 and Thr308 by phosphoinositide-dependent kinases and integrin-linked kinase. Recent studies have suggested that rafts are implicated in Akt activation.26–28
Given that cholesterol is an essential lipid component of rafts/caveolae implicated in Akt activation and that cholesterol is accumulated in several tumors, the present study investigated whether changes in cholesterol level alter raft/caveola levels, which are critical for Akt activation and tumor cell survival. We found that cholesterol depletion results in apoptosis with Akt inactivation and that this occurs in parallel with reduced raft/caveola formation. The present study shows that cancer cell types with higher membrane cholesterol levels have more rafts/caveolae and are more sensitive to the apoptosis induced by cholesterol-depleting agents. We also discuss the implication of cholesterol accumulation in tumors on raft/caveola formation and provide a biological basis for the potential therapeutic applications of cholesterol regulation in cancer therapy.
Materials and Methods
Cell Culture
The human epidermoid carcinoma cell line A431, human normal prostate and breast epithelial cell lines PZ-HPV7 and MCF-10A, human breast cancer cell lines MCF-7 and MDA-MB-231, and human prostate cancer cell lines PC-3 and LNCaP were obtained from the American Type Culture Collection (Rockville, MD). Dulbecco’s modified Eagles medium (DMEM) and RPMI 1640 with l-glutamine were purchased from JEIL Biotech Services, Inc. (Daegu, Korea); fetal bovine serum (FBS) and antibiotic-antimycotic (100×) were obtained from GIBCO Laboratories (Grand Island, NY). Human A431 cells were grown at 37°C in DMEM supplemented with 10% FBS and antibiotic-antimycotic (1×). PC-3, LNCaP, MCF-7, and MDA-MB-231 were grown in RPMI 1640 containing 10% FBS and antibiotic-antimycotic (1×). Human normal prostate epithelial cell line PZ-HPV7 was grown in keratinocyte serum-free medium with 5 ng/ml human recombinant epidermal growth factor (EGF) and 50 μg/ml bovine pituitary extract. MCF-10A was maintained in DMEM with 5% horse serum, 10 μg/ml insulin, 10 ng/ml EGF, 0.5 ng/ml hydrocortisone, and 100 ng/ml cholera toxin. The cells were grown to approximately 70% confluence and were then serum-starved for 4 hours using 0.1% bovine serum albumin (BSA) in medium before treatment.
Antibodies and Reagents
Alexa Fluor 568-conjugated cholera toxin subunit B and Texas Red-labeled goat anti-rabbit IgG were from Molecular Probes (Eugene, OR). Anti-caspase-3, anti-Bcl-xL, horseradish peroxidase-conjugated goat anti-mouse IgG, and goat anti-rabbit IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-phospho-Akt (Ser473) antibody was from Cell Signaling Technology (Beverly, MA); and anti-Erk1/2, anti-active Erk1/2, and anti-poly (ADP-ribose) polymerase were from Upstate (Lake Placid, NY). Annexin V-fluorescein isothiocyanate (FITC) was purchased from BD Pharmingen (San Diego, CA). 3,3′-Diethyloxacabocyanine iodide (DiOC6) and 4′6-diamidino-2-phenylindole·2HCl (DAPI) were from Molecular Probes. Recombinant human EGF was purchased from Upstate. Immobilion-P polyvinylidene difluoride membranes (0.45 μm) were from Millipore (Bedford, MA). Micro-BCA protein assay reagents were from Pierce (Rockford, IL). Chemiluminescent reagents were obtained from Kirkegaard & Perry Laboratories (Gaithersburg, MD). Methyl-β cyclodextrin, filipin, water-soluble cholesterol, and simvastatin were from Sigma-Aldrich (St. Louis, MO).
Cell Viability Assay
The cells were plated at 2 × 105/ml in 100 μl of culture medium in a 96-well microtiter plate, followed by treatment. The effects of the reagents on the cell viability were determined 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Promega, Madison, WI) as described in the manufacturer’s instruction. Absorbance was measured at 490 nm for MTS assay with an enzyme-linked immunosorbent assay plate reader from MTX Lab Systems, Inc. (Vienna, VA). Each experiment was performed in triplicate.
Western Blotting
After washing with ice-cold phosphate-buffered saline (PBS; 10 mmol/L Na2HPO4, pH 7.4, 145 mmol/L NaCl, and 2.7 mmol/L KCl), cells were lysed with 2× sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis sample buffer (20 mmol/L Tris, pH 8.0, 2% SDS, 2 mmol/L dithiothreitol, 1 mmol/L Na3VO4, 2 mmol/L ethylenediamine tetraacetic acid, and 20% glycerol) and boiled for 5 minutes. Protein concentration of each sample was determined using a Micro-BCA protein assay reagent as described by the manufacturer. In all, 30 μg of total cellular protein was separated by 10% SDS-polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membranes as described previously.29 The membranes were blocked overnight at 4°C in 20 mmol/L Tris, pH 8.0, 150 mmol/L NaCl, and 0.05% Tween 20 (TBST) containing either 5% BSA (for immunoblotting with anti-phospho-Akt antibody) or 5% nonfat dried milk (for immunoblotting with other antibodies). The membranes were then incubated with the primary antibody for 1 hour at 37°C, washed three times with TBST, incubated with horseradish peroxidase-conjugated goat anti-mouse IgG or goat anti-rabbit IgG secondary antibodies for 1 hour at 37°C, and then washed with TBST three times. The labeled proteins were visualized using the enhanced chemiluminescence method. In the instances in which the same membrane was reprobed with a different primary antibody, the membrane was incubated in a stripping buffer (62.5 mmol/L Tris, pH 6.8, 2% SDS, and 100 mmol/L dithiothreitol) at 70°C for 30 minutes, washed extensively, reblocked with 5% nonfat milk, and then reprobed with another antibody as described above.
Fluorescence Activated Cell Sorting (FACS) Analysis
Cells were trypsinized and suspended in PBS containing 2.5 mmol/L ethylenediamine tetraacetic acid, 2.5 mmol/L ethylene glycol tetra acetic acid (EGTA), and 1% BSA. For the measurement of mitochondrial membrane potential changes, cells were incubated with 20 nmol/L DiOC6 in PBS for 15 minutes at 37°C. To determine apoptosis, cells were incubated with 10 μl/ml annexin V-FITC and 6 μl/ml propidium iodide (PI) for 10 minutes at room temperature, followed by FACS analysis (Coulter, Marseille, France). For FACS analysis of sub-G1 DNA contents, cells were fixed with 70% ethanol overnight at −20°C and stained with 0.5 ng/ml PI plus 0.5 mg/ml RNase A.
DNA Fragmentation Assay
After methyl-β cyclodextrin (MBCD) treatment, DNA was extracted using the ApopLadder Ex kit (TAKARA BIO INC., Tokyo, Japan) as directed by manufacturer. Fragmented DNA was separated by electrophoresis in a 2% (w/v) agarose gel, followed by ethidium bromide staining.
Immunofluorescence and Confocal Microscopy Imaging
Cells grown on coverslips were fixed with 2% paraformaldehyde in PBS at room temperature for 10 minutes, rinsed with PBS, and treated with 1.5 mg/ml glycine in PBS to quench free aldehyde groups. Cells were then stained either with Texas Red-phalloidin (0.04 μg/ml) for actin staining or with filipin (0.05 mg/ml) and cholera toxin B subunit (CTXB)-Alexa 568 (0.04 μg/ml) for plasma membrane cholesterol and GM1 staining, respectively. For nucleus staining, cells were incubated with DAPI (0.5 μg/ml). After washing with PBS, cells were examined using Carl Zeiss fluorescence microscopy or inverted laser-scanning microscopy (Thornwood, NY).
Data Analysis
All data points represented the mean value of at least three independent experiments, with triplicates for each. Statistical significance was determined by Student’s t-test with P < 0.05.
Results
Cholesterol Depletion Induced Apoptosis in A431 Cells
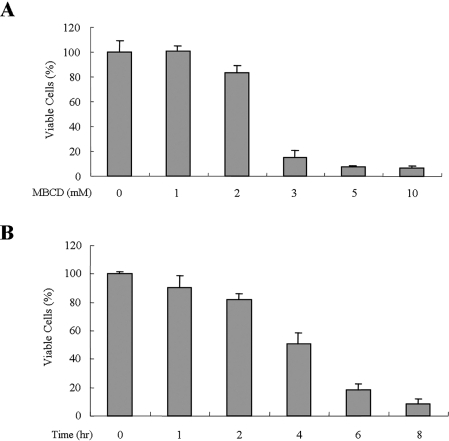
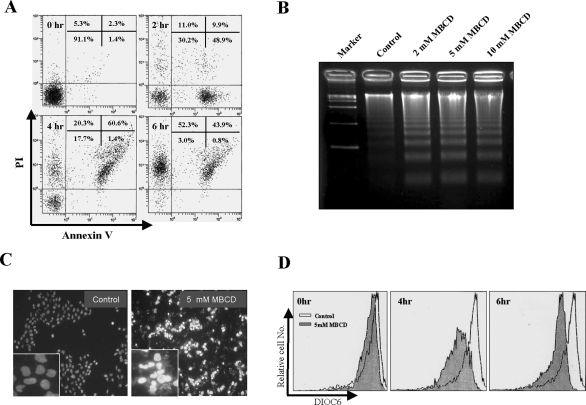
Although previous reports have demonstrated that the cholesterol depletion of A431 cells results in the activation of epidermal growth factor receptor,30,31 which is well known for survival signaling, the effect of cholesterol depletion on cell proliferation and/or cell viability has not been addressed. To investigate whether cholesterol depletion has an effect on cell viability, A431 cells were treated with various concentrations of MBCD, a cholesterol-depleting agent, for 24 hours, and then cell viability was determined using MTS assays. Interestingly, MBCD treatment dose-dependently inhibited cell proliferation from 3 mmol/L MBCD (Figure 1A). To assess the kinetics of cell death induced by MBCD, cells were treated with 5 mmol/L MBCD for 1 to 8 hours, and then cell viability was accessed by MTS assay. Within 4 hours of 5 mmol/L MBCD treatment, 50% cell death was observed (Figure 1B). To investigate whether MBCD-induced cell death is accompanied by apoptotic features, we examined the distribution of phosphatidyl serine on the plasma membrane, chromosomal DNA fragmentation, nuclei condensation, and the loss of mitochondrial membrane potential by annexin-V/PI staining, DNA laddering assay, DAPI staining, and the uptake of the mitochondria-specific dye DiOC6, respectively. MBCD-treated cells displayed an increased annexin-V-positive staining (early apoptosis) at 2 hours, and the annexin-V-positive and PI-positive (late apoptosis) population was increased at 4 hours, suggesting that MBCD-induced cell death follows an ordered apoptotic process (Figure 2A). In addition, analysis by agarose gel electrophoresis revealed DNA fragmentation in MBCD-treated cells (Figure 2B). Condensed and segmented nuclei were detected only in MBCD-treated cells (Figure 2C), and MBCD treatment also induced mitochondrial membrane potential changes (Figure 2D). These findings indicate that cholesterol depletion induces apoptosis in A431 cells. Therefore, we used A431 cells to further investigate the mechanism of MBCD-induced apoptosis.
Figure 1.
The effects of MBCD on cell viability. A: Serum-starved A431 cells were treated with 0 to 10 mmol/L MBCD for 24 hours, and cells were subjected to MTS assay as described in Materials and Methods. B: Serum-starved A431 cells were treated with 5 mmol/L MBCD for 0 to 8 hours, and cells were subjected MTS assay. Error bars represent the means ± SD of three independent experiments. Similar results were observed in two independent experiments.
Figure 2.
Apoptotic features of MBCD-induced cell death. A: A431 cells were treated with 5 mmol/L MBCD, stained with annexin V-FITC and PI, and subjected to flow cytometry analysis. Fluorescence dot blots of annexin V-positive (horizontal axis) and PI-positive (vertical axis) cells are shown. Cells that were positively stained by annexin V-FITC only (early apoptosis) and positive for both annexin V-FITC and PI (late apoptosis) were quantitated, and both subpopulations were considered as overall apoptotic cells. B: A431 cells were treated with 2, 5, and 10 mmol/L MBCD for 4 hours, and DNA was isolated and analyzed by 2% agarose gel electrophoresis. C: Cells were treated with 5 mmol/L MBCD for 4 hours, stained with DAPI, and analyzed by fluorescence microscopy. D: A431 cells were treated without or with 5 mmol/L MBCD for 4 and 6 hours and then incubated with DiOC6 to monitor ΔΨm by FACS. The loss of mitochondrial membrane potential was equated with decreased fluorescence and shift to the left. These experiments were repeated three separate times with comparable results.
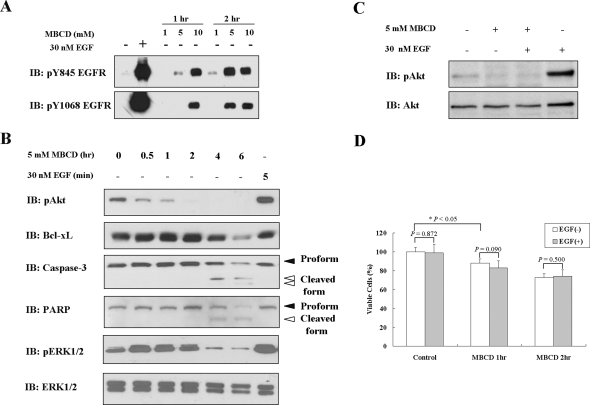
Cholesterol Depletion Activated Death-Related Pathways and Akt Inactivation, Which Could Not Be Restored by EGF Stimulation
Because A431 cells are known to overexpress the EGF receptor32 and because MBCD treatment has been reported to activate the EGF receptor, which is a well-known survival molecule, we also looked for MBCD-induced EGF receptor activation in our system. As shown in Figure 3A, cholesterol depletion increased phosphorylation of EGF receptor at its 845 and 1068 tyrosine residues time- and dose-dependently, which concurs with previous results.31 Because apoptosis was induced by MBCD despite EGF receptor activation and ERK1/2 activation (Figure 3B), we further explored the signaling pathways underlying MBCD-induced apoptosis. First, we investigated the status of Akt phosphorylation on cholesterol depletion because Akt plays a critical role in the cell survival signaling pathway.33 Akt activation, as assessed by its phosphorylation, was decreased time-dependently after MBCD treatment (Figure 3B). In addition, Bcl-xL, an anti-apoptotic molecule, was also decreased at protein (Figure 3B) and mRNA levels (Supplementary Figure S1 at http://ajp.amjpathol.org) in MBCD-treated cells. Based on the reduced Bcl-xL protein level and the loss of mitochondrial membrane potential after MBCD treatment, we examined whether caspase-3 is activated in MBCD-treated cells. As shown in Figure 3B, MBCD treatment resulted in caspase-3 activation as illustrated by a decrease in its proform (32 kd, black arrow) and a concomitant increase in its active form (17 and 19 kd, white arrow) as assessed by immunoblotting analysis using an anti-caspase-3 antibody that recognizes both forms. Subsequently, we also observed that MBCD caused the time-dependent proteolytic cleavage of PARP, a caspase-3 substrate, with accumulation of 85-kd fragments (white arrow) and the concomitant disappearance of the full-length 116-kd protein (black arrow) (Figure 3B).
Figure 3.
Effect of MBCD on basal and EGF-induced Akt activation. A: Serum-starved A431 cells were treated with 1, 5, or 10 mmol/L MBCD for 1 or 2 hours or with 30 nmol/L EGF for 5 minutes, followed by cell lysis with 2× sample buffer. Aliquots (30 μg of protein) from each treatment were subjected to immunoblotting analysis using anti-phospho-EGF receptor antibodies that specifically recognize tyrosine-phosphorylated EGF receptors at 845 or 1068 residues. B: Serum-starved A431 cells were treated with 5 mmol/L MBCD for 0 to 6 hours or with 30 nmol/L EGF for 5 minutes, and cells were lysed with 2× sample buffer. Thirty micrograms of protein from each treatment was subjected to immunoblotting analysis using antibodies specific for phospho-Akt, Bcl-xL, caspase-3, PARP, active-ERK1/2, and ERK1/2 (loading control). C: Serum-starved A431 cells were pretreated with 5 mmol/L MBCD and then washed with medium, followed by 30 nmol/L EGF treatment for 10 minutes. Thirty micrograms of protein from each treatment was subjected to immunoblotting analysis using anti-phospho Akt and anti-total Akt antibodies. D: Serum-starved A431 cells were pretreated with 5 mmol/L MBCD for 1 or 2 hours and then washed with medium, followed by 30 nmol/L EGF treatment for 2 hours. Cell viability was measured by MTS assay. Values represent the means ± SD of three independent experiments. These experiments were performed two separate times with comparable results.
Because EGF is a potent survival factor, we tested whether EGF stimulation can rescue cell viability after cholesterol depletion. EGF was able to activate Akt in serum-starved cells but not in MBCD-pretreated cells (Figure 3C). Consistent with this result, the differences between EGF-free and EGF-treated cells was not significant with respect to MBCD effect on cell viability, which means that EGF treatment could not reverse the MBCD effect on cell survival (Figure 3D). These data indicate that the integrity of lipid rafts is critical for both basal Akt activity and EGF-induced Akt activation for cell survival.
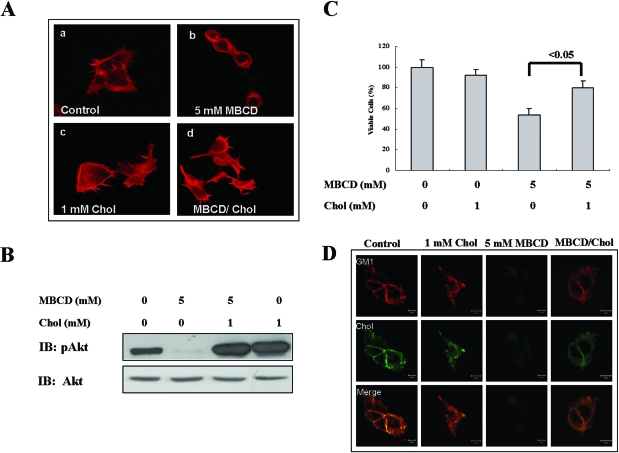
Reconstitution of Rafts/Caveolae by Cholesterol Addition Reactivated Akt and Restored Cell Viability
Because several studies have shown that cholesterol alters integrin-mediated cell adhesion,34,35 we examined whether the apoptosis induced by cholesterol depletion accompanies alteration of cell adhesion. MBCD-treated cells changed their shape from a spread shape to a round shape, and finally they detached from plates after 6 hours of treatment (Supplementary Figure S2 at http://ajp.amjpathol.org). This change was correlated with actin cytoskeletal reorganization in MBCD-treated cells, which was reversed by cholesterol addition (Figure 4A). Because we observed that cholesterol addition reverted MBCD-induced cell morphology change, we tested whether cholesterol replenishment also reactivates Akt and affects MBCD-induced apoptosis. As shown in Figure 4B, cholesterol addition after MBCD treatment was able to reactivate Akt, and this relatively rapid reactivation of Akt in cholesterol-treated cells appears to be correlated with an attenuated apoptosis induced by MBCD (Figure 4C). MBCD is known to deplete cholesterol from plasma membranes, thereby disrupting rafts/caveolae. However, we cannot rule out the possibility that the depletion of cholesterol results in decreased raft/caveola formation on the cell surface. To test this possibility, we stained cells with filipin for cholesterol and Alexa 568-conjugated CTXB for GM1, a marker for rafts/caveolae. Compared with control cells, cholesterol-treated cells showed strong GM1 staining patterns, whereas MBCD treatment resulted in a decrease in cholesterol staining and the GM1 disappearance from plasma membranes. However, cholesterol replenishment reversed these phenomena (Figure 4D). Taken together, these data indicate that cholesterol levels are critical for the maintenance and formation of rafts/caveolae and Akt activation.
Figure 4.
Effect of cholesterol repletion on cell morphology, Akt activation, cell viability, and raft/caveolae distribution. A: Serum-starved A431 cells were treated either without or with 5 mmol/L MBCD or 1 mmol/L cholesterol for 1 hour. Some MBCD-treated cells were washed with medium, and then cells were incubated without or with 1 mmol/L cholesterol for 1 hour. Cells were then stained with Texas Red-labeled phalloidin to visualize actin. B: Serum-starved A431 cells were treated as described in A, and then cell lysates were subjected to immunoblotting analysis using anti-phospho-Akt antibodies. C: Serum-starved A431 cells were treated as described in A, and cell viability was measured by MTS assay. Values represent the means ± SD of three independent experiments. D: Serum-starved A431 cells were treated as described in B, and cells were stained with filipin and Alexa Fluo568-CTXB and then subjected to confocal microscopy analysis. Similar results were obtained in three different experiments.
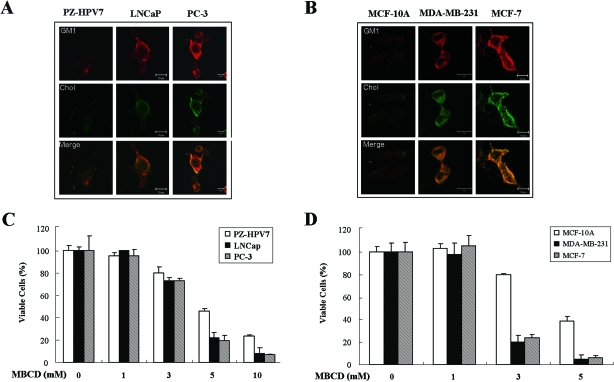
Breast and Prostate Cancer Cell Lines Contain Elevated Levels of Rafts/Caveolae and Were More Sensitive to Cholesterol Depletion-Induced Cell Death Than Their Normal Counterparts
Although cholesterol accumulation has been reported in some cancers, its implications in raft/caveolae formation have not been investigated. Based on our observation that cholesterol depletion decreases GM1 levels and that cholesterol repletion induced GM1 reappearance, it appeared possible that cholesterol accumulation is correlated with enhanced raft/caveolae formation and cell viability. To explore this possibility, we stained cholesterol and GM1 of human normal prostate epithelial cell line PZ-HPV7, human prostate cancer cell lines PC-3 (caveolin-1 positive) and LNCaP (caveolin-1 negative), human normal breast epithelial cell line MCF-10A, and human breast cancer cell lines MDA-MB-231 (caspase-3 positive) and MCF-7 (caspase-3 negative and very low levels of caveolin-1) without cell permeabilization.36 Although caveolin-1 has been proposed to be a tumor suppressor,37 caveolin-1 expression has been positively correlated with prostate cancer development.38 Interestingly, the cancer cell lines (PC-3, LNCaP, MCF-7, and MDA-MB-231) showed stronger cholesterol and GM1 staining than the normal cell lines (PZ-HPV7 and MCF-10A) (Figure 5, A and B). Accordingly, overlay data revealed a greater colocalization of cholesterol and GM1 in cancer cells, suggesting higher levels of rafts/caveolae in the cancer cell lines we tested in this study. When we purified lipid rafts based on the same cell number, about five times more lipid rafts were purified from PC-3 cells when compared with PZ-HPV7 (unpublished data). Next, we tested whether these cancer cells are more sensitive to MBCD-induced cell death. Interestingly, MBCD inhibited proliferation in prostate cancer and breast cancer cell lines in a dose-dependent manner, whereas their normal counterparts showed resistance to MBCD-induced cell death (Figure 5, C and D). To investigate whether this inhibition of cell proliferation reflects apoptosis, apoptotic cells were counted as cells containing sub-G1 DNA contents, and cancer cell lines were found to be more sensitive to MBCD-induced apoptosis (Supplementary Figure S3 at http://ajp.amjpathol.org). The cells with a DNA content less than a G1 cellular content via any possible DNA loss due to DNA fragmentation during apoptosis were classified sub-G1 population and considered as apoptotic cells.39 We also used human squamous cell carcinoma cell lines SCC-1 and SCC-6 that overexpress the EGF receptor32 and mouse fibroblast cell line B82L-CT97329 that was transfected with an oncogenic form of the EGF receptor to evaluate the effect of MBCD. Consistently, MBCD treatment caused Akt inactivation and apoptosis in those cell lines (Supplementary Figure S4 at http://ajp.amjpathol.org). These data indicate that cholesterol depletion can induce cell death regardless of deficiency of caveolin-1 and caspase-3 and activation of EGF receptor. Taken together, these data suggest that prostate and breast cancer cell lines contain elevated levels of rafts/caveolae, probably as a result of cholesterol accumulation, and that they are more susceptible to apoptosis caused by decreasing or disrupting raft/caveolae levels.
Figure 5.
Levels of rafts/caveolae in prostate cancer and breast cancer cell lines and their normal cell lines and their sensitivity to MBCD-induced apoptosis. A and B: Cells were cultured on aseptic coverslip overnight, and cells were stained with CTXB-Alexa 568 and filipin to detect plasma membrane cholesterol and lipid rafts, followed by confocal microscopy analysis. C and D: Serum-starved cells were treated with various concentrations of MBCD (0, 1, 3, 5, and 10 mmol/L) for 24 hours, and cells were subjected to MTS assay as described in Material and Methods. Values represent the means ± SD of three independent experiments. Similar observations were made in three different experiments.
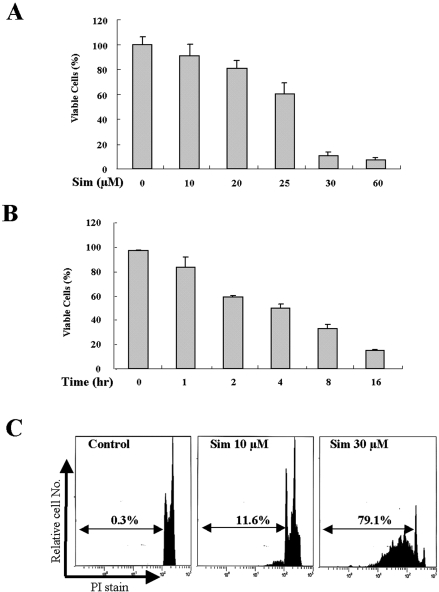
Decreased Levels of Cholesterol and Rafts by Simvastatin Treatment Correlate with Akt Inactivation and Apoptosis in A431 Cells
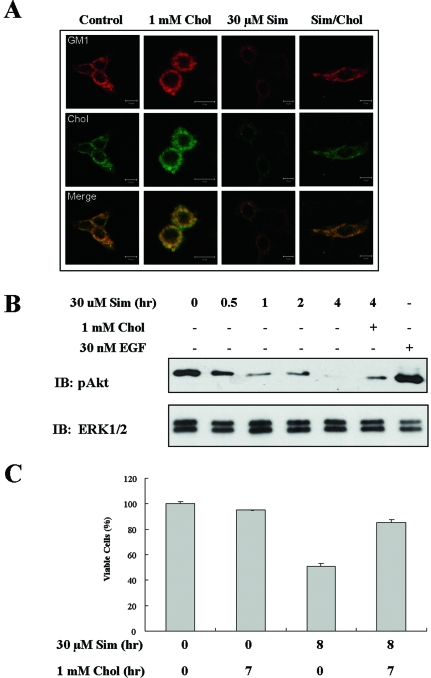
HMG-CoA reductase is a key enzyme in cholesterol synthesis that produces mevalonic acid, a precursor for cholesterol biosynthesis. Because cholesterol-lowering drugs such as the HMG-CoA reductase inhibitors, the statins, have been shown to have anticancer effects in many models,40–43 we tested whether simvastatin-induced cell death is related to lipid raft alterations. As shown in Figure 6A and B, the simvastatin treatment of A431 cells resulted in decreased cell viability in dose- and time-dependent manners. This reduced cell viability by simvastatin was further assessed by examining changes in cell morphology (data not shown) and increased sub-G1 population (Figure 6C). Next, to examine whether cholesterol synthesis inhibition can mediate lipid raft alterations, we investigated whether simvastatin treatment reduces lipid raft formation by confocal microscopic analysis of GM1 and cholesterol-stained cells. Interestingly, simvastatin treatment reduced the degree of both staining of cholesterol and GM1 and colocalization of those two in the plasma membrane, which could indicate decreased raft levels. However, cholesterol addition enhanced cholesterol levels and GM1 staining as well as their colocalization (Figure 7A). In addition, these decreased raft levels by simvastatin were recovered by cholesterol addition (Figure 7A). We further investigated the possibility that raft/caveolae levels decreased by simvastatin are associated with the down-regulation of Akt activity, and this is correlated with simvastatin-induced cell death. As shown in Figure 7B, pAkt detection was decreased in a time-dependent manner, and pAkt was almost undetectable at 4 hours of simvastatin treatment. However, cholesterol addition after simvastatin treatment restored Akt activity and rescued cells from simvastatin-induced cell death (Figure 7C). These data indicate that the cholesterol synthesis inhibitor simvastatin induces cell death, probably through reduction of raft formation, consequently down-regulating Akt activity.
Figure 6.
Simvastatin-induced apoptosis in A431 cells. A: Serum-starved A431 cells were treated with 0 to 60 μmol/L simvastatin for 24 hours, and cells were subjected to MTS assay as described in Materials and Methods. B: Serum-starved A431 cells were treated with 30 μmol/L simvastatin for 0 to 16 hours, and cells were subjected to MTS assay. Values represent the means ± SD of three independent experiments. C: Serum-starved A431 cells were treated either without or with 10 and 30 μmol/L simvastatin for 24 hours, and then cells were fixed and stained with propidium iodide, followed by sub-G1 analysis (values indicate the percentage of cells with sub-G1 DNA content). Values represent the means ± SD of three independent experiments. Similar results were obtained in three different experiments.
Figure 7.
The effect of simvastatin on raft/caveolae levels and Akt activity in A431 cells. A: A431 cells were cultured on aseptic coverslip overnight. Serum-starved A431 cells were treated without or with 1 mmol/L cholesterol for 2 hours, 30 μmol/L simvastatin for 4 hours, or 1 mmol/L cholesterol for 2 hours after pretreatment with 30 μmol/L simvastatin for 4 hours. Cells were then stained with CTXB-Alexa 568 and filipin to detect plasma membrane cholesterol and lipid rafts, followed by confocal microscopy analysis. B: Serum-starved A431 cells were treated without or with 30 μmol/L simvastatin for indicated times, or 1 mmol/L cholesterol for 2 hours after pretreatment with 30 μmol/L simvastatin for 4 hours or with 30 nmol/L EGF for 5 minutes. Cells were lysed with 2× sample buffer, and 30 μg of protein from each treatment was subjected to immunoblotting analysis using anti-phospho-Akt and anti-ERK1/2 antibodies (loading control). C: Serum-starved A431 cells were treated without or with 30 μmol/L simvastatin for indicated times, 1 mmol/L cholesterol for 2 hours, or 1 mmol/L cholesterol for 2 hours after pretreatment with 30 μmol/L simvastatin for 4 hours. MTS assay was performed to assess cell viability. Values represent the means ± SD of three independent experiments. Similar results were obtained in three different experiments.
Discussion
Many studies have revealed that rafts/caveolae are abundant in various signaling molecules and that they have been associated with a number of biological functions, including cell survival, proliferation, and migration.1 Cholesterol is a major lipid component of raft/caveola, and thus membrane cholesterol levels are an important factor for raft/caveolae stability and organization.8,9 Raft/caveolae disruption by cholesterol depletion from the plasma membrane has been reported to deregulate a number of intracellular signaling pathways and cross-talk between different receptor systems, indicating that raft/caveolae integrity is critical for intracellular signaling triggered by cell surface receptors.2 Although cholesterol accumulation has been reported in solid tumors,12 the effects of altered cholesterol levels on raft/caveolae formation and cell viability have not been explored. The present study demonstrates that cholesterol depletion of plasma membrane results in a reduction in raft/caveolae levels and leads to the anoikis-like apoptosis of various cancer cell types along with down-regulation of Akt activity and Bcl-xL and caspase-3 activation. This study also provides evidence that cells possessing higher levels of rafts/caveolae are more sensitive to the apoptosis induced by cholesterol depletion.
Akt, also known as PKB, is a major regulator of the survival-signaling cascade mediated by growth factors and cytokines. Akt activity has most commonly been described to be regulated by phosphorylation and the constitutive activation of Akt is a characteristic of various malignant tumors.44 Akt activation promotes cell survival via the phosphorylation and inactivation of pro-apoptotic proteins, including caspase-9 and Bad, as well as via activation of nuclear factor κB and thereby transcriptional up-regulation of anti-apoptotic genes such as Bcl-xL and FLIP.18–25 Our study showed that the disruption of rafts/caveolae by cholesterol depletion induced Bcl-xL down-regulation and Akt inactivation without changes in Akt protein levels (Figure 3, B and C). Correlating with Akt inactivation and Bcl-xL down-regulation, MBCD induced apoptosis through a mitochondrial pathway, including mitochondrial membrane potential change and caspase-3 activation. Because a recent study has shown that caspase-3 proenzyme and its active counterpart are co-purified with caveolin-enriched microdomains, we cannot exclude the possibility that alterations in cholesterol levels by MBCD treatment allow for the translocation of caspase-3 from rafts/caveolae, which results in enhanced caspase-3 activation as well as promiscuous characteristics of caspase-3, thereby inducing cell death.45
Chen et al30 have demonstrated that cholesterol depletion triggers signal transduction including EGF receptor activation in a ligand-independent manner. We also observed activation of EGF receptor, mitogen-activated protein kinase (MAPK), and Src (Figure 3, A and B; data not shown) after cholesterol depletion. Although survival factors, such as EGF, induce the activation of the phosphatidylinositol 3-kinase/Akt signaling pathway to promote cell survival, MBCD-induced EGF receptor activation per se might not be enough to rescue cells from apoptosis. EGF stimulation after raft/caveola disruption resulted in the further activation of EGF receptor and MAPK (data not shown) but not Akt (Figure 3C). It is intriguing that EGF administration could not restore Akt activation once rafts/caveolae were disrupted, but cholesterol addition reactivated Akt in the absence of EGF (Figure 4C). Consistent with these phenomena, cholesterol repletion but not EGF administration rescued cells from the apoptosis induced by raft/caveola disruption. Internalization-defective, oncogenic EGF receptors are known to reside in caveolae even after ligand binding,46 and this prolonged EGF receptor activity in caveolae generates altered signaling pathways such as the enhanced tyrosine phosphorylation of the caveolae molecules, caveolin-1 and dynamin.32 B82L cells that were transfected with noninternalizing, oncogenic EGF receptor construct also underwent apoptosis after MBCD treatment (Supplementary Figure S4 at http://ajp.amjpathol.org). These data indicate that the cholesterol depletion can induce apoptosis regardless of normal or oncogenic EGF receptor activation.
Adhesion to the extracellular matrix is necessary for the survival of adherent cell types and the disturbance of cell anchorage frequently leads to the immediate initiation of the suicide program known as anoikis.47,48 Focal adhesion kinase (FAK), when activated by integrins, can suppress anoikis, and PI3-kinase/Akt and MAPK may mediate this anoikis-suppressing effect in cells.49 In addition, certain Bcl-2 and Bcl-2-related proteins may also participate in the regulation of anoikis. Detachment of epithelial cells from the extracellular matrix has been reported to induce rapid caspase-3 activation and FAK degradation.50,51 In our study, MBCD treatment provoked rapid morphological change and cell detachment from plates, followed by cell death, indicating an anoikis-like cell death. We also observed that cholesterol depletion leads to FAK down-regulation (unpublished observations). It has been reported that cholesterol enrichment in the plasma membrane increases α5β1 integrin-mediated adhesion to fibronectin, indicating that membrane cholesterol can modify the function of adhesion molecules.34 It is possible that raft/caveola disruption and/or low levels of membrane cholesterol modulate affinity of integrins to extracellular matrix, leading to cell detachment and FAK down-regulation, which can promote apoptosis.
Cholesterol and sphingolipids are the two most important lipid components of rafts/caveolae that influence the formation and stability of rafts/caveolae. Membrane cholesterol levels and sphingolipid GM1, a marker for rafts/caveolae, can be evaluated by staining cells with filipin and CTXB, respectively. We noticed that both cholesterol and GM1 are enriched in raft/caveola fractions and that cholesterol depletion relocates GM1 from raft/caveola fractions to nonraft fractions (Supplementary Figure S5 at http://ajp.amjpathol.org). Therefore, in this study, we regarded the levels of rafts/caveolae as the levels of filipin and CTXB co-localization on cell surface. Interestingly, cholesterol addition recruited more GM1 to the cell surface and thus up-regulated raft/caveola formation (Figure 4E), whereas cholesterol depletion resulted in the disappearance of GM1 from the cell surface and consequently less co-localization of cholesterol and GM1. However, cholesterol repletion after MBCD treatment recovered cholesterol and GM1 co-localization levels, indicating raft/caveola re-formation. This raft/caveola reconstitution by cholesterol addition Akt activity and thus cell survival were restored. These data further support the notion that cholesterol is a critical component for raft/caveola formation and stability and that levels and/or integrity of rafts/caveolae are important for cell survival.
Rapidly proliferating tumor cells presumably require cholesterol for new membrane synthesis. Cholesterol accumulation may be a more general property of cancer, and it has been especially well correlated with prostate cancer progression. Although raft disruption is reported to inactivate Akt and to induce apoptosis in LNCaP cells, the association of raft/caveolae levels with cell survival has not been addressed.52 Freeman and Solomon12 have suggested a model for how increases in membrane cholesterol alter signal transduction in cancer cells. They postulated that elevated cholesterol levels lead to raft coalescence, which might serve to sequester and thus stimulate “on” signals to oncogenic pathway. It is very interesting to find elevated levels of membrane cholesterol in prostate cancer cell lines and breast cancer cell lines compared with their normal counterparts (Figure 5, A and B). These increased membrane cholesterol levels were positively correlated with raft/caveola levels as assessed by the degree of GM1 and cholesterol co-localization (Figure 5, A and B) and by raft/caveola fractionation (Supplementary Figure S5 at http://ajp.amjpathol.org). These data suggest that prostate cancer cells and breast cancer cells contain more rafts/caveolae than their normal cell lines. Accordingly, it is likely that cells with higher levels of rafts/caveolae would undergo cell death if membrane cholesterol is depleted. Moreover, it is very intriguing that prostate cancer cells and breast cancer cells are more sensitive to MBCD-induced apoptosis than their normal cell lines (Figure 5, C and D). Recently, Zhuang et al53 have also reported that cholesterol depletion inhibits Akt phosphorylation but does not induce apoptosis in normal prostate epithelial cells. In addition, they demonstrated that cholesterol elevation promoted tumor growth, increased Akt activation, and induced tumor aggressiveness in subcutaneous inoculated xenograft tumors.
Cholesterol metabolism is dysregulated in many hematopoietic malignancies including acute myeloid leukemia, and high cellular cholesterol may also improve leukemia cell survival and impart relative resistance to therapy.54,55 HMG-CoA reductase produces mevalonate, a precursor for cholesterol biosynthesis, and generates isoprenoids that posttranslationally modify specific proteins for membrane targeting.56 It is known that malignant cells have elevated rates of mevalonate synthesis because of higher levels and activity of HMG-CoA reductase.57 Mevalonate has also been reported to promote tumor growth. HMG-CoA reductase inhibitors, more commonly known as statins, are cholesterol-lowering drugs that have been widely used to lower serum cholesterol levels. Statins have also been demonstrated to exert potent anti-cancer effects in model systems, and recently, several reviews have summarized this topic.42,56–58 However, the effect of cholesterol synthesis inhibition on raft/caveola formation has not been addressed so far. In our study, we demonstrate that simvastatin treatment causes reduction of raft/caveolae formation, Akt inactivation, and apoptosis. Moreover, cholesterol addition was able to rescue cells from simvastatin-induced cell death along with raft/caveolae re-formation and Akt reactivation. Therefore, it is conceivable that simvastatin induces apoptosis by disrupting and/or lowering rafts/caveolae in addition to inhibition of lipid modifications of membrane targeting of signaling molecules.
Caveolin-1 expression and caspase-3 deletion have been linked to malignant transformation in prostate and breast cancers, respectively.59,60 Recently, Lisanti et al61,62 have also demonstrated that caveolin-1 promotes tumor progression in mouse model of prostate cancer and clearly established the role for caveolin-1 function as a tumor and/or metastasis modifier gene. However, we found that cholesterol depletion induced apoptosis in caveolon-1-expressing PC-3, caveolin-1-deficient LNCaP, caspase-3-positive MDA-MB-231, and caspase-3-deficient MCF-7 cell lines, further supporting the concept that cholesterol content is more important for cell survival than caveolin-1 or caspase-3 status.
In conclusion, cholesterol depletion from the plasma membrane results in anoikis-like apoptosis, and this type of cell death is remarkable in prostate and breast cancer cell lines that possess higher levels of membrane cholesterol. In addition, raft/caveolae disruption induced apoptosis regardless of EGF receptor activation, caveolin-1 expression, and caspase-3 deficiency. Cholesterol accumulation and higher activities of HMG-CoA reductase in multiple cancers have been reported, but their implications on tumor progression have not been intensively addressed. However, it has been proposed that progressive increases in membrane cholesterol contribute to the expansion of rafts/caveolae, which may potentiate oncogenic pathways of cell signaling. Therefore, the targeted therapy for rafts/caveolae would be one of the possible cancer chemotherapy, such as changing cell membrane cholesterol contents, thereby modulating raft/caveola levels. The findings of this study provide a biological basis for the potential therapeutic applications of cholesterol regulation in cancer therapy.
Supplementary Material
Footnotes
Address reprint requests to Yong-Nyun Kim, Division of Specific Organs Cancer, Pediatric Oncology Division, National Cancer Center, 809 Madu 1-dong, Ilsan-gu, Goyang-si, Gyeonggi-do, 411-769, Korea. E-mail: ynk@ncc.re.kr.
Supported by the Korea Science and Engineering Foundation (grant C00524).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–411. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- Waugh MG, Minogue S, Anderson JS, dos Santos M, Hsuan JJ. Signalling and non-caveolar rafts. Biochem Soc Trans. 2001;29:509–511. doi: 10.1042/bst0290509. [DOI] [PubMed] [Google Scholar]
- Harder T. Lipid raft domains and protein networks in T-cell receptor signal transduction. Curr Opin Immunol. 2004;16:353–359. doi: 10.1016/j.coi.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Muppidi JR, Siegel RM. Ligand-independent redistribution of Fas (CD95) into lipid rafts mediates clonotypic T cell death. Nat Immunol. 2004;5:182–189. doi: 10.1038/ni1024. [DOI] [PubMed] [Google Scholar]
- Gniadecki R. Depletion of membrane cholesterol causes ligand-independent activation of Fas and apoptosis. Biochem Biophys Res Commun. 2004;320:165–169. doi: 10.1016/j.bbrc.2004.05.145. [DOI] [PubMed] [Google Scholar]
- Carpenter G. The EGF receptor: a nexus for trafficking and signaling. Bioessays. 2000;22:697–707. doi: 10.1002/1521-1878(200008)22:8<697::AID-BIES3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Zajchowski LD, Robbins SM. Lipid rafts and little caves: compartmentalized signalling in membrane microdomains. Eur J Biochem. 2002;269:737–752. doi: 10.1046/j.0014-2956.2001.02715.x. [DOI] [PubMed] [Google Scholar]
- Silvius JR. Role of cholesterol in lipid raft formation: lessons from lipid model systems. Biochim Biophys Acta. 2003;1610:174–183. doi: 10.1016/s0005-2736(03)00016-6. [DOI] [PubMed] [Google Scholar]
- London E, Brown DA. Insolubility of lipids in Triton X-100: physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim Biophys Acta. 2000;1508:182–195. doi: 10.1016/s0304-4157(00)00007-1. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Scheel-Toellner D, Wang K, Singh R, Majeed S, Raza K, Curnow SJ, Salmon M, Lord JM. The death-inducing signalling complex is recruited to lipid rafts in Fas-induced apoptosis. Biochem Biophys Res Commun. 2002;297:876–879. doi: 10.1016/s0006-291x(02)02311-2. [DOI] [PubMed] [Google Scholar]
- Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem. 2004;91:54–69. doi: 10.1002/jcb.10724. [DOI] [PubMed] [Google Scholar]
- Kolanjiappan K, Ramachandran CR, Manoharan S. Biochemical changes in tumor tissues of oral cancer patients. Clin Biochem. 2003;36:61–65. doi: 10.1016/s0009-9120(02)00421-6. [DOI] [PubMed] [Google Scholar]
- Li HY, Appelbaum FR, Willman CL, Zager RA, Banker DE. Cholesterol-modulating agents kill acute myeloid leukemia cells and sensitize them to therapeutics by blocking adaptive cholesterol responses. Blood. 2003;101:3628–3634. doi: 10.1182/blood-2002-07-2283. [DOI] [PubMed] [Google Scholar]
- Duncan RE, El-Sohemy A, Archer MC. Mevalonate promotes the growth of tumors derived from human cancer cells in vivo and stimulates proliferation in vitro with enhanced cyclin-dependent kinase-2 activity. J Biol Chem. 2004;279:33079–33084. doi: 10.1074/jbc.M400732200. [DOI] [PubMed] [Google Scholar]
- El-Sohemy A, Archer MC. Inhibition of N-methyl-N-nitrosourea- and 7,12-dimethylbenz[a] anthracene-induced rat mammary tumorigenesis by dietary cholesterol is independent of Ha-Ras mutations. Carcinogenesis. 2000;21:827–831. doi: 10.1093/carcin/21.4.827. [DOI] [PubMed] [Google Scholar]
- Bennis F, Favre G, Le Gaillard F, Soula G. Importance of mevalonate-derived products in the control of HMG-CoA reductase activity and growth of human lung adenocarcinoma cell line A549. Int J Cancer. 1993;55:640–645. doi: 10.1002/ijc.2910550421. [DOI] [PubMed] [Google Scholar]
- Shimamura H, Terada Y, Okado T, Tanaka H, Inoshita S, Sasaki S. The PI3-kinase-Akt pathway promotes mesangial cell survival and inhibits apoptosis in vitro via NF-kappa B and Bad. J Am Soc Nephrol. 2003;14:1427–1434. doi: 10.1097/01.asn.0000066140.99610.32. [DOI] [PubMed] [Google Scholar]
- Romashkova JA, Makarov SS. NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature. 1999;401:86–90. doi: 10.1038/43474. [DOI] [PubMed] [Google Scholar]
- Panka DJ, Mano T, Suhara T, Walsh K, Mier JW. Phosphatidylinositol 3-kinase/Akt activity regulates c-FLIP expression in tumor cells. J Biol Chem. 2001;276:6893–6896. doi: 10.1074/jbc.C000569200. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Edelstein LC, Gelinas C. The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L). Mol Cell Biol. 2000;20:2687–2695. doi: 10.1128/mcb.20.8.2687-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partovian C, Simons M. Regulation of protein kinase B/Akt activity and Ser473 phosphorylation by protein kinase Calpha in endothelial cells. Cell Signal. 2004;16:951–957. doi: 10.1016/j.cellsig.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Elhyany S, Assa-Kunik E, Tsory S, Muller T, Fedida S, Segal S, Fishman D. The integrity of cholesterol-enriched microdomains is essential for the constitutive high activity of protein kinase B in tumour cells. Biochem Soc Trans. 2004;32:837–839. doi: 10.1042/BST0320837. [DOI] [PubMed] [Google Scholar]
- Hill MM, Feng J, Hemmings BA. Identification of a plasma membrane Raft-associated PKB Ser473 kinase activity that is distinct from ILK and PDK1. Curr Biol. 2002;12:1251–1255. doi: 10.1016/s0960-9822(02)00973-9. [DOI] [PubMed] [Google Scholar]
- Kim YN, Wiepz GJ, Guadarrama AG, Bertics PJ. Epidermal growth factor-stimulated tyrosine phosphorylation of caveolin-1: enhanced caveolin-1 tyrosine phosphorylation following aberrant epidermal growth factor receptor status. J Biol Chem. 2000;275:7481–7491. doi: 10.1074/jbc.275.11.7481. [DOI] [PubMed] [Google Scholar]
- Chen X, Resh MD. Cholesterol depletion from the plasma membrane triggers ligand-independent activation of the epidermal growth factor receptor. J Biol Chem. 2002;277:49631–49637. doi: 10.1074/jbc.M208327200. [DOI] [PubMed] [Google Scholar]
- Westover EJ, Covey DF, Brockman HL, Brown RE, Pike LJ. Cholesterol depletion results in site-specific increases in epidermal growth factor receptor phosphorylation due to membrane level effects: studies with cholesterol enantiomers. J Biol Chem. 2003;278:51125–51133. doi: 10.1074/jbc.M304332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YN, Bertics PJ. The endocytosis-linked protein dynamin associates with caveolin-1 and is tyrosine phosphorylated in response to the activation of a noninternalizing epidermal growth factor receptor mutant. Endocrinology. 2002;143:1726–1731. doi: 10.1210/endo.143.5.8814. [DOI] [PubMed] [Google Scholar]
- Downward J. PI 3-kinase, Akt and cell survival. Semin Cell Dev Biol. 2004;15:177–182. doi: 10.1016/j.semcdb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna P, Rangaraj N, Pande G. Cholesterol alters the interaction of glycosphingolipid GM3 with alpha5beta1 integrin and increases integrin-mediated cell adhesion to fibronectin. Exp Cell Res. 2004;300:43–53. doi: 10.1016/j.yexcr.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Gopalakrishna P, Chaubey SK, Manogaran PS, Pande G. Modulation of alpha5beta1 integrin functions by the phospholipid and cholesterol contents of cell membranes. J Cell Biochem. 2000;77:517–528. [PubMed] [Google Scholar]
- Ravid D, Maor S, Werner H, Liscovitch M. Caveolin-1 inhibits cell detachment-induced p53 activation and anoikis by upregulation of insulin-like growth factor-I receptors and signaling. Oncogene. 2005;24:1338–1347. doi: 10.1038/sj.onc.1208337. [DOI] [PubMed] [Google Scholar]
- Hino M, Doihara H, Kobayashi K, Aoe M, Shimizu N. Caveolin-1 as tumor suppressor gene in breast cancer. Surg Today. 2003;33:486–490. doi: 10.1007/s10595-002-2538-4. [DOI] [PubMed] [Google Scholar]
- Li L, Ren CH, Tahir SA, Ren C, Thompson TC. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol. 2003;23:9389–9404. doi: 10.1128/MCB.23.24.9389-9404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- Denoyelle C, Albanese P, Uzan G, Hong L, Vannier JP, Soria J, Soria C. Molecular mechanism of the anti-cancer activity of cerivastatin, an inhibitor of HMG-CoA reductase, on aggressive human breast cancer cells. Cell Signal. 2003;15:327–338. doi: 10.1016/s0898-6568(02)00124-9. [DOI] [PubMed] [Google Scholar]
- Schmidmaier R, Baumann P, Simsek M, Dayyani F, Emmerich B, Meinhardt G. The HMG-CoA reductase inhibitor simvastatin overcomes cell adhesion-mediated drug resistance in multiple myeloma by geranylgeranylation of Rho protein and activation of Rho kinase. Blood. 2004;104:1825–1832. doi: 10.1182/blood-2003-12-4218. [DOI] [PubMed] [Google Scholar]
- Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9:10–19. [PubMed] [Google Scholar]
- Seeger H, Wallwiener D, Mueck AO. Statins can inhibit proliferation of human breast cancer cells in vitro. Exp Clin Endocrinol Diabetes. 2003;111:47–48. doi: 10.1055/s-2003-37501. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Park J, Hemmings BA. PKB binding proteins: getting in on the Akt. Cell. 2002;111:293–303. doi: 10.1016/s0092-8674(02)01083-8. [DOI] [PubMed] [Google Scholar]
- Oxhorn BC, Buxton IL. Caveolar compartmentation of caspase-3 in cardiac endothelial cells. Cell Signal. 2003;15:489–496. doi: 10.1016/s0898-6568(02)00149-3. [DOI] [PubMed] [Google Scholar]
- Mineo C, Gill GN, Anderson RG. Regulated migration of epidermal growth factor receptor from caveolae. J Biol Chem. 1999;274:30636–30643. doi: 10.1074/jbc.274.43.30636. [DOI] [PubMed] [Google Scholar]
- Grossmann J. Molecular mechanisms of “detachment-induced apoptosis–Anoikis”. Apoptosis. 2002;7:247–260. doi: 10.1023/a:1015312119693. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- Xia H, Nho RS, Kahm J, Kleidon J, Henke CA. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem. 2004;279:33024–33034. doi: 10.1074/jbc.M313265200. [DOI] [PubMed] [Google Scholar]
- Walden PD, Globina Y, Nieder A. Induction of anoikis by doxazosin in prostate cancer cells is associated with activation of caspase-3 and a reduction of focal adhesion kinase. Urol Res. 2004;32:261–265. doi: 10.1007/s00240-003-0365-7. [DOI] [PubMed] [Google Scholar]
- Wen LP, Fahrni JA, Troie S, Guan JL, Orth K, Rosen GD. Cleavage of focal adhesion kinase by caspases during apoptosis. J Biol Chem. 1997;272:26056–26061. doi: 10.1074/jbc.272.41.26056. [DOI] [PubMed] [Google Scholar]
- Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62:2227–2231. [PubMed] [Google Scholar]
- Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115:959–968. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksumova L, Ohnishi K, Muratkhodjaev F, Zhang W, Pan L, Takeshita A, Ohno R. Increased sensitivity of multidrug-resistant myeloid leukemia cell lines to lovastatin. Leukemia. 2000;14:1444–1450. doi: 10.1038/sj.leu.2401856. [DOI] [PubMed] [Google Scholar]
- Vitols S, Norgren S, Juliusson G, Tatidis L, Luthman H. Multilevel regulation of low-density lipoprotein receptor and 3-hydroxy-3-methylglutaryl coenzyme A reductase gene expression in normal and leukemic cells. Blood. 1994;84:2689–2698. [PubMed] [Google Scholar]
- Mo H, Elson CE. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp Biol Med (Maywood) 2004;229:567–585. doi: 10.1177/153537020422900701. [DOI] [PubMed] [Google Scholar]
- Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508–519. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- Splichal JE, Stamm JA, Ornstein DL. The statins: multifunctional antithrombotic and antineoplastic drugs. Semin Thromb Hemost. 2003;29:259–274. doi: 10.1055/s-2003-40964. [DOI] [PubMed] [Google Scholar]
- Thompson TC, Timme TL, Li L, Goltsov A. Caveolin-1, a metastasis-related gene that promotes cell survival in prostate cancer. Apoptosis. 1999;4:233–237. doi: 10.1023/a:1009612708099. [DOI] [PubMed] [Google Scholar]
- Devarajan E, Sahin AA, Chen JS, Krishnamurthy RR, Aggarwal N, Brun AM, Sapino A, Zhang F, Sharma D, Yang XH, Tora AD, Mehta K. Down-regulation of caspase 3 in breast cancer: a possible mechanism for chemoresistance. Oncogene. 2002;21:8843–8851. doi: 10.1038/sj.onc.1206044. [DOI] [PubMed] [Google Scholar]
- Williams TM, Hassan GS, Li J, Cohen AW, Medina F, Frank PG, Pestell RG, Di Vizio D, Loda M, Lisanti MP. Caveolin-1 promotes tumor progression in an autochthonous mouse model of prostate cancer: genetic ablation of Cav-1 delays advanced prostate tumor development in TRAMP mice. J Biol Chem. 2005;280:25134–25145. doi: 10.1074/jbc.M501186200. [DOI] [PubMed] [Google Scholar]
- Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494–C506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.