Abstract
Dystrophin deficiency is the cause of Duchenne muscular dystrophy, but the precise physiological basis for muscle necrosis remains unclear. To determine whether dystrophin-deficient muscles are abnormally susceptible to oxidative and nitric oxide (NO)-driven tissue stress, a hindlimb ischemia/reperfusion (I/R) model was used. Dystrophic mdx mice exhibited abnormally high levels of lipid peroxidation and protein nitration, which were preceded by exaggerated NO production during ischemia. Visualization of NO with the fluorescent probe 4,5-diaminofluorescein diacetate suggested that excess NO production during ischemia occurred within a subset of mdx fibers. In mdx muscles only, prior exposure to I/R dramatically increased the level of sarcolemmal damage resulting from stretch-mediated mechanical stress, indicating greatly exacerbated hyperfragility of the dystrophic fiber membrane. Treatment with NO synthase inhibitors (l-NG-nitroarginine methyl ester hydrochloride or 7-nitroindazol) effectively blocked the synergistic interaction between I/R and mechanical stress-mediated sarcolemmal damage under these conditions. Taken together, our findings provide direct ex-perimental evidence that several prevailing hypotheses regarding the cause of muscle fiber damage in dystrophin-deficient muscle can be integrated into a common pathophysiological framework involving interactions between oxidative stress, abnormal NO regulation, and hyperfragility of the sarcolemma.
Duchenne muscular dystrophy (DMD) is a fatal X-linked recessive neuromuscular disorder affecting approximately 1 in 3500 male births. The disease results from the absence of a 427-kd cytoskeletal protein, known as dystrophin,1 that is normally found at the inner aspect of the muscle fiber surface membrane, or sarcolemma. DMD patients develop severe skeletal muscle weakness due to progressive muscle fiber destruction and replacement of the muscle by noncontractile connective tissue elements. Patients are generally wheelchair bound by their early teens, and death most often occurs because of involvement of the diaphragm and other respiratory muscles. The most widely used animal model of DMD, the mdx mouse, lacks dystrophin because of a spontaneous nonsense mutation within exon 23 of the murine dystrophin gene.2 One of the most characteristic and early features of dystrophin deficiency in DMD patients and mdx mice is sarcolemmal damage, which can be ascertained at a morphological level3,4 and through the abnormal bidirectional passage of large molecules between muscle fibers and the bloodstream.5,6 In addition, both in vitro and in vivo studies have shown that dystrophin-deficient muscle cells are abnormally vulnerable to membrane damage when subjected to increased mechanical stress.7–10
In normal skeletal muscle, dystrophin exists as part of a multimolecular network (the dystrophin-glycoprotein complex [DGC]) that spans the sarcolemma and permits a physical linkage to be established between the intracellular cytoskeleton and the extracellular matrix.11,12 In addition to dystrophin itself, the DGC is composed of integral membrane (dystroglycan and sarcoglycan-sarcospan subcomplexes) and subsarcolemmal (syntrophins and dystrobrevins) components.13–15 Several potential signaling molecules are also associated with the DGC. In particular, the neuronal isoform of nitric oxide synthase (nNOS) has received major attention, because it is involved in multiple aspects of skeletal muscle function.16 In the setting of dystrophin deficiency, most members of the DGC are completely lost from the sarcolemma. In the case of nNOS, there is displacement of the enzyme away from the sarcolemma to an abnormal location within the myofiber cytosol, where its enzymatic activity is also significantly reduced.17,18 This has been shown to result in a failure of normal NO-mediated vasodilation during muscle contraction, which leads to episodes of relative muscle ischemia during muscle activity followed by reperfusion during rest.19,20
Despite great progress in identifying the genetic and biochemical abnormalities associated with DMD as outlined above, the precise mechanisms by which absence of DGC components from the sarcolemma causes progressive muscle pathology remain ill-defined. Leading candidate pathogenetic mechanisms implicated in the initiation of myofiber injury include 1) weakening of the sarcolemma due to a loss of mechanical reinforcement from dystrophin, 2) inappropriate calcium influx, 3) aberrant cell signaling, 4) increased oxidative stress, and 5) recurrent muscle ischemia related to abnormal NO regulation (for review, see Petrof et al21 ). It should be emphasized that the above mechanisms are in no way mutually exclusive and may interact with one another to a significant degree.22 However, a plausible model of disease pathogenesis in dystrophin deficiency should be able to account for the abnormal membrane permeability and vulnerability to sarcolemmal damage induced by increased mechanical stress,8–10 because these are characteristic functional aspects of the disease phenotype.
It is well established that oxidative stress is capable of causing significant modifications to the biophysical properties of cell membranes.23 Few data are available to support or refute the existence of a heightened susceptibility to oxidative stress-induced membrane damage in DMD patients, but it is interesting to note that transient limb ischemia of 10-minute duration followed by 1 minute of reperfusion caused increased serum creatine kinase levels in a group of DMD patients.24 Ischemia/reperfusion (I/R) has been implicated in the pathogenesis of dystrophin deficiency as noted earlier19,20,25 and is also a potent inducer of oxidative stress in muscle.26–29 In the present study, we hypothesized that dystrophin-deficient muscle could be abnormally susceptible to cellular damage induced by oxidative and nitrosative (NO-driven) modifications, which could in turn promote the hallmark increases in membrane fragility and mechanical stress-induced disruption of the sarcolemma. Accordingly, our specific objectives were as follows: 1) to directly compare indices of oxidative stress generated by I/R in wild-type and dystrophin-deficient muscles to determine whether the latter demonstrate an abnormal vulnerability; 2) to examine NO regulation by wild-type and dystrophic muscles in the setting of I/R to ascertain whether there is greater nitrosative stress in dystrophic muscles under these conditions; and 3) to determine whether I/R and the associated changes in oxidative/nitrosative stress have any effect on one of the most emblematic features of dystrophin deficiency, ie, the reduced ability of the sarcolemma to maintain its structural integrity when subjected to increased levels of mechanical stress.
Materials and Methods
Reagents and Mice
Polyclonal antibody directed against 4-hydroxy-2-nonenal (HNE) protein adducts was purchased from Calbiochem (San Diego, CA). A monoclonal antibody directed against 3-nitrotyrosine was obtained from Caymen Chemicals (Ann Arbor, MI). Monoclonal antibodies directed against nNOS (NOS 1), iNOS (NOS 2), and eNOS (NOS 3) were purchased from Transduction Laboratories (Lexington, KY). Horseradish peroxidase-conjugated goat anti-rabbit IgG was purchased from Cedarlane Laboratories Limited (Hornby, ON, Canada), and horseradish peroxidase-conjugated anti-mouse IgG was obtained from Transduction Laboratories. The enhanced chemiluminescence kit for detection of immunoblotted proteins was purchased from Amersham Pharmacia Biotech UK Limited (Buckinghamshire, UK). The nitric oxide synthase inhibitors l-NG-nitroarginine methyl ester hydrochloride (l-NAME) and 7-nitroindazol (7-NI) were obtained from Caymen Chemicals; the former is a general inhibitor of NOS isoforms, whereas the latter is selective for nNOS at the appropriate dose.30–32 The fluorescence probe 4,5-diaminofluorescein diacetate (DAF-2) was purchased from Calbiochem. All other chemical reagents were purchased from Sigma Chemical Co. (St. Louis, MO). Dystrophin-deficient (mdx) and normal wild-type mice control mice (C57) were purchased from The Jackson Laboratory (Bar Harbor, ME). Animals were studied at 6 to 8 weeks of age and were exclusively males. The mice were provided with food and water ad libitum, and all experimental protocols were approved by the institutional animal care and ethics committee.
I/R Protocol
To induce ischemia in the tibialis anterior (TA) muscle, a locking plastic pull-tie tourniquet (2 mm wide) was applied just above the knee of the right hindlimb of deeply anesthetized (ketamine, 130 mg/kg; and xylazine, 20 mg/kg intramuscularly) mdx and wild-type mice. A sponge pad was placed underneath the tourniquet to prevent skin injury. Cessation of blood flow was confirmed by a notable decrease in skin temperature and a loss of capillary refill in the foot of the involved extremity. After 90 minutes, the tourniquet was removed, and capillary refill quickly returned to normal. To study the effects of I/R, animals were euthanized at either 1 hour (for indices of oxidative and nitrosative stress) or 3 hours (for Evans Blue dye uptake) after the removal of the tourniquet (see below). The time periods of ischemia and reperfusion used here are similar to those in previous reports of short-term I/R injury in rodent hindlimb muscles.27,29 Control mice (sham-treated) were anesthetized in the same manner, but did not undergo ischemia. For the experimental groups that were treated with NOS inhibitors, mice received intraperitoneal injections of either l-NAME (100 mg/kg) or 7-NI (50 mg/kg) just before applying the tourniquet.
Indices of Oxidative and Nitrosative Stress
Measurement of Reduced (GSH)-to-Oxidized (GSSG) Glutathione Ratio
Total GSH was determined by Tietze’s glutathione reductase recycling method adapted for the Cobas Mira spectrophotometer (Roche Diagnostics, Indianapolis, IN). The reaction mixture was allowed to incubate at 37°C for 4 minutes, glutathione reductase enzyme (1.0 units/100 μl) was added, and the reaction was monitored at 405 nm every 24 seconds for a period of 12 minutes. Using these conditions, the glutathione reductase method is linear for GSH concentrations ranging from 0 to 6 μmol/L. The concentration of GSH in each sample was determined from a calibration curve produced using known glutathione standards. For the GSSG assay, the sample was derivatized with 45.5% triethanolamine, 0.4% 5-sulfosalicylic acid, and 9% 2-vinylpyridine in ddH2O. To quantify the amount of GSSG, known standards of 2.5 μmol/L GSH, 2.5 μmol/L GSH + 0.1 μmol/L GSSG, and 2.5 μmol/L GSH + 0.5 GSSG were similarly derivatized and assayed. The samples were analyzed as for total GSH above. A regression curve was used to obtain the value of the GSSG recovered in each assay, and from this, a correction factor was obtained to calculate the GSSG for each unknown.
Detection of Protein Modifications by Western Blotting
Proteins were extracted from muscle, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and transferred onto polyvinylidene difluoride membranes. Protein modification induced by the lipid peroxidation by-product 4-HNE, an unsaturated aldehyde that can bind to lysine, cysteine, and histidine residues of proteins,33 was evaluated by reacting the membranes with anti-HNE antibody at a dilution of 1:1000. For detection of 3-nitrotyrosine formation, which is considered a marker for the highly reactive radical peroxynitrite,34,35 membranes were reacted with anti-3-nitrotyrosine antibody at 1:3000 dilution. Immunoblotting for the neuronal, inducible, and endothelial isoforms of NOS was performed with anti-nNOS (1:700), anti-iNOS (1:700), and anti-eNOS (1:500) antibodies, respectively. Lysates from rat pituitary gland, cytokine-stimulated macrophages, and human endothelial cells served as positive controls, respectively. For all of the above, antibody-bound proteins were revealed by enhanced chemiluminescence, and Coomassie Blue staining was used to ensure equal protein loading across lanes. Quantification of immunoblot protein band intensities was performed by densitometric analysis using FluorChem 8000 software (Alpha Innotech, San Leandro, CA).
Measurement of Nitric Oxide Production
To evaluate the production of NO by muscle tissues, NO and its metabolites (collectively referred to as NOx) were assayed using a Sievers 280 Nitric Oxide Analyzer (NOA; Sievers Instruments Inc., Boulder, CO). Two muscles were pooled for each sample and quickly ground in liquid nitrogen, homogenized in ice-cold HEPES buffer, and centrifuged (5000 rpm at 4°C) for 30 minutes. Samples were adjusted to contain a uniform protein concentration of 8 μg/μl and an equal volume of 40% trichloroacetic acid was added to prevent foaming of the samples, followed by centrifugation at 15,000 rpm for 10 minutes. Each sample (40 μl) was then injected into a purge vessel containing the reducing agent VCl3 in HCl at 91°C. By this method, nitrite (NO2G) and nitrate (NO3G) present in the muscle homogenate are reduced to NO, which then produces a chemiluminescence signal.36 Each sample was assayed in triplicate, and the concentrations of NOx were calculated using a standard curve produced by assaying various concentrations of a sodium nitrate solution.
In Situ Detection of Nitric Oxide
The diaminofluorophore DAF-237 was used to visualize the site of NO production on cryostat muscle tissue sections, according to the protocol described by Heydemann et al.32 Muscle sections were incubated with DAF-2 (100 μmol/L diluted in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum) and 500 μmol/L l-arginine at 37°C for 30 minutes. To determine the effects of NOS inhibition, DAF-2-treated sections were co-incubated with 7-NI (100 μmol/L) or l-NAME (1 mmol/L). The slides were examined under fluorescence microscopy using fluorescein isothiocyanate filter settings, and the images were captured to computer with a digital camera, using the identical exposure parameters for all experimental conditions.
Myeloperoxidase (MPO) Assay
MPO activity was assayed according to the method of Koike et al38 with minor modifications, to serve as an index of muscle infiltration by neutrophils. In the setting of skeletal muscle I/R, polymorphonuclear neutrophils are a potential source of oxidant injury,39 and the presence of elevated MPO activity can also result in 3-nitrotyrosine formation.40,41 Muscle tissue was homogenized in 40 mmol/L potassium phosphate buffer (PPB), pH 7.4, and centrifuged (3000 rpm at 4°C) for 10 minutes. The resulting pellet was resuspended in 50 mmol/L PPB, pH 6.0, containing 0.5 g/dl centrimonium bromide, and sonicated for 3 minutes. Samples were incubated at 60°C for 2 hours and centrifuged at 13,000 rpm for 10 minutes. A 100-μl volume of supernatant was added to 2.9 ml of 50 mmol/L PPB, pH 6.0, containing 0.167 mg/ml o-dianisidine and 5 × 10−4% H2O2, and absorbance was measured at 460 nm for 3 minutes. MPO activity (units/g) = ΔA460 × 13.5/g, where ΔA460 equals the change in absorbance between 1 and 3 minutes. Pure MPO from human leukocytes was used as a positive control.
Assays for Sarcolemmal Fragility and Damage
Evans Blue dye (EBD), a vital dye that is unable to penetrate the sarcolemma of normal muscle fibers, has been used to evaluate sarcolemmal integrity in mouse models of muscular dystrophy.5 Accordingly, we used EBD penetration into the muscle fiber cytoplasm as an index of sarcolemmal disruption resulting from oxidative/nitrosative stress (induced by I/R), mechanical stress (induced by muscle stretch), or the two in combination. Hindlimb muscle ischemia was induced as described above, and EBD (5 μg/μl solution in PBS; 5 μl/g body wt) was injected into the jugular vein of anesthetized mice immediately before removal of the tourniquet. To allow sufficient time for the circulating EBD to enter damaged membranes at reproducible levels, the mice were euthanized 3 hours later.42 At the end of each experiment, the TA was quickly removed, weighed, and snap-frozen in isopentane precooled with liquid nitrogen. Transverse cryosections of frozen TA muscles were fixed in methanol and visualized by fluorescence microscopy under green light. A digital camera was used to acquire microscopic images to a computer, and quantitative analysis of the number of EBD-positive fibers was performed using a commercial software package (Image-Pro Plus, Media Cybernetics, Silver Springs, MD), as previously described in detail.42
To impose increased mechanical stress on the muscles, the distal tendon of the TA was attached to the lever arm of a computer-controlled force-length servomotor system (model 305B; Cambridge Technology, Watertown, MA) and then passively stretched in a repetitive fashion immediately before euthanasia. The experiments were timed to terminate the stretch protocol at approximately 10 minutes before killing of the animals. The muscles received 30 passive stretches through a distance equal to 50% of resting in situ length, which amounts to a maximal stretch of the TA to approximately 120% of its optimal length (Lo). Muscle force and length data generated during passive stretches were acquired to computer at a sampling rate of 1000 Hz, and muscle cross-sectional area was also determined. This allowed calculation of the levels of mechanical stress placed on muscle tissues during passive stretch.8
Statistical Analysis
All data were reported as means ± SEM and were analyzed with the statistical analysis program SigmaStat (SPSS Inc., Chicago, IL). Differences between groups were initially tested by analysis of variance, with post hoc application of the Tukey test where appropriate. Statistical significance was defined as P < 0.05.
Results
Abnormal Susceptibility of Dystrophic Muscles to Oxidative/Nitrosative Stress
In comparison with normal C57 muscles, the mdx muscles demonstrated significant reductions in total glutathione levels (0.67 ± 0.02 versus 0.80 ± 0.01; P < 0.05) as well as a significantly lower ratio of reduced (GSH) to oxidized (GSSG) glutathione (24.65 ± 1.46 versus 41.94 ± 1.32; P < 0.05), under basal conditions (n = 16 mice per group). These findings suggest that abnormally increased oxidative stress and/or a decreased ability to cope with normal levels of oxidative stress are present under basal conditions in dystrophin-deficient muscles.
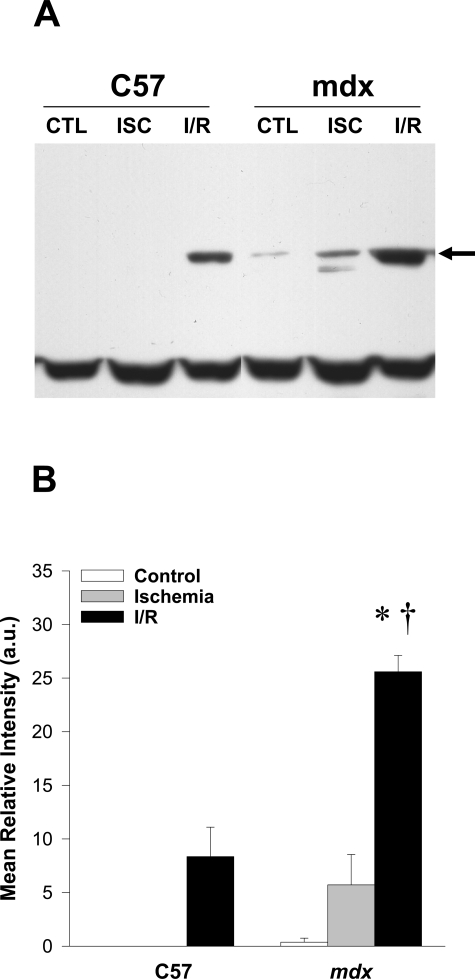
The unsaturated aldehyde HNE is generated during lipid peroxidation reactions, and once formed, it is capable of binding proteins to form HNE-protein adducts.33 Figure 1 shows the effects of ischemia alone and I/R on the generation of HNE-protein adducts, as detected by immunoblotting. As can be seen, a protein band of ∼70 kd appeared consistently at a low level in the I/R-treated C57 group, but was not detected in either sham- or ischemia-treated C57 muscle. In mdx muscles, immunoreactivity for this protein band was detected at low levels in the sham- and ischemia-treated groups, and was increased dramatically after I/R. Quantitative analysis by densitometry revealed that the mean relative intensity of this band was >300% higher in mdx than in C57 muscles after I/R. These findings indicate that dystrophin-deficient skeletal muscle is abnormally susceptible to lipid peroxidation after I/R-induced oxidative stress.
Figure 1.
Lipid peroxidation is increased in mdx muscle after I/R. A: Western blot analysis of hydroxynonenal protein adducts revealed significant increases in a protein band of approximately 70 kd (arrow) in the mdx group after I/R. Note that the lowest band is essentially constant across lanes, indicating equal loading. B: Densitometric quantification of the 70-kd band. Each lane was loaded with 30 μg of total protein; n = 4–5 for each experimental group (CTL, control; ISC, ischemia alone; I/R, ischemia/reperfusion). All values are group means ± SEM. *P < 0.05 versus CTL (sham-treated) within the same mouse strain; †P < 0.05 for C57 versus mdx within the same experimental condition.
Peroxynitrite, a highly reactive oxidant formed by the reaction of NO with superoxide, is a major mediator of 3-nitrotyrosine formation in tissues. Figure 2 shows the effects of ischemia alone and I/R on 3-nitrotyrosine levels in the TA muscle, as detected by immunoblotting in C57 and mdx mice. Although several protein bands demonstrated a degree of immunoreactivity at baseline as has been previously noted in skeletal muscle,43 a band of ∼58 kd was the only species to be consistently increased by I/R treatment in mdx muscles. The mean relative intensity of this band was >250% higher in mdx than in C57 muscle after I/R, suggesting greater exposure to peroxynitrite in the mdx group after I/R.
Figure 2.
Protein nitration is increased in mdx muscle after I/R. A: Western blot analysis for 3-nitrotyrosine revealed significant increases in a protein band of approximately 58 kd (arrow) in the mdx group after I/R. Note that other bands are essentially constant across lanes, indicating equal loading. B: Densitometric quantification of the 58-kd band. Each lane was loaded with 15 μg of total protein; n = 4–5 for each experimental group. All values are group means ± SEM. *P < 0.05 versus control within the same mouse strain; †P < 0.05 for C57 versus mdx within the same experimental condition.
To determine whether augmented NO production by mdx muscles could underlie the increased 3-nitrotyrosine formation associated with I/R, NOx levels were measured by chemiluminescence (Figure 3A). At baseline, NOx levels were lower in mdx than in C57 TA muscles, although this difference did not reach statistical significance. However, in mdx TA muscles subjected to ischemia alone, there was a significant increase in NOx levels, which then returned to baseline levels after reperfusion. In contrast, neither ischemia nor I/R affected NOx levels in C57 muscles. These results indicate that the exaggerated increases in I/R-induced lipid peroxidation and protein nitration in mdx muscles are preceded by an abnormal increase in NO production during ischemia.
Figure 3.
NO production, but not NOS expression, is increased in mdx muscle during ischemic challenge. A: In mdx muscles only, there was a significant rise in NOx levels during ischemia (ISC) over the baseline control (CTL) levels, which subsequently returned to baseline values after I/R. Levels of NO derivatives (NOx) were measured using a Sievers NOA 280i chemiluminescence NO analyzer (n = 6 for each experimental group; *P < 0.05versus CTL within the same mouse strain; †P < 0.05 for C57 versus mdx within the same experimental condition). B: Representative Western blotting for NOS isoforms: lanes were loaded with 50, 80, or 60 μg of total protein for nNOS, iNOS, and eNOS, respectively (+, rat pituitary gland, cytokine-stimulated macrophages, and human endothelial cells serving as positive controls, respectively). C: Densitometric quantification of nNOS expression within whole-muscle homogenates. Two-way analysis of variance revealed a significant reduction of nNOS levels in the mdx group compared with C57, but no effects of either ischemia or I/R (n = 3 per group; *P < 0.05 for C57 versus mdx within the same experimental condition).
To examine the potential role of altered NOS isoform expression in the abnormal NOx response found in mdx muscles under these conditions, immunoblotting experiments for nNOS, iNOS, and eNOS were performed on whole-muscle homogenates (Figure 3B). Although densitometric analysis revealed that nNOS expression was lower in mdx than in C57 muscles (Figure 3C), nNOS and eNOS expression levels were not altered by ischemia or I/R in either strain of mice. Because invading neutrophils can induce oxidative stress as well as iNOS and nitrotyrosine formation, MPO activity was also assessed. There was no detectable increase in MPO activity in muscles from either C57 or mdx mice after I/R (data not shown). Similarly, no histological evidence of increased neutrophil invasion of the muscles was noted by hematoxylin and eosin staining within the time frame of I/R used in our study. Along these same lines, iNOS expression was not detected in any of the experimental groups. Overall, these results indicate that altered expression levels of NOS isoforms do not account for the changes in NOx levels seen in dystrophin-deficient skeletal muscle during ischemia and instead suggest a possible up-regulation of constitutive NOS isoform (nNOS or eNOS) activity under these conditions.
To further address this issue, the fluorescence probe DAF-2 was used to detect NO on C57 and mdx muscle tissue cross-sections. As shown in Figure 4A, the pattern of fluorescence intensity differed greatly between the two mouse strains under baseline conditions. In C57 mice, there was a relatively uniform pattern across the tissue section, in which the intensity of staining appeared to be most intense along the sarcolemma. This pattern did not change with the imposition of ischemia. On the other hand, although mdx muscles seemed to demonstrate an overall decrease in background fluorescence intensity compared with C57, there were occasional foci of very intense fluorescent staining. These foci of intense fluorescence were scattered throughout the tissue section, appeared to be localized within the myofibers themselves (often concentrated near the sarcolemma), and were increased by the imposition of ischemia. Furthermore, the intensity of fluorescent staining found under these conditions was greatly diminished (but not completely abolished) by co-incubation with NOS inhibitors. This is shown in Figure 4B, in which it can be seen that both 7-NI and l-NAME exerted essentially equivalent effects in greatly diminishing the magnitude of fluorescence intensity within ischemic tissues.
Figure 4.
In situ detection and localization of NO. C57 and mdx muscle sections were treated with DAF-2, a fluorescent indicator of NO, and examined under blue light fluorescence. All images were obtained using the same exposure conditions. A: At higher magnification (×400), normal C57 fibers demonstrate more concentrated staining along the sarcolemma of many fibers. In mdx muscle, sarcolemmal staining as well as the overall background level of fluorescence intensity is generally lower, but there are foci of very intense staining within a subset of myofibers. These foci are increased in the mdx group after ischemia (ISC). B: At lower magnification (×200), the increase in DAF-2 staining after ISC in the mdx group is more evident. In addition, co-incubation with NOS inhibitors (7-NI or l-NAME) greatly reduces the intensity of the fluorescence signals.
Sarcolemmal Hyperfragility and Reversal by NOS Inhibition
Figure 5 shows the levels of peak tissue stress placed on mdx and C57 muscles subjected to repetitive stretch, both with and without the prior imposition of I/R. As can be seen, in both mouse strains, there was an initial decrease in peak tissue stress over the first five stretches, after which it remained fairly constant over the remainder of the protocol. Despite the same level of muscle stretch, the absolute magnitude of peak stress imposed on the muscles was consistently lower in the mdx group, which is in keeping with previous reports of increased tissue compliance (ie, lower stiffness) in dystrophin-deficient muscles at this age.44 Importantly, the prior imposition of I/R did not alter these relationships.
Figure 5.
Mechanical stress levels generated by repetitive stretch in wild-type and mdx muscles. Force and length were continuously monitored during the imposition of 30 consecutive stretches at 1-second intervals. Despite the same level of muscle stretch (50% in situ length), the magnitude of the peak stress imposed on the muscles was consistently lower in the mdx group. In addition, the levels of peak tissue stress placed on the tibialis anterior muscle of C57 and mdx mice were not altered by the prior imposition of I/R. Values are group means ± SEM; n = 10 per group.
Figure 6 demonstrates the effects of oxidative and mechanical stresses, applied either alone or in combination, on sarcolemmal integrity as assessed by EBD penetration into individual muscle fibers. Whereas ischemia alone did not increase sarcolemmal injury in either mouse strain (data not shown), the imposition of I/R produced substantial increases in sarcolemmal damage, but no significant differences were seen between the two mouse strains. Stretch alone also caused only very small increases in sarcolemmal damage in mdx as well as C57 muscles. In contrast, the combination of I/R and stretch produced a dramatic increase in the number of fibers showing EBD uptake in mdx muscles. A much more modest increase was observed in the C57 group exposed to I/R and stretch, despite the fact that levels of peak tissue stress placed on C57 muscles by the stretch protocol were actually higher than in the mdx group (as shown in Figure 5).
Figure 6.
The interaction of I/R and stretch-mediated mechanical stress greatly amplifies sarcolemmal injury in mdx muscles. Representative micrographs of tibialis anterior muscle cross-sections under green light fluorescence, demonstrating Evans Blue dye uptake by those muscle fibers that have suffered sarcolemmal damage. Note that when I/R was followed by repetitive muscle stretch to generate the levels of mechanical stress shown in Figure 5, the prevalence of muscle fibers exhibiting such dye uptake was markedly increased in the mdx group.
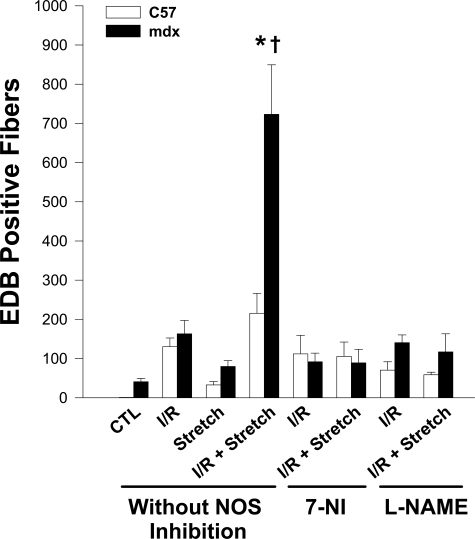
Figure 7 shows the group mean data for EBD uptake under the above conditions, as well as the effects of NOS inhibition by 7-NI or l-NAME. In the normal C57 muscles (without NOS inhibition), the interaction between I/R and stretch-mediated effects on sarcolemmal integrity was essentially additive in nature. In contrast, mdx muscles with prior exposure to I/R demonstrated a much greater increase in sarcolemmal damage after being subjected to stretch-mediated mechanical stress. Moreover, to the extent that the magnitude of the increase in sarcolemmal damage was approximately threefold greater than the sum of isolated I/R and stretch-mediated damage values, the interaction between I/R and stretch-mediated effects in the mdx group was synergistic in nature. Importantly, both 7-NI and l-NAME were able to completely abolish this synergistic interaction and thereby return EBD uptake values to the levels observed with I/R alone.
Figure 7.
The synergistic interaction of I/R and stretch-mediated sarcolemmal injury in mdx muscles is greatly reduced by NOS inhibition. EBD-positive fibers present on entire cross-sections of the tibialis anterior were quantified in C57 and mdx muscles under the following conditions: Control (CTL), I/R alone, stretch alone, and I/R followed by stretch (I/R + Stretch). The responses to I/R alone and stretch alone did not differ significantly between C57 and mdx muscles. However, the combination of I/R + Stretch dramatically increased the number of fibers demonstrating sarcolemmal damage in the mdx group, in a manner that greatly exceeded the additive effects of either intervention alone (synergistic effect). Treatment of mdx mice with the NOS inhibitors, 7-NI and l-NAME, prevented this synergistic interaction and restored sarcolemmal damage to the levels seen after I/R alone. Values are group means ± SEM; n = 6–8 per group. *P < 0.05 versus CTL within the same mouse strain; †P < 0.05 for C57 versus mdx within the same experimental condition.
Discussion
In the present study, we compared the responses of wild-type and mdx mouse muscles with I/R, a classical inducer of oxidative stress. Because sarcolemmal hyperfragility with an attendant reduction in the threshold for mechanical stress-induced plasma membrane rupture is one of the most characteristic hallmarks of dystrophin-deficient muscle fibers, we also tested the hypothesis that I/R would lead to mechanical weakening of the sarcolemma. The principal findings of our study can be summarized as follows. 1) Dystrophin-deficient muscles demonstrated an increased vulnerability to I/R-induced oxidative and nitrosative stress compared with the wild-type group. 2) During the ischemic phase, increased NOx production was observed in mdx but not in wild-type muscles, suggesting a major difference in NO regulation during ischemic challenge. 3) In dystrophic muscles only, the combination of I/R and mechanical stress acted in a synergistic fashion to dramatically increase the magnitude of sarcolemmal damage, indicating a large increase in sarcolemmal fragility. 4) Treatment with NOS inhibitors was able to block the synergistic interaction between I/R and mechanical stress-mediated sarcolemmal damage.
The use of I/R is not only well established as a model of oxidative stress, but it also has the additional advantages of being largely limited to the target muscle tissue of interest and a form of oxidative stress that is particularly relevant to DMD.19,20,25 The levels of oxidative stress and muscle injury produced by I/R in our study are greater than would be expected to occur in the mdx mouse during normal muscle activity, and hence the magnitude of differential responses between dystrophic and wild-type mice could be amplified by our acute model. Nonetheless, our results suggest that increases in oxidative/nitrosative stress triggered by I/R may greatly contribute to the mechanical damage suffered by dystrophin-deficient muscle fiber membranes. Therefore, our findings are consistent with a scenario in which an increased susceptibility of dystrophin-deficient muscle fibers to oxidative/nitrosative stress could play an important role in disease pathogenesis by way of attendant increases in mechanical fragility of the muscle cell membrane. Moreover, these findings provide direct experimental evidence that several prevailing hypotheses regarding the cause of muscle fiber damage in dystrophin-deficient muscle (ie, hyperfragility of the sarcolemma, oxidative stress, and abnormal NO regulation) can potentially be linked together and integrated into a common pathophysiological framework, as has been previously suggested.22
In principle, a greater susceptibility of dystrophin-deficient muscle to I/R-induced oxidative stress could be related to an increased inherent sensitivity to the damaging effects of free radicals (including NO) as well as a greater level of exposure to oxidants.22 In previous studies, markers of oxidative stress such as protein carbonyls,45 lipid peroxidation by-products,46 and lipofuscin47 were noted to be abnormally elevated in DMD patients or mdx mice. Some studies have also attempted to mitigate the disease process using antioxidant therapy, but the results have been inconclusive.48,49 An increased activation of endogenous antioxidant systems can also be demonstrated before the actual onset of necrosis in mdx mice.50 In addition, Rando et al51,52 found that cultured myotubes lacking dystrophin are abnormally vulnerable to cellular death after exposure to different oxidants.
To our knowledge, our findings constitute the first direct evidence that dystrophin-deficient muscle is abnormally susceptible to a form of acute oxidative stress imposed in vivo. In addition, we report here that abnormal increases in lipid peroxidation and protein nitration induced by I/R within mdx muscles are preceded by an exaggerated level of NO production during ischemia. The probe DAF-2, which is activated to emit a fluorescence signal when nitrosated, was used to visualize the location of NO production.37 In normal muscle, the DAF-2 fluorescence signal was most intense along the sarcolemma (corresponding well to the normal location of nNOS) and was almost completely suppressed by NOS inhibitors, including 7-NI. On the other hand, mdx muscles showed very intense DAF-2 fluorescence but only within certain fibers, and the remainder of the fibers exhibited virtually no signal. This suggests that increased NO production by mdx muscles during ischemia originates primarily from a subset of dystrophic fibers, perhaps reflecting exaggerated activation of the mislocalized nNOS in this myofiber subpopulation. It has also been observed that enhanced NO production during cardiac ischemia can result not only from increases in NOS activity, but from the nonenzymatic reduction of nitrite to NO.53 Under the above conditions, the majority of NO generation and associated contractile dysfunction induced by I/R can still be attributed to the enzymatic generation of NO.53 Nonetheless, a nonenzymatic contribution to NO production by ischemic mdx muscles cannot be excluded in our study and could account for the fact that NOS inhibitors (including l-NAME, which acts on all NOS isoforms) were not able to completely abolish DAF-2 fluorescence during ischemia.
The reaction product of NO and superoxide anion is peroxynitrite, and 3-nitrotyrosine formation has been used as an indirect index of the interaction of peroxynitrite with tissue components.34,35 In addition to peroxynitrite, other reactive nitrogen species can also nitrate tyrosine, and several proteins have been shown to be nitrated in skeletal muscle under normal conditions.43 In the present study, we observed increased 3-nitrotyrosine formation within mdx muscles during the period of reperfusion, which coincided with a fall in NOx levels. Although the fall in NOx could be caused by washout from the muscle during reperfusion, it is also consistent with a scenario in which the excess NO generated during ischemia was scavenged by the elevated levels of superoxide produced during reperfusion, with attendant formation of peroxynitrite. In most experimental systems, peroxynitrite and its derivatives such as peroxynitrous acid are considered to be noxious agents, with the capability of causing damage to multiple subcellular components, including the cell membrane.35,54 Moreover, we have previously reported a synergistic interaction between exposure of cultured myotubes to a peroxynitrite generator (SIN-1) and stretch-induced sarcolemmal injury in vitro,54 which closely resembles the in vivo interaction between I/R and stretch-induced sarcolemmal damage demonstrated within mdx muscles in the current study.
A major finding in the present investigation was that inhibition of NOS activity is able to effectively block the adverse effects of I/R on mechanical stability of the sarcolemma. This protective effect was observed with two different NOS inhibitors administered in vivo, l-NAME and 7-NI. l-NAME is a general inhibitor of NOS isoforms, whereas 7-NI is generally considered to be selective for nNOS at the doses used in our study.30–32 Accordingly, the fact that 7-NI was able to block I/R-induced sarcolemmal fragility to the same extent as l-NAME suggests that nNOS played a predominant role in these findings. Little is known about the regulation of nNOS activity in normal muscle, and even less is known about how this regulation might be altered when nNOS is displaced to the cytosol as in dystrophin-deficient muscles. Binding of nNOS to membrane-associated caveolin-3 inhibits its enzymatic activity,55,56 whereas the latter is up-regulated by intracellular calcium. Intracellular free calcium levels have been found to increase in normal muscle during ischemia.57–59 The lack of change in whole-muscle mechanical stiffness after I/R in our study argues against a generalized increase of intracellular calcium. However, it remains possible that large increases or even small but spatially sequestered (and hence concentrated) elevations in intracellular calcium occurred within a subset of fibers, thus triggering increased NO production by the mislocalized and hence dysregulated nNOS in the mdx group. These fibers could be responsible for a large proportion of the excess NO generated during ischemia in mdx muscles as noted previously. Such a scenario would also be consistent with the foci of intense DAF-2 fluorescence observed near the sarcolemma in certain mdx fibers, which could represent foci of higher calcium concentration within the cell.
It is interesting to relate the findings of the present study to previous investigations in which nNOS expression has been experimentally manipulated in mdx mice. Genetic crosses of mdx mice with nNOS knockout mice did not lead to obvious improvements in dystrophic histopathology,60,61 which would appear to argue against a role for mislocalized nNOS. However, several other previously described NO effects on skeletal muscle such as modulation of repair mechanisms,62,63 proteolysis,64 glucose uptake,65 and calcium regulation,66,67 may have also been affected. To the extent that NO plays myriad roles in skeletal muscle biology, its complete removal could produce a combination of beneficial and adverse effects in mdx muscles. Wehling et al68 found that transgenic overexpression of nNOS was able to mitigate dystrophic pathology in mdx mice. Although these authors hypothesized that protection was afforded by anti-inflammatory properties of NO, the observed beneficial outcome is also consistent with prevention of recurrent ischemia by the constitutive overexpression of nNOS. In addition, NO effects are very much dependent on specific aspects of the physiological context involved, such as the amount, timing, and location of NO production, as well as the availability of different molecular partners for interaction.32,69 Finally, it should be noted that in previous investigations, the skeletal muscles of mdx mice completely lacking or overexpressing nNOS were not dynamically stressed in any fashion, such as by exercise or I/R.
In conclusion, the results of the present study indicate that in dystrophic muscle fibers, the increase in oxidative/nitrosative stress induced by I/R acts together in a synergistic fashion with the loss of dystrophin to further compromise the ability of the sarcolemma to withstand mechanical stress. Interestingly, red blood cells from patients with underlying spectrin mutations demonstrate more intense lipid peroxidation than normal erythrocytes after being exposed to an oxidant,70 and exposure to NO/peroxynitrite can cause a decrease in red blood cell deformability as well as a reduced ability to withstand hypoosmotic challenge.71,72 Such adverse effects on membrane properties are likely related to the cross-linking and/or degradation of different protein and lipid moieties present within the membrane.23,33 There is evidence that membrane cytoskeleton proteins may be particularly sensitive to such oxidative modifications in red blood cells.73 In our study, the electrophoretic pattern revealed specific increases in HNE adducts at ∼70 kd and 3-nitrotyrosine formation at ∼58 kd. Although we have not determined the identity of these proteins or whether they bear any functional relationship to the sarcolemma, our findings suggest that distinct groups of proteins are preferentially targeted by oxidative/nitrosative stress in muscles lacking dystrophin. Therefore, identifying the individual proteins that undergo preferential oxidative and nitrosative modification in mdx muscles and elucidation of their relationships to the biophysical properties of muscle cell membranes should be areas of major interest in future investigations.
Footnotes
Address reprint requests to Basil J. Petrof, Royal Victoria Hospital, Room L411, 687 Pine Ave. West, Montreal, Quebec H3A 1A1, Canada. E-mail: basil.petrof@mcgill.ca.
Supported by Muscular Dystrophy Association, Canadian Institutes of Health Research, and Fonds de la Recherche en Sante du Quebec.
References
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science. 1989;244:1578–1580. doi: 10.1126/science.2662404. [DOI] [PubMed] [Google Scholar]
- Mokri B, Engel AG. Duchenne dystrophy: electron microscopic findings pointing to a basic or early abnormality in the plasma membrane of the muscle fiber. Neurology. 1975;25:1111–1120. doi: 10.1212/wnl.25.12.1111. [DOI] [PubMed] [Google Scholar]
- Carpenter S, Karpati G. Duchenne muscular dystrophy: plasma membrane loss initiates muscle cell necrosis unless it is repaired. Brain. 1979;102:147–161. doi: 10.1093/brain/102.1.147. [DOI] [PubMed] [Google Scholar]
- Straub V, Rafael JA, Chamberlain JS, Campbell KP. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J Cell Biol. 1997;139:375–385. doi: 10.1083/jcb.139.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilquin JT, Brussee V, Asselin I, Kinoshita I, Gingras M, Tremblay JP. Evidence of mdx mouse skeletal muscle fragility in vivo by eccentric running exercise. Muscle Nerve. 1998;21:567–576. doi: 10.1002/(sici)1097-4598(199805)21:5<567::aid-mus2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Menke A, Jockusch H. Decreased osmotic stability of dystrophin-less muscle cells from the mdx mouse. Nature. 1991;349:69–71. doi: 10.1038/349069a0. [DOI] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MFC, Khakee R, McNeil PL. Loss of cytoplasmic basic fibroblast growth factor from physiologically wounded myofibers of normal and dystrophic muscle. J Cell Sci. 1993;106:121–133. doi: 10.1242/jcs.106.1.121. [DOI] [PubMed] [Google Scholar]
- Brussee V, Tardif F, Tremblay JP. Muscle fibers of mdx mice are more vulnerable to exercise than those of normal mice. Neuromuscul Disord. 1997;7:487–492. doi: 10.1016/s0960-8966(97)00115-6. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Kahl SD, Campbell KP. Purification of dystrophin from skeletal muscle. J Biol Chem. 1991;266:9161–9165. [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Dystrophin and the membrane skeleton. Curr Opin Cell Biol. 1993;5:82–87. doi: 10.1016/s0955-0674(05)80012-2. [DOI] [PubMed] [Google Scholar]
- Blake DJ, Nawrotzki R, Peters MF, Froehner SC, Davies KE. Isoform diversity of dystrobrevin, the murine 87-kDa postsynaptic protein. J Biol Chem. 1996;271:7802–7810. doi: 10.1074/jbc.271.13.7802. [DOI] [PubMed] [Google Scholar]
- Sadoulet-Puccio HM, Rajala M, Kunkel LM. Dystrobrevin and dystrophin: an interaction through coiled-coil motifs. Proc Natl Acad Sci USA. 1997;94:12413–12418. doi: 10.1073/pnas.94.23.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Hama H, Ishikawa-Sakurai M, Imamura M, Mizuno Y, Araishi K, Wakabayashi-Takai E, Noguchi S, Sasaoka T, Ozawa E. Biochemical evidence for association of dystrobrevin with the sarcoglycan-sarcospan complex as a basis for understanding sarcoglycanopathy. Hum Mol Genet. 2000;9:1033–1040. doi: 10.1093/hmg/9.7.1033. [DOI] [PubMed] [Google Scholar]
- Reid MB. Role of nitric oxide in skeletal muscle: synthesis, distribution and functional importance. Acta Physiol Scand. 1998;162:401–409. doi: 10.1046/j.1365-201X.1998.0303f.x. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof BJ. Molecular pathophysiology of myofiber injury in deficiencies of the dystrophin-glycoprotein complex. Am J Phys Med Rehabil. 2002;81:S162–S174. doi: 10.1097/00002060-200211001-00017. [DOI] [PubMed] [Google Scholar]
- Rando TA. Role of nitric oxide in the pathogenesis of muscular dystrophies: a “two hit” hypothesis of the cause of muscle necrosis. Microsc Res Tech. 2001;55:223–235. doi: 10.1002/jemt.1172. [DOI] [PubMed] [Google Scholar]
- Richter C. Biophysical consequences of lipid peroxidation in membranes. Chem Phys Lipids. 1987;44:175–189. doi: 10.1016/0009-3084(87)90049-1. [DOI] [PubMed] [Google Scholar]
- Lanari A, Gonzalez PM, Semeniuk GB. Increased levels of serum creatine-phosphokinase after transient limb ischaemia in patients with muscular dystrophy. Lancet. 1970;1:217–218. doi: 10.1016/s0140-6736(70)90575-1. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Engel WK, Derrer EC. Duchenne muscular dystrophy: functional ischemia reproduces its characteristic lesions. Science. 1971;172:1143–1145. doi: 10.1126/science.172.3988.1143. [DOI] [PubMed] [Google Scholar]
- Choudhury NA, Sakaguchi S, Koyano K, Matin AF, Muro H. Free radical injury in skeletal muscle ischemia and reperfusion. J Surg Res. 1991;51:392–398. doi: 10.1016/0022-4804(91)90139-d. [DOI] [PubMed] [Google Scholar]
- Fantini GA, Yoshioka T. Deferoxamine prevents lipid peroxidation and attenuates reoxygenation injury in postischemic skeletal muscle. Am J Physiol. 1993;264:H1953–H1959. doi: 10.1152/ajpheart.1993.264.6.H1953. [DOI] [PubMed] [Google Scholar]
- Kadambi A, Skalak TC. Role of leukocytes and tissue-derived oxidants in short-term skeletal muscle ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2000;278:H435–H443. doi: 10.1152/ajpheart.2000.278.2.H435. [DOI] [PubMed] [Google Scholar]
- Grisotto PC, dos Santos AC, Coutinho-Netto J, Cherri J, Piccinato CE. Indicators of oxidative injury and alterations of the cell membrane in the skeletal muscle of rats submitted to ischemia and reperfusion. J Surg Res. 2000;92:1–6. doi: 10.1006/jsre.2000.5823. [DOI] [PubMed] [Google Scholar]
- Moore PK, Babbedge RC, Wallace P, Gaffen ZA, Hart SL. 7-Nitroindazole, an inhibitor of nitric oxide synthase, exhibits anti-nociceptive activity in the mouse without increasing blood pressure. Br J Pharmacol. 1993;108:296–297. doi: 10.1111/j.1476-5381.1993.tb12798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi N, Lei B, Soutani M, Arai T. Different roles of neuronal and endothelial nitric oxide synthases on ischemic nitric oxide production in gerbil striatum. Neurosci Lett. 2000;288:151–154. doi: 10.1016/s0304-3940(00)01222-2. [DOI] [PubMed] [Google Scholar]
- Heydemann A, Huber JM, Kakkar R, Wheeler MT, McNally EM. Functional nitric oxide synthase mislocalization in cardiomyopathy. J Mol Cell Cardiol. 2004;36:213–223. doi: 10.1016/j.yjmcc.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Halliwell RA, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford: Oxford University Press,; 1999 [Google Scholar]
- Ischiropoulos H. Biological tyrosine nitration: a pathophysiological function of nitric oxide and reactive oxygen species. Arch Biochem Biophys. 1998;356:1–11. doi: 10.1006/abbi.1998.0755. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, peroxynitrite: the good, the bad, and the ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Braman RS, Hendrix SA. Nanogram nitrite and nitrate determination in environmental and biological materials by vanadium (III) reduction with chemiluminescence detection. Anal Chem. 1989;61:2715–2718. doi: 10.1021/ac00199a007. [DOI] [PubMed] [Google Scholar]
- Kojima H, Sakurai K, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagano T. Development of a fluorescent indicator for nitric oxide based on the fluorescein chromophore. Chem Pharm Bull (Tokyo) 1998;46:373–375. doi: 10.1248/cpb.46.373. [DOI] [PubMed] [Google Scholar]
- Koike K, Moore FA, Moore EE, Trew CE, Banerjee A, Peterson VM. Endotoxin pretreatment inhibits neutrophil proliferation and function. J Surg Res. 1994;57:49–54. doi: 10.1006/jsre.1994.1108. [DOI] [PubMed] [Google Scholar]
- Kyriakides C, Austen W, Jr, Wang Y, Favuzza J, Kobzik L, Moore FD, Jr, Hechtman HB. Skeletal muscle reperfusion injury is mediated by neutrophils and the complement membrane attack complex. Am J Physiol. 1999;277:C1263–C1268. doi: 10.1152/ajpcell.1999.277.6.C1263. [DOI] [PubMed] [Google Scholar]
- van der Vliet A, Eiserich JP, Halliwell B, Cross CE. Formation of reactive nitrogen species during peroxidase-catalyzed oxidation of nitrite: a potential additional mechanism of nitric oxide-dependent toxicity. J Biol Chem. 1997;272:7617–7625. doi: 10.1074/jbc.272.12.7617. [DOI] [PubMed] [Google Scholar]
- Sampson JB, Ye Y, Rosen H, Beckman JS. Myeloperoxidase and horseradish peroxidase catalyze tyrosine nitration in proteins from nitrite and hydrogen peroxide. Arch Biochem Biophys. 1998;356:207–213. doi: 10.1006/abbi.1998.0772. [DOI] [PubMed] [Google Scholar]
- Danialou G, Comtois AS, Dudley R, Karpati G, Vincent G, Des Rosiers C, Petrof BJ. Dystrophin-deficient cardiomyocytes are abnormally vulnerable to mechanical stress-induced contractile failure and injury. FASEB J. 2001;15:1655–1657. doi: 10.1096/fj.01-0030fje. [DOI] [PubMed] [Google Scholar]
- Barreiro E, Comtois AS, Gea J, Laubach VE, Hussain SN. Protein tyrosine nitration in the ventilatory muscles: role of nitric oxide synthases. Am J Respir Cell Mol Biol. 2002;26:438–446. doi: 10.1165/ajrcmb.26.4.4634. [DOI] [PubMed] [Google Scholar]
- Kumar A, Khandelwal N, Malya R, Reid MB, Boriek AM. Loss of dystrophin causes aberrant mechanotransduction in skeletal muscle fibers. FASEB J. 2004;18:102–113. doi: 10.1096/fj.03-0453com. [DOI] [PubMed] [Google Scholar]
- Haycock JW, Mac NS, Mantle D. Differential protein oxidation in Duchenne and Becker muscular dystrophy. Neuroreport. 1998;9:2201–2207. doi: 10.1097/00001756-199807130-00010. [DOI] [PubMed] [Google Scholar]
- Ragusa RJ, Chow CK, Porter JD. Oxidative stress as a potential pathogenic mechanism in an animal model of Duchenne muscular dystrophy. Neuromuscul Disord. 1997;7:379–386. doi: 10.1016/s0960-8966(97)00096-5. [DOI] [PubMed] [Google Scholar]
- Nakae Y, Stoward PJ, Kashiyama T, Shono M, Akagi A, Matsuzaki T, Nonaka I. Early onset of lipofuscin accumulation in dystrophin-deficient skeletal muscles of DMD patients and mdx mice. J Mol Histol. 2004;35:489–499. doi: 10.1023/b:hijo.0000045947.83628.a7. [DOI] [PubMed] [Google Scholar]
- Bornman L, Rossouw H, Gericke GS, Polla BS. Effects of iron deprivation on the pathology and stress protein expression in murine X-linked muscular dystrophy. Biochem Pharmacol. 1998;56:751–757. doi: 10.1016/s0006-2952(98)00055-0. [DOI] [PubMed] [Google Scholar]
- Buetler TM, Renard M, Offord EA, Schneider H, Ruegg UT. Green tea extract decreases muscle necrosis in mdx mice and protects against reactive oxygen species. Am J Clin Nutr. 2002;75:749–753. doi: 10.1093/ajcn/75.4.749. [DOI] [PubMed] [Google Scholar]
- Disatnik MH, Dhawan J, Yu Y, Beal MF, Whirl MM, Franco AA, Rando TA. Evidence of oxidative stress in mdx mouse muscle: studies of the pre-necrotic state. J Neurol Sci. 1998;161:77–84. doi: 10.1016/s0022-510x(98)00258-5. [DOI] [PubMed] [Google Scholar]
- Rando TA, Disatnik MH, Yu Y, Franco A. Muscle cells from mdx mice have an increased susceptibility to oxidative stress. Neuromuscul Disord. 1998;8:14–21. doi: 10.1016/s0960-8966(97)00124-7. [DOI] [PubMed] [Google Scholar]
- Disatnik MH, Chamberlain JS, Rando TA. Dystrophin mutations predict cellular susceptibility to oxidative stress. Muscle Nerve. 2000;23:784–792. doi: 10.1002/(sici)1097-4598(200005)23:5<784::aid-mus17>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Zweier JL, Wang P, Samouilov A, Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- Ebihara S, Hussain SN, Danialou G, Cho WK, Gottfried SB, Petrof BJ. Mechanical ventilation protects against diaphragm injury in sepsis: interaction of oxidative and mechanical stresses. Am J Respir Crit Care Med. 2002;165:221–228. doi: 10.1164/ajrccm.165.2.2108041. [DOI] [PubMed] [Google Scholar]
- Kone BC. Protein-protein interactions controlling nitric oxide synthases. Acta Physiol Scand. 2000;168:27–31. doi: 10.1046/j.1365-201x.2000.00629.x. [DOI] [PubMed] [Google Scholar]
- Sunada Y, Ohi H, Hase A, Ohi H, Hosono T, Arata S, Higuchi S, Matsumura K, Shimizu T. Transgenic mice expressing mutant caveolin-3 show severe myopathy associated with increased nNOS activity. Hum Mol Genet. 2001;10:173–178. doi: 10.1093/hmg/10.3.173. [DOI] [PubMed] [Google Scholar]
- Tupling R, Green H, Senisterra G, Lepock J, McKee N. Effects of 4-h ischemia and 1-h reperfusion on rat muscle sarcoplasmic reticulum function. Am J Physiol Endocrinol Metab. 2001;281:E867–E877. doi: 10.1152/ajpendo.2001.281.4.E867. [DOI] [PubMed] [Google Scholar]
- Tupling R, Green H, Senisterra G, Lepock J, McKee N. Ischemia-induced structural change in SR Ca2+-ATPase is associated with reduced enzyme activity in rat muscle. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1681–R1688. doi: 10.1152/ajpregu.2001.281.5.R1681. [DOI] [PubMed] [Google Scholar]
- Ivanics T, Miklos Z, Ruttner Z, Batkai S, Slaaf DW, Reneman RS, Toth A, Ligeti L. Ischemia/reperfusion-induced changes in intracellular free Ca2+ levels in rat skeletal muscle fibers–an in vivo study. Pflugers Arch. 2000;440:302–308. doi: 10.1007/s004240000287. [DOI] [PubMed] [Google Scholar]
- Chao DS, Silvagno F, Bredt DS. Muscular dystrophy in mdx mice despite lack of neuronal nitric oxide synthase. J Neurochem. 1998;71:784–789. doi: 10.1046/j.1471-4159.1998.71020784.x. [DOI] [PubMed] [Google Scholar]
- Crosbie RH, Straub V, Yun HY, Lee JC, Rafael JA, Chamberlain JS, Dawson VL, Dawson TM, Campbell KP. mdx muscle pathology is independent of nNOS perturbation. Hum Mol Genet. 1998;7:823–829. doi: 10.1093/hmg/7.5.823. [DOI] [PubMed] [Google Scholar]
- Lee KH, Baek MY, Moon KY, Song WK, Chung CH, Ha DB, Kang MS. Nitric oxide as a messenger molecule for myoblast fusion. J Biol Chem. 1994;269:14371–14374. [PubMed] [Google Scholar]
- Anderson JE. A role for nitric oxide in muscle repair: nitric oxide-mediated activation of muscle satellite cells. Mol Biol Cell. 2000;11:1859–1874. doi: 10.1091/mbc.11.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TJ, Tidball JG. Nitric oxide inhibits calpain-mediated proteolysis of talin in skeletal muscle cells. Am J Physiol Cell Physiol. 2000;279:C806–C812. doi: 10.1152/ajpcell.2000.279.3.C806. [DOI] [PubMed] [Google Scholar]
- Roberts CK, Barnard RJ, Scheck SH, Balon TW. Exercise-stimulated glucose transport in skeletal muscle is nitric oxide dependent. Am J Physiol. 1997;273:E220–E225. doi: 10.1152/ajpendo.1997.273.1.E220. [DOI] [PubMed] [Google Scholar]
- Aghdasi B, Reid MB, Hamilton SL. Nitric oxide protects the skeletal muscle Ca2+ release channel from oxidation induced activation. J Biol Chem. 1997;272:25462–25467. doi: 10.1074/jbc.272.41.25462. [DOI] [PubMed] [Google Scholar]
- Reid MB, Kobzik L, Bredt DS, Stamler JS. Nitric oxide modulates excitation-contraction coupling in the diaphragm. Comp Biochem Physiol A Mol Integr Physiol. 1998;119:211–218. doi: 10.1016/s1095-6433(97)00417-0. [DOI] [PubMed] [Google Scholar]
- Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubbo H, Radi M, Trujilo M, Telleri R, Kalyanaraman B, Barnes S, Kisk M, Freeman BA. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation: formation of novel nitrogen-containing oxidized lipid derivatives. J Bio Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- Caprari P, Bozzi A, Ferroni L, Strom R, Salvati AM. Oxidative erythrocyte membrane damage in hereditary spherocytosis. Biochem Int. 1992;26:265–274. [PubMed] [Google Scholar]
- Ismail NH, Cohn J, Jr, Mollitt DL. Nitric oxide synthase inhibition negates septic-induced alterations in cytoplasmic calcium homeostasis and membrane dynamics. Am Surg. 1997;63:20–23. [PubMed] [Google Scholar]
- Kondo H, Takahashi M, Niki E. Peroxynitrite-induced hemolysis of human erythrocytes and its inhibition by antioxidants. FEBS Lett. 1997;413:236–238. doi: 10.1016/s0014-5793(97)00922-8. [DOI] [PubMed] [Google Scholar]
- Caprari P, Bozzi A, Malorni W, Bottini A, Iosi F, Santini MT, Salvati AM. Junctional sites of erythrocyte skeletal proteins are specific targets of tert-butylhydroperoxide oxidative damage. Chem Biol Interact. 1995;94:243–258. doi: 10.1016/0009-2797(94)03339-a. [DOI] [PubMed] [Google Scholar]