Abstract
Tyrosine kinases play a fundamental role in cell proliferation, survival, adhesion, and motility and have also been shown to mediate malignant cell transformation. Here we describe constitutive expression of the protein tyrosine kinase Brk in a large proportion of cutaneous T-cell lymphomas and other transformed T- and B-cell populations. The kinase is expressed in the nuclear localization and activated state. Brk expression was also induced in normal T cells on their activation. Introduced expression of the Brk gene resulted in markedly diminished cytokine and growth factor dependence of transfected BaF3 lymphocytes in regard to their in vitro proliferation and survival. Brk also conferred in vivo oncogenicity on the BaF3 cells. siRNA-mediated inhibition of the endogenous Brk in malignant T cells diminished their growth and survival capacity. These findings document inducible expression of Brk in normal T lymphocytes and persistent expression of the activated kinase in malignant T and B cells. Furthermore, our results indicate that Brk may play a key role in lymphomagenesis, hence identifying the kinase as a potential therapeutic target in lymphomas.
Tyrosine kinases play a fundamental role in cell biology during cell proliferation, survival, adhesion, and motility by regulating ligand-mediated signal transduction, cell-cycle progression, and cytoskeleton function. Many tyrosine kinases have also been shown to mediate malignant cell transformation. One such kinase, Brk (also known as PTK6) with its murine homolog being called Sik, is a member of the distinct Frk family of intracellular tyrosine kinases and is distantly related to src-type kinases.1–3 Brk is strongly expressed in a large subset of breast carcinomas1,4–6 and some metastatic melanomas,7 moderately overexpressed in some colon cancers,8 and overexpressed or underexpressed in various types of squamous cell carcinomas.9–11 Normal Brk expression is relatively restricted and has been analyzed in differentiating epithelial cells of the skin and gastrointestinal tract.8,11,12 Brk was also demonstrated to localize in the nuclei of normal and malignant prostate epithelial cells, with a transition to cytoplasmic localization being associated with increased tumor grade.13 The role of Brk in cell transformation remains poorly defined. Brk fosters anchorage-independent cell growth in NIH3T3,14 nontransformed mammary epithelial,14 and HEK 29315 cells; promotes the proliferation of nontransformed mammary epithelial,14 breast carcinoma,16 HEK 29315 and astrocytic17 cells; protects HEK 293 cells from apoptosis15; and mediates migration and epidermal growth factor-induced chemotaxis and matrix degradation by a variety of cell types.18 In contrast, in Rat1a fibroblasts, Brk expression does not result in enhanced proliferative properties, but sensitizes the cells to apoptotic stimuli,19 and Brk expression in keratinocyte cell lines promotes the expression of differentiation markers.11,12 Here we report that Brk can be induced in normal T lymphocytes after their activation and is constitutively expressed in their malignant counterparts as well as Epstein-Barr virus (EBV)-transformed and lymphoma-derived B cells. Brk is expressed in malignant lymphocytes in a nucleus-localized and continuously activated form and plays a key role in their proliferation and survival.
Materials and Methods
Transformed and Normal T and B Lymphocytes
Most cell lines used in this study have been described in detail previously.20–22 In brief, three cell lines (PB-1, 2A, 2B) were established from a patient with a progressive cutaneous T-cell lymphoma (CTCL). MyLa2056 and 3676 were derived from advanced CTCL. SUDHL-1, JB6, Karpas 299, and SUP-M2 lines were derived from ALK+ T-cell lymphoma and Jurkat from T-cell lymphoblastic lymphoma.
Raji, Ramos, and Daudi were obtained from patients with Burkitt lymphoma. MM and HH represent lymphoblastoid B-cell lines established in our laboratory by the EBV-mediated immortalization of peripheral blood B lymphocytes. Jeko-1 and Mino cell lines were developed from circulating malignant B cells from patients with mantle cell lymphoma. LY18, Ly7, and Val cell lines were derived from diffuse large B-cell lymphoma and were a kind gift of L. Pasqualucci from Columbia University, New York, NY. BaF3 is an interleukin (IL)-3-dependent murine pro-B-cell lymphoblastic cell line. Peripheral blood mononuclear cells (PBMCs) were obtained from healthy individuals, and circulating malignant (Sezary) cells from patients with advanced cases of CTCL after centrifugation on Ficoll/Paque gradient. PBMCs were activated by 3-day stimulation with a mitogen (PHA) or plastic-immobilized antibodies against CD3 and CD28. CTCL cells used in the study were >80% pure as determined by flow cytometric staining with anti-CD3, -CD4, -CD8, -CD7, and -CD26 antibodies. Cell lines and stimulated PBMCs were cultured at 37°C with 5% CO2 in standard RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 1% penicillin/streptomycin/fungizone mixture, 2 mmol/L l-glutamine, and, in the case of BaF3 cells, 1 ng/ml of IL-3 (R&D Systems, Minneapolis, MN).
Kinase-Specific Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)/Denaturing Gradient Gel Electrophoresis
The method has been described by us in great detail previously.20 In brief, RT-PCR reaction was performed under low-stringency conditions using two sets of PTK-specific degenerated primers in the buffer containing a 32P-dCTP (Amersham, Arlington Heights, IL). A parallel denaturing gradient gel was cast into the gel sandwich and had the targeted range of denaturating gradient of 20 to 75%. After sample loading and electrophoretic separation, the gel was subjected to autoradiography. The bands of interest were cut from the gel, and the cDNA was recovered, PCR reamplified, precipitated, and enzymatically digested and ligated into a pGEM vector (Promega, Madison, WI). The nucleotide sequence of the cloned cDNA fragment was determined using Big Dye Terminator (Applied BioSystems, Foster City, CA) with an ABI 377 autosequencer. Identity of the obtained sequence was established by BLAST sequence similarity search of the combined GenBank/EMBL/PDB files.
Northern Blot
Twenty-five μg of total RNA was fractionated by electrophoresis in a 1% agarose-formaldehyde denaturing gel and transferred to a nylon membrane (Amersham). After prehybridization, the membranes were hybridized overnight at 42°C with a 32P-dCTP-labeled, cloned Brk or β-actin cDNA fragment in a fresh hybridization solution that contained 50% formamide and 5× SSPE and washed in 0.1× standard saline citrate/0.1% sodium dodecyl sulfate. The filters were washed to a final stringency in 0.1× standard saline citrate/0.1% sodium dodecyl sulfate at 62°C, and exposed to X-ray film with an intensifying screen for 7 days at −70°C.
RT-PCR
Total RNA was isolated using RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The extracted RNA was treated with DNase I (Invitrogen, Carlsbad, CA) and reverse transcription was performed using the Thermoscript RT-PCR system (Invitrogen) with random hexamers as cDNA synthesis primers as recommended by the kit’s manufacturer. The following primer pairs were used for cDNA amplification: Brk 5′-TGTGGAGTGTCTGCGTCCAATACA-3′ (forward) and 5′-AGGCCAAGCTCTCAAGACACAAGA-3′ (reverse) and β-actin 5′-ACCATTGGCAATGAGCGGT-3′ (forward) and 5′-GTCTTTGCGGATGTCCACGT-3′ (reverse). PCR was performed using Platinum TaqDNA polymerase (Invitrogen) for 25 (for β-actin) and 30 (for Brk) cycles comprised of denaturation for 20 seconds at 94°C, annealing for 30 seconds at 58°C, and elongation for 30 seconds at 72°C.
Western Blot
These experiments were performed as described previously.21 In brief, the cells were washed and lysed in radioimmunoprecipitation assay buffer [50 mmol/L Tris-HCl (pH 7.4), 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid] supplemented with 0.5 mmol/L phenylmethyl sulfonyl fluoride, phosphatase inhibitor cocktails I and II (Sigma), and protease inhibitor cocktail (Roche, Nutley, NJ) according to the manufacturer’s specifications. For normalization of gel loading, the protein extracts were assayed by the Lowry method (Dc protein assay; Bio-Rad, Hercules, CA). Cell lysates were separated in a 10% polyacrylamide-sodium dodecyl sulfate gel and transferred to polyvinylidene difluoride membranes (Amersham). Proteins were detected with polyclonal antibodies against Brk (rabbit; Santa Cruz Biotechnology, Santa Cruz, CA), β-actin (goat; Santa Cruz), LCK (rabbit; Cell Signaling Technology, Beverly, MA), lamin A/C (rabbit, Cell Signaling Technology), monoclonal antibody against α-tubulin (Santa Cruz), and the appropriate peroxidase-conjugated secondary antibodies (Santa Cruz). Blots were developed using the SuperSignal West Dura from Pierce (Rockford, IL) and exposed to X-ray film (Kodak, Rochester, NY). Brk- or control, IL-21-reactive blocking oligopeptides (Santa Cruz) were used to confirm specificity of the Brk antibody.
Assay for Brk Kinase Activity
Kinase activity assay was performed as described previously.23 Briefly, cell lysates were immunoprecipitated with 40 μl of the polyclonal anti-Brk antibody (Santa Cruz) and 20 μl of Protein G agarose (Life Technologies, Inc., Grand Island, NY). To measure autophosphorylation of Brk,24 the precipitates were washed and incubated at room temperature in 25 mmol/L HEPES, pH 7.1, 10 mmol/L MnCl2, 1 μmol/L ATP, and 10 μCi γ-32[P] ATP (6000 μCi/mmol; DuPont NEN, Boston, MA). The reaction was stopped by the addition of 2× Laemmli sample buffer and boiling. Samples were analyzed by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, followed by autoradiography on Kodak X AR-5 film at −70°C.
Nuclear Extraction
Cells were centrifuged and washed, and cell pellet volume was measured using the graduation on the tube. Cells were washed with a 5× cell pellet volume of hypotonic buffer (10 mmol/L HEPES, 1.5 mmol/L MgCl2, 10 mM KCl, 0.2 mmol/L phenylmethyl sulfonyl fluoride, 0.5 mmol/L dithiothreitol). The pellets were then suspended in 3× cell pellet volume of hypotonic buffer and incubated 10 minutes on ice. Cells were transferred to a glass Dounce homogenizer and homogenized with 10 up-and-down stokes using a type B pestle. Nuclei were collected by centrifugation (15 minutes, 3300 × g), and supernatants were saved as cytoplasmic extracts. Volume of nuclei was measured using the graduation on the tube, and the nuclei were resuspended in a 0.5× volume of low-salt buffer (20 mmol/L HEPES, 25% glycerol, 1.5 mmol/L MgCl2, 0.02 mol/L KCl, 0.2 mmol/L ethylenediaminetetraacetic acid, 0.2 mmol/L phenylmethyl sulfonyl fluoride, 0.5 dithiothreitol), followed by a 30-minute incubation with continuous mixing in 0.5× volume of nuclei of high-salt buffer (20 mmol/L HEPES, 25% glycerol, 1.5 mmol/L MgCl2, 1.2 mol/L KCl, 0.2 mmol/L ethylenedia-minetetraacetic acid, 0.2 mmol/L phenylmethyl sulfonyl fluoride, 0.5 dithiothreitol). The extracted nuclei were pelleted by centrifugation for 30 minutes at 25,000 × g.
Cell Transfection
pRcCMV plasmids (Invitrogen) containing no Brk cDNA (empty vector), cDNA of wild-type (WT) Brk, or kinase-inactive mutant (K219M) expressed under control of the CMV promoter were described previously.14 The plasmids were stably expressed in murine IL-3-dependent lymphoid cell line BaF3 by standard electroporation. In brief, 107 BaF3 cells were exposed to electrical shock in the presence of 10 μg of the plasmids in Gene Pulser Xcell Total System (Bio-Rad) according to the manufacturer’s protocol. Cells were cultured in the complete RPMI medium supplemented with murine IL-3, as described above. Plasmids were washed away from the medium after 24 hours. The selection of Brk-expressing cells, uncloned or cloned by the single cell dilution method, was begun 48 hours later using 1 mg/ml of geneticin (Life Technologies, Inc.). The expression of Brk in the BaF3 cells was confirmed using Western blot with the anti-Brk antibody. BaF3 cell populations with a high level of Brk expression were chosen for further experiments.
Bromodeoxyuridine (BrdU) Incorporation
The detection of BrdU incorporation was performed using the cell proliferation enzyme-linked immunosorbent assay (Roche) according to the manufacturer’s protocol. In brief, cells were seeded for 16 hours in 96-well plates (Corning, Corning, NY) at a concentration of 4 × 106 cells/well in triplicate cell culture in RPMI medium supplemented (unless stated otherwise) with 10% fetal bovine serum or 2% bovine serum albumin (BSA) and/or murine IL-3 (R&D Systems) and labeled with BrdU (Roche) for 4 hours. After the plate centrifugation (10 minutes at 300 × g), supernatant removal, and plate drying, the cells were fixed and the DNA was denatured by adding 200 μl of FixDenat (Roche) reagent. The amount of incorporated BrdU was determined by colorimetric method using a specific, peroxidase-conjugated antibody and the enzyme-linked immunosorbent assay plate reader.
Cell-Cycle Analysis
After the cell culture at 0.5 × 106 cells/ml for 24 hours, the cells were washed and permeabilized with 75% ice-cold ethanol for at least 1 hour at 4°C. After additional washes the cells were resuspended in Master Mix [950 μl phosphate-buffered saline (PBS), 0.1 mg RNase; Roche] containing 40 μg of propidium iodide (Sigma, St. Louis, MO) and incubated for 15 minutes at 37°C. The cells were analyzed by flow cytometry (FACSort BD) using CellQuest Pro and ModFit software.
Terminal dUTP Nick-End Labeling (TUNEL) Staining
TUNEL staining was performed using ApoAlert DNA fragmentation assay kit from BD Bioscience, San Diego, CA, according to the manufacturer’s protocol. In brief, 3 to 5 × 106 cells were collected, washed, and fixed with 1% formaldehyde/PBS. After washing, permeabilization with 70% ice-cold ethanol for at least 2 hours, and additional washing, cells were exposed to TdT for 1 hour at 37°C with TdT-unexposed samples and DNase I-treated samples used as negative and positive controls, respectively. Reaction was stopped by adding 20 mmol/L ethylenediaminetetraacetic acid, and cells were washed in 0.1% Triton X-100/BSA/PBS. Finally, samples were resuspended in 0.5 ml of propidium iodide/RNase/PBS and analyzed by flow cytometry (FACSort BD) using CellQuest PRO software.
Annexin-V Staining
The staining was performed using Annexin-V-FLUOS staining kit from Roche according to the manufacturer’s protocol. In brief, the cells were collected, washed, and resuspended in 100 μl of Annexin-V-FLUOS labeling solution containing propidium iodide. The samples were incubated 15 minutes at room temperature and analyzed by flow cytometry.
In Vivo Tumorigenicity
Mice were inoculated as previously described.25 In brief, 5- to 7-week-old SCID-beige mice (Taconic Farms, Germantown, NY) were injected with 45 mg/kg of etoposide (Bedford Labs) to deplete macrophages. Four days later BaF3 cells that stably expressed Brk or contained empty vector (pRcCMV plasmid) were injected intraperitoneally at 107 per mouse. Mice were sacrificed when symptoms of severe morbidity (cachexia, lethargy, and/or ascites) occurred or at the conclusion of the experiment. All mice were necropsied, and the internal organs (liver, spleen, lungs, heart, kidney, bone marrow), enlarged lymph nodes, and any tumors that developed in the mice were further evaluated by histopathology.
siRNA
Brk-expressing Jurkat, Karpas 299, and SUDHL-1 cell lines were treated with siRNA twice at 24-hour intervals. A mixture of four Brk-specific or nontargeting siRNA (both purchased from Dharmacon) was introduced into cells by lipofection according to the manufacturer’s protocol. In brief, 16 μl of DMIRIE-C (Invitrogen) and 100 nmol/L of the siRNA duplexes were mixed and incubated for 30 minutes in OptiMEM medium (Invitrogen) to allow formation of complexes. Then the complexes were admixed with 5 × 105 cells cultured in medium and incubated 4 to 5 hours at 37°C in 5% CO2. Twenty-four hours after the second dose was administered, the cells were examined in the functional assays to determine their proliferative capacity (BrdU incorporation) and spontaneous apoptotic rate (TUNEL assay). Efficiency of Brk protein suppression was assessed by Western blotting with lck and β-actin proteins serving as internal controls.
Statistical Analysis
The analysis of variance was used to evaluate the effect on BaF3 cells of the transfected constructs: empty vector, WT Brk (with mixed, uncloned cells, and a clone designated 1 analyzed separately and as a group), and Brk WTcl1, BrkK219M mutant (clones designated 1 and 2 analyzed separately or grouped). A similar approach was used to examine the effect of the medium type (10% FBS + IL-3, 10% FBS and 2% BSA + IL-3) on the BaF3 cells expressing the above constructs as well as the effects of the Brk versus nontargeting siRNA on the malignant T cells and cell line type (Jurkat, Karpas, SUDHL1) on the sensitivity to the Brk siRNA-mediated Brk depletion. Tukey’s procedure with SAS version 9.1 was used to evaluate pair-wise comparisons to control for the experiment-wise error rate.
Results
Expression of Brk in Normal and Malignant Lymphocytes
To define better the role of tyrosine kinases in lymphomagenesis, we examined the mRNA expression pattern of the kinases in malignant and normal T cells using our previously described kinase-specific RT-PCR/denaturing gradient gel electrophoresis method.20 One of the differentially expressed mRNAs revealed 100% identity with the sequence of the Brk tyrosine kinase as determined by searching of the NCBI data bank. To confirm Brk expression in malignant T cells, we examined by Northern blotting RNA isolated from three T-cell lines derived from CTCL. Four breast carcinoma cell lines, two being Brk-negative and the other two strongly positive,1 served as negative and positive controls, respectively. As shown in Figure 1A, all three CTCL-derived cell lines constitutively expressed Brk mRNA in the amount comparable to the Brk-positive breast carcinoma lines. To determine whether Brk expression is also present in other types of malignant and normal T cells, we performed a RT-PCR analysis using RNA isolated from a larger panel of malignant T-cell lines representing three different types of lymphoma as well as normal, T-cell-rich PBMCs, either nonstimulated or stimulated with a mitogen (PHA). Whereas the normal, nonstimulated PBMCs did not express Brk message (Figure 1B depicts the result with PBMCs from one of the five different healthy individuals examined), PHA activation induced Brk expression (results with one of the four donors examined is displayed). In regard to the malignant T cells, Brk was constitutively expressed by an additional CTCL-derived cell line (MyLa2056), as well as cell lines from anaplastic lymphoma kinase (ALK)-expressing T/null-cell lymphoma (ALK + TCL) and T-cell acute lymphoblastic leukemia/lymphoblastic lymphoma (T-ALL).
Figure 1.
Expression of Brk in T and B lymphocytes. A: Northern blot: Brk mRNA expression in T-cell lymphoma cell lines (PB-1, 2A, and 2B) derived from a progressive CTCL. Breast carcinoma cell lines positive (MFC-7 and T47D) and negative (BTTY and MDA231) for Brk expression served as controls. B: RT-PCR: Brk mRNA expression in normal PBMCs, either resting or mitogen (PHA)-activated, and in T-cell lymphoma cell lines derived from CTCL, anaplastic lymphoma kinase-expressing T/null-cell lymphoma (ALK + TCL), and T-cell acute lymphoblastic leukemia (T-ALL). C: Western blot: Brk protein expression in T-cell lines derived from the above three different types of T-cell lymphoma. D: Western blot: abrogation of Brk protein detection by Brk-specific blocking peptide. E: Western blot: Brk expression in B-cell lines derived from Epstein-Barr virus-transformed cells (EBV + LCL), Burkitt lymphoma (BL), mantle cell lymphoma (MCL), and diffuse large B-cell lymphoma (DLBCL).
To determine whether Brk is expressed in the malignant T cells also at the protein level, we examined the CTCL-, ALK + TCL-, and T-ALL-derived cell lines by Western blotting (Figure 1C). All cell lines expressed Brk protein. The expression, however, varied from very strong in most to barely detectable in the remaining few, specifically in two of the four ALK + TCL cell lines examined. To confirm specificity of the detected band, we pretreated the anti-Brk antibody with a blocking oligopeptide corresponding to Brk or an irrelevant, IL-21 oligopeptide. As shown in Figure 1D, the former has completely abrogated the band, whereas the latter control peptide displayed no inhibitory effect. To determine whether Brk expression is confined to malignant lymphocytes of the T-cell lineage, we examined 10 B-cell lines with two being transformed by EBV and the others derived from Burkitt, mantle cell, and diffuse large B-cell lymphoma. All 10 cell lines expressed Brk whereas all diffuse large B-cell lymphoma and mantle cell lymphoma cell lines expressing the kinase protein very strongly (Figure 1E).
Activation Status of Brk in Malignant T Lymphocytes
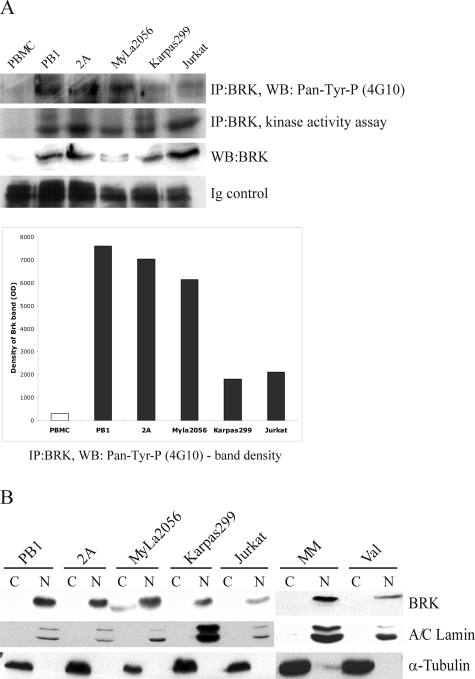
Although the mechanisms of Brk activation remain unknown, kinases typically, if not universally, require phosphorylation to become enzymatically active. To determine whether Brk is in an activated state in malignant T cells, we examined its phosphorylation status in such cells using the Brk antibody immunoprecipitates probed in Western blotting with a 4G10 antibody that reacts with phosphorylated (but not unphosphorylated) tyrosine moieties. As shown in the Figure 2A (top band and the accompanying densitometric tracing depicted below the panel), all T-cell lines examined displayed evidence of Brk phosphorylation, which was quite robust in some of the lines. As expected no signal could be demonstrated in the control, resting PBMCs that do not express detectable Brk mRNA (Figure 1B) and protein (Figure 2A; bottom middle panel).
Figure 2.
Activation status and subcellular localization of Brk in selected T- and B-cell populations. A: Kinase activity assay to determine activation status of Brk as detected by its autophosphorylation and Western blot to examine protein phosphorylation using anti-phosphotyrosine antibody (4G10). The protein phosphorylation is shown also schematically by depicting densitometric analysis data. B: Western blot: Brk protein expression in fractionated nuclei and cytoplasm with A/C lamin (nuclear protein) and a-tubulin (cytoplasmic protein) serving as controls.
Because it has been established that enzymatic activity of the kinase is enhanced through autophosphorylation,24 we examined the capacity of Brk precipitated from the malignant T cells to phosphorylate itself in vitro. As shown in Figure 2A (middle top panel), Brk from all T-cell lines examined displayed Brk autophosphorylation with some variation in its degree, documenting overall that the kinase is persistently activated in such cells.
Subcellular Localization of Brk in Malignant T and B Lymphocytes
Because Brk has been found expressed in cancer cells not only in the cell cytoplasm but also in the nucleus,6,8,10,11,13,26 we performed subcellular fractionations to determine the exact Brk localization in malignant lymphocytes. Two other proteins known to localize either in the nucleus (A/C lamin) or cytoplasm (α-tubulin) served as controls of the fractionation effectiveness. As shown in Figure 2B, similar to A/C lamin and in contrast to α-tubulin, Brk expression was essentially confined to the nucleus in all transformed T- and B-cell types examined.
Brk Protein Expression and Activation Status in Normal Activated and Native CTCL-Derived T Cells
Next, we evaluated Brk expression in circulating (Sezary) CTCL cells from patients who developed the leukemic phase and in normal T-cell-rich PBMCs, both resting and activated (Figure 3A). In addition to the PHA-mediated T-cell stimulation, we have used also alternative T-cell activation by the combination of immobilized anti-CD3 and anti-CD28 antibodies to directly determine whether engagement of the T-cell receptor complex leads to Brk expression. Whereas, none of the resting normal PBMCs expressed Brk (one of the performed five experiments with cells from different donors is presented), both PHA and CD3/CD28 stimulations induced expression of the kinase (the depicted result is representative for four and two separate experiments, respectively). In regard to the native CTCL cells, three of the seven cell isolates examined displayed moderate to strong expression of Brk protein.
Figure 3.
Brk expression and activation status in normal activated and malignant T-cell populations. A: Western blot: expression of Brk by T-cell-rich PBMCs, either resting or activated by mitogen (PHA) or anti-CD3/CD28 antibody combination and by patient-derived CTCL cells. B: Kinase activity assay: enzymatic activity of Brk immunoprecipitated from the resting and PHA-activated PBMCs and CTCL cells. Immunoprecipitates from the 2A T-cell line lysate untreated or treated with a Brk-blocking peptide served as controls.
To demonstrate that Brk expression in the normal activated and native malignant CTCL cells is associated with Brk activation, we performed the kinase autophosphorylation assay using the Brk immunoprecipitates as described above for the T-cell lines (Figure 2) with one of the cell lines (2A) now serving as a control. As can be seen in Figure 3B, normal PHA-activated PBMCs as well as the Brk-expressing CTCL cells contained the enzymatically active kinase, confirming its activation status. The Brk activity in some of the CTCL samples was quite robust and matched the signal intensity generated by the control 2A cells. Abrogation of the signal by pretreatment of the Brk antibody with the Brk-derived blocking peptide confirmed its identity as Brk.
In Vitro and in Vivo Oncogenic Properties of Brk Transfected into Brk-Negative Lymphocytes
The above-described, widespread expression in malignant lymphoid cells of Brk in an activated form strongly suggested that this tyrosine kinase may play a role in lymphomagenesis. To explore this supposition more directly, we either introduced constitutively expressed Brk into lymphocytes or depleted the kinase in malignant lymphocytes that expressed it constitutively. In the first approach, we stably transfected the B-cell line BaF3 with Brk. This murine, immature B-cell line broadly used for cell transfection experiments is cytokine (IL-3)-dependent and, as with most cell lines, serum-dependent in regard to its proliferation and survival. As shown in Figure 4A, the exogenously expressed Brk accumulated in the cell nucleus of BaF3 cells similar to the localization it displayed in the human lymphoma-derived T- and B-cell lines (Figure 2B). To test the impact of Brk on BaF3 cell biology, we challenged the Brk- and empty vector-transfected cells with deprivation of IL-3 or serum. In some experiments we additionally used BaF3 cells transfected with the Brk mutant K219M, which is devoid of kinase activity. As shown in Figure 4B, removal of IL-3 or serum markedly inhibited proliferation of the BaF3 transfected with the empty vector as determined by their BrdU uptake (P < 0.001 as determined in three separate experiments performed in triplicates). However, expression of Brk in its native WT form preserved to a large degree the proliferative capacity of BaF3 cells on withdrawal of serum and, in particular, IL-3 (P < 0.05). As shown in Figure 4, comparable results were obtained using antibiotic-resistant but uncloned (mixed) cells as well as a randomly selected clone. In contrast to the Brk WT, the Brk K219M kinase-silent mutant was unable to effectively compensate for the loss of IL-3 or serum-mediated stimuli as demonstrated using two different clones expressing this Brk variant. The Brk K219M-expressing BaF3 cells yielded results closely resembling the ones obtained with the cells transfected with empty vector. Similar to the BrdU uptake, another determinant of cell proliferation, cell-cycle progression (depicted as the percentage of cells in the S phase; Figure 4C), was preserved in the Brk WT-expressing cells as compared to the empty vector-transfected cells (P < 0.05).
Figure 4.
Brk expression and impact on in vitro proliferation and survival and in vivo tumorigenicity in the Brk-transfected lymphoid cells. A: Brk protein expression in nuclei versus cytoplasm in the IL-3-dependent BaF3 lymphoid cells transfected with either WT Brk construct or empty vector (E. vec). The depicted Brk-WT, Brk-kinase-inactive (K219M) mutant, and vector-expressing BaF3 cells cultured in the presence of either complete (10% FBS and IL-3), cytokine-depleted (10% FBS alone), or serum-depleted (IL-3 and 2% BSA) medium and evaluated for BrdU incorporation (B) or DNA fragmentation (TUNEL assay; D). The Brk-WT- and vector-expressing BaF3 cells cultured in the complete, cytokine-, or serum-depleted medium were evaluated for propidium iodide incorporation (C) and cell-surface annexin V expression (E). F: SCID-beige mice (five per group) were inoculated intraperitoneally with either Brk-WT- or vector-expressing BaF3 cells and followed for evidence of morbidity. The diagram depicts survival time of both groups with the mice injected with Brk-expressing BaF3 cells developing a wide-spread lymphoproliferative disorder within 5 weeks and the mice injected with vector-carrying BaF3 cells showing no sign of disease by week 17.
As shown in Figure 4D, cell deprivation of serum and, to a much lesser degree, IL-3 enhanced the apoptotic rate of the empty vector-carrying BaF3 cells (ninefold and twofold, respectively; P = 0.03 based on three separate experiments) as determined in the DNA fragmentation (TUNEL) assay. Expression of Brk WT almost completely abrogated the increase for both IL-3 and, more markedly, serum withdrawal (P < 0.05). In contrast, the apoptotic rates of K219M- and vector-carrying BaF3 cells were similar to each other for both IL-3 and serum deprivation. Evaluation of the early stage of cell apoptosis by detection of cell-surface binding of annexin V yielded results similar to the DNA fragmentation assays. Whereas withdrawal of either serum or IL-3 markedly increased annexin V binding in the empty vector-transfected BaF3 cells (P < 0.05), it had essentially no effect on the Brk WT-expressing cells (Figure 4E).
To determine the role of Brk in in vivo tumorigenesis, the Brk WT- or empty vector-transfected BaF3 cells were inoculated into immunodeficient SCID-beige mice. All mice injected intraperitoneally with the Brk-expressing cells became moribund within 5 weeks from the inoculation whereas the control mice, injected with the empty vector-carrying BaF3 cells, displayed no sign of the disease for the 17-week duration of the experiment (Figure 4F; P < 0.005). Autopsy performed on mice from the former group revealed the presence in all animals of intraperitoneal tumors and enlargement of spleen, liver, and/or intra-abdominal lymph nodes. Histological evaluation of the tumors and the enlarged organs confirmed the presence of malignancy comprised of large, noncohesive cells with atypical, hyperchromatic nuclei consistent with lymphoid malignancy (not shown). In addition, microscopic foci of the malignant cells were frequently found in the lungs and, occasionally, kidneys. No definitive involvement of the bone marrow and heart could be appreciated in the sections examined. Three randomly selected tumor samples revealed strong expression of Brk protein (not shown). In contrast, mice inoculated with the empty vector-carrying BaF3 cells that were sacrificed 17 weeks after the cell injection showed no macroscopic or histological evidence of malignancy in any of the organs.
Impact of siRNA-Mediated Brk Depletion on Malignant T Cells Constitutively Expressing the Kinase
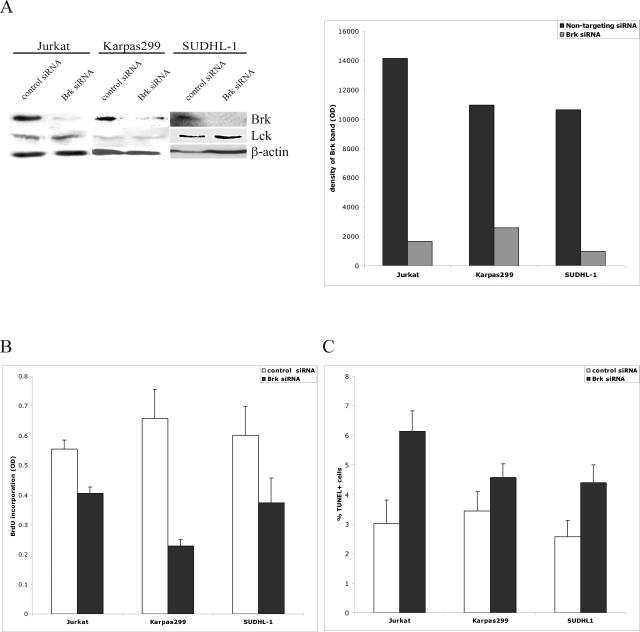
To determine the biological role of Brk in malignant T cells that express the kinase endogenously (Figure 1) in the persistently activated state (Figure 2A), we inhibited Brk expression in such cells using the siRNA technology. Treatment of three different malignant T-cell lines with the Brk-specific, but not control, siRNA diminished expression of Brk in these cells by 80 to 90% (Figure 5A depicts the representative result including the densitometry tracking of up to five independent experiments per cell line). Expression of two control proteins, Lck (brk-related, src-type kinase) and β-actin, remained unaffected by the Brk siRNA. As shown in Figure 5B, Brk depletion reproducibly inhibited proliferation of all three malignant T-cell lines (P < 0.001 determined from up to three separate experiments performed in triplicates) with one of the lines examined, Karpas 299, being the most and another, Jurkat, the least affected (P = 0.03). The Brk depletion impacted adversely also on cell survival (Figure 5C, P = 0.03 determined from three to five individual experiments). Interestingly, the effect in this assay was the most pronounced in Jurkat (P < 0.05) and the least visible in Karpas 299 cells.
Figure 5.
Effect of siRNA-mediated Brk depletion on proliferation and survival of malignant T-cells. A: Brk protein expression in Jurkat, Karpas 299, and SUDHL-1 T-cell lymphoma lines on treatment with Brk-specific and nontargeting siRNA. Lck and β-actin proteins served as controls. B and C: BrdU incorporation (B) and DNA fragmentation (TUNEL assay) (C) in the siRNA-treated cells.
Discussion
Although Brk is expressed in several types of normal and cancerous tissues, its role in cell physiology and malignant transformation remains rather poorly defined. On one hand, the studies in mammary cells suggest that Brk plays a key role in carcinogenesis. Accordingly, Brk expression is very low or undetectable in normal breast tissue and benign lesions, whereas 60% of breast carcinomas express the kinase at varying elevated levels.4 Furthermore, Brk expression promotes anchorage-independent growth and increases the proliferative rate of nontransformed mammary epithelial cells,14 and also promotes the proliferation of a breast tumor cell line.16 Roles for Brk in promoting proliferation in various other cell types have been described,15,17 as have roles in protection from apoptosis15 and increasing migration/chemotaxis/matrix degradation.18 On the other hand, in the skin, gastrointestinal tract, prostate, and upper respiratory tract, Brk is detected in normal epithelium in a pattern suggestive of its involvement in cell differentiation and senescence,8,10–13 and in some squamous cell carcinomas expression correlates inversely with the degree of malignant cell transformation.10,11 Interestingly, in some malignant epithelial cells in vivo Brk loses nuclear localization.10,13 Functional roles for Brk in promoting apoptosis19 and the expression of differentiation markers11,12 have also been found. The above findings suggest that the expression pattern and function of Brk are highly tissue-type-specific. Furthermore, the mechanisms and effects of Brk dysregulation also appear cancer-type and/or cell of origin-specific. Finally, it seems that these mechanisms are highly diverse and affect Brk expression as well as its activation and specific intracellular localization.
Our results demonstrate Brk expression in lymphocytes. Although we focused our analysis on malignant T-cell lymphoma cells, we have also documented the expression of Brk in normal T-cell-rich PBMCs on their activation with mitogenic or T-cell receptor-targeting stimuli as well as transformed B lymphocytes. We detected constitutively expressed Brk in essentially all T-cell lines derived from three different types of T-cell lymphoma, CTCL, ALK+, and lymphoblastic, with most expressing Brk very strongly. Almost half of the native CTCL samples but none of the control, nonstimulated PBMC populations derived from healthy donors expressed Brk. Similarly to the malignant T cells, B-cell lines from EBV-transformed normal B lymphocytes and three different types of lymphoma (Burkitt lymphoma, mantle cell lymphoma, and diffuse large B-cell lymphoma) expressed the kinase. The above findings strongly suggest that Brk expression is very common among malignant T and B cells, albeit its intracellular concentration seems to vary with some malignant cell populations clearly overexpressing the kinase. Our observation that expression of Brk can be induced in normal T cells suggests that Brk plays a role in the physiology of such cells seemingly in response to antigens, because the T-cell receptor perturbation but not cytokine (IL-2) stimulation (data not presented) was able to induce its expression. However, elucidation of the exact role of Brk in T and, possibly, B lymphocyte physiology as well as determination of the comprehensive expression pattern of the kinase in normal and malignant lymphocytes and other immune and hematopoietic cells requires further studies.
We have also documented that Brk is expressed in malignant T cells in the persistently phosphorylated, enzymatically active state, providing additional evidence that it may play a role in the pathogenesis of lymphomas. It is interesting in this context that Brk shows propensity for nuclear localization in lymphocytes as shown by us in several malignant T- and B-cell lymphoma cell lines, transfected BaF3 cells, and PHA-stimulated PBMCs (data not presented). The observed nuclear localization implies that the function of Brk in the T-cell lymphoma and other lymphoid cell populations is different from some other cell types in which Brk is located in the cytoplasm. In the latter location, it is implicated in the regulation of signaling by epidermal growth factor (EGF) and serum, via EGF-R, erbB3, and possibly other cell-surface receptors, to changes in phosphoinositide 3-kinase, Akt, paxillin, and Rac1 activities and interactions.14,18,27,28 The nuclear localization in lymphocytes suggests interactions of Brk in such cells with a different set of intracellular partners and may involve intranuclear proteins from the Star family, possibly impacting on cell proliferation.17,29
Our data provide strong direct evidence that in malignant lymphocytes Brk is involved in cell proliferation and survival, and hence by remaining constitutively expressed in the persistently active state may play a key role in the pathogenesis of lymphomas. Brk may, therefore, represent an attractive therapeutic target in T-cell lymphomas and, possibly, other lymphoid malignancies. In support of the Brk-mediated oncogenicity, we have shown that the kinase promoted proliferation and inhibited apoptotic cell death of the transfected BaF3 cells by compensating for the loss of cytokine (IL-3)- or serum (growth factor)-mediated signals and by conferring on the cells in vivo tumorigenicity. Noteworthy, these pro-oncogenic properties of Brk were dependent on its enzymatic activity because the kinase-inactive K219M mutant failed to preserve proliferative and survival capacities of the BaF3 cells on cytokine and serum withdrawal. In contrast, the Brk overexpression-mediated augmentation of T-47D breast carcinoma cell line proliferation is kinase activity-independent,16 providing additional evidence that Brk may play very different roles in malignant transformation of lymphoid and mammary cells. Interestingly, the proproliferative and anti-apoptotic properties of Brk may be differentially regulated judging from the seemingly distinct impact, in this regard, of Brk on the various malignant T-cell lines examined. However, the exact mechanisms responsible for the Brk-mediated control over these basic cell functions remain to be elucidated.
In summary, our data document expression of the Brk tyrosine kinase in lymphoma-derived and normal T cells as well as EBV-transformed and lymphoma-derived B cells. Our results also indicate that Brk may play an important role in malignant cell transformation of lymphocytes. Therefore, it may represent an attractive ther-apeutic target in lymphomas and, possibly, other malignancies.
Footnotes
Address reprint requests to Mariusz A. Wasik, University of Pennsylvania Medical Center, Department of Pathology and Laboratory Medicine, 3400 Spruce St., 7.106 Founders Pavilion, Philadelphia, PA 19104-4283. E-mail: wasik@mail.med.upenn.edu.
Supported in part by grants from the National Cancer Institute (R01-CA89194 and R01-CA96856 to M.A.W. and R01-CA108552 to A.P.).
References
- Mitchell PJ, Barker KT, Martindale JE, Kamalati T, Lowe PN, Page MJ, Gusterson BA, Crompton MR. Cloning and charactersation of cDNAs encoding a novel non-receptor tyrosine kinase, brk, expressed in human breast tumours. Oncogene. 1994;9:2383–2390. [PubMed] [Google Scholar]
- Vasioukhin V, Serfas MS, Siyanova EY, Polonskaia M, Costigan VJ, Liu B, Thomason A, Tyner AL. A novel intracellular epithelial cell tyrosine kinase is expressed in the skin and gastrointestinal tract. Oncogene. 1995;10:349–357. [PubMed] [Google Scholar]
- Serfas MS, Tyner AL. Brk, Srm, Frk, and Src42A form a distinct family of intracellular Src-like tyrosine kinases. Oncol Res. 2003;13:409–419. doi: 10.3727/096504003108748438. [DOI] [PubMed] [Google Scholar]
- Barker KT, Jackson LE, Crompton MR. BRK tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene. 1997;15:799–805. doi: 10.1038/sj.onc.1201241. [DOI] [PubMed] [Google Scholar]
- Zhao C, Yasui K, Lee CJ, Kurioka H, Hosokawa Y, Oka T, Inazawa J. Elevated expression levels of NCOA3, TOP1, and TFAP2C in breast tumors as predictors of poor prognosis. Cancer. 2003;98:18–23. doi: 10.1002/cncr.11482. [DOI] [PubMed] [Google Scholar]
- Born M, Quintanilla-Fend L, Braselmann H, Reich U, Richter M, Hutzler P, Aubele M. Simultaneous over-expression of the Her2/neu and PTK6 tyrosine kinases in archival invasive ductal breast carcinomas. J Pathol. 2005;205:592–596. doi: 10.1002/path.1720. [DOI] [PubMed] [Google Scholar]
- Easty DJ, Mitchell PJ, Patel K, Florenes VA, Spritz RA, Bennett DC. Loss of expression of receptor tyrosine kinase family genes PTK7 and SEK in metastatic melanoma. Int J Cancer. 1997;71:1061–1065. doi: 10.1002/(sici)1097-0215(19970611)71:6<1061::aid-ijc24>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Llor X, Serfas MS, Bie W, Vasioukhin V, Polonskaia M, Derry J, Abbott CM, Tyner AL. BRK/Sik expression in the gastrointestinal tract and in colon tumors. Clin Cancer Res. 1999;5:1767–1777. [PubMed] [Google Scholar]
- Lin HS, Berry GJ, Fee WE, Jr, Terris DJ, Sun Z. Identification of tyrosine kinases overexpressed in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004;130:311–316. doi: 10.1001/archotol.130.3.311. [DOI] [PubMed] [Google Scholar]
- Petro BJ, Tan RC, Tyner AL, Lingen MW, Watanabe K. Differential expression of the non-receptor tyrosine kinase BRK in oral squamous cell carcinoma and normal oral epithelium. Oral Oncol. 2004;40:1040–1047. doi: 10.1016/j.oraloncology.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Wang TC, Jee SH, Tsai TF, Huang YL, Tsai WL, Chen RH. Role of breast tumour kinase in the in vitro differentiation of HaCaT cells. Br J Dermatol. 2005;153:282–289. doi: 10.1111/j.1365-2133.2005.06604.x. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Tyner AL. A role for the epithelial-cell-specific tyrosine kinase Sik during keratinocyte differentiation. Proc Natl Acad Sci USA. 1997;94:14477–14482. doi: 10.1073/pnas.94.26.14477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry JJ, Prins GS, Ray V, Tyner AL. Altered localization and activity of the intracellular tyrosine kinase BRK/Sik in prostate tumor cells. Oncogene. 2003;22:4212–4220. doi: 10.1038/sj.onc.1206465. [DOI] [PubMed] [Google Scholar]
- Kamalati T, Jolin HE, Mitchell PJ, Barker KT, Jackson LE, Dean CJ, Page MJ, Gusterson BA, Crompton MR. Brk, a breast tumor-derived non-receptor protein-tyrosine kinase, sensitizes mammary epi-thelial cells to epidermal growth factor. J Biol Chem. 1996;271:30956–30963. doi: 10.1074/jbc.271.48.30956. [DOI] [PubMed] [Google Scholar]
- Kim HI, Lee ST. An intramolecular interaction between SH2-kinase linker and kinase domain is essential for the catalytic activity of protein-tyrosine kinase-6. J Biol Chem. 2005;280:28973–28980. doi: 10.1074/jbc.M504568200. [DOI] [PubMed] [Google Scholar]
- Harvey AJ, Crompton MR. Use of RNA interference to validate Brk as a novel therapeutic target in breast cancer: Brk promotes breast carcinoma cell proliferation. Oncogene. 2003;22:5006–5010. doi: 10.1038/sj.onc.1206577. [DOI] [PubMed] [Google Scholar]
- Lukong KE, Larocque D, Tyner AL, Richard S. Tyrosine phosphorylation of sam68 by breast tumor kinase regulates intranuclear localization and cell cycle progression. J Biol Chem. 2005;280:38639–38647. doi: 10.1074/jbc.M505802200. [DOI] [PubMed] [Google Scholar]
- Chen HY, Shen CH, Tsai YT, Lin FC, Huang YP, Chen RH. Brk activates rac1 and promotes cell migration and invasion by phosphorylating paxillin. Mol Cell Biol. 2004;24:10558–10572. doi: 10.1128/MCB.24.24.10558-10572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegebarth A, Nunez R, Tyner AL. The intracellular tyrosine kinase Brk sensitizes non-transformed cells to inducers of apoptosis. Cell Cycle. 2005;4:1239–1246. doi: 10.4161/cc.4.9.1965. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Zhang Q, Wilson J, Ratajczak MZ, Wasik MA. Detection of protein tyrosine-kinase (PTK) gene expression pattern in normal and malignant T lymphocytes by combined PTK-specific PCR and parallel denaturing gradient gel electrophoresis (DGGE). J Mol Diagn. 2003;5:113–120. doi: 10.1016/S1525-1578(10)60460-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Raghunath PN, Xue L, Majewski M, Carpentieri DF, Odum N, Morris S, Skorski T, Wasik MA. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J Immunol. 2002;168:466–474. doi: 10.4049/jimmunol.168.1.466. [DOI] [PubMed] [Google Scholar]
- Wlodarski P, Kasprzycka M, Liu X, Marzec M, Slupianek A, Wasik MA. Activation of mTOR in transformed B lymphocytes is nutrient-dependent but independent of Akt MEK, IGF-I, and serum. Cancer Res. 2005;65:7800–7808. doi: 10.1158/0008-5472.CAN-04-4180. [DOI] [PubMed] [Google Scholar]
- Ptasznik A, Nakata Y, Kalota A, Emerson SG, Gewirtz AM. Short interfering RNA (siRNA) targeting the Lyn kinase induces apoptosis in primary, and drug-resistant, BCR− ABL1(+) leukemia cells. Nat Med. 2004;10:1187–1189. doi: 10.1038/nm1127. [DOI] [PubMed] [Google Scholar]
- Qiu H, Miller WT. Regulation of the nonreceptor tyrosine kinase Brk by autophosphorylation and by autoinhibition. J Biol Chem. 2002;277:34634–34641. doi: 10.1074/jbc.M203877200. [DOI] [PubMed] [Google Scholar]
- Majewski M, Korecka M, Kossev P, Li S, Goldman J, Moore J, Silberstein L, Nowell PC, Schuler W, Shaw LM, Wasik MA. The immunosuppressive macrolide RAD inhibits growth of human Epstein-Barr virus-transformed B lymphocytes in vitro and in vivo: a potential approach to prevention and treatment of posttransplant lymphoproliferative disorders. Proc Natl Acad Sci USA. 2000;97:4285–4290. doi: 10.1073/pnas.080068597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry JJ, Richard S, Valderrama Carvajal H, Ye X, Vasioukhin V, Cochrane AW, Chen T, Tyner AL. Sik (BRK) phosphorylates Sam68 in the nucleus and negatively regulates its RNA binding ability. Mol Cell Biol. 2000;20:6114–6126. doi: 10.1128/mcb.20.16.6114-6126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamalati T, Jolin HE, Fry MJ, Crompton MR. Expression of the BRK tyrosine kinase in mammary epithelial cells enhances the coupling of EGF signalling to PI 3-kinase and Akt, via erbB3 phosphorylation. Oncogene. 2000;19:5471–5476. doi: 10.1038/sj.onc.1203931. [DOI] [PubMed] [Google Scholar]
- Zhang P, Ostrander JH, Faivre EJ, Olsen A, Fitzsimmons D, Lange CA. Regulated association of protein kinase b/akt with breast tumor kinase. J Biol Chem. 2005;280:1982–1991. doi: 10.1074/jbc.M412038200. [DOI] [PubMed] [Google Scholar]
- Haegebarth A, Heap D, Bie W, Derry JJ, Richard S, Tyner AL. The nuclear tyrosine kinase BRK/Sik phosphorylates and inhibits the RNA-binding activities of the Sam68-like mammalian proteins SLM-1 and SLM-2. J Biol Chem. 2004;279:54398–54404. doi: 10.1074/jbc.M409579200. [DOI] [PubMed] [Google Scholar]