Abstract
Remodeling of injured blood vessels is dependent on smooth muscle cells and matrix metalloproteinase activity. Doxycycline is a broad spectrum matrix metalloproteinase inhibitor that is under investigation for the treatment of acute coronary syndromes and aneurysms. In the present study, we examine the mechanisms by which doxycycline inhibits smooth muscle cell responses using a series of in vitro assays that mimic critical steps in pathological vascular remodeling. Doxycycline treatment dramatically increased smooth muscle cell adhesion to the substrate, as evidenced by interference reflection microscopy and immunostaining for paxillin and phosphotyrosine. Cell aggregation was also potentiated after treatment with doxycycline. Treatment with 104 μmol/L doxycycline reduced thymidine uptake by 58% compared with untreated cells (P < 0.05) and inhibited closure of a scrape wound made in a smooth muscle cell monolayer by 20% (P < 0.05). Contraction of a three-dimensional collagen gel was used as an in vitro model for constrictive vessel remodeling, demonstrating that treatment with 416 μmol/L doxycycline for 12 hours inhibited collagen gel remodeling by 37% relative to control (P < 0.05). In conclusion, we have shown that doxycycline treatment leads to dramatically increased smooth muscle cell adhesion, which in turn might limit responses in pathological vascular remodeling.
Intimal hyperplasia and inward constrictive remodeling are involved in vascular occlusive diseases including atherosclerosis, restenosis, and transplant arteriopathy. Smooth muscle cell (SMC) proliferation and migration contribute to intimal hyperplasia, whereas SMC contraction and reorganization of the vascular extracellular matrix lead to changes in vessel geometry. The matrix metalloproteinases (MMPs), a family of matrix-degrading enzymes with broad substrate specificity, have been shown to mediate pathological remodeling in many arterial diseases. During restenosis, the MMPs facilitate smooth muscle cell migration from media to intima, and also affect inward remodeling by permitting the cleavage and reorganization of the vascular extracellular matrix.1
The tetracyclines are potent inhibitors of the MMP enzyme family and have been used to reduce tissue degradation in periodontal disease and arthritis.2 Doxy-cycline, a tetracycline derivative, has been used experimentally to inhibit matrix degradation during abdominal aortic aneurysm formation,3–5 and recent clinical studies have investigated the use of doxycycline to limit MMP activity and aneurysm growth.6–9 Doxycycline is also currently under investigation as a prophylactic treatment for acute coronary syndromes.10,11
We have previously demonstrated the potent inhibitory effects of doxycycline treatment on intimal thickening and inward remodeling in a series of studies using rat and rabbit models of arterial injury. In the balloon-injured rat carotid artery, doxycycline inhibited SMC proliferation and migration, which led to an attenuation of intimal thickening.12 Moreover, CMT-3, a derivative of doxycycline that lacks antibiotic activity but retains anti-MMP activity, also inhibited intimal thickening, demonstrating that these effects were independent of the antibiotic activity of the drug.13 Furthermore, doxycycline attenuated inward remodeling of the rabbit abdominal aorta by preventing the organization of collagen fibers in the vessel wall.14 These results were in accord with previous studies that showed that treatment with doxycycline could inhibit outward vessel remodeling in response to increased blood flow15 and reduce intimal thickening in a vein graft model of stenosis16 and that minocycline inhibits neointimal thickening.17
Despite these promising findings using in vivo models of vascular disease, few studies have addressed the mechanisms of action of doxycycline on vascular SMCs. Tetracyclines inhibit cell proliferation, migration, and synthesis of matrix in several cell types studied in culture.18–20 At present, it is not entirely clear whether some or all of these effects are dependent on the anti-MMP actions of doxycycline. In addition to well-known effects degrading extracellular matrix molecules, recent research shows that MMPs can modulate cell-matrix and cell-cell adhesion by cleaving integrin,21 discoidin domain receptor,22 and cadherin adhesion molecules.23 Therefore inhibition of the MMPs with doxycycline may lead to changes in cell adhesion, motility, and proliferation that could be important in the pathogenesis of arterial occlusive disease. However, the effects of doxycycline on SMC adhesiveness have not been studied.
In the current report, we have examined the mechanisms by which doxycycline inhibits SMC responses to injury, using a series of in vitro assays that mimic critical steps in intimal hyperplasia and inward remodeling. These include SMC adhesion to substrate and focal contact formation, cell-cell adhesion, migration, proliferation, and collagen matrix reorganization. We show that doxycycline treatment leads to dramatically increased SMC adhesion, which in turn might limit SMC responses involved in vascular remodeling.
Materials and Methods
All chemicals were obtained from Sigma-Aldrich Canada (Oakville, ON, Canada) unless stated otherwise.
Smooth Muscle Cell Culture
SMCs were isolated from the carotid arteries of male Sprague-Dawley rats (Charles River, Montreal, QC, Canada). Vessels were cleared of adventitia and endothelial cells, and medial SMCs were dispersed by digestion with collagenase and elastase as previously described.24 SMCs were grown in Dulbecco’s modified Eagle’s medium, supplemented with 10% fetal calf serum (FCS) and 2% penicillin-streptomycin in T75 tissue culture flasks. SMCs were used for experiments between passages 4 and 10. In many of our experiments, SMCs were plated on uncoated surfaces to begin; however, the incubation times allowed ample time for synthesis, deposition, and remodeling of an endogenous matrix by the SMCs. To examine morphology, SMCs were incubated with standard medium (10% FCS) for 24 hours, followed by fixation and staining with Dif-Quick Stain Set (Dade Behring, Newark, DE) according to manufacturer’s directions.
Smooth Muscle Cell Adhesion
To measure adhesion, subconfluent cultures of SMCs were grown for 48 hours in 6-well tissue culture plates in standard culture medium (10% FCS). Cells were then growth-arrested by incubation in 0.5% FCS for 24 hours, after which fresh media containing 0.5% FCS and 0, 10, or 31 μmol/L doxycycline were added, and the cells underwent a second 24-hour incubation period. Growth was then stimulated by the addition of standard culture medium (10% FCS) containing the same concentration of doxycycline as was used in the pretreatment period. The addition of standard medium was designated as time 0 for the assay. Cell adhesion was assayed at time points 0, 2, 4, and 6 days after serum stimulation, when cells were washed with phosphate buffered saline (PBS) and then treated with trypsin (0.05%). Cells released into suspension after trypsinization were counted by hemocytometer. Cells remaining on the plate were rinsed with PBS and fixed with 4% paraformaldehyde for 5 minutes, and the number of adherent cells was quantified by staining with toluidine blue and measuring the absorbance at 595 nm in a spectrophotometer. We have verified that the OD595 nm is proportional to the number of adherent cells. These experiments were repeated four times. The data were analyzed by analysis of variance followed by Holm Sidak test for pairwise comparisons.
An inverted centrifugation adhesion assay was performed to measure cell adhesion after doxycycline treatment. SMCs were seeded on 24-well plates at a density of 1 × 104 cells per well and incubated with standard culture medium (10% FCS) for 16 hours. Cells were then growth-arrested by serum-deprivation (0.5% FCS) for 24 hours, after which cells were incubated in fresh medium (0.5% FCS) supplemented with 0, 10, or 31 μmol/L doxycycline for an additional 24 hours. At the end of the 48-hour preincubation, medium was replaced with standard culture medium (10% FCS) containing the same concentration of doxycycline, and the cultures were incubated for another 72 hours. The plates were washed with PBS to remove nonadherent cells and then inverted and spun in a plate centrifuge at 0, 100, 200, or 400 × g for 5 minutes. Cells remaining on the plate after centrifugation were quantified by fixing and staining with 0.5% toluidine blue as described above. These experiments were performed in triplicate and repeated three times. The data were analyzed by analysis of variance followed by Fisher’s protected least significant difference (PLSD) for pairwise comparisons.
Interference Reflection Microscopy
Cell contact with the substratum was visualized by interference reflection microscopy (IRM) using a confocal microscope (MRC-600; Bio-Rad, Hercules, CA) with a reflection module as previously described25 and with the following modifications. Smooth muscle cells were plated on poly-l-lysine-coated coverslips, at a density of 50,000 cells per well in 6-well plates, and cultured for 48 hours in standard culture medium (10% FCS). SMCs were then growth-arrested by serum deprivation (0.5% FCS) for 24 hours, after which they received fresh medium (0.5% FCS) supplemented with 0, 31, or 104 μmol/L doxycycline. After an additional 24 hours, cells were stimulated with standard culture medium (10% FCS) containing the same concentration of doxycycline. After an additional 48-hour incubation, SMCs were fixed in 4% paraformaldehyde for 20 minutes and then mounted in PBS:glycerol at 1:1 volume. IRM takes advantage of the interference pattern of monochromatic light reflected at the phase boundaries that exist between the thin layer of culture medium that separates the cell from the glass substratum. The resultant grayscale image demonstrates the relative degree of contact between the cell and the glass substrate. In general, white patches indicate a separation of >100 nm; broad areas of light to dark gray are produced by close contacts, with cell-substratum separations ranging from 70 to 20 nm; and focal contacts appear as discrete black patches with cell-substratum separations of <15 nm.
The formation of close and focal contacts was estimated by tracing each cell and measuring the average gray level in the cell from the IRM images using SIMPLE PCI imaging software (C-Imaging Inc., Mars, PA). On the gray level scale, white is set to a value of 255 and black to a value of 1. The area of focal contacts was measured by setting a threshold for black in the image and measuring the fractional area of the cell that was black using SIMPLE PCI imaging software. At least nine cells were analyzed for each concentration of doxycycline (0, 31, or 104 μmol/L). The data were analyzed by analysis of variance followed by Holm-Sidak test for pairwise comparisons.
Immunofluorescence Staining and Western Blotting
SMCs were plated on glass coverslips in 6-well tissue culture plates at a density of 50,000 cells per well and were grown in standard culture medium (10% FCS). After 72 hours, fresh culture medium supplemented with doxycycline (0, 31, or 104 mmol/L) was added to the cells, which were incubated for another 48 hours. SMCs were fixed with 4% paraformaldehyde for 8 minutes and immunostained with antibodies against paxillin (1:400; BD Biosciences, San Jose, CA) and phosphotyrosine (1:50; Santa Cruz Biotechnology, Santa Cruz, CA). F-actin was labeled with TRITC-phalloidin (1:400). Imaging was performed using a confocal imaging system (Bio-Rad Radiance). The fractional area in each cell that stained positive for paxillin was measured by setting a threshold for positive staining on the image and measuring positively stained areas within the cell using SIMPLE PCI Imaging Software, measuring at least four cells for each concentration of doxycycline (0 or 104 mmol/L). The extent of tyrosine phosphorylation in focal adhesion plaques was measured using similar methods, and at least 12 cells were measured for each condition (0, 31, or 104 mmol/L doxycycline). The data were analyzed by analysis of variance followed by Holm-Sidak test for pairwise comparisons.
Western blots containing cell lysates were probed with antibodies (paxillin, 1:10,000; phosphotyrosine, 1:1,000; and SMC α-actin, 1:500; or β-actin, 1:5,000) to determine changes in total levels of each protein in the cells. These experiments were repeated twice.
Cell Aggregation Assay
SMC cultures were grown to 80% confluence in T75 tissue culture flasks with standard culture medium (10% FCS). The cells were then pretreated for 24 hours with 0 or 31 μmol/L doxycycline added to the medium. SMCs were trypsinized and suspended in 6-well tissue culture plates precoated with 1% Sea Plaque Agarose (Cambrex Biosciences, Rockland, ME) at a density of 50,000 cells per well. Standard culture medium (10% FCS) ± 31 μmol/L doxycycline was added to each well, and the cultures were incubated at 37°C and shaken at 100 rpm for 4 hours to distinguish cell-cell adhesion from random clustering of cells. Duplicate experiments were performed in the presence of 4 mmol/L ethylenediamine tetraacetic acid (EDTA) to disrupt calcium-dependent cell-cell adhesions. The cells were then fixed with 4% paraformaldehyde, and aggregates were imaged under phase contrast microscopy. Clusters of cells were identified as aggregates if the cell membranes were tightly apposed.
Smooth Muscle Cell Proliferation
Incorporation of methyl-[3H]thymidine (GE Health Care, Baie d’Urfe, QC) was measured as an index of cell proliferation. SMCs were plated at a density of 10,000 cells per well in 24-well culture plates. They were allowed to attach for 48 hours in standard culture medium (10% FCS) and then growth-arrested in serum-free medium containing 2% bovine serum albumin for 24 hours, after which they received 0, 31, or 104 μmol/L doxycycline dissolved in the medium for another 24 hours. The medium was then replaced with standard culture medium (10% FCS) containing the same concentration of doxycycline and 2 μCi/ml [3H]thymidine. After 48 hours, cells were washed with PBS and then fixed with 10% trichloroacetic acid for 20 minutes. Precipitates were washed with 10% trichloroacetic acid and 95% ethanol, solubilized with 0.3 mol/L NaOH for 20 minutes, and then neutralized with 0.3 N HCl. [3H] counts were measured in a liquid scintillation counter. Experiments were repeated five times. The data were analyzed by analysis of variance followed by Fischer’s PLSD.
Smooth Muscle Cell Scrape Wounding Assay
Confluent cultures of SMCs grown in 6-well tissue culture plates were wounded with a P20 pipette tip and incubated in standard culture medium (10% FCS) containing 0, 31, or 104 μmol/L doxycycline. In this type of assay, cells close the wound by migrating and proliferating into the wounded area.26 Measurements of wound width were obtained every 12 hours over a period of 96 hours using Simple PCI Imaging software. Mean values were expressed as percent wound closure at each time point. These experiments were performed in triplicate and repeated four times. Repeated-measures analysis of variance was used to analyze the data, followed by Holm-Sidak test for pairwise comparisons.
Three-Dimensional (3-D) Collagen Gel Contraction Assay
Cell culture in hydrated collagen gels was performed using a solution of type I collagen (Vitrogen 100; Collagen Biomaterials, Mahwah, NJ) as previously described,27,28 with the following modifications. SMCs were trypsinized, counted with a hemocytometer, and resuspended in standard culture medium (10% FCS). Cell suspensions were added to a solution of neutralized type I collagen (1.5 mg/ml type I collagen) at a concentration of 9 × 105 cells/ml before gel polymerization. Aliquots of 0.5 ml (containing 1 × 105 cells and 1.0 mg/ml type I collagen) were added to 24-well tissue culture plates and polymerized for 1 hour at 37°C in a humidified incubator with 5% CO2. To assess the effects of doxycycline on collagen gel contraction, the collagen gels were impregnated with 0, 42, 104, 208, or 416 μmol/L doxycycline before polymerization, and culture medium was supplemented with the same concentration of doxycycline. These higher concentrations of doxycycline were necessary to ensure penetration of doxycycline into the thick three-dimensional gels. The collagen gels were released from the plate using a spatula and by pipetting medium at the gel-dish interface. Released gels were permitted to contract for 72 hours, and digital images were obtained at 12, 24, 48, and 72 hours after release using a Nikon Coolpix digital camera (Nikon, Mississauga, ON, Canada). Measurement of collagen gel diameter at the indicated time points was performed using Simple PCI imaging software. Gels were measured by tracing around the edges of the gel disk using a mouse. Maximum length of the gel was measured using an algorithm that calculates the maximum distance between any two points on the perimeter of the gel. This maximum length was normalized to the maximum breadth, a second line perpendicular to the first and also spanning the entire gel. If the gel was a perfect circle, the ratio of maximum length to maximum breadth would be 1.0, and either measurement would represent the true diameter of the gel. We analyzed only gels with ratios measuring between 1.0 and 1.2 and so used the maximum length measurement to indicate gel diameter. Gel contraction was expressed relative to control gel diameter (no doxycycline treatment) at each time point. All experiments were performed in triplicate and repeated at least three times. Data were analyzed by analysis of variance followed by Fischer’s PLSD.
Gelatin Zymography
Two-Dimensional (2-D) SMC Monolayer Culture
Type I collagen (Vitrogen 100) at a concentration of 50 mg/ml was used to form a thin layer of collagen on the wells of 24-well plates. SMCs were seeded at a density of 1 × 104 cells/well and incubated for 16 hours with standard culture medium (10% FCS). The medium was replaced with fresh medium containing 0.5% FCS and 0, 42, 104, 208, or 416 μmol/L doxycycline, and the cells were incubated for an additional 24 hours. Conditioned media was collected from the cultures and stored at −80°C (n = 2 for each concentration of collagen used). Aliquots (5 μl per lane) were electrophoresed on nonreducing sodium-dodecyl-sulfate polyacrylamide gels that contained 0.1% gelatin as substrate for the MMP digestion as previously described.29
SMCs Embedded in 3-D Collagen Gels
At 72 hours after release of the 3-D collagen gels, 1-ml samples of conditioned medium were collected from each well (n = 4 for each concentration of doxycycline used) and stored at −80°C. Aliquots of 5 μl were used for analysis by gelatin zymography. Densitometric analysis of the gelatin zymograms was performed using Scion Image (Scion Corporation). Values were normalized to the control sample in each gel and expressed as relative densitometric units.
Results
Doxycycline Increased SMC Adhesion to Substrate
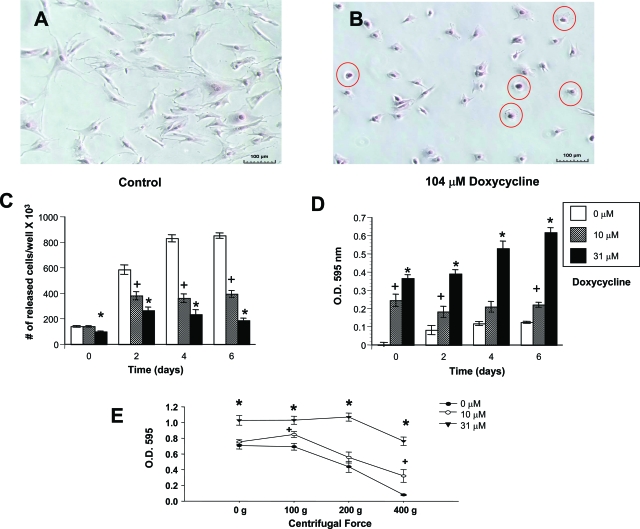
SMCs treated with doxycycline for 48 hours exhibited a pronounced change in cell morphology compared with untreated cells. Normal SMCs were elongated and spindle–or stellate–shaped (Figure 1A), whereas the doxycycline-treated cells were smaller, rounder, and often flattened (Figure 1B, circled in red). Control SMCs readily detached from the substratum when treated with trypsin, however, the doxycycline-treated cells were resistant to trypsinization. Quantification of the number of cells released into suspension after trypsinization revealed a marked decrease in the number of cells released in doxycycline-treated cultures (Figure 1C). Conversely, a greater number of cells remained attached to the plate in the doxycycline-treated cultures compared with controls (Figure 1D).
Figure 1.
Micrographs of control SMCs (A) or SMCs treated with 104 μmol/L doxycycline (B) stained with Dif-Quick. In the doxycycline-treated cultures, many cells were rounded and flattened (indicated by the red circles). C: The number of SMCs released from the tissue culture plate after trypsinization was decreased by doxycycline treatment at concentrations of 10 or 31 μmol/L. +, the value measured in the group treated with 10 μmol/L doxycycline was significantly less than the value in the untreated control group. *, the value in the group treated with 31 μmol/L doxycycline was significantly less than value in the control group (P < 0.05). D: Conversely, the number of cells left behind on the plate was increased after doxycycline treatment. E: An inverted centrifugation adhesion assay was performed, and this showed that more force was necessary to detach doxycycline-treated cells from the culture well. In both D and E, the number of cells left on the plate was estimated by staining with toluidine blue and measuring absorbance at 595 nm. Values are means ± SEM. +, the value measured in the group treated with 10 μmol/L doxycycline was significantly greater than the value in the untreated control group. *, the value measured in the group treated with 31 μmol/L doxycycline was significantly greater than the value in the untreated control group. (P < 0.05). Scale bars = 100 μm.
An inverted centrifugation adhesion assay was performed to measure the force required to detach cells from the culture plate. Cell attachment was significantly greater in cultures treated with 31 μmol/L doxycycline than in untreated cultures (Figure 1E). Furthermore, although untreated cells and cells treated with 10 μmol/L doxycycline were detached after inverted centrifugation at 200 × g, cells treated with 31 μmol/L doxycycline were detached only after centrifugation at 400 × g (Figure 1E).
The increased adhesion of SMCs treated with doxycycline was studied further using IRM. This technique uses the interference pattern of monochromatic light reflected from the cell-substrate boundary to approximate the distance between the cell membrane and the glass coverslip at contact sites. Areas of focal contact where there is less than 15 nm of separation between the cell membrane and the substratum are visualized as black patches on the IRM image. Treatment with 31 μmol/L doxycycline (Figure 2B) resulted in the formation of focal contacts that were larger and appeared to be more numerous than control untreated cells (Figure 2A). Focal contacts formed at the end of cell protrusions (arrowheads) and also were distributed over the ventral surface of the cell (arrows). Treatment with 104 μmol/L doxycycline resulted in the formation of a wide, peripheral band of focal contact circling the cell (Figure 2C, arrows). Measurement of the average gray level in the cell was used to confirm the visual observations, where a decrease in scale value corresponds to more black. The average gray level was significantly lower after treatment with 104 μmol/L doxycycline (Figure 2D). The fractional area of the cell that appeared black was also increased significantly after treatment with 104 μmol/L doxycycline (Figure 2E).
Figure 2.
Interference reflection microscopy images of a control cell (A) and a cell treated with 31 μmol/L (B) or 104 μmol/L (C) doxycycline. Each panel shows a single cell, although in B, the edge of a second cell is also evident in the lower right corner. Areas of focal contact where there is less than 15 nm of separation between the cell membrane and the substratum were visualized as black patches on the IRM image. There was increased focal contact formation with increased doxycycline concentration. These focal contacts formed at the end of cell protrusions (arrowheads) and on the ventral surface of the cell (arrows). A dark band ringing the cell, representing close apposition of plasma membrane to the substrate, often formed in cells treated with 104 μmol/L doxycycline (arrows; C). Magnification, ×90. D: Gray levels were calculated by tracing around the cell and measuring the average gray level within each cell. Higher values represent more white area; lower values, more black area. *, average gray level in cells treated with 104 μmol/L doxycycline was significantly different from the control group or the group treated with 31 μmol/L doxycycline. (P < 0.05). E: The fractional area of the cell occupied by focal contacts on the IRM image. *, the fractional area occupied by focal contacts was significantly greater in cells treated with 104 μmol/L doxycycline than untreated cells or those treated with 31 μmol/L doxycycline (P < 0.05).
Doxycycline Treatment Was Associated with Reorganization of the Actin Cytoskeleton and the Formation of Peripheral Focal Adhesions
Visualization of focal adhesions and the actin cytoskeleton was performed by immunostaining for paxillin and labeling F-actin with TRITC-phalloidin. In control SMCs, actin was arranged in thick central stress fibers (Figure 3A), whereas cells treated with 104 μmol/L doxycycline lacked these stress fibers, and instead, more actin fibers were distributed around the cell periphery in parallel with the cell membrane, and only a few fibers crossed the cell at oblique angles (Figure 3B). In control cells, paxillin staining was localized at the ends of the stress fibers, which were located at the edges of cell protrusions (Figure 3A, arrows). In doxycycline-treated cells, paxillin staining was still evident at the tips of actin filaments; however, because actin filaments were shorter and spread around the cell periphery, paxillin staining was prominent in a ring at the cell periphery (Figure 3B, arrows). The more spread distribution of paxillin staining in the doxycycline-treated cells led to an increase in the fractional area of the basal cell surface that stained positive for paxillin (14.7 ± 0.4%) compared with controls (8.4 ± 0.6%, P < 0.001). Western blots containing cell lysates were probed with antibodies against paxillin and actin, but there were no changes in the total levels of these proteins (data not shown), confirming that doxycycline affected the subcellular distribution but did not affect protein levels in the cells.
Figure 3.
A and B: Confocal micrographs of rat medial SMCs stained with phalloidin to label the actin cytoskeleton (red) and the focal adhesion protein paxillin (green). Several cells are evident in each micrograph. A: Control SMCs contained central actin stress fibers that terminated in paxillin-containing focal adhesions at the cell periphery (arrows). B: After 104 μmol/L doxycycline treatment, most actin filaments were found at the cell periphery, paralleling the cell membrane. Paxillin-rich focal adhesions were more concentrated around the peripheral edges of the cells (arrows). C and D: Confocal micrographs of rat medial SMCs stained with an antibody against phosphotyrosine. Control SMCs (C) exhibited phosphotyrosine staining at focal adhesions distributed evenly on the ventral surface of the cell (arrows) and also at the cell periphery (arrowheads). Doxycycline treatment (D) resulted in thicker focal adhesions that were more densely concentrated at the cell periphery (arrowheads). Magnification, ×60.
Immunostaining for phosphotyrosine was also performed. In control SMCs, phosphotyrosine staining appeared as punctate spots distributed along the basal surface of the cell (arrows) as well as broad bands localized at the edges of cells (arrowheads) (Figure 3C). After doxycycline treatment, phosphotyrosine staining was redistributed and was more localized to the peripheral edge of the cell membrane (Figure 3D, arrowheads). As a result of this redistribution and concentration of the staining at the extreme edges of the cells, there was a significant decrease in the fractional area of the basal cell surface occupied by phosphotyrosine staining from 17.4 ± 2.4% in control cells to 7.9 ± 0.5% in doxycycline-treated cells (P < 0.001). Western blots probed with the phosphotyrosine antibody failed to show a difference between levels of phosphorylation between control and doxycycline-treated cells (data not shown), again confirming redistribution of these proteins without change in the total levels of protein.
Doxycycline Promoted Smooth Muscle Cell-Cell Adhesion
In preliminary experiments, we observed that doxycycline-treated SMCs formed large aggregates after removal from the tissue culture plate with trypsin. Based on this observation, the effect of doxycycline on cell-cell adhesion was investigated using a cell suspension assay. Untreated SMCs formed small aggregates containing few cells (Figure 4A). After treatment with 31 μmol/L doxycycline, the SMCs formed numerous large three-dimensional aggregates, as indicated by darker regions in the micrograph (Figure 4B). Addition of EDTA disrupted the aggregates in both the doxycycline-treated and the control cell suspensions (Figure 4, C and D), confirming that cell aggregation was calcium–dependent.
Figure 4.
Phase contrast images of rat medial SMCs suspended on agarose in a cell aggregation assay. Cells untreated (A and C) or cells treated (B and D) with 31 μmol/L doxycycline, in the absence (A and B) or presence (C and D) of EDTA. Doxycycline treatment resulted in a dramatic increase in cell aggregation (B) compared with control (A). Addition of EDTA inhibited aggregation in both control (C) and doxycycline-treated SMCs (D). Scale bars = 100 μm.
Doxycycline Inhibited Smooth Muscle Cell Proliferation and Migration
Increases in cell-substrate and cell-cell adhesion may impact on the ability of SMCs to proliferate or migrate. Treatment with 104 μmol/L doxycycline significantly decreased SMC tritiated thymidine incorporation over 48 hours, from 136 ± 14 × 103 cpm in control cells to 58 ± 3 × 103 cpm in treated cells (P < 0.05). To assess the effects of doxycycline on SMC migration and proliferation, scrape wounding of a SMC monolayer was performed. Wound closure in this assay involves both SMC proliferation and migration into the wounded area. Photomicrographs taken 72 hours after wounding show delayed wound closure by SMCs treated with 31 or 104 μmol/L doxycycline, compared with nontreated controls (Figure 5A). Measurement of the percent wound closure versus time revealed a significant inhibitory effect of doxycycline treatment between 24 and 96 hours after wounding (Figure 5B).
Figure 5.
A: Micrographs of SMC monolayer cultures 72 hours after scrape wounding with a pipette tip. The original wound area is indicated by the dotted white lines. Magnification, ×100. B: Percent wound closure by SMCs treated with 0, 31, or 104 μmol/L doxycycline was measured at 0 to 96 hours after injury. Values are means ± SEM. The value measured in the group treated with 104 μmol/L doxycycline was significantly less than the control group (*P < 0.05) or the group treated with 31 μmol/L doxycycline (†P < 0.05).
Doxycycline Inhibited Remodeling of 3-D Collagen Gels
SMCs were cultured in 3-D, floating type I collagen gels to assess the effects of doxycycline on gel contraction and matrix reorganization. Doxycycline had a potent dose-dependent inhibitory effect on collagen gel contraction (Figure 6A). At 12 hours, doxycycline treatment at concentrations ranging from 42 to 416 μmol/L resulted in significant increases in gel diameter relative to control, indicating inhibition of gel contraction (Figure 6B). At later times (24 to 72 hours), gel contraction was inhibited by concentrations of doxycycline ranging from 104 to 416 μmol/L. Maximal inhibition of contraction was observed at 12 hours with 416 μmol/L doxycycline, resulting in a 63% increase in gel diameter relative to control.
Figure 6.
A: Photographs of SMCs embedded in 3-D collagen gel disks treated with increasing concentrations of doxycycline. B: Gel diameter relative to control (no doxycycline treatment) was measured from photographs obtained at various times during gel contraction. Values are means ± SEM. *, the value measured in the doxycycline-treated group was significantly greater than the value in the control group (P < 0.05).
Doxycycline Inhibited MMP-2 and -9 Activity
Samples of conditioned medium were collected from cultures and used to assess the effects of doxycycline on MMP activity. Medium from SMC monolayers plated on a thin layer of type I collagen (2-D cultures) was subject to gelatin zymography (Figure 7A). In control samples (no doxycycline treatment), three lytic bands were visualized: a 92-kd band corresponding to MMP-9 proenzyme and 63- and 55-kd bands corresponding to the proenzyme and active forms of MMP-2, respectively. After treatment with doxycycline at concentrations of 208 mmol/L or higher, there was a marked decrease in activity of the MMP-9 and -2 bands (Figure 7A). This was confirmed for the MMP-2 proenzyme and MMP-2 active by densitometric analysis (Figure 7B). Because it was difficult to reliably detect and measure the MMP-9 band by densitometry, it was not included in the analysis. MMP activity was also assessed in conditioned medium from 3-D collagen gel cultures (Figure 7C). Treatment with 416 μmol/L doxycycline resulted in decreased MMP activity in the pro-MMP-2 and active MMP-2 bands and also in the pro-MMP-9 band. This was confirmed by densitometric analysis (Figure 7D).
Figure 7.
A: Gelatin zymography of conditioned medium samples obtained from SMCs grown in monolayer 2-D culture on type I collagen. The cells were treated with 0 to 416 μmol/L doxycycline. Bands are evident for MMP-9 proenzyme (92 kd), MMP-2 proenzyme (63 kd), and MMP-2 active (55 kd). B: Densitometric analysis of MMP zymograms from SMC in 2-D culture. N = 2 per group. C: Gelatin zymography of conditioned medium samples obtained from contracted 3-D collagen gels 72 hours after release. D: Densitometric analysis of MMP zymograms from SMC in 3-D culture. N = 4 per group.
Discussion
This study was designed to assess the effects of doxycycline treatment on vascular smooth muscle cells, using a series of in vitro assays that mimic critical events in intimal thickening and inward remodeling. Doxycycline is a known inhibitor of MMP activity, and we have confirmed inhibition of MMP-2 and -9 activities in 2-D monolayer and 3-D collagen gel cultures. In this study, we have concentrated on MMP-2 and -9, because both are known to be important in mediating the SMC response to injury. The mechanisms by which the tetracyclines inhibit MMP activity are not completely understood, but they include binding to Zn2+ or Ca2+ associated with the enzyme and blocking the active site, resulting in decreased enzyme activity.2 One limitation of the current study is that MMP-2 and -9 are the only MMPs for which we can measure activity using the zymography technique. However, it is likely that the activities of other MMPs expressed by SMCs are inhibited by doxycycline. Furthermore, doxycycline can reduce the steady-state levels of mRNA for several MMPs.30,31 Therefore doxycycline may reduce the activity of several other MMPs that may contribute to the biological effects observed.
Cell-matrix adhesion complexes signal for cell survival and proliferation, provide traction to migrating SMCs, and transduce intracellular force to the matrix, permitting remodeling of the three-dimensional matrix structure. However, an optimum balance of cell adhesion must be achieved. Too little adhesion and the cells cannot generate the traction needed for forward movement. Too strong an adhesion prevents the cells from releasing from the substratum to translocate or proliferate.32 We found that doxycycline treatment increased SMC attachment to substrate, as evidenced by the round and flattened morphology of the cells, resistance to trypsinization, and resistance to detachment after inverted centrifugation of the plates. We investigated this attachment phenomenon further using interference reflection microscopy and demonstrated that doxycycline-treated SMCs possessed large peripheral focal contacts that were tightly apposed to the substratum. Immunostaining further revealed that these areas contained large mature focal adhesions rich in paxillin and phosphotyrosine. Doxycycline-treated SMCs also adopted a peripheral distribution of actin filament that paralleled the cell membrane. The redistribution of the proteins occurred without any change in total levels of the proteins. This distribution of focal adhesion and cytoskeletal components is characteristic of firmly attached nonmotile cells.33
Doxycycline treatment also caused increased smooth muscle cell-cell adhesion in suspension culture. Cell-cell adhesion is mediated through cadherins, which form calcium-dependent, homophilic interactions between cells. SMCs express several cadherin molecules, including N-, R-, and T-cadherins, and previous studies have implicated these cadherins in modulating SMC migration and proliferation, with both positive and negative effects.23,34–36 Cadherins are linked to the actin cytoskeleton via the catenins and paxillin, and the latter is present in both cell-matrix and cell-cell adhesions,37,38 thus doxycycline might affect the distribution of cytoplasmic proteins in both types of adhesion complexes.
In addition to their well-recognized role in degrading matrix molecules, recent work indicates that MMPs can cleave integrin,21 discoidin domain receptor,22 and cadherin23,39,40 extracellular domains, thereby destabilizing cell-substrate and cell-cell adhesions. The profound effects of doxycycline on cell-matrix and cell-cell adhesion are likely mediated via MMP inhibition, either directly by preventing MMP-mediated shedding of adhesion receptors or indirectly by impairing the clearance of matrix substrates thereby reinforcing cell-matrix contacts. However, the mechanisms of inhibition by doxycycline could be more complex. One study has shown that a chemically modified tetracycline, CMT-8, increased the expression of E-cadherin protein in breast cancer cells.19 Another study has suggested that MMP-9 may mediate disassembly of cadherins by a mechanism not dependent on proteolytic activity.41
Neointimal thickening that occurs in atherosclerosis and restenosis is dependent on the proliferation and migration of SMCs, so we next examined the effects of doxycycline on these parameters. Tritiated thymidine uptake during cell growth (an index of cell proliferation) was reduced after doxycycline treatment. Doxycycline also inhibited wound closure in a scratch wound assay, where closure is a result of both cell migration and proliferation. These in vitro findings are consistent with our previous in vivo studies demonstrating impaired SMC migration from media to intima and decreased SMC proliferation with doxycycline treatment after balloon catheter injury in the rat carotid artery.12,13 They are also consistent with numerous other studies describing the impairment of cell proliferation and migration after tetracycline treatment.17,19,42–46
Remodeling of vessel diameter is another important determinant of lumen loss after balloon angioplasty. The precise mechanisms of remodeling are not known, however, cell contraction, cell translocation in a circumferential direction within the vessel wall, and reorganization of the extracellular matrix with deposition of both fibrin and collagen have all been implicated.47,48 We investigated the mechanisms of inward remodeling using a 3-D collagen gel contraction assay, a model that is often used to study vessel remodeling and contraction.27,49,50 We found that doxycycline significantly inhibited contraction of collagen gels by SMCs. Our results are consistent with previous studies showing that gel contraction by fibroblasts is impaired by tetracyclines51 and with studies showing that treatment with MMP inhibitors prevented inward vessel remodeling in vivo.52–55 In the current study, we show that reductions in MMP-2 and -9 activities were correlated with reduced gel contraction after doxycycline treatment. However, it is important to note two recent studies that argue that collagen gel contraction is not dependent on the proteolytic activity of MMP-9 but is instead due to an interaction between MMP-9 and CD44 receptors on the cell surface.50,56 We cannot exclude the possibility that doxycycline may have effected MMP-9 independent of the inhibition of proteolytic activity, for example by reducing production of MMP-9, and this is an interesting possibility worthy of future study.
In addition, doxycycline may also impact on other mechanisms. Tension in floating collagen gels is dependent on the presence of serum or growth factors sequestered in the matrix, such as platelet-derived growth factor57 and transforming growth factor-β58; thus doxycycline may inhibit collagen gel remodeling by preventing the release or activation of growth factors sequestered in the extracellular matrix. Because both SMCs and fibroblasts use α1β1, α2β1 and αvβ3 integrins to translate intracellular force to the extracellular matrix in 3-D collagen gels,28,59–61 another possibility is that doxycycline acts by reinforcing integrin-mediated cell adhesion, causing an extremely strong attachment, thereby preventing cell movement within the gel and preventing the exertion of tractional forces on matrix fibers. Furthermore, because MMPs have recently been shown to activate big endothelin-1,62 it is possible that doxycycline is inhibiting collagen gel remodeling by attenuating the generation of bioactive endothelin and thereby limiting the amount of contractile force generated by the SMCs. Finally, we cannot rule out the possibility that doxycycline inhibited the proliferation of cells within the collagen gel, therefore limiting the number of cells present and able to contract the gel.
In this study, we have shown that SMCs treated with doxycycline demonstrate an extremely adhesive phenotype, exhibiting increases in both cell-cell and cell-matrix adhesion. Doxycycline inhibited SMC proliferation, migration, and collagen gel remodeling, all important events in the pathogenesis of postangioplasty stenosis. To date, a number of preclinical and clinical studies have demonstrated the safety and efficacy of doxycycline as a treatment for occlusive vascular diseases. Our study provides insight into the effects of doxycycline at a cellular level and demonstrates effects that may extend beyond the prevention of matrix degradation.
Acknowledgments
We thank Dr. Michal Opas (Department of Laboratory Medicine and Pathobiology, University of Toronto) for assistance with interference reflection microscopy.
Footnotes
Address reprint requests to Dr. Michelle P. Bendeck, Department of Laboratory Medicine and Pathobiology, University of Toronto, Medical Sciences Building, Room 6217, 1 King’s College Circle, Toronto, ON, Canada M5S 1A8. E-mail: michelle.bendeck@utoronto.ca.
Supported by the Heart and Stroke Foundation of Ontario grant T5339, a Career Investigator award (to M.P.B.), a doctoral research award from the Heart and Stroke Foundation (to C.F.), and a Canada Graduate Scholarship Master’s award from the Canadian Institutes for Health Research (to B.H.). C.F. and B.H. were also supported through a Premier’s Research Excellence award (to M.P.B.).
References
- Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005;85:1–31. doi: 10.1152/physrev.00048.2003. [DOI] [PubMed] [Google Scholar]
- Golub LM, Lee HM, Ryan ME, Giannobile WV, Payne J, Sorsa T. Tetracyclines inhibit connective tissue breakdown by multiple non- antimicrobial mechanisms. Adv Dent Res. 1998;12:12–26. doi: 10.1177/08959374980120010501. [DOI] [PubMed] [Google Scholar]
- Curci JA, Petrinec D, Liao S, Golub LM, Thompson RW. Pharmacologic suppression of experimental abdominal aortic aneurysms: a comparison of doxycycline and four chemically modified tetracyclines. J Vasc Surg. 1998;28:1082–1093. doi: 10.1016/s0741-5214(98)70035-7. [DOI] [PubMed] [Google Scholar]
- Prall AK, Longo GM, Mayhan WG, Waltke EA, Fleckten B, Thompson RW, Baxter BT. Doxycycline in patients with abdominal aortic aneurysms and in mice: comparison of serum levels and effect on aneurysm growth in mice. J Vasc Surg. 2002;35:923–929. doi: 10.1067/mva.2002.123757. [DOI] [PubMed] [Google Scholar]
- Manning MW, Cassis LA, Daugherty A. Differential effects of doxycycline, a broad-spectrum matrix metalloproteinase inhibitor, on angiotensin II-induced atherosclerosis and abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2003;23:483–488. doi: 10.1161/01.ATV.0000058404.92759.32. [DOI] [PubMed] [Google Scholar]
- Franklin IJ, Harley SL, Greenhalgh RM, Powell JT. Uptake of tetracycline by aortic aneurysm wall and its effect on inflammation and proteolysis. Br J Surg. 1999;86:771–775. doi: 10.1046/j.1365-2168.1999.01137.x. [DOI] [PubMed] [Google Scholar]
- Curci JA, Mao D, Bohner DG, Allen BT, Rubin BG, Reilly JM, Sicard GA, Thompson RW. Preoperative treatment with doxycycline reduces aortic wall expression and activation of matrix metalloproteinases in patients with abdominal aortic aneurysms. J Vasc Surg. 2000;31:325–342. doi: 10.1016/s0741-5214(00)90163-0. [DOI] [PubMed] [Google Scholar]
- Mosorin M, Juvonen J, Biancari F, Satta J, Surcel HM, Leinonen M, Saikku P, Juvonen T. Use of doxycycline to decrease the growth rate of abdominal aortic aneurysms: a randomized, double-blind, placebo-controlled pilot study. J Vasc Surg. 2001;34:606–610. doi: 10.1067/mva.2001.117891. [DOI] [PubMed] [Google Scholar]
- Baxter BT, Pearce WH, Waltke EA, Littooy FN, Hallett JW, Jr, Kent KC, Upchurch GR, Jr, Chaikof EL, Mills JL, Fleckten B, Longo GM, Lee JK, Thompson RW. Prolonged administration of doxycycline in patients with small asymptomatic abdominal aortic aneurysms: report of a prospective (phase II) multicenter study. J Vasc Surg. 2002;36:1–12. doi: 10.1067/mva.2002.125018. [DOI] [PubMed] [Google Scholar]
- Brown DL, Desai KK, Vakili BA, Nouneh C, Lee HM, Golub LM. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol. 2004;24:733–738. doi: 10.1161/01.ATV.0000121571.78696.dc. [DOI] [PubMed] [Google Scholar]
- Axisa B, Loftus IM, Naylor AR, Goodall S, Jones L, Bell PR, Thompson MM. Prospective, randomized, double-blind trial investigating the effect of doxycycline on matrix metalloproteinase expression within atherosclerotic carotid plaques. Stroke. 2002;33:2858–2864. doi: 10.1161/01.str.0000038098.04291.f6. [DOI] [PubMed] [Google Scholar]
- Bendeck MP, Conte M, Zhang M, Nili N, Strauss BH, Farwell SM. Doxycycline modulates smooth muscle cell growth, migration and matrix remodeling after arterial injury. Am J Pathol. 2002;160:1089–1095. doi: 10.1016/S0002-9440(10)64929-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MM, Franco CD, Courtman DW, Bendeck MP. A non-antibiotic chemically modified tetracycline (CMT-3) inhibits intimal thickening. Am J Pathol. 2003;163:1557–1566. doi: 10.1016/S0002-9440(10)63512-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtman DW, Franco CD, Meng Q, Bendeck MP. Inward remodeling of the rabbit abdominal aorta is blocked by the MMP inhibitor doxycycline. J Vasc Res. 2004;41:157–165. doi: 10.1159/000077145. [DOI] [PubMed] [Google Scholar]
- Tronc F, Mallat Z, Lehoux S, Wassef M, Esposito B, Tedgui A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: interaction with NO. Arterioscler Thromb Vasc Biol. 2000;20:120–126. doi: 10.1161/01.atv.20.12.e120. [DOI] [PubMed] [Google Scholar]
- Loftus IM, Porter K, Peterson M, Boyle J, London NJ, Bell PR, Thompson MM. MMP inhibition reduces intimal hyperplasia in a human vein graft stenosis model. Ann NY Acad Sci. 1999;878:547–550. doi: 10.1111/j.1749-6632.1999.tb07723.x. [DOI] [PubMed] [Google Scholar]
- Pinney SP, Chen HJ, Liang D, Wang X, Schwartz A, Rabbani LE. Minocycline inhibits smooth muscle cell proliferation, migration and neointima formation after arterial injury. J Cardiovasc Pharmacol. 2003;42:469–476. doi: 10.1097/00005344-200310000-00003. [DOI] [PubMed] [Google Scholar]
- Seftor RE, Seftor EA, De LJ, Kleiner DE, Leferson J, Stetler-Stevenson WG, McNamara TF, Golub LM, Hendrix MJ. Chemically modified tetracyclines inhibit human melanoma cell invasion and metastasis. Clin Exp Metastasis. 1998;16:217–225. doi: 10.1023/a:1006588708131. [DOI] [PubMed] [Google Scholar]
- Meng Q, Xu J, Goldberg ID, Rosen EM, Greenwald RA, Fan S. Influence of chemically modified tetracyclines on proliferation, invasion and migration properties of MDA-MB-468 human breast cancer cells. Clin Exp Metastasis. 2000;18:139–146. doi: 10.1023/a:1006732424102. [DOI] [PubMed] [Google Scholar]
- TeKoppele JM, Beekman B, Verzijl N, Koopman JL, DeGroot J, Bank RA. Doxycycline inhibits collagen synthesis by differentiated articular chondrocytes. Adv Dent Res. 1998;12:63–67. doi: 10.1177/08959374980120012201. [DOI] [PubMed] [Google Scholar]
- Deryugina EI, Bourdon MA, Jungwirth K, Smith JW, Strongin AY. Functional activation of integrin alpha V beta 3 in tumor cells expressing membrane-type 1 matrix metalloproteinase. Int J Cancer. 2000;86:15–23. doi: 10.1002/(sici)1097-0215(20000401)86:1<15::aid-ijc3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Vogel WF. Ligand-induced shedding of discoidin domain receptor 1. FEBS Lett. 2002;514:175–180. doi: 10.1016/s0014-5793(02)02360-8. [DOI] [PubMed] [Google Scholar]
- Uglow EB, Slater S, Sala-Newby GB, Aguilera-Garcia CM, Angelini GD, Newby AC, George SJ. Dismantling of cadherin-mediated cell-cell contacts modulates smooth muscle cell proliferation. Circ Res. 2003;92:1314–1321. doi: 10.1161/01.RES.0000079027.44309.53. [DOI] [PubMed] [Google Scholar]
- Hou G, Mulholland D, Gronska MA, Bendeck MP. Type VIII collagen stimulates smooth muscle cell migration and matrix metalloproteinase synthesis after arterial injury. Am J Pathol. 2000;156:467–476. doi: 10.1016/S0002-9440(10)64751-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel MP, Dziak E, Lo CM, Ferrier J, Mesaeli N, Michalak M, Opas M. Calreticulin affects focal contact-dependent but not close contact-dependent cell-substratum adhesion. J Biol Chem. 1999;274:15085–15094. doi: 10.1074/jbc.274.21.15085. [DOI] [PubMed] [Google Scholar]
- Selden SC, III, Schwartz SM. Cytochalasin B inhibition of endothelial proliferation at wound edges in vitro. J Cell Biol. 1979;81:348–354. doi: 10.1083/jcb.81.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RT, Berditchevski F, Cheng GC, Hemler ME. Integrin-mediated collagen matrix reorganization by cultured human vascular smooth muscle cells. Circ Res. 1995;76:209–214. doi: 10.1161/01.res.76.2.209. [DOI] [PubMed] [Google Scholar]
- Klein CE, Dressel D, Steinmayer T, Mauch C, Eckes B, Krieg T, Banker RB, Weber L. Integrin α2β1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates reorganization of collagen I fibrils. J Cell Biol. 1991;115:1427–1436. doi: 10.1083/jcb.115.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75:539–545. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- Hanemaaijer R, Visser H, Koolwijk P, Sorsa T, Salo T, Golub LM, van Hinsbergh VW. Inhibition of MMP synthesis by doxycycline and chemically modified tetracyclines (CMTs) in human endothelial cells. Adv Dent Res. 1998;12:114–118. doi: 10.1177/08959374980120010301. [DOI] [PubMed] [Google Scholar]
- Jonat C, Chung FZ, Baragi VM. Transcriptional downregulation of stromelysin by tetracycline. J Cell Biochem. 1996;60:341–347. doi: 10.1002/(sici)1097-4644(19960301)60:3<341::aid-jcb6>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Jones M, Sabatini PJB, Lee FSH, Bendeck MP, Langille BL. N-cadherin upregulation and function in response of smooth muscle cells to arterial injury. Arterioscler Thromb Vasc Biol. 2002;22:1972–1977. doi: 10.1161/01.atv.0000036416.14084.5a. [DOI] [PubMed] [Google Scholar]
- Ivanov D, Philippova M, Allenspach R, Erne P, Resink T. T-cadherin upregulation correlates with cell-cycle progression and promotes proliferation of vascular cells. Cardiovasc Res. 2004;64:132–143. doi: 10.1016/j.cardiores.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Ivanov D, Philippova M, Tkachuk V, Erne P, Resink T. Cell adhesion molecule T-cadherin regulates vascular cell adhesion, phenotype and motility. Exp Cell Res. 2004;293:207–218. doi: 10.1016/j.yexcr.2003.09.030. [DOI] [PubMed] [Google Scholar]
- Crawford BD, Henry CA, Clason TA, Becker AL, Hille MB. Activity and distribution of paxillin, focal adhesion kinase, and cadherin indicate cooperative roles during zebrafish morphogenesis. Mol Biol Cell. 2003;14:3065–3081. doi: 10.1091/mbc.E02-08-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano H, Mazaki Y, Kurokawa K, Hanks SK, Matsuda M, Sabe H. Roles played by a subset of integrin signaling molecules in cadherin-based cell-cell adhesion. J Cell Biol. 2004;166:283–295. doi: 10.1083/jcb.200312013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho AT, Voura EB, Soloway PD, Watson KL, Khokha R. MMP inhibitors augment fibroblast adhesion through stabilization of focal adhesion contacts and up-regulation of cadherin function. J Biol Chem. 2001;276:40215–40224. doi: 10.1074/jbc.M101647200. [DOI] [PubMed] [Google Scholar]
- Lochter A, Galosy S, Muschler J, Freedman N, Werb Z, Bissell MJ. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol. 1997;139:1861–1872. doi: 10.1083/jcb.139.7.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanceau J, Truchet S, Bauvois B. Matrix metalloproteinase-9 silencing by RNA interference triggers the migratory-adhesive switch in Ewing’s sarcoma cells. J Biol Chem. 2003;278:36537–36546. doi: 10.1074/jbc.M304300200. [DOI] [PubMed] [Google Scholar]
- Yao JS, Chen Y, Zhai W, Xu K, Young WL, Yang GY. Minocycline exerts multiple inhibitory effects on vascular endothelial growth factor-induced smooth muscle cell migration: the role of ERK1/2, PI3K, and matrix metalloproteinases. Circ Res. 2004;95:364–371. doi: 10.1161/01.RES.0000138581.04174.2f. [DOI] [PubMed] [Google Scholar]
- Cakir Y, Hahn KA. Direct action by doxycycline against canine osteosarcoma cell proliferation and collagenase (MMP-1) activity in vitro. In Vivo. 1999;13:327–331. [PubMed] [Google Scholar]
- Fife RS, Sledge GW, Jr, Roth BJ, Proctor C. Effects of doxycycline on human prostate cancer cells in vitro. Cancer Lett. 1998;127:37–41. doi: 10.1016/s0304-3835(98)00003-2. [DOI] [PubMed] [Google Scholar]
- Guerin C, Laterra J, Masnyk T, Golub LM, Brem H. Selective endothelial growth inhibition by tetracyclines that inhibit collagenase. Biochem Biophys Res Commun. 1992;188:740–745. doi: 10.1016/0006-291x(92)91118-a. [DOI] [PubMed] [Google Scholar]
- Lokeshwar BL, Selzer MG, Zhu BQ, Block NL, Golub LM. Inhibition of cell proliferation, invasion, tumor growth and metastasis by an oral non-antimicrobial tetracycline analog (COL-3) in a metastatic prostate cancer model. Int J Cancer. 2002;98:297–309. doi: 10.1002/ijc.10168. [DOI] [PubMed] [Google Scholar]
- Courtman DW, Schwartz SM, Hart C. Sequential injury of the rabbit abdominal aorta induces intramural coagulation and luminal narrowing independent of intimal mass: extrinsic pathway inhibition eliminates luminal narrowing. Circ Res. 1998;82:996–1006. doi: 10.1161/01.res.82.9.996. [DOI] [PubMed] [Google Scholar]
- Geary RL, Nikkari ST, Wagner WD, Williams JK, Adams MR, Dean RH. Wound healing: a paradigm for lumen narrowing following arterial reconstruction. J Vasc Surg. 1998;27:96–108. doi: 10.1016/s0741-5214(98)70296-4. [DOI] [PubMed] [Google Scholar]
- Travis JA, Hughes MG, Wong JM, Wagner WD, Geary RL. Hyaluronan enhances contraction of collagen by smooth muscle cells and adventitial fibroblasts: role of CD44 and implications for constrictive remodeling. Circ Res. 2001;88:77–83. doi: 10.1161/01.res.88.1.77. [DOI] [PubMed] [Google Scholar]
- Defawe OD, Kenagy RD, Choi C, Wan SY, Deroanne C, Nusgens B, Sakalihasan N, Colige A, Clowes AW. MMP-9 regulates both positively and negatively collagen gel contraction: a nonproteolytic function of MMP-9. Cardiovasc Res. 2005;66:402–409. doi: 10.1016/j.cardiores.2004.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers SA, Wolowacz RG. Tetracycline-based MMP inhibitors can prevent fibroblast-mediated collagen gel contraction in vitro. Adv Dent Res. 1998;12:86–93. doi: 10.1177/08959374980120012701. [DOI] [PubMed] [Google Scholar]
- de Smet BJ, de Kleijn D, Hanemaaijer R, Verheijen JH, Robertus L, Der Helm YJ, Borst C, Post MJ. Metalloproteinase inhibition reduces constrictive arterial remodeling after balloon angioplasty: a study in the atherosclerotic Yucatan micropig. Circulation. 2000;101:2962–2967. doi: 10.1161/01.cir.101.25.2962. [DOI] [PubMed] [Google Scholar]
- Sierevogel MJ, Pasterkamp G, Velema E, de Jaegere PP, de Smet BJ, Verheijen JH, de Kleijn DP, Borst C. Oral matrix metalloproteinase inhibition and arterial remodeling after balloon dilation: an intravascular ultrasound study in the pig. Circulation. 2001;103:302–307. doi: 10.1161/01.cir.103.2.302. [DOI] [PubMed] [Google Scholar]
- Margolin L, Fishbein I, Banai S, Golomb G, Reich R, Perez LS, Gertz SD. Metalloproteinase inhibitor attenuates neointima formation and constrictive remodeling after angioplasty in rats: augmentative effect of αvβ3 receptor blockade. Atherosclerosis. 2002;163:269–277. doi: 10.1016/s0021-9150(02)00035-7. [DOI] [PubMed] [Google Scholar]
- Sierevogel MJ, Velema E, van der Meer FJ, Nijhuis MO, Smeets M, de Kleijn DP, Borst C, Pasterkamp G. Matrix metalloproteinase inhibition reduces adventitial thickening and collagen accumulation following balloon dilation. Cardiovasc Res. 2002;55:864–869. doi: 10.1016/s0008-6363(02)00467-4. [DOI] [PubMed] [Google Scholar]
- Johnson C, Galis ZS. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. Arterioscler Thromb Vasc Biol. 2004;24:54–60. doi: 10.1161/01.ATV.0000100402.69997.C3. [DOI] [PubMed] [Google Scholar]
- Gullberg D, Tingstrom A, Thuresson A-C, Olsson L, Terracio L, Borg TK, Rubin K. β1 integrin mediated collagen gel contraction is stimulated by PDGF. Exp Cell Res. 1990;186:264–272. doi: 10.1016/0014-4827(90)90305-t. [DOI] [PubMed] [Google Scholar]
- Montesano R, Orci L. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci USA. 1988;85:4894–4897. doi: 10.1073/pnas.85.13.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke ME, Sakai T, Mosher DF. Contraction of collagen matrices mediated by alpha2beta1A and alpha(v)beta3 integrins. J Cell Sci. 2000;113:2375–2383. doi: 10.1242/jcs.113.13.2375. [DOI] [PubMed] [Google Scholar]
- Langholz O, Rockel D, Mauch C, Kozlowska E, Bank I, Krieg T, Eckes B. Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by α1β1 and α2β1 integrins. J Cell Biol. 1995;131:1903–1915. doi: 10.1083/jcb.131.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broberg A, Heino J. Integrin alpha2beta1-dependent contraction of floating collagen gels and induction of collagenase are inhibited by tyrosine kinase inhibitors. Exp Cell Res. 1996;228:29–35. doi: 10.1006/excr.1996.0295. [DOI] [PubMed] [Google Scholar]
- Fernandez-Patron C, Radomski MW, Davidge ST. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res. 1999;85:906–911. doi: 10.1161/01.res.85.10.906. [DOI] [PubMed] [Google Scholar]