Abstract
Hypoxic stress activates various signal transduction pathways including posttranslational modification with the ubiquitin-like SUMO protein (SUMOylation). However, the molecular mechanisms by which SUMOylation regulates hypoxic responses remain unclear. Here, we investigated the ability of rat salivary Pa-4 epithelial cells to resist cell injury elicited by 1% O2- or hypoxia-mimetic desferroxamine (DFO)-stimulated SUMOylation processes. By using Pa-4 cells stably transduced with lenti-SUMO-1 and a cell-permeant peptide harboring SUMO-binding motif to interfere with SUMO-dependent protein-protein interactions, we demonstrate that SUMOylation augments cell survival against DFO treatment. This appeared to be partly mediated through attenuation of Protein Kinase C (PKC)-δ activation and caspase-3 cleavage, hallmarks of pro-apoptotic signaling. Intriguingly, DFO-induced phosphorylation of DNA damage marker ataxia-telangiectasia-mutated protein S1981 preceded activation of PKCδ and caspase-3. Constitutive SUMOylation facilitated 1% O2- or DFO-induced nuclear factor κB transactivation, possibly via activation of genotoxic signaling cascade. In addition, we observed transient preservation of transepithelial electrical resistance during the early stage of hypoxia (1% O2) as well as enhanced transepithelial electrical resistance recovery after prolonged hypoxia in SUMO-1-expressing cell monolayers. In conclusion, our results unveil a previously unrecognized mechanism by which SUMOylation and activation of ataxia-telangiectasia-mutated protein, PKCδ, caspase-3, and nuclear factor κB signaling pathways modulate salivary adaptive responses to stress in cells exposed to either 1% O2 or DFO.
Small ubiquitin-like modifier (SUMO) is the best characterized member of a growing family of ubiquitin-related proteins. SUMO is conjugated to target proteins using an enzyme conjugation system similar to but distinct from that of ubiquitin.1,2 Importantly, SUMO-1, -2, -3, and -4 have emerged as important posttranslational modifiers that regulate diverse cellular functions including intracellular targeting, DNA repair, cell cycle progression, and responses to extracellular stimuli.1–3 A large number of proteins are modified by SUMO, and recent proteomic studies have shown that as many as 400 yeast proteins are modified by yeast SUMO homolog, and 2683 potential SUMO substrates are conserved in both humans and mice.4–6 The precise functional differences of various SUMO paralogs remain to be established. However, given the importance of SUMOylation, it is not surprising that SUMO plays important roles in the development of various diseases.7–9 Although the significance of SUMOylation in modulating cellular adaptive responses is well established, it is still not clear how SUMO modification regulates specific key cellular functions.
Accumulating evidence suggests the potential importance of SUMOylation in governing cellular hypoxic responses in that hypoxia up-regulates the steady-state level of SUMO-1 by as much as 100-fold.10,11 Hypoxia is a (patho)physiological condition that arises when cellular oxygen demand exceeds supply. Regions of hypoxia occur not only in disease states but also during normal development. For example, mammalian embryos are, for significant periods of time, in an almost entirely hypoxic environment before a blood circulatory system is established.12 Hypoxia is also a physiological inducer of the p53 tumor suppressor and provides selective pressure during tumor growth for the elimination of cells with wild-type p53 and the clonal expansion of cells with mutated p53.13 It has been established that hypoxic cells acquire genetic and adaptive changes to survive and proliferate in a hypoxic microenvironment, enabling eventual evasion from hypoxia-induced cell death.14
As an activator of pro-inflammatory and anti-apoptotic genes, the transcription factor nuclear factor κB (NF-κB) is a key factor in determining whether cells survive after being subjected to genotoxic stress. Hypoxia-elicited phenotypic manifestations have been reported to be a consequence of the induction of tumor necrosis factor-α (TNF-α), a known activator of NF-κB signaling.15,16 NF-κB transcriptional activity is stimulated by hypoxia.10,15,17 Hypoxia also appears to be a key factor involved in the development of genetic instability.18 Recent studies have suggested that hypoxia elicits increased DNA damage, enhanced mutagenesis, and functional impairment in DNA repair pathways (reviewed in Ref. 19,20). Because SUMOylation has been demonstrated to be involved in governing DNA repair and genomic stability,1 we postulated that SUMO-1 functions as a central player in the generalized hypoxic response. SUMOylation of Inhibitor of NF-κB (IκB) has been previously demonstrated to act in an anti-NF-κB fashion by attenuating the activation of NF-κB by various cytokines.21 In contrast, reports from a recent study by Huang et al22 suggest that both SUMOylation and ataxia-telangiectasia-mutated protein (ATM) activation enhance genotoxic stress-mediated NF-κB activation. Hence, SUMO-1 can function in both anti-NF-κB and pro-NF-κB manners, depending on the individual stimuli and specific pathway used for NF-κB activation. However, questions remain regarding the exact role of SUMOylation in modulating NF-κB transactivation in response to stress by hypoxia (1% O2) or hypoxia-mimetic desferroxamine (DFO).
In this report, we demonstrate that treatment with 1% O2 or DFO induces global SUMOylation, disrupts epithelial barrier function and ZO-1 assembly, is cytotoxic, and activates genotoxic signaling cascade. We next examined the effect of the augmented SUMOylation process on modulating the stress response in salivary epithelial Pa-4 cells by investigating the following biological events. Is enhanced SUMOylation capacity beneficial for cell survival under DFO treatment? Will SUMOylation elicit an inhibitory or stimulatory effect on 1% O2- or DFO-dependent NF-κB activation? Does SUMOylation protect cells against hypoxia-mediated decreases in transepithelial electrical resistance (TER) and alter tight-junctional protein distribution? To achieve this goal, we stably overexpressed SUMO-1 in rat salivary epithelial Pa-4 cells by lentivirus-mediated transduction to examine the effect of SUMOylation on governing epithelial homeostatic control mechanisms against exposure to 1% O2 or DFO. Together, we conclude that SUMOylation attenuates activation of pro-apoptotic protein kinase-Cδ and caspase-3 while promoting genotoxicity-induced NF-κB transactivation and facilitating TER restoration and assembly of tight junction-associated proteins in response to exposure to reduced oxygen tension or DFO.
Materials and Methods
Cell Culture and Treatment
The rat parotid epithelial cell lines Pa-4 and SUMO-1-transduced Pa-4 were cultured as previously described.23–26 Human embryonic kidney (HEK) 293T cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS) plus 1% penicillin/streptomycin and maintained at 37°C. HeLa cells were grown in Dulbecco’s modified Eagle’s medium (4.5 g/L glucose) supplemented with 10% FBS, 1 mmol/L sodium pyruvate, and 1% penicillin/streptomycin. DFO was purchased from Sigma (St. Louis, MO).
Vector Construction and Viral Production
The enhanced green fluorescent protein (EGFP)-SUMO-1 fragment obtained from digestion of pEGFP-C1-SUMO-1 was cloned into the PinAI and HincII sites of the pRRLsin.hCMV156 vector to construct pRRLsin.hCMV-EGFP-SUMO-1 (Figure 1A). HEK 293T cells (70% confluence) in 175-mm flasks were cotransfected by calcium phosphate precipitation with 10 μg of pRRLsin.hCMV-EGFP (a human immunodeficiency virus-based self-inactivating replication-defective lentiviral transfer vector expressing EGFP), -EGFP-SUMO-1, or -EGFP-SUMO-1aa (unconjugatable SUMO-1); 10 μg of pΔ8.7 (for viral packaging); and 6 μg of pVSV-G (for VSV-G pseudotyping). Chloroquine was added to a final concentration of 25 μmol/L, and cells were incubated in a 5% CO2 incubator at 37°C for 16 hours. Chloroquine-containing medium was replaced with culture medium containing 10 mmol/L sodium butyrate, and cells were incubated for at least another 8 hours before the addition of fresh culture medium and then incubated for an additional 16 hours. Viral supernatant was collected, centrifuged at 2500 rpm for 10 minutes, and stored immediately at 4°C. Supernatants were pooled and concentrated using a Macrosep Centrifuge Device with a 300-kd molecular mass cut-off (Millipore, Billerica, MA) and a 0.45-μm syringe filter. Aliquots of concentrated virus were stored at −80°C. Titers of concentrated (2 to 4 × 108 transducing units/ml) lentiviral stocks (pRRLsin.hCMV-EGFP and -EGFP-SUMO-1) were determined by infecting HEK 293T cells in the presence of 6 μg/ml polybrene with serial dilutions based on results of fluorescence-activated cell sorting (FACS).
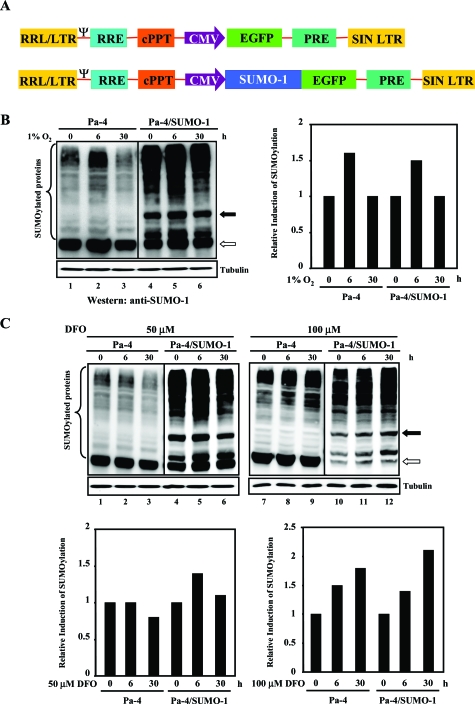
Figure 1.
Establishment of SUMO-1-transduced Pa-4 cells. A: Diagram of pRRLsin.hCMV-EGFP-SUMO-1 and parental constructs. B and C: Hypoxia or DFO induces global SUMOylation. Equal amounts of whole-cell lysates were subjected to Western analyses with an anti-GMP-1 (SUMO-1) antibody. Representative Western analysis data demonstrate overall increases in SUMOylation process after treatment with 1% O2 (B) or DFO (50 or 100 μmol/L) (C) for different time periods, as indicated. A filled arrow indicates the SUMO-modified RanGAP1, and an unfilled arrow depicts the unmodified RanGAP1. After normalizing with that of tubulin, the respective steady-state level of SUMOylated proteins in Pa-4 and Pa-4/SUMO-1 cells before treatment was designated as 1. The relative induction of SUMOylation by treatment with 1% O2 (B) or DFO (50 or 100 μmol/L) is shown. C: All Western analyses were repeated at least three times with comparable results.
NF-κB Transactivation Assays
Pa-4, Pa-4/SUMO-1, or HEK 293T cells were transiently transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) with 0.5 μg of pGL2-NF-κB luciferase reporter construct harboring an interleukin-6 promoter and two NF-κB binding sites (a generous gift of Dr. Yun Yen, City of Hope, Duarte, CA). For normalization of transfection efficiency, 0.1 μg of the Renilla luciferase pRL-TK plasmid was cotransfected. Six hours after the start of transfection, cells were recovered overnight in 0.05% stripped-serum medium and subsequently exposed to normoxic or hypoxic conditions (1% O2 or DFO treatment [see below]) for 24 hours before luciferase assays. Relative luciferase activity from the firefly luciferase reporter gene was determined and normalized to Renilla luciferase activity using the Dual Luciferase Reporter Assay System (Promega, Madison, WI). Fold induction by hypoxia was calculated after normalizing the pGL2-NF-κB reporter activities with the activities from cotransfected pRL-TK. The nuclear protein preparation and TransBinding NF-κB Assays (Panomics, Redwood City, CA) were performed according to manufacturer’s instructions.
Viral Transduction
Pa-4 cells (1 × 105) were plated to reach 50% confluence before transduction (multiplicity of infection [MOI] of 0.1 to 40) with lentivirus encoding SUMO-1 in the presence of 6 μg/ml polybrene to establish the optimal transduction efficiency. Twenty-four hours after transduction, the poly-brene-containing medium was replaced with fresh culture medium and incubated for an additional 16 hours before analyses. Seventy-two hours after transduction, cells were washed and trypsinized for further propagation. An aliquot of these cells was analyzed by FACS for determination of transduction efficiency. For FACS analyses, trypsinized cells were re-suspended in 0.5 ml of phosphate-buffered saline (PBS) (4 × 105 cells/ml). For generation of Pa-4/SUMO-1aa (unconjugatable form of SUMO-1) and Pa-4/EGFP, Pa-4 cells were transduced as described above at an MOI of 10.
Confocal Microscopy
For hypoxic exposure, cells were incubated in medium with reduced serum (0.05% FBS) for 24 hours before exposure to 1% O2 for time-course studies. At appropriate time points, cells maintained under normoxic and hypoxic conditions were washed with PBS, fixed with 4% paraformaldehyde in PBS for 30 minutes at room temperature, and quenched with 50 mmol/L NH4Cl in PBS. Fixed cells were then permeabilized with 0.5% Triton X-100 in PBS for 15 minutes, blocked with 1% BSA in PBS, and incubated with rhodamine-phalloidin (1:100; Chemicon International, Temecula, CA) for 1 hour for detection of F-actin and an anti-ZO-1 antibody (1:200; Zymed, South San Francisco, CA) to detect ZO-1, respectively. Processed cells were mounted with Prolong Antifade (Molecular Probes, Eugene, OR) and examined.
3-[4,5-Dimethylthiazol-2-yl]-2,5-Diphenyltetrazolium Bromide (MTT) Assays for Measurement of Cell Viability
Cells were seeded into 24-well plates to obtain a confluency of 35 to 50% on the day of the experiment. Cells were then treated with increasing concentrations of DFO (up to 100 μmol/L), and medium was changed daily for 2 days. Twenty-four to 48 hours after the start of DFO treatment (depending on cell types), 0.2 ml of 0.1 mg/ml MTT (Sigma) in OptiMEM I (Invitrogen, Carlsbad, CA) was added to each well, and the plate was incubated at 37°C for an additional 1.5 hours. The MTT solution was then aspirated, and 0.2 ml of isopropanol was added to each well to dissolve the formazan crystals. Absorbance was read immediately at 540 nm in a scanning multiwell spectrophotometer. The results were depicted as percentage cell viability and reported as the mean ± SD of three independent experiments performed in triplicate.
Western Analyses
Cells were washed twice with ice-cold 1× PBS, lysed in 2× sodium dodecyl sulfate lysis buffer, boiled for 5 minutes, and spun at 13,000 rpm for 10 minutes before being snap-frozen and stored at −80°C. For SUMOylation assays, 25 mmol/L N-ethylmaleimide (Sigma) was included in the lysis buffer. Cell lysates were collected, and protein concentrations were determined using the Bradford protein assay. Twenty to 40 μg of protein lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis analyses. Phosphorylated ATM protein extraction and Western analyses were performed according to manufacturer’s instructions. Western analyses were performed with the following antibodies: anti-GMP (SUMO-1) (Zymed), anti-phospho-ATM (Ser1981) (Upstate Biotechnology, Lake Placid, NY), anti-phospho-H2AX (Ser139) (Upstate), anti-PKCδ (Santa Cruz Biotechnology, Santa Cruz, CA), anti-caspase-3 (Cell Signaling Technology, Beverly, MA), anti-cyclin A (Santa Cruz Biotechnology), anti-IκBα (Santa Cruz Biotechnology), anti-green fluorescent protein (Santa Cruz Biotechnology), anti-actin (Chemicon), and anti-tubulin (Santa Cruz Biotechnology), as indicated. Images were visualized using an enhanced chemiluminescence detection kit (ECL Plus; Amersham Pharmacia Biotech, Piscataway, NJ) following the manufacturer’s instructions and the Versadoc 5000 Imaging System (BioRad, Hercules, CA). Quantitative analyses were performed using Quantity One software (BioRad) and normalized with the respective steady-state level of tubulin.
Flow Cytometry for Cell Cycle Analyses
Cells were seeded at 50 to 80% confluency in 35-mm dishes and serum-starved overnight to synchronize cell cycle. After the desired treatment, cells were fixed in 70% ethanol overnight and stained with propidium iodide (20 μg/ml). Flow cytometry was performed at the Norris Cancer Center Flow Cytometry Core Facility using FACSCaliber (Becton Dickinson, Franklin Lakes, NJ).
Hypoxic Treatment
Parental Pa-4 and transduced cells were seeded onto tissue culture-treated, 12-mm polycarbonate filters (Costar-Corning, Corning, NY) and cultured for 2 days at 35°C and 5% CO2 in air before being transferred to an exposure chamber, which was flushed with 1% O2 balanced with 5% CO2 and 94% N2. The chamber was then sealed airtight and kept at 35°C for the duration of hypoxia treatment. For NF-κB reporter assays, transiently transfected cells were exposed to 1% O2 for 24 hours as described above.
Measurement of TER
Bioelectrical parameters of epithelial cell monolayers were monitored using a MilliCell ERS screening device (Millipore). Spontaneous potential difference (expressed in millivolts [apical side as a reference]) and TER (expressed in kilo-ohms/centimeter2) were measured with a set of chopstick-style electrodes. Offset potential differences generated by the voltage-sensing electrodes and background electrical resistance contributed by both the bathing medium and the filter membrane were measured at the beginning and end of each set of bioelectric measurements using blank filters and corrected for. Spontaneous potential difference and TER were measured up to 6 days. Data are presented as mean ± SD from at least three separate experiments performed in triplicate.
Preparation of SUMO-Binding Motif (SBM) Peptide
The peptide that noncovalently binds to SUMO (PIASX) as well as a control peptide with scrambled amino acid sequence with identical composition27 was fused with human immunodeficiency virus-1 TAT nuclear localization signal. Peptides were synthesized by the Peptide Synthesis Core Facility at the City of Hope, purified by high performance liquid chromatography (HPLC), and verified by mass spectrometry. Pa-4/SUMO-1 cells were pre-incubated with SBM (10 μmol/L) for 24 hours, followed by treatment with varying concentrations of DFO in the presence or absence of 10 μmol/L SBM and incubated for 72 hours before determining cell viability by MTT assays.
Statistical Analysis
Experiments were independently performed in duplicate at least three times, unless stated otherwise. One representative data set from these three independent experiments is presented where appropriate. Reporter activity shown is the mean ± SD based on at least three independent transfection experiments. Error bars represent the SD of the mean. Statistical analyses were performed using one-way analysis of variance, followed by posthoc comparisons based on modified Newman-Keuls-Student procedure with P < 0.05 considered significant. Where appropriate, unpaired Student’s t-tests were also performed to determine the difference between two data groups.
Results
Exposure to Either 1% O2 or Hypoxia-Mimetic DFO Stimulated Global SUMOylation
Because the exact role of hypoxia-induced SUMO-1 overexpression in modulating hypoxic response is still unclear, we sought to develop a cell model system to examine the effect of augmented SUMO-1 expression in governing various hypoxia-elicited cellular responses, such as cell survival, NF-κB activation, and barrier integrity. Because there is a very limited (almost none) choice of pharmacological reagents that can be used to modulate SUMOylation capacity in cells, we used an EGFP-SUMO-1 (Figure 1A) lentivirus-mediated transduction system to enhance SUMOylation. First, by using FACS analyses to monitor EGFP expression, a dose-dependent transduction was established in Pa-4 cells by increasing MOIs from 0.1 to 40 to yield 21 to 99% EGFP-SUMO-1-positive cells (data not shown). To assess the stability of transgene expression, transduced cells were passaged, stored, and revived for FACS analyses, which showed that Pa-4/SUMO-1 cells remained >99% of EGFP-SUMO-1 positive after repeated passage at least eight times (data not shown). Western analyses were performed to confirm that the transduced EGFP-SUMO-1 was functionally conjugated to the target proteins by using whole-cell lysates prepared from Pa-4/SUMO-1 cells (data not shown). Consistent with previous reports in other cell types,10,11 1% O2 treatment stimulated overall SUMOylation profile by about 1.5-fold in both Pa-4 and Pa-4/SUMO-1 cells at 6 hours after treatment (Figure 1B). We next examined whether iron chelator DFO is able to mimic low O2 tension to induce SUMOylation process by treating both Pa-4 and Pa-4/SUMO-1 cells with 50 and 100 μmol/L DFO, respectively. As shown in Figure 1C, Pa-4 cells treated with 100 μmol/L DFO displayed an enhanced SUMOylation profile similar to that detected in Figure 1B, whereas both 50 and 100 μmol/L DFO treatment elicited a more sustained increase of overall SUMOylation pattern in Pa-4/SUMO-1 cells.
SUMO-1 Strengthens Passive Barrier Properties against Acute Hypoxia
After verifying that treatment of either 1% O2 or DFO increases global SUMOylation in both Pa-4 and Pa-4/SUMO-1 cells (Figure 1, B and C), we next investigated whether augmented SUMOylation process is (patho)physiologically relevant. Previously, we have demonstrated that hypoxic treatment causes a weakening of epithelial barrier properties in Pa-4 cells.28 To search for functional consequence of increased SUMOylation, TER was measured in Pa-4 and Pa-4/SUMO-1 cell monolayers under both normoxic and hypoxic conditions. When TER changes over time in normoxic Pa-4 and Pa-4/SUMO-1 cells were measured, a notably higher baseline TER was observed in Pa-4/SUMO-1 cells (Figure 2A).
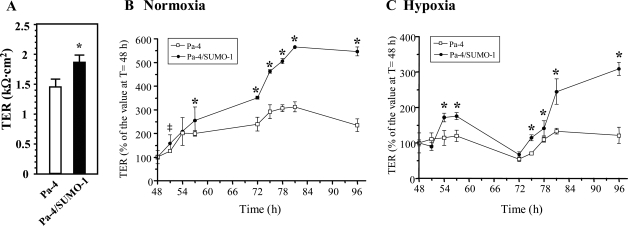
Figure 2.
Overexpression of SUMO-1 protects TER over time. A: Higher TER in Pa-4/SUMO-1 cells than that in Pa-4 cells. B: Effect of SUMO-1 on TER under normoxic condition. TER was measured in Pa-4 and Pa-4/SUMO-1 cells that were grown on Transwell filters at indicated time points. Time courses of TER for Pa-4 and Pa-4/SUMO-1 cells between 48 and 96 hours after seeding under normoxic condition as shown. C: Overexpression of SUMO-1 protects TER from being compromised by hypoxia. Cell culture and TER measurements were performed as described in B. Hypoxic exposure was initiated at t = 48 hours. Data are presented as mean ± SD from three independent experiments, each performed in triplicate. * and ‡, significant difference in TER percentages between Pa-4 and Pa-4/SUMO-1 cells at corresponding time points (P < 0.001 and P < 0.01, respectively).
Based on our previous experience that TER increases at day 2 after seeding in our experimental system,28 studies on TER time courses under parallel normoxic or hypoxic treatments were performed on cells starting from 48 to 96 hours after seeding to investigate the effect of SUMOylation on TER maintenance. Under normoxic conditions, TER of Pa-4/SUMO-1 monolayers at 96 hours displayed a more robust increase (up to almost fivefold) than that (∼twofold) observed in Pa-4 monolayers (Figure 2B), indicating that augmented SUMOylation strengthens passive barrier properties of Pa-4 cells under baseline conditions. By contrast, there was only a transient TER increase detected in Pa-4/SUMO-1 but not Pa-4 cells during the first 9-hour interval of hypoxia (48 to 57 hours posthypoxia; Figure 2C) when both Pa-4 and Pa-4/SUMO-1 monolayers were subjected to hypoxic 1% O2 treatment. However, TERs of both types of monolayers eventually decreased to less than 65% of the initial value at 24 hours posthypoxia (ie, at 72 hours in Figure 2C). Together, these data indicate that enhanced SUMOylation in Pa-4/SUMO-1 cells is able to transiently blunt hypoxia-induced deterioration of passive permeability barrier properties up to 9 hours posthypoxia, and the efficacy of SUMO-1 overexpression in attenuating hypoxia-induced TER reduction was not sustained at 24 hours after 1% O2 exposure as indicated at 72 hours in Figure 2C.
During 24 to 48 hours of treatment with 1% O2 (indicated as 72 to 96 hours in Figure 2C), we noted an increase in TER in Pa-4/SUMO-1 monolayers, recovering beyond the prehypoxia level (Figure 2C). As shown in Figure 2C, the TER reached its highest value (25% greater than its prehypoxia TER at 48 hours [100%]) at 33 hours posthypoxia onset (ie, at 81 hours in Figure 2C) in Pa-4 cells, and the TER in Pa-4/SUMO-1 cells also exceeded by 150% (compared with that at 48 hours) at 33 hours posthypoxia onset and sustained for another 15 hours. The highest TER value (at 48 hours posthypoxia) in Pa-4/SUMO-1 cells was 200% greater than that at the onset of hypoxia (Figure 2C). However, these values were still less than the control (normoxic) counterparts at each time point. Although the exact effects of SUMOylation on the restoration of TER after prolonged exposure to hypoxia in Pa-4/SUMO-1 cells are difficult to interpret at this point, these observations were reproducible. Collectively, these data indicate that overexpression of SUMO-1 in Pa-4/SUMO-1 cells plays a transient and partially protective role against injury to passive barrier properties exerted by early stage of hypoxic stress and renders a higher TER value after prolonged hypoxic exposure.
SUMOylation Facilitates the Reassembly of F-Actin and ZO-1 after Prolonged Hypoxic Exposure
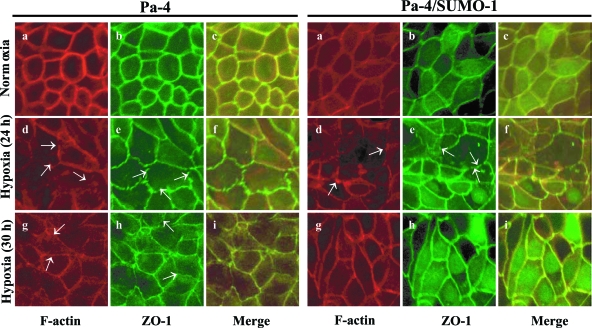
Higher TER value is a hallmark of polarized monolayers with a stabilized tight junction complex, which involves a network of occludin and claudins linked to actin via ZO proteins.29,30 Thus, a decrease in TER may reflect changes in epithelial integrity and junction formation. In this regard, we next examined whether the chronic hypoxia-induced decrease in TER was a consequence of altered F-actin and ZO-1 assembly in both Pa-4 and Pa-4/SUMO-1 cell monolayers under normoxia and hypoxia. Pa-4 and Pa-4/SUMO-1 monolayers were exposed to 1% O2 as described for TER measurements and processed for F-actin and ZO-1 visualization by confocal microscopy. Exposure of confluent Pa-4 (Figure 3, left panels) and Pa-4/SUMO-1 (Figure 3, right panels) monolayers to hypoxia for 24 hours resulted in irregular, discontinuous F-actin patterns at the cellular border and punctuate, gap-like appearances at points of cellular contacts (Figure 3d), as we have previously reported.28 Similar discontinuous appearances were also observed for ZO-1 after 24 hours of hypoxia exposure in both Pa-4 and Pa-4/SUMO-1 cells (Figure 3e) compared with a smooth pattern of ZO-1 in cells under normoxic conditions. However, the appearance of discontinuity of F-actin (Figure 3g) and ZO-1 (Figure 3h) was less pronounced at 30 hours of hypoxia. Notably, at 30 hours posthypoxic treatment, there were few irregular structures at cellular borders and gap-like appearances at cellular contacts detectable for both F-actin and ZO-1 in Pa-4/SUMO-1 cells, whereas gap-like structures at cellular borders were still apparent in Pa-4 monolayers (Figure 3, g and h). We concluded that chronic (or prolonged) hypoxia-induced TER decrease could be a consequence of altered F-actin and ZO-1 appearance and assembly pattern of tight junctions, correlating reasonably well with the decrease of TER at 24 hours and recovery at 30 hours posthypoxia onset (Figure 2C). Importantly, SUMOylation facilitates the restoration of F-actin and ZO-1 assembly after prolonged hypoxic exposure (30 hours) in Pa-4/SUMO-1 cells.
Figure 3.
SUMO-1 restores disrupted F-actin and ZO-1 assembly after prolonged hypoxia. The effects of hypoxia on the localization of F-actin (red) and ZO-1 (green) in Pa-4 and Pa-4/SUMO-1 cell monolayers were assessed by confocal microscopy at indicated time points. Arrows indicate gap-like (zagged) structures.
SUMO-1 Potentiates Cell Survival against Acute Hypoxia
Because hypoxia-mimetic DFO has been shown to induce global SUMOylation (Figure 1C) and is known to cause cell growth arrest and cell death,31 we treated parental Pa-4, Pa-4/EGFP, Pa-4/SUMO-1, and Pa-4/SUMO-1aa (unconjugatable form of SUMO-1) cells with increasing concentrations of DFO. As demonstrated in Figure 4A, DFO rendered a dose-dependent growth inhibition in all four cell types. Notably, Pa-4/SUMO-1 cells exhibited a more pronounced resistance to cytotoxicity elicited by 50 μmol/L DFO than the other three cell types examined (Figure 4A, lane 8). In addition, Pa-4/SUMO-1aa cells were somewhat more sensitive to DFO (50 μmol/L) treatment than parental Pa-4 cells or Pa-4/EGFP cells (Figure 4A, lane 11 versus lanes 2 and 5), suggesting that the protective effect observed in Pa-4/SUMO-1 cells is specific. Because there were no noticeable differences between Pa-4 and Pa-4/EGFP cells (Figure 4A, lanes 2 and 3 versus lanes 5 and 6) and both served as a control, only Pa-4 cells were used in the following studies.
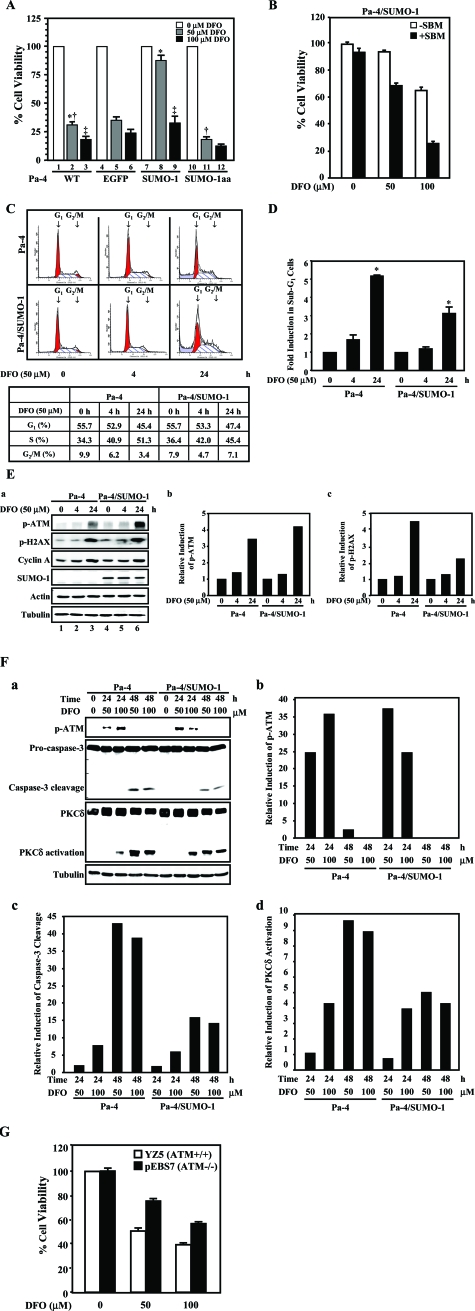
Figure 4.
SUMOylation attenuates DFO-induced PKCδ and caspase-3 activation. A: SUMOylation antagonizes DFO-induced cell death. Pa-4, Pa-4/EGFP, Pa-4/SUMO-1, and Pa-4/SUMO-1aa cells were treated with increasing concentrations of DFO, as indicated. Cell survival was measured by MTT assays at 24 hours after treatment. *, significant difference (P < 0.001) between lanes 2 and 8. ‡, significant difference (P < 0.01) between lanes 3 and 9. †, significant difference (P < 0.05) between lanes 2 and 11. B: SUMO-dependent protein-protein interaction is involved in hypoxic responses. Cell permeant, SBM peptide and scrambled peptide (control peptide) were introduced into Pa-4/SUMO-1 cells and treated with an increasing concentration of DFO (0, 50, and 100 μmol/L). C: Hypoxia renders S phase arrest. Synchronized Pa-4 and Pa-4/SUMO-1 cells were treated with either vehicle or 50 μmol/L DFO for 4 and 24 hours, respectively, and subjected to FACS analyses. The populations of G1 and G2/M cells were indicated by arrows. The percentage of cells in each phase of cell cycle was summarized in the table. One representative FACS analysis from three independent analyses is shown. D: SUMOylation protects Pa-4 cells against DFO-elicited apoptosis as reflected by a decrease in sub-G1 population of Pa-4/SUMO-1 cells. The populations of sub-G1 cells in C were normalized and expressed as fold induction compared with that of vehicle-treated Pa-4 and Pa-4/SUMO-1 cells. *, significant difference (P < 0.001) between lanes 3 and 6. E: Induction of ATM S1981 and H2AX S139 phosphorylation by DFO treatment. Equal amounts of protein lysates prepared from cells treated with DFO (50 μmol/L) for different time periods as indicated were subjected to Western analyses (a). After normalizing with that of tubulin, the respective steady-state levels of S1981-phosphorylated ATM (p-ATM) and S-139-phosphorylated H2AX (p-H2AX) in Pa-4 and Pa-4/SUMO-1 cells before treatment were designated as 1. The relative induction of p-ATM (b) and p-H2AX (c) by DFO is shown. F: ATM activation proceeds caspase-3 activation on DFO treatment. Pa-4 and Pa-4/SUMO-1 cells were treated with an increasing concentration of DFO for different time periods as indicated and subjected to Western analyses. The quantitative analyses on the relative induction of p-ATM (b), caspase-3 cleavage (c), and PKCδ (d) were performed as described in E. G: ATM-deficient cells are relative to resistant to DFO treatment. ATM-deficient pEBS7 and ATM-proficient YZ5 cells were exposed to different concentrations of DFO, followed by MTT assays as described in A.
To further ascertain the role of enhanced SUMOylation in protecting Pa-4/SUMO-1 cells against DFO-elicited cell growth inhibition, 10 μmol/L cell permeant peptide containing SBM27,32 was delivered into cells and treated with an increasing concentration of DFO (Figure 4B). Cells that took up scrambled peptide and were subsequently treated with the same concentration of DFO served as a control. There was not a notable decrease in cell viability in cells treated with SBM compared with control in the absence of DFO. However, in 100 μmol/L DFO-treated Pa-4/SUMO-1 cells, cell viability decreased by more than 40% in cells with 10 μmol/L SBM compared with control. This supported our postulation that SUMOylation and SUMO-dependent protein-protein interactions are essential for modulating the cellular adaptive responses to hypoxia.
DFO treatment has been reported to delay cells exiting from S phase of cell cycle transition.33 To explore the mechanism underlying SUMO-dependent enhancement of cell survival against DFO, we then assayed whether the effect of SUMOylation is cell cycle-dependent. As shown in Figure 4C, DFO (24-hour) treatment rendered 3.4% of Pa-4 and 7.1% of Pa-4/SUMO-1 cells to be accumulated in G2/M phase, decreasing from 9.9 and 7.9% of untreated Pa-4 and Pa-4/SUMO-1 cells, respectively. Notably, a substantially higher portion of Pa-4 cells was in the S phase of cell cycle, whereas overexpression of SUMO-1 rendered more cells entering cell cycle transition, in response to DFO (Figure 4C). We also quantified the apoptosis of Pa-4 and Pa-4/SUMO-1 cells after DFO exposure by assessing the sub-G1 cell population, a hallmark of cell apoptosis. A significant decrease in sub-G1 cell population was observed in Pa-4/SUMO-1 cells compared with that of Pa-4 cells (Figure 4D). Together, it is possible that enhanced SUMOylation allows Pa-4 cells to escape DFO-elicited cell cycle S phase arrest and apoptosis.
Effect of SUMOylation on DFO-Induced ATM, PKCδ, and Caspase-3 Activation
We further investigated whether DFO treatment induces DNA damage by examining the phosphorylation profiles of both ATM S1981 and H2AX S139 in DFO-treated Pa-4 cells (Figure 4E). The ATM S1981 phosphorylation was detected at 4 hours after treatment and was robustly stimulated at 24 hours after treatment in both Pa-4 and Pa-4/SUMO-1 cells (Figure 4E, lanes 5 and 6 versus lane 4, top panel). Consistent with the DFO-mediated ATM activation, a marked induction of H2AX S139 phosphorylation was also noticed in both Pa-4 and Pa-4/SUMO-1 cells (Figure 4E, a). Because PKCδ is one of the abundant PKC isoforms expressed in salivary cells and is proteolytically activated by caspase-3 to induce mitochondria-dependent apoptosis,34 we next performed additional experiments to address whether ATM S1981 phosphorylation and PKCδ/caspase-3 activation are a sequential event in cells being exposed to either 50 or 100 μmol/L DFO. DFO treatment led to the generation of a 40-kd fragment, a hallmark of PKCδ activation, at 48 hours after treatment, and augmented SUMOylation modestly attenuated the induced PKCδ cleavage in Pa-4/SUMO-1 cells (Figure 4F, d). Consistent with PKCδ cleavage pattern, DFO treatment (24 hours) elicited enhanced caspase-3 activation in Pa-4 cells compared with Pa-4/SUMO-1 cells (Figure 4F, c). We conclude that DFO treatment alone is sufficient to elicit DNA damage and caspase-3/PKCδ activation in both Pa-4 cells and Pa-4/SUMO-1 cells. Intriguingly, S1981-phosphorylated ATM was detected at 24 hours after DFO treatment and before activation of the PKCδ/caspase-3, which appeared at 48 hours after treatment in both Pa-4 and Pa-4/SUMO-1 cells (Figure 4F, a). Together with results shown in Figure 4, A and E, SUMOylation conceivably protected cells from DFO-elicited cell death by attenuating the activation of PKCδ and caspase-3 on exposure to DFO.
It was previously reported that there is a decreased PKCδ expression in ATM(−/−) cells.35 To examine the role of PKCδ activation in mediating DFO-elicited cell growth inhibition, we then treated pEBS7 (ATM(−/−)) and YZ5 (pEBS7+ATM) cells with DFO followed by MTT assays. As expected, the ATM-deficient pEBS7 cells are relatively resistant toward DFO treatment compared with ATM-proficient YZ5 cells (Figure 4G). It was conceivable that DFO-elicited PKCδ activation plays an essential role in conveying DFO-exerted cytotoxicity. Together, we postulate that enhanced SUMOylation attenuates PKCδ signaling cascade, resulting in an increased cell survival in Pa-4/SUMO-1 cells (Figure 4A).
Effect of SUMOylation on Hypoxia-/DFO-Induced NF-κB Activation
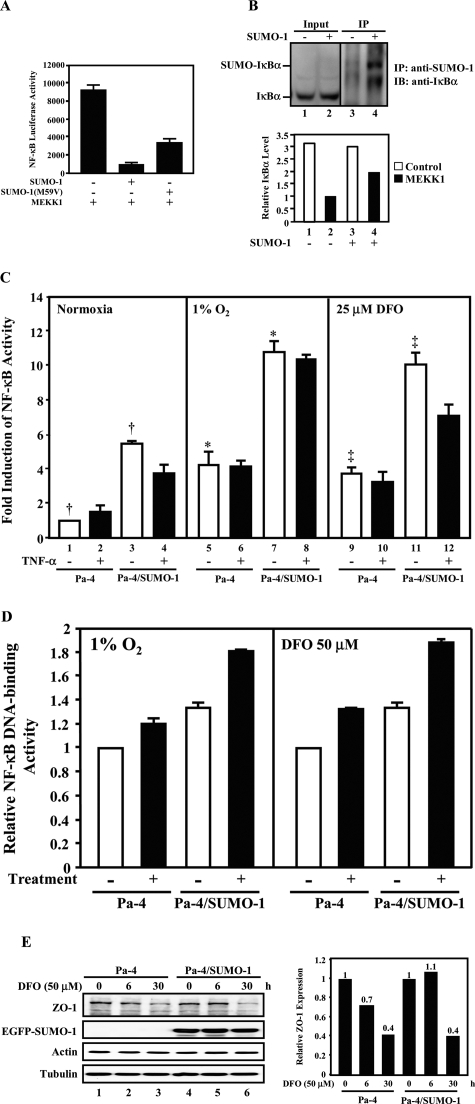
To determine whether SUMO-1 has a distinct biological effect on NF-κB activation in response to either 1% O2 or DFO exposure, a luciferase reporter construct containing NF-κB binding elements was used in transfection assays. We first tested whether SUMO-1 attenuates NF-κB activation by cytoplasmic pathways. MEKK1, which acts as an upstream activator of IκB Kinase (IKK),36,37 was chosen to activate NF-κB. It was clear that wild-type SUMO-1 repressed MEKK1-mediated NF-κB reporter activation (Figure 5A, lane 2 versus lane 1). Cotransfection with SUMO-1(M59V), harboring an M59V mutation analogous to the reported SUMO-4 M55V loss-of-function mutation,7,38 partially restored the transactivation of NF-κB induced by MEKK1 (Figure 5A, lane 3 versus lane 2). Immunoprecipitation followed by Western analyses were performed to confirm that the IκBα was SUMOylated (Figure 5B, top panel). The decreased MEKK1-mediated NF-κB activation in SUMO-1-transfected cells (Figure 5A, lane 2 versus lane 1) resulted, at least in part, from the higher IκBα in MEKK1-transfected cells (Figure 5B, bottom panel, lane 4 versus lane 2). These data confirm that SUMO-1 is involved in attenuating the MEKK1-mediated NF-κB transactivation.
Figure 5.
SUMOylation enhances genotoxicity-induced NF-κB transactivation. A: SUMO-1 attenuates NF-κB-dependent transcription by cytoplasmic pathway. HEK 293T cells were cotransfected with NF-κB reporter, pRL-TK indicator, and a combination of pEGFP-C1 (vector control), SUMO-1 (wild-type), SUMO-1(M59V), and MEKK1 expression constructs. The relative luciferase activities from the firefly luciferase reporter gene were determined and normalized with Renilla indicator luciferase activity, and the normalized NF-κB activity (×1000) is shown. SUMO-1 expression led to a 10-fold repression of the MEKK1-activated NF-κB transactivation, whereas SUMO-1(M59V) conveyed a 3.6-fold higher MEKK1-dependent NF-κB transactivation than did SUMO-1. B: SUMO-1 antagonizes MEKK1-mediated NF-κB activation by stabilizing the steady-state level of IκBα. HeLa cells (top panel) were transfected with or without pEGFP-SUMO-1. Equal amounts of cell lysates were subjected to immunoprecipitation with an anti-SUMO-1 antibody, followed by Western analyses with an anti-IκBα antibody (lanes 3 and 4). The inputs (5%; lanes 1 and 2) were also analyzed by an anti-IκBα antibody. HEK 293T cells (bottom panel) were transfected with a vector (pEGFP-C1) alone (lanes 1 and 2) or pEGFP-SUMO-1 (lanes 3 and 4) in the presence and absence of MEKK1. The effect of SUMO-1 on MEKK1-mediated IκBα degradation was determined by densitometry of signals obtained from Western analyses using an anti-IκBα antibody and anti-actin antibody (data not shown). After normalizing with that of actin, the lowest steady-state level of IκBα is designated as 1 (lane 2). The relative IκBα level, which represents one of three independent experiments, is shown. C: SUMO-1 enhances the hypoxia-stimulated NF-κB-dependent transcription. Pa-4 and Pa-4/SUMO-1 cells were transfected with an NF-κB reporter and pRL-TK indicator. After transfection, cells were exposed to nonhypoxia, 1% O2, or 25 μmol/L DFO for 24 hours in the presence and absence of TNF-α (25 ng/ml). The normalized NF-κB activity under nonhypoxia in the absence of TNF-α was designated as 1 (lane 1). The fold induction was calculated by dividing the normalized luciferase activity under indicated experimental conditions with the NF-κB activity observed under normoxic conditions in the absence of TNF-α. *, significant difference (P < 0.001) between lanes 5 and 7. ‡, significant difference (P < 0.01) between lanes 9 and 11. †, significant difference (P < 0.05) between lanes 1 and 3. D: SUMOylation enhances NF-κB DNA-binding activity. Pa-4 and Pa-4/SUMO-1 cells were treated with 1% O2 or 50 μmol/L DFO for 16 hours and subjected to TransBinding NF-κB Assays (Panomics). E: SUMOylation retards DFO-induced decrease on steady-state level of ZO-1. Pa-4 and Pa-4/SUMO-1 cells were treated with 50 μmol/L DFO for indicated time periods and subjected to Western analyses with anti-ZO-1, anti-actin, and anti-tubulin antibodies, respectively. After normalizing with that of tubulin, the respective steady-state level of ZO-1 in Pa-4 and Pa-4/SUMO-1 cells before treatment was designated as 1. The relative ZO-1 level, which represents one of three independent experiments, is shown.
We next performed the same NF-κB reporter assays in Pa-4 and Pa-4/SUMO-1 cells under a combination of hypoxia and TNF-α treatments. TNF-α (25 ng/ml) alone elicited a very modest stimulating effect on NF-κB activation in Pa-4 cells (Figure 5C, lane 2 versus lane 1), as we previously reported in endothelial cells.17 Although we observed an enhancement effect of 1% O2 or 25 μmol/L DFO on NF-κB transactivation in Pa-4 cells (Figure 5C, lanes 5 and 9 versus lane 1), we also noticed that the basal NF-κB activities in Pa-4/SUMO-1 cells were higher than those in Pa-4 cells (Figure 5C, lane 3 versus lane 1) and that TNF-α (25 ng/ml) failed to induce NF-κB activation in Pa-4/SUMO-1 cells under normoxic condition (Figure 5C, lane 4 versus lane 3). In addition, treatment of Pa-4/SUMO-1 cells with either 1% O2 or 25 μmol/L DFO was able to further enhance NF-κB activation, despite their high basal NF-κB level (Figure 5C, lanes 7 and 11 versus lane 3). Addition of TNF-α (25 ng/ml) to either 1% O2- or DFO-treated Pa-4 or Pa-4/SUMO-1 cells had no stimulatory NF-κB activation (Figure 5C, lanes 6, 8, 10, and 12 versus lanes 5, 7, 9, and 11). The lack of additional TNF-α-mediated NF-κB activation in Pa-4 cells under hypoxic conditions supported our notion that SUMOylation elicited differential regulation on NF-κB-dependent transactivation. Importantly, DFO/hypoxic treatment in the context of enhanced SUMOylation resulted in a markedly stronger NF-κB activation (Figure 5C, lanes 7 and 11 versus lanes 5 and 9, respectively). By using TransBinding NF-κB Assays (Panomics), we further confirmed that the increased NF-κB-dependent reporter transactivation resulted from enhanced NF-κB activity (Figure 5D).
We next examined the steady-state level of ZO-1 protein to determine the effect of SUMOylation on governing endogenous ZO-1 expression under DFO-stressed condition. Consistent with results demonstrated in Figures 2 and 3, there was a clear decrease of the steady-state level of ZO-1 in Pa-4 cells after DFO treatment, and enhanced SUMOylation presumably retarded such a DFO-elicited down-regulation of ZO-1 at 6 hours after treatment (Figure 5E). Together, we postulate that SUMOylation probably protects Pa-4 cells against hypoxia- and DFO-elicited cytotoxicity by attenuating pro-apoptotic signals, such as activation of PKCδ and caspase-3, and by augmenting pro-survival signals, such as NF-κB activation and sustained level of ZO-1, on hypoxic exposure.
Discussion
We have investigated alterations in sensitivity toward hypoxia (1% O2) or DFO as reflected by enhanced cell survival and accelerated reassembly of F-actin/ZO-1 and restoration of transepithelial electrical resistance on exposure of SUMO-1-overexpressing salivary Pa-4 cells to 1% O2 or DFO. Together, we propose that 1% O2- or DFO-induced SUMOylation partly accounts for these manifested phenotypes through reprogrammed adaptive responses to hypoxia. For example, salivary epithelial Pa-4 cells possess the ability to restore their TER and F-actin/ZO-1 assembly after undergoing prolonged hypoxic exposure, and this aspect of the “recovery” phase during the course of chronic exposure to 1% O2 is also potentiated by increased SUMOylation.
Hypoxia-mediated signaling pathways play key roles in critical developmental and physiological processes including angiogenesis, erythropoiesis, glucose transport, glycolysis, iron transport, cell survival, and proliferation (reviewed in Refs. 20 and 39). More importantly, clinical and laboratory studies have demonstrated that hypoxia-dependent events are involved in numerous human diseases, such as myocardial and cerebral ischemia, pulmonary hypertension, preeclampsia, intra-uterine growth retardation, and cancer.40 It gradually became appreciated that cells may respond in a more active/adaptive manner than a mere passive fashion after prolonged hypoxic exposure, correlating well with the hypoxia-induced SUMOylation. Herein, we addressed the role of induced SUMOylation in modulating hypoxic responses by demonstrating that SUMOylation promotes transient TER maintenance, attenuates caspase-3/PKCδ cleavage, and enhances genotoxicity-induced NF-κB transactivation, maintaining epithelial integrity of hypoxia and DFO-treated salivary Pa-4 cells. Conceivably, SUMOylation could function as an important posttranslational modification for protecting epithelial cells against DFO-elicited DNA damage and also accelerate “recovery” processes from chronic hypoxia-induced tight junction disruption on prolonged hypoxia.
What are the SUMOylation targets involved in modulating cellular responses to hypoxia or DFO? Bae et al41 and Shao et al11 have independently reported that HIF-1α can be modified by SUMOylation, leading to an increase in HIF-1α stability and its transcriptional activation during hypoxia. It was also indicated by Shao et al11 that the induction of SUMO-1 and SUMOylated HIF-1α by hypoxia is accompanied by an increased production of vascular endothelial growth factor, a known target of hypoxia/HIF-1α. The purposes of this report are to demonstrate that DFO treatment also induces SUMOylation and to identify and characterize additional signaling pathways that participate in salivary adaptive responses to stress by hypoxia or DFO. Our results clearly demonstrate that SUMO-1-attenuated activation of PKCδ and casapase-3 and SUMO-1-enhanced NF-κB activation in response to DFO are positively correlated with enhanced survival of DFO-treated Pa-4 cells.
Among various SUMOylation functions, the regulation of NF-κB activation by SUMOylation is perhaps one of the most intriguing ones. Cytokine-mediated NF-κB activation occurs through the signaling-induced proteolytic degradation of IκB, via an ubiquitination-mediated system, rendering the nuclear translocation of NF-κB. Polyubiquitination of IκBα, mainly at lysine residues 21 and 22, targets it for proteasomal degradation after extracellular stimuli-mediated IκBα phosphorylation. In contrast, SUMOylation at lysine 21 of IκBα appears to protect IκBα from proteasome-mediated degradation, thus inhibiting NF-κB-dependent transcription by cytokines.21 In addition, it is also postulated that SUMOylation of IκBα is a nuclear event to titrate out nuclear NF-κB.42 We first demonstrated that SUMO-1 antagonizes MEKK1-mediated NF-κB activation (Figure 5A), confirming the already published role of SUMO-1 in down-regulating cytokine-induced NF-κB activation.
However, a recent report by Huang et al22 suggested that SUMOylation of NF-κB essential modulator (NEMO), an essential NF-κB nuclear modulator, allows NEMO to be incorporated into ATM signaling complex in response to genotoxic stress. Modified NEMO then exits the nucleus and associates with and activates the IKK complex, resulting in release of NF-κB from its IκBα inhibitor. Hence, the SUMO modification of NEMO and the ATM activation work in concert to activate NF-κB and its downstream survival pathway(s) against genotoxic stress. Consistent with previous reports,33,43 we further demonstrated that hypoxia-mimetic DFO exposure leads to ATM activation and H2AX S139 phosphorylation in salivary epithelial cells (Figure 4E), hallmarks of DNA damage, and causes Pa-4 cell cycle arrest in S phase (Figure 4C). Importantly, by using SBM to interfere with presumably SUMO-dependent protein-protein interaction,27,32 we were able to partially reverse the observed resistance toward DFO-induced cytotoxicity in Pa-4/SUMO-1 cells (Figure 4B). Together with Figure 4E, we further concluded that hypoxia-/DFO-induced NF-κB activation (Figure 5, C and D) is mediated by a genotoxic signaling pathway, which is distinct from the aforementioned SUMOylation-sensitive NF-κB activation pathway used by cytokines. Moreover, we demonstrated that Pa-4/SUMO-1 cells exhibit a higher basal NF-κB activity (Figure 5, C and D), supporting the notion that SUMO-1 also acts in a pro-NF-κB manner to protect cells against genotoxicity generated endogenously, such as DNA replication. The higher basal and hypoxia-/DFO-induced NF-κB activity may, at least in part, result in the development of resistance against injuries caused by hypoxic exposure in Pa-4/SUMO-1 cells. The mechanism underlying SUMOylation-mediated attenuation of DFO-triggered PKCδ/caspase-3 cleavage is still unclear and currently under investigation.
The present study also provides evidence that overexpression of SUMO-1 leads to a transient protection of passive barrier properties (eg, TER) against acute hypoxic injury (Figure 2C). The increase in TER under baseline conditions indicates that SUMO-1 overexpression enhances epithelial integrity. However, we postulate that this is probably not by a direct effect by enhanced SUMOylation on TER per se but may take place via a regulatory mechanism used by SUMO-1 to enhance the expression of tight junction-associated proteins, subsequently leading to the observed higher TER under acute hypoxia (Figure 2C). For example, we presented evidence that there was a higher level of ZO-1 protein detected in Pa-4/SUMO-1 cells than that in Pa-4 cells at 6 hours after treatment (Figure 5E). Moreover, the increase in NF-κB activity by hypoxia has been proposed to protect against hypoxia-induced increase in paracellular permeability,44 a hallmark of deranged tight-junction properties, also lending credence to our results. Therefore, overexpression of SUMO-1 in Pa-4 cells may result in an enhancement of genotoxicity-induced NF-κB transactivation that subsequently induces transcription and translation of certain target genes, resulting in higher TER under nonhypoxia and acute hypoxia, and also leading to accelerated reassembly of F-actin/ZO-1 under prolonged hypoxia. There are also studies that identified loss of organization in the F-actin and ZO-1 organization as being a critical event leading to a lower TER and increased paracellular permeability in epithelial monolayers.28,44–48 Results from our studies supported this notion by showing that hypoxic stress causes a disruption of F-actin and ZO-1 organization and a decrease of steady-state level of ZO-1 (Figures 3 and 5E). In addition, TNF-α in hypoxia also functions in an autocrine manner, decreasing barrier function. Consequently, the failure of TNF-α to induce NF-κB transactivation in hypoxia-treated Pa-4/SUMO-1 cells (Figure 5C) would protect barrier function.
Emerging evidence has suggested that the maintenance or breakdown of epithelial integrity has significant impact on proper tissue and organ function.49 For example, disruption of epithelial or endothelial barrier formation may lead to altered pulmonary permeability and airway fluid accumulation, unregulated cancer cell growth and increased invasiveness, and perturbation of vascular permeability.50–52 Hence, it is not surprising to see that SUMOylation enhances both epithelial barrier function and cell survival in response to hypoxic stress. The exact nature of cross-talk between signaling pathways rendering the observed protection of F-actin/ZO-1 assembly and the increased resistance against hypoxia remains enigmatic. Notwithstanding the uncertainty concerning the detailed mechanism underlying these two processes, our results unequivocally demonstrate a crucial role of SUMOylation in augmenting salivary adaptive response(s) to exposure to either hypoxia or DFO. Although it is possible that these two events are indirectly linked, we postulate that the reorganization of tight junctions and the genotoxic signalings may functionally cooperate downstream from enhanced SUMOylation process. One plausible model is that genotoxicity-induced NF-κB transactivation is part of a central regulatory circuit in response to hypoxic stress.
Comerford et al10 recently proposed that SUMOylation represents a general “off switch” to hypoxia-induced inflammatory phenotypes. We have provided evidence herein to suggest that SUMO-1 overexpression protects salivary Pa-4 cells against epithelial injury by TER maintenance after acute hypoxia, hence providing some protection for hypoxic cells. We propose a possible mechanism by which enhanced SUMOylation might be protective via a “preconditioning” effect. Further studies will be needed to resolve many remaining questions about this system, including the identities of other SUMOylation targets, the role of NF-κB activation in controlling SUMO-dependent resistance to hypoxia-mediated cell killing, and the mechanisms underlying an accelerated restoration of passive barrier properties, eg, TER in SUMO-1-expressing cells, after prolonged hypoxia. It is also known that tumor cells are more resistant to chemotherapy and radiation therapy under hypoxic conditions.20,53 Hence, the activation of genotoxicity-induced NF-κB pathway and the SUMOylation process are likely to be important targets for hypoxia-mimetic cytotoxins, because they represent an oxygen-sensitive activation/induction mechanism and contribute to the altered signaling events induced by hypoxia.
Footnotes
Address reprint requests to David K. Ann, Ph.D., University of Southern California, Health Science Campus, PSC-209, 1985 Zonal Ave., Los Angeles, CA 90033-1049. E-mail: ann@usc.edu.
Supported in part by National Institutes of Health Research grants R01-DE-10742 and R01-DE-14183 (to D.K.A.), HL-38658 (to K.J.K.), HL-38621 and HL-64365 (to E.D.C.), HL-62569 and HL-38578 (to Z.B.), and CA-94595 (to Y.C.) and by the Hastings Foundation. E.D.C. is Kenneth T. Norris, Jr. Chair and Hastings Professor of Medicine.
H.-V.N. and J.-L.C. contributed equally to this work.
References
- Dohmen RJ. SUMO protein modification. Biochim Biophys Acta. 2004;1695:113–131. doi: 10.1016/j.bbamcr.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Johnson ES. Protein modification by SUMO. Annu Rev Biochem. 2004;73:355–382. doi: 10.1146/annurev.biochem.73.011303.074118. [DOI] [PubMed] [Google Scholar]
- Chen XL, Reindle A, Johnson ES. Misregulation of 2 microm circle copy number in a SUMO pathway mutant. Mol Cell Biol. 2005;25:4311–4320. doi: 10.1128/MCB.25.10.4311-4320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panse VG, Hardeland U, Werner T, Kuster B, Hurt E. A proteome-wide approach identifies sumoylated substrate proteins in yeast. J Biol Chem. 2004;279:41346–41351. doi: 10.1074/jbc.M407950200. [DOI] [PubMed] [Google Scholar]
- Wohlschlegel JA, Johnson ES, Reed SI, Yates JR., III Global analysis of protein sumoylation in Saccharomyces cerevisiae. J Biol Chem. 2004;279:45662–45668. doi: 10.1074/jbc.M409203200. [DOI] [PubMed] [Google Scholar]
- Zhou F, Xue Y, Lu H, Chen G, Yao X. A genome-wide analysis of sumoylation-related biological processes and functions in human nucleus. FEBS Lett. 2005;579:3369–3375. doi: 10.1016/j.febslet.2005.04.076. [DOI] [PubMed] [Google Scholar]
- Bohren KM, Nadkarni V, Song JH, Gabbay KH, Owerbach D. A M55V polymorphism in a novel SUMO gene (SUMO-4) differentially activates heat shock transcription factors and is associated with susceptibility to type I diabetes mellitus. J Biol Chem. 2004;279:27233–27238. doi: 10.1074/jbc.M402273200. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang H, Wang S, Quon D, Liu YW, Cordell B. Positive and negative regulation of APP amyloidogenesis by sumoylation. Proc Natl Acad Sci USA. 2003;100:259–264. doi: 10.1073/pnas.0235361100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffan JS, Agrawal N, Pallos J, Rockabrand E, Trotman LC, Slepko N, Illes K, Lukacsovich T, Zhu YZ, Cattaneo E, Pandolfi PP, Thompson LM, Marsh JL. SUMO modification of Huntingtin and Huntington’s disease pathology. Science. 2004;304:100–104. doi: 10.1126/science.1092194. [DOI] [PubMed] [Google Scholar]
- Comerford KM, Leonard MO, Karhausen J, Carey R, Colgan SP, Taylor CT. Small ubiquitin-related modifier-1 modification mediates resolution of CREB-dependent responses to hypoxia. Proc Natl Acad Sci USA. 2003;100:986–991. doi: 10.1073/pnas.0337412100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao R, Zhang FP, Tian F, Anders Friberg P, Wang X, Sjoland H, Billig H. Increase of SUMO-1 expression in response to hypoxia: direct interaction with HIF-1alpha in adult mouse brain and heart in vivo. FEBS Lett. 2004;569:293–300. doi: 10.1016/j.febslet.2004.05.079. [DOI] [PubMed] [Google Scholar]
- Chen EY, Fujinaga M, Giaccia AJ. Hypoxic microenvironment within an embryo induces apoptosis and is essential for proper morphological development. Teratology. 1999;60:215–225. doi: 10.1002/(SICI)1096-9926(199910)60:4<215::AID-TERA6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Koumenis C, Alarcon R, Hammond E, Sutphin P, Hoffman W, Murphy M, Derr J, Taya Y, Lowe SW, Kastan M, Giaccia A. Regulation of p53 by hypoxia: dissociation of transcriptional repression and apoptosis from p53-dependent transactivation. Mol Cell Biol. 2001;21:1297–1310. doi: 10.1128/MCB.21.4.1297-1310.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA, Tan TT, Rabson AB, Anderson D, Degenhardt K, White E. Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev. 2004;18:2095–2107. doi: 10.1101/gad.1204904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376. doi: 10.1152/ajpgi.00173.2003. [DOI] [PubMed] [Google Scholar]
- Taylor CT, Dzus AL, Colgan SP. Autocrine regulation of epithelial permeability by hypoxia: role for polarized release of tumor necrosis factor alpha. Gastroenterology. 1998;114:657–668. doi: 10.1016/s0016-5085(98)70579-7. [DOI] [PubMed] [Google Scholar]
- Chau CH, Clavijo CA, Deng HT, Zhang Q, Kim KJ, Qiu Y, Le AD, Ann DK. Etk/Bmx mediates the expression of stress-induced adaptive genes, VEGF, PAI-1, and iNOS via multiple signaling cascades in different cell systems. Am J Physiol Cell Physiol. 2005;289:C444–C454. doi: 10.1152/ajpcell.00410.2004. [DOI] [PubMed] [Google Scholar]
- Bindra RS, Schaffer PJ, Meng A, Woo J, Maseide K, Roth ME, Lizardi P, Hedley DW, Bristow RG, Glazer PM. Down-regulation of Rad51 and decreased homologous recombination in hypoxic cancer cells. Mol Cell Biol. 2004;24:8504–8518. doi: 10.1128/MCB.24.19.8504-8518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond EM, Giaccia AJ. The role of ATM and ATR in the cellular response to hypoxia and re-oxygenation. DNA Repair (Amst) 2004;3:1117–1122. doi: 10.1016/j.dnarep.2004.03.035. [DOI] [PubMed] [Google Scholar]
- Harris AL. Hypoxia: a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Desterro JM, Rodriguez MS, Hay RT. SUMO-1 modification of IkappaBalpha inhibits NF-kappaB activation. Mol Cell. 1998;2:233–239. doi: 10.1016/s1097-2765(00)80133-1. [DOI] [PubMed] [Google Scholar]
- Huang TT, Wuerzberger-Davis SM, Wu ZH, Miyamoto S. Sequential modification of NEMO/IKKgamma by SUMO-1 and ubiquitin mediates NF-kappaB activation by genotoxic stress. Cell. 2003;115:565–576. doi: 10.1016/s0092-8674(03)00895-x. [DOI] [PubMed] [Google Scholar]
- Chau CH, Chen KY, Deng HT, Kim KJ, Hosoya K, Terasaki T, Shih HM, Ann DK. Coordinating Etk/Bmx activation and VEGF upregulation to promote cell survival and proliferation. Oncogene. 2002;21:8817–8829. doi: 10.1038/sj.onc.1206032. [DOI] [PubMed] [Google Scholar]
- Li D, Lin HH, McMahon M, Ma H, Ann DK. Oncogenic raf-1 induces the expression of non-histone chromosomal architectural protein HMGI-C via a p44/p42 mitogen-activated protein kinase-dependent pathway in salivary epithelial cells. J Biol Chem. 1997;272:25062–25070. doi: 10.1074/jbc.272.40.25062. [DOI] [PubMed] [Google Scholar]
- Wen X, Lin HH, Shih HM, Kung HJ, Ann DK. Kinase activation of the non-receptor tyrosine kinase Etk/BMX alone is sufficient to transactivate STAT-mediated gene expression in salivary and lung epithelial cells. J Biol Chem. 1999;274:38204–38210. doi: 10.1074/jbc.274.53.38204. [DOI] [PubMed] [Google Scholar]
- Zentner MD, Lin HH, Deng HT, Kim KJ, Shih HM, Ann DK. Requirement for high mobility group protein HMGI-C interaction with STAT3 inhibitor PIAS3 in repression of alpha-subunit of epithelial Na+ channel (alpha-ENaC) transcription by Ras activation in salivary epithelial cells. J Biol Chem. 2001;276:29805–29814. doi: 10.1074/jbc.M103153200. [DOI] [PubMed] [Google Scholar]
- Song J, Durrin LK, Wilkinson TA, Krontiris TG, Chen Y. Identification of a SUMO-binding motif that recognizes SUMO-modified proteins. Proc Natl Acad Sci USA. 2004;101:14373–14378. doi: 10.1073/pnas.0403498101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm-Alvarez SF, Chang A, Wang Y, Jerdeva G, Lin HH, Kim KJ, Ann DK. Etk/Bmx activation modulates barrier function in epithelial cells. Am J Physiol Cell Physiol. 2001;280:C1657–C1668. doi: 10.1152/ajpcell.2001.280.6.C1657. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M. Occludin and claudins in tight-junction strands: leading or supporting players? Trends Cell Biol. 1999;9:268–273. doi: 10.1016/s0962-8924(99)01578-0. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Hammond EM, Denko NC, Dorie MJ, Abraham RT, Giaccia AJ. Hypoxia links ATR and p53 through replication arrest. Mol Cell Biol. 2002;22:1834–1843. doi: 10.1128/MCB.22.6.1834-1843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Zhang Z, Hu W, Chen Y. SUMO recognition of a SUMO-binding motif: a reversal of the bound orientation. J Biol Chem. 2005;280:40122–40129. doi: 10.1074/jbc.M507059200. [DOI] [PubMed] [Google Scholar]
- Hammond EM, Dorie MJ, Giaccia AJ. Inhibition of ATR leads to increased sensitivity to hypoxia/reoxygenation. Cancer Res. 2004;64:6556–6562. doi: 10.1158/0008-5472.CAN-04-1520. [DOI] [PubMed] [Google Scholar]
- Reyland ME, Anderson SM, Matassa AA, Barzen KA, Quissell DO. Protein kinase C delta is essential for etoposide-induced apoptosis in salivary gland acinar cells. J Biol Chem. 1999;274:19115–19123. doi: 10.1074/jbc.274.27.19115. [DOI] [PubMed] [Google Scholar]
- Li B, Wang X, Rasheed N, Hu Y, Boast S, Ishii T, Nakayama K, Nakayama KI, Goff SP. Distinct roles of c-Abl and Atm in oxidative stress response are mediated by protein kinase C delta. Genes Dev. 2004;18:1824–1837. doi: 10.1101/gad.1223504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee FS, Hagler J, Chen ZJ, Maniatis T. Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- Nakano H, Shindo M, Sakon S, Nishinaka S, Mihara M, Yagita H, Okumura K. Differential regulation of IkappaB kinase alpha and beta by two upstream kinases, NF-kappaB-inducing kinase and mitogen-activated protein kinase/ERK kinase kinase-1. Proc Natl Acad Sci USA. 1998;95:3537–3542. doi: 10.1073/pnas.95.7.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo D, Li M, Zhang Y, Yang P, Eckenrode S, Hopkins D, Zheng W, Purohit S, Podolsky RH, Muir A, Wang J, Dong Z, Brusko T, Atkinson M, Pozzilli P, Zeidler A, Raffel LJ, Jacob CO, Park Y, Serrano-Rios M, Larrad MT, Zhang Z, Garchon HJ, Bach JF, Rotter JI, She JX, Wang CY. A functional variant of SUMO4, a new I kappa B alpha modifier, is associated with type 1 diabetes. Nat Genet. 2004;36:837–841. doi: 10.1038/ng1391. [DOI] [PubMed] [Google Scholar]
- Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- Bae SH, Jeong JW, Park JA, Kim SH, Bae MK, Choi SJ, Kim KW. Sumoylation increases HIF-1alpha stability and its transcriptional activity. Biochem Biophys Res Commun. 2004;324:394–400. doi: 10.1016/j.bbrc.2004.09.068. [DOI] [PubMed] [Google Scholar]
- Hay RT, Vuillard L, Desterro JM, Rodriguez MS. Control of NF-kappa B transcriptional activation by signal induced proteolysis of I kappa B alpha. Philos Trans R Soc Lond B Biol Sci. 1999;354:1601–1609. doi: 10.1098/rstb.1999.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond EM, Dorie MJ, Giaccia AJ. ATR/ATM targets are phosphorylated by ATR in response to hypoxia and ATM in response to reoxygenation. J Biol Chem. 2003;278:12207–12213. doi: 10.1074/jbc.M212360200. [DOI] [PubMed] [Google Scholar]
- Brown RC, Mark KS, Egleton RD, Huber JD, Burroughs AR, Davis TP. Protection against hypoxia-induced increase in blood-brain barrier permeability: role of tight junction proteins and NfkappaB. J Cell Sci. 2003;116:693–700. doi: 10.1242/jcs.00264. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Lowenkopf T, Apatira D. Identification of a 160-kDa polypeptide that binds to the tight junction protein ZO-1. Proc Natl Acad Sci USA. 1991;88:3460–3464. doi: 10.1073/pnas.88.8.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht G, Pothoulakis C, LaMont JT, Madara JL. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988;82:1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carnes D, Lemichez E, Boquet P, Madara JL. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci USA. 1995;92:10629–10633. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun. 2001;69:1329–1336. doi: 10.1128/IAI.69.3.1329-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinacker AB, Vaszar LT. Acute respiratory distress syndrome: physiology and new management strategies. Annu Rev Med. 2001;52:221–237. doi: 10.1146/annurev.med.52.1.221. [DOI] [PubMed] [Google Scholar]
- Finigan JH, Dudek SM, Singleton PA, Chiang ET, Jacobson JR, Camp SM, Ye SQ, Garcia JG. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J Biol Chem. 2005;280:17286–17293. doi: 10.1074/jbc.M412427200. [DOI] [PubMed] [Google Scholar]
- Gon Y, Wood MR, Kiosses WB, Jo E, Sanna MG, Chun J, Rosen H. S1P3 receptor-induced reorganization of epithelial tight junctions compromises lung barrier integrity and is potentiated by TNF. Proc Natl Acad Sci USA. 2005;102:9270–9275. doi: 10.1073/pnas.0501997102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Li L, Backer J, Wong AS, Schwanke EL, Stewart BG, Pasdar M. Bcl-2 expression decreases cadherin-mediated cell-cell adhesion. J Cell Sci. 2003;116:3687–3700. doi: 10.1242/jcs.00644. [DOI] [PubMed] [Google Scholar]
- Harrison L, Blackwell K. Hypoxia and anemia: factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist. 2004;9(Suppl 5):31–40. doi: 10.1634/theoncologist.9-90005-31. [DOI] [PubMed] [Google Scholar]