Abstract
The synovial lining of diarthrodial joints is composed of a condensed network of synoviocytes that form an intact layer via cell-to-cell contacts with significant intercellular matrix spaces. However, the molecular basis for synovial lining formation and its structural integrity has not been previously defined. In this study, using a three-dimensional fibroblast-like synoviocyte in vitro organ culture system, we provide evidence that cadherin-11 expressed in fibroblast-like synoviocytes plays a determining role in establishing the synovial lining layer. Fibroblast-like synoviocytes that were grown in three-dimensional matrices demonstrated formation of a lining structure at the interface between the matrix and the fluid phase. Treatment of fibroblast-like synoviocyte organ cultures with a cadherin-11-Fc fusion protein efficiently abrogated lining layer organization. Moreover, because E-cadherin-expressing fibroblasts failed to organize a lining layer structure at the tissue boundary, this effect appears to be a distinct characteristic of fibroblasts expressing cadherin-11. We found that cadherin-11 mediated fibroblast-like synoviocyte cell-to-cell adhesion via formation of adherens junctions that were linked to and remodeled the actin cytoskeleton. Together, these studies implicate cadherin-11 in synovial tissue and lining layer formation and provide an in vitro system to model fibroblast-like synoviocyte behavior and function in organizing the synovial tissue.
The synovium of diarthrodial joints is a highly organized tissue that resides between the joint cavity and the fibrous joint capsule.1,2 In healthy states, the predominant cell type is of mesenchymal origin and demonstrates fibroblast-like features.3,4 These fibroblast-like synoviocytes (FLSs) condense and accumulate at the tissue-joint cavity interface to form a distinct structure called the synovial lining layer, which overlays the loosely packed stroma. Architecturally, the synovial lining is distinct from most other anatomical lining structures. In contrast to epithelium or endothelium, the synovial lining layer lacks specific adhesive structures such as tight junctions, desmosomes, and a discrete basement membrane.5 Instead, the cells of the synovial lining condense together through cell-to-cell contacts to form an intact layer with significant intercellular matrix spaces.2 Thus, the synovial lining layer is composed of a condensed network of synoviocytes within a lattice of extracellular matrix (ECM). The synovial lining is responsible for the synthesis of lubricants (eg, lubricin, hyaluronan) and for transudating nutrients in the synovial fluid that facilitate joint motion and support chondrocytes in the avascular cartilage.6–9 In addition, the lining FLSs synthesize both components of the ECM and degradative enzymes such as cathepsins, serine proteases, and matrix metalloproteinases that are necessary for matrix turnover and cellular movement.10–15
During the course of rheumatoid arthritis, the inflamed synovial tissue undergoes remodeling. The synovial lining becomes hyperplastic and forms a condensed tissue mass, called pannus, which attaches to and invades the cartilage from the periphery of the joint. FLSs and macrophages comprise the major cell population of the hyperplastic synovial lining in rheumatoid arthritis. Furthermore, FLSs are the predominant cellular lineage in the invasive pannus tissue. The molecular mechanisms contributing to condensation of FLSs and their aberrant behavior in the context of chronic inflammation remain obscure.
Both embryonic tissue morphogenesis and postnatal organization of tissues require stable cell-to-cell adhesion to integrate cells into three-dimensional structures that confer specialized tissue and cell functions.16 The cadherin family of integral membrane proteins mediates homotypic cell-to-cell adhesion via dimerization between ectodomains of identical cadherin molecules on adjacent cells.17–19 During development, the expression of a cadherin results in the aggregation and compaction of cells into a rudimentary tissue or lining.20
Cadherin engagement triggers cadherin clustering and association of the cadherin cytoplasmic tail with intracellular catenins to form a molecular complex called the adherens junction (AJ).21 At the cadherin cytoplasmic tail two distinct domains have been identified. The cytoplasmic juxta-membrane domain associates with p120-catenin whereas the distal domain binds β-catenin, which in turn binds α-catenin.21–23 α-Catenin is a scaffolding protein that functionally links the AJ to the actin cytoskeleton.24–27 It has been implicated in actin bundling and is required for the recruitment of signaling molecules to AJs, such as formin-1, which regulate the actin cytoskeleton.28 Actin cytoskeletal reorganization upon AJ formation induces changes in cell shape and stabilizes cell-to-cell contacts.29 Cadherin-mediated cell-to-cell contacts, however, are not static. These contacts are actively remodeled to allow cell rearrangements and the movement of cells along other cells.30–33 Thus, cadherins provide a molecular means for the stable organization, orderly turnover, and remodeling of tissues.
Recently, we identified cadherin-11 expression on FLSs by indirect immunohistochemistry of frozen synovial tissue sections derived from rheumatoid arthritis patients or healthy patients.34 Functional studies confirmed homophilic adhesion of cultured FLSs mediated by cadherin-11. In this report, we demonstrate that cadherin-11 expressed in human FLSs mediates formation of AJs that are linked to and actively remodel the actin cytoskeleton. Using a three-dimensional organ culture system, we find that the establishment of synovial lining architecture in vitro is critically dependent on cadherin-11 function.
Materials and Methods
Cell Culture
Discarded synovial tissues from rheumatoid arthritis patients (American College of Rheumatology criteria) were obtained with approval of the Brigham and Women’s Hospital Institutional Review Board from synovectomy or joint replacement procedures. Synoviocyte cell suspensions were prepared from synovial tissues by mincing followed by gently rocking for 1 hour at 37°C in 1 mg/ml of collagenase (type IV; Worthington Biochemicals, Lakewood, NJ) in HEPES-buffered saline (HBS) solution (20 mmol/L HEPES, 137 mmol/L NaCl, and 3 mmol/L KCl, pH 7.4) containing 2 mmol/L CaCl2. The cell suspension was passed through a 70-μm cell strainer (BD Biosciences, Bedford, MA) and placed in tissue culture flasks (BD Labware, Lincoln Park, NJ), in RPMI medium 1640 (Life Technologies, Inc., Gaithersburg, MD) supplemented with 10% heat-inactivated fetal bovine serum (HI-FBS) (Hyclone, Logan, UT), 2 mmol/L l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin sulfate, 50 μmol/L 2-mercaptoethanol, and amino acids (Life Technologies, Inc.) in a 37°C 5% CO2 incubator. After the third passage, confluent monolayers appeared fibroblast-like and were negative for CD68, suggesting they were composed mainly of FLSs, as previously described.35 For subsequent experiments, FLSs were released from the culture dish using 0.02% (w/v) TPCK-treated trypsin (Worthington Biochemicals) in HBS containing 1 mmol/L CaCl2. In the presence of CaCl2, cadherins are protected from trypsin cleavage, and the cells are released as multicellular aggregates as a result. Cadherin adhesive function is dependent on the presence of calcium. To disrupt cadherin-mediated cell-to-cell adhesive bonds, the cells were then washed in HBS without CaCl2. This procedure allowed generation of a single cell suspension while preserving cadherin-11 molecules on the cell surface. The murine fibroblast cell line L-M (CCL1.3, L cells; American Type Culture Collection, Rockville, MD) was grown in Dulbecco’s modified Eagle’s medium, high glucose, supplemented with 10% HI-FBS, 2 mmol/L l-glutamine, 10 μmol/L nonessential amino acids (Life Technologies, Inc.), 100 U/ml penicillin, and 100 μg/ml streptomycin sulfate at 10% CO2. L-cell transfectants were grown in the above medium with 0.8 mg/ml of hygromycin B (Invitrogen, Carlsbad, CA).
Plasmids and Generation of L-Cell-Cadherin-11 Stable Transfectants
The human cadherin-11 cDNA or E-cadherin cDNA was inserted into the expression plasmid pCEP4 (Invitrogen). L cells known to contain the catenins but no endogenous cadherins, were transfected with pCEP4/cadherin-11, pCEP4/E-cadherin, or with the empty pCEP4 vector as described before.34 Transfected cells were selected by culture in 0.8 mg/ml of hygromycin B, and cadherin expression was confirmed by flow cytometry (Supplemental Figure 1, see http://ajp.amjpathol.org).
Figure 1.
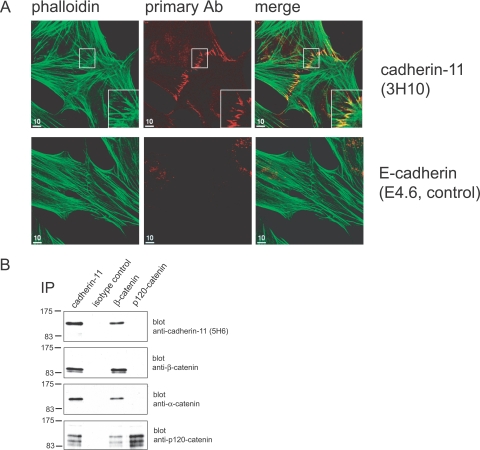
Formation of cadherin-11-mediated AJs in FLSs. A: Cadherin-11 localizes to cell-to-cell contacts in cultured FLSs. FLSs were cultured on coverslips for 3 days and processed for indirect immunofluorescence microscopy using phalloidin to label F-actin (green) and anti-cadherin-11 (3H10) to label AJs. At sites of cell-to-cell contact, FLSs demonstrated numerous filopodia-like processes that interdigitated with filopodia from neighboring cells to form an adhesion zipper (area in white box is magnified in inset). F-Actin fibers radiated internally from intercellular junctions (green). The antibody to cadherin-11 labeled clusters of AJs as short parallel lines (red). By contrast, an isotype control antibody did not label AJs. B: Formation of cadherin-11-mediated AJs in FLSs. Western blot detection of cadherin-11, β-catenin, α-catenin, and p120-catenin from FLS immunoprecipitates. Confluent FLSs were lysed with Brij 97. Components of AJs were immunoprecipitated with antibodies against cadherin-11 (3H10), β-catenin, or p120-catenin. An isotype antibody against an unknown antigen was used as a control. β-Catenin, α-catenin, and p120-catenin co-immunoprecipitated with cadherin-11. Multiple p120-catenin species co-immunoprecipitated with cadherin-11 represent known isoforms of p120-catenin. Cadherin-11, α-catenin, and p120-catenin were detectable in β-catenin precipitates. Cadherin-11, β-catenin, or α-catenin were not detectable in p120-catenin precipitates.
Antibodies and Other Reagents
The monoclonal antibodies (mAbs) cadherin-11–3H10, cadherin-11–5H6, anti-human E-cadherin (E4.6, IgG1) were developed previously in our laboratory. P3 (control mouse IgG1), affinity-purified goat anti-human IgG, and polyclonal anti-estrogen receptor-β antibody (rabbit Ig) were purchased from Zymed (San Francisco, CA). Anti-p120ctn mAb (pp120, clone 98, mouse IgG1), anti-β-catenin mAb (clone 14, mouse IgG1), and anti-mouse MHC class I (H-2Kk; 36-7-5, mouse IgG2a) were purchased from BD Biosciences. Anti-α-catenin (rabbit antiserum) was purchased from Sigma, St. Louis, MO. Human plasma fibronectin was purchased from Invitrogen. Cadherin-11-Fc protein and E-cadherin-Fc protein was produced in our laboratory and purified as previously described. The purity of the fusion protein was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie blue staining, and the concentration was determined by Bradford assay using bovine serum albumin (BSA) as the standard (Bio-Rad, Hercules, CA).
Cell Lysis and Immunoprecipitation
FLSs were grown to confluence, lysed, immunoprecipitated, and analyzed by Western blotting essentially as previously described except that the lysis buffer was 1% Brij 96 in HBS with 2 mmol/L CaCl2 and 5 mmol/L MgCl2.36
Immunofluorescence and Flow Cytometry
For immunofluorescence, cells were cultured on coverglass, fixed in 2% paraformaldehyde in phosphate-buffered saline (PBS) for 15 minutes, and permeabilized in 0.2% (w/v) saponin in PHEM-buffer (60 mmol/L Pipes, 25 mmol/L HEPES, 10 mmol/L EGTA, and 2 mmol/L MgCl, pH 6.9) for 30 minutes. Coverslips were then blocked for 1 hour in blocking buffer (PHEM-buffer supplemented with 5% fetal bovine serum and 2.5% donkey serum) and incubated with primary antibodies (5 μg/ml for each) for 1 hour at room temperature. After washing three times with blocking buffer, cells were incubated with Cy3-conjugated secondary antibody (diluted 1:800 in blocking buffer; Jackson ImmunoResearch Laboratories, West Grove, PA) and Alexa 488-conjugated phalloidin (1:2000 in blocking buffer; Molecular Probes, Eugene, OR) or Cy2-conjugated secondary antibody (1:200 in blocking buffer, Jackson ImmunoResearch Laboratories) for 1 hour at room temperature. Preparations were then washed three times with blocking buffer, mounted in vinol, and analyzed by confocal microscopy. For flow cytometry, cultured cells were released and incubated with primary antibodies in HBS with 1 mmol/L CaCl2 containing 2% human serum for 1 hour at 4°C. Cells were then washed twice, incubated with fluorescein isothiocyanate-conjugated secondary antibody [goat F(ab)2 anti-mouse IgG; Caltag Laboratories, Burlingame, CA] for 1 hour at 4°C, washed twice, and analyzed on a FACScan (Becton Dickinson, Mountain View, CA).
Synovial Organ Cultures
For cell organization experiments, we established a three-dimensional in vitro culture model. Passaged FLSs (passages 3 to 10) or L-cell transfectants were released from the culture flask using trypsin in HBS containing 1 mmol/L CaCl2 and washed once in HBS without CaCl2. Cells were resuspended in ice-cold Matrigel Matrix (BD Biosciences) or collagen matrix (Vitrogen; Cohesion, Palo Alto, CA) (2 × 106 cells/ml ECM for FLS cultures; 1 × 107 cells/ml for L-cell cultures). Twenty-five-μl droplets of the suspension were placed onto poly-2-hydroxyethyl-methacrylate (poly-HEMA; Aldrich Chemical Co., Milwaukee, WI)-coated culture dishes. Poly-HEMA prevents attachment of cells to the culture dish. Gelation was allowed for 45 minutes at 37°C for Matrigel cultures and 75 minutes for collagen cultures. After this, the gel was overlaid with culture medium [DME medium (Life Technologies, Inc.) supplemented with penicillin, streptomycin, l-glutamine, nonessential amino acid solution, ITS (BioWhittaker, Rockland, ME), and 0.1 mmol/L ascorbic acid, 10% HI-FBS]. The floating three-dimensional culture was maintained for various times as indicated; the medium was routinely replaced every 3 days. Thereafter, the three-dimensional culture was fixed with 2% (w/v) paraformaldehyde in HBS containing 1 mmol/L CaCl2 for 2 hours, rinsed with ethanol several times, and embedded in paraffin. Sections of the tissue-like structure were stained with hematoxylin and eosin or stained for reticular fibers using the Gomori silver impregnation technique.
Immunohistochemistry
Sections of synovial organ cultures were deparaffinized with xylene, followed by washing in ethanol and rehydration in PBS. Endogenous peroxidase activity was blocked with 0.5% hydrogen peroxide in PBS for 30 minutes at room temperature. Sections were rinsed and incubated for 1 hour at room temperature with 5% normal donkey serum in PBS. Cadherin-11 was detected by incubating sections with a polyclonal rabbit anti-cadherin-11 antibody (10 μg/ml, Zymed) for 1 hour. An isotype control antibody (polyclonal anti-estrogen receptor-β) was used as a control. Slides were washed, and the secondary antibody (donkey anti-rabbit horseradish peroxidase, Jackson ImmunoResearch Laboratories) was added for 1 hour at room temperature. Slides were washed, and the Vectastain ABC kit (Vector Laboratories, Burlingame, CA) was used to detect horseradish peroxidase activity. Sections were counterstained with hematoxylin and slides were mounted using Cytoseal 60 (Richard Allan Scientific, Kalamazoo, MI). Light microscopic images were captured using a Leica DM LB2 light microscope (Leica Microsystems, Wetzlar, Germany) using lens 20×/0.50 with a digital camera (model DFC 300, Leica) using Spot software. Image processing was done using Adobe Photoshop.
Results
Cadherin-11 Mediates AJ Formation in FLSs
A prerequisite for cadherin function in mediating stable cell-to-cell adhesion is association with cytoplasmic catenins and the actin cytoskeleton at the cadherin cytoplasmic tail. To visualize the components of AJs at sites of cell-to-cell contact in FLSs, immunofluorescence studies were performed. Cells were plated onto coverglasses at 50% confluence, cultured overnight, processed for indirect immunofluorescence and stained with antibodies to cadherin-11, catenins including p120-catenin, β-catenin, or α-catenin, and with phalloidin to label polymerized actin. Confocal microscopic analysis revealed actin fibers radiating internally from filopodia. The filopodia were particularly numerous at sites of intimate cell-to-cell contact (Figure 1A, top). At these sites, the anti-cadherin-11 antibody labeled a ladder-like series of lines, consistent with AJs at interdigitating filopodia that have formed an FLS cell-to-cell adhesion zipper (Figure 1A, top, middle). A similar staining pattern was observed for p120-catenin, β-catenin, and α-catenin, indicating localization of p120-catenin, β-catenin, and α-catenin to FLS AJs (Supplemental Figure 2, see http://ajp.amjpathol.org). By contrast, isotype control antibodies did not label AJs, indicating that the staining for cadherin-11 or intracellular catenins was specific (Figure 1A, bottom; and Supplemental Figure 2, see http://ajp.amjpathol.org).
Figure 2.
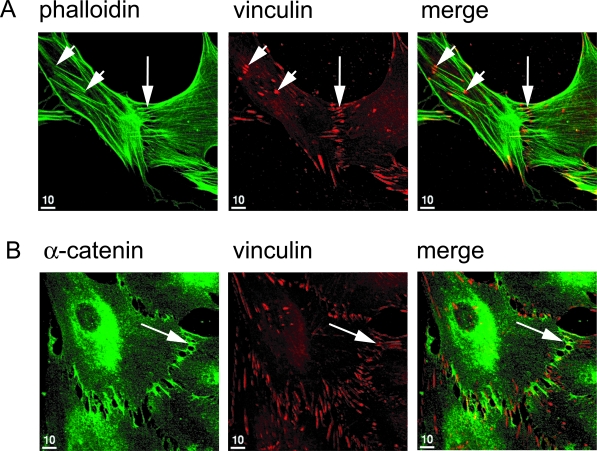
Vinculin is a component of AJs in FLSs. Indirect immunofluorescence was conducted on FLSs that were cultured for 3 days on coverslips using phalloidin (green) to label F-actin, an antiserum to α-catenin (green), or an antibody to vinculin (red). A: The antibody to vinculin (red) labeled focal contacts (short arrows) as well as AJs (long arrow points to adhesion zipper with multiple clusters of AJs labeled as short parallel lines). B: The antiserum to α-catenin labeled AJs but not focal contacts. Co-localization of vinculin and α-catenin at AJs resulted in yellow labeling at sites of cell-to-cell contact in the merged picture (long arrow).
To demonstrate that cadherin-11 forms a molecular complex with intracellular catenins, confluent FLSs were lysed and antibodies against cadherin-11, β-catenin, p120-catenin, or isotype control immunoprecipitates were then probed in Western blots with antibodies to the components of the cadherin-catenin complex. For example, Western blotting revealed that anti-cadherin-11 mAb had co-immunoprecipitated β-catenin, α-catenin, and p120-catenin (Figure 1B). Blotting with β-catenin antibody detected two distinct protein species that are consistent with phosphorylated and unphosphorylated β-catenin.37,38 Multiple p120-catenin species that co-immunoprecipitated with cadherin-11 represent the known isoforms of p120-catenin.39 As a specificity control, cadherin-11 as well as intracellular catenins were not detected from isotype control immunoprecipitates. In reciprocal experiments, we found that anti-β-catenin co-immunoprecipitated cadherin-11, α-catenin, and p120-catenin. By contrast, cadherin-11, β-catenin, and α-catenin were not detectable from p120-catenin immunoprecipitates. Because p120-catenin co-immunoprecipitated with cadherin-11 as well as β-catenin, this finding suggests that only a minor fraction of the cytoplasmic p120-catenin pool associates with the cadherin-11 complex. Together with the immunofluorescence studies, these experiments indicate that formation of AJs at sites of cell-to-cell contact in FLSs involves cadherin-11, intracellular catenins, and the actin cytoskeleton.
AJ Formation in FLSs Involves Recruitment of Vinculin
The intricate link between actin filaments and cadherin-11 AJs was evident by immunofluorescence microscopy, where cell-to-cell junctions linked to radial actin fibers (Figure 1A). Formation of stable cell-to-cell contacts involves clustering of cadherins and catenins and recruitment of molecules that regulate actin cytoskeletal dynamics. Vinculin is a cytoskeletal protein that serves as a scaffold for molecules required for actin cytoskeletal reorganization.40 Vinculin localizes to focal contacts (cell-to-matrix adhesion complex) as well as AJs (E-cadherin-mediated adhesion complex) in epithelial cells.40,41
To determine whether vinculin localizes to the cadherin-11 AJs, FLSs were plated onto coverglasses, cultured overnight, processed for indirect immunofluorescence, and co-stained with antibodies to vinculin and α-catenin or phalloidin. At sites of intimate cell-to-cell contact, confocal microscopy revealed vinculin-specific staining in a ladder-like series of lines similar to the cadherin-11 staining pattern (Figure 2A, long arrows). Like the cadherin-11 antibody, the vinculin antibody labeled adhesion zippers. However, in contrast to cadherin-11, the vinculin antibody also labeled other F-actin-associated structures at the cell periphery that are consistent with localization of vinculin to integrin-mediated focal contacts (Figure 2A, short arrows). Double-immunofluorescence staining using antibodies against α-catenin and vinculin revealed co-staining at sites of cell-to-cell contact, further demonstrating that vinculin localizes to the cadherin-11-β-catenin-α-catenin complex (Figure 2B, long arrows). Differential labeling of specific structures at the cell periphery that were labeled with the vinculin antibody but not the α-catenin antibody is consistent with localization of vinculin to focal contacts from which α-catenin is excluded. Thus, vinculin is found both in integrin-mediated focal contacts and in cadherin-11-mediated AJs.
The Cadherin-11 AJs Stimulate Actin Polymerization and Remodeling
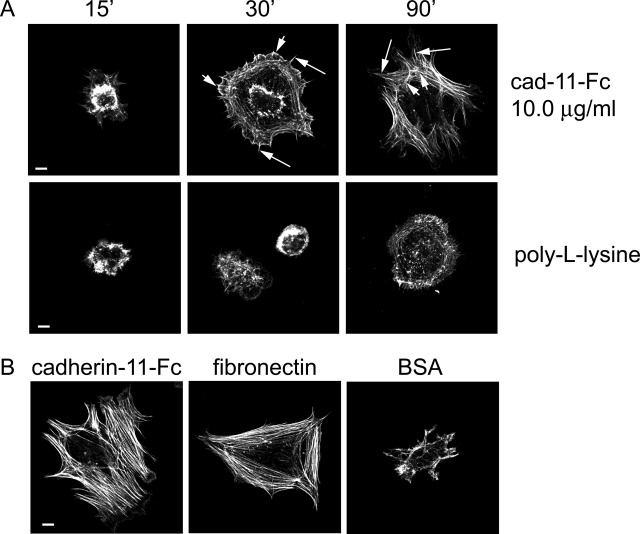
The fluorescence experiments above describe the formation of cadherin-11-mediated AJs in FLSs. Here, we probed the role of the cadherin-11-catenin complex in polymerizing actin and reorganizing the actin cytoskeleton, linked to the cadherin-11 complex. Importantly, actin polymerization and reorganization upon cell-to-cell contact provide the physical force necessary to stabilize cell-to-cell adhesion, to induce changes in cell shape, and promote movement of cells along other cells. We used the cadherin-11-Fc fusion protein to determine cadherin-11 function in mediating actin dynamics (Supplemental Figure 3, see http://ajp.amjpathol.org).34 Coverglasses were precoated with cadherin-11-Fc fusion protein or control protein (poly-l-lysine, fibronectin) and then blocked with BSA. Cultured FLSs were serum-starved for 18 hours, released from the culture dish, and seeded onto coverslips in the absence of serum to exclude any effect of integrin binding to serum components. When FLSs were seeded onto cadherin-11-Fc fusion protein (10 μg/ml)-coated coverglasses, changes in cell shape and cytoskeletal organization were observed as a function of time (Figure 3A, top). After 15 minutes, small polygonally shaped FLSs formed short cellular projections reaching out attached to the molecular stratum. After 30 minutes, further cell spreading characterized by numerous radial spikes extending out from the cell border was observed (Figure 3A, top, middle; long arrows). At the cell periphery, phalloidin labeled multiple, broad-based projections consistent with lamellipodial protrusions (Figure 3A, top, middle; short arrows). After 90 minutes, pronounced cell spreading was apparent. Numerous actin fibers radiated internally from multiple cellular extensions (Figure 3A, right; long arrows). Additionally, circumferential fibers became visible (Figure 3A, right; short arrows). In contrast to cadherin-11-Fc-induced cytoskeletal changes, poly-l-lysine failed to induce radial actin fibers as well as lamellipodial protrusions (Figure 3A, bottom).
Figure 3.
Cadherin-11 engagement stimulates actin polymerization and actin cytoskeletal reorganization. A: FLSs were plated on protein-coated coverslips as indicated in the absence of serum for the indicated times, fixed, permeabilized, stained for F-actin (phalloidin-Alexa 488, green), and analyzed by confocal microscopy. Note time-dependent cell spreading. After 30 minutes the cells plated on cadherin-11-Fc fusion protein demonstrated radial spikes (middle, long arrows) and lamellipodial protrusions (middle, short arrows). After 90 minutes pronounced cell spreading was accompanied by the formation of radial actin fibers (right, long arrows) and circumferential actin fibers (right, short arrows). In contrast, in FLSs plated on poly-l-lysine, radial actin fibers and lamellipodial protrusions were not detectable. B: FLSs were plated on cadherin-11-Fc fusion protein-, fibronectin-, or BSA-coated coverglasses. Cadherin-11-Fc induced pronounced cell spreading and actin fiber formation in a similar way as fibronectin, whereas BSA failed to induce cell spreading. Scale bars, 10 μm.
To compare the effect of cadherin-11 engagement on actin remodeling with that of fibronectin, FLSs were also plated on fibronectin-coated coverglasses. Fibronectin is a component of the ECM that can induce actin dynamics by binding to integrin receptors. As expected, FLSs seeded onto fibronectin demonstrated pronounced cell spreading and numerous actin fibers similar to the cadherin-11-Fc fusion protein after 90 minutes of incubation (Figure 3B). Because cadherin-11-Fc-, poly-l-lysine-, or fibronectin-coated coverglasses were blocked with BSA to prevent nonspecific attachment of FLSs to the coverglass, we plated FLSs on coverglasses that were solely coated with BSA to control for any attachment factors that might be present in the BSA preparation. In this situation, only a few cells adhered to the coverglass after 90 minutes of incubation. These FLSs appeared small in size with only a few short cellular extensions, indicating that BSA did not support FLS spreading (Figure 3B).
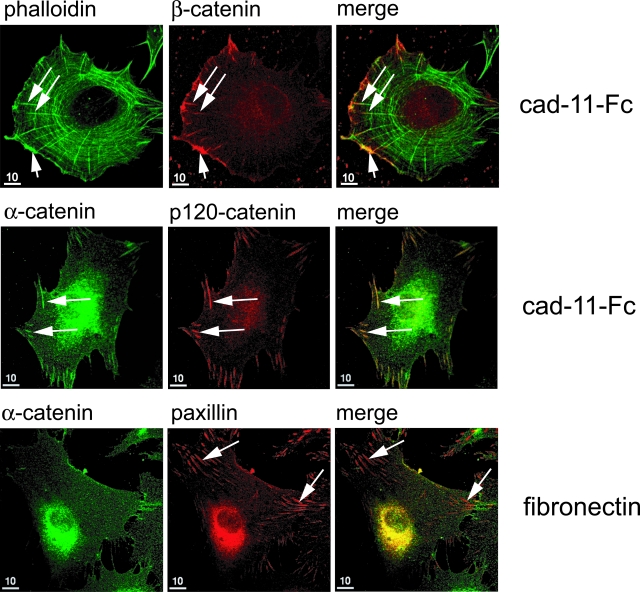
To illustrate further specific cadherin-11-induced actin cytoskeletal changes, FLSs were plated on cadherin-11-Fc- or fibronectin-coated coverglasses for 90 minutes and co-stained with phalloidin and antibodies to catenins. When plated on cadherin-11-Fc, the antibody to β-catenin labeled distinct broad-based structures at the cell periphery consistent with lamellipodia (Figure 4, top; short arrows). Underlying the cell border, circumferential actin fibers were visible. Additionally, multiple actin fibers radiated internally from the cell periphery. These fibers emanated from short line-like structures that were labeled with the antibody to β-catenin (Figure 4, top; long arrows). Staining with antibodies to α-catenin or p120-catenin revealed similar structures at the cell periphery, suggesting that these structures represent clustered cadherin-11-mediated AJs (Figure 4, middle; long arrows). To test whether or not these clusters are specifically induced by cadherin-11 engagement, FLSs were also plated on fibronectin and stained with antibodies to α-catenin or paxillin. Paxillin is a component of cell-to-matrix adhesion complex and labels integrin-mediated focal contacts. Indeed, paxillin staining revealed numerous focal contacts in FLSs that have spread on fibronectin (Figure 4, bottom; long arrows). These focal contacts were not detected by the antibody to α-catenin, indicating that α-catenin specifically localizes to cadherin-11-mediated AJs but not focal contacts. Thus, we conclude that cadherin-11 engagement in FLSs stimulates cell spreading, actin polymerization, and reorganization. This activity was accompanied by organized formation of clusters of cadherin-11 AJs, dependent on time, and specifically induced by homophilic cadherin interactions and not by ECM.
Figure 4.
Cadherin-11-stimulated cell spreading of FLSs is accompanied by clustering of AJs. FLSs were seeded on cadherin-11-Fc- or fibronectin-coated coverslips (10 μg/ml, respectively) and incubated for 90 minutes followed by indirect immunofluorescence using antibodies as indicated. Phalloidin staining revealed lamellipodia (top, short arrow), radial actin fibers (top, long arrows), and circumferential actin fibers (top). The antibody to β-catenin labeled lamellipodia (top, short arrow) and clusters of AJs (top, long arrows) at the cell periphery of FLSs seeded on cadherin-11-Fc-coated coverslips. Note radial actin fibers emanating from clusters of AJs. The α-catenin antiserum and the antibody to p120-catenin both detected clusters of AJs at the cell periphery similar to the β-catenin antibody (middle, long arrows). In FLSs plated on fibronectin, the antibody to paxillin labeled focal contacts that were not detected by the α-catenin antiserum (bottom, long arrows).
FLSs Cultured in Three-Dimensional Matrices Establish a Tissue Structure Resembling Synovial Architecture
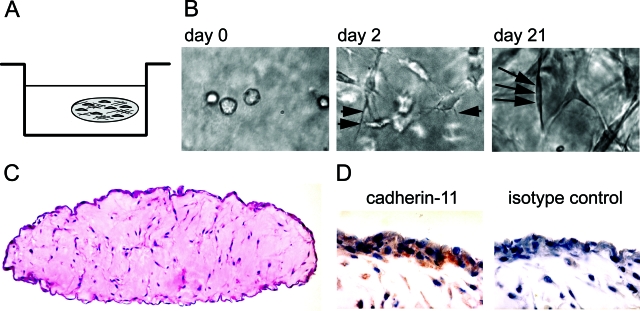
Cadherin-mediated cell-to-cell adhesion and cadherin-stimulated cytoskeletal reorganization are closely intertwined processes that allow cells to organize into a tissue structure. The capacity of cadherin-11 in FLSs to mediate cell-to-cell adhesion and provoke cytoskeletal dynamics led us to propose that cadherin-11 participates in establishing a synovial lining structure. To test this hypothesis, we developed an in vitro three-dimensional organ culture system to model the synovial lining of the joints. Human FLSs embedded in Matrigel were cultured in a floating micromass, simulating the connective tissue border with the fluid-filled joint cavity. Matrigel is a solubilized basement membrane preparation that is rich in matrix components such as laminin, collagen type IV, and heparan sulfate proteoglycans. It provides a scaffold to enable cultured, disaggregated FLSs to re-establish three-dimensional cellular organization present in the synovial lining. Human FLSs were homogeneously dispersed into liquid Matrigel at 4°C and drops were placed onto culture dishes. After warming to 37°C, solidification occurs, and the floating spheres were then cultured in media for 21 to 28 days (Figure 5A). Dramatic changes in cellular morphology were noted throughout time by phase contrast microscopy. Initially, the FLSs were isolated, single round cells dispersed evenly through the matrix (Figure 5B, left). After 2 days in culture, fine processes began to emanate from the cell bodies (Figure 5B, middle; short arrows). After 3 weeks, the FLSs were elongated and spindle-shaped, exhibiting typical fibroblast-like appearance (Figure 5B, right; long arrows). Strikingly, the three-dimensional organ culture revealed the condensed accumulation of FLSs forming a lining-layer-like structure at the interface between the matrix and the fluid phase when examined histologically (Figure 5C). This organized lining layer overlaid a sparsely populated matrix. This tissue-like structure remarkably resembled in vivo synovial lining, suggesting that FLSs have an intrinsic capacity to establish synovial architecture. Immunohisto-chemical analysis using anti-cadherin-11 antibody demonstrated cadherin-11-specific reactivity at the lining layer, as well as on cells in the sublining area (Figure 5D, red-brown staining). This staining pattern was strikingly similar to the ex vivo staining pattern we found for the normal human synovium.34
Figure 5.
FLSs establish a synovial lining-like structure when cultured in three-dimensions. A: Schematic representation of the three-dimensional organ culture system. FLSs are dispersed as single cells in liquid ECM and placed on a poly-HEMA-coated culture dish to prevent attachment of the micromass to the culture plastic. The ECM was allowed to solidify at 37°C, overlaid with medium, and cultured as a floating sphere. B: Phase contrast microscopic appearance of FLSs cultured in three-dimensional matrix. Immediately after plating, the FLSs were round shaped and isolated. After 2 days in culture, the cells demonstrated thin cellular extensions (short arrows) and became more elongated. At day 21, the FLSs had developed a spindle-like cell shape (long arrows depict a spindle-shaped FLS embedded within the matrix). C: Lining layer formation at the interface between the matrix and the fluid phase. After 3 weeks in culture, the three-dimensional structure was fixed with 2% paraformaldehyde and embedded in paraffin, and sections were stained with H&E. Strikingly, at the tissue interface, the FLSs established a lining structure one to three cell layers thick. The lining layer was overlaying a sparsely populated ECM. D: Immunohistochemistry on three-dimensional FLS synovial organ cultures. In Matrigel dispersed FLSs were cultured for 28 days. The tissue-like structure was fixed, embedded in paraffin, and sections were processed for immunohistochemistry using antiserum against cadherin-11 or control serum. Cadherin-11-specific reactivity was detected at the lining layer and in cells of the sublining area.
Cadherin-11 Localizes to Cell-to-Cell Contacts of FLSs Grown in Three-Dimensional Organ Culture
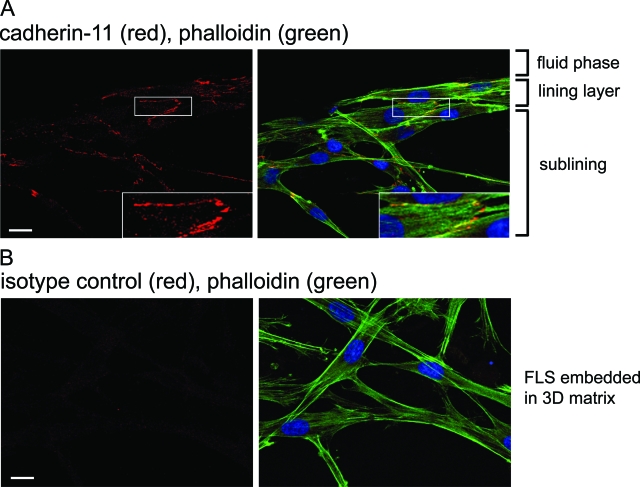
To test whether or not lining layer formation could be observed in a three-dimensional organ culture system that uses a second matrix composition as a scaffold instead of Matrigel, FLSs were dispersed in Vitrogen, a matrix preparation containing mainly collagen type I and a low percentage of collagen type III. Moreover, by use of Vitrogen instead of Matrigel, AJs were more readily accessible to antibody staining, thus allowing analysis of the structure of intercellular junctions of FLSs grown in three-dimensional organ cultures by immunofluorescence and confocal microscopy. We used phalloidin to label polymerized actin and antibodies to cadherin-11 and β-catenin to label AJs. We found that FLSs dispersed in Vitrogen established a lining structure at the interface between the matrix and the fluid phase similar to that seen in Matrigel FLS synovial organ cultures (Figure 8B, left). As determined by immunofluorescence, cellular condensation in the lining layer was associated with numerous cadherin-11- or β-catenin-labeled AJs, staining as a pattern of jagged lines (Figure 6A). Phalloidin staining revealed bundles of actin fibers that were oriented along the longitudinal axis of the cell. These fibers radiated internally from AJs. Beneath the lining layer FLSs were spindle shaped with long cellular extensions that were connected to neighboring cells (Figure 6A). As a control, an isotype antibody to E-cadherin did not label AJs (Figure 6B). These results demonstrate the formation of cadherin-11 mediated AJs that are linked to the actin cytoskeleton in FLSs grown in three-dimensional organ culture.
Figure 8.
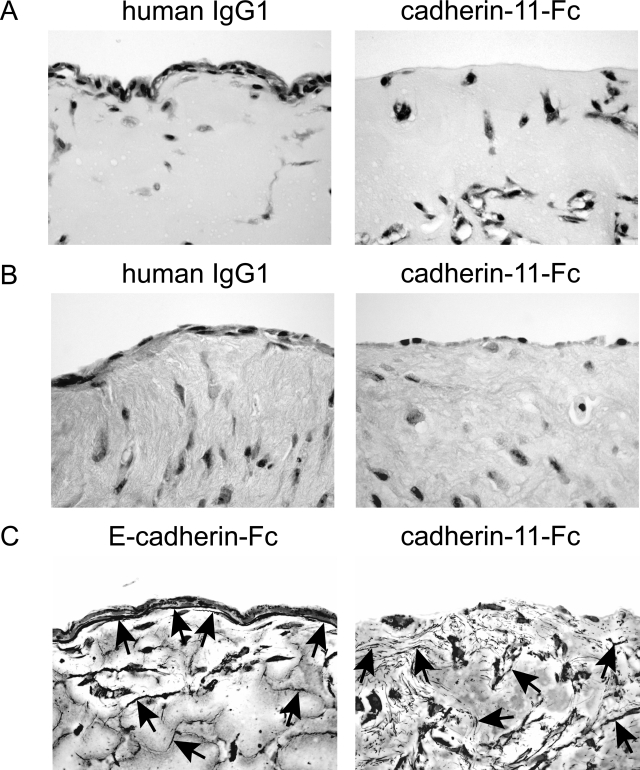
Cadherin-11 determines lining formation and matrix organization in synovial organ cultures. A and B: Cadherin-11-Fc disrupts FLS lining layer formation. FLSs were released from the culture dish and preincubated with cadherin-11-Fc fusion protein, control human IgG1, or E-cadherin-Fc fusion protein (100 μg/ml, respectively) for 15 minutes at 4°C. Cells and proteins were then dispersed in Matrigel (A) or Vitrogen (B), and cultured for 10 to 12 days in the presence of cadherin-11-Fc, human IgG1, or E-cadherin-Fc (100 μg/ml, respectively). FLSs treated with the cadherin-11-Fc fusion protein failed to establish lining architecture, whereas E-cadherin-Fc or IgG1 treatment had no effect on lining layer formation. C: Cadherin-11 determines organization of reticular fibers. Synovial organ cultures treated with either E-cadherin-Fc fusion protein or cadherin-11-Fc fusion protein (see above) were processed for histochemical analysis using the Gomori silver staining technique to label reticular fibers and nuclear fast red to label FLSs. Note orientation of reticular fibers (black linear structures, arrowspointing to examples) along the FLS lining layer at the tissue interface in E-cadherin-Fc-treated three-dimensional FLS organ cultures. In the sublining area, reticular fibers could be detected as well (irregular, black linear structures, often associated with nuclear fast red-labeled FLSs, arrowspointing to examples). By contrast, in cadherin-11-Fc-treated three-dimensional FLS organ cultures a more chaotic pattern of reticular fibers (short black linear structures throughout the tissue, arrowspointing to examples) could be observed. Specifically, a particular orientation of reticular fibers at the tissue interface could not be seen. Original magnifications: ×200 (A, B); ×400 (C).
Figure 6.
Cadherin-11 localization to cell-to-cell junctions. A: FLSs were dispersed in collagen (Vitrogen) and cultured for 5 days. Thereafter, the three-dimensional structure was fixed with 2% paraformaldehyde, permeabilized with 0.2% saponin, and stained with phalloidin (green), the 5H6 antibody to cadherin-11 (red), and DRAQ5 to label nuclei (blue). The FLSs were analyzed by confocal microscopy. Several z-sections were rendered together. Consistent with AJ formation, specific cadherin-11 reactivity was detected at sites of cell-to-cell contact (area in white box is magnified in inset). From AJs, actin fibers radiated internally along the longitudinal axis of the FLS. Note the condensed network of cells forming a lining structure at the interface between the matrix and the fluid phase. B: The isotype control antibody did not detect AJs at sites of cell-to-cell contact. Scale bars, 20 μm.
Lining Layer Formation Is a Characteristic Function of Cells Expressing Cadherin-11
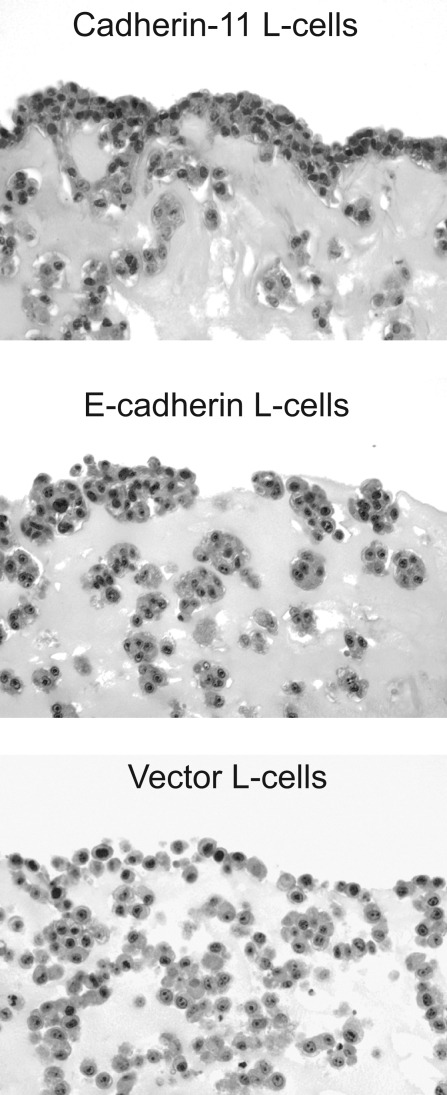
Given the role of cadherin-11 in AJ formation and actin remodeling noted above, we explored whether establishing the lining architecture is due to cadherin-11 expression. For this purpose, we used L-cell fibroblast clones stably expressing cadherin-11 and compared them to control L cells stably expressing E-cadherin or empty vector (no cadherin) (Supplemental Figure 1, see http://ajp.amjpathol.org). L cells are frequently used for expression of transfected cadherins because they lack expression of an endogenous cadherin, but they contain the intracellular catenins needed for proper cadherin function. Strikingly, when cultured in three-dimensional matrices for 7 days, cadherin-11-expressing L cells established a condensed cellular lining layer at the interface between the matrix and the fluid phase (Figure 7). E-cadherin-expressing L cells formed tight aggregates within the matrix but did not condense into a lining at the interface between the matrix and the fluid phase. The vector control L cells formed only loose aggregates without a lining structure. This comparison of otherwise identical L cells, except for the type of cadherin expression, underscores the role of cadherin-11 in lining formation.
Figure 7.
Cadherin-11 expression confers on cells the ability to establish a lining layer at the interface between the matrix and the fluid phase. L-cell transfectants as indicated were dispersed in Matrigel, cultured for 7 days, and processed for histochemistry. H&E-stained sections revealed condensed accumulation of cadherin-11 L cells at the tissue interface and cell aggregates in the sublining area. By contrast, E-cadherin L cells formed tight aggregates but failed to establish a lining structure. Vector control L cells formed loose aggregates without forming a lining layer at the tissue interface. Original magnifications, ×200.
Blockade of Cadherin-11 Abrogates Lining Formation by Human FLSs
We next examined whether lining layer formation in the synovial organ culture could be disrupted by a cadherin-11-Fc fusion protein. The studies above (Figures 3 and 4) showed that cadherin-11-Fc functioned in binding cellular cadherin-11. Thus, we examined its ability to compete with intercellular cadherin-11 binding. Cultured FLSs were released and preincubated with the cadherin-11-Fc fusion protein, human IgG1 (isotype matched to the cadherin-11-Fc fusion protein), or E-cadherin-Fc fusion protein (100 μg/ml, respectively). The cells and proteins were then dispersed in Matrigel or Vitrogen, and the three-dimensional culture was maintained in the presence of cadherin-11-Fc protein, human IgG1, or E-cadherin-Fc protein (100 μg/ml, respectively) for 10 to 16 days. Importantly, cadherin-11-Fc markedly impaired lining formation in the three-dimensional cultures. When the three-dimensional cultures were treated with control IgG1 or E-cadherin-Fc fusion protein, a lining layer at the interface of the three-dimensional culture was still detected (Figure 8, A and B; left). In contrast, when treated with cadherin-11-Fc, the FLSs embedded within the matrix were no longer able to condense into a lining layer at the interface between the matrix and the fluid phase (Figure 8, A and B; right). Together with the experiments using L-cell transfectants, these results emphasize that cadherin-11 function is critical for the ability of cells to establish a lining structure that resembles the in vivo synovial architecture. Moreover, this activity is specific for cadherin-11, because E-cadherin expression did not result in lining layer formation.
Cadherin-11-Orchestrated Lining Formation Determines Organization of Reticular Fibers
A primary function of fibroblasts is the production and organization of the ECM. FLSs are major participants in the continuous process of matrix remodeling by producing matrix components and matrix-degrading enzymes, and by directing fibrillogenesis. In particular, fibroblasts have been implicated in the formation of reticular fibers. Reticular fibers are supramolecular ECM structures characterized by a core of collagen fibrils (collagen type I, collagen type III) ensheathed by proteoglycans.42,43 These fibers provide a three-dimensional framework for the physical integrity of tissues. To address the question whether or not cadherin-11-directed cellular organization impacts organization of reticular fibers, we used the Gomori silver staining technique to label reticular fibers on three-dimensional FLS organ cultures. We find that cadherin-11-directed formation of a lining layer is associated with numerous reticular fibers at the lining layer that are oriented linearly along the condensed network of FLSs at the interface between matrix and the fluid phase in both Matrigel synovial organ cultures (Figure 8C, left) and Vitrogen synovial organ cultures (not shown). Reticular fibers were also found in the sublining area associated with FLSs in an ordered manner. The treatment of such cultures with E-cadherin-Fc had no effect on reticular fiber formation (Figure 8C, left). In contrast, in three-dimensional FLS cultures treated with the cadherin-11-Fc fusion protein, reticular fibers that were oriented along the tissue border were missing (Figure 8C, right). Moreover, although numerous reticular fibers within the matrix were present, they were not organized in any particular orientation. Together, these results indicate that cadherin-11-mediated lining formation determines organization of reticular fibers that are critical for the integrity of the tissue.
Discussion
Previously, the molecular basis for synovial lining architecture was not known. In this study, we provide evidence that cadherin-11 plays a critical role in directing FLS formation of the synovial lining layer. FLSs that were grown in three-dimensional matrices established a lining structure at the interface between the matrix and the fluid phase. Treatment of three-dimensional FLS organ cultures with the cadherin-11-Fc fusion protein inhibited lining layer formation. Because E-cadherin failed to induce a lining layer at the tissue interface, this effect appears to be a distinct characteristic of cadherin-11.
Cadherins have emerged as major morphogenic factors as they function to mechanically integrate cells into tissues.16 Cadherins are expressed in most solid tissues, and their expression is associated with distinct tissue structures, such as epithelial layers.44 During embryonic development, the epithelial (E-) cadherin controls the formation of epithelial structures, and, in adult tissues, it is required for the maintenance of epithelial lining integrity and architecture.45,46 Cadherin-11 is associated with mesenchymal tissues and is expressed in FLSs.34,47 In particular, it is abundantly expressed in FLSs of the synovial lining layer of the normal as well as diseased human synovium. Together with the results presented here, these findings suggest that cadherin-11 functions to integrate FLSs into the synovial lining layer, much as E-cadherin functions to integrate epithelial cells into epithelial layers.
Beyond cell-to-cell adhesion, cadherins are increasingly recognized as central players in a variety of cell functions including cell migration and cell invasion.48–50 By controlling these cellular activities, they are thought to differentially regulate the behavior of the respective cell types in which they are expressed.20 During embryonic development, cadherin-11 expression correlates with a migratory cellular phenotype.51,52 Evidence for cadherin-11 functionally modulating cell mobility derives from studies that demonstrate the requirement of cadherin conversion from E-cadherin to cadherin-11 to allow cells to dissociate from the tightly adherent epithelial layer and to rearrange to form new structures.52 Our results are consistent with the proposal that cadherin-11 influences the migratory behavior of cells differently from E-cadherin. E-cadherin-expressing L cells formed tight aggregates within the three-dimensional matrix but failed to accumulate at the periphery of the micromass. By contrast, cadherin-11 expression enabled cells to accumulate and condensate at the tissue boundary, suggesting increased mobility of cadherin-11-expressing cells when compared to E-cadherin-expressing cells.
In the context of inflammatory arthritis, FLSs participate in profound changes in synovial tissue organization. In particular, rheumatoid synovitis is characterized by the formation of a condensed mass of FLSs that encroaches over and invades the cartilage. It is intriguing that cadherin-11 has been implicated in the regulation of cell mobility by facilitating cellular rearrangements as a means for tissue extension or invasion into adjacent tissues. Therefore, cadherin-11 is most likely involved in a regulatory circuit that determines FLS condensation into an aggressive cell mass and their invasive behavior in rheumatoid synovitis.
Our findings implicate cadherin-11 AJs in actin reorganization and directional polymerization, processes key to stabilizing cell-to-cell contacts and tissue organization. Seeding of FLSs onto cadherin-11-Fc fusion protein-coated coverglasses induced FLS spreading that was accompanied by the generation of F-actin fibers. These F-actin fibers emanated from clusters of AJs as demonstrated by immunofluorescence staining for β-catenin, α-catenin, or p120-catenin (Figure 4). It is interesting to note the mechanistic similarities with AJ formed by E-cadherin in epithelial cells where E-cadherin engagement results in actin cytoskeletal reorganization that allows the formation of a continuous cell layer and seals the epithelial sheet.26,28 Our demonstration of vinculin localization to AJs in FLSs along with actin polymerization induced by cadherin-11 engagement is consistent with a similar function for the cadherin-11-catenin complex in recruiting molecules that drive actin polymerization and reorganization.
Electron microscopy studies on the synovial lining layer indicate significant matrix space between the lining cells and the lack of intercellular junctions such as desmosomes and tight junctions.2 Specifically, thickening of the plasma membrane, condensation of the submembranous cytoplasm at sites of apposing cell membranes, or increased density between two cell membranes that are characteristic ultrastructural features of desmosomes and tight junctions in epithelial cells are not present.53 Within the compacted cell layer, however, these studies revealed cytoplasmic processes closely apposed to adjacent cells with interdigitations in some areas of apposed cell membranes that are consistent with EM characteristics of cadherin-mediated cell-to-cell junctions.16,26 Thus, the synovial lining layer is composed of a condensed network of cells, one to three cells thick, within a specialized ECM.
Our confocal microscopic analyses of FLSs grown in three-dimensional organ culture reveal a lining organization at the interface between the matrix and the fluid phase that is strikingly similar to that seen in the human synovial lining layer. The lining layer found in vitro demonstrates intercellular matrix space and cellular extensions forming cell-to-cell contacts. The presence of cadherin-11 and β-catenin at sites of cell-to-cell contact from which bundles of actin filaments radiate internally along the longitudinal axis of the spindle-shaped FLSs is consistent with a functional role for cadherin-11 in establishing lining architecture (Figure 6). This suggests that cadherin-11-based AJs serve as spatial cues for actin cytoskeletal organization in FLSs. Given the importance of the actin cytoskeleton for cell shape, strength, and stability of cell interconnections, cadherin-11-directed actin cytoskeletal organization provides a molecular mechanism by which cadherin-11 regulates the structural integrity of the synovial lining layer.
Fibroblasts produce ECM components and direct their assembly to form fibrils.11,12,54,55 In particular, reticular fibers closely linked to fibroblasts provide a framework for organ structure and integrity.42,56 Consistent with FLS function in directing matrix organization, our analyses show reticular fibers at the tissue interface that are oriented along the condensed cellular lining layer. This fiber orientation suggests that cadherin-11, in mediating FLS lining formation, also determines organization of reticular fibers. This organization is critical for establishing a combined cellular-ECM scaffold that provides a functional barrier between the fluid phase and the underlying connective tissue. Finally, studies that used cadherin-11-Fc showed that it specifically blocks three-dimensional lining organization.
Thus, using a three-dimensional FLS culture system that faithfully reproduces features of synovial lining organization, we demonstrate the critical role of cadherin-11 in establishing synovial lining-like architecture involving both the organization of the cells of the lining as well as its matrix. Just as E-cadherin is integrally linked to the function of the epithelial lining, we propose that cadherin-11 may similarly be integrally linked to the function of FLSs in health and in conditions such as rheumatoid arthritis.
Supplementary Material
Acknowledgments
We thank Hui-Ya Gilbert, Jean Lai, Terry Bowman, and David Bowman for expert technical assistance; and Drs. Jonathan Higgins and Erika Noss for useful discussions.
Footnotes
Address reprint requests to Michael B. Brenner, M.D., Department of Medicine, Division of Rheumatology, Immunology, and Allergy, Brigham and Women’s Hospital, Harvard Medical School, Smith Building, Room 552, One Jimmy Fund Way, Boston, MA 02115. E-mail: mbrenner@rics.bwh.harvard.edu.
Supported by the National Institutes of Health (grants AR048114 to M.B.B. and K08-AR02214 to D.M.L.) and the Arthritis Foundation (to H.P.K. and D.M.L.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Castor CW. The microscopic structure of normal human synovial tissue. Arthritis Rheum. 1960;3:140–151. doi: 10.1002/art.1780030205. [DOI] [PubMed] [Google Scholar]
- Barland P, Novikoff AB, Hamerman D. Electron microscopy of the human synovial membrane. J Cell Biol. 1962;14:207–220. doi: 10.1083/jcb.14.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson LS, Pitsillides AA, Worrall JG, Edwards JC. Light microscopic characterization of the fibroblast-like synovial intimal cell (synoviocyte). Arthritis Rheum. 1992;35:1179–1184. doi: 10.1002/art.1780351010. [DOI] [PubMed] [Google Scholar]
- Stevens CR, Mapp PI, Revell PA. A monoclonal antibody (Mab 67) marks type B synoviocytes. Rheumatol Int. 1990;10:103–106. doi: 10.1007/BF02274823. [DOI] [PubMed] [Google Scholar]
- Lever JD, Ford EHR. Histological, histochemical and electron microscopic observations on synovial membrane. Anat Rec. 1958;132:525–539. doi: 10.1002/ar.1091320402. [DOI] [PubMed] [Google Scholar]
- Levick JR, McDonald JN. Fluid movement across synovium in healthy joints: role of synovial fluid macromolecules. Ann Rheum Dis. 1995;54:417–423. doi: 10.1136/ard.54.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yielding KL, Tomkins GM, Bunim JJ. Synthesis of hyaluronic acid by human synovial tissue slices. Science. 1957;125:1300. doi: 10.1126/science.125.3261.1300. [DOI] [PubMed] [Google Scholar]
- Jay GD, Britt DE, Cha CJ. Lubricin is a product of megakaryocyte stimulating factor gene expression by human synovial fibroblasts. J Rheumatol. 2000;27:594–600. [PubMed] [Google Scholar]
- Swann DA, Silver FH, Slayter HS, Stafford W, Shore E. The molecular structure and lubricating activity of lubricin isolated from bovine and human synovial fluids. Biochem J. 1985;225:195–201. doi: 10.1042/bj2250195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh JM, Wiebkin OW, Gale S, Muir H, Maini RN. Synthesis of sulphated proteoglycans by rheumatoid and normal synovial tissue in culture. Ann Rheum Dis. 1979;38:166–170. doi: 10.1136/ard.38.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapp PI, Revell PA. Fibronectin production by synovial intimal cells. Rheumatol Int. 1985;5:229–237. doi: 10.1007/BF00541341. [DOI] [PubMed] [Google Scholar]
- Schneider M, Voss B, Rauterberg J, Menke M, Pauly T, Miehlke RK, Friemann J, Gerlach U. Basement membrane proteins in synovial membrane: distribution in rheumatoid arthritis and synthesis by fibroblast-like cells. Clin Rheumatol. 1994;13:90–97. doi: 10.1007/BF02229873. [DOI] [PubMed] [Google Scholar]
- Unemori EN, Hibbs MS, Amento EP. Constitutive expression of a 92-kD gelatinase (type V collagenase) by rheumatoid synovial fibroblasts and its induction in normal human fibroblasts by inflammatory cytokines. J Clin Invest. 1991;88:1656–1662. doi: 10.1172/JCI115480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Boyle DL, Chang L, Bennett B, Karin M, Yang L, Manning AM, Firestein GS. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J Clin Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Ladner U, Gay S. MMPs and rheumatoid synovial fibroblasts: Siamese twins in joint destruction? Ann Rheum Dis. 2002;61:957–959. doi: 10.1136/ard.61.11.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- Yagi T, Takeichi M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 2000;14:1169–1180. [PubMed] [Google Scholar]
- Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grubel G, Legrand JF, Als-Nielsen J, Colman DR, Hendrickson WA. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380:360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Adams CL, Nelson WJ, Smith SJ. Quantitative analysis of cadherin-catenin-actin reorganization during development of cell-cell adhesion. J Cell Biol. 1996;135:1899–1911. doi: 10.1083/jcb.135.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120cas associates with E-cadherin complexes. Mol Cell Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Niessen CM, Gumbiner BM. The juxtamembrane region of the cadherin cytoplasmic tail supports lateral clustering, adhesive strengthening, and interaction with p120ctn. J Cell Biol. 1998;141:779–789. doi: 10.1083/jcb.141.3.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak A, Pasolli HA, Fuchs E. Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat Cell Biol. 2004;6:21–30. doi: 10.1038/ncb1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CL, Nelson WJ. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr Opin Cell Biol. 1998;10:572–577. doi: 10.1016/s0955-0674(98)80031-8. [DOI] [PubMed] [Google Scholar]
- Brieher WM, Gumbiner BM. Regulation of C-cadherin function during activin induced morphogenesis of Xenopus animal caps. J Cell Biol. 1994;126:519–527. doi: 10.1083/jcb.126.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Gumbiner BM. Disruption of gastrulation movements in Xenopus by a dominant-negative mutant for C-cadherin. Dev Biol. 1995;171:363–373. doi: 10.1006/dbio.1995.1288. [DOI] [PubMed] [Google Scholar]
- Uemura T, Oda H, Kraut R, Hayashi S, Kotaoka Y, Takeichi M. Zygotic Drosophila E-cadherin expression is required for processes of dynamic epithelial cell rearrangement in the Drosophila embryo. Genes Dev. 1996;10:659–671. doi: 10.1101/gad.10.6.659. [DOI] [PubMed] [Google Scholar]
- Niewiadomska P, Godt D, Tepass U. DE-Cadherin is required for intercellular motility during Drosophila oogenesis. J Cell Biol. 1999;144:533–547. doi: 10.1083/jcb.144.3.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia X, Higgins JM, Kiener HP, Lee DM, Podrebarac TA, Dascher CC, Watts GF, Mizoguchi E, Simmons B, Patel DD, Bhan AK, Brenner MB. Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes. J Exp Med. 2004;200:1673–1679. doi: 10.1084/jem.20041545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang XZ, Lang BJ, Issekutz AC. Adhesion molecule mechanisms mediating monocyte migration through synovial fibroblast and endothelium barriers: role for CD11/CD18, very late antigen-4 (CD49d/CD29), very late antigen-5 (CD49e/CD29), and vascular cell adhesion molecule-1 (CD106). J Immunol. 1998;160:467–474. [PubMed] [Google Scholar]
- Higgins JM, Cernadas M, Tan K, Irie A, Wang J, Takada Y, Brenner MB. The role of alpha and beta chains in ligand recognition by beta 7 integrins. J Biol Chem. 2000;275:25652–25664. doi: 10.1074/jbc.M001228200. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M, Matsuyoshi N, Ohnishi Y, Gotoh B, Takeichi M, Nagai Y. p60v-src causes tyrosine phosphorylation and inactivation of the N-cadherin-catenin cell adhesion system. EMBO J. 1993;12:307–314. doi: 10.1002/j.1460-2075.1993.tb05658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypta RM, Su H, Reichardt LF. Association between a transmembrane protein tyrosine phosphatase and the cadherin-catenin complex. J Cell Biol. 1996;134:1519–1529. doi: 10.1083/jcb.134.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiadis PZ, Reynolds AB. The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Sci. 2000;113:1319–1334. doi: 10.1242/jcs.113.8.1319. [DOI] [PubMed] [Google Scholar]
- DeMali KA, Barlow CA, Burridge K. Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J Cell Biol. 2002;159:881–891. doi: 10.1083/jcb.200206043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazan RB, Kang L, Roe S, Borgen PI, Rimm DL. Vinculin is associated with the E-cadherin adhesion complex. J Biol Chem. 1997;272:32448–32453. doi: 10.1074/jbc.272.51.32448. [DOI] [PubMed] [Google Scholar]
- Ushiki T. Collagen fibers, reticular fibers and elastic fibers. A comprehensive understanding from a morphological viewpoint. Arch Histol Cytol. 2002;65:109–126. doi: 10.1679/aohc.65.109. [DOI] [PubMed] [Google Scholar]
- Montes GS, Krisztan RM, Shigihara KM, Tokoro R, Mourao PA, Junqueira LC. Histochemical and morphological characterization of reticular fibers. Histochemistry. 1980;65:131–141. doi: 10.1007/BF00493161. [DOI] [PubMed] [Google Scholar]
- Boller K, Vestweber D, Kemler R. Cell-adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells. J Cell Biol. 1985;100:327–332. doi: 10.1083/jcb.100.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermiston ML, Gordon JI. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J Cell Biol. 1995;129:489–506. doi: 10.1083/jcb.129.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinkle CL, Lechler T, Pasolli HA, Fuchs E. Conditional targeting of E-cadherin in skin: insights into hyperproliferative and degenerative responses. Proc Natl Acad Sci USA. 2004;101:552–557. doi: 10.1073/pnas.0307437100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura Y, Matsunami H, Inoue T, Shimamura K, Uchida N, Ueno T, Miyazaki T, Takeichi M. Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Dev Biol. 1995;169:347–358. doi: 10.1006/dbio.1995.1149. [DOI] [PubMed] [Google Scholar]
- Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–193. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- Pishvaian MJ, Feltes CM, Thompson P, Bussemakers MJ, Schalken JA, Byers SW. Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res. 1999;59:947–952. [PubMed] [Google Scholar]
- Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147:631–644. doi: 10.1083/jcb.147.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers A, David R, Wedlich D. Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development. 2001;128:3049–3060. doi: 10.1242/dev.128.16.3049. [DOI] [PubMed] [Google Scholar]
- Locascio A, Nieto MA. Cell movements during vertebrate development: integrated tissue behaviour versus individual cell migration. Curr Opin Genet Dev. 2001;11:464–469. doi: 10.1016/s0959-437x(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- Wolf J, Carsons SE. Distribution of type VI collagen expression in synovial tissue and cultured synoviocytes: relation to fibronectin expression. Ann Rheum Dis. 1991;50:493–496. doi: 10.1136/ard.50.7.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danen EH, Sonneveld P, Brakebusch C, Fassler R, Sonnenberg A. The fibronectin-binding integrins alpha5beta1 and alphavbeta3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J Cell Biol. 2002;159:1071–1086. doi: 10.1083/jcb.200205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa M, Kobayashi M, Hoshino T. Microfibrils: a constitutive component of reticular fibers in the mouse lymph node. Cell Tissue Res. 1990;262:199–201. doi: 10.1007/BF00327763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.