The Tumor Microenvironment (TME) Study Section is one of the new study sections created as a result of a reorganization by the Oncological Sciences Integrated Review Group. The TME Study Section reviews grants focusing on basic mechanisms of bidirectional cancer cell and host interaction, including interactions between cancer-cell-secreted molecules; soluble and insoluble factors; and host immune, inflammatory, stromal, vascular, neural, and stem cells. The TME Study Section also reviews proposals on cancer cell and host organ interaction involving cell-cell, cell-growth factor, cell-extracellular matrix, and cell-adhesion/junctional communications with cancer cells and the progression of cancer cells to local invasion and distant metastasis (http://cms.csr.nih.gov/PeerReviewMeetings/CSRIRGDescription/ONCIRG/TME.htm). We present here a summary of the first TME Study Section Scientific Workshop held in Washington, DC, on February 27, 2005. This workshop focused primarily on recent developments in the study of cancer cell interaction with the bone microenvironment.
Vascular-Bone Interaction (Presenter, Dwight Towler)
Bone cannot be formed in vivo without adequate interaction with a surrounding vascular system. Recent studies in developmental biology, molecular genetics, and clinical pharmacology have highlighted the crucial role of the vasculature in providing 1) a sustentacular niche and source of adult mesenchymal stem cells, including osteoprogenitors; 2) an organizational structural and rate-limiting “point-of-reference” for bone growth, remodeling, and fracture repair; and 3) a conduit for the calcium, phosphate, hematopoietic, and nutrient supply necessary for matrix synthesis, mineralization, and calcium mobilization.1 During fracture repair, the paracrine endothelial-mesenchymal signaling interactions that are recruited are reminiscent of the epithelial-mesenchymal interactions controlling bone morphogenesis during development.
Angiogenic agents such as vascular endothelial growth factor (VEGF) synergize with bone morphogens to enhance mineralization and osteogenic differentiation in vivo and in vitro.1 Reciprocal endothelial-mesenchymal interactions between VEGF-producing osteogenic mesenchymal cells and bone morphogenetic protein-secreting endothelial cells control bone formation during fracture repair.1 Virtually all bone-forming agents are VEGF “secretagogues,” indicating that this is a central feature of bone formation. Microvascular smooth muscle cells from multiple venues exhibit osteogenic potential. The bone marrow stromal cell can be considered a tissue-specific pericyte because 1) it is juxtaposed in the anatomical venue between fenestrated endothelium and active bone-forming osteoblasts, and 2) it shares pluripotent osteogenic potential and several characteristic phenotypic markers (Stro-1, 3G5, VSMC and α-actin) with other microvascular smooth muscle cells (eg, retinal pericytes).2
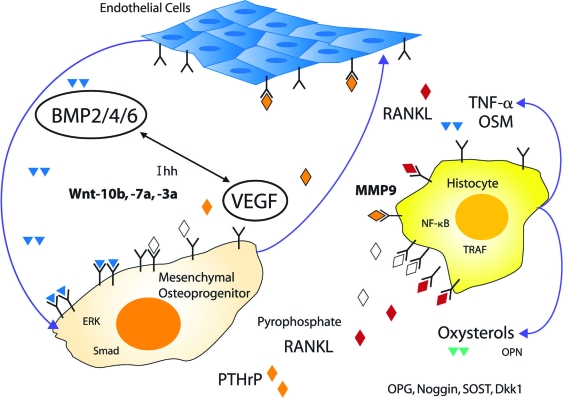
Detailed studies of orthotopic and heterotopic calcification have identified paracrine canonical wingless/int signaling as crucial to the osteogenic lineage allocation of vascular progenitors, modulated by regional signals provided by endothelial cells and cells of the monocytic/macrophage lineage.3 Thus, a useful working model emerges in which paracrine endothelial-mesenchymal-histiocyte interactions functionally define the osteogenic unit (Figure 1). This tripartite interaction is entrained to the morphogenetic, metabolic, mechanical, inflammatory, and endocrine demands placed on actively mineralizing tissues, whether it be orthotopic bone formation, valvular or vascular calcification, or calcified granuloma formation. Of note, age- and disease-specific strategies will be required to safely promote bone formation in individuals with underlying vasculopathies such as those associated with diabetes or renal failure that impair bone formation and fracture repair, extant osteoporosis that has removed trabecular templates for bone apposition, osteoporosis in the setting of childhood growth and open epiphyses, underlying malignancy, and the drug- or coagulopathy-related disorders that cause avascular necrosis. A fundamental understanding of the molecular mechanisms whereby vascular biology contributes to bone formation will provide insights useful for devising novel therapeutic strategies to address these unmet clinical needs.
Figure 1.
The osteogenic unit can be defined by paracrine endothelial-mesenchymal-histiocyte interactions. During postnatal life, osteogenic matrix calcification—-observed in the settings of trabecular bone remodeling, fracture repair, and atherosclerotic vascular calcification—requires the coordinated activities of endothelial cells, mesenchymal osteoprogenitors (eg, bone marrow stromal cells or pericytes), and tissue histiocytes derived from the monocyte-macrophage lineage. Paracrine endothelial-mesenchymal interactions activated by mechanical stimulation, injury, and inflammation recapitulate the epithelial-mesenchymal morphogenetic interactions that control bone and tooth formation during prenatal skeletal development. BMP, bone morphogenetic protein; Dkk, dickkopf; Ihh, Indian hedgehog; OPN, osteopontin; OSM, oncostatin M; PTHrP, parathyroid hormone-related protein; SOST, sclerostin; TNF, tumor necrosis factor; Wnt, wingless/int family member. Illustration by Jan Hurst.
Hematopoietic and Vascular Stem Cell Niches (Presenter, Shahin Rafii)
The hematopoietic and vascular stem cell niches support stem cells in their self-renewal, proliferation, differentiation, and mobilization to the circulation. Dr. Rafii provided evidence that most stem cells reside in the vicinity of the osteoblastic niche of the marrow. Stromal cells within the osteoblastic niche support the survival of stem cells. Physiological stress, such as the angiogenic switch induced by tumor growth, tissue ischemia, or marrow suppression, promote recruitment of stem cells from the osteoblastic niche to the “vascular niche” of the bone marrow.4–6 The vascular niche provides a conduit for the mobilization of stem and progenitor cells to the circulation and also establishes a cellular platform for the differentiation of stem cells.
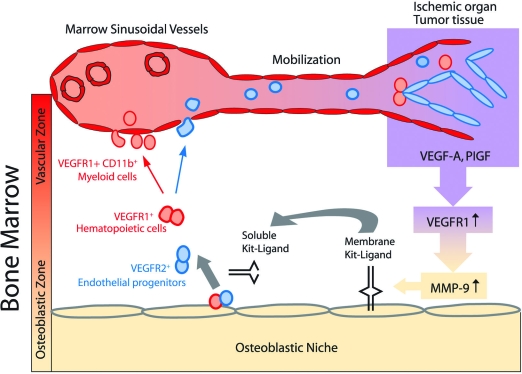
One pathway involved in recruiting stem cells is driven by activation of matrix metalloproteinase-9 (MMP-9). MMP-9 activation results in release of soluble kit-ligand from membrane kit-ligand.6 This results in increased motility of hematopoietic cells and their migration toward the marrow’s vascular niche (Figure 2). Subsequently, other stem and progenitor chemokines, including stromal derived factor-1 (SDF-1) and fibroblast growth factor 4 (FGF-4), up-regulate expression of adhesion molecules and localize stem cells to the vascular niche.
Figure 2.
Mobilization of stem cells is dependent on MMP-9-mediated release of soluble kit-ligand. Tumor growth or ischemic injury results in the release of angiogenic factors, including VEGF-A and PlGF. Activation of VEGFR1 results in MMP-9-mediated release of bio-available soluble Kit-ligand (sKitL). Increase in the level of sKitL enhances the cycling of VEGFR1+ hematopoietic and VEGFR2+ endothelial stem and progenitor cells, thereby permitting mobilization to the circulation where they contribute to neo-angiogenesis. Illustration by Jan Hurst.
To dissect the mechanism by which stem and progenitor cells are recruited into the marrow vascular niche, Dr. Rafii’s laboratory used thrombopoietin knock-out mice. Remarkably, SDF-1 and FGF-4 supported cytokine-independent localization of progenitors to the marrow vascular niche, promoting differentiation of progenitors into megakaryocytes and restoring platelet production. SDF-1 and FGF-4 induced up-regulation of adhesion molecules, including VLA4/VCAM1, facilitating localization to the vascular niche. Dr. Rafii concluded that chemokines support rapid reconstitution through localization of the stem cells to the vascular niche.
The cellular pathways involved in maintenance of the vascular niche of the marrow are unknown. VEGF-A, through interaction with its receptors VEGFR1 and VEGFR2, conveys signals that support the assembly of marrow neo-vessels. Angiopoietins, through activation of Tie2, support remodeling of neo-vessels, and marrow suppression results in the up-regulation of Tie2 expression in the regenerating neo-vessels.7
Similar to regeneration of hematopoietic cells, leukemic and myeloma cells may also take advantage of pro-survival signals conveyed by the osteoblastic and vascular niches to support their proliferation and invasive potential. There is an increase in neo-vessel density in the marrow of patients with leukemia and multiple myeloma. These data suggest that cross-talk between leukemic and myeloma cells and the activated vascular niche contribute to disease progression. Dr. Rafii and others have shown that inhibition of angiogenesis effectively blocks the progression of subsets of leukemias and myelomas. Clinical trials have been initiated to evaluate the use of anti-angiogenic agents in combination with standard chemotherapy to treat leukemias and multiple myeloma. However, whether the bone marrow vascular niche also provides a permissive microenvironment for the homing, engraftment, and growth of solid tumors is not known and will be closely scrutinized.
Host Stromal Reaction to Cancer (Presenter, David Rowley)
The stromal reaction is characterized by a change in the stromal cell phenotype to an activated myofibroblast/fibroblast population.8,9 These cells exhibit elevated synthesis of pro-collagen I, tenascin, and fibroblast activation protein. In human prostate tissue, reactive stroma originates immediately adjacent to premalignant prostatic intraepithelial neoplasia (PIN) foci. Many PIN foci also overexpress transforming growth factor (TGF)-β1. In addition to stimulating stromal cell wound repair responses, TGF-β1 also stimulates angiogenesis by regulating endothelial-pericyte interactions, differentiation of pericytes, and vessel stability. Moreover, TGF-β1 regulates expression of stromal growth factors important to wound repair and angiogenesis, including connective tissue growth factor (CTGF) and FGF-2. Reactive stroma in PIN and cancer is associated with elevated microvessel density and FGF-2 expression. TGF-β1 and downstream regulators appear critical for reactive stroma biology and angiogenesis in the premalignant PIN micro-environment.
Dr. Rowley hypothesized that epithelial overexpression of TGF-β1 in PIN induces a reactive stroma that co-evolves with epithelial cancer foci. He and his colleagues have developed the differential reactive stroma (DRS) xenograft model to address the role of reactive stroma, TGF-β1, CTGF, and FGF-2 in prostate cancer.10–12 Differential tumorigenesis and angiogenesis were observed using different human prostate stromal cell lines co-inoculated with LNCaP cancer cells in DRS model xenografts. Inoculated stromal cells were also localized in the vessel wall in a pericyte position, suggesting a direct role in angiogenesis. Gene expression analysis showed that several TGF-β1-regulated genes (including CTGF) were expressed in stromal cells capable of supporting tumors. Neutralization of TGF-β1 in DRS tumors inhibited tu-morigenesis with a significant (3.5-fold) reduction in microvessel density.11 Similarly, preliminary studies showed that DRS LNCaP tumors constructed with TGF-β receptor II-null or Smad3-dominant-negative prostate stromal cell lines exhibited decreased tumorigenesis and angiogenesis. To address the role of CTGF, FGF-2, and other TGF-β1-regulated genes, DRS tumors were constructed with stromal cells engineered to overexpress genes of choice, either in a regulated manner (Gene-Switch) with mifepristone (RU-486) or by retroviral infection. Prostate stromal cell lines engineered to overexpress CTGF showed elevated tumorigenesis and stimulation of angiogenesis during early LNCaP DRS tumor formation.12 Preliminary studies showed that overexpression of FGF-2 in prostate stromal cells resulted in differential tumor growth. In addition, the overexpression or inclusion of recombinant ps20 (an additional TGF-β1-regulated gene cloned by Dr. Rowley’s laboratory) in stromal cells also resulted in increased tumorigenesis and angiogenesis.13
These studies suggest that a principal function of carcinoma-expressed TGF-β1 in early prostate cancer is to induce a cascade of downstream factors, including CTGF and FGF-2, leading to a wound repair type of reactive stroma response and induction of angiogenesis in the immediate tumor microenvironment. Moreover, elevated TGF-β1 and FGF-2 in the reactive stroma microenvironment may promote epithelial to mesenchymal transition in adjacent carcinoma cells leading to tumor progression. Ongoing studies focus on dissecting key downstream mechanisms and co-mediators of TGF-β1, CTGF, and FGF-2 action in regulating reactive stroma and carcinoma cell biology in cancer progression.
Gap Junctions and Bone Metastasis (Presenter, Henry Donahue)
Gap junctional intercellular communication (GJIC) may contribute to breast cancer cell metastasis to bone. Gap junctions are membrane-spanning channels that allow passage of signaling molecules (<1 kd) from one cell to another. Each gap junction is composed of two hexameric hemichannels, termed connexons, that in turn are composed of 6 subunits termed connexins, at least 20 of which have been identified in mammals.
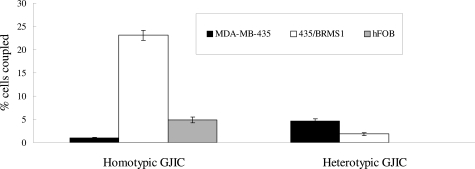
Dr. Donahue’s studies suggest that the highly metastatic breast cancer cell line MBA-MD 435 (435) does not express connexin 43 (Cx43) but does express Cx32, whereas normal breast epithelial cells express Cx43 but not Cx32.14 Additionally, 435 cells express the osteoblastic markers osteopontin, osteocalcin, and core binding factor A-1 (Cbfa-1), whereas normal breast epithelial cells do not. Furthermore, 435 cells expressing the metastasis-suppressing gene BRMS-1 (435/BRMS-1) display increased Cx43 and decreased Cx32 and osteopontin expression. 435 cells also display less homotypic GJIC communication with themselves than do 435/BRMS-1.15 On the other hand, 435 cells display more heterotypic GJIC with osteoblastic cells than do 435 cells expressing BRMS-1 (Figure 3). This suggests that the connexin expression profile and GJIC are intimately linked to breast cancer cell expression of osteoblastic genes and metastatic potential. This concept is supported by studies demonstrating that forced expression of Cx43 in 435 cells reduces Cx32, osteopontin, and Cbfa-1 expression and, more importantly, reduces the ability of 435 cells to metastasize in vivo. Thus, expressing Cx43 in 435 cells that normally expresses Cx32 but not Cx43 reduces their metastatic potential. Inhibiting expression of Cx32 in breast cancer cells reduces expression of Cbfa-1, a key osteoblast-specific transcription factor, and osteocalcin, a bone-specific extracellular matrix protein. Furthermore, invasion and migration were inhibited by decreasing the expression of Cx32. On the other hand, Cx43 and osteopontin mRNA levels were not affected by inhibiting the expression of Cx32 in breast cancer cells. Thus, both Cx43 and Cx32 expression appear linked to Cbfa-1 and osteocalcin, whereas only Cx43 appears linked to osteopontin.
Figure 3.
GJIC in breast cancer cells. Homotypic GJIC between breast cancer cells (left three bars) or heterotypic GJIC between breast cancer cells and osteoblastic hFOB cells was assessed by FACS analysis as described by Kapoor et al.15 Black bars represent homotypic communication between 435 cells or heterotypic communication between 453 cells and osteoblastic hFOB cells. White bars represent homotypic communication between 435/BRMS-1 or heterotypic communication between 435/BRMS-1 cells and osteoblastic hFOB cells. Gray bars represent homotypic communication between hFOB cells and are included as a positive control.
These results suggest that altering either Cx43 or Cx32 expression in breast cancer cells, so that levels are more similar to those in normal breast epithelial cells, reduces the expression of bone-specific genes and also diminishes migration and invasion of and, thereby, the metastatic potential of breast cancer cells. Additionally, inhibition of osteopontin expression in breast cancer cells decreases the ability of these cells to adhere to both osteoblastic and endothelial cells. This suggests that osteopontin contributes to metastatic potential by increasing breast cancer cell adhesion to endothelial and osteoblastic cells. The finding that inhibiting osteopontin expression did not restore Cx43 expression in 435 cells, together with previous studies demonstrating that restoring Cx43 expression decreases osteopontin expression, suggests that alterations in osteopontin expression with subsequent effects on metastatic potential are downstream of alterations of Cx43 expression. The findings that Cx32 expression was not consistently affected by inhibiting osteopontin expression but that breast cancer cell adhesion to osteoblastic cell and endothelial cells as well as breast cancer cell migration and invasion into matrix are affected by inhibiting osteopontin expression suggest that Cx32 is less strongly related to osteopontin expression and metastatic potential than Cx43 expression.
Proteases and Bone Metastasis (Presenter, Michael Cher)
Inhibition of MMP activity reduces skeletal tumor burden and bone degradation in animal models of metastasis.16,17 The findings from these types of preclinical studies suggest that protease inhibition should be revisited on the clinical level. One issue that needs to be resolved is determining the relative contribution of individual cell types and particular proteases to the process of bone metastasis. To gain further insight into this issue, Dr. Cher used specific assays to measure net tissue enzymatic activities of individual MMPs during colonization of bone by prostate cancer cells.18 These assays were considered clinically relevant in that net tissue enzymatic activity is the most appropriate parameter for assessing the potential utility of systemic pharmacological targeting with protease inhibitors. PC3 cells were injected into the marrow of human fetal femurs previously implanted in SCID mice. MMP-9 protein was found in both tumor cells and osteoclasts, with net MMP-9 activity in bone tissues peaking 2 weeks after tumor cell injection, coinciding with a wave of osteoclast recruitment. In contrast, MMP-2 and MT1-MMP activity did not change. siRNA targeting of MMP-9 expression in osteoclasts demonstrated that tumor-induced osteoclast motility was dependent on MMP-9 expression. Thus, osteoclast-derived MMP-9 may represent a potential therapeutic target in bone metastasis.
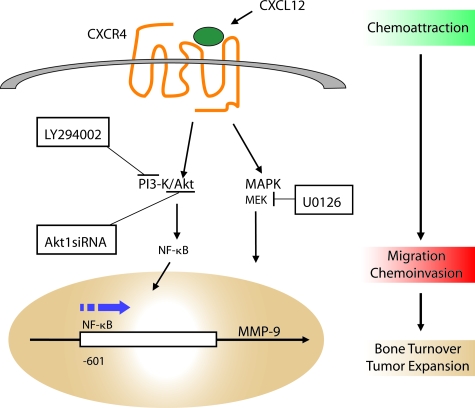
Tumor-associated MMPs may also contribute to bone metastasis. Because MMP-9 protein was found in tumor cells populating the marrow, Dr. Cher’s group further examined microenvironmental factors that induced MMP-9 production by tumor cells.19 The group hypothesized that the bone-derived chemokine CXCL12 interacts with the CXCR4 receptor on cancer cells and that this ligand-receptor event facilitates cancer cell invasion of bone by activating intracellular signaling pathways leading to the expression and release of MMP-9. Expression of the CXCR4 receptor by PC cells was shown in vitro as well as in bone in vivo. CXCL12 was expressed by many stromal cell types, especially bone stromal cells. Exogenous CXCL12 induced MMP-9 gene expression and protein secretion by PC cells. This pathway was found to be crucial for cancer cell migration because anti-CXCR4 blocking antibodies inhibited migration induced by bone stromal cells and bone tissue-conditioned media. Pharmacological inhibitors of cell signaling pathways were used to show that the phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways were activated on CXCL12-stimulated migration and invasion of PC cells. Finally, exogenous CXCL12 was shown to induce Akt1 phosphorylation and activation of the nuclear factor-κB transcription factor, and Akt1 depletion by siRNA transfection abrogated CXCL12-induced Akt phosphorylation, proMMP-9 secretion, and migration and invasion of PC cells. These data suggest that cell signaling induced by binding of the chemokine to its receptor leads to the activation of multiple signaling pathways and subsequent secretion of MMP-9 into the local environment. Together, these findings may provide a link between chemoattractive mechanisms, invasion of tumor cells in bone, and tumor-enhanced bone matrix turnover (Figure 4).
Figure 4.
Proposed link between chemoattraction of cancer cells into the bone marrow and subsequent tumor-associated bone remodeling. Binding of the chemokine CXCL12 to its receptor CXCR4 activates signaling pathways that ultimately lead to expression of proteases by cancer cells. Illustration by Jan Hurst.
Osteoclasts and Bone Metastasis (Presenter, Evan Keller)
Metastasis of prostate cancer to bone is a common complication of progressive prostate cancer. Skeletal metastases are often associated with severe pain and demand therapeutic intervention. Although often characterized as osteoblastic, prostate cancer skeletal metastases usually have an underlying osteoclastic component.20 Advances in osteoclast biology and pathophysiology have helped define putative therapeutic targets to attack tumor-induced osteolysis. Several factors are important for tumor-induced promotion of osteoclast activity. One key factor is the protein receptor activator of nuclear factor-κB ligand (RANKL), which induces osteoclastogenesis when it binds to its receptor RANK, which is expressed on the cell surface of the osteoclast precursor.21 RANKL is produced by prostate cancer bone metastases, enabling these metastases to induce osteolysis through osteoclast activation (Figure 5). Another endogenous factor, osteoprotegerin (OPG), is a soluble decoy receptor for RANKL that inhibits RANKL-induced osteoclastogenesis.22 OPG has been shown to inhibit tumor-induced osteolysis in murine models.23 Furthermore, inhibiting RANKL activity with a recombinant soluble form of RANK was shown to inhibit progression of prostate cancer growth in bone.24 In addition to RANKL, parathyroid hormone-related protein and interleukin-6 are produced by prostate cancer cells and can promote osteoclastogenesis. Finally, MMPs, which are secreted by prostate cancer cells, promote osteolysis primarily through degradation of the nonmineralized bone matrix. MMP inhibitors have been shown to diminish tumor establishment in bone in murine models.16 Thus, many factors derived from prostate cancer metastases can promote osteolysis, and these factors may be therapeutic targets.
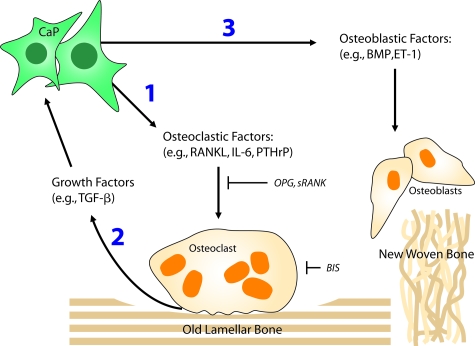
Figure 5.
Model for how prostate cancer induces bone remodeling and targets of cancer-induced osteoclastic activity. The prostate cancer cells initially1 induce osteoclastogenesis and resorption of mature lamellar bone. As the bone matrix is destroyed, it releases growth factors2 that induce growth of prostate cancer cells and alter their phenotype. The changing bone microenvironment enhances the production of osteoblastic factors by prostate cancer cells, resulting in production of immature woven bone that is weaker than lamellar bone. Targeting RANKL with molecules such as OPG or sRANK can inhibit osteoclastogenesis, whereas targeting osteoclasts with bisphosphonates can induce osteoclast apoptosis. BIS, bisphosphonate; BMP, bone morphogenetic protein; CaP, prostate cancer cell; ET-1, endothelin-1; IL-6, interleukin-6; PTHrP, parathyroid hormone-related peptide; sRANK, soluble RANK. Illustration by Jan Hurst.
The importance of osteoclasts in the establishment and progression of skeletal metastases has led to clinical evaluation of therapeutic agents targeting this critical cell type. Bisphosphonates are a class of compounds that decrease the osteoclast lifespan by promoting apoptosis. The bisphosphonate pamidronate has proven clinical efficacy for relief of bone pain associated with breast cancer metastases and shows similar promise for prostate cancer metastases.25 Another bisphosphonate, zoledronic acid, appears to target prostate cancer cells directly in addition to diminishing osteoclast activity at the metastatic site.26 In addition to bisphosphonates, other novel therapies based on studies delineating the mechanisms of skeletal metastases will certainly be developed in the near future.
Osteomimicry (Presenter, Leland Chung)
Osteomimicry is the ability of cancer cells to express highly restricted bone proteins (such as osteocalcin, osteopontin, bone sialoprotein, osteonectin, and RANKL CSF) that may support cancer cell growth and survival in the skeleton.1 Osteomimicry may be observed in cancer cells still residing in the primary tumor. This ability to mimic bone cells intensifies as cancer cells acquire increased malignancy potential in bone and visceral organs. In the lethal phenotype, prostate cancer cells metastasize to the skeleton and soft tissues and ultimately acquire androgen independence. Potential curative therapies for bone metastasis depend on understanding the biology of prostate cancer progression before rational targets can be developed and validated.
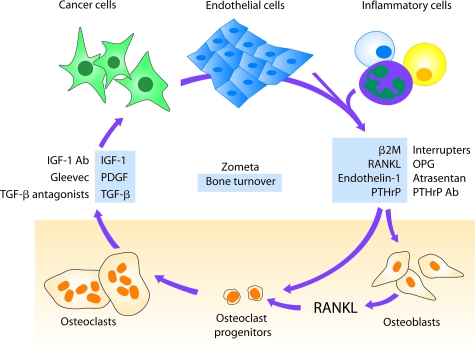
Dr. Chung and colleagues27 developed an LNCaP prostate cancer progression model and elucidated the molecular switch defining osteomimicry in prostate cancer cells. They found that prostate cancer cells have the ability to mimic osteoblasts by expressing osteocalcin, osteopontin, and bone sialoprotein. This was due exclusively to the ability of prostate cancer cells to respond to both autocrine and paracrine mediators released by cancer cells and host cells in their microenvironment. Cyclic AMP is a potent activator of the protein kinase A signaling pathway, which controls osteomimicry of prostate cancer cells.28 A known soluble factor, β2 microglobulin (β2M), secreted by prostate cancer, bone, and inflammatory cells, assumes a key regulatory role in stimulating prostate cancer growth in bone. By overexpressing β2M in human prostate cancer cells, Dr. Chung’s group observed increased angiogenesis and explosive growth of prostate cancer in bone. These results led to the conclusion that β2M is not only a key factor in controlling the presentation of major histocompatibility class I antigen on the cell surface of prostate cancer cells but is also a potent growth regulatory factor and a key signaling molecule mediating the vicious cycle between prostate cancer and bone cells (Figure 6). By understanding intercellular communications between prostate cancer and bone cells, new therapeutic drugs such as OPG, atrasentan, and growth factor receptor antibodies can be developed. Further therapeutic targeting of β2M-mediated growth factor and cell signaling activities may prevent prostate cancer growth and metastasis to bone.
Figure 6.
A proposed vicious cycle between prostate cancer and bone cells in which soluble factors serve as key mediators communicating among cancer cells, host endothelial cells, inflammatory cells, and bone cells. Understanding the key regulatory factors and their mechanisms of action could help the development of new therapeutic approaches (in blue) for the treatment of human prostate cancer and the protection of the normal host bone in patients with prostate cancer metastasis. Illustration by Jan Hurst.
Summary
In this workshop, members of the TME Study Section presented concepts relating to basic bone biology, tumor-stromal interaction, and biology of the bone microenvironment as it relates to the growing metastatic deposit. Recently, it has become clear that several defined vascular elements of the bone marrow are critical with regard to the processes of bone development, organization of the bone microenvironment, and the multifaceted response to perturbations such as fractures or the arrival of metastatic tumor cells. In fact, recent work has identified and even localized various types of stem cells residing in different microenvironmental niches. The stromal response to metastatic cancer cells, in the primary tumor as well as at the metastastic site, is beginning to be defined at the cellular and molecular level. Tumor-associated proteases, growth factors, chemokine receptors, and intracellular communication channels contribute to the tumor-bone interaction. Also, the reciprocal effect of the stroma on tumor cells is a target of investigation. Finally, the bone microenvironment can influence the phenotype of cancer cells in a phenomenon termed osteomimicry. Better definition of tumor-stromal interactions within the bone microenvironment may lead to new avenues of therapy.
Acknowledgments
We thank Jan Hurst from JanDesign Graphics for her expert illustrations of Figures 1, 2, 4–6.
Footnotes
Address reprint requests to Michael L. Cher, M.D., Departments of Urology and Pathology, Wayne State University School of Medicine, 4160 John R, Suite 1017, Detroit, MI 48201. E-mail: mcher@med.wayne.edu.
This Review article is based on material presented at the first Tumor Mircoenvironment (TME) Study Section Scientific Workshop held by the National Institutes of Health in Washington, DC, on February 27, 2005.
References
- Towler DA. Angiogenesis and marrow stromal cell fates: roles in bone strength. Osteoporos Int. 2003;14(Suppl 5):46–53. doi: 10.1007/s00198-003-1473-5. [DOI] [PubMed] [Google Scholar]
- Abedin M, Tintut Y, Demer LL. Mesenchymal stem cells and the artery wall. Circ Res. 2004;95:671–676. doi: 10.1161/01.RES.0000143421.27684.12. [DOI] [PubMed] [Google Scholar]
- Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avecilla ST, Hattori K, Heissig B, Tejada R, Liao F, Shido K, Jin DK, Dias S, Zhang F, Hartman TE, Hackett NR, Crystal RG, Witte L, Hicklin DJ, Bohlen P, Eaton D, Lyden D, De Sauvage F, Rafii S. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med. 2004;10:64–71. doi: 10.1038/nm973. [DOI] [PubMed] [Google Scholar]
- Hattori K, Heissig B, Wu Y, Dias S, Tejada R, Ferris B, Hicklin DJ, Zhu Z, Bohlen P, Witte L, Hendrikx J, Hackett NR, Crystal RG, Moore MA, Werb Z, Lyden D, Rafii S. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med. 2002;8:841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires mmp-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp HG, Avecilla ST, Hooper AT, Shmelkov SV, Ramos CA, Zhang F, Rafii S. Tie-2 activation contributes to hemangiogenic regeneration after myelosuppression. Blood. 2005;106:505–513. doi: 10.1182/blood-2004-11-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–2923. [PubMed] [Google Scholar]
- Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol. 2001;166:2472–2483. [PubMed] [Google Scholar]
- Tuxhorn JA, McAlhany SJ, Dang TD, Ayala GE, Rowley DR. Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. Cancer Res. 2002;62:3298–3307. [PubMed] [Google Scholar]
- Tuxhorn JA, McAlhany SJ, Dang TD, Rowley DR. Inhibition of TGF-β activity decreases angiogenesis in a human prostate cancer reactive stroma xenograft model. Cancer Res. 2002;62:6021–6025. [PubMed] [Google Scholar]
- Yang F, Tuxhorn JA, Ressler SJ, McAlhany SJ, Dang TD, Rowley DR. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005;65:8887–8895. doi: 10.1158/0008-5472.CAN-05-1702. [DOI] [PubMed] [Google Scholar]
- McAlhany SJ, Ressler SJ, Larsen M, Tuxhorn JA, Yang FY, Dang TD, Rowley DR. Promotion of angiogenesis by ps20 in the differential reactive stroma prostate cancer xenograft model. Cancer Res. 2003;63:5859–5865. [PubMed] [Google Scholar]
- Saunders MM, Seraj MJ, Li Z, Zhou Z, Winter CR, Welch DR, Donahue HJ. Breast cancer metastatic potential correlates with a breakdown in homospecific and heterospecific gap junctional intercellular communication. Cancer Res. 2001;61:1765–1767. [PubMed] [Google Scholar]
- Kapoor P, Saunders MM, Li Z, Zhou Z, Sheaffer N, Kunze EL, Samant RS, Welch DR, Donahue HJ. Breast cancer metastatic potential: correlation with increased heterotypic gap junctional intercellular communication between breast cancer cells and osteoblastic cells. Int J Cancer. 2004;111:693–697. doi: 10.1002/ijc.20318. [DOI] [PubMed] [Google Scholar]
- Nemeth JA, Yousif R, Herzog M, Che M, Upadhyay J, Shekarriz B, Bhagat S, Mullins C, Fridman R, Cher ML. Matrix metalloproteinase activity, bone matrix turnover, and tumor cell proliferation in prostate cancer bone metastasis. J Natl Cancer Inst. 2002;94:17–25. doi: 10.1093/jnci/94.1.17. [DOI] [PubMed] [Google Scholar]
- Winding B, NicAmhlaoibh R, Misander H, Hoegh-Andersen P, Andersen TL, Holst-Hansen C, Heegaard AM, Foged NT, Brunner N, Delaisse JM. Synthetic matrix metalloproteinase inhibitors inhibit growth of established breast cancer osteolytic lesions and prolong survival in mice. Clin Cancer Res. 2002;8:1932–1939. [PubMed] [Google Scholar]
- Dong Z, Bonfil RD, Chinni S, Deng X, Trindade Filho JC, Bernardo M, Vaishampayan U, Che M, Sloane BF, Sheng S, Fridman R, Cher ML. Matrix metalloproteinase activity and osteoclasts in experimental prostate cancer bone metastasis tissue. Am J Pathol. 2005;166:1173–1186. doi: 10.1016/S0002-9440(10)62337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinni SR, Sivalogan S, Dong Z, Filho JC, Deng X, Bonfil RD, Cher ML. CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: The role of bone microenvironment-associated CXCL12. Prostate. 2006;66:32–48. doi: 10.1002/pros.20318. [DOI] [PubMed] [Google Scholar]
- Keller ET, Brown J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J Cell Biochem. 2004;91:718–729. doi: 10.1002/jcb.10662. [DOI] [PubMed] [Google Scholar]
- Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, Mizokami A, Fu Z, Westman J, Keller ET. Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Invest. 2001;107:1235–1244. doi: 10.1172/JCI11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Dai J, Yao Z, Lu Y, Dougall W, Keller ET. Soluble receptor activator of nuclear factor kappaB Fc diminishes prostate cancer progression in bone. Cancer Res. 2003;63:7883–7890. [PubMed] [Google Scholar]
- Dawson NA. Therapeutic benefit of bisphosphonates in the management of prostate cancer-related bone disease. Expert Opin Pharmacother. 2003;4:705–716. doi: 10.1517/14656566.4.5.705. [DOI] [PubMed] [Google Scholar]
- Quinn JE, Brown LG, Zhang J, Keller ET, Vessella RL, Corey E. Comparison of Fc-osteoprotegerin and zoledronic acid activities suggests that zoledronic acid inhibits prostate cancer in bone by indirect mechanisms. Prostate Cancer Prostatic Dis. 2005;8:253–259. doi: 10.1038/sj.pcan.4500815. [DOI] [PubMed] [Google Scholar]
- Koeneman KS, Yeung F, Chung LW. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 1999;39:246–261. doi: 10.1002/(sici)1097-0045(19990601)39:4<246::aid-pros5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Huang WC, Xie Z, Konaka H, Sodek J, Zhau HE, Chung LW. Human osteocalcin and bone sialoprotein mediating osteomimicry of prostate cancer cells: role of cAMP-dependent protein kinase A signaling pathway. Cancer Res. 2005;65:2303–2313. doi: 10.1158/0008-5472.CAN-04-3448. [DOI] [PubMed] [Google Scholar]