Abstract
Ischemic preconditioning protects steatotic livers against ischemia-reperfusion (I/R) injury, but just how this is achieved is poorly understood. Here, I/R or preconditioning plus I/R was induced in steatotic and nonsteatotic livers followed by investigating the effect of pharmacological treatments that modulate heat shock proteins (HSPs) and mitogen-activated protein kinases (MAPKs). MAPKs, HSPs, protein kinase C, and transaminase levels were measured after reperfusion. We report that preconditioning increased HSP72 and heme-oxygenase-1 (HO-1) at 6 and 24 hours of reperfusion, respectively. Unlike nonsteatotic livers, steatotic livers benefited from HSP72 activators (geranylgeranylacetone) throughout reperfusion. This protection seemed attributable to HO-1 induction. In steatotic livers, preconditioning and geranylgeranylacetone treatment (which are responsible for HO-1 induction) increased protein kinase C activity. HO-1 activators (cobalt(III) protoporphyrin IX) protected both liver types. Preconditioning reduced p38 MAPK and c-Jun N-terminal kinase (JNK), resulting in HSP72 induction though HO-1 remained unmodified. Like HSP72, both p38 and JNK appeared not to be crucial in preconditioning, and inhibitors of p38 (SB203580) and JNK (SP600125) were less effective against hepatic injury than HO-1 activators. These results provide new data regarding the mechanisms of preconditioning and may pave the way to the development of new pharmacological strategies in liver surgery.
Steatotic livers tolerate ischemia-reperfusion (I/R) poorly.1,2 Results obtained under normothermic conditions indicate that heat shock preconditioning (whole body hyperthermia) induces a marked expression of heat shock protein 72 (HSP72) and heme-oxygenase-1 (HO-1) in steatotic livers, which protect against hepatic injury.3,4 The possible functions of HSPs in ischemic tissue include repair of damaged proteins, protection against oxidative stress, suppression of pro-inflammatory cytokines, and repair of the ion channel.5,6 However, despite the benefits of heat shock preconditioning, its application in the clinical setting is limited.
In common with heat shock preconditioning, ischemic preconditioning also involves the induction of organ stress to protect steatotic liver against I/R injury.7,8 There is some evidence in isolated hepatocytes and experimental models of perfused liver that mitogen-activated protein kinases (MAPKs) are involved in the protective mechanisms of ischemic preconditioning.9,10 Signaling pathways involved in HSP induction include MAPKs. HSP72 induction includes p38 MAPK (p38) and c-Jun N-terminal kinase (JNK) in heart in response to hypoxia.11 HO-1 overexpression by p38 and JNK has been reported in cultures of hepatocytes,12 isolated endothelial cells,13 and experimental models of lung I/R.14 The respective roles of HSPs and MAPKs in inducing the benefits of ischemic preconditioning on I/R injury in steatotic livers remain unclear.
Our experimental study aimed to evaluate whether 1) ischemic preconditioning, through HSP induction, protects steatotic livers against I/R injury and 2) HSP induction by preconditioning is related to MAPK activation. Ischemic preconditioning has been successfully used clinically under normothermic conditions for tumor hepatic resections.15–17 If ischemic preconditioning were understood at the molecular level, protective drugs that achieve similar protection could be developed, avoiding repeated vascular clamping and prolonged surgery.
Materials and Methods
Homozygous (obese, Ob) and heterozygous (lean, Ln) Zucker rats (Iffa-Credo, L′Abresle, France), aged 16–18 weeks, were used in the experiments. The animals were anesthetized with ketamine (100 mg/kg) and xylazine (8 mg/kg).7,18 The study followed European Union regulations (Directive 86/609 EEC) on animal experiments. All animals were randomized into groups.
Experimental Design
Protocol 1. HSPs in Ischemic Preconditioning
Protocol 1 is as follows: Effect of preconditioning on HSPs: Group 1: Sham (n = 12). Hepatic helium vessels of Ln and Ob animals (six in each group) were dissected. Group 2: ischemia-reperfusion (I/R) (n = 24). Group 2.1: A group of animals (n = 12, 6 Ln and 6 Ob) were subjected to 60 minutes of partial (70%) hepatic ischemia followed by 6 hours of reperfusion. Group 2.2: A second group of animals (n = 12, 6 Ln and 6 Ob) were subjected to 60 minutes of partial (70%) hepatic ischemia followed by 24 hours of reperfusion.7,18 Group 3: PC (n = 24). Animals were treated as in group 2, but with previous preconditioning induced by 5 minutes ischemia followed by 10 minutes reperfusion.7,18
The Role of NO involved in preconditioning on HSPs was carried out as follows: Group 4: PC plus NAME (n = 24). As in group 3, but with Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME) (Sigma Chemical, St. Louis, MO.), nonspecific nitric oxide synthase (NOS) inhibitor (10 mg/kg i.v.), 5 minutes before preconditioning.7,18 Group 5: PC plus NAME plus Arg (n = 24). As in group 3, but with l-NAME (10 mg/kg i.v.) 5 minutes before preconditioning and L-arginine (Sigma Chemical) (100 mg/kg i.v.) immediately before l-NAME.7,18,19 Group 6: PC plus NNA (n = 24). As in group 3, but with Nω-nitro-l-arginine (NNA; Sigma Chemical), nonspecific NOS inhibitor (10 mg/kg i.v.), 5 minutes before preconditioning.20 Group 7: PC plus AG (n = 24). As in group 3, but treated with aminoguanidine hemisulfate (AG; Sigma Chemical), an inducible NOS inhibitor (10 mg/kg, i.v.) 5 minutes before preconditioning.18
The Role of HSP72 and HO-1 in hepatic injury was as follows: Group 8: PC plus HSP72inh (n = 24). As in group 3, but with Quercetin (Sigma Chemical), a Hsp72 inhibitor (100 mg/kg, i.p.), 2 hours before preconditioning.21,22 Group 9: PC plus HO-1inh (n = 24). As in group 3 but with zinc(II) protoporphyrin IX (ZnPP) (Oxisresearch, Portland, Oregon), a HO-1 inhibitor (20 mg/kg, i.p.), 24 hours before preconditioning.23 Group 10: I/R plus HSP72act (n = 24). As in group 2, but with geranylgeranylacetone (GGA; Eisai Co., Tokyo, Japan), a Hsp72 activator (200 mg/kg, orally), 24 hours before ischemia.24 Group 11: I/R plus HSP72act plus HO-1inh (n = 6). I/R was induced in Ob animals (as in group 2.2), but with GGA, a HSP72 activator (200 mg/kg, orally) and ZnPP, a HO-1 inhibitor (20 mg/kg, i.p.), 24 hours before ischemia.23,24 Group 12: I/R plus HO-1act (n = 24). As in group 2.2, but with cobalt(III) protoporphyrin IX (Alexis Biochemicals, Lausen, Switzerland), a HO-1 activator at dose of 5 mg/kg, i.p. (n = 12, 6 Ln and 6 Ob) and 10 mg/kg, i.p. (n = 12, 6 Ln and 6 Ob), 24 hours before ischemia.23
Following reperfusion, liver and plasma samples corresponding to protocol 1 were collected. In the sham group, liver and plasma samples were obtained 24 hours after the dissection of helium vessels. HSPs (HSP90, HSP72, and HO-1) and protein kinase C (PKC) were analyzed in liver. Hepatic injury was evaluated by determination of transaminases. Liver was also analyzed histologically.
Protocol 2. Relation between MAPKs and HSPs
Protocol 2 is as follows: Effect of preconditioning on MAPKs. Group 13: I/R30 (n = 12, 6 Ln and 6 Ob): Animals were subjected to 60 minutes of partial (70%) hepatic ischemia (as in group 2), and liver samples were obtained 30 minutes after reperfusion (as opposed to 6 and 24 hours) to evaluate p38 and JNK.25,26 Group 14: PC30 (n = 12): As in group 13, but with previous preconditioning induced by 5 minutes ischemia followed by 10 minutes reperfusion.
Effect of MAPKs on HSPs and hepatic injury: Group 15: PC plus p38/JNKactiv (n = 24): As in group 3, but with anisomycin (Sigma Chemical), an activator of both p38 and JNK (0.1 mg/kg, i.p.), 24 hours before preconditioning.27 Group 16: I/R plus p38inh (n = 24): As in group 2, but with SB203580 (Sigma Chemical), a p38 inhibitor (1 mg/kg i.p), 24 hours before ischemia27 Group 17: I/R plus JNK inh (n = 24): As in group 2, but with SP600125 (Sigma Chemical), a JNK inhibitor (6 mg/kg s.c.), 1 hour before ischemia.28 Following reperfusion, liver and plasma samples were collected. MAPKs (p38 and JNK) and HSPs (HSP72 and HO-1) in liver were analyzed. Transaminases in plasma were measured.
Biochemical Determinations
Transaminase Assay
Plasma alanine aminotransferase (ALT) was measured using standard procedures.
HSPs and MAPKs Analyses
Liver tissue was homogenized, and proteins were separated by sodium docecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes.29 Western blotting was performed with primary antibodies against HSP72, HSP90, and HO-1 (BD Transduction Laboratories, San Jose, CA) and p38, JNK, phospho-p38, and phospho-JNK (Cell Signaling Technology Inc., Beverly, MA). Positive controls for HSPs and MAPKs were obtained from Stressgen Biotechnologies (Victoria, BC, Canada) and Cell Signaling Technology Inc., respectively. Signals were detected by enhanced chemiluminescence and quantified by scanning densitometry. All signals were standardized to the corresponding Ponceau S staining.
PKC Assay
Frozen liver samples were homogenized as described previously.30 Cytosolic and membrane extracts were then used in the biochemical assay of PKC activity and Western blotting. Total PKC activity in the membrane and cytosolic extracts were measured using an enzyme-linked immunosorbent assay kit from Stressgen Bioreagents. For Western blot assay, aliquots of the cytosol and membrane fractions were processed following a similar protocol to that described above for HSPs and MAPKs. Primary antibodies against PKC isozymes (α, β, γ, δ, and ε) were obtained from BD Transduction Laboratories. Rat cerebrum lysate was used as a positive control (BD Transduction Laboratories).
Histology
To appraise the severity of hepatic injury, hematoxylin and eosin-stained sections were evaluated using a point-counting method on an ordinal scale.7
Statistics
Data are expressed as means ± SE and are compared statistically by variance analysis, followed by Student-Newman-Keuls. P < 0.05 was considered significant.
Results
Role of HSPs in Inducing the Benefits of Preconditioning for Hepatic I/R Injury
Effect of Preconditioning on HSPs
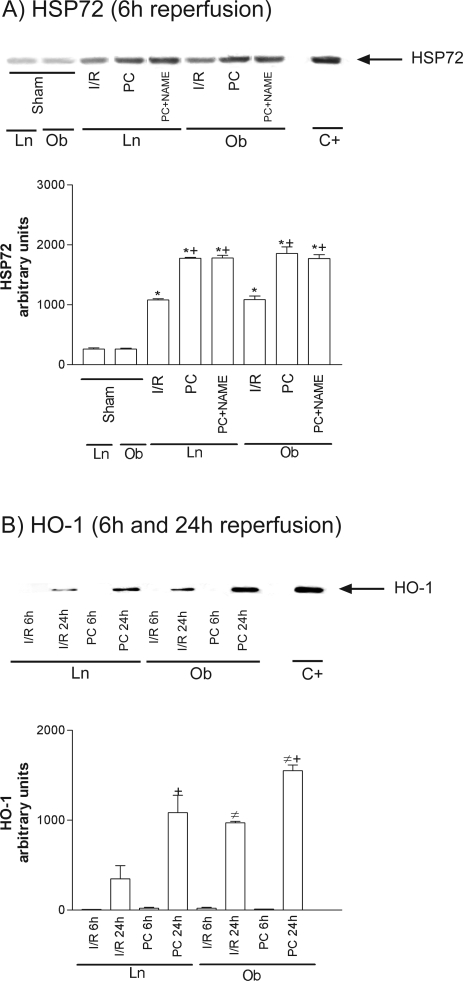
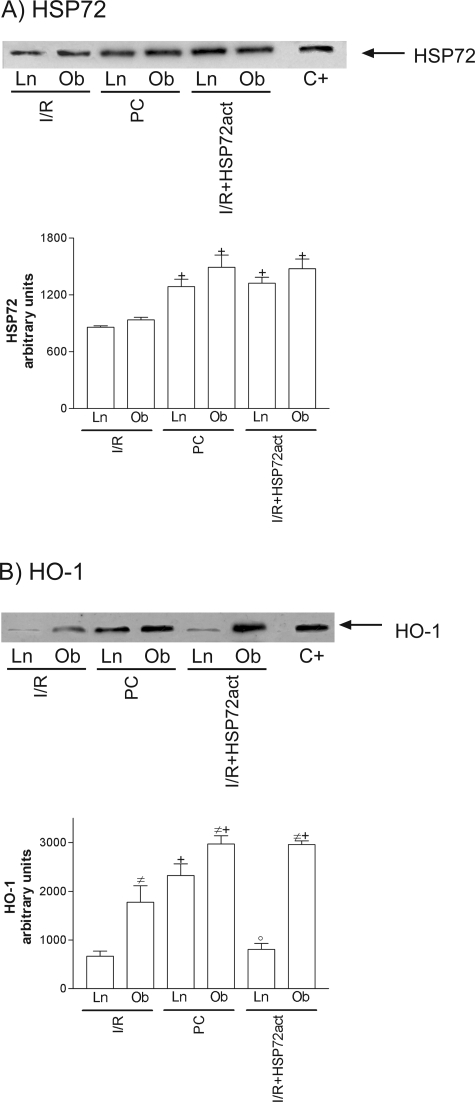
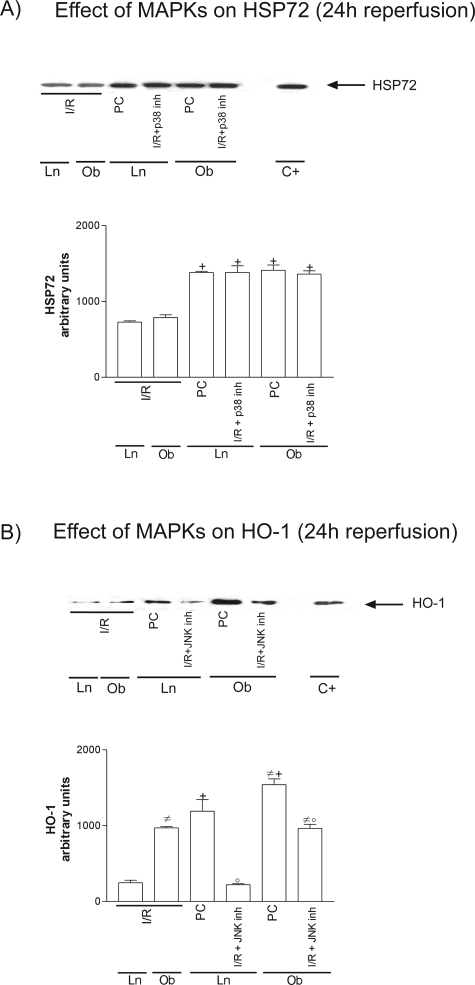
HSP90 was unchanged in all groups (data not shown). However, at 6 hours of reperfusion, increased HSP72 levels were seen in both steatotic and nonsteatotic livers as a consequence of I/R (Figure 1A), and similar results were observed at 24 hours (data not shown). Figure 1B shows HO-1 levels at 6 hours (I/R6 hours) and 24 hours (I/R24 hours) of reperfusion. HO-1 was only present at 24 hours of reperfusion (I/R 24 hours), but HO-1 levels were significantly higher in steatotic livers. Preconditioning (PC) increased HSP72 and HO-1 levels in both liver types more than in the I/R group (Figure 1, A and B).
Figure 1.
Effect of ischemic preconditioning on HSP72 (A) and HO-1 (B). Representative Western blots of three similar experiments at the top and densitometric analysis (area × density of the band) at the bottom. Steatotic livers had higher HO-1 levels compared with the nonsteatotic livers (#, P < 0.05). *, P < 0.05 versus sham; +, P < 0.05 versus I/R.
HSPs and Hepatic Injury
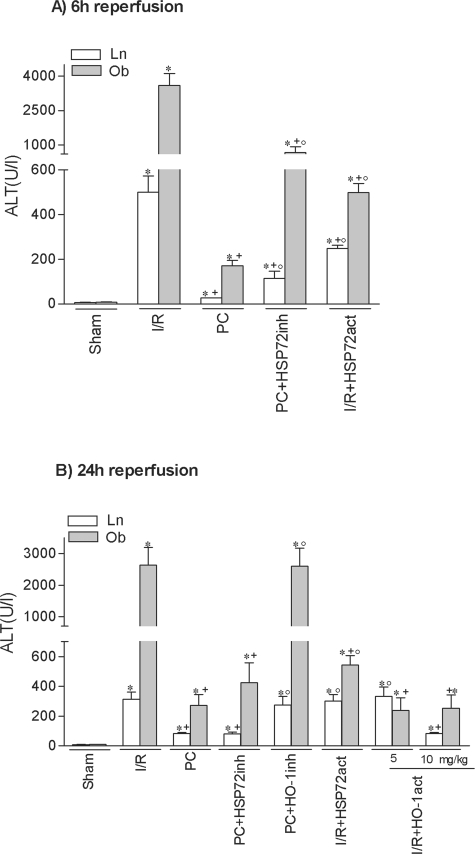
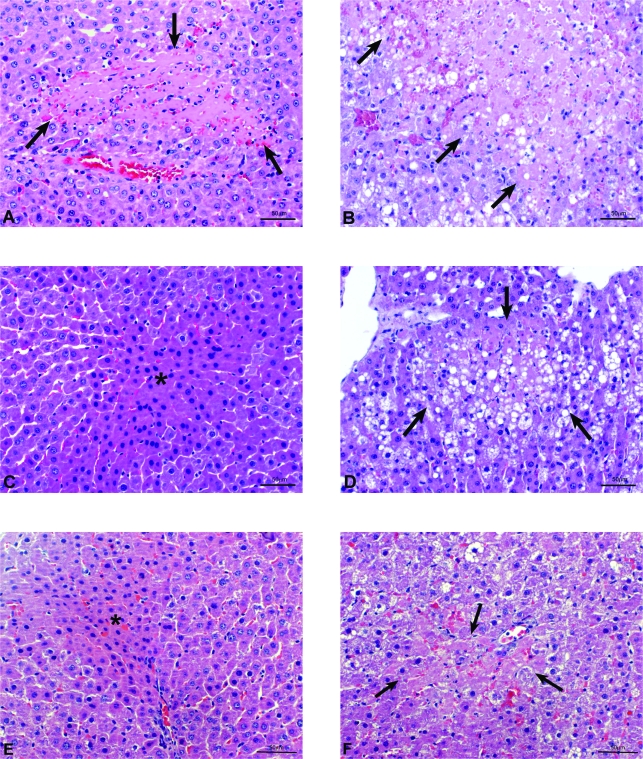
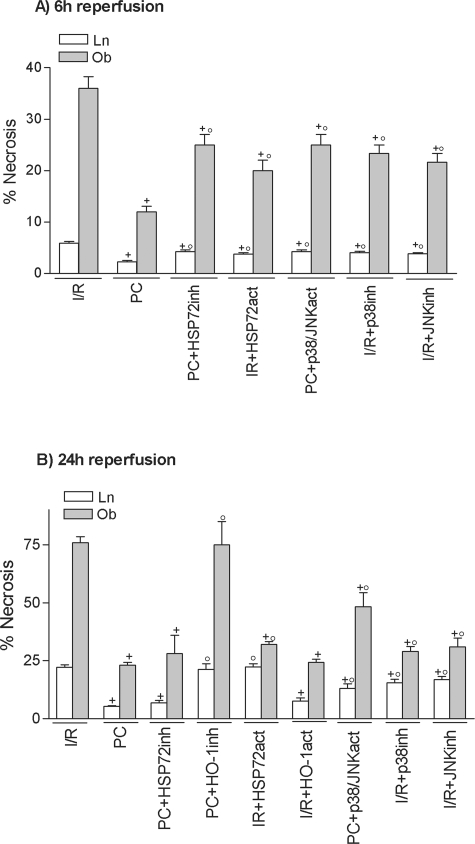
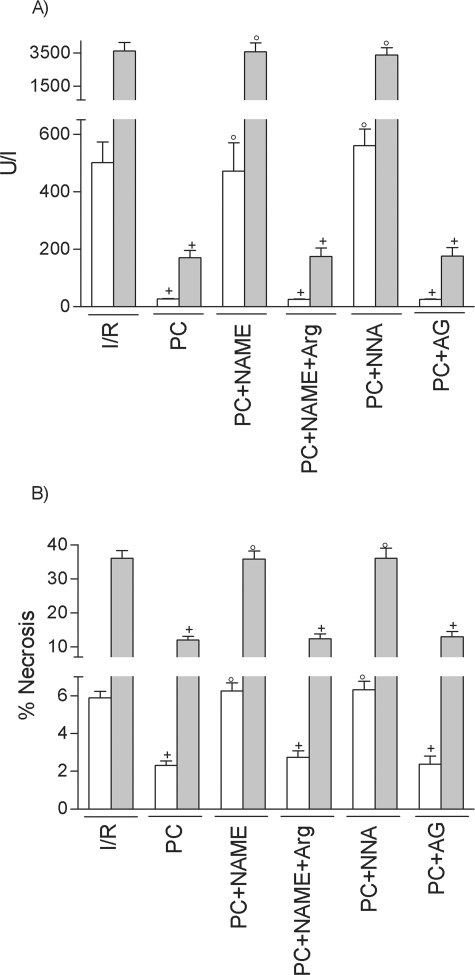
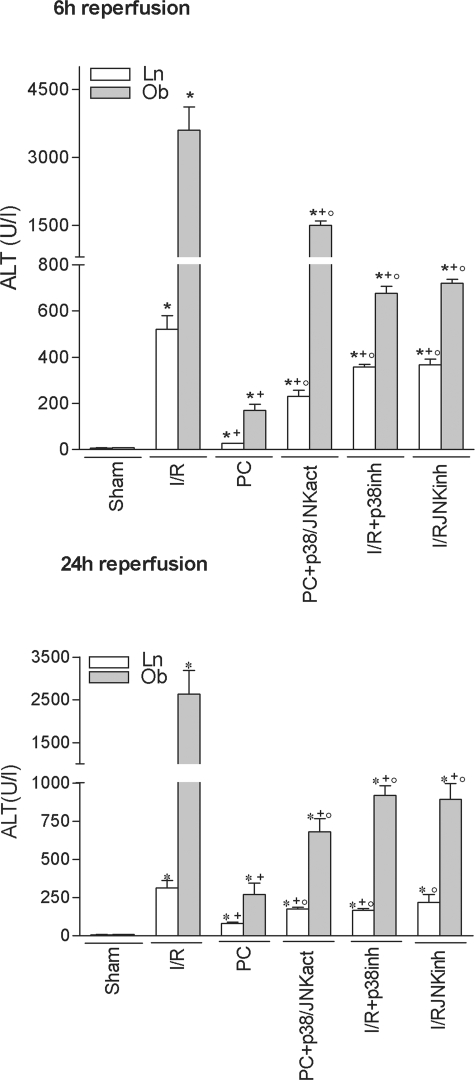
At 6 hours of reperfusion, HSP72 inhibition (PC plus HSP72inh) increased hepatic injury in both liver types but did not completely revert the effectiveness of preconditioning, because ALT levels did not rise as much as in the I/R group (Figure 2A). HO-1 inhibition (PC plus HO-1inh) did not modify the benefits of preconditioning (data not shown). At 24 hours of reperfusion (Figure 2B), HSP72 inhibition had no effect on the benefits of preconditioning. However, HO-1 inhibition (PC plus HO-1inh) abolished the protection conferred by preconditioning, resulting in transaminase levels comparable to those in the I/R group (see Figure 2B). The histological results were consistent with the biochemical parameters of hepatic injury. At 24 hours of reperfusion, nonsteatotic livers corresponding to I/R and PC plus HO-1inh groups showed multifocal areas of moderate coagulative necrosis and neutrophil infiltration, randomly distributed throughout the parenchyma (Figure 3A); in steatotic livers, extensive and confluent areas of coagulative necrosis with neutrophil infiltration were found (Figure 3B). By contrast, PC reduced the extent and number of necrosis areas in both liver types compared with the results obtained in the I/R group. In nonsteatotic livers (Figure 3C), these areas were mainly of incipient necrosis; in steatotic livers (Figure 3D), patchy areas of incipient hepatocyte necrosis and scattered multifocal areas of coagulative necrosis were observed. The percentage of grade 3 necrosis at 24 hours of reperfusion is shown in Figure 4B.
Figure 2.
Role of HSP72 and HO-1 on hepatic I/R injury at 6 hours (A) and 24 hours (B) of reperfusion. *, P < 0.05 versus sham; +, P < 0.05 versus I/R; °, P < 0.05 versus PC.
Figure 3.
Histological lesions in liver after 24 hours of reperfusion. PC plus HO-1inh (A, Ln; B, Ob). A: Small area of coagulative hepatic necrosis (arrows) with neutrophil infiltration. B: Widespread coagulative hepatic necrosis with neutrophil infiltration (arrows). PC (C, Ln; D, Ob). C: Irregular area of incipient necrosis (asterisk). D: Small area of coagulative hepatic necrosis (arrows) with neutrophil infiltration. I/R plus HO-1act (E, Ln; F, Ob). Histological lesions similar to the PC group.
Figure 4.
Percentage of grade 3 necrosis resulting from pharmacological modulation of HSPs and MAPKs. +, P < 0.05 versus I/R; °, P < 0.05 versus PC.
Effect of NO on Hepatic Injury and HSPs
At 6 hours of reperfusion, NO synthesis inhibition with l-NAME (PC plus NAME) or l-NNA (PC plus NNA) abolished the benefits of preconditioning on the biochemical and histological parameters of hepatic injury (Figure 5, A and B, respectively) in both liver types. The effects of l-NAME were reverted with previous l-arginine addition (PC plus NAME plus Arg). Inducible NOS inhibition in the preconditioned group (PC plus AG) did not modify the benefits of preconditioning on transaminase and grade 3 necrosis (see Figure 5). Similar results were observed at 24 hours of reperfusion (data not shown). In regards to HSPs, NO synthesis inhibition with l-NAME in preconditioned group (PC plus NAME) had no effect on either HSP72 (Figure 1A) or HO-1 (data not shown). Similar results were obtained with the others NOS inhibitors used, l-NNA and AG. These results confirm the data previously reported18,31,32 on the role of NO (derived mainly from constitutive NOS) in the benefits of preconditioning on hepatic I/R injury. However, it appears unlike that the protective mechanisms of NO could be dependent on HSP.
Figure 5.
Role of NO in steatotic livers undergoing I/R on ALT (A) and necrosis (B). +, P < 0.05 versus I/R; °, P < 0.05 versus PC.
Effect of HSP Inductors on Hepatic Injury and HSPs
Treatment with HSP72 activators, such as GGA (I/R plus HSP72 act), protected against hepatic I/R injury in both liver types at 6 hours of reperfusion (Figures 2A and 4A). However, at 24 hours of reperfusion (Figures 2B and 4B), the benefits of GGA were only found in steatotic livers. We then evaluated the effect of GGA on HSP72 and HO-1. GGA pretreatment (I/R plus HSP72act) increased HSP72, but no significant differences in HSP72 levels were found between the two liver types (Figure 6A). Interestingly, GGA pretreatment (I/R plus HSP72act) increased HO-1 levels in steatotic livers (Figure 6B) but not in nonsteatotic ones. HO-1 inhibition in steatotic livers pretreated with GGA (I/R plus HSP72act plus HO-1inh) abolished the benefits of GGA (I/R plus HSP72act) for hepatic I/R injury. Thus, at 24 hours of reperfusion ALT levels of 2398.3 ± 150.4 and 570.9 ± 52.5 were recorded in the I/R plus HSP72act plus HO-1inh and I/R plus HSP72act groups, respectively), which points to the importance of HO-1 in inducing benefits of GGA. Therefore, we evaluated the effect of HO-1 activators on hepatic I/R injury. In steatotic livers, pretreatment with HO-1 activators at a 5 mg/kg dose reduced transaminase levels; in nonsteatotic livers, a 10 mg/kg dose was needed to ameliorate the I/R injury (Figure 2B). The administration of HO-1 activator (5 and 10 mg/kg in steatotic and nonsteatotic livers, respectively) (Figure 3, E and F) resulted in histological finding similar to those observed in the PC group (Figures 3, C and D). The percentage of grade 3 necrosis is shown in Figure 4B.
Figure 6.
Effect of HSP72 activator on HSP72 (A) and HO-1 (B). Representative Western blot of three similar experiments at the top and densitometric analysis (area × density of the band) at the bottom. Steatotic livers had higher HO-1 levels compared with the nonsteatotic livers (#, P < 0.05). +, P < 0.05 versus I/R; °, P < 0.05 versus PC.
PKC and HO-1
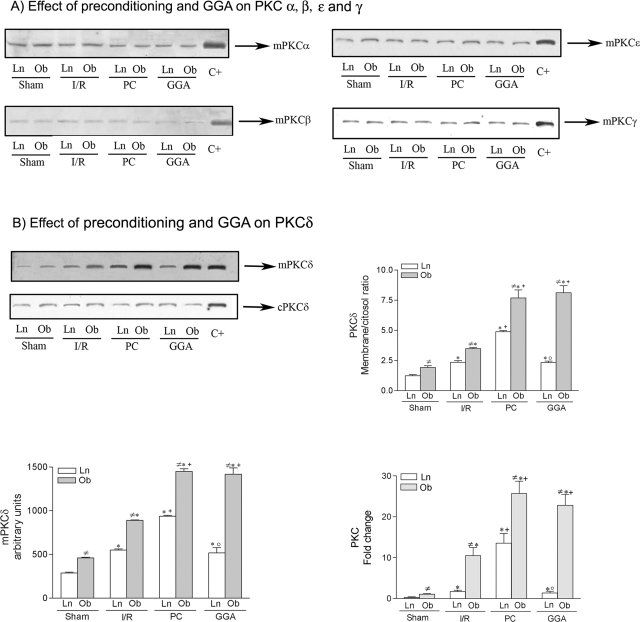
HO-1 induction by PKC has been previously reported in various cell types.33,34 Our results indicate that both preconditioning and GGA were responsible for HO-1 induction in steatotic livers. We then sought to ascertain whether preconditioning and GGA might modify PKC activity. It has been widely reported that the translocation of the PKC isoenzymes from cytosol to the membrane fraction is a key step in the activation of PKC.35 Analysis of the specific PKC isoenzymes in both liver types revealed that, in liver preconditioned or pretreated with GGA, PKC isoforms (α, β, ε, and γ) in membrane fraction (mPKC) remained unmodified (Figure 7A) and similar results were obtained for cytosolic fraction (cPKC) (data not shown). However, this was not the case for PKCδ. In steatotic livers, preconditioning and GGA pretreatment (which increased HO-1) did not modified PKCδ in cytosolic fraction (cPKCδ) but increased PKCδ in the membrane resulting in a higher PKCδ membrane/cytosol ratio (see Figure 7B). PKC membrane/cytosol ratio has been considered as an index of PKC activation.36 Furthermore, both procedures (preconditioning and GGA) increased mPKC activity from steatotic livers (Figure 7B). In nonsteatotic livers, preconditioning (which increased HO-1) resulted in high levels of mPKC activity, whereas in nonsteatotic livers, GGA pretreatment (which was unable to induce HO-1) had no effect on mPKC activity (see Figure 7B). Cytosolic PKC activity remained unaltered in all groups (data not shown). As previously reported,30,36 in all of the groups of study, PKC activity in steatotic livers was higher than in nonsteatotic ones (Figure 7B).
Figure 7.
Effect of preconditioning and GGA (HSP72 activator) on PKCα, -β, -ε, and -γ (A) and PKCδ (B). A: Analysis of PKCα, -β, -ε, and -γ protein expression in membrane fraction (mPKC) by Western blot. B: Analysis of PKCδ in membrane and cytosolic fractions (mPKC and cPKC, respectively) by Western blot and densitometric analysis for mPKCδ (area × density of the band), relative densities of the PKCδ bands in the membrane fraction compared with the cytosolic fraction (PKC membrane/cytosol ratio) and biochemical assay of PKC activity (the data represent the -fold change versus sham). Western blot of three similar experiments at the top and densitometric analysis (area × density of the band) at the bottom. Steatotic livers had higher HO-1 levels compared with the nonsteatotic livers (#, P < 0.05). *, P < 0.05 versus sham. +, P < 0.05 versus I/R; °, P < 0.05 versus PC.
Role of MAPKs in Inducing the Benefits of Preconditioning on HSP and Hepatic Injury
Effect of Preconditioning on MAPKs
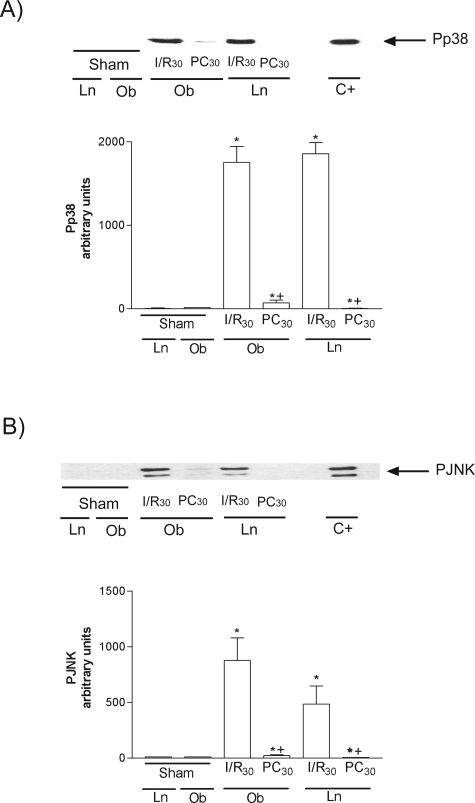
MAPKs are activated by dual phosphorylation on tyrosine and threonine in response to extracellular stimuli.25 Both p38 and JNK (Figure 8, A and B, respectively) were notably phosphorylated (Pp38 and PJNK) at 30 minutes of reperfusion (I/R30 group). Preconditioning reduced Pp38 and PJNK in both liver types. Total p38 and JNK were unchanged in all groups (data not shown).
Figure 8.
Effect of ischemic preconditioning on Pp38 (A) and PJNK (B). Representative Western blot of three similar experiments at the top and densitometric analysis (area × density of the band) of Pp38 and PJNK at the bottom. *, P < 0.05 versus sham; +, P < 0.05 versus I/R.
Effect of MAPKs on HSPs
The pretreatment with p38 or JNK inhibitors as well as p38/JNK activators modified HSP72 (see Figure 9A for the HSP72 results obtained with p38 inhibitor). Thus, HSP72 levels in I/R plus p38inh were significantly higher than those observed in I/R group. However, the various pharmacological treatments aimed at activating or inhibiting p38 and JNK had no effect on HO-1 (see Figure 9B for the HO-1 results obtained with JNK inhibitor). Thus, HO-1 levels in I/R plus JNKinh were similar to those observed in the I/R group.
Figure 9.
Effect of MAPKs on HSP72 (A) and HO-1 (B). Representative Western blot of three similar experiments at the top and densitometric analysis (area × density of the band) at the bottom. Steatotic livers had higher HO-1 levels compared with the nonsteatotic livers (#, P < 0.05). +, P < 0.05 versus I/R; °, P < 0.05 versus PC.
MAPKs and Hepatic Injury
Treatment with p38/JNK activators in the preconditioned group (PC plus p38/JNKact) increased transaminase levels (Figure 10) and grade 3 necrosis (Figure 4) but did not completely revert the benefits of preconditioning. MAPK inhibition (I/R plus p38inh and I/R plus JNKinh) reduced hepatic I/R injury in both liver types. In addition, the benefits resulting from MAPK inhibition were not as great as those obtained with HO-1 activators (Figures 2 and 10).
Figure 10.
Role of p38 and JNK on hepatic I/R injury. *, P < 0.05 versus sham; +, P < 0.05 versus I/R; °, P < 0.05 versus PC.
Discussion
Few studies have sought to evaluate the role of HSP72 in both steatotic and nonsteatotic livers undergoing I/R.3 The authors investigated the role of heat shock preconditioning under warm ischemia and revealed that, in steatotic livers, the maximum accumulation of HSP72 after heat exposure occurs earlier and is weaker than in nonsteatotic ones.3 Here, the induction of ischemic preconditioning resulted in the same response, in HSP72 levels, in both liver types. Our results indicate that the increase in HSP72 caused by preconditioning protected steatotic and nonsteatotic livers at 6 hours of reperfusion, whereas HO-1 induction seems essential for keeping both liver types protected throughout reperfusion. In addition, our results provide information concerning the action mechanisms of HSP activators in steatotic and nonsteatotic livers undergoing I/R and on their potential effectiveness in liver surgery.
The induction of HSP72 by GGA protected cultured gastric mucosal cells against ethanol-induced injury, and GGA has been widely used in clinical practice as an antiulcer drug for over 15 years.37–41 In liver surgery, GGA suppressed I/R injury in liver transplantation from nonsteatotic grafts.38,40,41 Our results indicate that, although pretreatment with HSP72 activators did protect nonsteatotic livers at 6 hours of reperfusion, this protection was transient. However, the pharmacological induction of HSP72 effectively protected steatotic livers against warm I/R injury for extended reperfusion periods.
Evidence from isolated hepatocytes suggests that GGA induces much higher levels of HSP72 in liver under stress conditions, such as ethanol or H2O2.37,40,42 Under our conditions, GGA induced similar HSP72 levels in both liver types. GGA increased HO-1 levels in steatotic livers but did not have the same effect in nonsteatotic livers. Thus, to determine whether the benefits of GGA in steatotic livers undergoing I/R might be explained by its effect on HO-1, we inhibited the action of HO-1 in steatotic livers pre-treated with GGA and found that, under these conditions the benefits of GGA were abolished. Similarly, it is reasonable to assume that the ineffectiveness of GGA in nonsteatotic livers is due to the fact that it fails to induce HO-1.
Our results reveal the potency of preconditioning and treatment with GGA in the activation of HO-1 and PKC in steatotic livers undergoing I/R. Our experiments based on the characterization of the PKC isoforms taking part in preconditioning and GGA signaling in steatotic livers have revealed that the classic PKCs (α, β, and γ), which require Ca2+ for their activation,43 are not involved. However, it seems not to be the case for PKCδ (Ca2+-independent). Although further investigations on ischemic preconditioning and GGA pretreatment are required to ascertain whether PKCδ regulates HO-1 expression in steatotic livers, a potential relationship between PKC and HO-1 should not be ruled out. In fact, the activation of PKC has been reported as being involved in the signaling pathways of preconditioning in myocardium and isolated hepatocytes,44,45 and data obtained in isolated-perfused heart indicate that GGA activates PKC.46 PKC has been reported as being responsible for HO-1 induction in different cell types.33,34
Studies examining the benefits of HO-1 in hepatic I/R injury have focused primarily on nonsteatotic livers47,48 or steatotic livers.4,23 To the best of our knowledge, there are no studies in hepatic I/R investigating the role of HO-1 in both liver types. Our results indicate that HO-1 activators, such as cobalt(III) protoporphyrin IX, might protect both liver types against warm I/R injury and, consequently, reduce the inherent risk of surgery with steatotic liver. However, a lower dose of HO-1 activator was required to protect steatotic livers effectively. Given these results, the fact that steatotic livers undergoing I/R showed higher HO-1 levels than nonsteatotic livers and the capacity of GGA to induce HO-1 in steatotic liver, steatotic livers might well be more predisposed to overexpressing HO-1. This might be explained by the increased reactive oxygen species generated in steatotic livers due to I/R7,18 and/or the increased PKC activity observed in steatotic livers compared with nonsteatotic ones. In fact, the induction of HO-1 could be a response to oxidative stress,49,50 and HO-1 induction has been associated with high PKC activity.33,34 However, further studies are required to provide a full explanation.
Exogenous NO supplementation protects liver from hepatotoxicity and oxidative stress inducing HSP72 or HO-1 overexpression.51,52 However, our results suggest that endogenous NO generated by preconditioning is not responsible for increased HSP. In fact, the mechanisms involved in the beneficial effects of the two sources of NO might well be different.53 Given the benefits of MAPK activation caused by preconditioning in hepatocytes and experimental models of perfused liver,9,10 and the relationship between MAPKs and HSPs,11–14 we posited that the increased HSP levels caused by preconditioning were mediated by MAPK activation. However, our results obtained with the pharmacological modulation of MAPKs, are in line with those reported in a study in transplantation from steatotic liver grafts indicating that JNK activation might be harmful to hepatocytes after reperfusion.54 In addition, under the conditions reported here, the levels of both p38 and JNK fell in both liver types following preconditioning. These apparently contradictory data on MAPKs in liver preconditioning have also been reported for the heart.55 Thus, apart from species differences, or differences between in vivo and in vitro models, specific MAPK isoforms might play a role. For example, in the case of p38, studies in heart indicate that while p38α isoform activation is believed to accelerate the death pathway, the p38β pathway may be antiapoptotic.55 Similarly, the differential activation of JNK isoenzymes could result in different functional roles.56 In addition, the importance of subcellular MAPKs should be considered, because ischemic preconditioning increased myocardial p38 activity in the cytosolic but not the nuclear fraction.56
To the best of our knowledge, no studies have established the effect of MAPKs on HO-1 induction in steatotic livers undergoing I/R. Our results suggest that HO-1 induction caused by preconditioning is not dependent on MAPKs. Indeed, preconditioning reduced MAPKs, which in turn increased HSP72 levels. Like HSP72, MAPKs do not seem to play a crucial role in the protective mechanisms of preconditioning. However, our results suggest that this does not exclude the potential benefits of MAPK inhibitor pretreatment.
Some of the inhibitors used in the present study, such as ZnPP and SB203580, were given 24 hours before ischemia, which may cast doubt on the efficacy of these drugs. However, in several experimental models of hepatic I/R,23,48,57 the pretreatment dose of ZnPP was given 24 hours before liver surgery, at a dose of 1.5 mg/kg48,57 or 20 mg/kg.23 Under our conditions, control experiments indicated that the effective dose of ZnPP (which inhibited HO-1) was 20 mg/kg. Although the exact half-life in the circulation of ZnPP is unknown,58 the plasma ZnPP concentration remained elevated for 2–3 days after a single dose of 25 mg/kg i.p.59 In regards to SB203580, the elimination half-life of this drug (2.1 mg/kg i.v.) was 3.5 hours.60 However, the data reported on SB203580, which have been mainly focused in myocardial I/R,27,61,62 indicate that the pretreatment dose was 24 hours at 1 mg/kg i.p.27,61 or 2.5 mg/kg.62 Under our conditions, SB203580 (1 mg/kg i.p. 24 hours before surgery) was enough to prevent phospho-p38 up-regulation. Moreover, the possibility that some of the effects ascribed to a particular inhibitor and pathway might be related to adaptation of other systems should not be ruled out. In addition to HO-1, ZnPP inhibits all HO activity mediated by HO-2 and HO-3.63,64 Similarly, protein kinase Raf and particular isoforms of JNK65,66 could be modified by SB20380, albeit less potently than p38.
The results of the present study point to a new pathway in the protective mechanisms of PC, which is not dependent on NO. The MAPKs-HSP72 pathway is less important than HO-1 in terms of the protection conferred by preconditioning throughout reperfusion. The results obtained with GGA, a HSP72 activator, indicate differences between the two liver types. GGA may be effective in the presence of steatosis, but its protection of nonsteatotic livers was transient. Both, preconditioning and GGA increased PKC activity in steatotic livers. Future investigations will be required to elucidate whether PKC is responsible for HO-1 induction in steatotic livers. Activators of HO-1 such as cobalt(III) protoporphyrin IX could well become an important pharmacological strategy for protecting both steatotic and nonsteatotic livers against I/R injury, but the effective dose may differ between the two liver types. Unlike HO-1 pretreatment, MAPK inhibitors (SB203580 and SP600125), at the same dose, protected against hepatic I/R injury, but their benefits in both liver types were not as great as those obtained with HO-1 activator pretreatment. These results may open the door to new pharmacological strategies for the effective protection of both steatotic and nonsteatotic livers from I/R injury.
Acknowledgments
We thank Robin Rycroft at the Language Advisory Service of the University of Barcelona for revising the English text. We are also grateful to Emma Puig-Oriol and Llorenç Quintó, of the Unit of Epidemiology and Biostatistics, University of Barcelona, for help and advice in conducting the statistical analyses and to Esai Co. for the generous gift of GGA.
Footnotes
Address reprint requests to Dr. Joan Roselló-Catafau; Experimental Hepatic Ischemia-Reperfusion Unit, Institut d′Investigacions Biomèdiques August Pi i Sunyer, C/ Rosellón 161, 7a planta, 08036 Barcelona, Spain. E-mail: jrcbam@iibb.csic.es.
Supported by the Ministerio de Ciencia y Tecnología (project grants SAF 2005-00385 and BFI 2003-00912, Ramón y Cajal research contract for C.P.), and Generalitat Catalunya (2005SGR/0078).
M.M.-S. and A.C.-R. contributed equally to this study.
References
- McCormack L, Clavien PA. Understanding the meaning of fat in the liver. Liver Transpl. 2005;11:137–139. doi: 10.1002/lt.20354. [DOI] [PubMed] [Google Scholar]
- Imber CJ, St Peter SD, Handa A, Friend PJ. Hepatic steatosis and its relationship to transplantation. Liver Transpl. 2002;8:415–423. doi: 10.1053/jlts.2002.32275. [DOI] [PubMed] [Google Scholar]
- Yamagami K, Yamamoto Y, Kume M, Kimoto S, Yamamoto H, Ozaki N, Yamamoto M, Shimahara Y, Toyokuni S, Yamaoka Y. Heat shock preconditioning ameliorates liver injury following normothermic ischemia-reperfusion in steatotic rat livers. J Surg Res. 1998;79:47–53. doi: 10.1006/jsre.1998.5403. [DOI] [PubMed] [Google Scholar]
- Mokuno Y, Berthiaume F, Tompkins RG, Balis UJ, Yarmush ML. Technique for expanding the donor liver pool: heat shock preconditioning in a rat fatty liver model. Liver Transplant. 2004;10:264–272. doi: 10.1002/lt.20014. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Santoro MG. Stress-inducible responses and heat shock proteins: new pharmacologic targets for cytoprotection. Nat Biotechnol. 1998;16:833–838. doi: 10.1038/nbt0998-833. [DOI] [PubMed] [Google Scholar]
- Chi NC, Karliner JS. Molecular determinants of responses to myocardial ischemia/reperfusion injury: focus on hypoxia-inducible and heat shock factors. Cardiovasc Res. 2004;61:437–447. doi: 10.1016/j.cardiores.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Serafin A, Rosello-Catafau J, Prats N, Xaus C, Gelpi E, Peralta C. Ischemic preconditioning increases the tolerance of fatty liver to hepatic ischemia-reperfusion injury in the rat. Am J Pathol. 2002;161:587–601. doi: 10.1016/S0002-9440(10)64214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koti RS, Yang W, Glantzounis G, Quaglia A, Davidson BR, Seifalian AM. Effect of ischaemic preconditioning on hepatic oxygenation, microcirculation and function in a rat model of moderate hepatic steatosis. Clin Sci. 2005;108:55–63. doi: 10.1042/CS20040130. [DOI] [PubMed] [Google Scholar]
- Carini R, Grazia De, Cesaris M, Splendore R, Domenicotti C, Nitti MP, Pronzato MA, Albano E. Signal pathway responsible for hepatocyte preconditioning by nitric oxide. Free Radic Biol Med. 2003;34:1047–1055. doi: 10.1016/s0891-5849(03)00039-x. [DOI] [PubMed] [Google Scholar]
- Schauer RJ, Gerbes AL, Vonier D, op den Winkel M, Fraunberger P, Bilzer M. Induction of cellular resistance against Kupffer cell-derived oxidant stress: a novel concept of hepatoprotection by ischemic preconditioning. Hepatology. 2003;37:286–295. doi: 10.1053/jhep.2003.50064. [DOI] [PubMed] [Google Scholar]
- Rafiee P, Shi Y, Pritchard KA, Jr, Ogawa H, Eis AL, Komorowski RA, Fitzpatrick C, Tweddell JS, Litwin SB, Mussatto K, Jaquiss RD, Baker JE. Cellular redistribution of inducible Hsp70 protein in the human and rabbit heart in response to the stress of chronic hypoxia: role of protein kinases. J Biol Chem. 2003;278:43636–43644. doi: 10.1074/jbc.M212993200. [DOI] [PubMed] [Google Scholar]
- Kietzmann T, Samoylenko A, Immenschuh S. Transcriptional regulation of heme oxygenase-1 gene expression by MAP kinases of the JNK and p38 pathways in primary cultures of rat hepatocytes. J Biol Chem. 2003;278:17927–17936. doi: 10.1074/jbc.M203929200. [DOI] [PubMed] [Google Scholar]
- Bulger EM, Garcia I, Maier RV. Induction of heme-oxygenase 1 inhibits endothelial cell activation by endotoxin and oxidant stress. Surgery. 2003;134:146–152. doi: 10.1067/msy.2003.215. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bedard EL, Potter R, Zhong R, Alam J, Choi AM, Lee PJ. Mitogen-activated protein kinases regulate HO-1 gene transcription after ischemia-reperfusion lung injury. Am J Physiol. 2002;283:L815–L829. doi: 10.1152/ajplung.00485.2001. [DOI] [PubMed] [Google Scholar]
- Clavien PA, Selzner M, Rudiger HA, Graf R, Kadry Z, Rousson V, Jochum W. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–852. doi: 10.1097/01.sla.0000098620.27623.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzzo G, Giuliante F, Vellone M, De Cosmo G, Ardito F, Murazio M, D’Acapito F, Giovannini I. Pedicle clamping with ischemic preconditioning in liver resection. Liver Transpl. 2004;10:S53–S57. doi: 10.1002/lt.20045. [DOI] [PubMed] [Google Scholar]
- Chouker A, Schachtner T, Schauer R, Dugas M, Lohe F, Martignoni A, Pollwein B, Niklas M, Rau HG, Jauch KW, Peter K, Thiel M. Effects of Pringle manoeuvre and ischaemic preconditioning on haemodynamic stability in patients undergoing elective hepatectomy: a randomized trial. Br J Anaesth. 2004;93:204–211. doi: 10.1093/bja/aeh195. [DOI] [PubMed] [Google Scholar]
- Serafin A, Rosello-Catafau J, Prats N, Gelpi E, Rodes J, Peralta C. Ischemic preconditioning affects interleukin release in fatty livers of rats undergoing ischemia/reperfusion. Hepatology. 2004;39:688–698. doi: 10.1002/hep.20089. [DOI] [PubMed] [Google Scholar]
- Koti RS, Yang W, Dashwood MR, Davidson BR, Seifalian AM. Effect of ischemic preconditioning on hepatic microcirculation and function in a rat model of ischemia reperfusion injury. Liver Transpl. 2002;8:1182–1191. doi: 10.1053/jlts.2002.36846. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Nonami T, Kurokawa T, Takeuchi Y, Harada A, Nakao A, Takagi H. Role of endogenous nitric oxide in ischemia-reperfusion injury in rat liver. J Surg Res. 1995;59:772–779. doi: 10.1006/jsre.1995.1238. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Baird NR, Greene AL. Induction of stress response proteins and experimental renal ischemia/reperfusion. Kidney Int. 2001;59:1798–1802. doi: 10.1046/j.1523-1755.2001.0590051798.x. [DOI] [PubMed] [Google Scholar]
- Shoskes DA. Effect of bioflavonoids Quercetin and Curcumin on ischemic renal injury: A new class of renoprotective agents. Transplantation. 1998;66:147–152. doi: 10.1097/00007890-199807270-00001. [DOI] [PubMed] [Google Scholar]
- Amersi F, Buelow R, Kato H, Ke B, Coito AJ, Shen XD, Zhao D, Zaky J, Melinek J, Lassman CR, Kolls JK, Alam J, Ritter T, Volk HD, Farmer DG, Ghobrial RM, Busuttil RW, Kupiec-Weglinski JW. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 1999;104:1631–1639. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooie T, Takahashi N, Saikawa T, Nawata T, Arikawa M, Yamanaka K, Hara M, Shimada T, Sakata T. Single oral dose of Geranylgeranylacetone induces Heat-shock protein 72 and renders protection against ischemia/reperfusion injury in rat heart. Circulation. 2001;104:1837–1843. doi: 10.1161/hc3901.095771. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Takeyoshi I, Yoshinari D, Matsumoto K, Morishita Y. P38 mitogen-activated protein kinase inhibition attenuates ischemia-reperfusion injury of the rat liver. Surgery. 2002;131:344–349. doi: 10.1067/msy.2002.121097. [DOI] [PubMed] [Google Scholar]
- Yoshinari D, Takeyoshi I, Kobayashi M, Koyama T, Iijima K, Ohwada S, Matsumoto K, Morishita Y. Effects of a p38 mitogen-activated protein kinase inhibitor as an additive to University of Wisconsin solution on reperfusion injury in liver transplantation. Transplantation. 2001;72:22–27. doi: 10.1097/00007890-200107150-00007. [DOI] [PubMed] [Google Scholar]
- Zhao TC, Taher MM, Valerie KC, Kukreja RC. p38 triggers late preconditioning elicited by anisomycin in heart: involvement of NF-κB and iNOS. Circ Res. 2001;89:915–922. doi: 10.1161/hh2201.099452. [DOI] [PubMed] [Google Scholar]
- Schwabe RF, Bradham CA, Uehara T, Hatano E, Bennett BL, Schoonhoven R, Brenner DA. c-Jun-N-terminal kinase drives cyclin D1 expression and proliferation during liver regeneration. Hepatology. 2003;37:824–832. doi: 10.1053/jhep.2003.50135. [DOI] [PubMed] [Google Scholar]
- Uchinami H, Yamamoto Y, Kume M, Yonezawa K, Ishikawa Y, Taura K, Nakajima A, Hata K, Yamaoka Y. Effect of heat shock preconditioning on NF-κB/I-κB pathway during I/R injury of the rat liver. Am J Physiol. 2002;282:G962–G971. doi: 10.1152/ajpgi.00466.2001. [DOI] [PubMed] [Google Scholar]
- Qu X, Seale JP, Donnelly R. Tissue and isoform-selective activation of protein kinase C in insulin-resistant obese Zucker rats–effects of feeding. J Endocrinol. 1999;162:207–214. doi: 10.1677/joe.0.1620207. [DOI] [PubMed] [Google Scholar]
- Peralta C, Closa D, Hotter G, Gelpi E, Prats N, Rosello-Catafau J. Liver ischemic preconditioning is mediated by the inhibitory action of nitric oxide on endothelin. Biochem Biophys Res Commun. 1996;229:264–270. doi: 10.1006/bbrc.1996.1790. [DOI] [PubMed] [Google Scholar]
- Peralta C, Hotter G, Closa D, Prats N, Xaus C, Gelpi E, Rosello-Catafau J. The protective role of adenosine in inducing nitric oxide synthesis in rat liver ischemia preconditioning is mediated by activation of adenosine A2 receptors. Hepatology. 1999;29:126–132. doi: 10.1002/hep.510290104. [DOI] [PubMed] [Google Scholar]
- Anwar AA, Li FY, Leake DS, Ishii T, Mann GE, Siow RC. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: role of mitogen-activated protein kinases and Nrf2. Free Radic Biol Med. 2005;39:227–236. doi: 10.1016/j.freeradbiomed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Terry CM, Clikeman JA, Hoidal JR, Callahan KS. TNF-alpha and IL-1alpha induce heme oxygenase-1 via protein kinase C, Ca2+, and phospholipase A2 in endothelial cells. Am J Physiol. 1999;276:H1493–H1501. doi: 10.1152/ajpheart.1999.276.5.H1493. [DOI] [PubMed] [Google Scholar]
- Beuers U, Throckmorton DC, Anderson MS, Isales CM, Thasler W, Kullak-Ublick GA, Sauter G, Koebe HG, Paumgartner G, Boyer JL. Tauroursodeoxycholic acid activates protein kinase C in isolated rat hepatocytes. Gastroenterology. 1996;110:1553–1563. doi: 10.1053/gast.1996.v110.pm8613063. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- Ikeyama S, Kusumoto K, Miyake H, Rokutan K, Tashiro S. A non-toxic heat shock protein 70 inducer, geranylgeranylacetone, suppresses apoptosis of cultured rat hepatocytes caused by hydrogen peroxide and ethanol. J Hepatol. 2001;35:53–61. doi: 10.1016/s0168-8278(01)00053-8. [DOI] [PubMed] [Google Scholar]
- Fudaba Y, Tashiro H, Ohdan H, Miyata Y, Shibata S, Shintaku S, Nishihara M, Ito H, Fukuda Y, Asahara T, Dohi K. Prevention of warm ischemic injury in rat liver transplantation by geranylgeranylacetone. Transplant Proc. 2000;32:1615–1616. doi: 10.1016/s0041-1345(00)01449-4. [DOI] [PubMed] [Google Scholar]
- Hirota K, Nakamura H, Arai T, Ishii H, Bai J, Itoh T, Fukuda K, Yodoi J. Geranylgeranylacetone enhances expression of thioredoxin and suppresses ethanol-induced cytotoxicity in cultured hepatocytes. Biochem Biophys Res Commun. 2000;275:825–830. doi: 10.1006/bbrc.2000.3392. [DOI] [PubMed] [Google Scholar]
- Fudaba Y, Tashiro H, Ohdan H, Miyata Y, Shibata S, Shintaku S, Nishihara M, Asahara T, Ito H, Fukuda Y, Dohi K. Efficacy of HSP72 induction in rat liver by orally administered geranylgeranylacetone. Transpl Int. 2000;13:S278–S281. doi: 10.1007/s001470050341. [DOI] [PubMed] [Google Scholar]
- Fudaba Y, Ohdan H, Tashiro H, Ito H, Fukuda Y, Dohi K, Asahara T. Geranylgeranylacetone, a heat shock protein inducer, prevents primary graft nonfunction in rat liver transplantation. Transplantation. 2001;72:184–189. doi: 10.1097/00007890-200107270-00003. [DOI] [PubMed] [Google Scholar]
- Yamagami K, Yamamoto Y, Ishikawa Y, Yonezawa K, Toyokuni S, Yamaoka Y. Effects of geranyl-geranyl-acetone administration before heat shock preconditioning for conferring tolerance against ischemia-reperfusion injury in rat livers. J Lab Clin Med. 2000;135:465–475. doi: 10.1067/mlc.2000.106806. [DOI] [PubMed] [Google Scholar]
- Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- Kawamura S, Yoshida K, Miura T, Mizukami Y, Matsuzaki M. Ischemic preconditioning translocates PKC-δ and -ε, which mediate functional protection in isolated rat heart. Am J Physiol. 1998;275:H2266–H2271. doi: 10.1152/ajpheart.1998.275.6.H2266. [DOI] [PubMed] [Google Scholar]
- Carini R, De Cesaris MG, Splendore R, Vay D, Domenicotti C, Nitti MP, Paola D, Pronzato MA, Albano E. Signal pathway involved in the development of hypoxic preconditioning in rat hepatocytes. Hepatology. 2001;33:131–139. doi: 10.1053/jhep.2001.21050. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Takahashi N, Ooie T, Kaneda K, Yoshimatsu H, Saikawa T. Role of protein kinase C in geranylgeranylacetone-induced expression of heat-shock protein 72 and cardioprotection in the rat heart. J Mol Cell Cardiol. 2003;35:785–794. doi: 10.1016/s0022-2828(03)00133-0. [DOI] [PubMed] [Google Scholar]
- Redaelli CA, Tian YH, Schaffner T, Ledermann M, Baer HU, Dufour JF. Extended preservation of rat liver graft by induction of heme oxygenase-1. Hepatology. 2002;35:1082–1092. doi: 10.1053/jhep.2002.33067. [DOI] [PubMed] [Google Scholar]
- Kato H, Amersi F, Buelow R, Melinek J, Coito AJ, Ke B, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant. 2001;1:121–128. [PubMed] [Google Scholar]
- Cantoni L, Valaperta R, Ponsoda X, Castell JV, Barelli D, Rizzardini M, Mangolini A, Hauri L, Villa P. Induction of hepatic heme oxigenase-1 by diclofenac in rodents; role of oxidative stress and cytochrome P-450 activity. J Hepatol. 2003;38:776–783. doi: 10.1016/s0168-8278(03)00095-3. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Morita K, Akagi R, Sassa S. Heme oxygenase-1: a novel therapeutic target in oxidative tissue injuries. Curr Med Chem. 2004;11:1545–1561. doi: 10.2174/0929867043365080. [DOI] [PubMed] [Google Scholar]
- Kim YM, de Vera ME, Watkins SC, Billiar TR. Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor-α-induced apoptosis by inducing heat shock protein 70 expression. J Biol Chem. 1997;272:1402–1411. doi: 10.1074/jbc.272.2.1402. [DOI] [PubMed] [Google Scholar]
- Motterlini R, Hidalgo A, Sammut I, Shah KA, Mohammed S, Srai K, Green CJ. A precursor of the nitric oxide donor SIN-1 modulates the stress protein heme oxygenase-1 in rat liver. Biochem Biophys Res Commun. 1996;225:167–172. doi: 10.1006/bbrc.1996.1148. [DOI] [PubMed] [Google Scholar]
- Peralta C, Rull R, Rimola A, Deulofeu R, Rosello-Catafau J, Gelpi E, Rodes J. Endogenous nitric oxide and exogenous nitric oxide supplementation in hepatic ischemia-reperfusion injury in the rat. Transplantation. 2001;71:529–536. doi: 10.1097/00007890-200102270-00008. [DOI] [PubMed] [Google Scholar]
- Lehmann TG, Wheeler MD, Froh M, Schwabe RF, Bunzendahl H, Samulski RJ, Lemasters JJ, Brenner DA, Thurman RG. Effects of three superoxide dismutase genes delivered with an adenovirus on graft function after transplantation of fatty livers in the rat. Transplantation. 2003;76:28–37. doi: 10.1097/01.TP.0000065299.29900.17. [DOI] [PubMed] [Google Scholar]
- Ravingerova T, Barancik M, Strniskova M. Mitogen-activated protein kinases: a new therapeutic target in cardiac pathology. Mol Cell Biochem. 2003;247:127–138. doi: 10.1023/a:1024119224033. [DOI] [PubMed] [Google Scholar]
- Ping P, Zhang J, Huang S, Cao X, Tang XL, Li RC, Zheng YT, Qiu Y, Clerk A, Sugden P, Han J, Bolli R. PKC-dependent activation of p46/p54 JNKs during ischemic preconditioning in conscious rabbits. Am J Physiol. 1999;277:H1771–H1785. doi: 10.1152/ajpheart.1999.277.5.H1771. [DOI] [PubMed] [Google Scholar]
- Amersi F, Shen XD, Anselmo D, Melinek J, Iyer S, Southard DJ, Katori M, Volk HD, Busuttil RW, Buelow R, Kupiec-Weglinski JW. Ex vivo exposure to carbon monoxide prevents hepatic ischemia/reperfusion injury through p38 MAP kinase pathway. Hepatology. 2002;35:815–823. doi: 10.1053/jhep.2002.32467. [DOI] [PubMed] [Google Scholar]
- Miyazono M, Garat C, Morris KG, Jr, Carter EP. Decreased renal heme oxygenase-1 expression contributes to decreased renal function during cirrhosis. Am J Physiol. 2002;283:F1123–F1131. doi: 10.1152/ajprenal.00363.2001. [DOI] [PubMed] [Google Scholar]
- Rodgers PA, Seidman DS, Wei PL, Dennery PA, Stevenson DK. Duration of action and tissue distribution of zinc protoporphyrin in neonatal rats. Pediatr Res. 1996;39:1041–1049. doi: 10.1203/00006450-199606000-00018. [DOI] [PubMed] [Google Scholar]
- Ward KW, Proksch JW, Azzarano LM, Mumaw JA, Roethke TJ, Stelman GJ, Walsch MJ, Zeigler KS, McSurdy-Freed JE, Kehler JR, Chokshi J, Levy MA, Smith BR. Preclinical pharmacokinetics of SB-203580, a potent inhibitor of p38 mitogen-activated protein kinase. Xenobiotica. 2001;31:783–797. doi: 10.1080/00498250110065621. [DOI] [PubMed] [Google Scholar]
- Fujimoto H, Ohno M, Ayabe S, Kobayashi H, Ishizaka N, Kimura H, Yoshida K, Nagai R. Carbon monoxide protects against cardiac ischemia-reperfusion injury in vivo via MAPK and Akt-eNOS pathways. Arterioscler Thromb Vasc Biol. 2004;24:1848–1853. doi: 10.1161/01.ATV.0000142364.85911.0e. [DOI] [PubMed] [Google Scholar]
- Joyeux M, Boumendjel A, Carroll R, Ribuot C, Godin-Ribuot D, Yellon DM. SB 203580, a mitogen-activated protein kinase inhibitor, abolishes resistance to myocardial infarction induced by heat stress. Cardiovasc Drugs Ther. 2000;14:337–343. doi: 10.1023/a:1007847111368. [DOI] [PubMed] [Google Scholar]
- Lai IR, Ma MC, Chen CF, Chang KJ. The protective role of heme oxygenase-1 on the liver after hypoxic preconditioning in rats. Transplantation. 2004;77:1004–1008. doi: 10.1097/01.tp.0000121507.84801.36. [DOI] [PubMed] [Google Scholar]
- Huang TJ, McCoubrey WK, Jr, Maines MD. Heme oxygenase-2 interaction with metalloporphyrins: function of heme regulatory motifs. Antioxid Redox Signal. 2001;3:685–696. doi: 10.1089/15230860152543023. [DOI] [PubMed] [Google Scholar]
- Davies SP, Reddy H, Caivano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada J, Sugimoto M. An inhibitor of p38 and JNK MAP kinases prevents activation of caspase and apoptosis of cultured cerebellar granule neurons. Jpn J Pharmacol. 1999;79:369–378. doi: 10.1254/jjp.79.369. [DOI] [PubMed] [Google Scholar]