Abstract
Neurofibrillary tangles form in a specific spatial and temporal pattern in Alzheimer’s disease. Although tangle formation correlates with dementia and neuronal loss, it remains unknown whether neurofibrillary pathology causes cell death. Recently, a mouse model of tauopathy was developed that reversibly expresses human tau with the dementia-associated P301L mutation. This model (rTg4510) exhibits progressive behavioral deficits that are ameliorated with transgene suppression. Using quantitative analysis of PHF1 immunostaining and neuronal counts, we estimated neuron number and accumulation of neurofibrillary pathology in five brain regions. Accumulation of PHF1-positive tau in neurons appeared between 2.5 and 7 months of age in a region-specific manner and increased with age. Neuron loss was dramatic and region-specific in these mice, reaching over 80% loss in hippocampal area CA1 and dentate gyrus by 8.5 months. We observed regional dissociation of neuronal loss and accumulation of neurofibrillary pathology, because there was loss of neurons before neurofibrillary lesions appeared in the dentate gyrus and, conversely, neurofibrillary pathology appeared without major cell loss in the striatum. Finally, suppressing the transgene prevented further neuronal loss without removing or preventing additional accumulation of neurofibrillary pathology. Together, these results imply that neurofibrillary tangles do not necessarily lead to neuronal death.
Neurofibrillary tangles, which are characteristic of Alzheimer’s disease (AD) and other tauopathies, consist of paired helical filaments of hyperphosphorylated tau protein.1–4 In AD, these lesions accumulate with well defined spatial and temporal progression and correlate with cognitive decline and neuron loss.5–9 Neurofibrillary pathology is therefore implicated in mediating neurodegeneration. However, although neurofibrillary tangles correlate with neuronal loss in AD, the amount of loss far exceeds the amount of tangle formation.8 Thus, whether tangles cause neuronal death remains unclear.
The P301L mutation in the tau protein is associated with a hereditary tauopathy characterized by dementia and development of neurofibrillary pathology.10 A recently developed reversible mouse model of tauopathy, the rTg4510 strain, exhibits progressive memory deficits that can be prevented and even ameliorated by transgene suppression.11 Initial investigations of these mice revealed spatial memory deficits, neurofibrillary lesions, and dramatic neuron loss in the CA1 area of the hippocampus.11 Although the transgene is ubiquitously expressed throughout the cortex, limbic system, and basal ganglia, we noticed that different regions appeared to accumulate neurofibrillary changes and neuronal loss differentially. In tauopathies, formation of neurofibrillary lesions and neuronal loss also occur with regional specificity. For example, in AD the entorhinal cortex and hippocampus are particularly vulnerable12 with pathology occurring later in other cortical and subcortical areas, whereas in frontotemporal dementias neuronal loss is most prominent in frontal and temporal neocortex. 13 Here, we describe characterization of region-specific neuronal loss and neurofibrillary changes in rTg4510 mice using stereological methods and find a region-specific dissociation between neuronal loss and the accumulation of neurofibrillary pathology.
Materials and Methods
Subjects
In this study, we used a recently developed mouse model of tauopathy (rTg4510) in which expression of human tau containing the frontotemporal dementia-associated P301L mutation can be suppressed with doxycycline (dox).11 The human four-repeat tau gene with the P301L mutation is downstream of a tetracycline-operon responsive element. To express tau, this tau responder gene must be co-expressed with an activator transgene consisting of the tet-off open reading frame,14 which is downstream of Ca2+-calmodulin kinase II promoter elements, resulting in P301L tau expression restricted to forebrain structures.15 Tau-expressing mice (transgenic for both a tau responder transgene and an activator transgene) and littermate control mice that do not express tau (lacking either the tau responder transgene or the activator transgene) between 2.5 and 10 months of age were used. No differences were observed between control mice lacking the tau responder transgene and those lacking the activator transgene. dox administration of 200 ppm in the food was used to suppress the tau transgene for 6 weeks. Control mice were also treated with dox. Animals were housed and treated according to institutional and National Institutes of Health standards.
Tissue from the temporal and frontal lobes of one human subject was obtained from the Alzheimer’s Disease Research Center at Massachusetts General Hospital. The subject had frontotemporal dementia and carried the P301L tau mutation (70-year-old male, postmortem interval of 12 hours).
Histology
Tg4510 brains drop-fixed in formalin were embedded in paraffin, and 16-μm parasaggital sections were cut on a Leica RM2155 microtome. Every tenth section (selected on a systematic random basis) was deparaffinized for immunochemistry and blocked in 5% milk for 1 hour. Sections were then immunostained to label tau phosphorylated at serine 396 and 404 with anti-PHF1 primary antibody (1:200 in 5% milk in Tris-buffered saline courtesy of Dr. Peter Davies, Albert Einstein College of Medicine) and horseradish peroxidase-conjugated secondary (1:500 in 5% milk in Tris-buffered saline) developed with diaminobenzidine (0.3 mg/ml in 100 mmol/L Tris plus 0.02% H2O2). Nuclei were counterstained using cresyl violet. Other sections were immunostained with PHF1, CP13 (Peter Davies), or MC1 (Peter Davies) and a secondary anti-mouse IgG conjugated to Cy3 dye (1:200; Jackson ImmunoResearch, West Grove, PA) and then counterstained with Thioflavine S (0.05% in 50% ethanol). After micrographs were obtained, the sections stained with PHF1 and Thioflavine S were restained with Bielchowski silver stain. To confirm that counting neurons based on morphological appearance on cresyl violet-stained sections was accurate as has been previously described,16,17 we stained two sections from 9 mice at 4 months of age with NeuN (1:200; Chemicon, Temecula, CA), and horseradish peroxidase secondary antibody binding was developed with diaminobenzidine and counterstained with cresyl violet. Paraffin-embedded human tissues were cut at 8 μm, and two sections were stained with PHF1 and cresyl violet as described above. Negative controls with no primary antibody added showed no positive reaction in any case. Micrographs of stained tissue were obtained on an upright Olympus BX51 (Olympus, Denmark) fluorescence microscope with a DP70 camera using DPController and DPManager software (Olympus).
Stereology
Gross atrophy, neuron loss, and PHF1-positive aggregate formation were assessed on PHF1/cresyl violet-stained sections from mice in five age groups: 2.5, 4, 5.5, 7, and 8.5 to 10 months. At each age, three untreated tau mice and three to six control mice were used. At all ages, three or four tau mice were treated with dox for 6 weeks before sacrificing (to suppress tau transgene expression).
Unbiased stereologic counting methods were used to determine neuron density and number, PHF1-positive neuron density and number, and volume in five regions of the brain (hippocampal areas CA1, CA2/3, and dentate gyrus (DG); cortex; and striatum). The optical disector method18 was used in a similar fashion to previously described work in transgenic mice.19 Briefly, an image analysis system (CAST; Olympus) mounted on an upright BX51 Olympus microscope with an integrated motorized stage (Prior Scientific, Rockland, MA) was used to outline regions, sample, and count neurons. Neurons and PHF1-positive neurons were counted in a 21.8- × 21.8- × 16-μm counting frame placed using a meander sampling paradigm with step lengths determined to sample 100–300 neurons per region in each hemisphere. Typical step lengths were 1250 μm in cortex, 650 μm in striatum, 250 μm in CA2/3, 200 μm in DG, and 350 μm in CA1. Region volumes were determined according to Cavalieri’s principle, and the total number of neurons in each region was calculated.
Western Blotting
Tissues from five rTg4510 hemispheres and three control hemispheres snap frozen in isopentane on dry ice were homogenized using a sonic dismembrator (Model 500; Fisher Scientific, Pittsburgh, PA) in buffer containing 50 mmol/L Tris/HCl (pH 7.4), 175 mmol/L NaCl, 5 mmol/L EDTA (pH 8.0), and proteinase inhibitor mixture (complete mini; Roche Applied Science, Manheim, Germany). Homogenates were diluted in 2× Tris/glycine sample buffer and run on 10 to 20% polyacrylamide gels using the BioRad mini system at 100 V for approximately 2 hours. Proteins were transferred from the gel to a polyvinylidene difluoride membrane overnight at 20 V and 4°C. Blots were probed with primary antibodies against tau (CP27, mouse monoclonal, 1:2000; Dr. Peter Davies,) and actin (rabbit polyclonal, 1:1000; Sigma-Aldrich, St. Louis, MO), followed by secondary anti-mouse IgG conjugated to Alexa 680 (1:1000; Molecular Probes, Carlsbad, CA) and anti-rabbit IgG conjugated to IR800 (1:1000; Molecular Probes). Blots were imaged using the Odyssey Infrared Imager (Licor BioSciences, Lincoln, NB), and the optical density of the lanes was quantified using Image J (National Institutes of Health, Bethesda, MD).
Statistics
Data are presented as mean ± SD from the mean. Statistical analyses were performed using StatView software (SAS Institute, Cary, NC). Three experimental conditions were used: nontransgenic (control) animals, untreated tau animals (tau), and tau animals treated with dox for 6 weeks to suppress transgene suppression (tau plus dox). To assess the effects of age and the expression of the tau transgene on neuron number and neurofibrillary pathology, two-way analysis of variances were run with age and condition as independent variables. Bonferroni-Dunn posthoc comparisons between different conditions were performed to assess differences between two conditions, for example, whether the tau group was statistically different from the tau plus dox group. To compare the conditions at individual ages, one-way analysis of variances were used with condition as the independent variable and the posthoc Bonferroni-Dunn tests were split by age. Student’s t-test (two-tailed equal variance not assumed) were performed to analyze the difference between one dox-treated age and the age that treatment began in non-dox-treated animals.
Results
rTg4510 Brain Suffers Dramatic Neuron Loss with Regional Vulnerability
In a previous study, marked atrophy of the rTg4510 brain was noted along with neuronal loss in the hippocampal CA1 region.11 Here we characterize neuronal loss in five different regions: hippocampal areas CA1, CA2/3, and dentate gyrus, neocortex, and neostriatum using stereological estimations of cresyl violet-labeled neuronal nuclei (Figure 1). Neurons were identified by nuclear morphology. Comparisons of previous studies that used cresyl violet or NeuN labeling of neurons show almost identical neuron counts in human entorhinal cortex.16,17
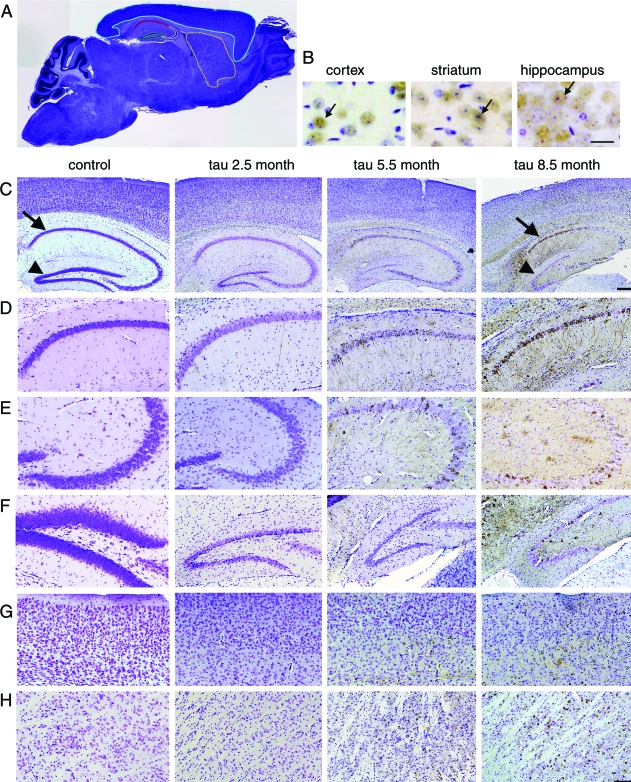
Figure 1.
Region-specific neuron loss in rTg4510 brain. A: We examined five brain regions for neuron loss and PHF1-positive neuron accumulation outlined in a ×1.25micrograph of a section: CA1 (outlined in red), CA2/3 (white), DG (green), neocortex (blue), and striatum (yellow). B: To confirm that cresyl violet-stained nuclei counted as neurons were identified correctly, NeuN immunostaining (brown) and cresyl violet staining were compared, showing colocalization of the neuronal marker with structures identified as neurons using nuclear morphology (arrows). C: At 4× magnification, age-related neuron loss (assessed with cresyl violet stain, blue) is obvious in the CA1 (arrows) and DG (arrowhead). D–H: Higher magnification (10×) reveals PHF1-positive neuron accumulation (brown) with regional differences in CA1 (D), CA2/3 (E), DG (F), cortex (G), and striatum (H). Scale bars, 25 μm (B); 250 μm (C); and 100 μm (D–H).
All regions examined exhibited significant neuronal loss (two-way analysis of variance, Bonferroni-Dunn post hoc test control versus tau, P < 0.0167). The extent and time course of neuronal loss in rTg4510 mice varied substantially by region, with the hippocampal formation exhibiting the most dramatic neuron loss compared to the average control neuron number (Figure 2 and Table 1). By 8.5 months, rTg4510 neuronal loss amounted to 85% in DG, 82% in CA1, 69% in CA2/3, and 52% in cortex. Although significant, the neuronal loss in striatum was minimal (Figure 2). In all areas except striatum, neuronal loss was age related. Bonferroni-Dunn posthoc analyses of one-way analysis of variances split by age were used as a conservative measure of significantly different numbers of neurons at each age (control versus tau), showing that neuron loss begins in CA1 and CA2/3 by 5.5 months, very early in DG (2.5 months), later in cortex (8.5 months), and no significant loss at any specific age in striatum (although as mentioned above, an analysis of variance that considers all age groups together indicates some neuronal loss in this area).
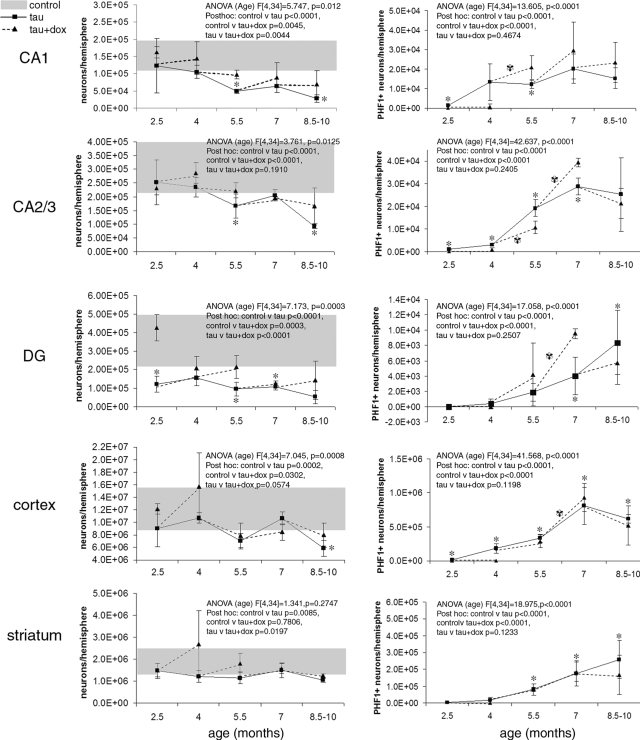
Figure 2 .
Region-specific neuronal loss (left) and accumulation of PHF1-positive neurofibrillary pathology (right) were examined in rTg4510 mice. All regions exhibited neuronal loss compared to control animals (average of controls at all ages ± SD, gray bars), and in all regions except striatum, this loss was age-related, as can be seen by the two-way analysis of variance results presented with each graph (age and condition [control, tau, and tau+dox] as independent variables, Bonferroni-Dunn posthoc tests). CA1 and DG undergo the most severe neuron loss. In CA1, 68% of neurons are lost by 5.5 months of age. At this same age, 25% of remaining neurons are PHF1-positive, growing to 56% PHF1-positive by 8.5 months (when 82% of neurons have died). In the dentate gyrus, neurons are lost early (by 2.5 months, P = 0.004) despite the complete absence of PHF1-positive neurons at this age, indicating that neuronal loss can occur before neurofibrillary lesions. rTg4510 CA2/3 and cortex also suffer significant neuronal loss, which reaches 69% and 52%, respectively, by 8.5 months. Suppression of the tau transgene with dox for 6 weeks prevented further neuron loss in all regions at all ages examined (dotted lines). All regions exhibited age-related PHF1-positive accumulation, which began by 2.5 months in the CA1, CA2/3, and cortex, by 5.5 months in striatum, and by 7 months in DG (the asterisks show posthoc comparison of control versus tau at individual ages). Tau transgene suppression did not remove existing neurofibrillary pathology in any region at any age examined. In fact, comparing the number of PHF1-positive neurons at the age when treatment began to the number after 6 weeks of suppression shows an increase in pathology in several cases (black and white asterisk symbols). In CA2/3, suppression from 4 to 5.5 months resulted in a significant prevention of further accumulation, although existing PHF1-positive neurons were not removed. This exception indicates a temporal and regional variability in the effects of transgene suppression.
Table 1.
Onset Ages
| Region | Age-related neuronal loss | Onset of neuronal loss (months) | Onset of PHF1 accumulation (months) | Prevention of loss with tau suppression | Prevention of PHF1 accumulation with tau suppression |
|---|---|---|---|---|---|
| CA1 | Yes | 5.5 | 2.5 | Yes | Only before 2.5 months |
| CA2/3 | Yes | 5.5 | 2.5 | Yes | Only before 5.5 months |
| DG | Yes | 2.5 | 7 | Yes | No |
| Cortex | Yes | 8.5 | 2.5 | Yes | Only before 4 months |
| Striatum | No | 5.5 | No |
Onset ages were determined by the first age at which posthoc analysis showed a difference between tau and control (for neuronal loss and PHF1 accumulation) and between tau and dox-treated tau (for prevention of PHF1 accumulation).
The more dramatic loss of neurons in hippocampus was not caused by higher levels of tau expression. Western blots from five rTg4510 mice and three control mice at 2.5 months show equal amounts of tau protein expressed in the hippocampus, cortex, and striatum of rTg4510 mice (Figure 3, A and B). These data also indicate that the early loss of neurons in the DG (beginning between 2.5 and 4 months) was not due to higher levels of tau in the hippocampal formation. Further, recent work from Ramsden et al showed that, in the forebrain, levels of soluble tau protein remain stable throughout the life of the transgenic animals, although Sarkosyl-insoluble tau increases with age.20
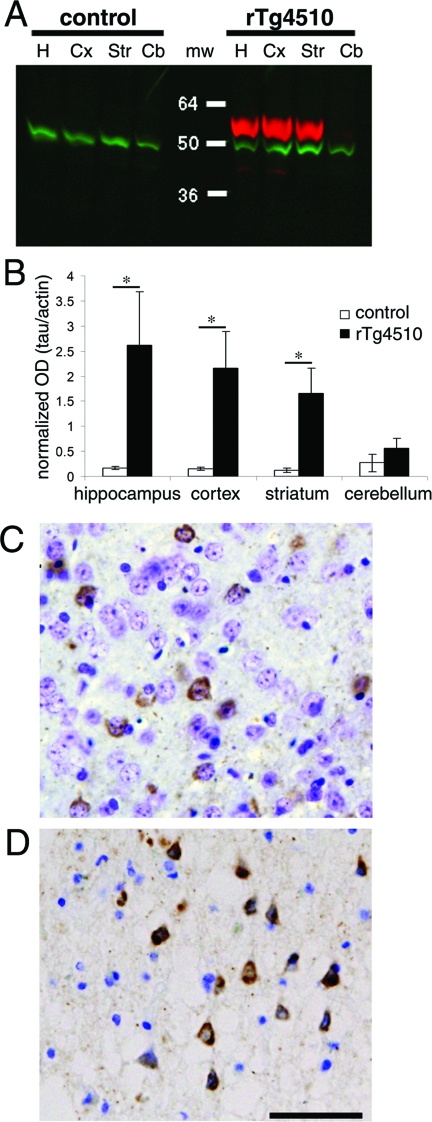
Figure 3.
Tau expression in rTg4510 mice. A: Western blot analysis of 5 rTg4510 mice and three control mice revealed that the neuronal loss already evident by 2.5 months in rTg4510 dentate gyrus is not induced by excessive tau protein (red) in hippocampus (lane H) compared to the cortex (lane Cx) and striatum (lane Str, mw = molecular weight). B: Using tau normalized to actin (green) as a loading control, quantitative analysis shows that none of the forebrain regions are significantly different from any of the others. Cerebellum (lane Cb) does not express the tau transgene and has significantly less tau expression than all forebrain areas (hippocampus P = 0.012, cortex P = 0.007, striatum P = 0.006). rTg4510 hippocampus, cortex, and striatum also have significantly more tau expression than the same areas of control brain (*, P < 0.01). C and D: PHF1-positive aggregates of tau (brown) in cortical neuronal soma of rTg4510 mice (C) appear morphologically very similar to those in a human case of tauopathy with the same P301L mutation (D). Scale bar, 50 μm.
Age-related Neurofibrillary Pathology Does Not Always Accompany Neuronal Loss
rTg4510 mice developed age-related PHF1-positive inclusions that closely resemble those from human tauopathy (Figure 3, C and D).11,12,21 In the temporal lobe of a 70-year-old patient with dementia associated with the P301L mutation, 4.4% of neurons were PHF1-positive, similar to the 10% of cortical neurons that were positive in P301L mice at an advanced age. In all of the regions examined in rTg4510 mice, intraneuronal PHF1-positive tau aggregates accumulated in an age-dependent manner (analysis of variances F[1,16] P < 0.0001 in all regions). Surprisingly, however, PHF1-positive tau staining did not always correlate with the pattern of neuronal loss. Although in CA1 there was both substantial accumulation of PHF1-positive cells and neuronal loss, other regions showed a clear dissociation. In DG, 53% of neurons were lost by 4 months of age, before PHF1-positive neurons appeared, and by 8.5 months, only 18% of the remaining neurons (ie, ∼3% of the original DG cells) were PHF1 immunoreactive (Figure 2). By contrast, in striatum, 11% of neurons at 7 months and 25% at 8.5 months of age exhibited PHF1 immunoreactivity, but there was no statistically significant loss of neurons at these time points (Figure 2). Because PHF1 is a relatively late marker of tau pathology, we also used CP13 (an early marker for tau phosphorylated at serine 20222) and MC1 (an early marker of conformational change23) on 2.5-month sections to determine whether the early loss of neurons in DG is preceded by these earlier markers. We found almost no cells positive for these markers in DG (three DG cells labeled with CP13 and one with MC1 in two sections each from four animals among thousands of unstained DG neurons), although there were some positive cells in CA1 and cortex at this age, as has been recently reported.20 Thus, the early loss of neurons in DG does appear to precede any detectable changes in tau confirmation, phosphorylation, or solubility.
Transgene Suppression Prevents Neuron Loss but Not Neurofibrillary Lesion Formation
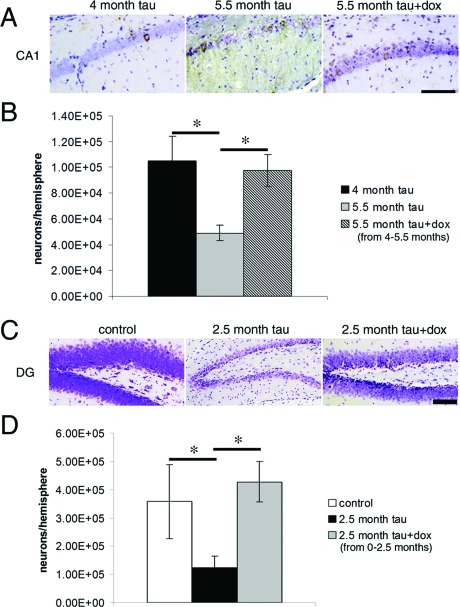
Suppression of tau transgene expression for 6 weeks using dox halted the progressive neuronal loss. In all five regions examined, the number of neurons per hemisphere was not significantly different between the age when suppression began and after 6 weeks of treatment (Figure 2, dotted lines, and Table 1), even when significant loss would be expected over this time period as was the case in CA1 and cortex between 4 and 5.5 months and in DG before 2.5 months. Figure 4 shows examples in CA1 and DG where transgene suppression with 6 weeks of dox treatment produced a qualitatively discernable, dramatic prevention of neuronal loss. In DG, the early drastic neuronal loss in rTg4510 mice was completely prevented by transgene suppression from 0 to 2.5 months, indicating that neuronal loss is degenerative and not due to developmental abnormalities.
Figure 4.
Micrographs demonstrating the dramatic prevention of neuronal loss with transgene suppression. A and B: Suppression of the P301L tau transgene with dox for 6 weeks, from 4 to 5.5 months of age, prevented further neuron loss in CA1 without significantly affecting neurofibrillary pathology. C and D: Similarly, the drastic early loss of neurons in DG between birth and 2.5 months could be prevented completely by transgene suppression implying that the neuronal loss is degenerative instead of developmental. Scale bars, 100 μm. *, P < 0.01; t-test.
In contrast, transgene suppression did not remove existing neurofibrillary pathology (eg, the number of PHF1-positive cells did not decrease from the levels at the beginning of treatment in any region in any age group tested (Figure 2)). In fact, in a few cases, the amount of PHF1-positive cells increased over 6 weeks of treatment despite transgene suppression. This was the case in CA1 and CA2/3 with suppression from 4 to 5.5 months and in CA2/3, DG, and cortex with treatment from 5.5 to 7 months (Figure 2). In CA2/3, suppression from 4 to 5.5 months prevented as many new PHF1-positive cells from appearing as would occur without treatment, indicating a selective differential fate of PHF1-immunoreactive tau deposition in different regions. Suppression beginning before pathology started to accumulate did prevent PHF1 accumulation. Animals that had been treated from birth to 2.5 months did not have any PHF1-positive cells in any region, even though in CA1, CA2/3, and cortex there are significant numbers of PHF1-positive cells at 2.5 months without treatment.
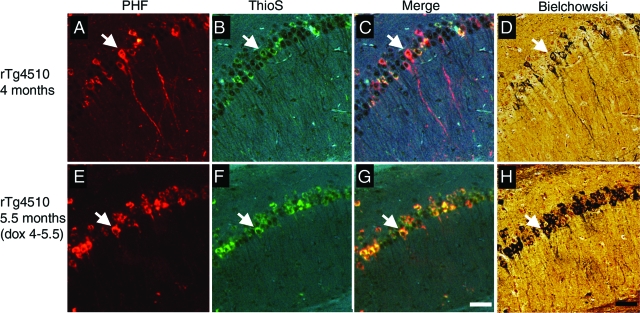
Counterstaining fluorescent immunostaining for PHF1 with Thioflavine S and subsequent staining with Bielchowski silver stain show that, after transgene suppression, Thioflavine S-positive and argyrophilic neurofibrillary lesions also persisted (Figure 5). This suggests that the persistence of neurofibrillary pathology after 6 weeks of transgene suppression is not merely conservation of the hyperphosphorylated state. Over this same time period of suppression, previous data showed a reduction of ∼85% in transgene mRNA and 70% in soluble protein levels.11 This shows that phosphorylated tau aggregates are relatively long-lived despite drastic reductions in tau production, but they do not necessarily lead to neuronal death.
Figure 5.
Persistent neurofibrillary pathology after transgene suppression. A and E: PHF1-positive accumulations in neurons persist after 6 weeks of transgene suppression. B and F: These lesions are also Thioflavine S (ThioS)-positive as can be seen in the merged images (C and G). D and H: Bielchowski silver staining shows that these same lesions are argyrophilic, indicating abnormal confirmation as well as hyperphosphorylation persists after transgene suppression. Arrows indicate cells positive for PHF1, Thioflavine S, and Bielchowski stains. Scale bars, 100 μm.
Discussion
In AD and other tauopathies, phospho-tau-immunoreactive inclusions and cell death occur differentially in different brain regions. Regional vulnerability has been extensively characterized in AD over the past decades. In AD layer II of the entorhinal cortex, CA1, and subiculum are particularly vulnerable to both neurofibrillary lesions and neuronal death, whereas CA2/3, DG, and presubiculum are far less affected.24,25 In the AD cortex, primary sensory and motor areas are less vulnerable than association areas.26–28 Frontotemporal dementia and Parkinsonism linked to chromosome 17 (FTDP-17) has been associated with mutations in tau, including the P301L mutation used in the rTg4510 mice.10 In P301L-associated FTDP-17, pretangles and neurofibrillary tangles have been observed in cortex, subiculum, and hippocampus.29 To some extent, our observations of the rTg4510 brain mimic the regional vulnerability observed in these tauopathies, because we see that the hippocampus is particularly vulnerable to neuronal death and neurofibrillary lesions. We find extensive, early neuronal loss of dentate gyrus granule cells in rTg4510 mice that is prevented by transgene suppression from birth. The DG does not undergo extensive cell loss in AD,30 although it does suffer dramatic biochemical changes that doubtless affect its function.31 In FTDP-17, the DG is vulnerable to accumulation of neurofibrillary pathology.32 In the case of rTg4510 mice, it cannot be ruled out that high levels of expression of any transgene could be neurotoxic and cause cell loss; however, transgenic models with similar levels of transgene expression do not exhibit extensive cell death such as we observe.19
Here we show that suppressing the mutant tau transgene with dox prevents further neuron loss without reducing the amount of neurofibrillary pathology in several brain regions. Minocycline, an antibiotic that like dox is a member of the tetracycline family, has well documented protective effects against neurodegeneration.33 It is possible that dox has similar neuroprotective effects that could contribute to some extent to the protection we observe when suppressing the transgene in rTg4510 mice. However, the literature on the protective effects of dox is inconclusive. In both adult and neonatal rat models of ischemia, dox treatment protects against ischemic degeneration.34,35 However, in a rat model of striatal degeneration, dox does not protect against quinolinic acid-induced neurodegeneration even though minocycline is protective.36 Although we cannot exclude some protective effect of dox in the current results, it is not likely to account for all of the protection we see with transgene suppression and, further, would not explain why neurofibrillary lesions are persistent with suppression.
The continued accumulation of PHF1-positive neurons without production of new tau is surprising. Transgene suppression lowers tau production ∼85% from untreated levels,11 leaving some minor amount of expression that could contribute to this phenomenon. Tau that has been produced before transgene suppression could also acquire the phosphorylation at serine 396 and 404 after suppression begins, thus making it recognizable by the PHF1 antibody. We also cannot exclude endogenous mouse tau contributing to neurofibrillary pathology.
The mechanism of cell death in these disorders remains elusive. Generally, neuronal death pathways are grouped based on changes in nuclear morphology and the presence of activated caspases as either apoptotic or necrotic.37 Elements of both apoptosis and necrosis have been found in AD,38 and several putative initiating agents have been identified, including reactive oxygen species and oligomeric amyloid-β,39–41 which relate to amyloid pathology. Tau pathology is also heavily implicated in cell death. Previous studies using transgenic mice expressing human tau showed accumulation of neurofibrillary pathology and neuron loss,42–45 implying a causal relationship between the two. In a recent study of mice expressing nonmutant human tau in the absence of mouse tau, Andorfer et al46 reported that age-related cell death did not correlate directly with the presence of tau filaments, thus implying that tau filaments do not directly cause cell death in accord with our present data. This same study showed some terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling staining and morphological evidence of apoptosis such as chromatin condensation and nuclear breakdown, but they did not find any activated caspases. Further, Andorfer et al46 found some dying cells that had morphological features of necrosis. In Drosophila, overexpression of human tau can result in neurodegeneration without tangle formation,47 and Shulman and Feany48 reported genetic modifiers implicating apoptosis in tau toxicity in fly models. Data from AD brains suggest that tangles correlate with neuron loss in hippocampal formation and often cortex,5–8 although neuronal loss exceeds tangle number in AD.8 The rTg4510 model provides the unique opportunity to tease out the relationship between neurofibrillary pathology and cell death and the regional vulnerability associated with them.
Our results show that 1) tau-induced neuron loss and neurofibrillary pathology accumulation can be dissociated, with CA1 and DG suffering the most dramatic neuron loss and CA1, CA2/3, and striatum accumulating the largest proportion of PHF1-positive neurons, 2) neuron loss can occur before, and therefore independently of, neurofibrillary pathology, 3) not all regions that develop PHF1 tau immunostaining undergo extensive neuron loss, and 4) tangles can be apparently long-lived, with neurons containing PHF1-immunoreactive inclusions remaining viable for at least 6 weeks after the tau transgene is suppressed. Together, these results dissociate vulnerability to P301L tau-induced neurofibrillary pathology and neuron death in a region-specific manner. Although some cells with tangles may be marked for death, these results coupled with previous work8,11 strongly suggest that cells without detectable neurofibrillary pathology are also vulnerable to degeneration. Importantly, the ability of transgene suppression to halt neuronal loss and the recent finding that suppression allows recovery of memory in these mice11 show the remarkable potential for recovery in the aged mammalian brain and lend hope that appropriate therapies may provide some recovery in tauopathies.
Acknowledgments
We thank Karlotta Fitch, Matthew Frosch, Cintia Conrad, Phill Jones, and Chris Conrad for valuable contributions to the manuscript and the Massachusetts Alzheimer Disease Research Center for access to tissue samples.
Footnotes
Address reprint requests to Bradley T. Hyman, MassGeneral Institute for Neurodegenerative Disease, Alzheimer Unit, 114 16th Street, Charlestown, MA 02129. E-mail: bhyman@partners.org.
Supported by National Institutes of Health grants AG26249, AG08487, and AG00277 and by an Alzheimer Association Pioneer Award.
T.L.S. and J.D.O. contributed equally to this work.
References
- Kidd M. Paired helical filaments in electron microscopy in Alzheimer’s disease. Nature. 1963;197:192–193. doi: 10.1038/197192b0. [DOI] [PubMed] [Google Scholar]
- Brion JP, Couck AM, Passareiro E, Flament-Durand J. Neurofibrillary tangles of Alzheimer’s disease: an immunohistochemical study. J Submicrosc Cytol. 1985;17:89–96. [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc Natl Acad Sci USA. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball MJ. Neuronal loss, neurofibrillary tangles and granulovacuolar degeneration in the hippocampus with ageing and dementia. A quantitative study. Acta Neuropathol (Berl) 1977;37:111–118. doi: 10.1007/BF00692056. [DOI] [PubMed] [Google Scholar]
- Bondareff W, Mountjoy CQ, Roth M, Hauser DL. Neurofibrillary degeneration and neuronal loss in Alzheimer’s disease. Neurobiol Aging. 1989;10:709–715. doi: 10.1016/0197-4580(89)90007-9. [DOI] [PubMed] [Google Scholar]
- Terry RD, Peck A, DeTeresa R, Schechter R, Horoupian DS. Some morphometric aspects of the brain in senile dementia of the Alzheimer type. Ann Neurol. 1981;10:184–192. doi: 10.1002/ana.410100209. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, Parisi JE, Hyman BT. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- Arriagada P, Growdon J, Hedley-Whyte E, Hyman B. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski JQ, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JB, Schofield PR, Andreadis A, Snowden JS, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, Van Swieten JC, Mann DM, Lynch T, Heutink P. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- SantaCruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Hyman BT. Ageing and dementia/Alzheimer’s disease. Graham DI, Lantos PL, editors. New York: Arnold,; Greenfield’s Neuropathology. 2002:pp 200–226. [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, Mufson EJ. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol. 2001;49:202–213. [PubMed] [Google Scholar]
- West MJ, Gundersen HJ. Unbiased stereological estimation of the number of neurons in the human hippocampus. J Comp Neurol. 1990;296:1–22. doi: 10.1002/cne.902960102. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. APPSw transgenic mice develop age-related A beta deposits and neurophil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997;56:965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, Guimaraes A, Yue M, Lewis J, Carlson G, Hutton M, Ashe KH. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J Neurosci. 2005;25:10637–10647. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizawa K, Ksiezak-Reding H, Davies P, Delacourte A, Tiseo P, Yen SH, Dickson DW. A double-labeling immunohistochemical study of tau exon 10 in Alzheimer’s disease, progressive supranuclear palsy and Pick’s disease. Acta Neuropathol (Berl) 2000;100:235–244. doi: 10.1007/s004019900177. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Weaver C, Lane E, Vianna C, Kress Y, Rockwood J, Davies P. cAMP-dependent protein kinase phosphorylations on tau in Alzheimer’s disease. J Neurosci. 1999;19:7486–7494. doi: 10.1523/JNEUROSCI.19-17-07486.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jicha GA, Bowser R, Kazam IG, Davies P. Alz-50 and MC-1, a new monoclonal antibody raised to paired helical filaments, recognize conformational epitopes on recombinant tau. J Neurosci Res. 1997;48:128–132. doi: 10.1002/(sici)1097-4547(19970415)48:2<128::aid-jnr5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Horsen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. The cellular basis of cortical disconnection in Alzheimer disease and related dementing conditions. Terry R, Katzman R, Bick KL, Sisoda SS, editors. Philadelphia: Lippincott Williams & Wilkins,; Alzheimer disease. 1999:pp 207–232. [Google Scholar]
- Pearson RC, Esiri MM, Hiorns RW, Wilcock GK, Powell TP. Anatomical correlates of the distribution of the pathological changes in the neocortex in Alzheimer disease. Proc Natl Acad Sci USA. 1985;82:4531–4534. doi: 10.1073/pnas.82.13.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Terry RD, Morrison JH. Laminar and regional distributions of neurofibrillary tangles and neuritic plaques in Alzheimer’s disease: a quantitative study of visual and auditory cortices. J Neurosci. 1987;7:1799–1808. doi: 10.1523/JNEUROSCI.07-06-01799.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Rosso SM, Kamphorst W, Ravid R, Van Swieten JC. Coexistent tau and amyloid pathology in hereditary frontotemporal dementia with tau mutations. Ann NY Acad Sci. 2000;920:115–119. doi: 10.1111/j.1749-6632.2000.tb06912.x. [DOI] [PubMed] [Google Scholar]
- West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Jones B, Kekonius L, Chin J, Yu G-Q, Raber J, Masliah E, Mucke L. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer’s disease-related cognitive deficits. Proc Natl Acad Sci USA. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Swieten JC, Stevens M, Rosso SM, Rizzu P, Joosse M, de Koning I, Kamphorst W, Ravid R, Spillantini MG, Niermeijer, Heutink P. Phenotypic variation in hereditary frontotemporal dementia with tau mutations. Ann Neurol. 1999;46:617–626. doi: 10.1002/1531-8249(199910)46:4<617::aid-ana10>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Blum D, Chtarto A, Tenenbaum L, Brotchi J, Levivier M. Clinical potential of minocycline for neurodegenerative disorders. Neurobiol Dis. 2004;17:359–366. doi: 10.1016/j.nbd.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Clark WM, Lessov N, Lauten JD, Hazel K. Doxycycline treatment reduces ischemic brain damage in transient middle cerebral artery occlusion in the rat. J Mol Neurosci. 1997;9:103–108. doi: 10.1007/BF02736854. [DOI] [PubMed] [Google Scholar]
- Jantzie LL, Cheung P-Y, Todd KG. Doxycycline reduces cleaved caspase-3 and microglial activation in an animal model of neonatal hypoxia-ischemia. J Cereb Blood Flow Metab. 2005;25:314–324. doi: 10.1038/sj.jcbfm.9600025. [DOI] [PubMed] [Google Scholar]
- Bantubungi K, Jacquard C, Greco A, Pintor A, Chtarto A, Tai K, Galas M-C, Tenenbaum L, Deglon N, Popoli P. Minocycline in phenotypic models of Huntington’s disease. Neurobiol Dis. 2005;18:206–217. doi: 10.1016/j.nbd.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Behl C. Apoptosis and Alzheimer’s disease. J Neural Transm. 2000;107:1325–1344. doi: 10.1007/s007020070021. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. Amyloid-beta oligomers: their production, toxicity and therapeutic inhibition. Biochem Soc Trans. 2002;30:552–557. doi: 10.1042/bst0300552. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Hickman SE, Thomas CA, Loike JD, Silverstein SC. Microglia, scavenger receptors, and the pathogenesis of Alzheimer’s disease. Neurobiol Aging. 1998;19:S81–S84. doi: 10.1016/s0197-4580(98)00036-0. [DOI] [PubMed] [Google Scholar]
- McLellan ME, Kajdasz ST, Hyman BT, Bacskai BJ. In vivo imaging of reactive oxygen species specifically associated with thioflavine S-positive amyloid plaques by multiphoton microscopy. J Neurosci. 2003;23:2212–2217. doi: 10.1523/JNEUROSCI.23-06-02212.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz J, Chen F, Barmettler R, Nitsch RM. Tau filament formation in transgenic mice expressing P301L tau. J Biol Chem. 2001;276:529–534. doi: 10.1074/jbc.M006531200. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Hong M, Zhang B, Nakagawa Y, Lee MK, Trojanowski JQ, Lee VM. Age-dependent emergence and progression of a tauopathy in transgenic mice overexpressing the shortest human tau isoform. Neuron. 1999;24:751–762. doi: 10.1016/s0896-6273(00)81127-7. [DOI] [PubMed] [Google Scholar]
- Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Murphy M Paul, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- Andorfer C, Kress Y, Espinoza M, de Silva R, Tucker KL, Barde YA, Duff K, Davies P. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem. 2003;86:582–590. doi: 10.1046/j.1471-4159.2003.01879.x. [DOI] [PubMed] [Google Scholar]
- Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci. 2005;25:5446–5454. doi: 10.1523/JNEUROSCI.4637-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann CW, Wszolek MF, Shulman JM, Salvaterra PM, Lewis J, Hutton M, Feany MB. Tauopathy in Drosophila: neurodegeneration without neurofibrillary tangles. Science. 2001;293:711–714. doi: 10.1126/science.1062382. [DOI] [PubMed] [Google Scholar]
- Shulman JM, Feany MB. Genetic modifiers of tauopathy in Drosophila. Genetics. 2003;165:1233–1242. doi: 10.1093/genetics/165.3.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]