Abstract
Platelet-activating factor (PAF), a potent lipid mediator with various biological activities, plays an important role in inflammation by recruiting leukocytes. In this study we used platelet-activating factor receptor (PAFR)-deficient mice to elucidate the role of PAF in inflammatory renal injury induced by folic acid administration. PAFR-deficient mice showed significant amelioration of renal dysfunction and pathological findings such as acute tubular damage with neutrophil infiltration, lipid peroxidation observed with antibody to 4-hydroxy-2-hexenal (day 2), and interstitial fibrosis with macrophage infiltration associated with expression of monocyte chemoattractant protein-1 and tumor necrosis factor-α in the kidney (day 14). Acute tubular damage was attenuated by neutrophil depletion using a monoclonal antibody (RB6-8C5), demonstrating the contribution of neutrophils to acute phase injury. Macrophage infiltration was also decreased when treatment with a PAF antagonist (WEB2086) was started after acute phase. In vitro chemotaxis assay using a Boyden chamber demonstrated that PAF exhibits a strong chemotactic activity for macrophages. These results indicate that PAF is involved in pathogenesis of folic acid-induced renal injury by activating neutrophils in acute phase and macrophages in chronic interstitial fibrosis. Inhibiting the PAF pathway might be therapeutic to kidney injury from inflammatory cells.
Inflammation is an important component of renal injury, in both acute renal failure1,2 and chronic renal damage that accompanies interstitial fibrosis.3,4 The infiltrating inflammatory cells contribute to renal damage through generation of reactive oxygen species (ROS), further recruitment of leukocytes, and production of proinflammatory and profibrotic cytokines. Therefore, the mechanism of directing circulating leukocytes to the kidney and maintaining them there is a potential target for therapeutic intervention for kidney diseases.
Platelet-activating factor (PAF; 1-O-alkyl-2-acethl-sn-glycero-3-phosphocholine) is a potent proinflammatory phospholipid mediator with various biological effects such as activating platelets, neutrophils, eosinophils, and macrophages. PAF binds to a G-protein-coupled seven transmembrane receptor, PAF receptor (PAFR); PAF plays an important role in the pathophysiology of inflammatory conditions.5,6 Synthesis of PAF has been demonstrated not only in blood cells but in the kidney under physiological conditions,7 ischemia reperfusion injury,8 and clipped kidney.9 Treatment with several PAF antagonists has been reported in acute renal failure10 and chronic renal failure animal models.11,12 However, problems exist regarding the lack of specificity of PAF antagonists.13–16 Therefore, we have developed genetically engineered PAFR-deficient (PAFR-KO) mice to confirm the role of PAF in vivo.17 The resultant PAFR-KO mice exhibit attenuated symptoms in several animal models such as chemical lung injury,18 bronchial asthma,19 and sponge-induced subcutaneous granuloma formation.20 It has been demonstrated that infiltrating inflammatory cells play important roles in these animal models.
The folic acid (FA)-induced renal injury model has been applied recently for evaluation of epithelial regeneration and interstitial fibrosis.21–24 Intraperitoneal administration of FA with sodium bicarbonate induced renal injury that showed acute tubular necrosis with transient elevations of blood urea nitrogen (BUN) and serum creatinine (Cr) followed by development of interstitial fibrosis with macrophage infiltration.21,25 Tubular obstructions by FA crystals and direct toxic effect of FA to tubular epithelial cells presumably cause renal damage,26 but the precise mechanism of injury remains unclear. Inflammatory cell infiltrations were remarkable in injured kidneys of this model.
Here we examined the role of PAF in inflammatory renal injury induced by FA administration. Using PAFR-KO mice, we show attenuation of renal injury not only in cases of acute tubular damage but also in cases of development of chronic interstitial fibrosis in vivo. In addition, we evaluated the effects of neutrophil depletion and PAF antagonist treatment after the acute phase to confirm the contribution of leukocytes to FA-induced renal injury. We also demonstrate the role of PAF as a strong chemoattractant for macrophages in vitro, indicating that PAF blockade can prevent progression of inflammatory damage in kidney.
Materials and Methods
Reagents
Folic acid was obtained from Sigma Chemical Co. (St. Louis, MO) and WEB2086 was a generous gift from Boehringer (Ingelheim, Germany). We used a mouse antibody to 4-hydroxy-2-hexenal (HHE)-modified protein, in which the aldehyde HHE is derived from the oxidative process of polyunsaturated fatty acid, obtained from NOF Corp. (Tokyo, Japan) and a mouse anti-smooth muscle α actin (α-SMA) monoclonal antibody (1A4) (DakoCytomation Co. Ltd., Glostrup, Denmark) for immunohistochemical and Western blot analyses. We used a rat anti-mouse neutrophil antibody (Caltag Laboratories, Burlingame, CA), a rat anti-mouse F4/80 macrophage antigen antibody (MCA497R) (Serotec, Raleigh, NC), and a goat anti-mouse MCP-1/JE antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA) for immunohistochemical analyses. The monoclonal antibody RB6-8C5 was used for neutrophil depletion. It is a rat IgG2b that selectively binds to and depletes mature neutrophils but not lymphocytes or macrophages.27–31 The hybridoma that secretes this antibody was a gift from Dr. R. Coffman (DNAX Research Inc., Palo Alto, CA); RB6-8C5 from hybridoma culture supernatants was purified through ammonium sulfate precipitation. All other chemicals were purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan) unless otherwise specified.
Animal Model
We established PAFR-KO mice as described previously.17 The PAFR-KO mice and the corresponding wild-type (PAFR-WT) mice were backcrossed for 10 generations onto a C57BL/6N genetic background. Mice were allowed food and water ad libitum, and experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals [Department of Health, Education and Welfare Publication No. (NIH) 86-23, Revised 1985, Office of Science and Health Reports, DRR/NIH, Bethesda, MD]. Male and female mice used for this study were 9 to 11 weeks of age, weighing 18 to 25 g. Within each experimental group, the sex ratio, age, and weight of animals did not differ significantly. The animals were anesthetized with diethyl ether and administered FA by intraperitoneal injection at the dose of 250 mg/kg in vehicle (0.5 ml of 0.3 mmol/L NaHCO3) or given vehicle alone. The dose of FA and NaHCO3 was critical for induction of severe renal damage and was determined by preliminary studies. Blood was drawn from the tail vein of each animal, and the levels of BUN and Cr were measured 3 days before and at 2, 7, and 14 days after FA administration by the urease-indophenol method with Urea N B (Wako Pure Chemical Industries Ltd.) and the colorimetric method based on hydrogen peroxide measurement with Nescoat VLII CRE (Alfresa Pharma Corp., Osaka, Japan), respectively. Kidneys were removed 2 and 14 days after FA administration and fixed in 10% buffered formalin for staining with hematoxylin and eosin, Masson’s trichrome, and immunochemical staining, except for mouse neutrophil detection for which methyl Carnoy’s solution was used. Parts of kidneys were snap-frozen for immunohistochemical testing of MCP-1; they were otherwise used for Western blot analysis and real-time polymerase chain reaction (PCR) assay.
For neutrophil depletion experiments, PAFR-WT mice were treated with 50 μg of monoclonal antibody RB6-8C5. Injection of FA was performed the following day, and the animals were sacrificed 2 days after FA administration. Injection of 25 μg of monoclonal antibody RB6-8C5 was reported to cause significant neutrophil depletion from the subsequent day up to 3 days.32–34 Blood collected at sacrifice was used for flow cytometry analyses to confirm neutrophil depletion. Mice that were untreated with RB6-8C5 served as control animals. To clarify the contribution of PAF for macrophage recruitment after initial damage, we intraperitoneally injected PAF antagonist (WEB2086) into PAFR-WT mice every day from day 2 to day 14 at the dose of 5 mg/kg and examined renal function (days 2, 7, and 14), macrophage infiltration, and interstitial fibrosis (day 14).
Flow Cytometry Analysis
Heparinized blood was incubated with anti-Gr-1 antibodies conjugated to fluorescein isothiocyanate (BD Pharmingen, San Diego, CA), and then Flowcount fluorospheres (Beckman Coulter Inc., Villepinte, France) were added. After red blood cell lysis with NH4Cl buffer, cell suspension was run on a FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA), and the absolute count of Gr-1-positive cells was calculated using standardized Flowcount beads.
Morphological Evaluation of Kidneys
The area of the interstitial fibrosis in the cortex was evaluated with Masson’s trichrome staining using a computer-aided evaluation program (AIS version 4.0; Fuji Photo Film Co. Ltd., Tokyo, Japan). Viewed at ×400 magnification, 10 randomly selected nonoverlapping fields from the cortical region were analyzed. The fibrotic areas stained in blue were depicted in digital images; then the percentage of the fibrotic area was calculated relative to the entire field area (percentage area). Glomeruli and large vessels were not included in the microscopic fields for image analysis. The degree of neutrophil and macrophage infiltration was measured as the average number of positive staining cells per field. The number of positively stained cells was determined in five randomly selected nonoverlapping fields at ×200 magnification in each section of the individual mouse renal cortex. Scores of respective kidneys as well as scores of each animal were averaged.
Western Blot Analysis
Western blot analyses were performed as described previously.35 Briefly, harvested kidneys were homogenized on ice in a radioimmunoprecipitation assay buffer with protease inhibitors. The lysates were separated electrophoretically on a 10% polyacrylamide gel. After transferring proteins from the gel to a nitrocellulose membrane, Western blot analyses were performed using HHE or α-SMA antibody. After incubation with horseradish peroxidase-conjugated appropriate second antibody, chemiluminescent signal detection (ECL Plus; Amersham Biosciences Corp., Uppsala, Sweden) was performed using a cooled charge-coupled device camera system (LAS-1000; Fuji Photo Film Co. Ltd.). The membrane was incubated at 50°C for 30 minutes in a stripping buffer to remove all probes. Then it was reprobed with the antibody to α-tubulin (Santa Cruz Biotechnology Inc.). Densitometric analyses of bands compared with the density of α-tubulin were performed using National Institutes of Health Image software, version 1.62.
Immunohistochemical Analysis
Immunohistochemical staining of 2-μm paraffin sections was performed using indirect immunohistochemical techniques. Biotin-free immunohistochemical staining using horseradish peroxidase-conjugated polymer system was conducted according to the manufacturer’s protocols (Histofine Mouse Stain kit and Histofine Simple Stain mouse MAX-PO (rat) kit; Nichirei Corp., Tokyo, Japan). The deparaffinized sections were preincubated with 0.3% hydrogen peroxide for 15 minutes and incubated with primary antibodies overnight at 4°C, followed by polymer-conjugated anti-mouse IgG. Protease K treatment was necessary for anti-F4/80 antibody; autoclaving in 10 mmol/L citrate-buffer (pH 6.0) was necessary for anti-HHE and anti-α-SMA antibody. For neutrophil staining, fixation with methyl Carnoy’s solution was indispensable.36 Diaminobenzidine tetrahydrochloride (Nichirei Corp.) was used for the substrate-chromogen reaction followed by counter staining with hematoxylin.
We used frozen sections of kidney to determine the immunohistochemistry of MCP-1, as described previously37 but with modifications. With 8-μm cryostat sections fixed in acetone and goat anti-mouse MCP-1 antibody (Santa Cruz Biotechnology Inc.), a Vectastain Elite ABC kit (Vector Laboratories Inc., Burlingame, CA) and diaminobenzidine tetrahydrochloride were applied in accordance with the manufacturers’ instructions. Sections were counterstained with methyl green (Vector Laboratories Inc.).
Real-Time PCR Assay for TNF-α and MCP-1 Expression
Harvested kidneys were homogenized with a homogenizer (Ultra-Turrax T8; IKA Labortechnik, Staufen, Germany) in Trizol total RNA isolation reagent (Invitrogen Corp., Carlsbad, CA). Total RNA was isolated by the acid guanidinium isothiocyanate-phenol-chloroform extraction method according to the manufacturer’s protocols and treated with DNase I to remove potential contamination of DNA (DNA-free; Ambion Inc., Austin, TX). The mRNA was then reverse-transcribed to cDNA using MLV reverse transcriptase (RiverTra Ace; Toyobo Co. Ltd., Osaka, Japan) with random hexamers. Transcripts encoding tumor necrosis factor (TNF)-α and monocyte chemoattractant protein (MCP)-1 were measured using TaqMan real-time quantitative PCR with Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA) using TaqMan universal PCR master mix (Applied Biosystems) according to the manufacturer’s specifications. The TaqMan probes and primers for TNF-α (assay identification number Mm00443258_m1) and MCP-1 (assay identification number Mm00441242_m1) were assay-on-demand gene expression products (Applied Biosystems). The PCR reaction conditions were 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. To normalize for variance in loaded cDNA, 18S ribosomal RNA were amplified in a separate tube. Amplification data were analyzed using Applied Biosystems’ Prism sequence detection software version 2.1 (Applied Biosystems). Standard curves were prepared for each gene and the 18S ribosomal RNA in each experiment to normalize the relative expression of the genes of interest to the 18S ribosomal RNA control.
Measurement of PAF Levels in Kidney
Harvested kidneys were frozen immediately with liquid nitrogen at days 2 and 14. The PAF levels in kidney were measured by reverse-phase high performance liquid chromatography-electrospray ionization-tandem mass spectrometry technique, as described in precedent studies.38,39
Migration Assay
Elicited peritoneal macrophages were harvested from PAFR-KO and PAFR-WT mice 3 days after injection of 4% thioglycolate. Peritoneal exudate cells were washed with phosphate-buffered saline and resuspended in RPMI 1640 medium supplemented with 0.1% bovine serum albumin. Cell migration was evaluated using a 96-well Boyden chamber as described previously, with minor modifications.40 The PAF at concentrations of 0, 1, and 10 nmol/L in 300 μl of RPMI 1640 containing 0.1% bovine serum albumin was added to the lower wells of a chemotaxis chamber (Neuro Probe, Inc., Gaithersburg, MD). A polycarbonate filter (8-μm-pore size; Neuro Probe, Inc.) was layered onto the wells; 65 μl of cell suspension (4.0 × 106/ml) were applied to the upper wells. The chamber was incubated at 37°C in a humidified atmosphere in the presence of 5% CO2 for 2 hours. At the end of incubation, the filter was removed, fixed with methanol, and stained with Diff-Quick (Sysmex Corp., Hyogo, Japan). Cells on the upper side of the filter were wiped away. The number of cells that had migrated to the lower side was determined by measuring optical densities at 595 nm using a 96-well microplate reader (model 3550; Bio-Rad Laboratories Inc., Hercules, CA). Each experiment was performed in triplicate.
Statistical Analysis
Results of statistical analyses are expressed as means ± SEM. Differences among groups at the same time point were examined using Student’s t-test. Differences among experimental groups at different time point were confirmed by one-way analysis of variance followed by the Tukey-Kramer test for individual comparison of group means. These calculations were performed using StatView-J5.0 (SAS Institute Inc., Cary, NC). The null hypothesis was rejected when P was <0.05.
Results
Attenuation of Renal Dysfunction in PAFR-KO Mice and Efficacy of PAFR Antagonist
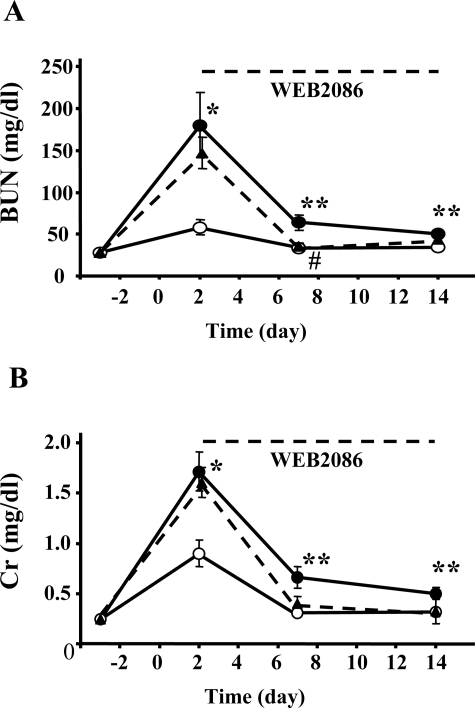
Administration of FA with sodium bicarbonate induced transient elevation of BUN and Cr at 48 hours after injection followed by subsequent renal dysfunction accompanied with interstitial fibrosis. Basal levels of BUN and Cr in PAFR-WT and PAFR-KO mice were similar [PAFR-WT: BUN 28.1 ± 0.8 mg/dl, Cr 0.25 ± 0.01 mg/dl (n = 16) versus PAFR-KO: BUN 27.8 ± 0.9 mg/dl, Cr 0.24 ± 0.01 mg/dl (n = 16)]. The measurement at 48 hours after FA injection showed statistically significant elevation of BUN and Cr compared with baseline in both PAFR-WT and PAFR-KO mice (PAFR-WT, P < 0.001; PAFR-KO, P < 0.005), and the levels of BUN and Cr in PAFR-WT mice were significantly higher compared with levels in PAFR-KO mice [PAFR-WT: BUN 180.0 ± 39.0 mg/dl, Cr 1.71 ± 0.20 mg/dl (n = 16) versus PAFR-KO: BUN 58.2 ± 8.9 mg/dl, Cr 0.90 ± 0.13 mg/dl (n = 16); P < 0.005] (Figure 1). The significant differences were also valid at days 7 and 14 [PAFR-WT: BUN 63.7 ± 9.4 mg/dl, Cr 0.66 ± 0.11 mg/dl (n = 8) versus PAFR-KO: BUN 33.1 ± 3.3 mg/dl, Cr 0.31 ± 0.04 mg/dl (n = 8); P < 0.05, at day 7] [PAFR-WT: BUN 50.2 ± 5.2 mg/dl, Cr 0.50 ± 0.06 mg/dl (n = 8) versus PAFR-KO: BUN 33.8 ± 3.3 mg/dl, Cr 0.31 ± 0.04 mg/dl (n = 8); P < 0.05, at day 14]. Treatment with WEB2086 after acute phase from day 2 to day 14 also showed partial protective effects for renal dysfunction [day 2: BUN 146.9 ± 18.7 mg/dl, Cr 1.60 ± 0.15 mg/dl; day 7: BUN 35.5 ± 3.6 mg/dl, Cr 0.39 ± 0.07 mg/dl; day 14: BUN 43.4 ± 3.9 mg/dl, Cr 0.32 ± 0.12 mg/dl (n = 5)]. In addition, there was a significant difference between WEB2086-treated and untreated PAFR-WT mice in BUN level at day 7 (P < 0.05) (Figure 1).
Figure 1.
Time course of BUN (A) and Cr (B) levels in PAFR-KO and PAFR-WT mice subjected to FA administration. Filled circles indicate PAFR-WT mice, open circles indicate PAFR-KO mice, and filled triangles indicate WEB2086-treated PAFR-WT mice. WEB2086 was injected every day from days 2 to 14. The numbers of animals were 16 at days −3 and 2, 8 at days 7 and 14 in PAFR-WT and PAFR-KO mice, and 5 in WEB2086-treated PAFR-WT mice. *P < 0.005, **P < 0.05 (PAFR-WT versus PAFR-KO). #P < 0.05 (PAFR-WT versus WEB2086-treated PAFR-WT).
Neutrophil and ROS Production in Acute Tubular Damage
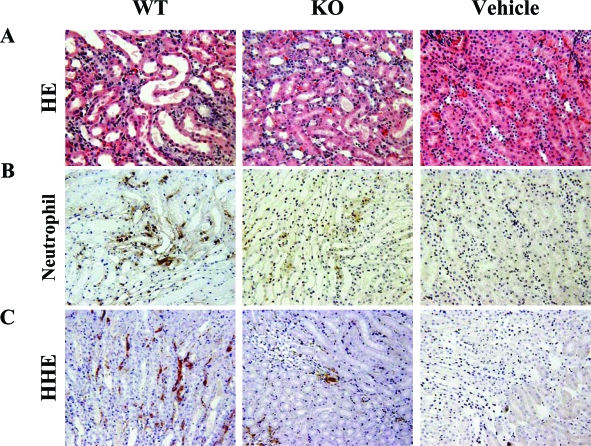
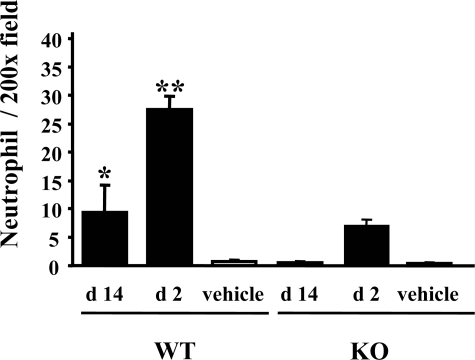
In addition to attenuation of renal dysfunction, morphological analyses demonstrated that acute tubular necrosis was significantly milder in PAFR-KO mice than in PAFR-WT mice at 48 hours after FA administration (Figure 2A). Immunohistochemical staining with anti-neutrophil antibody showed that infiltrating neutrophils decreased significantly in PAFR-KO mice compared with PAFR-WT mice at day 2 [PAFR-WT: 27.5 ± 2.3/×200 field (n = 4) versus PAFR-KO: 6.9 ± 1.0/×200 field (n = 4); P < 0.001] and were detected predominantly around the damaged tubules (Figure 2B and Figure 3).
Figure 2.
Histological findings at day 2 stained with H&E (A), immunohistochemistry using antibody to neutrophil (B), and antibody to HHE-modified proteins (C). Vehicle-treated PAFR-KO and PAFR-WT showed identical results. Therefore, only PAFR-WT mice are shown. Original magnifications, ×200.
Figure 3.
Number of infiltrating neutrophils in FA-injured kidney. Five animals were in the FA group; four received the vehicle. *P < 0.005 versus PAFR-KO mice at day 14 (d 14). **P < 0.00005 versus PAFR-KO mice at day 2 (d 2).
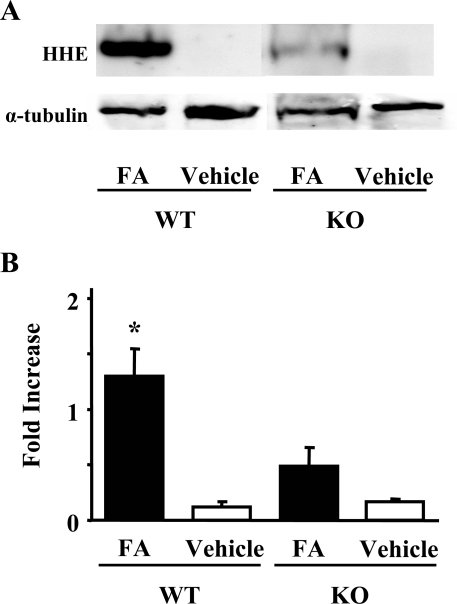
Through propagation of lipid peroxidation, ROS will damage tissue. Antibody to HHE, produced in the peroxidative metabolism of ω-3 polyunsaturated fatty acids in cell membranes, is a specific marker to detect lipid peroxidation.35 Immunohistochemical analyses dominantly detected HHE-modified proteins around peritubular capillaries of damaged kidney tissues (Figure 2C). Lipid peroxidation was also quantified in kidney homogenates by Western blot analysis. In PAFR-WT mice, kidney homogenates showed a higher intensity of immunoreactive bands of HHE-modified proteins compared with those from PAFR-KO mice (Figure 4).
Figure 4.
Western blot analysis of HHE-modified protein expression in kidney homogenates at day 2. A representative image is shown in A (top). B: The histogram depicts the relative density of bands in comparison with α-tubulin. Five animals were in the FA group; three received the vehicle. *P < 0.05 versus PAFR-KO mice with FA administration.
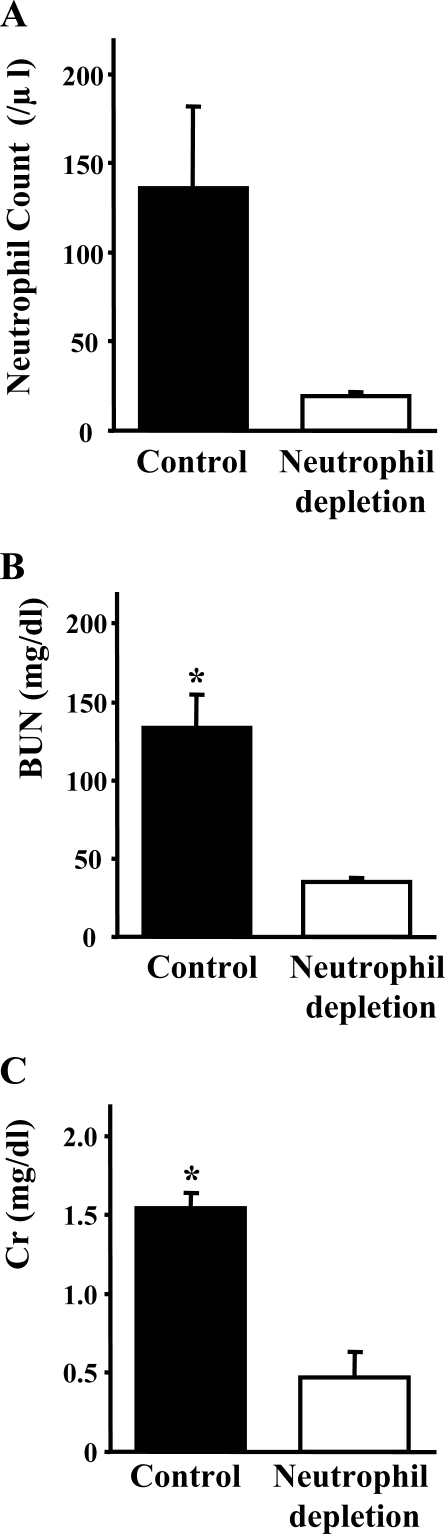
To evaluate the contribution of neutrophils for acute phase of FA-induced renal injury at day 2, we also injected FA into PAFR-WT mice pretreated with neutrophil depletion antibody. Neutrophil depletion was confirmed through flow cytometry analyses (Figure 5A). These animals showed remarkable amelioration of renal dysfunction [control: BUN 133.5 ± 21.1 mg/dl, Cr 1.54 ± 0.09 mg/dl (n = 5) versus neutrophil depletion: BUN 35.3 ± 2.4 mg/dl, Cr 0.47 ± 0.16 mg/dl (n = 5); P < 0.005] (Figure 5, B and C).
Figure 5.
Effects of neutrophil depletion on acute phase of FA-induced renal injury. Injection of a monoclonal neutrophil depletion antibody (RB6-8C5) at day −1 caused a significant decrease of neutrophil count in blood of PAFR-WT mice at day 2. A: Renal dysfunction at day 2 was attenuated with treated PAFR-WT mice compared with untreated PAFR-WT mice (B and C). *P < 0.005 versus untreated PAFR-WT mice. Five animals were in each group.
Development of Interstitial Fibrosis and Macrophage Infiltration
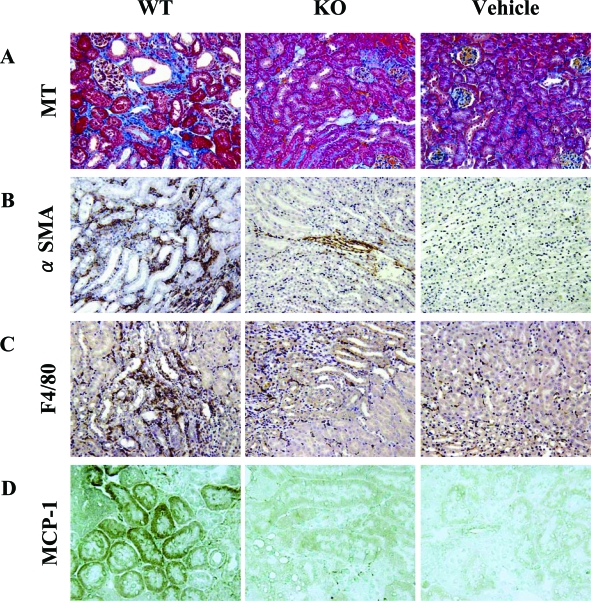
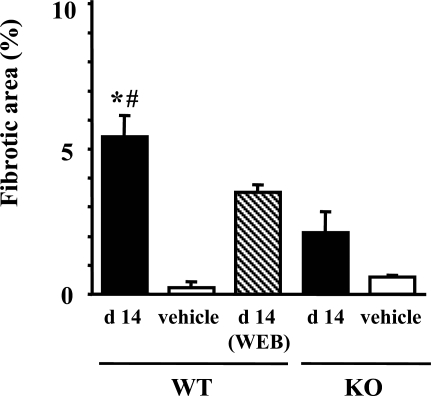
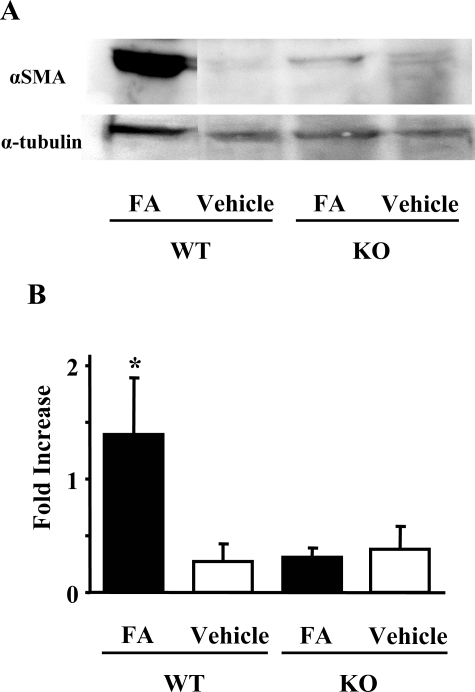
The FA-induced renal injury model showed development of segmental interstitial fibrotic lesions at day 14. Figure 6A shows that interstitial fibrotic areas stained in blue by Masson’s trichrome staining were more prominent in kidneys of PAFR-WT mice than in those of PAFR-KO mice. Quantitative analyses demonstrated that the fibrotic area in PAFR-WT mice was significantly larger than PAFR-KO mice [PAFR-WT: 5.4 ± 0.7% (n = 5) versus PAFR-KO: 2.1 ± 0.7% (n = 5); P < 0.05] (Figure 7). Immunohistochemical and Western blot analyses with anti-α-SMA antibody were performed to detect interstitial myofibroblasts. Positive staining of α-SMA was found in the identical site of fibrotic area stained blue with Masson’s trichrome staining (Figure 6B) and seen in vascular smooth muscle cells (data not shown). Kidney homogenates of PAFR-WT mice were shown by Western blot analysis to have a higher intensity of immunoreactive bands of α-SMA than those from PAFR-KO mice (Figure 8).
Figure 6.
Histological findings at day 14 stained with Masson’s trichrome (A), immunohistochemistry using antibody to α-SMA (B), antibody to F4/80-positive macrophage (C), and immunohistochemical analyses using antibody to MCP-1 with frozen sections. Vehicle-treated PAFR-KO and PAFR-WT showed identical results. Therefore, only PAFR-WT mice results are shown. Original magnifications: ×100 (A); ×200 (B, C); ×400 (D).
Figure 7.
The fibrotic area in the cortex from the kidney at day 14 (d 14) was calculated using a computer-aided evaluation program. Five animals were in the FA group; four received the vehicle. *P < 0.05 versus PAFR-KO mice. *P < 0.05 versus PAFR-KO mice with FA administration. #P < 0.05 versus WEB2086-treated PAFR-WT mice.
Figure 8.
Western blot analysis of α-SMA expression in kidney homogenates at day 14. A representative image is depicted in A (top). B: The histogram illustrates the relative density of bands in comparison with α-tubulin. Five animals were in the FA group; four received the vehicle. *P < 0.05 versus PAFR-KO mice with FA administration.
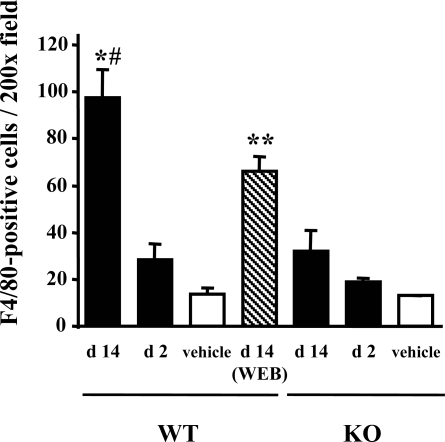
Macrophage infiltration was evaluated using immunostaining for F4/80, a specific macrophage antigen. Infiltrated F4/80-positive macrophages were detected in the peritubular interstitium around fibrotic areas in kidneys harvested 14 days after FA administration (Figure 6C). The number of infiltrated F4/80-positive macrophages at day 14 in PAFR-WT mice was significantly higher than that in PAFR-KO mice [PAFR-WT: 97.5 ± 11.9/×200 field (n = 5) vs. PAFR-KO: 32.1 ± 8.6/×200 field (n = 5); P < 0.05] (Figure 9). In contrast, the numbers of F4/80-positive cells in kidneys harvested at 48 hours after FA administration did not differ among groups [PAFR-WT: 28.5 ± 6.7/×200 field (n = 5) versus PAFR-KO: 18.7 ± 2.0/×200 field (n = 5); P = 0.21].
Figure 9.
Number of infiltrating F4/80-positive cells in the tubulointerstitium with FA-induced renal injury. Five animals were in the FA group; four received the vehicle. *P < 0.005 versus PAFR-KO mice at day 14 (d 14). #P < 0.05 versus WEB2086-treated PAFR-WT mice at day 14. **P < 0.05 versus PAFR-WT mice at day 14.
The PAFR-WT mice treated with WEB2086 after acute phase every day (from day 2 to day 14) showed significantly decreased interstitial fibrosis and macrophage infiltration at day 14 compared with untreated PAFR-WT mice [fibrotic area: 3.5 ± 0.3% (n = 5); P < 0.05 versus untreated PAFR-WT mice and macrophage counts: 66.0 ± 6.3/×200 field (n = 5); P < 0.05 versus untreated PAFR-WT mice] (Figures 7 and 9).
TNF-α and MCP-1 Expression in FA-Induced Renal Injury
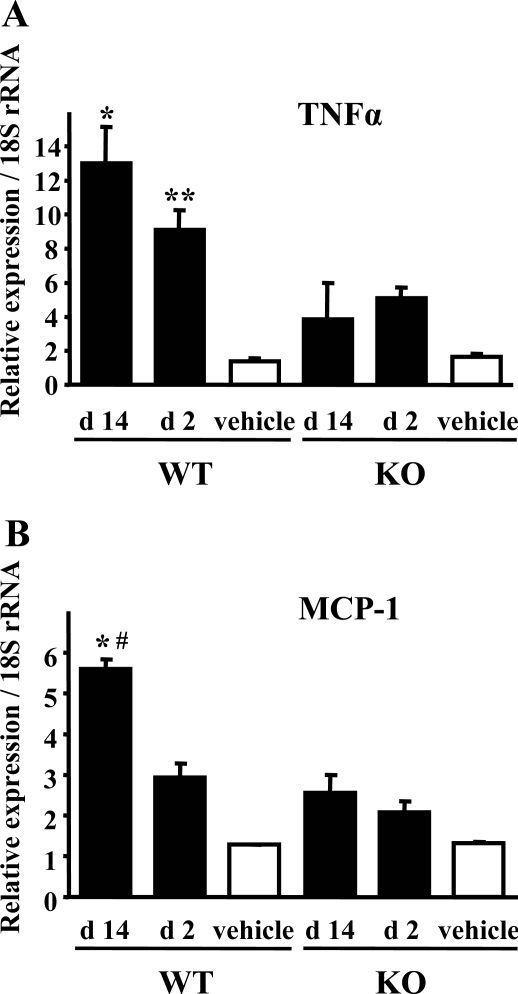
We examined TNF-α and MCP-1 mRNA expression using TaqMan real-time PCR assay to confirm the contribution of macrophages for FA-induced renal injury. Decreased expression of both TNF-α and MCP-1 were shown in the kidney of PAFR-KO mice compared with PAFR-WT mice (Figure 10). Furthermore, increased expression of these cytokines at day 14 was found only in PAFR-WT mice. These results indicate that macrophage accumulation to the injured kidney was amplified even after acute tubular damage at day 2. In addition, immunohistochemical analysis at day 14 revealed that MCP-1 protein was expressed dominantly in the proximal tubular epithelium (Figure 6D).
Figure 10.
Quantitative analyses of expression of TNF-α (A) and MCP-1 (B) using real-time PCR. Eight animals were in the FA group; three received the vehicle. A: TNF-α expression was higher in PAFR-WT mice, both at days 2 and 14. *P < 0.005 versus PAFR-KO mice at day 14 (d 14), **P < 0.05 versus PAFR-KO mice at day 2 (d 2). B: MCP-1 expression was higher in PAFR-WT mice than in PAFR-KO mice at day 14. *P < 0.05 versus PAFR-KO mice at day 14. Moreover, MCP-1 expression increased in PAFR-WT mice at day 14 greater than that at day 2. #P < 0.05 versus PAFR-WT mice at day 2.
PAF Levels in Kidney
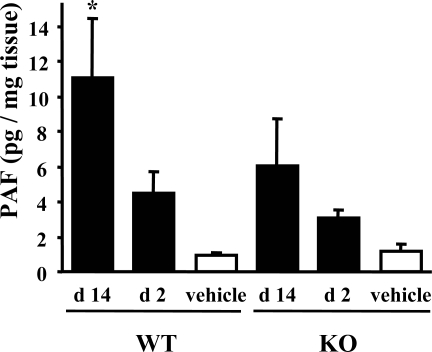
To clarify the presence of PAF and PAFR pathway in FA-induced renal injury, we examined the levels of PAF in the kidney. The renal PAF levels were virtually the same in normal kidney of PAFR-KO and PAFR-WT mice [PAFR-WT: 0.96 ± 0.15 pg/mg tissue (n = 4) versus PAFR-KO: 1.16 ± 0.39 pg/mg tissue (n = 4)] and increased by administration of FA, especially at day 14 in PAFR-WT mice [PAFR-WT at day 14, 11.08 ± 3.38 pg/mg tissue (n = 4) versus PAFR-WT normal kidney; P < 0.05] (Figure 11). The PAF levels in PAFR-WT mice were higher than those in PAFR-KO mice, especially at day 14, but those differences were not statistically significant.
Figure 11.
PAF level in FA-injured kidney. Four animals were in each group. *P < 0.05 versus PAFR-WT mice receiving the vehicle.
PAF-Induced Chemotaxis in Macrophage
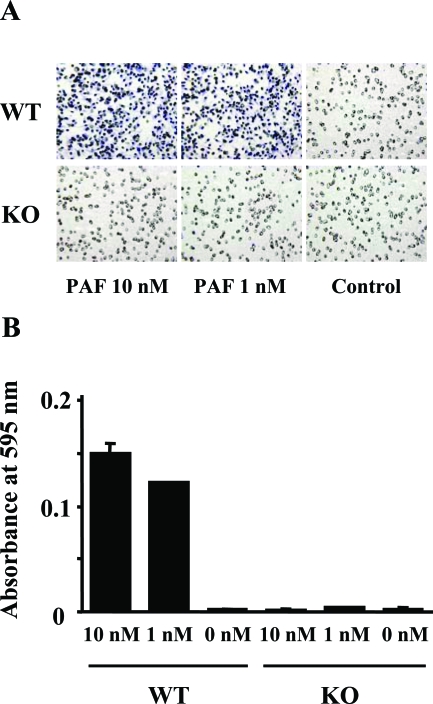
Infiltration of F4/80-positive macrophages in interstitial fibrosis of the kidney was attenuated in PAFR-KO mice; PAF is also known as a potent chemoattractant for leukocytes. For that reason, we examined the direct chemotactic activity of macrophages to PAF using a Boyden chamber system. Macrophages isolated from PAFR-WT mice showed a remarkable chemotactic activity to PAF at concentrations of 1 and 10 nmol/L. On the other hand, PAF induced no chemotaxis in macrophages isolated from PAFR-KO mice (Figure 12).
Figure 12.
PAF-induced chemotaxis in elicited peritoneal macrophages harvested from PAFR-KO and PAFR-WT mice. A: Light microscope analysis of the migration toward PAF in the Boyden chamber assay. B: Quantitative analysis with measured absorbance at 595 nm. Each experiment was performed in triplicate. Original magnifications, ×200.
Discussion
This study demonstrated that FA-induced renal injury comprises an acute and a subsequent chronic phase. Infiltration of ROS-producing neutrophils in acute tubular damage and macrophages in chronic interstitial fibrosis was attenuated significantly in PAFR-KO mice in com-parison with PAFR-WT mice. Moreover, treatments of PAFR-WT mice with neutrophil depletion antibody and PAFR antagonist, which were started after the acute phase, also showed significant amelioration of acute tubular damage and macrophage infiltration and interstitial fibrosis in the chronic phase. Therefore, our data indicate that leukocytes play a crucial role both in the acute phase and the chronic phase of this animal model and that PAF is involved in pathogenesis of FA-induced inflammatory renal injury. To our knowledge, this study is the first to demonstrate the attenuation of renal injury in PAFR-KO mice, although several precedent studies have shown the effects of PAFR antagonists on renal disease in animal models.10–12 However, those studies using PAFR antagonists often include problems related to drug specificity. Some PAFR antagonists inhibit the effect of histamine through interaction with its G protein-coupled receptor13,15; other antagonists inhibit the activity of PAF acetylhydrolase, which inactivates PAF.14,16 The present study demonstrates the pathological role of PAF in FA-induced renal injury using genetically engineered mice in which PAFR signaling was decreased congenitally by disrupting the PAFR gene. Several reports have used PAFR-KO mice to elucidate the role of PAF in various pathophysiologies. The PAFR-KO mice exhibited attenuation in animal models in which inflammatory injuries were crucial: chemical lung injury,18 bronchial asthma,19 and sponge-induced subcutaneous granuloma formation.20 On the other hand, PAFR-KO mice showed exacerbation of infection models such as pulmonary gram-negative bacteria infection41 and cardiac Trypanosoma infection.42 Those reports, along with the present study, indicate that PAF causes both beneficial and de-leterious effects by activating leukocytes in different circumstances.
The neutrophil infiltration and lipid peroxidation of HHE in kidney at day 2 in PAFR-KO mice were significantly lower than those of PAFR-WT mice in this study. Stimulation of neutrophils with PAF in vitro engenders numerous cellular responses including chemotaxis,43–45 degranulation,46 and ROS production.43,47 Priming neutrophils with PAF enhances ROS production by NADPH oxidase, which catalyzes superoxide production.48 Through in vivo analysis with ischemia-reperfusion kidney injury, Kelly and colleagues49 showed that PAFR antagonist reduced neutrophil infiltration. Lloberas and colleagues50 showed that antioxidant treatment with vitamin C decreased PAF release from kidney and neutrophil infiltration to kidney. Moreover, neutrophil depletion by a monoclonal antibody RB6-8C5 significantly ameliorated FA-induced acute tubular damage in this study. These findings indicate that ROS produced by activated infiltrating neutrophils play an important role in FA-induced acute tubular damage. On the other hand, incomplete recovery of FA-induced renal injury in PAFR-KO mice and better improvement in neutrophil-depleted PAFR-WT mice than PAFR-KO mice, despite the lack of statistical difference between them, indicates that additional factors related to neutrophil activation might contribute to FA-induced injury.
Recruitment and activation of macrophages and lymphocytes to the kidney play a central role in a final pathway of most injuries to chronic kidney damage. Therefore, regulating these immunocompetent cells might be a potential target for therapeutic intervention for kidney disease.4 The PAFR-KO mice showed significant amelioration of FA-induced renal injury that developed to remarkable interstitial fibrosis with macrophage infiltration accompanied by the increase in the expression of TNF-α and MCP-1. Contribution of TNF-α for interstitial fibrosis was demonstrated in unilateral ureter obstruction, another renal fibrosis model, with TNF-α receptor knockout mice.51 In PAFR-KO mice, reduced macrophage infiltration number and lack of a PAF receptor pathway might engender decreased TNF-α expression in the kidney and subsequent interstitial fibrosis. MCP-1 is presumed to play a potent role in macrophage chemotaxis and activation. Decreased expression of MCP-1 in PAFR-KO mice was in accordance with attenuated macrophage infiltration. MCP-1 production has been reported in tubular epithelial cells in unilateral ureter obstruction model52 and cultured renal proximal tubular epithelial cells.53 We also found MCP-1 production in tubular epithelial cells by immunohistochemistry that was decreased in PAFR-KO mice. Furthermore, PAF activates nuclear factor-κB,54 which is critical for production of MCP-1 in tubular epithelial cells.55 Expression of PAFR has been demonstrated in microdissected rat tubular cells56 and tubular cell line LLC-PK1.57 Taken together, it is quite plausible that PAF causes MCP-1 production in renal tubular cells. Indeed, Jocks and colleagues58 showed MCP-1 induction by PAF in glomerular immune injury model with isolated perfused rat kidney. Beaudeux and colleagues59 showed that PAF stimulated MCP-1 release in monocytes isolated from human peripheral blood. Further examination is required to clarify the role of PAF receptor pathway in renal tubular cells in this model.
We further examined the effect of blocking the PAF receptor pathway on macrophage recruitment with PAFR antagonist WEB2086 in vivo. It is noteworthy that treatment with WEB2086, even after acute tubular injury, also attenuated macrophage infiltration and interstitial fibrosis. Because we showed the chemotaxis activity of PAF to macrophages by Boyden chamber chemotaxis assay, PAF is likely to have a direct effect on the recruitment of macrophages to the injured kidney. However, renal functions and macrophage infiltration were not improved by WEB2086 to the degree of those of PAFR-KO mice. This might be because the degree of initial injury was so severe that WEB2086 could not improve renal function. Alternatively, WEB2086 could not suppress the PAF receptor pathway completely in this injury because of unfavorable pharmacokinetics and pharmacodynamics. Although several reports have described the effects of a single injection of WEB2086 at the dose of 5 to 10 mg/kg on mouse inflammation models,60,61 the optimal administration dose for chronic animal models and renal dysfunction models remains unclear. Further evaluation is required for effects of WEB2086 on FA-induced renal injury.
We measured PAF levels in the kidney and found higher PAF levels in the kidneys of PAFR-WT mice than in those of PAFR-KO mice. This result indicated that there was an additional portion of PAF produced by oxidative stress secondary to initial injury and this component was decreased in PAFR-KO mice. We also found higher PAF levels at the chronic phase (day 14) than in the acute phase (day 2), especially in PAFR-WT mice. Changes of PAF levels in the kidney were similar to those of macrophage counts and MCP-1 expression. Therefore, it is plausible that PAF produced by initial injury enhanced macrophage infiltration to the kidney through MCP-1 expression and that the recruited macrophages produced PAF in the kidney. Our data indicate a vicious cycle of an amplifying effect of macrophage recruitment that is exacerbated by the PAF pathway.
The present study demonstrates the involvement of PAF in FA-induced renal inflammatory injury. Blockade of the PAF receptor pathway significantly attenuates both acute tubular damage and subsequent chronic development of interstitial fibrosis by reducing infiltrating leukocytes. Such a blockade suggests a potent therapeutic approach to kidney injury caused by inflammatory cells.
Acknowledgments
We thank Dr. R. Coffman (DNAX Research Inc., Palo Alto, CA.) for providing RB6-8C5 hybridoma and Ms. Mami Haba for her skilled assistance.
Footnotes
Address reprint requests to Dr. Eisei Noiri, M.D., Ph.D., Department of Nephrology and Endocrinology, University of Tokyo, 7-3-1 Hongo, Bunkyo, Tokyo 113-8655, Japan. E-mail: noiri-tky@umin.ac.jp.
Supported in part by grants from the Cell Science Research Foundation, Osaka, Japan (to E.N.); the Araki Memorial Research Foundation for Medicine and Biochemistry, Tokyo, Japan (to E.N.); Takeda Medical Science, Osaka, Japan (to E.N.); and the Health and Labour Science research grants for Research on Human Genome, Tissue Engineering, and Food Biotechnology from the Ministry of Health, Labour, and Welfare of Japan (to E.N.).
K.D. and K.O. contributed equally to this work.
References
- Schrier RW, Wang W, Poole B, Mitra A. Acute renal failure: definitions, diagnosis, pathogenesis, and therapy. J Clin Invest. 2004;114:5–14. doi: 10.1172/JCI22353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- Strutz F, Neilson EG. The role of lymphocytes in the progression of interstitial disease. Kidney Int Suppl. 1994;45:S106–S110. [PubMed] [Google Scholar]
- Rodriguez-Iturbe B, Pons H, Herrera-Acosta J, Johnson RJ. Role of immunocompetent cells in nonimmune renal diseases. Kidney Int. 2001;59:1626–1640. doi: 10.1046/j.1523-1755.2001.0590051626.x. [DOI] [PubMed] [Google Scholar]
- Ishii S, Shimizu T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog Lipid Res. 2000;39:41–82. doi: 10.1016/s0163-7827(99)00016-8. [DOI] [PubMed] [Google Scholar]
- Imaizumi TA, Stafforini DM, Yamada Y, McIntyre TM, Prescott SM, Zimmerman GA. Platelet-activating factor: a mediator for clinicians. J Intern Med. 1995;238:5–20. doi: 10.1111/j.1365-2796.1995.tb00894.x. [DOI] [PubMed] [Google Scholar]
- Blank ML, Snyder F, Byers LW, Brooks B, Muirhead EE. Antihypertensive activity of an alkyl ether analog of phosphatidylcholine. Biochem Biophys Res Commun. 1979;90:1194–1200. doi: 10.1016/0006-291x(79)91163-x. [DOI] [PubMed] [Google Scholar]
- Lopez-Farre A, Torralbo M, Lopez-Novoa JM. Glomeruli from ischemic rat kidneys produce increased amounts of platelet activating factor. Biochem Biophys Res Commun. 1988;152:129–135. doi: 10.1016/s0006-291x(88)80689-2. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Imagawa M, Mimata H, Nakagawa M, Ogata J, Nomura Y. Role of platelet-activating factor in two-kidney, one-clip hypertension. Int J Urol. 1997;4:388–393. doi: 10.1111/j.1442-2042.1997.tb00213.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Novoa JM. Potential role of platelet activating factor in acute renal failure. Kidney Int. 1999;55:1672–1682. doi: 10.1046/j.1523-1755.1999.00450.x. [DOI] [PubMed] [Google Scholar]
- Thaiss F, Mihatsch MJ, Oberle G, Haberstroh U, Brecht HM, Stahl RA. Platelet-activating factor receptor antagonist improves renal function and glomerular lesions in renal ablation. J Lab Clin Med. 1994;124:775–781. [PubMed] [Google Scholar]
- Torras J, Cruzado JM, Riera M, Condom E, Duque N, Herrero I, Merlos M, Espinosa L, Lloberas N, Egido J, Grinyo JM. Long-term protective effect of UR-12670 after warm renal ischemia in uninephrectomized rats. Kidney Int. 1999;56:1798–1808. doi: 10.1046/j.1523-1755.1999.00724.x. [DOI] [PubMed] [Google Scholar]
- Carceller E, Merlos M, Giral M, Balsa D, Almansa C, Bartroli J, Garcia-Rafanell J, Forn J. [(3-Pyridylalkyl)piperidylidene]benzo-cycloheptapyridine derivatives as dual antagonists of PAF and histamine. J Med Chem. 1994;37:2697–2703. doi: 10.1021/jm00043a009. [DOI] [PubMed] [Google Scholar]
- Svetlov SI, Howard KM, Miwa M, Flickinger BD, Olson MS. Interaction of platelet-activating factor with rat hepatocytes: uptake, translocation, metabolism, and effects on PAF-acetylhydrolase secretion and protein tyrosine phosphorylation. Arch Biochem Biophys. 1996;327:113–122. doi: 10.1006/abbi.1996.0099. [DOI] [PubMed] [Google Scholar]
- Merlos M, Giral M, Balsa D, Ferrando R, Queralt M, Puigdemont A, Garcia-Rafanell J, Forn J. Rupatadine, a new potent, orally active dual antagonist of histamine and platelet-activating factor (PAF). J Pharmacol Exp Ther. 1997;280:114–121. [PubMed] [Google Scholar]
- Adachi T, Aoki J, Manya H, Asou H, Arai H, Inoue K. PAF analogues capable of inhibiting PAF acetylhydrolase activity suppress migration of isolated rat cerebellar granule cells. Neurosci Lett. 1997;235:133–136. doi: 10.1016/s0304-3940(97)00742-8. [DOI] [PubMed] [Google Scholar]
- Ishii S, Kuwaki T, Nagase T, Maki K, Tashiro F, Sunaga S, Cao WH, Kume K, Fukuchi Y, Ikuta K, Miyazaki J, Kumada M, Shimizu T. Impaired anaphylactic responses with intact sensitivity to endotoxin in mice lacking a platelet-activating factor receptor. J Exp Med. 1998;187:1779–1788. doi: 10.1084/jem.187.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase T, Ishii S, Kume K, Uozumi N, Izumi T, Ouchi Y, Shimizu T. Platelet-activating factor mediates acid-induced lung injury in genetically engineered mice. J Clin Invest. 1999;104:1071–1076. doi: 10.1172/JCI7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S, Nagase T, Shindou H, Takizawa H, Ouchi Y, Shimizu T. Platelet-activating factor receptor develops airway hyperresponsiveness independently of airway inflammation in a murine asthma model. J Immunol. 2004;172:7095–7102. doi: 10.4049/jimmunol.172.11.7095. [DOI] [PubMed] [Google Scholar]
- Ferreira MA, Barcelos LS, Campos PP, Vasconcelos AC, Teixeira MM, Andrade SP. Sponge-induced angiogenesis and inflammation in PAF receptor-deficient mice (PAFR-KO). Br J Pharmacol. 2004;141:1185–1192. doi: 10.1038/sj.bjp.0705731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DA, Woolf AS, Suda T, Yuan HT. Increased renal angiopoietin-1 expression in folic acid-induced nephrotoxicity in mice. J Am Soc Nephrol. 2001;12:2721–2731. doi: 10.1681/ASN.V12122721. [DOI] [PubMed] [Google Scholar]
- Surendran K, McCaul SP, Simon TC. A role for Wnt-4 in renal fibrosis. Am J Physiol. 2002;282:F431–F441. doi: 10.1152/ajprenal.0009.2001. [DOI] [PubMed] [Google Scholar]
- Yuan HT, Li XZ, Pitera JE, Long DA, Woolf AS. Peritubular capillary loss after mouse acute nephrotoxicity correlates with down-regulation of vascular endothelial growth factor-A and hypoxia-inducible factor-1 alpha. Am J Pathol. 2003;163:2289–2301. doi: 10.1016/s0002-9440(10)63586-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendran K, Simon TC, Liapis H, McGuire JK. Matrilysin (MMP-7) expression in renal tubular damage: association with Wnt4. Kidney Int. 2004;65:2212–2222. doi: 10.1111/j.1523-1755.2004.00641.x. [DOI] [PubMed] [Google Scholar]
- Byrnes KA, Ghidoni JJ, Suzuki M, Thomas H, Mayfield ED., Jr Response of the rat kidney to folic acid administration. II. Morphologic studies. Lab Invest. 1972;26:191–200. [PubMed] [Google Scholar]
- Fink M, Henry M, Tange JD. Experimental folic acid nephropathy. Pathology. 1987;19:143–149. doi: 10.3109/00313028709077125. [DOI] [PubMed] [Google Scholar]
- Rogers HW, Unanue ER. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan JW, North RJ. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski CJ, Brown JF, Wagner RD, Steinberg H. Administration of antigranulocyte monoclonal antibody RB6-8C5 prevents expression of acquired resistance to Listeria monocytogenes infection in previously immunized mice. Infect Immun. 1994;62:5161–5163. doi: 10.1128/iai.62.11.5161-5163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski CJ, Brown JF, Maroushek N, Wagner RD, Steinberg H. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J Immunol. 1994;152:1836–1846. [PubMed] [Google Scholar]
- Rakhmilevich AL. Neutrophils are essential for resolution of primary and secondary infection with Listeria monocytogenes. J Leukoc Biol. 1995;57:827–831. doi: 10.1002/jlb.57.6.827. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang Z, Sendo F. Neutrophils play a critical role in the pathogenesis of experimental cerebral malaria. Clin Exp Immunol. 2000;120:125–133. doi: 10.1046/j.1365-2249.2000.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Watanabe T, Watanabe H, Sendo F. Neutrophil depletion exacerbates experimental Chagas’ disease in BALB/c, but protects C57BL/6 mice through modulating the Th1/Th2 dichotomy in different directions. Eur J Immunol. 2001;31:265–275. doi: 10.1002/1521-4141(200101)31:1<265::AID-IMMU265>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Stephens-Romero SD, Mednick AJ, Feldmesser M. The pathogenesis of fatal outcome in murine pulmonary aspergillosis depends on the neutrophil depletion strategy. Infect Immun. 2005;73:114–125. doi: 10.1128/IAI.73.1.114-125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi K, Suzuki Y, Nakao A, Fujita T, Noiri E. Radical scavenger edaravone developed for clinical use ameliorates ischemia/reperfusion injury in rat kidney. Kidney Int. 2004;65:1714–1723. doi: 10.1111/j.1523-1755.2004.00567.x. [DOI] [PubMed] [Google Scholar]
- Ohse T, Ota T, Kieran N, Godson C, Yamada K, Tanaka T, Fujita T, Nangaku M. Modulation of interferon-induced genes by lipoxin analogue in anti-glomerular basement membrane nephritis. J Am Soc Nephrol. 2004;15:919–927. doi: 10.1097/01.asn.0000119962.69573.cc. [DOI] [PubMed] [Google Scholar]
- Hisada Y, Sugaya T, Yamanouchi M, Uchida H, Fujimura H, Sakurai H, Fukamizu A, Murakami K. Angiotensin II plays a pathogenic role in immune-mediated renal injury in mice. J Clin Invest. 1999;103:627–635. doi: 10.1172/JCI2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara Y, Ishii S, Kita Y, Toda A, Shimada A, Shimizu T. Dual phase regulation of experimental allergic encephalomyelitis by platelet-activating factor. J Exp Med. 2005;202:853–863. doi: 10.1084/jem.20050660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita Y, Takahashi T, Uozumi N, Shimizu T. A multiplex quantitation method for eicosanoids and platelet-activating factor using column-switching reversed-phase liquid chromatography-tandem mass spectrometry. Anal Biochem. 2005;342:134–143. doi: 10.1016/j.ab.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]
- Soares AC, Pinho VS, Souza DG, Shimizu T, Ishii S, Nicoli JR, Teixeira MM. Role of the platelet-activating factor (PAF) receptor during pulmonary infection with gram negative bacteria. Br J Pharmacol. 2002;137:621–628. doi: 10.1038/sj.bjp.0704918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talvani A, Santana G, Barcelos LS, Ishii S, Shimizu T, Romanha AJ, Silva JS, Soares MB, Teixeira MM. Experimental Trypanosoma cruzi infection in platelet-activating factor receptor-deficient mice. Microbes Infect. 2003;5:789–796. doi: 10.1016/s1286-4579(03)00146-1. [DOI] [PubMed] [Google Scholar]
- Ingraham LM, Coates TD, Allen JM, Higgins CP, Baehner RL, Boxer LA. Metabolic, membrane, and functional responses of human polymorphonuclear leukocytes to platelet-activating factor. Blood. 1982;59:1259–1266. [PubMed] [Google Scholar]
- Gaudreault E, Stankova J, Rola-Pleszczynski M. Involvement of leukotriene B4 receptor 1 signaling in platelet-activating factor-mediated neutrophil degranulation and chemotaxis. Prostaglandins Other Lipid Mediat. 2005;75:25–34. doi: 10.1016/j.prostaglandins.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Gambero A, Thomazzi SM, Cintra AC, Landucci EC, De Nucci G, Antunes E. Signalling pathways regulating human neutrophil migration induced by secretory phospholipases A2. Toxicon. 2004;44:473–481. doi: 10.1016/j.toxicon.2004.06.004. [DOI] [PubMed] [Google Scholar]
- O’Flaherty JT, Swendsen CL, Lees CJ, McCall CE. Role of extracellular calcium and neutrophil degranulation responses to 1-O-alkyl-2-O-acetyl-sn-glycero-3-phosphocholine. Am J Pathol. 1981;105:107–113. [PMC free article] [PubMed] [Google Scholar]
- Elzi DJ, Hiester AA, Silliman CC. Receptor-mediated calcium entry is required for maximal effects of platelet activating factor primed responses in human neutrophils. Biochem Biophys Res Commun. 1997;240:763–765. doi: 10.1006/bbrc.1997.7740. [DOI] [PubMed] [Google Scholar]
- Vercellotti GM, Yin HQ, Gustafson KS, Nelson RD, Jacob HS. Platelet-activating factor primes neutrophil responses to agonists: role in promoting neutrophil-mediated endothelial damage. Blood. 1988;71:1100–1107. [PubMed] [Google Scholar]
- Kelly KJ, Tolkoff-Rubin NE, Rubin RH, Williams WW, Jr, Meehan SM, Meschter CL, Christenson JG, Bonventre JV. An oral platelet-activating factor antagonist, Ro-24-4736, protects the rat kidney from ischemic injury. Am J Physiol. 1996;271:F1061–F1067. doi: 10.1152/ajprenal.1996.271.5.F1061. [DOI] [PubMed] [Google Scholar]
- Lloberas N, Torras J, Herrero-Fresneda I, Cruzado JM, Riera M, Hurtado I, Grinyo JM. Postischemic renal oxidative stress induces inflammatory response through PAF and oxidized phospholipids. Prevention by antioxidant treatment. FASEB J. 2002;16:908–910. doi: 10.1096/fj.01-0880fje. [DOI] [PubMed] [Google Scholar]
- Guo G, Morrissey J, McCracken R, Tolley T, Klahr S. Role of TNFR1 and TNFR2 receptors in tubulointerstitial fibrosis of obstructive nephropathy. Am J Physiol. 1999;277:F766–F772. doi: 10.1152/ajprenal.1999.277.5.F766. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, Takeya M, Kuziel WA, Matsushima K, Mukaida N, Yokoyama H. Blockade of CCR2 ameliorates progressive fibrosis in kidney. Am J Pathol. 2004;165:237–246. doi: 10.1016/S0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaya K, Koya D, Isono M, Sugimoto T, Sugaya T, Kashiwagi A, Haneda M. Involvement of ERK pathway in albumin-induced MCP-1 expression in mouse proximal tubular cells. Am J Physiol. 2003;284:F1037–F1045. doi: 10.1152/ajprenal.00230.2002. [DOI] [PubMed] [Google Scholar]
- Kravchenko VV, Pan Z, Han J, Herbert JM, Ulevitch RJ, Ye RD. Platelet-activating factor induces NF-kappa B activation through a G protein-coupled pathway. J Biol Chem. 1995;270:14928–14934. doi: 10.1074/jbc.270.25.14928. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rangan GK, Goodwin B, Tay YC, Harris DC. Lipopolysaccharide-induced MCP-1 gene expression in rat tubular epithelial cells is nuclear factor-kappaB dependent. Kidney Int. 2000;57:2011–2022. doi: 10.1046/j.1523-1755.2000.00051.x. [DOI] [PubMed] [Google Scholar]
- Asano K, Taniguchi S, Nakao A, Watanabe T, Kurokawa K. Distribution of platelet activating factor receptor mRNA along the rat nephron segments. Biochem Biophys Res Commun. 1996;225:352–357. doi: 10.1006/bbrc.1996.1179. [DOI] [PubMed] [Google Scholar]
- Jamil KM, Takano T, Nakao A, Honda Z, Shimizu T, Watanabe T, Kurokawa K. Expression of platelet activating factor receptor in renal tubular cell line (LLC-PK1). Biochem Biophys Res Commun. 1992;187:767–772. doi: 10.1016/0006-291x(92)91261-n. [DOI] [PubMed] [Google Scholar]
- Jocks T, Freudenberg J, Zahner G, Stahl RA. Platelet-activating factor mediates monocyte chemoattractant protein-1 expression in glomerular immune injury. Nephrol Dial Transplant. 1998;13:37–43. doi: 10.1093/ndt/13.1.37. [DOI] [PubMed] [Google Scholar]
- Beaudeux JL, Said T, Ninio E, Ganne F, Soria J, Delattre J, Soria C, Legrand A, Peynet J. Activation of PAF receptor by oxidised LDL in human monocytes stimulates chemokine releases but not urokinase-type plasminogen activator expression. Clin Chim Acta. 2004;344:163–171. doi: 10.1016/j.cccn.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Getting SJ, Flower RJ, Parente L, de Medicis R, Lussier A, Woliztky BA, Martins MA, Perretti M. Molecular determinants of monosodium urate crystal-induced murine peritonitis: a role for endogenous mast cells and a distinct requirement for endothelial-derived selectins. J Pharmacol Exp Ther. 1997;283:123–130. [PubMed] [Google Scholar]
- Perretti M, Flower RJ. Modulation of IL-1-induced neutrophil migration by dexamethasone and lipocortin 1. J Immunol. 1993;150:992–999. [PubMed] [Google Scholar]