Abstract
This study tests the hypothesis that invasion of partially transformed keratinocytes is initiated by diffusible, proinvasive signals provided by species-specific fibroblasts. In vitro organotypic cultures of neoplastic human oral mucosa were constructed by growing a partially transformed, nontumorigenic keratinocytic cell line isolated from a dysplastic human oral lesion (DOK-ECACC94122104) on top of various types of connective tissue equivalents. Cultured tissues were analyzed by histomorphometry (depth and area of invasion: Dinv, Ainv) and immunohistochemistry. Presence of human fibroblasts in the matrix induced a local invasion of DOK (Dinv = 95.6 ± 7.1 μm, Ainv = 45.8 ± 3.5%). Minimal invasion (P < 0.05) was observed when DOK grew on simple collagen matrix (Dinv = 14.1 ± 2.1 μm, Ainv = 3.7 ± 0.8%) or matrices containing fibroblasts from mouse (Dinv = 11.5 ± 4.0 μm, Ainv = 4.3 ± 1.0%) or rat (Dinv = 15.6 ± 1.2 μm, Ainv = 6.1 ± 0.5%). In these cultures, local invasion could be induced by the presence of human fibroblasts in a bottom layer of the collagen matrix (P < 0.05) or by conditioned medium from organotypic cultures of DOK on human fibroblast-containing matrix (P < 0.05) but not by conditioned medium from human fibroblast monocultures (P > 0.05). Deposition of human collagen IV was observed at epithelial-matrix interface only when DOK behaved invasively. In conclusion, invasion of partially transformed oral keratinocytes was triggered by keratinocyte-induced fibroblast-derived diffusible factor(s) in a species-specific manner and associated with de novo synthesis of collagen IV.
An increasing number of reports suggests that fibroblasts from tumor stroma actively contribute to malignant progression of epithelial neoplasms.1–5 Both normal6–8 and activated carcinoma-associated fibroblasts9–12 have been shown to enhance in vitro invasiveness of human squamous cell carcinoma (SCC) cell lines. Several reports of experiments done on monolayer cultures have suggested that this effect could be attributable to diffusible factors synthesized by fibroblasts.13–15 However, it has been suggested recently that the results from studies performed on conventional, two-dimensional monolayer cell culture models are difficult to extrapolate to the in vivo situation because they do not account for the much more complex mechanisms involved in the three-dimensional process of cancer development and invasiveness.4,16,17 Thus, further experimental evidence from more complex in vitro organotypic models that closely mimic the in vivo three-dimensional tissue structure and cell-to-cell interactions is needed to identify the specific fibroblast-related factor(s) of major importance for in vivo invasiveness of SCCs. On the other hand, the previous studies have used fully transformed cell lines with an established in vivo invasive phenotype. Whether the same fibroblast-dependent mechanism(s) of invasiveness takes place at earlier stages of keratinocyte transformation, when local invasion is initiated, it is not yet known, and therefore it has been the focus of this investigation.
Another fibroblast-related factor of importance for the in vivo invasiveness of carcinoma cells seems to be the origin of fibroblasts. A role for organ specificity of fibroblasts in promoting carcinoma cell invasion has been shown.11,18–20 Several studies have reported that only a limited number of tumor cells of human origin grew in nude mice.21,22 The sensitivity of the in vivo malignancy test for human neoplastic cells, especially those at early stages of malignancy has been questioned,23 and attempts to develop functionally reliable in vivo experimental models of human tissues in mice have been done by humanizing the mice microenvironment through addition of human fibroblasts before xenotransplantation of the human epithelial cells,24 but the issue of species specificity has not been further investigated partially because of the lack of appropriate experimental models.25 The later development of heterologous organotypic models has made possible such studies by construction of models harboring cell types from different species.26
In the present study we have used such organotypic models 1) to test the hypothesis that invasion of partially transformed oral epithelial cells is triggered by diffusible, proinvasive signal(s) provided by species-specific oral fibroblasts; 2) to investigate whether the growth factors suggested by monolayer culture studies to be important for SCC invasiveness play a key role for local invasiveness of partially transformed oral keratinocytes in organotypic three-dimensional cultures; and 3) to identify specific phenotypical changes of partially transformed oral keratinocytes associated with the transition from a noninvasive to an invasive behavior.
Materials and Methods
Cell Lines
Partially transformed human oral keratinocytes (DOK cell line, accession number 94122104) were obtained from The European Collection of Cell Cultures (Salisbury, Wiltshire, UK)27 and routinely grown in Dulbecco’s modified Eagle’s medium (Sigma, St. Louis, MO) supplemented with 10% fetal calf serum, 20 μg/ml l-glutamine, and 5 μg/ml hydrocortisone (all from Sigma). Primary normal human oral fibroblasts were isolated from six biopsies of human buccal mucosa, after surgical removal of wisdom teeth as previously described.28 The study, approved by the Ethics Committee of the University of Bergen, included clinically healthy donors only after informed consent. Research was performed at the Department of Odontology, Oral Pathology and Forensic Odontology, Faculty of Dentistry, University of Bergen, Bergen, Norway. Normal mouse oral fibroblasts were isolated from the buccal mucosa of six B6D2F mice (Jackson Laboratory, Bar Harbor, ME), and normal rat oral fibroblasts were isolated from the buccal mucosa of six BD IX rats (Charles River Laboratories, France) following the same procedure. Animal care was in accordance to national legislation and institutional guidelines. Primary human fibroblasts were routinely grown in minimum essential medium Eagle (Sigma). Primary mouse and rat fibroblasts were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, 20 μg/ml l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin B (all from Sigma). DOK in passage 29 and primary fibroblasts in early passages (2 to 4) were used in the study.
Organotypic Cell Culture
In vitro organotypic cell cultures of neoplastic oral mucosa were developed by seeding the DOK cells on top of connective tissue equivalents, as previously described.29 The study design implied construction of various connective tissue equivalents: simple collagen type I matrix (Figure 1, panel 1); primary human oral fibroblast-containing matrix (Figure 1, panel 2); primary mouse oral fibroblast-containing matrix (Figure 1, panel 3); primary rat oral fibroblast-containing matrix (Figure 1, panel 3); sandwich matrices, in which an intermediate layer (200 μl per culture) of either simple collagen matrix (Figure 1, panel 4) or of mouse fibroblast-containing matrix (Figure 1, panel 5) separated the epithelial compartment (0.5 × 106 DOK cells per culture) from the human fibroblast-containing matrix (500 μl per culture). Conditioned medium obtained from either monolayer cultures of human fibroblasts (Figure 1, panels 6 and 7) or parallel organotypic cultures of DOK on human fibroblast-containing matrix (Figure 1, panels 6 and 7) was mixed 1:1 vol with fresh culture medium and added to some of the organotypic cultures of DOK on top of either simple collagen matrix (Figure 1, panel 6) or mouse fibroblast-containing matrix (Figure 1, panel 7). Recombinant human growth factors and cytokines (hepatocyte growth factor, HGF; granulocyte-macrophage colony stimulation factor, GM-CSF; stem cell factor, SCF; epidermal growth factor, EGF; keratinocyte growth factor, KGF; transforming growth factor-α, TGF-α; interleukin 1, IL-1; all from Sigma) were tested alone or in various combinations by adding them to the culture media, in a concentration range from 0.1 to 100 ng/ml, at the time of tissue lifting at the liquid-air interface (day 3 of co-culture) (Figure 1, panels 8 and 9). The cultures were grown for 10 days in serum-free FAD medium (3 vol Dulbecco’s modified Eagle’s medium to 1 vol Ham’s F-12) supplemented with 0.4 μg/ml hydrocortisone, 5 μg/ml insulin, 20 μg/ml transferrin, 50 μg/ml l-ascorbic acid, 1 mg/ml linoleic acid-albumin, 20 μg/ml l-glutamine (all from Sigma). The cultured tissues were harvested at day 10 of co-culture. One half of each culture was snap-frozen in isopentane prechilled in liquid nitrogen, and the other half was fixed in 4% buffered formalin, pH 7.15, and embedded in paraffin. Experiments were run six times, in duplicates.
Figure 1.
Study design. Partially transformed human oral keratinocytic cell line (DOK) was organotypically grown on top of various connective tissue equivalents to investigate whether its growth pattern and invasive phenotype could be modulated by underlying mesenchymal stroma. The flow chart (1 to 9) of various models used in the study is presented.
Histomorphometry
Tissue sections (5 μm) from paraffin-embedded specimens, stained with hematoxylin and eosin, were morphometrically analyzed by a computer-based optical image analyzer (analySIS 11.0; Pro Soft Imaging System, GmbH, Munster, Germany). An arbitrary straight line was drawn between the epithelial compartment and the connective tissue compartment through the upper remnants of the collagen gel. Epithelial thickness (ET) was measured from that line to the surface of the epithelium. The degree of local invasiveness was determined by measuring two parameters: the depth of epithelial invasion (Dinv) and the area of epithelial nests that grew invasive in the collagen matrix (Ainv). Dinv was measured from the arbitrary line to the deepest pick of epithelial cells that migrated into the collagen matrix (two picks were measured per each field). Ainv was determined as percentage of the matrix area that was invaded by epithelial cells in a standard square of 30,000 μm2, measured in the matrix layer immediately under epithelium, starting from the arbitrary line described above. All measurements were done in six different fields per slide, situated 200 μm apart, at ×100 magnification.
Immunohistochemistry
Immunohistochemical staining was performed using the Autostainer universal staining system (DAKO-USA, Carpinteria, CA) as previously described.29 Five-μm formalin-fixed, paraffin-embedded sections were stained for Ki67 (MIB-1clone, IgG1, titration 1:50; DAKO, Glostrup, Denmark), α-smooth muscle actin (α-SMA, clone 1A4, IgG2a, titration 1:25; DAKO), and collagen IV (CIV221 clone, IgG1, titration 1:25; DAKO). Fresh-frozen, acetone-fixed samples were stained for c-met (NCL-cMET, IgM, titration 1:50; Novocastra Laboratories Ltd., Newcastle on Tyne, UK). Cell proliferation index (PI) was determined as percentage of Ki67-expressing cells among all cells of the epithelial compartment per microscopic field. At least 500 cells were counted per field in six fields per slide situated 200 μm apart, at ×200 magnification.
Statistical Analysis
Wilcoxon paired test was used with a level of significance set at 5% (SPSS 11.0; SPSS, Chicago, IL).
Results
Fibroblast-Derived Diffusible Factors Support Epithelial Growth of Neoplastic Epithelium Reconstructed in Vitro from Partially Transformed Keratinocytes
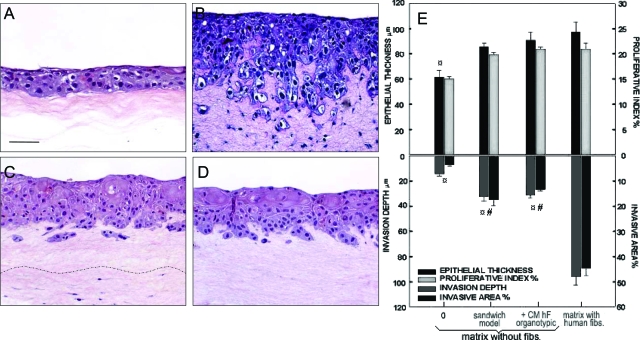
When grown organotypically, DOK formed a stratified squamous epithelium with a disorganized stratification, cellular atypia, and hyperchromatic nuclei (Figure 2, A–D). Epithelial thickness (ET = 61.1 ± 4.6 μm) and cell proliferation index (PI = 15.1 ± 0.4%) of the epithelium formed when DOK were grown on top of simple collagen matrices were significantly lower (P < 0.05) than the epithelium formed when DOK were grown on top of either human (ET = 97.3 ± 6.7 μm, PI = 21.2 ± 1.2%), mouse (ET = 84.6 ± 3.6 μm, PI = 19.9 ± 1.4%), or rat (ET = 81.1 ± 6.7 μm, PI = 18.1 ± 1.8%) fibroblast-containing matrices (Table 1). Diffusible factors from the bottom layer of human fibroblast-containing matrix of sandwich models supported the growth of DOK cells through the layer of simple collagen gel in a similar manner (P > 0.05) as the fibroblast-containing matrix in direct contact with DOK cells (Table 1 and Figure 2, C and E). Addition of conditioned media from monolayer cultures of human fibroblasts or from parallel organotypic co-cultures of DOK on human fibroblast-containing matrix induced an increase in ET and PI of DOK epithelium cultured on simple collagen gels that became comparable to DOK epithelium formed on human fibroblast-containing matrices (P > 0.05) (Table 1). Furthermore, addition of 10 ng/ml of TGF-α or of a cocktail of human growth factors and cytokines (10 ng/ml each of HGF, GM-CSF, SCF, EGF, KGF, TGF-α, and IL-1) increased ET and PI of DOK epithelium grown on top of simple collagen matrices up to values comparable to the DOK epithelium grown on human fibroblast-containing matrices (P > 0.05) (Table 1).
Figure 2.
Effects of human fibroblast-derived diffusible factors on growth and invasiveness of in vitro neoplastic oral epithelium reconstructed from partially transformed keratinocytes. Neoplastic oral epithelium was reconstructed by growing DOK cells on top of simple collagen gel (A), human fibroblast-containing matrix (B), or on sandwich models with a bottom layer of human fibroblast-containing matrix and an intermediate layer of simple collagen gel (C). Conditioned medium from parallel organotypic co-cultures of DOK on human fibroblast-containing matrices was added at day 3 of co-culture (D). H&E staining is shown (A–D). E: Epithelial growth (total epithelial thickness and cell proliferation index) and invasion (depth and area of invasion) were assessed. Data represent mean ± SEM of six different experiments. ¤Statistically significant difference when compared with DOK cultures on human fibroblast-containing matrix; #statistically significant difference when compared with DOK cultures on simple collagen gels. Scale bar, 50 μm.
Table 1.
Epithelial Growth and Invasion Analysis of Reconstituted Human Oral Neoplastic Epithelium from Partially Transformed Oral Keratinocytes Grown on Top of Various Types of Connective Tissue Equivalents and in Different Culture Conditions
| Type of matrix | Growth parameters
|
Invasion parameters
|
||
|---|---|---|---|---|
| Epithelial thickness (μm) | Proliferation index (%) | Depth of invasion (μm) | Area of invasion (%) | |
| Human fibroblasts (hF) in collagen type I | 97.3 ± 6.7 | 21.2 ± 1.2 | 95.6 ± 7.1 | 45.8 ± 3.5 |
| Simple collagen type I | 61.1 ± 4.6 | 15.1 ± 0.4 | 14.1 ± 2.1 | 3.7 ± 0.8 |
| Sandwich model with simple collagen and hF in bottom layer | 88.7 ± 5.1 | 19.5 ± 1.1 | 38.7 ± 3.2 | 18.1 ± 2.1 |
| Simple collagen and conditioned medium from hF in monolayer | 73.3 ± 3.2 | 18.1 ± 0.9 | 11.4 ± 2.8 | 3.9 ± 0.7 |
| Simple collagen and conditioned medium from hF in organotypic co-cultures | 93.1 ± 7.3 | 20.9 ± 1.2 | 37.5 ± 4.1 | 14.5 ± 1.2 |
| Simple collagen and human growth factors in cocktail | 90.2 ± 6.1 | 27.0 ± 1.9 | 13.2 ± 3.7 | 4.1 ± 0.7 |
| Mouse fibroblasts (mF) in collagen type I | 84.6 ± 3.6 | 19.9 ± 1.4 | 11.5 ± 4.0 | 4.3 ± 1.0 |
| Sandwich model with mF and hF in bottom layer | 94.1 ± 3.1 | 20.3 ± 1.0 | 31.5 ± 2.6 | 21.7 ± 2.3 |
| mF in collagen and conditioned medium from hF in monolayer | 88.5 ± 4.1 | 21.5 ± 1.1 | 16.3 ± 2.8 | 7.4 ± 1.0 |
| mF in collagen and conditioned medium from hF in organotypic co-cultures | 90.8 ± 5.2 | 21.8 ± 1.0 | 27.2 ± 1.9 | 19.3 ± 1.2 |
| mF in collagen and human growth factors in cocktail | 110 ± 5.8 | 23.8 ± 1.3 | 17.2 ± 2.3 | 6.7 ± 0.9 |
| Rat fibroblasts in collagen type I | 81.1 ± 6.7 | 18.1 ± 1.8 | 15.6 ± 1.2 | 6.1 ± 0.5 |
Epithelial growth (total epithelial thickness and cell proliferation index) and invasion (depth and area of invasion) were assessed by histomorphometry and immunohistochemical staining. Data represent mean ± SEM of six different experiments.
Species-Specific Keratinocyte-Induced Fibroblast-Derived Diffusible Factors Trigger Invasiveness of Neoplastic Epithelium Reconstructed in Vitro from Partially Transformed Keratinocytes
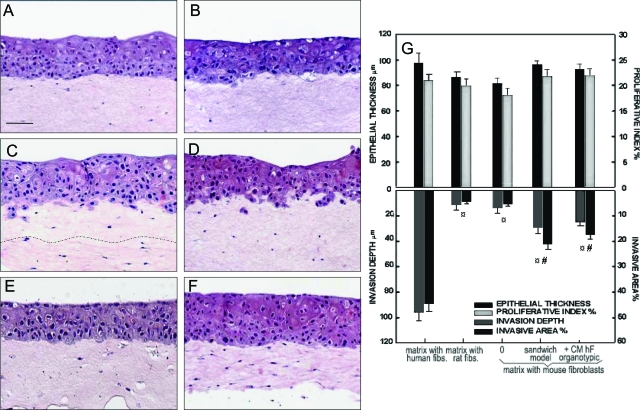
A minimal invasion was observed when DOK were grown on top of simple collagen matrices (Dinv = 14.1 ± 2.1 μm, Ainv = 3.7 ± 0.8%), on top of matrices containing fibroblasts from mouse (Dinv = 11.5 ± 4.0 μm, Ainv = 4.3 ± 1.0%) or rat (Dinv = 15.6 ± 1.2 μm, Ainv = 6.1 ± 0.5%) (Table 1; Figure 2A; and Figure 3, A and B). A significantly higher degree of local invasiveness into the collagen matrix was observed when DOK were grown on top of human fibroblast-containing matrix (Dinv = 95.6 ± 7.1 μm, Ainv = 45.8 ± 3.5%, P < 0.05) (Figure 2B). DOK invasion into the collagen gels was not impaired by growing DOK cells on top of collagen gels populated with a mixed population of mouse and human fibroblasts, in a range of 1:1 to 10:1 mouse to human fibroblasts (Dinv = 83.6 ± 8.7 μm, Ainv = 43.2 ± 3.7%, P > 0.05 when comparing a mixture ratio of 10:1 mouse:human with human fibroblasts only). Conditioned medium from mouse fibroblasts in monolayer or from parallel organotypic co-cultures of DOK on top of mouse fibroblast-containing matrices did not impair local invasiveness of DOK on human fibroblast-containing matrices (data not shown).
Figure 3.
Effects of species-specific fibroblasts on growth and invasion of in vitro neoplastic oral epithelium reconstructed from partially transformed keratinocytes. Neoplastic oral epithelium was in vitro reconstituted by growing DOK cell line on top of mouse fibroblast-containing matrix (A, D–F), rat fibroblast-containing matrix (B), or on sandwich models with a bottom layer of human fibroblast-containing matrix and an intermediate layer of mouse fibroblast-containing matrix (C). Conditioned medium from parallel organotypic co-cultures of DOK on human fibroblast-containing matrices (D), from human fibroblasts in monolayer (E), or a cocktail of known human growth factors (F) were added at day 3 of co-culture. H&E staining (A–F) is shown. Epithelial growth (total epithelial thickness and cell proliferation index) and invasion (depth and area of invasion) were assessed (G). Data represent mean ± SEM of six different experiments. ¤Statistically significant difference when compared with DOK cultures on human fibroblast-containing matrix; #statistically significant difference when compared with DOK cultures on mouse or rat fibroblast only-containing matrix. Scale bar, 50 μm.
Local invasion could be induced when DOK were grown on top of sandwich models in which the bottom layer of human fibroblast-containing matrix provided diffusible factors to the overlying epithelium through a layer of simple collagen gel (Dinv = 38.7 ± 3.2 μm, P < 0.05; Figure 2C) or through an intermediate layer of mouse fibroblast-containing matrix (Dinv = 31.5 ± 2.6 μm, Figure 3C). Conditioned medium from human fibroblasts in monolayer did not induce invasiveness of DOK on either simple collagen gels or on mouse fibroblast-containing matrices (P > 0.05) (Table 1 and Figure 3E). In contrast, conditioned medium from parallel organotypic co-cultures of DOK on top of human fibroblast-containing matrices triggered local invasiveness of DOK on both simple collagen gels and mouse fibroblast-containing matrices (P < 0.05) (Figures 2 and 3D). The local invasion induced in sandwich models or by conditioned medium from homologous co-cultures, however, did not reach the same extent as when the homologous cells were cultured in direct apposition (P < 0.05) (Table 1).
DOK cells were found to express HGF receptor (c-met) in all cultures. No difference in the pattern of expression of this receptor was observed between different types of cultures used (data not shown). However, neither HGF nor other human growth factors or cytokines tested alone or in various combinations induced DOK cell invasion (P > 0.05) (Table 1 and Figure 3F).
Invasiveness of DOK Cells Does Not Correlate with α-SMA Expression by Stromal Fibroblasts
Almost all fibroblasts stained positive for α-SMA in organotypic co-cultures (98.3 ± 0.5% for human, and 99.1 ± 2.3% for rat-derived fibroblasts), irrespective of the species of origin tested (P > 0.05). The fraction of α-SMA-positive fibroblasts grown organotypically in collagen gels was similar whether the fibroblasts were grown with or without DOK cells on top of the collagen gels (P > 0.05). Expression of α-SMA was significantly lower in fibroblasts grown in monolayer (63.5 ± 5.0% for human, and 83.7 ± 2.6% for rat-derived fibroblasts). The difference in the α-SMA expression observed between fibroblasts grown in monolayer and fibroblasts grown in organotypic co-culture with DOK cells more closely correlated with the culture method (monolayer on plastic versus organotypic in collagen gels) rather than with the presence or absence of an overlying epithelial cell compartment (DOK) to interact with. No correlation was found between the depth or area of DOK cell invasion and the proportion of α-SMA-positive fibroblasts in the collagen gels.
Occurrence of Local Invasiveness Is Associated with de Novo Synthesis and Deposition of Species-Specific Collagen IV
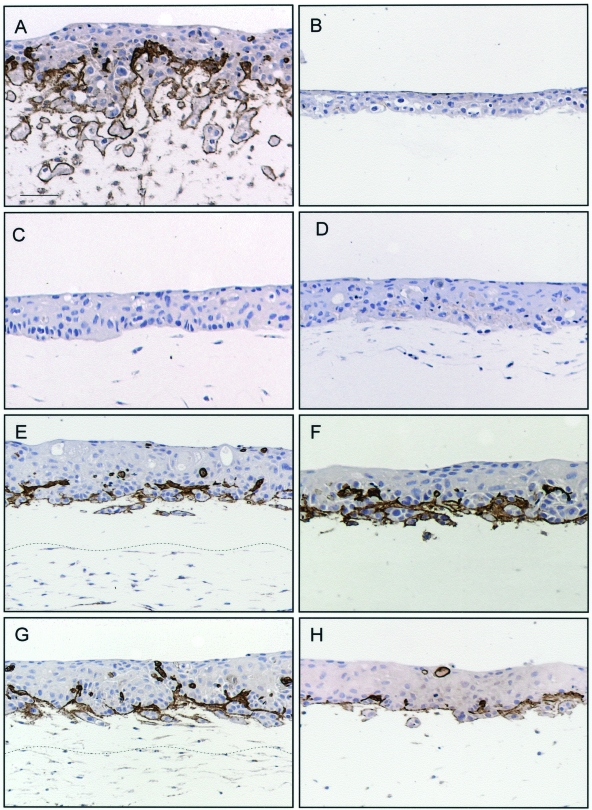
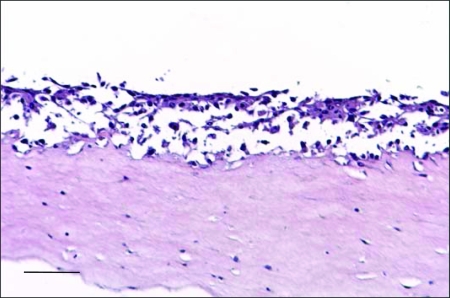
Deposition of collagen IV was observed at the epithelial-matrix interface in homologous organotypic co-cultures (Figure 4A), but not in DOK cultures on simple collagen gel (Figure 4B) or in heterologous co-cultures (Figure 4C). Deposition of collagen IV was observed at the invasion front in sandwich cultures (Figure 4, E and G) and in cultures treated with conditioned medium from homologous co-cultures (Figure 4, F and H), but not in cultures treated with conditioned medium from human fibroblasts in monolayer (Figure 4D). In addition, homologous co-cultures treated with collagen IV antibody (100 ng/ml of medium) showed loss of the invasive behavior of DOK cells (Figure 5).
Figure 4.
De novo synthesis and deposition of species-specific collagen IV at the epithelial-matrix interface is induced by species-specific diffusible factors and is associated with an invasive phenotype. Neoplastic oral epithelium was reconstructed by growing for 10 days DOK cells on top of human fibroblast-containing matrix (A), simple collagen gel (B, F), mouse fibroblast-containing matrix (C, D, H), or sandwich models with a bottom layer of human fibroblast-containing matrix and an intermediate layer of simple collagen gel (E), or of mouse fibroblast-containing matrix (G). Conditioned medium from human fibroblasts in monolayer (D) or from parallel organotypic co-cultures of DOK on human fibroblast-containing matrices (F, H) were added at day 3 of co-culture. Immunohistochemical staining for human collagen IV is shown. Scale bar, 50 μm.
Figure 5.
Invasive behavior of DOK cells is abolished by addition of monoclonal antibody against human collagen type IV in human homologous organotypic co-cultures. Human homologous organotypic co-cultures of neoplastic oral epithelium were constructed by growing for 10 days DOK cells on top of human fibroblast-containing matrices. The cultures were treated each second day starting from day 3 of co-culture with collagen IV antibody (100 ng/ml of culture medium). Parallel cultures treated with similar IgG subclass antibody served as negative controls. H&E staining is shown. Scale bar, 50 μm.
Discussion
As a model of partially transformed keratinocytes we have used a spontaneously immortalized cell line (DOK) isolated from a dysplastic lesion of oral mucosa. This cell line is aneuploid, has a complex karyotype,27 and harbors a mutated p53 gene,30 but it has been defined as partially transformed because it did not form tumors in nude mice.27 That DOK cells have a less transformed phenotype when compared to other oral neoplastic cell lines is also supported by our own in vitro findings from organotypic cultures,8 in which these cells formed an epithelium with a certain degree of epithelial maturation, a polarized proliferation to the basal cell layer, and a positive staining for CK 13 in the spinous cell layer. The present finding, that normal human fibroblasts could induce their invasiveness, thus brings additional proof to the role of fibroblasts on tumor progression. It suggests that the fibroblasts may have an important role on initiating the invasive behavior of keratinocytes at earlier stages of transformation and not only enhancing it in fully transformed malignant keratinocytes with already established invasive properties as previously shown.6,9,10,13
Of notice is the observation that conditioned medium from parallel organotypic co-cultures of DOK on human oral fibroblast-containing matrices, but not conditioned medium from human fibroblasts in monolayer, was able to trigger DOK invasiveness. This finding is in contrast to previous reports that found the conditioned medium from human fibroblasts in monolayer able to induce OSCC cell invasion.6,13 Conversion of stromal fibroblasts into myofibroblasts under the influence of transformed epithelial cells has been shown to occur at the invasion front of SSCs, and it has been suggested as an important factor for tumor progression and invasion.31 In this study the invasion of DOK cells into the stromal compartment did not correlate with the expression of the myofibroblast marker α-SMA in the stromal fibroblasts, and the expression of α-SMA by the fibroblasts in the collagen gel was not enhanced by epithelial-mesenchymal interactions. Nevertheless, our findings suggest an ability for the partially transformed oral keratinocytes to affect the spectrum of fibroblast-derived soluble factors. They also point to the interplay between partially transformed keratinocytes and stromal fibroblasts as an essential factor for the initiation of tumor invasiveness but do not indicate the myofibroblast conversion as the key factor in initiating invasion of partially transformed oral keratinocytes. This might be particular to OSCCs, if one keeps in mind that the myofibroblast transdifferentiation mechanisms have been described mainly for SCCs having an extensive desmoplastic stromal reaction, a feature not commonly found in OSCCs.32
Partially transformed oral keratinocytes were stimulated by species-specific fibroblast-derived diffusible factors to synthesize and deposit the basement membrane protein collagen IV extracellularly, at the epithelial-matrix interface, and this phenotypic property was found to be associated with the occurrence of an invasive behavior (Figure 4). Although this observation does not fit within the classical concept of loss or discontinuity of the basement membrane in epithelial cancers,33,34 it is compatible with recent evidence from both descriptive and experimental studies for de novo synthesis of basement membrane constituents in tumor front cells and adjacent stromal cells.35,36 A role in enhancing local invasion of transformed epithelial cells has been previously reported for certain basement membrane proteins and proteoglycans such as laminin 5 γ 2 chain, collagen VII, fibronectin, tenascin C, hyaluran, or perlecan.16,36–40 More recently, laminin 1 and type IV collagen were shown to promote intraepithelial expansion of transformed keratinocytes in similar organotypic models of skin.41 Our study addresses the intermediate step between the previous two, being the first one that brings experimental evidence for the involvement of basement membrane protein collagen IV in the initiation process of local invasion of partially transformed keratinocytes.
As an attempt to identify the specific diffusible factor(s) responsible for triggering DOK invasion in the organotypic models we have tested a panel of human growth factors and cytokines with a potential enhancing effect on epithelial tumor growth and invasiveness. Among these, HGF has been previously reported to promote OSCC cell migration in monolayer cultures.14 GM-CSF has been also shown to promote migration of neoplastic skin keratinocytes in such cultures.42 Although some of the factors tested in this study (eg, TGF-α) could enhance cell proliferation, none of them, used alone or in combination, was able to trigger DOK cell invasiveness in the organotypic models (Table 1). The finding that HGF in concentrations up to 100 ng/ml did not activate cellular migration of DOK cells into the collagen gels was rather unexpected, since c-met-positive OSCC cells have been previously reported to respond to HGF in monoculture.15 However, these results are in line with recent findings from organotypic models of colon carcinoma, in which HGF alone could not promote migration of tumor cells into collagen gels.16 It therefore seems appropriate to suggest that the mechanism(s) of invasion are more complex in the three-dimensional organotypic models and more diverse than could be suggested by the migration assays done in more simple conventional two-dimensional models as monolayers.
A novel finding of this study was that invasiveness of partially transformed oral keratinocytes was triggered by oral fibroblasts in a species-specific manner. Although the mouse and rat fibroblasts were able to stimulate DOK cell proliferation in a similar way as human fibroblasts did, local invasion was not observed in the presence of mouse or rat fibroblasts (Table 1, Figure 3). These in vitro results fit with previous reports of in vivo xenotransplantation tests, in which DOK cells did not form tumors in nude mice.27 The species specificity described above could be related to the differences in cytokine and collagenase production between mouse, rat, and human species.43,44 The fact that conditioned medium from co-cultures of DOK cells and human fibroblasts could induce DOK invasiveness in mouse fibroblast-containing matrix points more to the lack of appropriately induced growth factors in mouse fibroblasts by human DOK cells. This might be due to a deficiency in the cross-talk between human keratinocytes and mouse fibroblasts. The herein reported importance of species specificity of fibroblasts for acquisition of growth invasive properties by partially transformed keratinocytes brings some concern into the general use of animal models for testing human cell lines with respect to their malignant properties. We suggest that the in vitro organotypic human models may serve as additional test models for malignancy of human-derived neoplastic cell lines.
Acknowledgments
We thank Mrs. Eli Mossefinn and Mrs. Bjørg Svensson for the help in collecting the human samples, Dr. Oleg Tsinkalovsky for the generous gift of B6D2F mice, Mr. Tore Jakob Raa for the generous gift of BD IX rats and assistance with the animal handling, and Mrs. Gunnvor Øijordsbakken and Mrs. Gudveig Fjell for excellent technical assistance.
Footnotes
Address reprint requests to Daniela Elena Costea, Department of Odontology, Oral Pathology and Forensic Odontology, University of Bergen, The Gade Institute, Haukeland University Hospital, N-5021, Bergen, Norway. E-mail: daniela.costea@odfa.uib.no.
Supported by the Norwegian Government through Quota Program and the Norwegian Dental Depot Fund.
References
- Tlsty TD, Hein PW. Know thy neighbor: stromal cells can contribute oncogenic signals. Curr Opin Genet Dev. 2001;11:54–59. doi: 10.1016/s0959-437x(00)00156-8. [DOI] [PubMed] [Google Scholar]
- De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–447. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beacham DA, Cukierman E. Stromagenesis: the changing face of fibroblastic microenvironments during tumor progression. Semin Cancer Biol. 2005;15:329–341. doi: 10.1016/j.semcancer.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Horikoshi M, Rikimaru K, Enomoto S. A study of an in vitro model for invasion of oral squamous cell carcinoma. J Oral Pathol Med. 1989;18:498–501. doi: 10.1111/j.1600-0714.1989.tb01350.x. [DOI] [PubMed] [Google Scholar]
- Yamada S, Toda S, Shin T, Sugihara H. Effects of stromal fibroblasts and fat cells and an environmental factor air exposure on invasion of laryngeal carcinoma (HEp-2) cells in a collagen gel invasion assay system. Arch Otolaryngol Head Neck Surg. 1999;125:424–431. doi: 10.1001/archotol.125.4.424. [DOI] [PubMed] [Google Scholar]
- Costea DE, Johannessen AC, Vintermyr OK. Fibroblast control on epithelial differentiation is gradually lost during in vitro tumor progression. Differentiation. 2005;73:134–141. doi: 10.1111/j.1432-0436.2005.00017.x. [DOI] [PubMed] [Google Scholar]
- Atula S, Grenman R, Syrjanen S. Fibroblasts can modulate the phenotype of malignant epithelial cells in vitro. Exp Cell Res. 1997;235:180–187. doi: 10.1006/excr.1997.3676. [DOI] [PubMed] [Google Scholar]
- Berndt A, Hyckel P, Konneker A, Kosmehl H. 3-Dimensional in vitro invasion model for oral squamous epithelial carcinomas. Evaluation of tumor and stromal cell properties as well as extracellular matrix.] Mund Kiefer Gesichtschir. 1998;2:256–260. [Google Scholar]
- Shekhar MP, Werdell J, Santner SJ, Pauley RJ, Tait L. Breast stroma plays a dominant regulatory role in breast epithelial growth and differentiation: implications for tumor development and progression. Cancer Res. 2001;61:1320–1326. [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Shirasuna K, Hayashido Y, Sakai T, Matsuya T. Effects of human fibroblasts on invasiveness of oral cancer cells in vitro: isolation of a chemotactic factor from human fibroblasts. Int J Cancer. 1996;68:774–781. doi: 10.1002/(SICI)1097-0215(19961211)68:6<774::AID-IJC15>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T, Kramer RH. Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of focal adhesion kinase (p125FAK) and promotes migration and invasion by oral squamous cell carcinoma cells. J Biol Chem. 1994;269:31807–31813. [PubMed] [Google Scholar]
- Uchida D, Kawamata H, Omotehara F, Nakashiro K, Kimura-Yanagawa T, Hino S, Begum NM, Hoque MO, Yoshida H, Sato M, Fujimori T. Role of HGF/c-met system in invasion and metastasis of oral squamous cell carcinoma cells in vitro and its clinical significance. Int J Cancer. 2001;93:489–496. doi: 10.1002/ijc.1368. [DOI] [PubMed] [Google Scholar]
- De Wever O, Nguyen QD, Van Hoorde L, Bracke M, Bruyneel E, Gespach C, Mareel M. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 2004;18:1016–1018. doi: 10.1096/fj.03-1110fje. [DOI] [PubMed] [Google Scholar]
- Alt-Holland A, Zhang W, Margulis A, Garlick JA. Microenvironmental control of premalignant disease: the role of intercellular adhesion in the progression of squamous cell carcinoma. Semin Cancer Biol. 2005;15:84–96. doi: 10.1016/j.semcancer.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Fabra A, Nakajima M, Bucana CD, Fidler IJ. Modulation of the invasive phenotype of human colon carcinoma cells by organ specific fibroblasts of nude mice. Differentiation. 1992;52:101–110. doi: 10.1111/j.1432-0436.1992.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Kawai K, Iwashita T, Murakami H, Hiraiwa N, Yoshiki A, Kusakabe M, Ono K, Iida K, Nakayama A, Takahashi M. Tissue-specific carcinogenesis in transgenic mice expressing the RET proto-oncogene with a multiple endocrine neoplasia type 2A mutation. Cancer Res. 2000;60:5254–5260. [PubMed] [Google Scholar]
- Hsieh JT, Wu HC, Gleave ME, von Eschenbach AC, Chung LW. Autocrine regulation of prostate-specific antigen gene expression in a human prostatic cancer (LNCaP) subline. Cancer Res. 1993;53:2852–2857. [PubMed] [Google Scholar]
- Chang SE. In vitro transformation of human epithelial cells. Biochim Biophys Acta. 1986;823:161–194. doi: 10.1016/0304-419x(86)90001-6. [DOI] [PubMed] [Google Scholar]
- Mehta RR, Graves JM, Hart GD, Shilkaitis A, Das Gupta TK. Growth and metastasis of human breast carcinomas with Matrigel in athymic mice. Breast Cancer Res Treat. 1993;25:65–71. doi: 10.1007/BF00662402. [DOI] [PubMed] [Google Scholar]
- Fusenig NE, Breitkreutz D, Dzarlieva RT, Boukamp P, Bohnert A, Tilgen W. Growth and differentiation characteristics of transformed keratinocytes from mouse and human skin in vitro and in vivo. J Invest Dermatol. 1983;81:168s–175s. doi: 10.1111/1523-1747.ep12541032. [DOI] [PubMed] [Google Scholar]
- Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, Richardson A, Weinberg RA. Reconstruction of functionally normal and malignant human breast tissues in mice. Proc Natl Acad Sci USA. 2004;101:4966–4971. doi: 10.1073/pnas.0401064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Tumor-stroma interactions directing phenotype and progression of epithelial skin tumor cells. Differentiation. 2002;70:486–497. doi: 10.1046/j.1432-0436.2002.700903.x. [DOI] [PubMed] [Google Scholar]
- Stark HJ, Szabowski A, Fusenig NE, Maas-Szabowski N. Organotypic cocultures as skin equivalents: a complex and sophisticated in vitro system. Biol Proc Online. 2004;6:55–60. doi: 10.1251/bpo72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SE, Foster S, Betts D, Marnock WE. DOK, a cell line established from human dysplastic oral mucosa, shows a partially transformed non-malignant phenotype. Int J Cancer. 1992;52:896–902. doi: 10.1002/ijc.2910520612. [DOI] [PubMed] [Google Scholar]
- Costea DE, Dimba EA, Loro LL, Vintermyr OK, Johannessen AC. Proliferation and Differentiation in Organotypic Serum Free Cultures of Normal Human Oral Mucosa. 2002 Presented at the 8th International Congress on Oral Cancer, Rio de Janeiro, Brazil. [Google Scholar]
- Costea DE, Loro LL, Dimba EA, Vintermyr OK, Johannessen AC. Crucial effects of fibroblasts and keratinocyte growth factor on morphogenesis of reconstituted human oral epithelium. J Invest Dermatol. 2003;121:1479–1486. doi: 10.1111/j.1523-1747.2003.12616.x. [DOI] [PubMed] [Google Scholar]
- Burns JE, Clark LJ, Yeudall WA, Mitchell R, Mackenzie K, Chang SE, Parkinson EK. The p53 status of cultured human premalignant oral keratinocytes. Br J Cancer. 1994;70:591–595. doi: 10.1038/bjc.1994.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Marshall JF, Thomas GJ. Tumour-derived TGF-β1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer. 2004;90:822–832. doi: 10.1038/sj.bjc.6601611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuninger H, Schaumburg-Lever G, Holzschuh J, Horny HP. Desmoplastic squamous cell carcinoma of skin and vermilion surface: a highly malignant subtype of skin cancer. Cancer. 1997;79:915–919. doi: 10.1002/(sici)1097-0142(19970301)79:5<915::aid-cncr7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Hagedorn HG, Bachmeier BE, Nerlich AG. Synthesis and degradation of basement membranes and extracellular matrix and their regulation by TGF-β in invasive carcinomas. Int J Oncol. 2001;18:669–681. doi: 10.3892/ijo.18.4.669. [DOI] [PubMed] [Google Scholar]
- Ziober BL, Silverman SS, Jr, Kramer RH. Adhesive mechanisms regulating invasion and metastasis in oral cancer. Crit Rev Oral Biol Med. 2001;12:499–510. doi: 10.1177/10454411010120060401. [DOI] [PubMed] [Google Scholar]
- Berndt A, Hyckel P, Konneker A, Katenkamp D, Kosmehl H. Oral squamous cell carcinoma invasion is associated with a laminin-5 matrix re-organization but independent of basement membrane and hemidesmosome formation. Clues from an in vitro invasion model. Invasion Metastasis. 1997;17:251–258. [PubMed] [Google Scholar]
- Berndt A, Borsi L, Hyckel P, Kosmehl H. Fibrillary co-deposition of laminin-5 and large unspliced tenascin-C in the invasive front of oral squamous cell carcinoma in vivo and in vitro. J Cancer Res Clin Oncol. 2001;127:286–292. doi: 10.1007/s004320000205. [DOI] [PubMed] [Google Scholar]
- Hindermann W, Berndt A, Borsi L, Luo X, Hyckel P, Katenkamp D, Kosmehl H. Synthesis and protein distribution of the unspliced large tenascin-C isoform in oral squamous cell carcinoma. J Pathol. 1999;189:475–480. doi: 10.1002/(SICI)1096-9896(199912)189:4<475::AID-PATH462>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Jiang X, Multhaupt H, Chan E, Schaefer L, Schaefer RM, Couchman JR. Essential contribution of tumor-derived perlecan to epidermal tumor growth and angiogenesis. J Histochem Cytochem. 2004;52:1575–1590. doi: 10.1369/jhc.4A6353.2004. [DOI] [PubMed] [Google Scholar]
- Ortiz-Urda S, Garcia J, Green CL, Chen L, Lin Q, Veitch DP, Sakai LY, Lee H, Marinkovich MP, Khavari PA. Type VII collagen is required for Ras-driven human epidermal tumorigenesis. Science. 2005;307:1773–1776. doi: 10.1126/science.1106209. [DOI] [PubMed] [Google Scholar]
- Laurich C, Wheeler MA, Iida J, Neudauer CL, McCarthy JB, Bullard KM. Hyaluronan mediates adhesion of metastatic colon carcinoma cells. J Surg Res. 2004;122:70–74. doi: 10.1016/j.jss.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Andriani F, Garfield J, Fusenig NE, Garlick JA. Basement membrane proteins promote progression of intraepithelial neoplasia in 3-dimensional models of human stratified epithelium. Int J Cancer. 2004;108:348–357. doi: 10.1002/ijc.11525. [DOI] [PubMed] [Google Scholar]
- Mueller MM, Fusenig NE. Constitutive expression of G-CSF and GM-CSF in human skin carcinoma cells with functional consequence for tumor progression. Int J Cancer. 1999;83:780–789. doi: 10.1002/(sici)1097-0215(19991210)83:6<780::aid-ijc14>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- DeVore DP, Houchens DP, Ovejera AA, Dill GS, Jr, Hutson TB. Collagenase inhibitors retarding invasion of a human tumor in nude mice. Exp Cell Biol. 1980;48:367–373. doi: 10.1159/000163001. [DOI] [PubMed] [Google Scholar]