Abstract
Very low-density lipoprotein (VLDL) and LDL plasma levels are associated with cardiovascular mortality. Whereas VLDL/LDL lowering causes regression of early atherosclerotic lesions, less is known about the effects of aggressive lipid lowering on regression of advanced complex lesions. We therefore investigated the effect of VLDL/LDL lowering on pre-existing lesions in LDL receptor-deficient mice. Mice fed a high-fat diet for 16 weeks developed advanced lesions with fibrous caps, necrotic cores, and cholesterol clefts in the brachiocephalic artery. After an additional 14 weeks on a low-fat diet, plasma cholesterol levels decreased from 21.0 ± 2.6 to 8.4 ± 0.6 mmol/L, but lesions did not regress. Levels of VLDL/LDL were further lowered by using a helper-dependent adenovirus encoding the VLDL receptor (HD-Ad-VLDLR) under control of a liver-selective promoter. Treatment with HD-Ad-VLDLR together with a low-fat diet regimen resulted in reduced lesion size (cross-sectional area decreased from 146,272 ± 19,359 to 91,557 ± 15,738 μm2) and an 89% reduction in the cross-sectional lesion area occupied by macrophages compared to controls. These results show that aggressive VLDL/LDL lowering achieved by hepatic overexpression of VLDLR combined with a low-fat diet regimen induces regression of advanced plaques in the brachiocephalic artery of LDL receptor-deficient mice.
Dyslipidemia is a central risk factor for cardiovascular disease and is the primary target for prevention of cardiovascular mortality.1 Modification of low-density lipoproteins (LDL) is considered a key contributor to cardiovascular disease in the general population.2 Furthermore, levels of VLDL/triglycerides are often elevated in patients with type 2 diabetes or the metabolic syndrome,3 and increased level of triglycerides is considered a risk factor for atherosclerotic heart disease in patients with and without diabetes.4,5 The great majority of acute clinical cardiovascular events, myocardial infarction and stroke, are believed to be caused by unstable atherosclerotic lesions.6,7 Studies of human atherosclerotic lesions have indicated that most clinically relevant lesions are composed of necrotic cores covered by thin fibrous caps containing macrophage-rich regions.6,8,9 Several previous studies of regression in animal models of atherosclerosis have described the ability of lipid lowering to decrease the mass of fatty streak lesions with poorly developed necrotic cores.10–15 The relevance of this sort of regression to events occurring in more advanced lesions is less clear. Few small animal models are available for studies of regression of advanced lesions.
We therefore took advantage of the advanced lesions with necrotic cores, cholesterol clefts, and fibrous caps that form relatively quickly in the brachiocephalic artery (BCA) in LDL receptor-deficient (LDLR−/−) mice. This model was used to investigate regression of advanced lesions by using a combination of gene therapy to overexpress the VLDLR in the liver and a low-fat diet regimen to aggressively lower VLDL and LDL levels.
Materials and Methods
Generation of HD-Ad-mVLDLR and HD-Ad-0 Vectors
Helper-dependent (HD) adenoviral (Ad) vectors with all viral coding sequences deleted were generated as previously described.16 Unlike first, second, and third generation Ad vectors that express essentially all Ad viral protein genes, causing severe hepatitis in the recipient animals, HD-Ad vectors do not express any viral protein genes and have been shown to circumvent the problem of hepatitis. HD-Ad produce prolonged transgene expression without eliciting an inflammatory response. The HD-Ad-mVLDLR construct was subcloned into an expression cassette containing fragments of the phosphoenolpyruvate carboxykinase promoter, resulting in liver-selective expression of murine VLDLR (mVLDLR). An expression cassette devoid of the mVLDLR sequence, HD-Ad-0, was used as a control. Helper virus contamination was estimated to be <0.2% by real-time PCR.
Study Protocol
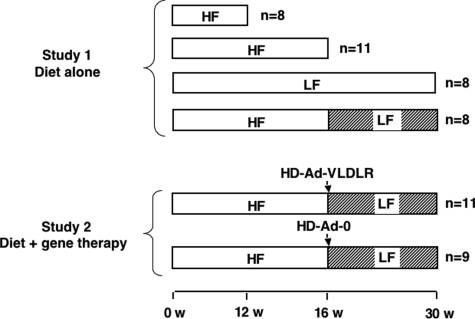
LDLR−/− littermates, 8 weeks of age (C57BL/6; Jackson Laboratories, Bar Harbor, ME), were housed in a modified specific pathogen-free, temperature-controlled facility that maintained a 12-hour light/dark cycle. Mice were given free access to food and water. Female mice, which do not develop diabetes when fed high-fat diets, were used.17 Mice were fed semipurified diets described previously17: a low-fat (LF) cholesterol-free diet containing 10.8% calories from fat and a high-fat (HF) 1.25% cholesterol diet (TD00241 and TD00244, respectively; Harlan Teklad, Madison, WI) containing 40.0% calories from fat. For the first study on effects of diet, mice were euthanized after 12 or 16 weeks on the HF diet. Other groups of mice were fed the LF diet for 30 weeks or were switched to the LF diet after 16 weeks on the HF diet (Figure 1). For the second study, after 16 weeks on the HF diet, groups of mice were switched to the LF diet and at the same time injected with HD-Ad-mVLDLR or HD-Ad-0 vectors (3 × 1011 particles per mouse) through a tail vein (Figure 1). The number of mice per group is indicated in Figure 1. The study was approved by the University of Washington Institutional Animal Care and Use Committee.
Figure 1.
Study design. For Study 1, analyzing the effects of diet alone, LDLR-deficient mice were fed the HF diet for 12 or 16 weeks, the LF diet for 30 weeks, or the HF diet for 16 weeks followed by the LF diet for an additional 14 weeks. For Study 2, analyzing the combined effects of diet and VLDLR gene therapy, LDLR-deficient mice were fed the HF diet for 16 weeks followed by the LF diet for an additional 14 weeks combined with injection of either control (HD-Ad-0) or VLDLR-expressing helper-dependent adenovirus (HD-Ad-VLDLR).
Measurements of Lipids and Glucose
Total blood and plasma cholesterol, blood glucose, plasma triglycerides, and lipoprotein profiles were evaluated as described previously.17
Measurements of Plasma Levels of sVCAM-1 and sICAM-1
Plasma levels of soluble vascular cell adhesion molecule-1 (sVCAM-1) and intercellular adhesion molecule-1 (sICAM-1) were measured by immunoassays (Quantikine; R&D Systems, Inc., Minneapolis, MN). Plasma samples were diluted 1:60 before analysis according to the manufacturer’s instructions.
Tissue Preparation and Histochemistry
Mice were perfused with 4% paraformaldehyde at physiological pressure, as described previously.17 The BCA, an artery susceptible to formation of advanced lesions, was fixed for an additional 3 hours in 4% paraformaldehyde. The entire BCA was paraffin-embedded and serially sectioned (5 μm). Every 20 μm, two sections were stained with a modified Movat’s pentachrome stain.17 The Movat’s pentachrome method stains nuclei and elastin black, collagen and reticular fibers yellow, glycosaminoglycans blue, muscle red, and fibrinoid and fibrin intensely red. The cross-sectional area at the point of maximum lesion thickness was determined using computer-assisted morphometry (Image-Pro Plus; Media-Cybernetics, Silver Spring, MD). Lesion morphology was scored in a masked fashion by two independent investigators. Calcification was defined by positive von Kossa staining for hydroxyapatite.18 Medial expansion was defined as enlargement of the medial layer, and lateral macrophage accumulation was defined as the presence of aggregates of foam cells situated on the lateral margins of the plaques. The presence of glycosaminoglycans within the lesion was defined as intense blue staining by the Movat’s pentachrome stain. Chondrocyte-like cells were defined as cells expressing collagen II. These parameters were recorded as binary outcomes. The frequency of each parameter was determined for serial sections representing each 20 μm along the full length of each BCA. These frequencies were averaged across animals in each group. Thus, a frequency of, eg, 0.2 indicates that an average of 20% of all BCA sections (up to 180 sections) from an individual mouse were positive for a certain morphological feature, such as cholesterol clefts.
To analyze aortic root lesion area, the heart was embedded in paraffin, and 5-μm-thick serial cross-sections were cut from each aortic root, beginning at the level of attachment of the aortic valve cusps. Serial cross-sections were stained with Movat’s pentachrome. Lesion areas, in three sections 20 μm apart, the middle section at the level of the ostium, were analyzed by ImageJ version 1.33 (National Institutes of Health (NIH)) and averaged for each mouse.
Quantification of Plaque Macrophage Content and Maximal Fibrous Cap Thickness
Maximal fibrous cap thickness at the site of maximal lesion area in the BCA was measured using NIH ImageJ 1.33 following immunostaining of smooth muscle α-actin according to the manufacturer’s instructions (DakoCytomation, Carpinteria, CA). The mouse anti-human smooth muscle α-actin antibody, clone 1A4, was coupled to horseradish peroxidase, as was the negative control antibody (EPOS Universal Negative Control). Most lesions had some areas that contained an acellular cap consisting of matrix/elastin, but no smooth muscle α-actin-positive cells, which is why minimal fibrous cap thickness was not evaluated in this study. Macrophages were identified as Mac-2-positive cells, as previously described.17 The lesion area occupied by Mac-2-positive macrophages at the site of maximal lesion area was analyzed using NIH ImageJ 1.33.
Detection of VCAM-1 and Intraplaque Hemorrhage by Immunohistochemistry
Immunoreactive VCAM-1 in the BCA was detected in paraffin sections using a rabbit anti-VCAM-1 antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at a final concentration of 4 μg/ml. A rabbit IgG (Bio-Rad Laboratories, Hercules, CA) negative control of the same concentration did not result in any staining.
We used three different criteria for detection of intraplaque hemorrhage. In addition to positive fibrin/fibrinoid stain using the Movat’s pentachrome procedure, the presence of intraplaque erythrocyte membranes was verified by using an antibody directed against the erythrocyte marker glycophorin A-associated protein (TER-119). BCA sections were incubated in citrate buffer (10 mmol/L citric acid, 0.05% Tween 20, pH 6.0) at 95°C for 10 minutes. After cooling for 20 minutes, the sections were incubated with the monoclonal rat anti-TER-119 antibody (eBioscience, San Diego, CA) at a 1:400 dilution, final concentration of 1.25 μg/ml for 1 hour at room temperature. The sections were then incubated with a biotinylated goat anti-rat IgG (Southern Biotechnology Associates, Inc., Birmingham, AL) at a 1:10,000 dilution for 30 minutes at room temperature. Rat IgG2b isotype-negative control was obtained from eBioscience. Adjacent sections were used for immunohistochemical detection of fibrin/fibrinogen. The sections were incubated in the citrate buffer antigen retrieval buffer described above. A rabbit anti-human fibrin/fibrinogen antibody (DakoCytomation California Inc.) was used at a 1:200 dilution. Rabbit IgG (Zymed Laboratories, Inc., Carlsbad, CA) was used as negative control. The slides were developed using the streptavidin-horseradish peroxidase complex (Vector Laboratories, Inc., Burlingame, CA) at a 1:5000 dilution for 30 minutes at room temperature, subsequent 10-minute incubation with diaminobenzidine, and 2–5 minutes counterstaining with methyl green. Clotted mouse blood fixed as described for the BCA was used as a positive control for the anti-TER-119 and anti-fibrin/fibrinogen antibodies. The blood was clotted for 30 minutes in Eppendorf tubes, fixed, embedded in paraffin, and sectioned. Areas that stained positive for fibrin/fibrinoid by the Movat’s pentachrome method, and also stained positive with the anti-fibrin/fibrinogen antibody and with the antibody against the erythrocyte membrane marker TER-119, were scored positive for intraplaque hemorrhage.
Western Blot Analysis of VLDLR
Analysis of hepatic VLDLR expression was performed as described previously.16 In brief, livers were rapidly frozen in liquid nitrogen at the end of the study. The tissue was homogenized using a Dounce homogenizer, and liver membranes were prepared. VLDLR was detected in membrane samples (60 μg/lane) using an affinity-purified rabbit polyclonal antibody generated against the VLDLR C-terminal peptide CTYPAISVVSTDDDLA.16
Statistical Analyses
Comparisons between two groups were performed by two-tailed unpaired Student’s t-test for the quantitative parameters (Prism 3.0; GraphPad Software, San Diego, CA). Comparisons between multiple groups were performed using one-way analysis of variance. Simultaneous multiple comparisons were based on posthoc comparison tests using a Student-Newman-Keuls test. Comparisons of the frequency of qualitative parameters within lesions were made using the Mann-Whitney U-test or Kruskal-Wallis test followed by Dunn’s posthoc comparisons.
Results
Lipid Lowering Achieved by Diet Alone Does Not Induce Regression of Pre-existing Lesions Despite Causing a Significant Reduction in Plasma Cholesterol
First, the effect of diet alone on advanced lesions was investigated (Figure 1). Plasma total cholesterol and VLDL plus LDL levels were increased in mice fed the HF diet compared to mice fed the LF diet (Table 1). The majority of cholesterol was found in VLDL+LDL both in mice fed the HF diet and LF diet (Figure 2A). There were no significant differences in levels of HDL, triglycerides, or blood glucose between the different groups (Table 1). Mice fed the LF diet for 12 weeks had a lower body weight than mice fed the same diet for 30 weeks due to the age difference, but there were no significant differences in body weights between mice fed the LF diet or HF diet (Table 1). Switching the mice from the HF diet to the LF diet at 16 weeks resulted in a significant reduction of plasma cholesterol and VLDL+LDL levels at the end of the study to levels that approached those of mice fed the LF diet for the entire 30-week study (Table 1). Switching the mice to an LF diet did not affect HDL, triglycerides, or body weights.
Table 1.
Lipids, Glucose, and Body Weights at the End of the Study
| Plasma cholesterol (mmol/L) | Plasma VLDL+LDL (μg chol/peak) | Plasma HDL (μg chol/peak) | Plasma triglycerides (mmol/L) | Blood glucose (mmol/L) | Body weight (g) | |
|---|---|---|---|---|---|---|
| 12-week HF | 14.8 ± 1.0 (8)* | 196.0 ± 8.8 (3)† | 30.9 ± 7.9 (3) | 2.6 ± 0.2 (8) | 7.9 ± 0.4 (8) | 20.4 ± 0.4 (8)* |
| 16-week HF | 21.0 ± 2.6 (11)‡ | 182.1 ± 66.5 (3)† | 31.3 ± 8.9 (3) | 2.8 ± 0.4 (11) | ND | 21.6 ± 0.3 (11) |
| 16-week HF plus | ||||||
| 14-week LF | 8.4 ± 0.6 (8) | 111.0 ± 15.6 (6) | 31.9 ± 6.9 (6) | 1.2 ± 0.1 (8) | 7.7 ± 0.5 (8) | 23.1 ± 0.6 (8) |
| 30-week LF | 6.6 ± 0.6 (8) | 62.8 ± 14.5 (5) | 20.1 ± 8.1 (5) | 1.5 ± 0.1 (8) | 6.9 ± 0.3 (8) | 22.8 ± 0.4 (8) |
The results are shown as means ± SE. The number of animals per group is indicated within parentheses. chol. cholesterol; ND, not determined.
P < 0.01 versus 30-week LF diet (ANOVA followed by Newman-Keuls multiple comparison test).
P < 0.05 versus 30-week LF diet (ANOVA followed by Newman-Keuls multiple comparison test).
P < 0.001 versus 30-week LF diet (ANOVA followed by Newman-Keuls multiple comparison test).
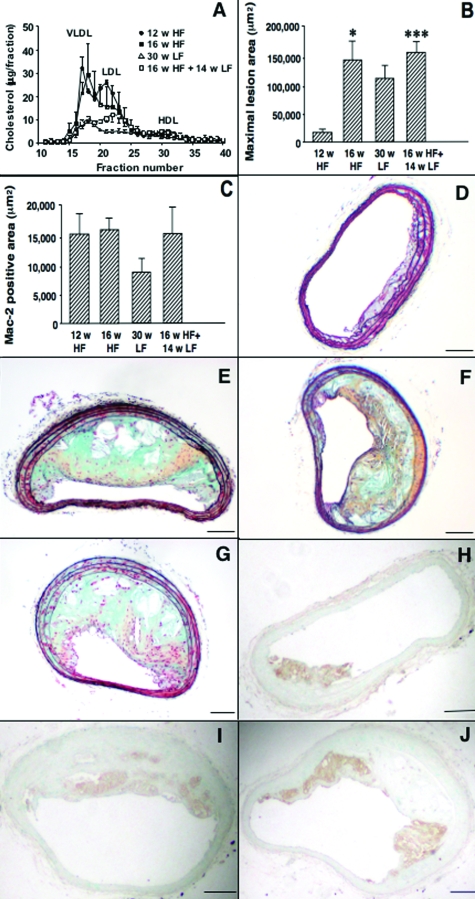
Figure 2.
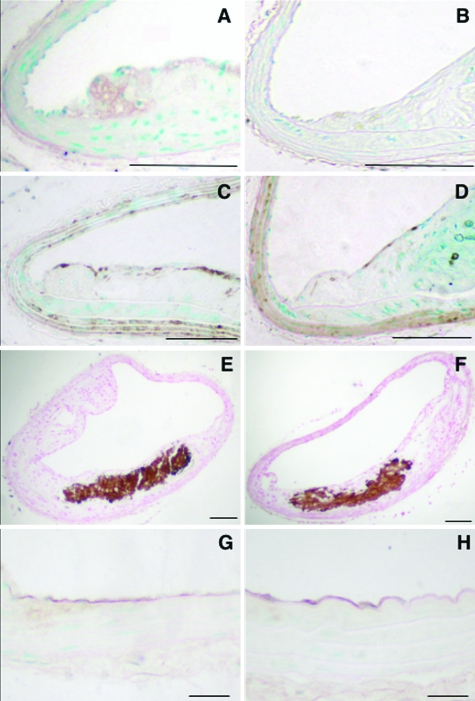
Lipid lowering achieved by diet alone does not inhibit progression of advanced lesions. Lipoprotein profiles (n = 3–6) were evaluated using 100 μl of plasma/mouse (A). Maximal BCA cross-sectional lesion area (B) is shown as mean + SEM. Between group differences were evaluated by one-way analysis of variance followed by Newman-Keuls posthoc analysis. *P < 0.05; ***P < 0.001 versus mice fed the HF diet for 12 weeks. Lesion area occupied by macrophages was measured as Mac-2-positive area (C). Representative BCA from a mouse fed the HF diet for 12 weeks (D and H), 16 weeks (E and I), the LF diet for 30 weeks (F), or the HF diet for 16 weeks and then the LF diet for an additional 14 weeks (G and J). Sections were stained using a Movat’s pentachrome procedure (D–G) or an anti-Mac-2 antibody (H–J). Scale bar = 100 μm.
The maximal cross-sectional area of lesions in the BCA was significantly larger in mice fed the HF diet for 16 weeks as compared to 12 weeks (Figure 2B). However, lipid lowering achieved by diet had no effect on lesion area (Figure 2B) or plaque macrophage content (Figure 2, C, I–J). After 12 weeks on the HF diet, lesions were small and consisted primarily of macrophages (Figure 2, D and H). Presence of cholesterol clefts, necrotic cores, lateral macrophage accumulation, collagen, or glycosaminoglycans were infrequent after 12 weeks on the HF diet (Figure 2D and Table 2) but were significantly increased after 16 weeks (Table 2 and Figure 2E). Switching the mice to the LF diet after 16 weeks did not result in significant reduction of these frequencies or a change in maximal fibrous cap thickness (Table 2 and Figure 2G, data not shown). Instead, the frequencies of chondrocyte-like cells and calcification present in the lesion were significantly higher in mice switched to the LF diet following 16 weeks on the HF diet, compared to mice fed the HF diet for 16 weeks (Table 2). Furthermore, lesions in mice fed the LF diet for the entire 30-week duration of the study showed all of the characteristics of advanced lesions (Table 2 and Figure 2F and data not shown). Thus, lowering of VLDL+LDL achieved by diet alone is not sufficient to induce regression of pre-existing lesions in the LDLR−/− mouse.
Table 2.
Plaque Morphology in Lesions from LDLR-deficient Mice Treated With Diet Only
| 12-week HF | 16-week HF | 30-week LF | 16-week HF plus 14-week LF | |
|---|---|---|---|---|
| Fibrous cap thickness (μm) | 1.91 ± 1.25 | 11.31 ± 1.84* | 6.41 ± 0.93 | 10.53 ± 2.51 |
| Medial expansion | 0.83 ± 0.07 | 1.00 ± 0.00* | 1.00 ± 0.00 | 1.00 ± 0.00 |
| Glycosaminoglycans | 0.49 ± 0.16 | 0.99 ± 0.01* | 0.96 ± 0.04 | 1.00 ± 0.00 |
| Collagen | 0.44 ± 0.17 | 0.99 ± 0.01* | 0.97 ± 0.03 | 0.92 ± 0.05 |
| Cholesterol clefts | 0.05 ± 0.05 | 0.46 ± 0.14† | 0.64 ± 0.14 | 0.88 ± 0.08 |
| Necrotic core | 0.24 ± 0.16 | 0.77 ± 0.07† | 0.74 ± 0.11 | 0.93 ± 0.06 |
| Lateral macrophage | ||||
| Accumulation | 0.08 ± 0.04 | 0.66 ± 0.11* | 0.77 ± 0.11 | 0.87 ± 0.07 |
| Chondrocyte-like cells | 0.11 ± 0.09 | 0.10 ± 0.07 | 0.62 ± 0.13 | 0.74 ± 0.12‡ |
| Calcification | 0.07 ± 0.07 | 0.10 ± 0.06 | 0.30 ± 0.10 | 0.38 ± 0.10§ |
The results are shown as frequencies (means ± SE), with the exception of cap thickness. For the 12-week time point, only two of eight mice exhibited fibrous caps. The presence or absence of each morphological characteristic was recorded as a binary outcome for each cross-section representing 20 μm along the full length of each BCA. These frequencies were averaged across animals in each group.
P < 0.05, 12-week HF group versus the 16-week HF group.
P < 0.01, 12-week HF group versus the 16-week HF group.
P < 0.001, 16-week HF group versus the 16-week HF plus 14-week LF group (Kruskal-Wallis test followed by Dunn’s Multiple Comparison test).
P < 0.05, 16-week HF group versus the 16-week HF plus 14-week LF group (Kruskal-Wallis test followed by Dunn’s multiple comparison test).
Overexpression of VLDLR Using a Helper-Dependent Adenoviral Vector Combined with a Low-Fat Diet Regimen Leads to Rapid and Stable VLDL+LDL Lowering beyond That Achieved by Diet Alone
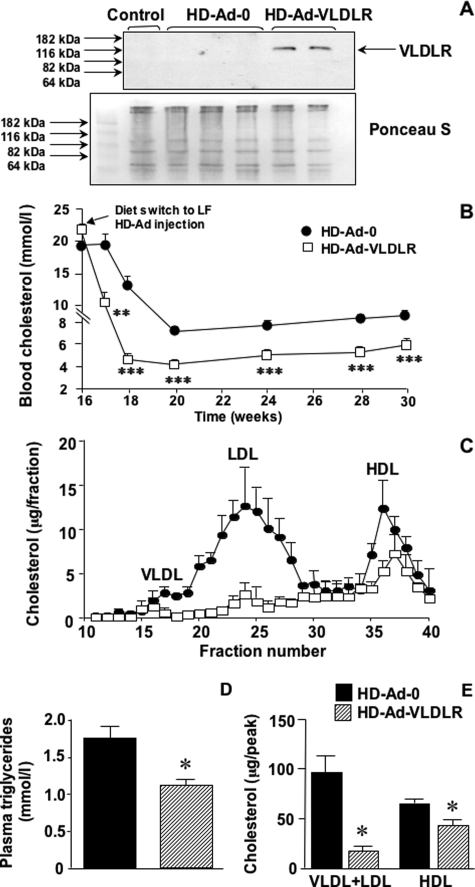
Injection of HD-Ad-VLDLR resulted in overexpression of VLDLR in the liver at the end of the study (Figure 3A) and a rapid and stable decrease in total plasma cholesterol levels beyond that achieved by diet alone (Figure 3B). Injection of the control HD-Ad-0 did not alter plasma cholesterol or triglyceride levels compared to untreated mice (compare Figure 3 and Table 1). HD-Ad vectors did not affect blood glucose levels or body weights. Body weights at the end of the study were 23.1 ± 0.6 g for HD-Ad-0-treated mice and 22.7 ± 0.5 g for HD-Ad-VLDLR-treated mice.
Figure 3.
HD-Ad-VLDLR treatment combined with a low-fat diet regimen results in a rapid and stable reduction in VLDL and LDL levels. Expression of hepatic VLDLR after 16 weeks of HF feeding and an additional 14 weeks after HD-Ad injection (A). Before probing the membrane with 2 μg/ml anti-VLDLR antibody (upper panel), the membrane was stained with Ponceau S (Sigma-Aldrich) as a loading control (lower panel). Saphenous blood cholesterol levels were measured at the indicated times (B). Statistical analysis was performed using unpaired Student’s t-test. C: Plasma cholesterol profiles. D: Plasma triglyceride levels at the end of the study. Statistical analysis was performed using unpaired Student’s t-test. E: Differences in the VLDL+LDL and HDL peaks. Statistical analysis was performed using one-way analysis of variance followed by Newman-Keuls posthoc analysis. Results are shown as means ± SE. *P < 0.05; **P < 0.01; and ***P < 0.001.
HD-Ad-VLDLR treatment also resulted in a marked reduction of VLDL, LDL, and triglyceride levels and a switch to a high-density HDL-rich lipoprotein profile at the end of the study, compared to HD-Ad-0 (Figure 3, C and D). Furthermore, total VLDL+LDL levels and HDL levels were significantly reduced in mice treated with HD-Ad-VLDLR (Figure 3E).
HD-Ad-VLDLR Treatment in Combination with a Low-Fat Diet Regimen Induces Regression of Pre-existing Lesions
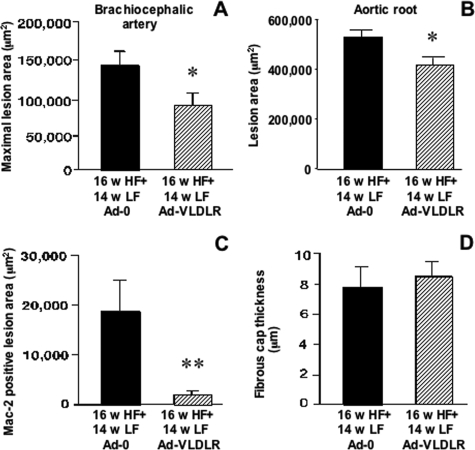
Injection of HD-Ad-VLDLR at the 16-week time point resulted in a significant decrease in maximal BCA lesion area at 30 weeks, compared to injection of HD-Ad-0 (Figure 4A). There was no significant difference in lesion size in mice injected with the HD-Ad-0 vector as compared to mice subjected to the LF diet regimen alone (compare Figures 2B and 4A). To verify that lesions were smaller in HD-Ad-VLDLR-treated mice compared to HD-Ad-0-treated mice, aortic root lesion areas were also evaluated. As shown by Figure 4B, the aortic root cross-sectional lesion area was significantly smaller in mice injected with HD-Ad-VLDLR- compared to HD-Ad-0-injected controls.
Figure 4.
HD-Ad-VLDLR combined with a low fat diet regimen results in reduced lesion area and reduced macrophage content. A: Maximal BCA cross-sectional lesion area. B: Aortic root lesion area. Macrophage content was evaluated as the Mac-2-positive BCA lesion area (C). Between group differences were evaluated by Student’s t-test. Thickness of the fibrous cap in the BCA was measured by smooth muscle α-actin immunohistochemistry (D). Results are shown as means ± SE. *P < 0.05; **P < 0.01.
A Combination of HD-Ad-VLDLR and Diet Treatment Results in Reduction of Macrophage Accumulation but Does Not Reduce the Frequency of Calcification, Cholesterol Clefts, or Other Features Associated with Advanced Lesions
Macrophage content in the BCA lesion was significantly reduced by HD-Ad-VLDLR compared to HD-Ad-0 (Figures 4C, 5A, and 5B). Interestingly, injection of HD-Ad-VLDLR did not affect the thickness of the fibrous cap, measured as maximal thickness of the subendothelial smooth muscle α-actin-positive layer (Figures 4D, 5C, and 5D), or the frequency of glycosaminoglycans, collagen, cholesterol clefts, necrotic cores, chondrocyte-like cells, or calcification (Figure 5, E and F, and data not shown). The finding that lipid lowering does not readily reduce calcification is in agreement with previous findings on lesions in monkeys and swine.19–21 These observations suggest that macrophage accumulation is particularly sensitive to aggressive lipid lowering, and that other features of advanced plaques, such as calcification and necrotic cores, are less sensitive.
Figure 5.
HD-Ad-VLDLR combined with a low fat diet regimen reduces lesion macrophage content. LDLR-deficient littermates were injected with HD-Ad-0 (A, C, E, and G) or HD-Ad-VLDLR (B, D, F, and H). Representative sections stained using anti-Mac-2 antibody (A and B), smooth muscle α-actin antibody (C and D), the von Kossa method for demonstration of calcification (E and F), and anti-VCAM-1 antibody (G and H) are shown. Scale bar = 100 μm (A–F); 20 μm (G and H).
It is possible that HD-Ad-VLDLR in combination with lipid lowering acts by improving endothelial dysfunction, thereby reducing monocyte adherence and recruitment. This possibility was investigated using two different approaches. First, levels of immunoreactive VCAM-1 were analyzed in BCA sections. VCAM-1 immunoreactivity was found in the endothelium, although the positive staining was not uniform (Figure 5, G and H). Other cells in the lesion also showed positive VCAM-1 staining in a similar fashion (data not shown), consistent with previous studies demonstrating VCAM-1 expression in lesion smooth muscle cells.22 There were no detectable differences in endothelial VCAM-1 staining between mice treated with HD-Ad-0 (Figure 5G) and HD-Ad-VLDLR (Figure 5H). Plasma levels of circulating soluble forms of VCAM-1 and ICAM-1 were also measured. HD-Ad-VLDLR treatment did not significantly alter plasma levels of sVCAM-1 or sICAM-1. Plasma levels of sVCAM-1 were 612.9 ± 46.9 ng/ml in HD-Ad-0-treated mice (n = 10) and 567.5 ± 35.2 ng/ml in HD-Ad-VLDLR-treated mice (n = 11). Plasma levels of sICAM-1 were 493.1 ± 53.7 and 448.1 ± 24.3 ng/ml, respectively.
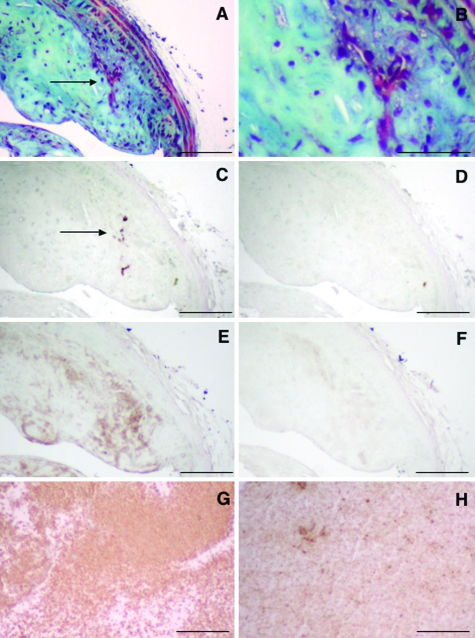
Finally, because intraplaque hemorrhage is associated with advanced plaques in humans23–26 and mice,27–31 we measured the frequency of intraplaque hemorrhage in these BCA lesions. Intraplaque hemorrhage was defined as lesion areas that stained positive for fibrin/fibrinoid by using the Movat’s pentachrome stain (Figure 6, A and B) and at the same time were positive for the erythrocyte membrane marker protein TER-119 (Figure 6C) and fibrin/fibrinogen (Figure 6E). As shown by Figure 6, the anti-fibrin/fibrinogen antibody resulted in staining that was more widespread than that of anti-TER-119. This is most likely due to the fact that anti-fibrin antibodies react with fibrinogen as well as with fibrin. The negative IgG controls for the anti-TER-119 and anti-fibrin/fibrinogen antibodies showed little staining (Figure 6, D and F), whereas a mouse blood clot-positive control stained positive for TER-119 (Figure 6G) and fibrin/fibrinogen (Figure 6H). Using the above criteria, intraplaque hemorrhage was infrequent in these 38-week-old LDLR-deficient mice. Two of the nine (22%) mice treated with HD-Ad-0 showed intraplaque hemorrhage, whereas none of the eleven mice treated with HD-Ad-VLDLR showed intraplaque hemorrhage.
Figure 6.
Intraplaque hemorrhage is infrequent in LDLR-deficient mice fed an HF diet for 16 weeks followed by an LF diet for 14 weeks. Intraplaque hemorrhage was defined as lesion areas staining intensely red using the Movat’s pentachrome stain (A and B) and staining positive for both erythrocyte membranes by an anti-TER-119 antibody (C) and for fibrin/fibrinogen (E). Negative-matched IgG controls of the same concentration are shown for TER-119 (D) and fibrin/fibrinogen (F). A mouse blood clot was used as a positive control for anti-TER-119 (G) and anti-fibrin/fibrinogen (H). B is a magnification of A. The arrow indicates area with intraplaque hemorrhage. Scale bar = 100 μm.
Discussion
The VLDLR is a peripheral lipoprotein receptor expressed primarily in heart, muscle, adipocytes, and brain.32 It shares structural homology to the LDLR, but in contrast to LDLR, does not bind LDL. Instead it binds apolipoprotein E (apoE)-containing lipoproteins such as VLDL, chylomicron remnants, and intermediate density lipoprotein. These findings, together with the fact that increased serum triglyceride/fatty acid levels might contribute to cardiovascular disease, have led to the development of gene therapies to reduce VLDL levels. Hepatic overexpression of VLDLR induced by a helper-dependent adenoviral vector has been shown to prevent atherosclerotic lesion formation and progression of fatty streak lesions in LDLR-deficient mice.16,33 This ability of HD-Ad-VLDLR treatment to reduce atherosclerosis is most likely due to the marked lowering of VLDL and intermediate density lipoprotein levels in mice overexpressing hepatic VLDLR. Because intermediate density lipoprotein is a precursor of LDL, LDL levels are also lowered as a result of VLDLR overexpression.16
In contrast to inhibition of lesions with poorly developed lipid cores,34–38 regression and associated morphological changes following lipid lowering have been less studied in advanced plaques. Swine and nonhuman primates develop advanced lesions with fibrous caps, necrotic cores, and calcification after prolonged fat feeding. Such models have shown that advanced lesions can regress, at least to some extent, following lipid lowering,19,39–40 and that regression is associated with a reduction in foam cells and with thicker fibrous caps,39 whereas calcification is less susceptible to regression.19,39 In the mouse, few models exist that allow studies on regression of advanced lesions following lipid lowering. In the study by Tsukamoto et al,41 regression of well characterized advanced lesions containing smooth muscle cells, necrotic cores, and extracellular lipid in the aortic root and arch of apoE-deficient mice was induced by hepatic overexpression of human apoE3. In the study of Reis et al,42 transplantation of thoracic aortas containing similar advanced lesions from apoE-deficient mice to wild-type mice was used to induce marked lipid lowering. This procedure led to a dramatic regression and loss of foam cells.42 The present study adds a new model for studies of the cellular and molecular mechanisms involved in regression of advanced plaques: regression of BCA lesions in LDLR-deficient mice by use of hepatic overexpression of VLDLR. The BCA is a site prone to more rapid development of advanced lesions containing fibrous caps, cholesterol clefts, and necrotic cores compared to, eg, the thoracic aorta. Our data show minimal effects of lipid lowering by diet alone on such advanced lesions. More aggressive VLDL+LDL lowering and a marked increase in HDL relative to VLDL+LDL induced by HD-Ad-VDLDR gene therapy in combination with an LF diet regimen decreased lesion mass and reduced macrophage content. This regression appears to be mediated by a reduction in lesion macrophage content, similar to what has been described in other models of regression of both earlier fatty streak-type lesions and more advanced lesions.34,36,38–44 Thus, treatment with HD-Ad-VLDLR reduced the content of plaque macrophages concomitant with a reduction in lesion size. Although it is possible that the reduced macrophage content is due to the reduced plaque size, it is perhaps more likely that the reduced macrophage content causes the smaller plaque size in HD-Ad-VLDLR-treated mice. Indeed, when the lesion area occupied by macrophages was subtracted from the maximal total lesion area, the statistical difference in lesion area between HD-Ad-VLDLR-treated mice and HD-Ad-0-treated mice was lost (data not shown). It is tempting to speculate that HD-Ad-VLDLR results in a reduced recruitment of macrophages to the lesion because of the dramatically reduced VLDL/LDL levels, perhaps in concert with an increased emigration of macrophages.14 One possible mechanism whereby reduced VLDL and LDL levels might reduce monocyte recruitment is by improving endothelial function. In the present study, we did not find evidence of reduced levels of immunoreactive VCAM-1 on the endothelium in HD-Ad-VLDLR-treated mice compared to controls. VCAM-1 is expressed on activated endothelial cells and mediates adhesion of monocytes, thereby playing a major role in atherosclerosis.45 Furthermore, plasma levels of soluble endothelial adhesion molecules (sVCAM-1 and sICAM-1), which are generally believed to reflect endothelial dysfunction, were not different between HD-Ad-VLDLR-treated mice and controls. These observations suggest that improved endothelial dysfunction might not mediate the effects of aggressive lipid lowering on plaque regression in this model. It has been shown that lesion regression is associated with decreased levels of oxidation epitopes present in LDL within the lesion.46 A reduced level of oxidized lipids within the arterial wall may result in plaque regression by reducing macrophage accumulation. Aggressive lipid lowering did not affect several other features of the advanced plaque, such as calcification and the presence of cholesterol clefts. It is possible that regression of these features require a more prolonged lipid lowering. Future studies will use this model to further investigate the mechanisms behind regression of advanced lesions.
The LDLR−/− mouse has elevated plasma cholesterol levels (6 to 8 mmol/L), and most of the cholesterol in VLDL+LDL even when fed an LF diet, as compared to C57BL/6 mice, in which most of the cholesterol is present in HDL. Treatment with HD-Ad-VLDLR in combination with an LF diet reduced plasma cholesterol to levels normally found in C57BL/6 mice and markedly increased HDL/LDL ratios. Conversely, a switch from the HF diet to the LF diet without treatment with HD-Ad-VLDLR did not result in significant lesion regression despite a reduction of plasma cholesterol levels from 21 to 8 mmol/L. Furthermore, treatment of fat-fed mice with HD-Ad-VLDLR did not result in significant lesion regression, using a similar study design (unpublished observations). Therefore, at least over this time interval and in this model, regression can only be induced following a severe reduction of plasma cholesterol to levels below those achieved by diet alone and/or improved lipoprotein profiles. This also appears to be the case in humans.47–53
Like all animal models of human disease, mouse models of atherosclerosis and lesion regression have several limitations. Importantly, although the mouse appears to be a suitable model for studies of events leading to formation of advanced plaques and even plaque rupture,54 mice seldom show signs of myocardial infarctions or strokes.55 Furthermore, there are differences in lipid metabolism between humans and LDLR-deficient mice. For example in contrast to humans, LDLR−/− mice have low levels of cholesteryl ester transfer protein and lipoprotein(a). However, overexpression of these proteins in mice does not result in more atherosclerosis.56,57 Although several of the limitations of mouse models have been overcome with the development of the LDLR−/− model, apoE−/− model, and several other models, it is clear that findings and treatment strategies based on studies in mouse models must be carefully evaluated in larger animal models and in humans.
In summary, the LDLR-deficient mouse treated with a low-fat diet regimen together with HD-Ad-VLDLR provides a new model to study the mechanisms involved in regression of advanced plaques. Our study shows that aggressive VLDL+LDL lowering beyond that achieved with diet alone induces regression of advanced lesions in LDLR-deficient mice. The ability of HD-Ad-VLDLR to reduce plaque appears to be due to its ability to inhibit macrophage accumulation in the plaque.
Footnotes
Address reprint requests to Dr. Karin E. Bornfeldt, Dept. of Pathology, 1959 NE Pacific St., University of Washington, Seattle, WA 98195-7470. E-mail: bornf@u.washington.edu.
Supported by grants from the National Institutes of Health (HL62887 and HL076719 to K.E.B.; HL03174 to S.M.S.; HL076748 to M.E.R.; HL073144 to K.O.; HL51586 and HL59314 to L.C.; and training grant T32 HL07312 to E.D.M. and the Juvenile Diabetes Research Foundation (1-2003-33 to K.E.B.). E.D.M. was also supported by a post-doctoral fellowship from the American Heart Association Northwest affiliate.
E.D.M. and F.K. contributed equally to this study.
References
- Aikawa M, Libby P. Lipid lowering therapy in atherosclerosis. Semin Vasc Med. 2004;4:357–366. doi: 10.1055/s-2004-869592. [DOI] [PubMed] [Google Scholar]
- Tabas I. Nonoxidative modifications of lipoproteins in atherogenesis. Annu Rev Nutr. 1999;19:123–139. doi: 10.1146/annurev.nutr.19.1.123. [DOI] [PubMed] [Google Scholar]
- Ginsberg HN. Lipoprotein physiology in nondiabetic and diabetic states. Relationship to atherogenesis. Diabetes Care. 1991;14:839–855. doi: 10.2337/diacare.14.9.839. [DOI] [PubMed] [Google Scholar]
- Faergeman O. Hypertriglyceridemia and the fibrate trials. Curr Opin Lipidol. 2000;11:609–614. doi: 10.1097/00041433-200012000-00007. [DOI] [PubMed] [Google Scholar]
- Malloy MJ, Kane JP. A risk factor for atherosclerosis: triglyceride-rich lipoproteins. Adv Intern Med. 2001;47:111–136. [PubMed] [Google Scholar]
- Kolodgie FD, Virmani R, Burke AP, Farb A, Weber DK, Kutys R, Finn AV, Gold HK. Pathologic assessment of the vulnerable human coronary plaque. Heart. 2004;90:1385–1391. doi: 10.1136/hrt.2004.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull W, Jr, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part II. Circulation. 2003;108:1772–1778. doi: 10.1161/01.CIR.0000087481.55887.C9. [DOI] [PubMed] [Google Scholar]
- Davies MJ, Richardson PD, Woolf N, Katz DR, Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993;69:377–381. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W, Jr, Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- Pick R, Prabhu R, Glick G. Diet-induced atherosclerosis and experimental hypertension in stumptail macaques (Macaca arctoides). Atherosclerosis. 1978;29:405–429. doi: 10.1016/0021-9150(78)90170-3. [DOI] [PubMed] [Google Scholar]
- Constantinides P. Overview of studies on regression of atherosclerosis. Artery. 1981;9:30–43. [PubMed] [Google Scholar]
- Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangirala RK, Tsukamoto K, Chun SH, Usher D, Pure E, Rader DJ. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation. 1999;100:1816–1822. doi: 10.1161/01.cir.100.17.1816. [DOI] [PubMed] [Google Scholar]
- Llodrá J, Angeli V, Liu J, Trogan E, Fisher EA, Randolph GJ. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc Natl Acad Sci USA. 2004;101:11779–11784. doi: 10.1073/pnas.0403259101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trogan E, Fayad ZA, Itskovich VV, Aguinaldo JG, Mani V, Fallon JT, Chereshnev I, Fisher EA. Serial studies of mouse atherosclerosis by in vivo magnetic resonance imaging detect lesion regression after correction of dyslipidemia. Arterioscler Thromb Vasc Biol. 2004;24:1714–1719. doi: 10.1161/01.ATV.0000139313.69015.1c. [DOI] [PubMed] [Google Scholar]
- Oka K, Pastore L, Kim IH, Merched A, Nomura S, Lee HJ, Merched-Sauvage M, Arden-Riley C, Lee B, Finegold M, Beaudet A, Chan L. Long-term stable correction of low-density lipoprotein receptordeficient mice with a helper-dependent adenoviral vector expressing the very low-density lipoprotein receptor. Circulation. 2001;103:1274–1281. doi: 10.1161/01.cir.103.9.1274. [DOI] [PubMed] [Google Scholar]
- Renard CB, Kramer F, Johansson F, Lamharzi N, Tannock LR, von Herrath MG, Chait A, Bornfeldt KE. Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J Clin Invest. 2004;114:659–668. doi: 10.1172/JCI17867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna LG, editor. New York: McGraw-Hill Book Company,; Manual of histologic staining methods of the Armed Forces Institute of Pathology. American Registry of Pathology. (ed 3) 1968:pp 176–177. [Google Scholar]
- Clarkson TB, Bond MG, Bullock BC, Marzetta CA. A study of atherosclerosis regression in Macaca mulatta. IV. Changes in coronary arteries from animals with atherosclerosis induced for 19 months and then regressed for 24 or 48 months at plasma cholesterol concentrations of 300 or 200 mg/dl. Exp Mol Pathol. 1981;34:345–368. doi: 10.1016/0014-4800(81)90052-6. [DOI] [PubMed] [Google Scholar]
- Daoud AS, Jarmolych J, Augustyn JM, Fritz KE. Sequential morphologic studies of regression of advanced atherosclerosis. Arch Pathol Lab Med. 1981;105:233–239. [PubMed] [Google Scholar]
- Stary HC. The development of calcium deposits in atherosclerotic lesions and their persistence after lipid regression. Am J Cardiol. 2001;88:16E–19E. doi: 10.1016/s0002-9149(01)01713-1. [DOI] [PubMed] [Google Scholar]
- Li H, Cybulsky MI, Gimbrone MA, Jr, Libby P. Inducible expression of vascular cell adhesion molecule-1 by vascular smooth muscle cells in vitro and within rabbit atheroma. Am J Pathol. 1993;143:1551–1559. [PMC free article] [PubMed] [Google Scholar]
- Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, Badimon JJ, O’Connor WN. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–2038. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- Kolodgie FD, Gold HK, Burke AP, Fowler DR, Kruth HS, Weber DK, Farb A, Guerrero LJ, Hayase M, Kutys R, Narula J, Finn AV, Virmani R. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med. 2003;349:2316–2325. doi: 10.1056/NEJMoa035655. [DOI] [PubMed] [Google Scholar]
- Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, Wrenn SP, Narula J. Atherosclerotic plaque progression and vulnerability to rupture angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- Takaya N, Yuan C, Chu B, Saam T, Polissar NL, Jarvik GP, Isaac C, McDonough J, Natiello C, Small R, Ferguson MS, Hatsukami TS. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation. 2005;111:2768–2775. doi: 10.1161/CIRCULATIONAHA.104.504167. [DOI] [PubMed] [Google Scholar]
- Rosenfeld ME, Polinsky P, Virmani R, Kauser K, Rubanyi G, Schwartz SM. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler Thromb Vasc Biol. 2000;20:2587–2592. doi: 10.1161/01.atv.20.12.2587. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Jackson CL. Atherosclerotic plaque rupture in the apolipoprotein E knockout mouse. Atherosclerosis. 2001;154:399–406. doi: 10.1016/s0021-9150(00)00515-3. [DOI] [PubMed] [Google Scholar]
- Williams H, Johnson JL, Carson KG, Jackson CL. Characteristics of intact and ruptured atherosclerotic plaques in brachiocephalic arteries of apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2002;22:788–792. doi: 10.1161/01.atv.0000014587.66321.b4. [DOI] [PubMed] [Google Scholar]
- de Nooijer R, von der Thusen JH, Verkleij CJ, Kuiper J, Jukema JW, van der Wall EE, van Berkel JC, Biessen EA. Overexpression of IL-18 decreases intimal collagen content and promotes a vulnerable plaque phenotype in apolipoprotein-E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:2313–2319. doi: 10.1161/01.ATV.0000147126.99529.0a. [DOI] [PubMed] [Google Scholar]
- Zadelaar AS, Thusen JH, Boesten M LS, Hoeben RC, Kockx MM, Versnel MA, van Berkel TJ, Havekes LM, Biessen L EA, van Vlijmen BJ. Increased vulnerability of pre-existing atherosclerosis in ApoE-deficient mice following adenovirus-mediated Fas ligand gene transfer. Atherosclerosis. 2005;138:244–250. doi: 10.1016/j.atherosclerosis.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Sakai J, Fujino T, Hattori H, Zenimaru Y, Suzuki J, Miyamori I, Yamamoto TT. The very low-density lipoprotein (VLDL) receptor: characterization and functions as a peripheral lipoprotein receptor. J Atheroscler Thromb. 2004;11:200–208. doi: 10.5551/jat.11.200. [DOI] [PubMed] [Google Scholar]
- Nomura S, Merched A, Nour E, Dieker C, Oka K, Chan L. Low-density lipoprotein receptor gene therapy using helper-dependent adenovirus produces long-term protection against atherosclerosis in a mouse model of familial hypercholesterolemia. Gene Ther. 2004;11:1540–1548. doi: 10.1038/sj.gt.3302310. [DOI] [PubMed] [Google Scholar]
- Aikawa M, Rabkin E, Okada Y, Voglic SJ, Clinton SK, Brinckerhoff CE, Sukhova GK, Libby P. Lipid lowering by diet reduces matrix metalloproteinase activity and increases collagen content in rabbit atheroma: a potential mechanism of lesion stabilization. Circulation. 1998;97:2433–2444. doi: 10.1161/01.cir.97.24.2433. [DOI] [PubMed] [Google Scholar]
- Aikawa M, Voglic SJ, Sugiyama S, Rabkin E, Taubman MB, Fallon JT, Libby P. Dietary lipid lowering reduces tissue factor expression in rabbit atheroma. Circulation. 1999;100:1215–1222. doi: 10.1161/01.cir.100.11.1215. [DOI] [PubMed] [Google Scholar]
- Kockx MM, De Meyer GR, Buyssens N, Knaapen MW, Bult H, Herman AG. Cell composition, replication, and apoptosis in atherosclerotic plaques after 6 months of cholesterol withdrawal. Circ Res. 1998;83:378–387. doi: 10.1161/01.res.83.4.378. [DOI] [PubMed] [Google Scholar]
- De Meyer GR, Hoylaerts MF, Kockx MM, Yamamoto H, Herman AG, Bult H. Intimal deposition of functional von Willebrand factor in atherogenesis. Arterioscler Thromb Vasc Biol. 1999;19:2524–2534. doi: 10.1161/01.atv.19.10.2524. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daoud AS, Jarmolych J, Augustyn JM, Fritz KE, Singh JK, Lee KT. Regression of advanced atherosclerosis in swine. Arch Pathol Lab Med. 1976;100:372–379. [PubMed] [Google Scholar]
- Strong JP, Bhattacharyya AK, Eggen DA, Stary HC, Malcom GT, Newman WP, 3rd, Restrepo C. Long-term induction and regression of diet-induced atherosclerotic lesions in rhesus monkeys. II. Morphometric evaluation of lesions by light microscopy in coronary and carotid arteries. Arterioscler Thromb. 1994;14:2007–2016. doi: 10.1161/01.atv.14.12.2007. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K, Tangirala R, Chun SH, Pure E, Rader DJ. Rapid regression of atherosclerosis induced by liver-directed gene transfer of ApoE in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 1999;19:2162–2170. doi: 10.1161/01.atv.19.9.2162. [DOI] [PubMed] [Google Scholar]
- Reis ED, Li J, Fayad ZA, Rong JX, Hansoty D, Aguinaldo JG, Fallon JT, Fisher EA. Dramatic remodeling of advanced atherosclerotic plaques of the apolipoprotein E-deficient mouse in a novel transplantation model. J Vasc Surg. 2001;34:541–547. doi: 10.1067/mva.2001.115963. [DOI] [PubMed] [Google Scholar]
- Harris JD, Graham IR, Schepelmann S, Stannard AK, Roberts ML, Hodges BL, Hill V, Amalfitano A, Hassall DG, Owen JS, Dickson G. Acute regression of advanced and retardation of early aortic atheroma in immunocompetent apolipoprotein-E (apoE) deficient mice by administration of a second generation [E1(−), E3(−), polymerase(−)] adenovirus vector expressing human apoE. Hum Mol Genet. 2002;11:43–58. doi: 10.1093/hmg/11.1.43. [DOI] [PubMed] [Google Scholar]
- Raffai RL, Loeb SM, Weisgraber KH. Apolipoprotein E promotes the regression of atherosclerosis independently of lowering plasma cholesterol levels. Arterioscler Thromb Vasc Biol. 2005;25:436–441. doi: 10.1161/01.ATV.0000152613.83243.12. [DOI] [PubMed] [Google Scholar]
- Cybulsky MI, Iiyama K, Li H, Zhu S, Chen M, Iiyama M, Davis V, Gutierrez-Ramos JC, Connelly PW, Milstone DS. A major role for VCAM-1, but not ICAM-1, in early atherosclerosis. J Clin Invest. 2001;107:1255–1262. doi: 10.1172/JCI11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsimikas S, Shortal BP, Witztum JL, Palinski W. In vivo uptake of radiolabeled MDA2, an oxidation-specific monoclonal antibody, provides an accurate measure of atherosclerotic lesions rich in oxidized LDL and is highly sensitive to their regression. Arterioscler Thromb Vasc Biol. 2000;20:689–697. doi: 10.1161/01.atv.20.3.689. [DOI] [PubMed] [Google Scholar]
- Taylor AJ, Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Vernalis MN. ARBITER: arterial biology for the investigation of the treatment effects of reducing cholesterol: a randomized trial comparing the effects of atorvastatin and pravastatin on carotid intima medial thickness. Circulation. 2002;106:2055–2060. doi: 10.1161/01.cir.0000034508.55617.65. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, Howard G, Cooper CJ, Brodie B, Grines CL, DeMaria AN, REVERSAL Investigators Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080. doi: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- Ornish D, Scherwitz LW, Billings JH, Brown SE, Gould KL, Merritt TA, Sparler S, Armstrong WT, Ports TA, Kirkeeide RL, Hogeboom C, Brand RJ. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280:2001–2007. doi: 10.1001/jama.280.23.2001. [DOI] [PubMed] [Google Scholar]
- von Birgelen C, Hartmann M, Mintz GS, Baumgart D, Schmermund A, Erbel R. Relation between progression and regression of atherosclerotic left main coronary artery disease and serum cholesterol levels as assessed with serial long-term (> or = 12 months) follow-up intravascular ultrasound. Circulation. 2003;108:2757–2762. doi: 10.1161/01.CIR.0000103664.47406.49. [DOI] [PubMed] [Google Scholar]
- Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T, Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) Study Investigators Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–1718. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM, Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22 Investigators Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK, Treating to New Targets (TNT) Investigators Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- Gough PJ, Gomez IG, Wille PT, Raines EW. Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen P, Baetta R, Bellosta S, Bernini F, Chinetti G, Cignarella A, von Eckardstein A, Exley A, Goddard M, Hofker M, Hurt-Camejo E, Kanters E, Kovanen P, Lorkowski S, McPheat W, Pentikainen M, Rauterberg J, Ritchie A, Staels B, Weitkamp B, de Winther M, MAFAPS Consortium Arterioscler Thromb Vasc Biol. 2003;23:535–542. doi: 10.1161/01.ATV.0000060200.73623.F8. [DOI] [PubMed] [Google Scholar]
- Berti JA, Salerno AG, Bighetti EJ, Casquero AC, Boschero AC, Oliveira HC. Effects of diabetes and CETP expression on diet-induced atherosclerosis in LDL receptor-deficient mice. APMIS. 2005;113:37–44. doi: 10.1111/j.1600-0463.2005.apm1130106.x. [DOI] [PubMed] [Google Scholar]
- Sanan DA, Newland DL, Tao R, Marcovina S, Wang J, Mooser V, Hammer RE, Hobbs HH. Low density lipoprotein receptor-negative mice expressing human apolipoprotein B-100 develop complex atherosclerotic lesions on a chow diet: no accentuation by apolipoprotein(a). Proc Natl Acad Sci USA. 1998;95:4544–4549. doi: 10.1073/pnas.95.8.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]