Abstract
Reduced synthesis of collagen types I and III is characteristic of chronologically aged skin. The present report provides evidence that both cellular fibroblast aging and defective mechanical stimulation in the aged tissue contribute to reduced collagen synthesis. The reduction in collagen synthesis due to fibroblast aging was demonstrated by a lower in vitro production of type I procollagen by dermal fibroblasts isolated from skin of young (18 to 29 years) versus old (80+ years) individuals (82 ± 16 versus 56 ± 8 ng/ml; P < 0.05). A reduction in mechanical stimulation in chronologically aged skin was inferred from morphological, ultrastructural, and fluorescence microscopic studies. These studies, comparing dermal sections from young and old individuals, demonstrated a greater percentage of the cell surface attached to collagen fibers (78 ± 6 versus 58 ± 8%; P < 0.01) and more extensive cell spreading (1.0 ± 0.3 vs. 0.5 ± 0.3; P < 0.05) in young skin compared with old skin. These features are consistent with a lower level of mechanical stimulation on the cells in old versus young skin. Based on the findings presented here, we conclude that reduced collagen synthesis in chronologically aged skin reflects at least two different underlying mechanisms: cellular fibroblast aging and a lower level of mechanical stimulation.
Reduction of fibrillar (types I and III) collagen is a characteristic feature of chronologically aged skin and is enhanced in photodamage. This has been well described using histological and ultrastructural approaches in the past,1–5 and our own recent studies have documented this biochemically in both chronological aging6 and photoaging.7 Collagen-degrading matrix metalloproteinases (MMPs) are up-regulated in skin by UV radiation.8,9 Repeated induction of these enzymes by exposure to solar radiation over years or decades is likely responsible for producing collagen fragmentation in sun-damaged skin. During natural or chronological aging of the skin, the same MMPs that are up-regulated acutely in response to UV radiation are gradually increased. This has been observed in monolayer culture with cells obtained from old versus young subjects,10–14 and our own studies have shown gradual up-regulation of MMPs in intact (old versus young) skin.15
Although destruction of existing collagen is, undoubtedly, central to the deleterious changes observed in aged/photoaged skin, failure to replace damaged collagen with newly synthesized material is also critical to the overall pathophysiology. There is a sustained down-regulation in collagen synthesis in photodamaged skin relative to what occurs in healthy sun-protected skin16 and in chronologically aged, sun-protected skin compared with what is seen in young skin.15
Mechanisms underlying the loss of collagen synthesis in photodamaged skin and chronologically aged skin have not been fully delineated. In a recent series of studies, we demonstrated that in severely photodamaged skin, the presence of fragmented collagen in the dermis inhibited collagen synthesis. Based on results from a variety of in vitro and in vivo approaches, we concluded that damaged collagen did not support a level of mechanical tension on resident fibroblasts necessary for efficient collagen synthesis.6,7,17,18 Whether a similar mechanism contributes to decreased collagen production in chronologically aged skin, and if so, to what extent it is responsible for the overall collagen reduction observed in aged skin, is not known. These issues are addressed in the present study.
Materials and Methods
Skin Biopsies
For this study, individuals between the ages of 18 and 29 years (young cohort) and individuals 80 years or older (old cohort) were recruited. Replicate 2- and/or 4-mm punch biopsies of sun-protected hip skin were obtained from each individual. The 4-mm punches were used for fluorescence microscopic and ultrastructural analysis and for assessment of type I procollagen levels. On arrival in the laboratory, one of the 4-mm biopsies was immediately frozen in optimal cutting temperature medium (OCT), and the other piece was fixed in glutaraldehyde. The 2-mm punch biopsies were used for routine histology at the light microscopic level and as a tissue source for the isolation of dermal fibroblasts. For routine histology, the biopsies were fixed in 10% buffered formalin. Fibroblast isolation was accomplished as indicated below. All procedures involving human subjects were approved by the Institutional Review Board, and biopsies were obtained after receiving informed consent.
Human Dermal Fibroblasts in Monolayer Culture
Fibroblasts were isolated from skin biopsies as described previously.15 Briefly, the biopsy was minced with a scissors and forceps, and tissue fragments were transferred to individual wells of a 24-well dish. Dulbecco’s modified minimal essential medium supplemented with nonessential amino acids and 10% fetal bovine serum (DMEM-FBS) was used as culture medium. Only a minimal amount of medium was included so that tissue pieces would adhere to the plastic surface. The dishes were maintained at 37°C in an atmosphere of 95% air and 5% CO2. Eventually, fibroblast proliferation around the edge of some of the tissue fragments was observed. When cells in sufficient number were available, they were harvested with trypsin/ethylenediamine tetraacetic acid (EDTA) and grown in monolayer culture using DMEM-FBS. Cells were subcultured by exposure to trypsin/EDTA one or two times and then used in experiments.
Type I Procollagen
Serial frozen sections were prepared from OCT-embedded skin biopsies as follows: 7, 200, and 7 μm. After hematoxylin and eosin staining, the areas of the sections from both ends of the 200-μm sample were measured using Image ProPlus software to calculate the volume of the 200-μm sample. Soluble protein extracts were prepared from the 200-μm samples, which were homogenized in ice-cold extraction buffer (50 mmol/L Tris-HCl, pH 7.4, 0.15 mol/L NaCl, 1% Triton X-100, and protease inhibitors [Complete Mini, Hoffmann-LaRoche, Nutley, NJ]), and vortexed in the presence of glass beads (Biospec, Bartlesville, OK). After centrifugation for 10 minutes at 10,000 × g and 4°C, supernatants were assayed for procollagen I using a commercial enzyme-linked immunosorbent assay kit (Panvera, Madison, WI) as described previously.15 The procollagen assay uses an antibody to the C-terminal propeptide region that is part of the collagen molecule as it is synthesized and secreted (before being proteolytically cleaved). As such, this assay is a measure of newly synthesized collagen. Type I procollagen concentrations were normalized to the volume of tissue used for the preparation of each sample.
Type I procollagen production was also assessed in dermal fibroblasts in monolayer culture. Fibroblasts were seeded at 4 × 104 cells per well in 24-well plates using DMEM-FBS as culture medium. The cells were allowed to attach overnight. The next day, they were washed and then incubated in keratinocyte growth medium (Cambrex Bioscience, Walkersville, MD) supplemented with 1.4 mmol/L Ca2+. Keratinocyte growth medium is a serum-free, low-Ca2+ modification of MCDB-153 medium supplemented with epidermal growth factor (EGF), insulin, hydrocortisone, and pituitary extract. Cell numbers were determined 2 days later by releasing the cells with trypsin/EDTA and enumerating them using a particle counter (Coulter Electronics, Hialeah, FL). At the time of harvest, the serum-free culture fluids were collected and assessed for type I procollagen using the same enzyme-linked immunosorbent assay procedure and then normalized to cell number.
Immunostaining and Confocal Microscopy
A mouse monoclonal antibody to vinculin was obtained from Chemicon (Temicula, CA). The primary antibody was visualized with rabbit anti-mouse IgG antibody bound to Alexa Fluor 488 (Invitrogen, Carlsbad, CA) and further amplified with Alexa Fluor 488 goat anti-rabbit IgG. Alexa Fluor 546-phalloidin (Invitrogen) was used as a probe for actin. Nuclei were counterstained with the nuclear dye 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) (Prolong Gold; Invitrogen). (Note that Alexa Fluor 488 is spectrally similar to fluorescein, whereas Alexa Fluor 546 is spectrally similar to rhodamine.)
OCT-embedded frozen skin biopsies from young and old individuals were used for staining. Briefly, the frozen tissue sections were fixed with 4% formaldehyde for 20 minutes. After fixation, the tissue sections were washed twice with wash buffer (0.05% Tween 20 in Dulbecco’s phosphate-buffered saline), followed by permeabilization with 0.1% Triton X-100 for 10 minutes. Tissue sections were again washed and then exposed to a blocking solution consisting of 1% bovine serum albumin in Dulbecco’s phosphate-buffered saline for 30 minutes. Next, the sections were treated with anti-vinculin antibody in blocking solution for 1 hour. After three subsequent washing steps (5 minutes each), each sample was treated with Alexa Fluor 488-conjugated secondary antibody in blocking solution and incubated for 45 minutes. Sections were simultaneously stained for actin expression using Alexa Fluor 546-phalloidin along with the secondary antibody. After three additional washing steps (5 minutes each), tissue sections were treated for 30 minutes with the amplification antibody (Alexa Fluor 488 goat anti-rabbit IgG). This was followed by two additional washing steps and treatment with DAPI (nuclei counterstain) for 3 minutes. After three final washing steps, tissue sections were rinsed once with water. Coverslips were mounted onto the microscope slides with Prolong Anti-Fade (Invitrogen). Stained tissue sections were examined by fluorescence microscopy. Microscopy was performed on a Zeiss LSM 510 confocal microscope using a 63× (C-Apochr) water immersion objective lens (numerical aperture (NA) = 1.2). Laser excitation wavelengths included 364, 488, and 543 nm scanned in sequence by the line method.
Light Microscopic and Transmission Electron Microscopic Studies
Skin biopsies were fixed overnight in 2% glutaraldehyde in 0.1 mmol/L cacodylate buffer (Sigma, St. Louis, MO) at pH 7.4. Glutaraldehyde-fixed specimens were treated with 2% osmium tetroxide buffered in 0.1 mmol/L cacodylate buffer. Specimens were dehydrated with graded ethanol to 2 × 100% ethanol and 2× propylene oxide (EM Sciences). The samples were embedded in pure epon resin. One-micrometer tissue sections were cut, stained with toluidine blue, and examined at the light microscopic level. Surface area occupied by individual cells was assessed quantitatively using NIH Image software on 1-μm sections obtained from glutaraldehyde-fixed, plastic-embedded tissue.
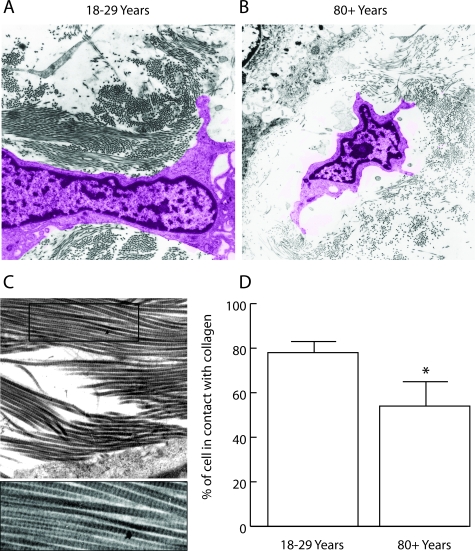
The same tissue sections used for quantification of surface area at the light microscopic level were also used to identify areas of interest for transmission electron microscopy. Ultrathin sections were cut from areas of interest, stained with lead citrate and uranyl acetate (both from EM Sciences), and observed using a Phillips 400 transmission electron microscope. Photographs were made from several areas of each specimen. Using the high-resolution photographs, interstitial cells were quantitatively evaluated for percentage of the cell boundary in contact with individual collagen fibrils or collagen fibril bundles. Although it is difficult to identify interstitial fibroblasts with 100% accuracy, obvious contaminants (mast cells, cells in vascular structures, glandular epithelial cells, and red blood cells) were not evaluated.
Statistical Analysis
Proliferation, type I procollagen production, two-dimensional surface area measurements and collagen-attachment data were compared between young and old skin. The data were analyzed using Student’s two-sample t-test (Microsoft Excel and SAS analytic software). Summary data are expressed as means ± SEM. All P values are two-tailed.
Results
Type I Procollagen Synthesis in Skin from Young and Old Individuals
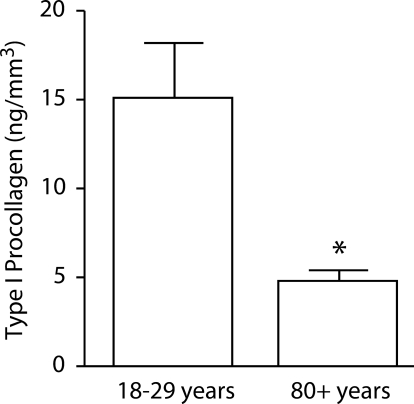
In the first series of experiments, type I procollagen content was measured in skin from young and old individuals. Type I procollagen content, a marker of ongoing collagen synthesis, was decreased by 68% in old skin versus young skin (Figure 1).
Figure 1.
Type I procollagen production in young and old skin. Values shown are averages ± SEM, based on six young and six old individuals. Statistical significance of the differences between young and old skin was determined using Student’s t-test. *Significance at the P < 0.05 level.
Type I Procollagen Synthesis by Fibroblast Isolates from Young and Old Skin
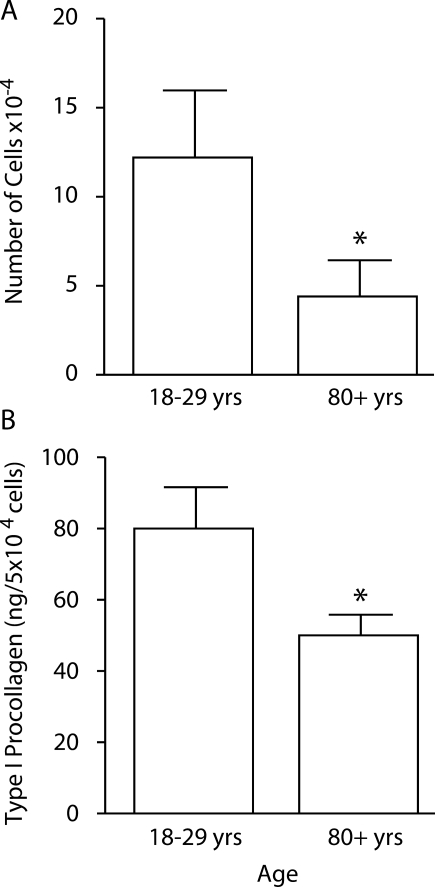
Next, conditioned medium from cultures of young and old fibroblasts was assessed for type I procollagen production. Isolates from the cohort of young individuals synthesized more type I procollagen than did the fibroblast isolates from the cohort of old individuals (Figure 2). Cells isolated from young skin also proliferated to a greater extent (Figure 2).
Figure 2.
Cellular proliferation and type I procollagen production in monolayer culture. Proliferation (A) and type I procollagen production (B) by fibroblasts isolated from young and old skin. Values shown are averages ± SEM, based on 26 fibroblast isolates from eight young individuals and 37 isolates from eight old individuals. Statistical significance of the differences between isolates from young and old skin was determined using Student’s t-test. *Significance at the P < 0.05 level.
Collagen Structure, Fibroblast-Collagen Interactions, and Cell Shape in Vivo: Comparison of Young and Old Skin
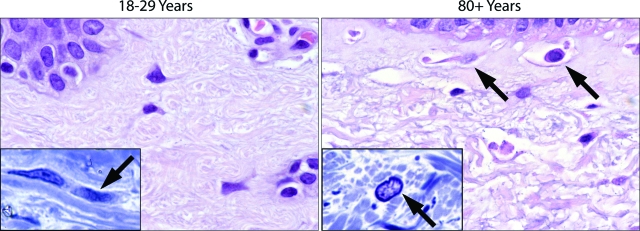
A series of related histological, ultrastructural, and fluorescence microscopic analyses were performed to determine the relationship between collagen structure, cell shape and adhesion site protein distribution. Differences in fiber bundle content were observed between young and old skin. Fiber bundles were thicker and there was less open space within and between bundles in the papillary dermis of the 18- to 29-year-old individuals than in the corresponding tissue from the 80+-year-old subjects (Figure 3). In sections of young skin, interstitial cells could be seen oriented in the plane of the collagen polymer (Figure 3, inset). In old skin, the papillary dermis was characterized by the presence of open space interspersed with criss-crossing, tangled, thin fibers. There was little evidence of fiber bundle orientation. Open space around interstitial cells was apparent (Figure 3B, arrows), and there was little evidence of cell orientation (Figure 3, inset).
Figure 3.
Histological features of sun-protected skin from young and old individuals as observed in 5-μm hematoxylin and eosin-stained sections from formalin-fixed tissue (main frames) and in 1-μm toluidine blue-stained sections from glutaraldehyde-fixed, plastic-embedded tissue (insets). Thick fiber bundles are present throughout the upper dermis of sun-protected young skin. Inset: Some fibroblasts can be seen oriented in the plane of the fiber bundles. In the old skin sample, the bundles have been replaced with thin, disorganized fibers. There is more open space in the dermis. Interstitial cells are round or oblong, and some are surrounded by open space (arrows). Inset: Fibroblast orientation (arrow) is not evident. Hematoxylin and eosin-stained sections, ×490; toluidine blue-stained sections (insets) ×980.
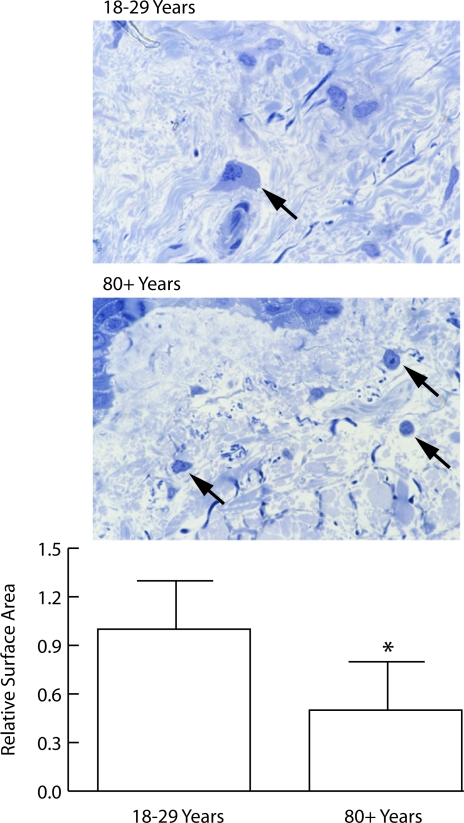
Cell shape (two-dimensional cross-sectional area of cells in 1-μm-thick sections from plastic embedded tissue) was quantitatively examined. Cells from young individuals were more spread than the corresponding cells from old individuals (Figure 4). The quantitative data shown in Figure 4 were obtained from sections of superficial (papillary) dermis. When the same analyses were done in the deeper layers of the (reticular) dermis, results were similar (not shown).
Figure 4.
Shape of fibroblasts in the papillary dermis of sun-protected hip skin from young and old individuals (1-μm toluidine blue-stained sections from glutaraldehyde-fixed, plastic-embedded tissue). Top panel: Cells in young skin are flattened, and cytoplasm and nucleus are visible (arrow). Cells are embedded in matrix. Cells in old skin appear round, and only the nucleus and a small amount of cytoplasm are visible (arrows). Bottom panel: Surface area measurements were made quantitatively as described in Materials and Methods. Values represent mean cross-sectional surface area ± SEM, based on 160 cells in sun-protected skin from six young individuals and 57 cells in sun-protected skin from six old individuals. Statistical significance was determined using Student’s t-test (two-tailed). *P < 0.01 (magnification, ×240).
Ultrastructural features of the matrix were compared in sections from young and old skin. Overall, there were no major differences in the appearance of the collagen polymers that could be used to distinguish young and old skin. There were, however, areas in sections of old skin where the density of collagen was reduced. Such areas were primarily (though not always) confined to the papillary dermis. Occasional unstriated fibers were present in some of the sections from the old skin samples, but we never detected the elastotic material characteristic of badly sun-damaged skin.2,3,5
As part of the analysis, the interaction between interstitial cells and the surrounding collagen was examined. For these studies, we avoided mast cells, epithelial cells in glandular structures, cells associated with the microvasculature and any inflammatory cell that might be present. Thus, a majority of the cells characterized were interstitial fibroblasts. Figure 5 demonstrates and quantifies the interaction of these cells with the surrounding collagen in sections from young and old individuals. In skin sections from the 18- to 29-year-old cohort, cells were in contact with intact collagen fibrils over a greater proportion of their surface (two-dimensional image) than were cells in sections from old skin.
Figure 5.
Ultrastructural appearance of dermal fibroblasts in healthy sun-protected hip skin from young and old individuals. A and B: The cell from the section of young skin (A) is flattened and well spread. The cell is in contact with collagen fibers over a high percentage of its surface. The cell in the old skin sample (B) is round and is in contact with collagen polymer over a smaller portion of its surface. There is more open space surrounding the cell. (The computer-generated coloring of the cells was done to aid in the demarcation of cells from extracellular material [magnification ×2050]). C: A high magnification (×3500) of old skin showing the striations in the fibers (typical of collagen). D: Quantification of contact between cells and collagen fibers. Values represent the percentage of the cell boundary in contact with collagen fibers ± SEM (P = 0.01; two-tailed Student’s t-test). Measurements are based on 33 cells in sections of healthy skin from six young individuals and 38 cells in sections from six old individuals.
Adhesion Site Protein Expression: Comparison of Cells in Young and Old Skin
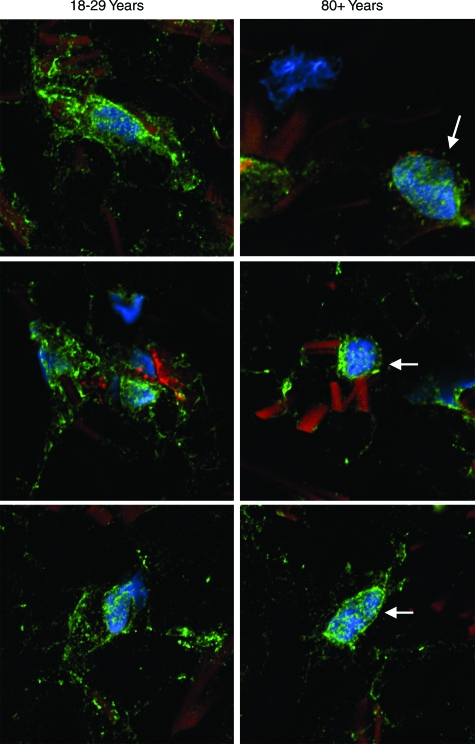
In a final set of experiments, anti-vinculin antibody was used to identify focal adhesion sites by fluorescence microscopy (Figure 6). In cells from both young and old skin, anti-vinculin staining was evident. Staining in skin sections from young individuals was associated with fiber bundles, which were evident by their dull orange appearance in the stained sections. In sections of old skin, there were fewer focal adhesions, and much of the staining was closely associated with nuclei (blue color). The differential pattern of adhesion site protein expression shown in Figure 6 correlated with differences in cell spreading, as observed at the light microscopic level (Figure 4), and with cell-collagen interactions, as observed at the transmission electron microscopic level (Figure 5). Alternatively, nuclear-associated fluorescence could indicate an intracellular (presumably, functionally inactive) pool of vinculin.
Figure 6.
Adhesion-site protein expression in sections of healthy sun-protected hip skin from young and old individuals. Tissue sections (OCT-embedded frozen) were stained using antibody to vinculin and concomitantly with phalloidin (actin stain) and DAPI (nuclear stain) as described in Materials and Methods. After staining, cells were examined by confocal fluorescence microscopy. Cells are identified by their blue (DAPI)-stained nuclei. Bright green punctate fluorescence identifies vinculin. In the 18- to 29-year-old skin samples, vinculin can be seen at a distance from the nucleus, and in many areas, the vinculin appears to be in close apposition to collagen fibers. In the 80+-year-old skin, blue-stained nuclei are apparent, but there is less vinculin than in the young skin samples. Where intense focal staining is evident, it is surrounding the nucleus (arrows). Away from the nucleus, staining is more diffuse than seen in cells from young skin. The sections presented are representative of young and old sun-protected skin from six individuals, respectively. In both young and old skin, collagen fibers are apparent by their dull orange fluorescence (magnification, ×1200).
Discussion
There is a large body of literature demonstrating a relationship between mechanical tension on cells in vitro and biological responses of cells to stress.19–23 When there is a sufficient level of mechanical tension on fibroblasts, production of collagen and other components of the extracellular matrix is high. When tension is reduced, matrix production falls, and elaboration of matrix-degrading enzymes is concomitantly stimulated.24–30 Lapiere and colleagues31 directly measured mechanical forces generated by fibroblasts in three-dimensional collagen lattices. In a series of studies, they demonstrated that the reduction in collagen synthesis occurring as a consequence of reduced mechanical tension reflected decreased transcription of genes for interstitial collagens, effects on enzymes involved in the posttranslational processing of procollagen peptides, and increased elaboration of collagen-degrading MMPs. It was shown, furthermore, that multiple signaling pathways were responsible for altered gene transcription.32,33 Of interest, changes in MMP production resulting from a loss of mechanical tension could be clearly separated from changes resulting from interleukin-1 stimulation.34
Consistent with these past observations, studies from our laboratory demonstrated that when human dermal fibroblasts were maintained on native three-dimensional collagen lattices, these cells produced type I procollagen. When the collagen in the lattice was fragmented by exposure to MMP-1 (interstitial collagenase) or to enzymes elaborated by UV-exposed skin (primarily MMP-1), a fall-off in collagen production occurred. Concomitant with the decrease in collagen production on the fragmented collagen lattices was a reduction in mechanical tension as evidenced by reduced cell spreading, decreased focal adhesions, and dissolution of actin stress fibers.6,7,17,18 Parallel studies showed that in severely photodamaged skin (with its extensive collagen degradation), resident fibroblasts were characterized by similar morphological, ultrastructural, and fluorescence microscopic findings as observed in vitro on fragmented collagen.18 Our interpretation of these findings is that degradation of collagen in severe photodamage produces an environment that is unable to support a level of mechanical tension required for efficient collagen-synthetic activity.
Collagen fragmentation, a reduction in total collagen, and decreased cell-collagen fiber interactions also characterize chronologically aged skin.1–3,5 The enzymes responsible for collagen degradation increase gradually over time in the skin.15 Collagen synthesis may also decline gradually, but the fall-off in new collagen production is most evident when skin damage is clinically evident.15 The present study was undertaken to determine what factors contribute to the loss of collagen-synthetic activity in chronological aging. Based on the results presented here, we suggest at least two mechanisms. In vitro studies indicate that reduced collagen synthesis in old skin reflects, at least in part, an age-related reduction in collagen-synthetic activity in the resident population of fibroblasts. Fibroblasts obtained from sun-protected skin of young adults (18 to 29 years of age) synthesized an average of 82 ng of type I procollagen per 5 × 104 cells, whereas cells from old individuals (80+ years of age) synthesized 56 ng per 5 × 104 cells under identical in vitro conditions. Coupled with this is the fact that there are fewer interstitial fibroblasts in aged skin compared with young skin,15 contributing to reduced growth capacity (Figure 2). Thus, even when all environmental factors that may contribute to differences in vivo are removed, there is still an age-dependent difference in collagen-synthetic capacity that explains at least part of the previously documented reduction in collagen content of aged skin.6
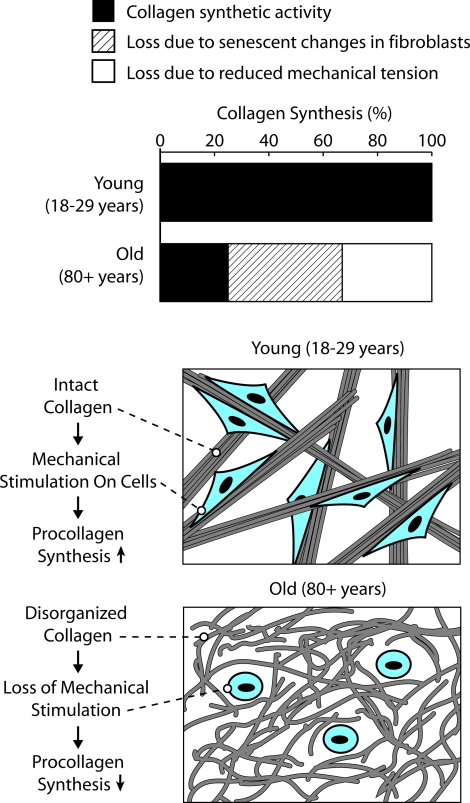
If a decrease in collagen synthetic capacity occurs as a function of fibroblast (cellular) aging, then what role does a reduction in mechanical tension play? Although it may be difficult to precisely estimate the percentage of the overall decrease in collagen production (in old skin relative to young skin) accounted for by decreased mechanical tension, the following analysis serves as a basis for comparison. Our past studies15 indicate that collagen production in sun-protected skin of old (80+ years) individuals is decreased by approximately 75% relative to production in corresponding skin of young (18 to 29 years) adults. Both Western blotting of skin extracts and immunostaining for type I procollagen are consistent in this regard. These past findings are also consistent with the direct measurements of type I procollagen in young and old skin presented here (ie, 68% reduction in old versus young skin; Figure 1). If the number of fibroblasts in skin from 80+-year-old individuals is reduced by approximately 35% relative to the number in skin of 18- to 29-year-old individuals as indicated by morphometric analysis15 and if type I procollagen synthesis is reduced by an average of 30% in fibroblasts from old skin as indicated in Figure 2 of the present study, then it is reasonable to suggest that age-dependent differences in fibroblast biosynthetic activity account for approximately 45% of the total decrease. Other factors (including loss of mechanical tension) account for the remaining 30%. This separation is shown schematically in Figure 7. How ultimate accuracy of the 45%/30% split is less important than the fact that while a loss of mechanical tension appears to be the major factor underlying decreased collagen synthesis in photodamaged skin,18 in chronologically aged skin, it is one of the two contributing mechanisms.
Figure 7.
Schematic representation of mechanisms underlying reduced collagen synthesis in aged skin.
Of interest, age-dependent alterations in fibroblast biosynthetic activity and the reduction in external mechanical tension on cells in the dermis of aged skin may not be independent. The same reduction in mechanical tension that lowers collagen production by resident fibroblasts may also indirectly contribute to permanent alterations in fibroblast function. It is generally accepted that phenotypic changes seen in aged fibroblasts are largely mediated by oxygen radical damage.35 This is potentially related to the issue at hand, because among the alterations that occur under conditions of reduced mechanical tension is increased oxidant stress as evidenced by increased levels of reactive oxygen species and altered expression of anti-oxidant enzymes (G.J. Fisher, unpublished observations). It might be inferred from this that environmental damage causally precedes changes in fibroblast function that are observed in the aged or senescent state.
The age-related decrease in collagen-synthetic activity may be, at least in part, reversible. It has been demonstrated that agents such as all-trans retinoic acid can stimulate collagen production in aged skin.16,36–38 Not surprisingly, topical retinoid use brings about an improvement in the appearance of aged skin39 and photodamaged skin.40
In summary, collagen synthetic capacity is low in aged (sun-protected) skin relative to that in healthy young sun-protected skin. Based on the findings presented here and by analogy with in vitro models and the findings of photoaging studies, we hypothesize that old fibroblasts have an age-dependent reduction in the capacity for collagen synthesis and simultaneously experience a loss of mechanical stimulation resulting from decreased intact collagen fibers.
Acknowledgments
We thank Suzan Rehbine for help with recruitment of volunteers, Robin Kunkel and Lisa Riggs for help with electron microscopy, Ted Hamilton for help with statistical analysis, Bruce Donohoe for help with fluorescence microscopy, and Laura Vangoor for help with the preparation of graphic materials.
Footnotes
Address reprint requests to James Varani, Ph.D., Department of Pathology, The University of Michigan, 1301 Catherine Rd./Box 0602, Ann Arbor, MI 48109 USA. E-mail: varani@umich.edu.
Supported in part by United States Public Health Service grants DK59169 and AG013964.
References
- Smith JG, Davidson EA, Clark WM. Alterations in human dermal connective tissue with age and chronic sun damage. J Invest Dermatol. 1962;39:347–356. doi: 10.1038/jid.1962.122. [DOI] [PubMed] [Google Scholar]
- Lavker RM. Structural alterations in exposed and unexposed aged skin. J Invest Dermatol. 1979;73:559–566. doi: 10.1111/1523-1747.ep12532763. [DOI] [PubMed] [Google Scholar]
- Pieraggi MT, Julian M, Bouissou H. Fibroblast changes in cutaneous aging. Virchows Arch A Pathol Anat Histopathol. 1984;402:275–287. doi: 10.1007/BF00695081. [DOI] [PubMed] [Google Scholar]
- Marks R. London: Martin Dunitz,; Sun-Damaged Skin. 1992 [Google Scholar]
- Lavker RM. Gilchrest BA, editor. Cambridge, MA: Blackwell Science,; Cutaneous agingchronologic versus photoaging. Photoaging. 1995:pp 123–135. [Google Scholar]
- Fligiel SEG, Varani J, Datta SH, Kang S, Fisher GJ, Voorhees JJ. Collagen degradation in aged/photoaged skin in vivo and after exposure to MMP-1 in vitro. J Invest Dermatol. 2003;120:842–848. doi: 10.1046/j.1523-1747.2003.12148.x. [DOI] [PubMed] [Google Scholar]
- Varani J, Spearman D, Perone P, Fligiel SEG, Datta SC, Wang ZQ, Shao Y, Kang S, Fisher GJ, Voorhees JJ. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am J Pathol. 2001;158:931–942. doi: 10.1016/S0002-9440(10)64040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, Voorhees JJ. The molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature (London) 1996;379:335–338. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- Fisher GJ, Wang Z-Q, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. New Eng J Med. 1997;337:1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- Millis AJ, Sottile J, Hoyle M, Mann DM, Diemer V. Collagenase production by early and late passage cultures of human fibroblasts. Exp Gerontol. 1989;24:559–575. doi: 10.1016/0531-5565(89)90060-0. [DOI] [PubMed] [Google Scholar]
- Millis AJ, Hoyle M, MCue HM, Martini H. Differential expression of metalloproteinase and tissue inhibitor of metalloproteinase genes in aged human fibroblasts. Exp Cell Res. 1992;201:373–379. doi: 10.1016/0014-4827(92)90286-h. [DOI] [PubMed] [Google Scholar]
- Burke EM, Horton WE, Pearson JD, Crow MT, Martin GR. Altered transcriptional regulation of human interstitial collagenase in cultured skin fibroblasts from older donors. Exp Gerontol. 1994;29:37–53. doi: 10.1016/0531-5565(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Bizot-Foulon V, Bouchard B, Hornebeck W, Dubertret L, Bertaux B. Uncoordinate expressions of type I and III collagens, collagenase and tissue inhibitor of matrix metalloproteinase 1 along in vitro proliferative life span of human skin fibroblasts: regulation by all-trans retinoic acid. Cell Biol Int. 1995;19:129–135. doi: 10.1006/cbir.1995.1053. [DOI] [PubMed] [Google Scholar]
- Ricciarelli R, Mini P, Ozer N, Zingg JM, Azzi A. Age-dependent increase of collagenase expression can be reduced by alpha-tocopherol via protein kinase c inhibition. Free Radic Biol Med. 1999;27:729–737. doi: 10.1016/s0891-5849(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Varani J, Warner RL, Gharaee-Kermani M, Phan SH, Kang S, Chung JH, Wang ZQ, Datta SC, Fisher GJ, Voorhees JJ. Vitamin a antagonizes decreased cell growth and elevated collagen-degrading matrix metalloproteinases and stimulates collagen accumulation in naturally aged human skin. J Invest Dermatol. 2000;114:480–486. doi: 10.1046/j.1523-1747.2000.00902.x. [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Russman AN, Majmudar G, Singer RS, Hamilton TA, Voorhees JJ. Restoration of collagen formation in photodamaged human skin by tretinoin (retinoic acid). N Engl J Med. 1993;329:530–535. doi: 10.1056/NEJM199308193290803. [DOI] [PubMed] [Google Scholar]
- Varani J, Perone P, Fligiel SEG, Fisher GJ, Voorhees JJ. Inhibition of type I procollagen production in photodamage: correlation between presence of high molecular weight collagen fragments and reduced procollagen synthesis. J Invest Dermatol. 2002;119:122–129. doi: 10.1046/j.1523-1747.2002.01810.x. [DOI] [PubMed] [Google Scholar]
- Varani J, Schuger L, Dame MK, Leonard C, Fligiel SEG, Kang S, Fisher GJ, Voorhees JJ. Reduced fibroblast interaction with intact collagen as a mechanism for depressed collagen synthesis in photodamaged skin. J Invest Dermatol. 2004;122:1471–1479. doi: 10.1111/j.0022-202X.2004.22614.x. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast-collagen matrix contraction: growth factor signalling and mechanical loading. Trends Cell Biol. 2000;10:362–365. doi: 10.1016/s0962-8924(00)01802-x. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechanoregulation of connective tissue remodeling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Grinnell F. Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol. 2003;13:264–269. doi: 10.1016/s0962-8924(03)00057-6. [DOI] [PubMed] [Google Scholar]
- Silver FH, Siperko LM, Seehra GP. Mechanobiology of force transduction in dermal tissue. Skin Res Technol. 2003;9:3–23. doi: 10.1034/j.1600-0846.2003.00358.x. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A, Pankow R, Yamada KM. Transmembrane: extracellular matrix-cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- Lambert CA, Soudant EP, Nusgens BV, Lapiere CM. Pretranslational regulation of extracellular matrix macromolecules and collagenase expression in fibroblasts by mechanical forces. Lab Invest. 1992;66:444–451. [PubMed] [Google Scholar]
- Geesin J, Brown LJ, Gordon JS, Berg RA. Regulation of collagen synthesis in human dermal fibroblasts in contracted collagen gels by ascorbic acid, growth factors and inhibitors of lipid peroxidation. Exp Cell Res. 1993;206:283–290. doi: 10.1006/excr.1993.1148. [DOI] [PubMed] [Google Scholar]
- Clark RAF, Nielsen LD, Welch MP, McPherson JM. Collagen matrices attenuate the collagen-synthetic response of cultured fibroblasts to TGF-β. J Cell Sci. 1995;108:1251–1261. doi: 10.1242/jcs.108.3.1251. [DOI] [PubMed] [Google Scholar]
- Chiquet M. Regulation of extracellular gene expression by mechanical stress. Matrix Biol. 1999;18:417–426. doi: 10.1016/s0945-053x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- Tamariz E, Grinnell F. Modulation of fibroblast morphology and adhesion during collagen matrix remodeling. Mol Biol Cell. 2002;13:3915–3929. doi: 10.1091/mbc.E02-05-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J, Rattner A, Chepda T, Frey J, Chamson A. Production of matrix metalloproteinase 2 in fibroblast reaction to mechanical stress in a collagen gel. Arch Derm Res. 2002;294:405–410. doi: 10.1007/s00403-002-0356-5. [DOI] [PubMed] [Google Scholar]
- Fluck M, Giraud M-N, Tunc V, Chiquet M. Tensile stress-dependent collagen XII and fibronectin production by fibroblasts requires separate pathways. Biochim Biophys Acta. 2003;1593:239–248. doi: 10.1016/s0167-4889(02)00394-4. [DOI] [PubMed] [Google Scholar]
- Delvoye P, Wiliquet P, Leveque J-L, Nusgens BV, Lapiere CM. Measurement of mechanical forces generated by skin fibroblasts embedded in a three-dimensional collagen gel. J Invest Dermatol. 1991;97:898–902. doi: 10.1111/1523-1747.ep12491651. [DOI] [PubMed] [Google Scholar]
- Lambert CA, Colige AC, Lapiere CM, Nusgens BV. Coordinated regulation of procollagens I and III and their post-translational enzymes by dissipation of mechanical tension in human dermal fibroblasts. Euro J Cell Biol. 2001;80:479–485. doi: 10.1078/0171-9335-00181. [DOI] [PubMed] [Google Scholar]
- Lambert CA, Colige AC, Munaut C, Lapeiere CM, Nusgens BV. Distinct pathways in the over-expression of matrix metalloproteinases in human fibroblasts by relaxation of mechanical tension. Matrix Biol. 2001;20:397–408. doi: 10.1016/s0945-053x(01)00156-1. [DOI] [PubMed] [Google Scholar]
- Lambert CA, Lapiere CM, Nusgens BV. An interleukin-1 loop I induced in human skin fibroblasts upon stress relaxation in a three-dimensional collagen gel but is not involved in the up-regulation of matrix metalloproteinase 1. J Biol Chem. 1998;273:23143–23149. doi: 10.1074/jbc.273.36.23143. [DOI] [PubMed] [Google Scholar]
- Jenkins G. Molecular mechanisms of skin aging. Mech Aging Develop. 2002;123:801–810. doi: 10.1016/s0047-6374(01)00425-0. [DOI] [PubMed] [Google Scholar]
- Kligman LH, Duo CH, Kligman AM. Topical retinoic acid enhances the repair of ultraviolet damaged dermal connective tissue. Connect Tissue Res. 1984;12:139–150. doi: 10.3109/03008208408992779. [DOI] [PubMed] [Google Scholar]
- Kligman LH. Effects of all-trans-retinoic acid on the dermis of hairless mice. J Am Acad Dermatol. 1986;15:779–785. doi: 10.1016/s0190-9622(86)70234-x. 884–847. [DOI] [PubMed] [Google Scholar]
- Kligman AM, Dogadkina D, Lavker RM. Effects of topical tretinoin on non-sun-exposed protected skin of the elderly. J Am Acad Dermatol. 1993;29:25–33. doi: 10.1016/0190-9622(93)70147-l. [DOI] [PubMed] [Google Scholar]
- Kligman AM, Grove GL, Hirose R, Leyden JJ. Topical tretinoin for photoaged skin. J Am Acad Dermatol. 1986;15:836–859. doi: 10.1016/s0190-9622(86)70242-9. [DOI] [PubMed] [Google Scholar]
- Weiss JS, Ellis CN, Headington JT, Tincoff T, Hamilton TA, Voorhees JJ. Topical tretinoin improves photoaged skin: a double-blind vehicle-controlled study. JAMA. 1988;259:527–532. [PubMed] [Google Scholar]