Abstract
Shallow invasion by extravillous trophoblast cells into the uterine wall reduces placental perfusion and causes placental dysfunction, but the one or more causes of shallow placental invasion are unknown. We hypothesized that infection with adeno-associated virus-2 (AAV-2) inhibits trophoblast invasion and is associated with preeclampsia, which is a common obstetric complication resulting from placental dysfunction. We determined that transformed extravillous trophoblast (HTR-8/SVneo) cells were susceptible to AAV-2 infection in the presence or absence of adenovirus, which provides helper function for AAV-2 replication, and that AAV-2 infection reduced invasion of HTR-8/SVneo cells through an extracellular matrix before cytopathic effects were detected. In a case-control study, AAV-2 DNA was found more frequently in trophoblast cells from cases of severe preeclampsia (22/40) than from normal term deliveries (5/27, P = 0.002). These results indicate that AAV-2 infection is a previously unidentified cause of placental dysfunction. Additional studies to determine the susceptibility of extravillous trophoblast to other viruses, and the mechanisms by which viral infection impairs placental function, are warranted.
The development of the human placenta is dependent on the differentiation of trophoblast cells along two pathways. In one pathway, mononucleated cytotrophoblast cells within the placental villi terminally differentiate into the multinucleated syncytiotrophoblast, which forms the only continuous layer separating the maternal intervillous space and the fetal capillary endothelium.1 In the other pathway, a subset of undifferentiated cytotrophoblast cells in anchoring villi invades maternal tissues and blood vessels within the uterine decidua and myometrium.2,3 These extravillous, or invasive, trophoblast cells mediate placental attachment to the maternal uterine wall and are responsible for establishing a high flow, low resistance maternal circulation supplying the placenta and fetus. Failed invasion by extravillous trophoblast cells leads to placental dysfunction and adverse obstetric outcomes associated with placental dysfunction, including preeclampsia, fetal growth restriction, and preterm delivery.4–7 Although some molecular abnormalities associated with failed placental invasion have been reported, the cause of shallow invasion by extravillous trophoblast cells has not been elucidated.3,8 We hypothesize that viral infections of the placenta induce pathological changes that impair trophoblast invasion into the uterine wall, possibly resulting in adverse obstetric outcomes due to placental dysfunction.
Adeno-associated virus (AAV) is a member of the parvovirus family, and several serotypes of AAV have been identified. Of these, types 2 and 3 infect humans, although type 3 is rare and is probably a laboratory contaminant that developed during construction of recombinant AAV vectors.9,10 AAV-2 is a common human isolate (40 to 80% of adults have been exposed), and infection involves hematogenous seeding.11,12 Thus, the placenta may be exposed to AAV-2 during primary or reactivated maternal infection.
Although productive AAV-2 infections usually require co-infection with helper viruses (including adenovirus, cytomegalovirus, human papillomavirus, and herpes simplex virus), other investigators demonstrated that AAV-2 can undergo full replicative cycles in keratinocytes, and we demonstrated that trophoblast cells are susceptible to AAV-2 in the presence or absence of helper virus co-infection.10,13–16 Additionally, there are several preliminary reports linking AAV-2 to adverse reproductive outcomes, including spontaneous miscarriage, gestational trophoblastic disease, and preterm labor.17–19 In pregnant mice, infection with AAV induces early abortion, and in humans early miscarriage has been associated with AAV-2 infection of trophoblast cells.18,20
Based on our previous findings and the observations of other investigators, we postulated that AAV-2 infection of the placenta may be relatively common and may be associated with an increased risk of obstetric complications associated with placental dysfunction, such as preeclampsia. Thus, we sought to determine 1) if infection of invasive trophoblast cells by AAV-2 reduces cell invasion and/or induces cell death and 2) if placental infection with AAV-2 is associated with severe preeclampsia.
Materials and Methods
Cell Culture
The HTR-8/SVneo trophoblast cell line was provided by C.H. Graham (Queen’s University, Ontario, Canada). HTR-8/SVneo cells originally were obtained from human first-trimester placenta and immortalized by transfection with a cDNA construct that encodes the simian virus 40 large T antigen.21 These cells exhibit a high proliferation index and share phenotypic similarities with nontransfected parent HTR-8 cells, including invasive characteristics.21 Cells were cultured in Roswell Park Memorial Institute 1640 medium (Invitrogen, Carlsbad, CA) supplemented with antibiotics and 5% fetal bovine serum as monolayers at 37°C in 5% CO2.
Viral Infections
Wild-type AAV-2 (wtAAV-2) and wild-type adenovirus-5 (wtAd-5) were purchased from the American Type Culture Collection. Replication-deficient AAV-2 vectors, purchased from the Institute of Human Gene Therapy at the University of Pennsylvania, contained transgenes encoding the following reporter proteins: green fluorescent protein (rAAV-2-GFP) and β-galactosidase (rAAV-2-LacZ).
HTR-8/SVneo cells were infected with AAV-2 vectors or wtAAV-2 in the presence or absence of wtAd-5 co-infection. After incubation at 4°C for 30 minutes, the infected cells were plated in 12-well culture plates (5 × 106 cells/well) coated with fibronectin (Invitrogen) for growth. Ninety-six hours after infection with wild-type viruses, the cells were examined for cytopathic effects.
Transduction Efficiencies
Reporter protein expression was detected by either β-galactosidase assay (rAAV-2-LacZ)16 or fluorescence microscopy to detect green fluorescent protein (rAAV-2-GFP). β-Galactosidase enzyme activity was assessed 24 hours after infection using a solution-based assay system and measuring absorbance at 420 nm (Promega Corp., Madison, WI).16 To detect GFP, cells were rinsed with phosphate-buffered saline 24 hours after viral infection, and transduction efficiency was determined by observing the cells under fluorescence microscopy.
Indirect Immunofluorescence Assays
HTR-8/SVneo cells (with or without 1500 particles of wtAAV-2/cell for 2 to 24 hours) were treated with normal goat serum to block nonspecific binding, after which the cells (at least 4 × 104 cells) were incubated with murine monoclonal antibodies against the AAV-2 receptors heparan sulfate (Seikagaku, Falmouth, MA) and αVβ5 integrin (Chemicon International, Temecula, CA).22–24 After treatment with primary antibody, the cells were incubated with fluorescein-conjugated goat antibody to mouse immunoglobulin, and fluorescence was measured using an Epics XL flow cytometer (Coulter Corp., Miami, FL).
Adhesion Assay
Adhesion of HTR-8/SVneo cells to fibronectin was measured as previously described.25 Briefly, 96-well tissue culture plates were coated with 10 μg/ml fibronectin. Infected and noninfected HTR-8/SVneo cells (4.0 × 104 cells/well) were added to the wells, and the mixtures were incubated at 37°C for 45 minutes. After rinsing the non-bound cells from the wells, the bound cells were quantified by measuring endogenous lactate dehydrogenase (LDH) using the CytoTox 96 nonradioactive cytotoxicity assay (Promega).
Invasion Assay
The invasiveness of HTR-8/SVneo cells through an extracellular matrix was measured using a commercially available cell invasion assay kit (Chemicon). Briefly, HTR-8/SVneo cells (1 × 106 cells/well) were infected with 15 to 1500 particles of wtAAV-2/cell in the presence or absence of 100 particles of wtAd-5/cell and plated on ECMatrix gel-coated layered cell culture inserts. After 24 hours, the noninvading cells and the ECMatrix gel from the interior of the inserts were removed using a cotton-tipped swab. Invasive cells on the lower surface of the membranes were stained by dipping the inserts in 0.2% crystal violet. After 20 minutes, the stained cells were dissolved in 10% acetic acid, and colorimetric absorbance was measured at 560 nm. Human embryonic kidney 293 cells (American Type Culture Collection CRL-1573), which are susceptible to AAV-2 infection and display an invasive phenotype, were used as controls.16
To determine whether decreased levels of adhesion and/or invasion could be attributed to cell death 24 hours after infection with wtAAV-2, the viability of HTR-8/SVneo cells was determined by measuring LDH leakage into the medium using the CytoTox 96 nonradioactive cytotoxicity assay. After collecting cell culture medium, HTR-8/SVneo cells were lysed with 1% Triton X-100, and equal volumes of medium and lysis buffer (containing LDH released from lysed cells) were transferred to separate wells in 96-well plates. Substrate mix provided with the kit was added, and the enzymatic reaction was measured by absorbance at 490 nm. Results were obtained after subtracting background values. The percentage of cells that remained viable was calculated as follows: 100 × (1 − spontaneous release of LDH/maximum release of LDH).
Apoptosis Assay
Apoptotic changes in HTR-8/SVneo cells were examined by detecting DNA fragmentation and measuring caspase-3 levels. Infected and noninfected HTR-8/SVneo cells (2.0 × 106 cells/well) were plated on 6-well plates in 2 ml of Roswell Park Memorial Institute 1640 medium. After 96 hours, adherent and floating cells were homogenized in lysis buffer, and DNA was isolated from the lysate by passage through filter tubes (Apoptotic DNA Ladder kit, Roche Diagnostics, Mannheim, Germany). DNA from each sample was electrophoresed in 1.8% agarose gels and visualized by ethidium bromide staining.
Case-Control Study
We conducted a case-control study comparing the rates of AAV-2 infection of trophoblast cells between cases of severe preeclampsia and controls who delivered at term without any medical or obstetric complications. The Institutional Review Board at the University of Pennsylvania approved this study (protocol 700943) in December 1999. We reviewed medical charts at the Hospital of the University of Pennsylvania to identify cases of severe preeclampsia requiring delivery before 37 weeks’ gestation in 1998 and 1999. Severe preeclampsia was defined according to criteria outlined by the American College of Obstetricians and Gynecologists: systolic blood pressure >160 mm Hg or diastolic blood pressure >110 mm Hg on two occasions at least 6 hours apart, or end-organ manifestations, including proteinuria (≥3+ by dipstick, ≥5 × g by 24-hour collection), oliguria, cerebral disturbances, pulmonary edema, thrombocytopenia, impaired liver function, or fetal growth restriction, in the setting of preeclampsia.26 Because preeclampsia frequently is not a straightforward diagnosis, only severe cases requiring iatrogenic preterm delivery were selected after review of the complete medical record by a certified Maternal-Fetal Medicine specialist (S.P.). Women were excluded if they had other risk factors for developing preeclampsia, including chronic hypertension, renal disease, diabetes, and multiple gestations. We also excluded women whose pregnancies were complicated by fetal malformations. All cases had placental specimens stored in the Pathology Laboratory at the Hospital of the University of Pennsylvania. Controls were selected at random from among uncomplicated term deliveries at the same hospital between July and December 2001, and placentas from these controls were sent to the Pathology Laboratory for storage for our research purposes. The control placentas were fixed and stored as paraffinized tissue blocks according to the same protocol that was used for routine clinical specimens.
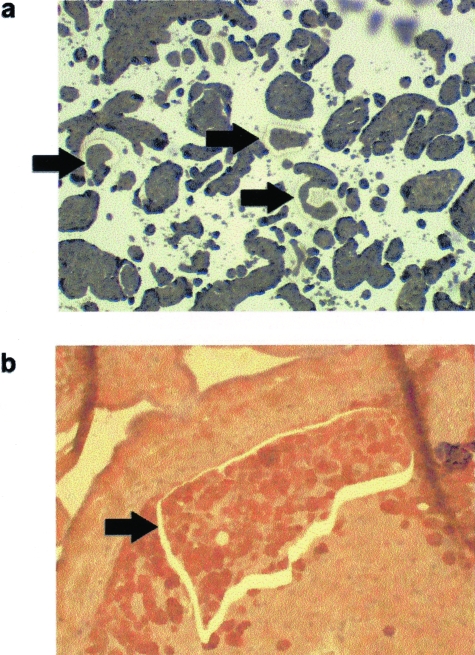
Histological sections from the basal plate region of placentas were placed on membrane slides for laser microdissection (Molecular Machines and Industries, Knoxville, TN), and trophoblast cells were microdissected from the membranes using the PixCell II Laser Capture Microdissection System (Arcturus Engineering, Mountain View, CA). The laser capture microscope was used to isolate extravillous trophoblast cells from invasive trophoblast columns and villous trophoblast cells from the periphery of chorionic villi (eg, cytotrophoblast and syncytiotrophoblast) in the basal plate region of placentas from cases and controls (Figure 1). DNA was extracted from laser-captured trophoblast cells using DNeasy minikits (Qiagen, Valencia, CA), and 2 ng of total DNA was used to amplify a 379-bp segment of the AAV-2 rep gene by nested PCR. The primers used for the first PCR were forward primer (nucleotide 1421) 5′-CGACTGTGTCGACAAGATGGTGAT-3′ and reverse primer (nucleotide 1885) 5′-TACCTGTCTGCGTAGTTGATCGAAG-3′. The primers used for the nested PCR were forward primer (nucleotide 1473) 5′-AAGGTCGTGGAGTCGGCCAAA-3′ and reverse primer (nucleotide 1851) 5′-GTCGATGGCTGCGCAACTGA-3′. PCR was performed under the following conditions: initial denaturation step at 95°C for 5 minutes, followed by 30 cycles at 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. The PCR products were visualized as a single band by 2% agarose gel electrophoresis, and PCR products were confirmed by sequencing. DNA extracted from wild-type AAV-2 was used as a positive control, whereas water was used as the negative control. To ensure that adequate amounts of DNA were extracted from all samples, we performed nested PCR to detect a 106-bp segment of the β-actin gene.
Figure 1.
Laser capture microdissection of trophoblast cells. a: Photomicrograph of placental villi from the placental basal plate region (magnification: 20×). b: Photomicrograph of extravillous trophoblast column from the placental basal plate region (magnification: 100×). Extravillous trophoblast cells were immunostained with anti-cytokeratin-18 antibodies for identification. Arrows indicate regions where cells were captured.
Statistical Analyses
In the viral transduction studies and adhesion, viability, and invasion assays, absorbance values were measured in triplicate samples for each experimental condition, and the experiments were performed three times. Average absorbance values were compared using two-tailed t-tests, and P values < 0.05 were considered statistically significant.
We calculated a sample size for a 3:2 case/control design, because placentas from controls had to be collected prospectively and prepared according to the same protocol used for routine clinical specimens (eg, cases). In previous preliminary studies, AAV-2 DNA was detected in 0 to 40% of placentas and amniocytes from early miscarriages, preterm deliveries, and controls.17,18,27 To demonstrate a 4-fold difference in AAV-2 detection rates (40 vs. 10%) between cases and controls, we determined that we needed to study placentas from 40 cases of preeclampsia and 27 controls (α = 0.05, 1 = β = 0.80). Rates of AAV-2 detection were compared between cases and controls by constructing two-by-two tables and performing χ2 or Fisher exact tests.
Results
Infection of HTR-8/SVneo Cells by AAV-2
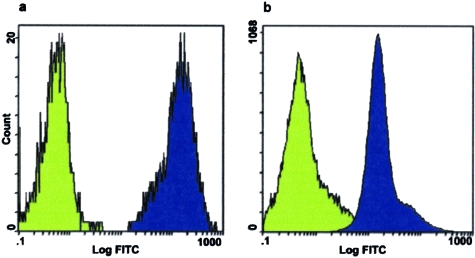
Flow cytometry studies demonstrated that 99.5 ± 0.5% of HTR-8/SVneo cells expressed the AAV-2 receptor heparan sulfate on cell surfaces, whereas 87.7 ± 2.3% of HTR-8/SVneo cells expressed αVβ5 integrin (Figure 2). Infection with 1500 particles of wtAAV-2/cell for 2 to 24 hours did not affect cell-surface expression of heparan sulfate or αVβ5 integrin.
Figure 2.
Indirect immunofluorescence assays indicating HTR-8/SVneo cell-surface expression of AAV-2 receptors. Fluorescence intensity was measured in cells that were incubated with monoclonal antibodies against heparan sulfate (a) or αVβ5 integrin (b) and fluorescein-conjugated secondary antibody (blue-shaded curves) and in cells that were incubated with fluorescein-conjugated secondary antibody alone (green-shaded curves). The y axis indicates cell number, and the x axis indicates logarithm of fluorescence intensity.
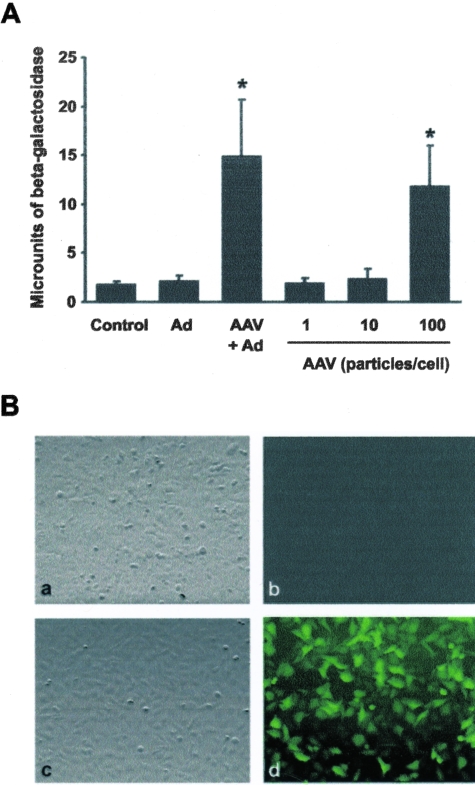
Based on the flow cytometry results, we anticipated that HTR-8/SVneo cells were susceptible to infection by AAV-2, and we observed that recombinant AAV-2 constructs (rAAV-2-LacZ or rAAV-2-GFP, 1 to 100 particles/cell) efficiently transduced HTR-8/SVneo cells in the presence or absence of 100 particles of wtAd-5/cell (Figure 3). The transduction efficiency of rAAV-2-GFP was determined by observing the cells from three separate experiments under fluorescence microscopy at 40× magnification. Fluorescent nuclei were detected in 0.67 ± 1.0% of uninfected cells and 43.7 ± 7.5% of HTR-8/SVneo cells transduced with 100 particles of rAAV-2-GFP/cell (P = 0.01, two-tailed t-test).
Figure 3.
Transduction of HTR-8/SVneo cells by recombinant AAV-2 constructs (rAAV-2-LacZ and rAAV-2-GFP). A: Successful transduction of the Lac-Z transgene was measured by β-galactosidase activity along the y axis. HTR-8/Svneo cells were infected for 24 hours with 1–100 viral particles/cell of rAAV-2-LacZ in the presence or absence of 100 viral particles/cell of wtAd-5 (Ad), and significantly greater transduction rates compared to uninfected controls are indicated by asterisks. B: Successful infection and production of the GFP transgene was detected by green fluorescent staining. HTR-8/SVneo cells were infected for 24 hours with 100 viral particles/cell of rAAV-2-GFP in the absence of wtAd-5. Results are: from a light microscope (a and c), from a fluorescence microscopy (b and d), uninfected cells (a and b), and cells infected with rAAV-2-GFP (c and d).
Adhesion and Invasion Assays
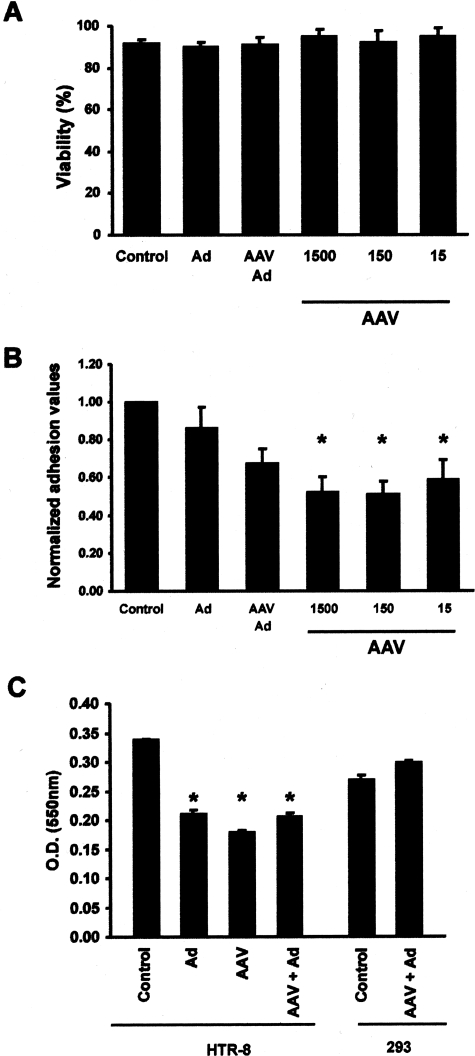
The pathological effects of AAV-2 infection were assessed by comparing adhesion of infected and noninfected HTR-8/SVneo cells to fibronectin-coated culture plates and invasion of the cells through an extracellular matrix. Viability assays performed 24 hours after infection with wtAAV-2 and/or wtAd-5 demonstrated that >90% of infected and noninfected HTR-8/SVneo cells remained viable (Figure 4A). Nevertheless, HTR-8/SVneo cells infected with 15–1500 particles of wtAAV-2/cell for 24 hours demonstrated significantly decreased adhesion to fibronectin-coated plates compared to controls (Figure 4B). Invasion assays were conducted by plating infected and noninfected HTR-8/SVneo cells and 293 cells in extracellular matrix on semipermeable membranes (Figure 4C). Baseline invasion rates were determined for noninfected HTR-8/SVneo cells and 293 cells. Infection of HTR-8/SVneo cells by wtAAV-2 or wtAd-5 reduced invasion by ∼40% but did not affect invasion through the extracellular matrix by 293 cells.
Figure 4.
Adhesion and invasion of HTR-8/SVneo cells. A: Viability of HTR-8/SVneo cells was determined by measuring LDH leakage into cell culture medium 24 hours after infection with 15 to 1500 viral particles/cell of wtAAV-2 and/or 100 viral particles/cell of wtAd-5. B: Adhesion of HTR-8/SVneo cells to fibronectin-coated plates 24 hours after infection with 15 to 1500 viral particles/cell of wtAAV-2 and/or 100 viral particles/cell of wtAd-5 was compared to noninfected controls. Significant differences (P < 0.05) are indicated by an asterisk. C: Invasion assays were performed 24 hours after viral infection. Invasion was measured spectrophotometrically according to the optical density for crystal violet at 550 nm (y axis). Baseline invasion rates were determined for noninfected HTR-8/SVneo cells and 293 cells, and infection of HTR-8/SVneo cells by wtAAV-2 (150 viral particles/cell) and/or wtAd-5 (100 viral particles/cell) reduced invasion by ∼40%. Significantly different invasion rates between infected cells and controls (P < 0.05) were indicated by an asterisk.
Cell Death Assays
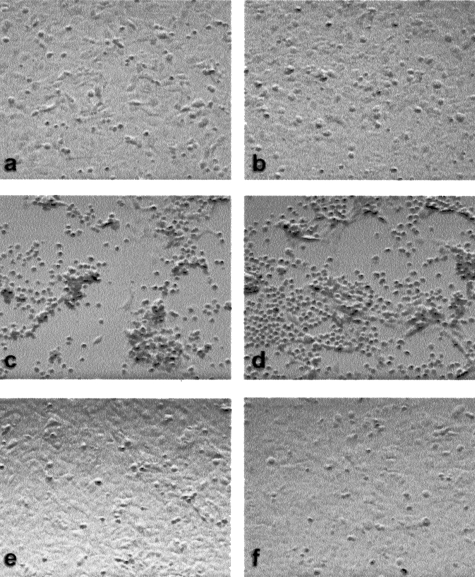
Although the viability of HTR-8/SVneo cells was not reduced 24 hours after infection by wtAAV-2, we sought to determine whether AAV-2 infection induced trophoblast cell death over a longer period of time. The cytopathic effects of AAV-2 infection on extravillous trophoblast cells were evaluated by direct microscopy. At 96 hours after infection with 15–1500 viral particles/cell of wtAAV-2 in the presence or absence of 100 particles/cell of wtAd-5, HTR-8/SVneo cells exhibited marked cytopathic changes, including cell rounding and detachment from the monolayer (Figure 5).
Figure 5.
Cytopathic effect of wtAAV-2. HTR-8/SVneo cells were infected with 15 to 1500 viral particles/cell of wtAAV-2 in the presence or absence of 100 viral particles/cell of wtAd-5. Cells were photographed at 10× magnification 96 hours after infection. Control (a); wtAd-5 (b); wtAAV-2 (1500 particles/cell) plus wtAd-5 (c); wtAAV-2 (1500 particles/cell) (d); wtAAV-2 (150 particles/cell) (e); and wtAAV-2 (15 particles/cell) (f).
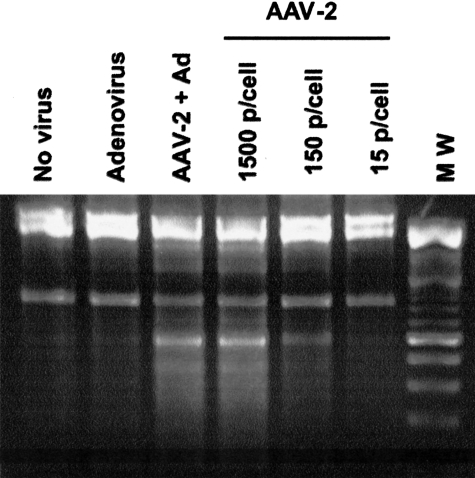
To determine whether the cytopathic effects were the result of apoptosis, DNA fragmentation was analyzed by agarose gel electrophoresis (Figure 6). In uninfected cells and cells infected with only wtAd-5, DNA fragments were not detected. However, in cells that were infected for 96 hours with wtAAV-2 with or without wtAd-5, the intensity of bands corresponding to DNA fragmentation was positively correlated with the amount of wtAAV-2 that was used to infect the HTR-8/SVneo cells.
Figure 6.
DNA ladder assay demonstrating nucleosomal fragments in apoptotic cells. HTR-8/SVneo cells were infected for 96 hours with 15 to 1500 viral particles/cell of wtAAV-2 and/or 100 viral particles/cell of wtAd-5. The intensity of bands corresponding to DNA fragmentation positively correlated with the amount of AAV-2 that was used to infect the HTR-8 cells.
Case-Control Study
The age and gravidity of preeclampsia cases and controls were similar, but a higher percentage of cases (28/40) than controls (6/27) were nulliparous (Table 1). Although the systolic and diastolic blood pressures of cases were greater than controls at their initial prenatal visits, these differences were clinically insignificant, and no subjects had blood pressures greater than 140/90 mm Hg early in pregnancy. As expected, the systolic and diastolic blood pressures of cases were greater than controls at the time of delivery, and the incidence of proteinuria and cesarean delivery was greater in cases than controls. Among cases, the average gestational age at delivery was 32.5 weeks, and 30% had intrauterine growth restriction.
Table 1.
Demographics and Clinical Outcomes for Cases and Controls
| Cases, N = 40 | Controls, N = 27 | P value* | |
|---|---|---|---|
| Age (year ± SD) | 25.2 ± 6.9 | 26.4 ± 6.1 | 0.48 |
| Gravidity (± SD) | 2.4 ± 1.6 | 3.0 ± 2.1 | 0.22 |
| Parity (± SD) | 0.5 ± 1.0 | 1.4 ± 1.4 | <0.01 |
| EGA† 1st visit (week ± SD) | 12.4 ± 5.4 | 15.9 ± 9.4 | 0.11 |
| Initial SBP‡ (mm Hg ± SD) | 116 ± 10.8 | 107.5 ± 12.0 | <0.01 |
| Initial DBP‡ (mm Hg ± SD) | 70.6 ± 8.5 | 58.3 ± 7.5 | <0.01 |
| Initial proteinuria (%) | 2/40 (5.0%) | 1/27 (3.7%) | 0.79 |
| SBP‡ delivery (mm Hg ± SD) | 159.7 ± 16.9 | 119.1 ± 10.4 | <0.01 |
| DBP‡ delivery (mm Hg ± SD) | 98.3 ± 10.4 | 68.2 ± 8.9 | <0.01 |
| Proteinuria delivery (%) | 39/40 (97.5%) | 0 | <0.01 |
| EGA† delivery (week ± SD) | 32.5 ± 2.3 | 39.8 ± 1.3 | <0.01 |
| BW§ (g ± SD) | 1588.1 ± 468.7 | 3376.9 ± 489.6 | <0.01 |
| IUGR (%) | 12/40 (30.0%) | 0 | <0.01 |
| Oligohydramnios∥ (%) | 4/40 (10.0%) | 0 | 0.11 |
| Cesarean delivery (%) | 25/40 (62.5%) | 6/27 (22.2%) | <0.01 |
IUGR, intrauterine growth restriction (birth weight < 10th percentile for gestational age at delivery).
Two-tailed t-tests were performed for continuous variables; χ-square (or Fisher’s exact) tests were performed for dichotomous variables.
EGA = estimated gestational age.
SBP = systolic blood pressure; DBP = diastolic blood pressure.
BW = birth weight.
Oligohydramnios = amniotic fluid index < 5 cm.
Two analyses were performed: 1) comparing rates of AAV-2 detection in extravillous or villous trophoblast cells between cases and controls and 2) comparing rates of AAV-2 detection in extravillous and villous trophoblast cells (possibly indicating greater viral load) between cases and controls. AAV-2 DNA was detected in trophoblast cells from the basal plate region in 27/67 placentas, and in 19/27 positives, AAV-2 DNA was detected in both villous trophoblast and extravillous trophoblast columns. AAV-2 DNA was detected significantly more frequently among cases of severe preeclampsia requiring delivery before 37 weeks’ gestation (22/40) than among controls (5/27, P = 0.002, OR = 5.38, 95% confidence interval (CI) = 1.70, 17.05; Table 2). If we compared rates of detection of AAV-2 DNA in both villous trophoblast and extravillous trophoblast between cases and controls, the incidence of AAV-2 infection was significantly greater among cases (15/40) than controls (4/27, P = 0.03, odds ratio [OR] = 3.45, 95% CI = 1.06, 11.92). Finally, when comparing the subgroup of preeclampsia cases who delivered remote from term (before 34 weeks’ gestation) to controls, the incidence of AAV-2 infection remained significantly greater among cases (15/30) than controls (5/27, P = 0.01, OR = 4.40, 95% CI = 1.32, 14.70; Table 2).
Table 2.
Detection of AAV-2 DNA in Trophoblast Cells from Cases and Controls
| Controls, N = 27 | PE* < 37 weeks, N = 40 | PE < 34 weeks,†N = 30 | |
|---|---|---|---|
| AAV-2 (+)‡ | 5 | 22 | 15 |
| AAV-2 (−) | 22 | 18 | 15 |
| %AAV-2 (+) | 18.5% | 55.0% | 50.0% |
| P value§ | 0.002 | 0.01 |
PE = preeclampsia.
PE < 34 weeks = subgroup of preeclampsia cases that delivered remote from term (<34 weeks’ gestation).
AAV-2 (+) = adeno-associated virus-2 DNA detected in trophoblast cells at basal plate of placentas.
P values determined by Fisher’s exact tests comparing case groups to controls.
Among nulliparous women (n = 34), the increased rate of AAV-2 detection in placentas from preeclampsia cases compared to controls was not statistically significant (P = 0.38, OR = 2.67), possibly because there were only six nulliparous controls. Among multiparous women (n = 33), AAV-2 was detected significantly more often in preeclampsia cases than controls (P = 0.04, OR = 6.0). However, we performed a stratified analysis based on parity, and the Mantel-Haenszel test of homogeneity was not significant (P = 0.52). Therefore, parity did not appear to be a significant confounder or effect modifier in the association between AAV-2 and preeclampsia.
Discussion
In this report, we demonstrated that AAV-2 efficiently infected extravillous trophoblast cells in the presence or absence of helper virus and that infection of extravillous trophoblast cells by AAV-2 induced decreased cell invasion before causing trophoblast cell death. The odds of detecting AAV-2 DNA in trophoblast cells from preeclampsia cases were more than 5-fold greater than the odds of detecting AAV-2 DNA in trophoblast cells from controls.
A limitation of our study is that an invasive trophoblast cell line was used to conduct the in vitro studies, so the results of these studies must be interpreted with caution. In addition, the timing of trophoblast infection in the case-control study could not be established. However, the significance of our observations is strengthened by the two-pronged approach we developed using in vitro and in vivo models to correlate AAV-2 infection with decreased trophoblast invasion, trophoblast cell death, and an adverse clinical outcome attributed to placental dysfunction. Our findings suggest that AAV-2 infection can induce placental dysfunction by inhibiting invasion of extravillous trophoblast cells and by causing trophoblast cell death. Finally, we were not surprised to detect such a large odds ratio (5.38) associated with the incidence of AAV-2 infection in cases of preeclampsia compared to controls because we excluded women with other pre-existing conditions that cause preeclampsia (ie, medical complications of pregnancy, multiple gestations) and because we studied only the worst cases of preeclampsia, which is otherwise a condition with subtle signs and symptoms that lead to frequent misdiagnoses.
The relatively high rate of placental infection by AAV-2 (27/67 subjects, or 40.3%) is consistent with the high rate of AAV-2 seropositivity in adults,11,12 and AAV-2 hematogenous shedding may occur with primary infections or with AAV-2 reactivation. We observed in another case-control study that 82.8% of preeclampsia cases and controls were seropositive for AAV-2 IgG antibodies, indicating exposure to AAV-2, but AAV-2 IgG antibodies were not associated with obstetric outcomes.28 However, among these IgG-positive subjects, seropositivity for AAV-2 IgM antibodies, indicating recent primary infection or reactivation, was associated with severe preeclampsia and other adverse obstetric outcomes. Hence, infection of the placenta may occur after primary maternal AAV-2 infection or reactivation of latent AAV-2, which seems to occur commonly in women during pregnancy.11
In comparison to other viruses such as adenovirus and herpes simplex virus, we observed in previous studies that trophoblast cells are uniquely susceptible to AAV-2 infection.16,29,30 This is the first report detailing AAV-2 infection of invasive trophoblast cells, and our findings are congruent with the findings of Meyers et al,15 who reported that AAV-2 autonomously replicates and induces cytopathic effects in differentiating keratinocytes. Although the HTR-8/SVneo cell line was transformed by replication-deficient simian virus, which might provide helper function for AAV-2 replication, we and other investigators did not observe successful transduction of other transformed cell lines by rAAV-2 vectors in the absence of helper virus.16,31
Molecular abnormalities associated with clinical conditions such as preeclampsia and fetal growth restriction have been reported, but the one or more causes of shallow invasion by trophoblast cells have not been elucidated.32–35 We postulate that viral infection may destroy these cells by inducing apoptosis or altering cell function so that normal invasion into the spiral arteries is limited. A report by Fisher et al,36 in which cytomegalovirus infection reduced cytotrophoblast invasion through an extracellular matrix, supports this hypothesis. In our experiments, we observed that lower doses of wtAAV-2 (15–150 viral particles/cell) were needed to significantly reduce adhesion and invasion compared to the dose required to induce cytopathic effects and apoptosis (1500 viral particles/cell), so decreased invasion may not be attributed exclusively to cell death. Collectively, our current findings illustrate how AAV-2 infections may impair the ability of extravillous trophoblast cells to invade uterine vessels and induce adverse pregnancy outcomes.
Future research will be directed toward studying the mechanisms by which AAV-2 infection may induce the apoptotic process and reduce invasion of trophoblast cells. For example, the interaction between αvβ5 integrin and AAV-2, which mediates endocytosis of AAV-2 into human cervical carcinoma (HeLa) cells, may block the integrin-extracellular matrix interactions that mediate trophoblast cell invasion.37 Integrins such as αVβ5 are essential for cell migration and invasion, not only because they directly mediate adhesion to the extracellular matrix, but also because they regulate intracellular signaling pathways that control the expression of proteolytic enzymes, cytoskeletal organization, and cell survival.38 In addition, AAV-2 integrates into the host cell genome and may be induced to replicate. The expression of Rep78, a multifunctional protein involved in AAV-2 integration and replication, induces apoptosis through activation of caspase-3, which may explain the results of our apoptosis assays.39,40 Finally, we intend to correlate AAV-2 infection of trophoblast cells with pathological abnormalities within the placenta and adverse outcomes associated with placental dysfunction. We believe our current findings and ongoing research provide novel insight into the role of viral infection in the pathogenesis of placental dysfunction and may inspire the development of clinical strategies to reduce the frequency of associated complications.
Footnotes
Address reprint requests to Samuel Parry, 1352 Biomedical Research Bldg. II/III, University of Pennsylvania School of Medicine, 421 Curie Blvd., Philadelphia, PA 19104-6160. E-mail: parry@mail.med.upenn.edu.
Supported by the National Institutes of Health (grant HD42100 to S.P.), by the Bill and Melinda Gates Foundation, and by the Fogarty International Center (grant D43-TW-00671 to F.A.-V.).
Current address of F.A.-V.: Research Unit in Reproductive Medicine, Hospital de Ginecobstetricia “Luis Castelazo Ayala,” Instituto Mexicano del Seguro Social, Mexico City, Mexico.
References
- Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004;25:114–126. doi: 10.1016/j.placenta.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Khong TY. Placental vascular development and neonatal outcome. Semin Neonatol. 2004;9:255–263. doi: 10.1016/j.siny.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Rama S, Rao AJ. Regulation of growth and function of the human placenta. Mol Cell Biochem. 2003;253:263–268. doi: 10.1023/a:1026076219126. [DOI] [PubMed] [Google Scholar]
- Meekins JW, Pijnenborg R, Hanssens M, McFadyen IR, van Asshe A. A study of placental bed spiral arteries and trophoblast invasion in normal and severe pre-eclamptic pregnancies. Br J Obstet Gynaecol. 1994;101:669–674. doi: 10.1111/j.1471-0528.1994.tb13182.x. [DOI] [PubMed] [Google Scholar]
- Salafia CM, Minior VK, Pezzullo JC, Popek EJ, Rosenkrantz TS, Vintzileos AM. Intrauterine growth restriction in infants of less than thirty-two weeks’ gestation: associated placental pathologic features. Am J Obstet Gynecol. 1995;173:1049–1057. doi: 10.1016/0002-9378(95)91325-4. [DOI] [PubMed] [Google Scholar]
- Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, Rotmensch S, Romero R. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- Germain AM, Carvajal J, Sanchez M, Valenzuela GJ, Tsunekawa H, Chuaqui B. Preterm labor: placental pathology and clinical correlation. Obstet Gynecol. 1999;94:284–289. doi: 10.1016/s0029-7844(99)00324-5. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25:127–139. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Berns KI, Giraud C. Biology of adeno-associated virus. Curr Top Microbiol Immunol. 1996;218:1–23. doi: 10.1007/978-3-642-80207-2_1. [DOI] [PubMed] [Google Scholar]
- Vihinen-Ranta M, Suikkanen S, Parrish CR. Pathways of cell infection by parvoviruses and adeno-associated viruses. J Virol. 2004;78:6709–6714. doi: 10.1128/JVI.78.13.6709-6714.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K, Sebokova P, Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J Med Virol. 1999;59:406–411. doi: 10.1002/(sici)1096-9071(199911)59:3<406::aid-jmv22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Grossman Z, Mendelson E, Brok-Simoni F, Mileguir F, Leitner Y, Rechavi G, Ramot B. Detection of adeno-associated virus type 2 in human peripheral blood cells. J Gen Virol. 1992;73:961–966. doi: 10.1099/0022-1317-73-4-961. [DOI] [PubMed] [Google Scholar]
- Fisher KJ, Gao GP, Weitzman MD, DeMatteo R, Burda JF, Wilson JM. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CJ, Berns KI. Adeno-associated virus type 2: a latent life cycle. Prog Nucleic Acid Res Mol Biol. 1994;48:29–52. doi: 10.1016/s0079-6603(08)60852-1. [DOI] [PubMed] [Google Scholar]
- Meyers C, Mane M, Kokorina N, Alam S, Hermonat PL. Ubiquitous human adeno-associated virus type 2 autonomously replicates in differentiating keratinocytes of a normal skin model. Virology. 2000;272:338–346. doi: 10.1006/viro.2000.0385. [DOI] [PubMed] [Google Scholar]
- Parry S, Holder J, Halterman MW, Weitzman MD, Davis AR, Federoff H, Strauss JF., 3rd Transduction of human trophoblastic cells by replication-deficient recombinant viral vectors. Promoting cellular differentiation affects virus entry. Am J Pathol. 1998;152:1521–1529. [PMC free article] [PubMed] [Google Scholar]
- Burguete T, Rabreau M, Fontanges-Darriet M, Roset E, Hager HD, Koppel A, Bischof P, Schlehofer JR. Evidence for infection of the human embryo with adeno-associated virus in pregnancy. Hum Reprod. 1999;14:2396–2401. doi: 10.1093/humrep/14.9.2396. [DOI] [PubMed] [Google Scholar]
- Tobiasch E, Rabreau M, Geletneky K, Larue-Charlus S, Severin F, Becker N, Schlehofer JR. Detection of adeno-associated virus DNA in human genital tissue and in material from spontaneous abortion. J Med Virol. 1994;44:215–222. doi: 10.1002/jmv.1890440218. [DOI] [PubMed] [Google Scholar]
- Kiehl K, Schlehofer JR, Schultz R, Zugaib M, Armbruster-Moraes E. Adeno-associated virus DNA in human gestational trophoblastic disease. Placenta. 2002;23:410–415. doi: 10.1053/plac.2002.0827. [DOI] [PubMed] [Google Scholar]
- Botquin V, Cid-Arregui A, Schlehofer JR. Adeno-associated virus type 2 interferes with early development of mouse embryos. J Gen Virol. 1994;75:2655–2662. doi: 10.1099/0022-1317-75-10-2655. [DOI] [PubMed] [Google Scholar]
- Graham CH, Hawley TS, Hawley RG, MacDougall JR, Kerbel RS, Khoo N, Lala PK. Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp Cell Res. 1993;206:204–211. doi: 10.1006/excr.1993.1139. [DOI] [PubMed] [Google Scholar]
- Kern A, Schmidt K, Leder C, Muller OJ, Wobus CE, Bettinger K, Von der Lieth CW, King JA, Kleinschmidt JA. Identification of a heparin-binding motif on adeno-associated virus type 2 capsids. J Virol. 2003;77:11072–11081. doi: 10.1128/JVI.77.20.11072-11081.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerford C, Bartlett JS, Samulski RJ. AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- Summerford C, Samulski RJ. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1998;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows TD, King A, Smith SK, Loke YW. Human trophoblast adhesion to matrix proteins: inhibition and signal transduction. Hum Reprod. 1995;10:2489–2500. doi: 10.1093/oxfordjournals.humrep.a136329. [DOI] [PubMed] [Google Scholar]
- American College of Obstetricians and Gynecologists Practice Bulletin #33 Diagnosis and Management of Preeclampsia and Eclampsia. Obstet Gynecol. 2002;99:159–167. [Google Scholar]
- Friedman-Einat M, Grossman Z, Mileguir F, Smetana Z, Ashkenazi M, Barkai G, Varsano N, Glick E, Mendelson E. Detection of adeno-associated virus type 2 sequences in the human genital tract. J Clin Microbiol. 1997;35:71–78. doi: 10.1128/jcm.35.1.71-78.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry S, Gomez L, Arechaveleta-Velasco FJ, Ma Y, Nelson DB. Maternal infection with adeno-associated virus-2 and adverse reproductive outcomes. J Soc Gynecol Invest. 2004;11:352A. doi: 10.1093/humrep/dem360. Abstract 821. [DOI] [PubMed] [Google Scholar]
- Koi H, Zhang J, Makrigiannakis A, Getsios S, MacCalman CD, Kopf GS, Strauss JF, 3rd, Parry S. Differential expression of the coxsackievirus and adenovirus receptor regulates adenovirus infection of the placenta. Biol Reprod. 2001;64:1001–1009. doi: 10.1095/biolreprod64.3.1001. [DOI] [PubMed] [Google Scholar]
- Koi H, Zhang J, Makrigiannakis A, Getsios S, MacCalman CD, Strauss JF, 3rd, Parry S. Syncytiotrophoblast is a barrier to maternal-fetal transmission of herpes simplex virus. Biol Reprod. 2002;67:1572–1579. doi: 10.1095/biolreprod.102.004325. [DOI] [PubMed] [Google Scholar]
- Laughlin CA, Cardellichio CB, Coon HC. Latent infection of KB cells with adeno-associated virus type 2. J Virol. 1986;60:515–524. doi: 10.1128/jvi.60.2.515-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Damsky CH, Chiu K, Roberts JM, Fisher SJ. Preeclampsia is associated with abnormal expression of adhesion molecules by invasive cytotrophoblasts. J Clin Invest. 1993;91:950–960. doi: 10.1172/JCI116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving JA, Lala PK. Functional role of cell surface integrins on human trophoblast cell migration: regulation by TGF-beta, IGF-II, and IGFBP-1. Exp Cell Res. 1995;217:419–427. doi: 10.1006/excr.1995.1105. [DOI] [PubMed] [Google Scholar]
- Caniggia I, Grisaru-Gravnosky S, Kuliszewsky M, Post M, Lye SJ. Inhibition of TGF-beta 3 restores the invasive capability of extravillous trophoblasts in preeclamptic pregnancies. J Clin Invest. 1999;103:1641–1650. doi: 10.1172/JCI6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman CW. Immunology of preeclampsia. Semin Perinatol. 1991;15:257–262. [PubMed] [Google Scholar]
- Fisher S, Genbacev O, Maidji E, Pereira L. Human cytomegalovirus infection of placental cytotrophoblasts in vitro and in utero: implications for transmission and pathogenesis. J Virol. 2000;74:6808–6820. doi: 10.1128/jvi.74.15.6808-6820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanlioglu S, Benson PK, Yang J, Atkinson EM, Reynolds T, Engelhardt JF. Endocytosis and nuclear trafficking of adeno-associated virus type 2 are controlled by rac1 and phosphatidylinositol-3 kinase activation. J Virol. 2000;74:9184–9196. doi: 10.1128/jvi.74.19.9184-9196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- Zhou C, Trempe JP. Induction of apoptosis by cadmium and the adeno-associated virus Rep proteins. Virology. 1999;261:280–287. doi: 10.1006/viro.1999.9882. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Afione S, Kotin RM. Adeno-associated virus type 2 Rep78 induces apoptosis through caspase activation independently of p53. J Virol. 2000;74:9441–9450. doi: 10.1128/jvi.74.20.9441-9450.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]