Abstract
Exposure of newborn male mice to estrogens is associated with age-related changes in prostate size and induction of epithelial hyperplasia and dysplasia. Whether these changes directly result from systemic estrogen administration or indirect effects of estrogens on systemic testosterone levels is unclear. We have addressed this question using aromatase-knockout (ArKO) mice that are estrogen-deficient during their lifespan but have elevated androgen levels and develop prostate enlargement and hyperplasia (McPherson SJ, Wang H, Jones ME, Pedersen J, Iismaa TP, Wreford N, Simpson ER, Risbridger GP: Endocrinology 2001, 142:2458–2467). Circulating testosterone and dihydrotestosterone levels were significantly decreased by neonatal diethylstilbestrol treatment, remained suppressed in adult wild-type mice, but rapidly returned to control levels in ArKO animals. However, adult prostate weight and luminal size were reduced in both wild-type and ArKO animals. Because both wild-type and ArKO mice developed epithelial hyperplasia and inflammation following neonatal diethylstilbestrol treatment, this validates that estrogens directly cause prostatic inflammation and epithelial hyperplasia. Furthermore, because ArKO mice are estrogen-deficient, this study demonstrates the sensitivity of the neonatal period to estrogen exposure and the long range and permanent nature of the prostatic responses that occur. Finally, this study establishes the ArKO mouse model of estrogen deficiency as a unique approach to study the effects of estrogens, estrogenic factors, and endocrine disruptors on prostate development.
The prostate gland is an androgen-dependent organ. Androgen ablation by castration results in involution of the prostate and activation of apoptosis, and these effects can be reversed following the restoration of androgens.1,2 Circulating testosterone entering the prostate can be metabolized to 5α-dihydrotestosterone (DHT) by local 5α-reductase activity.3,4 The effects of testosterone and DHT are mediated by activation of the intracellular androgen receptor.
Although primarily influenced by androgens, the prostate is also an estrogen-target organ. The biosynthesis of estrogens occurs via metabolism of an androgenic substrate, catalyzed by an enzyme complex known as aromatase.5 Detection of aromatase expression in the prostate is evidence for local estrogen synthesis,6–13 and the identification of estrogen receptor (ER) subtypes ERα and ERβ14,15 confirms that estrogen signaling pathways exist in the prostate.
Direct effects of estrogens are difficult to determine because the effects of exogenous estrogen administration are centrally mediated and disrupt the normal endocrine environment, eliciting negative feedback inhibition of endogenous gonadotropin production and depression of testicular androgen biosynthesis.16,17 However, we recently studied direct estrogen actions in the prostate using the hypogonadal mouse (hpg) model, deficient in gonadotropin and sex steroid synthesis.18 The effects of unopposed estradiol exposure were proliferative and included significant enlargement of prostate lobes. Aberrant growth was observed, including proliferation of stromal fibroblasts and epithelial basal cells accompanied by a disruption and reduction to smooth muscle and secretory epithelial cells. A prostatic inflammatory response was also identified in this model as has been previously shown using other models.18 Conversely, the effects of unopposed androgens on the prostate were also proliferative, as evident in the aromatase-knockout (ArKO) mouse model, which is estrogen-deficient.19 As a consequence of failure to metabolize androgens to estrogens, the ArKO mouse exhibited elevated peripheral and intraprostatic androgen levels as well as increased androgen receptor immunoexpression.20 Prostate lobes were enlarged and showed uniform volumetric expansion of stromal, epithelial, and luminal compartments.1 These data therefore provide evidence that both androgens and estrogens are proliferative in the prostate but in different ways. Coordinated growth and proliferation occur in response to androgens, whereas estrogens lead to uncoordinated and aberrant patterns of proliferation.
The brief exposure of newborn male rats to pharmacological doses of estrogens produces multiple changes to prostate growth, morphology, and function, including alterations to hormonal sensitivity in later life.21–25 This process is referred to as neonatal imprinting or developmental estrogenization. Exposure to estrogens during early life reduces androgen sensitivity in later life24,26 as a result of down-regulation and accelerated degradation of prostatic androgen receptor.21,22,27,28 The subsequent effects on the prostate include manifestation of atypical pathological changes with age, including inflammation, epithelial hyperplasia, and the emergence of dysplastic lesions.22,29–33 These changes are of major importance, because specific inflammatory pathologies may be positively associated with benign and malignant changes in the prostate.34–36 Dysplastic epithelial lesions, otherwise referred to as prostatic intraepithelial neoplasia (PIN), precede the emergence of prostatic carcinoma.37–41 The role of estrogens in mediating these changes is not well understood, because androgen production and action are suppressed, and it is difficult to distinguish between androgenic and estrogenic effects. However, it was shown using ER-deficient mouse models that the ERα subtype mediates acute and chronic pathological responses to developmental estrogenization, whereas ERβ is not required for these changes to occur.30 Furthermore, neonatal exposure to the androgen receptor antagonist flutamide was shown not to mimic any of the changes elicited by early estrogen exposure,22 supporting a direct effect of estrogen action on the prostate during estrogenization. To examine the long range effects of developmental estrogenization and assess the direct and indirect responses of the prostate gland, the estrogen-deficient (ArKO mouse) model was used.
Materials and Methods
Animals
The ArKO mouse colony was originally bred on a C57BL/6J background, generated by targeted disruption of the cyp19 gene as previously described.19 All mice were maintained under controlled conditions (lights on 7:00 AM to 7:00 PM; temperature 20 to 4°C), with free access to mouse feed and water. Soy-free mouse chow (Glen Forrest Stockfeeders, Glen Forrest, Australia) was provided for all animals in this study, because regular mouse chow has been shown to contain isoflavones.42 All studies were conducted with approval from the animal ethics committee of Monash University in accordance with guidelines of the National Health and Medical Research Council.
The mice used in this study were generated by breeding male and female mice heterozygous for the cyp19 gene, for the production of homozygous aromatase +/+ or −/− offspring. Pregnant mice were monitored daily for the delivery of pups, and the day of birth was designated day 0. Pups were sexed, and the genotyping of tail DNA from male offspring was determined by PCR analysis. Pups were allocated randomly to one of two treatment groups, administered either 2 μg of diethylstilbestrol (DES; Sigma Chemical Co., St. Louis, MO) in peanut oil (Sigma Chemical Co.) or peanut oil alone on postnatal days 1–5 via injection at the nape of the neck. For each group five to eight animals were used.
Monthly body weights were determined, and serum samples were collected by tail bleeding into capillary blood collection tubes (Microvette CB300KE; Sarstedt, Nümbrecht, Germany) from 50 days (∼7 weeks) of age. At 90 and 180 days of age, animals were weighed followed by sacrifice via cervical dislocation, and terminal serum samples were collected and stored at −20°C. To prevent variations in hormone concentrations as a consequence of diurnal hormonal secretion, sera were collected during a controlled period (1000 to 1400 hours). Testes, seminal vesicles (SV) and anterior (AP), ventral (VP), dorsal (DP), and lateral (LP) prostate lobes were excised and dissected free of extraneous fat. Organs were weighed and immersion-fixed in Bouin’s fixative before processing. Following dehydration, organs were embedded in paraffin wax and serially sectioned (5 μm).
Histopathology
Following serial sectioning of tissues, sections were sampled at 100-μm intervals (every 20th section) for AP and at 50-μm intervals (every 10th section) for remaining prostate lobes (VP, DP, and LP). Sections were stained with Mayer’s hematoxylin and eosin before unbiased blind histological examination by a pathologist.
Hormone Assays
Testosterone levels in plasma were assessed via organic extraction with a 3:2 mixture of hexane and ethyl acetate. Procedural recoveries were calculated with a parallel sample with tritiated testosterone added. The organic fraction was then dried overnight and reconstituted with a 1% gelatin phosphate-buffered saline buffer. The reconstituted aliquots were processed in a radioimmune precipitation assay with a specific antibody raised to testosterone (T3-125; Endocrine Sciences Laboratories, Calabasas, CA) and a liquid chromatography-purified tritiated testosterone. After 16-hour incubation at 4°C, free and bound hormones were separated with dextran T70-coated charcoal. The testosterone standard was calibrated against a World Health Organization testosterone preparation (coefficient of variation 3.1–7.5%). DHT was extracted, dried, and reconstituted as per the testosterone assay. The aliquots were then oxidized by exposure to 0.5% potassium permanganate for 30 minutes. Oxidation was terminated via a second organic extraction. The dried organic extract was reconstituted and assayed using antibody C0457 (Bioquest, North Ryde, Australia) and a liquid chromatography-purified tritiated DHT tracer. Procedural recoveries were calculated as per testosterone but with a DHT tracer (coefficient of variation 3.8–4.6%).
Stereological Analysis
All assessments were performed using a BX-51 microscope (Olympus Corp., Tokyo, Japan). The images were captured by a JVC TK-C1380 color video camera (Victor Co., Tokyo, Japan) coupled to an IBM computer and projected directly onto a video screen using an Integral Flashpoint 3Dx frame grabber video adaptor (Integral Technologies Inc., Indianapolis, IN). CAST software (version 2.1.4; Olympus Corp.) was used to generate a set of counting frames and a point grid (grid properties were assessed individually for each organ and treatment group; sampling was conducted at predetermined intervals along x- and y-axes). Fields were selected by a systematic uniform random sampling scheme, and volumes of tissue compartments were determined based on protocols modified from those previously used in the testis43 and prostate.20,44
Hematoxylin and eosin-stained serial sections 50 μm apart were examined under ×40 magnification. VP tissues were classified into three compartments: stroma, epithelium, and lumen. The relative volume of stroma, epithelium, and lumen per organ was determined by the sum of the number of points that landed on each compartment divided by the sum of the number of points contacting the entire organ. At least 100 counts per tissue compartment were obtained. The absolute volume of each tissue compartment was determined by multiplying the organ weight and the relative volume.
Immunohistochemistry
Proliferating cells were identified by immunostaining for proliferating cell nuclear antigen (PCNA; DAKO, Carpentaria, CA). Immunohistochemistry was performed by using a DAKO autostainer. Briefly, individual organs were sectioned longitudinally (5 μm) to reveal the proximo-distal orientation and deparaffinized, rehydrated, and treated with peroxidase block (DAKO) for 10 minutes. Antigen retrieval (0.01 mol/L citrate buffer, pH 6.0, as per the manufacturer’s specifications) was used before inactivation of endogenous peroxidase, and nonspecific binding was blocked using CAS block (Zymed, San Francisco, CA), followed by incubation with primary antibody for 30 minutes at room temperature. Primary antibody binding was detected using biotinylated rabbit anti-mouse IgG2A antibody (Zymed) followed by incubation with an avidin-biotin peroxidase kit (ABC Elite; Vector Laboratories, Burlingame, CA) for 15 minutes (PCNA). Antibody localization was visualized using diaminobenzidine tetrachloride as a chromogen. Finally, sections were counterstained with Mayer’s hematoxylin, gradually dehydrated with alcohol, cleared with Histolene, and covered with a coverslip and with DPX mounting solution.
PCNA Quantitation
A semiquantitative approach was used to determine the percentage of cells (stromal and epithelial) showing positive immunostaining for PCNA. Briefly, blocks of prostate tissues (four to five animals per group) were selected randomly and sectioned longitudinally to reveal the proximo-distal orientation; sections from each animal included paired lobes. Tissues from all groups were processed in the same immunohistochemical assay to eliminate interassay variation. Following PCNA immunostaining, 5-μm sections were subjected to systematic sampling commencing at a random point using an unbiased counting frame generated using CAST software (version 2.1.4; Olympus Corp.). Using ×40 magnification, stromal and epithelial cells were classified as PCNA-positive or -negative. Cell numbers were combined (minimum of 600 cells counted per organ), and percentages of PCNA-positive epithelial and stromal cells were determined.
Statistical Analysis
Data were compared among genotypes (wild-type (WT) and ArKO), treatment groups (oil and DES), and ages (90 and 180 days). Data were analyzed to determine normality, and significant differences were determined by t-test, with a significance threshold used at a level of 5% (P < 0.05), or by one-way analysis of variance analysis followed by the posthoc Tukey multiple comparison test. The analyses were conducted using Prism 4.00 software (GraphPad Software Inc., San Diego, CA). Data are expressed as mean ± SE.
Results
Neonatal DES Treatment Reduces Serum Androgen Levels in ArKO Mice but Not Below Normal Levels in Untreated WT Controls
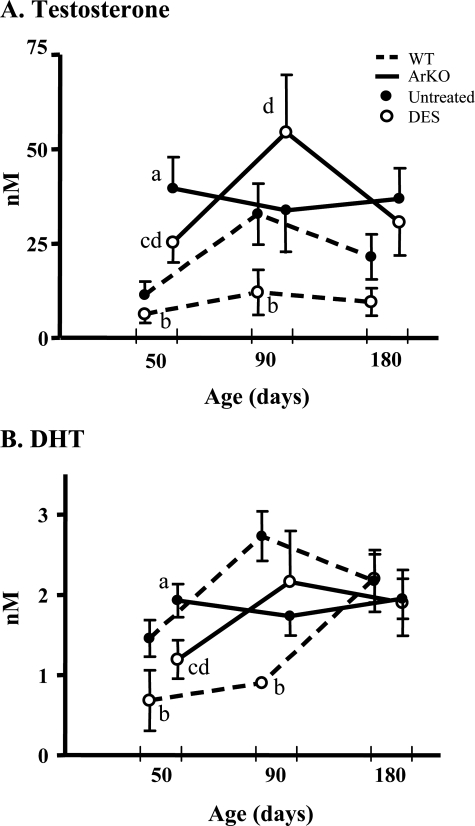
Circulating testosterone concentrations were significantly elevated in ArKO control versus WT control mice aged 50 days as previously reported (Figure 1A; P < 0.05). DES treatment to WT mice significantly reduced testosterone and DHT concentrations at ages 50 and 90 days (Figure 1, A and B; P < 0.05). DES treatment to ArKO mice reduced testosterone and DHT levels in mice 50 days old, although they were no different from WT controls (Figure 1, A and B; P < 0.05); no changes were identified after this time. Following DES treatment, the testosterone and DHT levels in ArKO mice were higher than or no different from (but never lower than) WT controls treated with DES (Figure 1, A and B; P = 0.08 and 0.08, respectively).
Figure 1.
Temporal analysis of serum androgens following neonatal exposure of WT and ArKO mice to DES. Circulating serum testosterone (A) and DHT (B) concentrations were significantly elevated in untreated ArKO (• and solid line) versus WT control (• and dashed line) mice at 50 days. WT mice given neonatal DES injections (○ and dashed line) showed significant reductions to testosterone (A) and DHT (B) at 50 and 90 days. DES treatment of ArKO mice (○ and solid line) resulted in reductions to testosterone (A) and DHT (B) only at 50 days (P < 0.05). After DES treatment, ArKO animals maintained significant elevations to testosterone (A) and DHT (B) versus WT animals at 50 and 90 days (P < 0.05), and nonsignificant elevations were identified at 180 days (P = 0.08 and 0.08, respectively). Data are expressed as mean ± SE; n = 5–8. Different letters indicate significant comparisons (P < 0.05): a, untreated ArKO versus untreated WT; b, DES-treated WT versus untreated WT; c, DES-treated ArKO versus untreated ArKO; d, DES-treated ArKO versus untreated WT. Some error bars were removed to simplify comparisons.
Body and Organ Weights
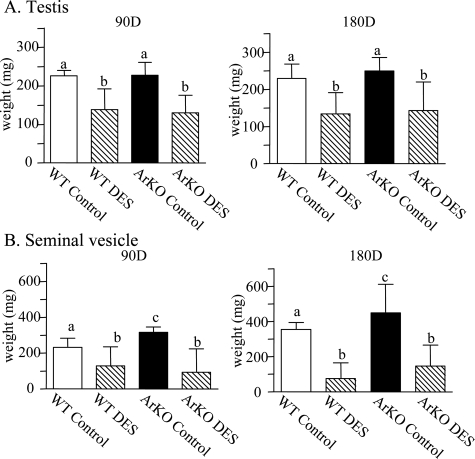
Body, testicular, and seminal vesicle weights were measured as indirect indicators of androgen status in DES-treated WT and ArKO mice. No changes to body weights were identified between WT and ArKO control animals, and DES treatment did not significantly alter body weights in WT or ArKO groups (data not shown). Although androgen levels were elevated in ArKO mice, the testis weights were not significantly different to WT; however, neonatal DES treatment significantly reduced testis weights of both WT and ArKO animals at 90 and 180 days of age (Figure 2A; P < 0.01), consistent with the initial decrease in androgen levels between 50 or 90 days of age (see Figure 1). Similarly, SV organ weights were significantly elevated (P < 0.05) in ArKO control versus WT control animals at 90 (P < 0.01) and 180 days (Figure 2B; P < 0.05). DES treatment significantly reduced WT and ArKO SV weights at both ages (P < 0.05 and P < 0.001), although by 180 days ArKO SVs had again become heavier than in WT littermates (162.9 ± 44.9 vs. 75.8 ± 40.07 mg).
Figure 2.
Effects of neonatal DES on nonprostatic reproductive organ weights. A: Testis weights of WT and ArKO control animals were not significantly different, but neonatal DES treatment significantly reduced testis weights of WT and ArKO animals at 90 and 180 days of age (P < 0.01). B: Seminal vesicle weights were significantly elevated (P < 0.05) in ArKO control versus WT control animals at 90 (P < 0.01) and 180 days (P < 0.05). DES treatment significantly reduced WT and ArKO SV weights at 90 (P < 0.05) and 180 days (P < 0.001). Different superscripts indicate significant comparisons.
The weights of the lobes of the prostate gland (VP, AP, LP, and DP) were determined as shown in Table 1. At 90 days of age, the weights of all of the prostate lobes in untreated ArKO mice were significantly increased (P < 0.05) compared to WT controls (Table 1). At 180 days of age, the differences remained statistically significant in only VP and LP lobes (Table 1; P < 0.05). Neonatal exposure to DES significantly reduced (P < 0.05) prostate lobe weights (AP, VP, LP, and DP) in both WT animals, as shown at 90 and 180 days of age (Table 1). Similarly, at 90 days of age, the weight of ArKO mouse tissues was significantly reduced, but in older ArKO mice (180 days old) only the ventral and lateral prostate lobes remained significantly lower (Table 1); the anterior and dorsal prostate lobes were no different from untreated ArKO controls (Table 1).
Table 1.
Analysis of Adult Prostate Lobe Weights after Neonatal DES
| Age (days) | Organ weight (mg) | ||||
|---|---|---|---|---|---|
| VP | AP | LP | DP | ||
| WT control | 90 | 7.883 ± 0.95 | 29.97 ± 1.61 | 1.46 ± 0.36 | 5.05 ± 0.38 |
| WT + DES | 90 | 4.26 ± 0.47*† | 14.09 ± 1.90*† | 0.33 ± 0.09*† | 3.51 ± 0.46*† |
| ArKO control | 90 | 11.47 ± 1.05* | 36.83 ± 2.15* | 3.72 ± 0.71* | 8.00 ± 0.71* |
| ArKO + DES | 90 | 5.76 ± 0.86† | 18.55 ± 2.36*† | 0.83 ± 0.36†‡ | 4.48 ± 0.55† |
| WT control | 180 | 9.50 ± 1.25 | 37.82 ± 2.95 | 3.18 ± 0.47 | 7.35 ± 0.65 |
| WT + DES | 180 | 4.04 ± 0.69*† | 25.80 ± 1.70*† | 0.88 ± 0.33*† | 5.24 ± 0.72*† |
| ArKO control | 180 | 14.85 ± 2.09* | 46.14 ± 5.32* | 8.70 ± 1.36* | 9.22 ± 1.35 |
| ArKO +DES | 180 | 7.76 ± 0.87†‡ | 37.76 ± 2.90†‡ | 3.27 ± 0.42†‡ | 8.56 ± 0.92‡ |
At 90 days of age, ArKO prostate lobe weights versus WT control (AP, VP, LP, and DP) were significantly elevated (P < 0.05). Similar patterns were recorded at 180 days of age, except in ArKO DP, which was not different from WT control. Following neonatal DES treatment, at 90 days of age all WT and ArKO prostate weights were significantly reduced compared to respective controls (P < 0.05), but ArKO VP, LP, and DP were no different to WT control weights. At 180 days of age, weights of DES-treated prostate lobes remained significantly reduced compared to WT or ArKO controls (P < 0.05), but DES-treated ArKO lobe weights were significantly elevated (P < 0.05) compared to WT counterparts and were not significantly different from WT control weights. Data are expressed as mean ± SE; n > 8.
Significant comparison (P < 0.05) versus WT control.
Significant comparison (P < 0.05) versus ArKO control.
Significant comparison (P < 0.05) versus WT DES.
Stereological Analysis
Because androgens regulate the secretory activity of the prostatic epithelia and are a significant factor in determining the weight of the prostate lobes, we used stereological analysis to quantify the changes to stromal, epithelial, and luminal volume in the VP lobes from 180-day-old mice. As previously shown,20 uniform increases in stroma, epithelia, and lumen volume were identified in ArKO compared to WT littermate control tissues that were significant for the epithelia (P < 0.05) and lumen (P < 0.05; Table 2; P = 0.0778). DES treatment of WT and ArKO mice reduced volume of lumen but not that of epithelia or stroma. The absolute volume of the VP lumen in WT mice was reduced by 86% following DES (vs. WT control; P = 0.0002); in ArKO mice the absolute volume of the VP lumen was reduced by 68% (vs. ArKO control; P = 0.0015) but remained significantly elevated compared to the lumen volume of WT DES mice (Table 2; P = 0.0001). Therefore, the changes to the lumen volume as determined by stereology correspond exactly with the observed changes in VP weights recorded in Table 1.
TAble 2.
Stereological Analysis of 180-Day-Old VP following Neonatal DES
| Compartmental volume (mm3) | |||
|---|---|---|---|
| Epithelium | Stroma | Lumen | |
| WT control | 2.10 ± 0.12 | 1.44 ± 0.16 | 6.83 ± 0.85 |
| WT + DES | 1.61 ± 0.30* | 1.49 ± 0.33 | 0.94 ± 0.14*† |
| ArKO control | 3.07 ± 0.41† | 2.12 ± 0.34 | 11.84 ± 1.66† |
| ArKO + DES | 2.75 ± 0.22†‡ | 2.53 ± 0.43†‡ | 3.74 ± 0.60*†‡ |
Compared to WT control tissue significant volumetric increases were detected in epithelial and luminal compartments (P < 0.05) of ArKO control VP, and a nonsignificant increase in stromal volume (P = 0.0778). After neonatal DES treatment, WT and ArKO stromal and epithelial volumes were not altered (compared to their respective controls), but luminal volumes were significantly reduced (P < 0.05 respectively; vs. controls). DES-treated ArKO volumes remaining significantly higher than in DES-treated WT and were significantly larger than WT controls (P = 0.05). Data are expressed as mean ± SE; n = 5–8.
Significant comparison (P < 0.05) versus ArKO control.
Significant comparison (P < 0.05) versus WT control.
Significant comparison (P < 0.05) versus WT DES.
PCNA Quantitation
Quantitation of PCNA staining in the epithelia is further evidence of the proliferative response to DES. There were no significant changes in the absolute volume of the epithelia from WT compared to ArKO mice after DES treatment (Table 3). However, analysis of the proportion of cells labeled with PCNA showed that neonatal DES treatment resulted in a significant increase in proliferating cells in the epithelium of WT and ArKO VP (Table 3), providing evidence of an increased proliferative response in the epithelium.
Table 3.
Quantitation of PCNA Activity in 180-Day-Old VP following Neonatal DES
| % proliferation | |||
|---|---|---|---|
| Epithelium | Stroma | Overall | |
| WT control | 24.91 ± 1.34 | 21.39 ± 5.52 | 25.15 ± 1.54 |
| WT + DES | 51.75 ± 6.03*† | 19.49 ± 4.81† | 44.55 ± 5.67* |
| ArKO control | 33.22 ± 3.28* | 34.77 ± 3.92* | 33.53 ± 3.32* |
| ArKO + DES | 48.38 ± 4.761*† | 21.03 ± 3.00† | 38.51 ± 3.91* |
ArKO VP showed significant increases in levels of epithelial and stromal cell proliferation compared WT controls (P < 0.05), which was reflected in a significant overall increase in proliferation. Neonatal DES treatment elevated PCNA localization significantly in the epithelium of both WT and ArKO VP compared to respective controls, but had no effect on WT stroma and significantly reduced proliferation in ArKO stroma (P < 0.05), resulting in a significant overall increase in WT proliferation (P < 0.05). Data expressed as mean ± SEM.
Significant comparison (P < 0.05) versus WT control.
Significant comparison (P < 0.05) versus ArKO control.
Histology
Regardless of changes to organ weight, histological examination of the tissues provided additional information about direct prostate tissue response to DES.
Wild-type Control versus ArKO Control
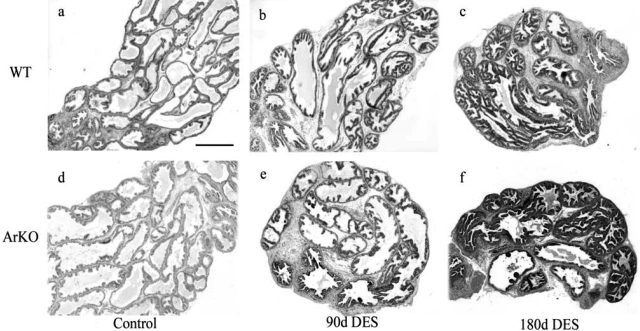
In the absence of DES exposure, differences in the histology of the prostate lobes from control WT and ArKO mice, including epithelial hyperplasia and distension of the ducts, were identified in VP (Figure 3d) and all other prostate lobes (data not shown) in ArKO mice. The epithelial hyperplasia in the VP was characterized by increased papillary infolding of the epithelium, and expansion of glandular lumen was associated with increased secretory fluid within the ducts (Figure 3d). Inflammation was not identified in control tissues at any age.
Figure 3.
Morphology of adult ventral prostate lobes following neonatal DES. Histological examination identified apparent increases in lumen size and epithelial infolding in ArKO control tissue (b) compared to WT (a). Following neonatal DES treatment VP from WT mice aged 90 (b) showed apparent ductal regression as indicated by narrowing of ducts and reduction in lumen size and an apparent increase in epithelial infolding, which became more pronounced at 180 days (c). DES-treated ArKO VPS at 90 (e) and 180 days (f) showed similar histological responses to those seen in WT tissue, although lumen appeared to be larger than those seen in WT tissues. Staining, H & E; magnification objective, ×4; scale bar = 200 μm.
Wild-type DES versus ArKO DES
The reduction in the weight of prostate lobes from DES-treated mice (see Table 1) was associated with distinctive histological changes. Following neonatal estrogenization, at 90 days of age, WT and ArKO animals demonstrated significant ductal regression, characterized by generally narrow ducts with smaller glands compared to control tissue (Figure 3, a and d) and an increase in epithelial infolding in VP (Figure 3, b and e) and all other prostate lobes (data not shown). ArKO tissues, however, appeared to retain larger lumen.
The characteristics identified in DES-treated WT and ArKO animals at 90 days appeared to be exacerbated at 180 days (Figures 3c, 3f, and 4), and reductions in weight (Table 1) were associated with reduced duct size and luminal fluid secretion. (Figure 3, c and f). However, ArKO tissues were all significantly (P < 0.05) heavier than the corresponding WT DES-treated mouse tissues with no difference in untreated WT controls (Table 1), the likely result of ArKO tissues having larger luminal volume compared to WT (Table 2).
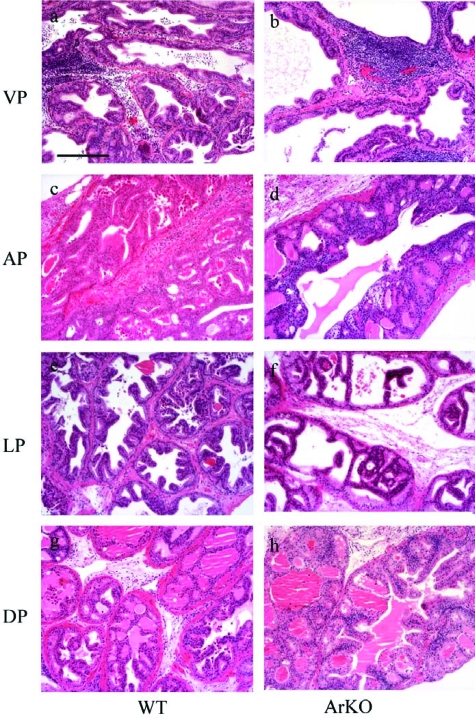
Figure 4.
Histological examination of adult ArKO and WT prostate lobes following neonatal DES. Treatment of both WT (a, c, e, and g) and ArKO (b, d, f, and h) with neonatal DES was associated with regressive changes to all prostate lobes, VP (a and b), AP (c and d), LP (e and f), and DP (g and h), including a significant reduction to luminal size within glands, although lumen from ArKO lobes remained larger compared to DES-treated WT tissues. Epithelial hyperplasia was identified in all prostate lobes from both DES-treated WT and ArKO mice and was characterized by increased papillary folding and the widespread identification of cribriform structures. Estrogenization was also associated with the manifestation of a local inflammatory response. Staining, H & E; magnification objective, ×10; scale bar = 200 μm.
Neonatal DES exposure also caused hyperplasia in the epithelium of all prostate lobes of both WT and ArKO mice that were characterized by increased papillary infolding of the epithelia and the presence of complex cribriform structures throughout glands (Figure 4). Nuclear changes were also observed within hyperplastic foci, including increased nuclear size and prominent. Significant increases in proliferative activity in VP tissues, from both WT and ArKO mice aged 180 days, was also observed as demonstrated by a significant increase in the percentage of PCNA-positive cells (Table 3).
Prostate Inflammation following Neonatal DES
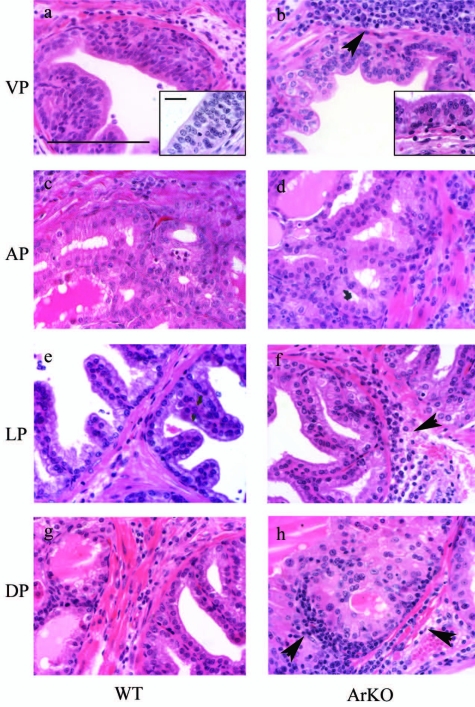
In addition to inducing histological changes, neonatal DES treatment of both WT and ArKO mice was associated with an inflammatory response in all of the prostate lobes at 180 days (Figure 5). Leukocytes were rarely observed in proximity to normal tissues; however, following DES exposure there was evidence of an inflammatory response in the ducts where there was also epithelial hyperplasia. Inflammation included an accumulation of neutrophils and lymphocytes, identified as forming lymphoid aggregates in the stroma but absent from control tissues (Figure 3). Immune cells were predominantly concentrated in the stroma and band of smooth muscle (Figure 5) but were also observed infiltrating the epithelial layer (Figure 5, a and b, inset). Evidence of inflammation was not detectable in any prostate lobe from control animals or at 90 days following neonatal DES treatment in either WT or ArKO mice.
Figure 5.
Induction of local inflammatory response in adult prostate following neonatal DES. DES treatment of animals was also associated with the manifestation of a local inflammatory response in all prostate lobes of both ArKO and WT. Inflammation was characterized by a significant concentration of leukocytes within the stroma and epithelium of tissues (b, f, and h, arrowheads). Inflammatory cells were also identified invading the epithelium (a and b, insets). Staining, H & E; magnification objective, ×40; scale bar = 100 μm; magnification, ×100 (inset), scale bar = 20 μm (inset).
Discussion
Since the administration of estrogen lowers androgens, the long range effects of neonatal estrogen treatment result from the combined effects of transient reduction in androgen and direct effects of estrogens per se. To assess the relative contributions of altering androgen and estrogen levels in neonatal mice, we used the estrogen-deficient ArKO mouse model, which provides a unique opportunity to study the effects of transient elevation of estrogen without complication due to endogenous estrogen synthesis thereafter. Androgen levels are elevated in ArKO male mice that subsequently develop prostate enlargement and hyperplasia.20 Treatment of mice with DES during the neonatal period significantly reduced circulating testosterone and DHT concentrations in mice of both WT and ArKO mice; however, the extent of this reduction differed between the genotypes and was reflected in the differing prostate weights and lumen size. Lumen size is indicative of the secretory activity of the prostate gland, which is known to be regulated by androgens. These results show that reduced prostate size in response to DES relates to the modification of androgen levels. However, despite variable serum hormone levels and a failure to consistently correlate them with changes in organ weights, prostatic epithelial hyperplasia and inflammation were consistently observed, confirming that this response is a direct effect of neonatal DES treatment. Furthermore, because the ArKO mice are estrogen-deficient, these results demonstrate prostatic susceptibility to transiently elevated estrogen levels during neonatal life and the permanent and long range nature of these effects.
This study shows that developmental estrogenization has dual actions on the epithelia leading to reduced secretory activity as well as epithelial cell proliferation and hyperplasia. The reduction in prostate size in both neonatal DES-treated ArKO and WT mice is almost entirely due to significant reductions in luminal volume, suggesting reduced activity of the secretory epithelial cells. In contrast, proliferation stimulated by DES treatment targets the basal cell layer, as previously described.45 Therefore the proliferative responses to transient neonatal estrogens differ from the hypertrophy and hyperplasia that occur in ArKO mice due to a sustained lifelong elevation of androgens. The presence of similar hyperplastic cribriform structures in both ArKO and WT mouse prostates indicates a direct biological effect of neonatal estrogen exposure on the prostate that is independent of, and unrelated to, subsequent serum androgen levels. These results provide additional evidence to implicate estrogens in the pathogenesis of human benign prostatic hyperplasia.46
Despite their reduced size and aberrant epithelial architecture, neonatally estrogenized prostates from both ArKO and WT mice show significant increases in the proliferative index of the epithelium compared to controls. The stimulus for proliferation may arise during neonatal estrogenization or occur later as inflammation occurs and cytokines derived from invading inflammatory cells stimulate aberrant prostatic epithelial proliferation within the basal cell compartment where cells are known to proliferate in response to stimulation by estrogen alone18 and in response to inflammatory cytokines.47
Prostate homeostasis depends on the hormonal milieu and requires a balance between the levels of androgens and estrogens. Perturbation of the androgen:estrogen ratio is associated with altered growth, behavior, and pathogenesis in the prostate, particularly when the ratio of androgens:estrogens is reduced on aging. In older men, peripheral androgen levels are reduced while estrogen levels remain unchanged or become elevated,48–50 and increasing circulating and intraprostatic estrogen:androgen ratios have been linked to prostate disease, including benign prostatic hyperplasia51–56. This study demonstrates the seminal importance of maintaining the appropriate balance between androgens and estrogens during neonatal life. Neonatal estrogenization decreases the androgen:estrogen ratio during neonatal life and is associated with the emergence of multiple pathological changes in the adult prostate as demonstrated in both WT and ArKO mice. If the androgen:estrogen ratio is significantly increased from birth to maturity, as it is in untreated ArKO mice, the response is equally profound but does not result in premalignant or malignant changes in adulthood.20 Neonatal imprinting by estrogens is associated with dysplasia, analogous to premalignant prostatic intraepithelial neoplasia in the human prostate.29,57,58 Dysplasia usually occurred 9–12 months after neonatal estrogenization in rats57 and mice,29 beyond the time frame of the current study. However, the presence of proliferative hyperplastic lesions and nuclear changes are consistent with the development of further premalignant lesions with time.
Regardless of the androgen status and the ratio of androgens:estrogens in WT or ArKO mice, immune cells were identified in prostates from estrogenized mice at 90 days of age, becoming increasingly prevalent after 180 days of age. Although inflammation frequently accompanied epithelial hyperplasia, it is not known if this was cause or effect, ie, the epithelial pathology caused a local inflammatory response, or whether increasing inflammation resulted in reactive epithelial hyperplasia. The coexistence of immune cell infiltration and hyperplasia is significant, because a positive correlation between inflammatory pathologies and benign and malignant changes has been previously reported in the human prostate.34–36
Disturbance of stromal-epithelial interactions occurs when homeostasis is disrupted and pathologies emerge.59 Chang et al60 reported periductal fibroblast proliferation in estrogenized prostates and proposed that this may contribute to formation of a physical barrier obstructing normal paracrine communications between the prostatic stroma and epithelium. In the current study, immune cells were identified invading the stroma and epithelium of estrogenized prostates as previously described,18,25,33,61 a response associated with alteration of stromal histology, disruption of normal stromal-epithelial signaling, and contribution to the development of pathologies, including proliferative hyperplastic lesions.18 We previously confirmed that both fibroblast proliferation and inflammation are direct biological effects of estrogen.18
In summary, these data demonstrate that neonatal DES exposure suppressed circulating testosterone and DHT concentrations in WT mice throughout adulthood but only reduced androgen concentrations in the ArKO mice to levels physiologically equivalent to those seen in untreated wild-type mice. The ArKO prostate showed resistance to several of the parameters associated with developmental estrogenization, particularly loss of epithelial secretory activity, which contributed to reduced prostate lobe weights in WT tissues. These data suggest that suppression of secretory activity occurring during estrogenization may be indirectly mediated through androgen antagonism. However, epithelial hyperplasia and development of prostatic inflammation were not altered by variable adult androgen or estrogen levels, implicating neonatal estrogens, and not androgens, in the pathogenesis of hyperplasia and inflammation.
Footnotes
Address reprint requests to Stephen John McPherson, Monash Institute of Reproduction and Development, Monash University, Clayton, Victoria 3168, Australia. E-mail: stephen.mcpherson@med.monash.edu.au.
Supported by a program grant from the National Health and Medical Research Council of Australia.
References
- Kyprianou N, Isaacs JT. Activation of programmed cell death in the rat ventral prostate after castration. Endocrinology. 1988;122:552–562. doi: 10.1210/endo-122-2-552. [DOI] [PubMed] [Google Scholar]
- Isaacs JT. Antagonistic effect of androgen on prostatic cell death. Prostate. 1984;5:545–557. doi: 10.1002/pros.2990050510. [DOI] [PubMed] [Google Scholar]
- Anderson KM, Liao S. Selective retention of dihydrotestosterone by prostatic nuclei. Nature. 1968;219:277–279. doi: 10.1038/219277a0. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N, Wilson JD. The conversion of testosterone to 5-alpha-androstan-17-beta-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968;243:2012–2021. [PubMed] [Google Scholar]
- Goto J, Fishman J. Participation of a nonenzymatic transformation in the biosynthesis of estrogens from androgens. Science. 1977;195:80–81. doi: 10.1126/science.831259. [DOI] [PubMed] [Google Scholar]
- Tsugaya M, Harada N, Tozawa K, Yamada Y, Hayashi Y, Tanaka S, Maruyama K, Kohri K. Aromatase mRNA levels in benign prostatic hyperplasia and prostate cancer. Int J Urol. 1996;3:292–296. doi: 10.1111/j.1442-2042.1996.tb00537.x. [DOI] [PubMed] [Google Scholar]
- Stone NN, Laudone VP, Fair WR, Fishman J. Aromatization of androstenedione to estrogen by benign prostatic hyperplasia, prostate cancer and expressed prostatic secretions. Urol Res. 1987;15:165–167. doi: 10.1007/BF00254430. [DOI] [PubMed] [Google Scholar]
- Negri-Cesi P, Colciago A, Poletti A, Motta M. 5α-Reductase isozymes and aromatase are differentially expressed and active in the androgen-independent human prostate cancer cell lines DU145 and PC3. Prostate. 1999;41:224–232. doi: 10.1002/(sici)1097-0045(19991201)41:4<224::aid-pros2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Matzkin H, Soloway MS. Immunohistochemical evidence of the existence and localization of aromatase in human prostatic tissue. Prostate. 1992;21:309–314. doi: 10.1002/pros.2990210407. [DOI] [PubMed] [Google Scholar]
- Kaburagi Y, Marino MB, Kirdani RY, Greco JP, Karr JP, Sandberg AA. The possibility of aromatization of androgen in human prostate. J Steroid Biochem. 1987;26:739–742. doi: 10.1016/0022-4731(87)91048-x. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Maehara I, Ozaki M, Harada N, Orikasa S, Sasano H. Aromatase in hyperplasia and carcinoma of the human prostate. Prostate. 1997;31:118–124. doi: 10.1002/(sici)1097-0045(19970501)31:2<118::aid-pros7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Harada N, Utsumi T, Takagi Y. Tissue-specific expression of the human aromatase cytochrome P-450 gene by alternative use of multiple exons 1 and promoters, and switching of tissue-specific exons 1 in carcinogenesis. Proc Natl Acad Sci USA. 1993;90:11312–11316. doi: 10.1073/pnas.90.23.11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellem SJ, Schmitt JF, Pedersen JS, Frydenberg M, Risbridger GP. Local aromatase expression in human prostate is altered in malignancy. J Clin Endocrinol Metab. 2004;89:2434–2441. doi: 10.1210/jc.2003-030933. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Birch L. Neonatal estrogen exposure up-regulates estrogen receptor expression in the developing and adult rat prostate lobes. Endocrinology. 1997;138:1801–1809. doi: 10.1210/endo.138.5.5106. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi IT, Warren DD, Catt KJ. Comparison of oestrogen and GnRH agonist analogue-induced inhibition of the pituitary-testicular function in rat. Acta Endocrinol (Copenh) 1983;103:163–171. doi: 10.1530/acta.0.1030163. [DOI] [PubMed] [Google Scholar]
- Karr JP, Wajsman Z, Kirdani RY, Murphy GP, Sandberg AA. Effects of diethylstilbestrol and estramustine phosphate on serum sex hormone binding globulin and testosterone levels in prostate cancer patients. J Urol. 1980;124:232–236. doi: 10.1016/s0022-5347(17)55383-5. [DOI] [PubMed] [Google Scholar]
- Bianco JJ, Handelsman DJ, Pedersen JS, Risbridger GP. Direct response of the murine prostate gland and seminal vesicles to estradiol. Endocrinology. 2002;143:4922–4933. doi: 10.1210/en.2002-220493. [DOI] [PubMed] [Google Scholar]
- Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson SJ, Wang H, Jones ME, Pedersen J, Iismaa TP, Wreford N, Simpson ER, Risbridger GP. Elevated androgens and prolactin in aromatase-deficient mice cause enlargement, but not malignancy, of the prostate gland. Endocrinology. 2001;142:2458–2467. doi: 10.1210/endo.142.6.8079. [DOI] [PubMed] [Google Scholar]
- Prins GS, Woodham C, Lepinske M, Birch L. Effects of neonatal estrogen exposure on prostatic secretory genes and their correlation with androgen receptor expression in the separate prostate lobes of the adult rat. Endocrinology. 1993;132:2387–2398. doi: 10.1210/endo.132.6.8504743. [DOI] [PubMed] [Google Scholar]
- Prins GS. Neonatal estrogen exposure induces lobe-specific alterations in adult rat prostate androgen receptor expression. Endocrinology. 1992;130:3703–3714. doi: 10.1210/endo.130.6.1597166. [DOI] [PubMed] [Google Scholar]
- Rajfer J, Coffey DS. Sex steroid imprinting of the immature prostate. Long-term effects. Invest Urol. 1978;16:186–190. [PubMed] [Google Scholar]
- Naslund MJ, Coffey DS. The differential effects of neonatal androgen, estrogen and progesterone on adult rat prostate growth. J Urol. 1986;136:1136–1140. doi: 10.1016/s0022-5347(17)45239-6. [DOI] [PubMed] [Google Scholar]
- Naslund MJ, Strandberg JD, Coffey DS. The role of androgens and estrogens in the pathogenesis of experimental nonbacterial prostatitis. J Urol. 1988;140:1049–1053. doi: 10.1016/s0022-5347(17)41924-0. [DOI] [PubMed] [Google Scholar]
- Rajfer J, Coffey DS. Effects of neonatal steroids on male sex tissues. Invest Urol. 1979;17:3–8. [PubMed] [Google Scholar]
- Prins GS, Birch L. The developmental pattern of androgen receptor expression in rat prostate lobes is altered after neonatal exposure to estrogen. Endocrinology. 1995;136:1303–1314. doi: 10.1210/endo.136.3.7867585. [DOI] [PubMed] [Google Scholar]
- Woodham C, Birch L, Prins GS. Neonatal estrogen down-regulates prostatic androgen receptor through a proteosome-mediated protein degradation pathway. Endocrinology. 2003;144:4841–4850. doi: 10.1210/en.2003-0035. [DOI] [PubMed] [Google Scholar]
- Santti R, Newbold RR, Pylkkanen MS, McLachlan JA. Developmental estrogenization and prostatic neoplasia. Prostate. 1994;24:67–78. doi: 10.1002/pros.2990240204. [DOI] [PubMed] [Google Scholar]
- Prins GS, Birch L, Couse JF, Choi I, Katzenellenbogen B, Korach KS. Estrogen imprinting of the developing prostate gland is mediated through stromal estrogen receptor alpha: studies with alphaERKO and betaERKO mice. Cancer Res. 2001;61:6089–6097. [PubMed] [Google Scholar]
- Pylkkanen L, Santti R, Newbold R, McLachlan JA. Regional differences in the prostate of the neonatally estrogenized mouse. Prostate. 1991;18:117–129. doi: 10.1002/pros.2990180204. [DOI] [PubMed] [Google Scholar]
- Pylkkanen L, Makela S, Valve E, Harkonen P, Toikkanen S, Santti R. Prostatic dysplasia associated with increased expression of c-myc in neonatally estrogenized mice. J Urol. 1993;149:1593–1601. doi: 10.1016/s0022-5347(17)36458-3. [DOI] [PubMed] [Google Scholar]
- Gilleran JP, Putz O, DeJong M, DeJong S, Birch L, Pu Y, Huang L, Prins GS. The role of prolactin in the prostatic inflammatory response to neonatal estrogen. Endocrinology. 2003;144:2046–2054. doi: 10.1210/en.2002-0038. [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzi MJ, De Marzo AM. Morphologic transitions between proliferative inflammatory atrophy and high-grade prostatic intraepithelial neoplasia. Urology. 2000;56:828–832. doi: 10.1016/s0090-4295(00)00776-7. [DOI] [PubMed] [Google Scholar]
- van Leenders GJ, Gage WR, Hicks JL, van Balken B, Aalders TW, Schalken JA, De Marzo AM. Intermediate cells in human prostate epithelium are enriched in proliferative inflammatory atrophy. Am J Pathol. 2003;162:1529–1537. doi: 10.1016/S0002-9440(10)64286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeal JE, Bostwick DG. Intraductal dysplasia: a premalignant lesion of the prostate. Hum Pathol. 1986;17:64–71. doi: 10.1016/s0046-8177(86)80156-3. [DOI] [PubMed] [Google Scholar]
- McNeal JE. Significance of duct-acinar dysplasia in prostatic carcinogenesis. Urology. 1989;34:9–15. [PubMed] [Google Scholar]
- McNeal JE, Villers A, Redwine EA, Freiha FS, Stamey TA. Microcarcinoma in the prostate: its association with duct-acinar dysplasia. Hum Pathol. 1991;22:644–652. doi: 10.1016/0046-8177(91)90286-x. [DOI] [PubMed] [Google Scholar]
- Bostwick DG, Brawer MK. Prostatic intra-epithelial neoplasia and early invasion in prostate cancer. Cancer. 1987;59:788–794. doi: 10.1002/1097-0142(19870215)59:4<788::aid-cncr2820590421>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Brawer MK. Prostatic intraepithelial neoplasia: a premalignant lesion. Hum Pathol. 1992;23:242–248. doi: 10.1016/0046-8177(92)90104-b. [DOI] [PubMed] [Google Scholar]
- Robertson KM, O’Donnell L, Simpson ER, Jones ME. The phenotype of the aromatase knockout mouse reveals dietary phytoestrogens impact significantly on testis function. Endocrinology. 2002;143:2913–2921. doi: 10.1210/endo.143.8.8957. [DOI] [PubMed] [Google Scholar]
- Meachem SJ, McLachlan RI, de Kretser DM, Robertson DM, Wreford NG. Neonatal exposure of rats to recombinant follicle stimulating hormone increases adult Sertoli and spermatogenic cell numbers. Biol Reprod. 1996;54:36–44. doi: 10.1095/biolreprod54.1.36. [DOI] [PubMed] [Google Scholar]
- Singh J, Zhu Q, Handelsman DJ. Stereological evaluation of mouse prostate development. J Androl. 1999;20:251–258. [PubMed] [Google Scholar]
- Risbridger GP, Wang H, Frydenberg M, Cunha G. The metaplastic effects of estrogen on mouse prostate epithelium: proliferation of cells with basal cell phenotype. Endocrinology. 2001;142:2443–2450. doi: 10.1210/endo.142.6.8171. [DOI] [PubMed] [Google Scholar]
- Griffiths K. Estrogens and prostatic disease. International Prostate Health Council Study Group. Prostate. 2000;45:87–100. doi: 10.1002/1097-0045(20001001)45:2<87::aid-pros2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Wang W, Bergh A, Damber JE. Chronic inflammation in benign prostate hyperplasia is associated with focal upregulation of cyclooxygenase-2, Bcl-2, and cell proliferation in the glandular epithelium. Prostate. 2004;61:60–72. doi: 10.1002/pros.20061. [DOI] [PubMed] [Google Scholar]
- Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991;73:1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- Deslypere JP, Vermeulen A. Leydig cell function in normal men: effect of age, life-style, residence, diet, and activity. J Clin Endocrinol Metab. 1984;59:955–962. doi: 10.1210/jcem-59-5-955. [DOI] [PubMed] [Google Scholar]
- Baker HW, Burger HG, de Kretser DM, Hudson B, O’Connor S, Wang C, Mirovics A, Court J, Dunlop M, Rennie GC. Changes in the pituitary-testicular system with age. Clin Endocrinol (Oxf) 1976;5:349–372. doi: 10.1111/j.1365-2265.1976.tb01964.x. [DOI] [PubMed] [Google Scholar]
- Krieg M, Nass R, Tunn S. Effect of aging on endogenous level of 5 alpha-dihydrotestosterone, testosterone, estradiol, and estrone in epithelium and stroma of normal and hyperplastic human prostate. J Clin Endocrinol Metab. 1993;77:375–381. doi: 10.1210/jcem.77.2.7688377. [DOI] [PubMed] [Google Scholar]
- Krieg M, Weisser H, Tunn S. Potential activities of androgen metabolizing enzymes in human prostate. J Steroid Biochem Mol Biol. 1995;53:395–400. doi: 10.1016/0960-0760(95)00085-e. [DOI] [PubMed] [Google Scholar]
- Gann PH, Hennekens CH, Longcope C, Verhoek-Oftedahl W, Grodstein F, Stampfer MJ. A prospective study of plasma hormone levels, nonhormonal factors, and development of benign prostatic hyperplasia. Prostate. 1995;26:40–49. doi: 10.1002/pros.2990260109. [DOI] [PubMed] [Google Scholar]
- Seppelt U. Correlation among prostate stroma, plasma estrogen levels, and urinary estrogen excretion in patients with benign prostatic hypertrophy. J Clin Endocrinol Metab. 1978;47:1230–1235. doi: 10.1210/jcem-47-6-1230. [DOI] [PubMed] [Google Scholar]
- Shibata A, Minn AY. Perinatal sex hormones and risk of breast and prostate cancers in adulthood. Epidemiol Rev. 2000;22:239–248. doi: 10.1093/oxfordjournals.epirev.a018036. [DOI] [PubMed] [Google Scholar]
- Partin AW, Oesterling JE, Epstein JI, Horton R, Walsh PC. Influence of age and endocrine factors on the volume of benign prostatic hyperplasia. J Urol. 1991;145:405–409. doi: 10.1016/s0022-5347(17)38353-2. [DOI] [PubMed] [Google Scholar]
- Prins GS. Naz R, editor. New York: CRC press,; Developmental estrogenization of the prostate gland. Prostate Basic and Clinical Aspects. 1997:pp 245–263. [Google Scholar]
- Arai Y, Mori T, Suzuki Y, Bern HA. Bourne GH, Danielle JF, editors. New York: Academic Press, Inc.,; Long-term effects of perinatal exposure to sex steroids and diethylstilbestrol on the reproductive system of male mammals. International Review of Cytology. 1983:pp 235–268. doi: 10.1016/s0074-7696(08)61019-0. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Hayward SW, Dahiya R, Foster BA. Smooth muscle-epithelial interactions in normal and neoplastic prostatic development. Acta Anat. 1996;155:63–72. doi: 10.1159/000147791. [DOI] [PubMed] [Google Scholar]
- Chang W, Wilson M, Birch L, Prins G. Neonatal estrogen stimulates proliferation of periductal fibroblasts and alters the extracellular matrix composition in the rat prostate. Endocrinology. 1999;140:405–415. doi: 10.1210/endo.140.1.6401. [DOI] [PubMed] [Google Scholar]
- Stoker TE, Robinette CL, Cooper RL. Perinatal exposure to estrogenic compounds and the subsequent effects on the prostate of the adult rat: evaluation of inflammation in the ventral and lateral lobes. Reprod Toxicol. 1999;13:463–472. doi: 10.1016/s0890-6238(99)00049-0. [DOI] [PubMed] [Google Scholar]