Abstract
Objective
To compare 2 hours of daily patching (combined with one hour of concurrent near visual activities) with a control group of spectacle wear alone (if needed) for treatment of moderate to severe amblyopia in children 3 to 7 years old.
Design
Prospective, randomized multicenter clinical trial (46 sites).
Participants
One hundred eighty children 3 to 7 years old with best-corrected amblyopic eye visual acuity of 20/40 to 20/400 associated with strabismus, anisometropia, or both who had worn optimal refractive correction (if needed) for at least 16 weeks or for two consecutive visits without improvement.
Intervention
Randomization either to 2 hours of daily patching with one hour of near visual activities or to spectacles alone (if needed). Patients were continued on the randomized treatment (or no treatment) until no further improvement was noted.
Main Outcome Measure
Best-corrected visual acuity in the amblyopic eye after 5 weeks.
Results
Improvement in visual acuity of the amblyopic eye from baseline to 5 weeks averaged 1.1 lines in the patching group and 0.5 lines in the control group (P=0.006), and improvement from baseline to best measured visual acuity at any visit averaged 2.2 lines in the patching group and 1.3 lines in the control group (P<0.001).
Conclusion
Following a period of amblyopia treatment with spectacles, two hours of daily patching combined with one hour of near visual activities modestly improves moderate to severe amblyopia in children 3 to 7 years old.
Precis
Following a period of amblyopia treatment with spectacles, two hours of daily patching combined with one hour of near visual activities modestly improves amblyopia associated with strabismus, anisometropia, or both in children 3 to 7 years old.
Introduction
Amblyopia is a leading cause of monocular visual impairment.1, 2 In previous studies, we reported similar effectiveness for 6 hours versus 2 hours of prescribed patching per day for moderate amblyopia and for full-time versus 6 hours of prescribed patching for severe amblyopia.3, 4
Despite expert opinion5, 6 and experimental evidence that amblyopia improves with patching,3, 4, 7 some authors8-10 have questioned whether there is benefit to patching, because few studies have included an untreated control group. Although results from randomized clinical trials comparing patching with control have been reported,11, 12 no study has been published to date that has done all of the following: (1) clearly defined amblyopia at enrollment, (2) incorporated a prolonged spectacles run-in phase with criteria for determining when maximum improvement has occurred, and (3) included a no-patching control group.
To address the question of whether patching improves amblyopia after a period of refractive correction, we conducted a randomized clinical trial to compare the effect of two hours of daily patching combined with one hour of near activities versus a control group in children age 3 to 7 years old with amblyopia caused by strabismus, anisometropia or both.
Patients and Methods
The study, supported through a cooperative agreement with the National Eye Institute of the National Institutes of Health, was conducted by the Pediatric Eye Disease Investigator Group at 46 clinical sites. The protocol and HIPAA-compliant informed consent forms were approved by institutional review boards, and the parent or guardian (referred to subsequently as “parent”) of each study patient gave written informed consent. Study oversight was provided by an independent data and safety monitoring committee.
Patient Selection
Eligibility criteria included age 3 to <7 years (at the time of enrollment) and the presence of or a history of an amblyogenic factor meeting study-specified criteria for strabismus and/or anisometropia (Table 1). At the time of randomization, the primary cohort required visual acuity in the amblyopic eye to be between 20/40 and 20/400 inclusive, visual acuity in the sound eye to be 20/40 or better, and an interocular acuity difference of 3 or more lines (0.3 logMAR, i.e., the logarithm of the minimum angle of resolution). A secondary cohort included patients who completed the spectacle run-in phase of the study (see below) with a 2-line (0.2 logMAR) interocular difference or with acuity of 20/32 in the amblyopic eye and 20/16 in the sound eye. Table 1 provides a complete listing of the eligibility and exclusion criteria.
Table 1.
Eligibility and Exclusion Criteria
Eligibility Criteria
|
Exclusion criteria
|
13 patients were <7 years old at study enrollment when spectacle wear started but were 7 years old at the time of randomization.
1 patient had visual acuity 20/32 in the amblyopic eye and 20/20 in the sound eye at the time of randomization.
D = diopter
Spectacle Run-in Phase
A detailed description of the spectacle phase of the study is in a separate publication.13 In brief, each patient who was prescribed spectacles for the first time, required a change in spectacles prescription, or had not worn optimal spectacles for at least 16 weeks entered the pre-randomization spectacle phase of the study. Following enrollment in the spectacle phase, the visual acuity in each eye was measured every 5 weeks until the amblyopic eye stopped improving, confirmed with a second test at the same visit (i.e., the better of the two acuities was the same or worse than the acuity at the prior visit). If there remained at least 2 lines of interocular acuity difference, the patient entered the randomized trial.
Patients who did not require spectacles or who had worn optimal spectacles for at least 16 weeks skipped the spectacle phase and entered the randomized trial phase directly.
Randomization and Treatment Protocol
Each patient was randomly assigned with equal probability to a control group or a patching group. Randomization was accomplished on the study's website using a permuted-blocks design of varying block sizes, with a separate sequence of computer-generated random numbers for each clinical site for the subgroup of patients with visual acuity 20/40 to 20/100. An additional sequence without stratification by site was used to randomize patients with visual acuity 20/125 to 20/400 and patients in the secondary cohort.
Patients in the control group wore spectacle correction only or continued without spectacles if none were required. Patients in the patching group similarly wore spectacles if needed and were prescribed two continuous hours of daily patching with at least one hour of near activities during patching. Adhesive skin patches provided by the study (Coverlet Eye Occlusors, Beiersdorf-Jobst, Inc., Rutherford College, NC) were used unless a skin allergy/irritation developed that was unresponsive to both local treatment with a skin emollient and a change in brand of patch, in which case a spectacle-mounted occluder was prescribed. In addition to patching, the parent was instructed orally and in writing to have the child spend at least one of the hours of patching time each day performing eye-hand coordination activities at near, such as crafts, coloring, tracing, cutting out shapes with scissors, completing workbook games (dot-to-dot, hidden pictures and word finds), computer-generated or video games (e.g., Game Boy/Nintendo/PlayStation), computer/internet, reading, written homework assignments, or other similar activities. Adherence to the treatment protocol was assessed by having the parent maintain a calendar on which the treatment (hours of occlusion and of near activities) completed each day was logged. The calendars were reviewed by the investigator at the 5-week visit, who then made an assessment of the patient's adherence to the prescribed treatment.
Examination Procedures
At baseline and at each follow-up visit, best-corrected visual acuity was measured in each eye by a study-certified vision tester using the Amblyopia Treatment Study visual acuity testing protocol14 (which uses single-surrounded HOTV optotypes) presented on the Electronic Visual Acuity Tester.15 Testing at baseline and at the 5-week outcome visit also included: (1) measurement of ocular alignment at distance and near fixation with a simultaneous prism and cover test, and (2) assessment of binocularity with the Randot Preschool Stereoacuity Test (Stereo Optical Company, Chicago, Illinois).
Five (+/−1) weeks after randomization, a best-corrected visual acuity primary outcome examination was performed by a study-certified tester who was masked to the patient's treatment assignment. If the amblyopic eye visual acuity had not improved at least one line from randomization, a retest of that eye was performed by the same or a different tester. Patients in whom amblyopic eye visual acuity improved at least one line at 5 weeks on the test or retest compared with baseline continued with the randomization-assigned treatment, with examinations at 10+/−1 weeks, 15+/−1 weeks, and every 3 months thereafter until visual acuity stopped improving or the amblyopic eye visual acuity matched or exceeded the sound eye visual acuity. At that time, study participation ended.
Statistical Methods
The trial was designed to assess whether amblyopic eye acuity improvement in the patching group was superior to the improvement in the control group (optical correction if needed) after 5 weeks. Using data from the 2 hour patching group in a previous study,3 the sample size was based on projecting a standard deviation of 0.15 logMAR (1.5 lines) for the 5-week amblyopic eye acuity and a mean difference between groups of 0.1 logMAR (one line), while assuming a type 1 error rate of 5%, a 5% loss to follow-up rate, and power of 90% for a subgroup analysis of patients with amblyopic eye acuity of 20/40 to 20/100. With these assumptions, a minimum sample size of 100 patients in this subgroup was planned. Because patients entered the aforementioned spectacle phase first, the actual sample size in the randomized trial exceeded this minimum.
The primary outcome was the 5-week amblyopic eye visual acuity score. The treatment groups were compared with an analysis of covariance model in which the primary outcome was adjusted for baseline acuity. Confounding was evaluated by including covariates of interest in the model, and interaction between baseline factors and treatment group on the 5-week outcome acuity was assessed by including interaction terms in the model. Methods used to analyze the best achieved amblyopic eye acuity during follow-up paralleled the analyses conducted on the 5-week data. For the primary 5-week analysis, there was no imputation for missing data. For the best achieved acuity analysis, patients with incomplete follow-up were excluded. A separate analysis including these patients provided similar results (data not shown). Similar methods were used in the analysis of the data of patients in the secondary cohort.
Treatment group comparisons for binary variables were made with Fisher's exact tests. For the stereoacuity scores, the treatment groups were compared with the exact Wilcoxon rank sum test.
All analyses followed the intention-to-treat principle (i.e., the treatment group data were based on the randomization assignments, not on the actual treatment received or whether the treatment protocol was followed). Within treatment groups, the change in acuity from baseline is reported in lines (0.1 logMAR). Treatment group comparisons are reported as differences in mean visual acuity. All reported P values are two-tailed. Analyses were conducted using SAS version 9.1 (SAS Institute, Cary, NC).
Results
Primary Cohort
Between March 2004 and June 2005, 180 patients with amblyopic eye acuity of 20/40 to 20/400 (mean 0.56 logMAR, approximately 20/80) and an interocular acuity difference of 3 or more lines (mean 5.3 lines) were randomly assigned to the patching group (N=87) or to the control group (N=93). The number of patients enrolled per site at the 46 sites ranged from 1 to 24 (median = 2.5). The average age of the patients at randomization was 5.4 years. The baseline characteristics of the 2 groups were similar (Table 2). The run-in spectacle-wear phase was completed by 155 (86%) of the 180 patients prior to being randomized whereas the other 25 patients skipped the run-in phase because optimal spectacles had been worn for greater than 16 weeks (N=20) or spectacle correction was not needed (N=5).
Table 2.
Baseline Characteristics of Treatment and Control Groups in Primary Cohort
| Patching (N=87) | Control (N=93) | |
|---|---|---|
| Gender: Female n (%) | 37 (43) | 52 (56) |
| Race / Ethnicity n (%) | ||
| White | 70 (80) | 75 (81) |
| African-American | 5 (6) | 6 (6) |
| Hispanic or Latino | 10 (11) | 6 (6) |
| Asian | 0 | 2 (2) |
| More than one race | 1 (1) | 4 (4) |
| Unknown/not reported | 1 (1) | 0 |
| Age at Randomization n (%) | ||
| 3 to <4 years | 11 (13) | 12 (13) |
| 4 to <5 years | 18 (21) | 23 (25) |
| 5 to <6 years | 37 (43) | 33 (35) |
| 6 to <7 years | 16 (18) | 17 (18) |
| 7 to <8 years | 5 (6) | 8 (9) |
| Mean (SD*) years | 5.4 (1.0) | 5.3 (1.1) |
| Prior treatment for amblyopia at enrollment n (%) | ||
| None | 78 (90) | 82 (88) |
| Patching | 6 (7) | 9 (10) |
| Atropine | 0 | 1 (1) |
| Patching and Atropine | 3 (3) | 1 (1) |
| Cause of Amblyopia n (%) | ||
| Strabismus | 21 (24) | 21 (23) |
| Anisometropia | 39 (45) | 45 (48) |
| Strabismus and anisometropia | 27 (31) | 27 (29) |
| Distance Visual Acuity in Amblyopic Eye n (%) | ||
| 20/400 | 1 (1) | 1 (1) |
| 20/320 | 0 | 2 (2) |
| 20/250 | 1 (1) | 4 (4) |
| 20/200 | 1 (1) | 3 (3) |
| 20/160 | 3 (3) | 2 (2) |
| 20/125 | 9 (10) | 6 (6) |
| 20/100 | 8 (9) | 6 (6) |
| 20/80 | 17 (20) | 7 (8) |
| 20/63 | 25 (29) | 25 (27) |
| 20/50 | 7 (8) | 17 (18) |
| 20/40 | 15 (17) | 19 (20) |
| 20/32 | 0 | 1 (1) |
| Mean (SD) logMAR† | 0.56 (0.20) | 0.55 (0.25) |
| Approximate Snellen Equivalent | 20/80+2 | 20/80+2 |
| Distance Visual Acuity in Sound Eye n (%) | ||
| 20/40 | 4 (5) | 3 (3) |
| 20/32 | 11 (13) | 6 (6) |
| 20/25 | 14 (16) | 24 (26) |
| 20/20 | 41 (47) | 34 (37) |
| 20/16 | 17 (20) | 26 (28) |
| Mean (SD) logMAR | 0.04 (0.11) | 0.02 (0.10) |
| Approximate Snellen Equivalent | 20/20-2 | 20/20-1 |
| Interocular Acuity Difference At Randomization | ||
| Mean (SD) lines | 5.3 (1.9) | 5.3 (2.3) |
| Refractive Error in Amblyopic Eye at Enrollment n (%) | ||
| <0 | 0 | 2 (2) |
| 0 to <+1.00D‡ | 3 (3) | 3 (3) |
| +1.00 to <+2.00D | 5 (6) | 3 (3) |
| +2.00 to <+3.00D | 6 (7) | 6 (6) |
| +3.00 to <+4.00D | 7 (8) | 9 (10) |
| >+4.00D | 66 (76) | 70 (75) |
| Mean (SD) spherical equivalent | 5.12 (2.16) | 4.74 (2.11) |
| Refractive Error in Sound Eye at Enrollment n (%) | ||
| <0 | 0 | 1 (1) |
| 0 to <+1.00D | 12 (14) | 13 (14) |
| +1.00 to <+2.00D | 23 (26) | 24 (26) |
| +2.00 to <+3.00D | 21 (24) | 23 (25) |
| +3.00 to <+4.00D | 6 (7) | 10 (11) |
| >+4.00D | 25 (29) | 22 (24) |
| Mean (SD) spherical equivalent | 2.89 (2.13) | 2.57 (1.74) |
| In Spectacle Phase Prior to Trial n (%) | 72 (83) | 83 (89) |
SD = Standard Deviation
logMAR = logarithm of the minimum angle of resolution
D = diopter
Patient Follow-up and Treatment
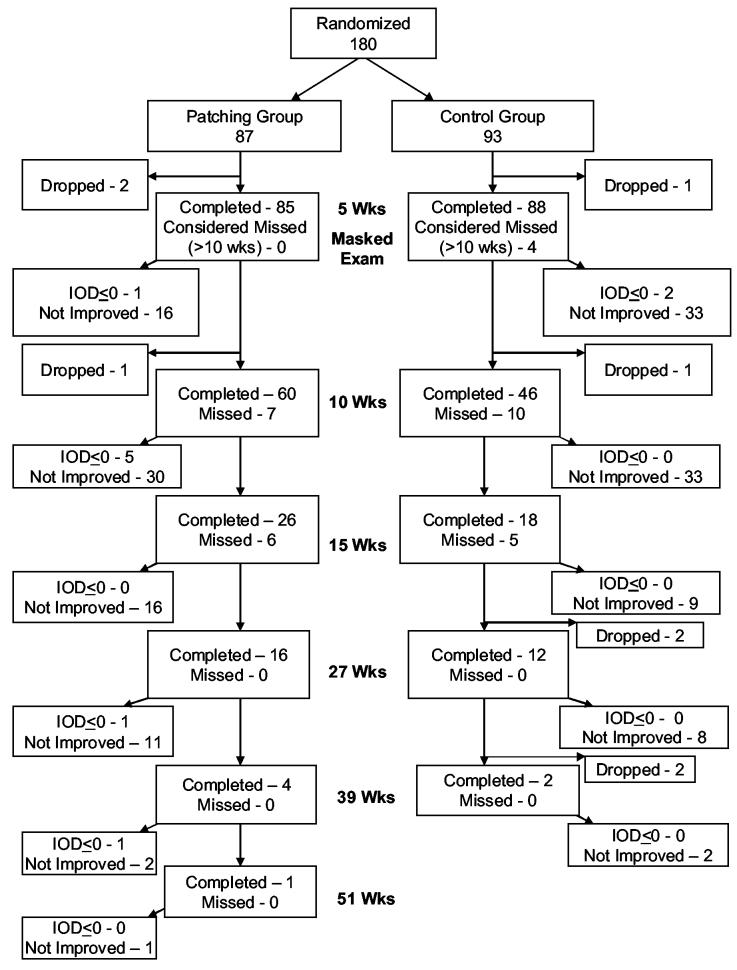
The 5-week outcome examination was completed by 98% of the patients in the patching group and 95% of patients in the control group (Figure 1). The vision tester was masked to treatment group for 97% of these examinations (95% in the patching group and 99% in the control group). During the initial 5 weeks of treatment, patient adherence with patching treatment was judged by the investigator to be excellent (76-100% compliance) in 68%, good (51-75% compliance) in 22%, fair (26-50% compliance) in 7%, and poor (0-25% compliance) in 2%. Two patients could not tolerate adhesive patches and switched to a spectacle-mounted occluder.
Figure 1.
Flowchart showing study completion for patching and control groups – primary cohort. Both patching and control groups wore spectacles if required, and the patching group was prescribed 2 hours of patching per day plus one hour of near activities. Patients continued with these treatments until there was no improvement or the IOD<=0. In the patching group, 83 patients completed the outcome examination within the time window (4 - 6 weeks), 0 were early (3 to <4 weeks), and 2 were late (>6 to 10 weeks). In the control group, 79 patients completed the outcome examination within the time window (4 - 6 weeks), 2 were early (3 to <4 weeks), and 7 were late (>6 to 10 weeks). IOD – Interocular acuity difference
After 5 weeks, 68 patients (80%) in the patching group and 53 patients (60%) in the control group met the criteria to continue in the study (amblyopic eye visual acuity improved at least one line from randomization but was still worse than sound eye acuity). Figure 1 depicts the follow up for all patients.
Visual Acuity in the Amblyopic Eye
At the 5-week primary outcome visit, visual acuity in the amblyopic eye had improved from baseline by an average of 1.1 lines in the patching group and 0.5 lines in the control group (mean difference in visual acuity between groups adjusted for baseline acuity= 0.07 logMAR; 95% CI, 0.02 to 0.12, P=0.006). The magnitude of effect was consistent in subgroups of moderate amblyopia (20/40 to 20/100) and severe amblyopia (20/125 to 20/400) (Table 3).
Table 3.
Visual Acuity in the Amblyopic Eye at the 5-Week Masked Outcome Examination Primary Cohort
| Overall | Baseline Amblyopic Eye Acuity |
|||||
|---|---|---|---|---|---|---|
| 20/40 to 20/100 | 20/125 to 20/400 | |||||
| Patching N=85 | Control N=88 | Patching N=71 | Control N=71 | Patching N=14 | Control N=17 | |
| Lines Change from Baseline n(%) | ||||||
| <=−3 | 2 (2) | 3 (3) | 1 (1) | 2 (3) | 1 (7) | 1 (6) |
| −2 | 2 (2) | 5 (6) | 2 (3) | 5 (7) | 0 | 0 |
| −1 | 5 (6) | 15 (17) | 5 (7) | 12 (17) | 0 | 3 (18) |
| 0 | 20 (24) | 22 (25) | 16 (23) | 17 (24) | 4 (29) | 5 (29) |
| +1 | 18 (21) | 25 (28) | 15 (21) | 20 (28) | 3 (21) | 5 (29) |
| +2 | 26 (31) | 13 (15) | 22 (31) | 12 (17) | 4 (29) | 1 (6) |
| +3 | 8 (9) | 2 (2) | 8 (11) | 1 (1) | 0 | 1 (6) |
| +4 | 2 (2) | 0 | 1 (1) | 0 | 1 (7) | 0 |
| +5 | 2 (2) | 2 (2) | 1 (1) | 2 (3) | 1 (7) | 0 |
| >+5 | 0 | 1 (1) | 0 | 0 | 0 | 1 (6) |
| Mean (SD*) lines change | 1.1 (1.6) | 0.5 (1.7) | 1.1 (1.5) | 0.4 (1.5) | 1.2 (1.9) | 0.6 (2.1) |
| Distribution of Visual Acuity n(%) | ||||||
| <20/125 | 5 (6) | 10 (11) | 2 (3) | 0 | 3 (21) | 10 (59) |
| 20/125 | 4 (5) | 2 (2) | 0 | 0 | 4 (29) | 2 (12) |
| 20/100 | 6 (7) | 9 (10) | 2 (3) | 5 (7) | 4 (29) | 4 (24) |
| 20/80 | 9 (11) | 11 (13) | 8 (11) | 11 (15) | 1 (7) | 0 |
| 20/63 | 13 (15) | 14 (16) | 12 (17) | 13 (18) | 1 (7) | 1 (6) |
| 20/50 | 9 (11) | 14 (16) | 9 (13) | 14 (20) | 0 | 0 |
| 20/40 | 24 (28) | 16 (18) | 23 (32) | 16 (23) | 1 (7) | 0 |
| 20/32 | 11 (13) | 10 (11) | 11 (15) | 10 (14) | 0 | 0 |
| 20/25 | 4 (5) | 1 (1) | 4 (6) | 1 (1) | 0 | 0 |
| 20/20 | 0 | 1 (1) | 0 | 1 (1) | 0 | 0 |
| Mean (SD) logMAR† | 0.44 (0.22) | 0.51 (0.28) | 0.38 (0.17) | 0.41 (0.16) | 0.74 (0.19) | 0.93 (0.26) |
| Approximate Snellen Equivalent | 20/50-2 | 20/63 | 20/50+1 | 20/50 | 20/100-2 | 20/160-1 |
| Difference Between Treatment Groups in Mean logMAR Acuity, Adjusted for Baseline Acuity‡ | 0.07 | 0.06 | 0.08 | |||
| (95% Confidence Interval) | (0.02, 0.12) | (0.01, 0.11) | (−0.09, 0.25) | |||
| Mean (SD) Interocular Difference at Masked Exam (lines) | 4.1 (2.4) | 4.8 (2.9) | 3.4 (1.9) | 3.9 (1.9) | 7.3 (2.3) | 8.6 (3.2) |
SD = Standard Deviation
logMAR = logarithm of the minimum angle of resolution
Adjusted for baseline visual acuity in analysis-of-covariance model. (overall P value = 0.006) A positive difference indicates that the patching group scores were better than control group scores.
2 patients in the patching group and 5 in the control group did not complete the 5-week masked exam
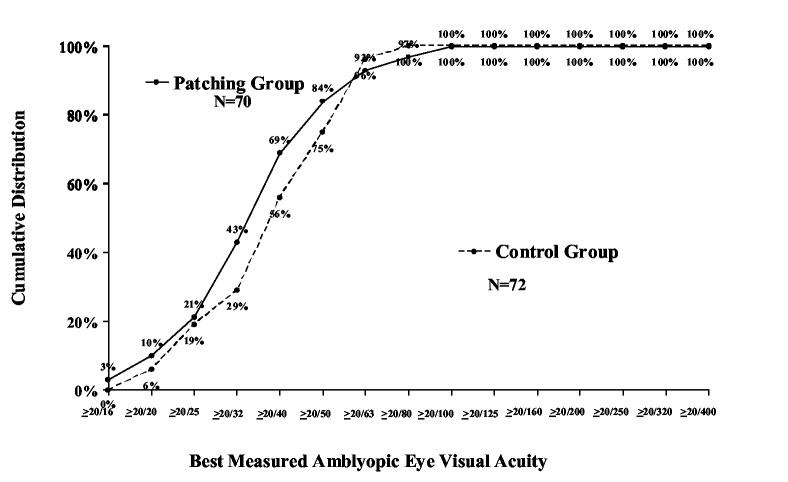
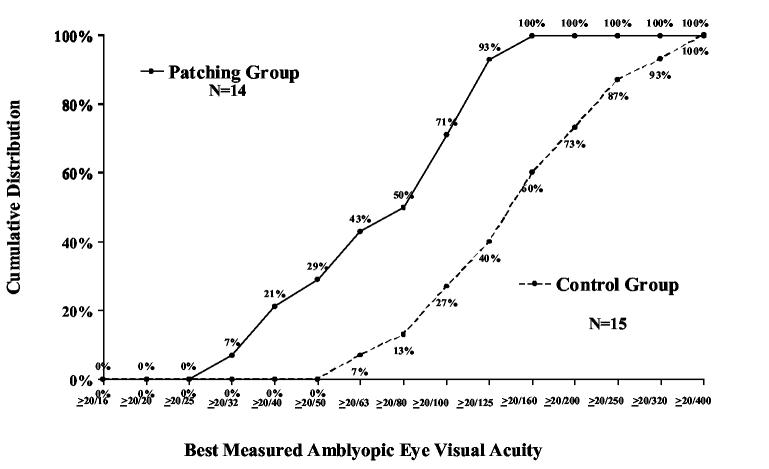
During follow up, the best measured visual acuity obtained at any visit was improved from baseline by an average of 2.2 lines in the patching group and 1.3 lines in the control group (mean difference in visual acuity between groups adjusted for baseline acuity=0.10 logMAR; 95% CI, 0.05 to 0.14, P <0.001, Table 4). Among patients with moderate amblyopia (20/40 to 20/100 at baseline), best measured acuity of 20/25 or better was achieved by 21% in the patching group and 19% in the control group (P=0.84). Among patients with severe amblyopia (20/125 to 20/400 at baseline), best measured acuity of 20/40 or better was achieved by 21% in the patching group and no patients in the control group (P=0.10, Figure 2).
Table 4.
Best Measured Visual Acuity During Follow-up Primary Cohort
| Overall | Baseline Amblyopic Eye Acuity |
|||||
|---|---|---|---|---|---|---|
| 20/40 to 20/100 | 20/125 to 20/400 | |||||
| Patching N=84 | Control N=87 | Patching N=70 | Control N=72 | Patching N=14 | Control N=15 | |
| Improvement from Baseline to Best Measured Acuity in Amblyopic Eye n(%) | ||||||
| 0 lines (no improvement or worsened) | 16 (19) | 34 (39) | 13 (19) | 27 (38) | 3 (21) | 7 (47) |
| 1 line improvement | 16 (19) | 22 (25) | 13 (19) | 18 (25) | 3 (21) | 4 (27) |
| 2 lines improvement | 19 (23) | 15 (17) | 18 (26) | 13 (18) | 1 (7) | 2 (13) |
| 3 lines improvement | 15 (18) | 12 (14) | 14 (20) | 11 (15) | 1 (7) | 1 (7) |
| ≥4 lines improvement | 18 (21) | 4 (5) | 12 (17) | 3 (4) | 6 (43) | 1 (7) |
| Mean (SD*) lines improvement | 2.2 (1.8) | 1.3 (1.4) | 2.1 (1.6) | 1.3 (1.3) | 2.7 (2.3) | 1.2 (1.9) |
| Interocular Difference† at Visit of Best Measured Acuity n(%) | ||||||
| ≥ 6 lines | 13 (15) | 18 (21) | 6 (9) | 6 (8) | 7 (50) | 12 (80) |
| 5 lines | 11 (13) | 17 (20) | 9 (13) | 15 (21) | 2 (14) | 2 (13) |
| 4 lines | 11 (13) | 9 (10) | 9 (13) | 9 (13) | 2 (14) | 0 |
| 3 lines | 14 (17) | 20 (23) | 12 (17) | 20 (28) | 2 (14) | 0 |
| 2 lines | 14 (17) | 13 (15) | 13 (19) | 12 (17) | 1 (7) | 1 (7) |
| 1 line | 14 (17) | 9 (10) | 14 (20) | 9 (13) | 0 | 0 |
| <=0 lines (amblyopic eye same or better than sound eye) | 7 (8) | 1 (1) | 7 (10) | 1 (1) | 0 | 0 |
| Mean (SD) lines | 3.3 (2.3) | 4.2 (2.6) | 2.8 (1.9) | 3.3 (1.6) | 5.9 (2.5) | 8.3 (2.9) |
| Time to Visit of Best Measured Acuity n(%) | ||||||
| Baseline | 16 (19) | 34 (39) | 13 (19) | 27 (38) | 3 (21) | 7 (47) |
| 5 weeks (3 to 7) | 36 (43) | 37 (43) | 30 (43) | 32 (44) | 6 (43) | 5 (33) |
| 10 weeks (8 to 12) | 20 (24) | 10 (11) | 19 (27) | 7 (10) | 1 (7) | 3 (20) |
| 15 weeks (13 to 17) | 5 (6) | 4 (5) | 4 (6) | 4 (6) | 1 (7) | 0 |
| >17 weeks | 7 (8) | 2 (2) | 4 (6) | 2 (3) | 3 (21) | 0 |
| Difference Between Treatment Groups in Mean Best logMAR‡ Acuity, Adjusted for Baseline Acuity§ | 0.10 | 0.07 | 0.20 | |||
| (95% Confidence Interval) | (0.05, 0.14) | (0.02, 0.12) | (0.01, 0.39) | |||
SD = Standard Deviation
Interocular difference evaluated using better of sound eye at baseline or at visit of best measured amblyopic eye acuity
logMAR = logarithm of the minimum angle of resolution
Adjusted for baseline visual acuity in analysis-of-covariance model. (overall P value < 0.001) A positive difference indicates that the patching group scores were better than control group scores.
3 patients in the patching group and 6 in the control group with incomplete follow up are not included
Figure 2.
Cumulative distribution of best measured amblyopic eye visual acuity scores obtained at any study visit according to group assignment at randomization – primary cohort. A. Patients with moderate amblyopia at baseline (20/40-20/100). B. Patients with severe amblyopia at baseline (20/125-20/400).
There was no significant interaction between treatment group and baseline visual acuity (P=0.07), gender (P=0.07), race (P=0.30), age at randomization (P=0.14), prior amblyopia treatment (P=0.80), participation in the spectacle phase of the study (P=0.22), or cause of amblyopia (strabismus > 5 prism diopters or anisometropia/microtropia, P=0.16) on the outcome visual acuity in the amblyopic eye.
Sound Eye Visual Acuity, Ocular Alignment, and Stereoacuity
At the 5-week primary outcome examination, visual acuity in the sound eye decreased from baseline by 2 or more lines in 2 patients (2%) in the patching group and 6 patients (7%) in the control group (P=0.28).
Assessment of distance ocular alignment at the outcome examination found that among 118 patients (55 in the patching group and 63 in control group) with no ocular deviation at baseline, 5 patients in the patching group and 3 patients in the control group were noted to have a small-angle strabismus (1 to 8 prism diopters (Δ) ) and one patient in the control group had a new strabismus measuring greater than 8Δ.
There was no difference between patching and control groups in performance on the Randot Preschool Stereoacuity test at the outcome examination when considering all patients (P = 0.60) or only patients without detectable strabismus (P = 0.76).
Secondary Cohort
The secondary cohort consisted of 63 patients (34 in the patching group and 29 in the control group) with either a 2 line (0.2 logMAR) interocular difference or acuity of 20/32 in the amblyopic eye and 20/16 in the sound eye.
At the 5-week outcome visit, amblyopic eye visual acuity had improved from baseline by an average of 0.8 lines in the patching group and 0.0 lines in the control group (P=0.003), and an interocular visual acuity difference of one line or less was achieved by 21 patients (64%) in the patching group and 5 patients (17%) in the control group (P<0.001, Table 5).
Table 5.
Visual Acuity in the Amblyopic Eye at Baseline and at the 5-Week Masked Outcome Examination for the Secondary Cohort*
| Patching N=33 | Control N=29 | |
|---|---|---|
| Distribution of Visual Acuity in the Amblyopic Eye at Baseline n(%) | ||
| 20/63 | 0 | 1 (3) |
| 20/50 | 2 (6) | 5 (17) |
| 20/40 | 9 (27) | 2 (7) |
| 20/32 | 16 (48) | 19 (66) |
| 20/25 | 6 (18) | 2 (7) |
| Distribution of Visual Acuity in the Amblyopic Eye at Masked Exam n(%) | ||
| 20/80 | 0 | 1 (3) |
| 20/63 | 0 | 2 (7) |
| 20/50 | 0 | 1 (3) |
| 20/40 | 7 (21) | 8 (28) |
| 20/32 | 8 (24) | 11 (38) |
| 20/25 | 12 (36) | 5 (17) |
| 20/20 | 5 (15) | 1 (3) |
| 20/16 | 1 (3) | 0 |
| Lines Change in Visual Acuity from Baseline to Masked Exam† mean (SD‡) in lines | 0.8 (1.0) | 0.0 (1.2) |
| Interocular Difference at Masked Exam (lines) n(%) | ||
| ≥3 | 3 (9) | 12 (41) |
| 2 | 9 (27) | 12 (41) |
| 1 | 14 (42) | 5 (17) |
| ≤0 (amblyopic eye same or better than sound eye) | 7 (21) | 0 |
Secondary cohort included those patients with 2 lines of interocular difference or visual acuity of 20/32 in the amblyopic eye and 20/16 in the sound eye at baseline
P value = 0.003 adjusted for baseline visual acuity in analysis-of-covariance model.
SD = Standard Deviation
1 patient in the patching group did not complete the 5-week exam
The best measured visual acuity for the secondary cohort obtained at any time during follow-up showed an average improvement from baseline of 1.4 lines in the patching group and 0.8 lines in the control group (P=0.006). Acuity of 20/25 or better was achieved by 25 patients (74%) in the patching group and 12 patients (43%) in the control group (P=0.02). Interocular difference at the visit with the best measured amblyopic eye acuity (using baseline sound eye acuity for one case in which sound eye at this visit tested worse than baseline) was one line or less in 23 patients (68%) in the patching group and 8 patients (29%) in the control group (P=0.005).
Discussion
We evaluated the effectiveness of 2 hours of daily patching combined with one hour of near visual activity versus a control group (wearing optical correction if needed) in the treatment of moderate and severe amblyopia in children 3 to 7 years old. All patients who required refractive error correction entered the trial only after having worn optimal spectacles for at least 16 weeks prior to enrollment or after having demonstrated no improvement in visual acuity from a study visit 5 weeks previously. The primary outcome was measured five weeks after randomization, with patients continuing in follow up as long as visual acuity was improving. After five weeks, the patching group had improved by an average of 0.6 lines more than the control group and at the time of study completion by an average of 0.9 lines more than the control group. Similar results were found for both moderate (20/40 to 20/100) and severe (20/125 to 20/400) amblyopia.
No previous study has compared patching with a control group after attempting to first maximize the visual acuity improvement that can be achieved with spectacle correction. In a randomized trial of children with reduced unilateral acuity, Clarke et al. found benefit for spectacles combined with patching compared with no treatment and compared with spectacles alone in children with a unilateral uncorrected acuity deficit of 20/60 to 20/120.11 The objective of Clarke's study was to assess the value of screening and their results are not directly comparable to ours. Since initial visual acuity in Clarke's study was tested without spectacle correction, some of these patients may not have had amblyopia. In addition, initiation of spectacle correction in Clarke's study occurred following randomization, whereas it occurred prior to randomization in our study. Awan et al. conducted a randomized trial of 52 strabismic children with amblyopic eye acuity of 20/40 to 20/160 that included 6 weeks of spectacle correction (if needed) prior to randomization.12 They reported amblyopic eye improvement after 12 weeks of 1.6 lines with no patching, 1.9 lines with 3 hours of prescribed daily patching and 2.3 lines with 6 hours of prescribed daily patching. The substantial treatment effect seen in the no patching group (1.6 lines) indicates that considerable improvement occurred with spectacles alone beyond the pre-randomization 6-week period of refractive correction. In a previous report evaluating the effectiveness of 2 hours of daily patching with one hour of near visual activity in patients with moderate amblyopia (20/40 to 20/80), we reported an average of 1.8 lines of improvement after 5 weeks of treatment 3 compared with 1.1 lines in the current study. However, our previous protocol only required optimal spectacle correction for 4 weeks prior to enrollment and thus some patients likely were still improving with spectacle treatment when patching was instituted.
Even with our more stringent requirements of either 16 weeks of spectacle wear or documentation of stability of acuity (including confirmation with a retest), about half of the patients in the control group improved one or more lines between the baseline and 5-week outcome visits. It is apparent, as noted by others,16-21 that amblyopia can improve with spectacles alone for a considerable length of time. This treatment with spectacles alone has been referred to as “refractive adaptation” by other research groups.21 There are several possible explanations for the continued improvement with spectacles alone after apparent stabilization of acuity. First, five weeks between one visit and the next may be too short of an interval to be certain that acuity is not improving. Second, the ATS visual acuity testing protocol used in our study provides acuity measurements in 0.1 logMAR (one line) steps. If acuity improved at a rate less than one line per five weeks, then it might have appeared to be stable when in fact it was still slowly improving. Third, although we required two acuity tests on the same day to confirm lack of improvement, some children may test below their true acuity on a given day due to lack of effort, fatigue, test-retest variability or other factors, which might give the impression of lack of improvement from the last visit. A final possibility to explain improvement in the control group is a learning effect. However, this seems unlikely since most patients had performed multiple visual acuity tests prior to randomization.
Although the magnitude of improvement with patching was modest, the trial was not designed to determine the maximum effect of patching. In order to minimize the duration of time that treatment (other than spectacle correction) was withheld from patients in the control group, the primary outcome was at 5 weeks, and patients whose amblyopia had not improved from baseline ended participation in the study at that time. Patients who showed improvement did continue in the study until there was no improvement at one visit, but for the reasons indicated above for the control group, many patients likely were discontinued from the study before maximal improvement attainable with patching was achieved.
The secondary cohort consisted of those patients with mild residual amblyopia following spectacle correction. In designing the trial, we decided not to include these patients in the primary analysis, because we thought that they had little room for further improvement with patching. We were surprised to find that a substantial proportion of these patients improved further with patching, with the magnitude of effect being similar to that in the primary cohort. Thus, it is apparent that many amblyopic children with mild residual amblyopia after improvement with spectacles can benefit from occlusion therapy.
In summary, following a period of amblyopia treatment with spectacles, two hours of daily patching combined with one hour of near visual activities modestly improves amblyopia in children 3 to 7 years old. Although not designed to determine the magnitude of benefit from patching, this trial provides conclusive evidence that occlusion of the sound eye improves amblyopia from strabismus, anisometropia or both.
Acknowledgements
Writing Committee: Lead authors: David K. Wallace, M.D.; Allison R. Edwards, M.S.; Susan A. Cotter, O.D.; Roy W. Beck, M.D., Ph.D.; Additional writing committee members (alphabetical): Robert W. Arnold, M.D.; William F. Astle, M.D.; Carmen N. Barnhardt, O.D.; Eileen E. Birch, Ph.D.; Sean P. Donahue, M.D., Ph.D.; Donald F. Everett, M.A.; Joost Felius, Ph.D.; Jonathan M. Holmes, B.M., B.Ch.; Raymond T. Kraker, M.S.P.H; B. Michele Melia, Sc.M.; Michael X. Repka, M.D.; Nicholas A. Sala, D.O.; David I. Silbert, M.D.; Katherine K. Weise, O.D., M.B.A.
Committees and sites that participated in this protocol are acknowledged in the separate spectacle phase publication.13
Footnotes
Supported through a cooperative agreement from the National Eye Institute EY11751
There are no conflicts of interest.
An address for reprints will not be provided.
References
- 1.Ederer F, Krueger DE. Report on the National Eye Institute's Visual Acuity Impairment Survey Pilot Study. Office of Biometry and Epidemiology, National Eye Institute, National Institutes of Health, Public Health Service, Department of Health and Human Services; Washington, DC: 1984. pp. 81–4. [Google Scholar]
- 2.Attebo K, Mitchell P, Cumming R, et al. Prevalence and causes of amblyopia in an adult population. Ophthalmology. 1998;105:154–9. doi: 10.1016/s0161-6420(98)91862-0. [DOI] [PubMed] [Google Scholar]
- 3.Pediatric Eye Disease Investigator Group A randomized trial of patching regimens for treatment of moderate amblyopia in children. Arch Ophthalmol. 2003;121:603–11. doi: 10.1001/archopht.121.5.603. [DOI] [PubMed] [Google Scholar]
- 4.Pediatric Eye Disease Investigator Group A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology. 2003;110:2075–87. doi: 10.1016/j.ophtha.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 5.American Academy of Ophthalmology . Preferred practice pattern: amblyopia. American Academy of Ophthalmology; San Francisco: 2002. pp. 1–25. [Google Scholar]
- 6.American Optometric Association . Care of the patient with amblyopia. Optometric clinical practice guideline. American Optometric Association; St. Louis, MO: 1994. pp. 1–51. [Google Scholar]
- 7.Pediatric Eye Disease Investigator Group A randomized trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol. 2002;120:268–78. doi: 10.1001/archopht.120.3.268. [DOI] [PubMed] [Google Scholar]
- 8.Snowdon SK, Stewart-Brown SL. Preschool Vision Screening. Health Technol Assess. 1997;1(8):1–83. [PubMed] [Google Scholar]
- 9.Lempert P. The effectiveness of patching for amblyopia should be tested with untreated control subjects (letter to editor) Arch Opthalmol. 2004;122:423–4. doi: 10.1001/archopht.122.3.423. [DOI] [PubMed] [Google Scholar]
- 10.Lempert P. The Pediatric Eye Disease Investigator Group report may be too optimistic about efficacy of treatment (letter to editor) Pediatrics. 2004;114:1366. doi: 10.1542/peds.2004-1333. [DOI] [PubMed] [Google Scholar]
- 11.Clarke MP, Wright CM, Hrisos S, et al. Randomised controlled trial of treatment of unilateral visual impairment detected at preschool vision screening. BMJ. 2003;327:1251. doi: 10.1136/bmj.327.7426.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Awan M, Proudlock FA, Gottlob I. A randomized controlled trial of unilateral strabismic and mixed amblyopia using occlusion dose monitors to record compliance. Invest Ophthalmol Vis Sci. 2005;46:1435–9. doi: 10.1167/iovs.04-0971. [DOI] [PubMed] [Google Scholar]
- 13.Pediatric Eye Disease Investigator Group Treatment of anisometropic amblyopia in children with refractive correction. Ophthalmology. 2005 Nov; doi: 10.1016/j.ophtha.2006.01.068. to be submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes JM, Beck RW, Repka MX, et al. The amblyopia treatment study visual acuity testing protocol. Arch Ophthalmol. 2001;119:1345–53. doi: 10.1001/archopht.119.9.1345. [DOI] [PubMed] [Google Scholar]
- 15.Moke PS, Turpin AH, Beck RW, et al. Computerized method of visual acuity testing: adaptation of the amblyopia treatment study visual acuity testing protocol. Am J Ophthamol. 2001;132:903–9. doi: 10.1016/s0002-9394(01)01256-9. [DOI] [PubMed] [Google Scholar]
- 16.Clarke WN, Noel LP. Prognostic indicators for avoiding occlusion therapy in anisometropic amblyopia. American Orthoptic Journal. 1990;40:57–63. [Google Scholar]
- 17.Kivlin JD, Flynn JT. Therapy of anisometropic amblyopia. J Pediatr Ophthalmol Strabismus. 1981;18:47–56. doi: 10.3928/0191-3913-19810901-12. [DOI] [PubMed] [Google Scholar]
- 18.Moseley MJ, Neufeld M, McCarry B, et al. Remediation of refractive amblyopia by optical correction alone. Ophthalmic Physiol Opt. 2002;22:296–9. doi: 10.1046/j.1475-1313.2002.00034.x. [DOI] [PubMed] [Google Scholar]
- 19.Krumholtz I, FitzGerald D. Efficacy of treatment modalities in refractive amblyopia. J Am Optom Assoc. 1999;70:399–404. [PubMed] [Google Scholar]
- 20.Moseley MJ, Fielder AR, Irwin M, et al. Effectiveness of occlusion therapy in ametropic amblyopia: a pilot study. Br J Ophthalmol. 1997;81:956–61. doi: 10.1136/bjo.81.11.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart CE, Moseley MJ, Fielder AR, et al. Refractive adaptation in amblyopia: quantification of effect and implications for practice. Br J Ophthalmol. 2004;88:1552–6. doi: 10.1136/bjo.2004.044214. [DOI] [PMC free article] [PubMed] [Google Scholar]