Abstract
The visual brain consists of many different visual areas, which are functionally specialized to process and perceive different attributes of the visual scene. However, the time taken to process different attributes varies; consequently, we see some attributes before others. It follows that there is a perceptual asynchrony and hierarchy in visual perception. Because perceiving an attribute is tantamount to becoming conscious of it, it follows that we become conscious of different attributes at different times. Visual consciousness is therefore distributed in time. Given that we become conscious of different visual attributes because of activity at different, functionally specialized, areas of the visual brain, it follows that visual consciousness is also distributed in space. Therefore, visual consciousness is not a single unified entity, but consists of many microconsciousnesses.
Keywords: visual brain, functional specialization, microconsciousness, motion system, colour system, reverse hierarchies
The cerebral cortex of the brain, which invests the cerebral hemispheres, has a deceptively simple structure. It is packed with nerve cells and their processes, the axons and the dendrites, which deliver signals to and from them. These cells are arranged according to a basic pattern almost everywhere in the cortex, a pattern that consists of layers of cells stacked upon each other (figure 1). So ubiquitous is this pattern that, apart from a few areas such as the primary visual or motor cortex, which have a more characteristic architecture, it takes experts with many years experience to tell the difference between one part of the brain and another in architectural terms.
Figure 1.
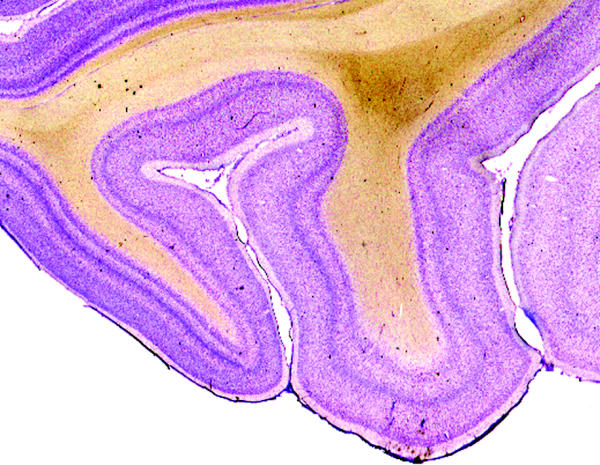
A section taken through the occipital lobe of the macaque monkey brain and stained with the Nissl method to reveal how cells are stacked upon one another to constitute the layered pattern of the cerebral cortex.
The daily preoccupations of a neurobiologist like myself involve trying to understand how this deceptively simple and yet infinitely complex organ functions. Perhaps the first question that arises is whether the essentially uniform anatomical pattern of the cerebral cortex is indicative of a basic operation that it performs everywhere, regardless of the specialization of its areas. Anatomy is powerless to answer this question, but the beginnings of an answer lie very much with anatomical methods, especially with learning how cells in one part of the cerebral cortex are connected with those in other parts and, indeed, with the rest of the brain. Such an indispensable study is usually only a prelude to other studies, of the physiology of cells in different brain areas, of what determines how they respond, the pharmacological and biophysical bases of their functions, and of the physiological relationship of single cell activity to perception. These are concerns that are wholly remote from the kind of question that taxpayers who subsidise this research would want to ask. They would probably want to know what kind of brain organization results in a Newton or a Michelangelo, why some are more intelligent or more mathematically or musically gifted than others, what dictates movements, actions, motives and desires. They would want to learn something about the neural basis of creativity as well as those atavistic impulses of love and compassion, but also of hatred and envy and greed, in the service of which mankind has achieved so much but also destroyed so much. Above all, they might want to know what consciousness, that entity that none can define adequately but all know exists, is and what constitutes its neural basis. This curiosity and disinterested interest of the layman was poetically and movingly summarized for generations of women and men by the genius of William Shakespeare, when he wrote in Hamlet:
What a piece of work is a man! How noble in reason! how infinite in faculty! in form, in moving, how express and admirable! in action how like an angel! in apprehension how like a god! the beauty of the world! the paragon of animals! And yet, to me, what is this quintessence of dust?(Hamlet, Act 2, Scene 2)
The Shakespearean question is a scientific question, but a scientific question that cannot be readily and properly addressed by today's scientific methods. However, we find that even when we study a relatively simple system such as the visual one, the question of consciousness—the quintessence of humans, which is commonly considered to be their defining characteristic—cannot be avoided. For the function of the visual brain, and indeed of much of the rest of the brain, is the acquisition of knowledge. A study of colour vision, one of the visual attributes to which I have given much emphasis, and which now occupies a central position in studies of the visual brain, aptly demonstrates this. Indeed, it is the study of colour vision that convinced me that, far from being a mere chronicler of external events in the visual world, the brain is actually a participant, along with the physical reality, in constructing that world (Zeki 1984). However, the brain can only construct the visual world from the ever-changing information that is available to it, and thus obtain knowledge of that world in the conscious state. Knowledge cannot be acquired in any significant way save in the conscious state, hence the importance of incorporating consciousness into one's studies of the visual brain.
When we thus define a key function of the visual brain, we are immediately led into a deeply philosophical world. For the problem of knowledge, of how we acquire it and of how sure we are of what we know, has preoccupied generations of philosophers since the time of Plato. This same problem preoccupies the visual neurobiologist today, even if this is not always explicitly acknowledged. In a sense, then, to study the neurobiology of the visual cortex is to pursue an age-old philosophical problem with new means. In this article, I aim to show that far-reaching conclusions for understanding the organization and functioning of the visual brain for acquiring knowledge, including conclusions about conscious experience, follow logically from relatively simple anatomical studies of the way in which it is wired. Indeed, there is a logical thread that leads ineluctably from the first anatomical studies of the organization of connections in the visual cortex to the organization of visual consciousness. Gradually, the conclusions drawn from one set of experiments and then the next lead us to a view of visual consciousness and perhaps of consciousness in general.
1. Functional specialization: the organizing principle of the visual brain
(a) The multiple visual areas of the primate brain
In approaching so lofty a problem, there are significant advantages in opting to study a relatively simple system such as the visual one, at least initially. A visual stimulus can be specified with more precision than auditory, olfactory or somatosensory stimuli; its precise position, luminous intensity, colour, shape and distance can be quantified very accurately. In trying to unravel the organization of the visual brain, and to understand how it obtains knowledge about the visual world, one can begin by asking how its cells respond to these different visual attributes, each one of which contributes to the brain's knowledge of the external world. This is far from a trivial task, although it may not have seemed so when Sir Gordon Holmes (1945) gave his Ferrier Lecture, where he emphasized a now outmoded view of the visual brain as consisting of a single visual area. Through the brilliant work that he and his two predecessors, Salomon Henschen in Sweden and Tatsuji Inouye in Japan, had undertaken, the consensus until the 1960s was that this was indeed so, the single visual area being usually referred to as the visuosensory cortex, or the calcarine cortex, or the ‘cortical retina’. More recently, it has become common to call it area V1, and I will use the latter term here. Writing some halfway through the twentieth century, Monbrun (1939) could state with authority that, ‘At present, all authors have rallied to the theory of a single [visual] cortical centre’.
V1 receives the major input from the retina through a subcortical centre known as the lateral geniculate nucleus (LGN; figure 2). Adjacent points on the retina connect with adjacent points in V1, thus creating a map of the retina (and therefore the visual field) in it. Damage to V1 leads to blindness, the extent and position of the blindness being in direct relation to the extent and position of the lesion in V1. It is therefore not surprising that V1 should have been considered and described by Henschen and others as the ‘cortical retina’, to which an image of the world, impressed on the retina, is relayed, thus enabling vision.
Figure 2.
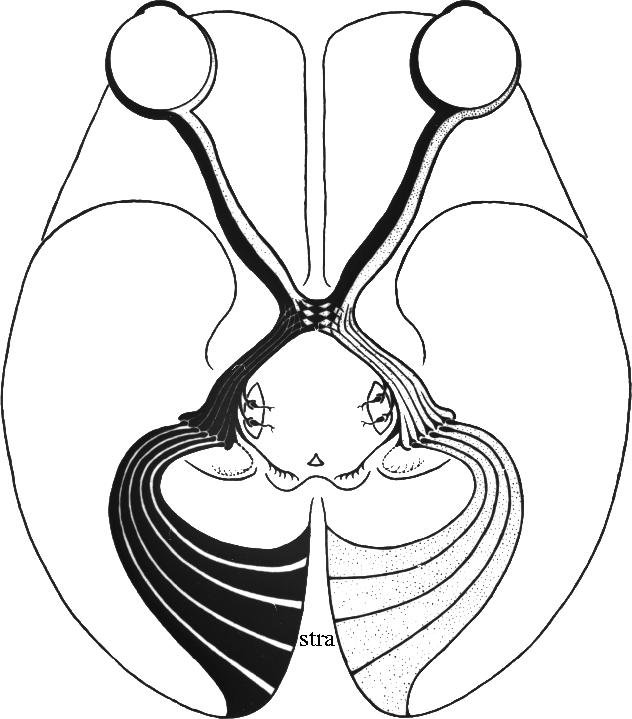
The projection from the retinas of the eyes to the striate cortex (stra; also known as area 17, primary visual cortex or V1). V1 is surrounded by a large expanse of cortex which was known as ‘association cortex’ but has been found to contain multiple visual areas. Reproduced from Polyak (1957).
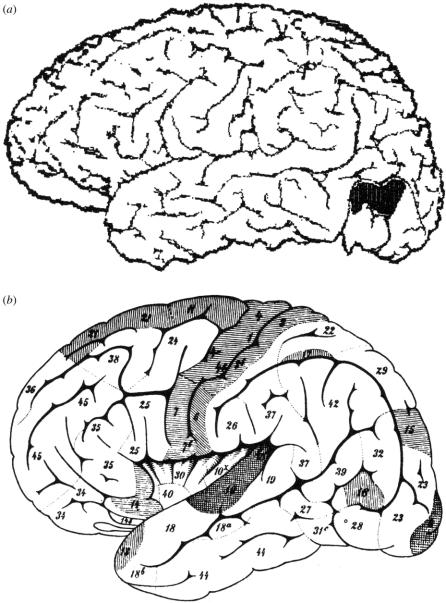
V1 is surrounded by a large expanse of cortex that was for a long time known as ‘association cortex’ (figure 2). This cortex has an anatomical architecture that is distinct from the architecture of V1. Flechsig (1901, 1905) believed from his developmental anatomical studies that the cerebral cortex could be subdivided into two broad divisions. The primary areas, among which he numbered V1, have a mature anatomical appearance at birth and are separated from each other by association cortex. The latter matures gradually after birth, as if its maturation depended upon the acquisition of experience. Flechsig's (1905) reading of this evidence had more profound implications, as he explained in an article that was somewhat grandly entitled Gehirnphysiologie und Willenstheorien. He came to believe that what he had designated as ‘association’ cortex had cognitive and ‘psychic functions’ (Cogitationszentren). Soon, the term association began to acquire its implied, rather than strictly anatomical, meaning more literally and explicitly. It came to mean the association of visual signals with one another or with other sensory signals derived from different cortical areas. The association cortex surrounding V1 became the visual ‘psychic centre’ (Bolton 1900), and was long popularly believed to be ‘constituted for the final elaboration and interpretation of these [visual] sensations’ (Campbell 1905).
The ‘association’ cortex extends to parietal and temporal cortex, with both of which it has uncertain cytoarchitectonic boundaries (figure 3). Given its sheer size, many thought it plausible and even probable that it may contain further areas, without necessarily supposing that these further areas are purely visual in function. But if so, how many areas could there be in this ‘association’ cortex? Campbell, one of the founders of cytoarchitectonic studies of the cerebral cortex, gave an answer that the Delphic Oracle would have approved of. He wrote that it contains ‘one or more areas’ (Campbell 1905). But however many areas it may contain, their function was presumed to be that of ‘associating’ signals derived from different sources. This view was prevalent well into the mid twentieth century. An example can be found in the work of Clare & Bishop (1954), who had tried to characterize the properties of association cortex in the cat. They considered the cortex that they were characterizing to be association cortex, although no associational activity was studied there. Instead, the area was ‘inferred to comprise an association area relating optic and acoustic activity’ because ‘it is usually taken for granted that impulses are propagated from an active projection area of cortex, for instance a sensory projection area such as primary optic cortex, to surrounding association cortex’ (Clare & Bishop 1954).
Figure 3.
The primordial areas (shaded) and the ‘association’ areas (white) of the cerebral cortex, as charted by Paul Flechsig. (a) A medial view; (b) a lateral view. The occipital lobe, situated at the right, has uncertain geographical boundaries with the temporal and parietal areas. Reproduced from Flechsig (1920).
The notion that areas located in ‘association’ cortex, assuming them to exist, may be purely visual in function was discounted until the 1960s, mainly because it seemed to call into question, either explicitly or implicitly, the doctrine that V1 was the only visual area in the brain, a supposition that was seemingly based on hard anatomical and pathological evidence (for a more extensive review, see Zeki 1993a,b). In the 1960s, the publication of a paper by Hubel & Wiesel (1965) showed that some areas of the visual association cortex in the cat could well be purely visual. They had studied cells in two of these areas, V2 and V3, and were able to excite them visually, without the use of other non-visual inputs. This led them to the conclusion that these two visual association areas were in fact associating visual signals with one another, in the process enlarging the receptive fields of cells and endowing them with more complex properties. The cells of areas in visual association cortex were, according to these studies, analysing the same information as antecedent cells, but at a more complex level. This was consistent with Hubel and Wiesel's more general view (derived principally from their detailed studies of the physiology of orientation-selective cells in V1) that the overall strategy used by the brain to analyse the visual environment is a hierarchical one. The hierarchical doctrine supposed that successive groups of cells analyse the same features of the visual environment as the antecedent cells from which they receive their input, but that they do so at a more complex and sophisticated level.
In the late 1960s and early 1970s, studies in the macaque (Cragg 1969; Zeki 1969, 1971a) and in the owl monkey (Allman & Kaas 1971) confirmed and enlarged the notion that there are purely visual areas outside V1. With this came a radical revision of how the visual brain is organized to analyse the visual world (Zeki 1978). For the new evidence showed that the visual areas of association cortex do not necessarily analyse the same features at an ever-increasing level of complexity. Rather, they seemed to be specialized to process different attributes of the visual scene, not the same ones at increasing levels of complexity (Zeki 1974, 1978). With this discovery, and with the incorporation of new studies, came the more general conclusion that the visual brain does not chronicle passively external events, but rather constructs the visual world from such information as reaches it (Zeki 1984). The latter does not represent as radical an innovation as may at first seem. It only appears radical when neurobiologists forget, as is often the case, the earlier, general view of Immanuel Kant and his successor, Arthur Schopenhauer, that to understand our knowledge of the external world, we must enquire not only into the nature of the physical world but also into the contribution that the mind (in our case the brain) makes, and the limitations that it imposes, upon the acquisition of such knowledge. We can therefore never know the thing in itself (das Ding ans sich) because our knowledge of it is obtained through the medium of the mind (brain).
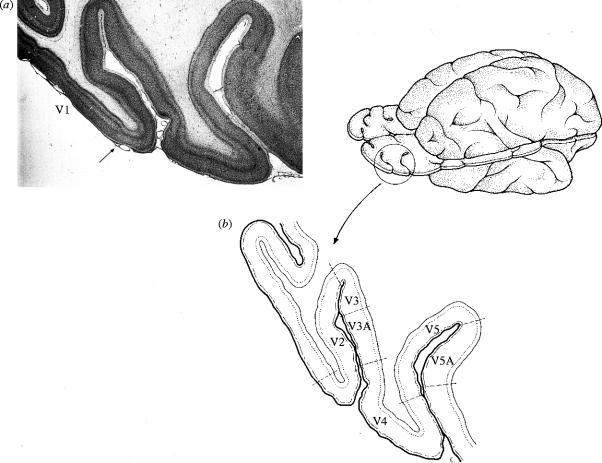
The evidence for a functional specialization of the visual brain was naturally obtained from functional studies. However, it is perhaps both important and pleasurable to emphasize that one could have concluded that this was so from observing the results of anatomical studies alone. In the macaque monkey, such studies had shown that V1 sends topographically organized outputs to two distinct areas (V2 and V3) within the architecturally uniform association cortex surrounding it, thus remapping the visual fields independently within them (Cragg 1969; Zeki 1969); it also sends a much less topographically organized output to another area, V5 (Zeki 1971b), which consequently has a much less precise map of the visual field in topographical terms (figure 4). Physiological mapping experiments, undertaken to map the manner in which the visual field is represented in the cortex surrounding V1 in owl monkey (Allman & Kaas 1971), showed that there are areas in association cortex of that species as well, with a topographic organization almost identical to what anatomical studies in the macaque monkey had predicted. These studies, together with the antecedent ones mentioned above, thus established the general principle of the multiplicity of visual areas in the cortex surrounding area V1. Since then, many more areas have been discovered in the cortex surrounding V1 in the primate, using different techniques. As with the era of ‘feverish map making’ (Sholl 1956) that the development of the cytoarchitectonic method ushered into cortical studies, so the general principle of multiplicity of areas acted as an inducement to the discovery of further areas, which some pursued with perhaps a little too much enthusiasm. Consequently, while some of the visual areas discovered since then are genuine, others are rather improbable (Zeki 2003a). However, every new genuine area charted has simply served to reinforce the general principle of the multiplicity of visual areas.
Figure 4.
(a) The cytoarchitecture of the striate cortex (area V1) and the cortex lying in front of it, the prestriate cortex (arrow marks transition), shown in this horizontal section taken through the occipital lobe of the macaque monkey brain. (b) The visual areas of the prestriate cortex in the macaque monkey shown at the level of the section in (a).
This relatively simple anatomical evidence told us more than that. Given that four of the visual areas at least (V2, V3, V3A and V4) reside in an area of uniform cytoarchitecture (area 18 of Brodmann; figure 4), it follows that the cytoarchitectectonic evidence cannot be a good guide to the number of areas that may exist within a single cytoarchitectonic field. This is not to say that architectural differences in general are not necessarily a good guide to functional subdivisions, but only that cytoarchitectural differences are not the best guide. In general, it seems that although architectural differences between cortical areas are a good guide to functional differentiation, as Vogt & Vogt (1919) emphasized, the absence of such differences using one or two methods only is not a safe guide that there is no further functional differentiation within cortex that is designated as being architecturally uniform. Over the past two decades, more novel architectonic methods have revealed striking architectural patterns that define functional compartments even within individual areas of the cerebral cortex (see below).
(b) A repetitive function of the visual cortex
There is something perhaps a little disturbing about this functional specialization, when viewed against the essentially uniform cytoarchitecture of the cerebral cortex and the visual areas of the brain in particular. As emphasized earlier, apart from V1 with its distinctive cytoarchitecture, there is little to distinguish V2, V3, V3A and V4, and even V5, from one another on the basis of cytoarchietctonics. Other architectonic methods however do differentiate these areas. Myeloarchitecture can set V5 apart fairly accurately, because of its heavy myelination (Jen & Zeki 1984); metabolic cytochrome oxidase (CO) architecture can set V2 apart because of its characteristic stripy appearance (Hubel & Livingstone 1985; Shipp & Zeki 1985). Although these methods show that cytoarchitecture is an imperfect guide to the organization of the cerebral cortex, they do not resolve a fundamental question—namely, why the cerebral cortex should have so uniform an architecture and what general property this could be indicative of. Answers are not easy to come by but I have tried to piece one together, in the context of the brain as a knowledge-acquiring system, by studying the results of physiological experiments from many different laboratories. From all these studies, I suggest that one such function is abstraction (Zeki 2001). Each of the visual areas contributes to our knowledge of the world according to its specialization. One of the first characteristics of an efficient knowledge-acquiring system is the capacity to abstract. By ‘abstraction’, I mean an emphasis on the general at the expense of the particular. This suggestion is not entirely speculative; there is much evidence in favour of it. We have all tended to ignore this general function because, naturally enough, we have tended to concentrate more on the specificities of the cells. But consider a directionally selective cell in V5, responding to motion towards 12 o'clock. It will do so regardless of the colour of the stimulus, its contrast and often its shape. It is essentially abstracting for the direction of motion, without being especially concerned with what it is that is moving in its preferred direction. Alternatively, take orientation-selective cells in the interblobs of V1 or in V3. The great majority will respond to the appropriate line, no matter how it is generated; they will therefore respond to a white line against a dark background or the reverse and will also respond to a coloured line that is equiluminant with its background (Kruger & Gouras 1980). They are abstracting for orientation, without being concerned with what it is that is appropriately oriented. The same is true for other, even non-visual areas. A cell in somatosensory cortex that responds to light touch is not especially concerned with the precise stimulus, but simply that a light touch should be produced in the appropriate place on the body surface. There is thus little doubt that cells in different parts of the brain do abstract the attribute for which they are specialized. Whether it is justified to see in this the cause of the cytoarchitectonic uniformity in the brain is quite another matter. The link between the two is not especially compelling, but there is no doubt that the uniformity itself impels us to ask questions about what uniform functions are performed by these different cerebral areas. Abstraction is one possibility and there may be many others.
(c) Parallel connections and parallelism in the primate brain
Another general and important principle emerged from these anatomical studies, and from the observation that not only V1 but other prestriate visual areas as well have multiple, parallel rather than serial, outputs to further cortical areas. This anatomical demonstration established the general principle of parallelism in cortical connections (Zeki 1976), not only in monkeys but also in humans (Zeki 1990a; Merigan et al. 1997). Indeed, it has since been shown that all cortical areas, be they visual or not, have multiple outputs, and that there is no cortical area that is recipient only. Each cortical area sends outputs and receives them. There is therefore no terminal station in the cerebral cortex, at least in anatomical terms (Zeki 1993a), an important observation when one comes to consider the nature of consciousness.
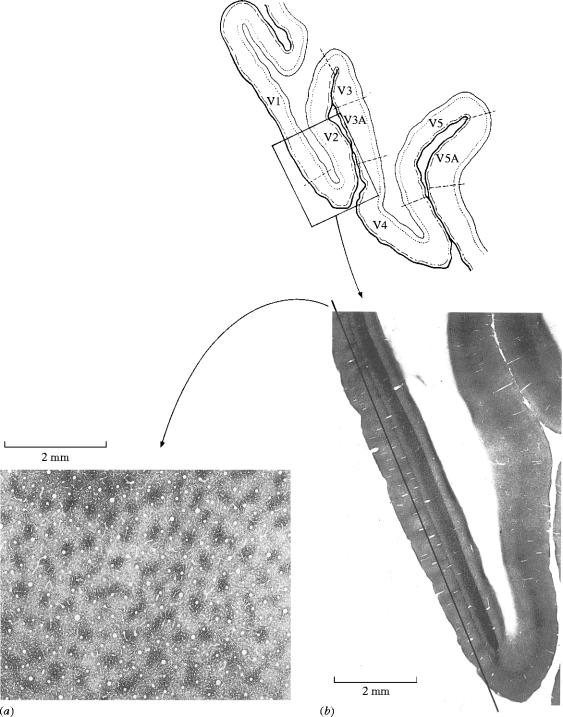
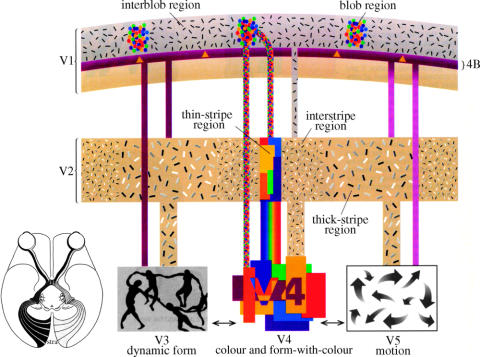
The observation that each cortical area has multiple parallel outputs has important implications, for it substantially increases the magnitude of the task facing the neurobiologist. However, parallelism also has great predictive value. The first prediction made from observing the parallel outputs from V1 is that the latter must be a segregator, pigeon-holing different signals and parcelling them out selectively to different visual areas for further processing (Zeki 1976). In other words, the functional specialization that is a hallmark of the visual brain must also be reflected in V1, even in spite of its uniform cytoarchitecture. At the time that this prediction was made, V1 was generally regarded to be a homogeneous area, at least judged from its uniform cytoarchitecture, which was thought to reflect its uniform functional architecture as well (Hubel & Wiesel 1977). However, this apparent cytoarchitectonic uniformity within a single visual area, V1, has turned out to be deceptive; it conceals several subdivisions, which are more adequately revealed by using other architectural methods, in this instance, by staining the cortex of V1 for the metabolic enzyme cytochrome oxidase (CO), a technique first used in the cat by (Wong-Riley 1979). Its use in the monkey has shown the cortex of V1 to have a repetitive pattern of blobs of high cytochrome oxidase content, especially evident in layers 2 and 3 (Horton 1984; Horton & Hedley-Whyte 1984; Livingstone & Hubel 1984; figure 5). These blobs are separated from one another by zones of lower metabolic activity, which stain less intensely for CO. Combining single cell physiological recordings with anatomical studies of the distribution of CO, Livingstone & Hubel (1984) found that wavelength-selective cells (those that respond to some wavelengths and not to others) are confined to the territory of CO rich compartments (blobs) within layers 2 and 3. By contrast, cells that are orientation selective and indifferent to the wavelength of the stimulus are preferentially distributed in the regions between the blobs, the ‘interblobs’. This functional segregation of cells into distinct, anatomically identifiable, compartments is also evident in area V2, which itself connects with the same visual areas of association cortex as V1 (DeYoe & Van Essen 1985; Hubel & Livingstone 1985; Shipp & Zeki 1985). In V2, cells that are wavelength selective are concentrated within the thin stripes, directionally selective cells are found predominantly within the thick stripes and orientation-selective cells distributed within both thick stripes and interstripes (figure 6).
Figure 5.
The cytochrome oxidase architecture of area V1 (a) is characterized by a set of darkly staining ‘blobs’, which are separated from each other by more lightly staining ‘interblob’ regions. This architecture is best revealed when a section that is parallel to the surface is taken through area V1 (b) and stained for the metabolic enzyme. The tracing above shows the region of the occipital lobe from which the section in (b) was taken. (From Zeki 1993a.)
Figure 6.
Area V2 of macaque monkey prestriate cortex surrounds area V1 and most of it lies buried within sulci. It is best revealed when the back of the brain is opened up. When a section through V2 is taken in the plane of the paper and stained for the metabolic enzyme cytochrome oxidase, the characteristic pattern of thick and thin stripes, separated by lightly staining interstripes, becomes evident. K=thick stripe; N=thin stripe; I=interstripe.
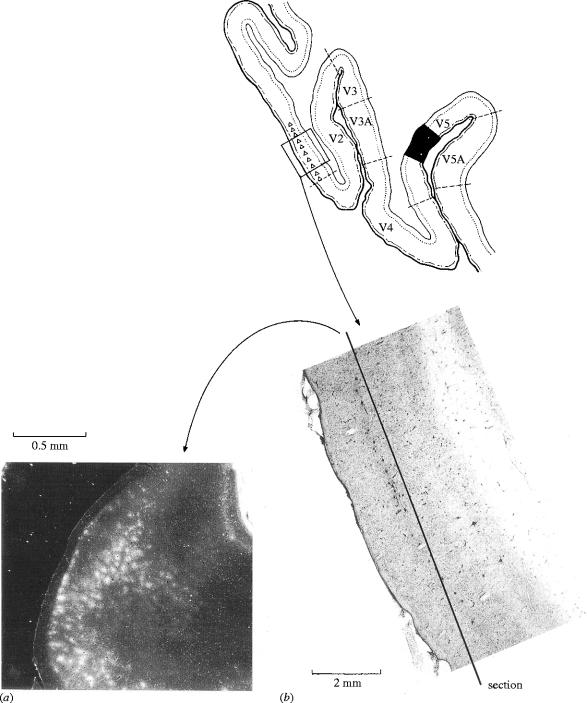
This compelling correlation between the anatomical picture and the functional segregation of cells within V1 has been disputed on the basis of indifferent and indeed unconvincing evidence. In particular, Lennie et al. (1990) and Leventhal et al. (1995) have supposed from their studies that there is no segregation within V1 of the kind demonstrated by Livingstone & Hubel (1984). Unfortunately, there is not a single anatomical reconstruction in the two works cited above, which diminishes their status when compared with the detailed combined anatomical and physiological evidence of Livingstone & Hubel (1984). In any case, the above is not the only evidence in favour of the segregatory function of V1. Lund et al. (1975) among others have shown that the output from area V1 to area V5 is restricted to two layers of the former area, layer 4B and upper layer 6. Hence, signals destined for V5 are not distributed throughout V1, but are restricted to certain layers within it, another strong sign of the segregatory role of V1. Even within these two layers, not every cell projects to V5. Rather, cells that project to V5 are separated from one another by cells that project elsewhere (Shipp & Zeki 1989a), another powerful testament to the segregatory role of V1 (figure 7).
Figure 7.
The patches of ‘direction selective’ cells in layer 4B of V1 projecting to area V5 seen in a section cut parallel to the cortical surface of V1 (a) and one perpendicular to it (b). This pattern is revealed when V5 is injected with the anatomical label horseradish peroxidase (in black on the tracing above). The label is transported retrogradely to V1, where it is seen within the projecting cells. (From Zeki 1993a.)
In summary, the present anatomical evidence strongly supports the view that, consistent with the principles of functional specialization, V1 acts as a segregator, which pigeon-holes different visual signals into different compartments and distributes the segregated signals in a specific way to different, specialized areas of the prestriate cortex. One can derive a general rule from this—namely, that all areas that have multiple parallel outputs (i.e. all cortical areas studied to date) have a segregatory function (Zeki & Shipp 1988). Whether signals are sent along these multiple parallel pathways in an indifferent way or whether recipient areas are only informed of what an area has processed on a ‘need to know’ basis remains a highly interesting but unanswered question.
Parallelism and computational neurobiology
Throughout the 1970s and the 1980s computational neurobiologists convinced themselves, as well as many others, that the key to understanding how so complex an organ as the brain functions lies in a computational approach, which is to say their approach. It is therefore a somewhat puzzling fact that computational neurobiologists, who are now so wedded to the idea of parallelism, should have been so late in realizing the power of parallel systems and thus understanding a key feature of the brain. It is even more surprising that the basic idea of parallelism in the brain should have come first from anatomists, traditionally regarded as the most boring of neurobiologists, and one to whom the computational neurobiologists, in what is regarded as their landmark book, Parallel Distributed Processing, make no reference. Yet one looks in vain in the pre-1975 literature of computational neurobiology for a clear, explicit, statement of the principle of parallelism. Even after the anatomical demonstration of parallel connections in the brain and the explicit use of the term parallelism (Zeki 1976), computational neurobiologists do not seem to have grasped its importance. David Marr's 1980 book, Vision, rightly prized by all, including anatomists, for its perceptive discussion of the problems confronting the visual brain and what solutions it could potentially bring to them, makes no mention of parallelism or of the multiplicity of visual areas even though these were demonstrated long before he published his book. I am not really competent to ask why this should have been so, but only to record the fact. Perhaps, as Minsky and Papert have argued in their 1992 book, Perceptrons, this may have been a result of the prevalence at the time of serial rather than parallel computers, and possibly as well (according to them) to the relative ignorance among computational neurobiologists of the precise characteristics and capabilities of parallel computers. Whatever the reasons, it perhaps shows that there is much insight to be derived from pedestrian anatomical studies.
(d) Functional specialization in the prestriate visual cortex
The notion of a functional specialization in the visual brain was put forward only after recordings from the newly defined visual areas of association (prestriate) cortex had been made (Zeki 1974, 1978) and was reinforced by recordings from the specialized compartments of V1 and V2 (Livingstone & Hubel 1988). However, a straightforward reading of the anatomical evidence derived from a study of the connections of monkey V1, without preconceptions about sensory and psychic functions and about a single visual area, V1, could also have led to the same conclusions, if one had been prescient enough or had thought about the evidence and its implications carefully enough. The anatomical results imposed an ineluctable logic: since it would be difficult to conceive of V1 as sending out the same signals in the distinct and parallel pathways emanating from it and terminating in the different visual areas of what was known as association cortex, it follows that V1 must be sending out different signals to these different visual areas. From this it follows that these different visual areas in the association cortex must be specialized to receive and process different attributes of the visual scene. Even had their function been that of merely associating incoming visual signals with previous ones, or relating present visual signals to past ones during the process of the ‘final elaboration and interpretation of these [visual] sensations’ (Campbell 1905), the role of these different visual areas would have been expected to be different from this anatomical evidence. It is a pity that we do not conduct more thought experiments!
As it is, early recording experiments showed that the brain uses the sort of strategy that one should have predicted from the anatomical picture given above, and therefore a strategy that is a radically different from the hierarchical one that had previously dominated our thinking about the functioning of the visual brain. Right from the start, it became obvious that the strategy that the brain uses is one of specialization, with different attributes of the visual scene being processed in different, and specialized, areas of the association cortex. The first prestriate area recorded from (Dubner & Zeki 1971; Zeki 1974) was located in the posterior bank of the superior temporal sulcus, and I later named it V5. It is an area that had previously been identified as one that receives a heavily myelinated input from V1 (Cragg 1969; Zeki 1969). Moreover, the projection from V1 to V5 is highly convergent (Zeki 1971b), leading one to suppose that its cells would have larger receptive fields than two other areas of the prestriate cortex, V2 and V3, which receive a more topical projection from V1 (Cragg 1969; Zeki 1969). Note that, if one were to restrict one's study of the physiology of V5 to coarse mapping only, determining solely the receptive field sizes of clusters of cells, without characterizing their response properties, one could well come to the conclusion that the brain does indeed employ a hierarchical strategy to analyse the visual world, with each set of cells reanalysing what had been analysed by antecedent cells, but at a higher level of complexity. This is because one of the suppositions of the hierarchical doctrine is that the receptive fields of cells become larger as their properties become more complex, thus enabling them to sample larger parts of the visual field (Hubel & Wiesel 1965). The physiological evidence instead gave a different picture; it showed that all cells in V5 are specifically responsive to motion and that the vast majority is directionally selective, responding to motion of a visual stimulus in one direction within their receptive fields and not in the opposite, null, direction. Moreover, the cells appear to be organized with a certain regularity in respect of their directional preferences, the shift in directional preferences encountered in long, oblique penetrations through the cortex being gradual and systematic rather than abrupt, leading to the suggestion that there is a columnar organization for directional preferences in V5 (Zeki 1974; Albright 1984) (figure 8).
Figure 8.
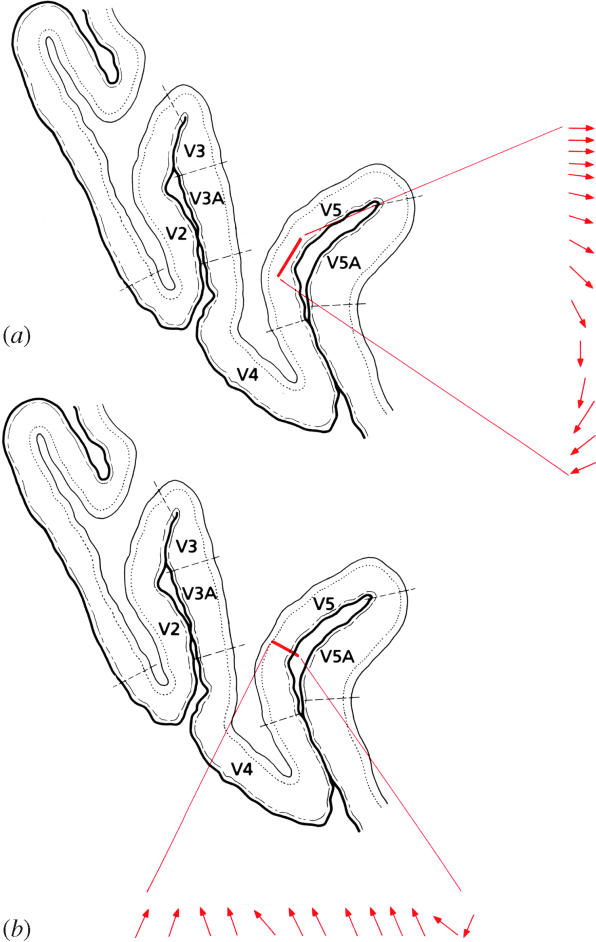
(a) The directional preferences of successive cells, separated from each other by small distances in a direction parallel to the cortical surface, in area V5. Note the orderly change in the successive directions of motion. (b) The directional preferences of successive cells lying perpendicular to the cortical surface of area V5. (From Zeki 1993a.)
It is perhaps not often realized that a detailed study of the physiology of V5 alone entitles one to state that there is a functional specialization in the visual brain even without recourse to further experimentation, but through a thought experiment alone. That statement would be true even if other experiments were to reveal that this is the only specialization in the visual brain. The monkey is an animal with good colour vision and V5 is a visual area in the sense that (a) it receives a strong projection from primary visual cortex and (b) all its cells are visually excitable. Yet, crucially, none of its cells is concerned with colour. This is not to say that the cells of V5 are incapable of responding to a coloured stimulus moving against a background of a different colour, even if the two are made isoluminant, that is differing in colour alone while being equally luminous (Saito et al. 1989; ffytche et al. 1995; Wandell et al. 1999). It means only that the cells of V5 are indifferent to the colour of the stimulus; they are capable of responding as well to a blue stimulus against a yellow background or a red stimulus against a green one. They are, in essence, colour blind. However, since the monkey has good colour vision, and since the cells of area V5 are indifferent to colour, it follows that colour must be processed in another area. From this it follows that there must be a functional specialization in the primate visual brain.
I note in passing that this interpretation of these results—that there is a functional specialization in the visual brain and that different visual areas of the association cortex undertake different tasks, not the same ones at more complex levels—is quite different from the interpretation given to more or less similar results obtained earlier by Hubel & Wiesel (1969). They had explored physiologically the Clare–Bishop area in the cat, an area lying in association cortex, and one whose properties bear a strong resemblance to those of monkey V5. They found that most of its cells were not only orientation selective but also directionally selective. Because of the impoverished colour vision of the cat, they had not studied colour selectivity there. Even in spite of the heavy presence of directional selectivity there, adherence to the hierarchical doctrine had puzzled them as to what the function of this area could be. They thought of it as executing ‘the same processes’ as earlier areas, ‘but with different degrees of refinement’, leaving them ‘…with the puzzling prospect of an area for which we can…assign no obvious function’ (Hubel & Wiesel 1969).
The picture that one derives from studying the functional properties of V5 is considerably different from that of V4, another area located in prestriate cortex. Because of the somewhat complex gyral configuration of the macaque monkey brain, the geography of V4 itself is complex (figure 9). Part of it lies in the anterior bank of the lunate sulcus and extends onto the prelunate gyrus. This part, V4, connects systematically with another area, V4A, lying just anterior to it. The two areas together constitute the V4 complex and I recorded from colour cells in both divisions, hence the term V4 complex (Zeki 1977). When traced ventrally, V4A extends into the inferior convolution of the temporal lobe. Some have called this ventral extension of V4A temporo-occipital (TEO), without seemingly realizing that it is in fact the ventral extension of V4A (see Zeki 1996). This has led to some confusion in the literature, to which I will refer below. It is, alas, not the only confusion regarding colour specialization in the primate brain. The upper part of V4 maps the lower contralateral hemifield and its ventral extension maps the upper contralateral hemifield, in both Cebus and Macaque (Gattass et al. 1988; A. Wade, A. Augath, N. Logothetis and B. Wandell, personal communication).
Figure 9.
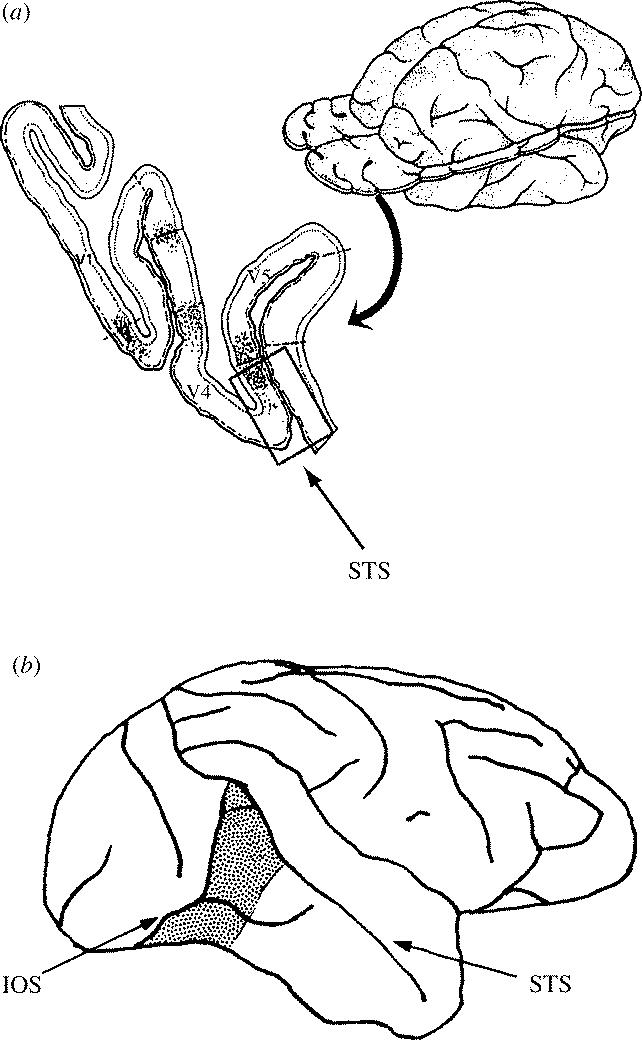
(a) The posterior part of the macaque monkey brain, as seen in a horizontal section taken at the level indicated. The boxed area is part of the V4 complex and has its distinctive callosal (interhemispheric) connections, shown by dots. At this level, it lies partly on the surface of the brain and extends onto the posterior bank of the superior temporal sulcus (STS). When traced ventrally, it appears on the surface of the brain anterior to the inferior occipital sulcus (IOS). (b) This area as it appears on the surface of the brain (stippling). This area is sometimes referred to as TEO, as if it were entirely separate from the V4 complex, which it is not. (From S. Zeki 1996.)
Cells in V4 were much more difficult to drive than those in V5 but very few, and then doubtfully, were directionally selective; indeed, visual motion was not an effective stimulus. This alone reinforces the view that there is a functional specialization in the visual brain. However, other evidence reinforces it further; colour seemed to be a much more effective stimulus for the cells of V4. In early penetrations, the great majority of cells were in one way or another selective for the colour of the stimulus; orientation-selective cells were less fussy about the precise orientation, but often showed some colour preference. With hindsight, I was perhaps much too timid in writing that ‘It would, of course, be premature to think of this area as dealing with colour exclusively…one may suggest tentatively that this is an area in which colour is emphasized’ (Zeki 1973), because subsequent physiological evidence—especially evidence derived from imaging experiments in both monkeys and humans—has shown the critical involvement of V4 in colour vision.
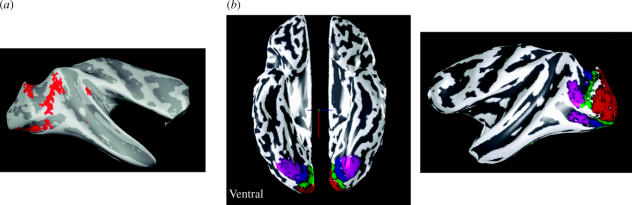
As well, there is evidence (Zeki 1983a) that shows that truly colour-coded cells—ones that respond to a colour regardless of the precise wavelength composition of the light reflected from it—are found in V4 but not in V1. The wavelength-selective cells of the latter are responsive to the presence and intensity of their preferred wavelength, without being concerned with the colour of the stimulus in their receptive field (but see also below). Similar recordings from wavelength-selective cells in area V2 have shown that they also code for the wavelength of the stimulus and are indifferent to its colour (Moutoussis & Zeki 2002a). The presence of true colour-coded cells in V4 has now been demonstrated in studies in the awake behaving monkey as well (Kusunoki et al. in preparation). Unlike the earlier studies in the anaesthetized monkey (Zeki 1983a), where the experimenter was the judge that the colour of the stimulus remained the same in spite of changes in the wavelength composition of the light reflected from it, in the behaving monkey experiments, it was the monkey that indicated that the colour remained the same. Moreover, the recordings in the behaving monkey were made in ventral V4 and the receptive fields of these colour cells were located in the upper contralateral quadrant. This finding reinforces the mapping and imaging experiments referred to above, that the lower part of V4 represents upper contralateral visual fields (Gattass et al. 1988; A. Wade, A. Augath, N. Logothetis and B. Wandell, personal communication). Perhaps most spectacularly of all, the recent imaging evidence from Alex Wade and colleagues shows that the strongest responses to coloured Mondrians (from which luminance differences had been subtracted) occurs in V2 and V4. Where the stimulation is in the upper contralateral quadrant, the responses are from lower V4 and vice versa (figure 10a,b). The pattern revealed by this imaging experiment is especially pleasing to me after such widespread and persistent doubt that monkey V4 has anything to do with colour. I am immensely grateful that their results came in time to be reproduced in the manuscript of my Ferrier Lecture.
Figure 10.
(a) The responses of macaque monkey brain to structured, multi-coloured Mondrians, revealed by imaging the activity in the brain of anaesthetized monkeys when they are presented with such stimuli, from which luminance differences have been eliminated. The activity (shown in red) is distributed in area V2 lying in the posterior bank of the lunate sulcus, and in both the upper and lower divisions of area V4. (b) While macaque V4 (purple, right) is distributed dorsoventrally, with the upper part representing lower visual fields and vice versa, human V4 (purple, left) is located within the ventral part of the occipital lobe, this being a ventral view. In human V4, located ventrally in the occipital lobe, lower visual fields are represented laterally and upper fields medially. It is as if the whole of macaque V4 has been displaced ventrally to give the human picture. (Both figures are from experiments of Alex Wade, Nikos Logothetis, A. Augath and Brian Wandell, and are reproduced here with permission of the authors.)
(i) Disputes in colour vision
The evidence for a specialization for colour within the visual association cortex, and more especially within area V4, was strongly disputed but has gained more general acceptance since the advent of imaging studies (see below). The disputes were three in kind. The first and more important one is related to whether there is any specialization for colour in the brain at all and, by extension, whether there is any functional specialization in the visual brain (Schein et al. 1982; Gulyas et al. 1994; Schiller 1997). This argument, to which I have responded elsewhere (Zeki 1983b), is perhaps best exemplified by the contrary view, which supposes that all visual cortical areas are multi-purpose. A recent version of this view runs like this: ‘Fueled [sic] in part by the pervasive belief in neuronal specificity, research in many brain areas using single-cell recordings ushered in a new age of phrenology’ (Schiller 1997). However, it is claimed that this cannot be so because ‘[n]eurons become increasingly multi-functional as one ascends from peripheral to central structures in the nervous system; this is an especially notable property of cortical neurons’. Through this ‘neuronal multi-functionality…general-purpose systems have evolved…[that] are able to perform a number of analyses concurrently’. This perceptual analysis ‘is performed interactively by areas and neurons with multi-purpose properties… In the course of evolution, the numerous extrastriate visual areas did not arise for the purpose of separately analyzing basic visual attributes such as color, motion, pattern, and depth’ (Schiller 1997). In fact, as so many physiological and imaging studies attest, multi-purpose cells and multi-purpose visual areas have been notoriously difficult to find, at least among the early visual areas, including the visual areas of the prestriate cortex. Given this, and the generally accepted principle of functional localization in the brain—which refers to the fact that different sensory modalities (vision, audition, somatosensation), as well as different faculties (e.g. the production of articulate language) are localized in anatomically distinct parts of the cerebral cortex, I was surprised that the proposal that I made in the early 1970s, that there is a functional specialization in the visual brain, should have attracted such resistance. The reasons for this are not clear. Perhaps the apparent unitary nature of our visual experience played a part, just as the ‘unity of mind’ was a factor in Karl Lashley's hostility towards the notion of functional localization, though even Lashley had to concede that because the mind is a unit the brain need not also be a unit. Perhaps the then dominant hierarchical doctrine played a role or perhaps other reasons intruded. Whatever the reasons, the argument against a specialization for colour and for motion, and hence against a functional specialization in the visual brain, suffered a serious blow with the advent of brain imaging techniques.
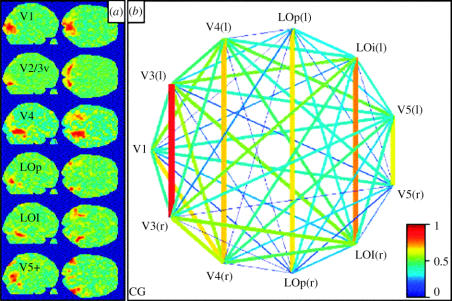
Human brain imaging techniques have been more effective than any other in showing a specialization for colour and motion in the brain. Our early human imaging experiments showed that the main area that is engaged when humans view an abstract multi-coloured display with no recognizable objects is located in the fusiform gyrus (Lueck et al. 1989; Zeki et al. 1991; McKeefry & Zeki 1997) and studies from many other laboratories have confirmed this. We named the relevant human area V4 (figure 11). Our relatively crude mapping experiments (Shipp et al. 1995), but especially the much more detailed and quantitative studies of Wade et al. (2002) showed that this is where the fourth representation of the visual field in the ventral occipital lobe is located and that, just like V4 in the macaque monkey, it represents both the lower and upper quadrants of the contralateral visual hemifield (see also McKeefry & Zeki 1997), even though it is located in the lower part of the occipital lobe—traditionally supposed (though without much supporting evidence) to represent upper visual field only. This tradition has its roots in the observation that in V1, and the two areas surrounding it, V2 and V3, the upper quadrant of the contralateral hemifield is mapped in the lower, ventral, part of each and hence in the ventral occipital lobe. However, results from other studies have demonstrated that an area located entirely in the dorsal part of the visual brain—namely, area V3A (Van Essen & Zeki 1978), can represent both lower and upper quadrants of the contralateral visual hemifield. Force of habit alone made some seek a dorsal counterpart for every ventral area discovered, without accepting—at least initially—the notion that a ventrally located visual area could represent both contralateral quadrants, as it clearly does in human V4 (see Zeki 2004 for a review).
Figure 11.
The human V4 complex, its two subdivisions and their retinotopic organization, as revealed in human imaging experiments. (a) The retinotopic organization in V4, with the lower field (green) being represented lateral to the upper field (red). This retinotopic organization is not evident in the anterior part of the V4 complex, V4α (bottom). (b) The results of an ICA, superimposed upon a brain imaging analysis. The ICA isolates the two subdivisions of the V4 complex as a single entity, showing that they form part of a single complex. (From Bartels & Zeki 2000.)
We were also able to locate V5 in the human brain (Zeki et al. 1991; Watson et al. 1993) and show that it is quite distinct in location to area V4. The location of V5 corresponds to an area of association cortex that is myelinated at birth (Flechsig 1905; figure 12). It is also worth noting that V5 seems to constitute a sort of ‘core’ area in a complex of areas which are involved in motion processing (Howard et al. 1996).
Figure 12.
(a) The position of area V5, the visual motion centre, of the human brain revealed by an imaging study using positron emission tomography. (b) The position of area V5 coincides with Field 16 of Flechsig, which is myelinated at birth.
Thus, imaging evidence may be said to have settled, as conclusively as is possible, the dispute about whether there is any specialization for colour and motion and, by extension, whether there is any functional specialization in the primate visual brain; there obviously is.
(ii) The location of the colour centre in the primate brain
The second dispute relates to where the colour centre is actually located. The very fact that the precise location of the colour centre should be disputed constitutes, of course, an open acknowledgement that there is a functional specialization for colour after all. There are those who have questioned whether it is the V4 complex in the macaque that is specialized for colour, or whether it is a more anterior region that is so specialized. Part of the evidence for the latter supposition was derived from carefully controlled behavioural lesion studies, in which the capacities of the monkey to discriminate colour is judged before and after the lesion. However, behavioural studies following lesions in the monkey have a dubious history, and this approach has served as one of the worst guides to the organization of the primate visual brain (see below).
In fact, the anatomical evidence speaks eloquently in favour of the V4 complex as constituting a colour centre in the macaque. It shows that the blobs of V1 as well as the thin stripes of V2 (in both, wavelength-selective cells are concentrated) connect with V4 and not with V5 (figure 13; Livingstone & Hubel 1984; DeYoe & Van Essen 1985; Hubel & Livingstone 1985; Shipp & Zeki 1985). V5 instead receives its input from layer 4B and layer 6 of V1 and the thick stripes of V2, both of them subdivisions that have few if any colour-coded cells. This anatomical evidence thus strongly suggests that colour signals are relayed to one cortical area and not to another, again establishing the principle of selective connections underlying functional specialization. The anatomical results also showed that the interstripes of V2, which contain mainly orientation but not wavelength-selective cells, also project to V4 (Zeki & Shipp 1989; figure 13), which is one reason for my referring to V4 as an area that is involved with colour and with form associated to colour (Zeki 1990b, 1993a). Perhaps more significantly, it is impossible to detach colour completely from form. This is because to calculate the ratio of light of any waveband reflected from one surface and from surrounding surfaces, we require a boundary between the relevant surface and its surround and that boundary will always have some form. In fact, there are orientation-selective cells within the V4 complex, but they have a wider acceptance angle and are less fussy than their counterparts in V1, V2 and V3 to the precise orientation of a stimulus (Zeki 1997). In addition, many of these have some kind of wavelength preference (Desimone & Schein 1987). Cells within the V4 complex that show variable degrees of orientation selectivity, often coupled to a wavelength bias, are grouped together and separated from each other by cells that are more strongly colour or wavelength selective (Zeki 1983b).
Figure 13.
A diagrammatic representation of areas V1 and V2 and their compartments, as well as three areas of the visual brain. Layers 2 and 3 of V1 are characterized by metabolically active ‘blobs’ in which wavelength-selective cells are concentrated and, between them, the interblobs, which contain the orientation-selective cells. Directionally selective cells are concentrated in layer 4B. These compartments project in an orderly way to specific compartments of V2 (thick, thin and interstripes) and also to the more specialized areas of the prestriate cortex—V3, V4 and V5. (From Zeki 1993a.)
One of the most extraordinary episodes in this dispute has been the result of poorly conducted experiments purporting to show an area in the human brain known as ‘V4v’. This area has always been a good candidate, perhaps the best, for being an improbable area. With a ventral location in the occipital lobe (in the fusiform gyrus), it supposedly represented upper visual fields only but no one has found a credible area that may constitute its dorsal counterpart, which should represent the lower part of the visual field for the same functions. In fact, many have found it impossible to locate such an area (McKeefry & Zeki 1997; Kastner et al. 1998; Bartels & Zeki 2000; Wade et al. 2002), and its existence must now be doubted. Wade et al. (2002) have shown that V4 (which represents both quadrants of the contralateral hemifield even though it is located in ventral occipital cortex) is also the fourth map in the ventral occipital lobe and that there is no intervening area V4v that represents the upper contralateral quadrant only between it and lower V3. The improbable and probably non-existent area V4v has nevertheless had interesting consequences. Hadjikhani et al. (1998) gave a location for the colour centre in the fusiform gyrus that was identical to ours (Lueck et al. 1989; McKeefry & Zeki 1997) but, believing that it is located in front of their improbable V4v, a supposition that was uncritically accepted (Heywood & Cowey 1998), called it ‘V8’. But since the colour area that they located is identical in position to the area that we had already charted, since ‘V4v’ has an improbable existence, and since the colour area in the fusiform gyrus that we and others have located constitutes the fourth visual map in the ventral occipital lobe (Wade et al. 2002), there seems little reason to accept this new terminology of ‘V8’, which, in any case, does not follow any logic, chronological or otherwise. It is hard to see why it should be retained as a term for something already demonstrated and named in a more logical and coherent way before the literature was confused by the improbable ‘V4v’.
Not the least interesting, and almost hilarious, aspect of the improbable ‘V4v’ is the belief that its hypothetical dorsal counterpart, which no one has yet managed to locate (because ‘V4v’ itself is an improbable area), may account for the fact that achromatopsic patients are able to discriminate equiluminant stimuli in motion (Cavanagh et al. 1998)!
(iii) A colour centre in the brain?
The third dispute relates to whether I was justified in calling the V4 complex a ‘colour centre’. This is a reasonable question given the fact that wavelength-selective cells are found not only in V4, but also in the areas that feed it—namely, V1 and V2. They are also found in more anterior parts of the inferior temporal cortex, in both monkeys and humans (Komatsu et al. 1992; Zeki & Marini 1998; Beauchamp et al. 2000). What then is the justification for referring to the V4 complex as the colour centre?
The colour of a surface depends upon the wavelength composition of the light reflected from it and from its surrounds (Land 1974). The brain is able to take a ratio of light of any waveband reflected from it and from its surrounds. This ratio never changes, no matter how the actual intensity of light of any given waveband reflected from the surface alone changes. It was Edwin Land who brought this ratio-taking system as one that enables the cerebral cortex to generate constant colours into focus. He also brought another feature into intellectual focus—namely, that the determination of the colour of a surface does not necessarily depend upon higher cognitive factors such as learning, judgment and memory which Helmholtz and Hering had invoked to account for colour constancy. In the Land system (Land & McCann 1971; Land 1974), colour is the result of a straightforward computational process that does not depend upon higher cognitive factors, which is not to say that such factors may not occasionally influence perceived colours. Although the precise details of the implementation that the brain uses to generate constant colours remains unknown, it is becoming increasingly clear that the implementation does depend upon a ratio-taking system and that the main seat of this ratio-taking system is within the V4 complex, without the mandatory involvement of cortical areas that are traditionally implicated in higher cognitive functions. In human imaging experiments (Bartels & Zeki 2000), we found that when subjects view a multi-coloured scene in which the wavelength composition of the light coming from every coloured patch changes continually without changing the perceived colour (because the ratios of the wavelength-intensity composition coming from one patch and from its surrounds remain the same), the main area of activity is the V4 complex, comprising two areas V4 and V4α. There is no involvement of frontal cortex or other areas associated with higher cognitive functions. Furthermore, when the ratio-taking system is overloaded by presenting humans with a stimulus in which the ratios are artificially and continuously changed, the main focus of activity is the V4 complex (Self & Zeki, unpublished results). Thus, V4 is the area that is most strongly involved in the ratio-taking system, which is at the heart of the cortical colour-generating mechanism. Just as often, we tend to neglect the negative evidence. The absence of any involvement of frontal cortex in colour processing is as significant as the strong engagement of the V4 complex, strongly suggesting that this process does not necessarily involve higher cognitive functions.
The above results, coupled to the clinical evidence reviewed briefly below, would seem to strengthen the belief that the V4 complex is a colour centre. Of course, such a centre does not work in isolation any more than does the motion centre. Instead it receives a highly selective input from wavelength-selective cells in V1 and V2 (Livingstone & Hubel 1984; DeYoe & Van Essen 1985; Shipp & Zeki 1985) and sends a return input to both areas (Zeki & Shipp 1988). Moreover, physiological and imaging evidence suggests that other, more anteriorly located, zones of the inferior temporal cortex are also involved in colour vision (Komatsu et al. 1992; Zeki & Marini 1998). Based on present evidence, the latter areas can be eliminated from constituting part of the colour centre, because they have not been shown to be activated in imaging studies in which colour remained constant while the wavelength composition of the light changed continually, or when the ratio-taking system was overloaded. The issue is less clear where V1 and V2 are involved. The argument in favour of their being part of the colour centre would be strengthened if it could be shown that their wavelength-selective cells behave like the colour-coded cells of V4. However, to date, the only work that has shown such an effect is that of Wachtler et al. (2003), whose results show that the effects obtained in V1 are weak by comparison to the vigorous effects obtained in V4. Moreover, the absence of any correction for multiple comparisons in the Wachtler study weakens the case further. Finally, both V1 and V2 are known to contain different functional types of cells that are grouped into specific compartments. They could therefore be more appropriately considered as heterogeneous centres. Thus, whereas there is still a possibility that cells of V1 and V2 may code for colours instead of wavelengths, the case for their being part of the colour centre is currently weak.
In summary, a specialization for colour is now generally acknowledged, just as a specialization for motion is accepted. Hence, the statement that there is a functional specialization in visual cortex remains true. Moreover, there is general agreement that a region located in the fusiform gyrus and in which both quadrants of the contralateral hemifield are mapped—the V4 complex—is critical for colour vision. Whether the V4 complex is the only colour centre or whether V1 and V2 also form part of it remains to be settled but, on present evidence, the claim of the latter two areas is weak.
(e) The clinical evidence for a functional specialization in the human visual brain
In principle, the final verdict in favour of a functional specialization in the brain should come from behavioural evidence. One approach consists of making a lesion in a specific area of the monkey visual brain—hoping that the lesion completely destroys the relevant area and is restricted to its territory. One can then note the behavioural capacities of the monkey in the relevant domain before and after the lesion. An alternative approach is to study the effects of either naturally occurring lesions in the human brain—for example, resulting from strokes—or lesions induced by unfortunate accidents such as gunshot wounds. Naturally, the latter lesions (unlike the carefully controlled lesions in the monkey that are often supplemented by sham-operated controls) do not usually respect the territory of an area and often spread beyond it, hence the rarity of adequate pathological material. Yet paradoxically, and in spite of their rarity, the evidence from patients has been a far more powerful guide to the organization of the visual brain than that obtained from monkey lesion experiments. It often does not take that much time for one set of results obtained with this latter approach to be contradicted by another set. In terms of colour vision, it is interesting to consider two results. The first one, by Heywood et al. (1995), seemed to suggest that strong deficits in colour vision could only be obtained after lesions anterior to V4, although it is significant that their lesions encroached heavily upon V4A, the anterior part of the V4 complex, which extends ventrally, and is often referred to as TEO, as if it were a separate area from V4A (see Zeki 1996; see above). They also involved V4 itself. This implied to the authors that V4 was not concerned with colour, a conclusion that was in direct contradiction to the earlier results of Walsh et al. (1993). In any case, within a short time, another study (Huxlin et al. 2000) showed that such anterior lesions do not lead to pronounced defects in colour vision or to colour blindness after all. Or take the example of motion vision; early studies seemed to indicate that lesions in V5 did not lead to motion imperception, producing no effect worth documenting (Collin & Cowey 1980). But subsequent evidence showed that such lesions did produce a defect in motion perception, though a transient one (Newsome et al. 1985). Perhaps even more surprisingly, extensive lesions of V1 were reported to have only marginal, indeed trivial, long-term effects on the discrimination of orientation, a surprising finding given the high concentration of orientation-selective cells there (Pasik & Pasik 1971; Dineen & Keating 1981). Why this is the case is problematic. Perhaps the monkey brain is more plastic than the adult brain. An early onset of plasticity following chemical lesions in V5 has indeed been demonstrated (Wurtz et al. 1990). Perhaps, the paradigms designed in these monkey lesion experiments are better tailored for studying the human brain, being designed by humans. Whatever the reason, it seems best to approach such monkey behavioural evidence with caution. Reliance on human clinical evidence is more profitable. Unfortunately, much of the clinical evidence was maligned and ignored when first put forward. It only gained acceptance after the demonstration of functional specialization in the monkey.
(i) Cerebral achromatopsia
It is now commonly agreed that the causative lesion in cases of acquired cerebral achromatopsia occurs in the fusiform gyrus, and usually includes the territory of the V4 complex as defined in imaging studies (Meadows 1974; Zeki 1990b). The number of studies showing this is too numerous to mention, but some interesting general principles emerge from a brief survey of the literature.
The most recent definitions of the colour centre in the human brain show that it is a complex of at least two areas, with the more posterior part (V4 proper) having separate representations of the superior and inferior contralateral hemifields and the anterior part (V4α) not being obviously topographically organized, although more detailed studies may reveal some kind of topography in the future (Bartels & Zeki 2000). Of course, much of this should have been clear or at least suspected from the first paper describing hemiachromatopsia and giving details of the causative lesion (Verrey 1888). However, not many took that paper seriously and it soon ‘vanished’ (Damasio 1985) from the published literature. Even Verrey himself does not appear to have understood the full implications of his paper (Zeki 1993b). The title of his paper is ‘Hémiachromatopsie droite absolue’ (total right hemiachromatopsia). The lesion is in the lingual and fusiform gyri (with another lesion in the cingulate cortex). Putting these two observations together, if anyone had bothered to consider them or undertaken a thought experiment, would have pointed inevitably to the possibility that an area located in the lower part of the occipital lobe is not only specialized for colour but also represents both quadrants of the contralateral hemifield. But no one took much notice of this work after Holmes and Henschen joined forces to dismiss it because it represented a threat to the doctrine of a single visual area in the brain (Zeki 1990b), the lingual and fusiform gyri lying outside the striate cortex, at that time considered to be the sole visual area in the brain.
Given the geography and extent of the colour centre in the brain, it is perhaps not surprising to note that the syndrome of acquired cortical colour blindness is itself somewhat complex. Some patients retain their achromatopsic status for a long time, whereas others recover after varying periods of time, which could be as little as a few days. In some patients, the colour loss is complete while in others it may affect a particular gamut of colours (Zeki 1990b). We have conjectured that these variations are possibly the result of unequal damage to the V4 complex (Bartels & Zeki 2000). This has never been formally tested, but it is the most plausible explanation currently available. Also worth noting is the close proximity to V4 of the area that is specialized for face processing (Tong et al. 1998), and damage to which leads to the syndrome of prosopagnosia or an incapacity to recognize faces. Not surprisingly therefore, many achromatopsic patients are also prosopagnosic.
What is perhaps worth noting as well is that achromatopsic patients have many visual capacities that are spared. They can read and write, have no object agnosia, can judge distance and perceive visual motion. To all intents and purposes, then, their incapacity is often largely limited to colour imperception. It would be surprising if there were not some involvement of form as well, simply because to assign constant colours to a surface, the brain must gauge the wavelength composition of the light coming from that surface and from its surrounds. But the surface will have a border with its surrounds. This is one reason, among others, why I proposed that V4 must process colour as well as form in association with colour (Zeki 1990b, 1993a). Even so, the effects of V4 lesions on form perception must be subtle since no one has so far given a convincing example of total form imperception associated with achromatopsia when the lesion is restricted to the territory of V4.
Where the damage leading to achromatopsia does not involve V1 and V2, the patient can still discriminate between wavelengths, though with elevated thresholds and without being able to assign colour to what is discriminated (Victor et al. 1989; Vaina 1994). There is at least one interesting example of a patient with a V4 lesion who was capable of discriminating colours but whose discrimination was very much wavelength based. The consequence was that he could not construct constant colours and could not perceive colours in a stable way (Kennard et al. 1995). This is not much different from what happens in monkeys with lesions in V4 who can also discriminate between light of different wavelength but with elevated thresholds (Heywood et al. 1992). Moreover, monkeys with V4 lesions, like their human counterparts, may have difficulty in discriminating the colour of surfaces when the wavelength composition of the light reflected from them is varied (Walsh et al. 1993).
(ii) Cerebral akinetopsia
The syndrome of achromatopsia associated with lesions of the V4 complex is quite different from the syndrome of akinetopsia (Zeki 1991), which is produced by lesions involving the territory of area V5. Unlike achromatopsia, for which there is now a respectable number of cases, there are far fewer examples of cerebral akinetopsia but there is at least one compelling example of a much studied patient first reported by Zihl et al. (1983, 1991). This patient could only see objects when they were stationary, not in motion. There are many other descriptions of this patient (Hess et al. 1989; Shipp et al. 1994). Of significance here is not only the generally selective nature of the imperception but, above all, the fact that it did not involve colour vision at all, the patient's chromatic sense being quite normal.
There are many more clinical examples of specific imperceptions resulting from damage to specific areas of the visual brain, and more are being regularly discovered. However, for the purposes of the present argument, it is sufficient that we use the two examples of colour and motion to press the point that there is a functional specialization in the visual brain. This evidence, together with the evidence from face imperception (prosopagnosia), object imperception (object agnosia) and other examples of specific imperceptions, shows that the specialization for function is in fact a much more general phenomenon, even if colour and motion have so far constituted the most powerful examples.
(iii) An historical aside
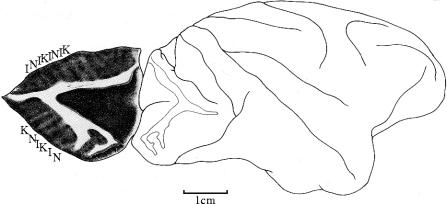
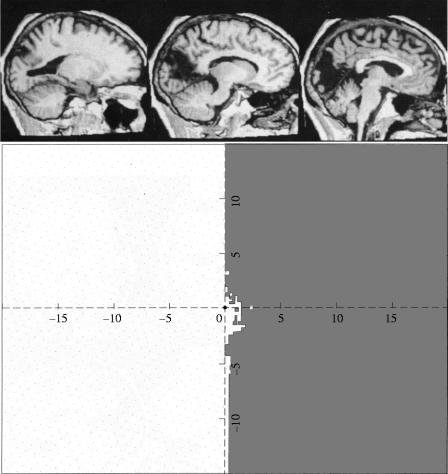
It is interesting to note that, historically, the dispute about whether there is any specialization in the visual brain is not new but runs from the late nineteenth century to the 1980s. It only appears to be new because the earlier evidence was successfully dismissed and vanished from the literature. Even today, few know about this evidence. That there is a specialization for colour in the visual brain should have been evident from an examination of the clinical–pathological evidence had anyone taken this evidence seriously. Wilbrand (1884) had supposed from his clinical studies that there is a specialization within V1 itself, a supposition that has turned out to be true (Livingstone & Hubel 1984). Just as impressive was the conclusion of Gowers (1888) that hemiachromatopsia ‘is proof of a separate centre for colour vision’. Even more precisely, he added that ‘It is, on the whole, probable that all impressions go first to the region of the apex of the occipital lobe, since disease here causes absolute hemianopia, and that a special half-vision centre for colour lies in front of this’ (Gowers 1887). Once again, reference to this suggestion cannot be found in any of the papers dealing with the subject (see Zeki 1990b, 1993a for reviews). Perhaps the most impressive evidence for a specialization for colour came from the Swiss ophthalmologist, Verrey (1888). He had studied a patient with a right hemiachromatopsia. He had also had the occasion to examine her brain post-mortem, and had located the lesion to the left lingual and fusiform gyri located in the lower half of the occipital lobe (figure 14). However, his evidence was so effectively dismissed by Henschen and Holmes that it vanished from the literature (Damasio 1985). It was Meadows (1974) who analysed all the clinical evidence available then, showing, for the first time, that the common causative lesion for the syndrome of acquired cerebral achromatopsia is located in the fusiform gyrus.
Figure 14.

A diagram to show the lesions (stippling) in the hemiachromatopsic patient of Louis Verrey (1888).
Why had this evidence disappeared from the literature? Very simply, because Verrey's results had implied, without his actually saying so, that the primary visual receptive centre was much larger than Henschen and Holmes had supposed and that one part of it extended beyond the calcarine cortex and was specialized for colour (see Zeki 1990b, 1993a). Henschen and Holmes believed instead that the visual centre was coterminous with an area of distinctive cytoarchitecture—the striate cortex—located within the calcarine cortex. Although this supposition was correct, its accepted corollary, that there is no other visual area, was incorrect. However, neither Henschen nor Holmes was prepared to consider this second alternative. In his Ferrier Lecture, Holmes (1945) had written that ‘…the perception of colour also depends on [V1]…there is no evidence that this is subserved by any other region of the brain’. Moreover, Holmes (1918) believed that ‘there is no dissociation of function after cortical lesions with an intact retinal sensibility’. In his eighth decade, Henschen spent considerable effort in trying to prove, like Holmes, that the striate cortex was also the visual centre for colour and that no separate visual receptive centre existed outside it (Henschen 1930). Holmes and Henschen had thus both successfully disputed whether there is any specialization for colour, but for reasons other than the ones advanced since 1973 (see Zeki 1990b, 1993a for reviews). They were, jointly, as well as with others like von Monakow (who had other reasons for dismissing the evidence for a colour specialization), successful in dismissing this evidence. It would be difficult to find any reference to colour specialization in the cerebral cortex in papers published between 1918 and 1973.
Holmes also successfully disputed whether there is any specialization for motion. Such a specialization within V1 had been posited by Riddoch (1917), who had examined patients rendered partially blind by gunshot wounds sustained during the Great War. He had found that his patients, though blind when tested with static perimetry, could nevertheless perceive motion in their blind fields. A believer in a single visual area, V1, he accounted for this sparing of visual motion in patients blinded by lesions to V1 by supposing that there are separate visual mechanisms within V1, which the gunshot wounds had spared. Holmes (1918) lost no time in dismissing Riddoch's improbable explanation and his results along with it, relegating the whole package to total oblivion until after the discovery of a functional specialization within the prestriate cortex, including a specialization for visual motion. In fact, with hindsight, Riddoch's explanation seems both more and less improbable today. On the one hand, at the time V1 was considered to be the only visual area and no one had imagined that there may be a visual area specialized for visual motion and located outside it. Therefore, it was the only explanation available—however improbable it may have seemed. On the other hand, the discovery that V1 cells projecting to V5 are located in layer 4B and in upper layer 6 shows that there is a segregation of function within V1, though one cannot obviously credit Riddoch with either the discovery or the insight. Moreover, even given the facts of segregation within V1 as we know them today, it becomes even more improbable to suppose that a gunshot wound should selectively spare cells in layer 4B and layer 6. With the discovery of a visual area in prestriate cortex specialized for processing visual motion, a more compelling explanation for the residual visual motion described by Riddoch—one that is important for understanding the minimum conditions for obtaining conscious vision—can be advanced (see below).
The verdict of the evidence accumulated since 1970, therefore, is that both Henschen and Holmes before 1920, and all others who have disputed the concept of functional specialization since 1973, have been wrong.
(f) Why is there a functional specialization in the visual brain?
Whether one looks at the physiological or anatomical evidence, at imaging or clinical studies, one is led ineluctably to the same conclusion—that there is a functional specialization in the visual brain. Yet, why should the brain be so organized? Perhaps we should seek the answer from the nature of the visual world and in the computational problems that this imposes upon the visual brain. Different attributes of the visual world occur haphazardly and erratically; they do not co-occur. A bus could be red and moving to the right. However, it could equally be green and stationary. A green object could be a car, a leaf or a sheet of paper. If two or more attributes always co-occurred, then one attribute would be sufficient for unfailing identification. There is therefore a principle of functional independence in the visual world (Bartels & Zeki 2004a), and the organization of the visual brain merely reflects the organization of the visual world.
To this we add another reason—namely, that the computational problem imposed in trying to find a solution to the perceptually constant representation of one attribute is quite different from that for another (Zeki 1993a). For colour, the brain has to compare the reflectance of one surface for light of all wavebands with the reflectance of surrounding surfaces for light of the same wavebands simultaneously in time. The actual disposition of the surrounds, their shapes and sizes are of no importance. For motion, the brain has to integrate information from at least two points successively in time. Once again, the shape of the moving stimulus is immaterial. By contrast, for shape, the relationship of different elements to one another, simultaneously in time, is critical. One can then suppose that these different computational problems require different internal organizations. There is little doubt that on both counts different visual areas differ. A very precise topographic organization is the hallmark of V1 and V2; in both, all different categories of cell are represented, with a preponderance of orientation-selective cells. Though less precise, because the receptive fields of cells are larger, a strict topographic organization is no less a hallmark of areas V3 and V3A (Cragg 1969; Zeki 1969; Van Essen & Zeki 1978; Zeki 1978), both of them areas with a heavy concentration of orientation selective cells. The topography in both V4 and V5, though present, is radically different (Van Essen & Zeki 1978; Maunsell & Van Essen 1987). These relative degrees of topographic precision are also reflected in the callosal connection between the homologous areas of the two hemispheres. Whereas such connections are very precise when connecting topographically precise areas in the two hemispheres, they are much less so when connecting areas in which the topography is cruder (Zeki 1993a). Moreover, there is reason to suppose that the internal anatomical wiring of these different areas differs significantly, though the extent to which one can relate these levels of topographic organization and differences in internal wiring to the functions of these areas remains an unresolved problem.
2. The nature of visual consciousness
As I have tried to show in §1, there is an inevitable logic that leads from the first demonstration of parallel outputs from V1 to the demonstration of functional specialization in the visual brain. Functional specialization constitutes not only the indispensable basis for understanding the nature of visual consciousness, but it also imposes an ineluctable logic that leads from it to insights into conscious vision. The questions that functional specialization leads to may be summarized as follows: (i) To what extent are the specialized processing sites also perceptual sites? (ii) How do they interact to give us our unified picture of the visual world? (iii) To what extent is their activity dependent upon other cortical areas? The answer to each of these questions has turned out to be more counterintuitive than I had imagined. Collectively, they have led me to view consciousness in a somewhat different way, not as a unified entity whose neural correlate will one day be discovered, but as consisting of many different microconsciousnesses that are distributed in time and space.
(a) Processing sites are also perceptual sites
The first step in this enquiry is to ask whether processing sites such as V4 and V5 are also perceptual sites, or whether the perception of the attributes that they have processed (colour and motion, respectively) depends upon higher areas of the brain. Many have thought, either implicitly or explicitly, that a processing site is not a perceptual site. Although neither Herman von Helmholtz nor Ewald Hering wrote in terms of processing or perceptual sites, it is obvious that both thought of the two as different. To account for colour constancy, both invoked higher cognitive factors—judgment and learning in the case of Helmhotz and memory in the example of Hering. Their accounts could not be clearer. Hering (1920) wrote, ‘[a]ll the colours that we know or think that we know, we see through the spectacles of our memory colours and therefore quite differently from the way we should see them without these, provided always that we are not thinking about the colour’ while Helmholtz (1911) wrote that ‘colour is due to an act of judgement, not an act of sensation’. In both, a ‘top-down’ influence is implied. Of course, there is little doubt that higher cognitive factors often influence our perceptions, implying some sort of top-down effect from higher to lower areas. However, is colour one of them? Land (1974) has in fact proposed a theory of colour vision that is entirely computational and makes no use of memory or judgment, which is not to say that these factors do not occasionally play a role. Given this history, it becomes legitimate to ask whether the site that undertakes the processing is also a perceptual site, which is tantamount to asking whether the result of the processing is the percept, without the mandatory intervention of cognitive factors and therefore of other cortical areas.
The question becomes even more compelling when one considers a relatively simple stimulus such as the Kanizsa triangle (figure 15), about whose physiological processing one can perhaps say a little more than one can about the detailed implementation used in colour vision. It has been assumed that the completion of the figure and its perception as a triangle depends on higher cognitive factors (Gregory 1972). However, it has also been shown that there are cells in the brain, in areas V2 and V3, which are capable of responding to virtual lines (Peterhans & von der Heydt 1989). Why should the responses of such cells not constitute the percept? If they do, then there is good reason to suppose that a processing site may be a perceptual site and does not require the mandatory participation of higher cognitive areas for what has been processed to become perceptually explicit.
Figure 15.

The Kanizsa triangle.
In fact, imaging evidence has not shown any involvement of frontal cortex during the perception of colours. However, in some instances, there may be such involvement. When objects are dressed in unnatural colours, there is an activation of frontal cortex (Zeki & Marini 1998). Equally, many laboratories have now studied the brain activity during the perception of Kanizsa figures and they unanimously fail to show any involvement of frontal or parietal cortex (Hirsch et al. 1995; ffytche & Zeki 1996; Larsson et al. 1999; Stanley & Rubin 2003). The activity in these experiments is rather in early visual areas (V2, V3) and in areas specialized for form within the parahippocampal gyrus. This leaves us to entertain the possibility outlined above—namely, that a processing site is also a perceptual site. This seemed especially interesting given the evidence, reviewed below, that activity in a cortical area may acquire a conscious correlate if it reaches a certain strength (Zeki & ffytche 1998). Konstantinos Moutoussis and I (Moutoussis & Zeki 2002b) therefore thought it worthwhile to devise an experiment to study this directly. In relatively simple psychophysical experiments, we asked subjects to view faces or houses. We chose these two attributes because they are known to be processed in separate areas of the brain lying within the fusiform gyrus in ventral occipital cortex (Tong et al. 1998). In the dichoptic viewing condition that we used, the stimulus is delivered to each eye alternately at intervals of 100 ms. If the identical stimulus is delivered to each eye (in our case, either a red outline house or face against a green background to each eye), then subjects will unfailingly identify the stimulus as a house or a face (figure 16). If, however, the same stimuli are delivered to each eye but in opposite colour contrast (e.g. a green outline house or face against a red background to one eye and the opposite colour contrast to the other eye), then the two stimuli will cancel each other out and the subjects will report seeing yellow, without being able to identify whether the stimulus was a face or a house. Here, then, are two conditions, in both of which the same signals are fed into the eye and relayed to specific brain areas, one specialized for processing faces, the other objects, including houses. In one condition, in spite of the reception of signals, subjects cannot not perceive the stimuli, whereas in the other they can. The imaging results showed that, whether they perceived the stimuli correctly or not, the same specific brain areas were active, the area specialized for faces when the stimulus was a face, and the area specialized for processing objects when the stimulus was a house. The difference between the perceived condition and the unperceived one was that activity in the relevant area was higher in the former instance compared with the latter. We thus conclude that a processing site is also a perceptual site, and that activity in a processing site can have a conscious correlate when it is sufficiently strong. The same conclusion about the relationship between activation strength in an area and conscious perception of a visual attribute can be reached by studying the conscious vision of blind subjects (Zeki & ffytche 1998; see below). We have not yet been able to determine whether the strengthened activity is a result of the recruitment of new cells in the relevant area or due to a heightened activity of already active cells.
Figure 16.
The experimental methods and the results from the experiments of Moutoussis & Zeki (2002b). (a) The dichoptically presented stimuli, with (on the left) the identical stimuli presented to the two eyes and (on the right) the same stimuli presented in opposite colour contrast. (b) Bars show that subjects were able to identify the stimuli correctly when the identical stimuli were delivered to the two eyes but were not able to do so when the two stimuli were of opposite colour contrast. (c) The average results from seven subjects, which shows that there was area-specific activation of the visual brain with both perceived and unperceived stimuli. The contrast same house versus same face (SH–SF) shows bilateral stimulus-specific activation in the parahippocampal gyrus. The contrast opposite house versus opposite face (OH–OF) shows unilateral stimulus-specific activation in the same region. The contrast same face versus same house (SF–SH) shows a stimulus-specific activation in the fusiform gyrus, while the contrast opposite face versus opposite house (OF–OH) reveals stimulus-specific activation in the same region. This experiment shows that processing sites are also perceptual sites.
(i) Nodes and essential nodes in the visual brain
Evidently, a processing site can also double as a perceptual site. We refer to such sites as processing–perceptual sites or more simply as ‘essential nodes’ (Zeki & Bartels 1999), and this is a good place to describe them and distinguish them from nodes (figure 17). By ‘node’, we mean any station in the visual pathway. For example, the blobs in V1 constitute a node as do the thin stripes of V2, both of which contain heavy concentration of wavelength-selective cells. V4, which receives input from both of these stations, is another node. By essential node we mean a station or an area of the visual pathway at which activity becomes perceptually explicit and requires no further processing (Zeki 1993a; Zeki & Bartels 1999). In the example of colour vision, V4 is an essential node because activity in it apparently becomes perceptually explicit—leads to the perception of colour—without further processing. The concept of nodes and essential nodes is however more subtle and complex. In the example given above, some of what has been processed in V1 and V2 does become perceptually explicit and probably does not require further processing by V4. For example, we are usually aware of a sudden change in the wavelength composition of the illuminating light. Changes in wavelength composition are known to activate the wavelength-selective cells of V1 and V2 (Zeki 1983a; Moutoussis & Zeki 2002b) and, in this sense, V1 and V2 are essential nodes for certain visual attributes. Moreover, we surmise that when V4 is fully damaged, the nodes feeding it become essential nodes. In this condition, the patient can only become aware of what these earlier stations have processed, as is discussed below.
Figure 17.
A schematic of the motion (left) and colour (right) processing systems of primate visual cortex. Each system consists of at least three nodes. In the motion system, the cells of layer 4B of V1 that project, directly or through the thick stripes of V2, to V5 constitute one node. The thick stripes of V2 constitute another node and V5 the third node. Of these, V5 is the essential node for the perception of motion, but when destroyed, a residual motion vision can be signalled through the first two nodes, which then become essential nodes. In the colour system, the cells of the blobs in V1 that project directly or through the thin stripes of V2–V4 constitute one node, the thin and interstripes of V2 another node and V4 is yet another one. The latter is an essential node for colour vision, but when destroyed, the nodes projecting to it may assume this role.
There are sound theoretical reasons why activity at any given essential node should potentially become perceptually explicit. If what is processed in a given area A is simply relayed to another area B to be reprocessed at a higher level of complexity, then what has been processed in area A becomes lost unless it is made perceptually explicit. It would make sense for the brain not to lose the information thus gained. The anatomical observation that there are no terminal stations in the cortex (Zeki 1993a) implies that there is no final perceptual centre, to which all antecedent areas report in a chain. The absence of such a terminal station makes perceptual sense, for such a hypothetical final perceptual area would have to code the results of the processing in a perceptually explicit way at each node, separately and in combinations. A more economical way would be to render the activity at each processing site perceptually explicit, and that activity can then be bound with the perceptually explicit activity at other processing sites.
The function of many nodes in a processing system is to discard some information in order to extract more global information. For example, a picture of a face composed of small dots will activate areas with cells that respond to dots and other areas whose cells respond to faces. Neither of the two stages explicitly codes information that the other stage explicitly codes for. The only way to preserve both types of information—dots and faces—is to make activity at both areas perceptually explicit. It would be wasteful for the brain to make only the information at a hypothetical final centre perceptually explicit. If the supposition that there are multiple essential nodes (and hence multiple perceptual sites) is true, then one would expect that what is processed at any given essential node should be made available to many other essential nodes, so that the perceptually explicit activity therein can be bound with the perceptually explicit activity at other essential nodes. Indeed, anatomical evidence shows that not only are there many connections between nodes, but that such connections start at the earliest stages in the visual pathway—namely, in area V1. Here, as in V2, lateral connections exist between blobs and interblobs or between thin stripes, thick stripes and interstripes (Rockland & Lund 1983; Hubener & Bolz 1992; Lund et al. 1993; Levitt et al. 1994; Yoshioka et al. 1996). Moreover, there are feedback connections from areas such as V4 and V5 to both V1 and V2 (Zeki & Shipp 1989; Shipp & Zeki 1989a,b). Interestingly, these feedback connections are not modular, in the sense that they do not project back solely to the cells of V1 and V2 that feed V5. Instead they are widespread, and encompass the territory of cells that project to other areas, for example to V4 or V3. Such feedback connections are thus good anatomical candidates for binding the activity at different nodes.
(ii) Is V1 an essential node?
Are all visual areas of the brain capable of acting as essential nodes or is this a privilege of some only? Crick & Koch (1995), supposed that, of all visual areas, V1 is not privileged in this regard because it does not have direct connections to the frontal lobes, a region thought by some to be critical for generating a conscious correlate. However, current evidence is not sympathetic to this view. Ingenious experiments from three laboratories suggest strongly that V1 is an essential node for some kinds of visual stimuli. Logothetis (1998) and his colleagues have shown that all visual areas, including area V1, have cells whose responses follow, or at least correlate, with the percept rather than the physical stimuli, even if the proportion of such cells increases as one records successively from V1, from visual areas of the prestriate cortex and then the inferior temporal cortex. Moreover, imaging experiments show that changes in activity within V1, as measured by the blood oxygen level dependent (BOLD) response, correlates with the percept (Ress & Heeger 2003; Zenger-Landolt & Heeger 2003) rather than the stimulus. This works in both directions, in that giving subjects a task in which the salience of a stimulus is reduced by surround masking also leads to a suppression in V1 activity. The evidence obtained from human imaging experiments is in good agreement with the evidence obtained from physiological recordings in the awake behaving monkey. Using a paradigm similar to the one used in the imaging experiments, Lamme et al. (2002) found that masking (which reduces the salience of a target stimulus) also selectively suppresses V1 signals optimally at target-mask intervals that make the target stimulus invisible. Finally, the recording experiments of Lamme and his colleagues have shown that activity of cells in V1 corresponds with figure–ground segregation. Taken together, these experiments make a strong case in favour of regarding V1 as an essential node.
There are conditions where V1 is not an essential node but becomes one in certain pathological conditions. A good example of this is provided by colour vision. Although the wavelength-selective cells of V1 connect, both directly and through the thin stripes of V2, with V4, the human evidence shows that the V4 complex is the only centre, or at least the principal one, for constructing constant colours (Bartels & Zeki 2000). Damage to V4 leads to cerebral achromatopsia, as described above. However, achromatopsic patients remain capable of distinguishing one waveband from another, although they cannot assign colours to them. In cases of incomplete achromatopsia, the colour vision of patients has been described as being at the mercy of the wavelength composition of the light reflected from a stimulus (Kennard et al. 1995; see above). This suggests that, under these conditions, what the subject becomes aware of is strictly tailored to the physiological capacities of the cells that are not damaged by the lesion. This accounts for the capacity of achromatopsic patients to discriminate wavelengths and yet be unable to generate constant colours because their intact V1 has wavelength cells which, collectively, allow the patient to discriminate different wavelengths of light. In fact, there is one experiment that has tested this supposition directly, an experiment that has its roots in a highly interesting observation made by Wechsler (1933), which others have since confirmed.
As first reported by Wechsler, subjects rendered blind by carbon monoxide poisoning can sometimes retain the capacity to discriminate colours consciously, in spite of their otherwise total blindness. I have explained this relative sparing of colour vision in blind subjects by supposing that the relatively rich vasculature of the CO-rich blobs and interblobs in V1 and V2 (Zheng et al. 1991) spares them from the effects of hypoxia (Zeki 1993a). Whatever the correct explanation for this somewhat counter-intuitive phenomenon—the chromatopsia of carbon monoxide poisoning—it can also manifest itself in other conditions, especially heart attacks, and the explanation that I have given above may apply to these cases too. My colleagues and I (Zeki et al. 1999) have had the occasion to examine a patient who, after stepping on a high-voltage cable, suffered a severe heart attack that left him unconscious for several months. Upon recovery, he was found to be blind though with a spared capacity for colour vision, much like the patient of Wechsler. Psychophysical examination of this patient with the Land Mondrian experiment (which was exceedingly difficult owing to his severe blindness for form) showed that his colour vision was strictly wavelength based. For example, unlike normals, a green surface that reflected 60, 30 and 10 mW Sr−1 m−2 of long-, middle- and short-wave light appeared white to him (which is what it appears to normals if the green patch is viewed in isolation, without a surround). If the amount of long-wave light was increased further, then the patch was described as red. On the other hand, he was unfailingly correct in reporting a stimulus of, say, 620 nm as red, just like normals. His colour vision, therefore, was very much wavelength dependent and he had an incapacity to construct constant colours. When the patient viewed coloured stimuli of high phosphor purity generated on the monitor in the scanner, he could name them correctly and the activity in his brain was restricted to V1 (figure 18). Even when the significance thresholds for detecting brain activity were dropped severely, no activity appeared in V4 of his abnormal brain. The obvious conclusion from these experiments, just like the conclusion from the experiments described above, is that, under conditions of normal colour perception, V1 is simply a node—that is, activity within it requires further processing, in this case by V4. When V4 is damaged, V1 becomes an essential node. Now, activity in it not only does not require further processing but is actually incapable of further processing, because there is no V4 to which the cells of V1 and V2 can now project. The patient now simply experiences consciously what the cells in his intact V1 allow him to experience.
Figure 18.
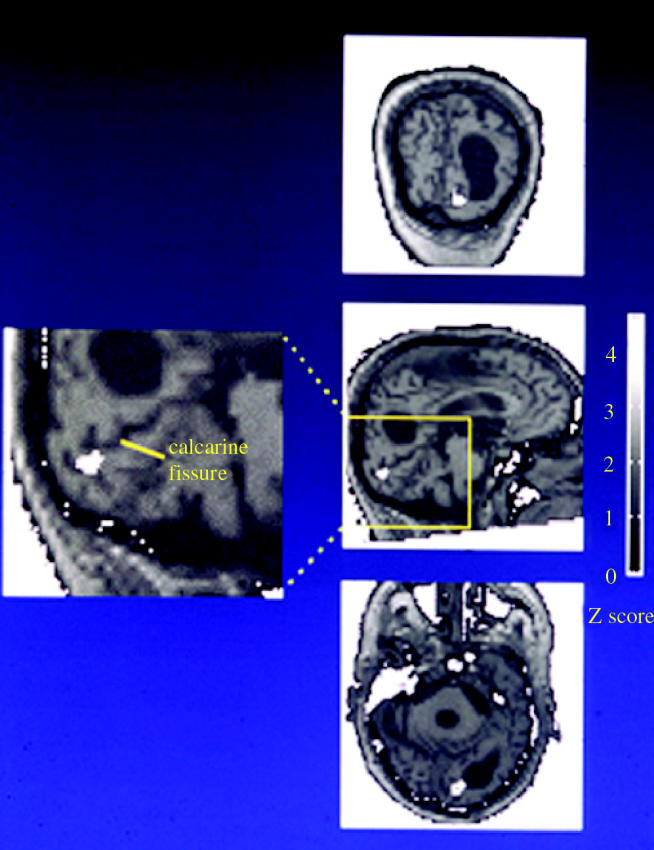
The activity (in white) in the brain of a patient who became blind after suffering a severe heart attack, but who could nevertheless discriminate colours. The activity is restricted to the territory of the calcarine sulcus (area V1) and is shown in saggital (centre), coronal (above) and horizontal sections (below). (From Zeki et al. 1999.)
On balance, therefore, it would seem that, in certain conditions of stimulation and in some pathological conditions, V1 can become an essential node.
(b) Binding as a multi-stage process
A corollary of having multiple essential nodes, where activity at each can become perceptually explicit, is that there must be many connections between different essential nodes so that the perceptually explicit activity at one becomes accessible to other nodes. A multiple essential-node system would be useless if each processing system led to a hypothetical integrator or perceptual area through one-way, feed-forward connections only.
If activity at each processing-perceptual site (essential node) can potentially become explicit, no matter where in the hierarchical chain within one of the parallel processing system it may be located, then it follows that that activity must be potentially capable of being bound to activity at other essential nodes, again, no matter where they are located. It thus becomes necessary to suppose that binding in the visual brain must be a multi-stage process (Zeki 1990a,b,c; Bartels & Zeki 1998). In other words, it is not only activity at the hypothetical end-station of a given specialized pathway that is ripe for binding with activity at the hypothetical end-station of another pathway. Instead, activity at any given node of a specialized pathway, in theory, should be capable of being bound with activity at any other given node. The only requirements here are that activity at both nodes is perceptually explicit and that there is some kind of requirement for them to be bound. It follows that binding must be a post-conscious and a multi-stage phenomenon.
(c) The visual brain as an asynchronous organ – functional specialization projected in time
To demonstrate that binding is post-conscious required us to undertake simple psychophysical experiments, which in fact constitute the next step in following up the consequences of functional specialization in the visual brain, because they directly address the question of how activity in different, specialized visual areas is bound together to give us our unitary experience of the visual world. This would not be such a problem, nor would there be any need for such an enquiry, if all visual signals were analysed by a single visual area (area V1), as Henschen, Holmes and others had believed. Nor would it constitute a problem if all the information in the visual world were to be analysed hierarchically by the same sets of cells, with one set located in one visual area analysing all the information analysed by the previous set, located in an antecedent area, but at a more complex level.
Any study of binding must, however, ascertain the time at which the processing activity at any given essential node becomes perceptually explicit. This is a difficult task; one way around it is to ask whether different attributes, processed by different essential nodes, reach a perceptual endpoint at the same time. One supposition, commonly made both implicitly and explicitly, is that all the areas start and terminate processing at the same time and can then communicate the results of their processing to one another either through direct connections or through feedback ones. This supposition has solid foundations in ordinary perception because it is a common, daily experience that, when we open our eyes and view a scene, all the different attributes of the visual world—colour, depth, motion, form—seem to be in precise spatial and temporal registration. However, the supposition must first be tested, to demonstrate the truth of the statement that we see all the different attributes of the visual world at the same precise time and in precise spatial registration. When we say that we see all the attributes of the visual scene at the same precise moment, what measure of time are we taking? Indeed, what standard can we use as a time reference where the nervous system is concerned? The question is not easy to answer, but a generally valid metric would be 0.5–1 ms. This is the time that it takes for the nervous impulse to cross from one nerve cell to the next through the synapse and it seems to be much the same wherever one looks in the brain.
In relatively simple psychophysical experiments, Konstantinos Moutoussis and I undertook to test the above proposition, starting with a study of colour and motion—the two visual attributes that are known to be most separate from each other in terms of their cortical representation. We were surprised to find that colour is perceived before motion by about 80 to 100 ms (Moutoussis & Zeki 1997a,b; Zeki & Moutoussis 1997). In terms of the unit of nervous system time given above, this is extraordinarily long, and has interesting consequences as far as the untested supposition—of seeing all attributes in perfect spatial and temporal registration—is concerned. The result, reinforced by other results (Arnold et al. 2001; Viviani & Aymoz 2001), tells us that there is a perceptual asynchrony in vision. This statement would be true even if it could be shown that all the other attributes besides colour and motion are seen simultaneously. In fact, it turns out that orientation is perceived before motion and after colour, so that the statement has more general validity (Moutoussis & Zeki 1997b).
Our interpretation of these differences in perceptual times is that they reflect differences in processing times. There are other good reasons for this supposition, in addition to the original demonstration of an asynchrony in perception. The experiments of Arnold & Clifford (2002) have shown that colour–motion pairings can be affected by the magnitude of motion changes. If the change in the direction of motion is 180° then the asynchrony is maximal, but if the change is less (e.g. 45°), then the difference in perceptual time between colour and motion is reduced, although never abolished. Inspired by these results, we decided to take our early experiments a step further. In the earlier ones, we had compared the perceptual times required to perceive up–down versus left–right motion, and found no difference in perceptual time. In the new experiments, we wanted to learn whether subjects would perceive up–down motion at the same time as, say, motion upwards and to the right. The rationale behind the experiment was that cells excited by motion in one direction are inhibited by motion in the opposite direction, and their response latencies also become elevated. Hence, motion in one direction will exert an inhibitory effect on cells whose preference is the opposite direction. By contrast, when motion between, say, 12 o'clock and 6 o'clock is being paired with motion between 3 o'clock and 12 o'clock, the inhibition in the latter condition will be trivial. Our results show that, with such pairings, subjects perceive motion towards 3 o'clock first, with an advantage of 40 ms (Roulston & Zeki, unpublished results). Another result that can be easily explained by supposing that the asynchronous perception is the result of differences in processing times is the comparison of the motion of dots generated from luminance differences with that of dots generated from equiluminant stimuli. In this instance, the motion generated from the former is perceived before the latter (Zeki, unpublished results). It is known that V5 neurons respond more sluggishly to equiluminant stimuli, which may be taken as a reason for the relatively delayed perception (Saito et al. 1989). Such an interpretation is corroborated by the observation that the intensity of activity in a given human visual area is directly related to the intensity of the subjective experience of the attribute for which that area is specialized (Bartels & Zeki 2004a).
The interpretation that we have given for the asynchronous perception of different visual attributes is not the only one. Nishida & Johnston (2002) propose a simpler one based on the fact that a first order change such as a change in colour requires only two frames on the screen whereas a second order change requires three. Although tempting in its simplicity, this explanation does not account for why two first order changes (orientation and colour) are perceived at different times, with colour leading orientation by about 40 ms (Moutoussis & Zeki 1997b). Nor does it account for why it is that two second order changes (up–down motion of a black–white checkerboard pattern versus left–right motion of a an equiluminant red–green checkerboard) are also seen at different times, with the equiluminant motion lagging by about 40 ms. Another interpretation, also by Nishida & Johnston (2002) is that these perceptual asynchronies result from a vague and hypothetical comparison stage in the brain, to which signals are relayed more slowly from some areas than from others or that signals from two different areas may be differently tagged temporally. Although possible, this interpretation is not compelling because it is difficult to believe that signals from V4 and V5 reach this hypothetical comparison centre with such big differences in time, and that manipulating the characteristics of stimuli can reduce or increase the temporal asynchrony. In summary, the simpler explanation, that differences in processing time lead to differences in perceptual time, is currently the more compelling one.
(i) Misbinding of attributes and post-conscious binding
Our psychophysical experiments gave other interesting results, which are of importance in understanding how the brain combines the activity of different, spatially separate visual areas to give us our unitary view of the visual world. Over very brief time windows, in the range of 100 ms, not only do subjects not perceive all the different attributes at the same time but, as a direct consequence of this perceptual asynchrony, are not able to combine them correctly as far as veridical reality is concerned. Instead, they bind the colour that they perceive at time t to the direction of motion that they perceive at time t−1 (Moutoussis & Zeki 1997a). In other words, they misbind in time. From this misbinding, we can draw an important conclusion—namely, that where a visual scene or stimulus contains a number of attributes, the brain does not wait for a given area, or for one of its processing systems, to complete its processing task and thus reach a perceptual endpoint, before binding the activity of cells in that area with the activity of cells in another. Rather, the brain simply binds what has it has already processed and is therefore perceptually available. In other words, it simply binds the perceptual endpoints reached by the processing systems. This explicitly shows that we become conscious of visual attributes before binding them. Hence binding must be post-conscious (Zeki & Bartels 1999). Of course, this view is substantially different from the view that supposes that it is the binding itself that generates the conscious experience (Crick & Koch 1990; Engel et al. 1999; Engel & Singer 2001; Tallon-Baudry 2004).
Another indication that binding may be post-conscious comes from recent psychophysical experiments which demonstrate that binding of colour to motion occurs after the binding of colour to colour or motion to motion (Bartels & Zeki, unpublished results). Subjects therefore become conscious of the bound percept after they become conscious of the attributes that are to be bound, suggesting another temporal hierarchy in visual perception, in addition to the temporal hierarchy that is implicit in the observation that we perceive certain visual attributes before others. I refer to consciousness of a stimulus that is compound, in that it consists of more than one attribute, as a ‘macroconsciousness’ to distinguish it from consciousness of a single attribute (e.g. colour), which I designate as a ‘microconsciousness’ (Zeki 2003b).
These psychophysical experiments are, I believe, important in giving us powerful clues as to how the brain operates but do not give us the whole picture. In the longer term, in excess of 500 ms, the different attributes are of course brought together into perfect registration but how this is done remains elusive.
(ii) The asynchronous brain and asynchronous computers
These results lead to another important conclusion, which of course should have been hinted at when functional specialization was first demonstrated. That neither I nor anyone else did so for a long time is indicative of how long it takes for unforeseen results (in this case, functional specialization) to sink in and its consequences understood. In a modular system, it is important to ask whether all the different modules have to continually, and simultaneously, reset themselves to some arbitrary zero value simultaneously, or whether each module can reset itself autonomously without having to wait for the other systems to do so. The more efficient way of organizing a multi-modular system is to make it asynchronous, as computer scientists have realized. They have designed the new generation of computers in which asynchronous components (in our case modules) process data only when it becomes available, thus consuming power only when doing useful work. By contrast, a ‘clocked’ system will consume power on every clock cycle, regardless of whether it has done useful work or not. Hence, power consumption is reduced in asynchronous computers. It has been argued as well that modular asynchronous computer systems enjoy the advantage of relying on local communication between components as compared with circuits with global clocking, thus making them easier to design. Whether the brain asynchrony is also designed, at least in part, to allow the brain to use energy more efficiently and to favour local communication may be debated. However, there is no denying that an asynchronous system has greater versatility. At the very least, it does not require the availability of a central clock that checks constantly that all the modules have reset. There may indeed be other, biologically more compelling, reasons for why evolution should have opted for a modular, functionally specialized, design, and a by-product of that design may have been the conferment of other advantages which the brain may share with asynchronous computers.
(d) The chronoarchitecture of the cerebral cortex
If we see different attributes asynchronously and if this asynchrony is the result of different processing speeds in different areas of the visual brain, then it becomes intuitively attractive to suppose that the time course of neural activity in one visual area may also be asynchronous with respect to that in another. This should be relatively easy to demonstrate, if one can locate the activity time course (ATC) in different areas and relate them to one another. Put more simply, one should find that when we view a complex scene containing many visual attributes, the intensity and time course of activity in the different visual areas of the brain should not increase and decrease, or otherwise change, simultaneously. This would be especially so if, as is the usual case, the intensity of the different attributes (e.g. colour or motion) not only occur but also vary independently from one another and if differences in the intensity of response in cortical areas varies in proportion to the intensity of the stimulus (Rees et al. 2000a). It might also be the case if the intensity of the response in different visual areas varies with the intensity of the subjective experience, as indeed it does (Bartels & Zeki 2004a). In the latter instance, it is entirely plausible to suppose that the subjective intensity with which, say, colour is experienced may be quite different and independent of the intensity with which another attribute, say motion, is experienced. In fact, the independent occurrence of the different attributes within a complex scene and the independence of the subjective intensity with which different attributes are experienced constitute good reasons for the functional specialization of the visual brain (Bartels & Zeki 2004a).
The development of the technique of independent component analysis (ICA) by Bell & Sejnowski (1995) and its successful application by others (Makeig et al. 1997; McKeown 2000; Calhoun et al. 2001a,b) encouraged Andreas Bartels and myself to address the questions raised above, by applying the same technique to study the ATC of individual visual areas when subjects view complex action scenes. The task in these experiments was to locate brain activity in time and space and thus determine the ATC in each area and its relation to the ATC of other areas (figure 19). We therefore asked subjects to view the first 22 min of an action movie, the James Bond film Tomorrow Never Dies, in a scanner. A straightforward expectation, given the psychophysical results described above, was that different visual areas would have different ATCs. However, this is not the only reason why we undertook such a study. One potential, and indeed actual, criticism of the doctrine of functional specialization in the visual cortex is that the experiments done to demonstrate it, be they physiological or imaging ones, are highly artificial. In a sense, they of course are. To some extent, artificiality is a characteristic of almost all experiments. After all, nothing could be more artificial than dropping a rock and a sheet of paper in a vacuum to see whether they fall with the same velocity. The physiological experiments use an electrode to isolate a single cell or a small cluster of cells in a given cortical area and stimulate these cells in a highly artificial way, with spots or bars of light, either white or of different colour and either stationary or moving, being flashed against a dim or dark background. Imaging experiments set up a hypothesis, often based on the belief that one or a small set of areas will be especially engaged when subjects are stimulated in the same artificial way as the cells in an electrophysiological experiment. On the other hand, the ICA method has no presuppositions and is not theoretically biased, save to the extent that it supposes that there is activity in the brain and that this activity can be located in time. It thus simply indicates that, during a finite time (corresponding in our case to the time taken to view the opening of the James Bond movie), there will be several episodes of high and low activity. The coupling of such results to fMRI experiments, which locate activity in space, means effectively that, in this new guise, the complex of the two approaches is also not hypothesis driven. The two together thus simply indicate that different, anatomically localized, areas have either the same ATCs or different ones. If their ATCs are the same, then the activities in them should be strongly correlated; if not they should remain uncorrelated. The experiment does not give any indication of what the function of each area that it localizes in time and space may be. That is something that more hypothesis-driven experiments or clinical studies will determine. The situation is not unlike that of anatomical architectonic studies. These, too, are not hypothesis driven, their only assumption being that any architectonic differences that may be revealed would be indicative of functional differences, without specifying necessarily what these functional differences are. The latter is left to functional or clinical studies.
Figure 19.
Independent components (ICs) containing visual areas; (a) BOLD signals correlations between their most active voxels (b) during the viewing of the first 22 min of the James Bond movie Tomorrow Never Dies. Data are from a single subject. (a) The glass-brain saggital and horizontal views of the positions of the isolated occipital ICs. All regions were stimulus driven and had significant and specific intersubject correlations. Labels indicate the locations of the ICs. The colouring of the ICs shows the relative voxel contribution, using the colour scale of (b), with red, green and blue indicating positive, neutral and negative contributions, respectively. (b) Correlogram to show the correlation (r) of BOLD signals between the most active voxels of areas identified in (a). The strength of correlation is indicated by the colour code and thickness of the line. LOp: posterior lateral occipital complex; LOI: lateral part of lateral occipital complex. (From Bartels & Zeki 2005.)
The results that we obtained using this approach were beyond our expectations (Bartels & Zeki 2004a,b). They showed in the first place that different areas do indeed have different ATCs, and thus that the correlation between them is low. In fact, the highest correlation between areas is when the brain is at rest with the eyes shut and without the subject undertaking any task. The minute the brain is exposed to the action movie, the areas become decorrelated, each one pursuing its own activity and displaying its independent ATC. Thus, the best indication of functional specialization in the visual brain, both spatially and temporally, comes from complex stimulation in free-viewing conditions, the very condition that, in the supposition of Schiller (1997) and others, would reveal that all areas are multi-purpose, each undertaking the same activity that all other areas undertake.
The second interesting result is that some areas have highly correlated ATCs. Bartels and I (Bartels & Zeki 2005) have assumed that areas that are highly correlated in their ATCs are the ones that are probably directly connected anatomically, while those that have disjunctive, uncorrelated, ATCs are probably less so. We see evidence for this supposition in the observation that homologous areas in the two hemispheres, known from anatomical studies in both monkeys and humans to be anatomically connected through the corpus callosum (Zeki 1970; Pandya et al. 1971; Clarke & Miklossy 1990) are always highly correlated in their ATCs. We have thus suggested that, when there is so little detailed knowledge of the anatomical connections in the human brain, the observation of areas with highly correlated ATCs when subjects undertake specific and complex tasks may be a quick and rough guide to their anatomical connections (Bartels & Zeki 2005).
The combination of techniques that locate cortical activity in time and space when subjects undertake complex tasks or view complex scenes led us to a picture of the overall cortical activity at any given moment, which we refer to as its ‘chronoarchitecture’. The chronoarchitecture of different subjects viewing the same complex scene is remarkably similar at any given moment in time. With hindsight, one may even conjecture that chronoarchitectonic maps should have been guessed a long time ago, from observing myeloarchitectonic maps. These show the pattern of myelination of different cortical zones. Because more heavily myelinated fibres conduct faster, it makes sense to suppose that the heavier myelination of a cortical zone is an indirect indication that it either receives, transmits or processes signals faster than less heavily myelinated areas. Indeed, a simple thought experiment would have convinced anyone that myeloarchitectonic maps were the prelude to chronoarchitectonic maps. But, collectively, we do not seem to be as advanced as physicists in conducting thought experiments. Chronoarchitectonic maps are in fact a good deal more informative than myeloarchitectonic maps, because they show a greater variety of cortical areas than the latter. But it is hard to believe that the basis of the chronoarchitectural picture does not lie in myeloarchitectonic differences, themselves indicative of different conduction velocities.
(e) Reverse hierarchies in the visual brain
The chronoarchitecture of the visual brain does not show any predictable temporal hierarchical order in the activation of areas. Indeed, at first viewing the pattern of activation seems more or less chaotic. This constitutes yet another reason for enquiring more deeply into the question of hierarchy in the visual brain. The hierarchy that one reads into the organization of each of the parallel systems is based on observing the increasing complexity of cell properties or of their receptive field sizes as one proceeds from one area to the next in a chain, for example, from V1 to V2 to V4 or V5. The concept of a hierarchy was indeed derived principally from a study of the increasing complexity of one category of cell, the orientation-selective cells in area V1 (Hubel & Wiesel 1977). It was based on the observation that a set of orientation-selective cells receiving input from an antecedent set of such cells has more complex properties and larger receptive fields, that the orientation-selective cells of V2 have larger receptive fields and are more sophisticated than their counterparts in V1 and that the same category of cells in V3 have yet larger receptive fields (Hubel & Wiesel 1965). It is only when systems other than the form system, based on orientation-selective cells, began to be studied that evidence for another principle dictating the organization of the visual brain—that of functional specialization—began to emerge (Zeki 1978). Indeed, Livingstone & Hubel's (1988) re-examination of the functional architecture of V1, but this time placing an equal emphasis on colour vision and on visual motion as well, led them also to the conclusion that functional specialization is a critical feature of the organization of the primary visual cortex as well, and that its importance as a general organizing principle extends to the whole of vision, just as the earlier studies on the functional specialization of prestriate cortex had postulated (Zeki 1978).
It is this functional specialization that ushers in a different set of hierarchies. In fact, at a certain level of observation, one could easily obtain an impression of hierarchy as an organizing principle if one were to restrict one's study to any one of the specialized parallel systems, not just the form system based on orientation-selective cells. Among these is the observation that, in the colour system, the wavelength-selective cells of V1 are simpler in their response properties than the colour cells of V4 and that the latter are probably simpler than the kind of colour-coding cell found in inferior temporal cortex (Zeki 1983a). It is also true that, unlike the directionally selective cells of V5, the orientation and direction-selective cells of V1 do not seem to be capable of distinguishing the true direction of motion of a stimulus from the motion of its constituent parts (Movshon & Newsome 1996). Yet even accepting that, at a certain level of observation, a hierarchical organization is characteristic of each of the parallel, specialized systems, doubts as to whether each of these systems is strictly hierarchical are raised by a number of recent studies, of which the asynchronous nature of visual perception and the chronoarchitecture of the visual brain are but two. This makes it interesting to enquire whether such a one-way hierarchy is a general principle dictating the organization of each of the parallel systems, or whether, superimposed on it, is another set of hierarchies operating in other temporal directions.
Implicit in the term hierarchy is a temporal order, with one set of cells receiving their visual input before another, in a sequential chain. In terms of temporal order, however, the succession of input from the retina to the LGN, to V1 and then on to, for example, V2 and V5 is not the only one. Another pathway appears to function in the reverse order temporally, activating V5 first and then V1. The evidence in favour of this temporal order is based in part on inactivating areas V1 and V5 with transcranial magnetic pulses (Beckers & Zeki 1995) and observing the effects on the perception of visual motion; it is also based on electroencephalographic (EEG) recordings from areas V1 and V5 after stimulating subjects with fast and slow motion (ffytche et al. 1995a). The former shows that, to compromise the perception of visual motion, the inactivating pulse has to be delivered to V5 some 30–10 ms before the stimulus appears. By contrast, to obtain the same effect from inactivating V1, the inactivating pulse has to be delivered some 50–60 ms after the appearance of the stimulus. The clear implication, verified by EEG recordings, is that signals reach the cortex of V5 before they reach the cortex of V1. The EEG evidence shows further that it is signals from fast moving stimuli (>6° s−1) that reach the cortex of V5 first, whereas those from slow-moving stimuli (<2° s−1) reach the cortex of V1 first (see also Morand et al. 2000). It is worth mentioning in passing that our EEG results confirm two subsequent studies in a significant way: first are the results of Rao et al. (2001) which show that the fast input of signals to the cortex (from fast moving visual stimuli) cannot be observed if the electrodes are placed outside V5, even at nearby sites. For example, at electrode positions 5 and 10 cm dorsal to V5, neither we (ffytche et al. 1995a) nor Rao et al. (2001) could identify a fast visual motion input. This is significant in showing that the fast input is channelled specifically to V5 or at any rate is not widely distributed throughout the cortex. Next are the subsequent results of Schoenfeld et al. (2002), which show, like our earlier ones, that fast delivery of signals to the cortex does not occur with stimuli that move at speeds of 5° s−1 or less. Thus the fast input is also specific as regards the speed of the moving visual stimulus.
Because of the results described above, I have suggested that there is a reverse hierarchy that operates in visual perception (Zeki 2001) and that it is manifest in both physiological and perceptual terms. Physiologically, it is based on the observation that, within a given system, such as, for example, the motion system, signals can reach a station that has been traditionally considered to be ‘higher’ than V1 at a significantly earlier time than they reach V1. One of two pathways, or both, neither of which passes through V1, could account for the fast delivery of signals to V5 and therefore for this reversal. Both had been charted anatomically long before we undertook our experiments. One pathway proceeds from the retina to the superior colliculus, from the latter to the pulvinar and from the pulvinar to V5 (Cragg 1969; Standage & Benevento 1983). The other pathway reaches the cortex of V5 directly from the LGN (Fries 1981; Yukie & Iwai 1981; Sincich et al. 2004). Whatever pathway is used, observation of an input to V5 that is faster than the one to V1 is nevertheless of great interest, for it implies a reversal of the assumed temporal order in visual processing. In addition, it has interesting implications for learning more about the residual vision of the blind and the minimal conditions for visual consciousness (see below).
Perceptually, the reverse hierarchy crosses the boundary between the specialized parallel processing-perceptual systems. Here we can speak of a reverse hierarchy because the EEG evidence shows that motion signals reach the cortex of V5 significantly before colour signals reach the cortex of V4 (Buchner et al. 1994; ffytche et al. 1995a). One would have imagined that, even allowing for the relative independence of these processing-perceptual systems, subjects would therefore perceive motion before they perceive colour. In fact, as the psychophysical experiments that I undertook with Konstantinos Moutoussis demonstrate, the reverse is the case. However one accounts for it—and we have done so by supposing that the processing time to perceive colour is shorter than the one required to perceive motion—it constitutes a reverse hierarchy in physiological terms because of the shorter latencies with which signals reach V5 compared with the latencies with which colour signals reach V4. And it constitutes a reverse hierarchy in perceptual terms because, contrary to one's expectation from the physiology, colour leads motion perceptually.
The set of experiments, both physiological and psychophysical, showing that there is a reverse temporal hierarchy in the visual brain naturally undermines the hierarchical doctrine further. The latter would be inconsistent with the anatomical results which show that the sequential pathway leading to a prestriate visual area is not the only one; inconsistent with the fact that signals arrive in some areas situated higher up the chain before they arrive in others lying lower in the chain, and inconsistent with the facts of perceptual asynchrony.
My proposed reverse hierarchy should not be confused with the reverse hierarchy in visual perception proposed by Hochstein & Ahissar (2002) or the proposals of Lamme and his colleagues (Lamme 2004). Both suppose that the fast input to the higher areas of the visual brain are not capable of leading to a conscious percept without feedback from them to lower areas such as V1. Based on such evidence as my colleagues and I have accumulated, I make the opposite supposition, namely that a direct input to the higher areas may be capable of eliciting a crude but conscious visual percept from an essential node without the mandatory participation of lower areas such as V1 and therefore without the necessity of a feedback input to V1. This is not to suggest that I do not, in broad outline, agree with the notion that a return input to V1 is important for conscious vision in general and may indeed be critical for certain kinds of phenomenal visual experience. If nothing else, the importance of a reverse input is attested by the massive reverse connections from V4 and V5 to V1 and V2 (Shipp & Zeki 1989a,b; Zeki & Shipp 1989) and by studies in patient LM, whose cortical lesions involved the territory of V5 bilaterally, rendering her akinetopsic. Imaging studies show that, when stimulated with visual motion, her apparently normal V1 is much less active than that of normal subjects with similar stimulation, leading us to suggest that a reverse input to V1 is critical (Shipp et al. 1994). However, once again, suggestive evidence is just that—it still must be tested. And the crucial, and indeed, final test of the supposition that a return input to V1 is essential for conscious vision is to test the visual capacities of a patient who has no V1. If my supposition that feedback to V1 is not essential for conscious vision is true, then a patient blinded by a lesion to V1 should be able to experience some sort of vision consciously. That indeed was the next step in the chain of experiments that resulted from the demonstration of functional specialization in the visual brain. The experiment revolves around the more general question of the extent to which these perceptual-processing systems (essential nodes) are dependent upon other areas in generating a conscious visual experience.
(f) The autonomy of the processing-perceptual systems
The differences in the timing of arrival of visual signals in different visual areas, the perceptual asynchrony, as well as the chronoarchitecture of the visual brain described above, all suggest that the visual areas of the brain are not wholly dependent upon one another and indeed enjoy a certain degree of autonomy. This makes it important to learn something about the extent of this autonomy. The question is an awkward one, for it is clear that, in the extreme condition, a given visual area would not be able to function if disconnected from the rest of the brain or indeed the visual brain. Given the extent of the brain, such an experiment must, moreover, remain in the realm of thought only, at least for the foreseeable future. However, we can simplify our search by asking whether a direct, hierarchically organized input from V1 or a return, feedback input to V1, are necessary conditions for the generation of a phenomenal awareness for the attribute for which a given processing-perceptual system is specialized. Data are not abundant, but there is one cortical area at least which delivers a compelling answer, that neither a feed-forward input through V1 nor a return feedback input to it are mandatory for conscious experience. That area is V5, an essential node for the perception of visual motion.
The motion system is a good one to look at, for several reasons. The motion centre in the human visual brain, area V5, is well defined and has been the subject of numerous experiments and demonstrations. The connections of V5 with V1 and with subcortical visual centres are relatively well known and it can be inferred that similar connections also exist in the human. In particular, it is well known that V5 receives a heavily myelinated, convergent, anatomical input from V1 whose major characteristics have been described above (Cragg 1969; Zeki 1969, 1971b), and sends back a diffuse projection to it (Shipp & Zeki 1989a,b).
It is these anatomical results, and especially the return input to V1, that led us to postulate that feedback to V1 is important for integration and consciousness (Zeki & Shipp 1988; Shipp & Zeki 1989a,b). Direct connections between the two areas that provided the best evidence for functional specialization in the visual brain, namely V4 and V5, are much too sparse (Shipp & Zeki 1995). The feedback connections from either area to V1 and V2 looked a good deal more promising. One feature of these feedback connections is that, unlike the forward ‘like-with-like’ connections, the return ones distribute to the territories of all cells in V1, regardless of their specificities (Zeki & Shipp 1989; Shipp & Zeki 1989a,b). Thus, the return input from V5 to V2, for example, distributes to the territory of thin, thick and interstripes, not just the thick stripes from which it receives input. We therefore looked to these return projections as providing the basis for the binding, and accepted, temporarily at least, the supposition that this binding is at the basis of our conscious visual experience (Crick & Koch 1990; Engel et al. 1999; Engel & Singer 2001; Tallon-Baudry 2004), since it is through this binding that all the attributes are brought together and we experience a whole consciously. Indeed, computer experiments by Finkel & Edelman (1989) had shown that a feedback input from V5 to V1 is mandatory for conscious vision, at least in the world of computers. I began to regard feedback as essential for conscious experience of the visual world, and others have since followed. In fact, the unexpected results of further experiments showed that this thinking was faulty.
Our predictions and conjectures have been well verified in recent electrophysiological experiments by Victor Lamme, Hans Super and their colleagues (Lamme 2001, 2004). They have in particular shown that the feed-forward input to V1 is not sufficient to mobilize a figure–ground differential response from V1 cells; to be able to do so, these cells require a further input, the feedback from further visual areas. This in turn has been interpreted to mean that a feedback to V1 is mandatory for visual consciousness (Lamme 2004). However, the demonstration that the receptive field properties of cells in V1 can be enhanced by a feedback input does not tell us whether the latter is essential for conscious vision. Return inputs may be important for elaborating receptive field properties and may indeed be important elements in conscious vision, but whether they are essential is not determined by such experiments. To do so, one has to rely on other experiments, which essentially turn out to be much simpler ones. In fact, there is no better way to begin than by asking whether the feed-forward input from V1 and the return pathway back to V1 are essential for conscious vision than by studying the extent to which a V5 that is isolated from V1 can sustain a conscious awareness of visual motion, however crude. To exclude the feed-forward output from V1 to V5, as well as the reciprocal connections between the two, from being essential for conscious experience of visual stimuli amounts to excluding a lot, and thus bringing us slightly closer to determining the minimum requirements for conscious experience.
It sometimes happens that humans blinded by damage to V1 can still see motion and are aware of having seen the moving stimuli consciously. This was first demonstrated by Riddoch (1917; see above). We have made EEG recordings from area V5 of normal humans and one subject, GY, who was blinded by a lesion in V1 (figure 20). We used this particular patient because we had ascertained in a previous study that he perceives visual motion consciously, even when it was presented to his blind field (Barbur et al. 1993). The recordings showed that traces obtained from his V5 from stimulation of his blind field with high contrast and fast visual motion were similar, though not identical, to traces obtained from normals. By contrast when control conditions, including slow motion, were used to stimulate his blind field the traces were highly abnormal compared with that obtained from normals (ffytche et al. 1996).
Figure 20.
The hemianopic (left) hemifield of patient GY, with macular sparing (centre), and the causative lesion in the left occipital lobe (right) that he sustained during childhood.
I chose GY also because he had been studied in great detail and had been described as a ‘blindsight’ patient, that is, one who can discriminate visual stimuli correctly without being consciously aware of having seen them (Weiskrantz 1986). GY had sustained a massive lesion to his left hemisphere, including especially the occipital lobe, in a car accident during childhood. This left him blind in one hemifield except for a macular sparing. By contrast, he can see stimuli presented to his intact hemifield well. I imagined, according to the doctrine current at the time, that when GY was able to discriminate visual stimuli presented to his blind hemifield correctly without being consciously aware of them, the activity in his brain would be restricted to the subcortex. By contrast, stimuli presented to his healthy hemifield, which he could discriminate correctly and was aware of, would activate his cortex. Thus, through imaging techniques, one would be able to demonstrate the dissociation between correct discrimination and awareness, with the latter being the province of the cerebral cortex, or so I imagined. However, the experiment took a different and unexpected turn. When we started examining him in 1992, I found to my great surprise that GY was able, right from the start, to report correctly—either verbally or through a keypad—the correct direction of motion of high contrast, fast-moving stimuli. That he was able to do so verbally shows at once that he was conscious of these visual stimuli. In our experience, which differs significantly from that of Weiskrantz and his colleagues (Weiskrantz 1986), GY was always good at discriminating the direction of motion of high contrast, fast moving stimuli when he was aware of them, and usually at chance in his discrimination when he was not aware of them. We found no evidence for a dissociation of awareness from correct discrimination (Zeki & ffytche 1998). His description of what he experienced was interesting: he invariably described the moving stimulus as a shadow, a description very much reminiscent of what Riddoch's patients had given. We therefore concluded that GY was conscious of the visual stimulus, that he experienced it phenomenally and that this capacity was not the result of the long time that had elapsed between the dates of cortical damage and our examination of its effects (Barbur et al. 1993; Zeki & ffytche 1998). This critical result was later confirmed by Weiskrantz et al. (1995), but arguments followed as to whether his conscious experience, though triggered by a visual stimulus, was indeed visual and, for a time, a strong position taken was that it was not possible to have a conscious experience without the participation of V1 (Stoerig & Cowey 1995), thus upholding earlier views that the prestriate cortex is not conscious without the active participation of V1 (Weiskrantz 1990). More recently, in elegant psychophysical experiments, Stoerig & Barth (2001) have shown that GY's conscious experience is indeed visual in nature, however crude it may be.
In conclusion, neither a feed-forward output from V1 to V5 nor a feedback return connection from V5 to V1 are essential for conscious vision.
These psychophysical observations were supplemented by imaging experiments which showed that, when GY could discriminate moving stimuli correctly and was aware of them, the activity in his brain was confined to area V5, the area that is specialized for visual motion (figure 21). There was obviously no activity in the ipsilateral V1, which was damaged (Barbur et al. 1993; Zeki & ffytche 1998). It follows that activity in V5, without the participation of V1, is enough to generate a conscious experience of visual motion. The experiment obviously also shows that a return input to V5 from V1 is not necessary for conscious experience, since in the absence of V1 there can be no return input to it. The visual experience is deeply impoverished (see above) but it is nevertheless conscious. This constitutes a direct imaging demonstration that a feedback to V1 from V5 is not essential for conscious vision, from which it follows that a direct input to V5 that bypasses V1 is enough to generate a crude but conscious visual experience. Note that I am not pretending that feedback in general is not essential for conscious vision, but only that feedback to V1 is not essential for it.
Figure 21.
The results of imaging experiments on patient GY and normals. (a) The activity produced in the contrast fast motion (which GY is conscious of and can discriminate correctly) versus slow motion (which he is not conscious of and cannot discriminate). The activity, shown in yellow on horizontal sections (left) and coronal ones (right), is restricted to the territory of V5. (b) In normal subjects (who, unlike GY, have a V1), the activity is found in both V1 and V5. (c) The results of presenting GY with slow motion (which he is not aware of and cannot discriminate) and contrasting that with the grey screen. Now, activity is found again in V5. This suggests that, although signals reach V5 in the slow motion condition, they are not potent enough to elicit activity in V5 that is strong enough to result in a conscious correlate. (From Zeki & ffytche 1998.)
The issue is important enough to dwell briefly on dissenting experiments. An experiment by Pascual-Leone & Walsh (2001) has been interpreted by its authors and others to mean that a return input to V1 from V5 is necessary for a conscious visual experience, in contradiction to the conclusion reached above. These authors reported that if an inactivating transcranial magnetic pulse is delivered to V5 before stimulation of V1, then subjects cannot experience the moving phosphenes that are produced by stimulation of V1 alone. Although interesting, these experiments do not prove that a return input from V5 to V1 is necessary for visual experience as so many have assumed (e.g. Nguyen 2001; Rees et al. 2002). In the first place, it is very doubtful that the inactivating stimulus was delivered to V5. The coordinates chosen are given as 4 cm lateral and 2 cm rostral to the inion, a position that does not coincide with V5. Indeed, in a previous experiment, this position was chosen as a control position, since delivery of an inactivating pulse there did not have any effects on visual motion (Beckers & Zeki 1995). This is consistent with the results obtained in the Pasquale–Leone and Walsh study, since they could not obtain any effects in 18 out of 26 subjects; in the remaining eight, the effects were not total in five. It is more likely that they were delivering inactivating pulses to area V3, which is often found to be engaged when subjects view moving stimuli. All this makes it doubtful that the stimuli were being delivered to V5. And, given that phosphenes are an artificially induced phenomenon usually ascribed to V1, it also becomes doubtful whether the result can be interpreted to mean that activity in prestriate cortex can only achieve conscious status if the relevant area feeds its input back to V1. I repeat once again: feedback connections to V1 may be important, they may enrich conscious vision, but they are not essential for generating a crude visual consciousness, and therefore not essential for visual consciousness.
More recent experiments using transcranial magnetic stimulation to induce phosphenes in subjects have also concluded that V1 is essential for conscious visual awareness (Silvanto et al. 2005a,b). One criticism of these studies is that the phosphenes are artificially induced. Another criticism is that neither study confronts the serious issue of how it is that a patient without a V1 can experience visual motion consciously. The latter, after all, is the ultimate experiment in this domain.
(g) The relationship of conscious awareness to strength of activity at an essential node
In fact, the imaging experiments on GY revealed another critical feature. Although the contrast of fast motion versus slow motion (that is what he could consciously perceive and discriminate versus what he could neither discriminate nor see) revealed activity in V5 alone, the contrast of slow motion versus grey screen revealed some activity in V5 (Zeki & ffytche 1998; figure 21). This suggested to us that whereas signals from slowly moving stimuli reach the cortex of V5 even in the absence of V1, they are not potent enough to either enable GY to discriminate their direction or become consciously aware of them This in turn led us to the conclusion that the difference between processing that has a conscious correlate and one that does not is simply a difference in strength of activation of the relevant cortical area, though we do not yet know whether this added strength is due to the recruitment of previously inactive cells or the heightened responses of already active ones.
In fact, a positive correlation between strength of activation at a given essential node or nodes has now been confirmed in other studies. These include not only our direct demonstration that processing sites are also perceptual sites (Moutoussis & Zeki 2002a) but also studies in both the visual (Rees et al. 2000b) and non-visual (Dehaene et al. 2001) domains.
(h) The parieto-frontal network of areas
If an area such as V5, though crippled by being disconnected from V1, can still function sufficiently for activity within it to result in a conscious correlate, it is worth extending the search to enquire what other areas may be necessary for activity in it to have a conscious correlate. Since we are dealing with a knowledge-acquiring system, it is natural to want to learn how essential the frontal cortex is for the generation of a conscious correlate. In fact, the so-called fronto-parietal network of areas, together with the cingulate cortex, has been considered to be critical and essential for conscious vision, constituting a sort of ill-defined conscious ‘work space’ (Dehaene & Naccache 2001). The somewhat vague nomenclature, of a network of areas, underlies the fact that the precise number of areas involved, their disposition and interrelations are not known. The evidence for their involvement is, moreover, largely derived from imaging studies. One difficulty with such studies is that, in the contrasts made, an area that may be active to a certain low level may not show up; excluding such an area from involvement may therefore be a mistake. Within these constraints, the mandatory involvement of this network of areas is not very promising. Human imaging experiments which have compared brain activity in eyes open versus eyes closed condition, when one would expect that a sudden conscious experience of the visual world would engage the frontal cortex or other areas in this putative work space, have not detected activity in the frontal-parietal network (Zeki et al. 1991; Marx et al. 2004). Moreover, in dichoptic viewing experiments that are so arranged that subjects sometimes consciously see the stimulus and sometimes not, imaging experiments show that it is only when subjects do not see the stimulus (though it is processed) that the frontal cortex is active; when they see it and can report it correctly, the frontal cortex is not active (Moutoussis & Zeki 2002b). Similar results have been obtained by ffytche & Pins (2003). It therefore is entirely possible that, in generating a phenomenal awareness, the specialized visual areas of the brain are not dependent upon the hypothetical work space.
Thus, present evidence indicates that activity in the essential nodes can generate a phenomenal conscious correlate without involvement of either V1 or the fronto-parietal cortex. This does not take into account reportability, which may (and probably does) involve further cortical areas and especially the frontal cortex. Indeed, recent imaging experiments suggest that the frontal cortex is recruited during access consciousness (Marois et al. 2004).
It needs to be emphasized, however, that in our experiments on the neural correlates of conscious visual motion, V5 was not acting in total isolation. When we used the contrast aware versus unaware, to show the activity in GY's brain when he reported himself to be aware of the stimulus (irrespective of whether there was actually a stimulus there), we found activity to be mainly in the pontine reticular formation (Zeki & ffytche 1998; figure 22). We regard this as an enabling system, without which V5 (and presumably other cortical areas) could not function.
Figure 22.
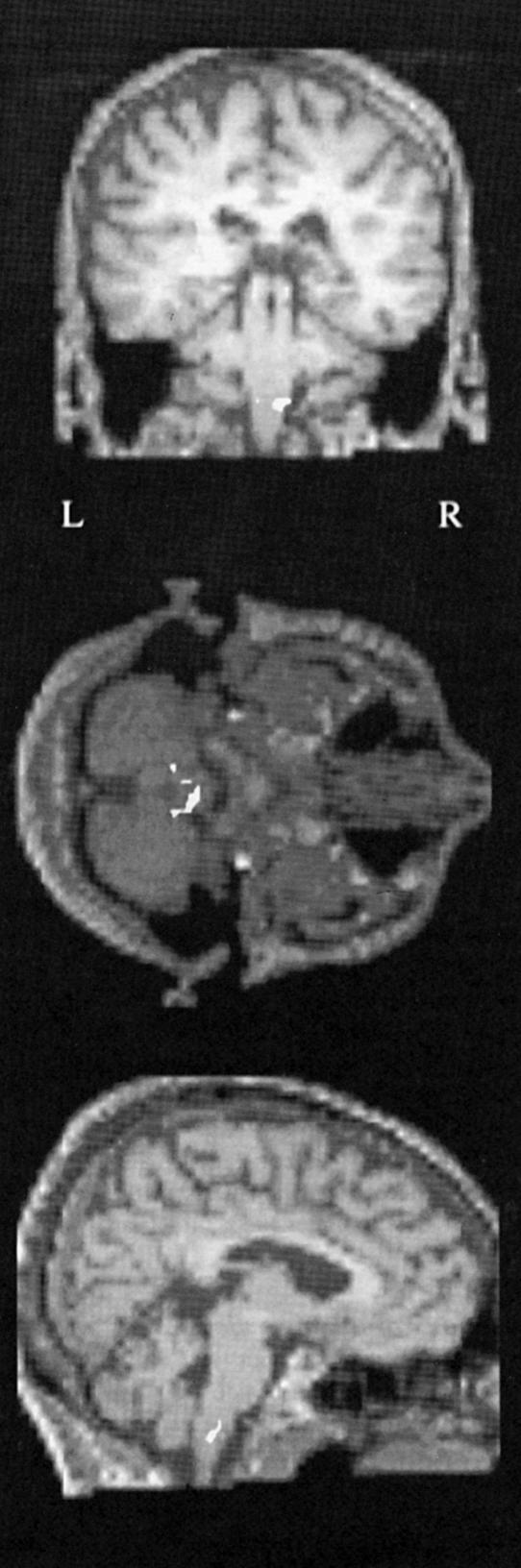
The results of comparing the activity produced in the brain of GY for stimuli that he reported to be aware of versus stimuli that he reported to be unaware of. The activity is located in the pontine reticular formation (in white) and is shown in transverse, coronal and saggital section, from above downwards. (From Zeki & ffytche 1998.)
Whether these observations on the motion system can be generalized to other processing–perceptual systems, even including the colour system, remains unknown. There is one unverified suggestion from the work of Blythe et al. (1987) that a patient blinded by lesions to V1 could still experience colours consciously, but the result has not been confirmed by others and must remain conjectural.
This gives us a fair hint of the degree of autonomy of the individual visual areas (essential nodes). The processing that each undertakes, and the speed of that processing, is largely independent of the processing that other areas undertake, with speeds that are uniquely tailored to the demands of each. Damage to one of the essential nodes leads to a deficit restricted very largely, if not exclusively, to the attribute for which it is specialized. Each one terminates its perceptual task at a different, and characteristic, time. And one of them (V5) at least can generate a crude but conscious visual experience for the attribute for which it is specialized, without participation of V1 with which it is reciprocally connected. The exclusion of other areas in the hypothetical work space is not based on as solid a foundation as the exclusion of a return input to V1. Future experiments may yet show that one or perhaps more components of this hypothetical work space are involved, but present evidence does not.
(i) The theory of microconsciousnesses
Pulling these results together leads us to the following conclusions about the operations of the visual brain. Again, I illustrate the point by reference to the colour and motion systems, the two systems with which I am most associated. The conclusions drawn from these two systems, if true, have general validity in that the statements that we may make using them cannot be easily falsified by discoveries about other systems.
That there is a functional specialization in the brain is more than adequately proven by the demonstration that two geographically distinct areas are engaged, respectively, when subjects view colour or visual motion stimuli.
Since we perceive colour before we perceive motion by a significant amount of time, it follows that there is a temporal hierarchy in visual perception.
Since perceiving something is tantamount to being conscious of it, and since we perceive different attributes at different times, it becomes necessary to suppose that we become conscious of different attributes at different times. From which it follows that consciousness is distributed in time.
Since consciousness of different attributes is due to activity at different, geographically distinct, essential nodes, it follows that consciousness is distributed in space as well.
We may thus say that visual consciousness is not a single unified entity, implicit in the term ‘unity of consciousness’ that is so often used. Visual consciousness consists instead of different microconsciousnesses, each one due to activity at a specific cortical site.
Microconsciousnesses for different attributes, e.g. form and colour, can be bound to constitute a macroconsciousness. There is therefore a hierarchy of visual consciousnesses with the unified consciousness, that of myself as the perceiving person, sitting at the apex.
The above leads us to the theory of microconsciousness (Zeki 2003b), which supposes that activity at each of the many essential nodes (see above) can have a conscious correlate, if that activity is strong enough. Although vague in terms of whether the heightened activation is due to a more vigorous response of cells that are already engaged in processing a stimulus or whether it is due to the recruitment of new cells, there is nevertheless compelling evidence in favour of this activation strength hypothesis, which is reviewed above.
It is always dangerous to read into past descriptions insights gained from novel experiments and thinking, but it is worth drawing attention to a critical passage in Kant's (1781) Critique of Pure Reason (Trans. Pluhar 1996) which can easily be interpreted as an older version of the theory of microconsciousness. In a footnote to the 1781 edition of his book, he wrote: ‘All presentations have perhaps a necessary relation to empirical consciousness…but all empirical consciousness has a necessary relation to the transcendental synthetic consciousness, viz of myself as the source of the perceptions’. Here I disagree only with the suggestion that the empirical (micro) consciousness has a necessary reference to the unified, transcendental, consciousness.
It follows from what I have described that consciousness is not the preserve or characteristic of the human race; animals are conscious as well. However, only humans are conscious of being conscious and that faculty is only achieved through the use of language and communication. Moreover, there is a law of exclusivity that applies to the synthetic consciousness just as much as it does to microconsciousness, in that one cannot be simultaneously conscious of say the colour of an object and assign the consciousness to oneself as the perceiving person.
3. Conclusion
Thus from simple anatomical beginnings profound conclusions follow and the whole hangs together in a compelling way.
This brings us a little, but not much, closer to understanding the nature of that ‘quintessence of dust’ that preoccupied Shakespeare, and then only in a negative sense. It brings us closer in the sense that we begin to understand that it may be quite illusory to pursue the dream of understanding the nature of consciousness, and thus of humans themselves, by supposing that there is a unified consciousness; it may be quite illusory to suppose that, even within a single system such as the visual, charting the detailed neural paths that result in a conscious correlate for colour would lead to an understanding of the neural correlates of conscious vision in general, although it is of course entirely plausible that, in addition to differences, some common neural mechanism is used in generating a conscious correlate in different, specialized, areas. It leads us, therefore, to separate that characteristic of mankind, the quintessence of dust, from the characteristic that we share with other animals, namely to become microconscious of discrete events in our world and thus to acquire knowledge about them and guide our actions accordingly. It removes the Shakespearean question to a different and higher dimension, one that can only be resolved through understanding the neural basis of language, thought and communication.
Although I delivered my Ferrier Lecture in 1995, I thought it worthwhile to compete with David Hubel and Torsten Wiesel, who submitted the typescript of their lecture 5 years after delivering their lecture. Conveniently for me, my appointment as Editor of Philosophical Transactions in 1997 made it inappropriate that I should submit my lecture to the journal of which I was then editor, or so I liked to pretend. Fortunately, this meant that I could include a lot of material that my colleagues and I gathered since 1995, which makes the story of the visual brain even more exciting and challenging. It also meant that I could submit the manuscript after a delay that is twice as long as that of David Hubel and Torsten Wiesel.
Acknowledgments
I would like to record my great appreciation to colleagues with whom I have discussed the work covered here or with whom I have collaborated. I owe special thanks to the following colleagues, for the inspiration that I derived from being able to work with them: Andreas Bartels, Georg Beckers, Dominic ffytche, Wolfgang Fries, Konstantinos Moutoussis, David Sandeman and Stewart Shipp. I thank Brian Wandell, Alex Wade and Nikos Logothetis for permission to illustrate their recent imaging experiments. I thank Anton Burdakov for his generous help in preparing the manuscript for publication. My work has been generously supported over many years by the Wellcome Trust, London.
References
- Albright T.D. Direction and orientation selectivity of neurons in visual area MT of the macaque. J. Neurophysiol. 1984;52:1106–1130. doi: 10.1152/jn.1984.52.6.1106. [DOI] [PubMed] [Google Scholar]
- Allman J.M, Kaas J.H. Representation of the visual field in striate and adjoining cortex of the owl monkey (Aotus trivirgatus) Brain Res. 1971;35:89–106. doi: 10.1016/0006-8993(71)90596-8. [DOI] [PubMed] [Google Scholar]
- Arnold D.H, Clifford C.W. Determinants of asynchronous processing in vision. Proc. R. Soc. B. 2002;269:579–583. doi: 10.1098/rspb.2001.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold D.H, Clifford C.W, Wenderoth P. Asynchronous processing in vision: color leads motion. Curr. Biol. 2001;11:596–600. doi: 10.1016/s0960-9822(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Barbur J.L, Watson J.D, Frackowiak R.S, Zeki S. Conscious visual perception without V1. Brain. 1993;116:1293–1302. doi: 10.1093/brain/116.6.1293. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The theory of multistage integration in the visual brain. Proc. R. Soc. B. 1998;265:2327–2332. doi: 10.1098/rspb.1998.0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The architecture of the colour centre in the human visual brain: new results and a review. Eur. J. Neurosci. 2000;12:172–193. doi: 10.1046/j.1460-9568.2000.00905.x. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. Functional brain mapping during free viewing of natural scenes. Hum. Brain Mapp. 2004;21:75–85. doi: 10.1002/hbm.10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The chronoarchitecture of the human brain—natural viewing conditions reveal a time-based anatomy of the brain. Neuroimage. 2004;22:419–433. doi: 10.1016/j.neuroimage.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. Brain dynamics during natural viewing conditions—a new guide for mapping connectivity in vivo. Neuroimage. 2005;24:339–349. doi: 10.1016/j.neuroimage.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Beauchamp M.S, Haxby J.V, Rosen A.C, DeYoe E.A.A. MRI functional case study of acquired cerebral dyschromatopsia. Neuropsychologia. 2000;38:1170–1179. doi: 10.1016/s0028-3932(00)00017-8. [DOI] [PubMed] [Google Scholar]
- Beckers G, Zeki S. The consequences of inactivating areas V1 and V5 on visual motion perception. Brain. 1995;118:49–60. doi: 10.1093/brain/118.1.49. [DOI] [PubMed] [Google Scholar]
- Bell A.J, Sejnowski T.J. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Blythe I.M, Kennard C, Ruddock K.H. Residual vision in patients with retrogeniculate lesions of the visual pathways. Brain. 1987;110:887–905. doi: 10.1093/brain/110.4.887. [DOI] [PubMed] [Google Scholar]
- Bolton J.S. The exact histological localisation of the visual area of the human cerebral cortex. Phil. Trans. R. Soc. B. 1900;193:165–222. [Google Scholar]
- Buchner H, Weyen U, Frackowiak R.S, Romaya J, Zeki S. The timing of visual evoked potential activity in human area V4. Proc. R. Soc. B. 1994;257:99–104. doi: 10.1098/rspb.1994.0100. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D, Adali T, McGinty V.B, Pekar J.J, Watson T.D, Pearlson G.D. fMRI activation in a visual-perception task: network of areas detected using the general linear model and independent components analysis. Neuroimage. 2001;14:1080–1088. doi: 10.1006/nimg.2001.0921. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D, Adali T, Pearlson G.D, Pekar J.J. Spatial and temporal independent component analysis of functional MRI data containing a pair of task-related waveforms. Hum. Brain Mapp. 2001;13:43–53. doi: 10.1002/hbm.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A.W. Cambridge University Press; 1905. Histological studies on the localisation of cerebral function. [Google Scholar]
- Cavanagh P, Henaff M.A, Michel F, Landis T, Troscianko T, Intriligator J. Complete sparing of high-contrast color input to motion perception in cortical color blindness. Nat. Neurosci. 1998;1:242–247. doi: 10.1038/688. [DOI] [PubMed] [Google Scholar]
- Clare M.H, Bishop G.H. Responses from an association area secondarily activated from optic cortex. J. Neurophysiol. 1954;17:271–277. doi: 10.1152/jn.1954.17.3.271. [DOI] [PubMed] [Google Scholar]
- Clarke S, Miklossy J. Occipital cortex in man: organization of callosal connections, related myelo- and cytoarchitecture, and putative boundaries of functional visual areas. J. Comp. Neurol. 1990;298:188–214. doi: 10.1002/cne.902980205. [DOI] [PubMed] [Google Scholar]
- Collin N.G, Cowey A. The effect of ablation of frontal eye-fields and superior colliculi on visual stability and movement discrimination in rhesus monkeys. Exp. Brain Res. 1980;40:251–260. doi: 10.1007/BF00237789. [DOI] [PubMed] [Google Scholar]
- Cragg B.G. The topography of the afferent projections in the circumstriate visual cortex of the monkey studied by the Nauta method. Vision Res. 1969;9:733–747. doi: 10.1016/0042-6989(69)90011-x. [DOI] [PubMed] [Google Scholar]
- Crick F, Koch C. Towards a neurobiological theory of consciousness. Semin. Neurosci. 1990;2:263–275. [Google Scholar]
- Crick F, Koch C. Are we aware of neural activity in primary visual cortex? Nature. 1995;375:121–123. doi: 10.1038/375121a0. [DOI] [PubMed] [Google Scholar]
- Damasio A. Disorders of complex visual processing: agnosias, achromatopsia, Balint's syndrome, and related difficulties of orientation and construction. In: Mesulam M.M, editor. Principles of behavioral neurology. Davis; Philadelphia: 1985. [Google Scholar]
- Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition. 2001;79:1–37. doi: 10.1016/s0010-0277(00)00123-2. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Bihan D.L, Mangin J.F, Poline J.B, Riviere D. Cerebral mechanisms of word masking and unconscious repetition priming. Nat. Neurosci. 2001;4:752–758. doi: 10.1038/89551. [DOI] [PubMed] [Google Scholar]
- Desimone R, Schein S.J. Visual properties of neurons in area V4 of the macaque: sensitivity to stimulus form. J. Neurophysiol. 1987;57:835–868. doi: 10.1152/jn.1987.57.3.835. [DOI] [PubMed] [Google Scholar]
- DeYoe E.A, Van Essen D.C. Segregation of efferent connections and receptive field properties in visual area V2 of the macaque. Nature. 1985;317:58–61. doi: 10.1038/317058a0. [DOI] [PubMed] [Google Scholar]
- Dineen J, Keating E.G. The primate visual system after bilateral removal of striate cortex. Survival of complex pattern vision. Exp. Brain Res. 1981;41:338–345. doi: 10.1007/BF00238891. [DOI] [PubMed] [Google Scholar]
- Dubner R, Zeki S.M. Response properties and receptive fields of cells in an anatomically defined region of the superior temporal sulcus in the monkey. Brain Res. 1971;35:528–532. doi: 10.1016/0006-8993(71)90494-x. [DOI] [PubMed] [Google Scholar]
- Engel A.K, Singer W. Temporal binding and the neural correlates of sensory awareness. Trends Cogn. Sci. 2001;5:16–25. doi: 10.1016/s1364-6613(00)01568-0. [DOI] [PubMed] [Google Scholar]
- Engel A.K, Fries P, Konig P, Brecht M, Singer W. Temporal binding, binocular rivalry, and consciousness. Conscious Cogn. 1999;8:128–151. doi: 10.1006/ccog.1999.0389. [DOI] [PubMed] [Google Scholar]
- ffytche D, Pins D. Are neural correlates of visual consciousness retinotopic? Neuroreport. 2003;14:2011–2014. doi: 10.1097/00001756-200311140-00001. [DOI] [PubMed] [Google Scholar]
- ffytche D.H, Zeki S. Brain activity related to the perception of illusory contours. Neuroimage. 1996;3:104–108. doi: 10.1006/nimg.1996.0012. [DOI] [PubMed] [Google Scholar]
- ffytche D.H, Guy C.N, Zeki S. The parallel visual motion inputs into areas V1 and V5 of human cerebral cortex. Brain. 1995;118:1375–1394. doi: 10.1093/brain/118.6.1375. [DOI] [PubMed] [Google Scholar]
- ffytche D.H, Skidmore B.D, Zeki S. Motion-from-hue activates area V5 of human visual cortex. Proc. R. Soc. B. 1995;260:353–358. doi: 10.1098/rspb.1995.0104. [DOI] [PubMed] [Google Scholar]
- ffytche D.H, Guy C.N, Zeki S. Motion specific responses from a blind hemifield. Brain. 1996;119:1971–1982. doi: 10.1093/brain/119.6.1971. [DOI] [PubMed] [Google Scholar]
- Finkel L.H, Edelman G.M. Integration of distributed cortical systems by reentry: a computer simulation of interactive functionally segregated visual areas. J. Neurosci. 1989;9:3188–3208. doi: 10.1523/JNEUROSCI.09-09-03188.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flechsig P. Developmental (mylogenetic) localisation of the cerebral cortex in the human subject. Lancet. 1901;ii:1027–1029. [Google Scholar]
- Flechsig P. Fifth International Psychology Congress. 1905. Gehirnphsyiologie und Willenstheorien. pp. 73–89. [Google Scholar]
- Flechsig P. Thieme; Leipzig: 1920. Anatomie des menschlichen Gehirns und Ru¨ckenmarks. [Google Scholar]
- Fries W. The projection from the lateral geniculate nucleus to the prestriate cortex of the macaque monkey. Proc. R. Soc. B. 1981;213:73–86. doi: 10.1098/rspb.1981.0054. [DOI] [PubMed] [Google Scholar]
- Gattass R, Sousa A.P, Gross C.G. Visuotopic organization and extent of V3 and V4 of the macaque. J. Neurosci. 1988;8:1831–1845. doi: 10.1523/JNEUROSCI.08-06-01831.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowers W. J. & A. Churchill; London: 1887. Lectures on the diagnosis of diseases of the brain. [Google Scholar]
- Gowers W. J. & A. Churchill; London: 1888. A manual of diseases of the brain. [Google Scholar]
- Gregory R.L. Cognitive contours. Nature. 1972;238:51–52. doi: 10.1038/238051a0. [DOI] [PubMed] [Google Scholar]
- Gulyas B, Heywood C.A, Popplewell D.A, Roland P.E, Cowey A. Visual form discrimination from color or motion cues: functional anatomy by positron emission tomography. Proc. Natl Acad. Sci. USA. 1994;91:9965–9969. doi: 10.1073/pnas.91.21.9965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Liu A.K, Dale A.M, Cavanagh P, Tootell R.B. Retinotopy and color sensitivity in human visual cortical area V8. Nat. Neurosci. 1998;1:235–241. doi: 10.1038/681. [DOI] [PubMed] [Google Scholar]
- Helmholtz Hv. L. Voss; Leipzig: 1911. Handbuch der Phsyiologischen Optik. [Google Scholar]
- Henschen S. Verlag des Verfassers; Stockholm: 1930. Pathologie des Gehirns, 8, I. Lichtsinn—und Farbensinnzellen im Gehirn. [Google Scholar]
- Hering E. Springer; Berlin: 1920. Gründzuge der Lehre vom Lichtsinn. [Google Scholar]
- Hess R.H, Baker C.L, Jr, Zihl J. The ‘motion-blind’ patient: low-level spatial and temporal filters. J. Neurosci. 1989;9:1628–1640. doi: 10.1523/JNEUROSCI.09-05-01628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood C, Cowey A. With color in mind. Nat. Neurosci. 1998;1:171–173. doi: 10.1038/617. [DOI] [PubMed] [Google Scholar]
- Heywood C.A, Gadotti A, Cowey A. Cortical area V4 and its role in the perception of color. J. Neurosci. 1992;12:4056–4065. doi: 10.1523/JNEUROSCI.12-10-04056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood C.A, Gaffan D, Cowey A. Cerebral achromatopsia in monkeys. Eur. J. Neurosci. 1995;7:1064–1073. doi: 10.1111/j.1460-9568.1995.tb01093.x. [DOI] [PubMed] [Google Scholar]
- Hirsch J, DeLaPaz R.L, Relkin N.R, Victor J, Kim K, Li T, Borden P, Rubin N, Shapley R. Illusory contours activate specific regions in human visual cortex: evidence from functional magnetic resonance imaging. Proc. Natl Acad. Sci. USA. 1995;92:6469–6473. doi: 10.1073/pnas.92.14.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S, Ahissar M. View from the top: hierarchies and reverse hierarchies in the visual system. Neuron. 2002;36:791–804. doi: 10.1016/s0896-6273(02)01091-7. [DOI] [PubMed] [Google Scholar]
- Holmes G. Disturbances of vision by cerebral lesion. Br. J. Ophthalmol. 1918;2:353–384. doi: 10.1136/bjo.2.7.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes G. The Ferrier Lecture. The organization of the visual cortex in man. Proc. R. Soc. B. 1945;132:348–361. [Google Scholar]
- Horton J.C. Cytochrome oxidase patches: a new cytoarchitectonic feature of monkey visual cortex. Phil. Trans. R Soc. B. 1984;304:199–253. doi: 10.1098/rstb.1984.0021. [DOI] [PubMed] [Google Scholar]
- Horton J.C, Hedley-Whyte E.T. Mapping of cytochrome oxidase patches and ocular dominance columns in human visual cortex. Phil. Trans. R. Soc. B. 1984;304:255–272. doi: 10.1098/rstb.1984.0022. [DOI] [PubMed] [Google Scholar]
- Howard R.J, Brammer M, Wright I, Woodruff P.W, Bullmore E.T, Zeki S. A direct demonstration of functional specialization within motion-related visual and auditory cortex of the human brain. Curr. Biol. 1996;6:1015–1019. doi: 10.1016/s0960-9822(02)00646-2. [DOI] [PubMed] [Google Scholar]
- Hubel D.H, Livingstone M.S. Complex-unoriented cells in a subregion of primate area 18. Nature. 1985;315:325–327. doi: 10.1038/315325a0. [DOI] [PubMed] [Google Scholar]
- Hubel D.H, Wiesel T.N. Receptive fields and functional architecture in two nonstriate visual areas (18 and 19) of the cat. J. Neurophysiol. 1965;28:229–289. doi: 10.1152/jn.1965.28.2.229. [DOI] [PubMed] [Google Scholar]
- Hubel D.H, Wiesel T.N. Visual area of the lateral suprasylvian gyrus (Clare-Bishop area) of the cat. J. Physiol. 1969;202:251–260. doi: 10.1113/jphysiol.1969.sp008808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D.H, Wiesel T.N. The Ferrier lecture. Functional architecture of macaque monkey visual cortex. Proc. R. Soc. B. 1977;198:1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Hubener M, Bolz J. Relationships between dendritic morphology and cytochrome oxidase compartments in monkey striate cortex. J. Comp. Neurol. 1992;324:67–80. doi: 10.1002/cne.903240106. [DOI] [PubMed] [Google Scholar]
- Huxlin K.R, Saunders R.C, Marchionini D, Pham H.A, Merigan W.H. Perceptual deficits after lesions of inferotemporal cortex in macaques. Cereb. Cortex. 2000;10:671–683. doi: 10.1093/cercor/10.7.671. [DOI] [PubMed] [Google Scholar]
- Jen L.S, Zeki S. High cytochrome oxidase content of the V5 complex of macaque monkey visual cortex. J. Physiol. 1984;348:23. [Google Scholar]
- Kant I. Hackett; Indianapolis: 1781. Kritik der reinen Vernunft. [Trans. W.S. Pluhar (1996) as Critique of Pure Reason.] [Google Scholar]
- Kastner S, De Weerd P, Desimone R, Ungerleider L.G. Mechanisms of directed attention in the human extrastriate cortex as revealed by functional MRI. Science. 1998;282:108–111. doi: 10.1126/science.282.5386.108. [DOI] [PubMed] [Google Scholar]
- Kennard C, Lawden M, Morland A.B, Ruddock K.H. Colour identification and colour constancy are impaired in a patient with incomplete achromatopsia associated with prestriate cortical lesions. Proc. R. Soc. B. 1995;260:169–175. doi: 10.1098/rspb.1995.0076. [DOI] [PubMed] [Google Scholar]
- Komatsu H, Ideura Y, Kaji S, Yamane S. Color selectivity of neurons in the inferior temporal cortex of the awake macaque monkey. J. Neurosci. 1992;12:408–424. doi: 10.1523/JNEUROSCI.12-02-00408.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger J, Gouras P. Spectral selectivity of cells and its dependence on slit length in monkey visual cortex. J. Neurophysiol. 1980;43:1055–1069. doi: 10.1152/jn.1980.43.4.1055. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Moutoussis K, Zeki S. The effect of background colors on the tuning of color senstive cells in monkey area V4. Submitted doi: 10.1152/jn.00597.2005. [DOI] [PubMed] [Google Scholar]
- Lamme V.A. Blindsight: the role of feedforward and feedback corticocortical connections. Acta Psychol. (Amst.) 2001;107:209–228. doi: 10.1016/s0001-6918(01)00020-8. [DOI] [PubMed] [Google Scholar]
- Lamme V.A. Separate neural definitions of visual consciousness and visual attention; a case for phenomenal awareness. Neural Netw. 2004;17:861–872. doi: 10.1016/j.neunet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Lamme V.A, Zipser K, Spekreijse H. Masking interrupts figure-ground signals in V1. J. Cogn. Neurosci. 2002;14:1044–1053. doi: 10.1162/089892902320474490. [DOI] [PubMed] [Google Scholar]
- Land E. The retinex theory of colour vision. Proc. R. Inst. Gt Britain. 1974;47:23–58. [Google Scholar]
- Land E.H, McCann J.J. Lightness and retinex theory. J. Opt. Soc. Am. 1971;61:1–11. doi: 10.1364/josa.61.000001. [DOI] [PubMed] [Google Scholar]
- Larsson J, Amunts K, Gulyas B, Malikovic A, Zilles K, Roland P.E. Neuronal correlates of real and illusory contour perception: functional anatomy with PET. Eur. J. Neurosci. 1999;11:4024–4036. doi: 10.1046/j.1460-9568.1999.00805.x. [DOI] [PubMed] [Google Scholar]
- Lennie P, Krauskopf J, Sclar G. Chromatic mechanisms in striate cortex of macaque. J. Neurosci. 1990;10:649–669. doi: 10.1523/JNEUROSCI.10-02-00649.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal A.G, Thompson K.G, Liu D, Zhou Y, Ault S.J. Concomitant sensitivity to orientation, direction, and color of cells in layers 2, 3, and 4 of monkey striate cortex. J. Neurosci. 1995;15:1808–1818. doi: 10.1523/JNEUROSCI.15-03-01808.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt J.B, Yoshioka T, Lund J.S. Intrinsic cortical connections in macaque visual area V2: evidence for interaction between different functional streams. J. Comp. Neurol. 1994;342:551–570. doi: 10.1002/cne.903420405. [DOI] [PubMed] [Google Scholar]
- Livingstone M.S, Hubel D.H. Anatomy and physiology of a color system in the primate visual cortex. J. Neurosci. 1984;4:309–356. doi: 10.1523/JNEUROSCI.04-01-00309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone M, Hubel D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- Logothetis N.K. Single units and conscious vision. Phil. Trans. R. Soc. B. 1998;353:1801–1818. doi: 10.1098/rstb.1998.0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueck C.J, Zeki S, Friston K.J, Deiber M.P, Cope P, Cunningham V.J, Lammertsma A.A, Kennard C, Frackowiak R.S. The colour centre in the cerebral cortex of man. Nature. 1989;340:386–389. doi: 10.1038/340386a0. [DOI] [PubMed] [Google Scholar]
- Lund J.S, Lund R.D, Hendrickson A.E, Bunt A.H, Fuchs A.F. The origin of efferent pathways from the primary visual cortex, area 17, of the macaque monkey as shown by retrograde transport of horseradish peroxidase. J. Comp. Neurol. 1975;164:287–303. doi: 10.1002/cne.901640303. [DOI] [PubMed] [Google Scholar]
- Lund J.S, Yoshioka T, Levitt J.B. Comparison of intrinsic connectivity in different areas of macaque monkey cerebral cortex. Cereb. Cortex. 1993;3:148–162. doi: 10.1093/cercor/3.2.148. [DOI] [PubMed] [Google Scholar]
- Makeig S, Jung T.P, Bell A.J, Ghahremani D, Sejnowski T.J. Blind separation of auditory event-related brain responses into independent components. Proc. Natl Acad. Sci. USA. 1997;94:10 979–10 984. doi: 10.1073/pnas.94.20.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois R, Yi D.J, Chun M.M. The neural fate of consciously perceived and missed events in the attentional blink. Neuron. 2004;41:465–472. doi: 10.1016/s0896-6273(04)00012-1. [DOI] [PubMed] [Google Scholar]
- Marx E, Deutschlander A, Stephan T, Dieterich M, Wiesmann M, Brandt T. Eyes open and eyes closed as rest conditions: impact on brain activation patterns. Neuroimage. 2004;21:1818–1824. doi: 10.1016/j.neuroimage.2003.12.026. [DOI] [PubMed] [Google Scholar]
- Maunsell J.H, Van Essen D.C. Topographic organization of the middle temporal visual area in the macaque monkey: representational biases and the relationship to callosal connections and myeloarchitectonic boundaries. J. Comp. Neurol. 1987;266:535–555. doi: 10.1002/cne.902660407. [DOI] [PubMed] [Google Scholar]
- McKeefry D.J, Zeki S. The position and topography of the human colour centre as revealed by functional magnetic resonance imaging. Brain. 1997;120:2229–2242. doi: 10.1093/brain/120.12.2229. [DOI] [PubMed] [Google Scholar]
- McKeown M.J. Detection of consistently task-related activations in fMRI data with hybrid independent component analysis. Neuroimage. 2000;11:24–35. doi: 10.1006/nimg.1999.0518. [DOI] [PubMed] [Google Scholar]
- Meadows J.C. Disturbed perception of colours associated with localized cerebral lesions. Brain. 1974;97:615–632. doi: 10.1093/brain/97.1.615. [DOI] [PubMed] [Google Scholar]
- Merigan W, Freeman A, Meyers S.P. Parallel processing streams in human visual cortex. Neuroreport. 1997;8:3985–3991. doi: 10.1097/00001756-199712220-00027. [DOI] [PubMed] [Google Scholar]
- Monbrun A. Les affections des voies optiques rétrochiasmatiques et de l'écorce visuelle. Traité d'Ophtalmologie. 1939;6:903–905. [Google Scholar]
- Morand S, Thut G, de Peralta R.G, Clarke S, Khateb A, Landis T, Michel C.M. Electrophysiological evidence for fast visual processing through the human koniocellular pathway when stimuli move. Cereb. Cortex. 2000;10:817–825. doi: 10.1093/cercor/10.8.817. [DOI] [PubMed] [Google Scholar]
- Moutoussis K, Zeki S. A direct demonstration of perceptual asynchrony in vision. Proc. R. Soc. B. 1997;264:393–399. doi: 10.1098/rspb.1997.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutoussis K, Zeki S. Functional segregation and temporal hierarchy of the visual perceptive systems. Proc. R. Soc. B. 1997;264:1407–1414. doi: 10.1098/rspb.1997.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutoussis K, Zeki S. Responses of spectrally selective cells in macaque area V2 to wavelengths and colors. J. Neurophysiol. 2002;87:2104–2112. doi: 10.1152/jn.00248.2001. [DOI] [PubMed] [Google Scholar]
- Moutoussis K, Zeki S. The relationship between cortical activation and perception investigated with invisible stimuli. Proc. Natl Acad. Sci. USA. 2002;99:9527–9532. doi: 10.1073/pnas.142305699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movshon J.A, Newsome W.T. Visual response properties of striate cortical neurons projecting to area MT in macaque monkeys. J. Neurosci. 1996;16:7733–7741. doi: 10.1523/JNEUROSCI.16-23-07733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsome W.T, Wurtz R.H, Dursteler M.R, Mikami A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J. Neurosci. 1985;5:825–840. doi: 10.1523/JNEUROSCI.05-03-00825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen P.V. Tracking the cortical signals that mediate visual awareness. Trends Neurosci. 2001;24:371–372. [Google Scholar]
- Nishida S, Johnston A. Marker correspondence, not processing latency, determines temporal binding of visual attributes. Curr. Biol. 2002;12:359–368. doi: 10.1016/s0960-9822(02)00698-x. [DOI] [PubMed] [Google Scholar]
- Pandya D.N, Karol E.A, Heilbronn D. The topographical distribution of interhemispheric projections in the corpus callosum of the rhesus monkey. Brain Res. 1971;32:31–43. doi: 10.1016/0006-8993(71)90153-3. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V. Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science. 2001;292:510–512. doi: 10.1126/science.1057099. [DOI] [PubMed] [Google Scholar]
- Pasik T, Pasik P. The visual world of monkeys deprived of striate cortex: effective stimulus parameters and the importance of the accessory optic system. Vision Res. 1971;Suppl. 3:419–435. doi: 10.1016/0042-6989(71)90055-1. [DOI] [PubMed] [Google Scholar]
- Peterhans E, von der Heydt R. Mechanisms of contour perception in monkey visual cortex. II. Contours bridging gaps. J. Neurosci. 1989;9:1749–1763. doi: 10.1523/JNEUROSCI.09-05-01749.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak S. Chicago University Press; 1957. The vertebrate visual system. [Google Scholar]
- Rao A, Nobre Ac, Cowey A. Disruption of visual evoked potentials following a V1 lesion: implications for blindsight. In: de Gelder B, de Haan E, Heywood C.A, editors. Out of mind. Oxford University Press; 2001. pp. 69–86. [Google Scholar]
- Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat. Neurosci. 2000;3:716–723. doi: 10.1038/76673. [DOI] [PubMed] [Google Scholar]
- Rees G, Wojciulik E, Clarke K, Husain M, Frith C, Driver J. Unconscious activation of visual cortex in the damaged right hemisphere of a parietal patient with extinction. Brain. 2000;123:1624–1633. doi: 10.1093/brain/123.8.1624. [DOI] [PubMed] [Google Scholar]
- Rees G, Kreiman G, Koch C. Neural correlates of consciousness in humans. Nat. Rev. Neurosci. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- Ress D, Heeger D.J. Neuronal correlates of perception in early visual cortex. Nat. Neurosci. 2003;6:414–420. doi: 10.1038/nn1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddoch G. Dissociation of visual perception due to occipital injuries, with especial reference to appreciation of movement. Brain. 1917;40:15–17. [Google Scholar]
- Rockland K.S, Lund J.S. Intrinsic laminar lattice connections in primate visual cortex. J. Comp. Neurol. 1983;216:303–318. doi: 10.1002/cne.902160307. [DOI] [PubMed] [Google Scholar]
- Saito H, Tanaka K, Isono H, Yasuda M, Mikami A. Directionally selective response of cells in the middle temporal area (MT) of the macaque monkey to the movement of equiluminous opponent color stimuli. Exp. Brain Res. 1989;75:1–14. doi: 10.1007/BF00248524. [DOI] [PubMed] [Google Scholar]
- Schein S.J, Marrocco R.T, de Monasterio F.M. Is there a high concentration of color-selective cells in area V4 of monkey visual cortex? J. Neurophysiol. 1982;47:193–213. doi: 10.1152/jn.1982.47.2.193. [DOI] [PubMed] [Google Scholar]
- Schiller P. Plenum Press; New York: 1997. Past and present ideas about how the visual scene is analyzed by the brain. [Google Scholar]
- Schoenfeld M.A, Heinze H.J, Woldorff M.G. Unmasking motion-processing activity in human brain area V5/MT+ mediated by pathways that bypass primary visual cortex. Neuroimage. 2002;17:769–779. [PubMed] [Google Scholar]
- Shipp S, Zeki S. Segregation of pathways leading from area V2 to areas V4 and V5 of macaque monkey visual cortex. Nature. 1985;315:322–325. doi: 10.1038/315322a0. [DOI] [PubMed] [Google Scholar]
- Shipp S, Zeki S. The organization of connections between areas V5 and V1 in macaque monkey visual cortex. Eur. J. Neurosci. 1989;1:309–332. doi: 10.1111/j.1460-9568.1989.tb00798.x. [DOI] [PubMed] [Google Scholar]
- Shipp S, Zeki S. The organization of connections between areas V5 and V2 in Macaque monkey visual cortex. Eur. J. Neurosci. 1989;1:333–354. doi: 10.1111/j.1460-9568.1989.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Shipp S, Zeki S. Segregation and convergence of specialised pathways in macaque monkey visual cortex. J. Anat. 1995;187:547–562. [PMC free article] [PubMed] [Google Scholar]
- Shipp S, de Jong B.M, Zihl J, Frackowiak R.S, Zeki S. The brain activity related to residual motion vision in a patient with bilateral lesions of V5. Brain. 1994;117:1023–1038. doi: 10.1093/brain/117.5.1023. [DOI] [PubMed] [Google Scholar]
- Shipp S, Watson J.D, Frackowiak R.S, Zeki S. Retinotopic maps in human prestriate visual cortex: the demarcation of areas V2 and V3. Neuroimage. 1995;2:125–132. doi: 10.1006/nimg.1995.1015. [DOI] [PubMed] [Google Scholar]
- Sholl D. Methuen; London: 1956. The cerebral cortex. [Google Scholar]
- Silvanto J, Cowey A, Lavie N, Walsh V. Striate cortex (V1) activity gates awareness of motion. Nat. Neurosci. 2005;8:143–144. doi: 10.1038/nn1379. [DOI] [PubMed] [Google Scholar]
- Silvanto J, Lavie N, Walsh V. Double dissociation of V1 and V5/MT activity in visual awareness. Cereb. Cortex. 2005 doi: 10.1093/cercor/bhi050. [DOI] [PubMed] [Google Scholar]
- Sincich L.C, Park K.F, Wohlgemuth M.J, Horton J.C. Bypassing V1: a direct geniculate input to area MT. Nat. Neurosci. 2004;7:1123–1128. doi: 10.1038/nn1318. (Epub 2004 Sep 1119.) [DOI] [PubMed] [Google Scholar]
- Standage G.P, Benevento L.A. The organization of connections between the pulvinar and visual area MT in the macaque monkey. Brain Res. 1983;262:288–294. doi: 10.1016/0006-8993(83)91020-x. [DOI] [PubMed] [Google Scholar]
- Stanley D.A, Rubin N. fMRI activation in response to illusory contours and salient regions in the human lateral occipital complex. Neuron. 2003;37:323–331. doi: 10.1016/s0896-6273(02)01148-0. [DOI] [PubMed] [Google Scholar]
- Stoerig P, Barth E. Low-level phenomenal vision despite unilateral destruction of primary visual cortex. Conscious Cogn. 2001;10:574–587. doi: 10.1006/ccog.2001.0526. [DOI] [PubMed] [Google Scholar]
- Stoerig P, Cowey A. Visual perception and phenomenal consciousness. Behav. Brain Res. 1995;71:147–156. doi: 10.1016/0166-4328(95)00050-x. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C. Attention and awareness in synchrony. Trends Cogn. Sci. 2004;8:523–525. doi: 10.1016/j.tics.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Tong F, Nakayama K, Vaughan J.T, Kanwisher N. Binocular rivalry and visual awareness in human extrastriate cortex. Neuron. 1998;21:753–759. doi: 10.1016/s0896-6273(00)80592-9. [DOI] [PubMed] [Google Scholar]
- Vaina L.M. Functional segregation of color and motion processing in the human visual cortex: clinical evidence. Cereb. Cortex. 1994;4:555–572. doi: 10.1093/cercor/4.5.555. [DOI] [PubMed] [Google Scholar]
- Van Essen D.C, Zeki S.M. The topographic organization of rhesus monkey prestriate cortex. J. Physiol. 1978;277:193–226. doi: 10.1113/jphysiol.1978.sp012269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrey L. Hémiachromatopsie droite absolue. Arch. Ophtalmol. (Paris) 1888;8:289–301. [Google Scholar]
- Victor J.D, Maiese K, Shapley R, Sidtis J, Gazzaniga M.S. Acquired central dyschromatopsia: analysis of a case with preservation of colour discrimination. Clin. Vision Sci. 1989;4:183–196. [Google Scholar]
- Viviani P, Aymoz C. Colour, form, and movement are not perceived simultaneously. Vision Res. 2001;41:2909–2918. doi: 10.1016/s0042-6989(01)00160-2. [DOI] [PubMed] [Google Scholar]
- Vogt O, Vogt C. Allgemeinere Ergebnisse unserer Hirnforschung. J. Psychol. Neurol. Lpz. 1919;25:399–462. [Google Scholar]
- Wachtler T, Sejnowski T.J, Albright T.D. Representation of color stimuli in awake macaque primary visual cortex. Neuron. 2003;37:681–691. doi: 10.1016/s0896-6273(03)00035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade A.R, Brewer A.A, Rieger J.W, Wandell B.A. Functional measurements of human ventral occipital cortex: retinotopy and colour. Phil. Trans. R. Soc. B. 2002;357:963–973. doi: 10.1098/rstb.2002.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh V, Carden D, Butler S.R, Kulikowski J.J. The effects of V4 lesions on the visual abilities of macaques: hue discrimination and colour constancy. Behav. Brain Res. 1993;53:51–62. doi: 10.1016/s0166-4328(05)80265-7. [DOI] [PubMed] [Google Scholar]
- Wandell B.A, Poirson A.B, Newsome W.T, Baseler H.A, Boynton G.M, Huk A, Gandhi S, Sharpe L.T. Color signals in human motion-selective cortex. Neuron. 1999;24:901–909. doi: 10.1016/s0896-6273(00)81037-5. [DOI] [PubMed] [Google Scholar]
- Watson J.D, Myers R, Frackowiak R.S, Hajnal J.V, Woods R.P, Mazziotta J.C, Shipp S, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb. Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- Wechsler I. Partial cortical blindness with preservation of color vision: report of a case following asphyxia (carbon monoxide poisoning.) Arch. Ophthalmol. 1933;9:957–965. [Google Scholar]
- Weiskrantz L. Clarendon Press; Oxford: 1986. Blindsight. [Google Scholar]
- Weiskrantz L. The Ferrier Lecture, 1989. Outlooks for blindsight: explicit methodologies for implicit processes. Proc. R. Soc. B. 1990;239:247–278. doi: 10.1098/rspb.1990.0016. [DOI] [PubMed] [Google Scholar]
- Weiskrantz L, Barbur J.L, Sahraie A. Parameters affecting conscious versus unconscious visual discrimination with damage to the visual cortex (V1) Proc. Natl Acad. Sci. USA. 1995;92:6122–6126. doi: 10.1073/pnas.92.13.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbrand H. J. F. Bergmann; Wiesbaden: 1884. Ophalmiatrische Beiträge zur Diagnostik der Gehirnkrankheite. [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Wurtz R.H, Yamasaki D.S, Duffy C.J, Roy J.P. Functional specialization for visual motion processing in primate cerebral cortex. Cold Spring Harb. Symp. Quant. Biol. 1990;55:717–727. doi: 10.1101/sqb.1990.055.01.067. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Blasdel G.G, Levitt J.B, Lund J.S. Relation between patterns of intrinsic lateral connectivity, ocular dominance, and cytochrome oxidase-reactive regions in macaque monkey striate cortex. Cereb. Cortex. 1996;6:297–310. doi: 10.1093/cercor/6.2.297. [DOI] [PubMed] [Google Scholar]
- Yukie M, Iwai E. Direct projection from the dorsal lateral geniculate nucleus to the prestriate cortex in macaque monkeys. J. Comp. Neurol. 1981;201:81–97. doi: 10.1002/cne.902010107. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. Representation of central visual fields in prestriate cortex of monkey. Brain Res. 1969;14:271–291. doi: 10.1016/0006-8993(69)90110-3. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. Interhemispheric connections of prestriate cortex in monkey. Brain Res. 1970;19:63–75. doi: 10.1016/0006-8993(70)90237-4. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. Cortical projections from two prestriate areas in the monkey. Brain Res. 1971;34:19–35. doi: 10.1016/0006-8993(71)90348-9. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. Convergent input from the striate cortex (area 17) to the cortex of the superior temporal sulcus in the rhesus monkey. Brain Res. 1971;28:338–340. doi: 10.1016/0006-8993(71)90665-2. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. Colour coding in rhesus monkey prestriate cortex. Brain Res. 1973;53:422–427. doi: 10.1016/0006-8993(73)90227-8. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. Functional organization of a visual area in the posterior bank of the superior temporal sulcus of the rhesus monkey. J. Physiol. 1974;236:549–573. doi: 10.1113/jphysiol.1974.sp010452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S.M. The functional organization of projections from striate to prestriate visual cortex in the rhesus monkey. Cold Spring Harb. Symp. Quant. Biol. 1976;40:591–600. doi: 10.1101/sqb.1976.040.01.055. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. Colour coding in the superior temporal sulcus of rhesus monkey visual cortex. Proc. R. Soc. B. 1977;197:195–223. doi: 10.1098/rspb.1977.0065. [DOI] [PubMed] [Google Scholar]
- Zeki S.M. Functional specialisation in the visual cortex of the rhesus monkey. Nature. 1978;274:423–428. doi: 10.1038/274423a0. [DOI] [PubMed] [Google Scholar]
- Zeki S. Colour coding in the cerebral cortex: the reaction of cells in monkey visual cortex to wavelengths and colours. Neuroscience. 1983;9:741–765. doi: 10.1016/0306-4522(83)90265-8. [DOI] [PubMed] [Google Scholar]
- Zeki S. The distribution of wavelength and orientation selective cells in different areas of monkey visual cortex. Proc. R. Soc. B. 1983;217:449–470. doi: 10.1098/rspb.1983.0020. [DOI] [PubMed] [Google Scholar]
- Zeki S. The construction of colours by the cerebral cortex. Proc. R. Inst. Gt Britain. 1984;56:231–257. [Google Scholar]
- Zeki S. Parallelism and functional specialization in human visual cortex. Cold Spring Harb. Symp. Quant. Biol. 1990;55:651–661. doi: 10.1101/sqb.1990.055.01.062. [DOI] [PubMed] [Google Scholar]
- Zeki S. A century of cerebral achromatopsia. Brain. 1990;113:1721–1777. doi: 10.1093/brain/113.6.1721. [DOI] [PubMed] [Google Scholar]
- Zeki S. A theory of multi-stage integration in the visual cortex. In: Eccles J.C, Creutzfeldt O, editors. The principles of design and operation of the brain. Pontifical Academy of Sciences; Vatican City: 1990. pp. 137–154. [Google Scholar]
- Zeki S. Cerebral akinetopsia (visual motion blindness). A review. Brain. 1991;114:811–824. doi: 10.1093/brain/114.2.811. [DOI] [PubMed] [Google Scholar]
- Zeki S. Blackwell Scientific; Oxford: 1993. A vision of the brain. [Google Scholar]
- Zeki S. The mystery of Louis Verrey (1854–1916) Gesnerus. 1993;50:96–112. [PubMed] [Google Scholar]
- Zeki S. Are areas TEO and PIT of monkey visual cortex wholly distinct from the fourth visual complex (V4 complex)? Proc. R. Soc. B. 1996;263:1539–1544. doi: 10.1098/rspb.1996.0225. [DOI] [PubMed] [Google Scholar]
- Zeki S. The color and motion systems as guides to conscious visual perception. Cereb. Cortex. 1997;12:777–809. [Google Scholar]
- Zeki S. Localization and globalization in conscious vision. Annu. Rev. Neurosci. 2001;24:57–86. doi: 10.1146/annurev.neuro.24.1.57. [DOI] [PubMed] [Google Scholar]
- Zeki S. Improbable areas in the visual brain. Trends Neurosci. 2003;26:23–26. doi: 10.1016/s0166-2236(02)00008-5. [DOI] [PubMed] [Google Scholar]
- Zeki S. The disunity of consciousness. Trends Cogn. Sci. 2003;7:214–218. doi: 10.1016/s1364-6613(03)00081-0. [DOI] [PubMed] [Google Scholar]
- Zeki S. Improbable areas in color vision. In: Chalupa L.M, Werner J.S, editors. The visual neurosciences. MIT Press; Cambridge, MA: 2004. [Google Scholar]
- Zeki S, Bartels A. Toward a theory of visual consciousness. Conscious Cogn. 1999;8:225–259. doi: 10.1006/ccog.1999.0390. [DOI] [PubMed] [Google Scholar]
- Zeki S, ffytche D.H. The Riddoch syndrome: insights into the neurobiology of conscious vision. Brain. 1998;121:25–45. doi: 10.1093/brain/121.1.25. [DOI] [PubMed] [Google Scholar]
- Zeki S, Marini L. Three cortical stages of colour processing in the human brain. Brain. 1998;121:1669–1685. doi: 10.1093/brain/121.9.1669. [DOI] [PubMed] [Google Scholar]
- Zeki S, Moutoussis K. Temporal hierarchy of the visual perceptive systems in the Mondrian world. Proc. R. Soc. B. 1997;264:1415–1419. doi: 10.1098/rspb.1997.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Shipp S. The functional logic of cortical connections. Nature. 1988;335:311–317. doi: 10.1038/335311a0. [DOI] [PubMed] [Google Scholar]
- Zeki S, Shipp S. Modular connections between areas V2 and V4 of Macaque monkey visual cortex. Eur. J. Neurosci. 1989;1:494–506. doi: 10.1111/j.1460-9568.1989.tb00356.x. [DOI] [PubMed] [Google Scholar]
- Zeki S, Watson J.D, Lueck C.J, Friston K.J, Kennard C, Frackowiak R.S. A direct demonstration of functional specialization in human visual cortex. J. Neurosci. 1991;11:641–649. doi: 10.1523/JNEUROSCI.11-03-00641.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki S, Aglioti S, McKeefry D, Berlucchi G. The neurological basis of conscious color perception in a blind patient. Proc. Natl Acad. Sci. USA. 1999;96:14 124–14 129. doi: 10.1073/pnas.96.24.14124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenger-Landolt B, Heeger D.J. Response suppression in V1 agrees with psychophysics of surround masking. J. Neurosci. 2003;23:6884–6893. doi: 10.1523/JNEUROSCI.23-17-06884.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D, LaMantia A.S, Purves D. Specialized vascularization of the primate visual cortex. J. Neurosci. 1991;11:2622–2629. doi: 10.1523/JNEUROSCI.11-08-02622.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zihl J, von Cramon D, Mai N. Selective disturbance of movement vision after bilateral brain damage. Brain. 1983;106:313–340. doi: 10.1093/brain/106.2.313. [DOI] [PubMed] [Google Scholar]
- Zihl J, von Cramon D, Mai N, Schmid C. Disturbance of movement vision after bilateral posterior brain damage. Further evidence and follow up observations. Brain. 1991;114:2235–2252. doi: 10.1093/brain/114.5.2235. [DOI] [PubMed] [Google Scholar]