Abstract
The physiological role of the mannitol cycle in the wheat pathogen Stagonospora nodorum (glume blotch) has been investigated by reverse genetics and metabolite profiling. A putative mannitol 2-dehydrogenase gene (Mdh1) was cloned by degenerate PCR and disrupted. The resulting mutated mdh1 strains lacked all detectable NADPH-dependent mannitol dehydrogenase activity. The mdh1 strains were unaffected for mannitol production but, surprisingly, were still able to utilize mannitol as a sole carbon source, suggesting a hitherto unknown mechanism for mannitol catabolism. The mutant strains were not compromised in their ability to cause disease or sporulate. To further our understanding of mannitol metabolism, a previously developed mannitol-1-phosphate dehydrogenase (gene mpd1) disruption construct [Solomon, Tan and Oliver (2005) Mol. Plant–Microbe Interact. 18, 110–115] was introduced into the mutated mdh1 background, resulting in a strain lacking both enzyme activities. The mpd1mdh1 strains were unable to grow on mannitol and produced only trace levels of mannitol. The double-mutant strains were unable to sporulate in vitro when grown on minimal medium for extended periods. Deficiency in sporulation was correlated with the depletion of intracellular mannitol pools. Significantly sporulation could be restored with the addition of mannitol. Pathogenicity of the double mutant was not compromised, although, like the previously characterized mpd1 mutants, the strains were unable to sporulate in planta. These findings not only question the currently hypothesized pathways of mannitol metabolism, but also identify for the first time that mannitol is required for sporulation of a filamentous fungus.
Keywords: mannitol, metabolomics, primary fungal metabolism, sporulation, Stagonospora nodorum (glume blotch), wheat fungal pathogen
Abbreviations: dpi, days post-inoculation; MAD1, mannitol dehydrogenase in Uromyces fabae; MM, minimal medium
INTRODUCTION
Mannitol is an acyclic hexitol that is found in most higher fungi and is usually the most abundant of all the soluble carbohydrates within the mycelium [1]. The physiological role of mannitol has been the widely studied over many years. Diverse roles in different species have been postulated using a range of genetic and biochemical methods. These roles include carbohydrate storage [2,3], a reservoir of reducing power [4,5], stress tolerance [6–8] and spore dislodgement/dispersal [9,10]. Two additional roles attributed to mannitol in phytopathogenic fungi are for quenching reactive oxygen species produced by plants in response to attack [11], and the sequestering of host fructose as mannitol, following cleavage of sucrose by host and/or fungal invertase [12]. This latter role depends on the fact that only a small number of plants have the ability to metabolize mannitol [13] and the fungus becomes a carbohydrate sink within the host.
There are only two proposed metabolic pathways for mannitol synthesis described in fungi (see Figure 1 of [17]). The first is the direct reduction of fructose to mannitol by NADP+-dependent D-mannitol:NADP+ 2-oxidoreductase (EC 1.1.1.138). Trivial names for this enzyme include mannitol 2-dehydrogenase, D-mannitol dehydrogenase and mannitol dehydrogenase, and it is the last of these names that will be used here. The second pathway is via reduction of fructose 6-phosphate to mannitol 1-phosphate by an NAD+-dependent D-mannitol-1-phosphate:NAD+ 5-oxido-reductase (EC 1.1.1.17). Trivial names for this enzyme include hexose reductase, fructose-6-phosphate reductase, D-mannitol-1-phosphate dehydrogenase, mannitol-1-phosphate 5-dehydrogenase and mannitol-1-phosphate dehydrogenase, and it is the last of these names which will be used here. The reduction step is followed by hydrolysis of mannitol 1-phosphate to mannitol and Pi by D-mannitol-1-phosphate phosphatase (EC 3.1.3.22) [10,14]. Both of these pathways have been demonstrated in a large number of fungal species, and it was proposed that they formed an enzymic cycle [15]. While this theory has been disputed [16], there is growing evidence that this cycle does exist, but is awaiting positive proof [8].
Figure 1. Southern analysis using a probe homologous to Mdh1 (A) and Mpd1 (B).
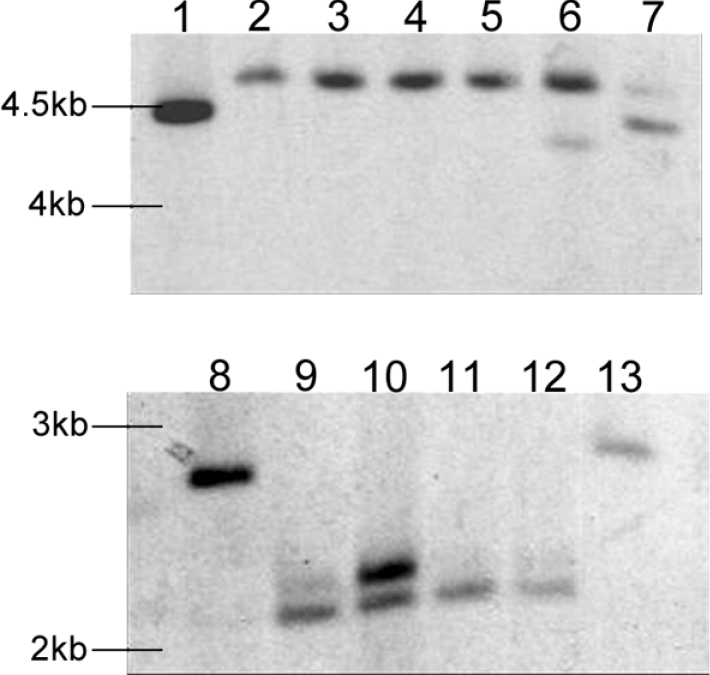
Digested genomic DNA of representative strains is shown: lanes 1 and 8, SN15; lane 2, mdh1-71; lane 3, mdh1-73; lane 4, mdh1-79; lanes 5 and 11, mpd1mdh1-102; lanes 6 and 12, mpd1mdh1-107; lane 7, mdh1-63; lane 9, mpd1-1; lane 13, mpd1mdh1-101.
One part of this proposed cycle has been recently disrupted by the inactivation of the mannitol-1-phosphate dehydrogenase (Mpd1) gene in the wheat pathogen Stagonospora nodorum [17] and the saprobe Aspergillus niger [8]. A. niger mutants contained no mannitol in growing mycelium and 30% less mannitol in conidiospores than in the wild-type [8], indicating this is the major pathway for mannitol production in A. niger. Spore viability was comparable with the wild-type even after prolonged storage, which indicated that mannitol is not an essential carbohydrate reserve. Spores were, however, extremely sensitive to heat and oxidative stress, indicating a role for mannitol in stress tolerance of spores in A. niger [8]. Mutant strains in S. nodorum had reduced levels of mannitol, consistent with mannitol-1-phosphate dehydrogenase being the major pathway for mannitol synthesis. Reduced levels of arabitol, but more trehalose, when grown on glucose, were noticed, indicating profound alterations to central metabolism. The mutants did, however, have normal levels of mannitol when grown on fructose, and were otherwise phenotypically equivalent to the wild-type strain. Lesion development on wheat was unaffected, but the strains could not sporulate on the host, suggesting a role for mannitol metabolism in sporulation in planta.
mdh genes have been previously investigated in two other fungal phytopathogens. Recently a mannitol dehydrogenase (MAD1) was cloned and characterized from the obligate rust pathogen Uromyces fabae [6]. It is not possible to perform gene-manipulation experiments in U. fabae. As MAD1 transcripts were only detected in haustoria and in spores, the enzyme was therefore postulated to be involved in mannitol production in haustoria and utilization in spores. Mannitol dehydrogenase has also been characterized in the cereal head blight fungus Gibberella zeae [10]. Circumstantial evidence suggested a role of the enzyme in forcible ascospore discharge.
In an attempt to determine the physiological function of mannitol metabolism and, in particular, the mannitol cycle in S. nodorum, the gene encoding mannitol dehydrogenase, mdh1, has been disrupted and the resulting strain characterized. A strain lacking both mannitol dehydrogenase and mannitol 1-phosphate dehydrogenase (mpd1mdh1) has also been developed and characterized. A detailed study on the role of the mannitol cycle and its requirement for pathogenicity is reported.
EXPERIMENTAL
Fungal growth and media
S. nodorum SN15 was provided by the Department of Agriculture and Food, Government of Western Australia, South Perth, WA, Australia. The fungus was routinely grown on CzV8CS sporulation medium and MM (minimal medium) as previously described [18].
Degenerate PCR
The amino acid sequence corresponding to the Cladosporium fulvum (leaf-mould fungus) NADP+-dependent mannitol dehydrogenase gene, MtDH, (GenBank® accession no. AAK67169) was retrieved from the NCBI (National Center for Biotechnology Information) protein database. A BLASTP [19] search of this sequence was used to retrieve similar sequences [Gibberella zeae (accession no. AAP33281) and Botrytis cinerea (botrytis spur and blossom blight; accession no. AL110741)], and aligned using ClustalW [20]. On the basis of conserved regions, the following primers were designed to amplify a product from the S. nodorum genome: Mdh1F1a (5′-GGNATGGGNATHGARGCNGC-3′) and Mdh1R1a (5′-GTYTGYTCYTGNGGRTARTT-3′). Degenerate PCR conditions used were an initial denaturation of 95 °C for 2 min, followed by 40 cycles of 95 °C for 10 s, 48 °C for 20 s, 72 °C for 30 s and a final extension for 5 min at 72 °C.
The Mdh1 open reading frame was sequenced by primer walking. The primers used were Mdh1F1b (5′-TGTTGAGCAGTGGAACGAG-3′), Mdh1F2 (5′-AACGTCTTTGATCATCGACC-3′), Mdh1F3 (5′-CGATGTTCCAGAGCCATATAG-3′), Mdh1F4 (5′-TCCAACCAAATCATAGCTCAC-3′), Mdh1R1b (5′-ACGAAGTCGGAAAGACCAG-3′), Mdh1R2 (5′-TGACAATGACGACCTTGCC-3′) and Mdh1R3 (5′-CACATCTCAGAAGAGCTATGC-3′). The complementary strand of the gene was sequenced using the primers MdhcompF1 (5′-CATGAATACATCACTGGAGGG-3′) and MdhcompF2 (5′-CAGACATGGAGGCAGTGATAAC-3′).
Vector construction and transformation of S. nodorum SN15
The genomic clone containing the Mdh1 gene was disrupted by insertional mutagenesis with the plasmid pGPS-phleo (encoding phleomycin resistance) as previously described [21]. The resulting plasmid, pGPSP-Mdh1, was linearized with the restriction endonuclease PstI and used to transform SN15 protoplasts as previously reported [22]. The development of a double mutant lacking mannitol dehydrogenase and mannitol 1-phosphate dehydrogenase was achieved by transforming a previously generated mpd1 construct encoding hygromycin resistance into the mdh1 background [17].
The primers used for the screening of the transformants were as follows: ActinF (5′-CTGCTTTGAGATCCACAT-3′), ActinR (5′-GTCACCACTTTCAACTCC-3′), Mdh1F (5′-ATGCCCATCCAAGTTCCCCA-3′), Mdh1R (5′-GGGGAACGAAGTCGGAAAGA-3′), Mpd1F (5′-AACGCCATCAACGCCACAAC-3′) and Mpd1R (5′-CAGGCGAAGAAGCAGTACATCC-3′).
Preparation of cell-free extracts and enzyme assays
Spores were harvested from each of the strains under investigation and 107 spores were inoculated into 100 ml of MM in a 250 ml flask and incubated for 3 days in the dark at 20 °C with shaking at 140 rev./min. Cultures were divided into two sterile 50 ml Falcon tubes and centrifuged for 10 min at 3000 g at 4 °C. Pellets were combined for each strain and washed once in 50 mM Tris/HCl, pH 7.5. The supernatant was discarded and mycelia were snap-frozen by placing the tubes in liquid nitrogen. Samples were freeze-dried overnight in a Maxi Dry Lyo (Heto Holten, Allerød, Denmark) freeze-drier prior to being disrupted in a mortar using a pestle. The tissue was then resuspended in 2 ml of 50 mM Tris/HCl, pH 7.5, centrifuged at 20000 g for 5 min and the supernatant was used for subsequent analysis.
Mannitol dehydrogenase activity was assayed as previously described [23]. The reaction mixture contained 0.25 mM NADPH, 0.8 M fructose and protein extract. The volume was made up to 1 ml with 20 mM Tris/HCl, pH 7.5. One unit of enzyme activity is defined as that needed to oxidize 1 μmol of NADPH/min at 30 °C. The mannitol-1-phosphate dehydrogenase assay contained 0.25 mM NADH and 50 μl of protein extract in 50 mM Tris/HCl, pH 7.5, in a volume of 1 ml. The reaction was started with the addition of fructose 6-phosphate to a final concentration of 2 mM unless otherwise stated. One unit of enzyme activity was defined as that needed to oxidize 1 μmol of NADH/min at 30 °C. Glucose-6-phosphate dehydrogenase activity was assayed as follows. The reaction mixture contained 0.9 mM NADP+, 14 mM MgCl2, 50 mM Tris/HCl, pH 7.5, and protein extract. The reaction was started by the addition of glucose 6-phosphate to a final concentration of 2 mM and the increase in A340 was monitored. One unit of enzyme activity was defined as that needed to reduce 1 μmol of NADP+/min at 25 °C. Total protein content was determined using the bicinchoninic acid (BCA) technique as previously described [24].
Fungal growth assays
Fungal growth assays were performed in 96-well microtitre plates. To each well, 160 μl of MM was added together with 20 μl of either 100 mM mannitol or glucose. The wells were inoculated with the addition of 20 μl of an inoculum containing 1×106 spores/ml, and the attenuance (D600) of the plates was read using a microplate reader (model 3550-UV; Bio-Rad). The microtitre plates were then wrapped in Parafilm and incubated without agitation at 20 °C. After 7 days, a second D600 reading was taken. To calculate growth over the 7-day period, the initial absorbance reading was subtracted from the final reading. Negative control experiments, using MM without added carbon, were also performed.
Growth assays during osmotic and reactive oxygen stress were undertaken essentially as previously described [22] with the exception that 20 μl of spores at a concentration of 106 spores/ml was used to inoculate the plates.
In vitro sporulation assays
Sporulation assays were performed as previously described [17]. To deplete the intracellular stores of mannitol, sporulation assays were also undertaken using inocula consisting of spores previously subjected to three rounds of continual single-spore sub-culturing on MM plates in the absence of mannitol. These spores were then used to inoculate MM plates for the subsequent sporulation assay.
Plant material and infection conditions
Pots of diameter 10 cm, containing Perlite (P500) and Grade 2 vermiculite (The Perlite and Vermiculite Factory, Jandakot, Perth, WA, Australia) were sown with five wheat (Triticum aestivum cv. Amery) seeds and grown at 20 °C in a 12 h day/12 h night cycle. Whole-plant spray infection was induced as previously described [21]. At 7 days after infection the plants were accorded a score between 0 and 10 depending on the severity of the infection, with 0 meaning that the plant was uninfected and showed no symptoms of disease, and 10 meaning a completely necrotic dead plant.
The ability of the strains to sporulate in planta was assessed using both a latent period assay and a detached-leaf assay [25]. For the latent period assay, the first true leaves of the seedling in the pathogenicity assay were harvested at 7 dpi (days post-inoculation) and embedded in 0.015% benzimidazole plates. The plates were then wrapped in parafilm and incubated at 22 °C in a 12 h day/12 h night cycle. The leaves were examined daily and scored when the number of emerging pycnidia at stage 3 or 4 exceeded 50. Further information detailing the various stages of pycnidial development for latent-period scoring can be found in the online supplementary material (http://www.BiochemJ.org/bj/399/bj3990231add.htm).
The assessment of sporulation using a detached-leaf assay was essentially as previously described [25]. The distal 2 cm of the detached wheat leaves was removed. The next 4–5 cm portion was embedded on benzimidazole agar, adaxial side up. The leaves were inoculated with 5 μl drops containing 5000 spores and 0.02% Tween 20 and incubated for 12 h light/12 h dark periods at 22 °C to enable disease development. Sporulation was typically assessed after 7 dpi unless otherwise stated.
Quantitative PCR
To quantify gene expression, 5 μl of 1:10-diluted cDNA was combined with 5 μl of the appropriate primer pair (1.2 μM each) and 10 μl of the iSybr PCR mix (Bio-Rad). The PCR conditions used for the actin (ActinqPCRf 5′-CTGCTTTGAGATCCACAT-3′, ActinqPCRr 5′-GTCACCACTTTCAACTCC-3′) and glyceraldehyde-3-phosphate dehydrogenase (GpdqPCRf 5′-CGTCAAGGTTGGTATCAACGGC-3′, GpdqPCRr 5′-TGGACGCATGTTAGCCCGTA-3′) primer pairs were an initial denaturing step of 95 °C for 3 min, followed by 35 cycles of 94 °C for 10 s, 57 °C for 20 s and 72 °C for 30 s using a RotorGene instrument (Corbett Life Science, Sydney, NSW, Australia). The conditions for the mpd1 (MpdqPCRf 5′-GCTTCGTTGCCGAGTTCCTT-3′, MpdqPCRr 5′-AGCGATCTCCTGGACGACCT-3′) and mdh1 (Mdhq-PCRf 5′-GCTGAGTTCGGCGCTGATGT-3′, MdhqPCRr 5′-ATGAGCTTCTCGCACGACGA-3′) primers were identical, except that 59 °C was used for annealing. Data was acquired during extension with the data obtained from the unknown samples compared with standard curves generated using known concentrations of DNA. All gene-expression data were normalized against actin.
Other molecular techniques
Total RNA was isolated from plant material using TRIzol Reagent (Invitrogen). A 1 μg portion of RNA was reverse-transcribed using iScript reverse transcriptase (Bio-Rad) as recommended by the manufacturer. Sequencing, genomic library screening, Southern analysis and other general molecular-biological techniques were all performed as previously described [22].
Metabolite analysis
The analyses of metabolites in vitro and also in planta were undertaken as previously described [26].
RESULTS
Cloning and sequencing of a gene encoding a mannitol dehydrogenase
Degenerate PCR was used to clone a gene encoding a mannitol dehydrogenase from S. nodorum. Using the primer pair and conditions listed in the Experimental section, a band approximating the predicted size of 480 bp was obtained. The band was ligated into pGEM®-T Easy vector (Promega) and sequenced using the generic SP6 primer. BLASTX [19] analysis revealed 65–88% sequence identity for the clone with a putative mannitol dehydrogenase gene in Alternaria alternata (tobacco brown spot; accession no. AAN28666), a fungal pathogen of the same class and order as S. nodorum, and 67–73% sequence identity with the characterized MtDH gene of Cladosporium fulvum (accession no. AAK67169). On this basis, the open reading frame was named Mdh1.
The full-length genomic copy of Mdh1 was extracted from a genomic library and sequenced. Sequence analysis suggested that Mdh1 consisted of 905 nucleotide bases (accession no. AY788902). Alignment of the nucleotide base sequence and predicted amino acid sequence suggested that the Mdh1 gene contains two introns of 58 and 46 bp. The three exons gave rise to a deduced protein of 266 amino acids. BLASTP [19] analysis of the predicted amino acid sequence returned high sequence identity with putative or proven mannitol dehydrogenase genes in A. alternata (accession no. AAN28666; 92% sequence identity), C. fulvum (accession no. AAK67169; 71% sequence identity) and G. zeae (accession no. AAP33281; 65% sequence identity), as well as a number of hypothetical fungal proteins, sorbitol-utilization proteins and reductases. Analysis of the S. nodorum mannitol dehydrogenase amino acid sequence against the PRINTS database of conserved protein motifs [27] returned significant matches with the glucose/ribitol dehydrogenase family signature, 2,3-dihydro-2,3-dihydroxybenzoate dehydrogenase signature and short-chain dehydrogenase/reductase superfamily signature. An RPS-BLAST against the Entrez database of conserved domains [28] produced significant alignments with domains of short-chain dehydrogenases which are part of a large protein family and Fab1, an enoyl-(acyl carrier protein) reductase.
Development of the S. nodorum mdh1 and mpd1mdh1 strains
Mdh1 was disrupted by insertional mutagenesis using the technique given in the Experimental section. A total of 306 colonies were obtained after transformation of the transposon-insertion reaction, of which 168 were screened by restriction-enzyme analysis. Positive clones were confirmed by sequencing from the TnsL end of the transposon. A construct in which the transposon had been inserted 335–336 bp downstream of the predicted translation start site was named pGPSP-Mdh1. For transformation, pGPSP-Mdh1 was linearized and transformed into S. nodorum. A total of 91 transformants were recovered and screened by PCR for homologous recombination. Of these, eight were confirmed by PCR as being insertional mutants (results not shown). Southern analysis confirmed the mutations, with strains S. nodorum mdh1-71, S. nodorum mdh1-73 and S. nodorum mdh1-79 used for further analysis (Figure 1). S. nodorum mdh1-63 was also selected as an ectopic control.
Development of the S. nodorum mpd1mdh1 double-knockout strain was undertaken by transforming the previously described mpd1 insertional inactivation construct, pGPSH-Mpd1 [17], into S. nodorum mdh1-71 and selecting for both phleomycin and hygromycin resistance. A total of 75 transformants were recovered, with three confirmed by PCR as having undergone homologous recombination at the mpd1 locus. Single integration for two of the three strains was confirmed by Southern analysis. These double mutants were subsequently named S. nodorum mpd1mdh1-102 and mpd1mdh1-107. The genome of a third mutant, S. nodorum mpd1mdh1-51, appeared to contain multiple insertions of the disruption construct and was not used for further analysis. mpd1mdh1-101 was retained as an ectopic control. Note that, for completeness, the previously characterized mpd1-1 strain was included in most of the subsequent analyses [17].
Enzymatic analysis of the mdh1 and mpd1mdh1 strains
To confirm that the inactivation of Mdh1 abolished mannitol dehydrogenase activity, the transformants were grown in liquid culture and analysed for their ability to reduce fructose using NADPH as a cofactor. Specific enzyme activities are shown in Supplementary Table 1 (http://www.BiochemJ.org/bj/399/bj3990231add.htm). Significant levels of fructose-reducing activity were demonstrated in both S. nodorum SN15 and the ectopic control, whereas none was detectable in mdh1 strains. The reverse reaction was also analysed, with NADP+-dependent mannitol-oxidizing activity detected in the wild-type, but not in the mdh1 mutants. To ensure that the lack of enzymatic activity was due to impairment of the Mdh1 gene and not to a lack of an active protein extract, mannitol 1-phosphate dehydrogenase enzyme activity was analysed. All strains tested showed comparable levels of activity to S. nodorum SN15, confirming that the lack of activity in the mdh1 strains is due to the impairment of the Mdh1 gene. NADH-dependent fructose-reducing (producing sorbitol) and NAD+-dependent sorbitol-oxidizing (producing fructose) activities were unaffected in the mdh1 background confirming that the impaired gene encodes for a mannitol:fructose NADPH oxido-reductase.
Similarly, mannitol-1-phosphate dehydrogenase enzyme assays were undertaken in the mpd1mdh1 transformants to confirm the success of the double inactivation. No mannitol-1-phosphate dehydrogenase activity was detected in the S. nodorum strains mpd1mdh1-102 or mpd1mdh1-107, whereas normal levels were recorded for both the wild-type and ectopic controls. To ensure that the mdh1 mutation was still intact, mannitol dehydrogenase activity was screened for in the double mutants, with none being detected. Wild-type levels of glucose-6-phosphate dehydrogenase activity were observed in the double mutants, confirming that the lack of mannitol-1-phosphate dehydrogenase activity was due to impairment of the Mpd1 gene and not an inactive protein extract.
In vitro characterization of the mdh1 and mpd1mdh1 strains
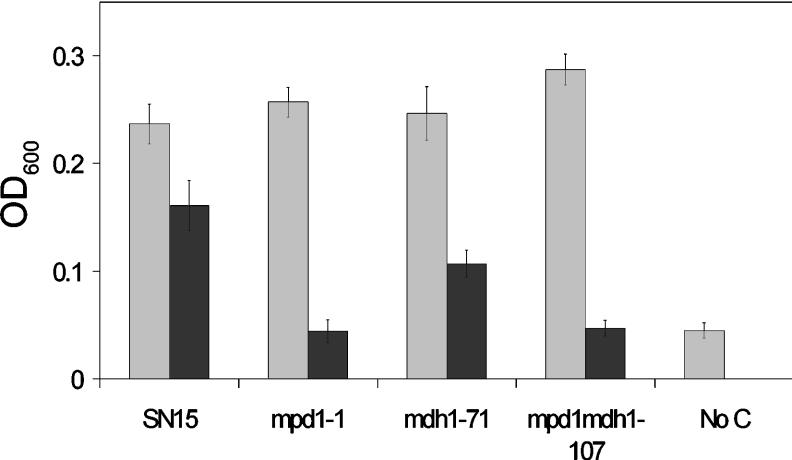
The transformants were examined for their ability to grow on different sole carbon sources (10 mM each of glucose, fructose, sucrose, mannitol, trehalose and glycerol). With the exception of mannitol, the growth of all strains was comparable on all carbon sources (results not shown). On mannitol, wild-type S. nodorum exhibited approx. 60% of the growth observed on glucose (Figure 2). The growth of the S. nodorum mdh1 strains on mannitol was significantly lower than that observed for S. nodorum SN15 on mannitol, whereas no growth was evident for either S. nodorum mpd1 or mpd1mdh1, indicating that strains lacking mannitol-1-phosphate dehydrogenase are unable to utilize mannitol as a sole carbon source.
Figure 2. Growth assays for strains SN15, mpd1-1, mdh1-71, mpd1mdh1-102 of S. nodorum on 10 mM glucose (grey bars) and 10 mM mannitol (black bars).
S. nodorum SN15 also grown in the absence of a carbon source is shown as a control (‘No C’). The growth data shown is the average of eight attenuances (OD600=D600) collected for each strain growing on each carbon source. The experiment was performed in triplicate with the S.E.M. bars shown.
The growth of the mutants under various stress conditions was examined. The ability of the mannitol mutants to resist reactive oxygen species was examined by measuring growth in the presence of H2O2. The growth of the mannitol mutants was comparable with that of the wild-type over the concentration range 0.33 mM–0.1 nM, inferring that neither mannitol-1-phosphate dehydrogenase nor mannitol dehydrogenase are required for the defence against reactive oxygen species (see Supplementary Figure 1 at http://www.BiochemJ.org/bj/399/bj3990231add.htm). Similarly, growth was also examined in NaCl to concentrations up to 1 M with no significant differences in growth apparent for any of the strains tested, indicating that mannitol metabolism is not involved in coping with osmotic stress (see Supplementary Figure 1 at http://www.BiochemJ.org/bj/399/bj3990231add.htm).
Only mannitol 1-phosphate dehydrogenase is required for sporulation
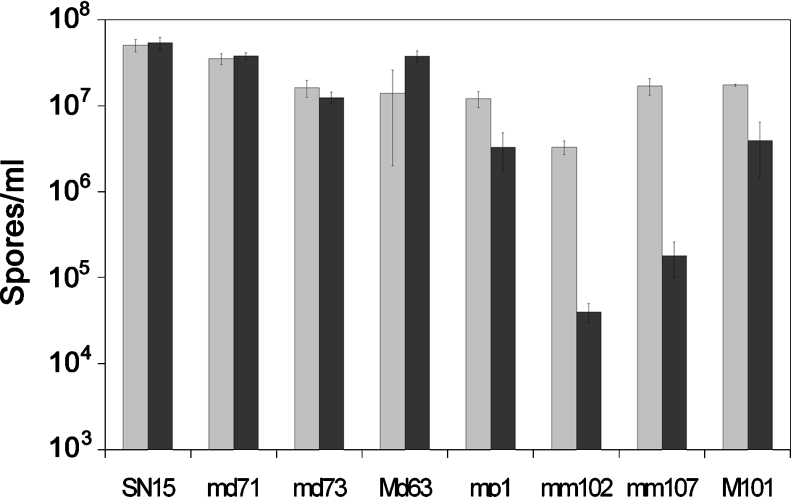
Sporulation assays were undertaken on the mdh1, mpd1 and mpd1pmdh1 strains to determine the role of the mannitol cycle during sporulation (Figure 3). Assays on complex CzV8CS agar plates showed that the sporulation was largely unaffected in the mutant strains compared with the wild-type. Subsequent metabolite analysis of the V8 component of the complex medium revealed the presence of mannitol at significant concentration (results not shown). Consequently, the sporulation assays were repeated on defined MM in the absence of mannitol. Spore counts revealed that growth on MM had no effect on the sporulation of either SN15 or the mdh1 strains. Strains lacking mannitol-1-phosphate dehydrogenase demonstrated a 4-fold decrease in spores produced on defined medium, whereas the effect on sporulation in the double mutant was striking, with a 200-fold decrease in spores produced compared with strains grown on the complex medium.
Figure 3. Sporulation assays on complex CzV8CS medium (light grey) and defined MM (dark grey).
Strains are as follows: SN15, S. nodorum SN15; md71, mdh1-71; md73, mdh1-73; Md63, mdh1-63; mp1, mpd1-1; m102, mpd1mdh1-102; m107, mpd1mdh1-107; M101, mpd1mdh1-101. The assays were performed in triplicate with S.E.M. bars shown.
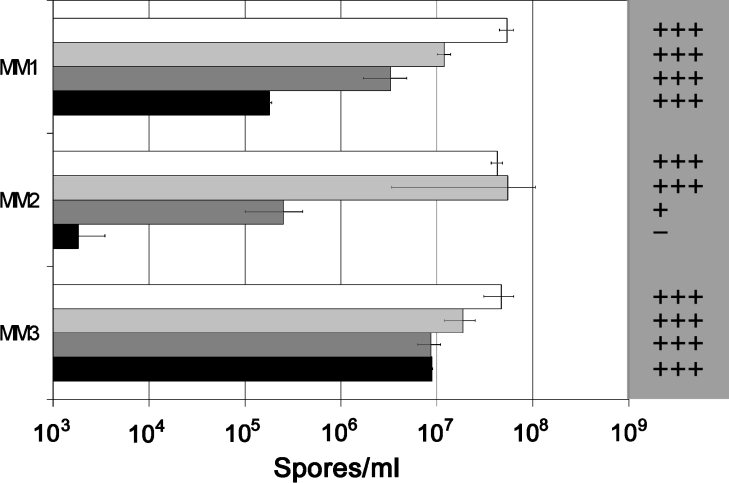
An artefact of the sporulation assay as described was that the spores used to inoculate the MM plates were harvested from CzV8CS plates that are likely to contain mannitol. Consequently, the mannitol present in the medium could be taken up by the growing fungus and either stored or utilized if required. To definitively determine the role of mannitol for sporulation, S. nodorum SN15 and each of the mannitol cycle mutants were subjected to progressive subculturing on MM as described in the Experimental section. The spores harvested at the end of the procedure were used to inoculate MM plates in the presence and absence of mannitol. These plates were subsequently used to determine spore numbers (Figure 4). Under these experimental conditions, there was no effect on sporulation for either S. nodorum SN15 or the mdh1 strains when plated on to MM with or without added mannitol. In contrast, the progressive subculturing resulted in 170-fold fewer mpd1-1 spores when grown in the absence of mannitol. The transfer of these mpd1-1 spores to MM containing 3 mM mannitol resulted in the immediate restoration of sporulation to wild-type levels. Similarly, with the mpd1mdh1-107 strain, sporulation was scarcely detectable in the absence of mannitol. However, the inoculation of these spores on to MM plates containing mannitol resulted in spore numbers returning to near wild-type levels, providing strong evidence that mannitol is required for sporulation.
Figure 4. Sporulation assays using inoculum sourced from progressive subculturing on MM.
MM1 refers to spore counts on MM plates inoculated with spores harvested from complex CzV8CS plates, MM2 represents MM plates not containing mannitol that were inoculated with spores harvested from progressive subculturing on MM plates, and MM3 is identical with MM2 with the exception that the MM plates contained 3 mM mannitol. The white bars represent S. nodorum SN15, light-grey bars represent mdh1-71, dark-grey bars represent mpd1-1, and the black bars represent mpd1mdh1-102. The grey shaded area to the right of the graph represents the mannitol abundance of the spores harvested from the plates for each of the strains under the different conditions: +++, high abundance; ++, medium abundance; +, low abundance; –, trace amounts detected.
In an attempt to correlate mannitol levels with sporulation, the concentration of mannitol in the spores harvested above was determined using GC-MS (Figure 4). However, as only such a low amount of material was available, particularly for the mpd1 and mpd1mdh1 strains, exact concentrations could not be determined. Instead, the levels could be divided into high, medium, low and trace abundance. Using this scale, mannitol was clearly highly abundant in both wild-type and the mdh1-71 background regardless of the presence of exogenous mannitol. In the absence of added mannitol, mannitol levels in the mpd1-1 mutant were much lower than either the wild-type or mdh1-71 strains. The addition of 3 mM mannitol to the MM plates restored mannitol in the mpd1-1 spores to levels similar to those of the Mpd1 strains. For the double mutant, mannitol was present in only trace amounts in spores harvested from MM agar plates. Nonetheless, wild-type levels of mannitol were restored in the double-mutant spores when harvested from mannitol-supplemented MM plates.
To determine the specificity for mannitol, sporulation assays were also undertaken using sorbitol. Sporulation was not restored in the presence of sorbitol, implying that the complementation of sporulation via the addition of exogenous mannitol occurs through a mechanism other than osmotic stress protection (results not shown). These results strongly suggest that mannitol is required for asexual sporulation in S. nodorum.
Determination of the metabolite levels in vitro
The alteration of mannitol metabolism has previously been shown to have profound effects on the concentrations of not only mannitol, but also other key metabolites [17]. Therefore metabolite levels in the SN15, mdh1, mpd1 and mpd1mdh1 strains were determined from glutamate-grown liquid cultures using GC-MS. The cultures were prepared for metabolite analysis as described above. As the cultures were grown on small pieces of foam, the wet weight of the harvested material was not indicative of the amount of fungus being assayed. Metabolite levels were normalized by comparison with actin gene copy number determined using quantitative PCR as previously described [26].
A large range of metabolites were separated and analysed. Of these, several were of particular interest (Table 1). The metabolite with the highest concentration in the wild-type samples was mannitol, and unaltered levels were determined for the mdh1 strains. The amount of mannitol present in the mpd1 extract was significantly reduced, with approx. 5-fold less detected. Only trace amounts of mannitol were detected in the double mutant. The most predominant metabolite identified in the mpd1 and mpd1mdh1 strains was trehalose, exceeding the levels measured in the Mpd1 strains by up to 23-fold. Arabitol and xylitol are almost identical five-carbon sugar alcohols that cannot be differentiated using the techniques used in the present study. A peak corresponding to these compounds was present for both the SN15 and the mpd1-1 extracts, although 6-fold less for the mutant. Arabitol or xylitol were not detected in either the mdh1-71 or the mpd1mdh1-102 extracts, suggesting a possible role for mannitol dehydrogenase in the synthesis of either of these polyols. Other metabolites differentially abundant during growth in culture were glucose 6-phosphate and fructose 6-phosphate, the substrate for mannitol 1-phosphate dehydrogenase. Glucose 6-phosphate was present in the mpd1-1 and mpd1mdh1 extracts, but was found in only negligible amounts in SN15 and was not detectable in the mdh1-71 extracts. Fructose 6-phosphate was only identified in extracts of the mpd1 strains.
Table 1. Normalized levels of relevant intracellular metabolites detected in vitro from S. nodorum strains SN15, mpd1-1, mdh1-71 and mpd1mdh1-102.
The metabolite levels were determined from each strain using three biological replicates. The normalized metabolite levels are arbitrary units that are calculated by dividing the area of the specific metabolite with that of the internal standard (ribitol), which is then divided by the fresh weight (g) and the amount of determined actin/g of fresh weight; the resulting number is then multiplied by 100 for simplification. The results are expressed as the means±S.D. nd, not detected.
| Normalized metabolite level | |||||
|---|---|---|---|---|---|
| Metabolite | Strain… | SN15 | mpd1-1 | mdh1-71 | mpd1mdh1-102 |
| Mannitol | 15.1±1.9 | 3.37±0.3 | 12.8±2.8 | 0.14±0.08 | |
| Glycerol | 0.03±0.008 | 0.02±0.003 | 0.04±0.003 | nd | |
| Trehalose | 0.4±0.1 | 9.3±0.6 | 0.6±0.1 | 5.92±1.64 | |
| Glucose 6-phosphate | 0.005±0.001 | 0.2±0.02 | nd | 0.05±0.01 | |
| Fructose 6-phosphate | nd | 0.02±0.001 | nd | nd | |
| Sorbitol | nd | 0.1±0.02 | nd | nd | |
| Arabitol/xylitol | 0.4±1.8 | 0.06±0.02 | nd | nd | |
Pathogenicity assays
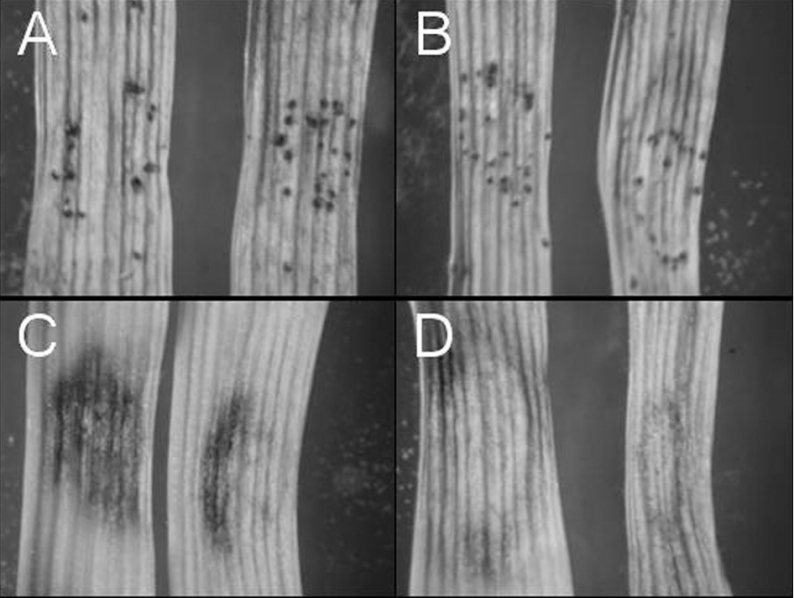
The ability of the mannitol cycle mutants to cause infection on wheat leaves was examined using a seedling assay. Pathogenicity assays showed that neither the mdh1 strains nor the mpd1mdh1 strains were compromised for lesion development (results not shown). Subsequent latent-period assays and detached-leaf assays investigating sporulation further determined that the Mdh1 gene was also unnecessary for S. nodorum to produce asexual pycnidia in planta (Figure 5). Sporulation assays using wheat leaves infected with the double mutants revealed the mpd1mdh1 strains were unable to sporulate at the completion of the infection cycle. The sporulation defect could be chemically complemented. Addition of exogenous mannitol by adding drops of 3 mM mannitol resulted in the production of a small, but significant, number of pycnidia (Figure 6).
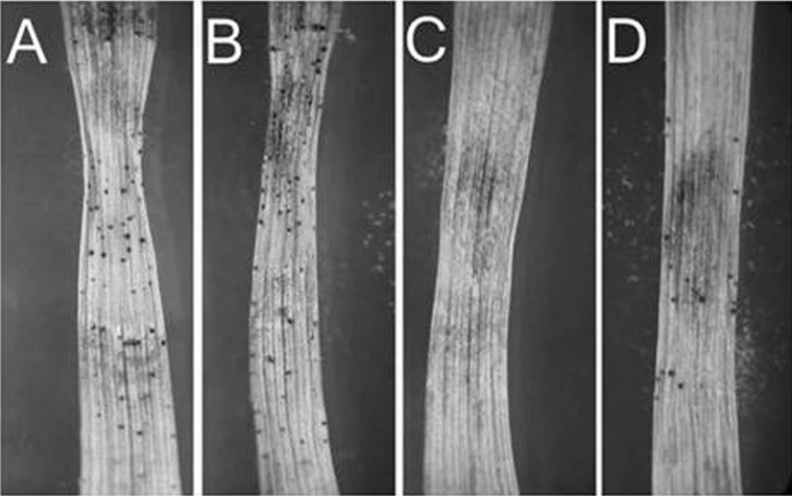
Figure 5. Infected wheat leaves at 8 dpi in a detached-leaf assay.
(A) S. nodorum SN15; (B) S. nodorum mdh1-71; (C) S. nodorum mpd1-1; (D) S. nodorum mpd1mpd1-102.
Figure 6. Detached wheat leaves infected with S. nodorum SN15 in the absence (A) or presence (B) of 3 mM mannitol, and S. nodorum mpd1mdh1-102 in the absence (C) or presence (D) of 3 mM mannitol.
Photographs were taken when the plants were at 19 dpi.
Determination of the mannitol and trehalose content during sporulation in planta
We previously showed that the mannitol cycle, and in particular the Mpd1 gene, appears to have a role in sporulation during infection [17]. To investigate this role further, the metabolome of the mutants was investigated during sporulation in a detached-leaf assay system (Table 2). We focused primarily on the abundance of mannitol and trehalose in planta, as these are presumed to be exclusively fungal. The levels were normalized for fungal biomass by quantitatively determining the amount of S. nodorum actin transcript in the infected leaf samples as previously described [26].
Table 2. Normalized levels of relevant intracellular metabolites detected during sporulation in planta from S. nodorum strains SN15, mpd1-1, mdh1-71 and mpd1mdh1-102.
For details, see the legend for Table 1.
| Normalized metabolite level* | ||||||
|---|---|---|---|---|---|---|
| Metabolite | Strain… | SN15 | mpd1-1 | mdh1-71 | mpd1mdh1-102 | Tween control |
| Mannitol | 34.3±4.5† | 10.1±5.2 | 29.9±9.5 | 0.5±0.1 | nd‡ | |
| Trehalose | 5.0±1.0 | 6.9±3.2 | 4.6±1.9 | 4.4±2.0 | nd | |
The abundance of mannitol in the infected leaf extracts during sporulation was lower than that observed for the in vitro metabolite analysis, but did follow the same relative pattern for each of the strains. The highest levels of mannitol were determined for both the SN15 and the mdh1-71 strain. Mannitol concentrations in the mpd1-1 strain were approx. 4-fold less than those for the wild-type. Only trace amounts of mannitol were detected for the double mutant. The levels of trehalose were comparable for all strains examined.
Expression of Mpd1 and Mdh1 during sporulation in planta
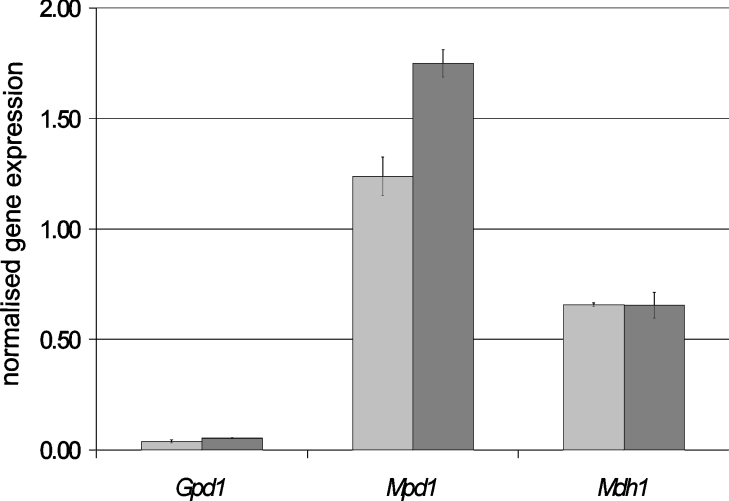
The results described thus far show that the mannitol cycle has a clear but elusive role during sporulation in planta. Whereas enzyme activities can be measured, specific activities cannot be calculated, owing to the presence of both wheat and fungal proteins. To investigate potential differential gene expression within the mannitol cycle during vegetative growth and sporulation, transcript levels were quantified (Figure 7). Gene expression was also measured for the constitutively expressed glyceraldehyde 3-phosphate dehydrogenase as a control. No significant difference in gene expression was measured for either GpdA or Mdh1 when comparing 4 with 8 dpi. In contrast, Mpd1 expression was significantly up-regulated during sporulation.
Figure 7. Normalized gene expression for each of Mpd1, Mdh1 and Gpd1 at 3 (mid-grey bars) and 8 (dark-grey bars) dpi on detached wheat leaves.
The scale shown represents the calculated number of copies of each gene divided by the number of determined actin transcripts for that particular sample. The experiment was performed in triplicate on biologically independent samples and average values with S.D. values are shown.
DISCUSSION
A previous report on mannitol metabolism in S. nodorum identified that mannitol-1-phosphate dehydrogenase was required for the pathogen to sporulate in planta [17]. We have extended the characterization of the mannitol cycle by cloning and characterizing a mannitol-2-dehydrogenase and also developing a double-mutant strain whereby both mannitol-2-dehydrogenase and mannitol-1-phosphate dehydrogenase activities are lacking.
The creation and subsequent characterization of these additional mannitol mutant strains has revealed three key findings. The first concerns the pathway of mannitol synthesis in S. nodorum. Previous qualitative analyses of mannitol concentrations have now been verified by quantitative metabolite profiling, revealing that strains carrying the single mpd1 mutation have significantly lower mannitol concentrations (Table 1). The reduced mannitol content strongly suggests that synthesis of this polyol occurs primarily via mannitol 1-phosphate, consistent with previous reports for A. niger [8]. Strains lacking NADPH-dependent fructose-reducing activity continued to produce wild-type levels of mannitol, both during growth in vitro and also during infection. Combining the mutations resulted in strains in which mannitol synthesis was effectively abolished, a finding that suggested that, although mannitol is synthesized predominately through mannitol 1-phosphate, as suggested by the mpd1 strains, mannitol synthesis can occur through the reduction of fructose. It may be that synthesis of mannitol via fructose only occurs when the mannitol-1-phosphate dehydrogenase enzyme is absent. Alternatively, operation of both mannitol biosynthesis pathways may depend on intracellular factors such as NADPH/NADH ratios [4]. It is also significant that the fungus still produces mannitol in the absence of mannitol-1-phosphate dehydrogenase, attesting to the importance of this dominant polyol.
The second finding concerns the pathway of mannitol catabolism. Mannitol supports growth of the wild-type strains to 60% of the level supported by glucose. Ablation of Mdh1 resulted in a 50% growth reduction, whereas ablation of Mpd1 resulted in zero growth (Figure 2). The prevailing hypothesis is that mannitol can only be catabolized via oxidation to fructose [17,29]. This hypothesis is consistent with the finding that mannitol utilization in A. niger mpd1 mutants was unaffected [8]. The simple interpretation of our results is that mannitol catabolism occurs primarily or even exclusively via mannitol 1-phosphate. However mannitol-1-phosphate phosphatase, which is responsible for the synthesis of mannitol from mannitol 1-phosphate, is thought to act irreversibly in the direction of mannitol. Attempts to direct the reverse activity within the present study were unsuccessful (results not shown). Other groups have also tried to detect mannitol phosphorylation activity and have been unsuccessful [30]. Detection of this activity is difficult, and it may be that the activity exists and a suitable assay has yet to be developed. Many Grampositive bacterial species possess a combined sugar-transport and phosphotransferase (protein-Nπ-phosphohistidine:sugar phosphotransferase; EC 2.7.1.69) system that can result in mannitol 1-phosphate, but there is no evidence for this system in fungi.
The putative mannitol cycle has been postulated to be involved in many processes in fungi. Operation of the cycle [17] involves the consumption of ATP and the conversion of reducing equivalents from NADH into NADPH. Ablation of either mannitol-1-phosphate dehydrogenase or mannitol dehydrogenase would abolish the operation of the cycle. However, S. nodorum strains in which either or both genes were disrupted were remarkably free from obvious phenotypic defects. This strongly suggests that the cycle does not operate, and that a primary role in transhydrogenation can be discounted. Instead, the primary role of mannitol-1-phosphate dehydrogenase or mannitol dehydrogenase must be to produce and consume mannitol.
The third result from the present study is that mannitol is required for asexual sporulation of S. nodorum. Sporulation assays in vitro showed that Mdh1 is not required for sporulation. Metabolite analysis of the spores produced contained wild-type levels of mannitol, consistent with the idea that the reduction of fructose is not the critical step for mannitol synthesis. Previous analysis of the mpd1 mutants had also shown that the mutants were able to sporulate normally in vitro. However, by altering the experimental design of the sporulation assay to remove any possible endogenous mannitol reserves from the source of the inoculum, the ability of the mpd1 strains to sporulate in vitro was compromised. Wild-type sporulation in the mpd1 strains could be restored with the inclusion of mannitol in the medium, providing the first evidence that mannitol is required for sporulation. Definitive proof of the requirement of mannitol for sporulation was gained through analysis of the S. nodorum strain lacking both Mpd1 and Mdh1. After progressive sub-culturing on MM in the absence of mannitol, the ability of the double mutants to sporulate was severely compromised, with the number of spores harvested being several orders of magnitude less than from MM plates inoculated with wild-type spores. Mannitol was present in only trace amounts from the spores that were harvested. The inclusion of 3 mM mannitol completely restored both spore numbers and mannitol concentrations to wild-type levels, providing unequivocal proof that mannitol is required for S. nodorum to sporulate. The requirement for multiple subculturing to remove endogenous mannitol stores shows that the polyol exists in a remarkably stable pool.
Sporulation assays in planta revealed a pattern with the wild-type and mdh1 strains sporulating normally, and the mpd1mdh1 strain unable to sporulate, similar to that observed in vitro. A previous report had shown that the mpd1 strain was unable to sporulate in planta, and this was confirmed in the present study. The fact that the mpd1 strains are able to sporulate in vitro, but not in planta, is interesting. Clearly, the wheat leaf at the time of sporulation is likely to provide a more challenging environment to the fungus than an agar plate in vitro. Although yet to be shown for the S. nodorum–wheat interaction during sporulation, both reactive oxygen species and osmotic stress are likely to be encountered, and it is possible that mannitol is being produced to counter a stress of some form. The stress may only be at such a level in vitro that the reduced amount of mannitol produced by the mpd1 strains can cope. However, this reduced mannitol content may not be sufficient for sporulation in a more challenging environment. The precise role of mannitol during sporulation is at present unclear, although we can state that mannitol is specifically required.
Mannitol has been postulated to have several roles, including acting as a carbohydrate storage compound [6,29], scavenger of reactive oxygen species [6], translocation of carbohydrates [29,31], a mechanism for storing reducing power [4] and as a source of turgor during ascospore discharge [10]. Growth and sporulation assays of the S. nodorum mutant strains under various stress conditions imply that mannitol has no critical role in responding to stress. Furthermore, the failure of sorbitol to complement sporulation in vitro appears to exclude the possibility that mannitol is simply acting as an osmoprotectant. To completely dismiss the possibility of mannitol playing any of the above roles, though, would be premature, and experiments are ongoing to determine its precise function.
Other than being involved in a stress response, mannitol may have a role as a signalling molecule to induce sporulation. Low-molecular-mass molecules have been postulated to play a role in the asexual sporulation of some fungi [32–34]. Of particular note is the role of the developmental activator protein FluG in A. nidulans, which is proposed to have an enzymatic role in the production of a sporulation factor. The factor has yet to be identified, although it is believed to be needed to initiate the Flb developmental pathway leading to conidiation [33]. It could be suggested that mannitol may have a comparable signalling role in initiating asexual conidiation in S. nodorum, but this is highly speculative, and further experimentation is required.
In conclusion, the present study has identified, for the first time, that mannitol is required for asexual sporulation in S. nodorum. This finding influences both our current understanding of this developmental phase in S. nodorum and also the role of mannitol metabolism itself. The significance of this finding must also not be underestimated when considering strategies to prevent fungal diseases, particularly polycyclic ones (diseases in which the pathogens produce new infectious inocula during the growing season) such as that caused by S. nodorum [35].
Online data
Acknowledgments
We thank the Grains Research and Development Council of Australia for financially supporting this project and acknowledge the technical assistance of Ms Kasia Rybak.
References
- 1.Lewis D., Smith D. Sugar alcohols in fungi and green plants. New Phytol. 1967;66:143–184. [Google Scholar]
- 2.Corina D. L., Munday K. A. Studies on polyol function in Aspergillus clavatus: a role for mannitol and ribitol. J. Gen. Microbiol. 1971;69:221–227. doi: 10.1099/00221287-69-2-221. [DOI] [PubMed] [Google Scholar]
- 3.Jennings D. H. Cambridge: Cambridge University Press; 1995. The Physiology of Fungal Nutrition. [Google Scholar]
- 4.Hult K., Veide A., Gatenbeck S. The distribution of the NADPH regenerating mannitol cycle among fungal species. Arch. Microbiol. 1980;128:253–255. doi: 10.1007/BF00406168. [DOI] [PubMed] [Google Scholar]
- 5.Dütsch G. A., Rast D. Biochemische beziehung zwischen mannitbildung und hexosemonophosphatzyklus in Agaricus bisporus. Phytochemistry. 1972;11:2677–2681. [Google Scholar]
- 6.Voegele R. T., Hahn M., Lohaus G., Link T., Heiser I., Mendgen K. Possible roles for mannitol and mannitol dehydrogenase in the biotrophic plant pathogen Uromyces fabae. Plant Physiol. 2005;137:190–198. doi: 10.1104/pp.104.051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoop J. M. H., Mooibroek H. Cloning and characterization of NADP mannitol dehydrogenase cDNA from the button mushroom, Agaricus bisporus, and its expression in response to NaCl stress. Appl. Environ. Microbiol. 1998;64:4689–4696. doi: 10.1128/aem.64.12.4689-4696.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruijter G. J. G., Bax M., Patel H., Flitter S. J., van de Vondervoort P. J. I., de Vries R. P., van Kuyk P. A., Visser J. Mannitol is required for stress tolerance in Aspergillus niger conidiospores. Eukaryotic Cell. 2003;2:690–698. doi: 10.1128/EC.2.4.690-698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Webster J., Davey R. A., Smirnoff N., Fricke W., Hinde P., Romos D., Turner J. C. R. Mannitol and hexoses are components of Buller's drop. Mycol. Res. 1995;99:833–838. [Google Scholar]
- 10.Trail F., Xu H. X. Purification and characterization of mannitol dehydrogenase and identification of the corresponding cDNA from the head blight fungus, Gibberella zeae (Fusarium graminearum) Phytochemistry. 2002;61:791–796. doi: 10.1016/s0031-9422(02)00430-2. [DOI] [PubMed] [Google Scholar]
- 11.Smirnoff N., Cumbes Q. J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry. 1989;28 [Google Scholar]
- 12.Joosten M. H. A. J., Hendrickx L. J. M., de Wit P. J. G. M. Carbohydrate composition of apoplastic fluids isolated from tomato leaves inoculated with virulent or avirulent races of Cladosporium fulvum syn. Fulvia fulva. Neth. J. Plant Pathol. 1990;96:103–112. [Google Scholar]
- 13.Jennings D. B., Ehrenshaft M., Pharr D. M., Williamson J. D. Roles for mannitol and mannitol dehydrogenase in active oxygen-mediated plant defense. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15129–15133. doi: 10.1073/pnas.95.25.15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boonsaeng V., Sullivan P. A., Shepherd M. G. Mannitol production in fungi during glucose catabolism. Can. J. Microbiol. 1976;22:808–816. doi: 10.1139/m76-117. [DOI] [PubMed] [Google Scholar]
- 15.Hult K., Gatenbeck S. Production of NADPH in the mannitol cycle and its relation to polyketide formation in Alternaria alternata. Eur. J. Biochem. 1978;88:607–612. doi: 10.1111/j.1432-1033.1978.tb12487.x. [DOI] [PubMed] [Google Scholar]
- 16.Singh M., Scrutton N. S., Scrutton M. C. NADPH generation in Aspergillus nidulans: is the mannitol cycle involved? J. Gen. Microbiol. 1988;134:643–654. doi: 10.1099/00221287-134-3-643. [DOI] [PubMed] [Google Scholar]
- 17.Solomon P. S., Tan K.-C., Oliver R. P. Mannitol 1-phosphate metabolism is required for sporulation in planta of the wheat pathogen Stagonospora nodorum. Mol. Plant–Microbe Interact. 2005;18:110–115. doi: 10.1094/MPMI-18-0110. [DOI] [PubMed] [Google Scholar]
- 18.Solomon P. S., Lee R. C., Wilson T. J. G., Oliver R. P. Pathogenicity of Stagonospora nodorum requires malate synthase. Mol. Microbiol. 2004;53:1065–1073. doi: 10.1111/j.1365-2958.2004.04178.x. [DOI] [PubMed] [Google Scholar]
- 19.Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solomon P. S., Thomas S. W., Spanu P., Oliver R. P. The utilisation of di/tripeptides by Stagonospora nodorum is dispensable for wheat infection. Physiol. Mol. Plant Pathol. 2003;63:191–199. [Google Scholar]
- 22.Solomon P. S., Tan K.-C., Sanchez P., Cooper R. M., Oliver R. P. The disruption of a Gα subunit sheds new light on the pathogenicity of Stagonospora nodorum on wheat. Mol. Plant–Microbe Interact. 2004;17:456–466. doi: 10.1094/MPMI.2004.17.5.456. [DOI] [PubMed] [Google Scholar]
- 23.Noeldner P. K. M., Coleman M. J., Faulks R., Oliver R. P. Purification and characterization of mannitol dehydrogenase from the fungal tomato pathogen Cladosporium fulvum (syn Fulvia fulva) Physiol. Mol. Plant Pathol. 1994;45:281–289. [Google Scholar]
- 24.Smith P. K., Krohn R. I., Hermanson G. T., Maliia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 25.Benedikz P. W., Mappledoram C. J., Scott P. R. A laboratory technique for screening cereals for resistance to Septoria nodorum using detached seedling leaves. Trans. British Mycol. Soc. 1981;77:667–668. [Google Scholar]
- 26.Solomon P. S., Lowe R. G. T., Trengove R. D., Rechberger J., Oliver R. P. Normalisation of metabolites in heterogenous systems using genomics. Anal. Biochem. 2006;350:156–158. doi: 10.1016/j.ab.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 27.Attwood T. K., Croning M. D., Flower D. R., Lewis A. P., Mabey J. E., Scordis P., Selley J. N., Wright W. PRINTS-S: the database formerly known as PRINTS. Nucleic Acids Res. 2000;28:225–227. doi: 10.1093/nar/28.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchler-Bauer A., Anderson J. B., DeWeese-Scott C., Fedorova N. D., Geer L. Y., He S., Hurwitz D. I., Jackson J. D., Jacobs A. R., Lanczycki C. J., et al. CDD: a curated Entrez database of conserved domain alignments. Nucleic Acids Res. 2003;31:383–387. doi: 10.1093/nar/gkg087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jennings D. H. Polyol metabolism in fungi. Adv. Microb. Physiol. 1984;25:149–193. doi: 10.1016/s0065-2911(08)60292-1. [DOI] [PubMed] [Google Scholar]
- 30.Schmatz D. M., Baginsky W. F., Turner M. J. Evidence for and characterization of a mannitol cycle in Eimeria tenella. Mol. Biochem. Parasitol. 1989;32:263–270. doi: 10.1016/0166-6851(89)90075-3. [DOI] [PubMed] [Google Scholar]
- 31.Koide R. T., Shumway D. L., Stevens C. M. Soluble carbohydrates of red pine (Pinus resinosa) mycorrhizas and mycorrhizal fungi. Mycol. Res. 2000;104:834–840. [Google Scholar]
- 32.Roncal T., Cordobes S., Sterner O., Ugalde U. Conidiation in Penicillium cyclopium is induced by conidiogenone, an endogenous diterpene. Eukaryotic Cell. 2002;1:823–829. doi: 10.1128/EC.1.5.823-829.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee B. N., Adams T. H. The Aspergillus nidulans fluG gene is required for production of an extracellular developmental signal and is related to prokaryotic glutamine synthetase I. Gene Dev. 1994;8:641–651. doi: 10.1101/gad.8.6.641. [DOI] [PubMed] [Google Scholar]
- 34.D'Souza C. A., Lee B. N., Adams T. H. Characterization of the role of the FluG protein in asexual development of Aspergillus nidulans. Genetics. 2001;158:1027–1036. doi: 10.1093/genetics/158.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon P. S., Lowe R. G. T., Tan K.-C., Waters O. D. C., Bailey A., Oliver R. P. Stagonospora nodorum; cause of Septoria nodorum blotch of wheat. Mol. Plant. Pathol. 2006;7:147–156. doi: 10.1111/j.1364-3703.2006.00326.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.