Abstract
Refeeding a carbohydrate-rich meal after a fast produces a co-ordinated induction of key glycolytic and lipogenic genes in the liver. The transcriptional response is mediated by insulin and increased glucose oxidation, and both signals are necessary for optimal induction of FAS (fatty acid synthase). The glucose-regulated component of FAS promoter activation is mediated in part by ChREBP [ChoRE (carbohydrate response element)-binding protein], which binds to a ChoRE between −7300 and −7000 base-pairs in a carbohydrate-dependent manner. Using in vivo footprinting with nuclei from fasted and refed rats, we identify an imperfect DR-1 (direct repeat-1) element between −7110 and −7090 bp that is protected upon carbohydrate refeeding. Electrophoretic mobility-shift assays establish that this DR-1 element binds HNF-4α (hepatocyte nuclear factor 4α), and chromatin immunoprecipitation establishes that HNF-4α binding to this site is increased approx. 3-fold by glucose refeeding. HNF-4α transactivates reporter constructs containing the distal FAS promoter in a DR-1-dependent manner, and this DR-1 is required for full glucose induction of the FAS promoter in primary hepatocytes. In addition, a 3-fold knockdown of hepatocyte HNF-4α by small interfering RNA produces a corresponding decrease in FAS gene induction by glucose. Co-immunoprecipitation experiments demonstrate a physical interaction between HNF-4α and ChREBP in primary hepatocytes, further supporting an important complementary role for HNF-4α in glucose-induced activation of FAS transcription. Taken together, these observations establish for the first time that HNF-4α functions in vivo through a DR-1 element in the distal FAS promoter to enhance gene transcription following refeeding of glucose to fasted rats. The findings support the broader view that HNF-4α is an integral component of the hepatic nutrient sensing system that co-ordinates transcriptional responses to transitions between nutritional states.
Keywords: hepatocyte nuclear factor 4α (HNF-4α), fatty acid synthase (FAS), direct repeat-1 (DR-1), carbohydrate response element-binding protein (ChREBP), primary hepatocyte, transcriptional activation
Abbreviations: AOX, acyl-CoA oxidase; ChIP, chromatin immunoprecipitation; ChoRE, carbohydrate response element; ChREBP, ChoRE-binding protein; DR-1, direct repeat-1; EMSA, electrophoretic mobility-shift assay; FAS, fatty acid synthase; HEK-293 cell, human embryonic kidney cell; HNF-4α, hepatocyte nuclear factor 4α; L-PK, liver pyruvate kinase; NF-Y, nuclear factor-Y; PEPCK, phosphoenolpyruvate carboxykinase; PKA, protein kinase A; RT, reverse transcriptase; Sp1, stimulatory protein 1; SREBP, sterol-regulatory-element-binding protein; Xu-5P, xylulose 5-phosphate; siRNA, small interfering RNA
INTRODUCTION
Consumption of a high-carbohydrate diet activates transcription of genes involved in glycolysis and de novo fatty acid synthesis in the mammalian liver. One of these genes, FAS (fatty acid synthase), is induced 20–30-fold when fasted rats are refed a high-carbohydrate meal [1]. In addition to carbohydrate, full induction of FAS transcription requires both insulin and glucocorticoids [2,3]. Conversely, FAS transcription is suppressed by glucagon and growth hormone [4,5] and by dietary polyunsaturated fatty acids and sterols [6,7]. Analysis of the FAS proximal promoter (−118 and +65) identified an insulin-responsive region that contains several cis-acting elements that interact with SREBP-1c (sterol-regulatory-element-binding protein-1c), Sp1 (stimulatory protein 1), NF-Y (nuclear factor-Y) and upstream stimulatory factor [8–10] to regulate promoter activity. Insulin increases expression of SREBP-1c [11], which enhances binding of NF-Y and Sp1 to their respective recognition sites [12,13]. Although it plays an important role in enhancement of FAS transcription, insulin accounts for only part of the increase in hepatic FAS mRNA in carbohydrate-refed rats [2,14]. For example, overexpression of SREBP-1c does not reproduce the induction of FAS mRNA observed with the combination of high glucose and insulin in hepatocytes [15]. Clearly, additional cis-acting regions contribute to transcriptional induction of FAS during this nutritional transition.
We reported previously that a 5′-flanking region of the FAS gene (−7300 to −6900 bp) combined with the proximal promoter region reproduced rates of transcriptional activation observed in fasted–refed rats [14]. Systematic analysis of this distal enhancer identified a ChoRE (carbohydrate response element) between −7214 and −7198 [16] with strong sequence similarity to those identified in glucose-responsive genes such as L-PK (liver pyruvate kinase), acetyl-CoA carboxylase and S14 [17–19]. ChREBP (ChoRE-binding protein) binds to ChoRE sites in promoters of these genes [20,21], and is activated by the glucose metabolite Xu-5P (xylulose 5-phosphate) through activation of a Xu-5P-activated protein phosphatase [22,23]. Thus increased ChREBP binding activity depends on increased glucose flux through the proximal portion of the glycolytic pathway to produce metabolites from the pentose shunt. Several lines of evidence link activation of ChREBP by carbohydrate to up-regulation of FAS transcription. For example, hepatic FAS mRNA levels are significantly lower in ChREBP−/− versus wild-type mice [24], and gene silencing of hepatic ChREBP compromises induction of endogenous FAS expression by glucose [25]. Moreover, FAS mRNA is unresponsive to glucose in hepatocytes from ChREBP−/− mice [21]. Collectively, these findings make a compelling case that ChREBP directly activates FAS gene expression in response to high glucose.
In the present study we analysed a region of the FAS distal enhancer downstream of the ChoRE (−7150 to −7050) by in vivo footprinting using nuclei prepared from rats that were refed glucose after a fast. Carbohydrate refeeding protected several bands and sequence analysis revealed that one protected region contained an imperfect DR-1 (direct repeat-1). Using a combination of EMSA (electrophoretic mobility-shift assay) and ChIP (chromatin immunoprecipitation) assays, we determined that HNF-4α (hepatocyte nuclear factor 4α) binding to this DR-1 was enhanced by carbohydrate refeeding after a fast. Complementary findings that knockdown of HNF-4α by siRNA (small interfering RNA) compromises FAS gene induction by glucose in conjunction with demonstration of a physical interaction between HNF-4α and ChREBP support an important complementary role for HNF-4α in the transcriptional regulation of FAS by glucose in liver. Together, these novel findings make a compelling case that HNF-4α is an important component of the nutrient-sensing system regulating FAS transcription during transition between nutritional states.
EXPERIMENTAL
Dietary method
All animal studies were approved by the Institutional Animal Care and Use Committee of the Pennington Biomedical Research Center. Two-month-old male Harlan Sprague–Dawley rats (150–200 g) were housed in plastic cages on a 12 h light/dark cycle and provided Purina rodent chow (Diet no. 5001) on a free choice basis. In the experiment proper, randomly selected rats were divided into two groups. Both groups were fasted for 48 h, after which the rats in group 1 were anaesthetized with pentobarbital for harvesting of livers and killed. After the 48 h fast, the rats in group 2 were refed a high glucose, fat-free diet for 6 h before harvesting their livers as previously described [16].
Plasmids
The luciferase reporter plasmids −250FAS.PGL2, −7382FAS.LUC (where LUC is luciferase), PK(−96)LUC and −7382/−6970FAS.PK were described previously [7,16,26]. Of note, the distance between the carbohydrate-responsive region (−7382 to −6970 of FAS) and the insulin-responsive region (−250 to +65 of FAS) of the −7382FAS.LUC reporter construct is 2877 bp, while the carbohydrate-responsive region (−7382 to −6970 of FAS) and the insulin-responsive region (−96 to +12 of L-PK) of −7382/−6970FAS.PK is separated by 45 bp. The −7382FAS.LUC mut DR1 and −7382/−6970FAS.PK mut DR1 constructs were created by site-directed mutagenesis (QuikChange®; Stratagene) to contain a mutation in the DR1 site as indicated by boldface letters in AGGTTCCTGACCT (wild-type AGGCCTTTGACCT). The expression vector containing HNF-4α was generated by inserting a PCR product containing the rat HNF-4α coding sequence into the EcoRI and BglII sites of pSG5 (Stratagene). A FLAG (DYKDDDDK)-tagged ChREBP expression construct was obtained from Dr Howard Towle (Department of Biochemistry, Molecular Biology, and Biophysics, University of Minnesota, Minneapolis, Minnesota, U.S.A.) [27].
Cell culture conditions, transient transfections and luciferase assays
A total of 1.5×105 COS-1 cells were plated in 12-well dishes containing 1 ml of F-12K medium (Invitrogen) supplemented with 10% (v/v) fetal bovine serum. The cells were transfected, 24 h after plating, using FuGENE (Roche Applied Science) with reporter plasmid (0.25 μg), internal control plasmid pRL-TK (0.15 μg), HNF-4α expression vector (0.25 μg) or empty expression vector (0.25 μg) as indicated in the Figure legends. Following 40 h incubation, the cells were harvested with passive lysis buffer (Promega) and luciferase assays were performed using the Dual Luciferase Reporter Assay system (Promega). Light intensity was measured for 5 s by a Micro Lumat Plus auto-injector luminometer (Berthold Technologies).
Primary hepatocyte culture and transfection
Primary hepatocytes were isolated from male Harlan Sprague–Dawley rats (180–220 g) using the collagenase perfusion method [28]. A total of 1.5×106 cells were plated in 6-well Primaria dishes (Invitrogen) containing 2 ml of M199 medium containing 5.5 mM glucose and supplemented with 5% fetal bovine serum. After a 4–6 h attachment period, hepatocytes were transfected using Lipofectamine™ 2000 (Invitrogen) with reporter plasmid (2 μg) and internal control plasmid pRL-TK (0.2 μg) in M199 medium containing 5.5 mM glucose, 100 nM dexamethasone and 100 nM insulin for 12–14 h. Following transfection, cells were incubated in M199 medium containing either 5 or 27.5 mM glucose, with 100 nM dexamethasone and 100 nM insulin. Cells were harvested, and luciferase activity was quantified.
In vivo DNase I footprinting analysis
Nuclei from fasted and refed rat liver were isolated by the method of Jump et al. [29] and stored at −80 °C until use. In vivo DNase I footprinting analysis was conducted as previously outlined in detail [16]. The sequences of the primer sets are: 5′-TCCTTCACAGAGAGGCTTGCTG-3′, 5′-CCTGAGTGAACACAGCCATGGCCTTG-3′ and CCTGAGTGAACACAGCCATGGCCTTGTGTG-3′ for the reverse reaction.
EMSA
Hepatic nuclear protein extracts were prepared from fasted and refed rats [26,30]. The following single-stranded oligonucleotides containing the candidate FAS DR-1 were synthesized for use in the gel shift assay: FAS −7110/−7074, 5′-GAGCAGGCCTTTGACCTGGCCAGCCGCCCATGATGTC-3′, 3′-CTCGTCCGGAAACTGGACCGGTCGGCGGGTACTACAG-5′; FAS −7110/−7089, 5′-GAGCAGGCCTTTGACCTGGCC-3′, 3′-CTCGTCCGGAAACTGGACCGG-5′; FAS–7110/−7074 mut DR1, 5′-GAGCAGGTTCCTGACCTGGCCAGCCGCCCATGATGTC-3′, 3′-CTCGTCCAAGGACTGGACCGGTAGGCGGGTACTACAG-5′; AOX (acyl-CoA oxidase) DR-1, 5′-GATCCTCCCGAACGTGACCTTTGTCCTGGTCCA-3′, 3′-CTCGGAGGGCTTGCACTGGAAACAGGACCAGGT-5′. Complementary strands were annealed by combining equal amounts of each oligonucleotide, heating to 95 °C for 5 min, and cooling to 25 °C. The annealed oligonucleotides were end-labelled with [γ-32P]ATP using T4 polynucleotide kinase and incubated on ice with nuclear protein extract for 30 min at room temperature in 10 mM Hepes (pH 7.9), 75 mM KCl, 1 mM EDTA, 5 mM MgCl2, 5 mM dithiothreitol, and 10% (v/v) glycerol. A typical reaction contained 50000 c.p.m. of labelled oligonucleotide (0.5–1.0 ng). For competition assays, 100× unlabelled AOX DR1 or FAS −7110/−7074 mut DR-1 probe was used as a specific competitor, and for supershift experiments, HNF-4α antibody (Santa Cruz Biotechnology; sc-6556) was included in the binding reactions. After incubation, samples were subjected to electrophoresis on a 5% non-denaturing polyacrylamide gel in a Tris/glycine buffer system. DNA–protein complexes were visualized by autoradiography after drying the gel.
ChIP assay
Cross-linked chromatin was prepared from rat liver using the method of Roder et al. [31]. ChIP was performed as described in [32] with some modifications. Chromatin samples of A260 8 were diluted to a final volume of 1000 μl, incubated with 100 μl of blocked Protein G beads (Roche) for 2 h on a rotating wheel at 4 °C, and beads were collected by centrifugation at 15000 g for 1 min. The beads were incubated with 500 μg of sheared salmon sperm DNA (Invitrogen) and BSA (Roche) for 30 min at 4 °C to block non-specific binding. The supernatant was transferred to new tubes and incubated with 50 μl of HNF-4α antibody (Santa Cruz Biotechnology; sc-6556) or 50 μl of goat IgG (Santa Cruz Biotechnology; sc-2028) overnight at 4 °C on a rotating wheel. The samples were centrifuged for 10 min at 15000 g and then the supernatant was transferred to a new tube and incubated with 100 μl of blocked Protein G beads for 90 min at 4 °C on a rotating wheel. The samples were washed extensively, eluted, decross-linked, and treated with proteinase K (Roche) as described in [32]. The samples were extracted with equal volume of phenol/chloroform/3-methylbutan-1-ol (25:24:1 by vol.) (Amresco) and precipitated with 40 μg of glycogen (Roche) and 2.4 vol. of ethanol at −20 °C overnight. Samples were washed once with 70% ethanol and resuspended in 40 μl of water.
Primers (5′-AGCTGAGACCTGAGTGAACAC-3′ and 5′-GACAGCTGAGTGAGGCAATG-3′) were designed to amplify a 337 bp fragment of the distal enhancer region of rat FAS (−7314 to −6980) and a 223 bp fragment (−489/−267 bp) spanning the HNF-4α binding site in the PEPCK (phosphoenolpyruvate carboxykinase) promoter (5′-CTAGAAGTTTCACGTCTCAGAGC-3′ and 5′-GACCGTGACTGTTGCTGATGC-3′. Primers were also designed to amplify a 227 bp fragment spanning the HNF-4α binding site (−216 to −195, 5′-CTTTGATCCAGGCTCTGCAGAC-3′ and −90 to −112, 5′-TGAGCTCTGGTTAAAGTATAACC-3′) in the enhancer region of rat L-PK. Standard PCR reactions were performed with PCR Core System I reagents (Promega M7660) with 4 μl of sample as template and 25 μM of each primer in a total volume of 50 μl. The PCR conditions were: 96 °C 2 min, 1 cycle; 96 °C 1 min, 59 °C 1 min, 72 °C 45 s, 30 cycles; and 72 °C 1 min 1 cycle. PCR products were separated on a 1.5% agarose gel and analysed by ethidium bromide staining. The stained gels were visualized and the PCR products from three to six individual experiments were quantified using a Versadoc imaging system (Bio-Rad). The numbers are derived from the average intensity of the PCR products from the refed liver samples compared with the average intensity of those from the fasted liver samples, which are set at 1.0.
Immunoblot analysis
Liver from fasted and refed rats was removed and placed in liquid nitrogen. Approximately 0.3 g of tissue was ground in a pestle in the presence of liquid nitrogen, resuspended in 3 ml of whole cell lysis buffer (50 mM KCl, 25 mM Hepes, pH 7.8, 1.0% Nonidet P40, 10 μg/ml leupeptin, 20 μg/ml aprotinin, 125 μM dithiothreitol, 1 mM PMSF and 1 mM Na3VO4), subjected to ten strokes in a Dounce homogenizer and centrifuged at 16000 g at 4 °C for 10 min. Protein concentrations were determined using the BCA (bicinchoninic acid) protein assay (Pierce) and lysates were stored at −80 °C. Prior to electrophoresis, an appropriate volume of cell lysate was diluted in 6× Laemmli loading buffer [375 mM Tris/HCl (pH 6.8), 10% SDS, 30% glycerol, 0.012% Bromophenol Blue and 30% 2-mercaptoethanol] and boiled for 5 min. Total cell protein (25 μg) was subjected to SDS/PAGE on 10% gels and electrotransferred on to a PVDF membrane. Membranes were probed with antibodies directed against HNF-4α (Santa Cruz Biotechnology; sc-6556 and sc-8987), actin (Abcam, ab8226-200), and ChREBP (Novus Biologicals, NB 400-135) and visualized by standard ECL® (enhanced chemiluminescence) procedure. Quantification of immunoblot signals was performed by densitometry using the Versadoc system (Bio-Rad Laboratories).
siRNA transfection method
Two validated Stealth™ siRNAs for rat HNF-4α (RSS304141 and RSS304142) and one scrambled siRNA (Stealth RNAi Negative Control Med GC) were purchased from Invitrogen. Primary hepatocytes were isolated from male Harlan Sprague–Dawley rats (180–220 g) using the collagenase perfusion method [28]. A total of 1.5×106 cells were plated in 6-well Primaria dishes (Invitrogen) containing 2 ml of M199 medium, supplemented with 5% fetal bovine serum, in the presence of 5 mM glucose. After a 4 h attachment period, the medium was replaced with M199 medium supplemented with 100 nM dexamethasone and 100 nM insulin, in the presence of 5 mM glucose. Replicate wells of hepatocytes were then transfected with 100 pmol of each HNF-4α siRNA or scrambled siRNA using Lipofectamine™ 2000 (Invitrogen). After transfection (14 h), the medium was changed and the hepatocytes were cultured for 48 h in M199 medium supplemented with 100 nM dexamethasone, in the presence of 5 mM glucose. Following the 48 h incubation, a sample of each HNF-4α siRNA- and scrambled siRNA-transfected wells were assessed for HNF-4α protein expression. To examine the effect of HNF-4α knockdown on regulation of endogenous FAS transcription, the hepatocytes were cultured in M199 medium containing 5 or 25 mM glucose, with or without 100 nM insulin, and supplemented with 100 nM dexamethasone. After 24 h, total RNA was extracted by TRIzol® (Invitrogen) reagent according to the manufacturer's instructions.
Analysis of mRNA expression by real-time quantitative PCR
Total RNA (2 μg) was reverse-transcribed for 1 h at 37 °C in a 25 μl final volume reaction containing 1 μg of oligo(dT) (Integrated DNA Technologies), 2.5 mM of each dNTPs, 40 units of RNasin (Promega) and 200 units of MMLV (Moloney-murine-leukaemia virus) RT (reverse transcriptase) (Promega). Real-time quantitative PCR analysis was performed starting with 20 ng of reverse-transcribed total RNA, in a final volume of 25 μl of PCR reaction, 0.4 mM of each primer (Integrated DNA Technologies) and 1× Ex Taq RT–PCR mix (TaKaRa) with SYBR Green I (Molecular Probes) in a Smart Cycler instrument (Cepheid). Samples were incubated for an initial denaturation at 95 °C for 30 s, followed by 35 cycles. Each cycle consisted of 95 °C for 3 s, 56 °C for 10 s, and 72 °C for 15 s. The primers used were as follows: for FAS, sense, 5′-GGCATCATTGGGCACTCCTT-3′; antisense, 5′-ACCAACAGCTGCCATGGATC-3′; for L-PK, sense, 5′-CTCGTAGCACCAGCATCATTG-3′; antisense, 5′-GGATGTTGGCGATGGATTCTG-3′; for cyclophilin, sense, 5′-AAGGTGAAAGAAGGCATGAGCA-3′; antisense, 5′-AGTTGTCCACAGTCGGAGATGG-3′. Relative FAS and L-PK mRNA levels were determined by using the Comparative Ct method (User Bulletin no. 2, PE Applied Biosystems). Cyclophilin mRNA was used as the invariant control.
Immunoprecipitation and immunoblot analysis
HEK-293 cells (human embryonic kidney cells) were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. HEK-293 cells were transfected using FuGENE (Roche Applied Science) with empty pSG5, pSG5/HNF-4α and FLAG-tagged ChREBP expression constructs as indicated. Nuclear extracts were generated, 42 h after transfection, using the NE-PER (Pierce Chemical) nuclear and cytoplasmic extraction kit as outlined by the manufacturer. Samples (200 μg) of nuclear proteins from each sample were pre-cleared with Protein G for 2 h, incubated overnight with HNF-4α (Santa Cruz Biotechnology; sc-6556) antibodies at 4 °C, and then incubated with Protein G for an additional 2 h. The samples were centrifuged for 1 min at 4000 g at 4 °C and the supernatants were removed and retained. The remaining pellets were washed four times with cold PBS containing 0.1% Nonidet P40, denatured and loaded on to SDS/10% polyacrylamide gels along with 5% input from the corresponding samples. Gels were transferred and probed with anti-FLAG (Sigma; FLAG-M2) antibody. The detected bands were visualized by the standard ECL® procedure, and band intensity was analysed using the Versadoc system (Bio-Rad).
For co-immunoprecipitation of endogenous HNF-4α and ChREBP, nuclear protein extracts were prepared from livers of fed rats by the method described previously [16]. Samples (350 μg) of nuclear protein were pre-cleared with Protein G for 2 h, and then incubated overnight with either HNF-4α (Santa Cruz Biotechnology; sc-6556), ChREBP (Novus Biologicals, NB 400–135), or preimmune IgG (Santa Cruz Biotechnology) overnight. The remaining pellets were washed four times with cold PBS containing 0.1% Nonidet P40, denatured and loaded on to SDS/10% polyacrylamide gels along with 50 μg of nuclear protein as input (13.5%). Gels were transferred and probed with antibodies directed against HNF-4α (Santa Cruz Biotechnology; sc-6556 and sc-8987) and ChREBP (Novus Biologicals; NB 400-135).
RESULTS
The distal glucose enhancer region of the FAS gene contains an imperfect DR-1
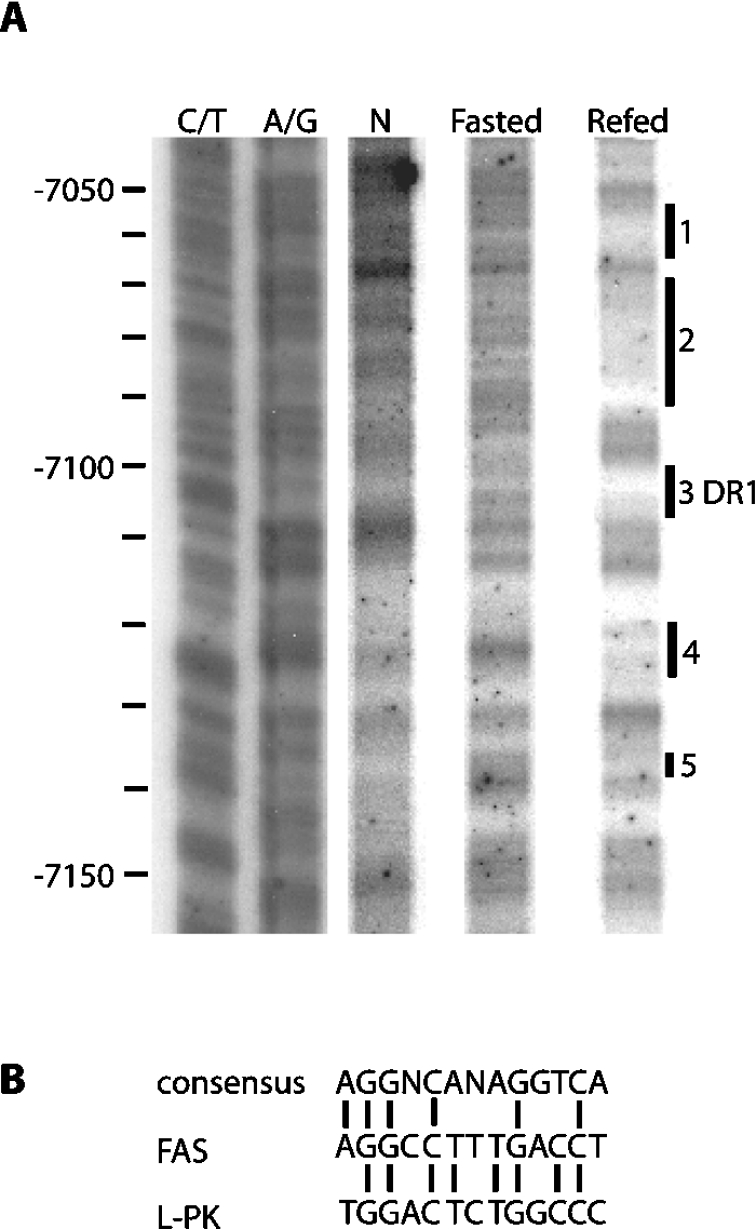
Previous analysis upstream of the insulin-responsive FAS proximal promoter identified a region from −7300 to −6900 that confers carbohydrate responsiveness [14,16]. Further inspection of this distal region through in vivo footprint, gel shift and sequence analysis uncovered a ChoRE element between −7240 and −7120 [16]. In the present study, an additional in vivo footprint downstream of the ChoRE, between −7150 and −7050 was performed, and revealed that refeeding fasted rats a high glucose diet protected several regions from DNase I cleavage (Figure 1A). Sequence analysis established that the protected region from −7105 to −7094 (Figure 1A, site 3) contains an imperfect DR-1 element with significant homology to the DR-1 element in the carbohydrate-responsive region of the L-PK promoter (Figure 1B) [33,34]. This finding supports the concept that refeeding carbohydrate initiates binding of nuclear receptor transcription factor(s) to this newly discovered DR-1 element in the FAS distal enhancer.
Figure 1. In vivo DNase I footprint analysis of the rat liver FAS gene region of −7150/−7050.
(A) Nuclei isolated from liver of fasted and fasted-refed rats were digested with DNase I as described in the Experimental section. The Figure represents the DNase I footprint of the sense strand of the FAS promoter from −7150 to −7050. The end points of the protected regions were identified by chemical sequencing (C/T and G/A lanes). Areas affected by refeeding are labelled as 1–5. Sequences 1, 2, 4 and 5 bind unknown factors, while sequence 3 represents the DR1. (B) The sequence of the FAS DR-1 element was compared with that of the consensus DR-1 and L-PK gene.
HNF-4α from rat liver extract binds the DR-1 element in the FAS enhancer in vitro
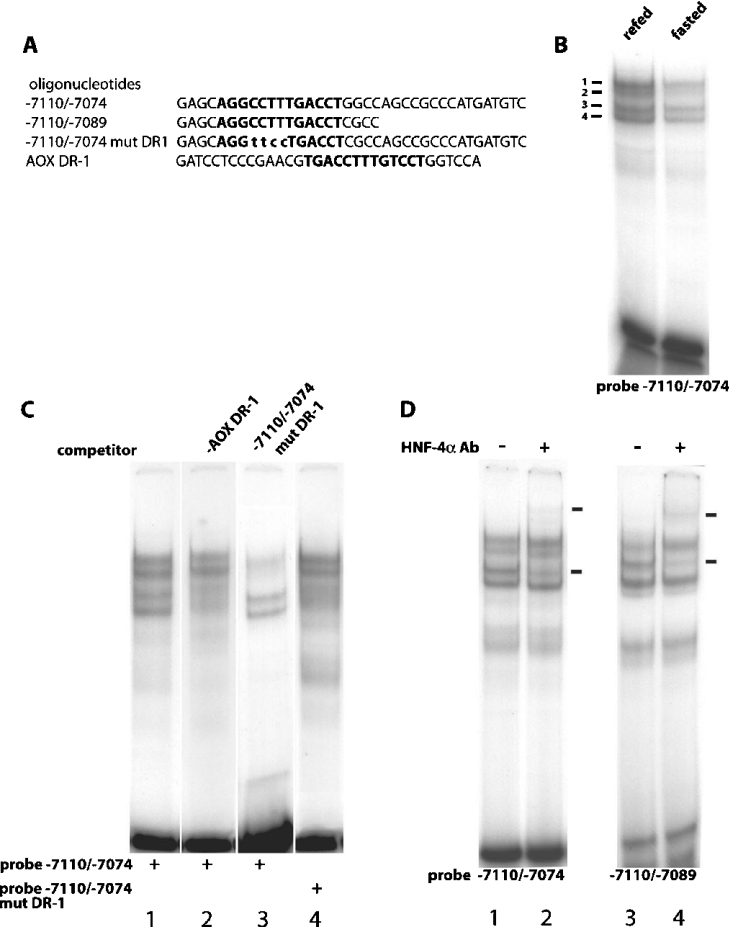
To identify factors that bind the potential DR-1 element in the carbohydrate-responsive region of the FAS distal promoter, EMSAs were used to probe nuclear extracts from fasted and refed rats. An oligonucleotide probe containing the putative DR-1 element and 20 bp of downstream sequence (−7110/−7074) (Figure 2A) produced four complexes with rat liver nuclear extracts (Figure 2B), supporting an interaction of this region with multiple proteins. In addition, binding of the four bands to the DR-1 probe was significantly increased in nuclear extracts from refed versus fasted rats (Figure 2B). Together, the observations support the hypothesis that the transition between fasting and refeeding regulates binding of nuclear proteins to the 37 bp sequence spanning the putative DR-1. To identify which of the four bands correspond to the protein(s) binding the putative DR-1 element and to test whether other known DR-1 elements could compete for binding to the identified band(s), oligonucleotides containing the DR-1 element of AOX (AOX DR-1) [35] and a mutated FAS DR-1 element (−7110/−7074 mut DR1) (Figure 2A) were used as competitors. Incubation with 100-fold excess of unlabelled AOX DR-1 oligonucleotide resulted in a significant reduction in the ability of protein bands 3 and 4 to bind probe −7110/−7074 (Figure 2C, lane 2). In contrast, an oligonucleotide with the DR-1 mutated (−7110/−7074 mut DR1) was unable to decrease the intensity of bands 3 and 4 (Figure 2C, lane 3), demonstrating that the proteins in these complexes are unable to interact with a mutated DR-1. To further establish that the factors responsible for bands 3 and 4 bind the DR-1 element, probe −7110/−7074 mut DR1 was labelled and incubated with protein extracts from the hepatic nuclei of fasted rats. Binding of protein bands 1 and 2 to the mutated probe were unaffected by the DR-1 site mutation, but the binding of bands 3 and 4 was almost completely eliminated (Figure 2C, lane 4). Collectively, these findings make a compelling case that protein bands 3 and 4 bind to a newly identified DR-1 element in the glucose-responsive region of the FAS promoter in a manner regulated by nutritional state.
Figure 2. HNF-4α binds the FAS DR-1 element.
(A) Sequences of the oligonucleotides used as probes and competitors in the gel-shift reactions. The sequence of the DR-1 elements are highlighted in boldface and the mutated bases in the −7110/−7074 mut DR-1 are displayed in lower-case. (B) 1.0 ng of −7110/−7074 radiolabelled oligonucleotide was incubated with either 5 μg of fasted or refed liver nuclear extract and subjected to electrophoresis on a 5% non-denaturing polyacrylamide gel. (C) All gel-shift reactions were incubated with 5 μg of refed liver nuclear extract and employed the following radiolabelled oligonucleotides: −7110/−7074 (lanes 1, 2 and 3) and −7110/−7074 mut DR1 (lane 4). The competing oligonucleotides AOX DR-1 (lane 2) and −7110/−7074 mut DR1 (lane 3) were added at 100× molar excess to the binding reaction. (D) Immunological characterization of HNF-4α binding to the DR-1 element. Gel-shift reactions included 5 μg of refed liver nuclear extract and employed the following radiolabelled oligonucleotides: −7110/−7074 (lanes 1 and 2) and −7110/−7089 (lanes 3 and 4). Antibody (Ab) directed against HNF-4α was added and arrows indicate positions of specific complexes.
Based on previous reports that glucose-induced activation of the L-PK promoter involves HNF-4α binding to a DR-1 element in the promoter [33,36], we tested the hypothesis that one of the two proteins represented by bands 3 and 4 was HNF-4α. Incubation of liver nuclear extract from refed rats with probe −7110/−7074 and HNF-4α antibody produced a super-shifted band and decreased abundance of band 3 (Figure 2D, lane 2). An identical result was obtained when a shorter oligonucleotide (−7110/−7089) was used as a probe with nuclear extract from refed rats (Figure 2D, lane 4). These findings demonstrate that HNF-4α binds the DR-1 element of the FAS distal enhancer and is the transcription factor responsible for the formation of band 3 when either oligonucleotide sequences −7110/−7074 or −7110/−7089 are used as probes. At present, the protein responsible for formation of band 4 has not been identified.
Refeeding glucose to fasted rats increases HNF-4α binding to the DR-1 element
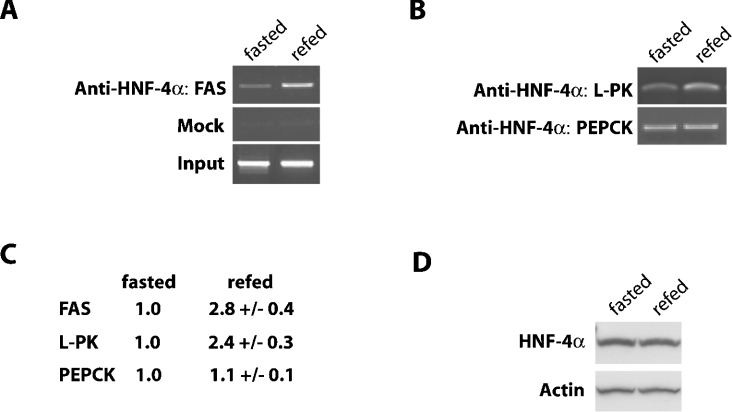
ChIP assays were employed to test the hypothesis that fasting and refeeding regulate binding of HNF-4α to the identified FAS DR-1 element in vivo. Following immunoprecipitation of cross-linked chromatin from replicate samples from each treatment with an HNF-4α-specific antibody, a region of genomic DNA spanning the DR-1 element of the FAS promoter (−7314 to −6980) was amplified by PCR. No amplification was observed when HNF-4α antibody was omitted from the immunoprecipitation reaction (Figure 3A). However, inclusion of HNF-4α antibody immunoprecipitated 2.8-fold more chromatin containing the DR-1 site in samples from rats refed glucose after fasting (Figures 3A and 3C). Stated another way, the amount of HNF-4α bound to the FAS DR-1 site was low during fasting, but was increased approx. 3-fold upon refeeding.
Figure 3. In vivo binding of HNF-4α to the FAS gene promoter is increased following the refeeding of carbohydrate to fasted rats.
(A) ChIP assay of FAS promoter using nuclei isolated from liver of fasted or carbohydrate-refed rats. Chromatin fragments immunoprecipitated with HNF-4α antibodies were amplified by PCR with primers spanning the distal carbohydrate region of the rat FAS gene promoter (−7314 to −6980). Immunoprecipitation with normal goat IgG (mock) was used as a negative control and 1% purified input DNA (input) was used a positive control for the PCR reaction. The data shown are representative of three to six individual experiments. (B) Chromatin immunoprecipitated by the HNF-4α antibody was amplified using primers spanning the DR-1 sites of the L-PK and PEPCK promoters. (C) The results of three to six individual experiments were quantified by measuring the density of the PCR products separated on an agarose gel. The numbers are derived from the average density of the PCR products from the refed liver samples compared with the average intensity of those from the fasted liver samples, which are set at 1.0. (D) Whole cell protein extracts from fasted and refed rat liver were analysed by immunoblotting for HNF-4α and actin as described in the Experimental section.
The L-PK gene is similar to FAS in the sense that it has a DR-1 site adjacent to a ChoRE in its promoter [36,37]. L-PK gene transcription is also induced by carbohydrate refeeding after a fast [38]. To determine whether HNF-4α binding to its promoter is also increased upon refeeding, chromatin immunoprecipitated by the HNF-4α antibody was amplified using primers spanning the DR-1 site of L-PK (−216 to −90 bp). Figure 3(B) shows that HNF-4α binding to the L-PK DR-1 site was also increased (2.4-fold, Figure 3C) in hepatic nuclei of rats refed glucose after a fast. This finding is consistent with previous EMSA data showing increased HNF-4α binding to an oligonucleotide probe spanning this DR-1 in hepatic nuclei from refed rats [39]. Together, these findings support a common mechanism which utilizes differential HNF-4α binding to DR-1 elements to facilitate nutritional regulation of promoters of carbohydrate-responsive genes.
To determine whether increased HNF-4α binding to the FAS and L-PK DR-1s is indicative of a general increase in HNF-4α binding to promoters in livers of refed rats, HNF-4α binding to the PEPCK promoter was also evaluated. Chromatin immunoprecipitated by the HNF-4α antibody was amplified using primers spanning the DR-1 site of the PEPCK promoter. Figure 3(B) shows that HNF-4α binding to this site was unaffected in samples from fasted versus refed rats, demonstrating that HNF-4α binding to the PEPCK promoter is not sensitive to changes in nutritional state.
To rule out changes in HNF-4α expression as a basis for fed state-dependent binding to the FAS promoter, Western blots were used to compare hepatic HNF-4α expression in samples from fasted versus refed rats. Figure 3(D) shows that HNF-4α expression did not differ between the groups, supporting the argument that increased HNF-4α binding to the FAS DR-1 element in liver of refed rats is the result of increased DNA binding activity rather than expression.
Interaction of HNF-4α with the DR-1 element in the FAS distal promoter is required for full responsiveness to carbohydrate
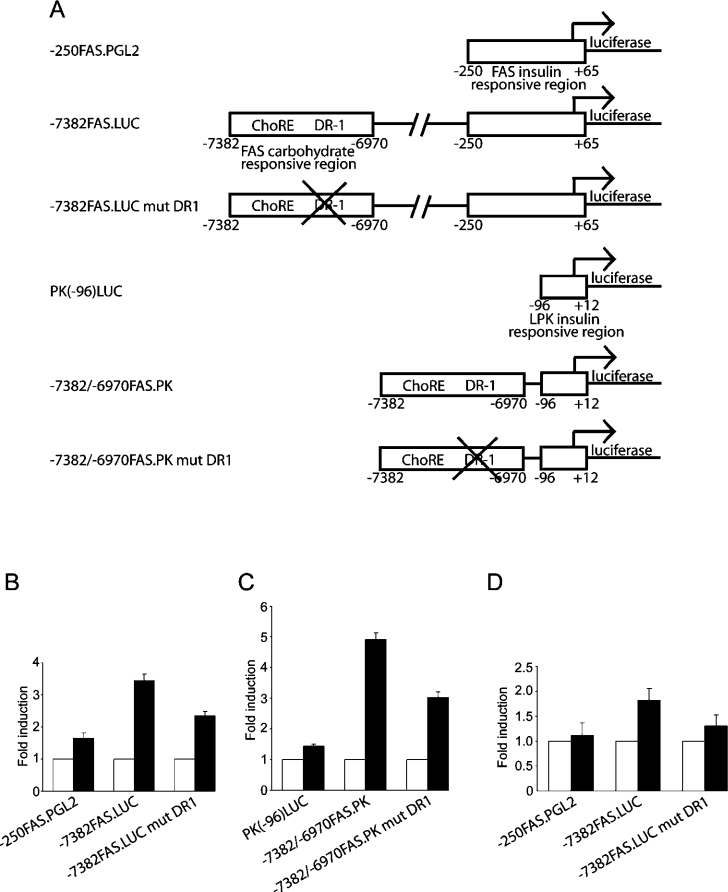
Our findings led us to propose that increased HNF-4α binding to the DR-1 element plays a significant role in enhancement of FAS transcription following refeeding of glucose. To isolate the contribution of HNF-4α to FAS transcriptional activation, promoter constructs containing the proximal insulin-responsive region alone or in tandem with the distal carbohydrate-responsive region were fused to a luciferase reporter (Figure 4A). Co-transfection of HNF-4α with the reporter constructs in COS-1 cells showed that HNF-4α produced a 3.4-fold activation of the promoter construct with both proximal and distal elements (−7382FAS.LUC) (Figure 4B). HNF-4α was far less effective in activating the FAS promoter containing only the proximal promoter region (−250FAS.PGL2) (1.7-fold) (Figure 4B). Thus the use of COS-1 cells, which lack endogenous HNF-4α [39], provided a low background and sensitive readout of HNF-4α effects on FAS promoter activation. Since the −250FAS.PGL2 construct does not contain a known binding site for HNF-4α, we addressed the basis for its 1.7-fold activation by HNF-4α in a parallel experiment with the minimal promoter region of L-PK (PK-96LUC). Co-transfection of HNF-4α with the L-PK minimal promoter produced a similar 1.4-fold activation of promoter activity (Figure 4C). The modest comparable activation of the −250FAS.PGL2 and PK-96LUC constructs suggests that HNF-4α may be acting through additional transcription factors that bind and transactivate the minimal insulin-responsive promoter regions of FAS and L-PK.
Figure 4. The DR-1 element of FAS is required for full glucose responsiveness.
(A) Schematic representation of the reporter constructs used in the subsequent luciferase assays. (B) COS-1 cells were transfected with the listed FAS luciferase reporter constructs and empty vector (open bars) or vector expressing HNF-4α (solid bars). Data are normalized to the luciferase activity of the luciferase constructs in the presence of vector alone, which is equal to 1. The results shown represent the means±S.E.M. for four independent experiments with three replicate transfections per experiment. (C) COS-1 cells were transfected with the listed FAS/L-PK luciferase reporter constructs as outlined in (B). (D) Rat primary hepatocytes were transfected with the listed luciferase reporter constructs. Cells were cultured in 5.5 mM (open bars) or 27.5 mM glucose (solid bars) for 48 h in the presence of 100 nM insulin and 100 nM dexamethasone. The data are expressed as fold increase in luciferase activity over 5.5 mM glucose. The results shown represent the means±S.E.M. for four independent experiments with three replicate transfections per experiment.
To establish that the identified DR-1 element was responsible for observed effects of HNF-4α on FAS promoter activation, we mutated the DR-1 element in the FAS reporter containing both the distal and proximal regions (−7382FAS.LUC mut DR1). Mutation of this site reduced promoter activation by HNF-4α from 3.4-fold to 2.4-fold (Figure 4B). For direct comparison, a reporter containing the distal region of FAS and the proximal region of L-PK (−7382/−6970FAS.PK) produced a 4.9-fold activation by HNF-4α that was similarly reduced to 3.0-fold when the DR-1 was mutated (−7382/−6970FAS.PK mut DR1) (Figure 4C). Notwithstanding some residual HNF-4α-sensitive activation independent of the distal DR-1 (Figures 4B and 4C), these findings establish collectively that HNF-4α binding to this DR-1 plays an important role in regulating FAS promoter activity. In particular, HNF-4α binding to this site is essential for optimal activation of the FAS promoter during the transition from fasted to fed states.
Using primary rat hepatocytes, we previously showed that reporter constructs containing both the distal carbohydrate-responsive region and the proximal insulin elements of the FAS promoter express approx. 2-fold more luciferase when the medium contained 27.5 mM glucose than when it contained only 5.5 mM glucose [16]. To assess the role of the FAS DR-1 in this response, primary cultures of rat hepatocytes were transfected with the promoter/reporter constructs described above. As predicted, the reporter containing only the insulin-responsive region (−250FAS.PGL2) was minimally responsive to changes in media glucose (1.1-fold), while the construct that included the distal carbohydrate-responsive region (−7382FAS.LUC) yielded a robust (1.8-fold) increase in promoter activity (Figure 4D). Interestingly, the reporter containing the mutated DR-1 element showed a blunted response to the increase in glucose concentration (1.3-fold). These results show that binding of HNF-4α to the FAS distal promoter is required for full transcriptional activation by increased media glucose, suggesting a functional interaction between ChREBP and HNF-4α during glucose-mediated activation of the distal FAS promoter.
Inhibition of HNF-4α gene expression reduces FAS gene induction by glucose in primary hepatocytes
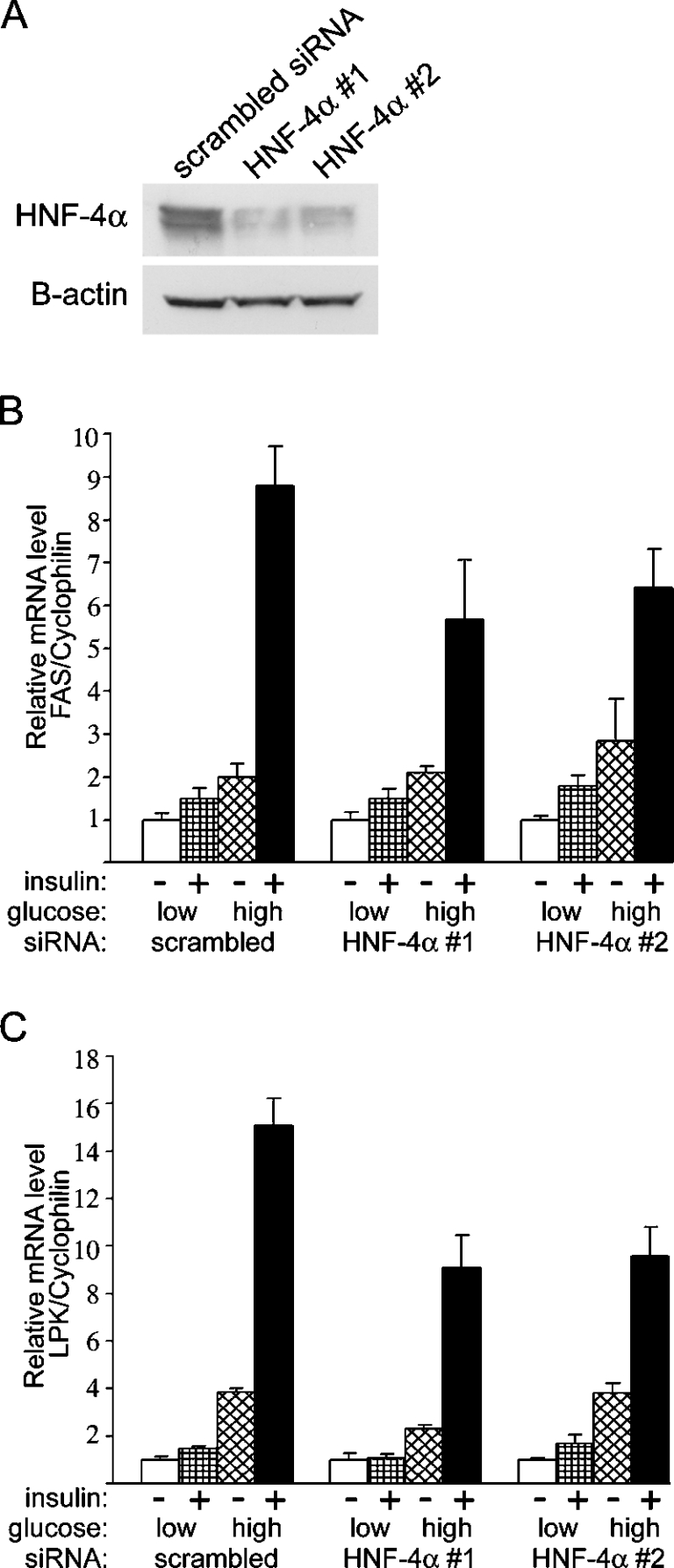
To determine the direct implication of HNF-4α in glucose action on endogenous FAS gene expression, we next performed a series of experiments using siRNA to deplete HNF-4α gene expression in primary cultures of rat hepatocytes. Primary hepatocytes were transfected with 100 pmol of either scrambled siRNA or one of two separate HNF-4α siRNAs (HNF-4α no. 1 and 2) and cultured for 48 h at low (5 mM) glucose concentration. Following the 48 h incubation, a sample of HNF-4α and scrambled siRNA transfected hepatocytes were harvested for immunoblot analysis of HNF-4α protein levels (Figure 5A). We observed an approx. 3-fold reduction in HNF-4α protein levels in the hepatocytes transfected with either HNF-4α siRNA. After the knockdown in HNF-4α protein, the hepatocytes were incubated for 24 h in the presence of 5 or 25 mM glucose with or without insulin. In the scrambled siRNA transfected hepatocytes, low glucose and insulin (1.5-fold) as well as high glucose alone (2.0-fold) had a stimulatory effect on FAS gene expression. When these hepatocytes were incubated with both high glucose concentration (25 mM) and insulin, FAS gene expression was stimulated by 8.8-fold (Figure 5B). This induction in FAS mRNA was similar to that observed in untransfected primary hepatocytes (results not shown). In contrast, FAS gene induction by high glucose and insulin concentration was reduced by 25–35% when hepatocytes were transfected with the HNF-4α siRNAs (Figure 5B). These results show that HNF-4α is required for optimal induction of FAS gene expression by increased media glucose and insulin. Interestingly, knockdown of HNF-4α protein had little effect on the stimulation of FAS gene expression with low glucose and insulin and high glucose alone (Figure 5B). These results suggest that HNF-4α may play a role in the co-ordination of signalling inputs from both insulin and high glucose in order to maximize the transcription of FAS. We next measured the expression of L-PK, a similarly insulin- and glucose-regulated gene. In scrambled siRNA transfected hepatocytes, the induction of L-PK was greatly induced (15.1-fold) by incubation in medium containing insulin and 25 mM glucose (Figure 5C). Similar to the decrease in expression observed in FAS, induction of L-PK by high glucose and insulin concentration was reduced by 35–40% when hepatocytes were transfected with the HNF-4α siRNAs (Figure 5C). Together, these findings support a common mechanism that utilizes HNF-4α to facilitate nutritional regulation of promoters of carbohydrate responsive genes.
Figure 5. Effects of HNF-4α gene silencing on FAS gene expression in primary hepatocytes.
After plating, hepatocytes were then transfected with 100 pmol of either HNF-4α siRNA or scrambled siRNA and incubated for 48 h in medium supplemented with 100 nM dexamethasone, in the presence of 5 mM glucose. (A) Following the 48 h incubation, a sample of both HNF-4α siRNA and scrambled siRNA transfected hepatocytes were harvested for immunoblot analysis of HNF-4α protein levels. Actin was used as a loading control. After knockdown of HNF-4α protein, the hepatocytes were cultured in M199 medium containing 5 or 25 mM glucose, with or without 100 nM insulin, and supplemented with 100 nM dexamethasone. After 24 h, total RNA was extracted and analysed for FAS (B) and LPK (C) gene expression by real-time quantitative PCR. Results are the means±S.E.M for four independent cultures.
Physical interaction of HNF-4α with ChREBP
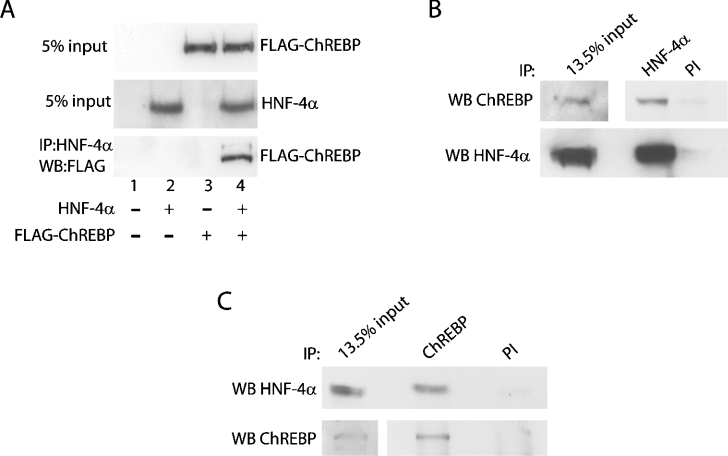
To explore the mechanism underlying the functional interaction of HNF-4α and ChREBP with respect to regulation of the FAS distal promoter, we tested the hypothesis that the two proteins physically interact to form a protein complex. In HEK-293 cells transiently expressing HNF-4α and FLAG-tagged ChREBP, protein–protein interactions between ChREBP and HNF-4α were assessed by immunoprecipitation in conjunction with Western blotting to determine whether the two proteins were co-immunoprecipitated. Initial control experiments with HEK-293 cells transiently expressing a different FLAG-fusion protein and HNF-4α were performed in order to eliminate the outside possibility that the FLAG domain interacts with HNF-4α (results not shown). Figure 6(A) show that both proteins were expressed and that co-expression of both proteins did not alter the relative expression of either protein. In nuclear extracts immunoprecipitated with HNF-4α antibody and probed with FLAG antibody by Western blotting, FLAG-ChREBP was only detected in immunoprecipitates from cells co-transfected with HNF-4α (Figure 6A, lane 4). To gain further evidence that the two proteins physically interact, we next determined whether endogenous HNF-4α and ChREBP could be co-immunoprecipitated. Using nuclear protein isolated from fasted rats refed a fat-free diet (350 μg), we performed immunoprecipitations with antibodies specific for HNF-4α and ChREBP as well as preimmune serum as a negative control. We found that ChREBP co-immunoprecipitated with anti-HNF-4α antiserum but only minimally with preimmune serum (Figure 6B, top panel). Regarding the input for this experiment (13.5%), we found that ChREBP is difficult to detect when only 50 μg of nuclear protein is loaded per lane. Therefore, a longer exposure is included in the Figure. When the inverse of the experiment displayed in Figure 6(B) was performed, we detected HNF-4α in ChREBP immunoprecipitates (Figure 6C, top panel). As far as we know, this is the first demonstration of a physical interaction between these two transcription factors. Collectively, these results demonstrate a physical interaction of HNF-4α and ChREBP, which may be related to the functional interaction of these two proteins in their regulation of the FAS distal promoter.
Figure 6. HNF-4α physically interacts with ChREBP in vivo.
(A) HEK-293 cells were co-transfected with expression vectors for HNF-4α and FLAG-tagged ChREBP. Nuclear protein extracts were prepared, 42 h following transfection, and subjected to co-immunoprecipitation with anti-HNF-4α antibody. After incubation at 4 °C overnight and washing three times with PBS containing 0.1% Nonidet P40, the samples were analysed on a 10% gel by SDS/PAGE and Western blotted with anti-FLAG antibody. The top and middle panels are Western blots of 5% of the nuclear extract before immunoprecipitation, and the bottom panel is a Western blot of the immunoprecipitated proteins. (B) Nuclear protein extracts from the liver of refed rats were prepared, subjected to immunoprecipitation with anti-HNF-4α antibody or preimmune IgG (PI), and Western blotted with anti-ChREBP antibody (top panel). The blot was then stripped and immunoblotted with a separate anti-HNF-4α antibody (bottom panel). (C) The inverse of the experiment displayed in (B) was performed, with nuclear protein extracts subjected to immunoprecipitation with anti-ChREBP antibody, and immunoblotted with anti-HNF-4α antibody (top panel). The blot was stripped and immunoblotted with anti-ChREBP antibody (bottom panel).
DISCUSSION
The co-ordinated regulation of FAS expression in response to changes in nutrient availability involves integration of transcriptional inputs from proximal and distal elements of its promoter. The existence of additional factors in the distal FAS promoter was explored using in vivo footprint analysis, and this approach identified a preferentially protected region in hepatic nuclei from carbohydrate-refed rats with sequence homology to a DR-1 element. EMSAs were used to test for complex formation, dependency of this putative DR-1 for binding, and identify proteins binding at this site. An oligonucleotide spanning the putative DR-1 element produced four complexes with rat liver nuclear extracts, two of which were absent when the DR-1 site was mutated. An oligonucleotide containing the known DR-1 site from AOX reduced interaction of the FAS DR-1 with the later two proteins. Supershift analysis identified HNF-4α as one of the two proteins whose binding to this element was regulated by nutritional state and required an intact DR-1. The identity of the other protein (band 4) bound to the FAS DR-1 is unknown, but its behaviour in EMSAs is consistent with that of a nuclear receptor. Although ChREBP binding to the distal FAS promoter would increase under these conditions [21], the absence of the ChoRE in the EMSA probe makes it unlikely that band 4 is ChREBP.
Our finding that carbohydrate increased HNF-4α binding to this imperfect DR-1 in the distal FAS promoter was complemented by our observation that HNF-4α activated a reporter construct containing the distal FAS promoter in a DR-1-dependent manner. Glucose regulated the same reporter constructs in isolated hepatocytes in a DR-1-dependent manner, supporting the idea that carbohydrate regulates FAS in part through modulation of HNF-4α binding to the FAS promoter. This finding extends our previous work showing that partial glucose responsiveness was retained in FAS promoter constructs in which the ChoRE had been mutated [16]. Together, the results demonstrate a functional interaction between HNF-4α and ChREBP in regulating the distal FAS promoter. We also found that the two factors physically interact to form a complex that can be detected by co-immunoprecipitation. This finding raises the interesting possibility that formation of this complex underlies the functional interaction that is required for full glucose-responsiveness of the FAS distal promoter. Although the underlying mechanism of their complimentary effects on promoter activation is unclear, previous [21] and present data demonstrate that glucose refeeding of fasted rats increases binding of both HNF-4α and ChREBP to the FAS and L-PK promoters. The specific increase in HNF-4α binding to the promoters of these and perhaps other carbohydrate-sensitive genes is consistent with the hypothesis that physical interaction of HNF-4α and ChREBP facilitates, directs, or stabilizes their binding to the distal FAS promoter. This protein–protein interaction could be a way of regulating the delivery of HNF-4α to promoters of specific subsets of genes. It will be important to pursue this possibility in future studies, and examine how changes in nutritional state regulate this complex.
Using both EMSA and ChIP approaches, we show that HNF-4α binding to an imperfect DR-1 in the FAS distal promoter is increased by refeeding glucose after a fast. An important unanswered question is how the transition from fasted to fed state increases HNF-4α binding to this promoter element in the absence of changes in its expression. HNF-4α also plays a key role in regulating gluconeogenic genes during fasting, so it is not surprising that its expression would be stable and changes in HNF-4α binding to target gene promoters would be targeted through post-translational mechanisms linked to changes in nutritional state. AMPK (AMP-activated protein kinase) and PKA (protein kinase A) are likely candidates in that hepatic AMPK is rapidly activated by fasting and decreased by glucose refeeding [40,41]. PKA activity is similarly modulated through the reciprocal effects of glucagon (fasting) and insulin (refeeding) on hepatic cAMP levels. More importantly, both kinases phosphorylate HNF-4α [39,42] and modulate the transcriptional activity of the FAS promoter [2,43–45].
Post-translational mechanisms are clearly important in regulating DNA binding activity of HNF-4α and ChREBP, but the binding of HNF-4α to the promoters of many genes is unresponsive to signals linked to nutritional state [46,47]. For instance, we show that HNF-4α binding to the PEPCK promoter is unaffected in samples where significant increases in HNF-4α binding to the FAS distal promoter were found. Thus a significant unresolved issue is how nutrient-sensing mechanisms target transcription factors to specific subsets of genes to produce adaptive transcriptional responses. In addition to FAS, we found increased binding of HNF-4α to the DR-1 in L-PK in liver samples from rats refed glucose after a fast. A common structural feature of carbohydrate-responsive regions of each promoter is a DR-1 site adjacent to a ChoRE. This raises the interesting possibility that promoters of these two, and perhaps other genes with similar configurations are sensitive to a protein complex containing both HNF-4α and ChREBP.
To determine the direct implication of HNF-4α in glucose action on endogenous FAS gene expression, we depleted HNF-4α protein levels in primary cultures of rat hepatocytes using siRNA and assessed the impact on induction of endogenous FAS mRNA by glucose. As predicted, knockdown of HNF-4α compromised the ability of high glucose and insulin to increase FAS mRNA (Figure 5). This finding illustrates that HNF-4α is required for optimal induction of FAS gene expression by increased media glucose and insulin. Interestingly, knockdown of HNF-4α protein had little effect on the stimulation of FAS gene expression with low glucose and insulin and high glucose alone. These results suggest that HNF-4α may play a role in the coordination of signalling inputs from both insulin and glucose in order to maximize the transcription of FAS. A recent report showing that HNF-4α and SREBP-1c physically interact [48] and our results showing an interaction between HNF-4α and ChREBP hint at the interesting possibility that HNF-4α may serve as a molecular scaffold to assemble the transcriptional machinery activated by glucose and insulin. If this were the case, the insulin- and carbohydrate-response elements of the FAS promoter could be brought together in a single complex through the interaction of ChREBP and SREBP-1c with HNF-4α.
In summary, we have identified a DR-1 element in the FAS distal promoter and shown for the first time that (i) HNF-4α binding to this site regulates transcriptional activity of the FAS promoter, (ii) HNF-4α binding to this site is regulated in vivo by glucose refeeding after a fast, and (iii) HNF-4α and ChREBP physically interact to form a complex that may be important in targeting HNF-4α to glucose-responsive genes. Collectively, these findings extend our understanding of hepatic nutrient-sensing systems and make a compelling case that HNF-4α plays an important, previously unappreciated role in mediating glucose-dependent effects on the FAS promoter.
Acknowledgments
Supported by the NIH (National Institutes of Health) DK53872 (T.W.G.), DK06156 (T.W.G.), P30 DK072476 (A.W.A.) and a Research Grant from the ADA (American Diabetes Association) (1-03-RA-26). A.W.A. is supported by NIH Training Grant T32 DK064584-02. We thank Dr Tom Dreesen for insightful comments and suggestions.
References
- 1.Clarke S. D., Armstrong M. K., Jump D. B. Dietary polyunsaturated fats uniquely suppress rat liver fatty acid synthase and S14 mRNA content. J. Nutr. 1990;120:225–231. doi: 10.1093/jn/120.2.225. [DOI] [PubMed] [Google Scholar]
- 2.Paulauskis J. D., Sul H. S. Hormonal regulation of mouse fatty acid synthase gene transcription in liver. J. Biol. Chem. 1989;264:574–577. [PubMed] [Google Scholar]
- 3.Armstrong M. K., Blake W. L., Clarke S. D. Arachidonic acid suppression of fatty acid synthase gene expression in cultured rat hepatocytes. Biochem. Biophys. Res. Commun. 1991;177:1056–1061. doi: 10.1016/0006-291x(91)90645-n. [DOI] [PubMed] [Google Scholar]
- 4.Rangan V. S., Oskouian B., Smith S. Identification of an inverted CCAAT box motif in the fatty-acid synthase gene as an essential element for modification of transcriptional regulation by cAMP. J. Biol. Chem. 1996;271:2307–2312. doi: 10.1074/jbc.271.4.2307. [DOI] [PubMed] [Google Scholar]
- 5.Yin D., Clarke S. D., Peters J. L., Etherton T. D. Somatotropin-dependent decrease in fatty acid synthase mRNA abundance in 3T3-F442A adipocytes is the result of a decrease in both gene transcription and mRNA stability. Biochem. J. 1998;331:815–820. doi: 10.1042/bj3310815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jump D. B., Clarke S. D., Thelen A., Liimatta M. Coordinate regulation of glycolytic and lipogenic gene expression by polyunsaturated fatty acids. J. Lipid Res. 1994;35:1076–1084. [PubMed] [Google Scholar]
- 7.Bennett M. K., Lopez J. M., Sanchez H. B., Osborne T. F. Sterol regulation of fatty acid synthase promoter. Coordinate feedback regulation of two major lipid pathways. J. Biol. Chem. 1995;270:25578–25583. doi: 10.1074/jbc.270.43.25578. [DOI] [PubMed] [Google Scholar]
- 8.Roder K., Wolf S. S., Beck K. F., Sickinger S., Schweizer M. NF-Y binds to the inverted CCAAT box, an essential element for cAMP-dependent regulation of the rat fatty acid synthase (FAS) gene. Gene. 1997;184:21–26. doi: 10.1016/s0378-1119(96)00568-9. [DOI] [PubMed] [Google Scholar]
- 9.Wang D., Sul H. S. Upstream stimulatory factors bind to insulin response sequence of the fatty acid synthase promoter. USF1 is regulated. J. Biol. Chem. 1995;270:28716–28722. doi: 10.1074/jbc.270.48.28716. [DOI] [PubMed] [Google Scholar]
- 10.Magana M. M., Koo S. H., Towle H. C., Osborne T. F. Different sterol regulatory element-binding protein-1 isoforms utilize distinct co-regulatory factors to activate the promoter for fatty acid synthase. J. Biol. Chem. 2000;275:4726–4733. doi: 10.1074/jbc.275.7.4726. [DOI] [PubMed] [Google Scholar]
- 11.Foretz M., Pacot C., Dugail I., Lemarchand P., Guichard C., Le Liepvre X., Berthelier-Lubrano C., Spiegelman B., Kim J. B., Ferre P., Foufelle F. ADD1/SREBP-1c is required in the activation of hepatic lipogenic gene expression by glucose. Mol. Cell Biol. 1999;19:3760–3768. doi: 10.1128/mcb.19.5.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiong S., Chirala S. S., Wakil S. J. Sterol regulation of human fatty acid synthase promoter I requires nuclear factor-Y- and Sp-1-binding sites. Proc. Natl. Acad. Sci. U.S.A. 2000;97:3948–3953. doi: 10.1073/pnas.040574197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roder K., Wolf S. S., Larkin K. J., Schweizer M. Interaction between the two ubiquitously expressed transcription factors NF-Y and Sp1. Gene. 1999;234:61–69. doi: 10.1016/s0378-1119(99)00180-8. [DOI] [PubMed] [Google Scholar]
- 14.Rufo C., Gasperikova D., Clarke S. D., Teran-Garcia M., Nakamura M. T. Identification of a novel enhancer sequence in the distal promoter of the rat fatty acid synthase gene. Biochem. Biophys. Res. Commun. 1999;261:400–405. doi: 10.1006/bbrc.1999.1034. [DOI] [PubMed] [Google Scholar]
- 15.Stoeckman A. K., Towle H. C. The role of SREBP-1c in nutritional regulation of lipogenic enzyme gene expression. J. Biol. Chem. 2002;277:27029–27035. doi: 10.1074/jbc.M202638200. [DOI] [PubMed] [Google Scholar]
- 16.Rufo C., Teran-Garcia M., Nakamura M. T., Koo S. H., Towle H. C., Clarke S. D. Involvement of a unique carbohydrate-responsive factor in the glucose regulation of rat liver fatty-acid synthase gene transcription. J. Biol. Chem. 2001;276:21969–21975. doi: 10.1074/jbc.M100461200. [DOI] [PubMed] [Google Scholar]
- 17.Liu Z., Thompson K. S., Towle H. C. Carbohydrate regulation of the rat L-type pyruvate kinase gene requires two nuclear factors: LF-A1 and a member of the c-myc family. J. Biol. Chem. 1993;268:12787–12795. [PubMed] [Google Scholar]
- 18.O'Callaghan B. L., Koo S. H., Wu Y., Freake H. C., Towle H. C. Glucose regulation of the acetyl-CoA carboxylase promoter PI in rat hepatocytes. J. Biol. Chem. 2001;276:16033–16039. doi: 10.1074/jbc.M101557200. [DOI] [PubMed] [Google Scholar]
- 19.Shih H. M., Liu Z., Towle H. C. Two CACGTG motifs with proper spacing dictate the carbohydrate regulation of hepatic gene transcription. J. Biol. Chem. 1995;270:21991–21997. doi: 10.1074/jbc.270.37.21991. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita H., Takenoshita M., Sakurai M., Bruick R. K., Henzel W. J., Shillinglaw W., Arnot D., Uyeda K. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc. Natl. Acad. Sci. U.S.A. 2001;98:9116–9121. doi: 10.1073/pnas.161284298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishii S., Iizuka K., Miller B. C., Uyeda K. Carbohydrate response element binding protein directly promotes lipogenic enzyme gene transcription. Proc. Natl. Acad. Sci. U.S.A. 2004;101:15597–15602. doi: 10.1073/pnas.0405238101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawaguchi T., Takenoshita M., Kabashima T., Uyeda K. Glucose and cAMP regulate the L-type pyruvate kinase gene by phosphorylation/dephosphorylation of the carbohydrate response element binding protein. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13710–13715. doi: 10.1073/pnas.231370798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabashima T., Kawaguchi T., Wadzinski B. E., Uyeda K. Xylulose 5-phosphate mediates glucose-induced lipogenesis by xylulose 5-phosphate-activated protein phosphatase in rat liver. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5107–5112. doi: 10.1073/pnas.0730817100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iizuka K., Bruick R. K., Liang G., Horton J. D., Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7281–7286. doi: 10.1073/pnas.0401516101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dentin R., Pegorier J. P., Benhamed F., Foufelle F., Ferre P., Fauveau V., Magnuson M. A., Girard J., Postic C. Hepatic glucokinase is required for the synergistic action of ChREBP and SREBP-1c on glycolytic and lipogenic gene expression. J. Biol. Chem. 2004;279:20314–20326. doi: 10.1074/jbc.M312475200. [DOI] [PubMed] [Google Scholar]
- 26.Koo S. H., Dutcher A. K., Towle H. C. Glucose and insulin function through two distinct transcription factors to stimulate expression of lipogenic enzyme genes in liver. J. Biol. Chem. 2001;276:9437–9445. doi: 10.1074/jbc.M010029200. [DOI] [PubMed] [Google Scholar]
- 27.Stoeckman A. K., Ma L., Towle H. C. Mlx is the functional heteromeric partner of the carbohydrate response element-binding protein in glucose regulation of lipogenic enzyme genes. J. Biol. Chem. 2004;279:15662–15669. doi: 10.1074/jbc.M311301200. [DOI] [PubMed] [Google Scholar]
- 28.Salati L. M., Clarke S. D. Fatty acid inhibition of hormonal induction of acetylcoenzyme A carboxylase in hepatocyte monolayers. Arch. Biochem. Biophys. 1986;246:82–89. doi: 10.1016/0003-9861(86)90451-0. [DOI] [PubMed] [Google Scholar]
- 29.Jump D. B., Wong N. C., Oppenheimer J. H. Chromatin structure and methylation state of a thyroid hormone-responsive gene in rat liver. J. Biol. Chem. 1987;262:778–784. [PubMed] [Google Scholar]
- 30.Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986;47:767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- 31.Roder K., Latasa M. J., Sul H. S. Murine H-rev107 gene encoding a class II tumor suppressor: gene organization and identification of an Sp1/Sp3-binding GC-box required for its transcription. Biochem. Biophys. Res. Commun. 2002;293:793–799. doi: 10.1016/S0006-291X(02)00274-7. [DOI] [PubMed] [Google Scholar]
- 32.Bennett M. K., Osborne T. F. Nutrient regulation of gene expression by the sterol regulatory element binding proteins: increased recruitment of gene-specific coregulatory factors and selective hyperacetylation of histone H3 in vivo. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6340–6344. doi: 10.1073/pnas.97.12.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergot M. O., Diaz-Guerra M. J., Puzenat N., Raymondjean M., Kahn A. Cis-regulation of the L-type pyruvate kinase gene promoter by glucose, insulin and cyclic AMP. Nucleic Acids Res. 1992;20:1871–1877. doi: 10.1093/nar/20.8.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Z., Towle H. C. Functional synergism in the carbohydrate-induced activation of liver-type pyruvate kinase gene expression. Biochem. J. 1995;308:105–111. doi: 10.1042/bj3080105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tugwood J. D., Issemann I., Anderson R. G., Bundell K. R., McPheat W. L., Green S. The mouse peroxisome proliferator activated receptor recognizes a response element in the 5′ flanking sequence of the rat acyl CoA oxidase gene. EMBO J. 1992;11:433–439. doi: 10.1002/j.1460-2075.1992.tb05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vaulont S., Puzenat N., Levrat F., Cognet M., Kahn A., Raymondjean M. Proteins binding to the liver-specific pyruvate kinase gene promoter. A unique combination of known factors. J. Mol. Biol. 1989;209:205–219. doi: 10.1016/0022-2836(89)90273-8. [DOI] [PubMed] [Google Scholar]
- 37.Shih H. M., Towle H. C. Definition of the carbohydrate response element of the rat S14 gene. Evidence for a common factor required for carbohydrate regulation of hepatic genes. J. Biol. Chem. 1992;267:13222–13228. [PubMed] [Google Scholar]
- 38.Vaulont S., Munnich A., Decaux J. F., Kahn A. Transcriptional and post-transcriptional regulation of L-type pyruvate kinase gene expression in rat liver. J. Biol. Chem. 1986;261:7621–7625. [PubMed] [Google Scholar]
- 39.Viollet B., Kahn A., Raymondjean M. Protein kinase A-dependent phosphorylation modulates DNA-binding activity of hepatocyte nuclear factor 4. Mol. Cell Biol. 1997;17:4208–4219. doi: 10.1128/mcb.17.8.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suchankova G., Tekle M., Saha A. K., Ruderman N. B., Clarke S. D., Gettys T. W. Dietary polyunsaturated fatty acids enhance hepatic AMP-activated protein kinase activity in rats. Biochem. Biophys. Res. Commun. 2005;326:851–858. doi: 10.1016/j.bbrc.2004.11.114. [DOI] [PubMed] [Google Scholar]
- 41.Munday M. R., Milic M. R., Takhar S., Holness M. J., Sugden M. C. The short-term regulation of hepatic acetyl-CoA carboxylase during starvation and re-feeding in the rat. Biochem. J. 1991;280:733–737. doi: 10.1042/bj2800733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong Y. H., Varanasi U. S., Yang W., Leff T. AMP-activated protein kinase regulates HNF4alpha transcriptional activity by inhibiting dimer formation and decreasing protein stability. J. Biol. Chem. 2003;278:27495–27501. doi: 10.1074/jbc.M304112200. [DOI] [PubMed] [Google Scholar]
- 43.Foretz M., Carling D., Guichard C., Ferre P., Foufelle F. AMP-activated protein kinase inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J. Biol. Chem. 1998;273:14767–14771. doi: 10.1074/jbc.273.24.14767. [DOI] [PubMed] [Google Scholar]
- 44.Leclerc I., Kahn A., Doiron B. The 5′-AMP-activated protein kinase inhibits the transcriptional stimulation by glucose in liver cells, acting through the glucose response complex. FEBS Lett. 1998;431:180–184. doi: 10.1016/s0014-5793(98)00745-5. [DOI] [PubMed] [Google Scholar]
- 45.Woods A., Azzout-Marniche D., Foretz M., Stein S. C., Lemarchand P., Ferre P., Foufelle F., Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol. Cell Biol. 2000;20:6704–6711. doi: 10.1128/mcb.20.18.6704-6711.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duong D. T., Waltner-Law M. E., Sears R., Sealy L., Granner D. K. Insulin inhibits hepatocellular glucose production by utilizing liver-enriched transcriptional inhibitory protein to disrupt the association of CREB-binding protein and RNA polymerase II with the phosphoenolpyruvate carboxykinase gene promoter. J. Biol. Chem. 2002;277:32234–32242. doi: 10.1074/jbc.M204873200. [DOI] [PubMed] [Google Scholar]
- 47.Gautier-Stein A., Mithieux G., Rajas F. A distal region involving hepatocyte nuclear factor 4alpha and CAAT/enhancer binding protein markedly potentiates the protein kinase A stimulation of the glucose-6-phosphatase promoter. Mol. Endocrinol. 2005;19:163–174. doi: 10.1210/me.2004-0105. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto T., Shimano H., Nakagawa Y., Ide T., Yahagi N., Matsuzaka T., Nakakuki M., Takahashi A., Suzuki H., Sone H., et al. SREBP-1 interacts with hepatocyte nuclear factor-4 alpha and interferes with PGC-1 recruitment to suppress hepatic gluconeogenic genes. J. Biol. Chem. 2004;279:12027–12035. doi: 10.1074/jbc.M310333200. [DOI] [PubMed] [Google Scholar]