Abstract
We determined MICs for 28 Candida isolates recovered from patients who developed breakthrough candidemia while receiving fluconazole. MICs correlated with daily and cumulative doses of fluconazole. Patients receiving ≥2,000 mg cumulatively or >200 mg daily were more likely to be infected with isolates that were not fluconazole susceptible.
Fluconazole prophylaxis is effective for the prevention of candidiasis in selected high-risk populations (3, 16). The practice is limited, however, by the potential for breakthrough candidiasis due to isolates that exhibit diminished susceptibilities to fluconazole (2, 4-9). This phenomenon of relative resistance to fluconazole has been well documented in particular cases (19, 20). In larger studies, the literature is conflicting. Some studies have shown that breakthrough candidemia is associated with the emergence of non-Candida albicans spp. and isolates of diminished fluconazole susceptibility (2, 4-9, 14), but others have not (10, 11, 15, 17). Studies have assessed different patient populations, associated risk factors, and fluconazole regimens. In most of the studies, patients received a fixed daily dose of fluconazole (3, 4, 11, 17). In others, doses differed (2, 14), but the relationship between dose and fluconazole susceptibility was not assessed.
We previously demonstrated that the fluconazole MIC and the fluconazole daily dose/MIC ratio correlated with therapeutic responses among patients with candidemia (1). As such, we predicted that MICs of breakthrough Candida bloodstream isolates would correlate with fluconazole doses used for prophylactic or empirical therapy. To test this hypothesis, we studied 28 isolates that caused consecutive episodes of breakthrough candidemia among unique patients. All of the patients received fluconazole daily for at least 3 days prior to the development of candidemia. Thirteen of the patients were enrolled in a prospective, multicenter, observational study of candidemia (1, 8). The remaining 15 were identified from 2004 to 2006 at the Shands Teaching Hospital at the University of Florida.
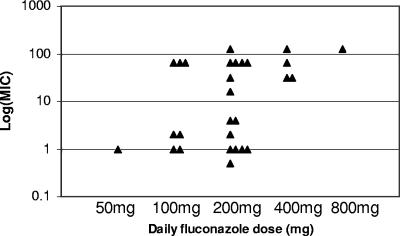
MICs were measured at 24 h using a standardized broth dilution method (1) (Table 1). Reference strains (C. albicans ATCC 90028 and ATCC 90029, C. parapsilosis ATCC 90018, and C. glabrata ATCC 90030) were incorporated into each set of experiments as quality controls. There was a correlation between the MIC and the daily fluconazole dose (P = 0.03, linear regression) (Fig. 1). Fifty-seven percent (13/23) of isolates recovered from patients who received ≤200 mg/day were in vitro susceptible to fluconazole (13) compared to 0% (0/5) of isolates recovered from patients who received >200 mg/day (P = 0.04, Fisher's exact test) (Table 2). In our earlier study, a daily fluconazole dose/MIC ratio of ≤50 was associated with a significantly greater likelihood of therapeutic failure (1). In this study, 20 of the 28 isolates demonstrated such ratios (Table 1).
TABLE 1.
MICs and doses for breakthrough Candida bloodstream isolates
| Candida sp. | Daily fluconazole dose (mg) | Cumulative fluconazole dose (mg) | MIC (μg/ml) | Daily dose/MIC ratio |
|---|---|---|---|---|
| C. albicans | 50 | 250 | 1 | 50 |
| C. parapsilosis | 100 | 1,300 | 1 | 100 |
| C. albicans | 100 | 700 | 1 | 100 |
| C. tropicalis | 100 | 400 | 2 | 50 |
| C. glabrata | 100 | 1,200 | 2 | 50 |
| C. glabrata | 100 | 2,100 | 64 | 1.56 |
| C. krusei | 100 | 2,200 | 64 | 1.56 |
| C. glabrata | 100 | 2,400 | 64 | 1.56 |
| C. parapsilosis | 200 | 5,000 | 0.5 | 400 |
| C. parapsilosis | 200 | 2,200 | 1 | 200 |
| C. albicans | 200 | 1,400 | 1 | 200 |
| C. albicans | 200 | 2,400 | 1 | 200 |
| C. parapsilosis | 200 | 800 | 1 | 200 |
| C. glabrata | 200 | 1,800 | 2 | 100 |
| C. tropicalis | 200 | 13,200 | 4 | 50 |
| C. parapsilosis | 200 | 3,600 | 4 | 50 |
| C. glabrata | 200 | 2,400 | 16 | 12.5 |
| C. lusitaniae | 200 | 3,600 | 32 | 6.25 |
| C. krusei | 200 | 2,000 | 64 | 3.12 |
| C. krusei | 200 | 5,000 | 64 | 3.12 |
| C. albicans | 200 | 3,600 | 64 | 3.12 |
| C. glabrata | 200 | 600 | 64 | 3.12 |
| C. tropicalis | 200 | 2,800 | 128 | 1.56 |
| C. glabrata | 400 | 5,400 | 32 | 12.5 |
| C. glabrata | 400 | 2,400 | 32 | 12.5 |
| C. krusei | 400 | 4,400 | 64 | 6.25 |
| C. albicans | 400 | 7,200 | 128 | 3.12 |
| C. glabrata | 800 | 12,400 | 128 | 6.25 |
FIG. 1.
Relationships between daily fluconazole dose and MIC. Each symbol represents an isolate recovered from a patient treated with the dose of fluconazole indicated on the x axis.
TABLE 2.
Fluconazole susceptibility patterns stratified by daily dose
| % (No.) of isolatesa
|
||||
|---|---|---|---|---|
| Daily dose | S | S-DD | R | P valueb |
| 50 to 200 mg | 57 (13) | 9 (2) | 35 (8) | |
| >200 mg | 0 (0) | 40 (2) | 60 (3) | 0.04 |
The total percentages for the 50-to-200-mg group exceed 100% due to rounding. S, susceptible; S-DD, susceptible and dose dependent; R, resistant.
Three-by-two comparison; chi-square test.
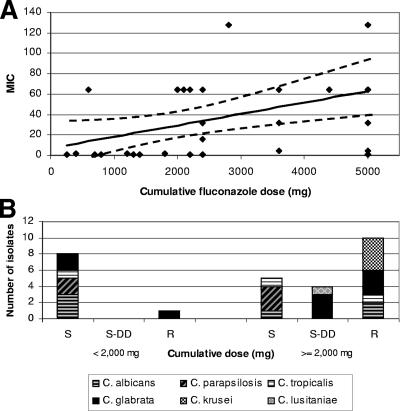
There was also a correlation between MIC and the cumulative fluconazole dose (P = 0.02, linear regression) (Fig. 2A). Eighty-nine percent (8/9) of isolates recovered from patients who received <2,000 mg were in vitro susceptible to fluconazole compared to 26% (5/19) of isolates recovered from patients who received ≥2,000 mg (P = 0.004, Fisher's exact test) (Table 3; Fig. 2B). There was no correlation between the duration of fluconazole therapy prior to the development of candidemia and the MIC of the breakthrough isolate.
FIG. 2.
Relationships between cumulative fluconazole dose, MICs, and susceptibility patterns. (A) The distribution of MICs stratified by cumulative fluconazole dose. (B) The association between cumulative doses and susceptibility patterns of the breakthrough Candida isolates.
TABLE 3.
Fluconazole susceptibility patterns stratified by cumulative dose
| % (No.) of isolatesa
|
||||
|---|---|---|---|---|
| Cumulative dose | S | S-DD | R | P valueb |
| <2,000 mg | 89 (8) | 0 | 11 (1) | |
| ≥2,000 mg | 26 (5) | 21 (4) | 53 (10) | 0.008 |
S, susceptible; S-DD, susceptible and dose dependent; R, resistant.
Three-by-two comparison; chi-square test.
The breakthrough species were C. glabrata (32%, 9/28), C. albicans (21%, 6/28), C. parapsilosis (18%, 5/28), C. krusei (14%, 4/28), C. tropicalis (11%, 3/28), and C. lusitaniae (4%, 1/28) (Table 1). All C. krusei and C. parapsilosis isolates were fluconazole resistant and fluconazole susceptible, respectively; the breakthrough isolates of other Candida spp. were susceptible, susceptible and dose dependent, or resistant.
To our knowledge, this is the first study to demonstrate a relationship between the daily and cumulative doses of fluconazole and the MICs of breakthrough Candida bloodstream isolates. Our demonstration that MICs correlated directly with the fluconazole dose received prior to the development of breakthrough candidemia appears to be clinically relevant, since the percentage of isolates susceptible to fluconazole decreased as the dose increased. Our data set differed in important ways from those of earlier studies, which likely accounts for the success of this study. Most notably, we collected isolates from a relatively large number of patients who had received wide ranges of fluconazole doses; these isolates, in turn, exhibited diverse MICs, including a large percentage that was not susceptible to fluconazole.
The results are important because of their implications for the management of breakthrough candidemia. Indeed, we have incorporated our data into a management strategy at our institution. Prior to species identification and antifungal susceptibility testing, we assume that yeasts isolated from the blood of patients receiving prophylactic or empirical fluconazole are not susceptible to the drug. Patients, therefore, are switched to an amphotericin B formulation or echinocandin. If susceptibility testing is performed, these results can be used to guide subsequent therapy. If susceptibility testing is not available or not performed, then treatment decisions must be made based upon species identification. C. glabrata breakthrough isolates, like C. krusei, should be considered resistant to fluconazole. Since C. parapsilosis breakthrough isolates are generally fluconazole susceptible (12; our unpublished data), the agent is a therapeutic option, provided vascular catheters are removed. For C. albicans and C. tropicalis isolates in our study, the cumulative fluconazole dose better predicted the susceptibility pattern than the daily dose did. Isolates recovered from patients who received <2,000 mg remained susceptible to fluconazole (Fig. 2B). Therefore, once the patient is stable on antifungal therapy and species identification is available, we believe it is reasonable to consider switching to fluconazole to complete the course of therapy. For cumulative doses of ≥2,000 mg, C. albicans and C. tropicalis susceptibility patterns are difficult to predict; in such cases, we complete treatment with amphotericin B or an echinocandin.
The design of this study does not allow us to draw any conclusions about the efficacy of empirical or preventive fluconazole therapy, appropriate doses for such therapy, or the potential role of fluconazole in selecting for non-C. albicans spp. It is also important to recognize that our data cannot be extrapolated to assess the use of the fluconazole dose/MIC ratio (or, alternatively, the area under the exposure curve/MIC ratio) as a pharmacodynamic tool to prevent the emergence of resistance (18). Carefully designed in vitro studies would be the first step toward addressing this important issue.
Acknowledgments
We thank Victor Yu, Arthur Morris, and David Snydman for their contributions to our previous study of candidemia (1). Experiments were conducted in the laboratories of C. J. Clancy and M. H. Nguyen at the North Florida/South Georgia Veterans Health System, Gainesville, FL. This project was conducted as part of the University of Florida Mycology Research Unit.
This project was supported by the Medical Research Service of the U.S. Department of Veterans Affairs.
REFERENCES
- 1.Clancy, C. J., V. L. Yu, A. J. Morris, D. R. Snydman, and M. H. Nguyen. 2005. Fluconazole MIC and the fluconazole dose/MIC ratio correlate with therapeutic response among patients with candidemia. Antimicrob. Agents Chemother. 49:3171-3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girmenia, C., P. Martino, F. De Bernardis, G. Gentile, M. Boccanera, M. Monaco, G. Antonucci, and A. Cassone. 1996. Rising incidence of Candida parapsilosis fungemia in patients with hematologic malignancies: clinical aspects, predisposing factors, and differential pathogenicity of the causative strains. Clin. Infect. Dis. 23:506-514. [DOI] [PubMed] [Google Scholar]
- 3.Goodman, J. L., D. J. Winston, R. A. Greenfield, P. H. Chandrasekar, B. Fox, H. Kaizer, R. K. Shadduck, T. C. Shea, P. Stiff, D. J. Friedman, et al. 1992. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N. Engl. J. Med. 326:845-851. [DOI] [PubMed] [Google Scholar]
- 4.Marr, K. A., K. Seidel, T. C. White, and R. A. Bowden. 2000. Candidemia in allogeneic blood and marrow transplant recipients: evolution of risk factors after the adoption of prophylactic fluconazole. J. Infect. Dis. 181:309-316. [DOI] [PubMed] [Google Scholar]
- 5.Marr, K. A., T. C. White, J. A. van Burik, and R. A. Bowden. 1997. Development of fluconazole resistance in Candida albicans causing disseminated infection in a patient undergoing marrow transplantation. Clin. Infect. Dis. 25:908-910. [DOI] [PubMed] [Google Scholar]
- 6.Mori, T., M. Matsumura, Y. Kanamaru, S. Miyano, T. Hishikawa, S. Irie, K. Oshimi, T. Saikawa, and T. Oguri. 1997. Myelofibrosis complicated by infection due to Candida albicans: emergence of resistance to agents during therapy. Clin. Infect. Dis. 25:1470-1471. [DOI] [PubMed] [Google Scholar]
- 7.Myoken, Y., T. Kyo, M. Fujihara, T. Sugata, and Y. Mikami. 2004. Clinical significance of breakthrough fungemia caused by azole-resistant Candida tropicalis in patients with hematologic malignancies. Haematologica 89:378-380. [PubMed] [Google Scholar]
- 8.Nguyen, M. H., J. E. Peacock, Jr., A. J. Morris, D. C. Tanner, M. L. Nguyen, D. R. Snydman, M. M. Wagener, M. G. Rinaldi, and V. L. Yu. 1996. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100:617-623. [DOI] [PubMed] [Google Scholar]
- 9.Nolte, F. S., T. Parkinson, D. J. Falconer, S. Dix, J. Williams, C. Gilmore, R. Geller, and J. R. Wingard. 1997. Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob. Agents Chemother. 41:196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nucci, M., and A. L. Colombo. 2002. Risk factors for breakthrough candidemia. Eur. J. Clin. Microbiol. Infect. Dis. 21:209-211. [DOI] [PubMed] [Google Scholar]
- 11.Pelz, R. K., C. W. Hendrix, S. M. Swoboda, M. Diener-West, W. G. Merz, J. Hammond, and P. A. Lipsett. 2001. Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann. Surg. 233:542-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller, M. A., S. A. Messer, L. Boyken, R. J. Hollis, C. Rice, S. Tendolkar, and D. J. Diekema. 2004. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS global antifungal surveillance program. Diagn. Microbiol. Infect. Dis. 48:201-205. [DOI] [PubMed] [Google Scholar]
- 13.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, and A. L. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, intraconzale, and Candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin. Infect. Dis. 24:248-249. [DOI] [PubMed] [Google Scholar]
- 14.Safdar, A., F. van Rhee, J. P. Henslee-Downey, S. Singhal, and J. Mehta. 2001. Candida glabrata and Candida krusei fungemia after high-risk allogeneic marrow transplantation: no adverse effect of low-dose fluconazole prophylaxis on incidence and outcome. Bone Marrow Transplant. 28:873-878. [DOI] [PubMed] [Google Scholar]
- 15.Shorr, A. F., K. Chung, W. L. Jackson, P. E. Waterman, and M. H. Kollef. 2005. Fluconazole prophylaxis in critically ill surgical patients: a meta-analysis. Crit. Care Med. 33:1928-1935. [DOI] [PubMed] [Google Scholar]
- 16.Slavin, M. A., B. Osborne, R. Adams, M. J. Levenstein, H. G. Schoch, A. R. Feldman, J. D. Meyers, and R. A. Bowden. 1995. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation—a prospective, randomized, double-blind study. J. Infect. Dis. 171:1545-1552. [DOI] [PubMed] [Google Scholar]
- 17.Swoboda, S. M., W. G. Merz, and P. A. Lipsetta. 2003. Candidemia: the impact of antifungal prophylaxis in a surgical intensive care unit. Surg. Infect. (Larchmt) 4:345-354. [DOI] [PubMed] [Google Scholar]
- 18.Thomas, J. K., A. Forrest, S. M. Bhavnani, J. M. Hyatt, A. Cheng, C. H. Ballow, and J. J. Schentag. 1998. Pharmacodynamic evaluation for factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob. Agents Chemother. 42:521-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wingard, J. R., W. G. Merz, M. G. Rinaldi, C. B. Miller, J. E. Karp, and R. Saral. 1993. Association of Torulopsis glabrata infections with fluconazole prophylaxis in neutropenic bone marrow transplant patients. Antimicrob. Agents Chemother. 37:1847-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wingard, J. R., W. G. Merz, M. G. Rinaldi, C. B. Miller, J. E. Karp, and R. Saral. 1991. Increase in Candida krusei infection among patients with bone marrow transplantation and neutropenia treated prophylactically with fluconazole. N. Engl. J. Med. 325:1274-1277. [DOI] [PubMed] [Google Scholar]