Abstract
Candida albicans remains the leading causative agent of invasive fungal infection. Although the importance of filamentation in C. albicans pathogenesis has been extensively investigated, in vivo studies to date have been unable to dissect the role of this developmental process in the establishment of infection versus the development of active disease as characterized by damage to the host leading to mortality. To address this issue, we genetically engineered a C. albicans tet-NRG1 strain in which filamentation and virulence can be modulated both in vitro and in vivo simply by the presence or absence of doxycycline (DOX): this strain enabled us, in a prior study, to demonstrate that yeast-form cells were able to infect the deep organs but caused no disease unless filamentation (induced by the addition of DOX) was allowed to occur. In the present study, we examined whether inhibiting filamentation (by withdrawing the DOX) at 24 or 48 h postinfection could serve as an effective therapeutic intervention against candidiasis. The results obtained indicate that DOX removal led to an alteration in the morphology of the infecting fungal cells and a dramatic increase in survival, but as with conventional antifungal drug therapy regimens, mortality rates increased markedly the longer this intervention was delayed. These observations reinforce the importance of invasive filamentous growth in causing the damage to the host and the lethality associated with active disease and suggest this process could be fruitfully targeted for the development of new antifungal agents.
Candida albicans, the main causative agent of invasive candidiasis, has become increasingly important in an expanding population of immunocompromised patients, with high morbidity and mortality rates and significant economic sequelae (1, 14-16). Management of these types of infections is complicated by the limited arsenal of antifungal drugs and the emergence of resistance to the existing classes of antifungal agents (10). Thus, there is a critical need for the development of new therapeutic approaches to combat invasive fungal infections.
The role of morphogenetic changes in fungal physiology and pathogenesis has long been an active area of research, particularly in C. albicans. This opportunistic fungus grows as ovoid yeast-form cells under many laboratory conditions but transitions to a filamentous form of growth upon exposure to a mammalian host or host-like conditions (e.g., neutral pH or at 37°C in the presence of serum). The role of morphology in C. albicans pathogenesis has been explored using a number of defined mutants defective in this process and which therefore remain locked in either the nonfilamentous (yeast) or filamentous forms of growth. In general, mutants that are locked in either the yeast or filamentous forms are significantly reduced in virulence (2, 3, 5, 6, 9, 12).
Here, we utilize an engineered C. albicans strain that is able to filament and cause mortality when mice are fed doxycycline (DOX), yet remains nonfilamentous in the absence of DOX because of elevated levels of expression of NRG1, a negative regulator of filamentation (11). The use of this strain in an earlier study enabled us to directly evaluate the role of filamentation and hyphal transitions in the early stages of infection and in the progression to active disease (host damage leading to mortality) within an animal host. Preventing the transition from occurring resulted in a nonlethal infection with no obvious adverse effects on the infected mice (11). In the present study, we again employed this strain to investigate the opposite scenario: that is, whether inhibition of NRG1-regulated filamentation after the establishment of a deep-seated infection would prove effective against the disease and therefore have potential as a novel therapeutic approach for the treatment of hematogenously disseminated candidiasis.
MATERIALS AND METHODS
C. albicans strain.
The C. albicans SSY50-B strain has been previously described (11). In this strain, morphogenetic conversions can be modulated both in vitro and in vivo by the presence or absence of DOX. In the absence of the antibiotic, high levels of NRG1 expression block the yeast-to hypha transition, whereas the presence of DOX inhibits expression of the tet-NRG1 allele and allows filamentation to occur normally in response to the appropriate stimuli.
Animal experiments.
Cultures of strain SSY50-B for injection were grown overnight at 25°C in yeast extract-peptone-dextrose (YPD). Cells were harvested by centrifugation and washed three times in sterile pyrogen-free saline. After counting using a hemocytometer, appropriate dilutions were made and 200 μl of the resulting cell suspension was injected into the lateral tail veins of each of the 6- to 8-week-old female BALB/c mice (10 mice per group). Animals in a control group did not receive DOX in their drinking water; all other groups received 2 mg/ml DOX in their water, starting 3 days prior to infection. Of these latter groups of mice, one received DOX throughout the experiment and in two other groups DOX was discontinued at either 24 or 48 h postinfection. An additional DOX-treated group also received fluconazole by intraperitoneal injection (20 mg/kg of body weight/day) starting 1 day postinfection. Mice were monitored for survival for 28 days postinfection. Days on which mice died were recorded; moribund animals were euthanized and recorded as dying the following day. To allow determination of fungal organ burdens and histological evaluation to be performed over the course of the infection for selected treatments, two parallel groups of mice (DOX/fluconazole treated and DOX discontinued at 24 h) were included for timed sacrifices at 4 and 10 days postinfection.
For all animals, upon death or sacrifice, brain, spleen, and kidneys were removed for the determination of fungal burden and histology (kidney) at time of death. One kidney from each animal was put aside for histological analysis. Briefly, kidneys for histological analysis were fixed in 10% buffered formalin and embedded in paraffin, and thin tissue slices were removed and stained with Grocott-Gomori methenamine-silver. Cell counts were determined from the remaining samples by weighing and homogenizing the tissues and then plating them onto solid YPD medium to determine viable CFU. All animal experiments were performed in accordance with institutional regulations. Mice were allowed a 1-week acclimatization period before infection experiments started.
Statistical analysis.
Survival data and differences between groups were analyzed using the Kaplan-Meier log rank test. Organ fungal burdens were monitored by determining the total CFU per gram of tissue in kidney, brain, and spleen. Thereafter, logarithmic values for the different groups were obtained and results were expressed as medians and their respective interquartile ranges. The Mann-Whitney test was used to determine statistical significance for differences in CFU data. Analyses were performed using Prism by GraphPad Software, Inc. (San Diego, CA).
RESULTS
Doxycyline withdrawal profoundly affected the outcome of the infection.
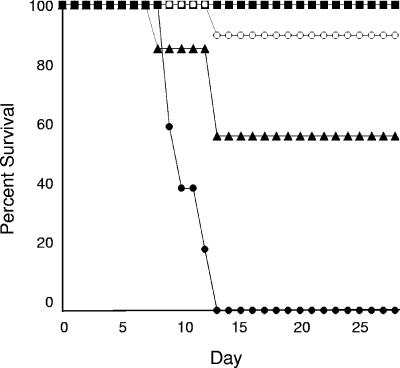
Several groups of animals were evaluated for survival following infection with the tet-NRG1 strain grown in the yeast form. As shown in Fig. 1, one control group of mice was not supplemented with DOX in their drinking water, and as a result, the infecting strain remained nonhyphal and was incapable of producing lethal disease, leading to 100% survival, as expected from our previous report (11). A second control group received DOX throughout the duration of the experiment, which rendered this strain essentially wild type with regard to virulence, leading to 100% mortality, with death occurring between days 9 and 13 (median survival time, 10 days) with this infecting inoculum. Withdrawal of the DOX 24 h after initiating an invasive infection led to a significant increase in survival (90%) compared to the continuously DOX-treated group (P < 0.0001). These survival rates decreased noticeably, although they remained statistically significant versus continuously DOX-treated animals (P = 0.0346), when the removal of the DOX was delayed for an additional 24 h (48 h postinfection), however. In fact, to more directly compare the effects of this treatment alongside a currently used drug therapy, an additional group of mice maintained on DOX throughout the experiment started receiving fluconazole daily, starting 24 h after infection, to simulate treatment with a clinically approved antifungal drug. As expected, all mice in this fluconazole-treated group survived the infection (Fig. 1).
FIG. 1.
Effect of DOX withdrawal on tet-NRG1 strain virulence. Groups of mice were infected with a suspension containing 5.8 × 105 CFU of the tet-NRG1 strain SSY50-B and monitored for survival. One group was never exposed to DOX (closed squares). All of the other groups started receiving DOX 3 days prior to infection. Of these latter groups, two remained on DOX throughout the experiment: one had no further additions (closed circles), whereas the other started receiving daily injections with fluconazole (closed squares). For the two remaining groups, DOX was removed from their drinking water either 24 h (open circles) or 48 h (closed triangles) postinfection to simulate intervention with an antifilamentation agent. Statistically significant differences were observed between groups of mice which remained on DOX throughout the infection and those from which it was removed 24 h (P < 0.0001) and 48 h (P = 0.0346) postinfection.
Doxycyline withdrawal altered the morphology of the fungal cells in the infected tissues.
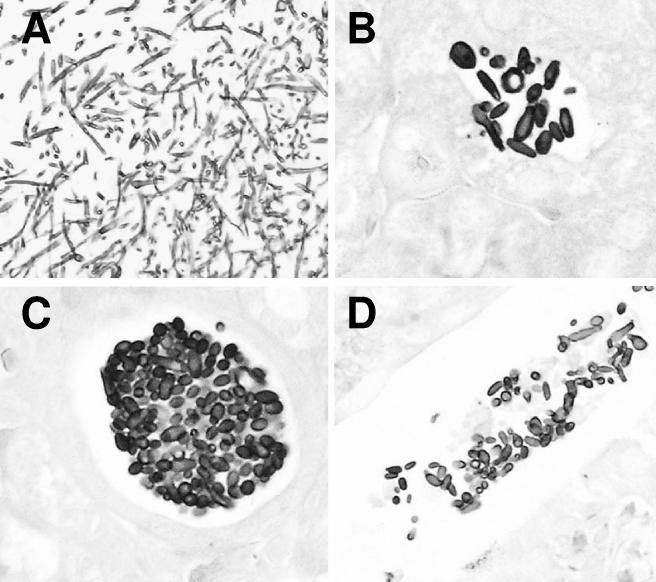
As seen in Fig. 2, DOX withdrawal resulted in the conversion of the fungal cells infecting the tissues into a yeast morphology, which was manifested as early as 4 days postinfection (3 days after therapeutic intervention). The data clearly show that, after establishment of a fungal infection, the subsequent host damage that leads to active disease and eventual mortality can be halted by inhibiting filament formation, leading to survival rates comparable to those achieved with conventional antifungal therapy.
FIG. 2.
Histopathological analysis of kidneys of mice from which DOX was withdrawn postinfection. Organs from the DOX withdrawal animals appear to contain only yeast-like or partially elongated fungal cells as opposed to the long filaments seen in kidneys from continuously DOX-treated animals which succumb to the infection (shown in panel A). This alteration in morphology is apparent as early as 3 days after DOX withdrawal (4 days postinfection [B]) and throughout the duration of infection (10 days [C] and 28 days [D]).
Doxycyline withdrawal did not rapidly kill the infecting fungal cells.
To ascertain whether the DOX withdrawal regimen had similar effects on the infecting fungal cells as the currently used conventional drug therapies, parallel groups of mice for the group with DOX withdrawal 24 h postinfection and the fluconazole-treated group were used to determine the tissue fungal burdens for brain, spleen, and kidney at prescheduled time points (4, 10, and 28 days) after infection (Table 1). Confirming the histological observations, these results clearly show that high fungal bioburdens are maintained throughout the course of infection in those animals from which DOX treatment was removed 24 h postinfection. Yet, these organisms do not (apparently) cause overt disease and, as a result, infection with them does not lead to mortality. In contrast, a clear trend towards decreasing viable fungal burden in all of the organs examined was observed in the fluconazole-treated mice: kidney and spleen cultures were predominantly negative at the end of the trial, although brain burdens remained significant even after 28 days (Table 1). The results from the DOX withdrawal group indicate that, while up-regulation of NRG1 gene expression postinfection inhibits filamentation and significantly decreases the virulence of resident organisms, these nonfilamentous cells are not immediately cleared from the infected tissues.
TABLE 1.
Organ fungal burdens in mice challenged with C. albicans SSY50-B
| Treatment and time of sacrifice (day postinfection)a | Fungal burden (log CFU/g) inb:
|
||
|---|---|---|---|
| Kidney | Brain | Spleen | |
| DOX removal | |||
| 4 | 5.8 ± 1.4** | 4.7 ± 0.8* | 2.4 ± 0.5 |
| 10 | 6.5 ± 1.5** | 4.6 ± 0.1** | 3.1 ± 0.9* |
| 28 | 6.6 ± 3.6** | 3.8 ± 0.9** | 3.0 ± 2.3** |
| Fluconazole | |||
| 4 | 3.3 ± 0.9 | 4.1 ± 0.8 | 2.3 ± 0.3 |
| 10 | 2.6 ± 0.6 | 3.4 ± 0.4 | 1.2 ± 0.6 |
| 28 | 1.2 ± 0.1 | 3.0 ± 0.4 | 1.2 ± 0.1 |
Groups of animals received DOX in their water. At 24 h postinfection, DOX was removed from the drinking water in one group (DOX removal), and the other group (Fluconazole) started daily treatment with fluconazole. Five animals from each group were sacrificed at different times postinfection for determination of organ fungal burdens.
Results are expressed as medians and interquartile ranges. Asterisks denote statistically significant differences between both groups (DOX removal and fluconazole treated) at the same time of scheduled sacrifice. *, P < 0.05; **, P < 0.01.
DISCUSSION
A series of experiments were designed to directly address the importance of the NRG1-regulated filamentation program for active disease progression and the pathogenesis of established C. albicans infections. For this, we took advantage of the manipulability of the tet-NRG1 strain (SSY50-B) we had previously constructed in which filamentation and virulence can be controlled in vivo by DOX (11). With this strain, the presence of DOX in the animal's drinking water blocks expression of the engineered tet-NRG1 allele and allows filamentation and disease to occur normally inside the animal, whereas absence of DOX from the drinking water results in high levels of expression of NRG1 with a concomitant inhibition of the yeast-to-hypha transition in the infecting fungal cells. To evaluate whether targeting the filamentation program by increasing NRG1 gene expression could represent a novel therapeutic avenue for the treatment of established C. albicans infections, two groups of mice were inoculated with the tet-NRG1 strain while on DOX (mimicking a wild-type infection) but subsequently had this removed from their drinking water at different time points (24 or 48 h) postinfection. The results clearly indicate that DOX removal led to a dramatic increase in survival. Importantly, this timeline of treatment initiation and the resulting survival rates closely mirror those seen in animal models using any of the three major classes of antifungal drugs currently available (7). These data also reinforce the observation that, irrespective of the chosen treatment regimen, rapid diagnosis and intervention remain the major determinants in the successful resolution of candidiasis.
In order to determine whether the increased survival observed in the DOX withdrawal animals correlated with an alteration in the morphology of the Candida cells within the organs, a histological examination was performed on the kidneys retrieved from animals from which DOX was withdrawn at 24 h postinfection and which were sacrificed at various time points thereafter. Our data indicate that this intervention led to the conversion of the fungal cells infecting the tissues into an almost exclusively yeast morphology. Together with the survival data, these observations validate that the NRG1-regulated filamentation program would likely represent a useful target for antifungal drug development.
The fact that, unlike the fungicidal effects of the fluconazole treatment, the removal of doxycycline left significant numbers of viable fungi within the organs fits well with our original study. In this previous work, we showed that mice injected with the tet-NRG1 strain in the absence of DOX (which normally survive) can be made to succumb to the infection through the addition of the DOX to their water up to several weeks after the initial challenge (11). While this would seem to rule out any likelihood of developing this into a possible therapeutic avenue, it should be noted that examination of the tissue fungal burdens of several mice at time points beyond the 28-day cutoff indicates that these resident, nonpathogenic yeast cells do not persist and are eventually cleared from the infected organs (S. P. Saville and J. L. Lopez-Ribot, unpublished results). This suggests that any therapeutic compound which mimics NRG1 overexpression by inhibiting filamentation and virulence would not need to be continually administered to repress the disease. Despite these observations, intervention with any such compound would likely find its greatest utility when employed as an adjunctive therapy in combination with a second (conventional) antifungal agent.
Despite more than 50 years of modern pharmaceutical research and development, few antifungal drugs are available. Filamentation, because of its presumed role in virulence, represents an attractive target for the development of new antifungal agents. However, progress on this front has been hampered by an incomplete understanding of the role of this fungal developmental program during the different stages (establishment of infection versus active disease) of C. albicans infections. Our experiments provide compelling evidence of the importance of filamentation in the progression to active disease and validate this pivotal physiological process as a potential target for the development of a novel class of therapeutic agents against established fungal infections. To this end, small-molecule inhibitors that effectively inhibit this transition in vitro have already been identified and underscore the potential to develop compounds specifically targeting this key developmental process (13; A. P. Bryant, C. M. Butler, A. Fretzen, A. L. Lazzell, A. W. Monreal, C. Monteagudo, E. O. Solberg, J. L. Lopez-Ribot, S. P. Saville, and G. T. Milne, Abstr. 44th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-222, p. 399, 2004). From the results presented here, it would seem that such a filamentation inhibitor would likely need to be employed as an adjunctive therapy to conventional antifungal regimens and not as monotherapy. In support of similar observations with conventional antifungal agents in animal models (7), and also with patients with disseminated candidiasis (4, 8), our data reinforce the need for prompt diagnosis and therapeutic intervention in at-risk patients.
Acknowledgments
The research carried out in this study was financially supported by the NIH (R03 A1054447 to J.L.L.-R.) and a sponsored research grant (Microbia, Inc., to S.P.S.) and Microbia, Inc., Cambridge, Mass.
Part of the laboratory work was performed at the University of Texas Health Science Center at San Antonio (UTHSCSA) while S.P.S., A.L.L., and J.L.L.-R. were employed at that institution.
REFERENCES
- 1.Beck-Sague, C., W. R. Jarvis, et al. 1993. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. J. Infect. Dis. 167:1247-1251. [DOI] [PubMed] [Google Scholar]
- 2.Braun, B. R., and A. D. Johnson. 2000. TUP1, CPH1 and EFG1 make independent contributions to filamentation in Candida albicans. Genetics 155:57-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun, B. R., D. Kadosh, and A. D. Johnson. 2001. NRG1, a repressor of filamentous growth in C. albicans, is down-regulated during filament induction. EMBO J. 20:4753-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garey, K. W., M. Rege, M. P. Pai, D. E. Mingo, K. J. Suda, R. S. Turpin, and D. T. Bearden. 2006. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin. Infect. Dis. 43:25-31. [DOI] [PubMed] [Google Scholar]
- 5.Liu, H., J. Kohler, and G. R. Fink. 1994. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266:1723-1726. [DOI] [PubMed] [Google Scholar]
- 6.Lo, H. J., J. R. Kohler, B. DiDomenico, D. Loebenberg, A. Cacciapuoti, and G. R. Fink. 1997. Nonfilamentous C. albicans mutants are avirulent. Cell 90:939-949. [DOI] [PubMed] [Google Scholar]
- 7.MacCallum, D. M., and F. C. Odds. 2004. Need for early antifungal treatment confirmed in experimental disseminated Candida albicans infection. Antimicrob. Agents Chemother. 48:4911-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morrell, M., V. J. Fraser, and M. H. Kollef. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 49:3640-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murad, A. M., P. Leng, M. Straffon, J. Wishart, S. Macaskill, D. MacCallum, N. Schnell, D. Talibi, D. Marechal, F. Tekaia, C. d'Enfert, C. Gaillardin, F. C. Odds, and A. J. Brown. 2001. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J. 20:4742-4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanglard, D., and F. C. Odds. 2002. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2:73-85. [DOI] [PubMed] [Google Scholar]
- 11.Saville, S. P., A. L. Lazzell, C. Monteagudo, and J. L. Lopez-Ribot. 2003. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot. Cell 2:1053-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoldt, V. R., A. Sonneborn, C. E. Leuker, and J. F. Ernst. 1997. Efg1p, an essential regulator of morphogenesis of the human pathogen Candida albicans, is a member of a conserved class of bHLH proteins regulating morphogenetic processes in fungi. EMBO J. 16:1982-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toenjes, K. A., S. M. Munsee, A. S. Ibrahim, R. Jeffrey, J. E. Edwards, Jr., and D. I. Johnson. 2005. Small-molecule inhibitors of the budded-to-hyphal-form transition in the pathogenic yeast Candida albicans. Antimicrob. Agents Chemother. 49:963-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viudes, A., J. Peman, E. Canton, P. Ubeda, J. L. Lopez-Ribot, and M. Gobernado. 2002. Candidemia at a tertiary-care hospital: epidemiology, treatment, clinical outcome and risk factors for death. Eur. J. Clin. Microbiol. Infect. Dis. 21:767-774. [DOI] [PubMed] [Google Scholar]
- 15.Wey, S. B., M. Mori, M. A. Pfaller, R. F. Woolson, and R. P. Wenzel. 1988. Hospital-acquired candidemia. The attributable mortality and excess length of stay. Arch. Intern. Med. 148:2642-2645. [DOI] [PubMed] [Google Scholar]
- 16.Wilson, L. S., C. M. Reyes, M. Stolpman, J. Speckman, K. Allen, and J. Beney. 2002. The direct cost and incidence of systemic fungal infections. Value Health 5:26-34. [DOI] [PubMed] [Google Scholar]