Abstract
The consequences of inactive empiric antimicrobial therapy are not well-described and may cause prolonged hospitalization or infection-related mortality. In vitro susceptibility results for 884 patients hospitalized at an academic medical center with gram-negative bloodstream infections (GNBI) from 2001 to 2003 were matched to antimicrobial orders within 24 h of culture. Clinical characteristics, organism, inpatient mortality, and length of stay after culture for patients with GNBI were compared between patients receiving active versus inactive empiric antimicrobial therapy. A total of 14.1% of patients with GNBI received inactive empiric therapy, defined as no antimicrobial therapy within 24 h of the culture active against the identified organism based on in vitro microbiology reports. Patients who received inactive therapy were more likely to be younger, to be infected with Pseudomonas aeruginosa, to have a nosocomial infection, and to receive antimicrobial monotherapy but less likely to be bacteremic with Escherichia coli or to have sepsis (P < 0.05). There were no significant differences in mortality between patients receiving active versus inactive empiric therapy (16.1% versus 13.6%, respectively) or in length of stay after positive culture (11.5 days versus 12.6 days, respectively). Only 45 patients had greater than 2 days of exposure to inactive therapy; however, 8/30 patients (26.7%) who never received active antimicrobial therapy died while in the hospital. Inactive empiric therapy was more common in healthier patients. Inactive antimicrobial therapy in the first 24 h did not significantly impact average outcomes for GNBI among hospitalized patients but may have caused harm to specific individuals.
The effect of time to appropriate antimicrobial administration and the impact of active empiric antimicrobial therapy upon clinical outcome are well-studied for various infections and pathogens. Delay in treatment for bloodstream infections due to Candida spp. (25), extended-spectrum beta-lactamase-producing organisms (14), and Pseudomonas aeruginosa (13, 15, 24) and pneumonia due to P. aeruginosa (1, 7, 10, 12, 17, 21, 23, 27) has been associated with worse outcomes. However, the relationship between appropriateness of initial therapy and mortality has not been demonstrated consistently in the literature (3, 5, 18). Furthermore, in several studies that showed comparable outcomes regardless of empiric therapy, outcomes depended upon definitive therapy once the organism was identified (4, 30).
Inactive therapy may result from lack of prescriber knowledge of institution-specific resistance patterns or suboptimal dosing based on pharmacokinetic and pharmacodynamic properties of each drug class, but the greatest driver of inactive therapy is probably antimicrobial resistance. A pilot study at our institution identified high utilization of fluoroquinolones, and a review of antimicrobial susceptibilities demonstrated that resistance was higher than would be expected based on inherent resistance (28). In 1999, fluoroquinolone resistance in Escherichia coli and Klebsiella pneumoniae was uncommon; however, by 2003, resistance among these organisms increased to 18% and 10%, respectively. These findings led us to examine antimicrobial resistance in more detail and to evaluate if the emergence of increasing resistance was impacting clinical outcomes.
The purpose of this investigation was to examine the rates of inactive empiric antimicrobial therapy and the clinical outcomes of patients with gram-negative bloodstream infections (GNBI) who received initial treatment to which the organism was resistant. The primary objective of the study was to determine whether inactive empiric antimicrobial selection increased the likelihood of mortality among hospitalized patients or the length of hospitalization following the positive blood culture.
MATERIALS AND METHODS
This retrospective, observational study examined data collected from inpatient admissions from 1 January 2001 to 30 November 2003 at Northwestern Memorial Hospital (NMH) in Chicago, Illinois. NMH is a 725-bed academic medical center with a full range of clinical services. This project, undertaken as part of ongoing infection control and quality improvement efforts, was approved by the Northwestern University Institutional Review Board.
Microbiology laboratory data.
Computerized microbiology laboratory results identified all blood cultures isolating a gram-negative organism between 1 January 2001 and 30 November 2003. All cultures were assumed to represent actual infection, since the presence of gram-negative organisms in blood cultures rarely represents contamination, and patients were defined as having a GNBI. In order to minimize the likelihood that another organism was responsible for the patients' outcomes, we excluded all patients with two or more different organisms recovered in blood culture during the same hospitalization. Additionally, patients were excluded if susceptibility testing was not performed.
Pharmacy antimicrobial database.
Computerized records for all dispensed antimicrobials on the hospital formulary with activity against gram-negative bacilli (amikacin, ampicillin, ampicillin-sulbactam, aztreonam, cefazolin, cefepime, cefotaxime, ceftazidime, ceftizoxime, ceftriaxone, cefuroxime, ciprofloxacin, gatifloxacin, gentamicin, imipenem-cilastatin, levofloxacin, meropenem, piperacillin, piperacillin-tazobactam, streptomycin, ticarcillin-clavulanate, and tobramycin) were matched to GNBI microbiology results for study patients. There were no antimicrobial use policy changes that occurred during this study period which would have impacted the antimicrobial selected. Empiric antimicrobial therapy was defined as the first antibacterial regimen received on the date of blood culture or within 24 h thereafter. Empiric-therapy activity was determined by organism susceptibility results based on the 1999 National Committee for Clinical Laboratory Standards guidelines (26) which defined antimicrobial susceptibility during the study time period. Therapy was considered active if study patients received an antimicrobial drug that met the following criteria: it was dosed according to standard dosing guidelines, it was administered within 24 h of a culture, and it was efficacious by in vitro susceptibility testing.
For all patients identified as receiving inactive empiric antimicrobial therapy, a manual review of the inpatient pharmacy records was performed by investigators to ensure that there were no data errors or deaths prior to drug administration that would have inaccurately classified these patients as receiving inactive therapy. During this review, the number of days from the first date of positive GNBI culture to the receipt of active antimicrobial therapy was recorded for each patient.
Specific dosages of antimicrobial agents were not recorded for this evaluation. A full pharmacokinetic consultation is prospectively performed by a trained clinical pharmacist for all patients in our institution initiated on antimicrobial therapy requiring drug level monitoring, including aminoglycosides. The patient is monitored throughout the full antimicrobial course to ensure appropriate dosing. All other antimicrobials initiated are prospectively reviewed for appropriateness of dosing based on patient-specific, disease-specific, pharmacokinetic, and pharmacodynamic parameters.
Clinical and demographic characteristics and patient outcomes.
Microbiology and pharmacy data for all study patients were matched to hospital administrative case mix data. Administrative data provided patient age, sex, discharge status, postculture length of stay as determined from date of discharge, and ICD-9-coded diagnoses and procedures. Since the analysis was designed to measure whether inactive therapy increased length of stay, patients who died in the hospital were excluded from length-of-stay comparisons. Patient age was expressed as a continuous rather than a categorical variable. Diagnosis-related group assignments were used to identify surgical admissions. Nosocomial infection was defined as an organism cultured more than 2 days after admission.
As no single measure of severity of illness has been shown to be superior for analyzing infectious outcomes, Deyo modification of the Charlson comorbidity score (6) was used to measure patient comorbidity. The burden of chronic conditions was divided into three levels based on Charlson scores of 0, 1 or 2, and ≥3. A sepsis diagnosis code was also noted as an additional measure of infection severity. Intensive care unit (ICU) admission at the time of GNBI was also included as a covariate and used as an additional proxy for illness severity. Markers for immunosuppression in each group, including any diagnosis code indicating human immunodeficiency virus infection, cancer, or solid-organ or bone marrow transplant, were compared between groups.
Statistical analysis.
Differences between patients who received inactive versus active empiric antimicrobials were evaluated by t test for continuous variables and chi-square test for categorical variables. Odds ratios (OR) derived from multiple logistic regression were estimated to test the significance of inactive empiric antimicrobials in predicting the likelihood of inpatient death, controlling for all clinically relevant factors. Multiple linear regression was used to determine the association of inactive therapy with postculture length of stay, controlling for the same covariates. Inpatient deaths were excluded from this analysis (although results were virtually identical for all patients) to avoid confounding. Because using log-transformed length of stay yielded the same results, only untransformed data are presented to assist in interpretation of regression coefficients for postculture length of hospital stay. All analyses were performed using SPSS for Windows (version 13.0; Chicago, IL).
The study was predicated on 90% power to compare a 10% death rate for approximately 800 patients with active antimicrobial therapy to a 20% death rate for approximately 150 patients with inactive therapy, with an alpha level of 0.05 in a two-sided chi-square test of proportions. The study similarly had 87% power to detect a mean postculture-length-of-stay difference of 10 versus 15 days for 700 surviving active-therapy versus 100 surviving inactive-therapy patients, respectively, with an overall standard deviation of 15 days, in a two-sided t test with an alpha level of 0.05.
RESULTS
Incidence of inactive therapy and associated patient characteristics.
Of the 949 patients with single-organism GNBI cultures, 33 (3.6%) could not be matched with inpatient pharmacy orders, and 32 (3.5%) neonates were excluded, leaving a final study sample of 884 patients. Principal diagnoses for these 884 patients with GNBI were wide-ranging and were evenly distributed between groups. The organisms identified in the study population were diverse. E. coli represented 40.0% of the infections, K. pneumoniae accounted for 21.7% of infections, and P. aeruginosa represented 14.9%. Other organisms constituted the remaining 23.4% of GNBI cultures, most commonly Enterobacter cloacae, Proteus mirabilis, Serratia marcescens, and Acinetobacter baumannii.
A total of 125 (14.1%) study patients with single-organism GNBI received inactive initial treatment. Charlson scores and medical conditions predisposing the patients to immunosuppression were similar between groups (Table 1). There were 237 patients (26.8%) with nosocomial GNBI, which were more common in the inactive-treatment group (P = 0.001). As a whole, there were 217 patients (24.5%) with sepsis and 230 (26.0%) who were in the ICU at the time of empiric antimicrobial therapy; both of these characteristics were more common in the active-therapy group (P = 0.004 and P = 0.06, respectively) (Table 1).
TABLE 1.
Characteristics of patients with GNBI receiving active versus inactive empiric therapya
| Patient characteristic | No. (%) of patients with characteristic
|
P valueb | |
|---|---|---|---|
| Active empiric therapy (n = 759) | Inactive empiric therapy (n = 125) | ||
| Male sex | 397 (52.3) | 58 (46.4) | 0.25 |
| Diagnosis code | |||
| Transplant | 38 (5.0) | 9 (7.2) | 0.31 |
| Cancer | 125 (16.5) | 17 (13.6) | 0.42 |
| HIVc | 11 (1.4) | 1 (0.8) | 0.56 |
| Organism | |||
| P. aeruginosa | 94 (12.4) | 38 (30.4) | <0.001 |
| E. coli | 321 (42.3) | 33 (26.4) | 0.001 |
| K. pneumoniae | 172 (22.7) | 20 (16.0) | 0.09 |
| Other organism | 172 (22.7) | 34 (27.2) | 0.27 |
| Nosocomial infectiond | 188 (24.8) | 49 (39.2) | 0.001 |
| Charlson score | |||
| 0 | 320 (42.2) | 57 (45.6) | 0.47 |
| 1 or 2 | 312 (41.4) | 51 (40.8) | 0.95 |
| ≥3 | 127 (16.7) | 17 (13.6) | 0.38 |
| Surgical admission | 483 (63.6) | 81 (64.8) | 0.80 |
| Sepsis | 199 (26.2) | 18 (14.4) | 0.004 |
| ICU admissione | 206 (27.1) | 24 (19.2) | 0.06 |
Total of 884 admissions from 2001 to 2003. The mean age of patients with active empiric therapy was 57.3 years (standard deviation, 18.3 years), and the mean age of patients with inactive empiric therapy was 53.2 years (standard deviation, 18.1 years) (P value of 0.02, determined by a nonpaired t test).
Determined by a χ2 test.
HIV, human immunodeficiency virus.
Defined as a culture date greater than 2 days after the hospital admission date.
Defined as ICU admission at the time of the index culture.
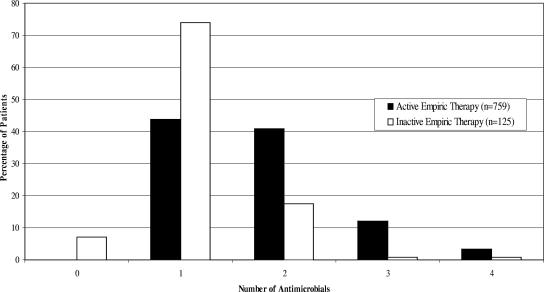
Fluoroquinolones were the most frequently used class of initial antimicrobial treatment (34.1%), followed by aminoglycosides (24.3%) and antipseudomonal penicillins (18.7%). Patients who received active empiric therapy received more antimicrobial agents within 24 h of blood culture (Fig. 1). Among patients who received inactive empiric treatment, 73.9% were administered a single antimicrobial agent, 19.0% received multiple antimicrobials, and 7.1% received no antimicrobials within 24 h of culture.
FIG. 1.
Numbers of empiric antimicrobials administered to patients receiving active or inactive empiric therapy (n = 884).
Patient sex, Charlson score, and admission service were not associated with the likelihood of inactive empiric antimicrobial therapy (Table 1). However, patients infected with P. aeruginosa or with a nosocomial pathogen were more likely to have received inactive therapy (P < 0.001 and P = 0.001, respectively). Conversely, infection with E. coli (P = 0.001), older age (P = 0.02), and a diagnosis of sepsis (P = 0.004) were associated with a higher likelihood of receiving active treatment.
Inpatient mortality.
Regardless of initial empiric therapy, the crude mortality rates were similar for both inactive- and active-therapy groups (13.6% and 16.1%, respectively; P = 0.48). No significant mortality difference was found between patients receiving inactive versus active therapy after controlling for other clinically significant mortality risk factors (OR = 0.61, P = 0.14) (Table 2) . Significant independent risk factors for inpatient mortality included P. aeruginosa infection (OR = 2.48, P = 0.004), nosocomial infection (OR = 4.84, P < 0.001), sepsis (OR = 3.27, P < 0.001), and ICU care at the time of empiric therapy (OR = 4.23, P < 0.001). A Charlson score of 1 or 2 (OR = 2.39, P = 0.001) and a score of 3 or greater (OR = 4.36, P < 0.001) were also associated with increased mortality. Interestingly, men who received inactive therapy were less likely to die than women (OR = 0.63, P = 0.03), as were patients admitted to the surgical service (OR = 0.27, P < 0.001) compared to those admitted to other hospital services.
TABLE 2.
Multiple logistic regression results for the effects of active versus inactive empiric therapy on inpatient mortality for patients with GNBI (n = 884)
| Patient characteristic | OR (95% CIa) | P value |
|---|---|---|
| Male sex | 0.63 (0.42, 0.96) | 0.03 |
| Age (yr) | 1.0 (0.99, 1.02) | 0.30 |
| Organismb | ||
| P. aeruginosa | 2.48 (1.34, 4.60) | 0.004 |
| E. coli | 0.96 (0.54, 1.70) | 0.88 |
| K. pneumoniae | 0.90 (0.48, 1.67) | 0.73 |
| Nosocomial infection | 4.84 (2.90, 8.09) | <0.001 |
| Charlson scorec | ||
| 1 or 2 | 2.39 (1.46, 3.92) | 0.001 |
| ≥3 | 4.36 (2.42, 7.86) | <0.001 |
| Surgical admission | 0.27 (0.16, 0.47) | <0.001 |
| Sepsis | 3.27 (1.70, 6.27) | <0.001 |
| ICU admissiond | 4.23 (2.72, 6.59) | <0.001 |
| Inactive empiric antimicrobial therapy | 0.61 (0.31, 1.18) | 0.14 |
95% CI, 95% confidence interval.
Compared to other organisms.
Compared to Charlson score of 0.
Defined as ICU admission at the time of the index culture.
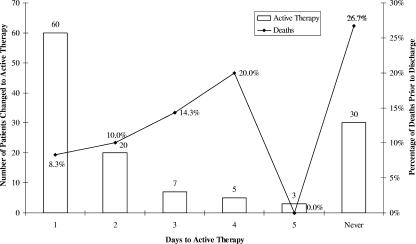
Upon manual review of pharmacy records, 80 patients (64.0%) receiving inactive empiric treatment received an appropriate agent within 2 days and 95 (76.0%) patients received active therapy within 5 days after the index culture. However, 30 of the 125 (24.0%) patients who received inactive therapy, including eight decedents, never received active therapy against the GNBI. The highest death rate (26.7%) was among the 30 patients who never received active antimicrobials (Fig. 2).
FIG. 2.
Times to active therapy and percentages of inpatient deaths for patients with GNBI receiving inactive empiric therapy (n = 127).
Postculture length of stay.
Comparisons of postculture lengths of stay were limited to 745 surviving patients, including 637 patients who received active therapy and 108 patients who received inactive empiric therapy. Patients with inactive antimicrobials had a modestly higher length of stay following the culture (12.6 versus 11.5 days, P = 0.18). Multiple linear regression results confirm that inactive therapy was not a significant predictor of postculture length of stay after controlling for the same covariates (1.8 days, P = 0.11) (Table 3). Nosocomial infection (6.72 days, P < 0.001), ICU care at the time of initial treatment (9.66 days, P < 0.001), a Charlson score of ≥3 (2.76 days, P = 0.02), surgical admission (2.26 days, P = 0.02), and male sex (1.98 days, P = 0.01) were all independent predictors of increased length of stay following positive blood cultures. Results including decedents were virtually identical (not shown).
TABLE 3.
Impact of patient characteristics on length of stay for 745 patients discharged alive after gram-negative bloodstream isolate culture (R2 = 0.27)
| Patient characteristic | β (days [standard error]) | P valuea |
|---|---|---|
| Male sex | 1.98 (0.79) | 0.01 |
| Age (yr) | 0.02 (0.02) | 0.30 |
| Organismb | ||
| P. aeruginosa | 2.22 (1.35) | 0.10 |
| E. coli | −0.85 (1.02) | 0.40 |
| K. pneumoniae | 0.02 (1.15) | 0.99 |
| Nosocomial infection | 6.72 (1.07) | <0.001 |
| Charlson scorec | ||
| 1 or 2 | 1.65 (0.84) | 0.51 |
| ≥3 | 2.76 (1.18) | 0.02 |
| Surgical admission | 2.26 (1.18) | 0.018 |
| Sepsis | −1.97 (1.08) | 0.068 |
| ICU admissiond | 9.66 (1.00) | <0.001 |
| Inactive empiric antimicrobial therapy | 1.80 (1.12) | 0.11 |
Determined by multiple linear regression.
Compared to other organisms.
Compared to a Charlson score of 0.
Defined as ICU admission at the time of the index culture.
DISCUSSION
These findings indicate that whereas initial inactive antimicrobial therapy was relatively frequent, there was little apparent association between therapy and key outcomes for patients with GNBI. While these findings appear contrary to studies evaluating the effect of inactive therapy for patients with pneumonia (1, 7, 10, 12, 17, 21, 23, 27), differences in study definitions, patient populations, and infecting organisms may preclude direct comparison. Previous studies of inactive therapy have used multiple classifications for appropriateness of antimicrobials, time to appropriate treatment, and classification of antimicrobial activity. Our findings contrast with prior investigations on the impact of GNBI but are consistent with similar findings for patients with nonfatal underlying diseases (3, 14). This suggests that patients with intact immune systems may not require immediate antimicrobial therapy and that guided therapy once culture results are obtained may be sufficient. Other published subgroup analyses have shown that the early receipt of active empiric therapy is less important in nonneutropenic patients (13).
Higher severity of illness among patients receiving active therapy.
This study evaluated all hospitalized patients with GNBI, regardless of organism and clinical status. There is the possibility that study results were biased by comparing outcomes for patients receiving inactive therapy with outcomes for apparently much sicker patients who received active therapy. Patients who received active antimicrobials were more acutely ill, as indicated by the fact that clinicians responded with a much higher rate of multidrug treatment in the group receiving active empiric antimicrobials. The patients who received dual active therapy had a significantly longer length of stay and a higher inpatient mortality rate than patients who received either single active therapy or even inactive therapy. However, it is unlikely that overall outcome differences related to initial antimicrobial therapy of any type would have been significant, even if the active-therapy comparison group had been limited to a more comparable subsample. The analysis described above indicates that empiric therapy was at best a marginal predictor of mortality and length of stay compared to measures of illness severity such as sepsis, ICU care, and Charlson score.
Since previous publications have established the importance of effective antimicrobial treatment for patients in a critical care setting (11, 16, 17), we also evaluated the subgroup of patients whose cultures were drawn in the ICU. The 24 patients who received inactive therapy in the ICU had an overall mortality rate of 41.7%, while the 206 patients receiving active therapy had a 29.6% mortality rate. While not statistically significant, this trend indicates the potential importance of active therapy in this critically ill patient population.
Gram-negative-organism virulence.
This study examined GNBI in all patients admitted to the hospital, including some who may not have been critically ill at the time of infection. Individuals infected with certain organisms may benefit more from timely therapy than others, as differences with regard to pathogenicity of the infecting organism exist. P. aeruginosa bacteremia has been shown to be associated with increased mortality; in contrast, E. coli infection is fatal less frequently than other gram-negative bloodstream infections (3, 15). K. pneumoniae and Enterobacter species infections are more likely to be treated appropriately initially (15), and K. pneumoniae infection is associated with less-frequent death (19). GNBI resulting from lung, peritoneal, and unidentified sources are associated with increased morbidity and mortality (2, 15). Additionally, appropriate antimicrobial therapy has a greater effect on outcomes for high-risk sources of bacteremia (lung, abdominal, and unidentified sources) than on outcomes for lower-risk bacteremias (urinary tract, intravenous catheter, and pancreatobiliary tract) (15, 20).
The source of bacteremia could not be evaluated fully in this retrospective investigation. However, patients with ICD-9 codes associated with any urinary tract infection and codes associated with a pulmonary source of infection were compared between the study groups. The frequencies of these diagnoses were not significantly different between the two groups (frequencies of urinary tract infection were 19.2% versus 23.3% and frequencies of pulmonary infection were 12.8% versus 11.9% for inactive- versus active-treatment groups, respectively); therefore, the original infection source did not likely bias the outcome of this study.
Time to active therapy.
Published studies have classified appropriate empiric treatment as therapy initiated within 24 h of (8-9, 15, 29), 48 h of (19), 72 h of (22), or prior to (11, 16, 31) culture result, while others have not factored a time component into appropriateness (18, 20). Our findings corroborate the previous work of Bryan et al. in which choice of antimicrobial on the initial day of infection was not related to outcome but subsequent selection of antimicrobial therapy was a more important predictor (3). Moreover, in that study patients who received no therapy within 24 h of culture but later received correct active therapy had lower overall mortality than patients who received correct initial therapy on the first day. Other investigators have also shown that mortality increases proportionally to the amount of time until active therapy is administered (13, 19).
Of the 125 patients who received inactive antimicrobial therapy, only 45 (36%) were not changed to active therapy within 2 days. The fact that so few patients in this study had prolonged exposure to inactive therapy may have blunted the potential effect on patient outcomes. However, despite small numbers, we did observe that the death rate among patients receiving inactive therapy increased with prolonged exposure. As Fig. 2 suggests, certain patients may indeed have experienced harm from inactive therapy, despite the lack of mean differences between groups.
Limitations.
Since this was a retrospective review of electronic data, no review of actual medical records pertaining to the patient's clinical status was performed. We were not able to determine the source of the GNBI, the severity of infection at the time of culture, or whether the cause of death was related specifically to the GNBI. The observational nature of the study design inevitably creates bias in that all of the potential confounding factors associated with patient outcomes could not be evaluated; therefore, it is difficult to completely verify that both active- and inactive-therapy groups were similar. Our observation can document only the overall harm, all-cause mortality, and total length of stay associated with patients receiving inactive therapy; it cannot address infection-related deaths and outcomes. The comparison presented here indicates that harm was marginal for the GNBI hospital population as a whole, but it was not powered to address specific subgroups that may still benefit from timely and appropriate antimicrobial selection.
Antimicrobial agents were considered active or inactive based on available national guidelines (26); yet, these guidelines are constantly evolving and the clinical efficacy of the antimicrobial may not be determined so easily. This may confound the definition of active therapy and its impact on patient outcomes. Individual drug selection or combination of drug therapy was not evaluated as an independent risk factor for patients receiving active treatment. Finally, pharmacy records of dispensed medications were used for this analysis. While returned doses were also analyzed, it is not possible to ensure that the dispensed antimicrobials were administered to the patient within the specified time frame, as delays and missed doses were not recorded in this database.
Finally, we cannot be certain that the groups were truly identical considering severity of illness and all comorbid conditions. While a well-established measure of comorbidity failed to note a difference between the groups, it must be acknowledged that the Charlson score was not established to predict acute morbidity and mortality (6). Therefore, other surrogate measures, such as diagnosis of sepsis, ICU admission, and diagnoses of comorbid conditions that would predispose patients for immunosuppression, were used in this study to account for the current acuity of illness for these patients.
Conclusion.
This evaluation of all patients with GNBI showed that inactive empiric therapy had no significant overall impact on postculture length of stay or inpatient mortality. However, some patient populations, such as those in the ICU, may indeed be affected adversely by inactive empiric therapy. Prompt change to active antimicrobials based on susceptibility results was common in this study population and is likely key to preventing excess morbidity and mortality. Institutions should be encouraged to develop interventions and educational programs to increase prescriber knowledge about the importance of appropriate empiric antimicrobial choices for populations of patients most vulnerable to the effects of inactive therapy.
Acknowledgments
This work was supported by the Ralph and Marion Falk Medical Research Trust.
The sponsor had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, and approval of the manuscript. No authors have conflicts of interest to report.
We acknowledge Carol Sullivan for providing the administrative hospital case mix data, Lana Gerzenshtein for assisting with the microbiology database, and Matt Scarsi for assisting with data processing.
REFERENCES
- 1.Alvarez-Lerma, F., et al. 1996. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unit. Intensive Care Med. 22:387-394. [DOI] [PubMed] [Google Scholar]
- 2.Blot, S., K. Vandewoude, D. De Bacquer, and F. Colardyn. 2002. Nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in critically ill patients: clinical outcome and length of hospitalization. Clin. Infect. Dis. 34:1600-1606. [DOI] [PubMed] [Google Scholar]
- 3.Bryan, C. S., K. L. Reynolds, and E. R. Brenner. 1983. Analysis of 1,186 episodes of gram-negative bacteremia in non-university hospitals: the effects of antimicrobial therapy. Rev. Infect. Dis. 5:629-638. [DOI] [PubMed] [Google Scholar]
- 4.Chamot, E., E. Boffi El Amari, P. Rohner, and C. Van Delden. 2003. Effectiveness of combination antimicrobial therapy for Pseudomonas aeruginosa bacteremia. Antimicrob. Agents Chemother. 47:2756-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatzinikolaou, I., D. Abi-Said, G. P. Bodey, K. V. Rolston, J. J. Tarrand, and G. Samonis. 2000. Recent experience with Pseudomonas aeruginosa bacteremia in patients with cancer: retrospective analysis of 245 episodes. Arch. Intern. Med. 160:501-509. [DOI] [PubMed] [Google Scholar]
- 6.Deyo, R. A., D. C. Cherkin, and M. A. Ciol. 1992. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J. Clin. Epidemiol. 45:613-619. [DOI] [PubMed] [Google Scholar]
- 7.Dupont, H., H. Mentec, J. P. Sollet, and G. Bleichner. 2001. Impact of appropriateness of initial antibiotic therapy on the outcome of ventilator-associated pneumonia. Intensive Care Med. 27:355-362. [DOI] [PubMed] [Google Scholar]
- 8.Garnacho-Montero, J., J. L. Garcia-Garmendia, A. Barrero-Almodovar, F. J. Jimenez-Jimenez, C. Perez-Paredes, and C. Ortiz-Leyba. 2003. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit. Care Med. 31:2742-2751. [DOI] [PubMed] [Google Scholar]
- 9.Harbarth, S., J. Garbino, J. Pugin, J. A. Romand, D. Lew, and D. Pittet. 2003. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am. J. Med. 115:529-535. [DOI] [PubMed] [Google Scholar]
- 10.Houck, P. M., D. W. Bratzler, W. Nsa, A. Ma, and J. G. Bartlett. 2004. Timing of antibiotic administration and outcomes for Medicare patients hospitalized with community-acquired pneumonia. Arch. Intern. Med. 164:637-644. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim, E. H., G. Sherman, S. Ward, V. J. Fraser, and M. H. Kollef. 2000. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 118:146-155. [DOI] [PubMed] [Google Scholar]
- 12.Kahn, K. L., W. H. Rogers, L. V. Rubenstein, M. J. Sherwood, E. J. Reinisch, E. B. Keeler, D. Draper, J. Kosecoff, and R. H. Brook. 1990. Measuring quality of care with explicit process criteria before and after implementation of the DRG-based prospective payment system. JAMA 264:1969-1973. [PubMed] [Google Scholar]
- 13.Kang, C. I., S. H. Kim, H. B. Kim, S. W. Park, Y. J. Choe, M. D. Oh, E. C. Kim, and K. W. Choe. 2003. Pseudomonas aeruginosa bacteremia: risk factors for mortality and influence of delayed receipt of effective antimicrobial therapy on clinical outcome. Clin. Infect. Dis. 37:745-751. [DOI] [PubMed] [Google Scholar]
- 14.Kang, C.-I., S.-H. Kim, W. B. Park, K.-D. Lee, H.-B. Kim, E.-C. Kim, M.-D. Oh, and K.-W. Choe. 2005. Bloodstream infections caused by antibiotic-resistant gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob. Agents Chemother. 49:760-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang, C.-I., S.-H. Kim, W. B. Park, K.-D. Lee, H.-B. Kim, E.-C. Kim, M.-D. Oh, and K.-W. Choe. 2004. Bloodstream infections due to extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for mortality and treatment outcome, with special emphasis on antimicrobial therapy. Antimicrob. Agents Chemother. 48:4574-4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kollef, M. H., G. Sherman, S. Ward, and V. J. Fraser. 1999. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462-474. [DOI] [PubMed] [Google Scholar]
- 17.Kollef, M. H., and S. Ward. 1998. The influence of mini-BAL cultures on patient outcomes: implications for the antibiotic management of ventilator-associated pneumonia. Chest 113:412-420. [DOI] [PubMed] [Google Scholar]
- 18.Krobot, K., D. Yin, Q. Zhang, S. Sen, A. Altendorf-Hofmann, J. Scheele, and W. Sendt. 2004. Effect of inappropriate initial empiric antibiotic therapy on outcome of patients with community-acquired intra-abdominal infections requiring surgery. Eur. J. Clin. Microbiol. Infect. Dis. 23:682-687. [DOI] [PubMed] [Google Scholar]
- 19.Leibovici, L., I. Shraga, M. Drucker, H. Konigsberger, Z. Samra, and S. D. Pitlik. 1998. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J. Intern. Med. 244:379-386. [DOI] [PubMed] [Google Scholar]
- 20.Leone, M., A. Bourgoin, S. Cambon, M. Dubuc, J. Albanese, and C. Martin. 2003. Empirical antimicrobial therapy of septic shock patients: adequacy and impact on the outcome. Crit. Care Med. 31:462-467. [DOI] [PubMed] [Google Scholar]
- 21.Luna, C. M., P. Vujacich, M. S. Niederman, C. Vay, C. Gherardi, J. Matera, and E. C. Jolly. 1997. Impact of BAL data on the therapy and outcome of ventilator-associated pneumonia. Chest 111:676-685. [DOI] [PubMed] [Google Scholar]
- 22.MacArthur, R. D., M. Miller, T. Albertson, E. Panacek, D. Johnson, L. Teoh, and W. Barchuk. 2004. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clin. Infect. Dis. 38:284-288. [DOI] [PubMed] [Google Scholar]
- 23.Meehan, T. P., M. J. Fine, H. M. Krumholz, J. D. Scinto, D. H. Galusha, J. T. Mockalis, G. F. Weber, M. K. Petrillo, P. M. Houck, and J. M. Fine. 1997. Quality of care, process, and outcomes in elderly patients with pneumonia. JAMA 278:2080-2084. [PubMed] [Google Scholar]
- 24.Micek, S. T., A. E. Lloyd, D. J. Ritchie, R. M. Reichley, V. J. Fraser, and M. H. Kollef. 2005. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob. Agents Chemother. 49:1306-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morrell, M., V. J. Fraser, and M. H. Kollef. 2005. Delaying the empiric treatment of Candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob. Agents Chemother. 49:3640-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial susceptibility testing, ninth informational supplement. M100-S9. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 27.Rello, J., M. Gallego, D. Mariscal, R. Sonora, and J. Valles. 1997. The value of routine microbial investigation in ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 156:196-200. [DOI] [PubMed] [Google Scholar]
- 28.Scheetz, M. H., M. K. Bolon, T. R. Zembower, J. Warren, and G. A. Noskin. 2005. Real-time, unit-specific antimicrobial susceptibility identifies significant differences among gram-negative bacteria. Presented at the IDSA Annual Meeting, San Francisco, Calif.
- 29.Valles, J., J. Rello, A. Ochagavia, J. Garnacho, and M. A. Alcala. 2003. Community-acquired bloodstream infection in critically ill adult patients: impact of shock and inappropriate antibiotic therapy on survival. Chest 123:1615-1624. [DOI] [PubMed] [Google Scholar]
- 30.Vidal, F., J. Mensa, M. Almela, J. A. Martinez, F. Marco, C. Casals, J. M. Gatell, E. Soriano, and M. T. Jimenez de Anta. 1996. Epidemiology and outcome of Pseudomonas aeruginosa bacteremia, with special emphasis on the influence of antibiotic treatment. Analysis of 189 episodes. Arch. Intern. Med. 156:2121-2126. [PubMed] [Google Scholar]
- 31.Zaragoza, R., A. Artero, J. J. Camarena, S. Sancho, R. Gonzalez, and J. M. Nogueira. 2003. The influence of inadequate empirical antimicrobial treatment on patients with bloodstream infections in an intensive care unit. Clin. Microbiol. Infect. 9:412-418. [DOI] [PubMed] [Google Scholar]