Abstract
The histidine-rich amphipathic cationic peptide LAH4 has antibiotic and DNA delivery capabilities. Here, we explore the interaction of peptides from this family with model membranes as monitored by solid-state 2H nuclear magnetic resonance and their antibiotic activities against a range of bacteria. At neutral pH, the membrane disruption is weak, but at acidic pH, the peptides strongly disturb the anionic lipid component of bacterial membranes and cause bacterial lysis. The peptides are effective antibiotics at both pH 7.2 and pH 5.5, although the antibacterial activity is strongly affected by the change in pH. At neutral pH, the LAH peptides were active against both methicillin-resistant and -sensitive Staphylococcus aureus strains but ineffective against Pseudomonas aeruginosa. In contrast, the LAH peptides were highly active against P. aeruginosa in an acidic environment, as is found in the epithelial-lining fluid of cystic fibrosis patients. Our results show that modest antibiotic activity of histidine-rich peptides can be dramatically enhanced by inducing membrane disruption, in this case by lowering the pH, and that histidine-rich peptides have potential as future antibiotic agents.
With the dramatic rise of antibiotic resistance in pathogenic bacteria, there is no doubt that there is an urgent requirement for the development of novel antibiotics. The lungs of cystic fibrosis patients are chronically infected by lineages of Pseudomonas aeruginosa with high mutation rates that can readily become resistant to current antibiotics (20, 25), while the impact of methicillin-resistant Staphylococcus aureus (MRSA) has been well documented (2). These bacteria, therefore, represent significant targets for new classes of antibiotics.
Cationic antimicrobial peptides play a central role in preventing the onset of infection in many organisms, and following the introduction of a number of such peptides in clinical trials (16), it seems likely that development of these peptides will provide the basis for a new class of antibiotics. LAH4, a histidine-rich amphipathic α-helical peptide (4), has been shown to possess both antibiotic (34) and DNA delivery (17, 18) capabilities. This dual functionality could be exploited in therapeutic strategies for a number of diseases, a notable example being cystic fibrosis, where LAH4 or a derivative thereof could form part of a gene delivery vehicle while concomitantly fighting microbial infections that result from the underlying condition. Therefore, a fundamental understanding of the antibiotic strategies employed by cationic antimicrobial peptides such as LAH4 will aid the design of more powerful antibiotics that may eventually become frontline treatments for a variety of infectious diseases.
To understand in greater detail how histidine-rich peptides operate against their microbial targets, we synthesized a series of derivatives of LAH4 and combined biophysical studies of their interactions using model membranes from solid-state nuclear magnetic resonance (NMR) of chain-deuterated lipids with in vitro observations of their properties against a variety of pathogenic bacterial targets. The original design of LAH4 (4) was inspired by the antibiotic peptide magainin, isolated from Xenopus laevis (37), which is thought to exert most of its bactericidal effects through disruption of the cell membrane (7, 38). In contrast, a number of cationic antimicrobial peptides, while also disrupting bacterial membranes, have been shown to operate through intracellular modes of killing, such as binding nucleic acids (26, 36) or inhibiting nucleic acid or protein synthesis (10, 27, 32).
Here, we show that LAH4 derivatives do display antibiotic activity at neutral pH but do not strongly disrupt model membranes, nor do they have a bacteriolytic effect on Escherichia coli or Bacillus megaterium, even at concentrations well above the determined MICs. At acidic pH, however, where the antibiotic activities of the peptides are enhanced and a bacteriolytic effect is observed at and above the MIC, the peptides adopt an in-plane orientation in the membrane and destabilize the anionic lipid component of model membranes. The LAH peptides show activity against a variety of pathogenic strains of Staphylococcus aureus at neutral pH. Furthermore, the LAH peptides, though ineffective against Pseudomonas aeruginosa at neutral pH, are highly active at acidic pH. These results indicate that these histidine-rich cationic peptides can operate with at least two distinct modes of bacterial killing and have the potential to be developed into effective antimicrobial agents.
MATERIALS AND METHODS
Peptides and lipids.
The peptides (Table 1) were synthesized using standard 9-fluorenylmethoxy carbonyl solid-state chemistry (24) on a Millipore 9050 synthesizer. In crude peptide preparations, a predominant peak was observed when they were analyzed by high-performance liquid chromatography with acetonitrile-water gradients. During high-performance liquid chromatography purification, the main peak was collected and the identity of the product was confirmed by matrix-assisted laser desorption ionization mass spectrometry, in which a single molecular ion peak was observed in addition to the monosodium and monopotassium peaks. The purities of the crude peptides were estimated to be approximately 80%, while the purified peptides were >95% pure. The lipids 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylethanolamine (POPE), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylglycerol (POPG), 1-palmitoyld31-2-oleoyl-sn-glycero-3-phosphatidylethanolamine (POPE-d31), and 1-palmitoyld31-2-oleoyl-sn-glycero-3-phosphatidylglycerol (POPG-d31) were obtained from Avanti Polar Lipids, Inc. (Alabaster, AL), and used without further purification. All other reagents were analytical grade or better.
TABLE 1.
sequences of LAH4 isomers, derivatives, and magainin 2 used in this study and their MIC50s for two model bacteria
| Peptidea | Sequenceb | Nominal charge at pH 5.5c | MIC (μM)
|
|||
|---|---|---|---|---|---|---|
|
E. coli
|
B. megaterium
|
|||||
| pH 7.2 | pH 5.5 | pH 7.2 | pH 5.5 | |||
| LAH4-L0 | KKALLAHALAHLALLALHLALHLKKA | +9 | 5 | 0.5 | 5 | 1 |
| LAH4-L1 | KKALLAHALHLLALLALHLAHALKKA | +9 | 5 | 0.5 | 5 | 5 |
| LAH4-L2 | KKALLALALHHLALLALHLAHALKKA | +9 | 5 | 0.5 | 5 | 5 |
| LAH4-AL6 | KKALLHLALALLALHAHALALHLKKA | +9 | 5 | 0.5 | 5 | 1 |
| LAH4-L1-F4 | KKALLAHFFHLLALLALHFFHALKKA | +9 | 20 | 0.5 | 10 | 5 |
| LAH6-L1 | KKALLAHALHHLALLAHHLAHALKKA | +11 | 1 | 0.5 | 1 | 1 |
| Magainin 2 | GIGKFLHSAKKFGKAFVGEIMNS | +5 | 15 | 0.5 | 10 | 5 |
The peptides are all C-terminally amidated, contributing one extra positive charge overall.
Histidine residues in the peptides are marked in bold.
At neutral pH, the LAH peptides and magainin 2 have nominal charges of +5 and +4, respectively.
Antibacterial assays.
The following strains were tested: Bacillus megaterium MA, Escherichia coli D22 and ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and Staphylococcus aureus NCIM 2079, S2070, S5751, S121, and S4820. The antibacterial activities of the peptides were tested at two different pHs (7.2 and 5.5), using a Mueller-Hinton broth medium (Difco Laboratories). The bacteria were first grown aerobically with vigorous shaking at 37°C in the appropriate medium, and aqueous peptide solutions (10 μl) were incubated in a 96-well microplate with 100 μl of a mid-logarithmic-phase culture of bacteria with a calculated starting optical density at 620 nm (OD620) of 0.001. Microbial growth was assessed by the increase of the OD620 after 18 h of incubation at 37°C, and the value of control cultures growing without peptide was taken as 100%. Each assay was performed in triplicate, and the antibacterial activity was evaluated with the MIC50 or MIC100.
In order to determine whether bacterial lysis was implicated in the bacterial growth inhibition mechanism, 1 ml of a mid-logarithmic-phase culture of bacteria with a starting OD600 of 0.4 (pH 7.2) or 0.2 (pH 5.5) was incubated with peptide under the usual experimental conditions, and the OD600 was measured after 10 min (T0) and 2 h (T1). Statistical analysis was by analysis of variance (19), with the one-tailed Bonferonni post hoc t test. After 2 h, the bacterial culture medium was diluted (10 μl/1 ml of Mueller-Hinton broth medium), and serial 10-fold dilutions were prepared and spread out on Mueller-Hinton agar. For each experiment, after 18 h, the colonies were counted.
Sample preparation for solid-state NMR.
Samples with different lipid compositions were prepared for 2H echo NMR: POPE-POPE-d31-POPG and POPE-POPG-d31, with respective molar ratios of 2:1:1 and 3:1. A total of around 5 mg lipids per sample was dissolved, mixed in chloroform, and dried under rotor evaporation at room temperature. The lipid films were exposed to a vacuum overnight and were then rehydrated with 5 ml of a suspension of LAH peptide in 0.1 M Tris-0.1 M KCl buffer, pH 7.5, at room temperature to give a final concentration of 2.5% by mole relative to the lipids. The samples were briefly sonicated in a bath sonicator and subjected to five rapid freeze-thaw cycles and then centrifuged at 21,000 × g for 20 min at room temperature. Previous determination of a binding constant of 4.2 × 103 (34) for LAH4 and phosphatidylcholine vesicles at pH 7.5 indicated that very little peptide would be free in solution after this process. The pellets, containing lipid vesicles and associated LAH peptides, were transferred to Bruker 4-mm magic angle spinning (MAS)rotors for NMR measurements. Lipid vesicles were also prepared in this way in the absence of peptide. After solid-state NMR measurements were recorded for these samples at pH 7.5, the pellets were resuspended in a large excess of buffer (0.1 M Tris-HCl, 0.1 M KCl) at pH 5. The samples were then sonicated as described above for 5 min and again centrifuged and loaded into MAS rotors for further NMR measurements.
Solid-state NMR.
2H quadrupole experiments (13) for samples containing POPG-d31 or POPE-d31 were performed at 46.10 MHz on a Bruker Avance 300 NMR spectrometer using a 4-mm MAS probe and a spectral width of 200 KHz with recycle delay, echo delay, acquisition time, and 90° pulse lengths of 0.3 s, 42 μs, 2 ms, and 5 μs, respectively. The temperature was maintained at 298 K. Between 50,000 and 200,000 transients were acquired to obtain a signal-to-noise ratio greater than 50:1. During processing, the first 40 points were removed in order to start Fourier transformation at the beginning of the echo. Spectra were zero filled to 8,192points, and 50-Hz exponential line broadening was applied. Smoothed deuterium order parameter profiles were obtained from symmetrized and dePaked 2H NMR powder spectra using published procedures (29, 30, 31).
RESULTS
Antibacterial assays; E. coli and B. megaterium.
The MIC50s for the gram-negative bacterium E. coli and the gram-positive bacterium B. megaterium are shown for a range of LAH4 isomers and derivatives and magainin 2 (Table 1), a peptide derived from Xenopus laevis (37) with well-characterized antibiotic activity (39).
The series of LAH4 isomers displayed antibiotic activity against both E. coli and B. megaterium at neutral pH, with a MIC of 5 μM, and were all more effective than magainin 2. There were practically no differences in antibiotic activity between the LAH4 isomers at either neutral or acidic pH, indicating that the positioning of the histidine residues does not significantly influence antimicrobial activity. Increasing the number of histidine residues in the peptide sequence slightly enhanced the antibiotic activity at neutral pH (Table 1). In contrast, the addition of phenylalanine residues to the sequence (LAH4-L1-F4) noticeably reduced the antibiotic activity of the peptide, reaching the level observed for magainin 2, which is also rich in phenylalanine. When the pH in the growth medium was lowered to 5.5, the activities of the peptides against both test bacteria were enhanced (Table 1); indeed, for the LAH4 isomers, a peptide concentration as low as 0.5 μM had an inhibitory effect against E. coli.
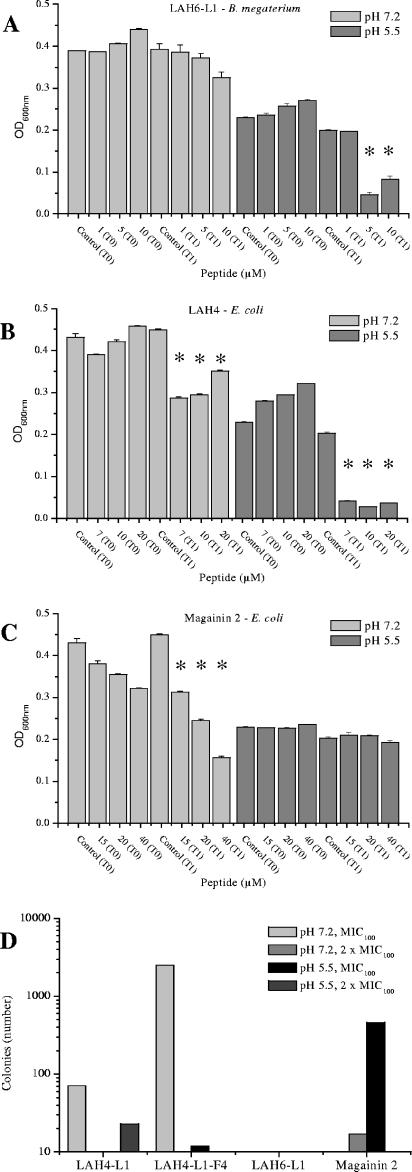
To gain some understanding of the mechanism of antibacterial action of the LAH peptides, two assays were performed to determine whether the peptides were bactericidal or bacteriostatic and, if bactericidal, whether the cell membrane was disrupted. After an initial period of growth of the two test bacterial strains, peptide was added at concentrations corresponding to the MIC50, the MIC100, and two times the MIC100. Four peptides were selected for this comparative study; LAH4-L1, LAH6-L1, and the original LAH4 were compared with magainin 2 at both neutral and acidic pHs (Fig. 1). Examples are shown for the action of LAH6-L1 on B. megaterium (Fig. 1A) and LAH4 and magainin 2 on E. coli (Fig. 1B and C). At acidic pH, when the histidine peptides are charged, the LAH peptides are expected to adopt an orientation, like magainin 2 (9), parallel to the membrane surface (1, 4). As seen for LAH4 and LAH6-L1 (Fig. 1A and B), under these conditions, the optical densities of the cultures were dramatically reduced after 2 h of incubation (T1) by the addition of the peptides at their effective antibacterial concentrations compared with the optical densities of control bacteria or bacteria incubated with peptide for only 10 min (T0). The reduction in optical density indicates that the bacterial cells were disrupted by the presence of the peptide and suggests that the mechanism of action, under acidic conditions, for all of the LAH peptides studied involves disruption of the bacterial membrane, inducing bacterial lysis. At neutral pH, however, addition of LAH peptides, even at concentrations twice the MIC100, caused, proportionally, a much more modest drop in optical density. This indicates that disruption of the bacterial membrane is not a crucial determinant of the peptide activity when the histidines are not charged. This result is in stark contrast with that for magainin 2, which can be seen to be lytic at pH 7.2 (Fig. 1C), and it is known that disruption of the bacterial membrane forms a large part of the killing strategy of that peptide (7, 38). To determine whether the LAH peptides were bacteriostatic or also bactericidal at neutral pH, the cultures were grown as described above, incubated with peptides for 2 hours, washed, and then plated. A large number of colonies were observed after incubation with LAH4-L1-F4 at pH 7.2 for 2 hours, indicating that bacteria remain viable even after treatment with this peptide at its MIC100. Very few colonies, however, were observed after 2 hours of incubation with LAH4-L1, LAH6-L1, or magainin 2 at either pH (Fig. 1D), indicating that the LAH peptides are also bactericidal at neutral pH. In summary, the results of these two assays indicate that the LAH peptides are bactericidal at both acidic and neutral pHs but operate through different mechanisms, depending on the pH.
FIG. 1.
Effects of two LAH antibiotic peptides and magainin 2 on culture OD600 at both acidic and neutral pHs. The peptides were added at concentrations corresponding to their MIC50s, MIC100s, and two times the MIC100s. The optical densities of the test bacteria are shown after 10 min (T0) and 2 h (T1) of incubation for LAH6-L1 against B. megaterium (A) and LAH4 (B) and magainin 2 (C) against E. coli. The error bars represent the standard deviations of the mean, while an asterisk denotes a P value of <0.01 for T1 measurement compared with both control and T0 measurements. Bacterial (E. coli) viability after incubation with peptide under the same conditions is shown by the number of colonies produced on a solid support (D). LAH4-L1 and LAH6-L1 were effective bactericidal peptides despite the apparent lack of membrane disruption, but a large number of cells remained viable after treatment with LAH4-L1-F1 at neutral pH. More than 3,000 colonies were counted on plates with no peptide treatment.
Solid-state NMR measurements.
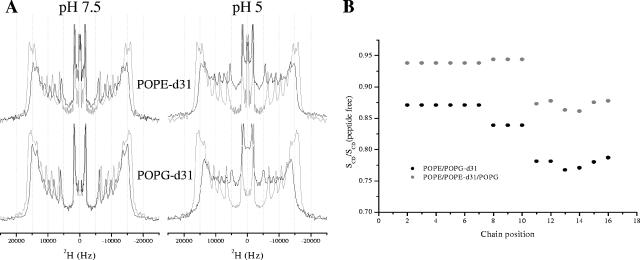
A detailed picture of the effects of the LAH peptides on the lipid chains in model membranes can be obtained by acquiring wide-line 2H echo spectra of PE-PG lipid mixes in the absence and presence of peptide at acidic and neutral pHs. By keeping the peptide concentration constant, at a level (2.5 mol%) where an effect on the fatty acyl chain order could be observed by solid-state NMR methods but without disintegration of the membrane, as previously observed using static 31P NMR on similarly prepared vesicles (23), and by varying the lipid conditions, we could obtain an accurate measure of the roles that the different lipids play in the peptide-lipid interactions. 2H echo spectra were acquired for hydrated powder samples pelleted by centrifugation from excess buffered solutions, which facilitated control of the sample pH. Solid-state 2H echo spectra of either POPE-d31 or POPG-d31 (Fig. 2A) in mixed POPE-POPE-d31-POPG or POPE-POPG-d31 vesicles revealed the strength of the interaction between LAH4-L1 and either the zwitterionic or anionic lipid component and the ability of the peptide to disrupt the lipid acyl chains (23, 21).
FIG. 2.
Comparison of 2H quadrupolar echo spectra of POPG-d31 or POPE-d31 in lipid vesicles containing POPE-POPG-d31 (75:25) or POPE-POPE-d31-POPG (50:25:25) in the absence (gray lines) or presence (black lines) of 2.5 mol% LAH4-L1 at either pH 7.5 or 5 (A). The spectra were recorded on a Bruker Avance 300 spectrometer at 298 K. A comparison of order parameter profiles for LAH4-L1/lipid vesicle complexes at pH 5 containing POPE-d31 or POPG-d31 as a reporter of mobility (B) revealed the response of the lipid acyl chains in either the zwitterionic or anionic lipid to the presence of the peptide. There was a slight reduction in order throughout the POPE acyl chain, with a light enhancement of the effect in the lower third, but this effect was noticeably enhanced when anionic POPG was used as a reporter.
At pH 7.5, LAH4-L1 induces a slight reduction of acyl chain order reflected in the spectra by reduced quadrupolar splittings (Fig. 2A), yet it is small compared with the effect of the same peptide at pH 5.5 (Fig. 2A). The disruption of the lipid chain order induced by 2.5 mol% LAH4-L1 at pH 5.5 can be seen clearly in the spectra (Fig. 2A) and compared in the calculated order parameter profiles, which are displayed relative to the peptide-free data (Fig. 2B). It can be seen that, particularly further down the acyl chain, the anionic PG acyl chains were very strongly disrupted by the presence of LAH4-L1, while the acyl chains of the zwitterionic PE lipids were much less affected (Fig. 2B).
Similar spectra of chain-deuterated lipids were obtained in the presence of LAH6-L1, LAH4-L1-F4, and the remaining LAH4 isomers (not shown). Importantly, LAH6-L1 caused no disruption of either PE or PG acyl chains at pH 7.5 but was extremely disruptive at acidic pH, while LAH4-L1-F4 and the remaining LAH4 isomers behaved similarly to LAH4-L1 in terms of their effects on the mobilities of the acyl chains of both labeled PE and PG probes.
Antibacterial assays; pathogenic bacteria.
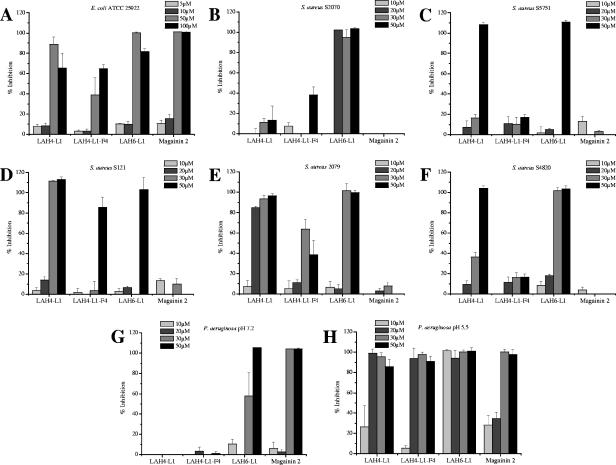
Next, three of the LAH peptides were selected to be tested against a range of pathogenic bacteria. LAH6-L1 was selected because it had shown the highest activity against both test bacteria, LAH4-L1 was selected because it is the most effective transfection agent and is representative of the LAH4 isomer series, LAH4-L1-F4 was selected to assess the role of the hydrophobic surface, and magainin 2 was selected to provide a useful comparison. The efficiencies of inhibition of the peptides can be readily compared (Fig. 3). Of the LAH peptides, in each case, LAH6-L1 was the most effective antibiotic, and it was the only LAH peptide to have any activity against the gram-negative bacterium Pseudomonas aeruginosa at neutral pH, albeit only at higher concentrations (Fig. 3G). In comparison, magainin 2 was as effective against P. aeruginosa as against E. coli (Fig. 3A and G). Magainin 2, however, had almost no detectable activity against the S. aureus strains (gram positive) used here, but a number of the LAH peptides did demonstrate considerable activity (Fig. 3B to F). These results indicate that the antibiotic efficiencies of the peptides are strongly dependent on the organism and that those peptides rich in histidine may succeed where other antibiotic peptides have previously struggled. Our study compared the effects of the four peptides selected above to those of five strains of S. aureus, including both MRSA and methillicin-sensitive S. aureus strains (Fig. 3B to F). S. aureus 2079 is sensitive to multiple antibiotics, and it and S4820 are sensitive to methillicin. The remaining three strains are resistant to methicillin, while all except strain 2079 exhibit resistance to one or more of the aminoglycoside antibiotics and erythromycin. The performances of the LAH peptides varied according to the strain tested, but it can be seen that LAH4-L1 was efficient at killing four out of five of the strains tested, while LAH4-L1-F4 again performed poorly in comparison. LAH6-L1 was in some cases highly effective, showed some killing capacity against all the strains tested (Fig. 3B to F), and was the most effective peptide overall, displaying some attractive antibiotic capabilities. As observed above, the LAH peptides were poorly active against P. aeruginosa at neutral pH (Fig. 3G), but when the cultures were grown in an acidic environment, the LAH peptides became highly effective antibiotic agents, with activities well in excess of that of magainin 2 (Fig. 3H). Again, LAH6-L1 was the most effective antibiotic and was effective at concentrations as low as 10 μM.
FIG. 3.
Charts comparing the inhibitory efficiencies of selected LAH peptides and magainin 2 on the growth of the pathogenic bacteria Escherichia coli 25922 (A); MRSA strains S2070 (B), S5751 (C), and S121 (D); and methillicin-sensitive S. aureus strains 2079 (E) and S4820 (F) at pH 7.2. P. aeruginosa 27853 was challenged with the same peptides at both pH 7.2 (G) and pH 5.5 (H).
DISCUSSION
Mechanism of LAH peptide antibacterial action.
Given the major problems being experienced with resistance among classical antibiotics, there seems to be considerable promise for the development of effective antibiotics from cationic peptides. However, for the full potential of these peptides to be realized, it is crucial that their mechanism of action be understood, since only then will more effective peptides be designed or peptides with either broader or targeted spectra of activity be conceivable.
For this reason, we have sought to understand in more detail the mechanism of antimicrobial action of histidine-rich cationic amphipathic peptides that have previously been shown to possess antibiotic activity (34). The structural design of LAH4 was inspired by amphipathic helical peptides, such as magainin (34). Magainins have been shown to induce channel-like activity in biological membranes (12, 14), and while a number of different mechanisms have been proposed for its killing strategy, disruption of the bacterial membrane is believed by most to be the main cause of cell death (7, 8, 38).
LAH4, however, is noticeably different from magainin 2 in that the positively charged lysines are located at the N and C termini, as anchors, rather than dispersed along the helix. The result of this is that LAH4 adopts a transmembrane orientation at neutral pH (pH 5), in contrast to magainin and other cationic helical peptides, which are oriented parallel to the membrane surface (5, 6, 21). Consistent with this, our data show that at neutral pH, the LAH peptides use a rather different killing strategy than magainin 2, since at peptide levels even double the MIC100, no cell lysis was observed. In addition, increasing the hydrophobicity of the already hydrophobic face of the peptide by introducing four phenylalanine residues reduced activity against all bacteria tested. While the mechanism of antibiotic activity of the LAH peptides at neutral pH is obscure and is an interesting line of further study, its strategy may center on nucleic acid binding or the inhibition of nucleic acid and/or protein synthesis, as has been shown for buforin 2, tachyplesin, and pleurocidin, as well as other cationic peptides (26, 27, 36).
Solid-state NMR.
At acidic pH, the observed activities of the LAH peptides were altered in a number of respects. First, the activities of the LAH peptides against E. coli and, to a lesser extent, B. megaterium were enhanced. Secondly, the LAH peptides were lytic at acidic pH, as evidenced by a drop in the optical density when the peptides were added to bacterial cultures at their effective concentrations. LAH4 has been shown to adopt an in-plane orientation at acidic pH when the histidine residues become protonated below a pH of approximately 6.1 (4). In this orientation, the peptides disrupt the membrane much more efficiently (34) and have been shown to interact strongly with anionic lipids (23). Recently, we used solid-state NMR as a tool to probe the lipid chain order of 2H-labeled lipids in mixed-membrane environments (21-23). Briefly, synthetic lipids carrying 2H labels throughout the length of, in this case, the saturated acyl chain were incorporated into lipid vesicles. The observed quadrupolar splittings for each CD2 group (Fig. 2) were scaled according to the order profile of the membrane (30), allowing a measure of membrane disorder to be determined on binding and insertion of the peptide (21-23). This method is particularly effective at discriminating between anionic and zwitterionic lipid components when membrane-active peptides are added to the system, and recently, we have shown that pleurocidin, a cationic amphipathic helical and histidine-rich peptide from winter flounder, disturbs anionic lipids in mixed PE-PG model membranes, without affecting the overall morphology of the bilayer, at concentrations at which channel-like activity has been observed (21). Using the same solid-state NMR method, we saw that at acidic pH, the disruption of the chain order in anionic PG in the presence of each of the LAH4 isomers was rather dramatic and was of a much greater magnitude than that of the zwitterionic PE lipid chains. Hence, we propose a model for LAH peptide-induced disruption of the membrane at acidic pH in which the observed changes in the spectra can be interpreted as a general increase in the mobility of the lipid chain, where the strongly cationic LAH peptides insert into the membrane and bind preferentially, but not exclusively, to the anionic PG in the head group interface region without causing an overall change in the morphology of the membrane. The resulting increase in the separation of the anionic lipids allows an increase in the disorder, or cis-trans isomerizations, at the level of the fatty acyl chains that is reflected in the observed reduction-of-order parameter throughout the acyl chain. This model can be compared with a previous study of the membrane insertion of ethanol at the hydrocarbon-water interface (3), where the hydrophobic core of the membrane also becomes disordered, albeit at rather high ethanol concentrations, and a very recent study of the effect of both melittin- and magainin-derived peptides on deuterated phosphatidylcholine(28), where both these peptides adopt an in-plane orientation and disrupt the acyl chain order in a fashion similar to that shown here for the LAH peptides at acidic pH.
Interestingly, the disruption of the PG acyl chains at acidic pH is much greater for LAH4-L1 than the effect of pleurocidin at the same concentration (21). This may reflect the nature of the charged residues responsible for the interaction with the anionic lipids since, with pleurocidin, the interaction is likely mediated by lysine residues with relatively long, flexible side chains, while histidine residues are uniquely responsible for this interaction for LAH4-L1.
Activities of LAH peptides against pathogenic bacteria.
At neutral pH, the LAH peptides demonstrate clear antibiotic activity against both methicillin-sensitive and -resistant strains of S. aureus but are almost completely ineffective against P. aeruginosa. Importantly, however, we have shown that lowering the pH of the medium activates the peptides and that LAH4-L1 and LAH6-L1 become highly active under acidic conditions against P. aeruginosa, a pathogen that commonly infests the airways of cystic fibrosis patients. The high antimicrobial activities of the peptides at acidic pH in vitro may be directly relevant to in vivo applications, since the epithelial-lining fluid can become rather acidic as a result of certain conditions. Exhaled human breath condensate has a pH of approximately 6.15, but in patients with cystic fibrosis, for example, this value can fall as low as 5.32 (33). Certain antimicrobial peptides have been shown recently to be effective against P. aeruginosa in vivo (40), a fact that increases the likelihood that histidine-rich peptides may be similarly applicable to combat lung infections.
The mechanism of disruption of the bacterial membrane has been the target of a large number of studies that sought to understand how cationic helical peptides function, yet it has been shown that, for a number of cationic peptides, membrane depolarization is incomplete even when 90% or more of the bacteria have been killed. Hence, although the membrane interactions of cationic antibiotic peptides are an important factor, they may be important for entry into the cell but not constitute the main killing mechanism among many classes of peptides (11, 15, 35). This finding is consistent with our demonstration of antibiotic activity of the LAH peptides despite the absence of significant membrane disruption. Nevertheless, the antibacterial activities of LAH peptides are modulated strongly by the pH of the media, and the enhanced activities of LAH peptides at acidic pH against the test bacteria, E. coli and B. megaterium, and the activation of the peptides at low pH against P. aeruginosa indicate that the effective disruption of the membrane is a key component of the antibiotic mechanism.
Acknowledgments
A.J.M. thanks Gérard Nullans for mass spectrometry analysis.
This work was supported by Vaincre la Mucoviscidose (TG0501), Inserm, Hôpitaux Universitaires de Strasbourg (PHRC3150 to MHMB), Université Louis Pasteur (a postdoctoral grant to A.J.M.), and the CNRS (Poste Rouge for A.J.M.).
REFERENCES
- 1.Aisenbrey, C., R. Kinder, E. Goormaghtigh, J.-M. Ruysschaert, and B. Bechinger. 2006. Interactions involved in the realignment of membrane-associated helices: an investigation using oriented solid-state NMR and ATR-FTIR spectroscopies. J. Biol. Chem. 281:7708-7716. [DOI] [PubMed] [Google Scholar]
- 2.Bal, A. M., and I. M. Gould. 2005. Antibiotic resistance in Staphylococcus aureus and its relevance in therapy. Exp. Opin. Pharmacother. 6:2257-2269. [DOI] [PubMed] [Google Scholar]
- 3.Barry, J. A., and K. Gawrisch. 1994. Direct NMR evidence for ethanol binding to the lipid-water interface of phospholipid bilayers. Biochemistry 33:8082-8088. [DOI] [PubMed] [Google Scholar]
- 4.Bechinger, B. 1996. Towards membrane protein design: pH-sensitive topology of histidine-containing polypeptides. J. Mol. Biol. 263:768-775. [DOI] [PubMed] [Google Scholar]
- 5.Bechinger, B. 1997. Structure and functions of channel-forming polypeptides: magainins, cecropins, melittin and alamethicin. J. Membr. Biol. 156:197-211. [DOI] [PubMed] [Google Scholar]
- 6.Bechinger, B. 1999. The structure, dynamics and orientation of antimicrobial peptides in membranes by solid-state NMR spectroscopy. Biochim. Biophys. Acta 1462:157-183. [DOI] [PubMed] [Google Scholar]
- 7.Bechinger, B. 2004. Membrane-lytic peptides. Crit. Rev. Plant Sci. 23:271-292. [Google Scholar]
- 8.Bechinger, B. 2005. Detergent-like properties of magainin antibiotic peptides: a 31P solid-state NMR spectroscopy study. Biochim. Biophys. Acta 1712:101-108. [DOI] [PubMed] [Google Scholar]
- 9.Bechinger, B., M. Zasloff, and S. J. Opella. 1993. Structure and orientation of the antibiotic peptide magainin in membranes by solid-state nuclear magnetic resonance spectroscopy. Protein Sci. 2:2077-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boman, H. G., B. Agerberth, and A. Boman. 1993. Mechanisms of action on Escherichia coli of cecropin P1 and PR-39, two antibacterial peptides from pig intestine. Infect. Immun. 61:2978-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brogden, K. A. 2005. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3:238-250. [DOI] [PubMed] [Google Scholar]
- 12.Cruciani, R. A., J. L. Barker, G. Raghunathan, H. R. Guy, M. Zasloff, and E. F. Stanley. 1992. Magainin 2, a natural antibiotic from frog skin, forms ion channels in lipid bilayer membranes. Eur. J. Pharmacol. 226:287-296. [DOI] [PubMed] [Google Scholar]
- 13.Davis, J. H. 1983. The description of membrane lipid conformation order and dynamics by 2H-NMR. Biochim. Biophys. Acta 737:117-171. [DOI] [PubMed] [Google Scholar]
- 14.Duclohier, H., G. Molle, and G. Spach. 1989. Antimicrobial peptide magainin I from Xenopus skin forms anion-permeable channels in planar lipid bilayers. Biophys. J. 56:1017-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedrich, C. L., D. Moyles, T. J. Beveridge, and R. E. W. Hancock. 2000. Antibacterial action of structurally diverse cationic peptides on gram-positive bacteria. Antimicrob. Agents Chemother. 44:2086-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hancock, R. E. W., and A. Patrzyat. 2002. Clinical development of cationic antimicrobial peptides: from natural to novel peptides. Curr. Drug Targets 2:79-83. [DOI] [PubMed] [Google Scholar]
- 17.Kichler, A., C. Leborgne, J. März, O. Danos, and B. Bechinger. 2003. Histidine-rich amphipathic peptide antibiotics promote efficient delivery of DNA into mammalian cells, Proc. Natl. Acad. Sci. USA 96:1564-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kichler, A., A. J. Mason, and B. Bechinger. 2006. Cationic amphipathic histidine-rich peptides for gene delivery, Biochim. Biophys. Acta 1758:301-307. [DOI] [PubMed] [Google Scholar]
- 19.Lindman, H. R. 1974. Analysis of variance in complex experimental designs. W. H. Freeman & Co., San Francisco, Calif.
- 20.Macia, M. D., D. Blanquer, B. Togores, J. Sauleda, J. L. Perez, and A. Oliver. 2005. Hypermutation is a key factor in development of multiple-antimicrobial resistance in Pseudomonas aeruginosa strains causing chronic lung infections, Antimicrob. Agents Chemother. 49:3382-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason, A. J., I. N. H. Chotimah, P. Bertani, and B. Bechinger. 2006. A spectroscopic study of the membrane interaction of the antimicrobial peptide pleurocidin. Mol. Membr. Biol. 23:185-194. [DOI] [PubMed] [Google Scholar]
- 22.Mason, A. J., J. J. Lopez, M. Beyermann, and C. Glaubitz. 2005. A spectroscopic study of the membrane interaction of tuberoinfundibular peptide of 39 residues (TIP39). Biochim. Biophys. Acta 1714:1-10. [DOI] [PubMed] [Google Scholar]
- 23.Mason, A. J., A. Martinez, C. Glaubitz, O. Danos, A. Kichler, and B. Bechinger. 2006. The antibiotic and DNA transfecting peptide LAH4 selectively associates with, and disorders, anionic lipids in mixed membranes. FASEB J. 20:320-322. [DOI] [PubMed] [Google Scholar]
- 24.Merrifield, R. B. 1963. Solid phase peptide synthesis: synthesis of a tetrapeptide. J. Am. Chem. Soc. 85:2149-2154. [Google Scholar]
- 25.Oliver, A., R. Cantón, P. Campo, F. Baquero, and J. Blázquez. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 288:1251-1253. [DOI] [PubMed] [Google Scholar]
- 26.Park, C. B., H. S. Kim, and S. C. Kim. 1998. Mechanism of action of the antimicrobial peptide buforin II: buforin II kills microorganisms by penetrating the cell membrane and inhibiting cellular functions. Biochem. Biophys. Res. Commun. 244:253-257. [DOI] [PubMed] [Google Scholar]
- 27.Patrzykat, A., C. L. Friedrich, L. Zhang, V. Mendoza, and R. E. Hancock. 2002. Sublethal concentrations of pleurocidin derived antimicrobial peptides inhibit macromolecular synthesis in Escherichia coli. Antimicrob. Agents Chemother. 46:605-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramamoorthy, A., S. Thennarasu, D.-K. Lee, A. Tan, and L. Maloy. 2006. Solid-state NMR investigation of the membrane-disrupting mechanism of antimicrobial peptides MSI-78 and MSI-594 derived from magainin 2 and melittin. Biophys. J. 91:206-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schäfer, H., B. Mädler, and F. Volke. 1995. De-PAKE-ing of NMR powder spectra by nonnegative least-squares analysis with Tikhonov regularization. J. Magn. Reson. 116:145-149. [Google Scholar]
- 30.Seelig, A., and J. Seelig. 1974. Dynamic structure of fatty acyl chains in a phospholipid bilayer measured by deuterium magnetic-resonance. Biochemistry 13:4839-4845. [DOI] [PubMed] [Google Scholar]
- 31.Sternin, E., M. Bloom, and A. L. MacKay. 1983. De-PAKE-ing of NMR Spectra. J. Magn. Reson. 55:274-282. [Google Scholar]
- 32.Subbalakshmi, C., and N. Sitaram. 1998. Mechanism of antimicrobial action of indolicidin. FEMS Microbiol. Lett. 160:91-96. [DOI] [PubMed] [Google Scholar]
- 33.Tate, S., G. MacGregor, M. Davis, J. A. Innes, and A. P. Greening. 2002. Airways in cystic fibrosis are acidified: detection by exhaled breath condensate. Thorax 57:926-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogt, T. C. B., and B. Bechinger. 1999. The interactions of histidine-containing amphipathic helical peptide antibiotics with lipid bilayers. J. Biol. Chem. 274:29115-29121. [DOI] [PubMed] [Google Scholar]
- 35.Wu, M., E. Amier, R. Benz, and R. E. W. Hancock. 1999. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 38:7235-7242. [DOI] [PubMed] [Google Scholar]
- 36.Yonezawa, A., J. Kuwahara, N. Fujii, and Y. Sugiura. 1992. Binding of tachyplesin I to DNA revealed by footprinting analysis: significant contribution of secondary structure to DNA binding and implication for biological action. Biochemistry 31:2998-3004. [DOI] [PubMed] [Google Scholar]
- 37.Zasloff, M. 1987. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. USA 84:5449-5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]
- 39.Zasloff, M., M. Martin, and H.-C. Chen. 1988. Antimicrobial activity of synthetic magainin peptides and several analogues. Proc. Natl. Acad. Sci. USA 85:910-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang, L., J. Parente, S. M. Harris, D. E. Woods, R. E. W. Hancock, and T. J. Falla. 2005. Antimicrobial peptide therapeutics for cystic fibrosis. Antimicrob. Agents Chemother. 49:2921-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]