Abstract
Ambruticin S, an antifungal cyclopropyl-pyran acid, showed curative effects against murine coccidioidal infection. Two analogs of this compound with greater in vitro potency were tested against lethal murine Coccidioides infection. Both improved the survival of mice over that of controls; one resulted in near-sterilization of infection.
The ambruticins are a family of cyclopropyl-pyran acids with antifungal activity that appears to be the result of interfering with the osmoregulatory system (2), resulting in increased intracellular osmolarity. Although three classes of fungicides with this mechanism of action have been used in agriculture, only ambruticin S has been examined as a treatment for experimental fungal infection of animals (3, 5, 6, 8). When mice were infected with lethal doses of Coccidioides spp. and treated with ambruticin S for times ranging from 17 to 56 days, 73% to 100% of the animals were free of fungus (3).
Derivatives of ambruticin with improved in vitro potency against Coccidioides spp. have been developed. This study reports an initial assessment of the efficacies of two compounds for Coccidioides-infected mice. Pharmacokinetic (PK) parameters and toxicity profiles for mice were also determined.
MICs were determined by the CLSI (formerly NCCLS) M38-A method (4). Arthroconidia from three strains each of Coccidioides posadasii (Silveira, C735, and RMSCC2127) and Coccidioides immitis (RS, S46, and RMSCC2281) were harvested into RPMI medium, and 0.9 ml was dispensed into tubes at 0.4 × 105 to 5 × 105 spores/ml. The ambruticin derivatives, as well as the controls ambruticin S and amphotericin B deoxycholate, were solubilized in dimethyl sulfoxide. Fluconazole was dissolved in RPMI medium. Serial dilutions were made in RPMI and 100 μl added to cells. Control wells received 100 μl of dimethyl sulfoxide. Tubes were incubated at 35°C for 48 h and then examined visually for inhibition of fungal growth. The MIC was reported as the dilution at which optical clarity occurred in tubes. Duplicate assays confirmed that both derivatives were more potent in vitro (MICs, 0.25 μg/ml for KOSN-2079 and 0.5 μg/ml for KOSN-2089) than ambruticin S, fluconazole, and amphotericin B (MICs, 4.0 μg/ml, 16.0 μg/ml, and 1.0 μg/ml, respectively).
For assessment of the PK parameters, the compounds were dissolved as follows: KOSN-2079 in 12% hydroxypropyl-β-cyclodextrin (HPBC)-1× phosphate-buffered saline (pH 7.5) and KOSN-2089 in 12% HPBC-50 mM citrate (pH 4.0). The compounds were administered to 6- to 8-week-old male C57BL/6 mice (Harlan Sprague Dawley, San Diego, CA) via the routes and at the doses indicated in Table 1. Plasma was drawn at 15-min intervals up to 90 min and at 2, 3, 4, 6, 10, and 24 h after drug administration, using three mice for each time point. Drug plasma levels were determined by liquid chromatography-mass spectrometry analysis employing epothilone C as a reference standard. As can been seen in Table 1, both compounds showed good oral bioavailability, but they differed dramatically in their PK behaviors. Both drugs are 90% protein bound in the serum. Orally administered, KOSN-2079 shows modest plasma accumulation, a ca. 5-h half-life, and a modest volume of distribution. KOSN-2089 is not accumulated in the plasma but shows a very high volume of distribution and a calculated plasma half-life of >24 h. No ill effects of the ambruticin analogs were observed.
TABLE 1.
Pharmacokinetic parametersa of KOSN-2079 and KOSN-2089 in mouse plasma
| Compound and routeb | Dose (mg/kg) | Cmax (μg/ml) | Tmax (h) | AUC (μg/ml · h) | t1/2 (h) | Clearance (liters/h/kg) | Vss (liters/kg) | F | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KOSN-2079
|
||||||||||||||||
| p.o. | 2.5 | 2.18 | 2.00 | 23.47 | 3.7 | 0.11 | 0.85 | 0.91 | ||||||||
| i.v. | 1.0 | 2.05 | 0.03 | 10.28 | 4.7 | 0.10 | 0.75 | |||||||||
| KOSN-2089
|
||||||||||||||||
| p.o. | 2.5 | 0.12 | 0.50 | 5.89 | 29.1 | 0.42 | 18.34 | 0.67 | ||||||||
| i.v. | 1.0 | 0.19 | 0.03 | 3.50 | 37.4 | 0.29 | 14.82 | |||||||||
Cmax, maximum concentration of drug in plasma; Tmax, time to maximum concentration of drug in plasma; AUC, area under the concentration-time curve; t1/2, half-life; Vss, volume of distribution at steady state.
p.o., oral (per os); i.v., intravenous.
To determine the in vivo efficacies of the two compounds, 18-g female C57BL/6 mice (Harlan Sprague Dawley, Indianapolis, IN) were infected intranasally with a lethal dose of C. posadasii strain Silveira arthroconidia (54/mouse) as previously described (7). On day 6 postinfection, twice-daily oral gavage was begun with KOSN-2079 and KOSN-2089 at 20 or 50 mg/kg of body weight (10 mice per group). Control mice (n = 8) were gavaged with the vehicle only (HPBC) on the same schedule. Mice were treated for 19 days and then monitored daily for an additional 24 days, after which time all remaining mice were sacrificed (at day 48 postinfection). Any mouse considered moribund at any time during the experiment was sacrificed at that time. Lungs and spleen were removed aseptically for quantitative organ culture; 10× serial dilutions of tissue homogenates were plated and CFU recorded after 3 days' growth at 37°C. Two mice from different groups (KOSN-2089 at 20 mg/kg and KOSN-2089 at 50 mg/kg) that died of gavage injury within 48 h of the beginning of treatment were excluded from analysis. Five additional animals (two treated with HPBC, one treated with KOSN-2079 at 20 mg/kg, and two treated with KOSN-2089 at 50 mg/kg) showed neither cultural nor gross evidence of infection at the time of sacrifice and were also excluded from analysis.
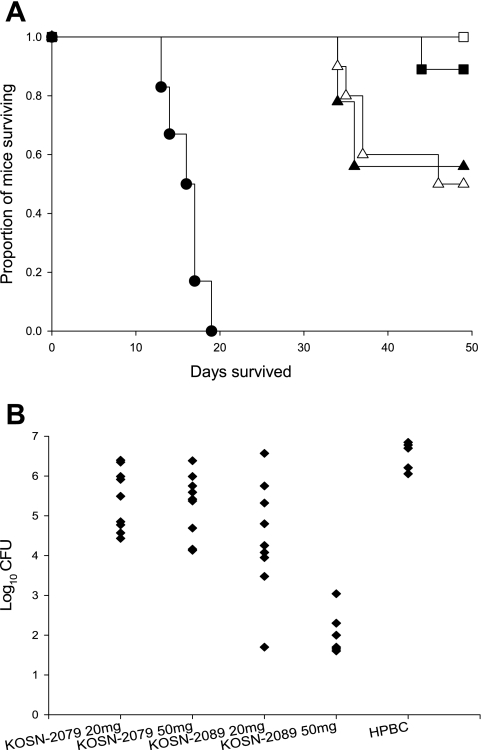
All mice treated with KOSN-2089 at 50 mg/kg (n = 7 included in analysis; n = 9 total) survived until the end of the study, and 8/9 treated with KOSN-2089 at 20 mg/kg survived (Fig. 1A). Losses occurred in both KOSN-2079 groups after treatment with the drug was discontinued. All treatments improved survival over that with HPBC only (P < 0.05 by the Mann-Whitney test). Moreover, KOSN-2089 at 50 mg/kg was significantly better than KOSN-2079 at either dose (P = 0.039 and P = 0.018 at 20 and 50 mg/kg, respectively). Comparing lung fungal burdens, all drug treatments produced significantly lower burdens than sham treatment, and KOSN-2089 at 50 mg/kg reduced CFU significantly more than all other treatments (P < 0.01 by the Kruskal-Wallis test) (Fig. 1B). Furthermore, treatment with KOSN-2089 at 50 mg/kg resulted in sterile cultures from the spleens, livers, and kidneys of all mice, while lung cultures were sterile for two mice and low numbers of CFU (100 to 1,100) were recovered from the other five. Growth from all organs was common in cultures from the other treatment groups.
FIG. 1.
Survival and quantitative organ culture of mice infected intranasally with 54 spores of C. posadasii and treated for 19 days with 20 mg/kg or 50 mg/kg of KOSN-2079 or KOSN-2089. (A) Survival curve for treated mice. All treatments provide prolonged survival compared to the vehicle alone (P < 0.01), and KOSN-2089 at 50 mg/kg results in significantly improved survival compared to KOSN-2079 at either dose (P < 0.05). KOSN-2089 at 20 mg/kg was not significantly different from the KOSN-2079 doses. Solid triangles, KOSN-2079 at 20 mg/kg (n = 9); open triangles, KOSN-2079 at 50 mg/kg (n = 10); solid squares, KOSN-2089 at 20 mg/kg (n = 9); open squares, KOSN-2089 at 50 mg/kg (n = 7); solid circles, vehicle only (n = 6). (B) Quantitative lung cultures for treated mice. KOSN-2089 at 50 mg/kg yielded significantly lower lung fungal burdens than all other treatments (P ≤ 0.006). All treatments reduced lung fungal burdens compared to the vehicle control (HPBC) (P < 0.01).
The fact that KOSN-2089 at 50 mg/kg twice daily for 19 days effected fungal cures for some mice is similar to what was reported for ambruticin S in the original studies, despite the differences in their PK profiles (3). The volume of distribution and long half-life may contribute to the greater efficacy of KOSN-2089; additional studies are required to confirm this. Extending the treatment time with KOSN-2089 might increase its cure rate, as such extension did previously for ambruticin S (3). These observations, though from a small study, suggest that confirmatory studies with larger numbers of animals and comparator drugs are merited. This family of drugs acts via a mechanism entirely different from those of the currently available antifungal medications for coccidioidomycosis, most of which are fungistatic in vivo (1, 3). If KOSN-2089 effects cures in repeated mouse studies, as well as for naturally infected larger species, it has potential to add significantly to the therapeutic armamentarium against coccidioidomycosis.
Acknowledgments
We thank Zhan Wang for assistance with the chemical synthesis of the ambruticin derivatives and Darren Craig for assistance in the PK studies.
This work was supported in part by the U.S. Office of Veterans Affairs.
REFERENCES
- 1.Georgopapadakou, N. H. 2001. Update on antifungals targeted to the cell wall: focus on β-1,3-glucan synthase inhibitors. Expert Opin. Investig. Drugs 10:269-280. [DOI] [PubMed] [Google Scholar]
- 2.Knauth, P., and H. Reichenbach. 2000. On the mechanism of action of the myxobacterial fungicide ambruticin. J. Antibiot. (Tokyo) 53:1182-1190. [DOI] [PubMed] [Google Scholar]
- 3.Levine, H. B., S. M. Ringel, and J. M. Cobb. 1978. Therapeutic properties of oral ambruticin (W7783) in experimental pulmonary coccidioidomycosis. Chest 73:202-206. [DOI] [PubMed] [Google Scholar]
- 4.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 5.Ringel, S. M. 1978. In vitro and in vivo studies on ambruticin (W7783): new class of antifungal antibiotics. Antimicrob. Agents Chemother. 13:762-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shadomy, S., C. J. Utz, and S. White. 1978. In vivo studies with ambruticin in murine histoplasmosis. Antimicrob. Agents Chemother. 14:95-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shubitz, L., T. Peng, R. Perrill, J. Simons, K. Orsborn, and J. N. Galgiani. 2002. Protection of mice against Coccidioides immitis intranasal infection by vaccination with recombinant antigen 2/PRA. Infect. Immun. 70:3287-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams, D. M., J. R. Graybill, and D. J. Drutz. 1979. Experimental chemotherapy of histoplasmosis in nude mice. Am. Rev. Respir. Dis. 120:837-842. [DOI] [PubMed] [Google Scholar]