Abstract
A leading bisthiazolium drug, T16, designed to mimic choline, was shown to exert potent antibabesial activity, with 50% inhibitory concentrations of 28 and 7 nM against Babesia divergens and B. canis, respectively. T16 accumulated inside Babesia-infected erythrocytes (cellular accumulation ratio, >60) by a saturable process with an apparent Km of 0.65 μM. Subcellular fractionation of Babesia parasites revealed the accumulation of T16 into a low-density fraction, while in malaria-infected erythrocytes a significant fraction of the drug was associated with heme malaria pigment. T16 exerts an early and specific inhibition of the de novo biosynthesis of phosphatidylcholine both in B. divergens- and Plasmodium falciparum-infected erythrocytes. Choline accumulation into isolated Babesia parasites was highly sensitive to inhibition by T16. These data are consistent with the hypothesis that bisthiazolium drugs target the de novo phosphatidylcholine biosynthesis of intraerythrocytic hematozoan parasites. In malaria parasites, which generate ferriprotoporphyrin IX during hemoglobin digestion, T16 binding to heme may enhance the accumulation and activity of the drug. The selectivity of accumulation and potent activity of this class of drug into parasite-infected erythrocytes offers unique advantages over more traditional antihematozoan drugs.
Babesia, which, like Plasmodium, belongs to the Apicomplexa phylum, is a genus of tick-transmitted protozoan parasites that can cause malaria-like symptoms and hemolytic anemia (19, 20, 24, 26, 27, 46, 48). The parasite is injected into the circulatory system of its vertebrate host and reproduces asexually inside red blood cells. There are many species of Babesia, affecting livestock (B. bovis and B. divergens), horses (B. equi), dogs (B. canis), and rodents (B. microti). Some species, e.g., B. microti and B. divergens, can spread to humans. As members of the Apicomplexa, Babesia and Plasmodium spp. possess the specific organelles rhoptries, micronemes, and dense granules of the apical complex involved in host cell invasion (29). Although related (14), there are some significant differences; for example, intraerythrocytic Babesia does not grow within a parasitophorous vacuole, which is rapidly lost after host cell invasion (32), and it lacks both cytostome and a digestive vacuole (23, 30, 31, 36).
The lipid content and biosynthetic machinery of Plasmodium, the genus of a malaria-causing parasite, have been widely characterized (43). During its intraerythrocytic development, the parasite synthesizes considerable amounts of membranes with a quasi-absence of cholesterol. The most abundant lipid is phosphatidylcholine (PC), the content of which increases sixfold after infection (41, 42). A major route for the synthesis of this phospholipid requires host choline (4). In this context, compounds which mimic choline structure have been developed to target phospholipid metabolism for possible use as novel class of antimalarials. Special attention has been given to the bisquaternary ammonium G25, since this compound has exceptional in vitro and in vivo antimalarial activity (2, 3, 44, 47). We recently designed expanded-spectrum compounds, bisthiazolium salts and their nonionic precursors, which are enzymatically converted to thiazolium drugs in vivo (22, 34, 45). These molecules exert a very rapid cytotoxic effect against malarial parasites. They are able to cure highly infected mice and retain full activity after a single injection as well as against P. falciparum and P. cynomolgi in primate models with no recrudescence (45).
We have recently shown that the antimalarial activity of bisquaternary ammonium (47) or bisthiazolium salts (45) relies primarily on their high intracellular accumulation (cellular accumulation ratio [CAR], ∼500), which partially results from accumulation in the malarial digestive vacuole and subsequent interaction with ferriprotoporphyrin IX (FPIX) and hemozoin, two hemoglobin degradation metabolites (7).
Although little has been described concerning the lipid composition and metabolism of Babesia (16, 40), PC emerges as the most abundant phospholipid, with 30% in B. bovis-parasitized erythrocytes compared to <5% in noninfected erythrocytes (17).
In this work, we report that the bisthiazolium choline analog T16 possesses potent antibabesial activity in the low-nanomolar range, possibly due to an inhibition of the intraparasitic entry of choline and inhibition of the de novo PC biosynthesis. We show that this compound accumulates selectively in Babesia-infected erythrocytes. In contrast to Plasmodium falciparum infection, erythrocytes infected with Babesia bovis do not contain heme. Therefore, the accumulation and activity of T16 in Babesia are mediated by a heme-independent mechanism. Our results highlight the unique ability of this class of compounds to selectively accumulate within and kill Apicomplexa-infected red blood cells.
MATERIALS AND METHODS
Chemicals.
T16 [1,12-dodecanemethylene bis(4-methyl-5-ethylthiazolium) diodide) and [3H]T16 (69 Ci/mmol) were synthesized in house. (The methods for the synthesis of the compounds and their structural analysis can be found in the supplemental material.) [3H]chloroquine diphosphate (26 Ci/mmol) was purchased from Amersham Biosciences (Orsay, France). [Me-3H]choline, [1-3H]ethan-1-ol-2-amine, [G-3H]hypoxanthine, and [3H]thymidine were purchased from ICN Biomedicals (Orsay, France). Streptolysin O (SLO) was obtained from La Technique Biologique (Paris, France). Pentamidine was provided by Roger Bellon's Laboratory (Neuilly/Seine, France). Imidocarb was supplied by Virbac Laboratories (Carros, France). RPMI 1640 and choline-free RPMI 1640 were obtained from Invitrogen (Cergy Pontoise, France). Complete medium consisted of RPMI 1640 supplemented with 25 mM HEPES (pH 7.4) and 10% human AB+ serum.
Biological materials.
Human blood and AB+ human serum were obtained from the Montpellier blood bank. Babesia divergens (strain Rouen 1987, clone 4) (18) was cultured by serial passages in O+ human erythrocytes at 5% hematocrit in complete medium in a 5% CO2 incubator. Babesia canis (isolate A) (39) was cultured by serial passages in dog erythrocytes suspended at 5% hematocrit in HEPES-buffered RPMI 1640 supplemented with 30 mg/ml glutathione and 10% dog serum (Bio2M, France). The P. falciparum strain 3D7 was maintained in human erythrocytes cultured at 7% hematocrit in complete medium, using the petri dish candle jar method (25). Human Jurkat lymphoblast cell lines were cultured in HEPES-buffered RPMI 1640 medium complemented with 50 μM β-mercaptoethanol, 1 mM fresh glutamine, and 10% fetal calf serum (Invitrogen) (21).
In vitro antiparasitic activities.
The activity of the compounds against the in vitro growth of B. divergens was determined in 96-well microtiter plates at 2% final hematocrit and 1.2% initial parasitemia. The final volume in each well was 200 μl of complete medium without (controls) or with drugs. After 8 h of incubation at 37°C, 30 μl of complete medium containing 0.7 μCi [3H]hypoxanthine was added to each well. After 8 h at 37°C, cells were lysed using an automatic cell harvester, and the parasite macromolecules were harvested onto glass fiber filters. The filters were counted for radioactivity in a liquid scintillation spectrometer (TopCount NXT; Packard).
For Babesia canis, the effects of drugs on parasite viability were evaluated as described above for Babesia divergens, except with an initial parasitemia of 0.7%. Antimalarial activity of the compounds against the in vitro growth of P. falciparum was determined after 48 h of incubation in the presence of the drugs at 1.5% final hematocrit and 0.6% parasitemia before adding hypoxanthine for a further 18-h incubation according to the Desjardin method (3, 13).
IC50s, i.e., the concentration of drug inhibiting parasite growth by 50%, were evaluated from the plotted parasite growth (expressed as a percentage of control) versus the log dose.
In vitro toxicity against a human Jurkat lymphoblast cell line.
The effect of drugs was measured in microtiter plates following [3H]thymidine incorporation into nucleic acids of the cell suspension. The final volume in each well was 200 μl of medium culture with or without drug and containing 6,000 cells. After 24 h of incubation at 37°C, 20 μl of medium culture containing 0.4 μCi [3H]thymidine was added for an additional 6-h period. Cells were then lysed, and the macromolecules were recovered onto glass fiber filters using the cell harvester.
T16 and chloroquine (CQ) uptake into parasitized erythrocytes and T16 reversibility.
Uninfected or infected erythrocytes (5 to 10% parasitemia, 5% hematocrit, 3 × 108 to 5 × 108 total cells for P. falciparum-synchronized cultures, 22 to 41% parasitemia, 5% hematocrit, 1 × 108 to 2 × 108 cells for B. divergens cultures) suspended in culture medium were incubated with 50 nM of [3H]T16 or [3H]CQ at 37°C or 4°C. At specific time intervals, aliquots of the suspension were taken, washed with cold (∼4°C) complete medium, and overlaid onto oil (400 μl of dibutylphtalate) in a microcentrifuge tube and centrifuged at 10,000 × g for 30 s, thereby sedimenting the cells below the oil. The tube tip containing the cell pellet was cut off, and the cell radioactivity was counted by liquid scintillation counting (8). In all our studies, we assumed that noninfected cells in an infected population would behave as their counterparts in an uninfected sample, as has been shown for P. falciparum (47). The cellular accumulation ratio (CAR) is the ratio of the amount of labeled drug in the cells to the amount of labeled drug in a similar volume of buffer after incubation. The volumes of uninfected and infected erythrocytes were assumed to be equal (75 fl) (33). [3H]T16 accumulation within human Jurkat lymphoblasts (1 × 106 cells) was measured according to the same procedure.
For T16 retention studies, unpararasitized or parasitized erythrocytes were incubated with 50 nM of [3H]T16 for 2 h at 37°C and then washed with cold complete medium as described above. An aliquot of the cell suspensions was then centrifuged through dibutylphtalate to measure the cellular accumulation. The suspension was then divided in two parts (200 nM of nonradioactive T16 was added to one of them), and they were incubated at 37°C or 4°C. At specific time intervals, aliquots of the suspension were overlaid onto dibutylphtalate, centrifuged, and treated as described above for radioactive counting.
Electron microscopy.
Parasitized erythrocytes were resuspended in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) at room temperature for 2 h, followed by two washes in the same buffer. The cells were then postfixed with osmic acid for 2 h in 1% OsO4 in the same buffer. After dehydration in a graded series of ethanol followed by propylene oxide, the cells were embedded in Epon. Sections were prepared on a Leica Ultracut microtome, contrasted with uranyle acetate and lead citrate, and observed with a JEOL 1200 EX electron microscope operated at 80 kV.
Subcellular fractionation using discontinuous sucrose gradients.
The subcellular localization of accumulated radioactive chemicals was analyzed on isolated parasites homogenized by sonication. Uninfected or infected erythrocyte suspensions (7 to 12% parasitemia, 5% hematocrit, 18 × 108 to 22 × 108 total cells for P. falciparum, 19 to 36% parasitemia, 5% hematocrit, 14 × 108 to 18 × 108 total cells for B. divergens) were incubated for 2 h at 37°C with 50 nM of [3H]T16 or [3H]CQ in complete medium and then washed in complete medium. A fraction of the uninfected or infected cell suspension was then centrifuged through dibutylphtalate to measure the cellular accumulation. Parasites were then isolated using either saponin or streptolysin O lysis. For saponin lysis, infected cells were resuspended at a hematocrit of 10% in 0.06% (wt/vol) saponin for 5 min at 4°C and centrifuged at 4,000 × g for 10 min. For SLO lysis, cells were resuspended at an SLO/cell ratio of 3 hemolytic units and incubated for 6 min at room temperature (6). The pellet with parasites was washed twice and finally resuspended in 2 ml of homogenization buffer (TE-S; 5 mM triethanolamine-HCl, pH 7.5, 1 mM ethylenediaminetetracetic acid, 0.2 M sucrose). One milliliter of parasite suspension was sonicated for 30 s on ice in a probe-type sonicator (Vibra Cell; Bioblock Scientific) with 15 pulses of 1 second (300 W each) at 1-s intervals. Homogenates (1 ml) were then applied to a discontinuous sucrose gradient composed of five cushions (1 ml each) of 0.2, 0.8, 1.2, 1.6, and 2.0 M sucrose prepared in TE buffer. After centrifugation for 1 h at 100,000 × g, the phases were collected and processed for radioactive counting. Hemozoin crystals but not FPIX were sedimented to the 2.0 M fraction (8).
Assay of phospholipid biosynthesis in Babesia- and Plasmodium-infected erythrocytes.
The effect of T16 on the incorporation of [3H]choline or [3H]ethanolamine into phospholipids of P. falciparum- and B. divergens-infected erythrocytes was monitored in microtiter plates as previously described (5). Infected erythrocyte suspensions (100 μl, 2 × 106 to 3 × 106 infected cells/assay, 10% parasitemia) were mixed with 50 μl of modified RPMI 1640 (without choline) with or without the drug. After 30 min at 37°C, 20 μM [3H]choline (2 μCi/well) or 2 μM [3H]ethanolamine (1 μCi/well) was added (50 μl) for 3 h using the candle jar method (38). Incorporation of [3H]hypoxanthine (0.7 μCi/well) into nucleic acids was monitored in the same experiment to determine specific effects.
RESULTS
The bisthiazolium T16 has potent antibabesial and antimalarial activities.
We determined the in vitro antiparasitic activity after a full cycle of contact between the compounds and parasite-infected erythrocytes (Table 1). After 8 h of contact, the bisthiazolium compound T16 exhibited potent antibabesial activity, with IC50s of 28.3 nM and 7 nM against B. divergens and B. canis, respectively. T16 also had potent antimalarial activity against the erythrocytic stage of P. falciparum, with an IC50 of 1.9 nM. Imidocarb and pentamidine, the two drugs currently used against Babesia, had an IC50 of 100 nM against B. divergens and also presented a higher potency against P. falciparum, with IC50s of 30 nM and 17 nM, respectively. On the other hand, chloroquine (CQ) had very weak activity against B. divergens (IC50 of 8 μM) while exerting potent activity against P. falciparum (IC50 of 51 nM). Both T16 and CQ exert moderate toxic effect against the human Jurkat lymphoblast cell lines, with IC50s of 34 μM and 22 μM, respectively.
TABLE 1.
In vitro growth inhibition of B. divergens, B. canis, and P. falciparum parasites as well as human Jurkat lymphoblasts against antihematozoan drugsa
| Drug | IC50 (nM) against:
|
|||
|---|---|---|---|---|
| B. divergens | B. canis | P. falciparum | Jurkat lymphoblasts | |
| T16 | 28.3 ± 2 (3) | 7 | 1.9 ± 0.3 (5) | 34,330 ± 3,330 (3) |
| Chloroquine | 8,000 | ND | 51.0 ± 0.7 (3) | 21,600 ± 1,600 (5) |
| Pentamidine | 100 | ND | 17 | ND |
| Imidocarb | 100 | ND | 30 | ND |
Cells were incubated with drugs for 8 h (B. divergens and B. canis), 48 h (P. falciparum), or 24 h (human Jurkat lymphoblasts) before adding [3H]hypoxanthine for parasite-infected cells or [3H]thymidine for Jurkat lymphoblasts to determining cell viability. Values are means of at least three independent experiments (indicated in parentheses) conducted at least in duplicate, using different stock drug solutions and different batches of infected erythrocytes (standard errors of the means are indicated). Otherwise, values are means of two independent experiments conducted in triplicate, differing by less than 40%. ND, not done.
T16 is specifically accumulated in Babesia- and Plasmodium-infected erythrocytes.
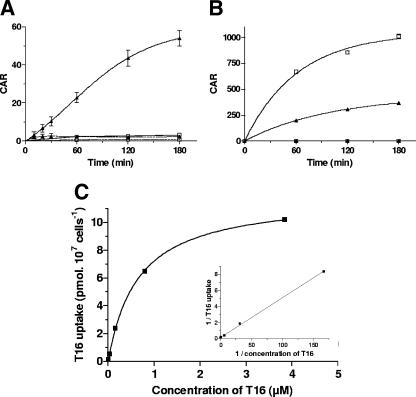
We evaluated the interaction of T16 with the parasitized erythrocytes by measuring the drug concentration in the infected cells. At 37°C, T16 (50 nM) was highly accumulated into B. divergens-infected erythrocytes (Fig. 1A), reaching a CAR of 54 after 3 h. No significant uptake of T16 could be measured at 4°C. At 37°C, [3H]T16 uptake gradually increased until 2 h, after which it reached a plateau indicating that entry of the bisthiazolium was saturable. T16 was not significantly accumulated into uninfected erythrocytes, which showed a CAR of ∼2.2, suggesting that the process responsible for drug accumulation is due to the intracellular parasite. CQ did not accumulate significantly into B. divergens-parasitized erythrocytes, the CAR being lower than 3.0 after 3 h of incubation (Fig. 1A).
FIG. 1.
Kinetics of T16 and CQ accumulation. Human erythrocytes parasitized (solid lines) by (A) B. divergens or (B) P. falciparum and control human uninfected erythrocytes (dashed lines) were incubated with 50 nM [3H]T16 (▴) or [3H]CQ (□) at 37°C. Cellular accumulation ratios (CAR) are the means ± standard errors of the means of two independent experiments (small standard errors of the means are hidden by the symbols), each measure being performed in triplicate. (C) Concentration dependence of T16 accumulation into B. divergens-infected erythrocytes. Cells were incubated for 1 h at 37°C in the presence of T16 concentrations ranging from 5 nM to 4 μM. The insert shows the Lineweaver-Burk representation of drug accumulation.
For comparison, we measured T16 and CQ accumulation in P. falciparum-parasitized erythrocytes. Plasmodium-infected cells accumulated T16 to a large extent, with a CAR of 365 after 3 h of incubation. CQ was accumulated into P. falciparum-parasitized erythrocytes to the largest extent, with a CAR higher than 1,000 after 3 h of incubation (Fig. 1B). These accumulations were also strictly temperature dependent (data not shown). We finally measured the interaction of T16 with a mammalian cell line. When incubated for 2 h with 50 nM [3H]T16, there was no significant accumulation in the Jurkat lymphoblasts (CAR of 1.3), emphasizing the selectivity of T16 for the hematozoan-parasitized erythrocytes.
[3H]T16 accumulation in Babesia-infected erythrocytes followed a Michaelis-Menten kinetic, with an apparent Km of 0.65 μM and a maximum velocity (Vmax) for [3H]T16 accumulation of 11.9 pmol/107 infected cells/h (Fig. 1C).
A chase of [3H]T16 by untritiated T16 was only observed in P. falciparum-parasitized erythrocytes.
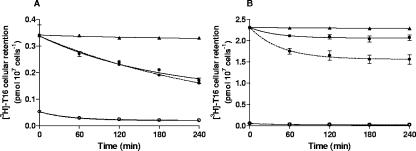
To investigate if the T16 uptake was reversible, we incubated infected cells at 37°C for 2 h with 50 nM [3H]T16. After preloading, cells were washed twice and resuspended in prewarmed complete medium with or without 200 nM untritiated T16. [3H]T16 cellular retention was measured for various times. In B. divergens, a fraction of accumulated T16 was slowly and partially released under all conditions (Fig. 2A). Infected cells retained 73% ± 2% and 57% ± 3% of the accumulated radioactive T16 after 2 and 4 h at 37°C. At 4°C, no T16 release was observed. The addition of 200 nM unlabeled T16 in the medium did not induce further loss of the intracellular radioactivity; infected erythrocytes still retained 55% ± 3% of [3H]T16 after 4 h (Fig. 2A).
FIG. 2.
Cellular retention of [3H]T16 in hematozoan-parasitized (•) or unparasitized (○) erythrocytes. (A) B. divergens- and (B) P. falciparum-parasitized erythrocytes or control unparasitized erythrocytes were preloaded with [3H]T16 (50 nM for 2 h at 37°C) and then washed and resuspended in complete culture medium with (dashed lines) or without (solid lines) 200 nM of nonradioactive T16. Plots show [3H]T16 remaining in the erythrocytes at the specified times. [3H]T16 cellular retention at 4°C in parasitized erythrocytes (▴) is also shown. Data are the means ± standard errors of the means of three independent experiments (small standard errors of the means are hidden by the symbols), each measure being performed in triplicate. For P. falciparum-infected erythrocytes, cellular retention of preaccumulated [3H]T16 at each time point was significantly altered by the presence of 200 nM external nonradioactive T16 (P < 0.1, Student's t test).
For P. falciparum-infected erythrocytes, in the absence of T16 in the medium a very small release of radioactive T16 was observed (Fig. 2B), since the infected cells retained 83% ± 3% of the loaded radioactivity after 4 h of incubation. Contrasting with Babesia, the addition of 200 nM T16 in the medium significantly increased the loss of cellular radioactivity by almost 40%.
Subcellular distribution of T16 is different between B. divergens and P. falciparum parasites.
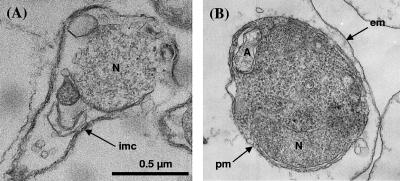
In order to compare [3H]T16 distribution in Babesia and Plasmodium, similar methods for parasite isolation were adopted. Saponin and SLO have been the most common substances used to rupture or to permeabilize the malaria-infected erythrocyte, whereas little has been published for Babesia-infected red blood cells. Saponin lysis destroyed Babesia and therefore could not be used (Fig. 3A). In contrast, the SLO procedure developed for Plasmodium-infected erythrocytes (6) was very effective for isolating intact B. divergens parasites. Light and electron microscopy of SLO-freed Babesia showed the integrity of free parasites (Fig. 3B), and spectrophotometric analysis at 412 nm showed that more than 97% of the hemoglobin had been released (data not shown). We therefore routinely applied SLO lysis of the erythrocytes to study the intracellular distribution of the compounds in both Babesia- and Plasmodium-infected erythrocytes.
FIG. 3.
Electron microscopy of intraerythrocytic B. divergens released from parasitized erythrocytes. Infected cell suspensions were treated by (A) saponin or (B) streptolysin O. (A) The parasite pellicle has been damaged by the saponin treatment, and the cytosol has been lost. (B) The parasite is intact within the hemolyzed erythrocyte. pm, parasite plasma membrane; em, erythrocyte membrane; imc, inner membrane complex; N, parasite nucleus; A, apicoplast.
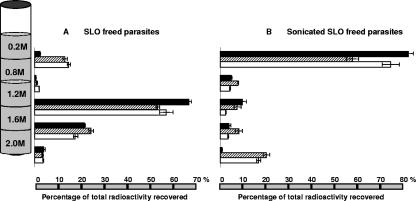
We then analyzed the distribution on discontinuous sucrose gradients of the accumulated radioactive compounds in free parasites isolated by SLO (Fig. 4A) and in parasites homogenized by sonication (Fig. 4B). The gradient using intact SLO-freed parasites showed a dense layer of parasites and a sedimentation of radioactivity at the 1.2 to 1.6 M and 1.6 to 2.0 M interfaces (Fig. 4A).
FIG. 4.
Distribution on discontinuous sucrose gradients of the accumulated compounds after incubation with 50 nM of T16 or CQ for 2 h at 37°C. B. divergens-infected erythrocytes were incubated with [3H]T16 (solid bars), and P. falciparum erythrocytes were incubated with [3H]T16 (hashed bars) or [3H]chloroquine (blank bars). Parasitized erythrocytes were then permeabilized with streptolysin O, and freed parasites collected by centrifugation. (A) SLO-freed parasites were directly overlaid onto discontinuous sucrose gradients (depicted schematically at the left of the figure). (B) Streptolysin O-freed parasites were sonicated and overlaid onto gradients. Each value is the means ± standard errors of the means of three independent experiments, each measure being performed in triplicate.
Most of the [3H]T16 radioactivity (81% ± 2.1%) associated with sonicated Babesia was recovered in the upper fraction, meaning that it was in a soluble form, free form, or protein-associated form, or it was associated with very light membranes floating over 0.8 M sucrose (Fig. 4B). Minor amounts of radioactivity were found at the other interfaces, likely arising from contamination from the upper interface for the 0.8 to 1.2 M interface and from incompletely lysed parasites for the 1.2 to 1.6 M and 1.6 to 2.0 M interfaces.
Performing the same fractionation with sonicated P. falciparum parasites that had been preincubated with [3H]T16 led to a different pattern. A large part of the radioactivity was recovered in the upper fraction (57% ± 2.7%), but another significant part of the radioactive drug (20% ± 1.4%) was found in the bottom of the tube associated with a dark pellet of hemozoin (Fig. 4B). Less than 10% of the radioactivity was recovered at each of the other interfaces, probably resulting from contamination or incomplete lysis as explained above. When Plasmodium-infected erythrocytes were incubated with [3H]CQ, the distribution of radioactivity in the gradient after sonication of free parasites was similar to the one described above for T16: 74% ± 3.7% of CQ was found in the overlay, and 17% ± 1.8% was found in the pellet where CQ was associated with a dark pellet of hemozoin, as described previously (37).
T16 selectively inhibits de novo phosphatidylcholine biosynthesis of both parasites.
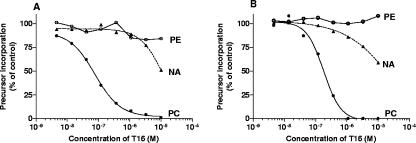
We investigated the effects of T16 on the de novo biosynthesis of PC and phosphatidylethanolamine and also on the biosynthesis of nucleic acids to determine a possible specific effect. Figure 5A shows a typical dose-response curve of T16 for B. divergens which quite specifically inhibits choline incorporation into PC. PC50, the drug concentration that reduces the amount of synthesized PC by 50%, is 69 nM after 3 h of incubation. At 1 μM of T16, incorporation of labeled choline into PC was nearly totally inhibited. By contrast, no inhibition of nucleic acids and phosphatidylethanolamine biosynthesis was observed until 1 μM. This strongly suggests that T16 acts as a choline analog by inhibiting choline incorporation into PC.
FIG. 5.
Effects of T16 on choline, ethanolamine, and hypoxanthine incorporation into phosphatidylcholine (PC), phosphatidylethanolamine (PE), and nucleic acids (NA), respectively. (A) B. divergens-infected erythrocytes or (B) P. falciparum-infected erythrocytes were incubated for 3.5 h at 37°C in the absence (control) or in the presence of T16 at the indicated concentrations. Radioactive precursors were added at 30 min: •, 2 μCi of [3H]choline (at 20 μM); ○, 1 μCi of [3H]ethanolamine (at 2 μM); ▴, 0.7 μCi of [3H]hypoxanthine.
For P. falciparum, the dose-response curve was very similar (Fig. 5B). Once again, T16 specifically inhibits choline incorporation, and with 1 μM of T16, PC biosynthesis was totally inhibited. Similar to B. divergens, we observed no inhibition of ethanolamine and hypoxanthine incorporation until 1 μM of T16. Together, these results indicate that T16 specifically inhibits the de novo PC biosynthesis (the Kennedy pathway) both in B. divergens parasites (PC50 of 69 nM after 3 h of exposure) and in Plasmodium (PC50 of 180 nM after 3 h of exposure) parasites.
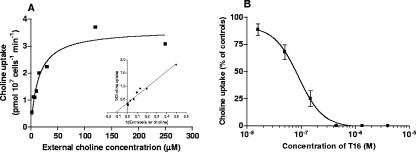
In erythrocyte-freed Babesia parasites, choline uptake was temperature dependent and linear as a function of time for at least 20 min. When measured as a function of concentration, the saturable uptake of choline was observed to exhibit Michaelis-Menten kinetics with an apparent Km for choline of 8.5 ± 1.8 μM (n = 4), reaching a maximum velocity (Vmax) of 3.47 ± 1.22 pmol choline/107 cells/min. At a physiological choline concentration of 15 μM, T16 was shown to exhibit a potent and concentration-dependent inhibition of choline uptake, with an IC50 of 89.4 ± 20.0 nM (n = 3) (Fig. 6).
FIG. 6.
Kinetics of choline uptake into free parasites of B. divergens and inhibition by T16. (A) SLO-freed parasites were incubated for 10 min at 37°C into special RPMI with [14C]choline (6.72 to 50.4 nCi/nmol) (2.107 cells in a final volume of 200 μl) and then isolated using a dibutylphthalate/dioctylphthalate oil cushion. Kinetics parameters were means of four independent experiments, each conducted in duplicate. Data shown are from a typical experiment fitted by nonlinear regression analysis for a Michaelis-Menten plot and by linear regression for a Lineweaver-Burke plot. (B) Concentration-dependent inhibition of choline uptake by T16. T16 compound was preincubated with free parasites for 5 min before the addition of 15 μM [14C]choline over 10 min at 37°C. Data are means ± standard errors of the means from three individual experiments, each in duplicate.
DISCUSSION
Bisquaternary ammonium compounds are potent inhibitors of choline transporters in a variety of eukaryotic cells (8, 15, 47). A structure-optimized combinatorial chemistry approach has led to the design of compounds with great potency against malaria (11, 12) and promising therapeutic potential for treatment of multidrug-resistant malaria (2, 45, 47). Leading molecules are bisthiazolium salts (and related prodrugs) with optimum structures for antimalarial activity, tolerance, and specificity (22, 34, 45).
Radiolabeled T16, a bisthiazolium compound, was recently shown to accumulate several hundredfold in malaria-infected erythrocytes (7). T16 was shown to accumulate in the parasite food vacuole by virtue of its interaction with heme. The interaction of T16 with heme was also found to be important for the antimalarial activity of the drug (7). It is possible that T16 might have dual modes of action against malarial parasites, because many earlier studies have shown that similar bisquaternary ammoniums (1, 3, 47) as well as the bisthiazoliums (8, 45) directly inhibit choline transport and parasite phospholipid biosynthesis. At the moment it is not clear whether the antiparasitic activity of these compounds is due to an interaction with heme or due to the inhibition of phospholipid biosynthesis.
With this in mind, we investigated possible activity of T16 against Babesia, a malaria-like protozoan that also infects erythrocytes and may synthesize phospholipid de novo but does not digest hemoglobin, lacks a central food vacuole, and does not produce hemozoin (23, 30, 31, 36).
T16 displays potent antibabesial activity.
We first examined the effect of CQ against B. divergens. CQ is one of the most widely used antimalarial drugs, with low-nanomolar activity against susceptible strains of malaria. The interaction of CQ with the hemoglobin degradation metabolites FPIX and hemozoin in the malarial food vacuole is considered to be the fundamental feature of its antimalarial specificity (10, 35, 37). Not surprisingly, CQ displays poor activity against B. divergens (IC50 = 8 μM). This is consistent with the absence of a hemoglobin-degrading food vacuole in these parasites.
In contrast to CQ, T16 exerts potent activity against Babesia during its asexual intraerythrocytic cycle. The antibabesial activity (IC50s of 28 and 7 nM against B. divergens and B. canis, respectively) is similar but slightly less potent than the activity against the human malarial parasite P. falciparum (IC50 of 1.9 nM) (Table 1). Since the activity of T16 against the mammalian Jurkat lymphoblast cell line is in the micromolar range (IC50 of 34 μM), T16 clearly exerts selective toxicity against the erythrocyte-invading hematozoans. The in vitro therapeutic indices of T16 are ∼860 and ∼20,000 for B. divergens and P. falciparum, respectively. Most remarkably, antibabesial activity of T16 was higher than that of the index antibabesial drugs, such as imidocarb and pentamidine (IC50 = 100 nM against B. divergens; data not shown).
One prominent feature of bisammoniums is their ability to accumulate several hundredfold in the malaria-infected erythrocyte. This was observed both with VB5, a tritium-labeled bisquaternary ammonium salt analog of G25 (47), and with the bisthiazolium T16 (Fig. 1B). Significant amounts of the drug were found in the Plasmodium food vacuole, where the radiolabel was found to associate with FPIX and hemozoin. This binding was shown to be an important factor in driving high levels of drug accumulation in this parasite (7).
Here we show that accumulation of T16 also occurs in B. divergens-infected erythrocytes. The accumulation process is both temperature dependent and saturable, with an apparent Km of 0.65 μM and a Vmax of 11.9 pmol/107 cells/h. By contrast, T16 was not significantly accumulated in uninfected erythrocytes (CAR < 2.2) or in human Jurkat lymphoblasts (CAR of 1.3), indicating that the drug accumulation process is linked to the intraerythrocytic parasite.
Saturable T16 uptake: drug transport or intracellular binding?
The accumulation of T16 in B. divergens is significantly lower than in P. falciparum (CAR of ∼60 for Babesia versus ∼500 for Plasmodium). This is in good agreement with the reduced activity of the drug against B. divergens relative to that against P. falciparum (28 nM versus 1.9 nM). The greater accumulation of T16 that is seen in P. falciparum can probably be attributed to the high-affinity binding of the drug to FPIX: Babesia parasites do not have a food vacuole and do not produce FPIX or hemozoin. Clearly the saturable uptake of T16 in B. divergens is not driven by drug-FPIX binding as it is in P. falciparum.
We have shown recently that the transport of T16 across the plasma membrane of the intraerythrocytic P. falciparum parasite is mediated by a parasite plasma membrane organic cation transporter that also mediates the transport of choline (8, 28). Could a similar transporter be responsible for the saturable uptake of T16 in B divergans? Temperature dependence, saturability, and kinetic parameters (Table 2) of T16 uptake into Babesia could reflect carrier-mediated transport, but they could also reflect the binding of T16 to intracellular receptors. In this respect, the apparent association of T16 with the light membrane fraction (Fig. 4B) is interesting. This observation raises the possibility that T16 and similar compounds are intercalated into phospholipid membranes. If so, this interaction would be specific to hematozoan membranes, since it does not occur in other eukaryotic cells (data not shown). On the other hand, the inhibition of T16 accumulation by choline (50% at 1 mM choline; data not shown) suggests that uptake might possibly be mediated by a choline or organic cation transporter similar to that described for P. falciparum (8, 9). The experimental approach taken here does not at the moment allow us to distinguish between transport processes and other saturable processes that might lead to drug being accumulated. Further experiments are required to resolve these issues.
TABLE 2.
Summary of properties associated with the pharmacological activity of bisthiazoliums against the two hematozoan parasites
| Parasite | IC50 for growth inhibition (nM) | T16 accumulation (CAR) into infected erythrocytes | T16 uptake into infected erythrocytes
|
Exchangeability | Proportion of T16 (%)
|
Interaction with FPIX/hemozoin | PC50 after 3 h | Kinetics for choline transport into free parasites
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apparent Km (μM) | Vmax (pmol/107 infected cells/min) | Inside the intracellular parasite | In acidic food vacuole | Associated with hemozoinb | Apparent Km (μM) | Vmax (pmol 107 infected cells/min) | T16 inhibition (IC50) | ||||||
| Babesia divergens | 28 | >60 | 0.65 | 0.20 | No | ∼80 | Not applicable | Not applied (<1) | Not applied | Yes; specific (69 nM) | 8.5 ± 1.8 | 3.47 ± 1.22 | Yes (89 ± 20 nM) |
| Plasmodium falciparum | 1.9 | >600a | 0.73 ± 0.26 | Partial (until 40%) | 50-80 | ∼40a | >20 | Yes, likely to contribute to antimalarial activityc | Yes; specific (180 nM) | 25.0 ± 3.5c | 46 ± 2c | Yes (140 μM)c | |
In summary, in this study we have demonstrated the very potent antibabesial activity of the bisthiazolium salt T16. The interaction of T16 with Babesia was investigated and compared to that in Plasmodium-infected erythrocytes (Table 2). A saturable, choline-inhibitable high-affinity uptake process appears to underpin the potency and specificity of this drug for hematozoan parasites. The antibabesial effect and its accumulation inside the Babesia-infected erythrocytes cannot be explained by an interaction with FPIX or hemozoin. Inhibition of the choline transport into the intraerythrocytic parasite and early alteration of the de novo PC biosynthesis occurs into Babesia-parasitized erythrocytes as it does for Plasmodium. PC is an essential component of lipids and is the most abundant phospholipid synthesized during the development of Babesia (16, 40), suggesting that the observed antibabesial activity derives from the inhibition of PC biosynthesis. A formulation of this class of compound is now entering preclinical development as an antimalarial. Altogether, this class of compounds might offer some unique advantages over more traditional antimalarial drugs. For example, one might envisage that resistance to a drug that had two distinct modes of action against a single organism might develop more slowly. Application for antibabesial activity might prove to be an additional and fruitful avenue of opportunity for this compound.
Supplementary Material
Acknowledgments
J. L. Morgat, M. Maynadier, and Y. Bordat are gratefully acknowledged for their expertise and skillful work.
Studies were supported by Centre National de la Recherche Scientifique (CNRS) and the European Union (QLK2-CT-2000-01166, FP6 Network of Excellence BioMalPar and the Integrated Project Antimal). P.G.B. and S.A.W. are supported by the BBSRC and the Wellcome Trust. G.A.B. is an Early Career Leverhulme Fellow.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Ancelin, M. L., M. Calas, J. Bompart, G. Cordina, D. Martin, M. Ben Bari, T. Jei, P. Druilhe, and H. Vial. 1998. Antimalarial activity of 77 phospholipid head analogs: close correlation between inhibition of phospholipid metabolism and in vitro Plasmodium falciparum growth. Blood 91:1426-1437. [PubMed] [Google Scholar]
- 2.Ancelin, M. L., M. Calas, A. Bonhoure, S. Herbute, and H. Vial. 2003. In vivo antimalarial activities of mono- and bisquaternary ammonium salts interfering with Plasmodium phospholipid metabolism. Antimicrob. Agents Chemother. 47:2598-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ancelin, M. L., M. Calas, V. Vidal-Sailhan, S. Herbute, P. Ringwald, and H. Vial. 2003. Potent inhibitors of Plasmodium falciparum metabolism with a broad spectrum of in-vitro antimalarial activities. Antimicrob. Agents Chemother. 47:2590-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ancelin, M. L., M. Parant, M. J. Thuet, J. Philippot, and H. Vial. 1991. Increased permeability to choline in simian erythrocytes after Plasmodium knowlesi infection. Biochem. J. 273:701-709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ancelin, M. L., F. Vialettes, and H. Vial. 1991. An original method for rapid serial determination of phospholipid biosynthesis. Applications to mammalian lymphocytic cells and a lower eucaryote, Plasmodium falciparum. Anal. Biochem. 199:203-209. [DOI] [PubMed] [Google Scholar]
- 6.Ansorge, I., J. Benting, S. Bhakdi, and K. Lingelbach. 1996. Protein sorting in Plasmodium falciparum-infected red blood cells permeabilized with the pore-forming protein streptolysin O. Biochem. J. 315:307-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biagini, G., E. Richier, P. G. Bray, M. Calas, H. Vial, and S. Ward. 2003. Heme binding contributes to antimalarial activity of bis-quaternary ammoniums. Antimicrob. Agents Chemother. 47:2584-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biagini, G. A., E. M. Pasini, R. Hughes, H. P. De Koning, H. J. Vial, P. M. O'Neill, S. A. Ward, and P. G. Bray. 2004. Characterization of the choline carrier of Plasmodium falciparum: a route for the selective delivery of novel antimalarial drugs. Blood 105:3372-3377. [DOI] [PubMed] [Google Scholar]
- 9.Biagini, G. A., S. A. Ward, and P. G. Bray. 2005. Malaria parasite transporters as a drug-delivery strategy. Trends Parasitol. 21:299-301. [DOI] [PubMed] [Google Scholar]
- 10.Bray, P. G., O. Janneh, K. J. Raynes, M. Mungthin, H. Ginsburg, and S. Ward. 1999. Cellular uptake of chloroquine in dependant on binding to FPIX and is independent of NHE activity in Plasmodium falciparum. J. Cell Biol. 145:363-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calas, M., M. L. Ancelin, G. Cordina, P. Portefaix, G. Piquet, V. Vidal-Sailhan, and H. Vial. 2000. Antimalarial activity of compounds interfering with Plasmodium falciparum phospholipid metabolism: comparison between mono- and bisquaternary ammonium salts. J. Med. Chem. 43:505-516. [DOI] [PubMed] [Google Scholar]
- 12.Calas, M., G. Cordina, J. Bompart, M. Bari, T. Jei, M. L. Ancelin, and H. Vial. 1997. Antimalarial activity of molecules interfering with Plasmodium falciparum phospholipid metabolism. Structure-activity relationship analysis. J. Med. Chem. 40:3557-3566. [DOI] [PubMed] [Google Scholar]
- 13.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fast, N., L. Xue, S. Bingham, and P. Keeling. 2002. Re-examining alveolate evolution using multiple protein molecular phylogenies. J. Eukaryot. Microbiol. 49:30-37. [DOI] [PubMed] [Google Scholar]
- 15.Fischer, A., and I. Hanin. 1980. Choline analogs as potential tools in developing selective animal models of central cholinergic hypofunction. Life Sci. 27:1615-1634. [DOI] [PubMed] [Google Scholar]
- 16.Florin-Christensen, J., C. E. Suarez, M. Florin-Christensen, S. A. Hines, T. F. Mcelwain, and G. H. Palmer. 2000. Phosphatidylcholine formation is the predominant lipid biosynthetic event in the hemoparasites Babesia bovis. Mol. Biochem. Parasitol. 106:147-156. [DOI] [PubMed] [Google Scholar]
- 17.Florin-Christensen, J., C. E. Suarez, M. Florin-Christensen, M. Wainszelbaum, W. C. Brown, T. F. Mcelwain, and G. H. Palmer. 2001. A unique phospholipid organization in bovine erythrocyte membranes. Proc. Natl. Acad. Sci. USA 98:7736-7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorenflot, A., P. Brasseur, E. Precigout, M. L'Hostis, A. Marchand, and J. Schrevel. 1991. Cytological and immunological responses to Babesia divergens in different hosts: ox, gerbils, man. Parasitol. Res. 77:3-12. [DOI] [PubMed] [Google Scholar]
- 19.Gorenflot, A., K. Moubri, E. Precigout, B. Carcy, and P. Schetters. 1998. Human babesiosis. Ann. Trop. Med. Parasitol. 92:489-501. [DOI] [PubMed] [Google Scholar]
- 20.Gray, J., L. V. Von Stedingk, and M. Grandstom. 2002. Zoonotic babesiosis. Int. J. Med. Microbiol. 291:108-111. [DOI] [PubMed] [Google Scholar]
- 21.Gumila, C., M. L. Ancelin, G. Jeminet, A. M. Delort, G. Miquel, and H. Vial. 1996. Differentiel in-vitro activities of ionophore compounds against Plasmodium falciparum and mammalian cells. Antimicrob. Agents Chemother. 40:602-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamze, A., E. Rubi, P. Arnal, M. Boisbrun, C. Carcel, X. Salom-Roig, M. Maynadier, S. Wein, H. Vial, and M. Calas. 2005. Mono- and bis-thiazolium salts have potent antimalarial activity. J. Med. Chem. 48:3639-3643. [DOI] [PubMed] [Google Scholar]
- 23.Healy, G. R., and T. K. Ruebusch. 1980. Morphology of Babesia microti in human blood smears. Am. J. Clin. Pathol. 73:107-109. [DOI] [PubMed] [Google Scholar]
- 24.Homer, J. M., I. Aguilar-Delfin, S. R. Telford III, P. J. Krause, and D. H. Persing.2000. Babesiosis. Clin. Microbiol. Rev. 13:451-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen, J., and W. Trager. 1977. Plasmodium falciparum in culture: use of outdated erythrocytes and description of the candle jar method. J. Parsitol. 63:883-886. [PubMed] [Google Scholar]
- 26.Kjemtrup, A. M., and P. A. Conrad. 2000. Human babesiosis: an emerging tick-borne disease. Int. J. Parasitol. 30:1323-1337. [DOI] [PubMed] [Google Scholar]
- 27.Krause, P. J. 2003. Babesiosis diagnosis and treatment. Vector Borne Zoonotic Dis. 3:45-51. [DOI] [PubMed] [Google Scholar]
- 28.Lehane, A. M., K. J. Saliba, R. J. Allen, and K. Kirk. 2004. Choline uptake into the malaria parasite is energized by the membrane potential. Biochem. Biophys. Res. Commun. 320:311-317. [DOI] [PubMed] [Google Scholar]
- 29.Morrissette, N. S., and D. Sibley. 2002. Cytoskeleton of apicomplexan parasites. Microbiol. Mol. Biol. Rev. 66:21-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Olliaro, P., and D. E. Goldberg. 1995. The Plasmodium digestive vacuole: metabolic headquarters and choice drug target. Parasitol. Today 11:294-297. [DOI] [PubMed] [Google Scholar]
- 31.Rudzinska, M. A. 1976. Ultrastructure of intraerythrocytic Babesia microti with emphasis on the feeding mechanism. J. Protozool. 23:224-233. [DOI] [PubMed] [Google Scholar]
- 32.Rudzinska, M. A., W. Trager, S. Lewengrub, and E. Gubert. 1976. An electron microscopic study of Babesia microti invading erythrocytes. Cell Tissue Res. 169:323-334. [DOI] [PubMed] [Google Scholar]
- 33.Saliba, K. J., H. A. Horner, and K. Kirk. 1998. Transport and metabolism of the essential vitamin pantothenic acid in human erythrocytes infected with the malaria parasite Plasmodium falciparum. J. Biol. Chem. 273:10190-10195. [DOI] [PubMed] [Google Scholar]
- 34.Salom-Roig, X. J., A. Hamze, M. Calas, and H. J. Vial. 2005. Dual molecules as new antimalarials. Comb. Chem. High Throughput Screen. 8:49-62. [DOI] [PubMed] [Google Scholar]
- 35.Slater, A. F., and A. Cerami. 1992. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature 355:167-169. [DOI] [PubMed] [Google Scholar]
- 36.Slomianny, C., P. Charet, and G. Prensier. 1983. Ultrastructural localization of enzymes involved in the feeding process in Plasmodium chabaudi and Babesia hylomysci. J. Protozool. 30:376-382. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan, D. J., I. Y. Gluzman, D. G. Russell, and D. E. Goldberg. 1996. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. USA 93:11865-11870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trager, W., and J. Jensen. 1976. Human malaria parasites in continuous culture. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 39.Uilenberg, G., F. Franssen, N. Perie, and A. Spanjer. 1989. Three groups of Babesia canis distinguished and a proposal for nomenclature. Vet. Q. 11:33-40. [DOI] [PubMed] [Google Scholar]
- 40.Valentin, A., D. Rigomier, E. Precigout, B. Carcy, A. Gorenflot, and J. Schrevel. 1991. Lipid trafficking between high density lipoproteins and Babesia divergens-infected human erythrocytes. Biol. Cell. 73:63-70. [DOI] [PubMed] [Google Scholar]
- 41.Vial, H., M. L. Ancelin, J. Philippot, and M. J. Thuet. 1990. Biosynthesis and dynamics of lipids in Plasmodium-infected mature mammalian erythrocytes. Blood Cells 16:531-555. [PubMed] [Google Scholar]
- 42.Vial, H., P. Eldin, A. Tielens, and J. Hellemond. 2003. Phospholipids in parasitic protozoa. Mol. Biochem. Parasitol. 126:143-154. [DOI] [PubMed] [Google Scholar]
- 43.Vial, H., and C. Mamoun. 2005. Plasmodium lipids: metabolism and function, p. 327-352. In I. Sherman (ed.), Molecular approach to malaria. ASM Press, Washington, D. C.
- 44.Vial, H. J., and M. Calas. 2001. Inhibitors of phospholipid metabolism, p. 347-365. In P. Rosenthal (ed.), Antimalarial chemotherapy, mechanisms of action, modes of resistance, and new directions in drug development. Humana Press, Totowa, N. J.
- 45.Vial, H. J., S. Wein, C. Farenc, C. Kocken, O. Nicolas, M. L. Ancelin, F. Bressolle, A. Thomas, and M. Calas. 2004. Prodrugs of bisthiazolium salts are orally potent antimalarials. Proc. Natl. Acad. Sci. USA 101:15458-15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiss, L. M. 2002. Babesiosis in humans: a treatment review. Exp. Opin. Pharmacother. 3:1109-1115. [DOI] [PubMed] [Google Scholar]
- 47.Wengelnik, K., V. Vidal-Sailhan, M. L. Ancelin, A.-M. Cathiard, J.-L. Morgat, C. H. Kocken, M. Calas, S. Herrera, A. W. Thomas, and H. Vial. 2002. A class of potent antimalarials and their specific accumulation in infected erythrocytes. Science 295:1311-1314. [DOI] [PubMed] [Google Scholar]
- 48.Zintl, A., G. Mulcahy, H. E. Skerett, S. M. Taylor, and J. S. Gray. 2003. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin. Microbiol. Rev. 16:622-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.